Abstract
The effects of pergolide, a mixed D1/D2 receptor agonist, and bromocriptine, a selective D2 receptor agonist, were assessed in a visual delay task to further investigate the “dopamine link” of working memory in humans and to look for differential D1 versus D2 receptor contributions. Two groups of 32 healthy young adults (16 female) received either 0.1 mg of pergolide or 2.5 mg of bromocriptine in a placebo-controlled cross-over design. A pretreatment with domperidone, a peripherally active D2 antagonist, was performed in both groups to reduce side effects. Interindividual differences in pharmacokinetics were controlled by the time course of serum prolactin inhibition. The working memory paradigm was a visuospatial delayed matching task; the location of a randomly generated seven-point pattern had to be memorized and compared after 2, 8, or 16 sec with a second pattern that was either identical or slightly shifted within a reference frame. The task was designed with the intention to present unique stimuli at each trial and to require minimal motor demands. Practice effects between the two pharmacological test days were minimized by training sessions that preceded the tests. The paradigm showed significant error and reaction time increases with longer delays. After comparable doses, only pergolide, but not bromocriptine, facilitated visuospatial working memory performance as demonstrated by a significant drug-by-delay interaction. These findings are in accordance with the monkey literature as well as with neuroanatomical findings, and they confirm a preferential role of prefrontal D1 receptors for working memory modulation in humans.
Keywords: short-term memory, working memory, prefrontal cortex, dopamine, D1 receptor, D2 receptor, bromocriptine, pergolide, domperidone, prolactin, humans
There is converging evidence from studies in monkeys with cortical ablation, single-cell recording, and regional brain cooling (Funahashi and Kubota, 1994; Fuster, 1995), as well as from studies in humans with brain-lesioned patients, functional neuroimaging methods, and transcranial magnetic stimulation (von Cramon, 1996), that the prefrontal cortex plays a pivotal role in mediating working memory, i.e., the ability to hold an item of information transiently in mind in the service of comprehension, thinking, and planning (Goldman-Rakic, 1996). The performance and task-related neuronal activity of monkeys in visuospatial delay tasks can be specifically modulated with dopaminergic drugs (Goldman-Rakic et al., 1997); therefore a special “dopamine link” of working memory and prefrontal functions has been proposed (Desimone, 1995). A preferential modulation of working memory via cortical D1 receptors [according to a recent proposal the terms D1 or D1-like and D2 or D2-like are used to refer to the subfamilies of dopamine receptors, or they are used when the genetic subtype (D1 or D5; D2, D3, or D4) is uncertain (Goldman-Rakic et al., 1997)] has been shown in monkey studies (Sawaguchi and Goldman-Rakic, 1991;Williams and Goldman-Rakic, 1995), but so far not in studies with humans.
In the prefrontal cortex of monkeys, neurons have been identified around the sulcus principalis that increase their firing when specific stimuli are removed from view and sustain that firing until a trained behavioral response is initiated. In analogy to receptive fields in primary cortical areas, assemblies of such neurons with delay-related activity have been designated as memory fields. Iontophoretic application of the D1 antagonist SCH39166 at low ejection currents consistently enhanced the neuronal activity within spatially tuned memory fields without observable behavioral effects. This effect was pharmacologically reversible by low doses of SKF38393, a partial D1 agonist, but not by D2 antagonists (Sawaguchi et al., 1988, 1990;Sawaguchi and Goldman-Rakic, 1991; Williams and Goldman-Rakic, 1995). The D1 effects on memory fields together with the neuroanatomical findings of 3- to 10-fold higher cortical density of D1 receptors as compared with D2 receptors (Lidow et al., 1991) and of D1 receptor localization on pyramidal neurons (Bergson et al., 1995) suggest that dopamine may modulate the cell firing of cortical delay-neurons directly via D1 receptors (Goldman-Rakic et al., 1997).
Other monkey studies used local drug injections into the prefrontal cortex (Sawaguchi and Goldman-Rakic, 1994) or systemic application of dopaminergic drugs (Arnsten et al., 1994) and reported further evidence for an involvement of D1 receptors in visuospatial working memory modulation. However, there are also some controversial results with regard to D1 receptor specificity as well as dose and age dependency of cognitive drug effects (Schneider et al., 1994; Arnsten et al., 1995;Jackson et al., 1995; Arnsten, 1997). Most other drug effects on short-term memory have been observed only in the rat and will not be considered in this context because of the noncomparable cognitive tasks and interspecies differences in cerebral organization (Berger et al., 1991; Preuss, 1995).
Only a limited number of pharmacopsychological studies with healthy human subjects used working memory tasks with delay-dependent errors as performance criteria. In a study with eight male volunteers, performance in a 16 sec condition of a delayed matching task was impaired during whole-body cold exposure at 4°C and could be improved to normal by l-tyrosine, a catecholamine precursor (Shurtleff et al., 1994). Luciana et al. (1992) found a facilitating effect of 2.5 mg of bromocriptine, a D2 agonist, on task performance in a visuospatial delayed response task in eight female subjects. These findings have been partially replicated in a second study that observed some improvement of spatial but not object working memory after 1.25 mg of bromocriptine (Luciana and Collins, 1997). Two independent laboratories, however, failed to replicate these findings using somewhat different experimental designs. Kimberg et al. (1997) observed no effect of bromocriptine on a spatial delayed response task; however, other measures of prefrontal (executive) functions were improved in a subgroup of subjects with low verbal working memory capacity as determined by a reading span task. In a pilot study that was looking for dose dependency, we observed no significant effects of 1.25, 2.5, or 5 mg of bromocriptine on performance in a visuospatial delayed matching task.
To further evaluate the functional role of D1 receptors within prefrontal memory fields, we designed a visuospatial delay task with minimal motor demands as well as difficult-to-rehearse stimuli and performed a psychopharmacological study with healthy young adults. Because there were no selective D1 agonists available for human research, a pharmacological substraction design was applied with pergolide, a mixed D1/D2 receptor agonist, and bromocriptine, a selective D2 agonist, to differentiate for D1- versus D2-receptor contributions to working memory modulation in humans.
MATERIALS AND METHODS
Subjects
A total of 32 healthy young adults (most of whom were undergraduate students), who had no previous experience with the experimental task, completed either the pergolide or the bromocriptine study protocol. There were no significant differences between the two groups with respect to sex, age, handedness, and education. All volunteers entered the study with written informed consent and after medical examination to exclude cardiovascular, endocrinological, neurological, or psychiatric irregularities and pregnancy. None of the subjects took any psychotropic medication at the time of the study; a light breakfast was allowed 2 hr before the test sessions started. The subjects had normal or corrected-to-normal vision. There was a financial compensation of 200 Deutsche Mark for each participant. The protocols were approved by the regional ethics committee of the Saxonian Board of Physicians.
Pergolide study
Subjects. Sixteen volunteers (eight female) completed this study. Two additional subjects entered the study but had to be excluded on the first day because of side effects, namely mild faintness, one before and one after pergolide intake. The age of the volunteers ranged from 19 to 29 (23.8 ± 2.9) years. All were right-handed with a mean laterality quotient (LQ) of 81.9 ± 14.2 (Oldfield, 1971).
Drug administration. On both days an opaque gelatin capsule filled with either 0.1 mg (per 80 kg body weight) of pergolide (Parkotil; Lilly Deutschland, Giessen, Germany) or placebo was administered in a double-blind crossover design, so that half of the subjects received pergolide on day B and the other half on day C.
Bromocriptine study
Subjects. Another 16 volunteers (eight female), who had not participated in the pergolide study, completed this study. One subject had to be excluded because of poor performance near chance level after the first training session (day A). The age of this group ranged from 19 to 29 (23.1 ± 3.0); all were right-handed (LQ 72.1 ± 20.9).
Drug administration. All subjects received either 2.5 mg (per 80 kg body weight) of bromocriptine (Kirim; Hormosan-Kwizda, Frankfurt, Germany) or identical placebo tablets in a double-blind crossover design.
Experimental design
In both studies there were 3 experimental days, each separated by 1 week. The first day (day A) was reserved for instructions and a training session. Both pharmacological test days (days B and C) also included a training session immediately after the oral drug administration, when no pharmacological effect would be expected. The main experimental task was performed between 150 and 210 min after drug intake, i.e., the period around the mean peak plasma concentrations of both pergolide and bromocriptine (Wachtel, 1991; Markham and Benfield, 1997). A pretreatment with 3 × 10 mg of domperidone (Motilium; Byk Gulden, Konstanz, Germany), a peripherally active D2 antagonist, was performed at −12, −2, and −0.5 hr to reduce side effects, especially nausea. This pharmacological procedure is well established in the treatment of patients with Parkinson’s disease (Oertel and Quinn, 1996). To measure prolactin levels, blood samples were taken at 0, 60, 120, and 210 min after drug ingestion via a flexible venule that was connected to a slowly running isotonic NaCl infusion. Blood samples were centrifuged immediately at 4°C, and serum was stored at −80°C until the end of each study and analyzed with commercially available radioimmunoassays. The intra- and interindividual comparison of prolactin inhibition allows for control of biological efficacy and interindividual differences in pharmacokinetics. Blood pressure, heart rate, and sublingual temperature were monitored every 30 min (Fig.1). To further control for nonspecific drug effects on arousal and attention, two standardized paper–pencil tasks were performed twice, shortly before drug intake and before the main delayed matching task: the d2 test, a letter cancellation task (Brickenkamp, 1994), and theZahlenverbindungstest (ZVT), a German version of the trail-making task (Oswald and Roth, 1987). To evaluate changes in mood, two self-rating scales were administered: the Adjective Mood Scale (Befindlichkeits-Skala, Bf-S), which measures the extent of subjective impairment, and the State Trait Anxiety Inventory (STAI-1), to rate state changes in anxiety (AMDP and CIPS, 1990).
Fig. 1.
Time course of an experimental day (1 = training session; 2 = main session; 0 min = drug administration).
Delayed matching paradigm
The working memory paradigm was a visuospatial delayed matching task implemented on a personal computer using the ERTS software package (Experimental Run Time System, version 3.18; BeriSoft Cooperation, Frankfurt/Main, Germany), which provides millisecond accuracy in stimulus presentation and response registration. The task was designed to present unique stimuli at each trial and to require minimal motor demands. Subjects sat in front of a 17-inch monitor (1024 × 768 resolution) in dim room light; the eye-to-screen distance was 100 cm as controlled by a chin rest. They had to memorize the location (“where”) of a seven-point pattern (sample) and compare it after a delay of 2, 8, or 16 sec with a second pattern (match) that was either identical or slightly shifted (3 mm/0.17° or 6 mm/0.34°, left or right) within a frame of 155 × 145 mm using a two-alternative forced-choice procedure.
The point pattern was generated using a 9 × 8 × 8 matrix with random attribution of dots to seven out of nine fields. The use of unique random dot patterns allows the identical experimental setup to be used for the investigation of object (“what”) memory, which will be used in future studies. Unique stimuli also reduce between-trial interference, which has been shown to be a significant problem for animals with prefrontal lesions (Fuster, 1995; Van Hest and Steckler, 1996). Pharmacological modulation of proactive interference, therefore, should not be responsible for the observed drug effects. Sample and match stimuli in a single trial differed only by location (same or different) and had the same object features. Memory for nonspatial features of the dot patterns therefore should not influence the spatial memory performance.
Each sample was presented for 2 sec to get stable responses (Dale, 1973) and was followed by a mask pattern with randomly distributed frame-filling dots that was shown for 0.1 sec (Fig.2A,B). To avoid eye movement drifts the reference frame remained visible during the whole trial. There was an auditory feedback (peep) after each error response. The 8 and 16 sec delay lengths were taken from the literature (Shurtleff et al., 1994; Fuster, 1995). Pilot testing showed the 2 sec delay to be preferable to a nondelay (or 0.1 sec) control condition to prevent floor effects and interference between sample and match. The three delay lengths (2, 8, or 16 sec) and two match conditions (same or shifted) were balanced in quasi-random sequences with no more than three consecutive trials of equal delay length or “same/different” condition.
Fig. 2.
A, Location of frame and dot pattern on the screen. B, Visuospatial delayed matching task; time course of a single trial.
The training sessions (on days A, B, and C) consisted of 120 trials, with short interruptions after each series of 20 trials. Both experimental sessions (on day B and C) had 180 trials and lasted ∼50 min.
Data analysis
The main outcome criterion of the delayed matching task was the total error rate, i.e., the sum of incorrect “same” (false alarms) and incorrect “different” (misses) responses for each of the three delay lengths. Reaction times (RTs) were measured to control for speed–accuracy relationships. As a consequence of the training sessions, practice effects were minimized (Fig.3A,B). A repeated-measures ANOVA was performed to analyze memory-related drug effects. Within-subject factors were drug (drug or placebo) and delay length (2, 8, or 16 sec); between factors were order (drug on days B or C) and group (pergolide or bromocriptine group). Additionally the individual error rates were baseline-adjusted by calculating the difference: error rate8 or 16 sec − error rate2 sec. Adjusted error rates were used mainly to better visualize the effect sizes. For specific comparisons paired t tests were used. RTs were analyzed only for correct trials and corrected for outliers using individual median values. The index of variation presented on all figures is the SEM.
Fig. 3.
A, Practice effects in the pergolide study: total error rates in the training sessions of the 3 test days (A1, B1, C1). There was no significant difference between the two pharmacological test days (B1and C1). B, Practice effects in the bromocriptine study: total error rates in the training sessions of the 3 test days (A1, B1, C1). There was no significant difference between the 2 pharmacological test days (B1and C1).
RESULTS
Delayed matching task
Practice effects and group comparison
As predicted from pilot testing, there were significant practice effects resulting in fewer delay-dependent errors when the first training session (day A) was compared with the training sessions on days B and C [day(3) × delay(3) ANOVA] in both the pergolide [F(2,30) = 4.01; p = 0.029] and the bromocriptine group [F(2,30) = 8.01;p = 0.002] (Fig. 3A,B). As calculated for the two pharmacological test days B and C [day(2) × delay(3) ANOVA], there were no significant day effects or day × delay interactions. When the working memory performance of the placebo days was compared using a between-subjects design, there was no significant group effect for either the error rate [F(1,15)= 0.27; p = 0.611] or the RT analysis [F(1,15) = 0.87; p = 0.365]. In both groups delay-dependent error increases did not correlate with sex, age, or laterality quotient.
Pergolide study
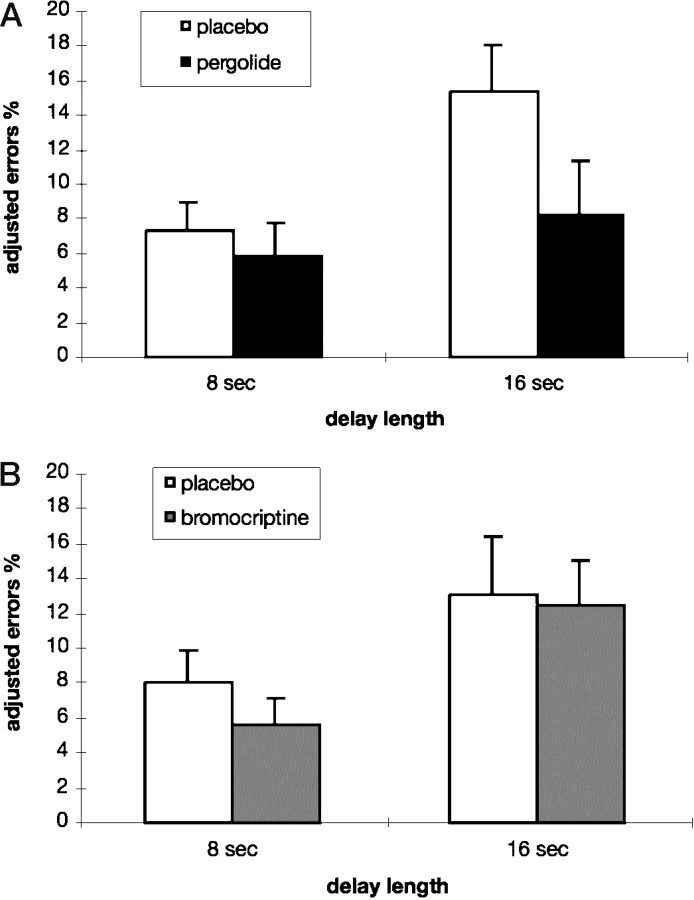
There were significant effects for the delay length [F(2,30) = 16.92; p < 0.001] and the drug × delay interaction [F(2,30)= 4.14; p = 0.026]. When error rates were adjusted for the performance in the 2 sec control condition, there was a significant effect of the D1/D2 agonist pergolide to improve performance in the 16 sec delay condition [t(15) = 2.20;p = 0.022], with a drug-induced error reduction of 47% [(adjusted error rateplacebo − adjusted error ratepergolide)/adjusted error rateplacebo] (Fig. 4A). Error reduction was not caused by slower RTs. The delay effect on RTs was again significant [F(2,30) = 10.39;p < 0.001], but there was no significant drug effect [F(1,15) = 0.10; p = 0.759] or drug × delay interaction [F(2,30) = 0.47;p = 0.632] (Table 1). Subjects with poor baseline performance, calculated as the mean error rate of the three training sessions, did not benefit more from dopaminergic stimulation, as demonstrated by nonsignificant correlations between baseline performance and drug effect.
Fig. 4.
A, Error rates after placebo and pergolide (0.1 mg/80 kg body weight) adjusted for the performance in the 2 sec delay control condition (error rate8 or 16 sec − error rate2 sec) in young adults. The D1/D2 agonist pergolide significantly improved delayed matching performance in the 16 sec delay condition. B, Error rates after placebo and bromocriptine (2.5 mg/80 kg) adjusted for the performance in the 2 sec delay control condition (error rate8 or 16 sec − error rate2 sec) in young adults. There was no significant effect of the D2 agonist bromocriptine in either the 8 sec or the 16 sec delay condition.
Table 1.
Delay-dependent error rates and RTs in the main delayed matching tasks of the pergolide and bromocriptine study (mean ± SD) and within group significances (p values) of drug effects (upper value) and drug × delay interactions (lower value)
| Study (dose) | Error rates (%) Delay length (sec) | p | RTs (msec) Delay length (sec) | p | ||||
|---|---|---|---|---|---|---|---|---|
| 2 | 8 | 16 | 2 | 8 | 16 | |||
| Placebo | 16.7 ± 7.9 | 24.1 ± 9.7 | 32.1 ± 11.0 | 0.206 | 731 ± 155 | 780 ± 186 | 822 ± 223 | 0.759 |
| Pergolide (0.1 mg) | 17.5 ± 8.6 | 23.3 ± 10.3 | 25.7 ± 10.6 | 0.026 | 736 ± 157 | 777 ± 184 | 802 ± 185 | 0.632 |
| Placebo | 16.0 ± 8.0 | 23.4 ± 6.7 | 29.0 ± 13.4 | 0.279 | 797 ± 142 | 842 ± 153 | 886 ± 183 | 0.233 |
| Bromocriptine (2.5 mg) | 18.3 ± 7.8 | 23.9 ± 7.0 | 30.6 ± 9.6 | 0.683 | 820 ± 153 | 885 ± 174 | 928 ± 219 | 0.577 |
Bromocriptine study
There was a significant main effect of delay length [F(2,30) = 15.46; p < 0.001] but no significant effect of drug [F(1,15) = 1.26; p = 0.279] or drug × delay interaction [F(2,30) = 0.39; p = 0.683]. There was no significant effect of the D2 agonist bromocriptine to reduce baseline-adjusted error rates in either the 8 sec [t(15) = 1.36; p = 0.097] or the 16 sec delay condition [t(15) = 0.30;p = 0.384] (Fig. 4B). For the RTs there was also a significant delay effect [F(2,30) = 18.24; p < 0.001], but no significant effect of drug [F(1,15) = 1.54; p = 0.233] or drug × delay interaction [F(2,30) = 0.56; p = 0.577] (Table 1).
Control parameters
Prolactin
In the bromocriptine study there were three missing data points on day B (placebo day) for subject 11, because only one blood sample could be taken. Basal as well as domperidone-stimulated prolactin serum concentrations were significantly lower in men than in women after pergolide [F(1,14) = 12.94; p = 0.003] and bromocriptine [F(1,14) = 11.04;p = 0.006], as is well known from the endocrinological literature (Hilland et al., 1981). In the overall group there were significant drug × time (of blood sample) interactions in both the pergolide [F(3,45) = 8.45;p < 0.001] and bromocriptine studies [F(3,42) = 3.10; p = 0.037] (Fig. 5A,B). The prolactin curves were not significantly different between the two study groups, comparing either the placebo or the drug days. Prolactin inhibition as calculated by the relative difference between baseline and task-related values [(prolactin0 + prolactin60)/2 − (prolactin120 + prolactin210)/2] did not correlate with delay-dependent error rates in either the pergolide or in the bromocriptine study.
Fig. 5.
A, Time course of prolactin responses to pergolide (0.1 mg/80 kg body weight) and placebo after pretreatment with 3 × 10 mg of domperidone. There was a significant drug × time interaction. B, Time course of prolactin responses to bromocriptine (2.5 mg/80 kg) and placebo after pretreatment with 3 × 10 mg of domperidone. There was a significant drug × time interaction.
Physiological parameters
There were no significant drug effects on blood pressure (systolic and diastolic), heart rate, or sublingual temperature, which were measured for safety and monitoring reasons.
Attention tasks
For the two paper–pencil tests of attention, there were no significant drug effect or drug × time (of task performance) interactions in the pergolide study, but there was a significant time effect for both the d2 test (letter cancellation) [F(1,15) = 21.91; p < 0.001] and the ZVT test (trail making) [F(1,15) = 7.76; p = 0.014], indicating a practice-related improvement during each test day. In the bromocriptine study there were no significant drug effect or drug × time interactions. A significant time effect was seen for the d2 test (letter cancellation) [F(1,15) = 9.90; p = 0.007] but not for the ZVT test.
Mood ratings
As compared with placebo, there were significant drug × time (of task application) interactions in the pergolide study for both mood ratings, the Adjective Mood Scale (Bf-S) [F(1,15) = 12.45; p = 0.003] and the State Trait Anxiety Inventory (STAI-1) [F(1,15) = 6.64; p = 0.031], i.e., a significant worsening of mood after pergolide application. There was also a significant time effect for the STAI-1 [F(1,15) = 7.45; p = 0.015], indicating a decrease in state anxiety in the course of both test days. In the bromocriptine study there were no significant effects on mood as measured with the Bf-S or STAI-1. Mood alterations as measured by the two self-rated questionnaires did not correlate with delay-dependent error rates in either the pergolide or the bromocriptine study.
DISCUSSION
The delayed matching task that was developed for this study showed significant error and RT increases, with longer delays in all drug and placebo conditions indicating a natural decay of the memorized visuospatial information. Pergolide, but not an equivalent dose of bromocriptine, specifically improved delayed matching performance, as compared with placebo, by reducing the error rate in the long delay condition. Because the two dopamine agonists differ mainly in their D1 agonistic properties—pergolide activates D1 receptors whereas bromocriptine does not—the findings of this study indicate that the decay of visuospatial information in working memory is modulated via D1 receptors. The pharmacological and cognitive specificity of this finding as well as topological and clinical implications will be discussed in detail.
Pharmacological specificity
This study could not replicate the findings of Luciana et al. (1992) who showed a facilitation of working memory by 2.5 mg of bromocriptine in a visuospatial delayed response task. The divergent bromocriptine findings can be explained either by population differences (subjects with high vs low working memory capacity) that might be related to different (high vs low) endogenous secretion of dopamine (Kimberg et al., 1997) or by different motor demands in delayed response (pointing movement) versus delayed matching tasks (button press). A third explanation is the small effect size provoked by selective D2 rather than D1 receptor stimulation.
In contrast to the bromocriptine study, we observed a facilitation of working memory after pergolide. Several assumptions have to be explained to claim a preferential role for the D1 receptor in working memory modulation. When a between-subjects design is used, it is important to demonstrate that there are no significant baseline differences between the two groups when the performance of the identical placebo sessions are compared. One could also argue about dosage differences, not only for the D1 agonistic but also for the D2 agonistic properties of the two substances, as indicated by apparent but not statistically significant differences in prolactin inhibition. The single doses of pergolide and bromocriptine we used are comparable in terms of biological and therapeutic efficacy (Wachtel, 1991; Pezzoli et al., 1994; Markham and Benfield, 1997).
The dose (and age) dependency of dopaminergic drug effects on working memory, however, is a crucial point. Findings from monkey studies that were looking for dose–response relationships can be summarized as biphasic effects of D1 antagonists, with low-dose facilitation and medium- to high-dose impairment (Williams and Goldman-Rakic, 1995; Arnsten, 1997), andtriphasic effects of D1 (and D2) agonists, with low-dose impairment, medium-dose facilitation, and high-dose deterioration (Arnsten et al., 1994, 1995; Arnsten, 1997). In this study the significant inhibition of prolactin secretion by both pergolide and bromocriptine indicates a medium dose D2 agonistic efficacy of both substances (at least at the level of the pituitary) during the period of cognitive task performance. Deteriorating effects of higher doses can be explained by unspecific toxicity (Murphy et al., 1996), whereas low doses might result in inverse net effects by actions via presynaptic feedback mechanisms (Altar et al., 1987).
As measured with self-rating scales, we did not observe mood improvements after a single dose of dopamine agonists as might be expected from the mild antidepressive efficacy of chronic bromocriptine (Weddell and Weiser, 1995) or pergolide (Boukoms and Mangini, 1993) treatment. The worsening of subjective well being after pergolide in this study can be explained by subliminal side effects that have not been reported, however, in the more explicit side effects questionnaire. As demonstrated by our small dropout rate, the pretreatment with domperidone seems to be an effective strategy for reducing side effects in dopamine agonist studies with normal volunteers.
Our findings suggest a preferential role of D1 receptor mechanism for visuospatial working memory modulation. This study, however, did not address the question of whether D1 receptor stimulation alone or the combination of D1 and D2 receptor stimulation is most effective. The functional role of other cortical dopamine receptors, especially D4 and D5, and of D2/D1 receptor interactions cannot be separated until more specific drugs are available for human research (Bergson et al., 1995). As for any neurochemically regulated behavior, neurotransmitter interactions (Ashby, 1996) and nondopaminergic mechanisms of working memory modulation have to be considered. Indeed, the pharmacological specificity of dopamine effects on visuospatial working memory has been investigated in only one study, which found delayed alternation impairment after dopamine depletion but not after noradrenaline or serotonin depletion in the prefrontal cortex (Brozoski et al., 1979). Other studies observed effects of α2-noradrenergic drugs on working memory function (Coull, 1994; Coull et al., 1996; Arnsten, 1997).
The finding that memory-related behavior and neural activity can be modulated by dopaminergic drugs must also be considered in the context of subcortical–cortical dopamine systems (LeMoal, 1995). Recent monkey studies showed that midbrain dopamine neurons (Mirenowicz and Schultz, 1996) and dorsolateral prefrontal neurons (Watanabe, 1996) are preferentially activated by appetitive and attractive stimuli, suggesting a dopamine link of working memory and subcortical reward mechanisms. Single-cell recording within the ascending mesocortical system revealed more dopaminergic activity during the learning phase of a delayed response task than with well established behavior (Schultz et al., 1993). In our study, all subjects entered both test days only after intensive training, and there was an aversive auditory feedback after each error, both suggesting low phasic activity of reward neurons with consequently low endogenous dopamine levels and a good chance for a dopamine agonist to stimulate the prefrontal cortex without intoxicating it (Murphy et al., 1996; Elliott et al., 1997).
Task specificity
The visuospatial delayed matching task of this study is similar to paradigms used in monkey research, as demonstrated by performance impairment with increasing delay length (for review, see Fuster, 1995;Goldman-Rakic, 1996). The linear relationship between delay length and error rate is in good correspondence with cognitive models of trace decay, i.e., the fading away of an internal representation that is supposed to be necessary for the matching operation (Dale, 1973; Hole, 1996). According to these models, memory improvement after pergolide administration can be explained by slowing the process of information decay or a more efficient inhibition of irrelevant information during the delay period or both. This effect of dopamine on cognitive signal-to-noise ratio has been conceptualized as a “focusing on relevant information” in the context of schizophrenic thought disorder and semantic priming experiments (Cohen and Servan-Schreiber, 1992; Kischka et al., 1996).
Topological and clinical implications
The prefrontal specificity of dopaminergic facilitation of working memory by pergolide is an assumption that is based on lesion data, functional neuroimaging studies, and neurophysiological and pharmacological findings, as well as dopamine receptor mapping. The most convincing evidence for an essential role of the prefrontal cortex in working memory regulation comes from studies with reversible lesions, because reorganization processes can be ignored. In normal human volunteers, delay-related memory functions were impaired by transcranial magnetic stimulation over the prefrontal but not over the motor cortex (Pascual-Leone and Hallet, 1994). In monkeys, intracranial cooling of the prefrontal but not of the parietal cortex worsened visual working memory in delayed response as well as in delayed matching tasks (Fuster, 1995). Several neuroimaging studies with PET or functional magnetic resonance imaging using either delay (Baker et al., 1996; Courtney et al., 1997) or monitoring (n-back) paradigms (Smith et al., 1995; Cohen et al., 1997) demonstrated an involvement of the dorsolateral prefrontal cortex in the neural networks of working memory (for review, see Owen, 1997).
After systemic drug administration, D1 agonists (or antagonists) have a greater probability to act on dopamine receptors in the prefrontal cortex than D2 compounds do, because the cortical density of D1 receptors is 3- to 10-fold higher as compared with D2 receptors, as has been shown consistently with autoradiographic and PET studies in both the monkey (Camps et al., 1989; Cortés et al., 1989; Lidow et al., 1991) and the human brain (Hall et al., 1994; Karlsson et al., 1995). Pharmacological studies with intracerebral drug application so far focused on prefrontal areas around the principal sulcus. Therefore, the claimed topological specificity is limited, because no drugs have been injected into other (namely parietal) neocortical areas that also possess a rich dopaminergic innervation (De Keyser et al., 1989) and show delay-related neuronal activity in visuospatial working memory tasks (Friedman and Goldman-Rakic, 1994; Constantinidis and Steinmetz, 1996).
Further evidence for a dopaminergic modulation of working memory comes from clinical studies. Patients with Parkinson’s disease show deficits in delay tasks (Partiot et al., 1996; Postle et al., 1997) and other prefrontal functions (Owen and Robbins, 1998) that are partially reversible with adequate dopaminergic treatment (Cooper et al., 1992;Lange et al., 1992). In patients with schizophrenia, there are two studies that found visuospatial working memory deficits using delayed response paradigms adapted from the monkey literature with registration of either eye movements (Park and Holzman, 1993) or pointing movements toward a memorized dot location (Spitzer, 1993). Preliminary clinical observations in patients with impaired executive and working memory functions after cerebrovascular lesions and traumatic brain injury showed dopamine agonists to be therapeutically helpful (Müller and von Cramon, 1994; McDowell, 1996).
This study found an improvement of visuospatial working memory caused by pergolide that might be mediated via prefrontal D1 receptors. Further research must now focus on the relationship of working memory deficits and reduced D1 receptor density in aging (De Keyser et al., 1990; Iyo and Yamasaki, 1993) and neuropsychiatric disorders (Okubo et al., 1997) as well as on the contribution of distinct prefrontal (Wilson et al., 1993) and subcortical structures (Schultz et al., 1995;Levy et al., 1997; Postle et al., 1997) to modality-dependent working memory functions in humans.
Footnotes
Presented at the 4th Annual Meeting of the Cognitive Neuroscience Society, Boston, March, 1997. We thank all subjects for their participation, Anke Pitzmaus and Nadja Saupe for technical assistance, Joachim Wiese for ERTS programming, Torsten Schubert for statistical advice, and Trevor Penney and two anonymous reviewers for helpful comments.
Correspondence should be addressed to Dr. Ulrich Müller, Max-Planck-Institute of Cognitive Neuroscience, Inselstrasse 22–26, 04103 Leipzig, Germany.
REFERENCES
- 1.Altar CA, Boyar WC, Oei E, Wood PL. Dopamine autoreceptors modulate the in vivo release of dopamine in the frontal, cingulate, and entorhinal cortices. J Pharmacol Exp Ther. 1987;242:115–120. [PubMed] [Google Scholar]
- 2.AMDP (Association for Methodology and Documentation in Psychiatry) and CIPS (Collegium Internationale Psychiatriae Scalarum) Rating scales for psychiatry: European edition. Beltz; Weinheim, Germany: 1990. [Google Scholar]
- 3.Arnsten AFT. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11:151–162. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- 4.Arnsten AFT, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult aged monkeys. Psychopharmacology. 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- 5.Arnsten AFT, Cai JX, Steere JC, Goldman-Rakic PS. Dopamine D2 receptor mechanisms contribute to age-related cognitive decline: the effects of quinpirole on memory and motor performance in monkeys. J Neurosci. 1995;15:3429–3439. doi: 10.1523/JNEUROSCI.15-05-03429.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashby CR. The modulation of dopaminergic neurotransmission by other neurotransmitters. CRC; Boca Raton, FL: 1996. [Google Scholar]
- 7.Baker SC, Frith CD, Frackowiak RSJ, Dolan RJ. Active representation of shape and spatial location in man. Cereb Cortex. 1996;6:612–619. doi: 10.1093/cercor/6.4.612. [DOI] [PubMed] [Google Scholar]
- 8.Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends Neurosci. 1991;14:21–27. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- 9.Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS. Regional, cellular, and subcellular variations in the distribution of D1 and D2 dopamine receptors in primate brain. J Neurosci. 1995;15:7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boukoms A, Mangini L. Pergolide: an antidepressant adjuvant for mood disorders? Psychopharmacol Bull. 1993;29:207–211. [PubMed] [Google Scholar]
- 11.Brickenkamp R. Test d2: Aufmerksamkeits-Belastungs-Test, Ed 8. Hogrefe; Göttingen, Germany: 1994. [Google Scholar]
- 12.Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- 13.Camps M, Cortés R, Gueye B, Probst A, Palacios JM. Dopamine receptors in human brain: autoradiographic distribution of D2 sites. Neuroscience. 1989;28:275–290. doi: 10.1016/0306-4522(89)90179-6. [DOI] [PubMed] [Google Scholar]
- 14.Cohen JD, Servan-Schreiber D. Context, cortex, and dopamine: connectionist approach to behavior and biology in schizophrenia. Psychol Rev. 1992;99:45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- 15.Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–607. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 16.Constantinidis C, Steinmetz MA. Neuronal activity in posterior area 7a during the delay periods of a spatial memory task. J Neurophysiol. 1996;76:1352–1355. doi: 10.1152/jn.1996.76.2.1352. [DOI] [PubMed] [Google Scholar]
- 17.Cooper JA, Sagar HJ, Doherty SM, Jordan N, Tidswell P, Sullivan EV. Different effects of dopaminergic and anticholinergic therapies on cognitive and motor function in Parkinson’s disease: a follow-up study of untreated patients. Brain. 1992;115:1701–1725. doi: 10.1093/brain/115.6.1701. [DOI] [PubMed] [Google Scholar]
- 18.Cortés R, Gueye B, Pazos A, Probst A, Palacios JM. Dopamine receptors in human brain: autoradiographic distribution of D1 sites. Neuroscience. 1989;28:263–273. doi: 10.1016/0306-4522(89)90178-4. [DOI] [PubMed] [Google Scholar]
- 19.Coull JT. Pharmacological manipulations of the α2-noradrenergic system effects on cognition. Drug Aging. 1994;5:116–126. doi: 10.2165/00002512-199405020-00005. [DOI] [PubMed] [Google Scholar]
- 20.Coull JT, Middleton HC, Robbins TW, Sahakian BJ. Contrasting the effects of clonidine and diazepam on tests of working memory and planning. Psychopharmacology. 1996;120:311–321. doi: 10.1007/BF02311179. [DOI] [PubMed] [Google Scholar]
- 21.Courtney SM, Ungerleider LG, Keil K, Haxby JV. Transient and sustained activity in a distributed neural system for human working memory. Nature. 1997;386:608–611. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- 22.Dale HCA. Short-term memory for visual information. Br J Psychol. 1973;64:1–8. doi: 10.1111/j.2044-8295.1973.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 23.De Keyser J, Ebinger G, Vauquelin G. Evidence for a widespread dopaminergic innervation of the human cerebral neocortex. Neurosci Lett. 1989;104:281–285. doi: 10.1016/0304-3940(89)90589-2. [DOI] [PubMed] [Google Scholar]
- 24.De Keyser J, de Backer JP, Vauquelin G, Ebinger G. The effect of aging on the D1 dopamine receptors in human frontal cortex. Brain Res. 1990;528:308–310. doi: 10.1016/0006-8993(90)91672-4. [DOI] [PubMed] [Google Scholar]
- 25.Desimone R. Is dopamine a missing link? Nature. 1995;376:549–550. doi: 10.1038/376549a0. [DOI] [PubMed] [Google Scholar]
- 26.Elliott R, Sahakian BJ, Matthews K, Bannerjea A, Rimmer J, Robbins TW. Effects of methylphenidate on spatial working memory and planning in healthy young adults. Psychopharmacology. 1997;131:196–206. doi: 10.1007/s002130050284. [DOI] [PubMed] [Google Scholar]
- 27.Friedman HR, Goldman-Rakic PS. Coactivation of prefrontal cortex and inferior parietal cortex in working memory tasks revealed by 2DG functional mapping in the rhesus monkey. J Neurosci. 1994;14:2775–2788. doi: 10.1523/JNEUROSCI.14-05-02775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Funahashi S, Kubota K. Working memory and prefrontal cortex. Neurosci Res. 1994;21:1–11. doi: 10.1016/0168-0102(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 29.Fuster JM. Memory in the cerebral cortex: an empirical approach to neural networks in the human brain and nonhuman primate. MIT; Cambridge, MA: 1995. [Google Scholar]
- 30.Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc Natl Acad Sci USA. 1996;93:13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldman-Rakic PS, Bergson C, Mrzljak L, Williams GV. Dopamine receptors and cognitive functions. In: Neve KA, Neve RL, editors. The dopamine receptors. Humana; Totowa, NJ: 1997. pp. 499–522. [Google Scholar]
- 32.Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L. Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology. 1994;11:245–256. doi: 10.1038/sj.npp.1380111. [DOI] [PubMed] [Google Scholar]
- 33.Hilland U, Bohnet HG, Blank M. Domperidone stimulated prolactin secretion in normal male and female volunteers. Endokrinologie. 1981;77:363–366. [PubMed] [Google Scholar]
- 34.Hole GJ. Decay and interference effects in visuospatial short-term memory. Perception. 1996;25:53–64. doi: 10.1068/p250053. [DOI] [PubMed] [Google Scholar]
- 35.Iyo M, Yamasaki T. The detection of age-related decrease of dopamine D1, D2 and serotonin 5-HT2 receptors in living human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:415–421. doi: 10.1016/0278-5846(93)90075-4. [DOI] [PubMed] [Google Scholar]
- 36.Jackson WJ, Buccafusco JJ, Terry AV, Turk DJ, Rush DK. Velnacrine maleate improves delayed matching performance by aged monkeys. Psychopharmacology. 1995;119:391–398. doi: 10.1007/BF02245854. [DOI] [PubMed] [Google Scholar]
- 37.Karlsson P, Farde L, Halldin C, Sedvall G, Ynddal L, Sloth-Nielsen M. Oral administration of NNC 756: a placebo controlled PET study of D1-dopamine receptor occupancy and pharmacodynamics in man. Psychopharmacology. 1995;119:1–8. doi: 10.1007/BF02246046. [DOI] [PubMed] [Google Scholar]
- 38.Kimberg DY, D’Esposito M, Farah MJ. Effects of bromocriptine on human subjects depend on working memory capacity. NeuroReport. 1997;8:3581–3585. doi: 10.1097/00001756-199711100-00032. [DOI] [PubMed] [Google Scholar]
- 39.Kischka U, Kammer T, Maier S, Weisbrod M, Thimm M, Spitzer M. Dopaminergic modulation of semantic network activation. Neuropsychologia. 1996;34:1107–1113. doi: 10.1016/0028-3932(96)00024-3. [DOI] [PubMed] [Google Scholar]
- 40.Lange KW, Robbins TW, Marsden CD, James M, Owen AM, Paul GM. l-dopa withdrawal in parkinson’s disease selectively impairs cognitive performance in tests sensitive to frontal lobe dysfunction. Psychopharmacology. 1992;107:394–404. doi: 10.1007/BF02245167. [DOI] [PubMed] [Google Scholar]
- 41.LeMoal M. Mesocorticolimbic dopaminergic neurons: functional and regulatory roles. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. Raven; New York: 1995. pp. 283–294. [Google Scholar]
- 42.Levy R, Friedman HR, Davachi L, Goldman-Rakic PS. Differential activation of the caudate nucleus in primates performing spatial and nonspatial working memory tasks. J Neurosci. 1997;17:3870–3882. doi: 10.1523/JNEUROSCI.17-10-03870.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P. Distribution of dopaminergic receptors in the primate cerebral cortex: quantitative autoradiographic analysis using (H3) raclopride, (H3) spiperone and (H3) SCH23390. Neuroscience. 1991;40:657–671. doi: 10.1016/0306-4522(91)90003-7. [DOI] [PubMed] [Google Scholar]
- 44.Luciana M, Collins PF. Dopaminergic modulation of working memory for spatial but not object cues in normal humans. J Cognit Neurosci. 1997;9:330–347. doi: 10.1162/jocn.1997.9.3.330. [DOI] [PubMed] [Google Scholar]
- 45.Luciana M, Depue RA, Arbisi P, Leon A. Facilitation of working memory in humans by a D2 receptor agonist. J Cognit Neurosci. 1992;4:58–68. doi: 10.1162/jocn.1992.4.1.58. [DOI] [PubMed] [Google Scholar]
- 46.Markham A, Benfield P. Pergolide: a review of its pharmacology and therapeutic use in Parkinson’s disease. CNS Drugs. 1997;7:328–340. doi: 10.2165/00023210-199707040-00005. [DOI] [PubMed] [Google Scholar]
- 47.McDowell SK. A role for dopamine in executive function deficits. J Head Trauma Rehabil. 1996;11:89–92. [Google Scholar]
- 48.Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- 49.Müller U, von Cramon DY. The therapeutic potential of bromocriptine in neuropsychological rehabilitation of patients with acquired brain damage. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:1103–1120. doi: 10.1016/0278-5846(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 50.Murphy BL, Arnsten AFT, Jentsch JD, Roth RH. Dopamine and spatial working memory in rats and monkeys: pharmacological reversal of stress-induced impairment. J Neurosci. 1996;16:7768–7775. doi: 10.1523/JNEUROSCI.16-23-07768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oertel WH, Quinn NP. Parkinsonism. In: Brandt T, Caplan LR, Dichgans J, Diener HC, Kennard C, editors. Neurological disorders: course and treatment. Academic; San Diego: 1996. pp. 715–772. [Google Scholar]
- 52.Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, Iyo M, Tateno Y, Toru M. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385:634–636. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- 53.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 54.Oswald WD, Roth E. Der Zahlen-Verbindungs-Test (ZVT), Ed 2. Hogrefe; Göttingen, Germany: 1987. [Google Scholar]
- 55.Owen AM. The functional organization of working memory processes within human lateral frontal cortex: the contribution of functional neuroimaging: Eur J Neurosci. 1997;9:1329–1339. doi: 10.1111/j.1460-9568.1997.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 56. Owen AM, Robbins TW. Attention and working memory in movements disorders. Neuropsychology of movement disorders Jahanashi M, Brown R. 1998. North-Holland; Amsterdam, in press. [Google Scholar]
- 57.Park S, Holzman PS. Association of working memory deficit and eye tracking dysfunction in schizophrenia. Schizophrenia Res. 1993;11:55–61. doi: 10.1016/0920-9964(93)90038-k. [DOI] [PubMed] [Google Scholar]
- 58.Partiot A, Vérin M, Pillon B, Teixeira-Ferreira C, Agid Y, Dubois B. Delayed response tasks in basal ganglia lesions in man: further evidence for a striato-frontal cooperation in behavioural adaptation. Neuropsychologia. 1996;34:709–721. doi: 10.1016/0028-3932(95)00143-3. [DOI] [PubMed] [Google Scholar]
- 59.Pascual-Leone A, Hallett M. Induction of errors in a delayed response task by repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex. NeuroReport. 1994;5:2517–2520. doi: 10.1097/00001756-199412000-00028. [DOI] [PubMed] [Google Scholar]
- 60.Pezzoli G, Martignoni E, Pacchetti C, Angeleri VA, Lamberti P, Muratorio A, Bonuccelli U, Demari M, Foschi N, Cossutta E, Nicoletti F, Giammona F, Canesi M, Scarlato G, Caraceni T, Moscarelli E. Pergolide compared with bromocriptine in Parkinson’s disease: a multicenter, crossover, controlled study. Mov Disord. 1994;9:431–436. doi: 10.1002/mds.870090409. [DOI] [PubMed] [Google Scholar]
- 61.Postle BR, Jonides J, Smith EE, Corkin S, Growdon JH. Spatial, but not object, delayed response is impaired in early Parkinson’s disease. Neuropsychology. 1997;11:171–179. doi: 10.1037//0894-4105.11.2.171. [DOI] [PubMed] [Google Scholar]
- 62.Preuss T. Do rats have prefrontal cortex: the Rose-Woolsey-Akert program reconsidered. J Cognit Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- 63.Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- 64.Sawaguchi T, Goldman-Rakic PS. The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J Neurophysiol. 1994;71:515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- 65.Sawaguchi T, Matsumura M, Kubota K. Dopamine enhances the neuronal activity of spatial short-term memory task in the primate prefrontal cortex. Neurosci Res. 1988;5:465–473. doi: 10.1016/0168-0102(88)90030-2. [DOI] [PubMed] [Google Scholar]
- 66.Sawaguchi T, Matsumura M, Kubota K. Effects of dopamine antagonists on neuronal activity related to a delayed response task in monkey prefrontal cortex. J Neurophysiol. 1990;63:1401–1412. doi: 10.1152/jn.1990.63.6.1401. [DOI] [PubMed] [Google Scholar]
- 67.Schneider JS, Sun ZQ, Roeltgen DP. Effects of dopamine agonists on delayed response performance in chronic low-dose MPTP-treated monkeys. Pharmacol Biochem Behav. 1994;48:235–240. doi: 10.1016/0091-3057(94)90522-3. [DOI] [PubMed] [Google Scholar]
- 68.Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci. 1993;13:900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schultz W, Romo R, Ljungberg T, Mirenowicz J, Hollerman JR, Dickinson A. Reward-related signals carried by dopamine neurons. In: Houk JC, Davis JL, Beiser DG, editors. Models of information processing in the basal ganglia. MIT; Cambridge, MA: 1995. pp. 234–248. [Google Scholar]
- 70.Shurtleff D, Thomas JR, Schrot J, Kowalski K, Harford R. Tyrosine reverses a cold-induced working memory deficit in humans. Pharmacol Biochem Behav. 1994;47:935–941. doi: 10.1016/0091-3057(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 71.Smith EE, Jonides J, Koeppe RA, Awh E, Schumacher EH, Minoshima S. Spatial versus object working memory: PET investigations. J Cognit Neurosci. 1995;7:337–356. doi: 10.1162/jocn.1995.7.3.337. [DOI] [PubMed] [Google Scholar]
- 72.Spitzer M. The psychopathology, neuropsychology, and neurobiology of associative and working memory in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 1993;243:57–70. doi: 10.1007/BF02191566. [DOI] [PubMed] [Google Scholar]
- 73.Van Hest A, Steckler T. Effects of procedural parameters on response accuracy: lessons from delayed (non-) matching procedures in animals. Cognit Brain Res. 1996;3:193–203. doi: 10.1016/0926-6410(96)00006-7. [DOI] [PubMed] [Google Scholar]
- 74.von Cramon DY. Neurobiologie des Arbeitsgedächtnisses. In: Möller HJ, Müller-Spahn F, Kurtz G, editors. Aktuelle Perspektiven der Biologischen Psychiatrie. Springer; Wien, Austria: 1996. pp. 1–11. [Google Scholar]
- 75.Wachtel H. Antiparkinsonian dopamine agonists: a review of the pharmacokinetic and neuropharmacology in animals and humans. J Neural Transm [PD Sect] 1991;3:151–201. doi: 10.1007/BF02259537. [DOI] [PubMed] [Google Scholar]
- 76.Watanabe M. Reward expectancy in primate prefrontal neurons. Nature. 1996;382:629–632. doi: 10.1038/382629a0. [DOI] [PubMed] [Google Scholar]
- 77.Weddell RA, Weiser R. A double blind cross over placebo controlled trial of the effects of bromocriptine on psychomotor function, cognition, and mood in de novo patients with parkinson’s disease. Behav Pharmacol. 1995;6:81–91. [PubMed] [Google Scholar]
- 78.Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 79.Wilson FAW, O’Scalaidhe SP, Goldman-Rakic PS. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993;260:1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]