Abstract
The Drosophila FMRFamide gene encodes multiple FMRFamide-related peptides. These peptides are expressed by neurosecretory cells and may be released into the blood to act as neurohormones. We analyzed the effects of eight of these peptides on nerve-stimulated contraction (twitch tension) ofDrosophila larval body-wall muscles. Seven of the peptides strongly enhanced twitch tension, and one of the peptides was inactive. Their targets were distributed widely throughout the somatic musculature. The effects of one peptide, DPKQDFMRFamide, were unchanged after the onset of metamorphosis. The seven active peptides showed similar dose–response curves. Each had a threshold concentration near 1 nm, and the EC50 for each peptide was ∼40 nm. At concentrations <0.1 μm, the responses to each of the seven excitatory peptides followed a time course that matched the fluctuations of the peptide concentration in the bath. At higher concentrations, twitch tension remained elevated for 5–10 min or more after wash-out of the peptide. When the peptides were presented as mixtures predicted by their stoichiometric ratios in the dFMRFamide propeptide, the effects were additive, and there were no detectable higher-order interactions among them. One peptide was tested and found to enhance synaptic transmission. At 0.1 μm, DPKQDFMRFamide increased the amplitude of the excitatory junctional current to 151% of baseline within 3 min. Together, these results indicate that the products of theDrosophila FMRFamide gene function as neurohormones to modulate the strength of contraction at the larval neuromuscular junction. In this role these seven peptides appear to be functionally redundant.
Keywords: Drosophila melanogaster, FMRFamide, neuromuscular junction, NMJ, muscle, neuropeptide, peptide hormone, propeptide, synapse
Many neuropeptides are cosynthesized as a cohort of closely related peptide isoforms that derive from a single propeptide precursor. These peptides include the vertebrate enkephalins (Höllt, 1989), FMRFamide-related peptides (Nambu et al., 1988; Schneider and Taghert, 1988; Greenberg and Price, 1992), myomodulins and buccalins (Lopez et al., 1993; Miller et al., 1993a,b;Kellett et al., 1996), allatostatins (Bendena et al., 1997), and the most extreme example of coordinated neuropeptide synthesis, the 37 related peptides derived from the sea anemone metamorphosin A gene (Leviev and Grimmelikhuijzen, 1995). Often, the specific peptide sequences and their relative positions within related neuropeptide precursors have been preserved over many millions of years of evolution (Taghert and Schneider, 1990; Schinkmann and Li, 1994; Bendena et al., 1997). Thus, related neuropeptides derived from single precursors may perform distinct and important functions, or they may contribute to coordinated signals (Březina and Weiss, 1997). In most cases, however, proof for such functional differences has been elusive.
To explore this issue, we examined the effects of DrosophilaFMRFamide-related peptides on the larval neuromuscular junction (NMJ). The Drosophila larval NMJ is well characterized and highly suited for studies of synapse development and synaptic physiology (Keshishian et al., 1996). Nevertheless, little is known about the effects of neuropeptide modulators or hormones on these synapses. Given the important roles of neuromodulators in the development and function of synapses in other systems (Hökfelt, 1991; Hall and Sanes, 1993), including the insect NMJ (O’Shea et al., 1985), the characterization of modulators at the Drosophila NMJ is necessary for a complete understanding of this system.
In Drosophila larvae the abdominal hemisegments A2–A7 all contain a stereotyped array of 30 muscles (Crossley, 1978; Johansen et al., 1989a,b). The entire motor neuron population is glutamatergic (Jan and Jan, 1976b; Johansen et al., 1989a,b), and subsets of the motor neuron terminals contain putative cotransmitters, including octopamine (Monastirioti et al., 1995), proctolin (Anderson et al., 1988), and peptides similar to insulin (Gorczyca et al., 1993) and pituitary adenylyl cyclase-activating peptide (PACAP) (Zhong and Peña, 1995). The functions of PACAP-like peptides at the NMJ are indicated by electrophysiological evidence (Zhong and Peña, 1995), but the functions of the other putative cotransmitters are unknown. The effects of circulating hormones on the larval NMJ have not been explored.
The Drosophila FMRFamide gene encodes as many as eight closely related neuropeptides (Nambu et al., 1988; Schneider and Taghert, 1988). These peptides may function as neurohormones (O’Brien et al., 1991; Schneider et al., 1991, 1993). Here, we found that several of these neuropeptides were excitatory when applied to a larval nerve–muscle preparation. This effect was mediated, at least in part, by a strong increase in synaptic efficacy. When the excitatory peptides were applied alone or as mixtures, based on their ratios in the dFMRFamide precursor, their effects were comparable. Thus, the various related neuropeptide products of the dFMRFamide propeptide were functionally equivalent at the larval NMJ.
MATERIALS AND METHODS
Animals. Wild-type Drosophila melanogaster (Oregon R, except as indicated below) were raised at 22–24°C on standard media. Third instar larvae were selected on the basis of the morphology of the anterior spiracles (Bodenstein, 1950). Feeding-stage third instar larvae were taken from the food, and wandering-stage third instar larvae were taken from the sides of the food bottle before eversion of the anterior spiracles. The wandering stage was used for all experiments, except where indicated.
Strain gauge apparatus. A semiconductor transducer (AE 801, Akers Electronics, Spånga, Sweden) was used as a strain gauge. The transducer consisted of a silicon beam (0.1 mm thick, 1 × 5 mm) with a diffused 100 Ω resistor mounted on each side; because of fragility the beam was encased to prevent excessive deflection. Nonlinearity of the gauge was negligible (approximately ± 0.25% full scale), and temperature effects were also negligible under the recording conditions that were used (sensitivity shift approximately −0.2% per degree Celsius; temperature variation within experiments <2°C). A sharpened tungsten needle with a bent tip was attached to the silicon beam with cyanoacrylate glue perpendicular to the plane of the beam. Signals were amplified by using a custom-built Wheatstone bridge amplifier (from Dr. Michael Dickinson, University of California, Berkeley); calibration was performed by using pieces of aluminum foil of known mass, based on the following calculations: 1 gm force = 9.807 × 10−3 Newton (N); weight of foil suspended from transducer arm = (foil mass)(acceleration due to gravity, g). The 10,000× analog output from the bridge amplifier was amplified further (200K final amplification) and low-pass-filtered at 20 Hz (Frequency Devices 902). Then the signal was digitized at 100 Hz on a Macintosh IIvx computer with a MacADIOS II/16 A/D daughterboard (GW Instruments, Somerville, MA) with a 64K buffer first-in-first-out (FIFO) for continuous data acquisition. The gain error and nonlinearity of the board were negligible (errors of ± 0.0005% full scale and ± 0.002% full scale, respectively). Recordings were stored and then analyzed off-line with SuperScope II software (GW Instruments).
Peptides. The peptides buccalin A, myomodulin A, Met-enkephalin, and pedal peptide were the generous gifts of Dr. Philip Lloyd (University of Chicago, IL). All other peptides (Fig.1) were synthesized, purified to >70% homogeneity by reverse-phase HPLC, and analyzed by ionspray mass spectrometry by Multiple Peptide Systems (M.P.S., San Diego, CA). The masses supplied by M.P.S. contained ∼75% peptide weight. No correction for the percentage of homogeneity or salt concentration was used in calculating the dose–response curves. Thus, the dose–response curves may underestimate the sensitivity of this assay (i.e., they may be shifted 0.3–0.5 log units to the right), although the magnitude of this error should be similar for each peptide. All peptides were lyophilized and stored at −20°C for several months without a detectable loss of activity. After storage for >1 year the peptides were heated in 5% β-mercaptoethanol and 0.1% trifluoracetic acid at 65°C for 1–2 hr and then lyophilized again. Before recording, the peptides were resuspended in recording saline on ice and used within a few hours.
Fig. 1.
Predicted organization of the Drosophila melanogaster FMRFamide prepropeptide. Left, The deduced dFMRFamide gene product is shown by avertical line with boxes to indicate the locations of several neuropeptide sequences. Center, Possible peptide intermediates were selected on the basis of the fact that each sequence was at least 70% identical to a sequence in a similar position in the Drosophila virilis FMRFamidegene (Taghert and Schneider, 1990). Right, Each of the eight peptides synthesized for this study is bordered by consensus sites for proteolytic cleavage from the precursor (Devi, 1991), contains a putative amidation signal at the C terminus (Bleakman et al., 1988), and contains two or more residues matching the FMRF consensus sequence common to this family of peptides (Greenberg and Price, 1992). *Purified from Drosophila tissue byNichols (1992); **purified from Drosophila tissue byNambu et al. (1988) and Nichols (1992). Hatched box, Signal sequence.
Strain gauge recording. Larvae were dissected in hemolymph-like recording saline [HL3; containing (in mm): 70 NaCl, 5 KCl, 1.5 CaCl2, 20 MgCl2, 10 NaHCO3, 5 trehalose, 115 sucrose, and 5 HEPES, pH 7.1 (Stewart et al., 1994)] by cutting along the dorsal midline and then removing most of the gut, the entire CNS, many of the imaginal disks, the salivary glands, and much of the fat body.
The larval nerve–muscle preparation was prepared as previously described (Jan and Jan, 1976a). Dissected larvae were pinned to a recording chamber at the anterior end only, and the posterior end of the animal was hooked to the tungsten arm of the strain gauge (Fig.2A). A suction electrode was placed on the cut end of one abdominal nerve, usually the left or right abdominal nerve 4 (L or RA4). The nerve was stimulated tonically at 1 Hz (20–40 msec, 2–8 V) to elicit muscle contractions in several of the muscles in one abdominal hemisegment (and occasionally muscles in adjacent segments). The volume of the recording chamber was ∼150 μl. Gravity-fed perfusion of the recording chamber (at a constant rate of 0.5–1.0 ml/min) was maintained throughout each recording, and the bath volume was maintained by a gravity- or vacuum-fed aspirator. In some preparations this perfusion method set up small, slow oscillations in the bath volume. Although these oscillations were almost imperceptible by eye, they were detected readily by the strain gauge, and they resulted in slow oscillations in baseline tension. These oscillations did not alter the amplitudes of individual nerve-stimulated contractions (see Fig. 5, small arrows).
Fig. 2.
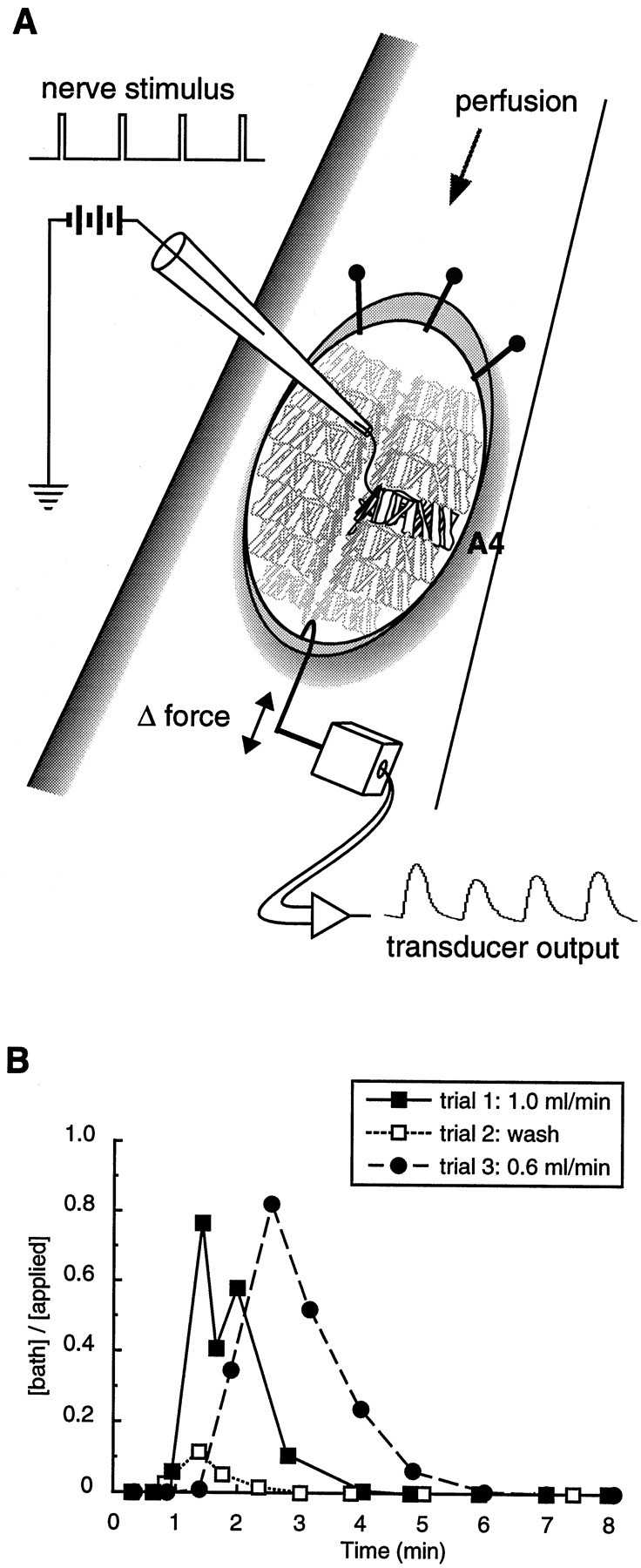
Schematic diagram of the neuromuscular preparation. A, After removal of the gut and CNS, the anterior of each larva was pinned to the recording dish while the posterior was tethered to the arm of a calibrated strain gauge. Stimulation of an abdominal nerve with a suction electrode (2–6 V, 25–35 msec, 1 Hz) elicited contractions in the muscles of one abdominal half-segment (black muscles). Occasionally, the nerve stimulus also elicited contractions in muscles of adjacent segments (nearest of the muscles shown ingray). B, Changes in the bath dye concentration/applied dye concentration as a function of time. Three successive trials were performed. In trials 1 and3, the dye (fast green) was applied to the recording chamber at time = 0 min. In trial 2, 1 ml of saline was used as a wash. The perfusion rate in trials 1 and2 (1.0 ml/min) was equal to the fastest rate used in the contraction assays. The perfusion rate in trial 3 (0.6 ml/min) was close to the slowest rate used in the assays.
Fig. 5.
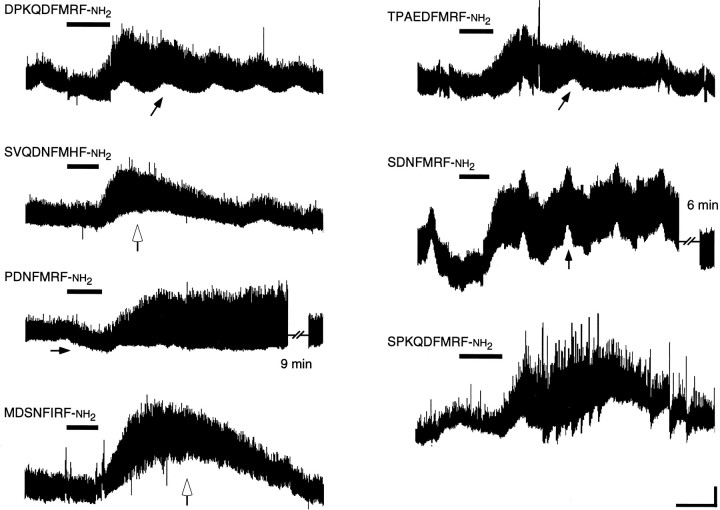
The excitatory effects of each of the seven active peptides are slow to develop and are long-lasting; the time courses of the responses are similar. Peptides (each at 10-7m) were applied to the preparations during the periods indicated by the horizontal bars. The illustrated differences in kinetics reflect the differences among different preparations rather than consistent differences among the effects of the different peptides. Baseline shifts such as those indicated (small arrows) are attributable to subtle changes in the bath volume and have no detectable effect on contraction amplitudes. Slower baseline shifts in some preparations accompanied peptide-mediated increases in nerve-stimulated contractions (open arrows), particularly at higher peptide concentrations. These shifts are attributable to a decrease in the relaxation rate, which causes summation (an apparent increase in baseline tension). In control preparations in which the frequency of nerve stimulation is varied, this summation does not alter the amplitudes of individual contractions. Calibration: 200 pN, 100 sec.
Recordings were initiated within ∼15 min or fewer of the start of the dissection, and the first application of peptide was at least 9 min after the beginning of the recording. Peptide solutions were applied by exchange with the recording saline. The duration of each peptide application was generally 1.1–1.8 min, depending on the flow rate, and the volume was 1 ml. The minimum interval between peptide applications was 9 min; this interval was increased to 15–25 min after the highest peptide concentrations to allow time for the nerve-stimulated muscle contractions (twitch tension) to return to baseline. Although most recordings lasted 2–2.5 hr, the randomized samples tested in Figure8D were presented within a 1–1.5 hr recording period.
Fig. 8.
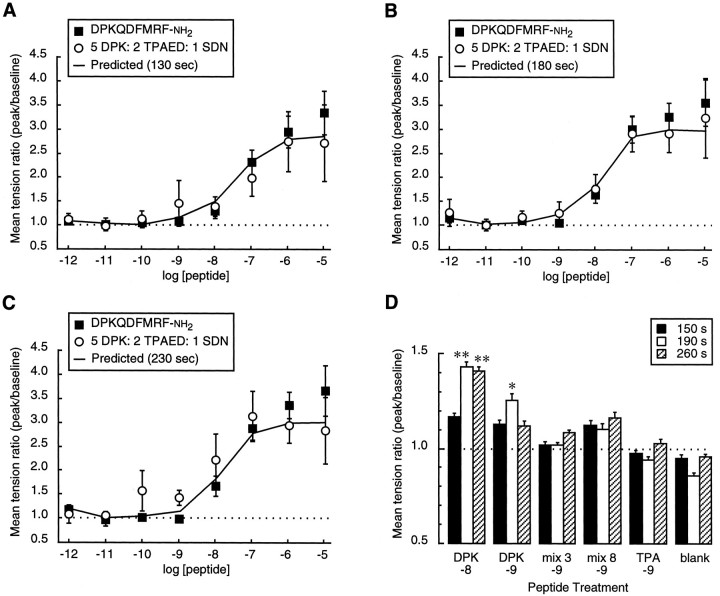
The effects of stoichiometric molar ratios of dFMRFamide-related peptides are additive. A–C, Mean tension ratio ± SEM is shown as a function of total peptide concentration, using a peptide mixture (“mix 3”) containing DPKQDFMRFamide, TPAEDFMRFamide, and SDNFMRFamide in a 5:2:1 molar ratio (“mix 3,” n = 10; DPKQDFMRFamide,n = 7). The predicted curve (solid line) was generated by averaging the results for DPKQDFMRFamide, TPAEDFMRFamide, and SDNFMRFamide in the same 5:2:1 ratio (data from the same recordings as in Fig. 6). Peak twitch tension measurements for “mix 3,” DPKQDFMRFamide, and the predicted curve were taken at 130 sec (A), 180 sec (B), or 230 sec (C) after the onset of peptide application. D, Mean tension ratio ± SEM measured at 150, 190, and 260 sec after the onset of peptide application. The six treatments were used in a double-blind experiment in a randomized order (n = 14 for all treatments). The differences in tension measured at 150, 190, and 260 sec with the blank control were not statistically significant (one-way ANOVA). DPK −8, 10-8mDPKQDFMRFamide; DPK −9, 10-9m DPKQDFMRFamide; mix 3 −9, 10-9m, 3 peptide mix in 5:2:1 ratio;mix 8 −9, 10-9m, 8 peptide mix in 5:2:1:1:1:1:1:1 ratio; TPA −9, 10-9m TPAEDFMRFamide;blank, saline control. *p < 0.05; **p < 0.01 (Spjotvoll–Stoline) [data inD, one-way ANOVA: 190 sec,F(5,78) = 5.091, p = 0.0004; 260 sec, F(5,78) = 3.637,p = 0.0052].
Dose–response curves were generated by applying the entire range of peptide concentrations to each preparation in the order of increasing concentration; thus the n represents the number of preparations used. In a small number of preparations the individual data points were omitted because of identifiable experimental errors such as the interruption of the perfusion. With few exceptions the FMRFamide-related peptides were applied at concentrations ranging from 10-12 to 10-5m at log unit intervals. The peptide applications were performed in this increasing order to minimize the potential effects of previous peptide applications on a given response. Such errors would result either because of peptide remaining in dead volume in the perfusion apparatus (equivalent to the application of peptide at 10% of the concentration of the preceding dose; see Fig. 2B) or because of long-term effects of peptides on the preparation. In control experiments we assayed eight repeated applications of 10-7m DPKQDFMRFamide rather than eight applications of the peptide at increasing concentrations (from 10-12 to 10-5m); the “dose–response curve” was flat at first, and then the slope of the curve displayed a small positive trend over the last four 10-7m peptide applications. However, this trend was not significant (n = 8; one-way ANOVA). In most preparations the baseline twitch tension amplitude declined rapidly during the first 5–10 min of recording and then declined at a slower, steady rate of <1% per minute after 10–20 min. Care was taken to apply each of the peptides at comparable baseline amplitudes, and the responses to the active peptides were not dependent on either the baseline twitch tension amplitude or the age of the recording, at least within the ranges used in these experiments (data not shown).
In a control experiment that used DPKQDFMRF-OH, buccalin A, myomodulin A, Met-enkephalin, and pedal peptide, each was applied at a single concentration (10-6 or 10-5m) in a random order. Likewise, the experiment shown in Figure 8D was performed double-blind, and the different peptides and peptide mixtures were applied in a random order.
During the contraction assays the peptides were applied to the preparation by switching perfusion sources (for 1 min) from the saline reservoir to a reservoir containing the saline plus peptide solution. There was a delay between the switch to a given peptide solution and exposure of the preparation to the peptide because of the saline present in the tubing (dead volume) between the reservoir and the preparation. To measure this delay and to examine the time course of the fluctuations of the peptide concentration in the bath, we performed control experiments with the dye fast green (Sigma, St. Louis, MO). We applied a 0.1% solution of fast green to the empty recording chamber, sampled from the chamber at regular intervals, and calculated the dye concentration in each sample by comparing its absorbance (at 614 nm) to a standard curve (Fig. 2B).
These control experiments demonstrated that the dye (peptide) first contacted the preparation after a 45–90 sec delay (Fig.2B). This latency was dependent on the perfusion flow rate, which varied according to the height of the saline reservoir. At the fastest rate used in the contraction assays (1 ml/min), the dye concentration in the bath reached 1% of the applied concentration at 45 sec and 10% of the applied concentration at 60 sec. At a flow rate near the slowest rate used in the contraction assays (0.6 ml/min), the dye concentration in the bath reached 1% of the applied concentration at 80 sec and 10% of the applied concentration at 90 sec. At both flow rate extremes the peak dye concentration in the recording chamber was ∼80% of the applied dye concentration. Thus, the assay underestimated the effects of applied peptides (i.e., the dose–response curves were shifted 0.1 log unit to the right—this was in addition to the 0.3–0.5 log unit shift noted above). Because this underestimate was not dependent on the flow rate (Fig.2B), within the range of flow rates that were used, the error was equal for all experiments.
The peak responses to each of the peptides and peptide mixtures were broad and poorly defined (see Fig. 5). Therefore, the peak response to each peptide occurred within a range of times (after the onset of peptide application) within which repeated measurements of twitch tension were not significantly different from each other. For most of the peptides (and peptide mixtures) this range was 130–260 sec, and the peak twitch tension amplitude for each of these peptides was measured at times near the middle of this range. For SDNFMRFamide the perfusion flow rate was faster than average, and the peak response ranged from 100 to 180 sec. Therefore, the peak twitch tension amplitude for SDNFMRFamide was measured at 120 sec. Importantly, the magnitudes of the responses to each of the different peptides were not correlated with flow rate (data not shown). Thus, the tension ratios obtained in different experiments could be compared despite differences in perfusion flow rates among individual trials.
Off-line analysis. The change in twitch tension (“tension ratio”) after a single peptide application was calculated as the mean contraction amplitude of five contractions at the time of peak response, divided by the mean contraction amplitude of 10 baseline contractions (five contractions 30 sec before peptide application and five contractions immediately before peptide application). This method corrected for changes in the baseline twitch tension between successive peptide applications and among preparations.
Voltage-clamp recording. The larval fillet preparation and voltage-clamp analysis were performed as described previously (Wu et al., 1978; Zhong and Wu, 1991; Stewart et al., 1994). In brief, third instar larvae (wild-type strain 2202u) were dissected in Ca2+-free HL3 saline, and recordings were performed in HL3 saline with 1 mm CaCl2. The voltage electrode was filled with 2.5 m KCl, and the current electrode was filled with a 2:1 mixture of 2.5 m KCl/2m potassium citrate. The resistances of the voltage electrodes and current injection electrodes were ∼25 and 10 MΩ, respectively. Recordings were made from muscle fiber 6, and the muscle was clamped at −80 mV to prevent the activation of voltage-gated ion channels (Zhong and Wu, 1991). Excitatory junctional currents (EJCs) were evoked by electrical stimulation of the abdominal nerve (0.02 Hz), and 0.1 μm DPKQDFMRFamide was applied to each preparation by removing 50% of the bath saline and adding an equal volume of 0.2 μm DPKQDFMRFamide.
Statistics. To satisfy the assumptions of the ANOVA, we log10-transformed the data before statistical analysis (Sokal and Rohlf, 1987). Before transformation the data were skewed (not shown), and the variances increased as a function of the mean (see Fig. 6). After transformation the data were verified to be normal by rankit plot (Bliss, 1967), and the variances were determined to be homoscedastic by using the Fmax test (Sokal and Rohlf, 1987). One-way ANOVAs and post hoc tests (Spjotvoll–Stoline) were performed with SuperANOVA (Abacus Concepts, Berkeley, CA). The data shown in the figures were plotted before transformation.
Fig. 6.
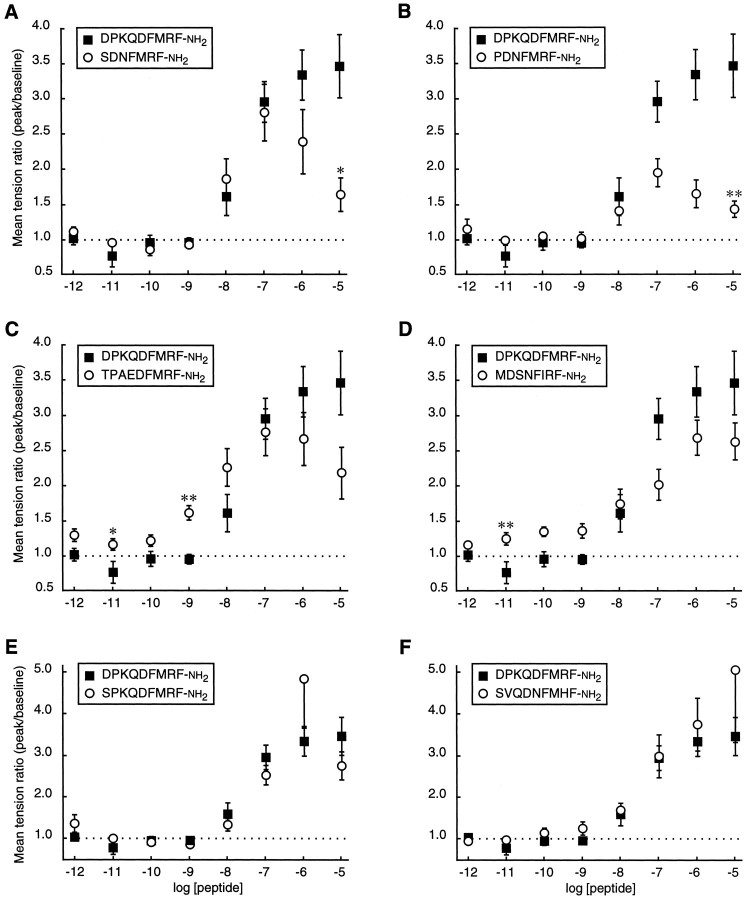
Mean tension ratio ± SEM as a function of peptide concentration for each of the predicted excitatory products of the FMRFamide gene (wandering third instar larvae). For clarity, the results were plotted in six sets, and the dose–response curve for DPKQDFMRFamide (see Fig. 3B,n = 7) was included in each set for reference. However, at each concentration a single ANOVA was performed on the complete data set, containing the results for all seven active peptides. Individual means were compared by post hoctesting subsequent to the ANOVA. *p < 0.05; **p < 0.01 (Spjotvoll–Stoline) [one-way ANOVA; 10-11m,F(6,55) = 3.164, p = 0.0097; 10-10m,F(6,55) = 4.810, p = 0.0005; 10-9m,F(6,55) = 7.896, p = 0.0001; 10-6m,F(6,55) = 3.319, p = 0.0073; 10-5m,F(6,55) = 7.353, p = 0.0001]. n = 10, SPKQDFMRFamide, SDNFMRFamide, and TPAEDFMRFamide; n ≥ 9, PDNFMRFamide;n ≥ 7, SVQDNFMHFamide; n ≥ 8, MDSNFIRFamide.
RESULTS
DPKQDFMRFamide is excitatory at the larval neuromuscular junction
The dFMRFamide gene is strongly expressed in neuroendocrine cells (O’Brien et al., 1991; Schneider et al., 1991,1993). In addition, there is a small number of motor neurons that appear to weakly express the FMRFamide gene (R. Benveniste and P. Taghert, personal communication). Thus, the products of the Drosophila FMRFamide gene have access to peripheral targets, including the skeletal muscles.
We used a sensitive strain gauge (Fig. 2A) to measure the muscle responses to eight synthetic peptides that either were known or were predicted to be products of the dFMRFamide gene (see Fig. 1). We first examined the effects of DPKQDFMRFamide in the muscle assay because this peptide is present in five copies in the dFMRFamide precursor (Nambu et al., 1988; Schneider and Taghert, 1988), has been purified from extracts of Drosophila tissue independently by two laboratories (Nambu et al., 1988; Nichols, 1992), and may be present in the neuroendocrine endings of the Tv cells (Nichols et al., 1995a). This peptide was excitatory. Bath application of 0.1 μm DPKQDFMRFamide for 105 sec caused a pronounced enhancement of nerve-stimulated muscle contraction (twitch tension; Fig. 3A). On average, the enhancement began ∼90 sec after the onset of peptide application and peaked after ∼3.6 min, and the contractions returned to baseline only after 10–15 min had elapsed (n = 13). These kinetics were mainly a function of the dynamics of the peptide concentration in the recording chamber (Figs. 2B, 3A; see below). The threshold concentration was 10 nm, and the dose–response curve reached a plateau at 1 μm (Fig.3B). Given the evidence for the neuroendocrine release of DPKQDFMRFamide (O’Brien et al., 1991; Schneider et al., 1991, 1993), these results suggest that DPKQDFMRFamide enhances body-wall muscle contractions in vivo.
Fig. 3.

DPKQDFMRFamide, the most abundant sequence in theDrosophila FMRFamide gene, enhances nerve-stimulated contractions of abdominal muscles. A, Recording of muscle contractions in a wandering third instar larva; 100 nm DPKQDFMRFamide was applied to the preparation during the period indicated by the shaded rectangle at a perfusion rate of 0.6 ml/min. Periods indicated by the black squares are shown at an expanded time scaleabove. The calculated changes in bath peptide concentration (derived from trial 3, Fig.2B) are plotted below at the same time scale. Before peptide application (baseline), each stimulation of the nerve elicited weak contractions in 5–10 of the 30 muscle fibers in one abdominal half-segment. At the time of peak response (peak), peak tension was increased by 456%. This increase in tension was accompanied by an increase in the number of strongly contracting fibers. B, Mean tension ratio ± SEM as a function of DPKQDFMRFamide (n = 7) or DPKQDFMRF-OH (free acid; n = 5) concentration in third instar larvae. Tension ratio = peak tension (the mean amplitude of five contractions 230 sec after the beginning of a given peptide application)/baseline tension (the mean amplitude of 10 contractions before peptide application—see Materials and Methods). **p < 0.01 (Student’s t test). Calibration: Top traces, 0.2 nN, 1 sec; bottom trace, 0.26 nN, 40 sec.
The excitatory effects of DPKQDFMRFamide in this assay were sequence-specific, and three unrelated peptides failed to elicit an excitatory response (Table 1). DPKQDFMRFamide was isolated from D. melanogaster tissue extracts as an amidated peptide (Nambu et al., 1988; Nichols, 1992). This C-terminal amide group was critical for normal activity in the contraction assay (Fig. 3B; Student’s t test,p = 0.0017), and the peptide DPKQDFMRF-OH was completely inactive when it was tested at 10 μm. A similar strong requirement for amidation has been observed for other FMRFamide-related peptides (Payza, 1987; Wang et al., 1995). The responses to DPKQDFMRFamide were not altered by the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX; Sigma) at a concentration of 10 μm (data not shown), suggesting that this response did not involve the action of cyclic nucleotides. A similar insensitivity to IBMX was observed for the effects of FMRFamide on the extensor tibiae muscle of the locust (Evans and Myers, 1986b).
Table 1.
Effects of non-FMRFamide-related peptides on nerve-stimulated muscle contraction
| Peptide (10−6m) | Sequence | n | Mean tension Ratio ± SEM | Group letters (p < 0.05) |
|---|---|---|---|---|
| Myomodulin A (Aplysia) | PMSMLRLamide | 3 | 0.30 ± 0.09 | A |
| Met-enkephalin | YGGFM | 5 | 0.71 ± 0.14 | A B |
| Buccalin A (Aplysia) | GMDSLAPSGGLamide | 5 | 0.80 ± 0.11 | B |
| Pedal peptide (Aplysia) | PLDSVYGTHGMSGFA | 4 | 1.19 ± 0.11 | B C |
| Drosophilia FMRFamide | DPKQDFMRFamide | 7 | 2.03 ± 0.25 | C |
Means with the same group letters are not significantly different (Spjotvoll–Stoline, one-way ANOVA, F(4,19) = 13.581; p = 0.0001).
Distribution of responsive muscles
Two observations indicate that the neuromuscular targets for DPKQDFMRFamide and the other predicted products of thedFMRFamide gene are distributed widely throughout the larval body-wall musculature. First, on the basis of visual inspection, DPKQDFMRFamide and the other predicted dFMRFamide-related peptides enhanced contractions in muscles located throughout the stimulated hemisegment. Because of the distribution of various muscle attachment points and the fact that many of the muscles are at oblique angles to each other, we could discern the effects of DPKQDFMRFamide on contractions in groups of external and internal muscles located along the entire dorsoventral axis. With the nerve stimulus that was used in these experiments, the contractions before adding DPKQDFMRFamide were visible but weak, and there were only small movements of the external cuticle. Weak contractions detected in the strain gauge recordings could be reliably observed visually, and vice versa. After the addition of ≥0.1 μm peptide, the contractions became very robust, and many of the muscles shortened to ∼50% of their unstimulated length with each twitch. With each muscle “group,” defined as adjacent muscles with similar points of attachment (such as muscles 6, 7, 12, and 13), we could not distinguish readily between active contractions or passive movements in individual muscles. Therefore, the specific muscle targets of DPKQDFMRFamide and the other dFMRFamide-related peptides were not defined.
The second observation in support of distributed targets was that high concentrations (≥1 μm) of several of the predicted dFMRFamide-related peptides often elicited spontaneous and irregular contractions that were not correlated with nerve stimulation. These spontaneous contractions were observed visually in muscles located in A4 (the stimulated segment) and throughout other thoracic and abdominal segments. They could be identified in the strain gauge recordings as twitch contractions that were not correlated with nerve stimulation and that were often of different magnitude from the adjacent nerve-stimulated events. Importantly, the nerves supplying most of the spontaneously contracting muscles did not receive direct electrical stimulation. Thus, the products of the dFMRFamide gene appeared to excite numerous targets located throughout the larval body-wall musculature.
Constancy in the muscle response to DPKQDFMRFamide at the onset of metamorphosis
The onset of metamorphosis in Drosophila is marked by a behavioral transition from the “feeding stage” to the “wandering stage” late during the third larval instar. Before this transition the feeding-stage larvae engage in relatively brief periods of locomotory behavior that are interrupted frequently by feeding behavior (Sokolowski, 1980). At the onset of the wandering stage the larvae cease feeding, leave the food source, and begin a period of constant locomotion. Wandering behavior continues until just before eversion of the anterior spiracles (Bainbridge and Bownes, 1981).
We used wandering-stage larvae in this study because their large size facilitated physiological recording with the strain gauge. However, the behavioral transition that marks the onset of the wandering stage may include changes in the actions of modulatory hormones and transmitters, including the dFMRFamide-related peptides. Such changes in the effects of these peptides could increase the variability of the assay, making quantitative analysis difficult. Importantly, the effects of DPKQDFMRFamide on twitch tension did not change after the transition to the wandering stage (Fig. 4; one-way ANOVA). The threshold concentration at each stage was 10 nm, and the EC50 at each stage was 25 nm. Therefore, the behavioral changes that occur at the onset of metamorphosis do not appear to involve changes in the effects of DPKQDFMRFamide at the neuromuscular junction.
Fig. 4.
The effects of DPKQDFMRFamide on muscle contraction do not change during progression from the feeding to the wandering larval stage. Dose–response curves (mean ± SEM) for DPKQDFMRFamide are plotted separately for feeding-stage (n ≥ 5) and wandering-stage (n= 7) larvae (wandering-stage data the same as shown in Fig.3B).
Experiment A: Effects of other dFMRFamide-related peptides
The Drosophila FMRFamide gene encodes several FMRFamide-related peptides. These peptides (some known, some predicted) are seven to nine amino acids in length, and each contains a near or complete match with the FMRFamide C-terminal consensus sequence characteristic of this peptide family. In contrast, these peptides display considerable sequence diversity in the Nterminal three to five amino acids.
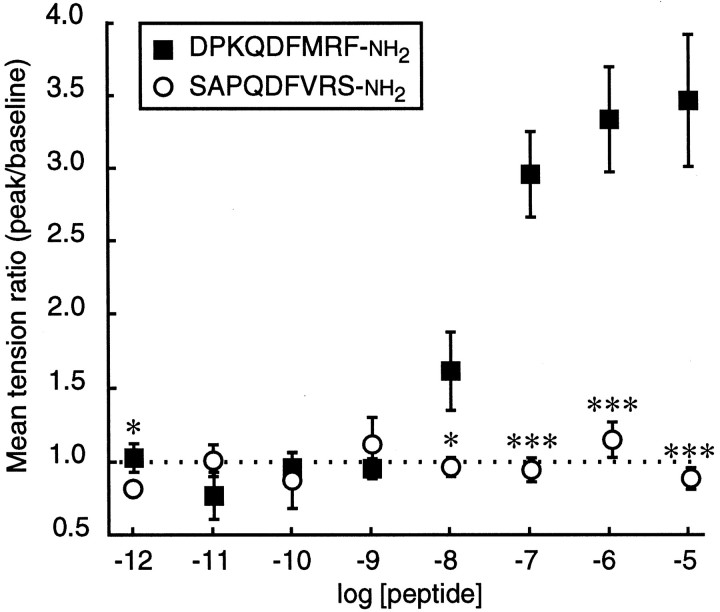
To assess whether the peptide sequence diversity results in a diversity of peptide functions, we compared the effects of eightDrosophila FMRFamide-related peptides on the muscles of wandering-stage larvae. Nerve-stimulated muscle contraction was enhanced by seven of the eight peptides (DPKQDFMRFamide, SPKQDFMRFamide, SDNFMRFamide, PDNFMRFamide, SVQDNFMHFamide, TPAEDFMRFamide, and MDSNFIRFamide; Fig.5). When the preparations were observed visually, each of the seven active peptides enhanced contractions in muscles located throughout segment A4, and there were no detectable peptide-dependent differences in the pattern of affected muscles. The dose–response curves for the seven active peptides also were similar (Fig. 6). For most of the active peptides the threshold excitatory concentration was 10 nm, the EC50 was ∼40 nm, the dose–response curve peaked or reached a plateau at 0.1–1 μm, and the dose–response curves displayed a negative slope at higher concentrations. Only a single peptide, SAPQDFVRSamide, displayed no detectable response (Fig. 7).
Fig. 7.
SAPQDFVRSamide did not enhance twitch tension. The mean tension ratio ± SEM (n = 6) at each concentration was measured at 230 sec (the average time to peak for DPKQDFMRFamide). The data for DPKQDFMRFamide are taken from Figure3B (n = 7). *p< 0.05; **p < 0.01; ***p < 0.001 [one-way ANOVA: 10-12m,F(1,11) = 6.329, p = 0.0287; 10-8m,F(1,11) = 9.444, p = 0.0106; 10-7m,F(1,11) = 72.601, p = 0.0001; 10-6m,F(1,11) = 37.506, p = 0.0001; 10-5m,F(1,11) = 72.170, p = 0.0001].
There were a few notable differences in the effects of the seven active peptides. The threshold excitatory concentration for five of the seven peptides was ∼10 nm (see Fig. 6). The remaining two peptides, MDSNFIRFamide and TPAEDFMRFamide, displayed threshold concentrations in the range of 1 nm. At 1 nm, the difference between the effects of TPAEDFMRFamide and DPKQDFMRFamide was statistically significant (p < 0.01; Spjotvoll–Stoline). The EC50 for TPAEDFMRFamide was 30 nm, or ∼10-fold lower than the other six active peptides. At 0.01 nm, TPAEDFMRFamide and MDSNFIRFamide both elicited a small excitatory response (see Fig. 6). These results suggest that TPAEDFMRFamide and MDSNFIRFamide enhance nerve-stimulated muscle contraction at lower concentrations than do the other five peptides. However, in a different experiment (Fig.8D), 1 nmTPAEDFMRFamide failed to elicit a detectable response. This apparent inconsistency likely is attributable to differences in experimental design (see below).
In addition to variations in threshold concentration, the effects of some of the active peptides also were distinct at concentrations ≥1 μm. At 1 μm, the response to SPKQDFMRFamide was greater than the response to PDNFMRFamide (p< 0.01; Spjotvoll–Stoline). At 10 μm, the responses to DPKQDFMRFamide and SVQDNFMHFamide were greater than the response to PDNFMRFamide (p < 0.01), and the responses to three additional pairs of peptides (DPKQDFMRFamide vs SDNFMRFamide, SPKQDFMRFamide vs PDNFMRFamide, and TPAEDFMRFamide vs SVQDNFMHFamide) were different at a significance level ofp < 0.05 (Spjotvoll–Stoline). However, these differences may not be functionally relevant, because it is likely that ≥1 μm concentrations of FMRFamide-related peptides are well beyond the normal physiological range in vivo (see Discussion).
Kinetics of the responses to dFMRFamide-related peptides
To examine the time course of the fluctuations of the peptide concentration in the bath, we performed control experiments with the dye fast green (see Fig. 2B and Materials and Methods). The latencies for the appearance of dye in the recording chamber and for the excitatory effects of dFMRFamide-related peptides on twitch tension were comparable at equal flow rates (see Figs.3A, 5). Therefore, the responses to the dFMRFamide-related peptides were first detectable within only a few seconds after the appearance of the peptide in the recording chamber. Similar, rapid-onset effects of several neuropeptides, including FMRFamide, have been observed in other systems (Jan and Jan, 1983; Norris and Calabrese, 1987; Shi and Belardetti, 1991; Březina et al., 1994a,b).
The times to peak for the responses to the dFMRFamide-related peptides were shortened by faster perfusion rates. In the control experiments with fast green (see Fig. 2B) the dye concentration peaked in the bath at 85 sec at the faster flow rate (1 ml/min) and peaked at 150 sec at the slower flow rate (0.6 ml/min). The twitch tension ratios obtained after the application of dFMRFamide-related peptides peaked later (see Figs. 3A, 5). At flow rates ≥0.9 ml/min, the time-to-peak response to the seven active dFMRFamide-related peptides was 176 ± 14 sec at 10 nm(n = 11; range 88–247 sec) and was 168 ± 19 sec at 10 μm (n = 16; range 63–285 sec). At flow rates ≤0.6 ml/min, the time-to-peak response was 262 ± 26 sec at 10 nm (n = 7; range 172–345 sec) and was 249 ± 32 sec at 10 μm (n = 13; range 105–466 sec). Thus, the excitatory effects of the dFMRFamide-related pep- tides on twitch tension peaked at ∼100 sec after the peptide concentration reached peak levels in the bath, and this delay was only weakly dependent on the perfusion flow rate (90 sec at ≥0.9 ml/min vs 105 sec at ≤0.6 ml/min). Responses to each peptide returned to baseline after a further 5–10 min; however, the responses to any of the peptides could at times last >30 min, particularly at high peptide concentrations (≥1 μm).
There was considerable variation among preparations in the responses to the same peptide. For example, we observed slow shifts in baseline tension (see Fig. 5, open arrows) or erratic twitch amplitudes (see Fig. 5, SPKQDFMRFamide) in some, but not all, preparations. This variation reflected differences among responses of different preparations and not reproducible effects of the distinct peptides. When the above flow rate-dependent variables were considered, there was no clear difference in the kinetics of the responses elicited by the seven active dFMRFamide-related peptides. Taken together with the dose–response data, these results suggest that the predicted products of the dFMRFamide gene (excluding SAPQDFVRSamide) elicited similar responses when applied alone to the larval neuromuscular junction.
Experiment B: Additive effects of dFMRFamide-related peptides
The results in the previous section show that the sequence diversity among the peptides derived from the dFMRFamidegene does not translate into clear functional diversity in the larval muscle assay. These experiments tested the effects of peptides applied separately, but it is likely that, in vivo, normal targets are exposed to multiple coreleased dFMRFamide peptides. To test for combinatorial or competitive interactions such as synergism or squelching, we examined the effects of peptide mixtures on nerve-stimulated muscle contraction.
We created two peptide mixtures in stoichiometric molar ratios that matched their predicted frequencies in the dFMRFamide propeptide (Nambu et al., 1988; Schneider and Taghert, 1988) and that matched their relative abundance in extracts of adult Drosophila (Nambu et al., 1988; Nichols, 1992). The first, “mix 3,” contained the three dFMRFamide peptides that have been purified from tissue: DPKQDFMRFamide, TPAEDFMRFamide, and SDNFMRFamide in a 5:2:1 molar ratio. To control for potential differences in the time to peak for different peptides or for the peptide mixture, we measured responses at 130, 180, and 230 sec. The dose–response curve generated with the mix 3 cocktail of peptides was similar to (1) the curves obtained with each of the three component peptides alone (see Fig. 6) and (2) the predicted curve obtained by taking the means of the responses to each of the three peptides according to the same 5:2:1 ratio (Fig.8A–C, solid lines). The threshold concentration for each curve was 1–10 nm, and each curve reached a plateau at 1–10 μm. The dose–response curve obtained with the mix 3 cocktail was not significantly different from the dose–response curve obtained with DPKQDFMRFamide at any of the three measurement times (Fig. 8A–C; one-way ANOVA). Furthermore, at each measurement time the dose–response curves for the mix 3 cocktail and DPKQDFMRFamide both overlapped the predicted dose–response curve (Fig. 8A–C). Also, the kinetics of the responses to each of the peptide treatments were similar (data not shown). Thus, we observed no evidence for synergism or squelching, and, further, the effects the mix 3 cocktail and DPKQDFMRFamide were indistinguishable in this assay.
The above observations suggest that mixtures of the dFMRFamide-related peptides elicit responses that are predicted accurately by simple summation of the effects of the individual peptide components. To test this hypothesis further, we applied single peptides and two peptide mixtures in the muscle assay at, or just below, the expected threshold peptide concentration. Because the peptide effects at these low concentrations were often difficult to discern from the background noise, we again measured the response at three times after the onset of peptide application (150, 190, and 260 sec); the five different peptide treatments and saline control were applied in random order and analyzed double-blind.
As expected (see Fig. 3B), 10 nm DPKQDFMRFamide enhanced nerve-stimulated muscle contraction by ∼50% (at 190–260 sec) as compared with the saline control (p < 0.01; Spjotvoll–Stoline; Fig. 8D). At 1 nm, DPKQDFMRFamide elicited a small but significant response at 190 sec only (p < 0.05; Spjotvoll–Stoline). In contrast, the responses to 1 nmconcentrations of TPAEDFMRFamide, the mix 3 cocktail, and the mix 8 cocktail (five copies of DPKQDFMRFamide; two copies of TPAEDFMRFamide; one copy of each of the remaining six peptides) were not significantly different from the saline control (Fig. 8D). Taken together, these results suggest that the FMRFamide-related peptides behave additively, not combinatorially, when applied as mixtures to the neuromuscular junction.
In contrast to these results, TPAEDFMRFamide elicited a significant response at 1 nm in a separate experiment (Experiment A; see Fig. 6C). As noted above, this difference in the effects of 1 nm TPAEDFMRFamide may be attributable to differences in experimental design. In Experiment A (Fig. 6), each preparation was exposed to eight repeated applications of a single peptide in the order of increasing concentration; cumulative effects of two of the peptides (TPAEDFMRFamide and MDSNFIRFamide) near the threshold concentration may have been observed. In Experiment B, each preparation was exposed to three of the six different peptide treatments (Fig.8D; each near the respective threshold concentration) that were presented in a random order. Thus, experiments A and B differed in both the total number of peptide applications and the duration of recording. Further, the randomization procedure in Experiment B may have obscured cumulative peptide effects, particularly if these cumulative effects either were elicited by a subset of the peptides or were dependent on the age of the preparation.
Cumulative effects were not observed with the mix 3 peptide cocktail, which contained 62.5% DPKQDFMRFamide and only 25% TPAEDFMRFamide (Fig. 8A–C); the dose–response curves obtained with the mix 3 cocktail and with DPKQDFMRFamide alone were the same. The effects of TPAEDFMRFamide at lower doses (applied alone at <10 nm; see Fig. 6) were obscured when DPKQDFMRFamide was the major component of the mixture. DPKQDFMRFamide is probably the most abundant FMRFamide-immunoreactive product of the dFMRFamidegene (Nambu et al., 1988), and the effects of this peptide are likely to predominate in vivo as well. Thus, the seven active FMRFamide-related peptides are functionally equivalent at theDrosophila larval neuromuscular junction.
Effects of a FLRFamide-related peptide on muscle contraction
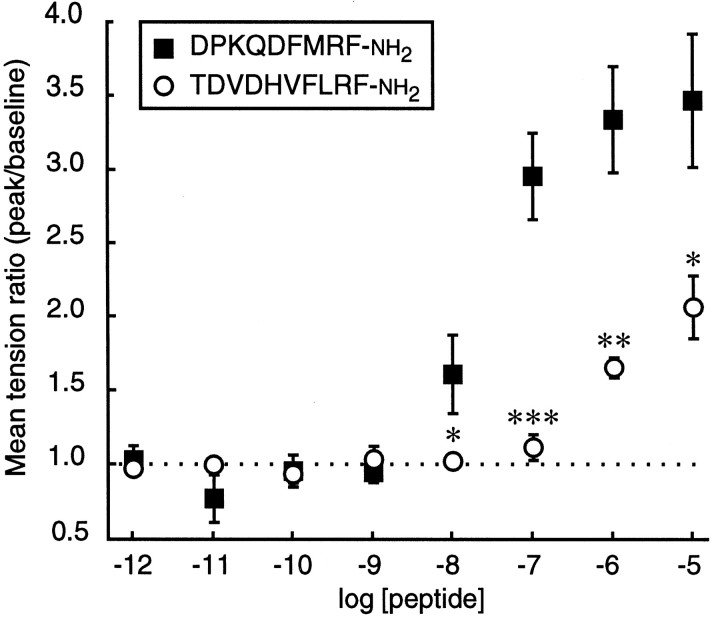
The results described above show that peptides with the C-terminal sequence F-M/I-R-Famide can enhance skeletal muscle contraction inDrosophila larvae despite considerable N-terminal sequence variation. In addition to these peptides, there are a number ofDrosophila peptides that end in the sequence -RFamide but that are not products of the dFMRFamide gene. These include dromyosuppressin, TDVDHVFLRFamide (Nichols, 1992). There is no apparent similarity between the N-terminal sequence of TDVDHVFLRFamide and any of the putative products of the dFMRFamide gene. However, at the C terminus these sequences differ by only one conservative substitution (Leu in place of Met or Ile). Therefore, we tested TDVDHVFLRFamide for activity in the larval nerve–muscle assay. As shown in Figure 9, TDVDHVFLRFamide enhanced nerve-stimulated muscle contraction, but only at concentrations that were 100-fold higher than those necessary to elicit the same response with DPKQDFMRFamide. Thus, by itself, this peptide appears unlikely to be a significant neuromodulator of this synapsein vivo.
Fig. 9.
A Drosophila myosuppressin, TDVDHVFLRFamide, enhances muscle contraction, but it is active only at micromolar concentrations. Mean tension ratio ± SEM is shown as a function of TDVDHVFLRFamide concentration in wandering third instar larvae (n = 6). The mean time-to-peak tension was 150.8 ± 7.4 (n = 18). The data for DPKQDFMRFamide are taken from Figure 3B(n = 7). *p < 0.05; **p < 0.001; ***p < 0.001 [one-way ANOVA: 10-8m,F(1,11) = 7.428, p = 0.0197; 10-7m,F(1,11) = 53.424, p = 0.0001; 10-6m,F(1,11) = 19.745, p = 0.0010; 10-5m,F(1,11) = 9.318, p = 0.0110].
DPKQDFMRFamide enhances synaptic transmission
We performed two-electrode voltage-clamp recordings to assess the effects of DPKQDFMRFamide on synaptic transmission at the larval neuromuscular junction. We recorded from muscle fiber 6, which usually is innervated by two motor neurons (RP3 and Mn7/6; Chang and Keshishian, 1996), because the morphology, development, and physiology of this synapse have been characterized extensively (Keshishian et al., 1996). In addition, muscle 6 is a target for dFMRFamide-related peptides; after the neighboring muscles had been severed, muscle 6 displayed increased twitch tension in response to SPKQDFMRFamide with kinetics that were similar to those of the aggregate muscle response in intact segments (data not shown). Similar results were obtained for muscle 12.
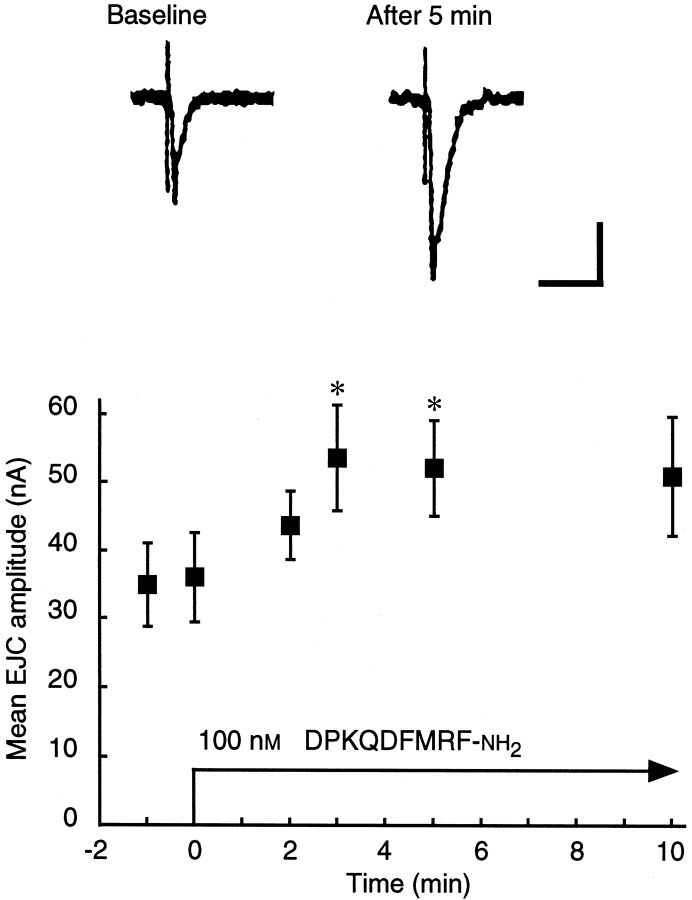
DPKQDFMRFamide caused a strong enhancement of synaptic currents in muscle fiber 6. After the bath application of 0.1 μmDPKQDFMRFamide, the mean EJC amplitude increased from a baseline value of 35.7 ± 4.2 nA (n = 12; mean ± SEM) to 43.9 ± 5.0 nA at 2 min (n = 4) and then reached a plateau at 53.7 ± 7.7 nA (n = 6), or 151% of the baseline value, at 3 min (Fig.10). On reaching this peak value, the EJC amplitude remained elevated (decreasing only 5%) for an additional 7 min in the continued presence of the peptide. Thus, DPKQDFMRFamide caused a robust and sustainable increase in EJC amplitude.
Fig. 10.
DPKQDFMRFamide enhances synaptic transmission at the larval neuromuscular junction. Excitatory junctional currents (EJCs) were evoked in muscle fiber 6 by electrical stimulation of the abdominal nerve. Representative EJCs (preceded by the stimulus artifact) are shown above. The mean EJC amplitude ± SEM as a function of time after the application of 100 nm DPKQDFMRFamide is plotted below(n = 4–6 at each point). *p < 0.05 (Student’s t test, compared with the baseline mean). Calibration: 20 nA, 20 msec.
The kinetics of the synaptic effects of DPKQDFMRFamide were consistent with two features of the kinetics of the response to dFMRFamide-related peptides in the contraction assays. First, the synaptic effects of DPKQDFMRFamide showed minimal decrement after 10 min (Fig. 10). Similarly, in the contraction assays the enhancement of twitch tension by dFMRFamide-related peptides often persisted for several minutes (see Fig. 5). Second, sustained exposure of the NMJ to DPKQDFMRFamide caused a gradual increase of the EJC amplitude that was observed in the voltage-clamp recordings during the first 2–3 min of peptide application (Fig. 10). In the contraction assays the effects of DPKQDFMRFamide and the other dFMRFamide-related peptides also were gradual; twitch tension increased over a period of 3–4 min and peaked ∼100 sec after the bath peptide concentration had peaked and declined to ∼25–35% of the highest level (see Fig. 3A). We cannot exclude the possibility that DPKQDFMRFamide has distinct effects on the EJC and on the contractile machinery, but with different kinetics. However, the differences in the method of peptide application between the voltage-clamp recordings and the contraction assays appear sufficient to account for the differences in kinetics. Taken together, these results indicate that the excitatory effects of DPKQDFMRFamide on twitch tension are mediated, at least in part, by a pronounced increase in synaptic efficacy.
DISCUSSION
Drosophila FMRFamide-related peptides are excitatory at the larval neuromuscular junction
We used a sensitive strain gauge to measure the effects of FMRFamide-related peptides on the Drosophila larval NMJ. Several predicted products of the Drosophila FMRFamidegene enhanced nerve-stimulated contractions of muscles located throughout the larval body wall. Given the probable hormonal broadcast of these neuropeptides, these results suggest that dFMRFamide-related peptides perform an important and general role in modulating muscle (twitch) tension in Drosophila larvae. One of these peptides, DPKQDFMRFamide, strongly potentiated synaptic transmission at the larval neuromuscular junction. Therefore, the dFMRFamide-related peptides enhance twitch tension, at least in part, via an increase in synaptic efficacy.
FMRFamide-related peptides enhance or inhibit nerve-stimulated muscle contraction in diverse invertebrates (Greenberg and Price, 1992). In insects, effects of FMRFamide-related peptides on visceral muscles of the heart, oviduct, foregut, and hindgut have been characterized (Nässel, 1996). As demonstrated here in Drosophila and in the locust (Walther et al., 1984; Evans and Myers, 1986a,b), FMRFamide-related peptides also are active on insect skeletal muscle. Thus, this system may be conserved evolutionarily among diverse insect orders.
Hemolymph concentrations of dFMRFamide-related peptides
Although we have not measured the normal physiological range of hemolymph concentrations of these peptides in vivo, comparative work in the stick insect, Carausius morosus (Miksys et al., 1997), the blood-feeding bug,Rhodnius prolixus (Elia et al., 1993), and the locust,Shistocerca gregaria (Robb and Evans, 1990), suggests that the active physiological range is between 1 nm and 0.1 μm. The dose–response curves obtained here with single peptides and with a peptide mixture peaked (or reached a plateau) at 0.1–1.0 μm, and the EC50 was ∼40 nm. Therefore, our results suggest that the active range of concentrations for the mixtures of dFMRFamide gene products is ∼10 nm to 0.1 μm. Because the peptides behaved additively in the mixtures, the active ranges for each of the peptides individually may be ∼10-fold lower (1–10 nm) than the range predicted above, except in the case of DPKQDFMRFamide, which is likely to be the predominant peptide species (Nambu et al., 1988; Schneider and Taghert, 1988). This model is supported by the fact that high concentrations of the dFMRFamide-related peptides (≥1 μm) often evoked strong spontaneous contractions in segments distant from the one receiving nerve stimulation. Similar spontaneous contractions are not observed along the body wall of intact wild-type larvae.
In many preparations the effects of the DrosophilaFMRFamide-related peptides were sustained for long periods (sometimes >30 min) after a single, relatively brief (<2 min) peptide application. In addition, there was no desensitization after repeated applications of the same concentration of peptide (see Materials and Methods). These results are consistent with two models (nonmutually exclusive) for the behavioral role of FMRFamide-related peptidesin vivo. First, the circulating levels of these peptides may be elevated for prolonged periods (several hours) to support general long-term states of behavioral “arousal,” such as those observed during diurnal activity rhythms (see Miksys et al., 1997). Second, FMRFamide-related peptides may be released acutely in response to environmental stimuli and/or during stereotyped behaviors.
Functional redundancy of DrosophilaFMRFamide-related peptides
We examined eight known or predicted products of the D.melanogaster FMRFamide gene for effects on nerve-stimulated muscle contraction. Seven of the peptides were excitatory, resulting in rapid, dramatic, and long-lasting enhancements of muscle tension, and one of the peptides was inactive. The active peptides showed similar dose–response curves and muscle targets, and, overall, the response kinetics for each of the seven peptides were similar. When the peptides were mixed in a “cocktail” predicted by their stoichiometric ratios in the dFMRFamide propeptide, the peptides behaved additively, and there were no detectable higher-order interactions. Taken together, these results indicate that the Drosophila FMRFamide-related peptides are functionally redundant in their effects on the larval body-wall muscles.
The ratio of dFMRFamide-related peptides present in the hemolymph ofDrosophila larvae may be sensitive to several factors and may differ substantially from the ratio predicted from the dFMRFamide propeptide. For example, there may be pre- or post-translational changes in the ratios of peptides derived from the precursor (Kaldany et al., 1985; Buck et al., 1987; Jung and Scheller, 1991; Benjamin and Burke, 1994)—there have been suggestions of differential processing of the dFMRFamide precursor (Nichols et al., 1995b)—or there may be differential sensitivities of these peptides to degradative enzymes (Rose et al., 1996; Bendena et al., 1997). Importantly, when they were used in the nerve–muscle assay, the FMRFamide-related peptides acted comparably when applied alone and behaved additively when applied together as peptide cocktails. Thus, it is unlikely that there were strong higher-order interactions (such as synergy) between these peptides, and the general conclusions of this study are likely to be valid even if the ratios of peptides present in vivo differ from the specific values used in the peptide cocktails.
Similar results were obtained in a study of the effects of myomodulins on the accessory radula closer (ARC) muscle of Aplysia. TheAplysia myomodulin gene encodes 10 copies of myomodulin A and one copy each of eight other related sequences (Miller et al., 1993b). All nine myomodulins increased net contraction amplitude and accelerated the relaxation rate of ARC at nanomolar concentrations, whereas at micromolar concentrations two of the peptides potentiated and the others abolished contraction (Březina et al., 1995). When the myomodulins were combined together in a cocktail representing the molar ratio likely to be released, the potentiating effects predominated, suggesting that the various myomodulin isoforms are functionally redundant.
Maintenance of sequence diversity in propeptide precursors
D. melanogaster and D. virilis are thought to have diverged ∼60 million years ago, and the FMRFamide genes of these species show strong conservation of neuropeptide sequence diversity (Taghert and Schneider, 1990). In such cases the occurrence of conserved peptide diversity suggests the potential for diverse functions; however, there are several other possible evolutionary constraints that may favor the conservation of peptide diversity in neuropeptide precursors (Březina et al., 1995; Březina and Weiss, 1997).
The Drosophila FMRFamide-related neuropeptides may have differential activities that are dependent on the target tissue, developmental stage, or interactions with other modulators. Evidence obtained from another fly, Calliphora vomitoria, suggests that this may be the case. Duve et al. (1992) purified 14 distinct FMRFamide-related sequences from this species, yet only 3 of the 14 peptides stimulated secretion by the larval salivary glands. Although the C. vomitoria FMRFamide gene has not been cloned, these results suggest that the effects of FMRFamide-related peptides on some targets in flies may be more sequence-dependent than on the NMJ. FMRFamide-related peptides can have common effects on some targets and differential effects on others (Lehman and Greenberg, 1987). Also, as in the case of cockroach allatostatins, the related products of a single precursor can exhibit a rank order of potencies that differs dramatically between different targets, although the effects of each of the peptides alone are qualitatively similar (Bendena et al., 1997). Therefore, target-dependent differences in the effects of the various FMRFamide-related peptides may contribute to the maintenance of sequence variation in the dFMRFamide gene.
In a few neuropeptide precursors there are “cryptic” neuropeptides that are encoded by spacer sequences—those separating recognizable peptide repeats—that also may have biological activity. For example, the vertebrate thyrotropin-releasing hormone (TRH) gene encodes multiple copies of the TRH tripeptide that are separated by connecting regions of varying length. Several of the connecting peptides have been purified from tissues that express the TRH gene, and at least one (Ps4) potentiates the effects of TRH (Ladram et al., 1994). Similarly, a 22-amino-acid peptide spacer sequence, called “SEEPLY,” from theLymnaea FMRFamide gene is expressed in Lymnaeaneurons and can modify the effects of the peptide FMRFamide (Benjamin and Burke, 1994). In other neuropeptide precursors, such as proenkephalin, certain endoproteolytic cleavage sites are used variably, resulting in the synthesis of larger peptide isoforms that are present in vivo and that are biologically active (Höllt, 1986). The Drosophila FMRFamide gene contains such spacer sequences and potential cryptic proteolytic cleavage sites. Therefore, it will be important in the future to consider the possible contributions of these to the overall function of dFMRFamide-related peptides.
Finally, there may be biosynthetic or energetic advantages to the maintenance of sequence repetition in neuropeptide precursors. For example, the propeptide intermediates may assume sequence-dependent secondary structures that are required for efficient processing. Furthermore, components necessary for the trafficking of propeptide intermediates may be rate-limiting or saturable, such that the presence of multiple peptides on a single precursor would allow for the production of more peptide. Through neutral selection, sequence diversity could arise secondarily (Březina and Weiss, 1997).
Clearly, the claim that multiple transmitters or hormones are functionally redundant must be made with caution. Our results indicate that the various predicted products of the dFMRFamide gene are functionally redundant at the neuromuscular junction at one developmental stage. Because the distinct peptides encoded by thedFMRFamide gene have been conserved evolutionarily, these results direct us to search for additional functions or processes in which these peptides may act differentially.
Footnotes
This work was supported by National Institutes of Health Grant NS21749 (P.H.T.) and American Cancer Society Postdoctoral Fellowship PF4212 (R.S.H.). E.C.S. was supported by a National Science Foundation Research Experience for Undergraduates Grant BIR 9531558 (to P.H.T.). We thank Dr. Philip Lloyd for peptides; Dr. Chi Hunt for the transducers; Dr. Chun-Fang Wu for the recording chamber; Dr. Michael Dickinson for assistance with the strain gauge apparatus and for the loan of equipment; Dr. Timothy Wooten for statistical assistance; Dr. Yi Zhong for assistance with recordings; and Drs. Andreas Burkhalter, Jeanne Nerbonne, Colin Nichols, and Larry Salkoff for additional equipment. We also thank Susan Renn for assistance with the data analysis.
Correspondence should be addressed to Dr. Randall S. Hewes, Department of Anatomy and Neurobiology, Box 8108, Washington University School of Medicine, 660 South Euclid Avenue, St. Louis, MO 63110.
REFERENCES
- 1.Anderson MS, Halpern ME, Keshishian H. Identification of the neuropeptide transmitter proctolin in Drosophila larvae: characterization of muscle fiber-specific neuromuscular endings. J Neurosci. 1988;8:242–255. doi: 10.1523/JNEUROSCI.08-01-00242.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bainbridge SP, Bownes M. Staging the metamorphosis of Drosophila melanogaster. J Embryol Exp Morphol. 1981;66:57–80. [PubMed] [Google Scholar]
- 3.Bendena WG, Garside CS, Yu CG, Tobe SS. Allatostatins: diversity in structure and function of an insect neuropeptide family. Ann NY Acad Sci. 1997;814:53–66. doi: 10.1111/j.1749-6632.1997.tb46144.x. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin PR, Burke JF. Alternative mRNA splicing of the FMRFamide gene and its role in neuropeptidergic signalling in a defined neural network. BioEssays. 1994;16:335–342. doi: 10.1002/bies.950160508. [DOI] [PubMed] [Google Scholar]
- 5.Bleakman A, Bradbury AF, Darby NJ, Maruthainar K, Smyth DG. Processing reactions in the later stages of hormone activation. Biochimie. 1988;70:3–10. doi: 10.1016/0300-9084(88)90152-6. [DOI] [PubMed] [Google Scholar]
- 6.Bliss CI. Statistics in biology: statistical methods for research in the natural sciences, Vol 1, pp 108–111. McGraw-Hill; St. Louis: 1967. [Google Scholar]
- 7.Bodenstein D. The postembryonic development of Drosophila. In: Demerec M, editor. Biology of Drosophila. Cold Spring Harbor Laboratory; Plainview, NY: 1950. pp. 275–367. [Google Scholar]
- 8.Březina V, Weiss KR. Analyzing the functional consequences of transmitter complexity. Trends Neurosci. 1997;20:538–543. doi: 10.1016/s0166-2236(97)01120-x. [DOI] [PubMed] [Google Scholar]
- 9.Březina V, Evans CG, Weiss KR. Enhancement of Ca current in the accessory radula closer muscle of Aplysia californica by neuromodulators that potentiate its contractions. J Neurosci. 1994a;14:4393–4411. doi: 10.1523/JNEUROSCI.14-07-04393.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Březina V, Evans CG, Weiss KR. Activation of K current in the accessory radula closer muscle of Aplysia californica by neuromodulators that depress its contractions. J Neurosci. 1994b;14:4412–4432. doi: 10.1523/JNEUROSCI.14-07-04412.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Březina V, Bank B, Cropper EC, Rosen S, Vilim FS, Kupfermann I, Weiss KR. Nine members of the myomodulin family of peptide cotransmitters at the B16–ARC neuromuscular junction of Aplysia. J Neurophysiol. 1995;74:54–72. doi: 10.1152/jn.1995.74.1.54. [DOI] [PubMed] [Google Scholar]
- 12.Buck LB, Bigelow JM, Axel R. Alternative splicing in individual Aplysia neurons generates neuropeptide diversity. Cell. 1987;51:127–133. doi: 10.1016/0092-8674(87)90017-1. [DOI] [PubMed] [Google Scholar]
- 13.Chang TN, Keshishian H. Laser ablation of Drosophila embryonic motoneurons causes ectopic innervation of target muscle fibers. J Neurosci. 1996;16:5715–5726. doi: 10.1523/JNEUROSCI.16-18-05715.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crossley CA. The morphology and development of the Drosophila muscular system. In: Ashburner M, Wright TRF, editors. The genetics and biology of Drosophila, Vol 2B. Academic; New York: 1978. pp. 499–560. [Google Scholar]
- 15.Devi L. Consensus sequence for processing of peptide precursors at monobasic sites. FEBS Lett. 1991;280:189–194. doi: 10.1016/0014-5793(91)80290-j. [DOI] [PubMed] [Google Scholar]
- 16.Duve H, Johnsen AH, Sewell JC, Scott AG, Orchard I, Rehfeld JF, Thorpe A. Isolation, structure, and activity of Phe-Met-Arg-Phe-NH2 neuropeptides (designated calliFMRFamides) from the blowfly Calliphora vomitoria. Proc Natl Acad Sci USA. 1992;89:2326–2330. doi: 10.1073/pnas.89.6.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elia AJ, Tebrugge VA, Orchard I. The pulsatile appearance of FMRFamide-related peptides in the haemolymph and loss of FMRFamide-like immunoreactivity from neurohaemal areas of Rhodnius prolixus following a blood meal. J Insect Physiol. 1993;39:459–469. [Google Scholar]
- 18.Evans PD, Myers CM. Peptidergic and aminergic modulation of insect skeletal muscle. J Exp Biol. 1986a;124:143–176. [Google Scholar]
- 19.Evans PD, Myers CM. The modulatory actions of FMRFamide and related peptides on locust skeletal muscle. J Exp Biol. 1986b;126:403–422. [Google Scholar]
- 20.Gorczyca M, Augart C, Budnik V. Insulin-like receptor and insulin-like peptide are localized at neuromuscular junctions in Drosophila. J Neurosci. 1993;13:3692–3704. doi: 10.1523/JNEUROSCI.13-09-03692.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg MJ, Price DA. Relationships among the FMRFamide-like peptides. Prog Brain Res. 1992;92:25–37. doi: 10.1016/s0079-6123(08)61162-0. [DOI] [PubMed] [Google Scholar]
- 22.Hall ZW, Sanes JR. Synaptic structure and development: the neuromuscular junction. Cell [Suppl] 1993;72:99–121. doi: 10.1016/s0092-8674(05)80031-5. [DOI] [PubMed] [Google Scholar]
- 23.Hökfelt T. Neuropeptides in perspective: the last ten years. Neuron. 1991;7:867–879. doi: 10.1016/0896-6273(91)90333-u. [DOI] [PubMed] [Google Scholar]
- 24.Höllt V. Opioid peptide processing and receptor selectivity. Annu Rev Pharmacol Toxicol. 1986;26:59–77. doi: 10.1146/annurev.pa.26.040186.000423. [DOI] [PubMed] [Google Scholar]
- 25.Höllt V. Opioid peptide genes: structure and regulation. In: Almeida OFX, Shippenberg TS, editors. Neurobiology of opioids. Springer; New York: 1989. pp. 11–51. [Google Scholar]
- 26.Jan LY, Jan YN. Properties of the larval neuromuscular junction in Drosophila melanogaster. J Physiol (Lond) 1976a;262:189–214. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jan LY, Jan YN. l-Glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. J Physiol (Lond) 1976b;262:215–236. doi: 10.1113/jphysiol.1976.sp011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jan YN, Jan LY. Electrophysiological techniques. In: Krieger DT, Brownstein MJ, Martin JB, editors. Brain peptides. Wiley; New York: 1983. pp. 547–563. [Google Scholar]
- 29.Johansen J, Halpern ME, Johansen KM, Keshishian H. Stereotypic morphology of glutamatergic synapses on identified muscle cells of Drosophila larvae. J Neurosci. 1989a;9:710–725. doi: 10.1523/JNEUROSCI.09-02-00710.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johansen J, Halpern ME, Keshishian H. Axonal guidance and the development of muscle fiber-specific innervation in Drosophila embryos. J Neurosci. 1989b;9:4318–4332. doi: 10.1523/JNEUROSCI.09-12-04318.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung LS, Scheller RH. Peptide processing and targeting in the neuronal secretory pathway. Science. 1991;251:1330–1335. doi: 10.1126/science.2003219. [DOI] [PubMed] [Google Scholar]
- 32.Kaldany RRJ, Nambu JR, Scheller RH. Neuropeptides in identified Aplysia neurons. Annu Rev Neurosci. 1985;8:431–455. doi: 10.1146/annurev.ne.08.030185.002243. [DOI] [PubMed] [Google Scholar]
- 33.Kellett E, Perry SJ, Santama N, Worster BM, Benjamin PR, Burke JF. Myomodulin gene of Lymnaea: structure, expression, and analysis of neuropeptides. J Neurosci. 1996;16:4949–4957. doi: 10.1523/JNEUROSCI.16-16-04949.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keshishian H, Broadie K, Chiba A, Bate M. The Drosophila neuromuscular junction: a model system for studying synaptic development and function. Annu Rev Neurosci. 1996;19:545–575. doi: 10.1146/annurev.ne.19.030196.002553. [DOI] [PubMed] [Google Scholar]
- 35.Ladram A, Bulant M, Delfour A, Montagne JJ, Vaudry H, Nicolas P. Modulation of the biological activity of thyrotropin-releasing hormone by alternate processing of pro-TRH. Biochimie. 1994;76:320–328. doi: 10.1016/0300-9084(94)90166-x. [DOI] [PubMed] [Google Scholar]
- 36.Lehman HK, Greenberg MJ. The actions of FMRFamide-like peptides on visceral and somatic muscles of the snail Helix aspersa. J Exp Biol. 1987;131:55–68. doi: 10.1242/jeb.131.1.55. [DOI] [PubMed] [Google Scholar]
- 37.Leviev I, Grimmelikhuijzen CJP. Molecular cloning of a preprohormone from sea anemones containing numerous copies of a metamorphosis-inducing neuropeptide: a likely role for dipeptidyl aminopeptidase in neuropeptide precursor processing. Proc Natl Acad Sci USA. 1995;92:11647–11651. doi: 10.1073/pnas.92.25.11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez V, Wickham L, Desgroseillers L. Molecular cloning of myomodulin cDNA, a neuropeptide precursor gene expressed in neuron L10 of Aplysia californica. DNA Cell Biol. 1993;12:53–61. doi: 10.1089/dna.1993.12.53. [DOI] [PubMed] [Google Scholar]
- 39.Miksys S, Lange AG, Orchard I, Wong V. Localization and neurohemal release of FMRFamide-related peptides in the stick insect Carausius morosus. Peptides. 1997;18:27–40. doi: 10.1016/s0196-9781(96)00245-8. [DOI] [PubMed] [Google Scholar]
- 40.Miller MW, Beushausen S, Cropper EC, Eisinger K, Stamm S, Vilim FS, Vitek A, Zajc A, Kupfermann I, Brosius J, Weiss KR. The buccalin-related neuropeptides: isolation and characterization of an Aplysia cDNA clone encoding a family of peptide cotransmitters. J Neurosci. 1993a;13:3346–3357. doi: 10.1523/JNEUROSCI.13-08-03346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller MW, Beushausen S, Vitek A, Stamm S, Kupfermann I, Brosius J, Weiss KR. The myomodulin-related neuropeptides: characterization of a gene encoding a family of peptide cotransmitters in Aplysia. J Neurosci. 1993b;13:3358–3367. doi: 10.1523/JNEUROSCI.13-08-03358.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monastirioti M, Gorczyca M, Rapus J, Eckert M, White K, Budnik V. Octopamine immunoreactivity in the fruit fly Drosophila melanogaster. J Comp Neurol. 1995;356:275–287. doi: 10.1002/cne.903560210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nambu JR, Murphy-Erdosh C, Andrews PC, Feistner GJ, Scheller RH. Isolation and characterization of a Drosophila neuropeptide gene. Neuron. 1988;1:55–61. doi: 10.1016/0896-6273(88)90209-7. [DOI] [PubMed] [Google Scholar]
- 44.Nässel DR. Neuropeptides, amines, and amino acids in an elementary insect ganglion: functional and chemical anatomy of the unfused abdominal ganglion. Prog Neurobiol. 1996;48:325–420. doi: 10.1016/0301-0082(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 45.Nichols R. Isolation and structural characterization of Drosophila TDVDHVFLRFamide and FMRFamide-containing neural peptides. J Mol Neurosci. 1992;3:213–218. doi: 10.1007/BF03380141. [DOI] [PubMed] [Google Scholar]
- 46.Nichols R, McCormick J, Lim I, Caserta L. Cellular expression of the Drosophila melanogaster FMRFamide neuropeptide gene product DPKQDFMRFamide. J Mol Neurosci. 1995a;6:1–10. doi: 10.1007/BF02736754. [DOI] [PubMed] [Google Scholar]
- 47.Nichols R, McCormick JB, Lim IA, Starkman JS. Spatial and temporal analysis of the Drosophila FMRFamide neuropeptide gene product SDNFMRFamide: evidence for a restricted expression pattern. Neuropeptides. 1995b;29:205–213. doi: 10.1016/0143-4179(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 48.Norris BJ, Calabrese RL. Identification of motor neurons that contain a FMRFamide-like peptide and the effects of FMRFamide on longitudinal muscle in the medicinal leech, Hirudo medicinalis. J Comp Neurol. 1987;266:95–111. doi: 10.1002/cne.902660108. [DOI] [PubMed] [Google Scholar]
- 49.O’Brien MA, Schneider LE, Taghert PH. In situ hybridization analysis of the FMRFamide neuropeptide gene in Drosophila. II. Constancy in the cellular pattern of expression during metamorphosis. J Comp Neurol. 1991;304:623–638. doi: 10.1002/cne.903040409. [DOI] [PubMed] [Google Scholar]
- 50.O’Shea M, Adams ME, Bishop C, Witten J, Worden MK (1985) Model peptidergic systems at the insect neuromuscular junction. Peptides 6[Suppl 3]:417–424. [DOI] [PubMed]
- 51.Payza K. FMRFamide receptors in Helix aspersa. Peptides. 1987;8:1065–1074. doi: 10.1016/0196-9781(87)90138-0. [DOI] [PubMed] [Google Scholar]
- 52.Robb S, Evans PD. FMRFamide-like peptides in the locust: distribution, partial characterization, and bioactivity. J Exp Biol. 1990;149:335–360. doi: 10.1242/jeb.149.1.335. [DOI] [PubMed] [Google Scholar]
- 53.Rose C, Vargas F, Facchinetti P, Bourgeat P, Bambal RB, Bishop PB, Chan SMT, Moore ANJ, Ganellin CR, Schwartz J-C. Characterization and inhibition of a cholecystokinin-inactivating serine peptidase. Nature. 1996;380:403–409. doi: 10.1038/380403a0. [DOI] [PubMed] [Google Scholar]
- 54.Schinkmann K, Li C. Comparison of two Caenorhabditis genes encoding FMRFamide (Phe-Met-Arg-Phe-NH2)-like peptides. Brain Res Mol Brain Res. 1994;24:238–246. doi: 10.1016/0169-328x(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 55.Schneider LE, Taghert PH. Isolation and characterization of a Drosophila gene that encodes multiple neuropeptides related to Phe-Met-Arg-Phe-NH2 (FMRFamide). Proc Natl Acad Sci USA. 1988;85:1993–1997. doi: 10.1073/pnas.85.6.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schneider LE, O’Brien MA, Taghert PH. In situ hybridization analysis of the FMRFamide neuropeptide gene in Drosophila. I. Restricted expression in embryonic and larval stages. J Comp Neurol. 1991;304:608–622. doi: 10.1002/cne.903040408. [DOI] [PubMed] [Google Scholar]
- 57.Schneider LE, Sun ET, Garland DJ, Taghert PH. An immunocytochemical study of the FMRFamide neuropeptide gene products in Drosophila. J Comp Neurol. 1993;337:446–460. doi: 10.1002/cne.903370308. [DOI] [PubMed] [Google Scholar]
- 58.Shi R, Belardetti F. Serotonin inhibits the peptide FMRFamide response through a cyclic AMP-independent pathway in Aplysia. J Neurophysiol. 1991;66:1847–1857. doi: 10.1152/jn.1991.66.6.1847. [DOI] [PubMed] [Google Scholar]
- 59.Sokal RR, Rohlf FJ. Introduction to biostatistics, pp 211–219. Freeman; New York: 1987. [Google Scholar]
- 60.Sokolowski MB. Foraging strategies of Drosophila melanogaster: a chromosomal analysis. Behav Genet. 1980;10:291–302. doi: 10.1007/BF01067774. [DOI] [PubMed] [Google Scholar]
- 61.Stewart BA, Atwood HL, Renger JJ, Wang J, Wu C-F. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol [A] 1994;175:179–191. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- 62.Taghert PH, Schneider LE. Interspecific comparison of a Drosophila gene encoding FMRFamide-related neuropeptides. J Neurosci. 1990;10:1929–1942. doi: 10.1523/JNEUROSCI.10-06-01929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walther C, Schiebe M, Voigt KH. Synaptic and non-synaptic effects of molluscan cardioexcitatory neuropeptides on locust skeletal muscle. Neurosci Lett. 1984;45:99–104. doi: 10.1016/0304-3940(84)90336-7. [DOI] [PubMed] [Google Scholar]
- 64.Wang Z, Orchard I, Lange AB, Chen X. Binding and activation regions of the decapeptide PDVDHVFLRFamide (SchistoFLRFamide). Neuropeptides. 1995;28:261–266. doi: 10.1016/0143-4179(95)90042-x. [DOI] [PubMed] [Google Scholar]
- 65.Wu C-F, Ganetzky B, Jan LY, Jan Y-N, Benzer S. A Drosophila mutant with a temperature-sensitive block in nerve conduction. Proc Natl Acad Sci USA. 1978;75:4047–4051. doi: 10.1073/pnas.75.8.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhong Y, Peña LA. A novel synaptic transmission mediated by a PACAP-like neuropeptide in Drosophila. Neuron. 1995;14:527–536. doi: 10.1016/0896-6273(95)90309-7. [DOI] [PubMed] [Google Scholar]
- 67.Zhong Y, Wu C-F. Altered synaptic plasticity in Drosophila memory mutant with altered cAMP cascade. Science. 1991;251:198–201. doi: 10.1126/science.1670967. [DOI] [PubMed] [Google Scholar]