Abstract
Previous psychophysical and neuroimaging studies suggest that perceiving the handedness of a visually presented hand depends on sensorimotor processes that are specific to the limb of the stimulus and that may be controlled by the cerebral hemisphere contralateral to the limb. Therefore, it was hypothesized that disconnection between cerebral hemispheres would disrupt mental simulation of a hand presented to the ipsilateral, but not the contralateral, hemisphere. This hypothesis was examined by the present study in which two callosotomy patients and eight healthy controls judged the handedness of drawings of left and right hands in various positions, without moving or inspecting their own hands. Stimuli were presented for 150 msec in the right or left visual hemifield. As predicted, for each hemisphere, patients’ accuracy was high when the hand was contralateral to the perceiving hemisphere, but it was not above chance when it was ipsilateral to the perceiving hemisphere. Controls’ accuracy was high in both conditions. Response time analyses indicate patients, like controls, mentally simulated reaching into stimulus postures. When the stimulus laterality was ipsilateral to the perceiving hemisphere, patients imagined the hand contralateral to the perceiving hemisphere reaching into the stimulus posture but did not detect the mismatch, guessing with a response bias or responding on the basis of shape similarity. We conclude that each hemisphere could represent the shape and movement of the contralateral hand but could not for the ipsilateral hand. Mentally simulating one’s action and discriminating body part handedness both depend on lateralized sensorimotor and somatosensory representations.
Keywords: motor imagery, shape recognition, cerebral lateralization, split brain, mental imagery, visual object discrimination
Humans can recognize or discriminate the shapes of objects from different viewpoints in many instances (Rock, 1973; Biederman, 1987; Tarr, 1995; Ullman, 1996). If the shapes to be discriminated are sufficiently similar, such as an object and its mirror image, we reorient the objects or ourselves by mental or physical means to compare the shapes at the same viewpoint (Shepard and Cooper, 1982; Hinton and Parsons, 1988). When deciding whether a hand is a left or right hand, psychophysical studies (Parsons, 1987b, 1994) indicate that observers imagine their own hand moving from its orientation during the task into the stimulus orientation for comparison. Typically, subjects imagine their left hand in the orientation of left stimuli and their right hand in the orientation of right stimuli. A rapid initial perceptual analysis of hand shape allows subjects to imagine moving first what often turns out to be the correct hand. The trajectory imagined for the left hand is strongly influenced by the biomechanical constraints on actual left-hand movements; likewise, the trajectories imagined for their right hand reflect its constraints.
Some of the principal data supporting this account are as follows. The time for the left–right hand judgments (without moving or seeing one’s own hands) is identical or proportional to time for actual movement from one’s position during that task into the stimulus orientation (without making a left–right judgment). Likewise, the time for imagining movement (without making a left–right judgment) is proportional to that for making the left–right judgment. The time for three tasks is specific to the body part involved, reflecting different joint constraints, i.e., response time (RT)-orientation functions for the left hand, right hand, left foot, and right foot are different from one another. Studies have documented such correspondences between the time to imagine other movements and the time required to perform them (Decety et al., 1989; Decety and Lindgren, 1991; Decety and Jeannerod, 1998). Moreover, in a positron emission tomography (PET) study of the hand judgment task, virtually all brain regions known to participate in the planning and execution of bodily movements were active (Parsons et al., 1995). Indeed, PET, functional MRI, and magnetoencephalogram studies of various tasks indicate that motor imagery can activate brain areas at least partially overlapping many or all of the brain areas activated by overt motor behavior (for review, see Jeannerod and Decety, 1995; Crammond, 1997).
Overall, these findings, among others, imply that judging whether a presented hand is a right or left hand depends on limb-specific sensorimotor mental simulation that uses limb-specific sensorimotor programs that would be expected to be in the sensorimotor cortex that controls the contralateral hand (Penfield and Jasper, 1954; Brinkman and Kuypers, 1972). If so, then a callosotomy individual with complete disconnection between hemispheres should be capable of the mental simulation necessary for a left–right judgment of a hand visually presented selectively to the contralateral hemisphere but not of a hand presented to the ipsilateral hemisphere. We tested this prediction with two callosotomy patients and eight healthy control subjects.
MATERIALS AND METHODS
Subjects. V.P., a 42-year-old female, has been in good health since 1979 when she underwent a two-stage callosotomy sparing the anterior commissure. The operation was treatment for pharmacologically intractable epilepsy caused by febrile illness at the age of 6 years. She regularly performs at chance on a number of tests of visual interhemispheric interaction, despite having a few spared fibers at the splenial and rostral tips (Sidtis et al., 1981;Gazzaniga, 1989). J.W., a 41-year-old male, also underwent in 1979 a two-stage callosal surgery with sparing of the anterior commissure. The surgery was treatment for pharmacologically intractable epilepsy brought on by concussive head trauma at the age of 13 years. Each subject’s right hemisphere has been shown to be capable of understanding simple verbal instructions (Sidtis et al., 1981), and so both hemispheres could be tested in this experiment. Both participants are right-handed. [More information on these two patients, including magnetic resonance scans, is available (Sidtis et al., 1981; Holtzman, 1984; Gazzaniga et al., 1985; Fendrich and Gazzaniga, 1989; Gazzaniga, 1989; Jha et al., 1997)]. In addition, four males and four females participated as control subjects. The eight control subjects were right-handed, were between the ages of 28 and 49 years (mean age, 37 years), and were not known to have any neurological disorders.
Stimuli. A left hand and right hand (Fig.1) were portrayed in 72 postures each (Parsons, 1987b, 1994). Left and right hands were mirror images of one another but were otherwise identical. A left or right hand shown from one of five views was oriented either upright, upside-down, clockwise 30, 60, 90, 120, or 150°, or counterclockwise 30, 60, 90, 120, or 150° (about the line-of-sight axis) from the pictures. All 120 stimuli (5 views × 12 orientations × 2 handed forms) were presented in each visual hemifield in random order in each half of the experiment. Thus, there were 480 trials, with each hemisphere judging each stimulus twice. Each stimulus was presented for 150 msec with its closest edge at 1.5° of visual angle to the left or right of a central fixation point. Digitized versions of hand-drawn stimuli were presented on an Apple Macintosh IIci computer, each subtending ∼5° of visual angle.
Fig. 1.
Each left-hand stimulus shown at lateral orientations in the top elliptical series, at medial postures in the bottom elliptical series, and at the endpoint postures of 0 and 180° orientations, which are neither medial nor lateral. Right-hand stimuli (data not shown) were exact mirror reflections of the left-hand stimuli.
Stimulus orientation effects on RT. Previous studies show that RT for the left–right judgment of a hand (without moving or seeing one’s own hand) is strongly influenced by the length of the trajectory to move the hand from its orientation during the task to the orientation of the stimulus (Parsons, 1987b, 1994). The length of this trajectory and the judgment RT are shorter when the hand faces the body’s midsaggital plane (termed a medial orientation) than when the hand is facing away from the body’s midsaggital plane (termed a lateral orientation). In Figure 1, the lateral orientations are shown in the top elliptical series of hands, and the medial orientations are shown in the bottom elliptical series. These different trajectory lengths are consequences of intrinsic joint constraints in the arm and hand. The left–right judgment RTs here are analyzed in terms of medial, lateral, and 0 and 180° orientations (which are neither medial nor lateral orientations). RT to stimuli are analyzed as RT orientation functions in which the mean RT across subject group and/or trial (as indicated in Results) is plotted for each lateral orientation, medial orientation, 0° orientation, and 180° orientation.
Experimental procedure. After studying left and right forms of each stimulus, each subject practiced on trials in which 48 stimuli (an equal number of right and left hands) were presented randomly to either hemifield until a response was made. Then, each participant performed the experiment in two sessions. In each trial, a stimulus was presented randomly in one or the other visual hemifield. Each patient understood that either hand could appear in either hemifield.
J.W. performed all trials by pressing a left key with his left index finger to indicate a left-hand stimulus and a right key with his right index finger to indicate a right-hand stimulus. In the first and last quarters of V.P.’s trials, she used the left middle finger to press the leftmost key for a stimulus showing a left-hand stimulus and the left index finger to press the rightmost key for a stimulus showing a right-hand stimulus. In the middle two quarters of her trials, V.P. used the right index finger to press the leftmost button for a left-hand stimulus and right middle finger to press the rightmost button for a right-hand stimulus.
Control subjects performed tasks by pressing a left key with their left index fingers to indicate a left-hand stimulus and a right key with their right index fingers to indicate a right-hand stimulus.
The computer recorded the time (±1 msec) from stimulus onset to button-press response and the accuracy of each response. Each subject was instructed to fixate the crosshair fixation point during a trial, to not to tilt his or her head, to keep his or her body still while performing a trial, and to respond as quickly and accurately as possible. An experimenter was seated behind the subject during the experiment.
RESULTS
Patients judged stimulus handedness accurately when the handedness of the stimulus was contralateral to the hemisphere perceiving it (contralateral trials) and judged it inaccurately when the handedness of the stimulus was ipsilateral to the hemisphere perceiving it (ipsilateral trials). As shown in Figure2, the performance of J.W. and V.P. on the ipsilateral trials was about one-third as accurate as that on contralateral trials (χ2(2) = 64.94;p < 0.001) and fell below the chance level of 50%. This pattern of effects was true for both the left and right cerebral hemispheres. There was lower accuracy for the right hemisphere than for the left one on ipsilateral trials (χ2(2) = 12.30; p < 0.001) and a trend (p < 0.20) toward the same effect on contralateral trials. There was no significant difference in the patients’ accuracy across the five stimuli (p> 0.20) or the seven orientations (p > 0.15).
Fig. 2.
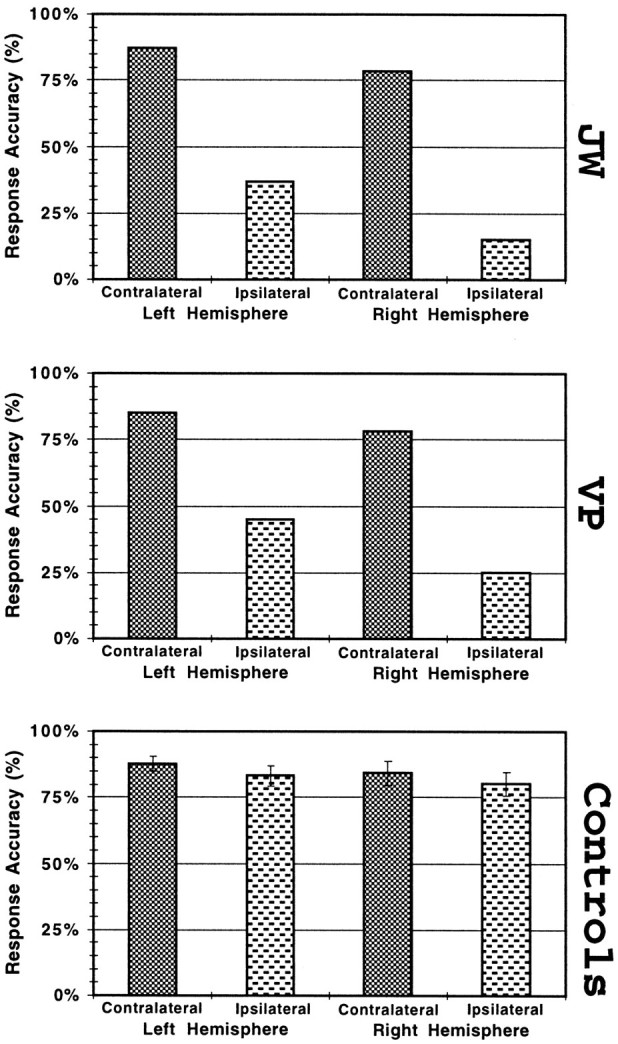
The accuracy of patients J.W. and V.P. and of healthy control subjects for the left–right judgment, plotted with respect to the cerebral hemisphere receiving the visual stimulus and the relationship between that stimulated hemisphere and the handedness of the stimulus. Error bars indicate SE.
Overall, the patients were more accurate and faster when the handedness of the stimulus was contralateral to the hemisphere perceiving it (82%, 683 msec for correct trials only) and less accurate and slower when the handedness of the stimulus was ipsilateral to the hemisphere perceiving it (30%, 979 msec). Statistical analysis of the experimental factor of response hand for V.P. revealed no significant interaction with any other experimental factors, so this factor was ignored in all subsequent analyses. In terms of overall accuracy and RT, the performances of V.P. and J.W. were highly correlated (r = +0.98 for a single test of the eight means in Figs. 1, 5), relative to which cerebral hemisphere was stimulated and to whether the presented hand was contralateral or ipsilateral to the stimulated hemisphere (F(1,6)= 154.40; p < 0.00001).
Fig. 5.
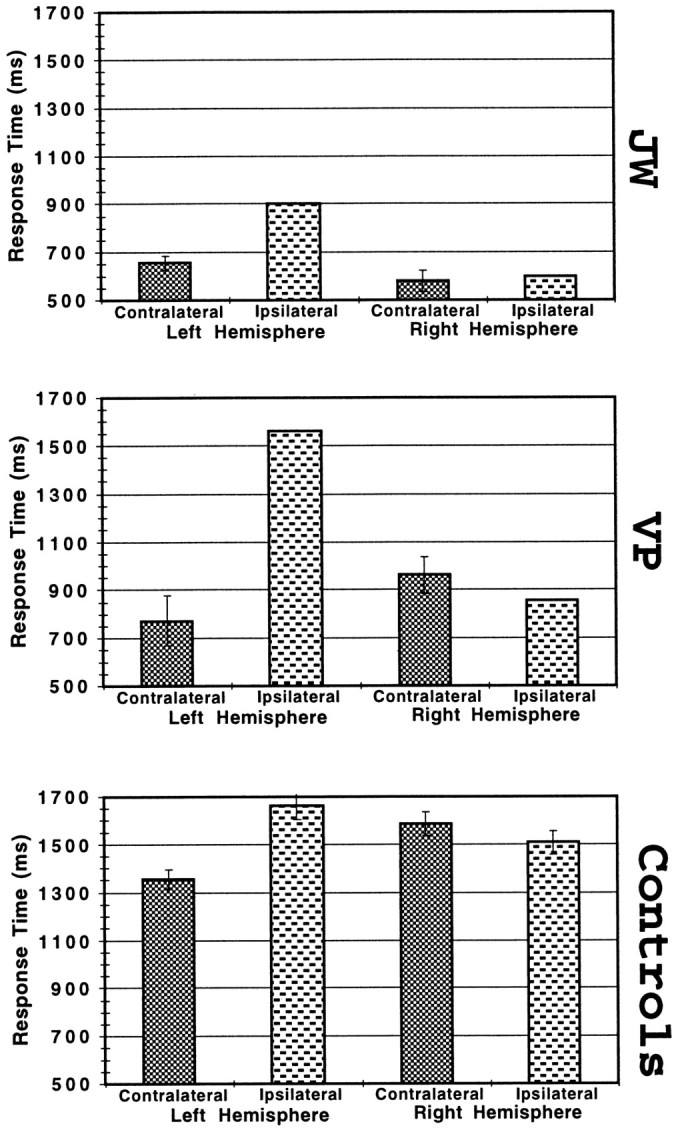
Mean RT on correct left–right judgments for patients J.W. and V.P. and for healthy control subjects plotted with respect to the cerebral hemisphere receiving the visual stimulus and the relationship between that stimulated hemisphere and the handedness of the stimulus. Error bars indicate SE.
The healthy control subjects, unlike the patients, performed quite accurately (Fig. 2) on both contralateral and ipsilateral trials. There was no significant difference (p > 0.05) between control subjects’ accuracy on contralateral and ipsilateral trials for either the left or right hemisphere. Nonetheless, there was a trend in controls’ data across those conditions such that accuracy was greatest for left hemisphere contralateral, less for right hemisphere contralateral, less still for left ipsilateral, and least for right ipsilateral. Patients showed this same pattern of accuracy (r = +0.91; F(1,2) = 9.59;p < 0.09). Thus, in terms of accuracy, patients’ single hemisphere performance may be a systematic distortion of trends in controls’ dual hemisphere performance. This observation suggests that both single and dual hemisphere performance is affected by the brief presentation of the stimulus to a single hemisphere. The tendency toward greater accuracy of the left hemisphere compared with right in both the controls and patients suggests some degree of hemispheric specialization for the task. The tendency toward greater error by control subjects on ipsilateral trials is likely attributable to the degradation of briefly glimpsed visual information as it is transferred from stimulated hemisphere to the other hemisphere (Hellige, 1993). The greater error by patients on ipsilateral trials, discussed in detail below, very likely has other causes.
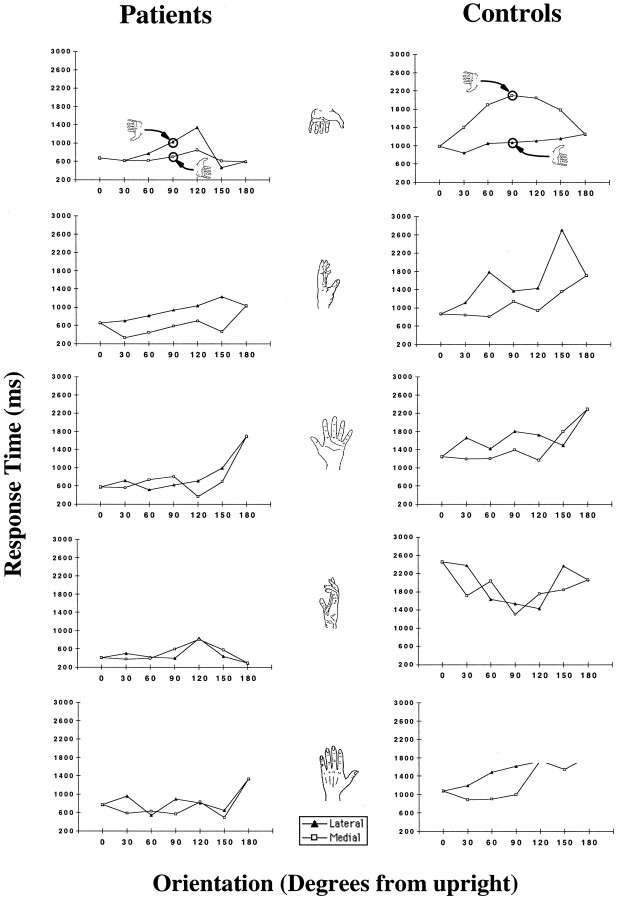
The patients performed the task using the same kind of strategy as healthy subjects. There was no significant difference between the accuracy of patients on the contralateral trials and that of healthy controls on either contralateral or ipsilateral trials (p > 0.20). Both controls and patients showed the effects of the stimulus posture (see Materials and Methods, Stimulus orientation effects on RT) such that medial orientations were longer than lateral ones (t(1,19) = 3.66;p < 0.0001; t(1,19) = 4.43;p < 0.0001) and such that among the 0 and 180° orientations, the more awkward orientations requiring longer trajectory paths to physically adopt required greater RT (t(1,4) = 3.66; p < 0.0001;t(1,4) = 2.38; p < 0.05). Moreover, the RT orientation function for correct contralateral trials, averaged across the patients, was well correlated with that of healthy controls; across all stimuli, except that of the side of the hand viewed from the little finger, the correlation wasr = +0.63 (F(1,46) = 30.67;p < 0.000001), and across all five stimuli, it wasr = +0.37 (F(1,58) = 9.06;p < 0.003). Figure 3shows the RT orientation function for each stimulus on contralateral trials for patients and controls as groups. Note that the previous studies of motor imagery and real movement that used these five stimuli observed more variability in RTs with the side of the hand viewed from the little finger than with the other four stimulus views (Parsons, 1987b, 1994). This is attributable to the fact that there is a greater variety of hand and arm movements available to move from the orientation of the hand in the task into the stimulus posture (in particular, to those orientations in which the fingers point within 60° of downward).
Fig. 3.
RT-orientation functions for patients and for healthy control subjects on contralateral trials for each stimulus.
Patient J.W.’s RT orientation function on contralateral trials was well correlated with that of V.P.: individual stimuli,r = +0.66 (F(1,10) = 7.90;p < 0.01); r = +0.62 (F(1,10) = 6.28; p < 0.03);r = +0.41 (F(1,10) = 2.01;p < 0.18); r = +0.40 (F(1,10) = 1.93; p < 0.19); andr = +0.38 (F(1,10) = 1.38;p < 0.26).
The RT orientation function for patients’ left hemisphere contralateral trials was well correlated with that for their right hemisphere trials (r = +0.48;F(1,48) = 14.07; p < 0.0004). Likewise, the control subjects’ RT-orientation functions for left and right visual hemifields were correlated to the same extent (r = +0.48; F(1,58) = 16.28;p < 0.0001). This good correspondence between patients’ left and right hemisphere RT orientation function is further evidence that the two hemisphere are performing the task in a similar manner on contralateral trials.
This variation in RT for the left–right hand judgment task is very likely primarily a function of variation in the time required to mentally simulate movements of the arm of different trajectory lengths to different stimulus hand postures (Parsons, 1987b, 1994). Although there is variation in the time for perceptual analysis (i.e., unfamiliar postures take longer than familiar ones), psychophysical studies with healthy subjects (Parsons, 1994) indicate that it is much smaller than the variation in time for movement and simulated movement. Patients’ RT orientation function on contralateral trials was well correlated with the real-movement time by orientation function when the time to perceive the stimulus was separated from the time to actually move (Parsons, 1994); across all stimuli, except that of the side of the hand viewed from the little finger, the correlation wasr = +0.49 (F(1,46) = 14.81;p < 0.0003), and across all five stimuli, the correlation was r = +0.30 (F(1,58) = 5.87; p < 0.01).
Patients’ accuracy was below chance (χ2(1) = 4.8; p < 0.05) on trials in which the handedness of the stimulus was ipsilateral to the hemisphere perceiving it. Close analysis of the RTs suggests that often on ipsilateral trials, subjects used the hemisphere receiving the stimulus to mentally simulate moving the hand contralateral to it into the stimulus orientation and then to either guess with a bias for the contralateral hand or respond on the basis of the similarity between the stimulus and the contralateral hand. Before discussing that analysis, we note that the observed data contradict three alternative models of performance.
First, the patients’ ipsilateral trial data are inconsistent with the possibility that they responded trivially on basis of hemifield of stimulation (e.g., pushing the left button for stimuli in left visual field). This model predicts a constant rapid RT, because one needs only to detect the hemifield of stimulation; imagining actions that possess trajectories of varying length is unnecessary. This prediction is clearly contradicted, because subjects’ RT-orientation functions are quite variable (Figs. 3, 4), in accordance with the model that mental simulation of reaching is used with the simulated path limited by actual joint constraints, a model to which both these callosotomy subjects (on contralateral trials) and healthy subjects conformed quite well. This model also predicts that all stimuli presented in a hemifield elicit the same response, a prediction contradicted by the patients’ error rate on ipsilateral trials (70%). Second, the below-chance accuracy and strong systematic variation in RT with stimulus orientation (Fig. 4, top left plot) contradict the model that states that instead of imagining reaching into the stimulus posture, the patients were just randomly guessing stimulus handedness (perhaps because they were at a loss without a representation of an ipsilateral stimulus). Third, patients’ poor accuracy is also inconsistent with the possibility that their comparison between ipsilateral and contralateral hands led them to disconfirm the stimulus handedness and then allowed them to infer the accurate alternative.
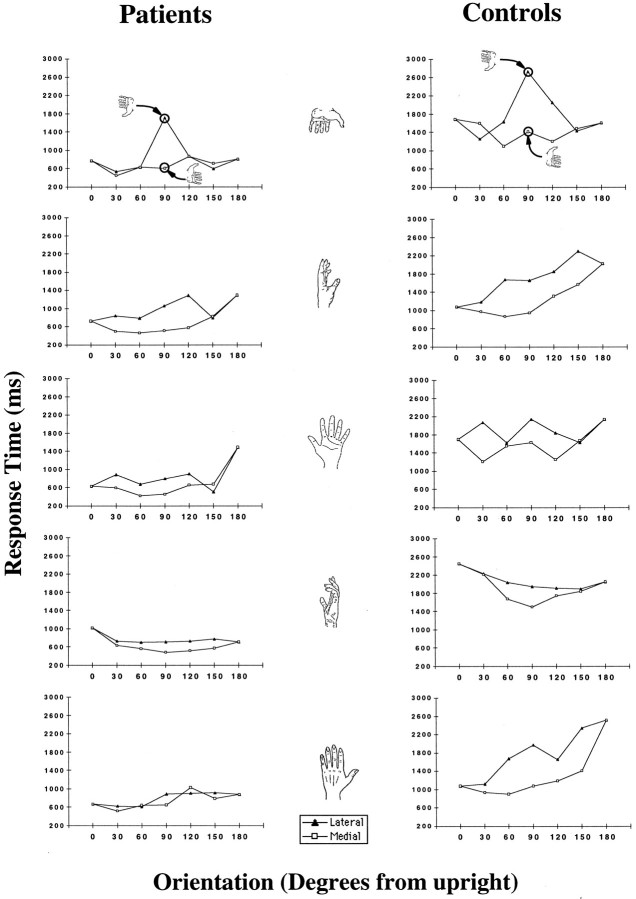
Fig. 4.
A test of the model that on ipsilateral trials patients used the hemisphere receiving the stimulus to mentally simulate moving the hand contralateral to it into the stimulus orientation, even when it was the inappropriate (ipsilateral) hand (see Results). Shown for each stimulus are the contralateral hand-based RT-orientation functions for patients on ipsilateral trials and the RT-orientation functions for healthy control subjects on ipsilateral trials.
The best model of performance on ipsilateral trials assumes that patients imagined moving the hand contralateral to the hemisphere receiving the visual stimulus so that its palm and fingers were oriented like those of the stimulus, but that they did not detect the mismatch in shape and either (1) guessed with a response bias for the contralateral hand or (2) responded on the basis of shape similarity (irrespective of laterality). To test the model on RTs, a special “contralateral hand-based” function was composed for all responses on ipsilateral trials (regardless of accuracy). If patients imagined their contralateral hand so that its fingers and palm were aligned with the fingers and palm of the ipsilateral stimulus, then RTs for ipsilateral stimuli should be strongly influenced by the joint constraints on motion of the contralateral hand rather than the ipsilateral one (Parsons, 1987b, 1994). Therefore, in the case of this contralateral hand-based function, because the left and right hands have joint constraints 180° out of phase in movement space (American Academy of Orthopedic Surgeons, 1965; Parsons, 1987b), trials in which stimuli showed lateral orientations of a hand were treated as medial trials and vice versa. The model is confirmed if this contralateral hand-based RT orientation function for ipsilateral trials is similar to the untransformed RT orientation function for corresponding contralateral trials (for correct responses only). This similarity is predicted because RTs on both kinds of trial are produced by mental simulations of a hand with the same joint constraints on movement.
This contralateral hand-based function composed of the two patients’ RT data on ipsilateral trials is well correlated over all stimuli with the RT orientation function for the controls. For the individual stimuli (Fig. 4), the correlation between the patient group’s contralateral hand-based RT-orientation function and the control group’s data was r = +0.79 (F(1,10) = 17.52; p < 0.001);r = +0.74 (F(1,10) = 11.98;p < 0.006); r = +0.74 (F(1,10) = 12.26; p < 0.005);r = +0.73 (F(1,10) = 11.68;p < 0.006); and r = +0.50 (F(1,10) = 3.45; p < 0.09). Over all stimuli, this correlation was r = +0.48 (F(1,58) = 17.50; p < 0.0001). The correlation between the patients’ contralateral hand-based function for ipsilateral trials and their untransformed function for contralateral trials across all stimuli was r = +0.56 (F(1,58) = 27.35; p < 0.00001). This statistical comparison is not guaranteed to yield a correlation; if RTs for medial and lateral stimulus orientations are switched in the healthy controls’ group data on ipsilateral trials to compose such a contralateral hand-based function, it is poorly correlated with the untransformed function on the controls’ contralateral trials (r = +0.007; F(1,58) = 0.003;p < 0.95). This contralateral hand-based RT orientation function is also well correlated with the real-movement time by orientation function when the time to perceive the stimulus is separated from the time to actually move (Parsons, 1994); across all stimuli, except that of the side of the hand viewed from the little finger, the correlation was r = +0.48 (F(1,46) = 14.20; p < 0.0004), and across all five stimuli, the correlation was r = +0.40 (F(1,58) = 11.58; p < 0.001). This indicates that the contralateral hand-based RT orientation function is strongly influenced by the imagined trajectory length from the patients’ task-specific hand position into the stimulus orientation. In addition, patient J.W.’s contralateral hand-based RT orientation function on ipsilateral trials was fairly well correlated with that of V.P.; across four of the stimuli, the correlation wasr = +0.33 (F(1,46) = 5.61;p < 0.02); individual stimuli, r = +0.51 (F(1,10) = 3.51; p < 0.09); r = +0.45 (F(1,10) = 2.52; p < 0.14); r = +0.37 (F(1,10) = 1.61; p < 0.23);r = +0.37 (F(1,10) = 1.57;p < 0.23); and r = +0.31 (F(1,10) = 1.07; p < 0.32). In combination, these analyses provide reasonably strong support for the model.
This contralateral hand-based RT orientation function for the patients’ ipsilateral trials can be averaged with their RT orientation function for contralateral trials to compose an overall RT orientation function for each stimulus. The resulting functions allow a comparison of the RT orientation function of patients and controls with a considerably larger sample size. These functions for patients are well correlated with controls’ RT orientation averaged over ipsilateral and contralateral trials; over all stimuli, except that showing the side of the hand from the little finger, the correlation was r= +0.69 (F(1,46) = 41.59; p < 0.0000001), and over all stimuli it was r = +0.50 (F(1,58) = 19.13; p < 0.00005). These close fits are further evidence that patients perform the task according to the process models described above.
The patients’ right hemisphere was faster overall to respond correctly (Fig. 5) than the left hemisphere (t(292) = 3.43; p < 0.001). There was no overall difference between left and right hemisphere trials for the controls in which the two cerebral hemispheres cooperatively perform the tasks; in addition, the controls showed no strong trend toward a speed–accuracy tradeoff. The patients’ right hemisphere advantage in RT is comparable to that of another commissurotomized subject (L.B.) in the related task of judging whether a misoriented letter is in its normal or mirror-reversed form (Corballis and Sergent, 1988). The right hemisphere speed advantage was present primarily on ipsilateral trials for which there was a nonsignificant trend for the left hemisphere to be more accurate; thus, although there were relatively few correct ipsilateral trials, there was a trend toward a speed-accuracy tradeoff in this regard.
The patients and controls show fairly similar patterns of overall RT across hemisphere and kind of trial, such that both show a tendency for much greater RT for ipsilateral than contralateral trials when the left hemisphere is stimulated and much less difference in RT for ipsilateral and contralateral trials when the right hemisphere is stimulated. This difference between RT in ipsilateral and contralateral trials was significant for controls only on left hemisphere trials (t(809) = 4.54; p < 0.001). There was no significant differences (p > 0.10) in either patient among RT means on contralateral trials for either hemisphere or on ipsilateral trials for either hemisphere.
There was trend in the patients’ RTs on the correct contralateral trials, suggesting that the right hemisphere performed the imagined movements more rapidly than the left hemisphere, although there was insufficient sample size to test statistically. This trend is found in RTs from a subset of the trials used to compose the RT means in Figure5. On contralateral trials with stimuli in which there was likely to be the shortest length of imagined movement, there was no difference in the patients’ mean RT for trials involving the left and right hemisphere; however, on contralateral trials with stimuli in which there was likely to be the longest length of imagined movement, the right hemisphere in the patient group was 210 msec faster than the left hemisphere. Thus, although our data above indicate that each hemisphere can mentally simulate action of their respective contralateral hands with the same biomechanical verisimilitude, the right hemisphere, apparently specialized for visual–spatial tasks (Ratcliff, 1979; DeRenzi, 1982; Deutsch et al., 1988), performed such simulations at an overall faster rate (although with somewhat less accuracy). It is worth noting that despite similarities in patients’ left and right hemisphere performance, various processes (e.g., attention, spatial analysis, and pragmatic knowledge of movements) may be involved in different ways or to different extents in the task performance by each hemisphere.
Finally, we note that the patients were faster in general than the control subjects. This difference in overall RT very likely reflects individual differences (i.e., V.P. was within the typical RT range indicated by our control subject group, whereas J.W. was more of an outlier), as well as much greater general familiarity and practice of the patients who have participated in many studies involving tachistoscopic hemifield presentation and speeded reaction time experiments, compared with the control subjects for whom this study was a novel experience.
DISCUSSION
The striking double dissociation in the callosotomy patients’ accuracy as a function of the lateralities of the stimulus and of the cerebral hemisphere receiving the stimulus confirms the hypothesis that mentally simulating one’s action and discriminating body part handedness both depend on lateralized motor and somatosensory representations. Our data suggest that within each cerebral hemisphere there are representations of the contralateral hand and its movement that are effective for spatial cognition and motor imagery, but such representations of the ipsilateral hand are absent altogether in the right hemisphere and are exceedingly ineffective in the left (dominant) hemisphere. Apparently, the left hemisphere is sufficient and necessary for the right-hand motor imagery, and the right hemisphere is sufficient and necessary for the left-hand motor imagery. Furthermore, our results imply that each hemisphere alone can support the mental simulation of an action with RT properties such as those in which the hemispheres can interact, which in turn closely correspond to real-movement time (Parsons, 1994). These data may be among the strongest evidence that the left hemisphere alone is capable of performing imagined spatial transformations and does them at least as accurately as the right hemisphere (for weaker evidence, see Corballis and Sergent, 1988).
The apparent presence of a very weak representation of the ipsilateral hand in the left hemisphere, but none in the right one, is consistent with earlier studies of motor behavior consequent to callosotomy in human and monkey (Gazzaniga, 1964; Gazzaniga et al., 1967; Volpe et al., 1982) and with the specialization of the left hemisphere for organizing bilateral motor activity (Kimura, 1977; Bradshaw and Nettleton, 1981; Bryden, 1982; MacNeilage et al., 1987; Fisk and Goodale, 1988; Harrington and Haaland, 1991). The right (nondominant) hemisphere apparently lacks a representation of the shape and movement of the right hand (and perhaps other right body parts), despite its possibly enhanced capacity for object recognition (Yin, 1970;Warrington and Taylor, 1973; Hecaen and Albert, 1978; Levy, 1983;Hamilton and Vermeire, 1988; Hellige, 1993; Ivry and Robertson, 1997). Thus, the right hemisphere may have representations of a hand (not coded explicitly for handedness) and definitely has representations of a left hand, but it is does not have one of a right hand; this is compatible with the view that humans do not in general represent object shape so as to discriminate a shape from its mirror image (Corballis and Beale, 1976; Hinton and Parsons, 1988).
The close link observed in callosotomy patients between the laterality of the imagined hand and of cerebral hemisphere is consistent with recent data from neuroimaging and neuropsychological studies. A PET study of this task in healthy subjects (Parsons and Fox, 1998) observed limb-contralateral neural structures activated by limb-specific motor imagery, including presupplementary motor area (pre-SMA), inferior premotor cortex [Brodmann area (BA) 44/46], and superior frontal sulcal premotor cortex (BA 4). These areas are implicated in higher-order aspects of motor control, movement preparation and selection, action recognition and copying, spatial working memory, and guidance and execution of self-paced movements. These brain areas would presumably be unable to support our callosotomy patients’ single hemisphere performance on ipsilateral trials, and their absence would in part account for the poor performance on those trials. The Parsons and Fox (1998) study also observed neural structures active in the dominant left hemisphere regardless of the limb involved in the motor imagery, including SMA proper, superior premotor (BA 6), and inferior parietal (BA 40). These areas are implicated in planning, guidance, and attention to motor performance, and their presumed absence during our patients’ right hemisphere performance would in part account for its diminished performance. In addition, there were also neural structures active in the nondominant right hemisphere regardless of the limb involved in motor imagery, including dorsal superior premotor (BA 6), insula, superior parietal (BA 7), and inferior occipitotemporal (BA 37) cortices. These areas have been implicated in motor planning, high-level somatic representation, the evaluation of visuospatial information, and the representation of the identity of objects and actions. These areas would presumably be unable to support the patients’ left hemisphere performance.
Furthermore, in patients with damage to motor cortex, mentally simulated movements reflect the decreased motor efficiency of the affected limb; executed and imagined hand movements are slowed to the same extent (Sirigu et al., 1995). There are similar laterality effects in the impairments of imagined and executed movements in patients with basal ganglia dysfunction attributable to Parkinson’s disease (Dominey et al., 1995). In addition, damage to parietal lobe disrupts the correlation in performance time between imagined movement and real movement (patients are either too fast or too slow in imagery or are inconsistent) (Sirigu et al., 1996). Patients with right parietal damage have impaired left-hand motor imagery (unimpaired left limb movement); patients with left parietal damage have impaired right- and left-hand motor imagery. The latter results suggest that motor imagery of the left hand may be weakly dependent on left (dominant) hemisphere support, perhaps consistent with the trend toward less accurate right hemisphere performance by patients and controls on contralateral trials in the present study. Indeed, the latter suggestions are consistent with the earlier studies of motor behavior in callosotomy subjects (Gazzaniga, 1964; Gazzaniga et al., 1967; Volpe et al., 1982) showing poorer ipsilateral hand control by the right hemisphere than the left one.
That common brain areas support both mentally simulated and actual movement is consistent with the fact that the physiological correlates of mental simulation of body movement are often similar to, although weaker than, those for executed body movement (Jeannerod, 1995).
The double dissociation of hand representation and cerebral hemisphere and the effect of actual joint constraints on the mental simulation of one’s action emphasize the domain specificity of imagined spatial transformation processes. This handedness task elicits operations on sensorimotor information rather than on visual representations alone, probably because the somatomotor system represents and operates on body part representations that specify handedness (unlike other representations of object shape).
It is also notable that each hemisphere of the callosotomy subjects could detect a match in shape to make the correct judgment but that neither hemisphere alone was able to detect a mismatch in shape between the left and right hand and infer it was the other hand. Thus, for this judgment to be performed accurately, the hemispheres must interact (Gazzaniga, 1989; Hellige, 1993; Ivry and Robertson, 1997). The inability of a hemisphere to disconfirm that an imagined hand is different from the stimulus is striking and worthy of some discussion. The avoidance of disconfirmation is consistent with the very strong bias against disconfirmation shown in the dual hemisphere performance of healthy individuals, in favor of exact match confirmation (Parsons, 1987a,b, 1994). People may prefer to use exact match confirmation when they are equally familiar with both mirror forms of a shape and to use disconfirmation when they are much more familiar with one of the two mirror forms. The former condition is consistent with the finding that in many judgment domains people prefer to use confirmation (Nisbett and Ross, 1980; Fischhoff and Beyth-Marom, 1983). This preference is attributed to the fact that in natural settings we often have independent evidence that the hypothesis we are attempting to confirm is more likely to be correct than incorrect (Klayman and Ha, 1987). Correspondingly, healthy subjects use disconfirmatory match comparisons and inferences in tasks when they have to judge whether a letter is presented in its normal (i.e., very familiar) or mirror-reversed (unfamiliar) form. Likewise, in callosotomy patients, the right hemisphere can disconfirm that a misoriented letter stimulus is a normal letter by first comparing it (as it appears when imagined to be upright) to the memorized normal form and then inferring that the stimulus is mirror-reversed (Corballis and Sergent, 1988). However, the right hemisphere cannot, as the present data indicate, perform the corresponding disconfirmation of shape and inference for a hand (both forms of which are equally familiar).
Of course, a preference for using exact match confirmation rather than disconfirmation is quite different from the inability to use disconfirmation whatsoever: that is, the inability to detect a mismatch between imagined and stimulus hands in the face of very frequent error. Healthy subjects do not appear to show such disability; although their introspections and RTs indicate a strong tendency to use exact match confirmation, they report using disconfirmation ∼6% of the time overall (Parsons, 1987b). The callosotomy patients’ inability to use disconfirmation on ipsilateral trials is unlikely to directly follow from their condition; disconnection of the cerebral hemispheres does not disrupt visual areas within each hemisphere, and the patients demonstrate their ability to detect matches between their imagined contralateral hand and a stimulus showing the contralateral hand. It is unclear at present why the patients avoided disconfirmation. It is conceivable that with training (e.g., feedback) or with a modified task they could begin to rely on disconfirmation on the ipsilateral trials. Further study of this surprising feature of their performance is necessary to understand its implications for callosotomy patients and for models of this task.
Footnotes
We thank Emilio Bizzi and Alan Baddeley for discussion in early phases of this project, Howard Hughes, Wendy Francis, and Michael Martinez for assistance with experiments, and an anonymous reviewer for thoughtful suggestions.
Correspondence should be addressed to Lawrence M. Parsons, Research Imaging Center, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78284-6240.
REFERENCES
- 1.American Academy of Orthopaedic Surgeons: Committee for the Study of Joint Motion. Joint motion: method of measuring and recording. American Academy of Orthopaedic Surgery; Chicago: 1965. [Google Scholar]
- 2.Biederman I. Recognition-by-components: a theory of human image understanding. Psychol Rev. 1987;94:115–147. doi: 10.1037/0033-295X.94.2.115. [DOI] [PubMed] [Google Scholar]
- 3.Bradshaw JL, Nettleton NC. The nature of hemispheric specialization in man. Behav Brain Sci. 1981;4:51–91. [Google Scholar]
- 4.Brinkman J, Kuypers HG. Splitbrain monkeys: cerebral control of ipsilateral and contralateral arm, hand, and finger movements. Science. 1972;176:536–539. doi: 10.1126/science.176.4034.536. [DOI] [PubMed] [Google Scholar]
- 5.Bryden MP. Laterality: functional asymmetry in the intact brain. Academic; New York: 1982. [Google Scholar]
- 6.Corballis MC, Beale IL. The psychology of left and right. Erlbaum; Hillsdale, NJ: 1976. [Google Scholar]
- 7.Corballis MC, Sergent J. Imagery in a commissurotomized patient. Neuropsychologia. 1988;26:13–26. doi: 10.1016/0028-3932(88)90027-9. [DOI] [PubMed] [Google Scholar]
- 8.Crammond DJ. Motor imagery: never in your wildest dream. Trends Neurosci. 1997;20:54–57. doi: 10.1016/s0166-2236(96)30019-2. [DOI] [PubMed] [Google Scholar]
- 9.Decety J, Jeannerod M (1998) Fitts’ law in mentally simulated movements. Behav Brain Res, in press. [DOI] [PubMed]
- 10.Decety J, Lindgren M. Sensation of effort and duration of mentally executed actions. Scand J Psychol. 1991;32:97–104. doi: 10.1111/j.1467-9450.1991.tb00860.x. [DOI] [PubMed] [Google Scholar]
- 11.Decety J, Jeannerod M, Parblanc C. The timing of mentally represented actions. Behav Brain Res. 1989;34:35–42. doi: 10.1016/s0166-4328(89)80088-9. [DOI] [PubMed] [Google Scholar]
- 12.DeRenzi E. Disorders of spatial exploration and cognition. Wiley; New York: 1982. [Google Scholar]
- 13.Deutsch G, Bourbon WT, Papanicolaou AC, Eisenberg HM. Visuospatial experiments compared via activation of regional cerebral blood flow. Neuropsychologia. 1988;26:445–452. doi: 10.1016/0028-3932(88)90097-8. [DOI] [PubMed] [Google Scholar]
- 14.Dominey P, Decety J, Broussolle E, Chazot G, Jeannerod M. Motor imagery of a lateralized sequential task is asymmetrically slowed in hemi-Parkinson patients. Neuropsychologia. 1995;33:727–741. doi: 10.1016/0028-3932(95)00008-q. [DOI] [PubMed] [Google Scholar]
- 15.Fendrich R, Gazzaniga MS. Evidence of foveal splitting in a commissurotomy patient. Neuropsychologia. 1989;27:273–281. doi: 10.1016/0028-3932(89)90018-3. [DOI] [PubMed] [Google Scholar]
- 16.Fischhoff B, Beyth-Marom R. Hypothesis evaluation from a Bayesian perspective. Psychol Rev. 1983;90:239–260. [Google Scholar]
- 17.Fisk JD, Goodale MA. The effects of unilateral brain damage on visually guided reaching: hemispheric differences in the nature of the deficit. Exp Brain Res. 1988;72:425–435. doi: 10.1007/BF00250264. [DOI] [PubMed] [Google Scholar]
- 18.Gazzaniga MS. Cerebral mechanisms involved in ipsilateral eye–hand use in split-brain monkeys. Exp Neurol. 1964;10:148–155. doi: 10.1016/0014-4886(64)90092-5. [DOI] [PubMed] [Google Scholar]
- 19.Gazzaniga MS. Organization of the human brain. Science. 1989;245:947–952. doi: 10.1126/science.2672334. [DOI] [PubMed] [Google Scholar]
- 20.Gazzaniga MS, Bogen JE, Sperry RW. Dyspraxia following division of the cerebral commissures. Arch Neurol. 1967;16:606–612. doi: 10.1001/archneur.1967.00470240044005. [DOI] [PubMed] [Google Scholar]
- 21.Gazzaniga MS, Holtzman JD, Deck MDK, Lee BCP. MRI assessment of human callosal function with neuropsychological correlates. Neurology. 1985;35:1763–1766. doi: 10.1212/wnl.35.12.1763. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton CR, Vermeire BA. Complementary hemispheric specialization in monkeys. Science. 1988;242:1691–1694. doi: 10.1126/science.3201258. [DOI] [PubMed] [Google Scholar]
- 23.Harrington DL, Haaland KY. Hemispheric specialization for motor sequencing: abnormalities in levels of programming. Neuropsychologia. 1991;29:147–163. doi: 10.1016/0028-3932(91)90017-3. [DOI] [PubMed] [Google Scholar]
- 24.Hecaen H, Albert ML. Human neuropsychology. Wiley; New York: 1978. [Google Scholar]
- 25.Hellige JB. Hemispheric asymmetry. Harvard UP; Cambridge, MA: 1993. [Google Scholar]
- 26.Hinton GE, Parsons LM. Scene-based and viewer-centered representations for comparing shape. Cognition. 1988;30:1–35. doi: 10.1016/0010-0277(88)90002-9. [DOI] [PubMed] [Google Scholar]
- 27.Holtzman JD. Interactions between cortical and subcortical visual areas: evidence from human commissurotomy patients. Vision Res. 1984;24:801–813. doi: 10.1016/0042-6989(84)90151-2. [DOI] [PubMed] [Google Scholar]
- 28.Ivry R, Robertson L. The two sides of perception. MIT; Cambridge, MA: 1997. [Google Scholar]
- 29.Jeannerod M. Mental imagery in the motor context. Neuropsychologia. 1995;33:1419–1432. doi: 10.1016/0028-3932(95)00073-c. [DOI] [PubMed] [Google Scholar]
- 30.Jeannerod M, Decety J. Mental motor imagery: a window into the representational stages of action. Curr Opin Neurobiol. 1995;5:727–732. doi: 10.1016/0959-4388(95)80099-9. [DOI] [PubMed] [Google Scholar]
- 31.Jha AP, Kroll NEA, Baynes K, Gazzaniga MS. Memory encoding following complete callosotomy. J Cogn Neurosci. 1997;9:143–159. doi: 10.1162/jocn.1997.9.1.143. [DOI] [PubMed] [Google Scholar]
- 32.Kimura D. Acquisition of a motor skill after left-hemisphere damage. Brain. 1977;100:527–542. doi: 10.1093/brain/100.3.527. [DOI] [PubMed] [Google Scholar]
- 33.Klayman J, Ha Y-W. Confirmation, disconfirmation, and information in hypothesis testing. Psychol Rev. 1987;94:211–228. [Google Scholar]
- 34.Levy J. Language, cognition, and the right hemisphere: a response to Gazzaniga. Am Psychol. 1983;38:538–541. doi: 10.1037//0003-066x.38.5.538. [DOI] [PubMed] [Google Scholar]
- 35.MacNeilage PF, Studdert-Kennedy MG, Lindblom B. Primate handedness reconsidered. Behav Brain Sci. 1987;10:247–303. [Google Scholar]
- 36.Nisbett R, Ross L. Human inference: strategies and shortcomings of social judgment. PTR Prentice Hall; Englewood Cliffs, NJ: 1980. [Google Scholar]
- 37.Parsons LM. Imagined spatial transformation of one’s body. J Exp Psychol. 1987a;116:172–191. doi: 10.1037//0096-3445.116.2.172. [DOI] [PubMed] [Google Scholar]
- 38.Parsons LM. Imagined spatial transformation of one’s hand and feet. Cogn Psychol. 1987b;19:178–241. doi: 10.1016/0010-0285(87)90011-9. [DOI] [PubMed] [Google Scholar]
- 39.Parsons LM. Temporal and kinematic properties of motor behavior reflected in mentally simulated action. J Exp Psychol. 1994;20:709–730. doi: 10.1037//0096-1523.20.4.709. [DOI] [PubMed] [Google Scholar]
- 40.Parsons LM, Fox PT (1998) The neural basis of the implicit movements used in recognizing hand shape. Cogn Neuropsychol, in press.
- 41.Parsons LM, Fox PT, Downs JH, Glass T, Hirsch TB, Martin CG, Jerabek PA, Lancaster JL. Use of implicit motor imagery for visual shape discrimination as revealed by PET. Nature. 1995;375:54–58. doi: 10.1038/375054a0. [DOI] [PubMed] [Google Scholar]
- 42.Penfield W, Jasper H. Epilepsy and the functional anatomy of the human brain. Little, Brown; Boston: 1954. [Google Scholar]
- 43.Ratcliff G. Spatial thought, mental rotation, and the right cerebral hemisphere. Neuropsychologia. 1979;17:49–54. doi: 10.1016/0028-3932(79)90021-6. [DOI] [PubMed] [Google Scholar]
- 44.Rock I. Orientation and form. Academic; New York: 1973. [Google Scholar]
- 45.Shepard RN, Cooper LA. Mental images and their transformations. MIT; Cambridge, MA: 1982. [Google Scholar]
- 46.Sidtis JJ, Volpe BT, Wilson DH, Rayport M, Gazzaniga MS. Variability in right hemisphere language function after staged callosal section: evidence for a continuum of generative capacity. J Neurosci. 1981;16:323–331. doi: 10.1523/JNEUROSCI.01-03-00323.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sirigu A, Cohen L, Duhamel JR, Pillon B, Dubois B, Agid Y, Pierrot-Deseilligny C. Congruent unilateral impairments for real and imagined movements. NeuroReport. 1995;6:997–1001. doi: 10.1097/00001756-199505090-00012. [DOI] [PubMed] [Google Scholar]
- 48.Sirigu A, Duhamel JR, Cohen L, Pillon B, Dubois B, Agid Y. The mental representation of hand movements after parietal cortex damage. Science. 1996;273:1564–1568. doi: 10.1126/science.273.5281.1564. [DOI] [PubMed] [Google Scholar]
- 49.Tarr MJ. Rotating objects to recognize them: on the role of viewpoint-dependency in the recognition of three-dimensional objects. Psychon Bull Rev. 1995;2:55–82. doi: 10.3758/BF03214412. [DOI] [PubMed] [Google Scholar]
- 50.Ullman S. High-level vision: object recognition and visual cognition. MIT; Cambridge, MA: 1996. [Google Scholar]
- 51.Volpe BT, Sidtis JJ, Holtzman JD, Wilson DH, Gazzaniga MS. Cortical mechanisms involved in praxis: observations following partial and complete section of the corpus callosum in man. Neurology. 1982;32:645–650. doi: 10.1212/wnl.32.6.645. [DOI] [PubMed] [Google Scholar]
- 52.Warrington EK, Taylor AM. The contribution of the right parietal lobe to object recognition. Cortex. 1973;9:152–164. doi: 10.1016/s0010-9452(73)80024-3. [DOI] [PubMed] [Google Scholar]
- 53.Yin RK. Face recognition by brain-injured patients: a dissociable ability? Neuropsychologia. 1970;8:395–402. doi: 10.1016/0028-3932(70)90036-9. [DOI] [PubMed] [Google Scholar]