Abstract
Serotonin (5-HT) is involved in the control of various behaviors inAplysia californica, including reproduction, feeding, locomotion, circadian rhythm, synaptic plasticity, and synaptic growth. The large variety of functions of 5-HT is mediated by different receptor subtypes that are coupled to different second-messenger systems. Here, we report the cloning of a cDNA coding for anAplysia G-protein-coupled 5-HT receptor (5-HTap1). Its deduced amino acid sequence resembles those of the 5-HT1 receptor subfamily. When expressed in stable cell lines, 5-HTap1 exhibits high-affinity binding for the serotonergic radioligand [N-methyl-3H]lysergic acid diethylamide. This binding is competed by several 5-HT agonists and antagonists, and the pharmacological profile of inhibition has some similarities with those of 5-HT1 and 5-HT7 receptors. Application of 5-HT or its agonists 5-carboxamidotryptamine maleate and (±)-8-hydroxy-2-(di-n-propyl-amino) tetralin hydrobromide on cells transformed with 5-HTap1produced a dose-dependent inhibition of forskolin-stimulated cAMP accumulation. 5-HTap1 is thus negatively coupled to adenylate cyclase. The production of antiserum against the 5-HTap1 receptor allowed us to examine its expression in animal tissues. The receptor protein is detected in every tissue examined, although it seems only weakly expressed in some samples. The receptor is also found in every ganglia of the nervous system, both in the sheath and in the neurons. 5-HTap1 mRNA is absent from the sheath, indicating that the protein observed there is probably located on the nerve terminals.
Keywords: Aplysia, 5-HT, 5-HT1 receptor, G-protein-coupled receptor, adenylate cyclase, inhibition
Serotonin (5-HT) has a large variety of functions both in vertebrates and invertebrates. It plays a role in feeding, circadian rhythm, locomotion, reproduction, and modulation of defensive behavior (Hen, 1993; Weiger, 1997). In mollusks, pharmacological and electrophysiological studies showed that 5-HT is a neuromodulatory neurotransmitter that acts on at least six different receptor subtypes to activate different postsynaptic responses (Gerschenfeld and Paupardin-Trich, 1974; Kadan and Hartig, 1988). In mammals, molecular cloning has shown the existence of at least 14 5-HT receptor subtypes (Hoyer et al., 1994). These receptors, which are members of the G-protein-coupled receptor family, can be classified on the basis of sequence identity and on the nature of the second messenger systems with which they are coupled. The 5-HT1,5and 5-HT4,6,7 subtypes inhibit or activate adenylate cyclase, respectively, whereas the 5-HT2 subtype stimulates phospholipase C. By contrast, the 5-HT3 subtype is a ligand-gated ion channel (Peroutka, 1995).
In the sensory neurons involved in defensive behavior, 5-HT increases the level of cAMP and activates protein kinase A (Bacskai et al., 1993), protein kinase C (Sugita et al., 1992; Byrne and Kandel, 1996), and MAP kinase (Martin et al., 1997). These actions of 5-HT are expected to be mediated by different 5-HT receptors (Braha et al., 1990; Mercer et al., 1991; Ghirardi et al., 1992; Emptage and Carew, 1993). In the neuroendocrine bag cell clusters, which are involved in the regulation of the egg-laying behavior, the application of 5-HT inhibits the bag cell afterdischarge that is itself dependent on the stimulation of adenylate cyclase (Jennings et al., 1981). 5-HT has also been reported to inhibit inhibitory interneurons of the tail withdrawal reflex pathway (Xu et al., 1995). Altogether, these results strongly suggest that the major mammalian 5-HT receptor types all have functional homologs in Aplysia. Recently, genes encoding two homologous 5-HT receptors have been cloned in Aplysia. Both receptors stimulate phospholipase C in response to 5-HT in a dose-dependent manner. However, they could not be readily grouped within any of the mammalian subgroups based on amino acid homologies (Li et al., 1995).
We have cloned and characterized cDNAs coding for an Aplysia5-HT receptor with significant sequence and functional homologies to the members of the vertebrate 5-HT1 receptor subfamily. Expression of 5-HTap1 in mammalian cells shows that the receptor is able to specifically bind 5-HT at nanomolar concentrations, as well as diverse serotonergic ligands. In addition, 5-HT and 5-HT agonists induce inhibition of adenylate cyclase in cells stably expressing 5-HTap1. Using RT-PCR and Western blot analysis, we detected expression of this receptor in the CNS and in peripheral tissues.
MATERIALS AND METHODS
Compounds. [2,8-3H]Adenine and [N-methyl-3H]lysergic acid diethylamide ([3H]LSD) were purchased from DuPont NEN (Mississauga, Ontario, Canada). Forskolin, ATP, cAMP, 5-HT, and isobutylmethylxanthine (IBMX) were from Sigma-Aldrich (Oakville, Ontario, Canada). 5-Carboxamidotryptamine maleate (5-CT) and methiothepin mesylate were from Research Biochemicals (Natick, MA). Alprenolol hydrochloride, clozapine, dopamine hydrochloride, (±)-8-hydroxy-2-(di-n-propyl-amino) tetralin hydrobromide (8-OH-DPAT), ketanserin tartrate, mesulergine hydrochloride, metergoline, methysergide maleate, NAN-190 hydrobromide,p-aminophenethyl-m-trifluoromethylphenyl piperazine, R-(+)-SCH-23390 hydrochloride, spiperone hydrochloride, and yohimbine hydrochloride were generous gifts from BioSignal Inc. (Montréal, Québec, Canada).
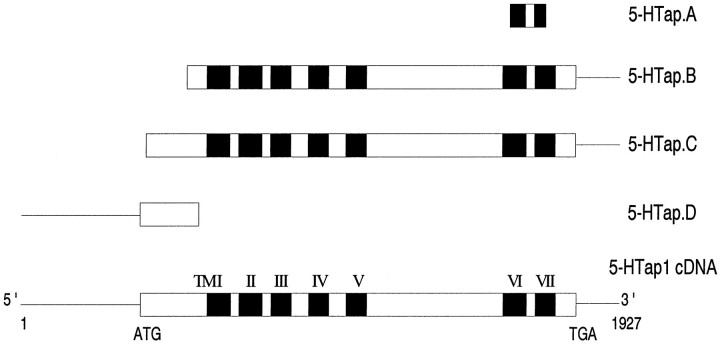
PCR amplification and screening of cDNA libraries. Phage DNAs (100 ng) isolated from Aplysia kidney and CNS cDNA libraries constructed in the λGT10 vector were PCR-amplified for 40 cycles (94°C for 1.5 min, 40°C for 2 min, and 72°C for 1 min) in the presence of two primers corresponding to highly conserved 5-HT receptor sequences located in transmembrane domains 6 and 7: 5′-(G,C)IGCITT(T,C)ITIITITG(C,T) TGG(C,T)TGG(C,T)TICCITT(C,T)TT-3′ and 5′-TCIGGII(A,T) (G,A)AAIATIG(T,C)(G,A)TA(G,A)ATIA(T,C)IGG(A,C)TT-3′. The PCR products were fractionated on a 4% agarose gel, and the 163 bp fragment (5-HTap.A), present in both the CNS and kidney samples, was subcloned and sequenced (Fig.1).
Fig. 1.
Molecular cloning of the 5-HTap1receptor cDNA. The 5-HTap.A fragment was PCR-amplified from DNA isolated from CNS and kidney cDNA libraries, using degenerate primers. The 5-HTap.B and 5-HTap.C cDNAs were isolated from a kidney cDNA library using a PCR-screening strategy (Israel, 1993). The 5-HTap.D was PCR-amplified from DNA isolated from the kidney cDNA library using nested antisense 5-HTap1-specific primers and a sense (GT10-specific) primer. The reconstituted full-length cDNA is schematized at thebottom. The open reading frame is represented byboxes, and the putative transmembrane domains are indicated by the dark regions. The linesrepresent the 5′ and 3′ untranslated regions of the transcript.
To clone the full-length cDNA, we used the PCR screening method previously described by Israel (1993). Briefly, an overnight culture ofEscherichia coli strain LE392 was infected with 4 × 106 phages from an Aplysia kidney cDNA library. After an incubation at 37°C for 20 min, the culture was diluted to 20 ml and used to fill a 64-well plate with 20,000 phage per well. The plate was incubated at 37°C until the phage titer reached ∼1 × 109 pfu/ml. Phage from eight wells across a row or eight wells down a column were pooled (25 μl/well) and diluted 1:1 with distilled water. The matrix of 64 wells was therefore reduced to 16 pools, which were used as templates for PCR analysis. PCR reactions were performed in a final volume of 25 μl using 0.5 μl of pooled phage culture as template and 200 ng of the degenerate primers described above. The reaction was held 10 min at 94°C to help phage denaturation before PCR amplification. PCR reaction products were electrophoresed through a 3% agarose gel, transferred to a Hybond N+ membrane (Amersham, Oakville, Ontario, Canada), and hybridized with the 5-HTap.A fragment at high stringency. Phage DNAs in wells located at the junction of positive rows and columns were individually PCR-amplified under the same conditions. Phages in single positive wells were plated and two clones, 5-HTap.B and 5-HTap.C (Fig. 1), were isolated using the plaque-lifting method (Sambrook et al., 1989). Their inserts were subcloned and sequenced. To clone the missing 5′ end of the transcript, we PCR-amplified the isolated phage DNA from the kidney cDNA library, using a λGT10-specific primer, 5′-AGCAAGTTCAGCCTGGTTAGTC-3′, and two nested 5-HTap1-specific primers, 5′-GATGAGACTCAGAGGATGAC-3′ and 5′-ATACAGCAACAGTTCAGG-3′. The product of the second PCR amplification (5-HTap.D) was subcloned in pCR 2.1 (Invitrogen, San Diego, CA) and sequenced (Fig. 1).
Cell lines. The coding region of 5-HTap1 was PCR-amplified with the primer pairs 5′-CAGC GAATTCCAGAGGATGGGAAGAAACG-3′ and 5′-CCGC GAATTCTCACTACGTAATTCGGTTCAC-3′ [nucleotides (nt) 320–1777], digested with EcoRI, and subcloned into the pBact-myc vector (Cravchik and Matus, 1993), in frame with the c-myc epitope EQKLISEEDLN (Degols et al., 1991). The pBact-myc/5-HTap1 construct was then partially digested with HindIII to remove the full-length c-myc/5-HTap1 fragment, and the resulting fragment was subcloned in pCDNA3/RSV (Jockers et al., 1996). The recombinant plasmid was introduced into HEK 293 cells by calcium phosphate-mediated transfection, and the transfected cells were selected in Geneticin (Life Technologies, Burlington, Ontario, Canada), to establish permanent cell lines. Isolated foci were amplified, and expression of the receptor gene was confirmed by indirect surface immunofluorescence using a monoclonal c-myc-specific antibody (a kind gift of M. Bouvier, Université de Montréal, Montréal, Québec, Canada) and fluorescein-conjugated rabbit anti-mouse Igs (Dako, Mississauga, Ontario, Canada) as the secondary antibody. Cell lines expressing the highest levels of the receptor protein at the cell surface were used in the functional assays.
Ligand binding analysis. HEK cells expressing 5-HTap1 were grown to 90–100% confluence, and membranes were prepared as described by Kohen et al. (1996). Membrane pellets were resuspended in (in mm): 75 Tris, pH 7.4, 5 MgCl2, and 2 EDTA buffer at a concentration of ∼250 μg protein/ml. For saturation experiments, 10 μg of membrane proteins were incubated in duplicate with increasing concentrations of tritiated lysergic acid diethylamide ([3H]LSD, 71.5 Ci/mmol; 1 Ci = 37 GBq) for 60 min at room temperature in a total volume of 200 μl. Competition binding assays were done in duplicate with 10 μg membrane proteins, in the presence of increasing concentrations of the competing agent (10−12–10−4m) and 1.5 nm [3H]LSD for 60 min at room temperature. Preliminary assays had shown that saturation was reached within 30 min and remained stable for at least 2 hr at room temperature (data not shown). All assays were terminated by rapid filtration over Whatman GF/C glass fiber filters (Xymotech Biosystems, Mt. Royal, Québec, Canada) and rinsed three times with 50 mmTris, pH 7.4. Nonspecific binding was defined with 10 μmmethiothepin. The amount of bound [3H]LSD was determined by scintillation spectrophotometry (Wallac 1409 liquid scintillation counter).
Adenylate cyclase activity. The cAMP content of cells stably expressing 5-HTap1 was measured by the prelabeling technique as described by Ansanay et al. (1992). Cells were cultured in 12-well plates. When apparent confluence was reached, cells were incubated with 2 μCi/ml [3H]adenine. After 2–3 hr, the cultures were washed and incubated with 2.5 mmIBMX, 2.5 μm forskolin, and the indicated drugs in a final volume of 1 ml PBS for 20 min at 37°C. The reaction was stopped by aspiration of the medium and addition of 1 ml of ice-cold 5% trichloroacetic acid. Cells were scraped with a rubber policeman, and 100 μl of a solution of 5 mm ATP and 5 mmcAMP were added to the mixture. Cellular proteins were centrifuged at 5000 × g. [3H]ATP and [3H]cAMP were separated by sequential chromatography on Dowex and alumina columns (Bio-Rad, Mississauga, Ontario, Canada) (Salomon et al., 1974). cAMP formation corresponded to the conversion: [3H]cAMP/([3H]ATP + [3H]cAMP) × 1000. Results are expressed as a percentage of the maximal cAMP accumulation.
Antibody production and immunoblotting. The 5-HTap1 sequence corresponding to the third cytoplasmic loop was PCR-amplified with the primer pair 5′-ATCA GAGCTCAGATATATCGCGCACGTCGG-3′ and 5′-AACA AAGCTTGCCAGACTTTCCTTTCTCGC-3′ (nt 1068–1523), digested with SacI and HindIII, and subcloned into the pQE30 prokaryote expression vector (Qiagen, Mississauga, Ontario, Canada), thus fusing a 6XHis tag to its N-terminal extremity. This domain of the protein was chosen because of the very low conservation of the primary structure of this region among different G-protein-coupled receptors. The fusion protein was purified on a nickel-nitrilo-tri-acetic acid resin column and used to inoculate rabbits for antibody production as described previously (Aloyz and DesGroseillers, 1995). For Western blotting, plasma membranes from freshly dissected tissues were prepared on a sucrose cushion as described in Bawab et al. (1992), electrophoresed on a 10% SDS polyacrylamide gel, and electroblotted onto nitrocellulose membranes. The nitrocellulose membranes were blocked with 5% nonfat dry milk for 1 hr at room temperature and then incubated with the primary antibody for 1 hr. After washing, the membrane was incubated with a horseradish peroxidase-conjugated secondary antibody (Dako) and immunoreactive bands were detected by a chemiluminescent substrate reaction (Pierce, Rockford, IL).
RT-PCR. Reverse transcription experiments were performed on total RNA extracted from the desheathed ganglia of the nervous system and from the sheath itself. RNA was purified using Trizol reagent (Life Technologies) according to the manufacturer’s instructions. RNA was reverse transcribed using an oligo-dT16 primer and Moloney murine leukemia virus reverse transcriptase (Perkin-Elmer, Foster City, CA) as described previously (Angers and DesGroseillers, 1998). A DNA fragment encompassing nt 1068–1523 was amplified using the same primers and conditions as described above. The presence of contaminating genomic DNA was monitored by amplification of the same samples without reverse transcription. The PCR products were fractionated on a 2% agarose gel and transferred to a Hybond N+ membrane (Amersham) for hybridization with a specific oligonucleotide probe. As a positive control, we used actin primers as described byDesGroseillers et al. (1994).
RESULTS
Isolation and structure of the gene encoding 5-HTap1
The striking sequence conservation of transmembrane domains six and seven of G-protein-coupled receptors was used in a PCR approach to isolate a DNA fragment encoding the corresponding region of a putative 5-HT receptor in Aplysia. Using degenerate oligonucleotide primers, we amplified a 163 bp fragment that shared 56% amino acid sequence identity with mammalian 5-HT1D receptors. These primers and the PCR-amplified fragment were then used to isolate two clones from a kidney cDNA library, using a PCR-based screening strategy (Fig. 1). The missing 5′ end of the transcript was PCR-amplified with DNA isolated from the cDNA library, using nested oligonucleotides derived from the cDNA and a paired primer derived from the λGT10 vector sequence.
The reconstituted cDNA is 1927 nt long. Its longest open reading frame of 1476 nt codes for a putative protein of 492 amino acids (Fig.2). Hydrophobicity analysis of the deduced amino acid sequence revealed the presence of seven stretches of hydrophobic residues that represent the seven transmembrane domains characteristic to all G-protein-coupled receptors (data not shown). Amino acid sequence identity between 5-HTap1 and other 5-HT receptors within the transmembrane domains and adjacent regions is 62.4% to Lymnaea 5-HTlym1; 54.7% toDrosophila 5-HTdro2A-B; 51.8% to human 5-HT1A; 49.6% to human 5-HT1D; 49.3% to human 5-HT1F; 47.8% to human 5-HT1E; 47.1% to mouse 5-HT1B; 42.7% to mouse 5-HT5A-B; 41.7% to mouse 5-HT7; 41.2% to 5-HTdro1; 35.6% to rat 5-HT2C; 35.5% to Lymnaea5-HTlym2; 35.2% to rat 5-HT4; 30.6% to Aplysia 5-HTapB1–2; and 29.6% to rat 5-HT6. A dendrogram analysis of amino acid sequence comparisons within the transmembrane domains indicates that 5-HTap1, along with other invertebrate 5-HT receptors, is associated with the mammalian 5-HT1 receptor family (Fig. 3).
Fig. 2.
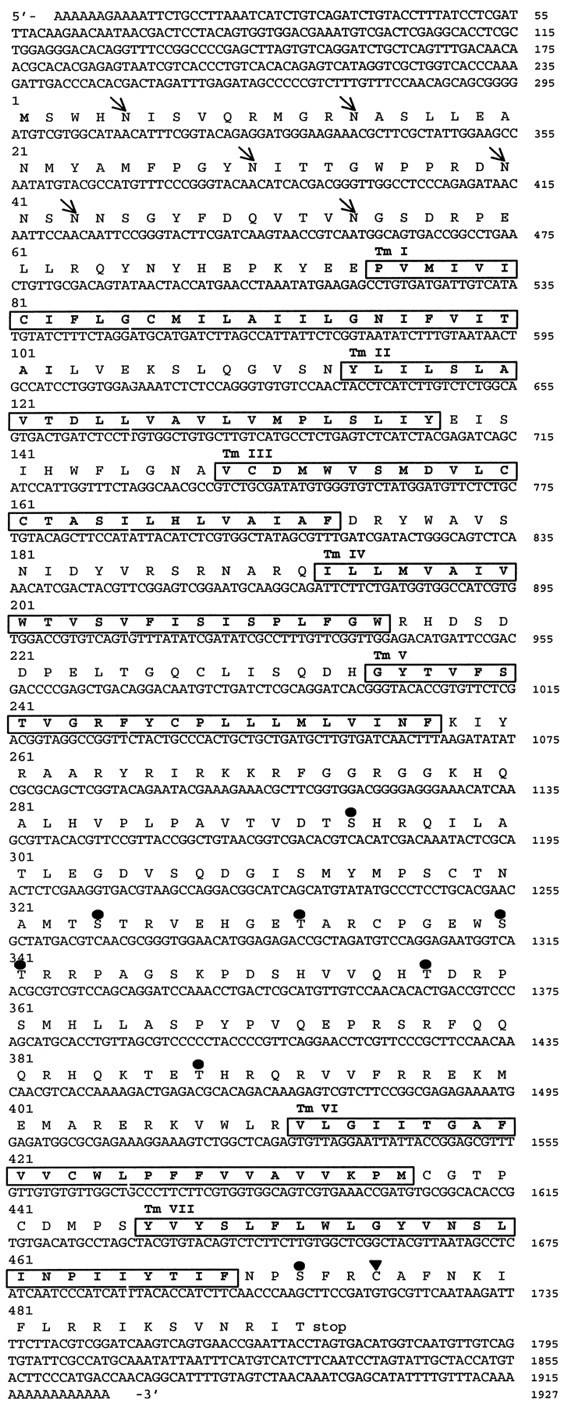
Nucleotide and deduced amino acid sequence of the 5-HTap1 cDNA. Putative transmembrane regions areboxed and numbered TMI–VII.Arrows indicate the position of consensus sites for N-linked glycosylation. Serines and threonines that are within a consensus sequence for phosphorylation by protein kinase C are indicated by circles (•). A potential palmitoylation site is indicated by a triangle (▴). The nucleotide sequence is available in the GenBank database under accession numberAF041039.
Fig. 3.
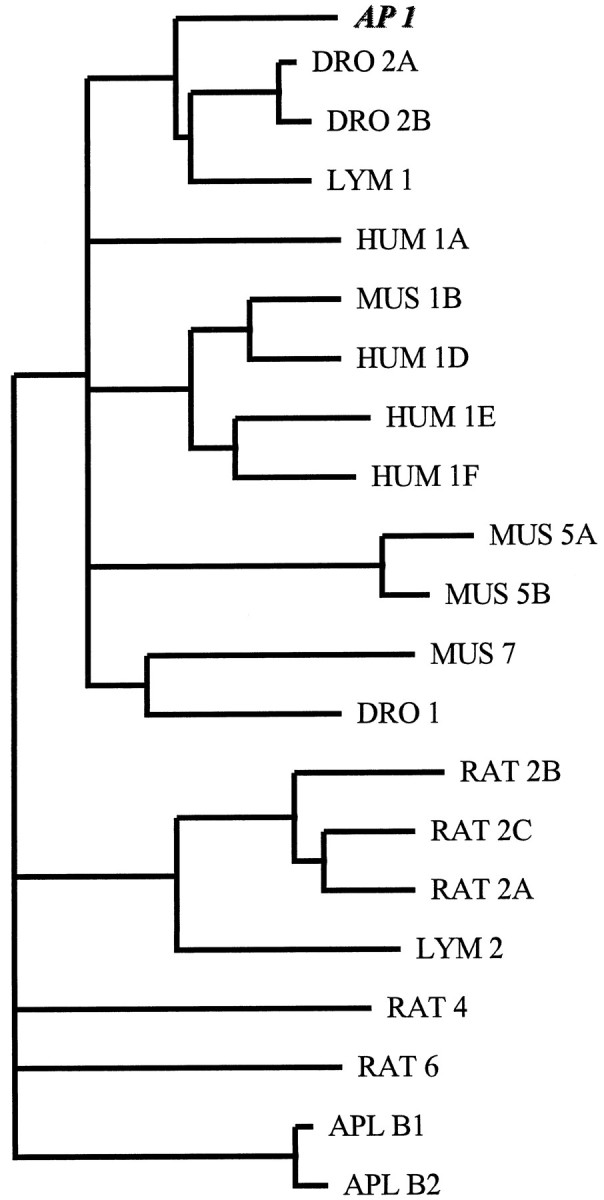
Phylogenetic analysis of 5-HT receptors. Amino acid sequences of 5-HT receptors were retrieved from the GenBank database. Their amino acid sequence, excluding their N termini and their third cytoplasmic loops, were aligned with the corresponding amino acid sequence of 5-HTap1, using the ClustalW package (Thompson et al., 1994). The alignment was then used for phylogenetic comparisons using the PHYLIP package (J. Felsenstein, 1993, PHYLIP 3.5.1c; University of Washington, Seattle, WA). Analysis was performed with a bootstrap procedure that computes the probability of occurrence of the branches for 100 possible trees. Branching order was determined using the Fitch-Margoliash algorithm included in the PHYLIP package. Only branches occurring in >80 trees are represented.HUM, human; MUS, mouse; RAT, rat.
Six consensus sites for N-linked glycosylation are present in the extracellular N terminus of 5-HTap1 (Fig. 2). Within the third cytoplasmic loop, seven serine or threonine residues may be used as a substrate for phosphorylation by protein kinase C. Another potential phosphorylation site for protein kinase C is found within the C-terminal intracellular domain. A cysteine residue is also found in this domain, suggesting that the receptor may be palmitoylated. The structure of the receptor, with its large third cytoplasmic loop and its short C-terminal tail, is similar to that of the vertebrate 5-HT1 receptor family, which groups receptors coupled to Gi (Boess and Martin, 1994).
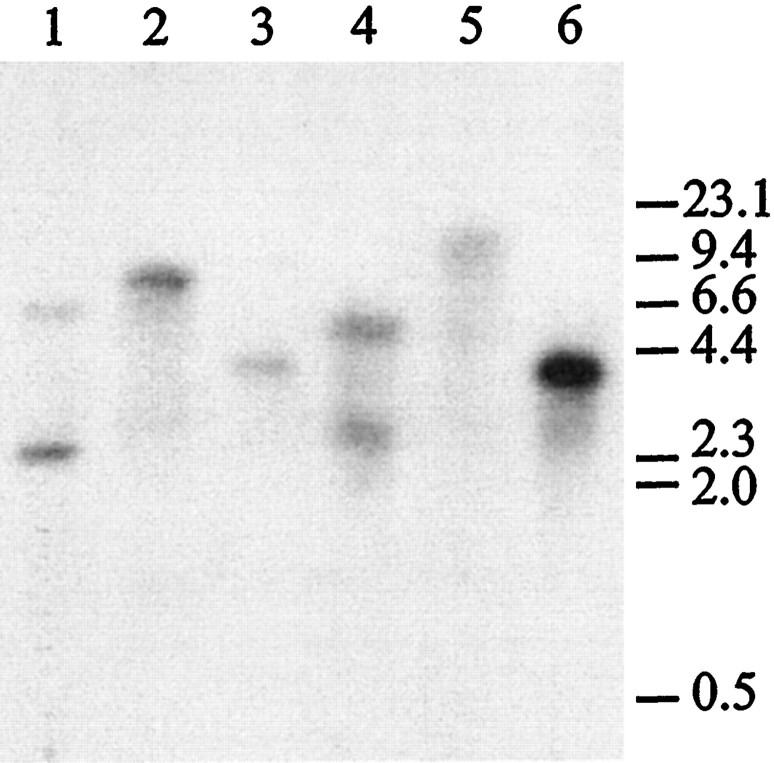
A Southern blot analysis of genomic DNA (Fig.4) reveals the presence of a single band when the restriction enzyme used to digest the DNA has no restriction site in the region covered by the probe and two bands when the restriction site is present in the cDNA probe sequence. This indicates that the gene is probably intronless. This conclusion was confirmed by partial sequencing of a genomic clone and PCR amplification of genomic DNA (data not shown). The Southern blot analysis also indicates that 5-HTap1 is unique in the genome and does not have a close homolog. This is in contrast to the Aplysia5-HTapB1 and 5-HTapB2 receptors, which share 86% identity at the nucleotide level (Li et al., 1995).
Fig. 4.
Southern blot analysis of the 5-HTap1gene. Genomic DNA was isolated from ovotestis, purified, and digested with BamHI (lane 1), BglII (lane 2), EcoRI (lane 3),HindIII (lane 4),SacI (lane 5), or XbaI (lane 6), as described previously (Wickham and DesGroseillers, 1991). Digested DNA was run on a 1% agarose gel, transferred to a Hybond N+ membrane, and hybridized with a32P-labeled 5-HTap.B cDNA fragment (nt 457–1927).BglII, EcoRI, SacI, andXbaI sites are not present in the probe sequence, whereas BamHI and HindIII appear once. The molecular weight marker is λ phage DNA digested withHindIII. The molecular weights are indicated in kilobase pairs.
Pharmacology
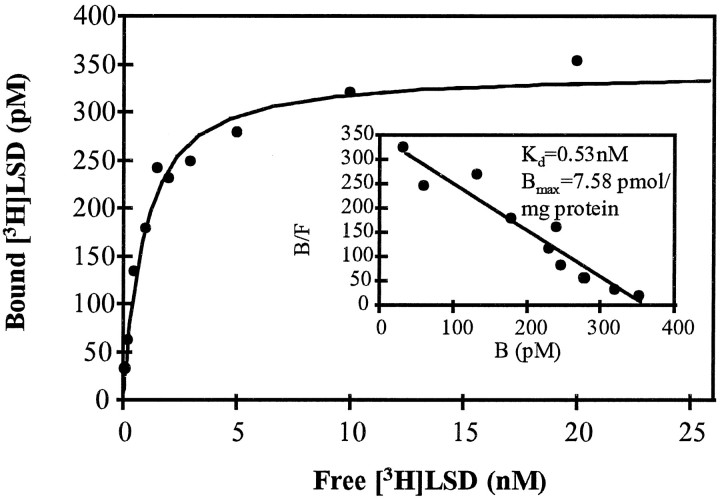
The coding region of 5-HTap1 was cloned in an expression vector in fusion with a c-myc epitope at its N terminus. The vector was transiently expressed in COS-7 and HEK 293 cells, and the expression of the receptor at the plasma membrane was monitored with the anti-myc antibodies (data not shown). The receptor with its myc tag was then used to transfect mammalian HEK 293 cells to establish permanent cell lines. Membranes isolated from one of these cell lines bound [3H]LSD in a saturable and dose-dependent manner with an estimated Kd of 0.56 (0.03 nm) and a receptor density of 6.89 (0.61 pmol/mg of protein; n = 3) (Fig. 5). Similar results were obtained from two independent cell clones and from transiently transfected COS-7 cells, for a meanKd of 0.51 (0.13 nm;n = 6). Of course, receptor density varied in each transfection experiment and in each clone tested.
Fig. 5.
Saturation analysis of [3H]LSD binding on the 5-HTap1receptor. Membranes harvested from stable cell lines expressing 5-HTap1 were incubated with increasing concentrations of [3H]LSD (0.1–20 nm) for 1 hr at room temperature. Nonspecific binding was defined in the presence of 10 μm unlabeled methiothepin. Results are those of a single experiment, but similar results were obtained in six different binding experiments. The Kd andBmax values were determined with the AllFit for Windows 2.1 computer program (C. De Léan and A. De Léan, 1993, Université de Montréal, Montréal, Québec, Canada). Inset, Scatchard plot of the same data.
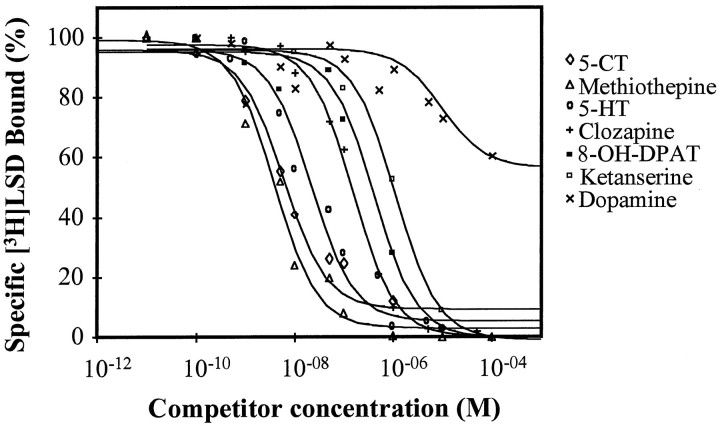
The rank order of potency of various serotonergic agonists and antagonists for the inhibition of [3H]LSD binding to HEK membranes is listed in (Table 1) and illustrated in Figure 6. Briefly, 5-HTap1 showed high-affinity binding to 5-HT, which is comparable to the affinity of 5-HT for the mammalian 5-HT1A,B,D-F, and 5-HT7 receptors (Hoyer et al., 1994). Compounds that show high binding affinity to the mammalian 5-HT1 receptors, such as 5-CT, methiothepin, PAPP, and methysergide, also bound 5-HTap1 in the nanomolar range. By contrast, metergoline, clozapine, mesulergine, and ketanserin, compounds that bind to 5-HT2 receptors with high affinity, showed a lower affinity for 5-HTap1. NAN-190, a very specific 5-HT1A antagonist, showed very poor affinity for 5-HTap1. Interestingly, 8-OH-DPAT, a 5-HT1A-specific agonist, bound 5-HTap1 with a better affinity than most other 5-HT receptors, except for 5-HT1A itself. Very high concentrations of dopamine, spiperone, or alprenolol were necessary to displace [3H]LSD binding, confirming the serotonergic nature of 5-HTap1. Overall, the pharmacological profile of 5-HTap1 seems related to the 5-HT1 and 5-HT7 receptor profiles, although not clearly associated with either one.
Table 1.
Affinities of various compounds that compete with the binding of 1.5 nM [3H]LSD to the membranes of HEK 293 cells stably transfected with the 5-HTap1 gene
| Drug | Ki (nM) |
|---|---|
| 5-CT | 1,04 |
| Methiothepin | 2,62 |
| PAPP | 9.50 |
| 5-HT | 13.23 |
| Methysergide | 15.01 |
| Clozapine | 56.63 |
| 8-OH-DPAT | 73.96 |
| Metergoline | 90.66 |
| Yohimbine | 222.37 |
| Mesulergine | 245.52 |
| Ketanserin | 288.18 |
| SCH 23390 | 616.14 |
| NAN 190 | >1000 |
| Dopamine | >1000 |
| Spiperone | >1000 |
| Alprenolol | >1000 |
Affinity estimates are given as Ki values in nanomolar concentrations and were determined by a computer-assisted nonlinear curve analysis (De Léan and De Léan, 1993).Ki values are expressed as the mean of at least three different determinations.
Fig. 6.
Inhibition of specific [3H]LSD binding to the Aplysia5-HTap1 receptor. Membranes from stable cell lines expressing 5-HTap1 were incubated with 1.5 nm[3H]LSD in the presence or absence of increasing concentrations of unlabeled competitors. Nonspecific binding was defined in the presence of 10 μm unlabeled methiothepin. Results at each concentration are presented as a percentage of the specific binding in the absence of the competitor. Results are from a single experiment but are representative of three such experiments. Data were analyzed by a computer-assisted nonlinear analysis (C. De Léan and A. De Léan, 1993, AllFit for Windows 2.1; Université de Montréal).
Functional coupling
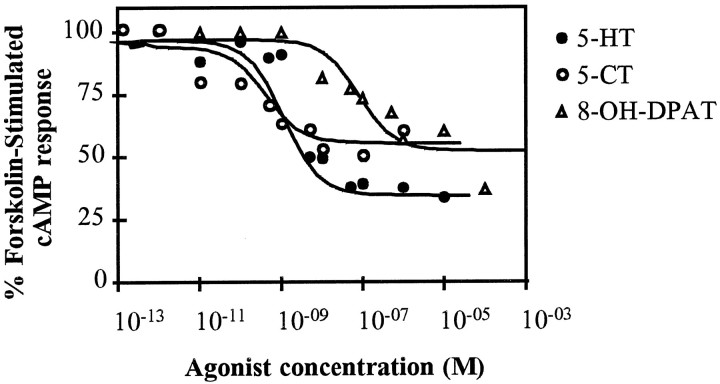
The molecular structure and pharmacological profile of 5-HTap1 suggested that this receptor is coupled to Gi and therefore could inhibit adenylate cyclase. To verify this hypothesis, we measured the accumulation of [3H]cAMP in the 5-HTap1-expressing HEK cells after application of 5-HT or agonists. As expected, forskolin-induced cAMP accumulation was efficiently inhibited by 5-HT and the other serotonergic agonists, 5-CT and 8-OH-DPAT, in a dose-dependent manner (Fig. 7). The concentrations of agonists at which inhibition of adenylate cyclase was effective corresponded to those that were shown to be necessary for competing [3H]LSD binding. 5-HT produced no inhibition of cAMP accumulation in the presence of 100 nmmethiothepin. 5-HT concentrations ranging from 10 nm to 10 μm had no effect on cAMP levels in untransfected HEK cells treated with forskolin (data not shown). Altogether, these experiments demonstrate that 5-HTap1 is functionally coupled to the mammalian Gi subunit, and that it inhibits adenylate cyclase and cAMP accumulation in the heterologous expression system.
Fig. 7.
5-HT- and agonist-induced decrease in cAMP levels in cell lines expressing the 5-HTap1 receptor. cAMP levels are expressed as a percentage of the value obtained with 2.5 μm forskolin (100%) in the absence of 5-HT or agonists. The values are the mean of an experiment done in triplicate and are representative of three such experiments. Data were analyzed by a computer-assisted nonlinear analysis (C. De Léan and A. De Léan, 1993, AllFit for Windows 2.1; Université de Montréal).
5-HTap1 protein distribution
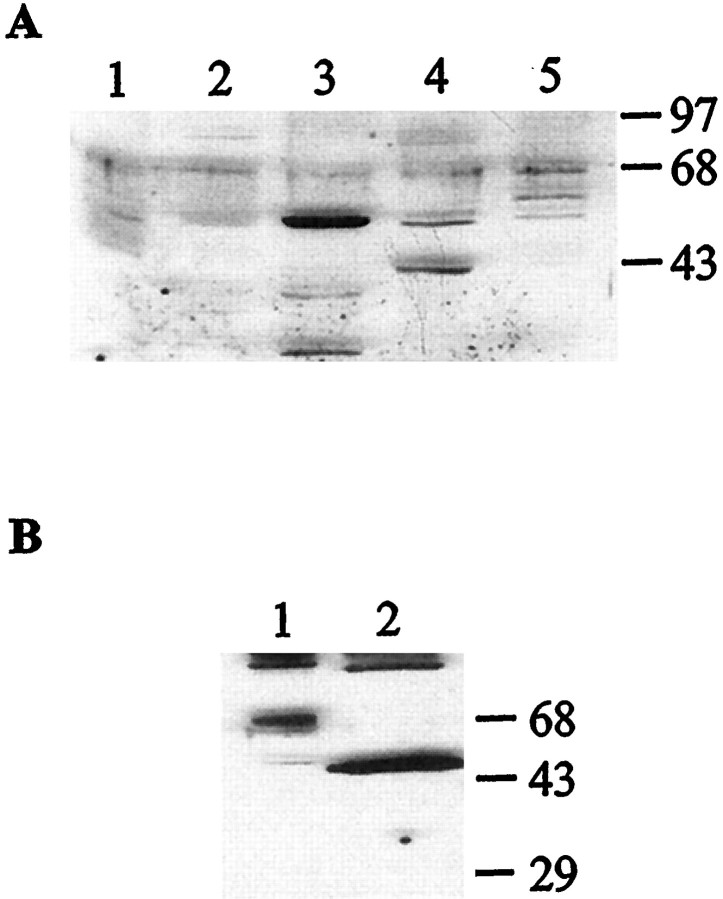
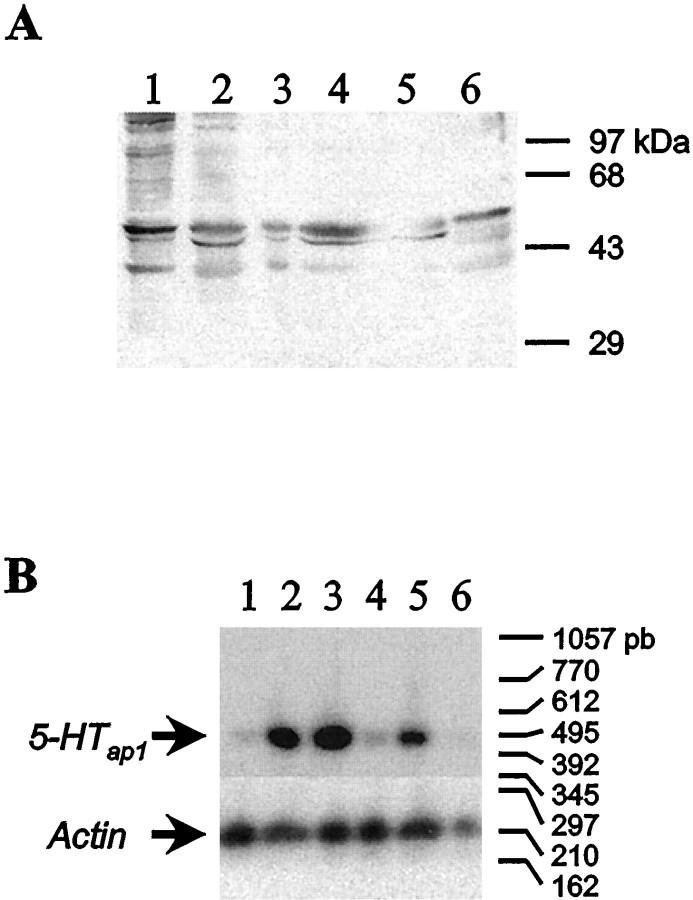
Plasma membrane extracts from various Aplysia tissues were prepared and used in an immunoblot analysis to detect the presence of the receptor. Different bands ranging in size from 45 to 71 kDa were detected in the gill, heart, hermaphroditic duct, kidney, and ovotestis extracts (Fig. 8A). To determine whether the differences in the size of the receptor can be explained by different patterns of glycosylation, kidney membrane extracts were treated with deglycosidase F (New England Biolabs, Mississauga, Ontario, Canada) and separated by SDS-PAGE. After treatment, the observed molecular weight of the receptor is decreased to 45 kDa, which is the size expected from the cDNA sequence (Fig.8B). In the CNS, the protein is detected in all ganglia, as well as in the sheath, with the strongest signal observed in the abdominal and pedal ganglia (Fig.9A). These results were confirmed by RT-PCR experiments. The mRNA could be amplified from all desheathed ganglia but not from the sheath itself (Fig. 9B). There is a poor correlation between the mRNA and protein levels, suggesting that the protein is expressed at the level of the nerve terminals.
Fig. 8.
Western blot analysis of the expression of the 5-HTap1 receptor in different Aplysiatissues. Proteins were resolved by SDS-PAGE and analyzed by immunoblotting with antiserum raised against the third cytoplasmic loop of the 5-HTap1 receptor. A, Thirty micrograms of cytoplasmic membrane protein extracts prepared from gill (lane 1), heart (lane 2), hermaphroditic duct (lane 3), kidney (lane 4), and ovotestis (lane 5). B, Fifty micrograms of kidney plasma membrane proteins before (lane 1) or after (lane 2) treatment with deglycosidase F to remove N-linked sugars. Molecular weight marker positions are indicated in kilodaltons.
Fig. 9.
Western blot and RT-PCR analysis of the expression of the 5-HTap1 receptor in the CNS. A, Fifty micrograms of total protein extracted from desheathed ganglia and sheath alone were resolved by SDS-PAGE and analyzed by immunoblotting with antiserum raised against the third cytoplasmic loop of the 5-HTap1 receptor. Abdominal (lane 1), buccal (lane 2), cerebral (lane 3), pedal (lane 4), and pleural (lane 5) ganglia from five animals were pooled. The sheath extract (lane 6) was prepared from a single animal. B, RT-PCR analysis was conducted on total RNA isolated from the same samples as in A. The PCR products were analyzed by Southern blotting probed with a 5-HTap1-specific oligonucleotide. The same procedure was followed for amplification of the control (actin). The sizes of the bands were estimated by comparison with the DNA size markers on the original ethidium bromide-stained gel.
DISCUSSION
We have isolated and characterized an Aplysia 5-HT receptor that belongs to the G-protein-coupled receptor family. Its structure and biochemical properties are closely related to those of the invertebrate 5-HTlym1 and 5-HTdro2A-2B and to those of the vertebrate 5-HT1 receptor subtypes. The absence of introns in the gene, the presence of a large third cytoplasmic loop and a short C-terminal domain in the protein, and a functional coupling to the inhibition of adenylate cyclase in stably transfected cells are all features of the 5-HT1 receptor subfamily. 5-HTap1 is therefore clearly associated with this vertebrate receptor subfamily. Two other 5-HT receptors have recently been cloned in Aplysia (Li et al., 1995). These receptors are closely related to each other and are linked to the phospholipase C pathway. They show very little sequence identity with 5-HTap1 and therefore are clearly members of another subfamily of 5-HT receptors. In Lymnaea, a 5-HT receptor with some similarities to vertebrate 5-HT1 receptors has been cloned (Sugamori et al., 1993). However, its functional coupling to a second messenger system has not been determined, and its pharmacological profile is different from that of 5-HTap1, suggesting that they may not be homologous.
Like other invertebrate G-protein-coupled receptors (Saudou et al., 1992; Li et al., 1995; Gerhardt et al., 1997), 5-HTap1 is efficiently coupled to mammalian G-proteins when expressed in mammalian cells. This underlines the high conservation among species of members of the G-protein-coupled receptor subfamily and the functional conservation of mechanisms that link these receptors to G-proteins. In this respect, it is not surprising to observe that the biochemical characterization of Gsα, Goα, and Giα, as well as Gβ, in theAplysia nervous system has demonstrated that these proteins are very similar to their mammalian counterparts (Critz et al., 1986;Vogel et al., 1989).
The pharmacological profile of 5-HTap1 is also reminiscent of those of 5-HT1-like receptors. TheKd and Ki values demonstrated the high affinity of the 5-HTap1 receptor for LSD, 5-CT, methiothepin, and 5-HT and are comparable to the values obtained with vertebrate 5-HT1 receptors (Boess and Martin, 1994). Interestingly, these ligands bind to all vertebrate 5-HT1 receptor subtypes with high affinity. By contrast, ligands that show a high specificity to the 5-HT1Areceptors, such as 8-OH-DPAT and NAN-190, do not bind 5-HTap1 efficiently. Their binding efficiency is more closely related to that of the 5-HT7 receptor than to those of other vertebrate 5-HT1 receptors (Ruat et al., 1993). These results suggest that the structure and function of the 5-HTap1 receptor are probably closely related to those of an ancestor gene, which existed before the divergence of the 5-HT receptor subtypes in vertebrates, and that it kept characteristics of more than one receptor subtype. Therefore, 5-HTap1 may be closer to the prototype of the early 5-HT1,7 receptors.
This view is in agreement with previous phylogenetic studies that suggested that the major subfamilies of 5-HT receptors diverged early in evolution to form three major subclasses: the 5-HT1(which includes 5-HT5 and 5-HT7), 5-HT2, and 5-HT6 receptor subclasses (Peroutka and Howell, 1994; also see Fig. 3). This divergence occurred before the evolution of vertebrates from invertebrates, and one can expect to find members of these major subclasses in invertebrate species (Peroutka, 1994). Further division within the 5-HT1and 5-HT2 subfamilies seems to have occurred after the separation of vertebrates and invertebrates, and homologs of these subtypes may not be found in mollusks. More than one 5-HT1receptor may nevertheless be found in Aplysia and in other invertebrates, but they will have emerged independently from vertebrate receptors.
The pharmacological profile of a receptor depends on the conservation of amino acids in and around the binding site of the receptor (Hibert et al., 1991; Ho et al., 1992; Chanda et al., 1993; van Rhee and Jacobson, 1996). Therefore, it is not surprising to find that the pharmacological profiles of mollusks and mammals are difficult to compare, considering the large phylogenetic distances. Nevertheless, whatever its pharmacological profile, the characterization of the second messenger pathway with which the receptor is linked clearly associates 5-HTap1 to the 5-HT1 subfamily and not to 5-HT7.
Very few 5-HT-dependent pathways have been characterized on a pharmacological basis in Aplysia. None of these generated a 5-HTap1-like pharmacological profile. In earlier works, binding experiments using crude membrane preparations of different tissues revealed a [3H]LSD binding site with a dissociation constant of 0.63 nm (Drummond et al., 1980). Similar results were obtained on tissue sections using [125I]LSD (Kadan and Hartig, 1988). Although this value is consistent with our data on the 5-HTap1 receptor expressed in mammalian cells, competition of the LSD binding sites in the crude tissue membranes with 5-HT gave Kivalues at least two orders of magnitude higher than the one observed for 5-HTap1 (Drummond et al., 1980; Kadan and Hartig, 1988). In addition, Drummond et al. (1980) demonstrated that there was a good correlation between the amount of [3H]LSD binding sites and the amount of 5-HT-induced adenylate cyclase activity in most tissues. Altogether, these results suggest that although 5-HTap1 is expressed in all these tissues, it was not recognized in these binding assays. It also suggests that more than one 5-HT receptor is present in most Aplysia tissues, and therefore, the binding assay with Aplysia tissue membranes probably recognized a heterogeneous population of receptors, with similar affinities for LSD.
More recently, Ram et al. (1994) reported the existence of anAplysia 5-HT1A-like receptor in theAplysia buccal muscle. In this system, 5-HT is known to potentiate the acetylcholine-elicited contractions. This effect was shown to be efficiently competed by NAN-190 in the nanomolar range and mimicked by 8-OH-DPAT in the micromolar range, thus defining a 5-HT1A-like site. However, this receptor is likely to be different from 5-HTap1, because we showed that 5-HTap1 does not bind NAN-190 at these concentrations. These last observations strongly suggest that at least one other 5-HT1-like receptor exists in Aplysia.
The application of 5-HT has generally been associated with an increase in cAMP levels in Aplysia. Nevertheless, application of 5-HT often triggers inhibitory responses, although the downstream pathway is not yet determined (Jennings et al., 1981; Xu et al., 1995). In addition, functional coupling of a receptor may vary in different cell types, and stimulation of a single receptor subtype may mediate different responses under different physiological conditions. For example, the mammalian 5-HT1A receptor has been shown to inhibit adenylate cyclase via its interaction to Gi protein and also to nonenzymatically mediate the opening of K+ channels via an interaction with a different, unidentified G-protein subunit (Andrade et al., 1986). Moreover, it is known that the stimulation of different Gi-coupled receptors in different cell types leads to the activation of MAP kinase through the Gβγ subunits (Koch et al., 1994). The presence of 5-HTap1 in many Aplysia tissues suggests that it may play a variety of roles linked to the inhibition of adenylate cyclase, activation of MAP kinase, and/or modulation of other pathways. It will be particularly interesting to determine whether 5-HTap1 is expressed in some sensory neuron clusters of the CNS (Storozhuk et al., 1998) and whether it is involved in the 5-HT-induced activation of MAP kinase in these cells. This activation is implicated in the establishment of long-term facilitation in cultured neurons (Martin et al., 1997). Further investigation of the roles of the 5-HTap1 receptor in this animal is susceptible to give interesting new data on this aspect of cell signaling.
Footnotes
This work was supported by Medical Research Council of Canada grants to L.D.G. and V.F.C. A.A. and T.D. received studentships from the Fonds pour la Formation de Chercheurs et l’Aide à la Recherche du Québec and the Natural Sciences and Engineering Research Council of Canada, respectively. We thank Roger Bossé, Michel Bouvier, Michael Dennis, Jean Labrecque, André Laperrière, Sandrine Nouet, and Graciella Piñeyro for generous help and discussion in the pharmacological experiments, Christelle Bouchard for help in the library screening, and Louise Wickham for critical reading of this manuscript.
Correspondence should be addressed to Luc DesGroseillers, Département de biochimie, Université de Montréal, C.P. 6128, Succursale “Centre-Ville,” Montréal, Québec, Canada H3C 3J7.
REFERENCES
- 1.Aloyz RS, DesGroseillers L. Processing of the L5–67 precursor peptide and characterization of LUQIN in the LUQ neurons of Aplysia californica. Peptides. 1995;16:331–338. doi: 10.1016/0196-9781(94)00140-5. [DOI] [PubMed] [Google Scholar]
- 2.Andrade R, Malenka RC, Nicall RA. A G-protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986;234:1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- 3.Angers A, DesGroseillers L. Alternative splicing and genomic organization of the L567 gene of Aplysia californica. Gene. 1998;208:271–277. doi: 10.1016/s0378-1119(98)00009-2. [DOI] [PubMed] [Google Scholar]
- 4.Ansanay H, Sebben M, Bockaert J, Dumuis A. Characterization of homologous 5-hydroxytryptamine4 receptor desensitization in colliculi neurons. Mol Pharmacol. 1992;42:808–816. [PubMed] [Google Scholar]
- 5.Bacskai BJ, Hochner B, Mahaut-Smith M, Adams SR, Kaang BK, Kandel ER, Tsien RY. Spatially resolved dynamics of cAMP and protein kinase A subunits in Aplysia sensory neurons. Science. 1993;260:222–226. doi: 10.1126/science.7682336. [DOI] [PubMed] [Google Scholar]
- 6.Bawab W, Querido E, Crine P, DesGroseillers L. Identification and characterization of aminopeptidases from Aplysia californica. Biochem J. 1992;286:967–975. doi: 10.1042/bj2860967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boess FG, Martin IL. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33:275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 8.Braha O, Dale N, Hochner B, Klein M, Abrams TW, Kandel ER. Second messengers involved in the two processes of presynaptic facilitation that contribute to sensitization and dishabituation in Aplysia sensory neurons. Proc Natl Acad Sci USA. 1990;87:2040–2044. doi: 10.1073/pnas.87.5.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrne JH, Kandel ER. Presynaptic facilitation revisited: state and time dependence. J Neurosci. 1996;15:425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chanda PK, Minchin MCW, Davis AR, Greenberg L, Reilly Y, McGregor WH, Bhat R, Lubeck MD, Mizutani S, Hung PP. Identification of residues important for ligand binding to the human 5-hydroxytryptamine1A serotonin receptor. Mol Pharmacol. 1993;43:516–520. [PubMed] [Google Scholar]
- 11.Cravchik A, Matus A. A novel strategy for the immunological tagging of cDNA constructs. Gene. 1993;137:139–143. doi: 10.1016/0378-1119(93)90262-2. [DOI] [PubMed] [Google Scholar]
- 12.Critz SD, Harper JF, Byrne JH. Evidence for the inhibitory subunit of adenylate cyclase (Ni) in nervous tissue of Aplysia. Neurosci Lett. 1986;64:145–150. doi: 10.1016/0304-3940(86)90090-x. [DOI] [PubMed] [Google Scholar]
- 13.Degols G, Leonetti JP, Mechti N, Lebleu B. Antiproliferative effects of antisense oligonucleotides directed to the RNA of c-myc oncogene. Nucleic Acids Res. 1991;19:935–938. doi: 10.1093/nar/19.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DesGroseillers L, Auclair D, Wickham L, Maalouf M. A novel actin cDNA is expressed in the neurons of Aplysia californica. Biochim Biophys Acta. 1994;1217:322–324. doi: 10.1016/0167-4781(94)90293-3. [DOI] [PubMed] [Google Scholar]
- 15.Drummond AH, Bucher F, Levitan IB. Distribution of serotonin and dopamine receptors in Aplysia tissues: analysis by [3H]LSD binding and adenylate cyclase stimulation. Brain Res. 1980;184:163–177. doi: 10.1016/0006-8993(80)90595-8. [DOI] [PubMed] [Google Scholar]
- 16.Emptage NJ, Carew TJ. Long-term synaptic facilitation in the absence of short-term facilitation in Aplysia neurons. Science. 1993;262:253–256. doi: 10.1126/science.8211146. [DOI] [PubMed] [Google Scholar]
- 17.Gerhardt CC, Leysen JE, Planta RJ, Vreugdenhil E, Van Heerikhuizen H. Functional characterization of a 5-HT2 receptor cDNA cloned from Lymnaea stagnalis. Eur J Pharmacol. 1997;311:249–258. doi: 10.1016/0014-2999(96)00410-4. [DOI] [PubMed] [Google Scholar]
- 18.Gerschenfeld HM, Paupardin-Tritsch D. On the transmitter function of 5-hydroxytryptamine at excitatory and inhibitory monosynaptic junctions. J Physiol (Lond) 1974;243:457–481. doi: 10.1113/jphysiol.1974.sp010762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghirardi M, Braha O, Hochner B, Montarlo PG, Kandel ER. Roles of PKA and PKC in facilitation of evoked and spontaneous transmitter release at depressed and nondepressed synapses in Aplysia sensory neurons. Neuron. 1992;9:479–489. doi: 10.1016/0896-6273(92)90185-g. [DOI] [PubMed] [Google Scholar]
- 20.Hen R. Structural and functional conservation of serotonin receptors throughout evolution. In: Pichon Y, editor. Comparative molecular neurobiology. Birkhäuser; Basel: 1993. pp. 266–278. [DOI] [PubMed] [Google Scholar]
- 21.Hibert MF, Trumpp-Kallmeyer S, Bruinvels A, Hoflack J. Three-dimensional models of neurotransmitter G-binding protein-coupled receptors. Mol Pharmacol. 1991;40:8–15. [PubMed] [Google Scholar]
- 22.Ho BY, Karschin A, Branchek T, Davidson N, Lester HA. The role of conserved aspartate and serine residues in ligand binding and in function of the 5-HT1A receptor: a site-directed mutation study. FEBS Lett. 1992;312:259–262. doi: 10.1016/0014-5793(92)80948-g. [DOI] [PubMed] [Google Scholar]
- 23.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PPA. VII. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 24.Israel DI. A PCR-based method for high stringency screening of DNA libraries. Nucleic Acids Res. 1993;21:2627–2631. doi: 10.1093/nar/21.11.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jennings KR, Host JJ, Kaczmarek LK, Strumwasser F. Serotonergic inhibition of afterdischarge in peptidergic bag cells. J Neurobiol. 1981;12:579–590. doi: 10.1002/neu.480120606. [DOI] [PubMed] [Google Scholar]
- 26.Jockers R, Da Silva A, Strosberg AD, Bouvier M, Marullo S. New molecular and structural determinants involved in (2-adrenergic receptor desensitization and sequestration. J Biol Chem. 1996;271:9355–9362. doi: 10.1074/jbc.271.16.9355. [DOI] [PubMed] [Google Scholar]
- 27.Kadan MJ, Hartig PR. Autoradiographic localization and characterization of [125I]lysergic acid diethylamide binding to serotonin receptors in Aplysia. Neuroscience. 1988;24:1089–1102. doi: 10.1016/0306-4522(88)90090-5. [DOI] [PubMed] [Google Scholar]
- 28.Koch WJ, Hawes BE, Allen LF, Lefkowitz RJ. Direct evidence that Gi-coupled receptor stimulation of mitogen-activated protein kinase is mediated by Gβγ activation of p21ras. Proc Natl Acad Sci USA. 1994;91:12709–12710. doi: 10.1073/pnas.91.26.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohen R, Metcalf MA, Khan N, Druck T, Huebner K, Lachowicz JE, Meltzer HY, Sibley DR, Roth BL, Hamblin MW. Cloning, characterization, and chromosomal localization of a human 5-HT6 serotonin receptor. J Neurochem. 1996;66:47–56. doi: 10.1046/j.1471-4159.1996.66010047.x. [DOI] [PubMed] [Google Scholar]
- 30.Li X-C, Giot J-F, Kuhl D, Hen R, Kandel ER. Cloning and characterization of two related serotonergic receptors from the brain and the reproductive system of Aplysia that activate phospholipase C. J Neurosci. 1995;15:7585–7591. doi: 10.1523/JNEUROSCI.15-11-07585.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin KC, Michael D, Rose JC, Barad M, Casadio A, Zhu H, Kandel ER. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron. 1997;18:899–912. doi: 10.1016/s0896-6273(00)80330-x. [DOI] [PubMed] [Google Scholar]
- 32.Mercer AR, Emptage NJ, Carew TJ. Pharmacological dissociation of modulatory effects of serotonin in Aplysia sensory neurons. Science. 1991;254:1811–1813. doi: 10.1126/science.1662413. [DOI] [PubMed] [Google Scholar]
- 33.Peroutka SJ. 5-Hydroxytryptamine receptors in vertebrates and invertebrates: why is there so many? Neurochem Int. 1994;25:533–536. doi: 10.1016/0197-0186(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 34.Peroutka SJ. 5-HT receptors: past, present, and future. Trends Neurosci. 1995;18:68–69. [PubMed] [Google Scholar]
- 35.Peroutka SJ, Howell TA. The molecular evolution of G-protein-coupled receptors: focus on 5-hydroxytryptamine receptors. Neuropharmacology. 1994;33:319–324. doi: 10.1016/0028-3908(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 36.Ram JL, Judge K, Jednak MA. Antagonists of cholinergic and serotonergic responses of Aplysia buccal muscle. Comp Biochem Physiol [C] 1994;107:235–242. doi: 10.1016/1367-8280(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 37.Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang JM, Schwartz JC. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc Natl Acad Sci USA. 1993;90:8547–8551. doi: 10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning, a laboratory manual, Ed 2. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 39.Salomon Y, Londos C, Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974;58:541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- 40.Saudou F, Boschert U, Amlaiky N, Plassat JL, Hen R. A family of Drosophila serotonin receptors with distinct intracellular signalling properties and expression patterns. EMBO J. 1992;11:7–17. doi: 10.1002/j.1460-2075.1992.tb05021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Storozhuk MV, Castellucci VF, DesGroseillers L (1998) Differential effect of 5-HT on synaptic connections of the RF sensory neurons in the abdominal ganglion ofAplysia. Soc Neurosci Abstr, in press.
- 42.Sugamori KS, Sunahara RK, Guan H-C, Bulloch AGM, Tensen CP, Seeman P, Niznik HB, Van Tol HHM. Serotonin receptor cDNA from Lymnaea stagnalis. Proc Natl Acad Sci USA. 1993;90:11–15. doi: 10.1073/pnas.90.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sugita S, Goldsmith JR, Baxter DA, Byrne JH. Involvement of protein kinase C in serotonin-induced spike broadening and synaptic facilitation in sensorimotor connections of Aplysia. J Neurophysiol. 1992;68:643–651. doi: 10.1152/jn.1992.68.2.643. [DOI] [PubMed] [Google Scholar]
- 44.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Rhee AM, Jacobson KA. Molecular architecture of G-protein-coupled receptors. Drug Dev Res. 1996;37:1–38. doi: 10.1002/(SICI)1098-2299(199601)37:1<1::AID-DDR1>3.0.CO;2-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vogel SS, Chin GJ, Mumby SM, Schonberg M, Schwartz JH. G-proteins in Aplysia: biochemical characterization and regional and subcellular distribution. Brain Res. 1989;478:281–292. doi: 10.1016/0006-8993(89)91508-4. [DOI] [PubMed] [Google Scholar]
- 47.Weiger WA. Serotoninergic modulation of behavior: a phylogenetic overview. Biol Rev Camb Philos Soc. 1997;72:61–95. doi: 10.1017/s0006323196004975. [DOI] [PubMed] [Google Scholar]
- 48.Wickham L, DesGroseillers L. A bradykinin-like neuropepide precursor gene is expressed in neuron L5 of Aplysia californica. DNA Cell Biol. 1991;10:249–258. doi: 10.1089/dna.1991.10.249. [DOI] [PubMed] [Google Scholar]
- 49.Xu Y, Pieroni JP, Cleary LJ, Byrne JH. Modulation of an inhibitory interneuron in the neural circuitry for the tail withdrawal reflex of Aplysia. J Neurophysiol. 1995;73:1313–1318. doi: 10.1152/jn.1995.73.3.1313. [DOI] [PubMed] [Google Scholar]