Abstract
A stretch-activated Cl− current (ICl) was investigated in cultured murine microglia using the whole-cell configuration of the patch-clamp technique. After application of membrane stretch, a Cl− current appeared within seconds, and its amplitude increased further within 3–8 min.ICl underwent rundown, which was prevented by addition of 4 mm ATP to the intracellular perfusing solution. The stretch-activated Cl− current exhibited outward rectification and did not show any voltage-dependent gating. Lowering the concentration of extracellular Cl− from 142 to 12 mm by equimolar substitution of Cl− with gluconate shifted the reversal potential of ICl by 41.6 ± 1.8 mV in the depolarizing direction. 4,4′-Diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS) and 4-acetamido-4′-isothiocyanatostilbene-2,2′-disulfonic acid (SITS) blocked ICl in a voltage- and time-dependent manner. At a test potential of +40 mV, a half-maximal blockade at 16.1 μm DIDS and at 71.0 μm SITS was determined for ICl. At a concentration of 200 μm, 5-nitro-2-(3-phenylpropylamino)benzoic acid or flufenamic acid blocked ICl by 88% and 75%, respectively. Each of these four Cl− channel blockers reversibly inhibited the ramification process of microglia, whereas blockers of voltage-gated Na+ and K+ channels did not affect the transformation of microglia from their ameboid into the ramified phenotype. It is suggested that in microglia functional stretch-activated Cl− channels are required for the induction of ramification but not for maintaining the ramified shape.
Keywords: brain macrophages, ramification, stretch-activated Cl− current, SITS, DIDS, NPPB, flufenamic acid
Microglia, the macrophages of the brain, can be distinguished morphologically into ameboid and ramified cells. Ameboid microglia appear in immature brain during late prenatal and early postnatal periods. These cells have round somata with short lamellopodial extensions. In contrast, microglia of normal adult CNS exhibit a ramified morphology, characterized by long secondary and tertiary branched processes arising from both poles of an elongated flattened cell body (Ling and Wong, 1993). Within the brain, ramified microglia are known to be in an immunologically resting state. During inflammation and several neurological disorders, microglia become activated; the activation process is correlated with a loss of the ramified shape of the cells (for review, see Kreutzberg, 1996; Streit, 1996).
The origin of ameboid and ramified microglia is still a matter of controversy (for review, see Ling and Wong, 1993). The transformation of ameboid into ramified microglia during development was first demonstrated by Ling (1979). In vitro studies have shown that the induction of ramification of microglia appears to be influenced by astrocytes. Thus, ameboid microglia differentiate into ramified cells during cultivation on top of an astrocytic monolayer (Sievers et al., 1994; Tanaka and Maeda, 1996) or in mixed microglia–astrocyte cultures (Liu et al., 1994). However, direct contact between microglia and astrocytes is not a prerequisite for these processes, because ramification can also be induced in microglia during treatment with astrocyte-conditioned medium (Eder et al., 1997a). Although the astrocytic factors responsible for triggering these shape changes in microglia have not been identified, the cytokines macrophage colony-stimulating factor and granulocyte/macrophage colony-stimulating factor seem to play an important role during processes of microglial transformation from their ameboid into the ramified phenotype (Liu et al., 1994; Fujita et al., 1996).
During depolarization of the cell membrane, ramified microglia exhibit a voltage-gated sodium current and a voltage-gated outward potassium current (Korotzer and Cotman, 1992; Sievers et al., 1994; Eder et al., 1996, 1997a) that are not seen in unstimulated ameboid microglia (for review, see Eder, 1998). However, it is not clear whether Na+ and outward K+ channels are required for shape changes in microglia. Expression of outward K+ channels that is not accompanied by shape changes of the cells can also be induced in microglia by treatment with low doses of the astrocyte-conditioned medium (Eder et al., 1997a).
In this study we investigated the effects of several ion channel blockers on the induction of ramification in cultured murine microglia. We suggest a participation of stretch-activated chloride channels in the ramification process of microglia. Properties of the stretch-activated chloride current were studied in detail.
MATERIALS AND METHODS
Cell culture. Microglia were obtained from brain cell cultures of newborn NMRI mice supplied by Hamann-Winkelmann (Borchen, Germany). Mixed brain cell cultures were prepared as described previously (Eder et al., 1995). Brain cortices were enzymatically dissociated (15 min at 37°C with 0.25% trypsin, type XI; Sigma, Deisenhofen, Germany), and a single-cell suspension was achieved by repeated triturations. Cells were seeded into tissue culture flasks at a density of 2–4 × 106/5 ml in DMEM (Life Technologies, Gaithersburg, MD) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Life Technologies) and 30% supernatant of L-929 fibroblasts as a source of macrophage colony-stimulating factor. After at least 10 d of incubation, microglia were harvested by shaking the cultures (30 min, 300 rpm) to detach weakly adherent cells from the astrocytic monolayer. Isolated microglia were seeded on glass coverslips in 24-well Costar plates (3 × 104/1 ml). Patch-clamp recordings were performed 1–5 d after the isolation procedure. To induce ramification of microglia, astrocyte-conditioned medium (ACM) was added to cell cultures.
Astrocytes from murine cortices were cultured as described by Hertz et al. (1982). Cells were seeded at a density of 3–4 × 105/ml and were grown in DMEM supplemented with 10% FCS. After 2 weeks in culture, the concentration of FCS was lowered to 2%. The supernatants of astrocytic cultures were collected twice per week and used as ACM.
In some cases one of the following ion channel blockers was added to ACM immediately before cells were treated with the ACM: 1–10 μm tetrodotoxin (TTX), 1 mm4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS), 1 mm 4-acetamido-4′-isothiocyanatostilbene-2,2′-disulfonic acid (SITS) (all from Sigma), 10–500 nm charybdotoxin (CTX), 10–500 nm kaliotoxin (KTX) (both from Latoxan, Rosans, France), 200 μm flufenamic acid, 200 μm 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) (both from Biotrend/RBI), and 1–4 μm chlorotoxin (Alomone Labs, Jerusalem, Israel). The effects of each drug were investigated in a minimum of four different cell cultures (four to eight coverslips per culture).
Electrophysiological recordings. Membrane currents were measured using the whole-cell configuration of the patch-clamp technique (Hamill et al., 1981). Current recordings were made with an EPC-7 patch-clamp amplifier (HEKA, Lambrecht/Pfalz, Germany). Data were recorded with a CED 1401 interface (Cambridge Electronic Design Ltd., Cambridge, UK) and stored on line using the software VCLAMP 6.0 (Cambridge Electronic Design) on an IBM-compatible computer for subsequent analyses. Data were sampled at 10 kHz and low-pass-filtered at 3 kHz using an 8-pole Bessel filter. Series resistance compensation was routinely used to reduce the effective series resistance by approximately 70%. Patch electrodes of 3–4 MΩ were fabricated on a two-stage puller (Narishige PP-83, Tokyo, Japan) from borosilicate glass (outer diameter, 1.5 mm, and inner diameter, 1 mm) (Hilgenberg, Malsfeld, Germany). For Cl− current recordings, the electrodes were filled with the following solution (in mm):N-methyl-d-glucamine chloride (NMGCl) 120, EGTA 11, CaCl2 1, MgCl2 2, HEPES 10, pH 7.35. The osmolarity was adjusted to 280 mOsm with D-glucose. In some cases NMG+ was substituted by K+. Mostly, the intracellular solution contained 4 mm NaATP (Sigma). The extracellular solutions contained (in mm): NaCl 130, KCl 5, CaCl2 2, MgCl2 1, HEPES 10, pH 7.35. To prevent spontaneous activation of swelling-induced Cl− currents in microglia, the osmolarity of the extracellular solutions was adjusted to 290–295 mOsm with D-glucose. In some experiments, NaCl was substituted by sodium gluconate (measurements were corrected for junction potentials). To study Cl− currents in isolation, 1 mmBaCl2 (blocker of inward rectifying K+currents), 1 μm TTX (blocker of voltage-gated Na+ currents), and 100 μmLaCl3 (blocker of voltage-gated H+currents) were routinely added to the extracellular solutions. At the concentrations given, neither of these drugs influenced stretch-activated Cl− currents of microglia (data not shown). In a few experiments, voltage-gated outward K+ currents were blocked by 100 nmkaliotoxin (Latoxan). All recordings were performed at room temperature (20–23°C). Data are presented as mean values ± SD of the number of experiments indicated.
Mechanical stimulation. In most experiments, membrane stretch was applied via the recording patch pipette. After the whole-cell configuration was established, the pipette was moved by 5 ± 1 μm. Microglia in culture are tightly attached to the glass coverslips, and movement of the pipette resulted in obvious stretching of the membrane. Displacement of the pipette for >5 μm did not significantly increase amplitudes of stretch-activated currents. In a few experiments, a fire-polished pipette was mounted on a second manipulator and used for mechanical stimulation according to the method described by Hu and Sachs (1996). Briefly, the pipette was pressed against the cell using either vertical or horizontal displacement of the pipette, which was <5 μm.
Pharmacological studies. Drugs were applied using a four-barrel microperfusion pipette, positioned at a distance of ∼30–50 μm from the recorded cell to permit a rapid exchange of solutions. The flow rate was adjusted by hydrostatic pressure.
We added 1 mm 4-aminopyridine, 5 × 10−7 to 1 × 10−3 M DIDS, 1 × 10−6 to 5 × 10−3 M SITS, 200 μm NPPB, or 200 μm flufenamic acid to the extracellular superfusing solution. Stock solution of 500 mm NPPB and 500 mm flufenamic acid (both dissolved in DMSO) were prepared and stored frozen at −20°C. Chlorotoxin (1 μm) was dissolved in a 0.1% bovine serum albumin containing extracellular solution.
The concentration–response curves for DIDS and SITS were approximated using a Hill equation of the following form:I[X] =Imax[X]n/([X]n+ IC50n), in whichI[X] is the current amplitude during superfusion with the drug, Imax is the maximal current amplitude (before superfusion with the drug), n is the Hill coefficient, and IC50 is the dissociation constant that determines the concentration of the drug for a half-maximal current inhibition.
RESULTS
Induction of stretch-activated Cl− currents
Voltage ramps from −100 to +90 mV were applied for a duration of 400 msec every 10 sec. The holding potential was set to −60 mV. Using a high K+-containing intracellular perfusing solution that did not contain ATP, voltage-gated K+currents were obtained in both ameboid and ramified microglia immediately after establishment of the whole-cell configuration. Figure1A illustrates current recordings of a microglial cell that expressed both inward and outward rectifier K+ currents. Inward rectifier K+ currents were observed at potentials negative to −75 mV, whereas outward K+ currents were detectable at potentials positive to −30 mV. The distribution of inward and outward K+ currents in murine microglia at various functional states as well as kinetic and pharmacological properties of these voltage-gated K+ currents has been described elsewhere (for review, see Eder, 1998).
Fig. 1.
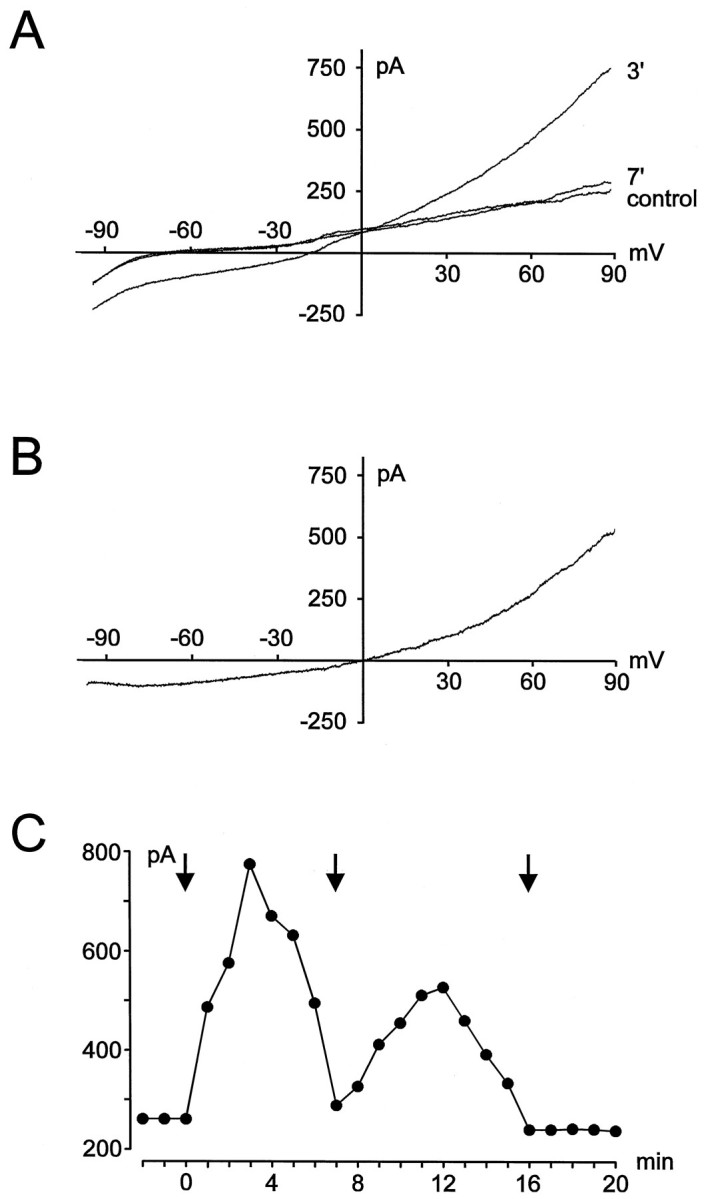
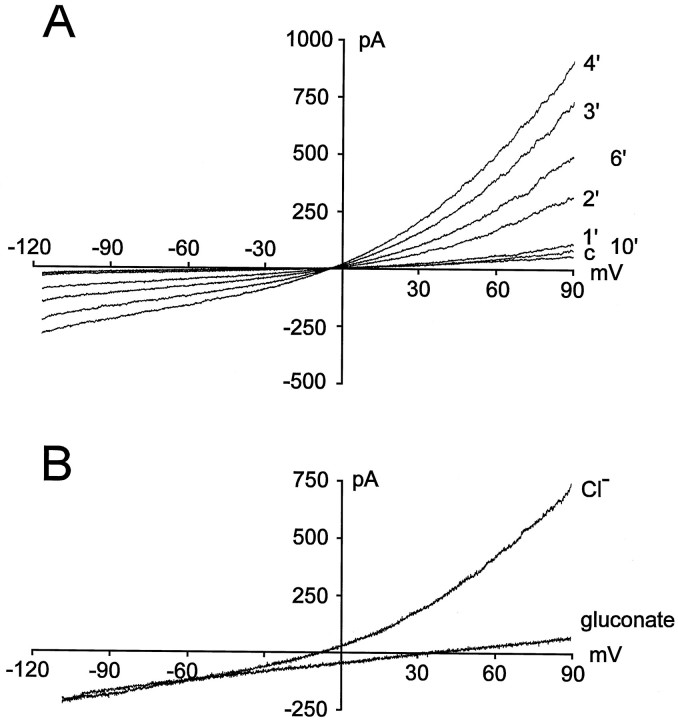
Induction of stretch-activated currents.A, Current recordings under control (before stretching of the membrane) and after application (3 and 7 min) of a membrane stretch using a K+-containing intracellular solution. B, Stretch-activated current isolated by substraction of the control trace from the trace measured 3 min after the stretch (shown in A). C, Time course of induction of the stretch-activated current for the example shown inA. Changes in amplitude (measured at +90 mV) are plotted against time after establishment of the whole-cell configuration.Arrows indicate the time at which the cell was stretched by moving the patch pipette.
Stretching of the cell membrane was induced by a movement of the recording patch pipette by a few micrometers. A membrane stretch resulted in the activation of an additional ionic current in both ameboid and ramified microglia (Fig. 1A,B). In a few experiments, stretching of the cell membrane was induced by a second fire-polished patch pipette. No differences were seen between currents evoked by both methods with respect to the time course of induction, kinetics, and pharmacological properties of the currents (see below). As shown for the example in Figure 1, stretch-activated currents activated very slowly. The amplitude of the currents increased steadily during the first 3–8 min after moving of the patch pipette. However, stretch-activated currents did not reach a steady state; rather the currents decreased and disappeared almost completely within 7–15 min after the membrane stretch. In contrast, recordings of voltage-gated inward and outward K+ currents were stable for more than 1 hr and did not show any kind of rundown. In Figure1C, the time course of current induction is illustrated for the example shown in Figure 1A. Amplitudes of the current were measured at a potential of +90 mV. As shown in Figure1C, in some cases stretch-activated currents could be activated again after their disappearance by application of a second membrane stretch. However, currents induced by a second stimulus mostly did not reach the amplitude of currents activated by a first stretch, and they also disappeared within a few minutes. Currents could only rarely be induced by a third membrane stretch. No differences were detected in the time course of current induction and rundown of stretch-activated currents between ameboid (n = 26) and ramified (n = 14) microglial cells.
Martin et al. (1995) described a stretch-activated K+ current in monocyte-derived macrophages that was completely blocked by 1 mm 4-aminopyridine (4-AP). To clarify whether a membrane stretch activates a similar K+ current in microglia, we investigated the effect of external 4-AP on stretch-activated currents in microglia. In these experiments, voltage-gated outward K+ currents were blocked by 100 nm KTX. The remaining stretch-activated current was unaffected by extracellular application of 1 mm4-AP (n = 11; data not shown). In further experiments, intracellular potassium ions were substituted by NMG+. In ameboid and ramified microglia, stretch-activated currents could also be evoked using K+-free intracellular solutions (Fig.2A). The K+-free intracellular solution was used in further studies. Moreover, to block inward rectifier K+currents the extracellular solution contained 1 mmBaCl2. Additionally, voltage-gated H+currents of microglia were routinely inhibited by 100 μmLaCl3, and voltage-gated Na+currents were blocked by 1 μm TTX. Because stretch-activated currents were not affected after the addition of BaCl2 (n = 8), LaCl3(n = 10), or TTX (n = 8) to the extracellular superfusing solution (data not shown), these currents could be investigated in isolation. In experiments using K+-free intracellular solutions, cells were held at −10 mV (a potential close to the equilibrium potential for Cl−) to prevent a continuous flux of Cl−. Voltage ramps were applied from −120 to +90 mV for a duration of 400 msec every 10 sec.
Fig. 2.
Recordings of stretch-activated currents using a K+-free intracellular solution. A, Induction of the currents. Time (in minutes) after the membrane stretch is indicated (c, control measurement before application of the membrane stretch). B, Current recording in the presence of 142 mm Cl−(Cl−) or after lowering concentration of extracellular Cl− to 12 mm by equimolar substitution with gluconate (gluconate).
To test the selectivity of the current that was activated in microglia in response to a membrane stretch, the concentration of extracellular Cl− ions ([Cl−]o) was lowered from 142 mm to 12 mm. As shown in Figure2B, lowering [Cl−]oby equimolar substitution with gluconate evoked a shift of the reversal potential of the stretch-activated current by 41.6 ± 1.8 mV (n = 8) to more positive potentials as well as a reduction of current amplitude. Thus, it appears that the stretch-activated current of microglia is carried mainly by chloride ions.
Effect of intracellular ATP
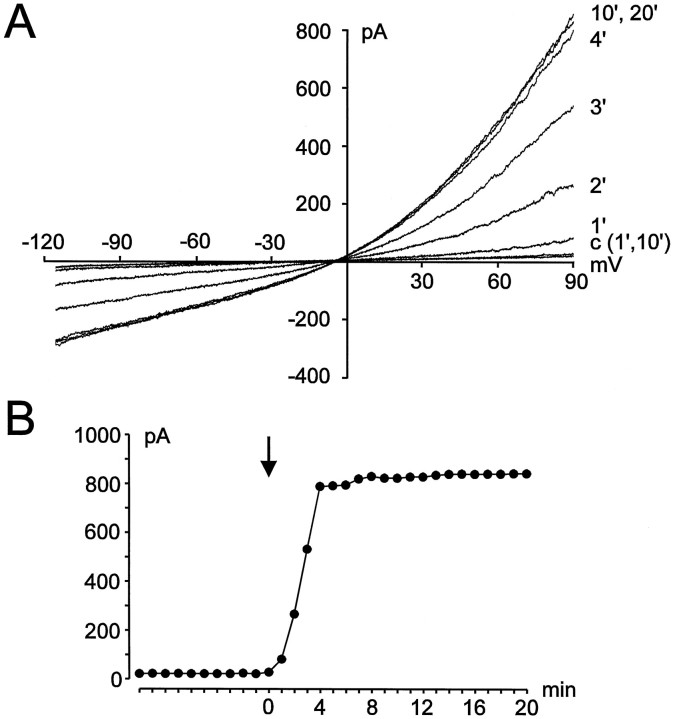
An influence of intracellular ATP on Cl−channels, namely an induction of current activation (Gill et al., 1992) or an inhibition of current rundown (Lewis et al., 1993), has been described in several cell preparations (for review, see Strange et al., 1996). In the presence of 4 mm intracellular ATP, Cl− currents did not appear spontaneously in murine microglia. A small leak current was the only conductance detected when no stretch was applied to the cell membrane (Fig.3A). After stretching the membrane, Cl− currents progressively increased in amplitude, reaching an apparent plateau within 4–8 min. The rundown of the stretch-activated Cl− current (ICl) observed without ATP was not observed in cells perfused with a 4 mm ATP pipette solution. ICl values reached their maximal amplitudes and did not change further within recordings of >1 hr. Figure 3A illustrates the development of Cl− currents with time after stretching of the cell membrane using an ATP-containing pipette solution. The time course of the changes in amplitudes of ICl is shown in Figure 3B, whereas amplitudes were determined at a potential of +90 mV.
Fig. 3.
Dependence of stretch-activated Cl− currents on intracellular ATP.A, Current recordings during perfusion of the cell with 4 mm ATP. Currents are shown at 1 and 10 min after establishment of the whole-cell configuration before the cell was stretched (c) and at several times (in minutes) after application of the membrane stretch. B, Corresponding amplitudes of the current shown in A(measured at +90 mV) are plotted against time after break-in. Membrane stretch is indicated by the arrow.
Pharmacology of stretch-activated Cl− currents
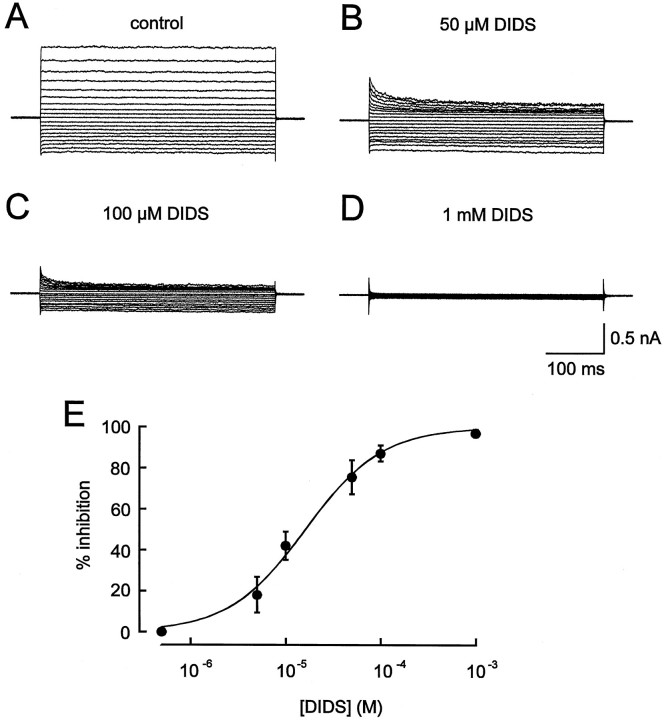
Several chloride channel blockers were tested for their ability to block stretch-activated Cl− currents of microglia. Voltage commands were applied from the holding potential of −10 mV for a duration of 400 msec. Cells were either depolarized to potentials between −10 and +80 mV or hyperpolarized to potentials between −10 and −110 mV. Under control conditions (Fig.4A), stretch-activated Cl− currents did not show any time-dependent activation or inactivation behavior at all test potentials. The stilbene disulfonate DIDS reduced the currents in the nanomolar–micromolar concentration range. As shown in Figure 4, 1 mm DIDS applied extracellularly abolished stretch-activated Cl− currents (Fig. 4D), whereas DIDS applied at lower concentrations induced a time- and voltage-dependent block of the currents (Fig. 4B,C). At hyperpolarizing potentials the block of IClby DIDS was less pronounced than at depolarizing potentials. To determine concentration–response relationship for DIDS, the drug was applied extracellularly to the cells at concentrations between 5 × 10−7 and 10−3 M. Amplitudes of Cl− currents evoked by a test pulse from the holding potential of −10 mV to a potential of +40 mV were measured at the end of the pulse where currents reached steady state. Current amplitudes were measured and normalized to the maximal current amplitudes for each cell, which were measured under control conditions before application of DIDS. A concentration–response curve was fitted by the Hill equation and a half-maximal effective concentration (IC50 value) of 16.1 μm DIDS (Hill coefficient 1.1) was determined for the stretch-activated Cl− current of microglia (n = 9) (Fig. 4E). The inhibitory effects of DIDS were reversible during washout.
Fig. 4.
Effect of extracellularly applied DIDS onICl. Measurements of stretch-activated Cl− currents before (A) and during superfusion of the cells with an extracellular solution containing 50 μm (B), 100 μm (C), or 1 mm(D) DIDS. E, Concentration–response curve for DIDS. At each DIDS concentration the percentage of current inhibition was determined in at least nine cells.
The inhibition of ICl by extracellularly applied SITS is demonstrated in Figure 5. Cl− currents were blocked by SITS in a similar voltage- and time-dependent manner as observed for the effects of DIDS on ICl. To determine the concentration–response relation for block of stretch-activated Cl− current by SITS, cells were superfused with SITS at concentrations between 10−6 and 5 × 10−3 M. An IC50 value of 71.0 μm SITS (Hill coefficient 1.0) was calculated from effects of SITS on amplitudes of the steady-state current evoked by a test pulse from −10 mV to +40 mV (n = 8) (Fig. 5C).
Fig. 5.
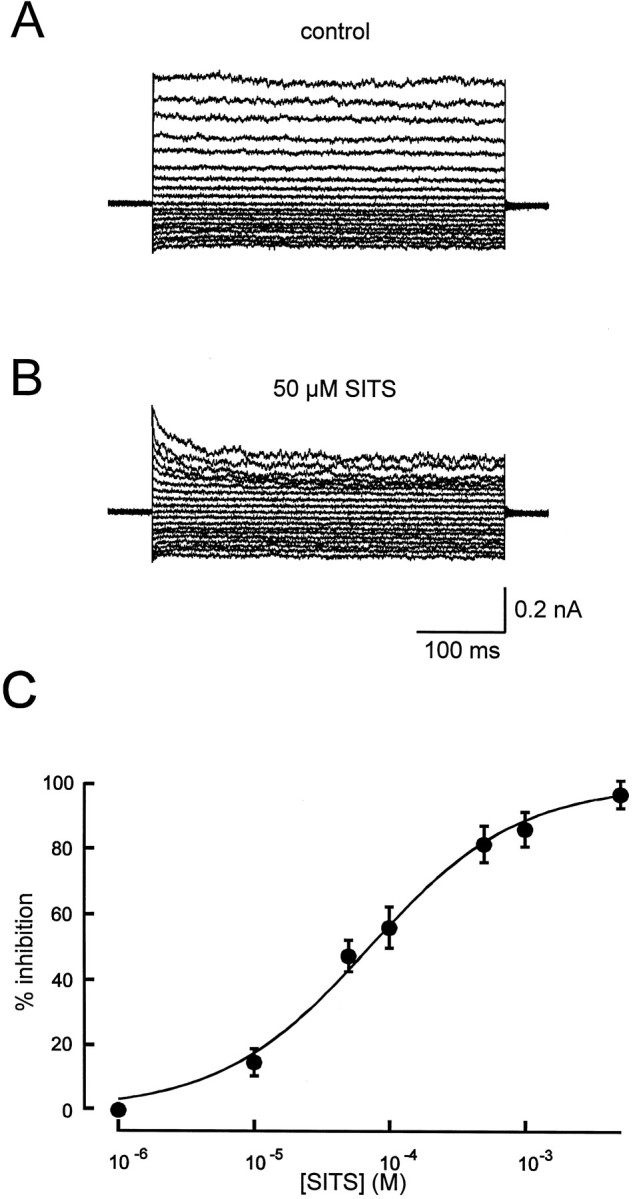
Blockade of stretch-activated Cl− currents by extracellularly applied SITS.A, Example of current recording using a voltage-step protocol while superfusing the cell with control solution (A) or a solution containing 50 μmSITS (B). C, Concentration–response curve for SITS (n = 8 for each concentration).
The effects of DIDS and SITS were also investigated using K+-containing intracellular solutions. In these experiments, 100 nm KTX was added to the superfusing solution to block voltage-gated outward K+ currents. Application of either 1 mm DIDS (n = 6) or 5 mm SITS (n = 5) completely blocked stretch-activated currents (data not shown), suggesting that microglia do not additionally express stretch-activated K+currents.
Block of chloride currents by NPPB and flufenamic acid appeared to be time- and voltage-independent (Fig.6A,B). At a concentration of 200 μm, NPPB reduced amplitudes ofICl by 87.5 ± 2.4% (n = 6), whereas flufenamic acid diminished amplitudes ofICl by 75.3 ± 7.1% (n = 6). The blocking effects of NPPB and flufenamic acid onICl were also reversible during prolonged washout of the drugs.
Fig. 6.
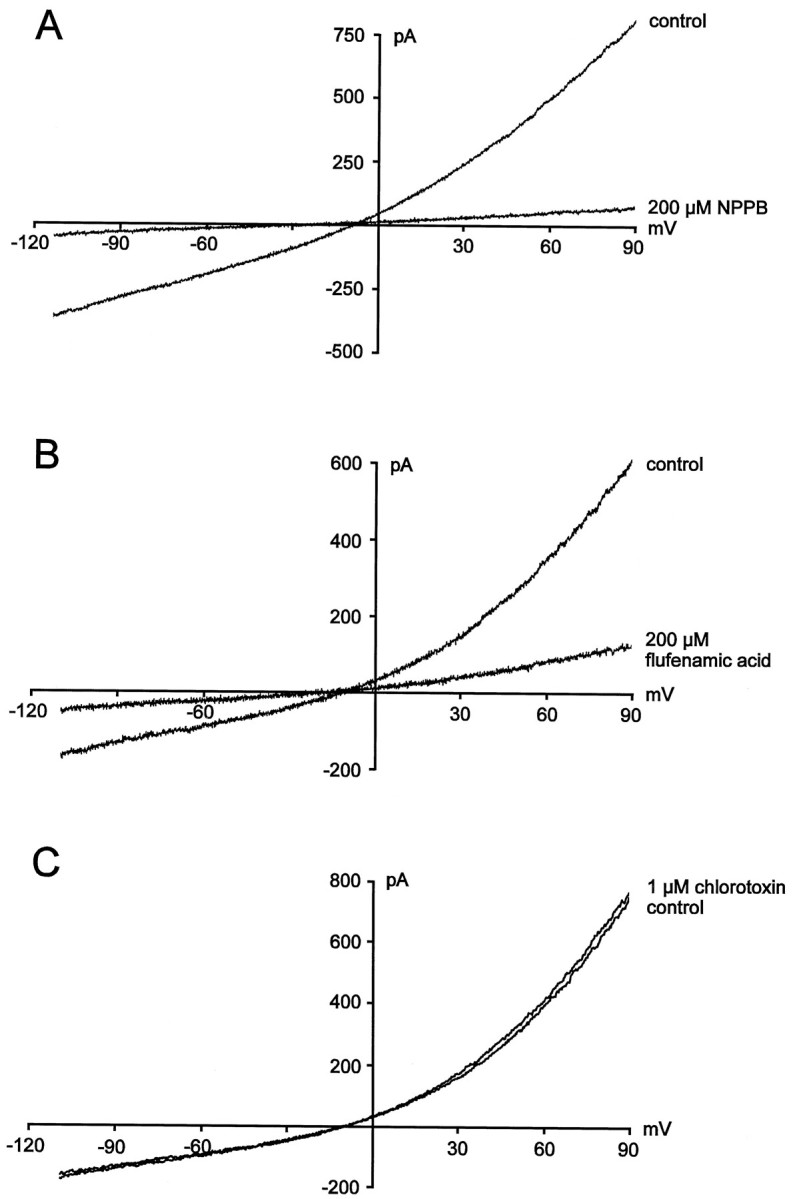
Effects of NPPB, flufenamic acid, and chlorotoxin on stretch-activated Cl− currents.A, Cl− current measurement in the absence and presence of 200 μm NPPB. B, Measurements of stretch-activated Cl− currents before and during superfusion of the cells with 200 μmflufenamic acid. C, Stretch-activated Cl− currents in the absence and presence of 1 μm chlorotoxin.
It has been reported previously that Cl− currents in glioma cells are potently inhibited on superfusion with chlorotoxin (Ullrich and Sontheimer, 1996; Ullrich et al., 1998). In contrast, stretch-activated Cl− currents of microglia were unaffected by extracellular application of 1 μmchlorotoxin. Neither kinetics (n = 6; data not shown) nor amplitude (n = 6) (Fig. 6C) of ICl was changed by the toxin.
Ramification of microglia
Cultured murine microglia were able to undergo dramatic shape changes after exposure to ACM. Figure 7shows examples of microglia that had been cultured either in normal culture medium (untreated) or in ACM. Untreated microglia exhibited an ameboid morphology during the whole time after isolation of the cells from the astrocytic monolayer (Fig. 7A). In contrast, addition of ACM to the cells evoked a transformation of microglia from their ameboid into a ramified phenotype within a few hours. After treatment with ACM, microglia exhibited long branched processes with lamellopodial tips as shown in Figure 7B,C. These ramified cells could be easily distinguished from ameboid cells by visual inspection. Ramified microglia were defined as cells with distinct processes at least greater than one cell diameter in length, as described previously (Korotzer and Cotman, 1992). In the presence of ACM, microglia retained their ramified morphology with time in culture. In contrast, ramified cells returned to their ameboid morphology by washing out ACM.
Fig. 7.
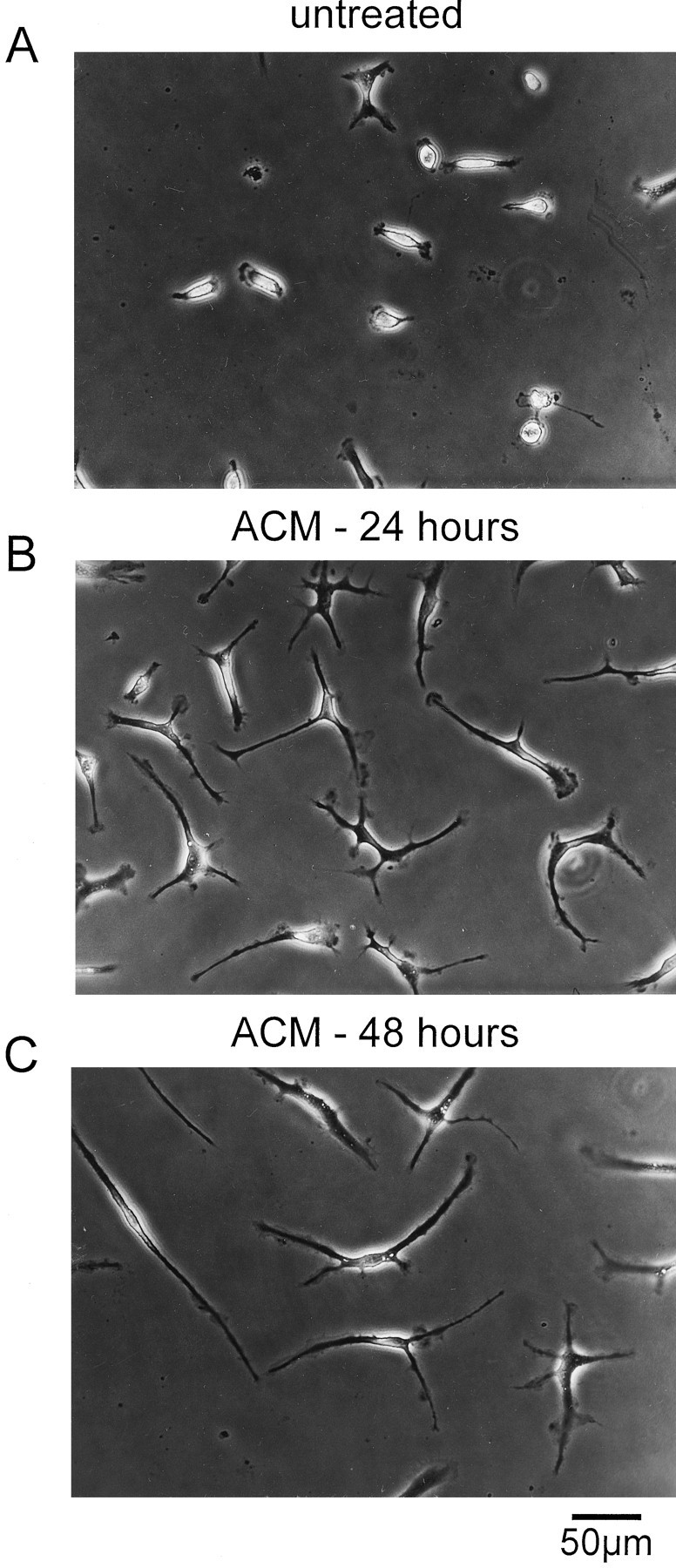
Cultures of ameboid and ramified murine microglia.A, Untreated cultured microglia exhibited an ameboid phenotype. B, C, Examples of ramified microglia that had been treated with astrocyte-conditioned medium for 24 (B) or 48 (C) hr. Cells were examined with a Zeiss Axioskop equipped with differential interference optics.
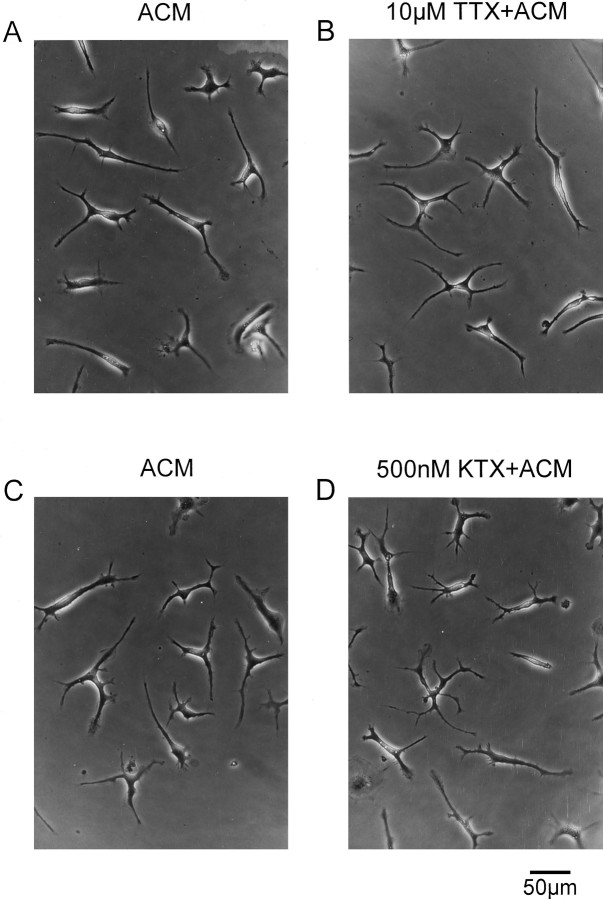
It has been demonstrated in previous studies that an expression of both voltage-gated sodium currents and voltage-gated outward potassium currents was induced in ramified microglia that had been co-cultured with astrocytes (Sievers et al., 1994). To test a possible involvement of either of these channels in the induction of ramification of microglia, the effects of specific blockers of these channels were investigated. TTX, a blocker of voltage-gated Na+currents, was added to the astrocyte-conditioned medium at concentrations between 1 and 10 μm. TTX did not influence ramification of microglia. As shown in Figure8A,B, microglia showed the same level of ramification as microglia treated with TTX-free ACM 24 hr after treatment with TTX-containing ACM.
Fig. 8.
Effect of TTX and KTX on microglia. Ameboid microglia were treated with ACM (A, C) and TTX-containing (B) or KTX-containing (D) ACM. Twenty-four hours after that treatment, microglia exhibited a ramified morphology in both toxin-free and toxin-containing ACM (A, same culture as inB; C, same culture as inD).
Microglial ramification could also not be inhibited by the addition of the potassium channel blockers KTX (Fig. 8C,D) or CTX (data not shown) to ACM at concentrations between 10 and 500 nm. No differences in the phenotype were detected between microglia cultured with ACM either in the presence or in the absence of these K+ channel blockers.
Effects of Cl− channel blockers on ramification of microglia
To investigate the role of stretch-activated Cl− channels in the process of ramification in microglia, the Cl− channel blockers DIDS, SITS, NPPB, or flufenamic acid were added to the astrocyte-conditioned medium. In the presence of either 1 mm DIDS or 1 mm SITS, ramification of microglia could not be induced. As demonstrated in Figure 9, 24 hr after the addition of DIDS- or SITS-containing ACM, microglia retained their ameboid phenotype (Fig. 9A,C). Twenty-four hours after the application of DIDS- or SITS-containing ACM, microglia were washed several times with culture medium and treated for another 24 hr with ACM that did not contain any Cl− channel blocker. Under these conditions, a transformation from their ameboid into a ramified phenotype was induced in microglia as illustrated in Figure9B,D. These microglial cells did not significantly differ in their morphology from microglia that had been treated for 24 or 48 hr exclusively with ACM. The level of ramification, i.e., the number and size of processes and lamellopodial tips, was not quantified in our study.
Fig. 9.
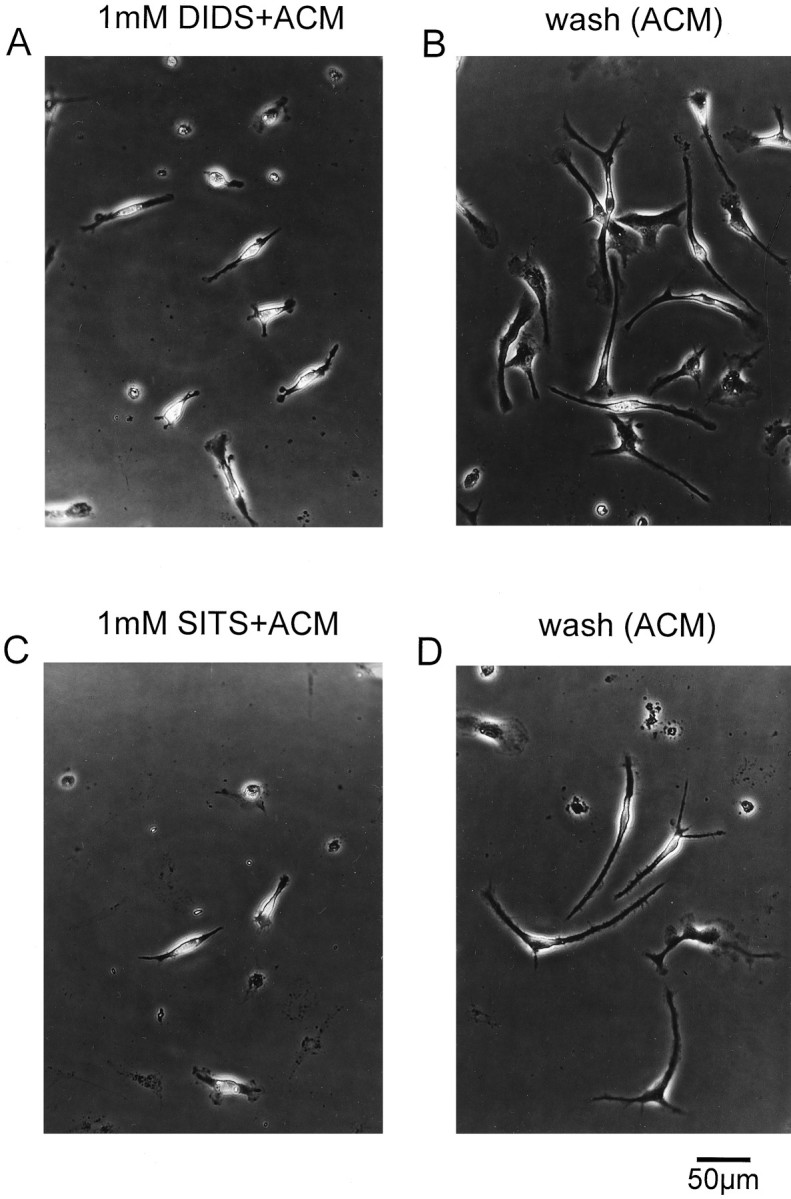
Effect of DIDS and SITS on ramification of microglia. A, Addition of 1 mm DIDS to ACM prevented the transformation of ameboid into ramified microglia.B, After washout of DIDS-containing ACM, the ramification of microglia was induced in the presence of ACM.C, SITS (1 mm) applied to ACM inhibited ramification of microglia. D, Microglia ramified in the presence of ACM after washout of SITS.
NPPB or flufenamic acid tested at a concentration of 200 μm also inhibited ramification of microglia, as shown in Figure 10A,C. Microglia treated with NPPB- or flufenamic acid-containing ACM showed an ameboid phenotype similar to that of untreated microglia. The inhibitory effect of these Cl− channel blockers on ramification of microglia was also reversible. As demonstrated in Figure 10B,D, after washout of NPPB- or flufenamic acid-containing ACM, microglia ramified in the presence of ACM to an extent similar to that of ACM-treated microglia that had not been pretreated with either of these Cl− channel blockers.
Fig. 10.
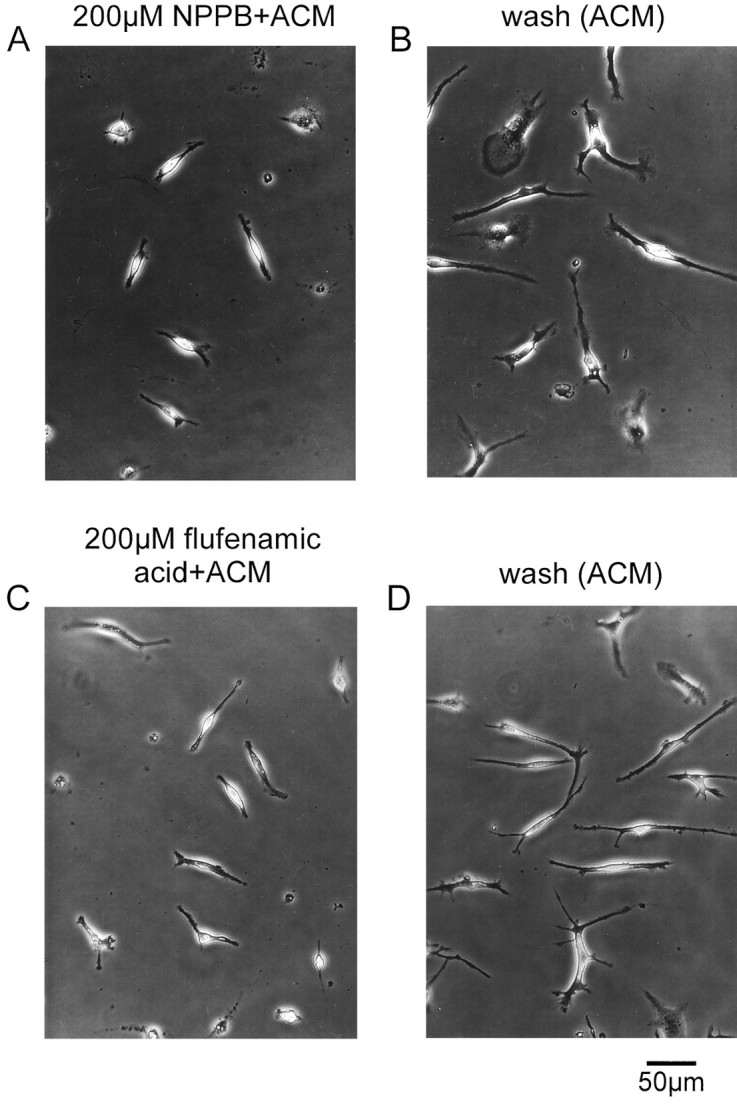
Inhibition of ramification of microglia by NPPB and flufenamic acid. Cells were treated with ACM that contained 200 μm NPPB (A) or 200 μmflufenamic acid (C). B, Microglia exhibited a ramified morphology 24 hr after substitution of NPPB-containing ACM with NPPB-free ACM. D, Ramified microglia 24 hr after washout of flufenamic acid in the presence of ACM.
In further experiments, microglia were exposed to ACM containing either 1 mm DIDS, 1 mm SITS, 200 μmNPPB, or 200 μm flufenamic acid for a duration of >24 hr. Microglial cells investigated for up to 5 d also did not change their ameboid morphology in the continuous presence of the Cl− channel blockers (data not shown), suggesting that these blockers did not delay morphological changes but prevented ramification of microglia.
In contrast to DIDS, SITS, NPPB, and flufenamic acid, chlorotoxin did not inhibit the process of ramification when added at concentrations of between 1 and 4 μm to the ACM. Microglial cells exposed to chlorotoxin-containing ACM ramified in a manner similar to microglia exposed to chlorotoxin-free ACM (data not shown).
The effects of Cl− channel blockers were also investigated in ramified microglia that had been treated first with ACM for 24 hr before Cl− channel blockers were added to the ACM. Ramified microglia were not influenced by each of the Cl− channel blockers (data not shown). Neither 1 mm DIDS-containing ACM nor 1 mm SITS-containing ACM evoked changes in morphological characteristics of ramified microglia. Similarly, microglia retained their ramified phenotype after the addition of either 200 μm NPPB or 200 μm flufenamic acid to the ACM.
DISCUSSION
Properties of stretch-activated Cl− currents in microglia
Stretching of the cell membrane activated an outwardly rectifying current in microglia, the macrophages of the brain. The existence of stretch-activated ion channels in macrophages has also been demonstrated by Martin and coworkers (1995). The stretch-activated K+ current described in monocyte-derived macrophages also exhibited an outwardly rectifying behavior (Martin et al., 1995). However, in contrast to observations made in monocyte-derived macrophages, substitution of intracellular K+ by NMG+ did not alter stretch-activated currents in microglia. Moreover, stretch-activated currents of monocyte-derived macrophages and of microglia differ also in their pharmacological profile (see below), suggesting the presence of different types of stretch-activated ion channels in these two types of macrophages.
In mammalian cells, a wide variety of stretch-activated ion channels has been described, namely nonselective ion channels, in which current is carried by several cations and channels that are selective to either potassium or chloride ions (for review, see Morris, 1995). Because in microglia reduction of extracellular Cl−ions resulted in a decrease in amplitude and a large shift of the reversal potential of stretch-activated currents to more depolarized potentials, these stretch-activated currents are mainly carried by chloride ions.
Chloride currents of microglia activated slowly with time after stretching of the cell membrane. Presumably, membrane stretch does not directly induce Cl− current activation in microglia, because the currents reached their maximal amplitudes only several minutes after the initial application of the stretch. It is more likely that a mechanical deformation of the cell membrane triggers some internal signals that are required for Cl−channel opening.
Stretch-activated Cl− currents of microglia showed a rundown immediately after reaching their maximal amplitudes. The rundown of the current was prevented by addition of ATP to the intracellular solution. A similar dependence on intracellular ATP has been described for Cl− channels of several cell preparations (for review, see Strange et al., 1996). However, in contrast to observations in NIH/3T3 fibroblasts (Gill et al., 1992) and pancreatic duct cells (Verdon et al., 1995), ATP was not required to induce Cl− current activation in microglia.
At least four different Cl− channel blockers potently inhibited stretch-activated Cl− currents of microglia. NPPB and flufenamic acid inhibited ICl in a time- and voltage-independent manner, whereas DIDS and SITS evoked a time- and voltage-dependent blockade of the current, with the deepest block seen at depolarizing potentials. This finding together with the IC50 values for current blockade are in good agreement with data reported for Cl− currents in T lymphocytes and osteoclasts (Lewis et al., 1993; Steinert and Grissmer, 1997). In contrast to observations made for Cl− currents in oocytes (Ackerman et al., 1994), extracellularly applied La3+ did not influence Cl−currents in microglia and could therefore be used to inhibit voltage-gated outward proton currents of the cells (Eder et al., 1995).
In previous studies, Cl− channels have been detected in rat (Visentin et al., 1995; Schlichter et al., 1996), bovine (McLarnon et al., 1995), and human (McLarnon et al., 1997) microglia. It is difficult to compare properties of Cl− currents described in the present paper with those of bovine and human microglia, because Cl−channels observed in these preparations were measured exclusively in excised patches, whereas corresponding whole-cell currents were not detected (McLarnon et al., 1995, 1997). In agreement, Cl− currents were also not seen in whole-cell measurements of murine microglia when no stretch was applied to the cell membrane. However, McLarnon and coworkers (1995, 1997) reported an inactivation of Cl− currents that became faster with increased cell depolarization, whereas we did not observe any time-dependent inactivation of the currents at test potentials up to +80 mV. Stretch-activated Cl− currents of murine microglia share similarities with Cl− currents that appeared spontaneously or after cell swelling in rat microglia (Visentin et al., 1995; Schlichter et al., 1996). These outwardly rectifying currents also do not show any voltage-dependent gating, and they are sensitive to the Cl− channel blockers NPPB and flufenamic acid. Thus, it might be possible that in microglia the same type of Cl− channels can be activated by osmotic or mechanical stimuli. It has been shown for T lymphocytes that the activation of mini Cl−channels, which was seen in the presence of either hypo-osmotic external or hyperosmotic internal solutions, could also be induced by application of positive pressure to the cell membrane via the patch pipette (Lewis et al., 1993). In contrast, distinct ion channels became activated in chick heart cells by either direct mechanical strain or hypotonic swelling (Hu and Sachs, 1996).
With respect to their kinetic and pharmacological properties, stretch-activated Cl− currents of microglia closely resemble Cl− currents that activated either spontaneously or in response to osmotic stress in other types of immune cells (for review, see Gallin, 1991; DeCoursey and Grinstein, 1998), including monocytes (Kim et al., 1996), monocyte-derived macrophages (Nelson et al., 1990), neutrophils (Stoddard et al., 1993), and lymphocytes (for review, see Garber and Cahalan, 1997).
Functional role of stretch-activated Cl−currents in microglia
In this study we demonstrate an involvement of chloride channels in ramification of microglia. At least four distinct chloride channel blockers reversibly inhibited the transformation of microglia from their ameboid into the ramified shape. Because ramified microglia were not affected by Cl− channel blockers, we conclude that Cl− channels are required for the induction of ramification, but they are less important for maintaining morphology of microglia. Membrane stretch during sprouting of processes might be the trigger for Cl− channel activation in microglia. Moreover, because morphological changes of microglia are accompanied by cytoskeletal reorganization (Abd-El-Basset and Fedoroff, 1995; Ilschner and Brandt, 1996), Cl− channels might also become activated or their rate of activation might be increased during the process of cytoskeletal reorganization. It has been demonstrated that disruption of F-actin leads to Cl− channel activation in astrocytes (Lascola and Kraig, 1996) and astrocytoma cells (Ullrich and Sontheimer, 1997) and potentiates the rate of Cl− channel activation under hypo-osmotic conditions in B lymphocytes (Levitan et al., 1995; Garber and Cahalan, 1997). Further investigations will be required to clarify whether Cl− currents in microglia can be activated or modulated by cytoskeletal disruptive agents.
A functional role of Cl− currents in microglia could also be related to changes in membrane potential. Conceivably, a shift of the resting membrane potential to more positive values near the Cl− reversal potential might be a prerequisite for the induction of ramification in microglia. Membrane depolarization induced by Cl− channel activation could lead to additional activation of other ion channels in microglia, e.g., voltage-gated K+, H+, or Ca2+ channels (for review, see Eder, 1998). It is thus possible that Ca2+ influx via voltage-gated Ca2+ channels triggers Ca2+-dependent intracellular processes necessary for microglial shape changes.
It is also possible that an activation of Cl−channels in microglia is required for triggering tyrosine phosphorylation signaling pathways as has been demonstrated for T lymphocytes (Phipps et al., 1996). It has been shown that tyrosine phosphorylation is induced during the process of ramification in microglia. Thus, herbimycin A can inhibit the induction of ramification (Liu et al., 1994). Furthermore, in the presence of the tyrosine kinase inhibitor genistein, ramified microglia shortened their processes (Tanaka and Maeda, 1996).
For other types of immune cells, an involvement of Cl− channels in regulatory volume decrease has been proven by several researchers (for review, see DeCoursey and Grinstein, 1998). Volume regulatory processes could also be involved in microglial ramification. Thus, cell volume of microglia might be adjusted by chloride efflux via stretch-activated Cl− channels followed by extrusion of water.
It is still unclear why voltage-gated Na+ and K+ channels become upregulated in ramified microglia. It has been shown that in ramified microglia voltage-gated Na+ currents can be effectively blocked by TTX (Korotzer and Cotman, 1992), and voltage-gated outward K+ currents are highly sensitive to CTX and KTX (Eder et al., 1996). However, in the presence of each of these blockers, microglia were also able to ramify, suggesting that neither Na+ nor voltage-gated outward K+currents are required for the regulation of shape changes in microglia. Because voltage-independent Ca2+-activated K+ channels of microglia are inhibited by CTX in a concentration range similar to that of voltage-gated outward K+ channels (Eder et al., 1997b), it is also unlikely that these channels play an important role during processes of ramification in microglia. An investigation of the role of inward rectifier K+ channels and outward H+ channels in microglia during processes of ramification is more difficult, because so far no specific inhibitor exists for each of these channels.
Footnotes
This work was supported by a fellowship of the Alexander von Humboldt Foundation (C.E.) and Grant SFB 507/C3 of the Deutsche Forschungsgemeinschaft (C.E., U.H.). We thank Sieglinde Latta for the excellent preparation of cell cultures and Astrid Düerkop for technical assistance. We are very grateful to Dr. Thomas E. DeCoursey for many helpful discussions and comments on this manuscript.
Correspondence should be addressed to Claudia Eder, Department of Neurophysiology, Institute of Physiology, Humboldt University, Tucholsky Strasse 2, D 10117 Berlin, Germany.
REFERENCES
- 1.Abd-El-Basset E, Fedoroff S. Effect of bacterial wall lipopolysaccharide (LPS) on morphology, motility, and cytoskeletal organization of microglia in cultures. J Neurosci Res. 1995;41:222–237. doi: 10.1002/jnr.490410210. [DOI] [PubMed] [Google Scholar]
- 2.Ackerman MJ, Wickman KD, Clapham DE. Hypotonicity activates a native chloride current in Xenopus oocytes. J Gen Physiol. 1994;103:153–179. doi: 10.1085/jgp.103.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DeCoursey TE, Grinstein S. Ion channels and carriers in leukocytes. Inflammation: basic principles and clinical correlates Gallin JI, Snyderman R, Fearon DT, Haynes BF, Nathan C. 1998. Raven; New York, in press. [Google Scholar]
- 4.Eder C (1998) Ion channels in microglia (brain macrophages). Am J Physiol, in press. [DOI] [PubMed]
- 5.Eder C, Fischer HG, Hadding U, Heinemann U. Properties of voltage-gated currents of microglia developed using macrophage colony-stimulating factor. Pflügers Arch. 1995;430:526–533. doi: 10.1007/BF00373889. [DOI] [PubMed] [Google Scholar]
- 6.Eder C, Klee R, Heinemann U. Blockade of voltage-gated outward K+ currents of ramified murine brain by scorpion peptide toxins. Neurosci Lett. 1996;219:29–32. doi: 10.1016/s0304-3940(96)13164-5. [DOI] [PubMed] [Google Scholar]
- 7.Eder C, Klee R, Heinemann U. Distinct soluble astrocytic factors induce expression of outward K+ currents and ramification of brain macrophages. Neurosci Lett. 1997a;226:147–150. doi: 10.1016/s0304-3940(97)00281-4. [DOI] [PubMed] [Google Scholar]
- 8.Eder C, Klee R, Heinemann U. Pharmacological properties of Ca2+-activated K+ currents of ramified murine brain macrophages. Naunyn Schmiedebergs Arch Pharmacol. 1997b;356:233–239. doi: 10.1007/pl00005046. [DOI] [PubMed] [Google Scholar]
- 9.Fujita H, Tanaka J, Toku K, Tateishi N, Suzuki Y, Matsuda S, Sakanaka M, Maeda N. Effects of GM-CSF and ordinary supplements on the ramification of microglia in culture: a morphometrical study. Glia. 1996;18:269–281. doi: 10.1002/(sici)1098-1136(199612)18:4<269::aid-glia2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 10.Gallin EK. Ion channels in leukocytes. Physiol Rev. 1991;71:775–811. doi: 10.1152/physrev.1991.71.3.775. [DOI] [PubMed] [Google Scholar]
- 11.Garber SS, Cahalan MD. Volume-regulated anion channels and the control of a simple cell behavior. Cell Physiol Biochem. 1997;7:229–241. [Google Scholar]
- 12.Gill DR, Hyde SC, Higgins CF, Valverde MA, Mintenig GM, Sepulveda FV. Separation of drug transport and chloride channel functions of the human multidrug resistance p-glycoprotein. Cell. 1992;71:23–32. doi: 10.1016/0092-8674(92)90263-c. [DOI] [PubMed] [Google Scholar]
- 13.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 14.Hertz L, Juurlink BHJ, Fosmark H, Schousboe A. Astrocytes in primary cultures. In: Pfeiffer SE, editor. Neuroscience approached through cell culture, Vol 1. CRC; Boca Raton, FL: 1982. pp. 175–186. [Google Scholar]
- 15.Hu H, Sachs F. Mechanically activated currents in chick heart cells. J Membr Biol. 1996;154:205–216. doi: 10.1007/s002329900145. [DOI] [PubMed] [Google Scholar]
- 16.Ilschner S, Brandt R. The transition of microglia to a ramified phenotype is associated with the formation of stable acetylated and detyrosinated microtubules. Glia. 1996;18:129–140. doi: 10.1002/(SICI)1098-1136(199610)18:2<129::AID-GLIA5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 17.Kim SY, Silver MR, DeCoursey TE. I. Ion channels in human THP-1 monocytes. J Membr Biol. 1996;152:117–130. doi: 10.1007/s002329900091. [DOI] [PubMed] [Google Scholar]
- 18.Korotzer AR, Cotman CW. Voltage-gated currents expressed by rat microglia in culture. Glia. 1992;6:81–88. doi: 10.1002/glia.440060202. [DOI] [PubMed] [Google Scholar]
- 19.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 20.Lascola CD, Kraig RP. Whole-cell chloride currents in rat astrocytes accompany changes in cell morphology. J Neurosci. 1996;16:2532–2545. doi: 10.1523/JNEUROSCI.16-08-02532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levitan I, Almonte C, Mollard P, Garber SS. Modulation of a volume-regulated chloride current by F-actin. J Membr Biol. 1995;147:283–294. doi: 10.1007/BF00234526. [DOI] [PubMed] [Google Scholar]
- 22.Lewis RS, Ross PE, Cahalan MD. Chloride channels activated by osmotic stress in T lymphocytes. J Gen Physiol. 1993;101:801–826. doi: 10.1085/jgp.101.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling EA. Transformation of monocytes into ameboid microglia and into microglia in the corpus callosum of postnatal rats, as shown by labelling monocytes by carbon particles. J Anat. 1979;128:847–855. [PMC free article] [PubMed] [Google Scholar]
- 24.Ling EA, Wong WC. The origin and nature of ramified and ameboid microglia: a historical review and current concepts. Glia. 1993;7:9–18. doi: 10.1002/glia.440070105. [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Brosnan CF, Dickson DW, Lee SC. Macrophage colony-stimulating factor mediates astrocyte-induced microglial ramification in human fetal central nervous system culture. Am J Pathol. 1994;145:48–53. [PMC free article] [PubMed] [Google Scholar]
- 26.Martin DK, Bootcov MR, Campbell TJ, French PW, Breit SN. Human macrophages contain a stretch-sensitive potassium channel that is activated by adherence and cytokines. J Membr Biol. 1995;147:305–315. doi: 10.1007/BF00234528. [DOI] [PubMed] [Google Scholar]
- 27.McLarnon JG, Sawyer D, Kim SU. Cation and anion unitary ion channel currents in cultured bovine microglia. Brain Res. 1995;693:8–20. doi: 10.1016/0006-8993(95)00664-c. [DOI] [PubMed] [Google Scholar]
- 28.McLarnon JG, Xu R, Lee YB, Kim SU. Ion channels of human microglia in culture. Neuroscience. 1997;78:1217–1228. doi: 10.1016/s0306-4522(96)00680-x. [DOI] [PubMed] [Google Scholar]
- 29.Morris CE. Stretch-sensitive ion channels. In: Sperelakis N, editor. Cell physiology source book. Academic; San Diego: 1995. pp. 483–489. [Google Scholar]
- 30.Nelson DJ, Jow B, Jow F. Whole-cell currents in macrophages. I. Human monocyte-derived macrophages. J Membr Biol. 1990;117:29–44. doi: 10.1007/BF01871563. [DOI] [PubMed] [Google Scholar]
- 31.Phipps DJ, Branch DR, Schlichter LC. Chloride-channel block inhibits T lymphocyte activation and signalling. Cell Signal. 1996;8:141–149. doi: 10.1016/0898-6568(95)02039-x. [DOI] [PubMed] [Google Scholar]
- 32.Schlichter LC, Sakellaropoulos G, Ballyk B, Pennefather PS, Phipps DJ. Properties of K+ and Cl− channels and their involvement in proliferation of rat microglial cells. Glia. 1996;17:225–236. doi: 10.1002/(SICI)1098-1136(199607)17:3<225::AID-GLIA5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 33.Sievers J, Schmidtmayer J, Parwaresch R. Blood monocytes and spleen macrophages differentiate into microglia-like cells when cultured on astrocytes. Anat Anz. 1994;176:45–51. doi: 10.1016/s0940-9602(11)80414-0. [DOI] [PubMed] [Google Scholar]
- 34.Steinert M, Grissmer S. Novel activation stimulus of chloride channels by potassium in human osteoblasts and human leukaemic T lymphocytes. J Physiol (Lond) 1997;500:653–660. doi: 10.1113/jphysiol.1997.sp022050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoddard JS, Steinbach JH, Simchowitz L. Whole cell Cl− currents in human neutrophils induced by cell swelling. Am J Physiol. 1993;265:C156–C165. doi: 10.1152/ajpcell.1993.265.1.C156. [DOI] [PubMed] [Google Scholar]
- 36.Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol. 1996;270:C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- 37.Streit W. The role of microglia in brain injury. Neurotoxicology. 1996;17:671–678. [PubMed] [Google Scholar]
- 38.Tanaka J, Maeda N. Microglial ramification requires nondiffusible factors derived from astrocytes. Exp Neurol. 1996;137:367–375. doi: 10.1006/exnr.1996.0038. [DOI] [PubMed] [Google Scholar]
- 39.Ullrich N, Sontheimer H. Biophysical and pharmacological characterization of chloride currents in human astrocytoma cells. Am J Physiol. 1996;270:C1511–C1521. doi: 10.1152/ajpcell.1996.270.5.C1511. [DOI] [PubMed] [Google Scholar]
- 40.Ullrich N, Sontheimer H. Cell cycle-dependent expression of a glioma-specific chloride current: proposed link to cytoskeletal changes. Am J Physiol. 1997;273:C1290–C1297. doi: 10.1152/ajpcell.1997.273.4.C1290. [DOI] [PubMed] [Google Scholar]
- 41.Ullrich N, Bordey A, Gillespie GY, Sontheimer H. Expression of voltage-activated chloride currents in acute slices of human gliomas. Neuroscience. 1998;83:1161–1173. doi: 10.1016/s0306-4522(97)00456-9. [DOI] [PubMed] [Google Scholar]
- 42.Verdon B, Winpenny JP, Whitfield KJ, Argent BE, Gray MA. Volume-activated chloride currents in pancreatic duct cells. J Membr Biol. 1995;147:173–183. doi: 10.1007/BF00233545. [DOI] [PubMed] [Google Scholar]
- 43.Visentin S, Agresti C, Patrizio M, Levi G. Ion channels in rat microglia and their different sensitivity to lipopolysaccharide and interferon-τ. J Neurosci Res. 1995;42:439–451. doi: 10.1002/jnr.490420402. [DOI] [PubMed] [Google Scholar]