Abstract
The 5HT3 receptor (5HT3R) is a serotonin-gated ion channel whose expression is restricted to a subset of cells within the central and peripheral nervous systems. In vitro analysis shows that a small proximal region of the TATA-less 5HT3R promoter is sufficient to direct neuronal-specific reporter gene expression. Three potential regulatory elements conserved between the mouse and human genes were identified within this proximal promoter, two of which are known sites for the ubiquitously expressed factors Sp1 and nuclear factor 1 (NF1). Surprisingly, mutation of the NF1 binding site abolished all reporter activity in cell transfection studies, suggesting that this element is essential for neuronal-specific transcriptional activity of the5HT3R. Furthermore, a complex of neuronal proteins that includes a member(s) of the NF1 family binds to this site, as shown by gel mobility super shift and DNaseI footprinting analyses. Although NF1 has been proposed to mediate basal transcription of many ubiquitously expressed genes, our data suggest that a member of the NF1 transcription factor family participates in neuronal-specific gene expression by promoting interactions with other regulatory factors found in sensory ganglia.
Keywords: gene expression, sensory neuron, NF1 transcription factor, ligand-gated ion channel, 5HT3R, TATA-less promoter
Serotonin (5HT) is a major neurotransmitter in both the CNS and peripheral nervous system. The wide array of physiological and behavioral responses to serotonin are mediated by multiple presynaptic and postsynaptic 5HT receptor subtypes, each exhibiting a unique pattern of expression within the nervous system (for review, see Tecott and Julius, 1993). Of the 14 mammalian 5HT receptor subtypes identified thus far, all but one are G-protein-coupled; the 5HT3 receptor (5HT3R) is the only known exception, belonging to the superfamily of ligand-gated ion channels that includes nicotinic acetylcholine (nAchR), GABAA, and glycine receptors (Unwin, 1993). In situ hybridization and radioligand binding studies have shown that 5HT3Rs are prominently expressed in a variety of peripheral ganglia, including a subset of neurons within dorsal root, trigeminal, cranial, and enteric ganglia (Tecott et al., 1993, 1995; Johnson and Heinemann, 1995b). As such, this receptor has been proposed to modulate nociception and pain responses, as well as enteric and cardiovascular reflexes. Within the CNS, 5HT3Rs are found primarily in limbic and brainstem structures, consistent with the actions of 5HT3R antagonists as antianxiety and antiemetic agents. Developmental studies have shown that 5HT3Rtranscripts serve as an early molecular marker for central and peripheral neurons, in many cases appearing while these cells are still dividing or migrating to their final destinations (Johnson and Heinemann, 1995; Tecott et al., 1995).
To date, the identity of transcription factors that specify differentiated neuronal phenotypes has remained elusive, especially with respect to the peripheral nervous system. Indeed, mechanisms governing the restricted expression of molecular markers of the sensory nervous system, such as high- and low-affinity neurotropin receptors, ATP-gated ion channels, and TTX-insensitive sodium channels have not been established. Although gene knock-out studies have implicated basic helix–loop–helix and POU-homeodomain transcription factors as being involved in the specification of mammalian neuronal phenotypes, downstream targets for these factors have not been identified (Ryan and Rosenfeld, 1997). It has been proposed that neural-specific gene expression may use a model of transcriptional repression rather than activation (Schoenherr and Anderson, 1995b), whereby the absence of a negative regulator in neuronal cells would promote transcription of neural-specific genes (Chong et al., 1995; Schoenherr and Anderson, 1995a; Schoenherr et al., 1996). However, the recent use of the nestin promoter to drive heterologous gene expression in a subset of neurons argues that at least some mechanisms of neuronal gene expression involve selective activation rather than repression (Zimmerman et al., 1994).
To delineate the molecular mechanisms controlling neural-specific gene expression, cis-acting regulatory elements within an array of target genes must first be characterized. We chose to define promoter elements within the 5HT3R gene because it is prominently expressed in a subset of sensory neurons, as well as in neuroblastoma cell lines, thereby facilitating promoter analysisin vitro. In this study, we have characterized the5HT3R promoter in cultured cell lines and found that a nuclear factor 1 (NF1) element is essential for5HT3R expression. Further in vitroanalyses suggest that a member of the NF1 gene family is bound by this element and is present in both neuroblastoma cell lines and in trigeminal ganglia.
MATERIALS AND METHODS
Plasmids and genomic constructs. Murine5HT3R genomic clones were isolated from a λ-FIX C57 genomic library (Stratagene, La Jolla, CA) using a radiolabeled 5HT3R cDNA probe containing 5′UTR and the first 20 bp of the published mouse cDNA (Maricq et al., 1991). Clones for the human 5HT3R were isolated from a commercial human genomic cosmid library (PWE15 vector; Stratagene) using a radiolabeled PvuII-HindIII fragment of the murine 5HT3 cDNA; this fragment contains 60 bp of 5′UTR, followed by 490 bp of coding sequence. The most proximal portion of the human5HT3R 5′ flanking sequence was determined (∼1.8 kb of a 3 kb BamHI-EcoRI fragment) and aligned with the mouse 5HT3R sequence using the Geneworks alignment program. GenBank accession numbers for complete sequenced genomic fragments are U73442 for murine and U73443for human.
Genomic regions upstream of exon 1 were subcloned into the pGL2-Basic Luciferase reporter plasmid (Promega, Madison, WI) from the two5HT3R genomic clones, −2534/−3 (λ18.2.1) and −1041/−3 (λ13.2.1), described above by using a convenientHindIII site located at position −3. Serial deletions of the upstream 5HT3R promoter regions were constructed with convenient restriction sites, StuI(−1544), NsiI(−893), and NsiI (−135), already present in the5HT3R gene or by an NdeI restriction site created at position −252 by site-directed mutagenesis (Muta-gene kit; Bio-Rad, Hercules, CA). Reporter constructs of mutated E-box, Pal-1, and NF1 elements (listed in Table1) were created in the −252 bpNdeI-HindIII 5HT3Rreporter luciferase construct by site-directed mutagenesis. Control plasmids included the minimal promoter -p36-LUC (Ingraham et al., 1988) and the PGL2 basic vector (Promega). All plasmids were sequenced to verify that the desired mutations were created.
Table 1.
Oligonucleotides used for gel mobility shift assays and competition studies
| Oligo name | Nucleotide sequence |
|---|---|
| E-box | 5′-TCCAGTCTAA-3′ |
| mE-box | 5′-TCacGcgTAA-3′ |
| Pal-1 | 5′-TGCCAGGCTGCAGCCTCACA-3′ |
| mPal-1 | 5′-TGCCcatCTGCActCTCACA-3′ |
| 5HT3R NF1 | 5′- TGGCGGCTC CCCA-3′ |
| AdNF1 | 5′- TGGCTTGAA GCCA-3′ |
| MBP NF1 | 5′- TGGCACTAT GCCA-3′ |
| NF1 consensus | 5′- TGGCNNNNN GCCA-3′ |
| 5HT3R mNF1 | 5′-aGtaGGCTCCCgA-3′ |
The core sequence is shown for all oligonucleotides used in this study for gel mobility shift assays and competition studies. Both the wild-type and mutated sequences are given. Specific residues were mutated by site directed mutagenesis within the context of the5HT3R proximal promoter as described in the Materials and Methods; mutated residues are depicted by lowercase type. In addition, the core consensus binding site of the NF1 protein is shown and compared with NF1 sites within the5HT3R promoter (this study), the myelin basic protein promoter (Tamura et al., 1990b), and the adenovirus type 5 origin of replication and enhancer (Gounari et al., 1990). MBP, Myelin basic protein; AdNF1, adenovirus NF1.
Northern blot and RNase protection assays. Total RNA was isolated from N18-TG2, NG108, and NCB-20 as described previously (Chirgwin et al., 1979). For Northern blot analysis, total RNA (10 μg) was immobilized on a Nylon N+ membrane and hybridized with a 500 bp radiolabeled fragment corresponding to the 3′ coding region of the mouse 5HT3R cDNA. Equivalent amounts of total RNA were loaded as judged by the intensity of 28 and 18 S ribosomal RNA. For the RNase protection assay, the −1041/−3 (λ13.2.1) genomic fragment of the 5HT3R gene was subcloned into pBKS+ (Stratagene), and the NdeI site generated at position −252 by site-directed mutagenesis was used to linearize the plasmid. Radiolabeled antisense cRNA was transcribed using T7 RNA polymerase and32P-UTP. cRNA probe (20 fmol) was hybridized overnight with 10 μg of total RNA, followed by digestion with RNases using buffers and directions according to the Ribonuclease Protection assay kit (Ambion). Protected fragments were analyzed on a 6% denaturing polyacrylamide gel in 1× TBE.
Cell culture and transfection studies. N18-TG2 cells were cultured in DMEM supplemented with 10% fetal calf serum (FCS), 60 nm 2-amino-6-mercaptopurine, and antibiotics. NCB-20 and NG108 cells were cultured in DMEM supplemented with 10% FCS, HeLa cells were cultured in DMEM supplemented with 10% calf serum, and HEK-293 cells were cultured in DMEM supplemented with 5% FCS, 5% bovine serum, and antibiotics. Cultured cells were grown in 60 mm dishes and transfected at 50% confluency with 3 μg of the pGL2 reporter DNA in triplicate using LipofectAMINE reagent (Life Technologies, Gaithersburg, MD). Cell lysates were harvested 36 hr later, and luciferase activity was assayed using the Promega Luciferase assay system and a Monolight 2010 system luminometer. Transfection efficiency was monitored by normalizing β-galactosidase activity (substrate, o-nitrophenyl β-scap[d]-galacto-pyranoside) to total protein (Bio-Rad protein determination kit).
Gel mobility shift assays. Nuclear extracts were prepared as described previously (Goyal et al., 1990). The promoter probe (−252/−3) was produced by PCR using primers specific for the mouse5HT3R sequence. The sense oligonucleotide was end-labeled using T4 polynucleotide kinase. Binding reactions were performed as previously described for 25 min at 25°C in binding buffer containing: 12% glycerol, 20 mm HEPES, pH 7.9, 60 mm KCl, 4 mm Tris-HCl, pH 8.0, 0.6 mm EDTA, 0.6 mm EGTA, 5 mm DTT, 0.5 μg/ml BSA, 5 ng/μl salmon sperm DNA, and 1 μg of poly(dI·dC) (Ingraham et al., 1988). Protein–DNA complexes were resolved on a nondenaturing 5–7% polyacrylamide gel in 0.5× TBE at 22°C. Preimmune or chick anti-NF1 serum was incubated for 30 min at 22°C before addition of the probe.
DNaseI protection assays. DNA–protein complexes were formed as described above using 1–7 μg of nuclear extract. Reactions were digested with 10 μl of Life Technologies RQ1 DNaseI (diluted 1:2500) in a 50 μl volume of 5 mm CaCl2, 10 mm MgCl2, and 0.2 mm EDTA for 3 min at 22°C. Reactions were then incubated in 100 μl of 2× Proteinase K buffer (in mm: 200 Tris-HCl, pH 7.5, 25 EDTA, 300 NaCl, and 2% SDS), 2 μl of tRNA (10 mg/ml), and 2 μl of Proteinase K (10 mg/ml) for 10 min at 4°C. Samples were phenol-extracted twice, precipitated, and separated on a denaturing 7% polyacrylamide gel in 0.5× TBE. The sequence of the DNaseI protected fragments was determined by comparison to a G plus A marker ladder generated by standard Maxim and Gilbert sequencing reaction.
RESULTS
Isolation of the mouse TATA-less 5HT3R gene
To determine the molecular mechanisms that give rise to the precise spatial pattern of 5HT3R expression, identification of cis-regulatory elements within the mouse and human 5HT3R promoter regions was undertaken. Comparison of 5′ upstream sequences among divergent species can be an extremely useful strategy for determining which potential regions are important for transcriptional regulation. Genomic clones containing the upstream region of the murine and human 5HT3Rgenes were isolated, and nucleotide sequences were obtained from ∼2.5 kb of the mouse gene and −1.8 kb of the human gene upstream of the ATG initiator methionine. Although no significant homology was observed between human and mouse clones in the region spanning −1.8 kb to −0.6 kb, a high degree of sequence similarity (62%) was detected within the region proximal to the initiation codon (ATG, +1). Direct nucleotide sequence comparison in this proximal region shows even greater sequence identity (>77%) between −230 and +1 (Fig.1). The striking similarity between the human and mouse 5HT3R genes in this area suggests that one or more regulatory elements critical for5HT3R expression might reside within this proximal 5′ flanking region. Indeed, a number of putative regulatory elements were found conserved, including Sp1, AP2, E-box, and NF1 sequences (Fig. 1).
Fig. 1.
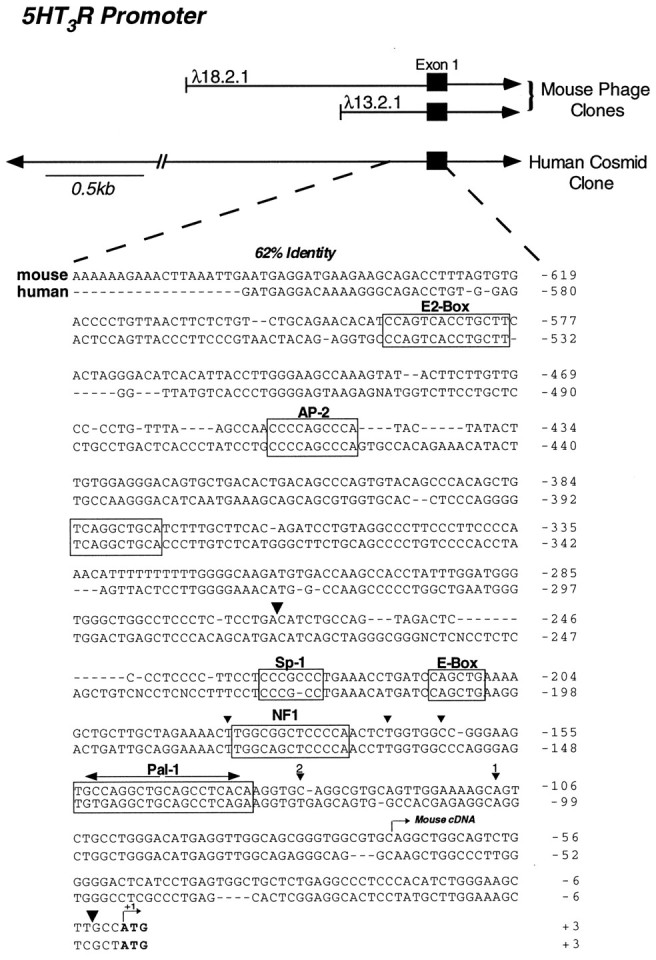
Comparison of the mouse and human5HT3R upstream promoter region. Two independent and overlapping mouse genomic clones containing 3 kb and 1.3 kb of genomic DNA, respectively, are shown with respect to the initiator methionine and exon 1 (black boxes) encoding the 5HT3R. The human 5HT3R cosmid clone of 20 kb is also depicted with arrowheadsto indicate that the clone continues. Sequence comparison of the proximal 5HT3R promoter region is shown for the mouse and human sequences with the first nucleotide in the ATG initiator codon assigned as position +1. Conserved elements are boxed and labeled, including the E-Box, Pal-1, and NF1 potential DNA binding recognition sites. The initiator methionine codon of the mouse and human cDNA clones is shown in bold, and the beginning of the mouse5HT3R cDNA is indicated by anarrow. Large arrowheads show the end positions of the fragment used for the RNase protection assay to determine the major start sites (Fig. 3B). Major start sites are shown with a smaller arrowhead, and thenumber given above each symbol corresponds to the assigned transcriptional start site, as shown in Figure2B.
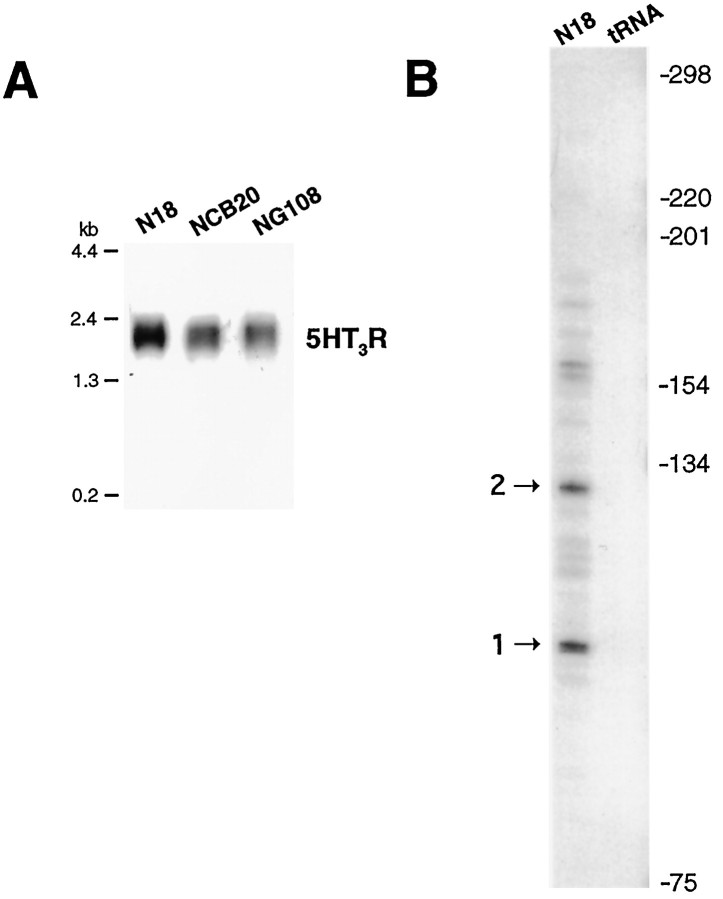
The absence of a canonical TATA box within this conserved 5′ region led us to map the approximate start site of the5HT3R gene, using an RNase protection assay. The5HT3R cDNA was originally cloned from the N18-TG2 neuroblastoma × Chinese hamster brain hybrid cell line referred to as NCB20 (Maricq et al., 1991). Among rodent neuroblastoma cell lines examined, the parental N18-TG2 mouse cell line exhibited the highest level of endogenous 5HT3R transcripts (Fig. 2A) and was therefore used to perform the RNase protection assays and cell transfection studies. Several distinct protected RNA species were observed using total RNA isolated from N18-TG2 cells (Fig.2B), suggesting that multiple start sites are used to initiate transcription of the mouse 5HT3R gene. These data infer that all 5HT3R major start sites are upstream of the reported 5′ end of the mouse cDNA but downstream of the conserved elements shared between the mouse and human5HT3R genes. The location of the two major start sites is indicated in Figures 1 and 2B. These same major start sites were also observed in a RNase protection assay performed with RNA isolated from the mouse neuroblastoma-derived cell line NG108-15 (data not shown). Our finding that transcription of the5HT3R gene is initiated at multiple start sites by a TATA-less promoter has also been described for other ligand-gated channel transcripts, such as the DrosophilaCa2+ activated K+ channel (slo) (Brenner et al., 1996) and the nAchR α3 subunit gene (Yang et al., 1994; Fornasari et al., 1997).
Fig. 2.
Mapping the 5HT3R transcriptional start site(s). A, Expression of5HT3R transcripts in three mouse neuroblastoma cell lines, N18, NCB20 and NG108, was determined by Northern blot analysis using 1 μg of poly(A+) mRNA. B, RNase protection analysis was performed by the method described in Shen et al. (1994), using a ∼300 bp 5HT3R fragment NdeI-Hd3 subcloned into pBKS+. An internal NdeI was introduced by site-directed mutagenesis at position −262, changing CATCTG to CATATG to allow for the generation of a conveniently sized radiolabeled cRNA probe. Antisense cRNA was transcribed using T3 polymerase and hybridized with N18 mRNA, as described in Materials and Methods. The patterns of protected species are shown for N18-TG2 total RNA compared with control yeast tRNA (tRNA).
Cell-specific 5HT3R gene expression requires a minimal promoter region
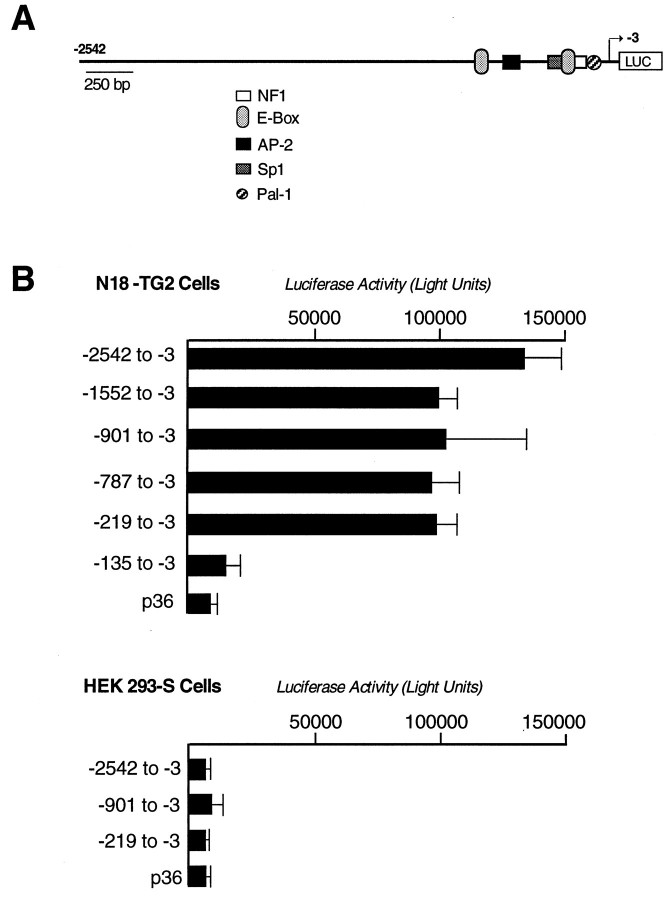
To determine whether a functional promoter for5HT3R resides within this proximal region, the −2583/−3 mouse 5HT3R genomic fragment was tested for transcriptional activity using standard luciferase reporter constructs in cell transfection studies. Robust reporter activity was observed with the −2.5 kb 5HT3R fragment when assayed in the neuroblastoma cell lines N18-TG2 (Fig.3A) or NCB-20 (data not shown), both of which express endogenous 5HT3Rtranscripts. By contrast, no significant reporter activity was seen in human embryonic kidney HEK-293 (Fig. 3B) or HeLa cells (data not shown), neither of which express endogenous5HT3R transcripts. Serial deletions of the −2542/−3 5HT3R promoter were constructed to further define regions required for neuronal-cell expression. Surprisingly, as little as −219 bp of the 5HT3Rpromoter region was required for robust reporter activity, and we note that this small proximal promoter was slightly less active than reporter constructs using the full −2542 bp of the5HT3R gene (Fig. 3A). Further deletion to −135 bp yielded background levels similar to that observed with the minimal rat prolactin promoter (p36). TATA-less promoters often contain Sp1 sites near the site of transcription initiation (Zenzie et al., 1993). Accurate transcription does occur in the absence of a TATA element in such promoters, and Sp1 has been proposed to facilitate the recruitment of TBP and the basal transcriptional machinery to these TATA-less promoters. Indeed, both the mouse and human promoters are TATA-less and contain a Sp1 motif; however, this element appears dispensable, because full luciferase activity is observed in 5HT3R constructs deleted for the Sp1 binding site (Fig. 3A,B). Together, these data suggest that cis elements critical for full transcriptional activity in neuronal cell lines are contained within −219 proximal regions of the 5HT3Rpromoter.
Fig. 3.
Functional analysis of the mouse5HT3R promoter and 5′ flanking region.A, Mouse 5HT3R promoter and upstream sequences. All conserved sites shared between the mouse and human 5HT3R genes are shown as labeled.B, A series of deletions were created in the5HT3R promoter and upstream regions and fused to the firefly luciferase reporter gene. The exact nucleotide location of the deletion is indicated on the side of the luciferase activity determined after transfection into either N18-TG2 or HeLa cells, as described in Materials and Methods. For each construct, triplicate samples were independently measured, and the entire experiment was performed at least four times. An additional plasmid, CMV-βGAL, was cotransfected in the transient transfections as an internal control for variation between transfection efficiencies. The p36 minimal prolactin promoter luciferase construct was used to define a basal level of transcription (Ingraham et al., 1988).
An NF1 element mediates DNA–protein interactions and is essential for 5HT3R expression
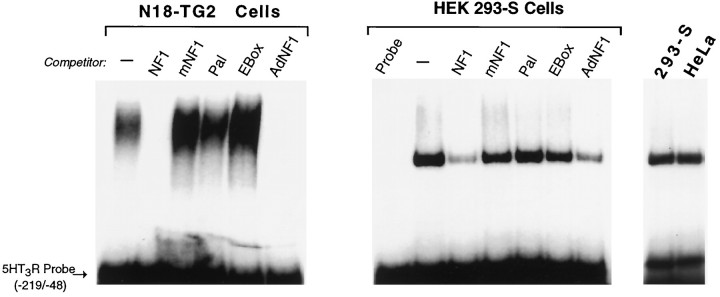
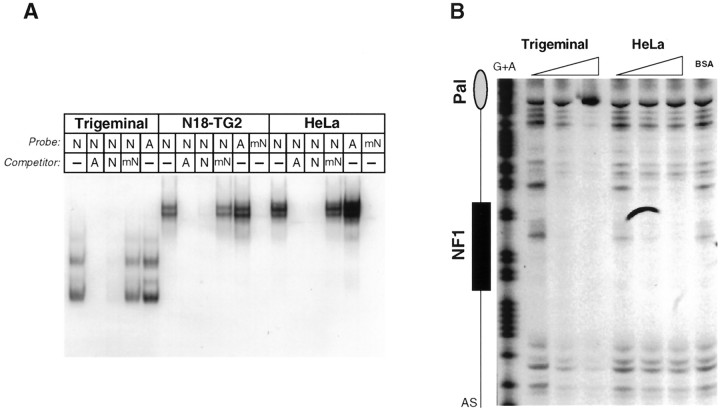
To detect DNA-binding proteins that might interact with the5HT3R proximal promoter, gel mobility shift assays were performed using nuclear protein extracts prepared from neural and non-neural cell lines. Although prominent DNA–protein interactions were formed with extracts from both N18-TG2 and HEK-293S cells, significant differences in the mobility of these complexes were observed (Fig. 4). Binding experiments with extracts from N18-TG2 cells produced a broad and slower migrating complex compared with the single and rapidly migrating complex observed with extracts from HEK-293 or HeLa cells. Several conserved sequences resembling known consensus DNA binding sites are located within the −219 bp 5HT3R promoter, including the conserved basic helix–loop–helix protein binding site of the E2-type box, an NF1 binding site, and a novel 12 bp conserved palindrome referred to as Pal-1 (Fig. 1, Table 1). In all cell lines tested, the dominant DNA–protein interaction appeared to involve the NF1 site, because addition of unlabeled competitor oligonucleotides specifying either the5HT3R NF1 site or the adenovirus NF1 (AdNF1) site reduced or eliminated complex formation (Fig. 4). Competition with a mutant NF1 binding site failed to abolish complex formation, as did excess 5HT3R E-box or Pal-1 elements. Thus, binding of a neuroblastoma-derived protein complex to the proximal5HT3R promoter is mediated, in part, by an NF1 element.
Fig. 4.
An NF1-like element is bound by a large protein complex in N18-TG2 cells. Gel mobility shift assays were performed using the entire −219 bp fragment of the5HT3R proximal promoter and nuclear extracts prepared from either the neuronal N18-TG2 cell line or the non-neuronal HEK-293S or HeLa cell lines. Nonradioactive annealed oligonucleotide competitors were added in 100-fold molar excess of the5HT3R proximal promoter probe; these sites include wild-type 5HT3R NF1 element (NF1), a mutant 5HT3R NF1 element (mNF1), the palindromic sequence (Pal-1), the E-box element (EBox), and the NF1 element in the adenovirus type 5 enhancer (AdNF1); refer to Table 1 for sequence information. Nuclear extracts (5 μg) were incubated together with unlabeled annealed oligonucleotides and radiolabeled5HT3R proximal promoter [5HT3R Probe (−219/−48)], as described in Materials and Methods. Identical band shift patterns are observed after incubation with either of the two non-neuronal cell extracts, HEK-293S or HeLa cells.
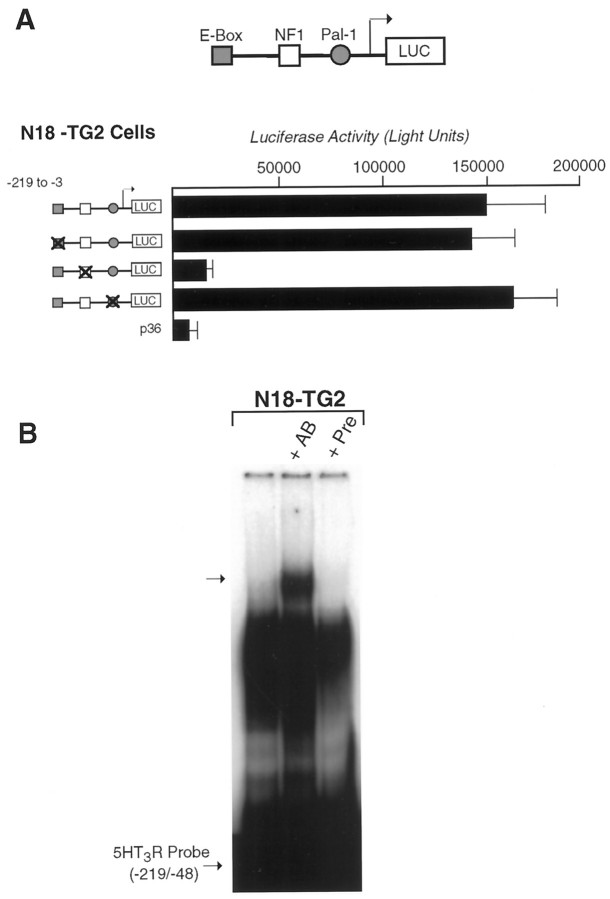
The functional role of these three conserved elements in the neuronal regulation of the 5HT3R gene was tested directly by mutating each element in the context of the −219/−3 promoter. Constructs bearing multiple point mutations in either the E-box or Pal-1 element exhibited full transcriptional activity compared with the wild-type 5HT3R proximal promoter (Fig.5A, Table 1). However, mutation of the NF1 element resulted in significant attenuation of reporter activity, demonstrating that this element is essential for5HT3R expression in neuroblastoma cell lines. Collectively, our in vitro transfection and binding studies show that a complex of neuroblastoma proteins interacts with an NF1 element to promote activation of the 5HT3Rgene.
Fig. 5.
Mutation of conserved elements in the5HT3R proximal promoter. A, Individual elements within the 5HT3R proximal promoter were mutated individually and compared with the wild-type promoter in N18-TG2 cell transfection assays. Each mutant promoter constructed is depicted in A, and the exact residues mutated are listed in Table 1. In all cases, either the palindromic nature of the site or the core residues proposed to mediate DNA binding was altered. Loss of the NF1 site results in a 10-fold loss of reporter activity of the 5HT3R proximal promoter and is equivalent to the lowered activity observed with the minimal 5HT3R (−135/−3) reporter construct (Fig. 3A). CMV-βGAL plasmid was cotransfected to control for transfection efficiencies, and the luciferase activity was corrected to reflect these values. B, Super-shift analysis of gel mobility shift assays was performed by preincubating the rabbit anti-chicken NFI antiserum (a gift from Dr U. Kruse, The Scripps Research Institute, La Jolla, CA) or preimmune serum with N18-TG2 nuclear extract before binding to the radiolabeled5HT3R proximal promoter probe. Super-shifted DNA–protein complexes are observed after preincubation with antibody (+AB) but are not present without or with preimmune serum (+Pre) in NG18-TG2 extract.
NF1 recognition sites are reported to bind a family of highly related transcription factors that are widely expressed (Kruse and Sippel, 1994a). The NF1 site in the 5HT3Rpromoter is similar, but not identical, to previously reported NF1 binding sites, raising the possibility that a neuronal-specific NF1 protein could bind to this site within the proximal5HT3R promoter. Using an antibody that cross-reacts with the DNA binding domain of all known members of the NF1 family (Schuur et al., 1995), a super-shifted DNA–protein complex was specifically observed (Fig.6B). A similar super-shifted complex was observed when the anti-NF1 antibody was incubated with nuclear extracts prepared from HEK-293 cells (data not shown). In both cases, only a small fraction of the DNA–protein complex was super-shifted, consistent with previous reports that the anti-chick NF1 antibody has low avidity for mouse NF1 proteins (Schuur et al., 1995).
Fig. 6.
NF1-like proteins from neuroblastoma and trigeminal ganglia bind to the 5HT3R NF1 site, conferring cell specific transcription. A, Gel mobility shift assays were performed with the radiolabeled5HT3R NF1 consensus site and ∼3–5 μg of nuclear extracts prepared from rat trigeminal ganglia, N18-TG2, and HeLa cultured cells. Various competitors were added in 100-fold molar excess of labeled probe and included the wild-type5HT3R NF1 site (N), a mutant 5HT3R NF1 site (mN), the adenovirus NF1 site (A), or no competitor (-). All major DNA–protein complexes are competed for with either the NF1 or AdNF1 annealed oligonucleotides but not with the mutated5HT3R NF1 site. The gel mobility shift patterns are nearly identical in both the N18-TG2 and HeLa cells.B, DNaseI footprinting patterns are shown for trigeminal and HeLa cell nuclear extracts on the sense strand of the5HT3R proximal promoter region. In a region centered on the NF1 consensus binding site, a large and prominent footprint region is observed with a small amount of trigeminal ganglia nuclear extracts (3 μg of protein). This pattern extends beyond the footprint observed with HeLa cells as more trigeminal ganglia protein is incubated. DNaseI footprinting analyses were performed on labeled sense and antisense strands and revealed no other protected footprints (data not shown).
N18-TG2 and trigeminal ganglia proteins bind to the NF1 site
Binding studies using the full −219 bp of the proximal5HT3R promoter suggested that a known NF1 protein or an NF1-like protein is bound to this promoter. We probed the nature of this NF1 binding activity by direct comparison of rat trigeminal, N18-TG2, and HeLa nuclear extracts in a gel shift assay. For these studies, an isolated NF1 element from the5HT3R promoter or adenovirus was used as the DNA probe (Fig. 6A). Because NF1 proteins have been well characterized in this cell line (Goyal et al., 1990), HeLa cell extract was used instead of HEK-293S cells. Similar to HEK-293S cells, HeLa cells do not support 5HT3R reporter activity but yield a nearly identical DNA–protein complex with the5HT3R proximal promoter (Fig. 4). Competition studies show that all DNA–protein complexes are readily competed by a 100-fold excess of either the 5HT3R NF1 or AdNF1 elements but not by the mutant 5HT3R NF1 (mN) element. These results suggest that NF1-like binding proteins that are found in trigeminal, N18-TG2, and HeLa cell nuclear extracts exhibit a similar affinity for NF-1 elements. Furthermore, mutating the5HT3R NF1 element (mN) abrogates all binding (Fig. 6A). However, we observed consistently that the overall DNA–protein pattern is distinctly different in extracts obtained from the cultured cell lines versus primary sensory ganglia tissue. It is plausible that these differences in gel shift patterns may reflect distinct sensory ganglia NF1 binding proteins or isoforms not present in cultured cells. Also, ternary complexes may form on the isolated NF1 element when using extracts made from cultured cell lines but not with extracts made from sensory ganglia protein. These potential ternary complexes could account for the observed differences in gel shift pattern, although we see little evidence of such complexes from competition experiments. Alternatively, this difference in the apparent size of NF1-bound proteins may simply be attributable to nonspecific proteolysis of trigeminal nuclear extract, despite our use of protease inhibitors during the preparation of nuclear extracts. Nonetheless, the grossly distinct gel shift patterns observed between the HEK-293S (or HeLa) and N18-TG2 cell lines when using the entire5HT3R proximal promoter as a probe (Fig. 4) versus the isolated NF1 site may arise from additional proteins binding to DNA regions adjacent to the core NF1 element.
To confirm the involvement of an NF1 site in the formation of a DNA–protein complex, DNaseI footprinting analysis was performed on complexes formed with nuclear extracts prepared from neuronal tissue (trigeminal sensory ganglia) or HeLa cells (Fig. 6B). DNaseI footprint patterns revealed that trigeminal ganglion nuclear proteins protected a large region extending well beyond the boundaries of the NF1 site. Consistent with the tight singular nature of the DNA–HEK-293S protein complex, a much smaller region was bound by HeLa nuclear proteins. Interestingly, although the DNaseI footprint pattern observed with trigeminal protein extracts extended into the region covered by both Pal-1 and E-box sites, neither of these sites effectively abrogated formation of a large DNA–protein complex using trigeminal ganglion extracts (data not shown). Collectively, these findings lead us to hypothesize that a large neuronal protein complex nucleated by NF1 or an NF1-like protein binds to the5HT3R proximal promoter region to activate5HT3R expression.
DISCUSSION
We report here that the proximal TATA-less promoter of the serotonin-gated ion channel is sufficient for neuronal-specific expression in cultured cell lines. Moreover, an element within this −219 bp fragment matching the known consensus binding site for the NF1 family serves as an essential cis-regulatory element for this restricted transcriptional activity.
To date, the NF1 family consists of four highly conserved genes, each giving rise to alternatively spliced transcripts, potentially encoding a number of the different isoforms that are able to heterodimerize and homodimerize (Kruse and Sippel, 1994a,b; Qian et al., 1995). The originally defined NF1 binding element was shown to be critical in adenovirus DNA replication and has subsequently been found in a number of promoters and enhancers of viral or cellular origin. Adenovirus and5HT3R NF1 sites are nearly identical (Table 1), and the strikingly similar gel shift patterns generated with either N18-TG2 or HeLa cells on an isolated NF1 site suggest that the same or closely related NF1 proteins are present in both neuronal and non-neuronal cell lines (Fig. 6A). Although particular NF1 isoforms (NF1B and NF1-related genes) appear to be enriched in the cerebellum and brain (Inoue et al., 1990; Sumner et al., 1996), they are also expressed in many other tissues and cell lines. Indeed, NF1 proteins exhibit ubiquitous patterns of expression, and it is therefore unlikely that they play a primary role in determining cell- or tissue-specific transcription. However, NF1 proteins have been implicated in cell-specific gene expression; examples include adipocyte-specific expression of adipocyte P2 gene (Graves et al., 1991, 1992), brain-specific transcription of myelin basic protein (Tamura et al., 1990b), and JC virus gene expression within the nervous system (Tamura et al., 1990a). More relevant to this study is the presence of an NF1 element in the TATA-less proximal promoter region of the human α3 nAchR subunit gene; this small promoter of 350 bp appears essential for expression in human neuroblastoma cell lines (Fornasari et al., 1997).
The obvious lack of restricted expression for the NF1 gene family has led to the hypothesis that NF1 may either activate or silence gene expression in a cell-specific manner by participating in a combinatorial code involving cofactors. This scenario is best exemplified by liver-specific vitellogenin gene expression. There, multiple NF1, cAMP response element-binding protein, and HNF3 binding sites are proposed to participate in the regulation of vitellogenin; however, of these three transcription factors, only HNF3 displays a tissue- or cell-type specific expression pattern (Cardinaux et al., 1994). This same type of mechanism may account for the distinct N18-TG2 gel shift patterns generated with the proximal5HT3R promoter in which additional regulatory factors not present in the HEK-293S extracts may directly interact with an NF1 protein or the residues adjacent to the NF1 core binding site. Alternatively, the nature of the binding to the5HT3R NF1 site by these NF1-like proteins may be different, although similar DNA–protein complexes generated in the N18-TG2 and HeLa cell lines would not readily support this argument.
Our data showing almost identical gel mobility shift patterns on an isolated NF1 binding site using either non-neuronal or neuronal proteins extracts support the notion that the5HT3R NF1 site is bound by a common NF1 isoform. Furthermore, the extended DNaseI footprint pattern beyond the NF1 site of the 5HT3R proximal promoter suggests that a combination of factors comprise the NF1-bound complex. In our in vitro system, it remains unclear whether the E-box and/or the unidentified palindromic sites that reside adjacent to the NF1 site are bound by neuronal-specific proteins, and attempts to show high-affinity and tissue-specific protein interactions with these isolated sites have been unsuccessful (data not shown). Moreover, disruption of either the E-Box or Pal-1 sites failed to significantly reduce5HT3R reporter activity. Together, these data imply that regulatory proteins may bind weakly to the 5HT3R proximal promoter, or they may exert control of cell-specific5HT3R expression via direct protein–protein interactions with an NF1 nucleated complex. Such large protein complexes containing NF1 have been described with brain nuclear extracts bound to the Asp aminotransferase gene promoter (Garlatti et al., 1996). Although specific cofactors have not been identified to date, the work described here introduces what may be a facile biochemical system for purifying novel components of NF1 transcription complexes.
In contrast to our findings showing regulation of the5HT3R by the proximal promoter region, expression of the rat neuronal nAchR β4 subunit gene is believed to involve an enhancer element positioned within the 3′UTR (McDonough and Deneris, 1997). Moreover, this element is also ∼2.5 kb upstream of the rat nAchR α3 subunit gene and has therefore been postulated to exert transcriptional control over this cluster of related ion channel genes. Two 37 bp repetitive elements are found within this region and are bound by a large protein complex from PC12 cell extracts; this distinctive 37 bp repeat is not present in the −219 bp5HT3R proximal promoter. Anothercis-acting element that has been identified in several neuron-specific genes is the neural-restrictive silencer element (NRSE) (Schoenherr and Anderson, 1995a), which is proposed to bind a repressor in non-neuronal cells referred to as RE1 silencing transcription factor 1 (REST). Based primarily on in vitro data, binding of REST to the NRSE site is predicted to silence expression of neuronal proteins outside of the nervous system (Chong et al., 1995; Schoenherr et al., 1996). Of genes containing a NRSE consensus sequence, many are expressed in the nervous system, including the nAchR β2-subunit and the type II sodium channel genes. Although the proximal or full −2.5 kb of 5′ flanking sequences in either the human or mouse 5HT3R promoter regions does not appear to contain a neural restrictive enhancer factor (NREF) or NREF-like element, it is possible that such an element is located further upstream or downstream of the region we have sequenced. Given that we observe robust activation in a neuroblastoma cell line, but not in non-neuronal cell lines, it seems likely that a model of activation rather than repression accounts for the expression pattern of the 5HT3R gene in sensory ganglia. Indeed, a silencing model may not account for all neuronal expression, because mutation of the NRSE in the nAchR β2 subunit gene dramatically reduces peripheral nervous system expression in vivo. Moreover, expression in nine independent transgenic lines show that the mutant NRSE transgene continues to be restricted to neuronal cell types (Bessis et al., 1997). Similarly, our results show that expression of the 5HT3R NF1 mutant reporter remains off in non-neuronal cell lines.
Candidate transcriptional activators have been proposed to specify sensory neuronal lineages or to activate the expression of ligand-gated ion channel genes. Such factors include members of the POU-domain gene family, such as Brn-3a and Brn-3b, Brn-3.0 and Brn-3.2, and SCIP/Tst-1 (Ninkina et al., 1993; McEvilly et al., 1996; Xiang et al., 1996; Smith et al., 1997). The 5HT3R proximal promoter region does not appear to contain binding sites for POU proteins (Li et al., 1993), making it unlikely that expression of this gene is directly regulated by such factors.
Apart from its role as a classical neurotransmitter, serotonin has also been suggested to exert morphogenic effects during neurogenesis and in the formation of craniofacial structures (Shuey et al., 1993). Indeed, during embryonic development, expression of5HT3R transcripts is high in certain non-neuronal tissues, particularly in regions where adjacent epithelial and mesenchymal tissues interact. This is best exemplified by the marked expression of the 5HT3R in the developing tooth bud, an embryonic structure in which reciprocal inductive signals between these adjacent epithelial and mesenchymal layers leads to the formation of bone and enamel structures (Johnson and Heinemann, 1995b; Tecott et al., 1995). It remains possible that the regulation of the 5HT3R gene in the peripheral nervous system relies on different cis elements or on a combination of different cofactors, distinct from those that may specify expression in embryonic non-neuronal tissues or in the CNS. Analysis of the 5HT3R proximal promoter in vivo will help determine whether this region by itself is sufficient to target expression to the sensory nervous system. Promoter analysis of genes encoding the 5HT3R and other cell surface markers (e.g., ATP receptors, capsaicin receptors, or TTX-insensitive sodium channels) whose expression is prominent in, or restricted to, sensory neuron subtypes will provide the molecular tools for analyzing or perturbing gene expression within the peripheral nervous system.
Footnotes
This work was supported by a postdoctoral fellowship from the National Alliance for Research into Schizophrenia and Depression (F.K.B.), and grants from National Institute of Mental Health (D.J.), National Institute of Diabetes and Digestive and Kidney Diseases, and the Lucille Markey Foundation (H.A.I.). We thank Dr. U. Kruse (The Scripps Research Institute, La Jolla, CA) for helpful discussions and for providing the anti-chick NF1A antibody. We also thank members of the Ingraham and Julius lab for suggestions throughout this project and Dr. Sherry Taylor for earlier work on this project.
Correspondence should be addressed to Dr. Holly A. Ingraham, Department of Physiology, University of California at San Francisco, San Francisco, CA 94143-0444.
Dr. Bedford’s present address: University College London, London WC1E 6BT, United Kingdom
REFERENCES
- 1.Bessis A, Champtiaux N, Chatelin L, Changeux JP. The neuron-restrictive silencer element: a dual enhancer/silencer crucial for patterned expression of a nicotinic receptor gene in the brain. Proc Natl Acad Sci USA. 1997;94:5906–5911. doi: 10.1073/pnas.94.11.5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner R, Thomas TO, Becker MN, Atkinson NS. Tissue-specific expression of a Ca(2+)-activated K+ channel is controlled by multiple upstream regulatory elements. J Neurosci. 1996;16:1827–1835. doi: 10.1523/JNEUROSCI.16-05-01827.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardinaux JR, Chapel S, Wahli W. Complex organization of CTF/NF-I, C/EBP, and HNF3 binding sites within the promoter of the liver-specific vitellogenin gene. J Biol Chem. 1994;269:32947–32956. [PubMed] [Google Scholar]
- 4.Chirgwin JJ, Przbyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 5.Chong JA, Tapia RJ, Kim S, Toledo AJ, Zheng Y, Boutros MC, Altshuller YM, Frohman MA, Kraner SD, Mandel G. REST: a mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell. 1995;80:949–957. doi: 10.1016/0092-8674(95)90298-8. [DOI] [PubMed] [Google Scholar]
- 6.Fornasari D, Battaglioli E, Flora A, Terzano S, Clementi F. Structural and functional characterization of the human alpha3 nicotinic subunit gene promoter. Mol Pharmacol. 1997;51:250–261. doi: 10.1124/mol.51.2.250. [DOI] [PubMed] [Google Scholar]
- 7.Garlatti M, Aggerbeck M, Bouguet J, Barouki R. Contribution of a nuclear factor 1 binding site to the glucocorticoid regulation of the cytosolic aspartate aminotransferase gene promoter. J Biol Chem. 1996;271:32629–32634. doi: 10.1074/jbc.271.51.32629. [DOI] [PubMed] [Google Scholar]
- 8.Gounari F, De Francesco R, Schmitt J, van der Vliet P, Cortese R, Stunnenberg H. Amino-terminal domain of NF1 binds to DNA as a dimer and activates adenovirus DNA replication. EMBO J. 1990;9:559–566. doi: 10.1002/j.1460-2075.1990.tb08143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goyal N, Knox J, Gronostajski RM. Analysis of multiple forms of nuclear factor I in human and murine cell lines. Mol Cell Biol. 1990;10:1041–1048. doi: 10.1128/mcb.10.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graves RA, Tontonoz P, Ross SR, Spiegelman BM. Identification of a potent adipocyte-specific enhancer: involvement of an NF-1-like factor. Genes Dev. 1991;5:428–437. doi: 10.1101/gad.5.3.428. [DOI] [PubMed] [Google Scholar]
- 11.Graves RA, Tontonoz P, Platt KA, Ross SR, Spiegelman BM. Identification of a fat cell enhancer: analysis of requirements for adipose tissue-specific gene expression. J Cell Biochem. 1992;49:219–224. doi: 10.1002/jcb.240490303. [DOI] [PubMed] [Google Scholar]
- 12.Ingraham HA, Chen R, Mangalam HJ, Elsholtz HP, Lin CR, Flynn SE, Simmons DM, Swanson L, Rosenfeld MG. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988;55:519–529. doi: 10.1016/0092-8674(88)90038-4. [DOI] [PubMed] [Google Scholar]
- 13.Inoue T, Tamura T, Furuichi T, Mikoshiba K. Isolation of complementary DNAs encoding a cerebellum-enriched nuclear factor I family that activates transcription from the mouse myelin basic protein promoter. J Biol Chem. 1990;265:19065–19070. [PubMed] [Google Scholar]
- 14.Johnson DS, Heinemann SF. Detection of 5-HT3R-A, a 5-HT3 receptor subunit, in submucosal and myenteric ganglia of rat small intestine using in situ hybridization. Neurosci Lett. 1995a;184:67–70. doi: 10.1016/0304-3940(94)11170-n. [DOI] [PubMed] [Google Scholar]
- 15.Johnson DS, Heinemann SF. Embryonic expression of 5-HT3 receptor subunit, 5-HT3R-A, in the rat, an in situ hybridization study. Mol Cell Neurosci. 1995b;6:122–138. doi: 10.1006/mcne.1995.1012. [DOI] [PubMed] [Google Scholar]
- 16.Kruse U, Sippel AE. The genes for transcription factor nuclear factor I give rise to corresponding splice variants between vertebrate species. J Mol Biol. 1994a;238:860–865. doi: 10.1006/jmbi.1994.1343. [DOI] [PubMed] [Google Scholar]
- 17.Kruse U, Sippel AE. Transcription factor nuclear factor I proteins form stable homo- and heterodimers. FEBS Lett. 1994b;348:46–50. doi: 10.1016/0014-5793(94)00585-0. [DOI] [PubMed] [Google Scholar]
- 18.Li P, He X, Gerrero MR, Mok M, Aggarwal A, Rosenfeld MG. Spacing and orientation of bipartite DNA-binding motifs as potential functional determinants for POU domain factors. Genes Dev. 1993;7:2483–2496. doi: 10.1101/gad.7.12b.2483. [DOI] [PubMed] [Google Scholar]
- 19.Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991;254:432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- 20.McDonough J, Deneris E. β43′:An enhancer displaying neural-restricted activity is located in the 3′-untranslated exon of the rat nicotinic acetylcholine receptor β4 gene. J Neurosci. 1997;17:2273–2283. doi: 10.1523/JNEUROSCI.17-07-02273.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McEvilly RJ, Erkman L, Luo L, Sawchenko PE, Ryan AF, Rosenfeld MG. Requirement for Brn-3.0 in differentiation and survival of sensory and motor neurons. Nature. 1996;384:574–577. doi: 10.1038/384574a0. [DOI] [PubMed] [Google Scholar]
- 22.Ninkina NN, Stevens GE, Wood JN, Richardson WD. A novel Brn3-like POU transcription factor expressed in subsets of rat sensory and spinal cord neurons. Nucleic Acids Res. 1993;21:3175–3182. doi: 10.1093/nar/21.14.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian F, Kruse U, Lichter P, Sippel AE. Chromosomal localization of the four genes (NFIA, B, C, and X) for the human transcription factor nuclear factor I by FISH. Genomics. 1995;28:66–73. doi: 10.1006/geno.1995.1107. [DOI] [PubMed] [Google Scholar]
- 24.Ryan AK, Rosenfeld MG. POU domain family values: flexibility, partnerships, and developmental codes. Genes Dev. 1997;11:1207–1225. doi: 10.1101/gad.11.10.1207. [DOI] [PubMed] [Google Scholar]
- 25.Schoenherr CJ, Anderson DJ. The neuron-restrictive silencer factor (NRSF): a coordinate repressor of multiple neuron-specific genes. Science. 1995a;267:1360–1363. doi: 10.1126/science.7871435. [DOI] [PubMed] [Google Scholar]
- 26.Schoenherr CJ, Anderson DJ. Silencing is golden: negative regulation in the control of neuronal gene transcription. Curr Opin Neurobiol. 1995b;5:566–571. doi: 10.1016/0959-4388(95)80060-3. [DOI] [PubMed] [Google Scholar]
- 27.Schoenherr CJ, Paquette AJ, Anderson DJ. Identification of potential target genes for the neuron-restrictive silencer factor. Proc Natl Acad Sci USA. 1996;93:9881–9886. doi: 10.1073/pnas.93.18.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuur ER, Kruse U, Iacovoni JS, Vogt PK. Nuclear factor I interferes with transformation induced by nuclear oncogenes. Cell Growth Differ. 1995;6:219–227. [PubMed] [Google Scholar]
- 29.Shen WH, Moore CC, Ikeda Y, Parker KL, Ingraham HA. Nuclear receptor steroidogenic factor 1 regulates the mullerian inhibiting substance gene: a link to the sex determination cascade. Cell. 1994;77:651–661. doi: 10.1016/0092-8674(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 30.Shuey DL, Sadler TW, Tamir H, Lauder JM. Serotonin and morphogenesis. Transient expression of serotonin uptake and binding protein during craniofacial morphogenesis in the mouse. Anat Embryol. 1993;187:75–85. doi: 10.1007/BF00208198. [DOI] [PubMed] [Google Scholar]
- 31.Smith MD, Morris PJ, Dawson SJ, Schwartz ML, Schlaepfer WW, Latchman DS. Coordinate induction of the three neurofilament genes by the Brn-3a transcription factor. J Biol Chem. 1997;272:21325–21333. doi: 10.1074/jbc.272.34.21325. [DOI] [PubMed] [Google Scholar]
- 32.Sumner C, Shinohara T, Durham L, Traub R, Major EO, Amemiya K. Expression of multiple classes of the nuclear factor-1 family in the developing human brain: differential expression of two classes of NF-1 genes. J Neurovirol. 1996;2:87–100. doi: 10.3109/13550289609146542. [DOI] [PubMed] [Google Scholar]
- 33.Tamura T, Aoyama A, Inoue T, Miura M, Mikoshiba K. A new transcription element in the JC virus enhancer. J Gen Virol. 1990a;71:1829–1833. doi: 10.1099/0022-1317-71-8-1829. [DOI] [PubMed] [Google Scholar]
- 34.Tamura T, Sumita K, Hirose S, Mikoshiba K. Core promoter of the mouse myelin basic protein gene governs brain-specific transcription in vitro. EMBO J. 1990b;9:3101–3108. doi: 10.1002/j.1460-2075.1990.tb07507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tecott L, Shtrom S, Julius D. Expression of a serotonin-gated ion channel in embryonic neural and nonneural tissues. Mol Cell Neurosci. 1995;6:43–55. doi: 10.1006/mcne.1995.1005. [DOI] [PubMed] [Google Scholar]
- 36.Tecott LH, Julius D. A new wave of serotonin receptors. Curr Opin Neurobiol. 1993;3:310–315. doi: 10.1016/0959-4388(93)90122-f. [DOI] [PubMed] [Google Scholar]
- 37.Tecott LH, Maricq AV, Julius D. Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proc Natl Acad Sci USA. 1993;90:1430–1434. doi: 10.1073/pnas.90.4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Unwin N. Neurotransmitter action: opening of ligand-gated ion channels. Cell. 1993;72:31–41. doi: 10.1016/s0092-8674(05)80026-1. [DOI] [PubMed] [Google Scholar]
- 39.Xiang M, Gan L, Zhou L, Klein WH, Nathans J. Targeted deletion of the mouse POU domain gene Brn-3a causes selective loss of neurons in the brainstem and trigeminal ganglion, uncoordinated limb movement, and impaired suckling. Proc Natl Acad Sci USA. 1996;93:11950–11955. doi: 10.1073/pnas.93.21.11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang X, McDonough J, Fyodorov D, Morris M, Wang F, Deneris ES. Characterization of an acetylcholine receptor alpha 3 gene promoter and its activation by the POU domain factor SCIP/Tst-1. J Biol Chem. 1994;269:10252–10264. [PubMed] [Google Scholar]
- 41.Zenzie GB, Khachi A, Garraway IP, Smale ST. Mechanism of initiator-mediated transcription: evidence for a functional interaction between the TATA-binding protein and DNA in the absence of a specific recognition sequence. Mol Cell Biol. 1993;13:3841–3849. doi: 10.1128/mcb.13.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimmerman L, Parr B, Lendahl U, Cunningham M, McKay R, Gavin B, Mann J, Vassileva G, McMahon A (1994) Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron [Erratum (1994) 12:1389] 12:11–24. [DOI] [PubMed]