Abstract
The functional identity of an olfactory receptor neuron is determined in part by its repertoire of responses to odorants. As an approach toward understanding the contributions of particular conductances to olfactory neuron excitability and odor discrimination, we have investigated the role of the putative cyclic nucleotide-modulated K+ channel subunit encoded by the ether a go-go (eag) gene in odorant responsiveness in Drosophila melanogaster. Four independent mutant eag alleles exhibited reduced antennal sensitivity to a subset of nine odorants, all having short aliphatic side chains: ethyl butyrate (EB), propionic acid, 2-butanone, and ethyl acetate. Significantly fewer eag antennal neurons responded to EB compared with control neurons; the proportion sensitive to 2-heptanone was similar to controls. Two aspects of the character of EB-induced excitability were affected by mutations ineag. First, fewer EB-induced inhibitory responses were observed in eag mutants, and second, fewer excitatory odorant responses dependent on extracellular Ca2+were observed. Furthermore, modulation of neuronal excitability by membrane-permeant cyclic nucleotide analogs was largelyeag dependent. Focal application of high K+ saline to sensillae altered the excitability of the majority of neurons from wild-type but not eagantennae, suggesting that Eag may have a dendritic localization.
Keywords: ether a go-go, eag, potassium channel, Drosophila, electroantennogram, specific, Ca2+, cyclic nucleotide analogs, mutant, olfaction
The ability to identify and discriminate among odorants is important for the survival of many animal species and depends in part on the contributions of odorant-modulated conductances to sensory cell excitability. Diverse mechanisms contribute to the initial events underlying the transduction of odorants in primary olfactory receptor neurons from vertebrates and invertebrates (Dionne and Dubin, 1994; Ache and Zhainazarov, 1995;Buck, 1996). In one mechanism, odorant binding to G-protein-coupled receptors expressed on the cilia and apical dendrites of sensory neurons activates adenylyl cyclase, which increases intracellular levels of the cyclic nucleotide (CN) cAMP (Pace et al., 1985; Breer et al., 1990; Buck and Axel, 1991; Firestein et al., 1991; Frings and Lindemann, 1991; Michel and Ache, 1992; Brunet et al., 1996). The repertoire of intracellular effectors modulated by cAMP determines the effect of cAMP on cell excitability. CNs directly activate CN-gated nonselective cation channels (CNGCs) in vertebrates (Nakamura and Gold, 1987) and voltage-insensitive K+ channels in lobster (Michel and Ache, 1992; Hatt and Ache, 1994), leading to excitation or inhibition of olfactory neurons, respectively. Mutations in invertebrate genes with homology to subunits of vertebrate CNGCs specifically disrupt olfactory processing of certain odorants inCaenorhabditis elegans (Coburn and Bargmann, 1996; Domatsu et al., 1996), and a homologous α subunit is expressed inDrosophila antennae (Baumann et al., 1994); however, there are no mutant Drosophila alleles available. CNs are important in Drosophila [e.g., learning and memory signaling pathways (Davis, 1996)]; however, a role for these second messenger systems in Drosophila odor transduction has not been identified.
Recently a semi-intact antennal preparation from Drosophilahas been described that appears to maintain the structural integrity of the main olfactory organ and is amenable to the study of single olfactory receptor neurons using extracellular electrophysiological recording techniques (Dubin and Harris, 1997). Using this in situ preparation, responses to membrane-permeable CN analogs can be examined in both wild-type neurons and those carrying mutations that disrupt potential CN-regulated transduction pathways. TheDrosophila voltage-activated K+ channelether a go-go (eag) (Kaplan and Trout, 1969;Drysdale et al., 1991; Warmke et al., 1991) contains a consensus CN-binding site (Guy et al., 1991). A number of independent mutations in eag appear to disrupt CN-dependent effects on K+ conductances in muscle (Zhong and Wu, 1993). We tested whether eag was involved in olfactory processing. Here we demonstrate that Drosophila eag is important for the transduction of a subset of odorants. The proportion of antennal neurons sensitive to odors, exogenous CNs, high concentrations of K+ focally applied to sensillae, and extracellular Ca2+ were reduced by mutations in eag, suggesting that Eag may be a component of the transduction machinery in some sensory neurons.
MATERIALS AND METHODS
Fly stocks. All fly strains were grown on standard cornmeal–agar medium at ∼21°C. waIn(1)sc29 (eagsc29),eagX6 stock (Df(1)eagX-6/Y/XX, yf; Dp(1:2)eagX-6/+), and the mutant g eaghd15 sd f (eaghd15), and the revertants g eaghd15-Rev2 sd f(eaghd15-Rev2) and g eaghd15-Rev3 sd f (eaghd15-Rev3) were kindly provided by Dr. Barry Ganetzky (University of Wisconsin, Madison, WI), andeag1 was obtained from the Bloomington Stock Center (Bloomington, IN). eagsc29, eag1, and eaghd15 were maintained as homozygous stocks. The eagX6 stock is maintained over an attached-X chromosome: only males carry the mutated X chromosome, and females carry the attached-X chromosome that lacks the eag mutation and serve as autosomal background control for their mutant male siblings. Two independently derived dysgenesis-induced revertants ofeaghd15 served as background controls for theeaghd15 mutant. eagsc29 andeagX6 express truncated transcripts and are presumed to be functional nulls (Drysdale et al., 1991).
Electroantennogram recordings. A detailed description of the assay method has been described (Dubin et al., 1995). Briefly, adult flies <1 week old were tested for extracellular electroantennogram responses to pure odorants (Fluka, Buchs, Switzerland) at the indicated dilutions in purified water. The odor solutions were made from concentrated (∼10 m) liquid stocks on the day of the experiment. Living flies were mounted in clay, and their heads were immobilized. A ground electrode was usually inserted into the thorax, and the recording electrode (tip diameter ∼20 μm) was pressed against the third antennal segment. Recording locations were restricted to the proximal anterior face of the third antennal segment (see Fig.1, inset). The peak of the smooth negative voltage deflection induced by odor application was measured. Propionic acid (Pro), ethyl butyrate (EB), ethyl acetate (EtAC), butyl acetate (ButAC), and benzaldehyde (BZ) were usually tested at a 10−3 dilution; 2-butanone (2-BT), 2-heptanone (2-HEPT), butanol (BUT), and 1-octanol (OCT) were tested at 10−2. Lower concentrations of some of the odorants were also tested: EB (10−6 − 10−4), Pro (10−8 − 10−4), and OCT (10−3). All experiments were performed at room temperature.
Fig. 1.
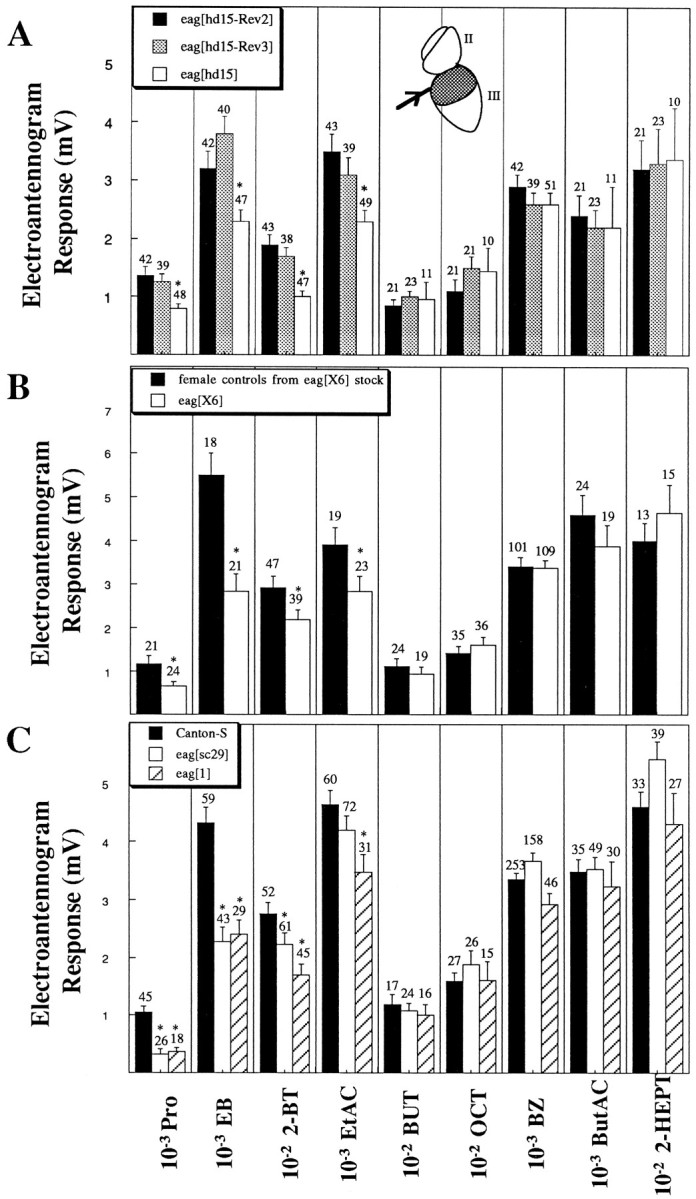
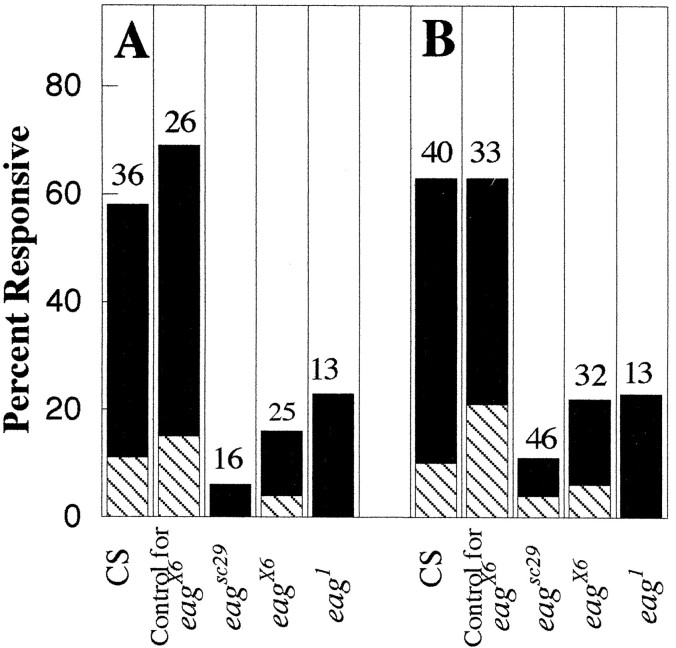
The eag mutant olfactory phenotype is a decreased responsiveness to four of nine tested odorants from five separate chemical classes. Extracellular voltage responses (in millivolts) were recorded from the dorsal region (stippled bars) of third antennal segments (A,inset, III, main olfactory organ) by the indicated odorants at the dilutions shown. Arithmetic means ± SEM are plotted with the number of observations indicated. Significant differences are indicated by the asterisks(*p < 0.001, Student’s t test).A, Electroantennogram responses elicited from the mutanteaghd15 (open bars) and two control revertants having identical genetic background (eaghd15-Rev2, solid bars;eaghd15-Rev3, stippled bars).B, Electroantennogram responses elicited from male mutant eagX6 (open bars) and female controls from the same stock (solid bars).C, Electroantennogram responses elicited from two mutant lines eagsc29 (open bars) andeag1 (hatched bars) and compared with Canton-S (solid bars).
Extracellular recording technique from single neurons. Patch pipettes were used to record extracellular currents driven by action potentials in neurons in a semi-intact antennal preparation described previously (Dubin and Harris, 1997), with a few exceptions. In the most recent method, antennae were mounted directly in periphery wax without the need for a coverslip. Antennae were perfused continuously with physiological saline (in mm: 130 NaCl, 2.5 KCl, 3 CaCl2, 1 MgCl2, 10 hemi Na-HEPES, 5 dextrose, and 5 Na-pyruvate, pH 7.4, 290–295 mOsm). In Ca2+ exchange experiments, 3 mmCa2+ and Ca2+-free Tyrode’s solution were used. The 3 mm Ca2+Tyrode’s solution contained (in mm): 130 NaCl, 4 KCl, 3 CaCl2, 1 MgCl2, 10 dextrose, and 10 HEPES, pH 7.4, 290–295 mOsm. Ca2+-free Tyrode’s solution was identical to 3 mm Ca2+Tyrode’s solution, with the following modifications (in mm): 0 CaCl2, 4.2 MgCl2, and 1 EGTA (the free divalent concentration was 4 mm). A few experiments used Ca2+-free Tyrode’s solution without increasing the Mg2+ concentration, and similar results were observed (data not shown). The increase in spontaneous activity observed in Ca2+-free salines containing the normal concentration of divalents is likely attributable to the inability of Mg2+ to completely substitute for Ca2+ (Hille, 1992). Free Ca2+ and Mg2+ concentrations were calculated using Chelator (Schoenmakers et al., 1992). Extracellular K+concentrations were altered by substituting KCl for NaCl and combining K+-free saline (in mm: 130 NaCl, 3 CaCl2, 1 MgCl2, 10 dextrose, and 10 hemi-Na HEPES) and 130 mm K+ saline (in mm: 130 KCl, 3 CaCl2, 1 MgCl2, 10 dextrose, and 10 hemi-Na HEPES) at the appropriate ratios. Osmolarities were determined experimentally using a Wescor 5500 vapor-pressure osmometer.
Recording electrodes fabricated from borosilicate capillary tubing (BF-100; Sutter Instruments) were fire-polished to have resistances of 10–20 MΩ when containing physiological saline. Current signals were sampled every 100–200 μsec, filtered (2 kHz) with an Axopatch-1C patch-clamp amplifier (Axon Instruments, Foster City, CA), and digitally recorded with an ITC-16 (Instrutech, Great Neck, NY) and Macintosh Power PC 7100. Data were acquired using HEKA Pulse programs. Odorants were applied from nearby puffer pipettes (BF-100 glass) at the indicated dilutions in extracellular saline.
Extracellular currents were recorded from single (and occasionally two) neurons when loose seal resistances were 50–100 MΩ. The observed currents were biphasic, comprising a fast transient positive-going phase followed by a negative-going current, and were very similar to those described previously (Frings and Lindemann, 1991). The duration of these transient currents was ∼1 msec (the time from peak to trough under normal recording conditions). They likely represent mainly capacitative transients driven by action potentials because their time course could be altered by experimental manipulations (e.g., increased in low extracellular Ca2+). The pipette potential was fixed at 0 mV, and no attempt was made at voltage clamping the patch of membrane beneath the electrode tip.
Apparent spontaneous activity was determined during the time before application of the stimulus (usually 500 msec or 1 sec); the average of at least three trials was calculated. The action potential frequency (APF) during odor application was determined either as the number of action potentials per unit time (usually over a duration of 1 sec) after onset of tonic responses or as the weighted average of the phasic (the initial high frequency burst, if present) and tonic components after onset of phasic–tonic responses. The weighted average of a phasic–tonic response was assigned as ([(APF during the initial phasic response) × time interval] + [(APF during tonic response) × time interval])/total time interval. Data are expressed as the fold increase (or decrease) in APF during exposure to the stimulus compared with the apparent spontaneous activity (APFstim/apparent spontaneous activity).
Statistical methods. The significance of the differences in percentages of responsive cells was determined using the χ2 test and Yate’s correction (Zar, 1996). Student’s two-tailed t test was used to compare parameters obtained from different populations. Values with uncertainties are expressed as mean ± SEM with the number of measurements indicated (n).
RESULTS
Mutations in eag caused decreased peripheral responsiveness to a subset of odorants
Initially, the response of large populations of antennal cells to a panel of single odorants was measured using electroantennogram recording techniques (Fig. 1) (Dubin et al., 1995). Drosophila is sensitive to a wide variety of volatile odorants produced in fermenting fruit, its natural food source, including organic acids, acetate esters, and alcohols. Peripheral sensitivity to four of nine tested odorants was reduced compared with controls in a P-element-induced mutant alleleeaghd15 (Fig. 1A), presumed null alleles of the eag locus [eagX6 andeagsc29 (Drysdale et al., 1991; Warmke et al., 1991)] (Fig. 1B,C), and an ethyl methanesulfonate (EMS)-induced allele [eag1(Lindsley and Zimm, 1992)] (Fig. 1C). Extracellular responses to low doses of the organic acid Pro, the ester EB, and the short-chain ketone 2-BT and acetate EtAC were significantly attenuated in mutant antennae with the rank order from most to least affected: EB ∼ Pro > 2-BT > EtAC. Sensitivity to two alcohols (BUT and OCT), an aldehyde (BZ), and a long-chain acetate (ButAC) and ketone (2-HEPT) were unaffected. All four alleles revealed similar qualitative and quantitative mutant phenotypes. The EtAC response obtained from eagsc29 was reduced compared with controls, but the difference was not significant.
Two lines of evidence indicate that defects in the eag gene are responsible for the observed mutant olfactory phenotype. First, four independent alleles with different genetic backgrounds (eagX6, eagsc29,eag1, and eaghd15) (Fig. 1) have similar mutant olfactory phenotypes. In particular,eaghd15 was significantly different from P-element excision revertants with identical genetic backgrounds (Fig.1A). Second, the mutant olfactory phenotype is observed in female eagX6/eagsc29heterozygotes (data not shown). The mutation conferring the olfactory phenotype is recessive; female heterozygotes (eagsc29/+, eagX6/+, andeag1/+) do not reveal an olfactory mutant phenotype (data not shown).
“Loose seal” extracellular recordings revealed fewer EB-sensitive neurons in eag mutants rather than a decreased responsiveness of all sensitive cells
Extracellular loose seal recordings were used to determine the apparent spontaneous activity and sensitivity to odors and CNs of individual eag mutant and wild-type control antennal neurons (Dubin and Harris, 1997). The antenna lumen as well as the sensillae, which contain the dendrites of olfactory neurons, were exposed to a low K+, Ca2+-containing extracellular physiological saline. Histograms of apparent spontaneous activity appeared normally distributed in both wild-type and eagneurons (data not shown). With the exception ofeagX6 mutant males, there were no significant differences in apparent spontaneous activity between eag and control neurons (spikes per second): controls, g eaghd15-Rev3 sd f, 2.9 ± 0.3 (n = 30); CS, 3.0 ± 0.1 (n = 255); female controls from the eagX6 stock, 3.0 ± 0.2 (n = 81); eag mutants: g eaghd15 sd f, 3.2 ± 0.3 (n = 31); eagsc29, 3.0 ± 0.2 (n = 89); eag1, 3.4 ± 0.2 (n = 107); eagX6, 3.9 ± 0.3 (n = 74). The difference (p < 0.05; Student’st test) between eagX6 mutant males and control females is likely attributable to genetic background differences present on the sex chromosomes (see Materials and Methods) because no differences were observed between mutanteaghd15 and control eaghd15-Rev3neurons.
EB modulated cell excitability in a dose-dependent manner. At low concentrations (10−10 and 10−8dilutions), only 20% (n = 15) and 40% (n = 16), respectively, of control antennal neurons responded, whereas half were sensitive at 10−6 and 10−4 dilutions (Figs.2, 3). The magnitudes of the responses were 22 ± 10% (n = 11; 10−10) and 65 ± 14% (n = 7; 10−8) of the response to 10−6 EB elicited from the same cell. Sensitive neurons could be observed in all regions of the third antennal segment (data not shown). At a nonphysiologically high EB concentration (10−1 dilution; data not shown), nearly all cells responded, indicating a nonspecific effect at these higher doses. EB (10−10 through 10−4 dilutions) and 2-HEPT (10−6 dilution) elicited excitatory, inhibitory, or no response from control and eag neurons (Fig. 2, Table 1). The majority of sensitive cells appeared to be excited by EB (Fig. 3, solid bars); the proportion of sensitive wild-type neurons inhibited by EB was ∼20–30% (Fig. 3, hatched bars).
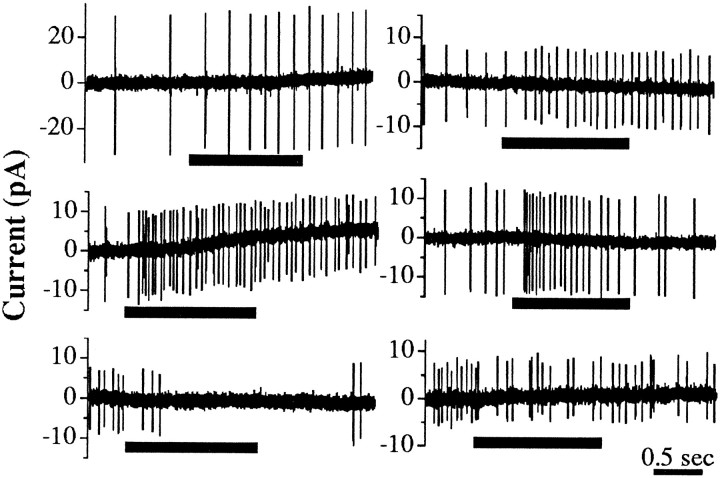
Fig. 2.
EB altered the excitability of Canton-S (left) and eag (right) antennal neurons. EB (10−4) applied from a nearby puffer pipette during the time indicated by thick barselicited sustained tonic increases (top), phasic–tonic increases (middle), and decreases (bottom) in action potential firing rate as determined from capacitative currents driven by action potentials. The latency for EB-induced (10−4) excitatory responses was 305 ± 36 msec (n = 42) for combined control neurons and 250 ± 24 msec (n = 32) for combinedeag alleles. The latency for EB-induced inhibitory responses was 255 ± 35 msec (n = 4) for controls and 243 ± 30 msec (n = 3) for combined eag alleles. There was a dose-dependent effect of EB on the latency that was significantly different at 10−8 EB. The latencies for excitatory responses to 10−8 EB were 381 ± 52 msec (n = 25) for combined controls and 379 ± 50 msec (n = 12) for combined eagalleles (p < 0.05 compared with responses to 10−4 EB, Student’s t test). The latencies for inhibitory responses were also longer, but the data are not significant (controls, 345 ± 88 msec, n = 7; eag, 400 msec, n = 1).
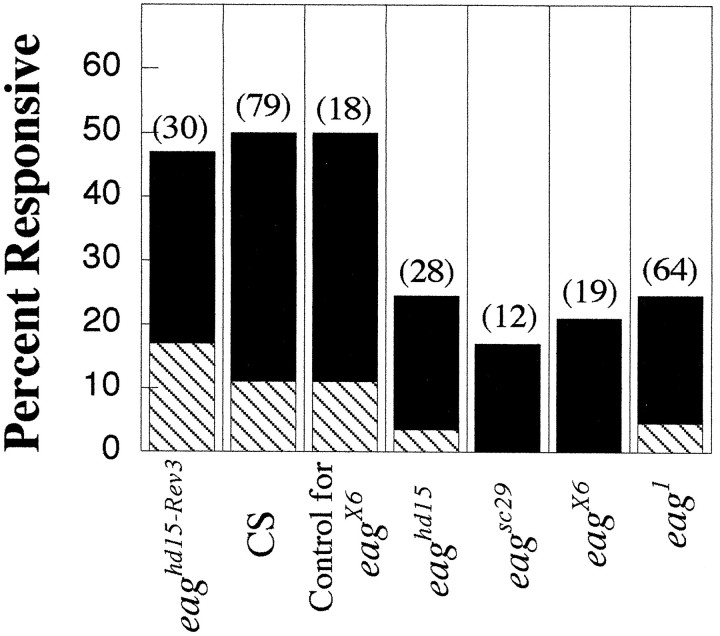
Fig. 3.
Significantly fewer EB-induced inhibitory and excitatory responses were observed in eag mutants. The percentage of neurons responding to EB (10−6dilution) with excitatory (solid bars) or inhibitory (hatched bars) responses is shown for the genotype indicated. Significant differences between all eag(combined eaghd15,eagsc29, eagX6, andeag1) alleles (n = 114) and controls (combined eaghd15-Rev3, Canton-S, and females from the eagX6 stock;n = 114) were observed for the proportions of EB-excited cells (p < 0.003) as well as the proportions of inhibitory responses [p = 0.0004, χ2 analysis (Zar, 1996)].
Table 1.
Mutations in eag specifically decreased the proportion of olfactory neurons responsive to the odorant EB
| Genotype | Response1-a to 10−6 EB (fold change compared with basal activity) | Response to 10−4 EB (fold change) | Response to 10−6 2-HEPT (fold change) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Increase1-b | Decrease | % (n) | Increase1-b | Decrease | % (n) | Increase1-b | Decrease | % (n) | |
| Control lines | |||||||||
| g eaghd15-Rev3 sd f | 3.2 ± 0.5 | 0.37 ± 0.06 | 47 | NT | NT | NT | NT | ||
| (n = 9) | (n = 5) | (n = 30) | |||||||
| CS | 4.0 ± 0.6 | 0.46 ± 0.06 | 52 | 7.5 ± 1.1 | 0.46 ± 0.15 | 47 | 3.1 ± 0.4 | 0.19 | 24 |
| (n = 31) | (n = 9) | (n = 79) | (n = 40) | (n = 4) | (n = 94) | (n = 14) | (n = 1) | (n = 53) | |
| Female controls from the eagX6stock | 6.4 ± 2.5 | 0.57 ± 0.05 | 50 | 6.7 ± 1.6 | 0.29 ± 0.1 | 62 | NT | NT | |
| (n = 7) | (n = 2) | (n = 18) | (n = 19) | (n = 4) | (n = 37) | ||||
| eag mutant lines | |||||||||
| g eaghd15 sd f | 3.1 ± 0.4 | 0.46 | 25 | NT | NT | NT | NT | ||
| (n = 6) | (n = 1) | (n = 28) | |||||||
| eagsc29 | 3.4 ± 1.1 | 17 | 7.0 ± 1.4 | 0.4 ± 0.07 | 39 | NT | NT | ||
| (n = 2) | (n = 0) | (n = 12) | (n = 17) | (n = 4) | (n = 54) | ||||
| eag1 | 2.7 ± 0.3 | 0.35 ± 0.05 | 23 | 5.0 ± 0.7 | 0.14 | 39 | 4.7 ± 0.8 | 0.55 ± 0.12 | 38 |
| (n = 13) | (n = 3) | (n = 52) | (n = 12) | (n = 1) | (n = 33) | (n = 20) | (n = 2) | (n = 58) | |
| eagX6 | 2.9 ± 0.7 | 21 | 7.8 ± 2.3 | 35 | NT | NT | |||
| (n = 4) | (n = 0) | (n = 19) | (n = 9) | (n = 0) | (n = 26) | ||||
Mutations in eag specifically decreased the proportion of olfactory neurons responsive to the odorant EB. Odorant-induced modulation of basal activity was determined during exposure of control and eag antennae to EB (10−6 and 10−4) dilutions) and 2-HEPT (10−6 dilution) using the loose patch recording technique. No significant difference in the magnitude of these odorant responses was observed among genotypes (Student’s t test).
Values indicate the action potential frequency during the odorant response (usually >1 sec) divided by the apparent spontaneous frequency.
Values indicate the fold-increase over basal levels for tonic and phasic–tonic responses (see Materials and Methods). Phasic–tonic responses were observed most often after 10−4 EB application in similar proportions of neurons from control and eag alleles (controls, 15%;eag alleles, 23%). NT, Not tested.
In the four eag alleles studied, fewer neurons were sensitive to EB compared with controls. There were 56 and 25% fewer responsive cells in eag antennae (data were combined for all alleles) compared with control (combined) antennae at 10−6 (p < 0.005) and 10−4 EB, respectively (Fig. 3, Table 1). Interestingly, fewer inhibitory as well as fewer excitatory responses were observed in eag alleles (Fig. 3). However, the magnitude and character of EB-induced responses from eagneurons that were sensitive to EB were similar to controls (Table 1). We tested whether EB-induced responses required odorant access to outer dendrites by occluding sensillar pores with wax (Dubin and Harris, 1997). Only 9% (1 of 11) of wild-type neurons in waxed antennae responded to 10−4 EB, compared with ∼50% of neurons from control antennae. The single responsive neuron may have been located in a region not exposed to the wax.
Electroantennogram studies (Fig. 1) revealed that most tested odorants, including the long-chain ketone 2-HEPT, produced normal responses ineag alleles. We tested whether responses to 2-HEPT were similarly unchanged using the loose seal procedure. As predicted from the electroantennogram studies, the proportion of neurons sensitive to 2-HEPT (10−6 dilution) was ∼30% in botheag and control antennae (Table 1). Responses to 2-HEPT appeared to be independent of responses to EB elicited in the same cell. Approximately 20% of wild-type neurons tested for their sensitivity to both EB and 2-HEPT (n = 51) responded to both odorants, and most of these were inhibited by EB and excited by 2-HEPT (data not shown). None of the cells from eag antennae (n = 51) could be inhibited by EB if they were excited by 2-HEPT, and there appeared to be an increase in the frequency of cells excited only by 2-HEPT (data not shown).
Dendritic K+ channels appeared to include Eag and may underlie most wild-type EB-induced inhibitory responses
In an attempt to understand how mutations in the putative CN-modulated K+ channel could affect excitatory as well as inhibitory EB odorant responses, we estimated theEK across the outer dendritic membrane for a population of wild-type as well as mutant antennal neurons. A dose-dependent increase in neuronal firing frequency was observed during focal application of a range of elevated external K+ solutions to sensillae close to the puffer pipettes (Fig. 4A) and approximately equidistant from either end of the third antennal segment. An increase in action potential frequency was observed during application of as low as 10 mm K+ to sensillae (seven of seven neurons) (Fig. 4B,top), whereas no effect was observed from these same neurons when 10 mm K+ saline was applied to either the cut end of antennae (n = 4) or the joint between the second and third segments (n = 2) (data not shown). The effect of increased K+ concentrations on neuronal excitability was dose dependent (Fig. 4B) up to 150 mm K+ (data not shown). At higher K+ concentrations, the latency for the increase in excitability was 50–300 msec. Based on these experiments, the concentration of K+ in the sensillar lymph under the imposed recording conditions is <10 mmK+ and is probably similar to that in the bathing solution. Assuming an intracellular K+ concentration of ∼140–150 mm, it is likely that the K+ equilibrium potential (EK) across dendritic membranes is more negative than the resting membrane potential (−70 mV) (Dubin and Harris, 1997) when antennae are perfused with 2.5–4 mmK+ saline, and that dendritic membranes have some permeability to K+. A control was performed to determine whether the sensillar lymph compartment maintained its integrity in this preparation. In vivo, the fluid surrounding the dendrites in the sensillar shaft is separate from the saline surrounding the cell bodies located in the lumen of the antenna. To test whether the excitatory responses to K+ were caused by the leak of K+ from the sensillar lymph space to neuronal cell bodies, olfactory neurons were exposed to TTX (1 μm) only from the sensillar space by focal application to sensillae located ∼100–150 μm from the cut end of the third antennal segment. In no case (five spontaneously active neurons from four separate antennae) was action potential frequency reduced; however, TTX could reversibly block action potentials in these neurons when applied to the cut end of the antenna in which TTX had access to axons. To assure that the recorded neurons were housed in sensillae that were exposed to TTX, EB applied from a second pipette was shown to modulate activity in these neurons.
Fig. 4.
In the in situ preparation used in this study, EK across the dendritic membrane appeared to be more negative than the resting membrane potential such that activation of a K+ conductance would inhibit cell activity. A, Schematic of the preparation. High K+ salines or TTX was applied from nearby puffer pipettes while activity was recorded from third antennal segment neurons housed in focally perfused sensillae. B, Increasing millimolar concentrations of K+(K+ replaced Na+; shown at theright) applied during the time indicated by thethick horizontal bar produced an increase in neuronal excitability (frequency of fast transient current spikes).
To test whether the increase in apparent spontaneous activity of control neurons in elevated external K+concentrations required wild-type eag, 50 mmK+ saline was applied from a nearby puffer pipette to control and eag antennae. Consistent with the hypothesis that K+ channels composed of Eag subunits exist in outer dendritic membrane of some neurons, significantly fewer neurons were modulated by high K+ saline in eagalleles (35%, n = 23; p < 0.05, χ2 analysis) compared with controls (70%,n = 27). The magnitude of the response to high K+ observed in sensitive eag neurons was similar to that of controls (data not shown).
Some wild-type EB-induced excitatory responses required extracellular Ca2+ and were dependent oneag
Activation of a dendritic K+ conductance would be expected to inhibit cell excitability under the conditions used in these single neuron studies. Surprisingly, mutations in eagdecreased the incidence of observing not only inhibitory responses but excitatory responses as well. It is possible that basal dendritic Eag activity could be decreased rather than increased by odorant in some cells, thus producing a depolarization and enhanced excitation. However, results using exogenously applied CN analogs (see below) suggested that excitatory as well as inhibitory responses may be mediated by activation of an eagK+ conductance. Because a previous report indicated that Eag homomultimers expressed in Xenopus oocytes were permeable to Ca2+ (Bruggemann et al., 1993; but see Discussion), we investigated whether a Ca2+-dependent process might underlieeag-dependent excitatory responses. The Ca2+ permeability of endogenous Eag-containing channels in Drosophila has not been examined. We tested the hypothesis that the excitatory EB-induced responses dependent oneag were caused by Ca2+ influx, perhaps by Ca2+ acting as a third messenger to regulate downstream excitatory conductances.
Residual extracellular Ca2+ was chelated with EGTA, and normal divalent cation levels were maintained with Mg2+. In control and eag alleles, removal of extracellular Ca2+ caused an increase in both the duration of the biphasic current driven by action potentials (control, 1.9 ± 0.2-fold, n = 15; eag, 2.0 ± 0.1-fold, n = 18) and apparent spontaneous activity (control, 1.9 ± 0.2-fold, n = 26; eag, 1.6 ± 0.2-fold, n = 25).
Control CS antennal neurons, which responded to 10−4 EB with an increased excitability in normal Ca2+, (Fig. 5,top row) were subsequently challenged with 10−4 EB in the absence of extracellular Ca2+ (Fig. 5, middle row). In Ca2+-free saline compared with Ca2+ Tyrode’s solution, EB-induced excitatory responses were attenuated (>25% reduction) in ∼75% of tested cells (20 of 26 cells) (Fig. 5). Interestingly, the EB-induced responses from five of these cells became inhibitory when extracellular Ca2+ was removed (Fig. 5, right panel). In all cases, the initial excitatory response was recovered after reintroduction of 3 mmCa2+ (Fig. 5, bottom row). The odorant response of some wild-type neurons was not altered in Ca2+-free saline, indicating that removing extracellular Ca2+ did not cause a general nonspecific effect (Fig. 5, left panel, middle row).
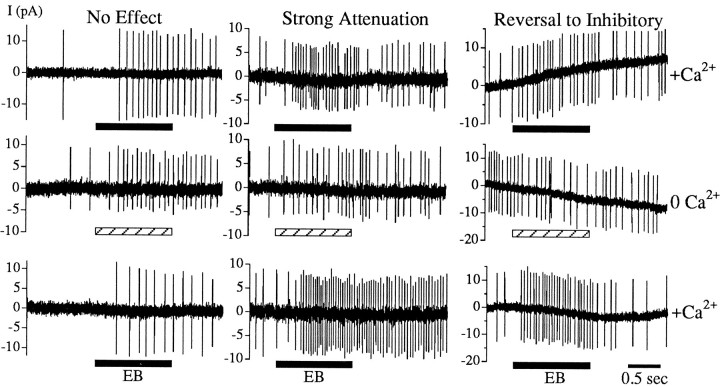
Fig. 5.
The majority of EB-induced excitatory responses from wild-type neurons depended on extracellular Ca2+. EB (10−4 dilution,horizontal bars) induced an increase in firing in the presence of 3 mm Ca2+ Tyrode’s solution (top row). After the removal of extracellular Ca2+ by bath exchange, subsequent exposure to EB (diluted to 10−4 in Ca2+-free Tyrode’s solution in a separate puffer) produced three types of effects (middle row). The responses of some neurons were not altered (left panel), were attenuated (center panel; example of a strongly attenuated response), and became inhibitory (right panel) after extracellular Ca2+ was removed. The excitatory response recovered after return to 3 mmCa2+ Tyrode’s solution (bottom row). Both attenuated and reversed effects were reproducible and reversible. The shift from an excitatory to an inhibitory effect was reproduced during three repetitions of the entire protocol for the neuron shown (right panel). Application of EB from each puffer produced similar responses in 3 mm Ca2+. The shifts in the baseline of some recordings are not stimulus dependent.
The distribution of the severity of the effects of removing extracellular Ca2+ on excitatory responses is shown in Figure 6. A wide range of effects was observed for wild-type neurons (Fig. 6, solid bars). The left-most column indicates those responses that became inhibitory in Ca2+-free saline (reversal to inhibitory); the remaining columns indicate the responses that were strongly attenuated (0–24% of the control response), less strongly attenuated (25–49%, 50–74%), and not significantly affected in 0 Ca2+ saline (75–99%, 100–124%). In rare cases, responses may have been enhanced (≥125%).
Fig. 6.
The distribution of Ca2+-dependent effects on control excitatory EB responses required a wild-type eag gene. Plotted is the percentage of neurons revealing different degrees of Ca2+-dependent EB responses (bins of 25% on abscissa). The distributions of the effects on control (solid bars; n = 26) and eag(hatched bars; n = 23) responses were significantly different (p < 0.0005, χ2 analysis). To obtain the percentage of control EB-induced response for each neuron tested, the magnitude of the EB-induced response was calculated in Ca2+-free saline and divided by the magnitude observed in 3 mmCa2+ Tyrode’s solution. In most cases the effect of Ca2+-free saline was fully reversible; in cases revealing slight rundown of the EB-induced effect, the control value was taken as the averaged EB responses observed in 3 mmCa2+ Tyrode’s solution before and after exposure to 0 Ca2+ saline (EB was applied at least twice before and after exposure of antennae to Ca2+-free saline at 45 sec intervals).
In contrast, most of EB-induced excitatory responses in eagneurons were not significantly altered in Ca2+-free saline (Fig. 6, hatched bars). No eagneurons that revealed an initial excitatory response were inhibited by EB in Ca2+-free saline. The dramatic attenuation of the EB-induced response in wild-type but not eag neurons does not appear to be a nonspecific effect caused by increased basal firing rate (likely caused by membrane depolarization) because the magnitude of the increased basal activity was similar among genotypes. Furthermore, the differential effects of 0 Ca2+ on EB-induced excitatory responses observed between control andeag neurons are not likely attributable to differential Ca2+ screening effects because it is unlikely that mutations in eag significantly altered the membrane composition (in particular, the negative charges) of the outer dendrite. However, the similar increase in spontaneous activity of both control and eag neurons in 0 Ca2+Tyrode’s solution may be attributable, in part, to the inability of Mg2+ to fully compensate for the loss of Ca2+ screening.
Fewer eag antennal neurons were sensitive to exogenous CN analogs compared with wild-type neurons
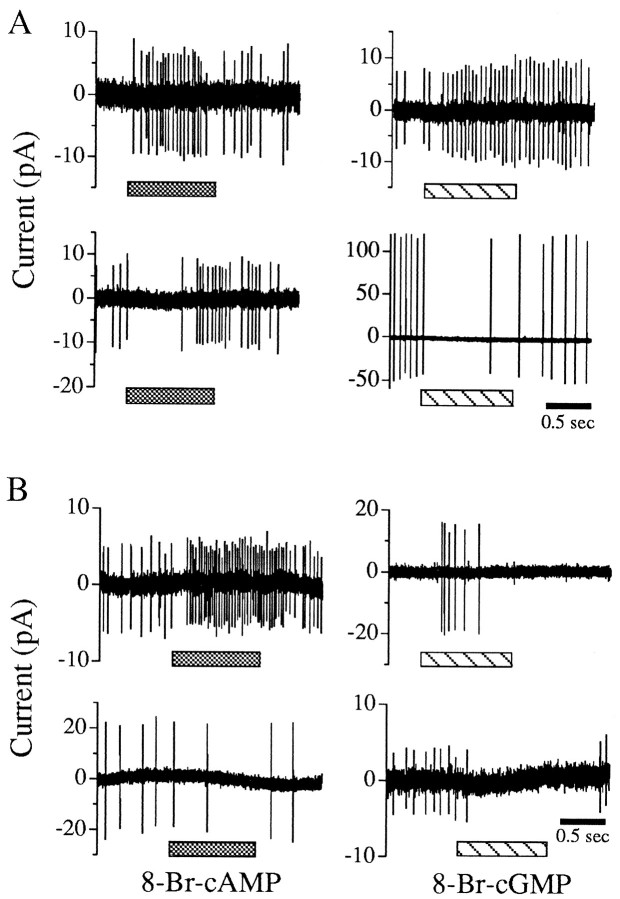
The gene product from the eag locus ofDrosophila contains a consensus intracellular CN-binding site (Guy et al., 1991), and CN modulation of K+currents in Drosophila muscle is altered in eagalleles (Zhong and Wu, 1993). If endogenous CNs mediate the modulation of Eag channels, then exogenous CN analogs should modulate wild-type cell excitability and be less effective on antennal neurons fromeag mutant lines. Wild-type antennal neurons in situ were challenged with either 8-bromo-cAMP (8-Br-cAMP) or 8-bromo-cGMP (8-Br-cGMP) (usually applied at 3 mm), and each could elicit a short-latency increase in excitability (Fig.7A, top; Table2, Increase) and inhibition (Fig.7A, bottom; Table 2, Decrease). The latency for the excitatory and inhibitory responses to the CN analogs (426 ± 54 msec, n = 17, and 425 ± 63 msec,n = 4, respectively) was similar to that observed for odorant application using the identical perfusion apparatus (Fig. 2, legend). Similar results were observed during application of each analog at 0.5 mm (data not shown). CNs also modulated the activity of antennal neurons from eag mutants (Fig.7B). However, there were significantly fewer CN-modulated neurons from eagsc29, eagX6, andeag1 antennae compared with controls (Fig.8, Table 2). Significantly fewer cells were inhibited (hatched) or excited (solid) by 8-Br-cAMP (Fig. 8A) and 8-Br-cGMP (Fig.8B). Exogenously applied 8-Br-cGMP (3 mm) did not elicit responses in control neurons in antennae with wax-occluded sensillar pores (n = 12), implicating the site of action at the apical dendrites.
Fig. 7.
Membrane-permeant cyclic nucleotide analogs rapidly modulated wild-type (A) andeag (B) neuronal excitability. 8-Br-cAMP (3 mm, left panel) and 8-Br-cGMP (3 mm, right panel) usually increased action potential frequency with latency ≤800 msec (73% of the responses). The latencies of responses were combined for all genotypes because they were similar. 8-Br-cAMP produced excitatory and inhibitory responses with latencies of 483 ± 75 msec (n = 11) and 400 ± 0 msec (n = 2), respectively. 8-Br-cGMP produced excitatory and inhibitory responses with latencies of 445 ± 57 msec (n = 11) and 376 ± 120 msec (n = 3), respectively.
Table 2.
Mutations in eag decreased the proportion of olfactory neurons responsive to membrane-permeant cycle nucleotide analogs
| Genotype | Response to 3 mm 8-Br-cAMP (fold change) | Response to 3 mm 8-Br-cGMP (fold change) | ||||
|---|---|---|---|---|---|---|
| Increase | Decrease | % (n) | Increase | Decrease | % (n) | |
| Control lines | ||||||
| CS | 3.4 ± 0.7 | 0.55 ± 0.03 | 58 (36) | 3.6 ± 0.7 | 0.52 ± 0.02 | 63 (40) |
| (n = 17) | (n = 4) | (n = 21) | (n = 4) | |||
| Female controls from the eagX6stock | 2.7 ± 0.4 | 0.57 ± 0.11 | 69 (26) | 5.0 ± 1.5 | 0.61 ± 0.04 | 64 (33) |
| (n = 14) | (n = 4) | (n = 14) | (n = 7) | |||
| eag mutant lines | ||||||
| eagsc29 | 1.9 | 6 (16) | 8.0 ± 3.1 | 0.41 ± 0.33 | 11 (46) | |
| (n = 1) | (n = 0) | (n = 3) | (n = 2) | |||
| eag1 | 2.1 ± 0.06 | 23 (13) | 3.0 ± 1.0 | 23 (13) | ||
| (n = 3) | (n = 0) | (n = 3) | (n = 0) | |||
| eagX6 | 2.7 ± 0.9 | 0.62 | 16 (25) | 2.3 ± 0.4 | 0.27 ± 0.23 | 22 (32) |
| (n = 3) | (n = 1) | (n = 5) | (n = 2) | |||
Mutations in eag decreased the proportion of olfactory neurons responsive to membrane-permeant cyclic nucleotide analogs. Cyclic nucleotide-induced modulation of basal activity was determined in loose patch recordings during exposure of control and eagantennae to either 8-Br-cAMP (3 mm) or 8-Br-cGMP (3 mm). No significant difference in the response magnitude was observed among genotypes, when comparisons were made between each control and eag allele separately, or between combined controls and eag alleles (Student’s t test). Although the average eagsc29 response was larger than that for controls, and the average eagX6response was smaller than the control value, individual values were within the range observed for control neurons.
Fig. 8.
Significantly fewer CN-induced inhibitory and excitatory responses were observed in eag mutants. The percentage of neurons responding to either 8-Br-cAMP (A) or 8-Br-cGMP (B) (each at 3 mm) with excitatory (solid bars) or inhibitory (hatched bars) responses is shown for the genotype indicated. Significant differences between all eag alleles (combined; n = 54 and 91) and combined controls (n = 62 and 124) were observed for the proportions of CN-sensitive cells (p < 2 × 10−6 and p < 0.005 for 8-Br-cAMP and 8-Br-cGMP, respectively) as well as the proportions of inhibitory responses [p < 0.05, for either 8-Br-cAMP or 8-Br-cGMP, χ2 analysis (Zar, 1996)].
DISCUSSION
Two principle findings are reported. First, mutations in theDrosophila eag gene encoding a distinct type of voltage-sensitive K+ channel (Warmke et al., 1991) that contains a consensus CN-binding domain (Guy et al., 1991) caused a specific adult olfactory mutant phenotype. Four independenteag mutant alleles, including an allele with matched controls having identical genetic backgrounds, exhibited decreased responsiveness of the main olfactory organ toward a subset of odorants. These included a short-chain ketone (2-BT), acetate esters (EB, EtAC), and an organic acid (Pro) but not long-chain ketones and acetate esters (2-HEPT, ButAC), alcohols, or benzaldehyde. Third instar larvae revealed a similar eag mutant olfactory phenotype in a behavioral assay (data not shown). Second, the Eag channel subunit appears to function in primary signal transduction events in a population of antennal neurons.
The Drosophila eag adult olfactory mutant phenotype is caused by mutations in the eag gene
Comparisons between a P-element-induced mutant allele (eaghd15) and revertants with identical genetic backgrounds (eaghd15-Rev2 andeaghd15-Rev3) demonstrate that mutations ineag caused the mutant phenotype; the specific reduction of responses to EB, Pro, 2-BT, and EtAC. Similarly reduced electroantennogram responses were observed in three other independenteag mutant alleles (eagX6,eagsc29, and eag1). The secondary mutations in eagX6 and eagsc29involve different loci (Drysdale et al., 1991) and are not likely to mediate the mutant olfactory phenotype. The EMS-inducedeag1 allele has a similar mutant phenotype. EB elicited dose-dependent changes in cell excitability in the range of 10−10 to 10−6 dilutions in both mutants and controls, and the mutant phenotype was observed in this dosage range. At higher concentrations EB elicited nonspecific effects: nearly all control and eag neurons responded to high EB concentrations (10−1), a finding incompatible with odor discrimination.
The reduced EB response could be accounted for by a decrease in the proportion of neurons sensitive to odorant, with no decrease in the magnitude of the elicited responses. Responses to 2-HEPT were similar to eag and controls. Data from experiments that investigated the dual responsivity of individual neurons to EB and 2-HEPT in wild-type and eag alleles argue against the specific death of a population of EB-sensitive neurons in eag antennae. Although 20% of wild-type neurons were responsive to both odorants and the proportion of neurons sensitive to 2-HEPT was similar ineag and control antennae, no eag neurons were sensitive to both odorants.
An olfactory mutant phenotype similar to eag has been reported for Sco (Dubin et al., 1995) and ota3, ota4, and ota5 (Woodard et al., 1989) mutants. In particular, Sco mutants reveal a decreased sensitivity to short-chain acetates and ketones (but not BZ), and theota mutants have reduced sensitivity toward EtAC and Pro (but not BZ). However, specific defects in responsiveness toward BZ are revealed in another group of mutants [ptg (Helfand and Carlson, 1989); olfA, olfB, and olfF (Ayyub et al., 1990); and smi (Anholt et al., 1996)]. Thus, odorant transduction likely occurs via diverse pathways inDrosophila.
Eag subunits appear to mediate transduction of the odorant EB
A population of eag antennal sensory neurons appears to be insensitive to a subset of odorants. Is this mutant phenotype caused by a defect in a signal transduction component (Eag channel subunits)? In support of this, Eag K+ channels appear to be located on the outer dendrites of antennal neurons. Two-thirds of wild-type neurons exhibited an increased excitability on focal exposure of sensillae to elevated K+, but only half as manyeag neurons were stimulated. Focally applied TTX had no effect on spontaneous activity, indicating that the effect of elevated external K+ concentrations was not attributable to leakage of K+ from the sensillar lymph to the antenna lumen containing neuronal somata. Experiments aimed at determining the sensillar K+ concentration revealed values <10 mm K+ under our recording conditions. Assuming a high intracellular K+concentration, the activation of a dendritic K+conductance would be inhibitory. Consistent with this, fewereag neurons were inhibited by EB compared with matched controls.
A second line of evidence that Eag plays a role in initial odor transduction derives from the observed differential effects of exogenously applied CNs in eag and control neurons. The incidence of observing short-latency excitatory and inhibitory responses to exogenous membrane-permeable CN analogs (8-Br-cAMP and 8-Br-cGMP) was significantly reduced in eag alleles compared with controls. A similar decrease in the percentage of responsive neurons was observed for EB and CNs (compare Figs. 3, 8). One report describing CN-induced increases in Eag currents expressed inXenopus oocytes (Bruggemann et al., 1993) has not been reproduced (Robertson et al., 1996); however, the latter study indicated that subtle changes in voltage dependence may not have been detected because of channel rundown. Because olfactory receptor neurons are very sensitive to small fluctuations in membrane currents because of their high membrane resistance (Lynch and Barry, 1989), small changes in Eag currents by CNs might substantially alter membrane potential. Although these data suggest the involvement of CNs in the modulation of Eag channel activity in vivo, CNs may act as odorants and modulate pathways used by a subset of odorants, including EB. Comparisons of responses induced by structurally dissimilar CN analogs may provide insights into this issue.
Interestingly, some CN-induced responses did not depend oneag. CNGCs may underlie the response to CN analogs in cells distinct from those affected by eag mutations. CNGCs with homology to vertebrate channels are expressed in Drosophilaantennae (Baumann et al., 1994), but their cellular location and presumptive role in olfactory transduction are unknown. In C. elegans, CNGC channels are expressed in only a subset of chemosensory cells, subserving the transduction of a subset of odorants (Coburn and Bargmann, 1996; Domatsu et al., 1996).
There were significantly fewer EB-induced excitatory responses ineag mutants, indicating that mutations in eagaffect a pathway leading to excitation in some, but not all, neurons. Most wild-type excitatory responses required extracellular Ca2+, and some became inhibitory in the absence of extracellular Ca2+, consistent with unmasking an underlying inhibitory conductance. This striking effect was not observed in eag neurons. These results are consistent with the existence of a Ca2+-dependent process in wild-type flies that is lacking in eag alleles. The initial electrophysiological characterization of Eag currents reported that Eag homomultimers expressed in Xenopus oocytes were permeable to Ca2+ (Bruggemann et al., 1993). In vertebrates, Ca2+ influx through CNGCs and subsequent activation of Ca2+-dependent chloride currents is largely responsible for altering membrane excitability (Kleene, 1993; Kurahashi and Yau, 1993; Lowe and Gold, 1993; Frings et al., 1995). A more recent study of Eag homomultimers expressed in Xenopus oocytes was unable to detect significant Ca2+ influx (Robertson et al., 1996); however, subtle Ca2+ permeabilities below the limit of detection may have profound in vivoeffects, and the subunit composition (Chen et al., 1996) and functional properties of Eag-containing channels in vivo may differ from those in heterologous expression systems (Zagotta et al., 1989). Thus, a third line of evidence supporting the role of Eag in initial transduction events is provided by the effects of eagmutations on Ca2+-dependent excitatory EB responses.
Alternatively, eag may indirectly affect the expression of olfactory neuron identity by increasing synaptic activity during development and retrogradely impacting the expression of the repertoire of signal transduction components (Farbman, 1994). However, whereas hyperexcitable Shaker eag and Hyperkinetic eag double mutants show striking morphological and functional abnormalities at peripheral synapses (Ganetzky and Wu, 1986; Budnik et al., 1990; Jia et al., 1993; Zhong et al., 1992), no detectable effect on neuromuscular junction morphology was reported for eaglarvae lacking the second mutation (Budnik et al., 1990; Zhong et al., 1992). However, central synapses may be more susceptible to activity-dependent developmental effects than the neuromuscular junction because of their lower safety factor for neurotransmission. Any developmental defect must account for a mutant phenotype in which (1) fewer neurons are excited by elevated K+concentrations, (2) fewer neurons are responsive to exogenous CNs and a subset of odorants in similar proportions, (3) responses of fewer neurons are dependent on external Ca2+, and (4) in the absence of external Ca2+, no excitatory odor responses become inhibitory. Although we cannot rule out the possibility of a developmental effect, the data are consistent with Eag mediating the transduction of a subset of odorants.
To mediate odor transduction, Eag must be expressed in outer dendrites. The localization of Eag subunits to dendrites will require immunohistochemical staining using a specific antibody, blocking Eag currents with specific antagonists (neither reagents are available), or electrophysiological access to dendritic currents (which has not yet been possible). Whole-cell odor-modulated conductances have been recorded from Drosophila antennal neurons (Dubin and Harris, 1997); however, the rarity of obtaining successful recordings prohibits this approach. Excised somata patches may reveal Eag-containing channels; however, not all ion channels expressed on soma are expressed in outer dendrites (McClintock and Ache, 1989).
In conclusion, Eag channel subunits may mediate the transduction of a subset of odorants in a population of Drosophila antennal neurons in a CN- and Ca2+-dependent manner. Eag may be the first K+ channel described in olfactory neurons that has the potential, when activated, to influence cellular activity dependent on the extracellular sensillar environment (e.g., Ca2+) and dendritic expression of downstream effectors.
Footnotes
This work was supported by National Institutes of Health Grant R03 DC02579 and the Whitehall Foundation Grant J94-11 to A.E.D., and the American Heart Association, California Affiliate, and the Muscular Dystrophy Association (G.L.H.). We thank Drs. Vincent Dionne, Barry Ganetzky, Blake Anson, Claudio Pikielny, and Sanford Bernstein for helpful comments on this manuscript, Dr. Stephen George for his help with the statistical analyses, and Dr. Richard Cripps and Michelle Mardahl-Dumesil for their advice concerning the genetics.
Correspondence should be addressed to Dr. Adrienne E. Dubin, R. W. Johnson Pharmaceutical Research Institute, 3535 General Atomics Court Suite 100, San Diego, CA 92121.
REFERENCES
- 1.Ache B, Zhainazarov A. Dual second-messenger pathways in olfactory transduction. Curr Opin Neurobiol. 1995;5:461–466. doi: 10.1016/0959-4388(95)80006-9. [DOI] [PubMed] [Google Scholar]
- 2.Anholt R, Lyman R, Mackay T. Effects of single P-element insertions on olfactory behavior in Drosophila melanogaster. Genetics. 1996;143:293–301. doi: 10.1093/genetics/143.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayyub C, Paranjape J, Rodrigues V, Siddiqi O. Genetics of olfactory behavior in Drosophila melanogaster. J Neurogenet. 1990;6:243–262. doi: 10.3109/01677069009107114. [DOI] [PubMed] [Google Scholar]
- 4.Baumann A, Frings S, Godde M, Seifert R, Kaupp U. Primary structure and functional expression of a Drosophila cyclic nucleotide-gated channel present in eyes and antennae. EMBO J. 1994;13:5040–5050. doi: 10.1002/j.1460-2075.1994.tb06833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breer H, Boekhoff I, Tareilus E. Rapid kinetics of second messenger formation in olfactory transduction. Nature. 1990;345:65–68. doi: 10.1038/345065a0. [DOI] [PubMed] [Google Scholar]
- 6.Bruggemann A, Pardo L, Stuhmer W, Pongs O. Ether-a-go-go encodes a voltage-gated channel permeable to K+ and Ca2+ and modulated by cAMP. Nature. 1993;365:445–448. doi: 10.1038/365445a0. [DOI] [PubMed] [Google Scholar]
- 7.Brunet L, Gold G, Ngai J. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron. 1996;17:681–693. doi: 10.1016/s0896-6273(00)80200-7. [DOI] [PubMed] [Google Scholar]
- 8.Buck L. Information coding in the vertebrate olfactory system. Annu Rev Neurosci. 1996;19:517–544. doi: 10.1146/annurev.ne.19.030196.002505. [DOI] [PubMed] [Google Scholar]
- 9.Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 10.Budnik V, Zhong Y, Wu C. Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J Neurosci. 1990;10:3754–3768. doi: 10.1523/JNEUROSCI.10-11-03754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen M, Hoshi T, Wu C. Heteromultimeric interactions among K+ channel subunits from Shaker and eag families in Xenopus oocytes. Neuron. 1996;17:535–542. doi: 10.1016/s0896-6273(00)80185-3. [DOI] [PubMed] [Google Scholar]
- 12.Coburn C, Bargmann C. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron. 1996;17:695–706. doi: 10.1016/s0896-6273(00)80201-9. [DOI] [PubMed] [Google Scholar]
- 13.Davis R. Physiology and biochemistry of Drosophila learning mutants. Physiol Rev. 1996;76:299–317. doi: 10.1152/physrev.1996.76.2.299. [DOI] [PubMed] [Google Scholar]
- 14.Dionne V, Dubin A. Transduction diversity of olfaction. J Exp Biol. 1994;194:1–21. doi: 10.1242/jeb.194.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Domatsu H, Mori I, Rhee J-S, Akaike N, Ohshima Y. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron. 1996;17:707–718. doi: 10.1016/s0896-6273(00)80202-0. [DOI] [PubMed] [Google Scholar]
- 16.Drysdale R, Warmke J, Kreber R, Ganetzky B. Molecular characterization of eag: a gene affecting potassium channels in Drosophila melanogaster. Genetics. 1991;127:497–505. doi: 10.1093/genetics/127.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubin A, Harris G. Voltage-activated and odor-modulated conductances in olfactory neurons of Drosophila melanogaster. J Neurobiol. 1997;32:123–137. doi: 10.1002/(sici)1097-4695(199701)32:1<123::aid-neu11>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 18.Dubin A, Heald N, Cleveland B, Carlson J, Harris G. Scutoid mutation of Drosophila melanogaster olfactory responses to short-chain acetate esters and ketones. J Neurobiol. 1995;28:214–233. doi: 10.1002/neu.480280208. [DOI] [PubMed] [Google Scholar]
- 19.Farbman A. Developmental biology of olfactory sensory neurons. Semin Cell Biol. 1994;5:3–10. doi: 10.1006/scel.1994.1002. [DOI] [PubMed] [Google Scholar]
- 20.Firestein S, Darrow B, Shepherd G. Activation of the sensory current in salamander olfactory receptor neurons depends on a G-protein-mediated cAMP second messenger system. Neuron. 1991;6:825–835. doi: 10.1016/0896-6273(91)90178-3. [DOI] [PubMed] [Google Scholar]
- 21.Frings S, Lindemann B. Current recording from sensory cilia of olfactory receptor cells in situ. I. The neuronal response to cyclic nucleotides. J Gen Physiol. 1991;97:1–16. doi: 10.1085/jgp.97.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frings S, Seifert R, Godde M, Kaupp U. Profoundly different calcium permeation and blockage determine the specific function of distinct cyclic nucleotide-gated channels. Neuron. 1995;15:169–179. doi: 10.1016/0896-6273(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 23.Ganetzky B, Wu C-F. Neurogenetics of membrane excitability in Drosophila. Annu Rev Genet. 1986;20:13–44. doi: 10.1146/annurev.ge.20.120186.000305. [DOI] [PubMed] [Google Scholar]
- 24.Guy H, Durell S, Warmke J, Drysdale R, Ganetzky B. Similarities in amino acid sequences of Drosophila eag and cyclic nucleotide-gated channels. Science. 1991;254:730. doi: 10.1126/science.1658932. [DOI] [PubMed] [Google Scholar]
- 25.Hatt H, Ache B. Cyclic nucleotide- and inositol phosphate-gated ion channels in lobster olfactory receptor neurons. Proc Natl Acad Sci USA. 1994;91:6264–6268. doi: 10.1073/pnas.91.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helfand S, Carlson J. Isolation and characterization of an olfactory mutant in Drosophila with a chemically specific defect. Proc Natl Acad Sci USA. 1989;86:2908–2912. doi: 10.1073/pnas.86.8.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hille B. Ionic channels of excitable membranes. Sinauer; Sunderland, MA: 1992. [Google Scholar]
- 28.Jia X, Gorczyca M, Budnik V. Ultrastructure of neuromuscular junctions in Drosophila: comparison of wild type and mutants with increased excitability. J Neurobiol. 1993;24:1025–1044. doi: 10.1002/neu.480240804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan W, Trout W. The behavior of four neurological mutants of Drosophila. Genetics. 1969;61:399–409. doi: 10.1093/genetics/61.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleene S. Origin of the chloride current in olfactory transduction. Neuron. 1993;11:123–132. doi: 10.1016/0896-6273(93)90276-w. [DOI] [PubMed] [Google Scholar]
- 31.Kurahashi T, Yau K. Co-existence of cationic and chloride components in odorant-induced current of vertebrate olfactory receptor cells. Nature. 1993;363:71–74. doi: 10.1038/363071a0. [DOI] [PubMed] [Google Scholar]
- 32.Lindsley D, Zimm G. The genome of Drosophila melanogaster. Academic; San Diego: 1992. [Google Scholar]
- 33.Lowe G, Gold G. Nonlinear amplification by calcium-dependent chloride channels in olfactory receptor cells. Nature. 1993;366:283–286. doi: 10.1038/366283a0. [DOI] [PubMed] [Google Scholar]
- 34.Lynch J, Barry P. Action potentials initiated by single channels opening in a small neuron (rat olfactory receptor). Biophys J. 1989;55:755–768. doi: 10.1016/S0006-3495(89)82874-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McClintock T, Ache B. Histamine directly gates a chloride channel in lobster olfactory receptor neurons. Proc Natl Acad Sci USA. 1989;86:8137–8141. doi: 10.1073/pnas.86.20.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michel W, Ache B. Cyclic nucleotides mediate an odor-evoked potassium conductance in lobster olfactory receptor cells. J Neurosci. 1992;12:3979–3984. doi: 10.1523/JNEUROSCI.12-10-03979.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura T, Gold G. A cyclic nucleotide-gated conductance in olfactory receptor cilia. Nature. 1987;325:442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- 38.Pace U, Hanski E, Salomon Y, Lancet D. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature. 1985;316:255–258. doi: 10.1038/316255a0. [DOI] [PubMed] [Google Scholar]
- 39.Robertson G, Warmke J, Ganetzky B. Potassium currents expressed from Drosophila and mouse eag cDNAs in Xenopus oocytes. Neuropharmacology. 1996;35:841–850. doi: 10.1016/0028-3908(96)00113-x. [DOI] [PubMed] [Google Scholar]
- 40.Schoenmakers T, Visser G, Flik G, Theuvenet P. CHELATOR: an improved method for computing metal ion concentrations in physiological solutions. Biotechniques. 1992;12:870–879. [PubMed] [Google Scholar]
- 41.Warmke J, Drysdale R, Ganetzky B. A distinct potassium channel polypeptide encoded by the Drosophila eag locus. Science. 1991;252:1560–1562. doi: 10.1126/science.1840699. [DOI] [PubMed] [Google Scholar]
- 42.Woodard C, Huang T, Sun H, Helfand S, Carlson J. Genetic analysis of olfactory behavior in Drosophila: a new screen yields the ota mutants. Genetics. 1989;123:315–326. doi: 10.1093/genetics/123.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zagotta W, Germeraad S, Garber S, Hoshi T, Aldrich R. Properties of ShB A-type potassium channels expressed in Shaker mutant Drosophila by germline transformation. Neuron. 1989;3:773–782. doi: 10.1016/0896-6273(89)90246-8. [DOI] [PubMed] [Google Scholar]
- 44.Zar J. Biostatistical analysis. Prentice Hall; Upper Saddle River, NJ: 1996. [Google Scholar]
- 45.Zhong Y, Budnik V, Wu C-F. Synaptic plasticity in Drosophila memory and hyperexcitable mutants: role of cAMP cascade. J Neurosci. 1992;12:644–651. doi: 10.1523/JNEUROSCI.12-02-00644.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhong Y, Wu C-F. Modulation of different K+ currents in Drosophila: a hypothetical role for the Eag subunit in multimeric K+ channels. J Neurosci. 1993;13:4669–4679. doi: 10.1523/JNEUROSCI.13-11-04669.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]