Abstract
Ionotropic ATP receptors are widely expressed in mammalian CNS. Despite extensive functional characterization of neuronal homomeric P2X receptors in heterologous expression systems, the subunit composition of native central P2X ATP-gated channels remains to be elucidated. P2X4 and P2X6 are major central subunits with highly overlapping mRNA distribution at both regional and cellular levels. When expressed alone in Xenopusoocytes, P2X6 subunits do not assemble into surface receptors responsive to ATP applications. On the other hand, P2X4 subunits assemble into bona fide ATP-gated channels, slowly desensitizing and weakly sensitive to the partial agonist α,β-methylene ATP and to noncompetitive antagonists suramin and pyridoxal-5-phosphate-6-azophenyl-2′,4′-disulfonic acid. We demonstrate here that the coexpression of P2X4 and P2X6subunits in Xenopus oocytes leads to the generation of a novel pharmacological phenotype of ionotropic ATP receptors. Heteromeric P2X4+6 receptors are activated by low-micromolar α,β-methylene ATP (EC50 = 12 μm) and are blocked by suramin and by Reactive Blue 2, which has the property, at low concentrations, to potentiate homomeric P2X4 receptors. The assembly of P2X4 with P2X6 subunits results from subunit-dependent interactions, as shown by their specific copurification from HEK-293 cells transiently transfected with various epitope-tagged P2X channel subunits. Our data strongly suggest that the numerous cases of neuronal colocalizations of P2X4 and P2X6 subunits observed in mammalian CNS reflect the native expression of heteromeric P2X4+6 channels with unique functional properties.
Keywords: purinoceptor; nucleotide; transmitter-gated cation channel; α,β methylene ATP; suramin; PPADS
Fast purinergic neurotransmission is mediated by nonselective cation channels gated by extracellular ATP. These transduction proteins, designated P2X receptors, constitute a distinct class of neurotransmitter-gated channels on the basis of their primary cDNA sequences and their predicted transmembrane protein topology. Currently, seven mammalian P2X genes have been identified with either expression or homology cloning assays (Buell et al., 1996). Among the neuronal P2X receptors, only P2X4 and P2X6 isoforms are predominantly expressed in the adult rat brain in which they show an overlapping pattern of regional and cellular distribution at the mRNA level (Collo et al., 1996). Homomeric rat P2X4 receptors expressed in HEK-293A cells orXenopus laevis oocytes and homomeric P2X6receptors silent in oocytes (Soto et al., 1996) but functional in HEK-293A cells (Collo et al., 1996) are weakly responsive to α,β-methylene ATP (αβmATP) and to P2 antagonists suramin and pyridoxal-5-phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS) (North and Barnard, 1997). Yet, native ionotropic purinergic responses from rat medial habenula, cerebellum, and hippocampus were blocked by P2 antagonists, and most native ATP receptors are activated by αβmATP (Edwards et al., 1992; Mateo et al., 1998; Ross et al., 1998). Moreover, high-affinity [3H]αβmATP autoradiographic binding sites have been localized in specific but widespread regions within the brain and spinal cord (Bo and Burnstock, 1994; Michel and Humphrey, 1994; Balcar et al., 1995). Discrepancies between pharmacological profiles of heterologously expressed homo-oligomeric P2X subunits and electrophysiological recordings from neuronal preparations likely reflect the existence of native heteromeric phenotypes of P2X receptors in peripheral nervous system as well as the CNS. Indeed, one such hybrid P2X phenotype was recorded in sensory neurons (Khakh et al., 1995; Lewis et al., 1995) and has been proposed to result from the association between coexpressed P2X2 and P2X3 subunits (Chen et al., 1995; Lewis et al., 1995; Radford et al., 1997). We describe in this report a novel P2X heteromeric receptor containing central P2X4 and P2X6 subunits. This phenotype of ATP-gated channel is endowed with a unique pharmacology characterized by increased sensitivities to αβmATP, 2-methylthio-ATP (2MeSATP), suramin, PPADS, and Reactive Blue 2 (RB-2) in Xenopusoocytes.
MATERIALS AND METHODS
Molecular biology. Wild-type full-length P2X6 subunit cDNA was obtained by RT-PCR using adult rat spinal cord RT-cDNA template, Expand DNA polymerase (Boehringer Mannheim, Indianapolis, IN), and exact match primers based on published primary sequences (Collo et al., 1996; Soto et al., 1996). Construction of P2X1-Flag and P2X4-Flag was reported previously (Lê et al., 1998). To generate epitope-tagged P2X6-Flag and P2X4-(His)6 subunits, an XhoI–XbaI cassette containing an in-frame His6 epitope followed by an artificial stop codon was grafted to the full-length HindIII–XhoI P2X4 construct. The P2X4-(His)6mutant was then subcloned directionally into the HindIII andXbaI sites of pcDNAI vector (Invitrogen, San Diego, CA) for cytomegalovirus-driven heterologous expression in mammalian cells andXenopus laevis oocytes. Epitope-tagged and RT-PCR constructs were subjected to dideoxy sequencing either manually with Sequenase (Upstate Biotechnology, Lake Placid, NY) or with an ALF DNA sequencer (Pharmacia, Piscataway, NJ).
Cell culture and protein chemistry. For cDNA transfections of epitope-tagged and wild-type P2X subunits into mammalian cells, HEK-293A cells (CRL 1573; American Type Culture Collection, Rockville, MD) were cultured in DMEM and 10% heat-inactivated fetal bovine serum (FBS) (Wisent, St-Bruno, Quebec, Canada) containing penicillin and streptomycin. Freshly plated cells reaching 30–50% confluency were used for transient cDNA transfections with the calcium phosphate method on 90 mm cell culture dishes (Falcon) with 10 μg of supercoiled plasmid cDNA/106 cells (Lê et al., 1998). For Western blots, transfected HEK-293A cells were lifted in Hank’s modified calcium-free medium with 20 mm EDTA, pelleted at low centrifugation, and homogenized in 10 volumes of 10 mm HEPES buffer, pH 7.4, containing protease inhibitors phenylmethylsulfonyl fluoride (0.2 mm) and benzamidine (1 mm). Cell lysates were pelleted at 14,000 ×g for 5 min, and membrane proteins in supernatants were solubilized with SDS-containing loading buffer. Approximately 150 μg of protein/lane were run on 12% SDS-PAGE and then transferred to nitrocellulose. Immunoprobing was performed with mouse mAb M2 (1 μg/ml, IBI) followed by peroxidase-labeled anti-mouse secondary antibodies for visualization by enhanced chemiluminescence (Amersham, Oakville, Ontario, Canada). Copurification of associated P2X subunits was performed as previously described for IRK channels (Tinker et al., 1996) with minor modifications. Cell lysates were solubilized with 5% Triton X-100 for 2 hr at 4°C. Unsolubilized materials were pelleted at 10,000 × g, and supernatants were incubated with 50 μl of 50% slurry of equilibrated Ni-NTA-Resin (Qiagen, Hilden, Germany) for 2 hr at 4°C. Nickel beads were then washed six times in TBS containing 25 mm imidazole and 1% Triton X-100. Bound proteins were eluted from Ni-NTA resin with 500 mm imidazole, diluted 1:1 (v/v) with SDS-containing loading buffer, and warmed for 10 min at 37°C. Samples were then loaded onto a 12% SDS-PAGE, transferred to nitrocellulose, and analyzed in Western blot using chemiluminescence as above.
Electrophysiology. For electrophysiological recordings in oocytes, ovary lobes were surgically removed from Xenopus laevis frogs anesthetized with Tricaine (Sigma, St. Louis, MO) and treated for 3 hr at room temperature with type II collagenase (Life Technologies, Gaithersburg, MD) in calcium-free Barth’s solution under vigorous agitations. Stage V–VI oocytes were then defolliculated chemically before nuclear microinjections of 5–10 ng of cDNA coding for each P2X channel subunit. After 2–5 d of incubation at 19°C in Barth’s solution containing 1.8 mm calcium chloride (CaCl2) and 10 μg/ml gentamicin, P2X currents were recorded in a two-electrode voltage-clamp configuration using an OC-725B amplifier (Warner Institute). Signals were low-pass-filtered at 1 kHz, acquired at 500 Hz using a Macintosh IIci equipped with an NB-MIO-16XL analog-to-digital card (National Instruments). Traces were postfiltered at 100 Hz in Axograph (Axon Instruments). Agonists, antagonists, and cofactors (zinc chloride, pH 6.5 and 8.0) were dissolved in Ringer’s solution containing (in mm): 115 NaCl, 2.5 KCl, and 1.8 CaCl2 in 10 HEPES, pH 7.4 standard at room temperature, and applied on oocytes at a constant flow rate of 12 ml/min. Dose–response curves and EC50 values were derived from fittings for the sigmoidal equation of Hill using Prism 2.0 software (Graphpad Software, San Diego, CA).
Statistical analysis. All comparisons involving two variances were performed with Fisher’s F values (variance homogeneity requirements) and with Student’s t tests for two unpaired groups. Two-tailed statistical thresholds, for both Fisher’s F and Student’s t critical values, were set at p < 0.05.
RESULTS
Functional impact of P2X6 subunit expression on ATP-induced currents
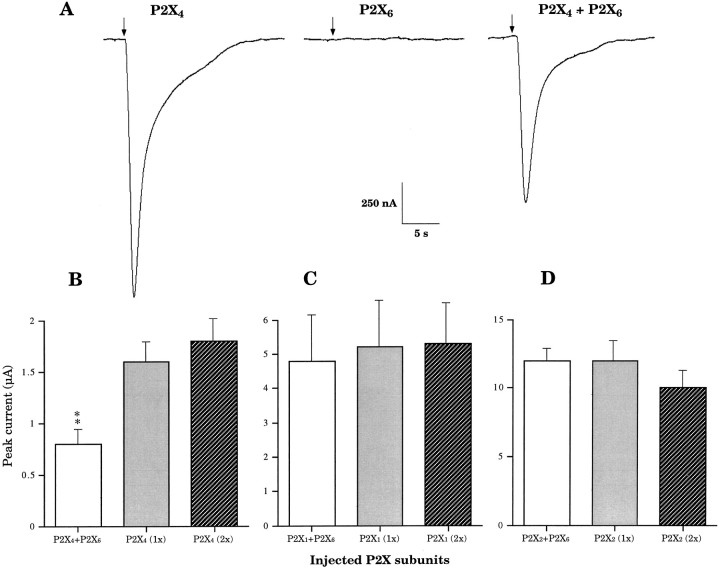
In response to 100 μm ATP, Xenopusoocytes microinjected with a mix of P2X4 and P2X6 cDNAs (1:1 molar ratio) gave rise to currents with kinetic profiles similar to those observed with oocytes expressing P2X4 alone (Fig.1A). P2X6by itself appeared to be silent in Xenopus oocytes, because no current was detected during ATP applications (Fig.1A), in agreement with what has been reported previously (Soto et al., 1996). Comparison of peak current amplitudes after 3 d of expression revealed, however, that currents from cells coexpressing P2X4 and P2X6 subunits were reproducibly and significantly smaller than currents from cells expressing only P2X4 receptors (Fig.1A,B), suggesting the possibility that the P2X6 channel subunit can heteropolymerize with other members of the P2X family. We coexpressed P2X6 together with P2X1 (Valera et al., 1994) or with P2X2(Brake et al., 1994). In response to 100 μm ATP, there were no differences between peak currents recorded from oocytes coexpressing P2X1 and P2X6 and those expressing P2X1 alone (Fig. 1C) after 3 d of expression. Similarly, we did not observe any functional impact of P2X6 on the expression of P2X2 under the same experimental conditions (Fig. 1D), eliminating the possibility of a general inhibitory effect of P2X6 on protein synthesis or on translocation. Thus these data indicate either that the subunit-specific interaction between P2X4 and P2X6 isoforms generates a heteromeric P2X4+6receptor, or that P2X6 subunits exert a specific inhibitory function on P2X4 receptor expression. If P2X4+6heteromers are expressed, smaller peak currents could result from a lower affinity for ATP or a smaller single conductance in comparison with homomeric P2X4 channels. Alternatively, smaller ATP responses at day 3 could simply reflect a slower kinetics of receptor expression.
Fig. 1.
Representative heteromeric P2X4+6channel current phenotype at day 3. A, ATP-induced currents after heterologous expression of P2X4, P2X6, and P2X4 + P2X6 (1:1 molar ratio) subunits recorded 3 d after corresponding cDNA nuclear microinjections in Xenopus oocytes.Arrows indicate beginnings of ATP applications (10 sec).B, P2X4-dependent functional impact of P2X6 on ATP-induced response (P2X4 expressed alone; 1x, 5 ng of cDNA; 2x, 10 ng).C, P2X1 receptor (P2X1;1x, 5 ng; 2x, 10 ng) functional expression is unaffected by coexpressed P2X6 subunits.D, P2X2-mediated (P2X2;1x, 5 ng; 2x, 10 ng) ATP-induced peak current amplitudes are unchanged in the presence of P2X6subunits. (Averages ± SEM from 3 to 15 oocytes in 2–4 independent experiments; double asterisks denote significant difference; p < 0.01).
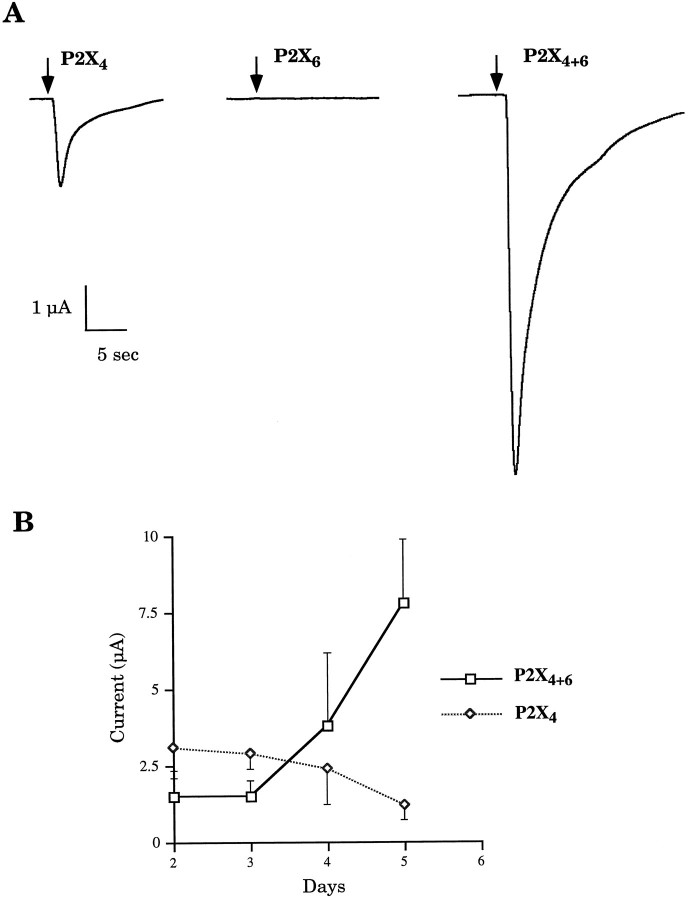
To further characterize a time-dependent effect, we studied the time course of expression, daily recording peak currents in response to 100 μm ATP from oocytes expressing either P2X4and P2X6 cDNAs or P2X4 cDNA alone. Figure2 demonstrates that ATP receptors in oocytes coexpressing P2X4 and P2X6 subunits, compared with P2X4 alone, needed a longer time to reach the same levels of ATP-induced currents. However, between days 2 and 5 after injection, there was a dramatic sevenfold increase in peak current amplitudes in oocytes coexpressing P2X4 and P2X6 subunits (Fig. 2A). This profile is in striking contrast with the time course of P2X4expression that slowly decayed over the same period (Fig.2B).
Fig. 2.
A, Potentiation of ATP response represented by P2X4+6 channel current phenotype at day 5.Arrows indicate beginnings of ATP applications (10 sec).B, Time course of heteromeric P2X4+6expression. Kinetics of appearance of functional ATP receptors on plasma membranes is strikingly different in oocytes coinjected with P2X4 and P2X6 subunits compared with those injected with P2X4 subunits (averages ± SEM from 3 to 15 oocytes in 2–8 independent experiments).
Agonist sensitivity profile of P2X4+6heteromeric receptors
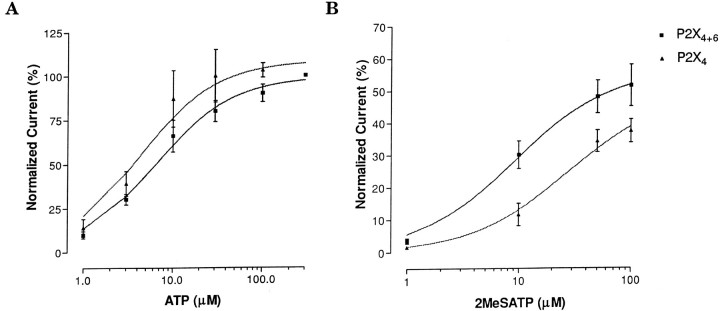
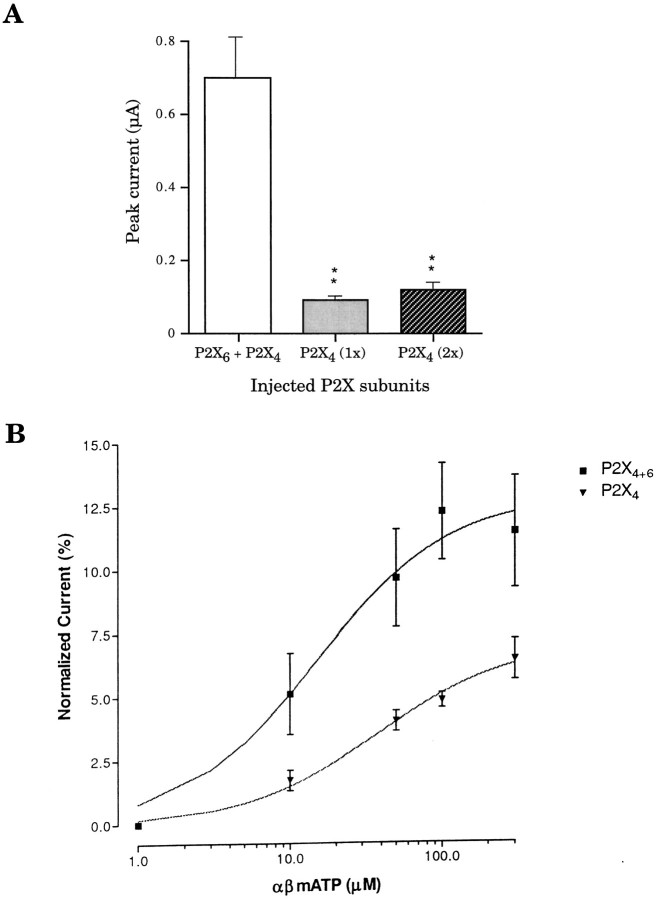
No significant difference was detected between the EC50 values derived from ATP dose–response profiles of P2X4+6 (6.3 ± 0.9 μm) channel phenotype and homomeric P2X4 (4.2 ± 1.1 μm) receptors (Fig. 3A) expressed in oocytes. However, the partial agonist 2MeSATP had EC50values of 7.67 ± 1.01 and 26 ± 1.8 μm for P2X4+6 and P2X4 receptors, respectively, a statistically significant difference (Fig. 3B). Even more striking, in response to 100 μm αβmATP on day 3 after injection, oocytes expressing P2X4+6 heteromeric channels gave rise to peak current amplitudes of 0.7 ± 0.13 μA compared with 0.12 ± 0.02 μA only from oocytes expressing P2X4 homomeric receptors, in marked contrast with the situation observed in response to ATP (compare Figs.4A, 1B). The αβmATP EC50 values were found to be 12 ± 2 μmfor P2X4+6 and 55 ± 2 μm for P2X4 channel phenotypes (Fig. 4B). Therefore, αβmATP shows more potency and has a higher affinity on P2X4+6 receptors than on P2X4 receptors. These different sensitivities to 2MeSATP and αβmATP constitute more experimental evidence for a functional association between P2X4 and P2X6 subunits coexpressed inXenopus oocytes.
Fig. 3.
Sensitivity of P2X4+6 receptors to the agonists ATP and 2MeSATP. A, Similar ATP dose–response profile between heteromeric P2X4+6 channels and homomeric P2X4 receptors in Xenopus oocytes.B, Heteromeric P2X4+6 receptors showed increased sensitivity to 2MeSATP compared with P2X4receptors. Values are normalized to the response to 300 μm ATP (averages ± SEM from 3 to 7 oocytes per point in 2 independent experiments).
Fig. 4.
Sensitivity of P2X4+6 receptors to the agonist αβmATP. A, Differential αβmATP responsiveness measured in peak current amplitudes betweenXenopus oocytes expressing either P2X4+6channels or P2X4 at day 3 after injection; see Figure1B for comparison of ATP-induced peak currents.B, Normalized dose–response curves of P2X4+6 and P2X4 receptor species for αβmATP. Values are normalized to the response to 100 μm ATP; double asterisks denote significant difference; p < 0.01 (averages ± SEM from 5 to 8 oocytes per point in 2 independent experiments).
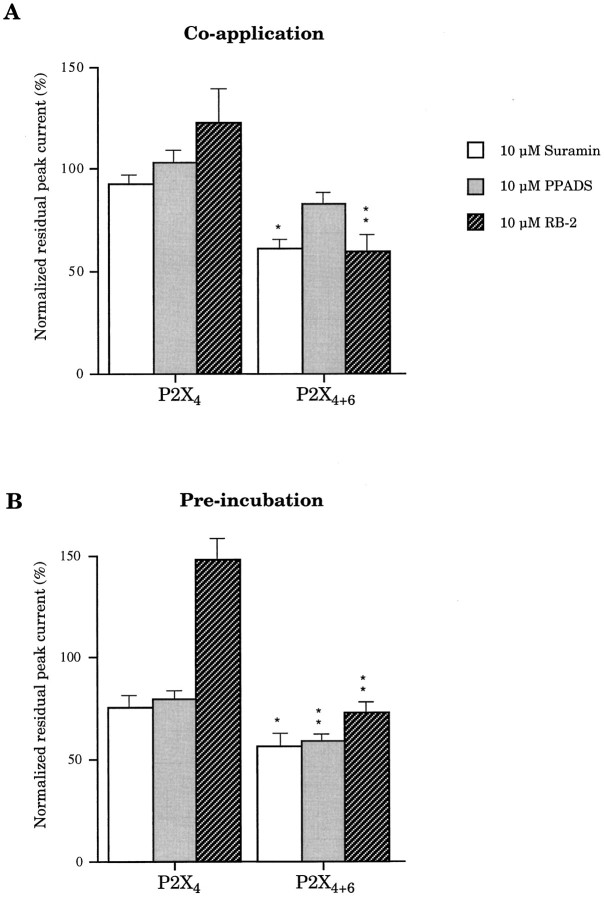
Sensitivity of P2X4+6 receptors to suramin, PPADS, and RB-2
It is widely recognized that neither P2X4 nor P2X6 homomeric receptors (in HEK-293 cells) are completely blocked by suramin or PPADS up to 100 μm without preincubation (Buell et al., 1996; Collo et al., 1996). In response to 100 μm ATP and 10 μm suramin coapplications without preincubation, oocytes expressing P2X4+6 gave rise to residual currents of 61 ± 3% (Fig.5) of the response to 100 μm ATP (100%). Under the same experimental conditions, oocytes expressing P2X4 receptors alone were almost unaffected (93 ± 3%; Fig. 5). We have also found that 10 μm PPADS coapplied with 100 μm ATP gave rise to peak current amplitudes of 83 ± 7 and 103 ± 6% for P2X4+6 and P2X4 receptor phenotypes, respectively (Fig. 5B), but we did not find any significant difference between this 17% inhibition on P2X4+6 and no effect on P2X4. We have also investigated the effects of RB-2 by coapplying 10 μm of the antagonist with 100 μm ATP: oocytes expressing P2X4+6 receptor phenotypes were characterized by residual peak currents of 60 ± 9% compared with potentiated peak currents of 123 ± 18% from oocytes expressing P2X4 receptors alone (Fig.5A). Preincubation of the cell with antagonist during 1 min before coapplication with ATP resulted in even more dramatic phenotypical differences between P2X4 and P2X4+6 for suramin (23% blockade vs 41%) and PPADS (19% blockade vs 38%) (Fig. 5B). Furthermore, in conditions of preincubation, 10 μm RB-2 blocked P2X4+6heteromeric channels by up to 26% but increased P2X4response by >45% (Fig. 5B). A potentiating effect of RB-2 on P2X4 homomeric receptors has been reported in oocytes, albeit to a smaller extent (Bo et al., 1995).
Fig. 5.
Sensitivity of P2X4+6 to P2X antagonists. Suramin, PPADS, and RB-2 were tested for their blocking properties on heteromeric P2X4+6 channels and on homomeric P2X4 receptors. Antagonists were coapplied with ATP (A) or preincubated before coapplication (B). Note that P2X4+6 receptors are inhibited, whereas P2X4 receptors are potentiated by 10 μm RB-2. Values are normalized to the response to ATP only (averages ± SEM from 5 oocytes per experiment;single and double asterisks denote significant difference; p < 0.05 andp < 0.01, respectively).
Sensitivity of P2X4+6 receptors to coagonists zinc ions and protons
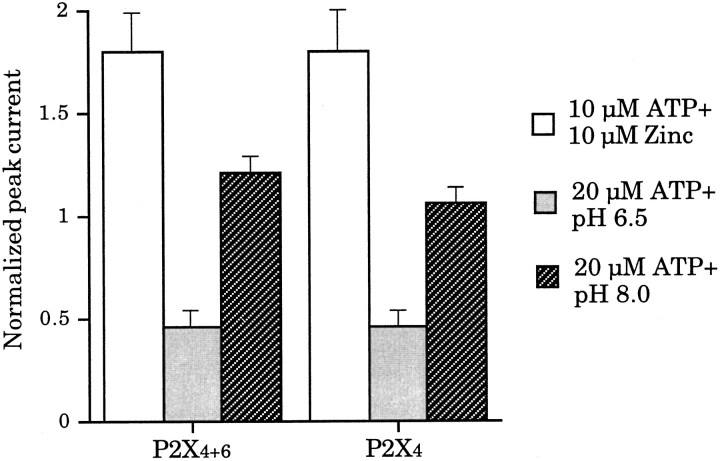
We have reported previously that 10 μm extracellular zinc ions coapplied with 10 μm ATP potentiated P2X4 peak currents by almost twofold (Séguéla et al., 1996). In addition, it has also been shown that the sensitivity to ATP of homomeric P2X4 channels is modulated by external pH: pH <7 inhibits ATP responses, whereas pH >8 has no significant effects (Stoop et al., 1997). Therefore, we checked whether these coagonists applied with ATP could discriminate between P2X4+6 and P2X4 receptor phenotypes. In response to 10 μm zinc ions and 10 μm ATP coapplications, there were no significant differences between potentiating factors of 1.8 ± 0.19 and 1.8 ± 0.21 for P2X4+6 heteromeric channels and P2X4 homomeric receptors, respectively (Fig.6A). There was also no significant difference between these two receptor phenotypes with respect to ATP (20 μm) applied at pH 6.5. In both cases, residual peak current amplitudes were 46 ± 4% of control values measured at pH 7.4 (Fig. 6B). When 20 μm ATP was applied at pH 8.0, it elicited peak currents of 121 ± 4 and 106 ± 4% for P2X4+6 heteromers and P2X4 homomers, respectively (Fig. 6C). Thus, contrary to αβmATP, 2MeSATP, and antagonists suramin, PPADS, and RB-2, cofactors zinc and protons did not discriminate between P2X4+6 and P2X4 receptors on a pharmacological basis.
Fig. 6.
Sensitivity of P2X4+6 to the extracellular cofactors zinc ions and pH. Extracellular Zn2+, pH 6.5 and 8.0, coapplied with ATP, did not allow differentiation between P2X4+6 and P2X4receptors. Values are normalized to the response to ATP only (averages ± SEM from 4 oocytes per experiment).
Subunit-specific association of P2X4 with P2X6 subunits
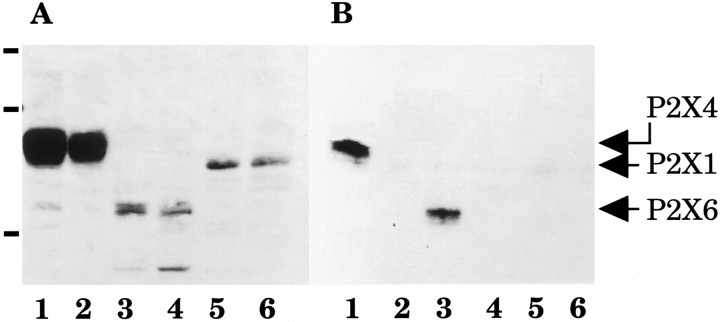
Before testing their biochemical interaction, the expression of Flag-tagged P2X1, P2X4, and P2X6 subunit proteins in transiently transfected HEK-293A cells was confirmed by immunoblot of total membrane proteins (Fig.7A, lanes 1–6). Homogenates from HEK-293A cells transiently cotransfected with cDNA templates encoding P2X4-(His)6 and either P2X1-Flag, P2X4-Flag, or P2X6-Flag constructs were analyzed for copurification. After solid-phase binding of P2X4-(His)6 proteins on poly His-binding resin, we detected the coprecipitation of P2X4-Flag subunits, confirming that P2X4 subunits interacted between themselves to generate a homomultimeric complex (positive controls, Fig.7B, lane 1). Coexpression of P2X4-(His)6 with P2X6-Flag subunits gave a positive band corresponding to the expected size of P2X6 (51 kDa; Fig. 7B, lane 3), demonstrating directly for the first time that P2X4 and P2X6 subunits do physically interact in a multimeric complex. Coexpression of P2X4-(His)6 with P2X1-Flag subunits did not give any signal when probed with anti-Flag M2 antibodies after purification, confirming that P2X4 and P2X1 subunits do not heteropolymerize (Fig. 7B, lane 5). All control coexpressions including wild-type P2X4 (lacking the poly-His motif) cotransfected with Flag-tagged P2X4, P2X6, or P2X1 subunits were negative after purification on poly His-binding resin (Fig. 7B,lanes 2, 4, 6).
Fig. 7.
Subunit specificity of P2X4 and P2X6 heteropolymerization. A, Immunoblot of Flag-tagged P2X1, P2X4, and P2X6 subunits probed with anti-Flag M2 monoclonal antibodies in total membrane proteins from transiently transfected HEK-293A cells. B, From the same samples, immunoblot of Flag-tagged P2X1, P2X4, and P2X6 subunits probed with M2 antibodies after copurification through P2X4-(His)6 subunits. Molecular weight markers (in A): 104, 82, and 48 kDa. Cotransfections: lane 1, P2X4-(His)6 + P2X4-Flag;lane 2, P2X4-wt + P2X4-Flag;lane 3, P2X4-(His)6 + P2X6-Flag; lane 4, P2X4-wt + P2X6-Flag; lane 5, P2X4-(His)6 + P2X1-Flag;lane 6, P2X4-wt + P2X1-Flag.
DISCUSSION
Functional identification of P2X4+6heteromeric receptors
In the present study, we first observed an apparent inhibition of P2X6 subunits on ATP-induced currents in oocytes expressing P2X4 subunits (Fig. 1B). However, neither ATP-induced currents mediated by P2X1 subunits (Fig.1C) nor currents mediated by P2X2 subunits (Fig.1D) were affected, strongly suggesting that P2X4 and P2X6 isoforms associate together, in a subunit-specific manner, into a novel heteromeric P2X channel. Functional P2X4+6 protein assembly and/or plasma membrane channel targeting appeared to be on a different time scale compared with P2X4 receptors. Indeed, in response to 100 μm ATP, P2X4+6 heteromultimers gave rise to increasing peak currents even after 5 d of expression (Fig.2A), whereas P2X4 homopolymers yielded decreasing peak current amplitudes under identical conditions (Fig.2B). We did not find any difference between the EC50 values of ATP for both homomeric and heteromeric receptor isoforms (Fig. 4A), so we concluded that the apparent inhibitory effect of P2X6 on P2X4recorded 3 d after injection was mainly attributed to a slower expression of P2X4+6 receptors on the cell surface, assuming similar channel conductance. These findings constituted our first set of experimental evidence demonstrating a heteropolymerization between P2X4 and P2X6 subunits. It has been noticed previously that P2X6 subunits and channels express poorly in HEK-293A cells (Collo et al., 1996) and are silent to ATP in the Xenopus oocyte expression system (Soto et al., 1996), as observed here. However, maximal ATP-induced peak currents were significantly larger at day 5 in the case of P2X4+6channels than in the case of P2X4 alone (Fig.2A). This situation is reminiscent of epithelial sodium-selective channels, belonging to another family of two-transmembrane-domain cation channels, whereby a fully functional channel requires the heteropolymerization of α subunits with β and γ subunits, both inactive when expressed alone (Canessa et al., 1994).
Unique pharmacological profile of P2X4+6heteromeric receptors
We made the assumption that the association between P2X4 and P2X6 subunits should be reflected in some unique aspects of the pharmacological profile of the resulting heteromeric receptor. Although P2X4 seemed the dominant subunit for the sensitivity to ATP in the heteromers, we observed a statistical difference between EC50 values of 2MeSATP for P2X4+6 heteromeric channels and P2X4 receptors (Fig. 4B). Furthermore, in response to 100 μm αβmATP applications, oocytes coexpressing P2X4 and P2X6 subunits gave rise to larger maximal peak currents than oocytes expressing P2X4 isoforms alone (Fig. 3A), despite slower kinetics of expression. Indeed, we measured a lower EC50 of αβmATP for P2X4+6 than for P2X4 channel species (Fig.3B). Therefore, in addition to opposite protein expression profiles between P2X4+6 and P2X4 channels, these observations strongly indicate that P2X4 and P2X6 subunits generate a novel receptor phenotype characterized by a unique agonist profile, namely increased 2MeSATP and αβmATP sensitivity. Moreover, these data provide for the first time experimental evidence for moderately desensitizing αβmATP-activated ionotropic responses.
Furthermore, we probed the sensitivity of P2X4+6 heteromers to P2 antagonists suramin, PPADS, and RB-2 coapplied with ATP. We found that suramin significantly blocked P2X4+6 activity without inhibiting significantly P2X4 homomeric receptors (Fig.5A); 10 μm suramin coapplied with 100 μm ATP decreased P2X4+6 heteromeric receptor peak current amplitudes by up to 40% compared with 7% for P2X4 homomeric channels. Coapplied PPADS inhibited P2X4+6 weakly, although it had no measurable effects on oocytes expressing P2X4 subunits alone (Fig.5A). After preincubation, low concentrations of RB-2 provided the most dramatic differential effect by inhibiting P2X4+6 heteromers while potentiating P2X4channel activity (Fig. 5B). Suramin and RB-2 would thus be useful pharmacological tools to investigate the expression of native P2X4+6 heteromers in αβmATP-sensitive neuronal preparations.
Biochemical evidence of P2X4+6 heteropolymers
We demonstrated direct interactions between the two predominant brain P2X4 and P2X6 isoforms through the use of an established copurification assay (Tinker et al., 1996). Based on our coprecipitation results with epitope-tagged subunits in nondenaturing conditions, P2X4 associates with P2X6 subunits (Fig. 7B). In Xenopus oocytes, this heteropolymerization underlies the specific pharmacological and electrophysiological phenotype of a novel heteromeric channel distinct from either P2X4 or P2X6 homomeric receptors. On the other hand, P2X4 and P2X1 subunits did not seem to interact significantly with each other (Fig.7B). Furthermore, the absence of obvious phenotypical differences between oocytes coexpressing P2X6 + P2X1 and P2X1 subunits alone (Fig.1C), or between P2X6 + P2X2 and P2X2 homomers (Fig. 1D), indicate that structural determinants of association between P2X4 and P2X6 isoforms are subunit-dependent. A similar biochemical approach using copurification of P2X4 with chimeric subunits based on P2X6 and P2X1 structures could lead to the identification of the domain(s) involved in specific heteropolymerization.
Functional correlates of native P2X4+6 heteromers
Purinergic responses from CA3 neurons in rat hippocampal slices have been shown recently to be activated by αβmATP and inhibited by suramin but not by PPADS (Ross et al., 1998). Based on in situ hybridization results (Collo et al., 1996), P2X4and P2X6 are the only P2X subunits expressed at significant levels in adult rat hippocampus, namely in CA1–CA4 hippocampal subfields and in the dentate gyrus. Thus, our functional data obtained from recombinant receptors are in close agreement with this native phenotype and suggest that the sensitivities to αβmATP and to suramin of rat CA3 neurons might be mediated through native P2X4+6 heteromeric channels.
Neonatal rat cerebellar Purkinje cells have been characterized as having purinergic receptors with a P2X2-like pharmacological profile in eliciting extracellular calcium influxes (Mateo et al., 1998). This conclusion rested on αβmATP insensitivity, the potency ratio of ATP to 2MeSATP, as well as suramin and PPADS blockade after preincubation. However, on recombinant P2X4+6 receptors, the concentration of αβmATP used byMateo et al. (1998) (50 μm) was ∼10% as efficacious as 50 μm ATP in eliciting ionotropic responses, so αβmATP-mediated intracellular calcium increases could have remained undetected and consequently interpreted as αβmATP unresponsiveness. The developmental regulation of expression levels of neuronal P2X genes in cerebellum is not established so far. Adult rat Purkinje neurons are known to transcribe P2X4 and P2X6 mRNA (Collo et al., 1996) and have been shown to translate high levels of P2X4 subunits (Lê et al., 1998), whereby P2X2 mRNAs (Collo et al., 1996) or subunits (Vulchanova et al., 1996) were reported previously to be absent (Kanjhan et al., 1996). It is also possible that native P2X receptors in neonatal Purkinje cells are composed of three subunits, namely P2X2, P2X4, and P2X6, assembled in a heteromeric complex in which P2X2 is pharmacologically dominant. We have recorded in oocytes purinergic currents mediated by P2X4+6 heteromeric channels that were significantly more sensitive to the agonists αβmATP and 2MeSATP, as well as to the antagonist suramin compared with P2X4 homomeric receptors. So it is likely that the moderately desensitizing αβmATP-activated and suramin-sensitive postsynaptic purinergic responses recorded from medial habenula (Edwards et al., 1992) could be accounted for by the expression of postsynaptic P2X4+6 receptors, because in situhybridization results demonstrate the exclusive presence of P2X4 and P2X6 transcripts in this region (Collo et al., 1996). The widespread distribution of high-affinity [3H]αβmATP binding sites within the rat CNS (Bo and Burnstock, 1994; Michel and Humphrey, 1994; Balcar et al., 1995) appears to correlate with in situ hybridization data on P2X4 and P2X6 mRNA distributions (Collo et al., 1996; Séguéla et al., 1996) as well as with the immunocytochemical localization of P2X4 protein (Lêet al., 1998). This neuroanatomical evidence strongly suggests that the P2X4+6 channel phenotype might be present in most rat brain and spinal cord regions. Moreover, we have shown that the P2X4 subunit is a major presynaptic purinoceptor component in laminae I and II of spinal cord and in olfactory glomeruli (Lêet al., 1998), two regions in which P2X6 is also expressed (Collo et al., 1996). Therefore, the heteromeric P2X4+6ATP-gated cation channel could play a significant role in the regulation of excitatory transmitter release in central sensory synapses.
Footnotes
K-T.L. holds a PhD studentship from the Savoy Foundation for Epilepsy; K.B. is a Medical Research Council-PMAC-Astra postdoctoral Fellow; and P.S. is a junior Scholar from the Fonds de la Recherche en Santé du Québec. We thank the Medical Research Council of Canada, the Fondation des Maladies du Coeur du Québec, and the Astra Research Center in Montreal for their operating support, as well as Michel Paquet for expert technical assistance.
Correspondence should be addressed to Dr. Philippe Séguéla, Cell Biology of Excitable Tissue Group, Montreal Neurological Institute, 3801 University Avenue, Room 778, Montreal, Quebec, Canada H3A 2B4.
REFERENCES
- 1.Balcar VJ, Li Y, Killinger S, Bennett MR. Autoradiography of P2X ATP receptors in the rat brain. Br J Pharmacol. 1995;115:302–306. doi: 10.1111/j.1476-5381.1995.tb15877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bo X, Burnstock G. Distribution of [3H]α,β-methylene ATP binding sites in rat brain and spinal cord. NeuroReport. 1994;5:1601–1604. doi: 10.1097/00001756-199408150-00015. [DOI] [PubMed] [Google Scholar]
- 3.Bo X, Zhang Y, Nassar M, Burnstock G, Schoepfer R. A P2X purinoceptor cDNA conferring a novel pharmacological profile. FEBS Lett. 1995;375:129–133. doi: 10.1016/0014-5793(95)01203-q. [DOI] [PubMed] [Google Scholar]
- 4.Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- 5.Buell G, Collo G, Rassendren F. P2X receptors: an emerging channel family. Eur J Neurosci. 1996;8:2221–2228. doi: 10.1111/j.1460-9568.1996.tb00745.x. [DOI] [PubMed] [Google Scholar]
- 6.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger J-D, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 7.Chen C-C, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- 8.Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the CNS. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- 10.Kanjhan R, Housley GD, Thorne PR, Christie DL, Palmer DJ, Luo L, Ryan AF. Localization of ATP-gated ion channels in cerebellum using P2X2R subunit-specific antisera. NeuroReport. 1996;7:2665–2669. doi: 10.1097/00001756-199611040-00051. [DOI] [PubMed] [Google Scholar]
- 11.Khakh BS, Humphrey PPA, Surprenant A. Electrophysiological properties of P2X-purinoceptors in rat superior cervical, nodose, and guinea pig coeliac neurones. J Physiol (Lond) 1995;484:385–395. doi: 10.1113/jphysiol.1995.sp020672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lê K-T, Villeneuve P, Ramjaun AR, McPherson PS, Beaudet A, Séguéla P. Sensory presynaptic and widespread somatodendritic immunolocalization of central ionotropic P2X ATP receptors. Neuroscience. 1998;83:177–190. doi: 10.1016/s0306-4522(97)00365-5. [DOI] [PubMed] [Google Scholar]
- 13.Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- 14.Mateo J, Garcia-Lecea M, Miras-Portugal MT, Castro E. Ca2+ signals mediated by P2X-type purinoceptors in cultured cerebellar Purkinje cells. J Neurosci. 1998;18:1704–1712. doi: 10.1523/JNEUROSCI.18-05-01704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michel AD, Humphrey PPA. Distribution and characterization of [3H]α,β-methylene ATP binding sites in the rat. Naunyn- Schmiedebergs Arch Pharmacol. 1994;348:608–617. doi: 10.1007/BF00167237. [DOI] [PubMed] [Google Scholar]
- 16.North RA, Barnard EA. Nucleotide receptors. Curr Opin Neurobiol. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- 17.Radford KM, Virginio C, Surprenant A, North RA, Kawashima E. Baculovirus expression provides direct evidence for heteromeric assembly of P2X2 and P2X3 receptors. J Neurosci. 1997;17:6529–6533. doi: 10.1523/JNEUROSCI.17-17-06529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross FM, Brodie MJ, Stone TW. Modulation by adenine nucleotides of epileptiform activity in the CA3 region of rat hippocampal slices. Br J Pharmacol. 1998;123:71–80. doi: 10.1038/sj.bjp.0701586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Séguéla P, Haghighi A, Soghomonian JJ, Cooper E. A novel neuronal P2X ATP receptor ion channel with widespread distribution in the brain. J Neurosci. 1996;16:448–455. doi: 10.1523/JNEUROSCI.16-02-00448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soto F, Garcia-Guzman M, Karschin C, Stühmer W. Cloning and tissue distribution of a novel P2X receptor from rat brain. Biochem Biophys Res Commun. 1996;223:456–460. doi: 10.1006/bbrc.1996.0915. [DOI] [PubMed] [Google Scholar]
- 21.Stoop R, Surprenant A, North RA. Differential sensitivities to pH of ATP-induced currents at four cloned P2X receptors. J Neurophysiol. 1997;78:1837–1840. doi: 10.1152/jn.1997.78.4.1837. [DOI] [PubMed] [Google Scholar]
- 22.Tinker A, Jan YN, Jan LY. Regions responsible for the assembly of inwardly rectifying potassium channels. Cell. 1996;87:857–868. doi: 10.1016/s0092-8674(00)81993-5. [DOI] [PubMed] [Google Scholar]
- 23.Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, Buell G. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- 24.Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. Differential distribution of two ATP-gated ion channels (P2X receptors) determined by immunocytochemistry. Proc Natl Acad Sci USA. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]