Abstract
Carbachol enhances rapid eye movement (REM) sleep when microinjected into the pontine reticular formation of the cat and rat. Carbachol elicits this REM sleep-like state via activation of postsynaptic muscarinic cholinergic receptors (mAChRs). The present study used in vitro autoradiography of carbachol-stimulated [35S]guanylyl-5′-O-(γ-thio)-triphosphate ([35S]GTPγS) binding to test the hypothesis that carbachol activates mAChRs to induce stimulation of G-proteins in brainstem nuclei contributing to REM sleep generation. The results demonstrate a heterogeneous increase in carbachol-stimulated G-protein activation across rat brainstem. Binding of [35S]GTPγS in the presence of carbachol, compared with basal binding, was significantly increased in the laterodorsal tegmental nucleus (75.7%), caudal pontine reticular nucleus (68.9%), oral pontine reticular nucleus (64.5%), pedunculopontine tegmental nucleus (55.7%), and dorsal raphe nucleus (54.0%) but not in the nucleus locus coeruleus. The activation of G-proteins by carbachol was concentration-dependent and antagonized by atropine, demonstrating that G-proteins were activated via mAChR stimulation. The results provide the first direct measures of mAChR-activated G-proteins in brainstem nuclei known to contribute to REM sleep generation.
Keywords: autoradiography, dorsal raphe nucleus, G-proteins, laterodorsal tegmental nucleus, locus coeruleus, pedunculopontine tegmental nucleus, pontine reticular formation
Cholinergic mechanisms within the brainstem contribute to rapid eye movement (REM) sleep generation (for review, see Baghdoyan, 1997a). Microinjection of cholinomimetics into the medial pontine reticular formation (mPRF) of cat or the homologous caudal (PnC) and oral (PnO) pontine reticular nuclei of rat causes a REM sleep-like state (Baghdoyan et al., 1984; Gnadt and Pegram, 1986;Baghdoyan et al., 1989; Bourgin et al., 1995). During REM sleep, neurons within the cholinergic laterodorsal tegmental (LDT) and pedunculopontine tegmental (PPT) nuclei increase their discharge rates (El Mansari et al., 1989; Kayama et al., 1992), some mPRF neurons are depolarized (Ito and McCarley, 1984), and mPRF acetylcholine (ACh) release is increased (Leonard and Lydic, 1997). LDT/PPT neurons send descending projections to the pontine reticular formation (Mitani et al., 1988; Shiromani et al., 1988; Jones, 1990; Semba et al., 1990), and electrical stimulation of LDT/PPT neurons enhances both ACh release in the mPRF (Lydic and Baghdoyan, 1993) and REM sleep (Thakkar et al., 1996). Cholinergic LDT/PPT neurons and cholinoceptive, noncholinergic neurons in the mPRF are modulated by monoaminergic projections from the dorsal raphe nucleus (DR) and locus coeruleus (LC) (McCarley et al., 1995). Together, the foregoing nuclei comprise key components of a pontine network generating REM sleep (for review, see Steriade and McCarley, 1990; Kryger et al., 1994).
Agonist activation of muscarinic cholinergic receptors (mAChRs) initiates REM sleep (Shiromani and Fishbein, 1986; Baghdoyan et al., 1989; Velazquez-Moctezuma et al., 1989; Imeri et al., 1994; Bourgin et al., 1995). Five subtypes of mAChRs (m1–m5) have been cloned, and all are members of a protein family containing seven transmembrane-spanning domains coupled to guanine nucleotide-binding proteins (G-proteins) (Felder, 1995). Currently, there is considerable interest in characterizing the molecular modulation of arousal states (Lydic, 1997) and in specifying the signal transduction pathways through which mAChRs generate REM sleep. Recent data have suggested that cholinergic REM sleep generation is mediated by an m2/m4-activated signal transduction pathway involving a Gi/Go-like G-protein, adenylyl cyclase, cAMP, and protein kinase A (Shuman et al., 1995; Capece and Lydic, 1997). No previous data, however, have provided a direct measure of mAChR-activated G-proteins in brainstem regions known to play a role in REM sleep generation.
Transmembrane signal transduction occurs for mAChRs when ligand binding leads to activation of G-protein α-subunits, which facilitates the binding of GTP. This fact has made it possible (Sim et al., 1995,1997) to visualize and quantify receptor-activated G-proteins in specific nuclei via autoradiography of agonist-stimulated [35S]guanylyl-5′-O-(γ-thio)-triphosphate ([35S]GTPγS) binding. The present study used the [35S]GTPγS assay to test the hypothesis that the cholinergic agonist carbachol activates mAChR-coupled G-proteins in brainstem nuclei known to be important for REM sleep generation. Direct measures of mAChR-activated G-proteins were obtained from LDT, PPT, PnO, PnC, LC, and DR, in which mAChRs have been localized (Baghdoyan, 1997b).
MATERIALS AND METHODS
Materials. Male Sprague Dawley rats (200–350 gm) were purchased from Charles River Laboratories (Wilmington, MA). [35S]GTPγS (1250 Ci/mmol) and Reflection autoradiography film were purchased from NEN Life Science Products (Boston, MA). Unlabeled GTPγS was obtained from Boehringer Mannheim (Indianapolis, IN). GDP, carbachol, and atropine were obtained from Sigma (St. Louis, MO). The μ-opiate agonist [d-Ala2,N-Me-Phe4,Gly-ol5]enkephalin (DAMGO) was purchased from Research Biochemicals International (Natick, MA). All other reagents were obtained from either Sigma or Fisher Scientific (Orangeburg, NY).
Tissue preparation. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Nine rats were killed by decapitation. Brains were removed quickly and immediately frozen in isopentane at −30°C. Brains were cut serially as 20 μm coronal or sagittal sections on a Hacker Bright OTF cryostat (Fairfield, NJ), thaw-mounted onto gelatin-coated glass slides, and placed in a vacuum desiccator on ice for 2 hr. Tissue sections were stored at −70°C until assayed.
In vitro [35S]GTPγS autoradiography.On the day of the assay, tissue sections were brought to room temperature in a vacuum desiccator. The in vitro[35S]GTPγS binding procedure was performed as described previously (Sim et al., 1995, 1997). Briefly, tissue sections were soaked in assay buffer (in mm: 50 Tris-HCl, 3 MgCl2, 100 NaCl, and 0.2 EGTA, pH 7.4) for 10 min at 25°C and preincubated in assay buffer containing 2 mm GDP for 15 min, pH 7.4, at 25°C. Brainstem sections from six rats were incubated with 0.04 nm [35S]GTPγS, 2 mm GDP, and either carbachol (1 mm) or, as a positive control to confirm assay conditions (Sim et al., 1996a, 1997), the μ-opiate agonist DAMGO (3 μm) for 2 hr, pH 7.4, at 25°C. Adjacent sections were incubated with carbachol (1 mm) in the presence of the muscarinic antagonist atropine (1 mm). Brainstem slices from three additional rats were incubated with 0.04 nm [35S]GTPγS, 2 mm GDP, and one of four concentrations of carbachol: 100 nm (10−7m), 10 μm (10−5m), 1 mm (10−3m), or 10 mm (10−2m). For all experiments, basal activity of G-proteins was determined by incubating tissue sections in the presence of 2 mm GDP and 0.04 nm [35S]GTPγS without agonist; nonspecific binding (NSB) was determined in the presence of 2 mm GDP and 10 μm unlabeled GTPγS without agonist. After the 2 hr incubation time, tissue sections were rinsed twice in ice-cold Tris buffer for 2 min, pH 7.0, at 25°C and once in ice-cold, deionized H2O for 30 sec. After assay completion, tissue sections were dried under a cool stream of air for 10 min and placed in a vacuum desiccator overnight (25°C). The next day tissue sections were packed in film cassettes with 14C microscale standards (Amersham, Arlington Heights, IL; 31–883 nCi/gm) and exposed to Reflection autoradiography film for 72 hr. Films were developed using a Kodak M35A X-OMAT autoprocessor. Tissue sections were fixed with paraformaldehyde vapors at 80°C (Herkenham and Pert, 1982) and stained with cresyl violet to aid in the localization of selected brainstem nuclei.
Data analysis. Cresyl violet-stained sections and corresponding autoradiograms were backlit with a Northern Light illuminator (Imaging Research, St. Catherines, Ontario, Canada) and digitized using a Cohu (San Diego, CA) CCD camera connected to a digitizing card (Data Translations, Marlboro, MA). Digitized images were analyzed using the NIH Image program (version 1.6) and an Apple Macintosh computer. G-protein activation was quantified by densitometric analysis of the digitized autoradiograms. Each brain region examined was localized on the cresyl violet-stained section according to the rat brain atlas of Paxinos and Watson (1986, 1997). The border of each brain region was outlined on the digitized cresyl violet-stained image, and that border was then transferred onto the matching autoradiographic image for quantification of density. Data were obtained as optical density measurements and converted to nanocuries of 35S per gram using 14C standards and a correction factor derived from densitometric measurements of brain paste slices containing known amounts of 35S (Sim et al., 1996a, 1997). Mean NSB values for each brain region were subtracted from total binding, and data are reported as specific [35S]GTPγS binding. These procedures made it possible to statistically compare G-protein activation in specified nuclei as a function of the different in vitro treatment conditions described above. The data were analyzed using one-way ANOVA for repeated measures and post hoc Tukey’s and Dunnett’s multiple comparison tests (p < 0.05). For coronal brainstem sections, the number of measurements acquired for each nucleus depended on the anteroposterior extent of the nucleus. For example, because the PnO ranges from bregma −7.30 to bregma −8.80 mm, the quantitative data included ∼30 measurements per rat per treatment condition. In the case of the LC, which ranges from bregma −9.16 to bregma −10.30 mm, measurements consisted of ∼10 values per rat per condition.
RESULTS
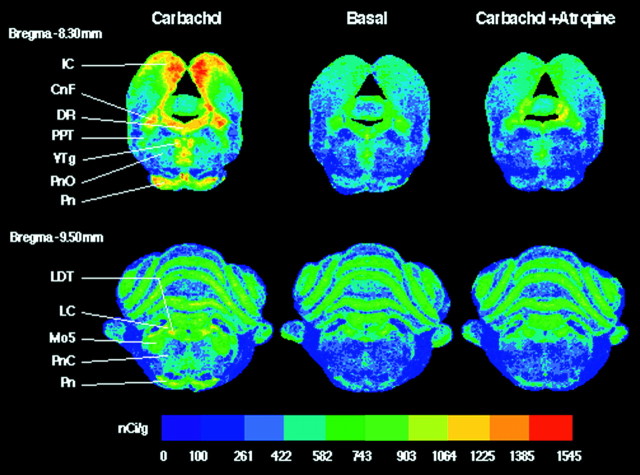
An example of carbachol-stimulated [35S]GTPγS binding and its antagonism by atropine is shown in Figure 1. Color-coded autoradiograms reveal that, compared with basal levels of [35S]GTPγS binding, carbachol increased G-protein activation in the following sleep-related nuclei: DR, PPT, PnO, LDT, and PnC. Carbachol also increased G-protein activation in several non-sleep-related brainstem nuclei: cuneiform nucleus (CnF; 68.8%), inferior colliculus (IC; 146.7%), motor trigeminal nucleus (Mo5; 224.4%), pontine nuclei (Pn; 226.4%), and ventral tegmental nucleus (VTg; 175.5%). Carbachol-stimulated [35S]GTPγS binding was blocked by the muscarinic antagonist atropine, consistent with the conclusion that G-protein activation was initiated by mAChR stimulation. Confirmation that the G-protein activation illustrated by Figure 1 was caused by mAChRs was also provided by the positive control tissue sections treated with the μ-opioid agonist DAMGO. Tissue sections treated with DAMGO showed significantly increased G-protein activation and a brainstem distribution of G-protein activation similar to that published previously (Sim et al., 1996a). DAMGO caused stimulation of G-proteins in different brainstem nuclei than carbachol.
Fig. 1.
Color-coded autoradiograms of coronal brainstem sections from the same rat show total [35S]GTPγS binding for three different treatment conditions: carbachol (1 mm), basal, and carbachol in the presence of atropine (1 mm). Color bar, Total [35S]GTPγS binding in nanocuries per gram. Adjacent sections from bregma −8.30 mm (top) and bregma −9.50 mm (bottom) show carbachol-stimulated G-protein activation in specific REM sleep-related nuclei (DR, PPT, PnO, LDT, and PnC) and in several non-sleep-related nuclei (IC, CnF, VTg, Pn, and Mo5). In all nuclei, this activation was blocked by the muscarinic antagonist atropine, confirming that carbachol-induced G-protein activation was mediated by mAChRs. CnF, Cuneiform nucleus;DR, dorsal raphe nucleus; IC, inferior colliculus; LC, locus coeruleus; LDT, laterodorsal tegmental nucleus; Mo5, motor trigeminal nucleus; Pn, pontine nuclei; PnC, nucleus pontis caudalis; PnO, nucleus pontis oralis;PPT, pedunculopontine tegmental nucleus;VTg, ventral tegmental nucleus.
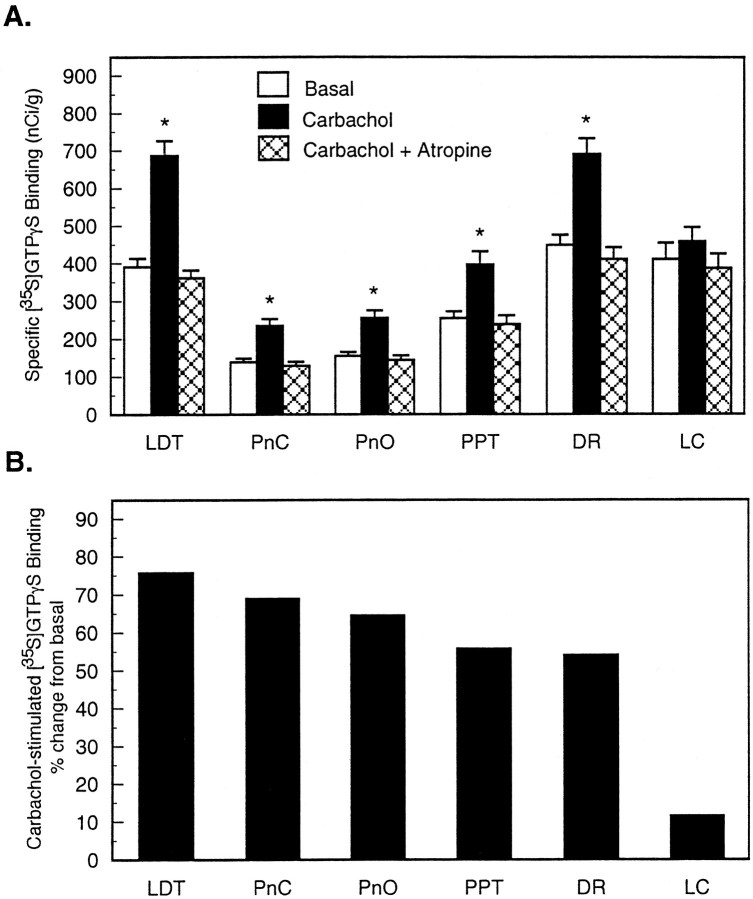
Figure 2 shows G-protein activation quantified for six brainstem nuclei. Data represent 1776 measurements from six rats. Figure 2A shows that incubating brainstem sections with 1 mm carbachol increased specific [35S]GTPγS binding (nanocuries per gram) in all nuclei measured, and atropine returned carbachol-stimulated [35S]GTPγS binding to basal levels. For the REM sleep-related nuclei, ANOVA revealed statistically significant differences in [35S]GTPγS binding in LDT (F(5,17) = 123.3; p < 0.0001), PnC (F(5,17) = 71.3; p < 0.0001), PnO (F(5,17) = 85.1; p< 0.0001), PPT (F(5,17) = 59.5;p < 0.0001), and DR (F(5,17) = 85.6; p < 0.0001).
Fig. 2.
Quantitative analysis of carbachol-stimulated G-protein activation in sleep-related brainstem nuclei from six animals. A, Bars represent mean specific [35S]GTPγS binding + SEM for each of the treatment conditions: basal binding assayed without agonist and carbachol-induced [35S]GTPγS binding assayed in the presence of 1 mm carbachol with or without atropine.B, Rank ordering of carbachol-stimulated [35S]GTPγS binding. Results are reported as percent increase from basal [35S]GTPγS binding. *Statistically significant increase in G-protein activation over basal levels (p < 0.05).
The effect of carbachol-stimulated [35S]GTPγS binding as a percent of basal binding for each brain region analyzed is shown in Figure 2B. ANOVA revealed a statistically significant region main effect of carbachol-stimulated [35S]GTPγS binding (F(5,35) = 35.3; p < 0.0001). The highest increase over basal [35S]GTPγS binding was in the LDT (75.7%), and the lowest was in the LC (11.5%). The PPT showed 55.7% stimulation over basal, and the DR revealed that [35S]GTPγS binding increased by 54.0%. Carbachol-stimulated G-protein activation in the PnC (68.9%) was not significantly different from G-protein activation in the PnO (64.5%).
Color-coded autoradiograms of sagittal brainstem sections (Fig.3) illustrate carbachol-stimulated and basal [35S]GTPγS binding. The sagittal plane clearly reveals the differential distribution of cholinergically activated G-proteins. Homogeneous G-protein activation within the reticular formation (PnO and PnC) in response to cholinergic stimulation also can be visualized in these sagittal sections (Fig. 3;L = 1.40 mm, L = 0.90 mm).
Fig. 3.
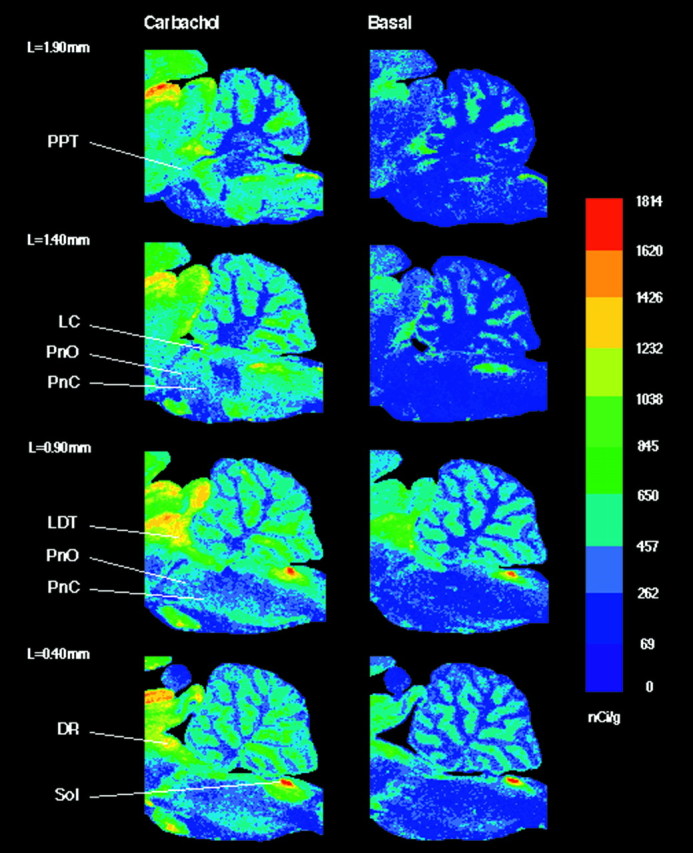
Color-coded autoradiograms of sagittal brainstem sections from four different lateralities (L =1.90–0.40 mm). Sections in the left column were treated with 1 mm carbachol, and sections in the right column were treated without agonist. Color bar, Total [35S]GTPγS binding in nanocuries per gram. In the medulla, the nucleus of the solitary tract (Sol) revealed a significant (33.3%) increase in carbachol-stimulated [35S]GTPγS binding compared with basal. Sagittal sections illustrate G-protein activation by carbachol across the rostrocaudal extent of the brainstem.DR, Dorsal raphe nucleus; LC, locus coeruleus; LDT, laterodorsal tegmental nucleus;PnC, nucleus pontis caudalis; PnO, nucleus pontis oralis; PPT, pedunculopontine tegmental nucleus; Sol, nucleus of the solitary tract.
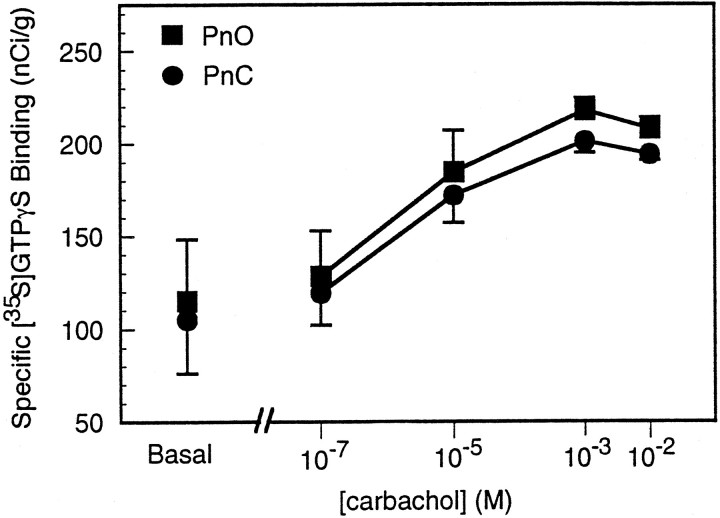
Figure 4 illustrates the concentration-dependent nature of carbachol-induced [35S]GTPγS binding in the reticular formation (PnO and PnC). These data summarize 792 measurements in three rats. Basal [35S]GTPγS binding was 115.0 nCi/g in the PnO and 105.1 nCi/g in the PnC. ANOVA and post hoc Tukey’s test revealed that 10−5, 10−3, and 10−2m carbachol caused a significant increase in G-protein activation compared with basal [35S]GTPγS binding (p < 0.05). The percent increase from basal binding for 10−7, 10−5, 10−3, and 10−2mcarbachol was 11.7, 60.6, 89.4, and 80.7%, respectively, in the PnO, and 13.7, 63.8, 91.3, and 84.7%, respectively, in the PnC. Maximum [35S]GTPγS binding was achieved with 10−3m (1 mm) carbachol. For each dose of carbachol, the increase in G-protein activation was not significantly different between the PnO and PnC.
Fig. 4.
Effects of four different concentrations of carbachol on [35S]GTPγS binding in areas of reticular formation: nucleus pontis oralis (PnO) and nucleus pontis caudalis (PnC). Data are plotted as mean specific [35S]GTPγS binding ± SEM. The concentration-dependent increase in G-protein activation indicates that carbachol stimulated G-proteins via mAChR activation.
DISCUSSION
The experimental procedures (Sim et al., 1995, 1997) used in this study took advantage of a transmembrane signal transduction process initiated by ligand binding to G-protein-coupled receptors (GPCRs). Before ligand activation of a GPCR, inactive G-proteins are bound to GDP. On carbachol activation of mAChRs and subsequent stimulation of G-proteins, GDP is exchanged for GTP. The GTP analog used in the present assay was a nonhydrolyzable and irreversible analog of GTP labeled with 35S. It should be clear that the results provide two different types of information. First, the autoradiographic data of Figures 1 and 3 represent total [35S]GTPγS binding, providing reliable and precise anatomical localization of G-proteins (Sim et al., 1995, 1997). Second, by subtracting nonspecific from total binding, the assay provides a quantitative measure of specific [35S]GTPγS binding (Figs. 2, 4).
Carbachol-induced G-protein activation was antagonized by atropine
Atropine, a competitive inhibitor of muscarinic receptors, blocked carbachol-stimulated G-protein activation in REM sleep-related brainstem nuclei (Fig. 2A), indicating that carbachol-induced G-protein activation is mediated by mAChRs. As demonstrated previously, atropine alone does not alter basal G-protein binding (Sim et al., 1996b). The present finding of atropine antagonism is consistent with other in vitro studies showing that carbachol-stimulated [35S]GTPγS binding can be blocked with atropine in porcine atrial membranes (Hilf et al., 1989), and with previous in vivo data showing that administration of muscarinic antagonists into the pontine reticular formation inhibits cholinergically induced REM sleep in cat (Baghdoyan et al., 1989;Velazquez-Moctezuma et al., 1989) and rat (Bourgin et al., 1995) and spontaneously occurring REM sleep in cat (Lee et al., 1995) and rat (Shiromani and Fishbein, 1986; Imeri et al., 1994).
Cholinergically activated G-proteins and muscarinic receptors: parallel distribution and different densities
Receptor autoradiography of cat (Baghdoyan et al., 1994) and rat (Baghdoyan, 1997b) brainstem has revealed the presence of M1, M2, and M3 mAChRs in REM sleep-related brainstem nuclei. The present overall distribution of G-protein activation (Figs. 1, 3) resembles the pattern of mAChR binding across rat brainstem (Baghdoyan, 1997b). The density of G-protein activation in specific brainstem nuclei, however, does not exactly correspond to levels of mAChRs in those same nuclei. For example, mAChR density in the rat was highest in LC and DR, intermediate in LDT and PPT, and lowest in the PnO and PnC (Baghdoyan, 1997b). In contrast, G-protein activation by carbachol was significantly greater in LDT, PnC, PnO, and PPT than in LC or DR (Fig.2B). The finding of parallel distribution and different density between receptors and G-proteins has been noted with other receptor systems (Sim et al., 1995). Such discrepancies may represent differences in the efficacy with which G-proteins couple to mAChRs (Sim et al., 1995) and raise intriguing questions for future studies of the transmembrane transduction mechanisms modulating REM sleep. Are mAChRs in the LC and DR linked to fewer G-proteins than mAChRs in the PnO and PnC? Does G-protein activation depend on which subtype of mAChR is activated? Whereas the LC and DR contain multiple mAChR subtypes, the PnO and PnC have predominantly M2 receptors (Baghdoyan, 1997b). Carbachol is known to bind with higher affinity to M2 receptors (Wess, 1993), and the signal transduction pathway for m2 and m4 receptors differs from the m1 and m3 transduction pathway (Felder, 1995). Activating m2 mAChRs decreases adenylyl cyclase activity, resulting in diminished cAMP production and inhibition of protein kinase A (PKA) (Caulfield, 1993). Recent data have demonstrated that mPRF administration of compounds facilitating adenylyl cyclase, cAMP, and PKA significantly decrease cholinergic REM sleep generation (Shuman et al., 1995; Capece and Lydic, 1997). Thus, carbachol activation of G-proteins in PnO and PnC is consistent with in vitro and in vivo data concerning cholinergic activation of M2 mAChRs and manipulation of signal transduction cascades known to be activated by mAChRs.
Carbachol-stimulated G-protein activation was homogeneous throughout the reticular formation
Within PnO and PnC regions of the reticular formation, there was a homogeneous activation of G-proteins by carbachol (Fig. 3). Considering the anatomical site specificity for carbachol-induced REM sleep enhancement in rat (Bourgin et al., 1995), one might have expected to see a localized area in the caudal PnO with significantly higher carbachol-stimulated [35S]GTPγS binding. Homogeneous [35S]GTPγS binding throughout PnO and PnC, however, is consistent with the homogeneous distribution of M2 mAChRs throughout PnO and PnC (Baghdoyan, 1997b). Perhaps the site specificity of carbachol for causing REM sleep enhancement from the caudal PnO (Bourgin et al., 1995) is not attributed to the number of muscarinic receptors or G-proteins present but may result from the ability of PnO G-proteins to differentially amplify transmembrane signaling (Felder, 1995).
Carbachol activation of G-proteins was concentration-dependent
In every experiment, [35S]GTPγS binding in rat brainstem was facilitated in the presence of carbachol. The concentration–response data (Fig. 4) revealed that 1 mm carbachol was most efficacious in stimulating [35S]GTPγS binding in rat brainstem. This finding is similar to previous studies (Hilf et al., 1989), showing that 1 mm carbachol stimulated G-protein activation in porcine atrial membranes, and to a previously reported dose–response curve for carbachol-stimulated [35S]GTPγS binding in chick optic tectum (Kurkinen et al., 1996). The ability of carbachol to enhance REM sleep when administered in vivointo the pontine reticular formation of cats and rats also has been shown to be dose-dependent. These microinjection studies have shown that 2.2 mm carbachol in cat (Baghdoyan et al., 1989) and 0.1–1.1 mm carbachol in rat (Bourgin et al., 1995) were sufficient to elicit the REM sleep-like state.
Limitations and conclusion
The ability to map the distribution of G-proteins coupled to populations of active receptors provides important information about the signal transduction pathways stimulated by specific agonists. The limitations of the [35S]GTPγS assay have been discussed in detail elsewhere (Sim et al., 1995, 1997). Three key limitations are of particular relevance to the current findings. First is the fact that the [35S]GTPγS assay procedure did not specify the type of G-protein stimulated. Second, although carbachol is known to bind with highest affinity to M2 receptors (Wess, 1993), the differential magnitude of mAChR subtype activation by carbachol in the nuclei studied is unknown. Thus, the present results do not differentiate subtypes of mAChRs or G-proteins. It should be possible, however, for future studies to overcome these two limitations through the use of relatively selective mAChR antagonists and G-protein toxins. A third limitation is that, although the results do quantify G-protein activation by cholinergic stimulation, these in vitro data may differ from G-protein activation during REM sleep.
In conclusion, this is the first study to map the distribution of cholinergically stimulated G-proteins in rat brainstem, with specific emphasis on nuclei known to contribute to the regulation of REM sleep. The data provide a novel perspective on the cholinergic transmembrane signal transduction cascade, consistent with recently emerging findings. The results demonstrate cholinergic activation of mAChR-coupled G-proteins that parallels the localization of mAChRs (Baghdoyan, 1997b). The pharmacological validation of the present [35S]GTPγS assay, demonstrating agonist concentration dependence and antagonist reversibility, also parallels the dose dependence and atropine blocking of cholinergic REM sleep generation (for review, see Baghdoyan, 1997a). The present [35S]GTPγS binding results are consistent with recent data showing that mAChRs, G-proteins, nitric oxide, adenylyl cyclase, cAMP, and protein kinase A in the medial pontine reticular formation modulate cholinergic REM sleep enhancement (Shuman et al., 1995; Capece and Lydic, 1997; Leonard and Lydic, 1997). The results encourage future in vivo studies and the exciting opportunity to quantify G-protein activation in relation to different states of electroencephalographic and behavioral arousal.
Footnotes
This work was supported by National Institutes of Health Grants HL-40881 (R.L.) and MH-45361 (H.A.B.) and the Departments of Anesthesia and Neuroscience and Anatomy of The Pennsylvania State University. We thank Jeri DiVittore and Pam Myers for excellent technical and secretarial assistance.
Correspondence should be addressed to Dr. Ralph Lydic, Department of Anesthesia, The Pennsylvania State University, College of Medicine, Hershey, PA 17033.
REFERENCES
- 1.Baghdoyan HA. Cholinergic mechanisms regulating REM sleep. In: Schwartz WJ, editor. Sleep science: integrating basic research and clinical practice. Karger; Basel: 1997a. pp. 88–116. [Google Scholar]
- 2.Baghdoyan HA. Location and quantification of muscarinic receptor subtypes in rat pons: implications for REM sleep generation. Am J Physiol. 1997b;273:896–904. doi: 10.1152/ajpregu.1997.273.3.R896. [DOI] [PubMed] [Google Scholar]
- 3.Baghdoyan HA, Monaco AP, Rodrigo-Angulo ML, Assens F, McCarley RW, Hobson JA. Microinjection of neostigmine into the pontine reticular formation of cats enhances desynchronized sleep signs. J Pharmacol Exp Ther. 1984;231:173–180. [PubMed] [Google Scholar]
- 4.Baghdoyan HA, Lydic R, Callaway CW, Hobson JA. The carbachol-induced enhancement of desynchronized sleep signs is dose dependent and antagonized by centrally administered atropine. Neuropsychopharmacology. 1989;2:67–79. doi: 10.1016/0893-133x(89)90009-2. [DOI] [PubMed] [Google Scholar]
- 5.Baghdoyan HA, Mallios VJ, Duckrow RB, Mash DC. Localization of muscarinic receptor subtypes in brain stem areas regulating sleep. NeuroReport. 1994;5:1631–1634. doi: 10.1097/00001756-199408150-00022. [DOI] [PubMed] [Google Scholar]
- 6.Bourgin P, Escourrou P, Gaultier C, Adrien J. Induction of rapid eye movement sleep by carbachol infusion into the pontine reticular formation in the rat. NeuroReport. 1995;6:532–563. doi: 10.1097/00001756-199502000-00031. [DOI] [PubMed] [Google Scholar]
- 7.Capece ML, Lydic R. cAMP and protein kinase A modulate cholinergic rapid eye movement sleep generation. Am J Physiol. 1997;273:1430–1440. doi: 10.1152/ajpregu.1997.273.4.R1430. [DOI] [PubMed] [Google Scholar]
- 8.Caulfield MP. Muscarinic receptors—characterization, coupling, and function. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- 9.El Mansari M, Sakai K, Jouvet M. Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep-waking cycle in freely moving cats. Exp Brain Res. 1989;76:519–529. doi: 10.1007/BF00248908. [DOI] [PubMed] [Google Scholar]
- 10.Felder CC. Muscarinic acetylcholine receptors: signal transduction through multiple effectors. FASEB J. 1995;9:619–625. [PubMed] [Google Scholar]
- 11.Gnadt JW, Pegram V. Cholinergic brainstem mechanisms of REM sleep in the rat. Brain Res. 1986;384:29–41. doi: 10.1016/0006-8993(86)91216-3. [DOI] [PubMed] [Google Scholar]
- 12.Herkenham M, Pert CB. Light microscopic localization of brain opiate receptors: a general autoradiographic method which preserves tissue quality. J Neurosci. 1982;2:1129–1149. doi: 10.1523/JNEUROSCI.02-08-01129.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilf G, Gierschik P, Jakobs KH. Muscarinic acetylcholine receptor-stimulated binding of guanosine 5′-O-(3-thiotriphosphate) to guanine-nucleotide-binding proteins in cardiac membranes. Eur J Biochem. 1989;186:725–731. doi: 10.1111/j.1432-1033.1989.tb15266.x. [DOI] [PubMed] [Google Scholar]
- 14.Imeri L, Bianchi S, Angeli P, Mancia M. Selective blockade of different brain stem muscarinic receptor subtypes: effects on the sleep-wake cycle. Brain Res. 1994;636:68–72. doi: 10.1016/0006-8993(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 15.Ito K, McCarley RW. Alterations in membrane potential and excitability of cat medial pontine reticular formation neurons during changes in naturally occurring sleep-wake states. Brain Res. 1984;292:169–175. doi: 10.1016/0006-8993(84)90903-x. [DOI] [PubMed] [Google Scholar]
- 16.Jones BE. Immunohistochemical study of choline acetyltransferase-immunoreactive processes and cells innervating the pontomedullary reticular formation in the rat. J Comp Neurol. 1990;295:485–514. doi: 10.1002/cne.902950311. [DOI] [PubMed] [Google Scholar]
- 17.Kayama Y, Ohta M, Jodo E. Firing of “possibly” cholinergic neurons in the rat laterodorsal tegmental nucleus during sleep and wakefulness. Brain Res. 1992;569:210–220. doi: 10.1016/0006-8993(92)90632-j. [DOI] [PubMed] [Google Scholar]
- 18.Kryger MH, Roth T, Dement WC. Principles and practice of sleep medicine, Ed 2. Saunders; Philadelphia: 1994. [Google Scholar]
- 19.Kurkinen K, Jokinen M, Saavedra JM, Laitinen JT. GTPγ[35S] autoradiography allows region-specific detection of G-protein activation in the chick optic tectum. Soc Neurosci Abstr. 1996;22:1042. [Google Scholar]
- 20.Lee LH, Friedman DB, Lydic R. Respiratory nuclei share synaptic connectivity with pontine reticular regions regulating REM sleep. Am J Physiol. 1995;268:251–262. doi: 10.1152/ajplung.1995.268.2.L251. [DOI] [PubMed] [Google Scholar]
- 21.Leonard TO, Lydic R. Pontine nitric oxide modulates acetylcholine release, rapid eye movement sleep generation, and respiratory rate. J Neurosci. 1997;17:774–785. doi: 10.1523/JNEUROSCI.17-02-00774.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lydic R, editor. Molecular regulation of arousal states. CRC; Boca Raton, FL: 1997. [Google Scholar]
- 23.Lydic R, Baghdoyan HA. Pedunculopontine stimulation alters respiration and increases ACh release in the pontine reticular formation. Am J Physiol. 1993;264:544–554. doi: 10.1152/ajpregu.1993.264.3.R544. [DOI] [PubMed] [Google Scholar]
- 24.McCarley RW, Greene RW, Rainnie D, Portas CM. Brainstem neuromodulation and REM sleep. Semin Neurosci. 1995;7:341–354. [Google Scholar]
- 25.Mitani A, Ito K, Hallanger AE, Wainer BH, Kataoka K, McCarley RW. Cholinergic projections from the laterodorsal and pedunculopontine tegmental nuclei to the pontine gigantocellular tegmental field in the cat. Brain Res. 1988;451:397–402. doi: 10.1016/0006-8993(88)90792-5. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, Ed 2. Academic; New York: 1986. [DOI] [PubMed] [Google Scholar]
- 27.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, Ed 3. Academic; New York: 1997. [DOI] [PubMed] [Google Scholar]
- 28.Semba K, Reiner PB, Fibiger HC. Single cholinergic mesopontine tegmental neurons project to both the pontine reticular formation and the thalamus in rat. Neuroscience. 1990;38:643–654. doi: 10.1016/0306-4522(90)90058-c. [DOI] [PubMed] [Google Scholar]
- 29.Shiromani PJ, Fishbein W. Continuous pontine cholinergic microinfusion via mini-pump induces sustained alterations in rapid eye movement (REM) sleep. Pharmacol Biochem Behav. 1986;25:1253–1261. doi: 10.1016/0091-3057(86)90120-6. [DOI] [PubMed] [Google Scholar]
- 30.Shiromani PJ, Armstrong DM, Gillin JC. Cholinergic neurons from the dorsolateral pons project to the medial pons: a WGA-HRP and choline acetyltransferase immunohistochemical study. Neurosci Lett. 1988;95:19–23. doi: 10.1016/0304-3940(88)90625-8. [DOI] [PubMed] [Google Scholar]
- 31.Shuman SL, Capece ML, Baghdoyan HA, Lydic R. Pertussis toxin-sensitive G-proteins mediate cholinergic regulation of sleep and breathing. Am J Physiol. 1995;269:308–317. doi: 10.1152/ajpregu.1995.269.2.R308. [DOI] [PubMed] [Google Scholar]
- 32.Sim LJ, Selley DE, Childers SR. In vitro autoradiography of receptor-activated G-proteins in rat brain by agonist-stimulated guanylyl 5′-[γ-[35S]thio]-triphosphate binding. Proc Natl Acad Sci USA. 1995;92:7242–7246. doi: 10.1073/pnas.92.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sim LJ, Selley DE, Dworkin SI, Childers SR. Effects of chronic morphine administration on μ opioid receptor-stimulated [35S]GTPγS autoradiography in rat brain. J Neurosci. 1996a;16:2684–2692. doi: 10.1523/JNEUROSCI.16-08-02684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sim LJ, Xiao R, Childers SR. Identification of opioid receptor-like (ORL1) peptide-stimulated [35S]GTPγS binding in rat bran. NeuroReport. 1996b;7:729–733. doi: 10.1097/00001756-199602290-00012. [DOI] [PubMed] [Google Scholar]
- 35.Sim LJ, Selley DE, Childers SR. Autoradiographic visualization in brain of receptor-G-protein coupling using [35S]GTPγS binding. Methods Mol Biol. 1997;83:117–132. doi: 10.1385/0-89603-495-X:117. [DOI] [PubMed] [Google Scholar]
- 36.Steriade M, McCarley RW. Brainstem control of wakefulness and sleep. Plenum; New York: 1990. [Google Scholar]
- 37.Thakkar M, Portas C, McCarley RW. Chronic low-amplitude electrical stimulation of the laterodorsal tegmental nucleus of freely moving cats increases REM sleep. Brain Res. 1996;723:223–227. doi: 10.1016/0006-8993(96)00256-9. [DOI] [PubMed] [Google Scholar]
- 38.Velazquez-Moctezuma J, Gillin JC, Shiromani PJ. Effect of specific M1 and M2 muscarinic receptor agonists on REM sleep generation. Brain Res. 1989;503:128–131. doi: 10.1016/0006-8993(89)91712-5. [DOI] [PubMed] [Google Scholar]
- 39.Wess J. Mutational analysis of muscarinic acetylcholine receptors: structural basis of ligand/receptor/G-protein interactions. Life Sci. 1993;53:1447–1463. doi: 10.1016/0024-3205(93)90618-d. [DOI] [PubMed] [Google Scholar]