Abstract
The effects of serotonin1B[5-hydroxytryptamine1B (5-HT1B)] receptor activation on cocaine reinforcement were investigated using intravenous cocaine self-administration by rats. The 5-HT1Breceptor agonists 5-methoxy-3-1,2,3,6-tetrahydro-4-pyridinyl-1H-indole (RU 24969) (0.3–3 mg/kg), 3-(1,2,5,6-tetrahydro-4-pyridyl)-5-propoxypyrrolo[3,2-b]pyridine (CP 94,253) (0.3–3 mg/kg), and 3-(1,2,5,6-tetrahydropyrid-4-yl)pyrrolo[3,2-b]pyridine (CP 93,129) (3 and 10 μg, i.c.v.) each dose-dependently reduced the self-administration of a cocaine dose on the descending limb of the fixed-ratio 5 (FR-5) cocaine dose–effect function, in a manner similar to the effect produced by increasing the unit dose of cocaine. In addition, each of these 5-HT1B agonists lowered the threshold dose of cocaine that supported self-administration. These results are consistent with a 5-HT1B agonist-induced potentiation of cocaine reinforcement. On a progressive ratio schedule of reinforcement, RU 24969 and CP 94,253 dose-dependently (0.3–3 mg/kg) increased the highest completed ratio for cocaine self-administration, again by producing behavioral alterations similar to those induced by increasing the unit dose of cocaine. The effect of CP 94,253 was dose-dependently blocked by the 5-HT1B/1D receptor partial agonist 2′-methyl-4′-(5-methyl[1,2,4]oxadiazol-3-yl)-biphenyl-4-carboxylic acid[4-methodoxy-3-(4-methyl-piperazin-1-yl)-phenyl]-amide (GR 127,935) (0.3–10 mg/kg) but was unaffected by the 5-HT1A receptor antagonist 4-iodo-N-[2-[4-(methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinyl- benzamide (p-MPPI; 1–10 mg/kg). Self-administration behavior was not maintained when either RU 24969 or CP 94,253 was substituted for cocaine, indicating that these 5-HT1B agonists do not produce significant reinforcing effects alone. Together, these findings indicate that 5-HT1B receptor stimulation facilitates the reinforcing properties of cocaine. These results are in opposition to recent findings with 5-HT1B receptor knock-out mice and may have important ontogenic implications in the area of drug abuse research.
Keywords: cocaine; self-administration; 5-HT1B; 5-HT1A; RU 24969; CP 94,253; CP 93,129; GR 127,935; 8-OH-DPAT; p-MPPI; progressive ratio
It is well established that the reinforcing effects of cocaine and other psychostimulants are dependent on the ability of these drugs to enhance extracellular dopamine (DA) concentrations in the mesocorticolimbic system (Wise, 1984; Koob and Bloom, 1988; Kuhar et al., 1991). However, cocaine also elevates extracellular serotonin [5-hydroxytryptamine (5-HT)] and norepinephrine levels (Chen and Reith, 1994; Li et al., 1996), suggesting that monoaminergic systems other than DA could play some role in the mediation of cocaine reinforcement.
There is increasing evidence implicating a serotonergic component in the behavioral effects of cocaine in rats and monkeys (Carroll et al., 1990a,b; Loh and Roberts, 1990; Spealman, 1993) as well as in humans (Walsh et al., 1994; Aronson et al., 1995; Satel et al., 1995;Buydens-Branchey et al., 1997). Acute cocaine administration dramatically alters the electrophysiological activity of both 5-HT and DA neurons (Cunningham, 1995; White et al., 1995), and chronic cocaine exposure produces pronounced decrements in both 5-HT and DA neuronal activity (Cunningham, 1995; White et al., 1995) and 5-HT and DA efflux (Parsons et al., 1991, 1995; Weiss et al., 1995). Moreover, the combined stimulation of 5-HT and DA receptors is required to mimic the effects of cocaine on striatal gene expression (Bhat et al., 1992; Bhat and Baraban, 1993) and on the firing rate of DA cells in the nucleus accumbens (White et al., 1993). However, in contrast to what is known about dopaminergic mechanisms, relatively little is known about the contribution of serotonergic mechanisms to the behavioral and physiological effects of cocaine.
Serotonin neurotransmission is mediated by at least 14 different 5-HT receptor subtypes (Hoyer et al., 1994). Recent findings suggest that serotonin1B (5-HT1B) receptors, in particular, play some role in the mediation of the reinforcing and subjective effects produced by psychostimulants. For example, 5-HT1B receptor agonists potentiate the reinforcing effects of a selective DA uptake inhibitor (Parsons et al., 1996), enhance the discriminative cue produced by cocaine, and partially substitute for the discriminative stimulus properties of cocaine (Callahan and Cunningham, 1995, 1997). In addition, 5-HT1B receptors mediate the cocaine-induced reduction in ventral tegmental area GABA release (Cameron and Williams, 1994) and contribute to cocaine-induced striatal c-fos expression (Lucas et al., 1997). These findings suggest an involvement of 5-HT1B receptors in the behavioral, neurochemical, and cellular effects produced by cocaine.
To test the hypothesis that 5-HT1B receptors play a role in the mediation and/or modulation of cocaine reinforcement, we investigated the effects of the putative 5-HT1B receptor agonists 5-methoxy-3-1,2,3,6-tetrahydro-4-pyridinyl-1H-indole (RU 24969), 3-(1,2,5,6-tetrahydro-4-pyridyl)-5-propoxypyrrolo[3,2-b]pyridine (CP 94,253), and 3-(1,2,5,6-tetrahydropyrid-4-yl)pyrrolo[3,2-b]pyridine (CP 93,129) on intravenous cocaine self-administration in rats. Alterations in cocaine reinforcement were assessed by examining shifts in the cocaine dose–effect function using both a low fixed-ratio schedule (FR-5) and a progressive ratio schedule of cocaine reinforcement. The ability of the selective 5-HT1B/1D receptor partial agonist 2′-methyl-4′-(5-methyl[1,2,4]oxadiazol-3-yl)-biphenyl-4-carboxylic acid[4-methodoxy-3-(4-methyl-piperazin-1-yl)-phenyl]-amide (GR 127,935) or the selective 5-HT1A receptor antagonist 4-iodo-N-[2-[4-(methoxy-phenyl)-1-piperazinyl]ethyl]-N-2-pyridinyl-benzamide (p-MPPI) to alter the effects of CP 94,253 on cocaine reinforcement was also investigated. The reinforcing effects of intravenous RU 24969 and CP 94,253 self-administration were also explored.
MATERIALS AND METHODS
Subjects
Male Wistar rats (Charles River Laboratories, Wilmington, MA; n = 80) weighing 250–275 gm after delivery were housed in groups of two to three in a humidity- and temperature-controlled (22°C) vivarium on a 12 hr light/dark cycle (lights off at 10 A.M.). Animals were provided with food and waterad libitum throughout the course of the study, with the exception of 1 week of food restriction during operant training (see self-administration training). All behavioral procedures began between 1 to 3 hr after the onset of the dark phase. All procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Drugs
RU 24969 was generously provided by Roussel UCLAF (Romainville, France). CP 94,253 and CP 93,129 were generously provided by Pfizer (Groton, CT). GR 127,935 was generously provided by GlaxoWellcome (Stevenage, United Kingdom). Cocaine HCl was obtained from the National Institute on Drug Abuse (Washington, DC). (±)-8-Hydroxy-dipropylaminotetralin (8-OH-DPAT) and p-MPPI were purchased from Research Biochemicals (Natick, MA). All drugs were dissolved in 0.9% saline (CP 94,253, GR 127,935, and p-MPPI required gentle heating). All doses refer to the weights of the respective salts, and all test drugs were administered subcutaneously in a volume of 0.6 ml/kg [with the exception of CP 93,129 that was administered intracerebroventricularly because this compound does not penetrate the blood–brain barrier].
Intracerebroventricular surgery
Animals that were to receive CP 93,129 pretreatments were anesthetized (n = 7; 1.0–1.5% halothane), mounted in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA), and implanted with a sterilized stainless steel guide cannula (made from a 23 gauge needle to a length of 7 mm) lowered to 1 mm above the lateral cerebral ventricle [from bregma: posterior, −0.6 mm; lateral +1.4 mm; and ventral −3.2 mm (from skull); atlas of Paxinos and Watson (1986)]. The cannula was secured to the skull with stainless steel screws and cranioplastic cement. Dummy stylets made of 0.011 inch wire that extended to the tip of the cannulae were inserted to prohibit cannula blockade and entry of foreign particles. All animals were allowed 7 d of recovery before returning to cocaine self-administration testing.
Intracerebroventricular drug administration
Injectors (made from 30 gauge needles; 8 mm in length) were connected to 70 cm lengths of polyethylene-10 tubing that were calibrated and marked for 2 μl volumes and filled with drug solution. Ten minutes before the cocaine self-administration sessions, the dummy stylet was removed from the cannula, and the injector was lowered into the ventricle via the guide cannula. By raising the tubing above the animal’s head, gravity was used to deliver 2 μl of drug solution over a 30–60 sec period. Injectors were left in place for 30 sec after drug delivery and were then replaced with a dummy stylet.
Intravenous cocaine self-administration
Surgery. Rats were anesthetized with a halothane/oxygen vapor mixture (1.0–1.5%) and prepared with chronic intravenous catheters as described previously (Caine et al., 1993) with minor modifications (Emmett-Oglesby and Lane, 1993; Parsons et al., 1996). All animals were allowed to recover for a minimum of 7 d before the start of cocaine self-administration training. Catheters were flushed daily with sterile physiological saline containing heparin (30 USP units/ml).
Apparatus. The self-administration chambers consisted of operant boxes enclosed in sound-attenuating, ventilated environmental cubicles (chambers and cubicles manufactured in-house). Each operant chamber was equipped with a single retractable lever (Model E21–03; Coulbourn Instruments, Allentown, PA) that was extended into the chamber at the start of the session. Drug infusions were delivered by a syringe pump equipped with a 5 rpm motor (Model A; Razel Scientific Instruments, Stanford, CT) activated for 4 sec to deliver drug in a volume of 0.1 ml via a stainless steel liquid swivel and a polyethylene tube attached to the catheter on the animal’s back.
Self-administration training. Before surgery, rats were food restricted (20 gm per rat per day) and allowed to press a lever for 0.45 mg food pellets (Bio-Serve, Frenchtown, NJ). After stable responding maintained by food on a fixed-ratio 5 (FR-5) schedule of reinforcement was achieved, the animals were given ad libitum access to food for the remainder of the experiment. The animals were then surgically prepared with chronic jugular catheter implants as described above. After a 7 d recovery period, cocaine self-administration training began in daily 3 hr sessions, 6 d per week, in which lever pressing was reinforced by infusion of 0.25 mg of cocaine on an FR-5 schedule of reinforcement. With the exception of the first self-administration session, all sessions commenced with two noncontingent infusions of cocaine. The lever was then extended, after which time the completion of each ratio requirement resulted in an injection signaled immediately by a cue light that was located above the lever and that remained lit for a 20 sec “timeout” period, during which responses were recorded but not reinforced. Self-administration training sessions continued until the total number of drug infusions per session stabilized to within ±10% for 3 consecutive days (baseline criterion). The integrity of the intravenous catheter was tested whenever an animal not receiving drug pretreatments deviated substantially from baseline self-administration performance. If intravenous blood could not be readily withdrawn via the catheter, 0.1 ml of the ultra short-acting barbiturate anesthetic Brevital Sodium (1% methohexital sodium; Eli Lilly, Indianapolis, IN) was administered through the catheter, and the animal was observed. Animals that did not exhibit prominent signs of anesthesia (pronounced loss of muscle tone) within 3 sec of intravenous injection were later anesthetized with halothane, and the faulty catheter was examined for leaks and repaired, or the animal was recatheterized in the contralateral jugular vein.
Experiment I: effects of the 5-HT1B agonists RU 24969, CP 94,253, and CP 93,129 on FR-5 cocaine self-administration. After the baseline criterion was met with the training dose of cocaine, the effect of RU 24969 pretreatment (1 mg/kg) on the cocaine self-administration dose–effect function was examined using cocaine doses of 0, 0.015, 0.03, 0.06, 0.125, and 0.25 mg per infusion. All animals (n = 8) were tested with each cocaine dose using a within-subject experimental design for dose, with different doses tested between sessions. The order of cocaine dose presentation was randomized between animals. A stability criterion of three consecutive self-administration sessions with less than ±10% variation in the total number of infusions earned was established for each cocaine dose [including the 0 dose (saline)] before pretreatment tests with RU 24969 or vehicle. To examine the dose dependency of the effects of RU 24969 on cocaine self-administration, we also pretreated animals with RU 24969 (0.3 and 3.0 mg/kg) before the self-administration of the 0.125 mg dose of cocaine per infusion (a dose on the descending limb of the dose–effect function). Thus, after completion of this experiment, all rats received both saline and RU 24969 (1.0 mg/kg) pretreatments before the self-administration of all cocaine doses in the dose–effect determination, in addition to receiving 0.3 and 3.0 mg/kg doses of RU 24969 before the self-administration of cocaine (0.125 mg/infusion). Pretreatments were administered 15 min before testing and were given in a randomized order between animals.
Two separate groups of animals (n = 7 per group) were used to investigate the dose-dependent effects of CP 94,253 and CP 93,129 on responding for a dose of cocaine on the descending limb of the dose–effect function (0.125 mg/infusion). In addition, a dose of cocaine found to be too low to maintain responding (0.03 mg/infusion) was made available after pretreatment with a midrange dose of either 5-HT1B agonist. Animals in the first group were pretreated with saline or CP 94,253 (0.3, 1, or 3 mg/kg) before the self-administration of cocaine (0.125 mg/infusion) and with CP 94,253 (1 mg/kg) before the self-administration of cocaine (0.03 mg/infusion). Animals in the second group received saline or CP 93,129 (3 or 10 μg, i.c.v.) before the self-administration of cocaine (0.125 mg/infusion) and CP 93,129 (3 μg) before the self-administration of cocaine (0.03 mg/infusion). Baseline criterion for stable self-administration was met before each test day (as described above). All animals in each group were tested with each of the 5-HT1B pretreatment doses, which were administered in a randomized order between animals. CP 94,253 was administered 15 min before testing, and CP 93,129 was administered 10 min before testing.
Experiment II: effects of the 5-HT1B agonists RU 24969 and CP 94,253 and of the 5-HT1A agonist 8-OH-DPAT on progressive ratio cocaine self-administration. For this schedule of reinforcement, the response requirement (i.e., the number of lever responses required to receive a drug infusion or “ratio”) was increased in the following manner. For each of the first eight cocaine infusions, the ratio was increased by 1; for each of the next eight infusions, the ratio was increased by 2; and for each of the next eight cocaine infusions, the ratio was increased by 4. Thereafter, the ratio was increased by 8 for each cocaine infusion (Caine and Koob, 1995). A 20 sec timeout period followed all cocaine infusions in which responses were recorded but had no programmed consequence. Sessions on this schedule were terminated when >1 hr had elapsed since the last self-administered cocaine infusion. The breaking point was defined as the highest completed ratio in a session (see Data analyses). The effect of the cocaine unit dose on the breaking point was tested with cocaine doses of 0.125, 0.25, and 0.5 mg/infusion using a within-subjects design (n = 7). Cocaine doses were tested in separate sessions, and the order of dose presentation was randomized between animals. A cocaine dose of 0.125 mg/infusion was used for all subsequent pretreatment tests. Separate groups of animals were then pretreated with either RU 24969 (0, 0.3, 1.0, and 3.0 mg/kg;n = 7), CP 94,253 (0, 0.3, 1.0, and 3.0 mg/kg;n = 9), or 8-OH-DPAT (0, 0.03, 0.1, and 1.0 mg/kg;n = 7) 15 min before the start of progressive ratio cocaine self-administration (0.125 mg/infusion). The 0 dose for each drug was 0.9% saline. The pretreatment order was randomized between animals. Before cocaine doses were changed or drug pretreatment tests were conducted, animals had to meet the baseline criterion of three consecutive self-administration sessions with less than ±10% variation in the total number of infusions earned.
Experiment III: effects of the 5-HT1B partial agonist GR 127,935 and of the 5-HT1A antagonist p-MPPI on CP 94,253-induced alterations in progressive ratio cocaine self-administration. Separate groups of animals received either GR 127,935 (0, 0.3, 1, 3, and 10 mg/kg; n = 9) or p-MPPI (0, 1, 3, and 10 mg/kg; n = 7) before either CP 94,253 (1 mg/kg) or saline, followed by cocaine self-administration (0.125 mg/infusion) on a progressive ratio schedule. The 0 dose for each drug was 0.9% saline. GR 127,935 and p-MPPI were administered 25 min before the self-administration sessions, and CP 94,253 was administered 15 min before the sessions. Pretreatment doses were randomized between animals, and all animals in each group received all pretreatments. Before cocaine doses were changed or drug pretreatment tests were conducted, animals were required to meet the stability criterion of three consecutive self-administration sessions with less than ±10% variation in the total number of infusions earned.
Experiment IV: intravenous self-administration of the 5-HT1B agonists RU 24969 and CP 94,253. Separate groups of animals (n = 6/group) were trained to self-administer cocaine (0.25 mg/infusion) as described above, and stable responding for cocaine was established. To determine “behaviorally active” intravenous doses of the 5-HT1Bagonists for use in subsequent self-administration tests, we added either RU 24969 or CP 94,253 to the self-administered cocaine solution of each respective group. Doses of 0.003 mg of each 5-HT1Bagonist per infusion were tested first and were increased in subsequent test sessions in half-log increments until 5-HT1B agonist doses were found that reduced cocaine self-administration by 50%. The test sessions were limited to 90 min and were separated by at least three daily sessions in which cocaine was self-administered alone.
After a behaviorally active dose of each agonist was established (i.e., a dose that reduced cocaine self-administration by 50%), the ability of each agonist alone to maintain FR-5 self-administration behavior was investigated. The doses tested were the behaviorally active dose, one dose below and two doses above the behaviorally active dose (incremented by half-log concentrations), and saline. 5-HT1B agonist test sessions were 3 hr in duration and were separated by at least three daily sessions in which cocaine was self-administered alone.
Data analyses. All data from the FR-5 cocaine self-administration experiments were subjected to repeated measures ANOVA with drug pretreatment as the within-subjects factor and with the total number of self-administered infusions per session as the dependent measure. For experiments examining RU 24969-induced changes in the cocaine self-administration dose–effect function, cocaine and RU 24969 pretreatment doses were used as the two factors in a two-way ANOVA with repeated measures on both factors. A significant interaction effect was explored using simple main effects to determine significant effects of RU 24969 at different cocaine doses. A leftward shift of the dose–effect function was defined by a significant cocaine dose × RU 24969 interaction, in the presence of both an RU 24969-induced increase in the self-administration of cocaine doses on the ascending limb and an RU 24969-induced decrease in the self-administration of cocaine doses on the descending limb of the dose–effect function. For the examination of the dose-dependent effects of 5-HT1Bagonist pretreatment (RU 24969, CP 94,253, and CP 93,129) on the self-administration of a single cocaine dose, the effects of agonist pretreatment were analyzed by one-way ANOVA. Comparisons of individual 5-HT1B agonist doses with saline pretreatment were subsequently determined by Fisher’s partial least squares post hoc test.
The dependent measure in the progressive ratio experiments was the total number of infusions obtained per session. Although the breaking point (i.e., the highest completed ratio) has been used as the dependent variable in progressive ratio studies (Hodos, 1961), this measure can be problematic for statistical analyses because the assumption of homogeneity of variance is often violated (Depoortere et al., 1993; Woolverton, 1995; Rowlett et al., 1996). Alternately, the number of infusions obtained per session is a natural logarithmic function of the highest completed ratio, and thus this measure does not violate the assumption of homogeneity of variance. Alterations in the breaking point for cocaine self-administration are also presented in the Results and in the figures as a qualitative measure of changes in behavioral output. However, breaking points were not used for statistical comparisons.
The effects of a drug pretreatment or various unit doses of self-administered cocaine on the total number of cocaine infusions obtained per session were analyzed by repeated measures ANOVA, followed where appropriate by Fisher’s partial least squares post hoc test. To examine the effects of the 5-HT1Bantagonist GR 127,935 on CP 94,253-induced alterations in progressive ratio cocaine self-administration, we used the antagonist and agonist doses as the two factors in a two-way ANOVA with repeated measures. A significant main interaction was explored using simple effects to determine significant effects of different GR 127,935 doses on CP 94,253-induced alterations in cocaine self-administration. This same approach was used to examine the effect of the 5-HT1Areceptor antagonist p-MPPI on CP 94,253-induced alterations in progressive ratio cocaine self-administration.
RESULTS
Experiment I: effects of the 5-HT1B agonists RU 24969, CP 94,253, and CP 93,129 on FR-5 cocaine self-administration
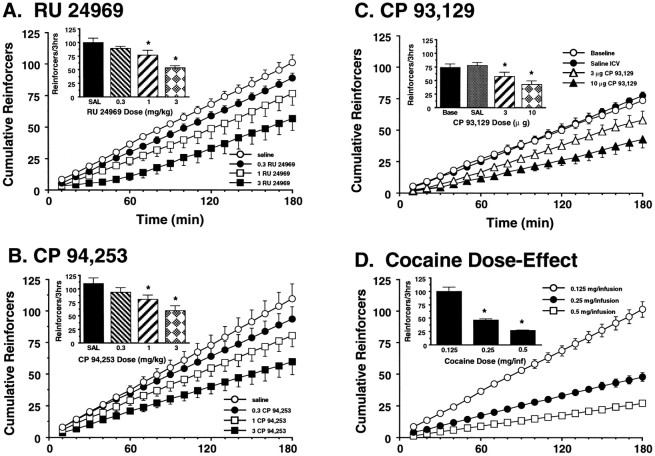
Cocaine self-administration on the FR-5 schedule produced a characteristic inverted U-shaped dose–effect function (Fig.1). Pretreatment with RU 24969 (1 mg/kg) produced a leftward shift of the cocaine dose–effect function. RU 24969 produced a significant overall main effect [F(1,7) = 12.8; p < 0.001] and a significant cocaine dose × RU 24969 interaction [F(4,28) = 12.71; p < 0.0001] when compared with saline pretreatment. The self-administration of cocaine doses on the ascending limb of the dose–effect function was increased by RU 24969 [F(1,7) = 14.5;p < 0.01 for the 0.03 mg of cocaine/infusion dose;F(1,7) = 8.35; p < 0.05 for the 0.06 mg of cocaine/infusion dose] (also Table1), whereas RU 24969 reduced the total intake of cocaine doses on the descending limb of the dose–effect function [F(1,7) = 13.6; p < 0.01 for the 0.125 mg of cocaine/infusion dose;F(1,7) = 5.5; p < 0.05 for the 0.25 mg of cocaine/infusion dose] by increasing the time interval between drug infusions. Self-administration was not reliably maintained when saline was substituted for cocaine (Fig. 1), and there was no significant effect of RU 24969 on responding for saline compared with that obtained with vehicle pretreatment [F(1,7)= 0.312; NS].
Fig. 1.
Effects of RU 24969 pretreatment (1 mg/kg, s.c.) on the cocaine self-administration dose–effect function on an FR-5 schedule of reinforcement (n = 8).Open circles are data obtained after saline pretreatment, and filled circles are data obtained after RU 24969 pretreatment. Symbols adjacent to thevertical axis represent the effects of either saline (open triangle) or RU 24969 (1 mg/kg; open square) pretreatment on lever pressing for intravenous saline infusions. Asterisks denote significant effects (p < 0.05) of RU 24969 versus saline pretreatments on the self-administration of individual cocaine doses.S.A., Self-administration.
Table 1.
Effect of 5-HT1B agonist pretreatment on the FR-5 self-administration of a subthreshold dose of cocaine (0.03 mg/infusion)
| Pretreatment | Number of cocaine infusions per 3 hr | |
|---|---|---|
| Vehicle | Agonist | |
| RU 24969 (1 mg/kg, s.c.) | 12 ± 3 | 135 ± 31* |
| CP 94,253 (1 mg/kg, s.c.) | 10 ± 2 | 133 ± 12* |
| CP 93,129 (10 μg, i.c.v.) | 11 ± 2 | 143 ± 13* |
Data are the total number of cocaine infusions (mean ± SEM) obtained per 3 hr session after pretreatment with either RU 24969 (1 mg/kg, s.c.; n = 8), CP 94,253 (1 mg/kg, s.c.;n = 7), or CP 93,129 (10 μg, i.c.v.;n = 7) along with the number of cocaine infusions obtained after vehicle pretreatment for each respective group.
*Significant 5-HT1B agonist-induced increase (p < 0.05) in responding for cocaine compared to saline pretreatment.
The dose-dependent effects of RU 24969 on FR-5 cocaine self-administration were examined using a cocaine dose on the descending limb of the self-administration dose–effect function (0.125 mg/infusion) (Fig. 2A). RU 24969 dose-dependently (0.3, 1, and 3 mg/kg) reduced the total number of cocaine infusions obtained in a 3 hr session [F(3,28) = 9.284; p < 0.0005] by increasing the interinfusion interval without disrupting the regular pattern of drug intake [shown as the slope of the cumulative reinforcers per unit-time curve in Fig. 2A;F(3,28) = 4.165; p < 0.05]. Although the 0.3 mg/kg dose of RU 24969 did not significantly reduce cocaine self-administration, both the 1 and 3 mg/kg doses produced significant reductions in cocaine intake compared with that obtained with saline-pretreated animals (p < 0.05 for each dose), with the 3 mg/kg dose producing significantly greater reductions than the 1 mg/kg dose (p < 0.05). Saline did not support self-administration when substituted for cocaine, and there was no significant effect of any RU 24969 dose on responding for saline compared with that obtained with saline pretreatment [F(3,28) = 0.031; NS; data not shown]. RU 24969 did not alter the number of responses made during the 20 sec timeout period that followed each cocaine infusion relative to that obtained with saline pretreatment [F(3,31)= 0.329; NS].
Fig. 2.
Dose-dependent effects of RU 24969 (A; n = 8), CP 94,253 (B; n = 7), and CP 93,129 (C; n = 7) pretreatment on the self-administration of a cocaine dose on the descending limb of the FR-5 cocaine dose–effect function (0.125 mg of cocaine/infusion). Each 5-HT1B agonist dose-dependently reduced the total number of self-administered cocaine infusions obtained in a 3 hr session (seeinsets) by producing stable increases in the time interval between infusions (shown by the decreasing slopes of the cumulative reinforcer per time curves; D).A, B, The pretreatment doses of RU 24969 and CP 94,253 are denoted by the following symbols:open circles, saline (SAL); filled circles, 0.3 mg/kg; open squares, 1 mg/kg; andfilled squares, 3 mg/kg. C, The followingsymbols denote data collected after intracerebroventricular pretreatment with the following doses of CP 93,129: open circles, no intracerebroventricular (ICV) injection; filled circles, 2 μl of saline; open triangles, 3 μg of CP 93,129; andfilled triangles, 10 μg of CP 93,129.Asterisks denote significant differences from saline pretreatment (p < 0.05). D, For comparison, the effect of increasing unit doses of cocaine on self-administration behavior in saline-pretreated animals is shown (n = 8). Inset, Increasing doses of cocaine dose-dependently reduced the total number of cocaine reinforcers obtained in a 3 hr session by increasing the interval between infusions. The different cocaine doses are denoted by the following symbols: open circles, 0.125 mg/infusion; filled circles, 0.25 mg/infusion; andopen squares, 0.5 mg/infusion. Asterisksdenote significant differences in cocaine intake compared with the intake of the 0.125 mg of cocaine/infusion dose (p < 0.05). Comparison of these findings to those in A–C suggests that 5-HT1B agonist pretreatment altered FR-5 cocaine self-administration in a manner similar to increasing the unit dose of cocaine.
CP 94,253 also altered cocaine self-administration in a manner consistent with a dose-dependent leftward shift of the cocaine dose–effect function. On the descending limb of the cocaine dose–effect function (0.125 mg of cocaine/infusion), CP 94,253 dose-dependently reduced cocaine intake [F(3,24) = 5.264; p < 0.01] by increasing the time interval between cocaine infusions [F(3,24) = 4.203; p < 0.01; Fig. 2B]. The total number of self-administered cocaine infusions was significantly lower after pretreatment with either 1 or 3 mg/kg CP 94,253 compared with that obtained with saline-pretreated controls (p < 0.05 for each dose). CP 94,253 (1 mg/kg) also significantly enhanced the self-administration of a subthreshold dose of cocaine on the ascending limb of the cocaine dose–effect function [0.03 mg of cocaine/infusion; F(1,13) = 109.502;p < 0.0001; see Table 1]. There was no significant effect of any CP 94,253 dose on the number of lever presses occurring during the 20 sec timeout period that followed each cocaine infusion [F(3,27) = 0.513; NS] or on responding for saline self-administration [F(3,24) = 0.085; NS; data not shown] relative to that obtained with saline pretreatment.
Similar effects were observed after the intracerebroventricular administration of CP 93,129 (3 and 10 μg). On the descending limb of the cocaine dose–effect function (0.125 mg of cocaine/infusion), CP 93,129 dose-dependently reduced cocaine intake [F(2,18) = 8.007; p < 0.005] by increasing the time interval between cocaine infusions [F(2,18) = 6.281; p < 0.01; Fig. 2C]. Both doses of CP 93,129 produced a significant reduction (p < 0.05) in the total number of cocaine infusions obtained relative to that obtained with saline-pretreated controls. CP 93,129 (10 μg, i.c.v.) also significantly enhanced the self-administration of a subthreshold dose of cocaine on the ascending limb of the cocaine dose–effect function [0.03 mg of cocaine/infusion; F(1,13) = 104.04;p < 0.0001; see Table 1]. There was no significant effect of the intracerebroventricular injection procedure on cocaine intake [F(1,12) = 0.217; NS], as demonstrated by the similar patterns of cocaine intake between animals given saline (2 μl, i.c.v.) and animals that received no intracerebroventricular infusion before the self-administration session (Fig. 2C). Neither dose of CP 93,129 altered the number of lever presses occurring during the 20 sec timeout period that followed each cocaine infusion [F(2,10) = 0.13; NS] or the responding for saline self-administration relative to saline pretreatment [F(2,18) = 0.02; NS; data not shown].
All doses of RU 24969, CP 94,253, and CP 93,129 produced stable reductions in cocaine self-administration (0.125 mg/infusion) for the duration of the 3 hr session, as shown by the slopes of the cumulative reinforcer records in Figure 2, A–C.
For comparison, the effect of increasing unit cocaine doses on the descending limb of the cocaine dose–effect function is shown in Figure2D. Cocaine intake was dose-dependently reduced [F(2,21) = 60.18; p < 0.0001] by increasing unit doses of cocaine (0.125, 0.25, and 0.5 mg/infusion). This was a result of a stable, dose-dependent increase in the time interval between cocaine infusions [F(2,21) = 46.062; p < 0.0001]. Thus, RU 24969, CP 94,253, and CP 93,129 each produced alterations in cocaine self-administration that were similar to the effects of increasing the unit dose of cocaine in saline-pretreated animals.
Experiment II: effects of the 5-HT1B agonists RU 24969 and CP 94,253 and the 5-HT1A agonist 8-OH-DPAT on progressive ratio cocaine self-administration
In saline-pretreated animals, increasing unit cocaine doses produced a dose-dependent increase in the total number of cocaine infusions obtained in a progressive ratio session [Fig.3C; main effect of cocaine dose; F(2,18) = 4.921; p < 0.05]. The breaking points (i.e., the highest completed ratio) per session were 90 ± 9, 148 ± 10, and 214 ± 15 for the 0.125, 0.25, and 0.5 mg of cocaine/infusion doses, respectively. Because the time required to reach the breaking point increased significantly with increasing cocaine doses [F(2,18) = 21.413; p < 0.0001], the 0.125 mg of cocaine/infusion dose was used for subsequent tests with 5-HT1 agonist pretreatments to ensure that the effects of the pretreatment drug persisted for the duration of the sessions (see FR-5 schedule results). With the 0.125 mg of cocaine/infusion dose, breaking points were reached in <2.5 hr in 40 of the 46 rats tested on this schedule.
Fig. 3.
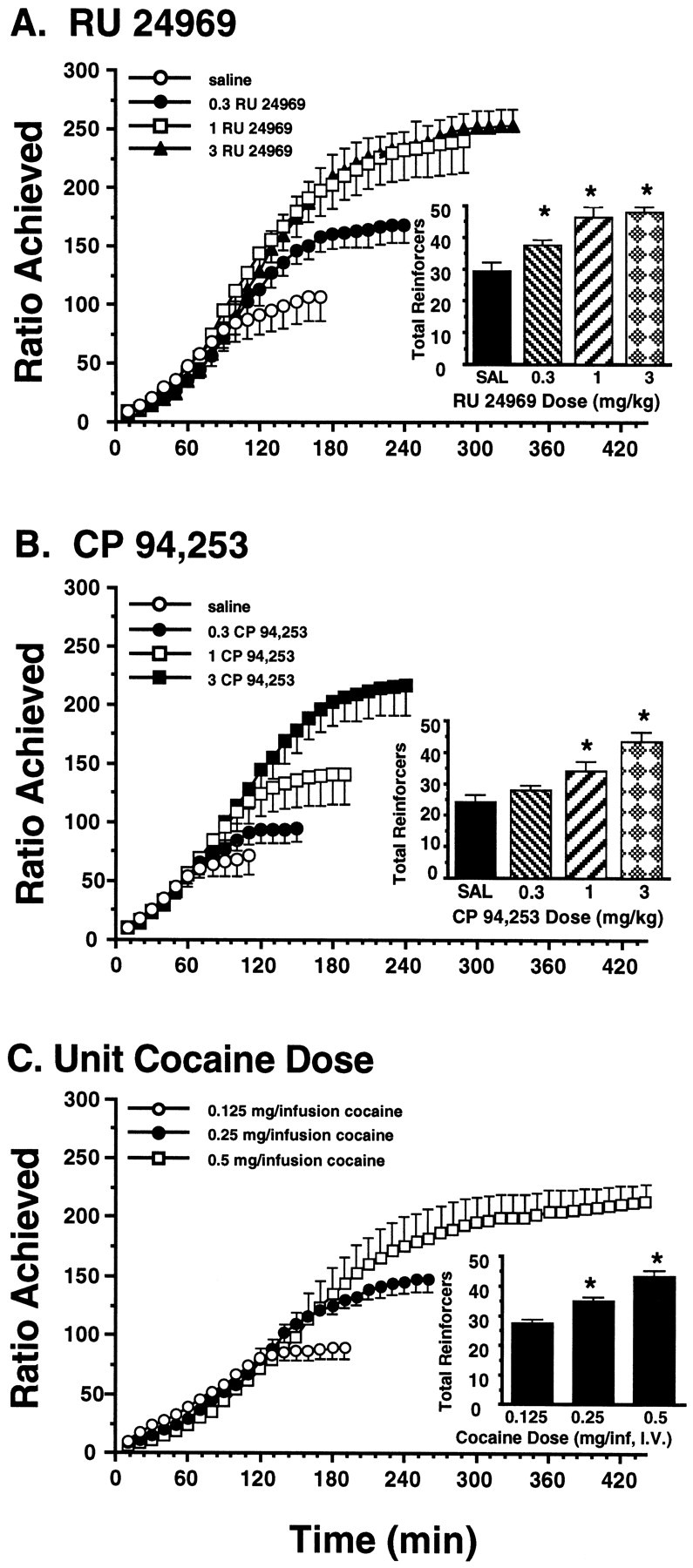
Effects of RU 24969 (A;n = 7) and CP 94,253 (B;n = 9) pretreatments on cocaine self-administration (0.125 mg/infusion) on a progressive ratio schedule of reinforcement. Both 5-HT1B receptor agonists dose-dependently increased the highest completed ratio of responding for cocaine.A, B, The RU 24969 and CP 94,253 pretreatment doses are denoted by the following symbols:open circles, saline (SAL); filled circles, 0.3 mg/kg; open squares, 1 mg/kg; andfilled triangles (A) andsquares (B), 3 mg/kg.Insets, The total number of cocaine infusions obtained per 3 hr session are shown. Asterisks denote significant differ ences in cocaine intake compared with saline (p < 0.05). C, For comparison, the effect of increasing unit doses of cocaine on progressive ratio schedule self-administration in saline-pretreated rats is shown (n = 7). Increasing unit doses of cocaine dose-dependently increased the highest completed ratio of responding for cocaine. The unit cocaine doses are denoted by the following symbols: open circles, 0.125 mg/infusion; filled circles, 0.25 mg/infusion; andopen squares, 0.5 mg/infusion. Inset, The total numbers of cocaine infusions obtained in a 3 hr session are shown. Asterisks denote significant differences in the total number of cocaine infusions obtained compared with that obtained with the 0.125 mg/infusion dose (p < 0.05). Comparison of these findings to those in A andB suggests that 5-HT1B agonist pretreatment altered cocaine self-administration on a progressive ratio schedule in a manner similar to increasing the unit dose of cocaine.
RU 24969 dose-dependently increased the total number of cocaine infusions (0.125 mg/infusion) obtained per session [Fig.3A, inset; main effect of RU 24969 dose;F(3,24) = 13.141; p < 0.0001;n = 7]. Relative to the control condition, the 0.3, 1, and 3 mg/kg doses of RU 24969 each significantly increased total cocaine intake (p < 0.05). Both the 1 and 3 mg/kg doses of RU 24969 produced significantly greater cocaine intake than did the 0.3 mg/kg dose (p < 0.05), although the 1 and 3 mg/kg doses were not significantly different from each other. The breaking point for this dose of cocaine was 108 ± 16 after saline pretreatment. RU 24969 increased the breaking point to 167 ± 14, 239 ± 25, and 252 ± 14 for the 0.3, 1, and 3 mg/kg doses, respectively. In addition to potentiating the breaking point of responding for cocaine, RU 24969 dose-dependently increased the time taken to reach the breaking point of responding [F(3,24) = 10.102; p < 0.0005], similar to the effect of increasing the unit dose of self-administered cocaine (compare Fig. 3A withC).
CP 94,253 also dose-dependently increased the total number of cocaine infusions (0.125 mg/infusion) obtained per session [Fig.3B, inset; main effect of CP 94,253 dose;F(3,32) = 10.833; p < 0.0001;n = 9]. Relative to the control condition, the 1 and 3 mg/kg doses of CP 94,253 produced significant elevations in total cocaine intake (p < 0.05). The 3 mg/kg dose of CP 94,253 produced significantly greater effects than did either the 0.3 or 1 mg/kg doses (p < 0.05). The breaking point of responding was 71 ± 16 after saline pretreatment. CP 94,253 increased the breaking point to 94 ± 12, 141 ± 26, and 216 ± 25 for the 0.3, 1, and 3 mg/kg doses, respectively. CP 94,253 also dose-dependently increased the time taken to reach the breaking point of responding [F(3,32) = 28.266;p < 0.0001], similar to the effect of increasing the unit dose of self-administered cocaine (compare Fig. 3B withC).
To investigate a potential contribution of 5-HT1A receptor activation to the effects produced by either RU 24969 or CP 94,253, we treated a separate group of animals (n = 7) with the selective 5-HT1A agonist 8-OH-DPAT (Glennon and Dukat, 1991; Hoyer et al., 1994) before progressive ratio cocaine self-administration sessions. In contrast to RU 24969 and CP 94,253, 8-OH-DPAT produced a significant reduction in cocaine intake (Fig.4, inset). There was a significant main effect of 8-OH-DPAT on the total number of cocaine infusions per session [F(3,20) = 4.294;p < 0.05], with a significant reduction in cocaine intake after the 0.3 mg/kg dose of 8-OH-DPAT (p< 0.05) and trends toward reductions with the 0.03 and 1.0 mg/kg doses. The breaking points were 97 ± 17, 73 ± 7, 41 ± 4, and 58 ± 11 for the 0, 0.03, 0.1, and 1 mg/kg doses of 8-OH-DPAT, respectively. Unlike the effects produced by different unit cocaine doses, there were no significant effects of 8-OH-DPAT pretreatment on the time taken to reach the breaking point of responding [F(3,24) = 0.272; NS].
Fig. 4.
Effects of pretreatment with the selective 5-HT1A receptor agonist 8-OH-DPAT on progressive ratio cocaine self-administration (0.125 mg/infusion; n = 7). In contrast to the effects of 5-HT1B agonist pretreatment, 8-OH-DPAT reduced the highest completed ratio of responding for cocaine. The pretreatment doses of 8-OH-DPAT are denoted by the following symbols: open circles, saline (SAL); filled circles, 0.03 mg/kg;open squares, 0.3 mg/kg; and filled squares, 1 mg/kg. Inset, The total number of cocaine infusions obtained in a 3 hr session is shown. Anasterisk denotes a significant difference in the number of cocaine infusions compared with that obtained with saline (p < 0.05). Five of the seven saline-pretreated animals reached the breaking point of responding for cocaine in <2 hr, whereas two animals took substantially longer to reach the breaking point.
Experiment III: effects of the 5-HT1B partial agonist GR 127,935 and the 5-HT1A antagonist p-MPPI on CP 94,253-induced alterations in progressive ratio cocaine self-administration
The selective 5-HT1B/1D receptor partial agonist GR 127,935 (Skingle et al., 1996; Pauwels, 1997) dose-dependently (0.3, 1, 3, and 10 mg/kg) blocked the ability of 1 mg/kg CP 94,253 to increase responding for cocaine on a progressive ratio schedule [significant interaction between CP 94,253 pretreatment and GR 127,935 dose;F(4,32) = 10.997; p < 0.0001;n = 9]. The increase in cocaine intake induced by CP 94,253 was significantly reduced (p < 0.05) by pretreatment with the 3 and 10 mg/kg doses of GR 127,935 (Fig.5A). In addition, GR 127,935 dose-dependently reduced the CP 94,253-induced increase in the time taken to reach the breaking point of responding [Fig. 5B;F(4,40) = 10.571; p < 0.0001] in a manner similar to that of decreasing the unit dose of self-administered cocaine (compare Figs. 5B with 3C). There was no significant effect of GR 127,935 pretreatment alone (0.3, 1, and 10 mg/kg) on cocaine intake [Fig.5A; F(4,32) = 1.948; NS] or on the time taken to reach the breaking point of responding [Fig.5C; F(4,40) = 1.642;p = NS].
Fig. 5.

Effects of the selective 5-HT1B/1Dreceptor partial agonist GR 127,935 on the potentiation of progressive ratio cocaine self-administration induced by CP 94,253 (n = 9). A, GR 127,935 dose-dependently reversed the enhancement of cocaine self-administration produced by 1 mg/kg CP 94,253 but did not alter cocaine self-administration when coadministered with saline.Asterisks denote a significant effect of CP 94,253 on cocaine intake in the presence of GR 127,935 compared with the cocaine intake after pretreatment with the respective dose of GR 127,935 alone.Number signs denote a significant GR 127,935-induced reduction in the effect of CP 94,253 on cocaine intake compared with that obtained by CP 94,253 pretreatment alone. B, The effect of coadministration of GR 127,935 and CP 94,253 on the profile of responding for cocaine is shown. CP 94,253 pretreatment increased both the highest completed ratio of responding for cocaine and the duration of responding for cocaine. Both effects of CP 94,253 were dose-dependently blocked by GR 127,935. The pretreatment doses are denoted by the following symbols:filled circles, saline (SAL) and saline;filled squares, saline and 1 mg/kg CP 94,253;open inverse triangles, 0.3 mg/kg GR 127,935 and 1 mg/kg CP 94,253; open triangles, 1 mg/kg GR 127,935 and 1 mg/kg CP 94,253; open diamonds, 3 mg/kg GR 127,935 and 1 mg/kg CP 94,253; and open leftward triangles, 10 mg/kg GR 127,935 and 1 mg/kg CP 94,253. C, GR 127,935 itself produced no alteration in either the breaking point of responding for cocaine or in the duration of cocaine self-administration. The pretreatment doses are denoted by the following symbols:filled circles, saline; open inverse triangles, 0.3 mg/kg GR 127,935; open triangles, 1 mg/kg GR 127,935; open diamonds, 3 mg/kg GR 127,935; and open leftward triangles, 10 mg/kg GR 127,935.
Pretreatment with the selective 5-HT1A receptor antagonist p-MPPI (Kung et al., 1994; Thielen et al., 1996) induced a dose-dependent (1, 3, and 10 mg/kg) decrease in cocaine self-administration both when animals were pretreated with 1 mg/kg CP 94,253 [F(3,18) = 8.082; p < 0.001] and when animals were pretreated with saline [F(3,18) = 24.188; p < 0.0001; Fig. 6; n = 7]. However, p-MPPI produced no significant effect on the CP 94,253-induced potentiation of cocaine self-administration [interaction between CP 94,253 pretreatment and p-MPPI dose; F(3,18) = 0.123; NS; Fig. 6A]. Although animals self-administered significantly less cocaine when pretreated with a combination of 10 mg/kg p-MPPI and 1 mg/kg CP 94,253 than when pretreated with 1 mg/kg CP 94,253 alone (p < 0.05), the 10 mg/kg dose of p-MPPI also produced a significant reduction in cocaine self-administration when this antagonist was administered alone (p < 0.05). Thus, relative to the cocaine intake after pretreatment with each respective dose of p-MPPI alone, CP 94,253 potentiated cocaine intake to a similar degree regardless of the dose of coadministered p-MPPI. When coadministered with CP 94,253, p-MPPI produced no significant alteration in the time taken to reach the breaking point relative to pretreatment with CP 94,253 alone [F(3,24) = 1.459;p = 0.2507; Fig. 6B]. However, when administered alone, p-MPPI induced a dose-dependent increase in the time taken to reach the breaking point [F(3,24)= 3.903; p < 0.05], despite a dose-dependent reduction in breaking point (Fig. 6C).
Fig. 6.

Effects of the selective 5-HT1Areceptor antagonist p-MPPI on the potentiation of progressive ratio cocaine self-administration induced by 1 mg/kg CP 94,253 (n = 7). A, p-MPPI pretreatment induced a significant and dose-dependent decrease in cocaine self-administration in both saline- and CP 94,253-pretreated animals (denoted by number signs). However, p-MPPI did not alter the ability of CP 94,253 to potentiate cocaine self-administration as shown by a significant increase in cocaine intake after coadministration of p-MPPI and CP 94,253 compared with the cocaine intake obtained after pretreatment with the respective dose of p-MPPI alone (denoted byasterisks). B, The effect of coadministration of p-MPPI and CP 94,253 on the profile of responding for cocaine is shown. CP 94,253 pretreatment increased both the highest completed ratio of responding for cocaine and the duration of responding for cocaine. p-MPPI did not alter the effects of CP 94,253 on the duration of responding for cocaine but decreased the CP 94,253-induced potentiation of responding for cocaine relative to saline/CP 94,253 pretreatment [note, however, that p-MPPI also reduced responding for cocaine when administered alone (seeC)]. The symbols in Bcorrespond to the following pretreatment doses: filled circles, saline (SAL) and saline;filled squares, saline and 1 mg/kg CP 94,253;open inverse triangles, 1 mg/kg p-MPPI and 1 mg/kg CP 94,253; open diamonds, 3 mg/kg p-MPPI and 1 mg/kg CP 94,253; and open triangles, 10 mg/kg p-MPPI and 1 mg/kg CP 94,253. C, p-MPPI itself dose-dependently reduced the breaking point of responding for cocaine and dose-dependently increased the duration of cocaine self-administration. The pretreatment doses are denoted by the following symbols: filled circles, saline; open inverse triangles, 1 mg/kg p-MPPI; open diamonds, 3 mg/kg p-MPPI; and open triangles, 10 mg/kg p-MPPI.
Experiment IV: intravenous self-administration of the 5-HT1B agonists RU 24969 and CP 94,253
Inclusion of either RU 24969 or CP 94,253 (0.01–0.1 mg/infusion) in the self-administered cocaine solution (0.25 mg/infusion) resulted in a dose-dependent decrease in the total number of drug infusions obtained in a 90 min test session (data not shown). This reduction in the number of cocaine infusions obtained per time resulted from a stable increase in the time interval between cocaine infusions, similar to the effects produced when either 5-HT1B agonist was administered as an intraperitoneal pretreatment (Fig. 2). A dose of 0.1 mg/infusion of either 5-HT1B agonist was required to reduce cocaine intake by 50%.
In subsequent test sessions, the ability of saline or 0.03, 0.1, 0.3, and 1.0 mg/infusion of either RU 24969 or CP 94,253 alone to maintain FR-5 self-administration was examined in 3 hr sessions in separate groups of animals (n = 6/group). Neither agonist, at any dose, supported self-administration behavior for longer than 90 min (Fig. 7). Moreover, of all doses of both agonists tested, only the 0.1 mg/infusion dose of CP 94,253 produced a significant increase in the total number of self-infusions obtained relative to that obtained with saline self-administration (p < 0.05). Both RU 24969 and CP 94,253 dose-dependently increased the duration of lever-pressing activity relative to saline [F(4,29) = 6.585;p < 0.001 for RU 24969; F(4,29)= 9.469; p < 0.0001 for CP 94,253] and decreased the high rates of burst-like responding observed during the first minutes of saline self-administration (i.e., “extinction”-like responding). These alterations in the patterning of responses suggest that these agonist doses were behaviorally active. Informal visual observations made during self-administration indicated that infusions of either RU 24969 or CP 94,253 at doses of 0.1 mg/infusion and higher induced fairly robust motor activation during the early portion of the sessions (t = 20–40 min). However, as self-administration progressed (t = 40–90 min) this motor activation was supplanted by mild flat body posture and spontaneous tail flicks.
Fig. 7.

The intravenous self-administration of RU 24969 (A; n = 6) and CP 94,253 (B; n = 6) on an FR-5 schedule of reinforcement. Both 5-HT1B agonists dose-dependently reduced the burst-like extinction behavior observed with saline self-administration (t = 10 min) and dose-dependently increased the duration of operant responding. The infusion doses of RU 24969 and CP 94,253 are denoted by the followingsymbols: open circles, saline;filled circles, 0.03 mg; open squares, 0.1 mg; filled squares, 0.3 mg; and open triangles, 1.0 mg. Insets inA, B, Although CP 94,253 produced an inverted U-shaped drug intake dose–effect function, RU 24969 did not. However, neither agonist supported self-administration behavior for longer than 90 min. Asterisks denote significant differences in drug intake compared with that obtained with saline self-administration (p < 0.05).
DISCUSSION
RU 24969, CP 94,253, and CP 93,129 each altered FR-5 cocaine self-administration in a manner consistent with a leftward shift of the cocaine dose–effect function. Pretreatment with each 5-HT1B agonist lowered the threshold dose of cocaine that supported cocaine self-administration (Table 1). Moreover, each 5-HT1B agonist produced a dose-dependent decrease in the intake of cocaine doses on the descending limb of the dose–effect function by increasing the interval between infusions (Fig.2A–C), similar to the effect produced by increasing the unit dose of self-administered cocaine (Fig. 2D). This latter finding, coupled with the observation that none of the agonists altered responding for saline self-administration or the number of lever presses occurring during a 20 sec timeout period after each cocaine infusion, indicates that the effects of the 5-HT1B agonists were not produced by a nonspecific increase in lever-pressing activity. This observation is particularly important given that 5-HT1B receptor activation increases motor activity (Callaway and Geyer, 1992; Cheetham and Heal, 1993; Ramboz et al., 1996). Thus, RU 24969, CP 94,253, and CP 93,129 each induced alterations in FR-5 cocaine self-administration that are consistent with an enhancement of the reinforcing properties of cocaine (Woods et al., 1978, 1987; Bergman et al., 1990; Caine and Koob, 1995).
The effects of RU 24969 and CP 94,253 on progressive ratio cocaine self-administration were also evaluated. The breaking point of responding on this schedule is presumed to reflect the maximum effort an animal will expend to receive a drug infusion and thus serves as a measure of the reinforcing efficacy of the drug (Depoortere et al., 1993; Markou et al., 1993; Richardson and Roberts, 1996; Rowlett et al., 1996). Treatments that increase the breaking point of responding have been interpreted as enhancing the rewarding efficacy of the self-administered drug (Roberts et al., 1989; Ranaldi et al., 1996;Rodefer and Carroll, 1996). Both RU 24969 and CP 94,253 dose-dependently increased the breaking point of responding for cocaine self-administration (Fig. 3A,B) and produced alterations in the pattern of drug intake that were similar to the effect produced by increasing the unit dose of cocaine in saline-pretreated animals (Fig. 3C). Thus, RU 24969 and CP 94,253 each altered progressive ratio cocaine self-administration in a manner consistent with a potentiation of cocaine reinforcement.
There are at least three lines of evidence that suggest a specific involvement of 5-HT1B receptors in the potentiation of cocaine reinforcement. First, the selective 5-HT1B/1Dreceptor partial agonist GR 127,935 dose-dependently attenuated the effect of CP 94,253 on responding for cocaine (Fig. 5). Second, although CP 94,253 displays a moderate affinity for 5-HT1Areceptors, the selective 5-HT1A receptor antagonist p-MPPI did not alter the ability of CP 94,253 to increase the breaking point of responding for cocaine (Fig. 6). In addition, the selective 5-HT1A receptor agonist 8-OH-DPAT induced opposite effects on cocaine self-administration from those produced by the 5-HT1B receptor agonists (Figs. 3, 4). These findings indicate that 5-HT1A receptors are not involved in the potentiation of cocaine reinforcement by CP 94,253. It is interesting, however, that both the 5-HT1A agonist 8-OH-DPAT and the 5-HT1A antagonist p-MPPI decreased the breaking point of responding for cocaine. An explanation for this is presently unclear, although it is possible that this reflects a differential selectivity of these ligands for distinct 5-HT1A receptor subtypes (De Vry, 1995) and/or for 5-HT7 receptors (Tsou et al., 1994;Ying and Rusak, 1997). It is noteworthy that each of the 5-HT1B agonists altered the pattern of cocaine self-administration in a manner similar to the effect produced by increasing the unit cocaine dose, whereas the 5-HT1Areceptor ligands produced effects that were dissimilar to those produced by changes in cocaine dose. This provides qualitative evidence that 5-HT1B receptor stimulation alters the reinforcing properties of cocaine, whereas 5-HT1A receptor manipulations produce nonspecific effects on cocaine self-administration. Third, the three putative 5-HT1Bagonists presently tested display similar affinities for and potencies at 5-HT1B receptors, but they differ considerably in their activities at other 5-HT receptor subtypes (Macor et al., 1990; Koe et al., 1992a,b). Thus, the comparable effects of these agonists on cocaine self-administration point to an involvement of 5-HT1B receptors. Moreover, RU 24969, CP 94,253, CP 93,129, and GR 127,935 have extremely low affinity for dopaminergic, adrenergic, muscarinic, benzodiazepine, or opiate receptors (Tricklebank et al., 1986; Van Wijngaarden et al., 1990; Koe et al., 1992a,b; Skingle et al., 1996). Together, these observations suggest a specific involvement of 5-HT1B receptors in the presently observed potentiation of cocaine reinforcement.
Although the neural mechanisms via which 5-HT1B agonists facilitate cocaine reinforcement are unknown, at least two hypotheses can be proposed. First, because serotonergic lesions increase the reinforcing effects of cocaine (Carroll et al., 1990b; Loh and Roberts, 1990; Richardson and Roberts, 1991), it is possible that 5-HT1B receptor agonists facilitate cocaine reward by reducing 5-HT release via 5-HT1B autoreceptor activation. However, doses of the 5-HT1A agonist 8-OH-DPAT that significantly reduce 5-HT levels (Blier and De Montigny, 1987;Lum and Piercey, 1988; Chen and Reith, 1995) did not enhance the reinforcing effects of either cocaine (Fig. 4) or the dopamine uptake inhibitor 1-[2-[bis(4-fluorphenyl)methoxy]ethyl-4-(3-phenylpropyl)piperazine (GBR 12909) (Parsons et al., 1996). This suggests that acute reductions in 5-HT release do not potentiate stimulant reinforcement. A second potential mechanism is a 5-HT1B receptor-mediated regulation of neurotransmitter systems that modulate the activity of mesoaccumbens dopamine neurons. Examples of this mechanism are the 5-HT1B receptor regulation of both the GABAergic projections to the ventral tegmental area and the substantia nigra (Johnson et al., 1992; Cameron and Williams, 1994;Gongora-Alfaro et al., 1997; Parsons et al., 1998) and the hippocampoaccumbens glutamate projection (Boulenguez et al., 1996). In addition, the reinforcing properties of cocaine may be altered independently of mesolimbic dopamine neurotransmission (Zito et al., 1985; Hubner and Koob, 1990; Robledo and Koob, 1993) by a 5-HT1B receptor regulation of the activity of the accumbopallidal GABA projection (Bruinvels et al., 1993, 1994).
It cannot be determined from the present experiments whether the stimulation of 5-HT1B receptors by the indirect 5-HT agonist properties of cocaine contributes to the reinforcing effects produced by this psychostimulant. Although GR 127,935 was developed as an antagonist at 5-HT1D/1B receptors (Skingle et al., 1993), recent evidence suggests that this ligand displays partial agonist properties both in vitro and in vivo (for review, see Pauwels, 1997). Thus, although GR 127,935 reduced the effect of a full 5-HT1B agonist on cocaine self-administration (Fig. 5A), it may not have induced sufficient 5-HT1B receptor inactivation itself to eliminate a contribution of these receptors to cocaine reinforcement. This hypothesis is supported by the recent finding that the 5-HT1B receptor partial agonist 7-trifluromethyl-4(4-methyl-1-piperazinyl)-pyrrolo[1,2-a]quinoxaline (CGS 12066B) (Cheetham and Heal, 1993) enhances the reinforcing effects of the selective DA uptake inhibitor GBR 12909 but not the reinforcing effects of cocaine (Parsons et al., 1996). This latter observation suggests that the stimulation of multiple 5-HT receptor subtypes during cocaine self-administration masks or counters a mild enhancement of reinforcement produced by partial 5-HT1Breceptor activation. Thus, although it is clear from the present experiments that 5-HT1B receptor agonists potentiate the reinforcing effects of cocaine, final conclusions on the relative contribution of 5-HT1B receptors in the mediation of cocaine reinforcement await the development of selective full-efficacy 5-HT1B receptor antagonists.
It is interesting that neither RU 24969 nor CP 94,253 supported self-administration behavior when they were substituted for cocaine (Fig. 7), suggesting that 5-HT1B receptor stimulation alone is not sufficiently rewarding to support operant responding. Thus the effects of RU 24969 and CP 94,253 on cocaine self-administration may reflect a facilitation of cocaine reinforcement rather than a summation of independent reinforcement produced by the 5-HT1Bagonists and cocaine, respectively. This interpretation is supported by recent reports that 5-HT1B agonists potentiate the interoreceptive cue produced by cocaine at doses that do not produce substantial cocaine-appropriate responding on their own (Callahan and Cunningham, 1995, 1997). Analogously, RU 24969 was recently found to enhance the ability of cocaine to elevate nucleus accumbens dopamine levels at doses that when administered alone do not alter accumbens dopamine (Parsons et al., 1998). Thus, rather than mediating reward, 5-HT1B receptors may play a modulatory role with regard to stimulant reinforcement. It should be noted, however, that behaviors characteristic of 5-HT1A receptor activation were apparent during the 5-HT1B agonist self-administration sessions [i.e., flat body posture and spontaneous tail flicks (Smith and Peroutka, 1986; O’Connell and Curzon, 1996)]. Thus it is possible that the effects of 5-HT1B receptor stimulation were confounded by concurrent 5-HT1A receptor activation. Accordingly, selective 5-HT1B receptor agonists are required to fully assess the efficacy of 5-HT1Breceptor-mediated reinforcement.
In an effort to circumvent the pharmacological limitations of the 5-HT1B receptor ligands currently available, two recent reports have examined cocaine self-administration in transgenic mice lacking 5-HT1B receptors and in their wild-type counterparts (Rocha et al., 1997, 1998). Although no major differences between strains were observed with self-administration on a fixed-ratio schedule, the breaking point of responding for cocaine on a progressive ratio schedule was significantly higher in the 5-HT1B“knock-out” animals than in the wild-types. This latter finding suggests that cocaine has a greater reward value in mice lacking 5-HT1B receptors. However, as noted by Rocha et al. (1998), behaviors observed in 5-HT1B knock-out mice do not always reflect behaviors observed in wild-type mice treated with 5-HT1B receptor antagonists. This implies that compensatory mechanisms may develop in the 5-HT1B knock-out animals that render them more sensitive to the effects of cocaine. Accordingly, it may be prudent to use caution when interpreting results obtained with knock-out mice. It is important, however, to recognize that differences observed between knock-out and wild-type mice may have ontogenic implications in the area of drug abuse.
In conclusion, 5-HT1B receptor activation was found to potentiate the reinforcing properties of cocaine. It is likely that the serotonergic regulation of stimulant reinforcement is dependent on the net balance of effects produced by individual 5-HT receptor subtypes (Parsons et al., 1996; Walsh and Cunningham, 1997). Because recent reports indicate that extended cocaine exposure alters the function of various 5-HT receptor subtypes (Cunningham et al., 1992; Baumann et al., 1993; Baumann and Rothman, 1995, 1996; Simms and Gallagher, 1996), including an upregulation of 5-HT1B receptor function (Levy et al., 1992; L. H. Parsons, P. Sanna, and G. F. Koob, unpublished observations), the present observations allow for a potential involvement of 5-HT1B receptors in the development of cocaine dependence.
Footnotes
This work was supported by National Institute on Drug Abuse Grants DA 11004 (L.H.P.), DA 07348 (F.W.), and DA 08467 (G.F.K.). This manuscript is publication 10916-NP from The Scripps Research Institute. We gratefully acknowledge the gifts of RU 24969 from Roussel UCLAF, CP 94,253 and CP 93,129 from Pfizer, and GR 127,935 from GlaxoWellcome.
Correspondence should be addressed to Dr. Loren H. Parsons, Department of Neuropharmacology, Division of Psychopharmacology, CVN-7, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037.
REFERENCES
- 1.Aronson SC, Black JE, McDougle CJ, Scanley BE, Jatlow P, Kosten TR, Heninger GR, Price LH. Serotonergic mechanisms of cocaine effects in humans. Psychopharmacology (Berl) 1995;119:179–185. doi: 10.1007/BF02246159. [DOI] [PubMed] [Google Scholar]
- 2.Baumann MH, Rothman RB. Repeated cocaine administration reduces 5-HT1A-mediated prolactin secretion in rats. Neurosci Lett. 1995;193:9–12. doi: 10.1016/0304-3940(95)11652-d. [DOI] [PubMed] [Google Scholar]
- 3.Baumann MH, Rothman RB. Chronic cocaine exposure potentiates prolactin and head shake responses to 5-HT2 receptor stimulation in rats. Neuropharmacology. 1996;35:295–301. doi: 10.1016/0028-3908(95)00166-2. [DOI] [PubMed] [Google Scholar]
- 4.Baumann MH, Brockington AM, Rothman RB. Withdrawal from chronic cocaine enhances behavioral sensitivity to the 5-HT2/1C agonist DOI. Biol Psychiatry. 1993;34:576–577. doi: 10.1016/0006-3223(93)90204-q. [DOI] [PubMed] [Google Scholar]
- 5.Bergman J, Kamien JB, Spealman RD. Antagonism of cocaine self-administration by selective D1 and D2 antagonists. Behav Pharmacol. 1990;1:355–363. doi: 10.1097/00008877-199000140-00009. [DOI] [PubMed] [Google Scholar]
- 6.Bhat RV, Baraban JM. Activation of transcription factor genes in striatum by cocaine: role of both serotonin and dopamine systems. J Pharmacol Exp Ther. 1993;267:496–505. [PubMed] [Google Scholar]
- 7.Bhat RV, Cole AJ, Baraban JM. Role of monoamine systems in activation of zif268 by cocaine. J Psychiatr Neurosci. 1992;17:94–102. [PMC free article] [PubMed] [Google Scholar]
- 8.Blier P, De Montigny C. Modification of 5-HT neuron properties by sustained administration of the 5-HT1A agonist gepirone: electrophysiological studies in the rat brain. Synapse. 1987;1:470–480. doi: 10.1002/syn.890010511. [DOI] [PubMed] [Google Scholar]
- 9.Boulenguez P, Rawlins JNP, Chauveau J, Joseph MH, Mitchell SN, Gray JA. Modulation of dopamine release in the nucleus accumbens by 5-HT1B agonists: involvement of the hippocampo-accumbens pathway. Neuropharmacology. 1996;35:1521–1529. doi: 10.1016/s0028-3908(96)00099-8. [DOI] [PubMed] [Google Scholar]
- 10.Bruinvels AT, Palacios JM, Hoyer D. Autoradiographic characterisation and localisation of 5-HT1D compared to 5-HT1B binding sites in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:569–582. doi: 10.1007/BF00166939. [DOI] [PubMed] [Google Scholar]
- 11.Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, Hoyer D, Palacios JM. Localization of 5-HT1B, 5-HT1Dα, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994;33:367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 12.Buydens-Branchey L, Branchey M, Fergeson P, Hudson J, McKerin C. Craving for cocaine in addicted users. Role of serotonergic mechanisms. Am J Addict. 1997;6:65–73. [PubMed] [Google Scholar]
- 13.Caine SB, Koob GF. Pretreatment with the dopamine agonist 7-OH-DPAT shifts the cocaine self-administration dose–effect function to the left under different schedules in the rat. Behav Pharmacol. 1995;6:333–347. [PubMed] [Google Scholar]
- 14.Caine SB, Lintz R, Koob GF. Intravenous drug self-administration techniques in animals. In: Sahgal A, editor. Behavioural neuroscience: a practical approach, Vol 2. Oxford UP; Oxford: 1993. pp. 116–143. [Google Scholar]
- 15.Callahan PM, Cunningham KA. Modulation of the discriminative stimulus properties of cocaine by 5-HT1B and 5-HT2C receptors. J Pharmacol Exp Ther. 1995;274:1414–1424. [PubMed] [Google Scholar]
- 16.Callahan PM, Cunningham KA. Modulation of the discriminative properties of cocaine: comparison of the effects of fluoxetine with 5-HT1A and 5-HT1B receptor agonists. Neuropharmacology. 1997;36:373–381. doi: 10.1016/s0028-3908(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 17.Callaway CW, Geyer MA. Tolerance and cross-tolerance to the activating effects of 3,4-methylenedioxymethamphetamine and a 5-hydroxytryptamine1b agonist. J Pharmacol Exp Ther. 1992;263:318–326. [PubMed] [Google Scholar]
- 18.Cameron DL, Williams JT. Cocaine inhibits GABA release in the VTA through endogenous 5-HT. J Neurosci. 1994;14:6763–6767. doi: 10.1523/JNEUROSCI.14-11-06763.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carroll ME, Lac ST, Asencio M, Kragh R. Fluoxetine reduces intravenous cocaine self-administration in rats. Pharmacol Biochem Behav. 1990a;35:237–244. doi: 10.1016/0091-3057(90)90232-7. [DOI] [PubMed] [Google Scholar]
- 20.Carroll ME, Lac ST, Asencio M, Kragh R. Intravenous cocaine self-administration is reduced by dietary l-tryptophan. Psychopharmacology (Berl) 1990b;100:293–300. doi: 10.1007/BF02244596. [DOI] [PubMed] [Google Scholar]
- 21.Cheetham SC, Heal DJ. Evidence that RU 24969-induced locomotor activity in C57/Bl/6 mice is specifically mediated by the 5-HT1B receptor. Br J Pharmacol. 1993;110:1621–1629. doi: 10.1111/j.1476-5381.1993.tb14010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen NH, Reith MEA. Effects of locally applied cocaine, lidocaine, and various uptake blockers on monoamine transmission in the ventral tegmental area of freely moving rats: a microdialysis study on monoamine interrelationships. J Neurochem. 1994;63:1701–1713. doi: 10.1046/j.1471-4159.1994.63051701.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen NH, Reith MEA. Monoamine interactions measured by microdialysis in the ventral tegmental area of rats treated systemically with (±)-8-hydroxy-2-(di-n-propylamino)tetralin. J Neurochem. 1995;64:1585–1597. doi: 10.1046/j.1471-4159.1995.64041585.x. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham KA. Modulation of serotonin function by acute and chronic cocaine: neurophysiological analyses. In: Hammer RP Jr, editor. The neurobiology of cocaine: cellular and molecular mechanisms. CRC; Boca Raton, FL: 1995. pp. 288–321. [Google Scholar]
- 25.Cunningham KA, Paris JM, Goeders NE. Chronic cocaine enhances serotonin autoregulation and serotonin uptake binding. Synapse. 1992;11:112–123. doi: 10.1002/syn.890110204. [DOI] [PubMed] [Google Scholar]
- 26.Depoortere RY, Li DH, Lane JD, Emmett-Oglesby MW. Parameters of self-administration of cocaine in rats under a progressive-ratio schedule. Pharmacol Biochem Behav. 1993;45:539–548. doi: 10.1016/0091-3057(93)90503-l. [DOI] [PubMed] [Google Scholar]
- 27.De Vry J. 5-HT1A receptor agonists: recent developments and controversial issues. Psychopharmacology (Berl) 1995;121:1–26. doi: 10.1007/BF02245588. [DOI] [PubMed] [Google Scholar]
- 28.Emmett-Oglesby MW, Lane JD. Tolerance to self-administration of cocaine in rats: time course and dose–response determination using a multi-dose method. Drug Alcohol Depend. 1993;32:247–256. doi: 10.1016/0376-8716(93)90089-9. [DOI] [PubMed] [Google Scholar]
- 29.Glennon RA, Dukat M. Serotonin receptors and their ligands: a lack of selective agents. Pharmacol Biochem Behav. 1991;40:1009–1017. doi: 10.1016/0091-3057(91)90121-h. [DOI] [PubMed] [Google Scholar]
- 30.Gongora-Alfaro JL, Hernandez-Lopez S, Flores-Hernandez J, Galarraga E. Firing frequency modulation of substantia nigra reticulata neurons by 5-hydroxytryptamine. Neurosci Res. 1997;29:225–231. doi: 10.1016/s0168-0102(97)00092-8. [DOI] [PubMed] [Google Scholar]
- 31.Hodos W. Progressive-ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 32.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PPA. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 33.Hubner CB, Koob GF. The ventral pallidum plays a role in mediating cocaine and heroin self-administration in the rat. Brain Res. 1990;508:20–29. doi: 10.1016/0006-8993(90)91112-t. [DOI] [PubMed] [Google Scholar]
- 34.Johnson SW, Mercuri NB, North RA. 5-Hydroxytryptamine1b receptors block the GABAb synaptic potential in rat dopamine neurons. J Neurosci. 1992;12:2000–2006. doi: 10.1523/JNEUROSCI.12-05-02000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koe BK, Lebel LA, Fox CB, Macor JE. Characterization of [3H]CP-96,501 as a selective radioligand for the serotonin 5-HT1B receptor: binding studies in rat brain membranes. J Neurochem. 1992a;58:1268–1276. doi: 10.1111/j.1471-4159.1992.tb11338.x. [DOI] [PubMed] [Google Scholar]
- 36.Koe BK, Nielsen JA, Macor JE, Heym J. Biochemical and behavioral studies of the 5-HT1B receptor agonist CP-94,253. Drug Dev Res. 1992b;26:241–250. [Google Scholar]
- 37.Koob GF, Bloom FE. Molecular and cellular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 38.Kuhar ML, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- 39.Kung M-P, Zhuang Z-P, Frederick D, Kung HF. In vivo binding of [123I]4-(2′-methoxy-phenyl)-1-[2′-(N-2′′-pyridinyl)-p-iodobenzamidol-ethyl-piperazine, p-MPPI, to 5-HT1A receptors in rat brain. Synapse. 1994;18:359–366. doi: 10.1002/syn.890180412. [DOI] [PubMed] [Google Scholar]
- 40.Levy AD, Rittenhouse PA, Li Q, Bonadonna AM, Alvarez Sanz MC, Kerr JE, Bethea CL, Van de Kar LD. Repeated injections of cocaine inhibit the serotonergic regulation of prolactin and renin secretion in rats. Brain Res. 1992;580:6–11. doi: 10.1016/0006-8993(92)90920-5. [DOI] [PubMed] [Google Scholar]
- 41.Li M-Y, Yan Q-S, Coffey LL, Reith MEA. Extracellular dopamine, norepinephrine, and serotonin in the nucleus accumbens of freely moving rats during intracerebral dialysis with cocaine and other monoamine uptake blockers. J Neurochem. 1996;66:559–568. doi: 10.1046/j.1471-4159.1996.66020559.x. [DOI] [PubMed] [Google Scholar]
- 42.Loh EA, Roberts DCS. Break-points on a progressive ratio schedule reinforced by intravenous cocaine increase following depletion of forebrain serotonin. Psychopharmacology (Berl) 1990;101:262–266. doi: 10.1007/BF02244137. [DOI] [PubMed] [Google Scholar]
- 43.Lucas JJ, Segu L, Hen R. 5-Hydroxytryptamine1B receptors modulate the effect of cocaine on c-fos expression: converging evidence using 5-hydroxytryptamine1B knockout mice and the 5-hydroxytryptamine1B/1D antagonist GR127935. Mol Pharmacol. 1997;51:755–763. doi: 10.1124/mol.51.5.755. [DOI] [PubMed] [Google Scholar]
- 44.Lum JT, Piercey MF. Electrophysiological evidence that spiperone is an antagonist of 5-HT1A receptors in the dorsal raphe nucleus. Eur J Pharmacol. 1988;149:9–15. doi: 10.1016/0014-2999(88)90035-0. [DOI] [PubMed] [Google Scholar]
- 45.Macor JE, Burkhart CA, Heym JH, Ives JL, Lebel LA, Newman ME, Nielsen JA, Ryan K, Schulz DW, Torgesen LK, Koe BK. 3-(1,2,5,6-Tetrahydropyrid-4-yl)pyrrolo[3,2-b]pyrid-5-one: a potent and selective serotonin (5-HT1B) agonist and rotationally restricted phenolic analogue of 5-methoxy-3-(1,2,5,6-tetrahydropyrid-4-yl)indole. J Med Chem. 1990;33:2087–2093. doi: 10.1021/jm00170a007. [DOI] [PubMed] [Google Scholar]
- 46.Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology (Berl) 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- 47.O’Connell MT, Curzon G. A comparison of the effects of 8-OH-DPAT pretreatment of different behavioural responses to 8-OH-DPAT. Eur J Pharmacol. 1996;312:137–143. doi: 10.1016/0014-2999(96)00496-7. [DOI] [PubMed] [Google Scholar]
- 48.Parsons LH, Smith AD, Justice JB., Jr Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence from chronic cocaine. Synapse. 1991;9:60–65. doi: 10.1002/syn.890090109. [DOI] [PubMed] [Google Scholar]
- 49.Parsons LH, Koob GF, Weiss F. Serotonin dysfunction in the nucleus accumbens of rats during withdrawal after unlimited access to intravenous cocaine. J Pharmacol Exp Ther. 1995;274:1182–1191. [PubMed] [Google Scholar]
- 50.Parsons LH, Weiss F, Koob GF. Serotonin-1B receptor stimulation enhances dopamine-mediated reinforcement. Psychopharmacology (Berl) 1996;128:150–160. doi: 10.1007/s002130050120. [DOI] [PubMed] [Google Scholar]
- 51.Parsons LH, Koob GF, Weiss F (1998) RU 24969, a 5-HT1B/1A receptor agonist, potentiates cocaine-induced increases in nucleus accumbens dopamine. Synapse, in press. [DOI] [PubMed]
- 52.Pauwels PJ. 5-HT1B/D receptor antagonists. Gen Pharmacol. 1997;29:293–303. doi: 10.1016/s0306-3623(96)00460-0. [DOI] [PubMed] [Google Scholar]
- 53.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 2nd Edition. Academic; San Diego: 1986. [Google Scholar]
- 54.Ramboz S, Saudou F, Amara DA, Belzung C, Segu L, Misslin R, Buhot MC, Hen R. 5-HT1B receptor knock out–behavioral consequences. Behav Brain Res. 1996;73:305–312. doi: 10.1016/0166-4328(96)00119-2. [DOI] [PubMed] [Google Scholar]
- 55.Ranaldi R, French E, Roberts DCS. Systemic pretreatment with MK-801 (dizocilpine) increases breaking points for self-administration of cocaine on a progressive-ratio schedule in rats. Psychopharmacology (Berl) 1996;128:83–88. doi: 10.1007/s002130050113. [DOI] [PubMed] [Google Scholar]
- 56.Richardson NR, Roberts DCS. Fluoxetine pretreatment reduces breaking points on a progressive ratio schedule reinforced by intravenous cocaine self-administration. Life Sci. 1991;49:833–840. doi: 10.1016/0024-3205(91)90248-a. [DOI] [PubMed] [Google Scholar]
- 57.Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 58.Roberts DCS, Loh E, Vickers G. Self-administration of cocaine on a progressive ratio schedule in rats: dose–response relationship and effect of haloperidol pretreatment. Psychopharmacology (Berl) 1989;97:535–538. doi: 10.1007/BF00439560. [DOI] [PubMed] [Google Scholar]
- 59.Robledo P, Koob GF. Two discrete nucleus accumbens projection areas differentially mediate cocaine self-administration in the rat. Behav Brain Res. 1993;55:159–166. doi: 10.1016/0166-4328(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 60.Rocha BA, Ator R, Emett-Oglesby MW, Hen R. Intravenous cocaine self-administration in mice lacking 5-HT1B receptors. Pharmacol Biochem Behav. 1997;57:407–412. doi: 10.1016/s0091-3057(96)00444-3. [DOI] [PubMed] [Google Scholar]
- 61.Rocha BA, Scearce-Levie K, Lucas JJ, Hiroi N, Castanon N, Crabbe JC, Nestler JC, Hen R. Increased vulnerability to cocaine in mice lacking the serotonin-1B receptor. Nature. 1998;393:175–178. doi: 10.1038/30259. [DOI] [PubMed] [Google Scholar]
- 62.Rodefer JS, Carroll ME. Progressive ratio and behavioral economic evaluation of the reinforcing efficacy of orally delivered phencyclidine and ethanol in monkeys: effects of feeding conditions. Psychopharmacology (Berl) 1996;128:265–273. doi: 10.1007/s002130050134. [DOI] [PubMed] [Google Scholar]
- 63.Rowlett JK, Massey BW, Kleven MS, Woolverton WL. Parametric analysis of cocaine self-administration under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology (Berl) 1996;125:361–370. doi: 10.1007/BF02246019. [DOI] [PubMed] [Google Scholar]
- 64.Satel SL, Krystal JH, Delgado PL, Kosten TR, Charmey DS. Tryptophan depletion and attenuation of cue-induced craving for cocaine. Am J Psychiatry. 1995;152:778–783. doi: 10.1176/ajp.152.5.778. [DOI] [PubMed] [Google Scholar]
- 65.Simms D, Gallagher JP. Modification of serotonin responses in rat dorsolateral septal nucleus neurons by acute and chronic cocaine. J Pharmacol Exp Ther. 1996;276:1292–1303. [PubMed] [Google Scholar]
- 66.Skingle M, Scopes DIC, Feniuk W, Connor HE, Carter MC, Clitherow JW, Tyers MB. GR 127935: a potent orally active 5-HT1D receptor antagonist. Br J Pharmacol. 1993;110:9P. [Google Scholar]
- 67.Skingle M, Beattie DT, Scopes DIC, Starkey SJ, Connor HE, Feniuk W, Tyers MB. GR 127935: a potent and selective 5-HT1D receptor antagonist. Behav Brain Res. 1996;73:157–161. doi: 10.1016/0166-4328(96)00089-7. [DOI] [PubMed] [Google Scholar]
- 68.Smith LM, Peroutka SJ. Differential effects of 5-hydroxy-tryptamine1A selective drugs on the 5-HT behavioral syndrome. Pharmacol Biochem Behav. 1986;24:1513–1519. doi: 10.1016/0091-3057(86)90477-6. [DOI] [PubMed] [Google Scholar]
- 69.Spealman RD. Modification of behavioral effects of cocaine by selective serotonin and dopamine uptake inhibitors in squirrel monkeys. Psychopharmacology (Berl) 1993;112:93–99. doi: 10.1007/BF02247368. [DOI] [PubMed] [Google Scholar]
- 70.Thielen RJ, Fangon NB, Frazer A. 4-(2′-Methoxyphenyl)-1-[(2′′-pyridinyl)-p-iodobenzamido]piperazine and 4-(2′-methoxyphenyl)-1-[2′-[N(2′′-pyridinyl)-p-fluorobenzamido]ethyl]piperazine, two new antagonists at pre- and postsynaptic serotonin-1A receptors. J Pharmacol Exp Ther. 1996;277:661–670. [PubMed] [Google Scholar]
- 71.Tricklebank MD, Middlemiss DN, Neill J. Pharmacological analysis of the behavioral and thermoregulatory effects of the putative 5-HT1 receptor agonist, RU-24969, in the rat. Neuropharmacology. 1986;25:877–886. doi: 10.1016/0028-3908(86)90014-6. [DOI] [PubMed] [Google Scholar]
- 72.Tsou AP, Kosaka A, Bach C, Zuppan P, Yee C, Tom L, Alvarez R, Ramsey S, Bonhaus DW, Stefanich E, Jakeman L, Eglen RM, Chan HW. Cloning and expression of a 5-hydroxytryptamine7 receptor positively coupled to adenylyl cyclase. J Neurochem. 1994;63:456–464. doi: 10.1046/j.1471-4159.1994.63020456.x. [DOI] [PubMed] [Google Scholar]
- 73.Van Wijngaarden I, Tulp MThM, Soudijn W. The concept of selectivity in 5-HT receptor research. Eur J Pharmacol. 1990;188:301–312. doi: 10.1016/0922-4106(90)90190-9. [DOI] [PubMed] [Google Scholar]
- 74.Walsh SL, Cunningham KA. Serotonergic mechanisms involved in the discriminative stimulus, reinforcing and subjective effects of cocaine. Psychopharmacology (Berl) 1997;130:41–58. doi: 10.1007/s002130050210. [DOI] [PubMed] [Google Scholar]
- 75.Walsh SL, Preston KL, Sullivan JT, Fromme R, Bigelow GE. Fluoxetine alters the effects of intravenous cocaine in humans. J Clin Psychopharmacol. 1994;14:396–407. [PubMed] [Google Scholar]
- 76.Weiss F, Parsons LH, Markou A. Neurochemistry of cocaine withdrawal. In: Hammer RP Jr, editor. The neurobiology of cocaine: cellular and molecular mechanisms. CRC; Boca Raton, FL: 1995. pp. 163–180. [Google Scholar]
- 77.White FJ, Hu X-T, Henry DJ. Electrophysiological effects of cocaine in the rat nucleus accumbens: microiontophoretic studies. J Pharmacol Exp Ther. 1993;266:1075–1084. [PubMed] [Google Scholar]
- 78.White FJ, Hu X-T, Henry DJ, Zhang X-F. Neurophysiological alterations in the mesocorticolimbic dopamine system with repeated cocaine administration. In: Hammer RP Jr, editor. The neurobiology of cocaine: cellular and molecular mechanisms. CRC; Boca Raton, FL: 1995. pp. 99–120. [Google Scholar]
- 79.Wise RA. Neural mechanisms of the reinforcing action of cocaine. In: Grabowski J, editor. Cocaine: pharmacology, effects and treatment of abuse, Vol 50, National Institute on Drug Abuse Research Monograph Series. United States Government Printing Office; Washington, DC: 1984. pp. 15–33. [PubMed] [Google Scholar]
- 80.Woods JH, Herling S, Winger G. Chlorpromazine- and haloperidol-induced changes in some behavioral effects of cocaine and amphetamine. In: Deniker P, Radauco-Thomas D, Villeneuve A, editors. Proceedings of the 10th Congress Collegium Internationale Neuropsychopharmacologicum. Pergamon; New York: 1978. pp. 1485–1502. [Google Scholar]
- 81.Woods JH, Winger G, France CP. Reinforcing and discriminative stimulus effects of cocaine: analysis and pharmacological mechanisms. In: Fisher S, Raskin A, Uhlenhuth EH, editors. Cocaine: clinical and biobehavioral aspects. Oxford UP; New York: 1987. pp. 21–65. [Google Scholar]
- 82.Woolverton WL. Comparison of the reinforcing effects of cocaine and procaine in rhesus monkeys responding under a progressive-ratio schedule. Psychopharmacology (Berl) 1995;120:296–302. doi: 10.1007/BF02311177. [DOI] [PubMed] [Google Scholar]
- 83.Ying SW, Rusak B. 5-HT7 receptors mediate serotonergic effects on light-sensitive suprachiasmatic nucleus neurons. Brain Res. 1997;755:246–254. doi: 10.1016/s0006-8993(97)00102-9. [DOI] [PubMed] [Google Scholar]
- 84.Zito KA, Vickers G, Roberts DCS. Disruption of cocaine and heroin self-administration following kainic acid lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1985;23:1029–1036. doi: 10.1016/0091-3057(85)90110-8. [DOI] [PubMed] [Google Scholar]