Abstract
The electric organ (EO) of the weakly electric fishSternopygus macrurus derives from striated myofibers that fuse and suppress many muscle properties. Mature electrocytes are larger than muscle fibers, do not contain sarcomeres, or express myosin heavy chain (MHC) or tropomyosin. Furthermore, electrocytes express keratin, a protein not expressed in muscle. In S. macrurus the EO is driven continuously at frequencies higher than those of the intermittently active skeletal muscle. The extent to which differences in EO and muscle phenotype are accounted for by activity patterns, or innervation per se, was determined by assessing the expression of MHC, tropomyosin, and keratin 2 and 5 weeks after the elimination of (1) activity patterns by spinal transection or (2) all synaptic input by denervation.
Immunohistochemical analyses showed no changes in muscle fiber phenotypes after either experimental treatment. In contrast, the keratin-positive electrocytes revealed an upregulation of MHC and tropomyosin. Nearly one-third of all electrocytes expressed MHC (35%) and tropomyosin (25%) 2 weeks after spinal transection, whereas approximately two-thirds (61%) expressed MHC 2 weeks after denervation. After 5 weeks of denervation or spinal transection, all electrocytes contained MHC and tropomyosin. Newly formed sarcomere clusters also were observed in denervated electrocytes. The MHC expressed in electrocytes corresponded to that present in a select population of muscle fibers, i.e., type II fibers. Thus, the elimination of electrical activity or all synaptic input resulted in a partial reversal of the electrocyte phenotype to an earlier developmental stage of its myogenic lineage.
Keywords: phenotypic conversion, sarcomere formation, electrocytes, sarcomeric proteins, neural influence of electrocyte phenotype, myogenesis
Electric organs (EOs) are formed from a large variety of skeletal muscles in different species that have evolved independently at least six times (Darwin, 1859; Bennett, 1971;Bass, 1986). For example, EOs form from extraocular, brachial, pectoral, axial, or tail muscles (Bennett, 1971). In each case, light and electron microscopy studies have shown that mesodermal cells differentiate into myoblast-like cells that subsequently form multinucleated myotubes. In most fish, further maturation of the EO results in the disintegration of all myofilaments in parallel with alterations in the morphology of myotubes (Bennett, 1971). Nevertheless, fully developed electrocytes, the current-producing cells of the EO, still share many features with muscle cells (Bennett, 1971).
In the weakly electric fish Sternopygus macrurus, mature electrocytes express some muscle proteins like desmin, actin, sarcomeric α-actinin, and acetylcholine receptors but do not contain sarcomeres nor express tropomyosin or myosin heavy chain (MHC) (Patterson and Zakon, 1997). Furthermore, electrocytes express keratin, a protein not found in mature muscle fibers (Patterson and Zakon, 1996). The regulatory factors that generate and maintain distinct patterns of gene expression in muscle and EO are unknown.
The EO in S. macrurus is innervated by a distinct population of electromotoneurons, whereas skeletal muscle is innervated by somatomotoneurons (Bennett, 1971). Electromotoneurons are driven by medullary neurons in the pacemaker nucleus and activate the EO at a continuous rate of 50–200 Hz (Mills et al., 1992). In contrast, somatomotoneurons in teleost fish innervate muscle fibers that are driven intermittently and at frequencies of <8 Hz (Rome et al., 1992,1996). We wished to determine whether differences in activation patterns, or innervation per se, are responsible for maintaining phenotypic differences between muscle fibers and the myogenically derived EO.
Evidence that innervation might play an important role in determining the phenotypic properties of muscle and EO has been documented in the elasmobranch Torpedo. For example, developmental studies showed that the conversion of muscle to EO did not occur before innervation by the electromotor nerve (Fox and Richardson, 1978, 1979). In addition, Gautron (1974) reported the appearance of myofibril bundles in a sarcomeric-like arrangement throughout the cytoplasm of electrocytes 24 d after the transection of a nerve branch near its entry into the EO in adult Torpedo. Whether this is unique to Torpedo or whether it also occurs in other groups of electric fish is not known.
In the present study, immunohistochemistry and electron microscopy were used to analyze tails from adult S. macrurus that had been electrically “silenced” or denervated for up to 5 weeks. Specifically, changes in tropomyosin, MHC, and keratin expression were assessed after (1) elimination of motoneuronal activity patterns by spinal transection or (2) elimination of all synaptic input by removal of spinal cord segments, resulting in the degeneration of axons innervating muscle and EO. Differentiated muscle fiber phenotypes appeared unaltered by changes in neural input. In contrast, the elimination of neural activity or denervation resulted in the reexpression of sarcomeric proteins and the formation of sarcomere clusters in mature electrocytes.
MATERIALS AND METHODS
S. macrurus, a fresh-water species of knifefish native to South America, was obtained commercially from various fish importers. Adult fish of both sexes, 20–35 cm in length, were housed individually in 15–20 gallon aerated aquaria maintained at 25–28°C and were fed three times weekly. Fish were anesthetized with 2-phenoxy ethanol (1:1500 in tank water) for all surgical procedures, returned to their tanks, and monitored until fully recovered from anesthesia. All surgical wounds were sutured immediately and treated with Woundex (a topical fungicide/antibiotic).
Elimination of neural input
The electromotoneurons that innervate the EO are driven by pacemaker neurons located in the medulla (Fig.1A). Figure 1,B and C, illustrates the two surgical procedures performed in this study. A spinal cord transection at the level of the pectoral fin severs the connection between the pacemaker nucleus and the electromotoneurons, rendering the electromotoneurons silent. Inputs to the electromotoneurons, other than supraspinal, have not been reported in this species. If present, nonsupraspinal connections are not likely to comprise a major source of electrical input, because the EO discharge (EOD) is absent after spinal transection. A spinal cord transection at this level also eliminates all supraspinal activation to somatomotoneurons that are innervating skeletal muscles. Furthermore, it has been reported that the neuromuscular system of teleost fishes lacks muscle spindles and γ-motoneurons (van Asselt et al., 1990). Spinal transection in S. macrurus renders the fish immobile, indicating a substantial reduction in motoneuronal activation of muscle fibers.
Fig. 1.
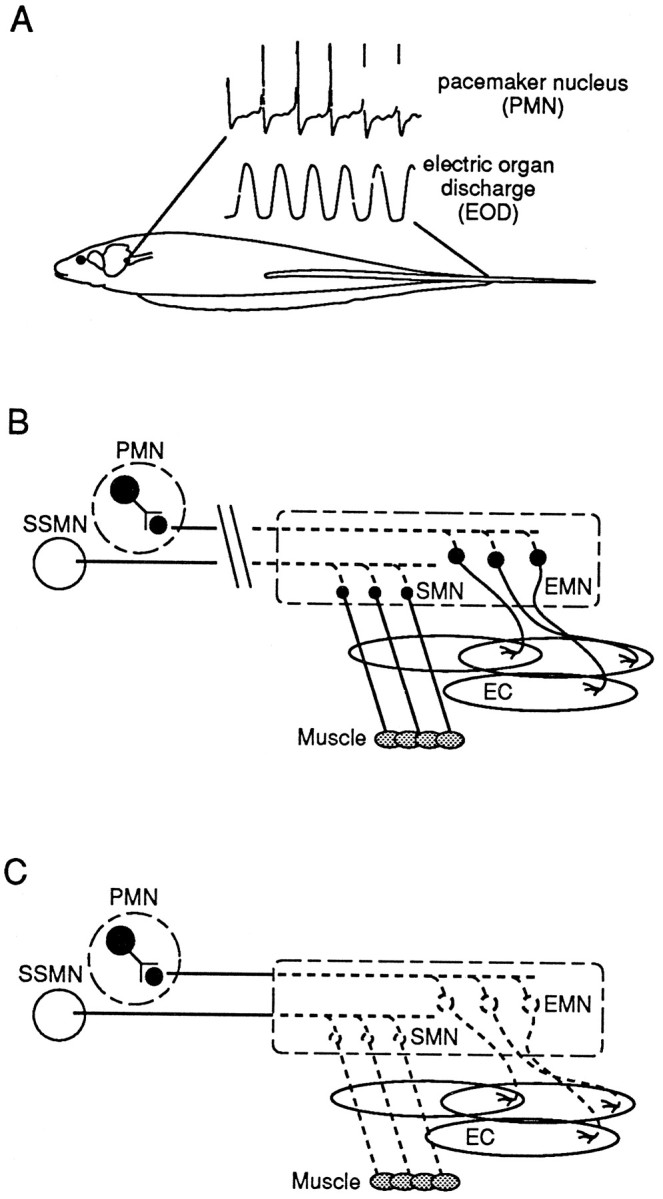
Schematic illustration of the electric organ discharge (EOD)-generating circuitry in S. macrurus and its manipulation in the present study.A, Extracellularly recorded field potentials from the medullary pacemaker nucleus (PMN) demonstrate its extremely regular firing frequency. A pulse from the electric organ follows each spike by a few milliseconds. B, Diagram showing the organization of the EOD-generating circuitry and the site of spinal cord transection that removes supraspinal input to both electromotoneurons (EMN) and somatomotoneurons (SMN) and renders the EO and muscle fibers inactive. Axons from the PMN and the summed supraspinal inputs to SMNs (SSMN) distal to the cut degenerate (boxed region), as depicted by the dotted lines. The EMNs and SMNs remain intact (see Results). EC, Electrocytes. C, Diagram showing the elimination of EMNs and SMNs by the removal of a segment of the spinal cord (boxed region), i.e., denervation. Denervation results in the degeneration of motoneuronal axons, leaving the target muscle fibers and electrocytes denervated.
Despite the absence in EOD and locomotor activity, the possibility that some neurotransmitter or trophic factors still might continue to be released to the target cells and influence their phenotype cannot be excluded. To eliminate all motoneural influence on the distalmost portions of both muscle and EO, we removed the distal 15–18 segments of the spinal cord. Extraction of spinal motoneurons results in the degeneration of their distal axons, leaving target muscle fibers and electrocytes denervated (Fig. 1C). Two survival periods were studied: (1) 2 weeks was the minimum period allowed to ensure the complete degeneration of nerve branches after denervation, and (2) 5 weeks was chosen on the basis of reports that the regeneration of descending axons in other fish does not occur within this survival time (van Asselt et al., 1990).
Spinal cord transection. An incision in the skin and through the underlying muscles on the dorsal side was made at the level of the pectoral fin (proximal one-third of body), and a partial laminectomy was performed to expose the spinal cord. Once exposed, the spinal cord was transected with a scalpel and fine forceps. In sham controls a partial laminectomy was performed, but the spinal cord was left intact. To ensure that the EO was rendered inactive, we placed recording electrodes next to each fish in its aquarium (Mills and Zakon, 1987); the EOD was measured immediately after spinal cord transection or sham surgery and daily thereafter throughout the 2 and 5 week survival periods.
Fish with no EOD also lacked all muscle contraction distal to the transection and lay immobile on their ventral side at the bottom of the tank. Fish generally resumed eating within 1 week after surgery and remained in good health throughout each survival period. Although the EOD was absent in these fish, most fish maintained some mobility of their frontal fins, which allowed them to barely move around their aquaria 2–3 weeks after spinal cord transection. The distal 5–6 cm segment of the tail was amputated 2 or 5 weeks after spinal transection or sham transection for histological examination.
Denervation of muscle and electric organ. To remove the synaptic contacts from motoneurons onto electrocytes and muscle fibers in the distalmost region of the tail, we completely removed the distalmost segment of the spinal cord. An incision was made through the skin on the dorsal surface of the distalmost one-third of the tail. A partial laminectomy was performed to expose the spinal cord, and 5–6 cm of spinal cord (equivalent to 15–18 spinal cord segments) was removed. Care was taken to minimize bleeding and injury to the surrounding tissues. The fish were returned immediately to their tanks and monitored until they had recovered fully from anesthesia.
Because only the distalmost 5–6 cm of the tail was denervated, all fish continued to produce an EOD by using the remaining intact EO (proximal to the site of denervation). Hence, unlike spinally transected fish, EOD was not used as a criterion to assess the extent of denervation of electrocytes throughout their survival period. Instead, the absence of spinal cord and neurofilaments innervating EO and muscle was used to determine a successful denervation (see below). These denervation procedures caused no detrimental effects to the health of the fish. In general, fish maintained normal feeding behavior and motility up to 5 weeks after denervation. In sham-operated fish the distalmost segment of the spinal cord also was exposed, but not removed. The denervated segment of the tail was amputated 2 or 5 weeks after spinal cord removal for histological examination.
Immunohistochemistry
Five fish from each control and each experimental group from each survival period were used for histochemical analysis. Fish were anesthetized, and the distal 5–6 cm tail segment was amputated, mounted on cork, frozen in liquid nitrogen-cooled isopentane, and stored at −80°C until further processing. Immunohistochemical analyses were performed on serial cryostat cross sections and longitudinal sections (12 μm thick) cut at −20°C, mounted on glass slides, and air-dried at room temperature. Tissue sections then were rehydrated in PBS for 5 min, incubated in blocking solution (PBS, 2% BSA, and 5% horse serum) for 30 min, and subsequently incubated overnight at 4°C in the appropriate dilution of specific monoclonal antibodies raised against tropomyosin (CH1), all sarcomeric MHC (MF20), desmin (D76), neurofilament-associated protein (3A10), acidic keratin (AE1), sarcoplasmic reticulum-associated protein (12-101), α-acetylcholine receptor (88b), IIb MHC (BF-F3), I/IIa MHC (N2.261), developmental MHC (DEV), and anti-IIa/IIx MHC (A4.74). The specificity of each anti-MHC antibody used in this study has been determined previously for S. macrurus (Unguez and Zakon, 1998). Sections incubated without primary antibody were used as controls to visualize nonspecific staining.
After incubation in the primary antibody, the sections were washed three times for 5 min in BSA/PBS and were processed for antigen–antibody visualization. Primary antibody was visualized by using either a fluorescein-conjugated secondary antibody (Cappel, ICN Pharmaceuticals, Aurora, OH) or a biotinylated secondary antibody (Vectastain ABC kit, Vector Laboratories, Burlingame, CA). For the latter, an HRP reaction was run to amplify the signal, using diaminobenzidine (DAB) and hydrogen peroxide (H2O2). Similar immunolabeling of the antigens was obtained with both secondaries. Images of labeled cells were recorded with a Nikon Diaphot epifluorescence microscope connected to a Cohu 4915 video camera and a Colorado Video frame store and interfaced to a Macintosh Quadra 800 running National Institutes of Health Image 1.47 software (Bethesda, MD). Based on the immunohistochemical staining with each of the antibodies listed above, the phenotype of muscle fibers (50 per fish) and electrocytes (at least 15 per fish) was assessed by using the tissue sections near the middle of the distal 5–6 cm segment of the tail.
Morphological measurements
The cross-sectional areas (CSA) of electrocytes in tails of fish from each control (n = 5) and each experimental (n = 5) group were measured with National Institutes of Health Image 1.44 processing system. At least 15 electrocytes per fish were sampled from cross sections stained for desmin taken from the middle of the distal 5–6 cm segment of the tail. The mean electrocyte CSA was calculated for each animal, and this number then was used to compute differences among different groups with a twoway ANOVA. Data are presented as mean ± SEM. Significance was set atp < 0.05.
Electron microscopy
Muscle and EO from two adult unoperated and two 5 week denervated tails were examined under a transmission electron microscope (TEM). After surgical removal the tails were fixed in 4% paraformaldehyde/2.5% glutaraldehyde overnight, transferred to sodium phosphate buffer, post-fixed in osmium (2% osmium in sodium phosphate buffer) for 1 hr, dehydrated in an alcohol series and propylene oxide, and embedded in Spurrs’ plastic resin (Polysciences, Warrington, PA). Ultrathin (∼90 nm) tissue cross sections were cut with a diamond knife, stained with uranyl acetate and lead citrate, and examined with the Hitachi HU 11-E TEM (Cell Research Institute of the University of Texas at Austin).
RESULTS
Phenotypic differences between muscle fibers and electrocytes in control S. macrurus
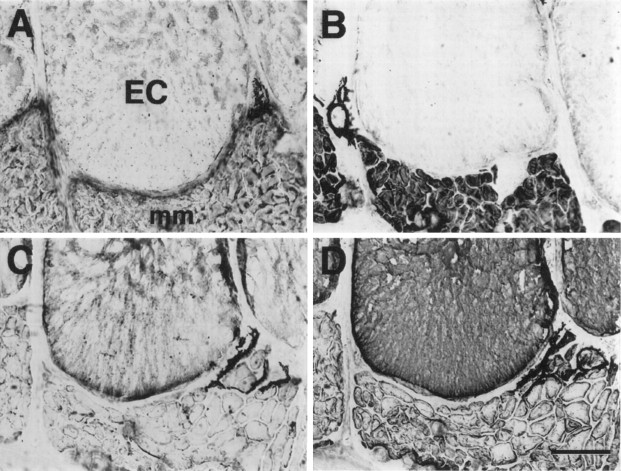
Muscle fibers and electrocytes in adult S. macrurus can be differentiated on the basis of morphology and biochemical properties. For example, mature electrocytes have a cross-sectional area (range, 7181–43,292 μm2) that is remarkably larger than that of muscle fibers (range, 175–1990 μm2; Unguez and Zakon, 1998). Furthermore, unlike muscle fibers, electrocytes express keratin (Fig.2A) but do not react with either anti-MHC (Fig. 2B) or anti-tropomyosin (Fig. 2C) antibodies. Therefore, although electrocytes have a myogenic lineage, differences in morphology and biochemical properties clearly distinguish electrocytes from muscle fibers in the mature tail.
Fig. 2.
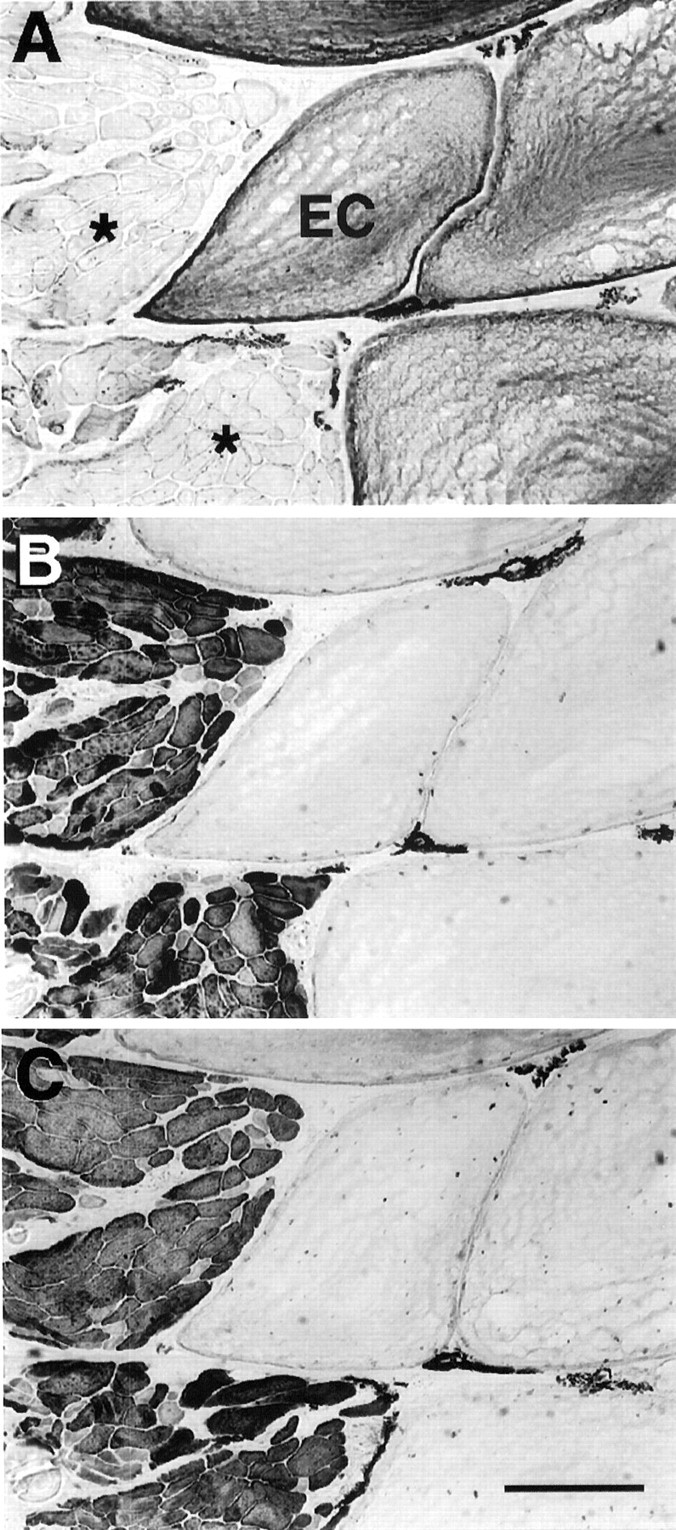
Phenotypic properties of electrocytes and muscle fibers in normal adult tail. Serial cross sections (12 μm thick) of a fish tail immunolabeled with AE1 (anti-keratin; A), MF20 (anti-MHC; B), and CH1 (anti-tropomyosin;C). Keratin (A) was found only in electrocytes (EC). Muscle fibers (asterisks) expressed sarcomeric MHC (B) and tropomyosin (C), two proteins not detected immunohistochemically in electrocytes. Scale bar, 100 μm.
Electrocytes atrophy in the absence of neural input
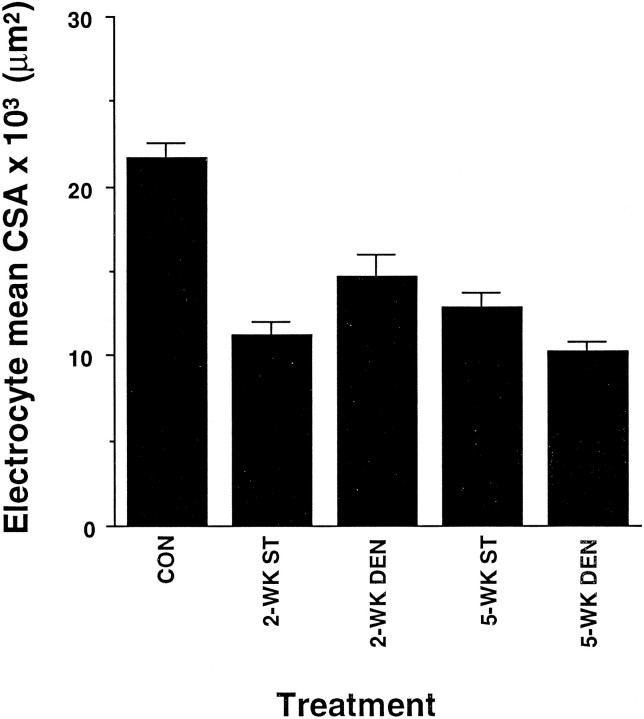
The mean CSA of electrocytes in the distal tail region of fish after 2 or 5 weeks of spinal transection (see Fig.1B) or denervation (see Fig. 1C) was assessed quantitatively. Electrocytes of unoperated and all sham-control fish tails had similar mean CSAs. Thus, these data were combined and used as one control group. In contrast, both spinal transection and denervation resulted in a significant reduction in the mean CSA of adult electrocytes (Fig. 3). The extent to which the mean CSA of electrocytes decreased after either spinal transection or denervation was not significantly different. Furthermore, the reduction in mean CSA found 2 weeks after either surgery was similar to that found after 5 weeks (Fig. 3).
Fig. 3.
Atrophy of electrocytes 2 and 5 weeks after spinal transection or denervation. Each column represents the mean cross-sectional area ± SEM of electrocytes in five tails from unoperated, spinal-transected (ST), and denervated (DEN) fish. The mean cross-sectional area of electrocytes in sham-operated control tails was not significantly different from that of unoperated tails. Hence, the control group includes data from unoperated adult tails and sham-operated controls. The mean values from 2 and 5 week spinal transection and denervated groups were significantly lower than controls (p < 0.05). However, the mean cross-sectional areas of electrocytes from spinally transected and denervated fish groups did not show a significant difference.
Time-dependent expression of MHC and tropomyosin in electrocytes after spinal transection EO phenotype after 2 weeks of inactivity
At 2 weeks after spinal transection the electrocytes had innervation patterns similar to those observed in unoperated fish (Patterson and Zakon, 1996) and sham-operated controls (data not shown). For example, axons were present at the posterior end of the electrocyte where the endplate is found (Fig.4A,B). The spinal cord and spinal nerves were also present and appeared intact within and near the vertebrae, respectively (Fig. 4C). Furthermore, although physiological changes in motoneurons were not assessed after spinal transection, the absence of an EOD indicates a decrement in electrical output by electromotoneurons.
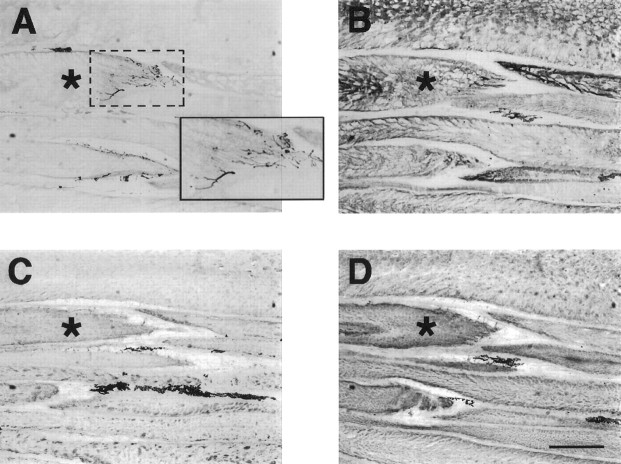
Fig. 4.
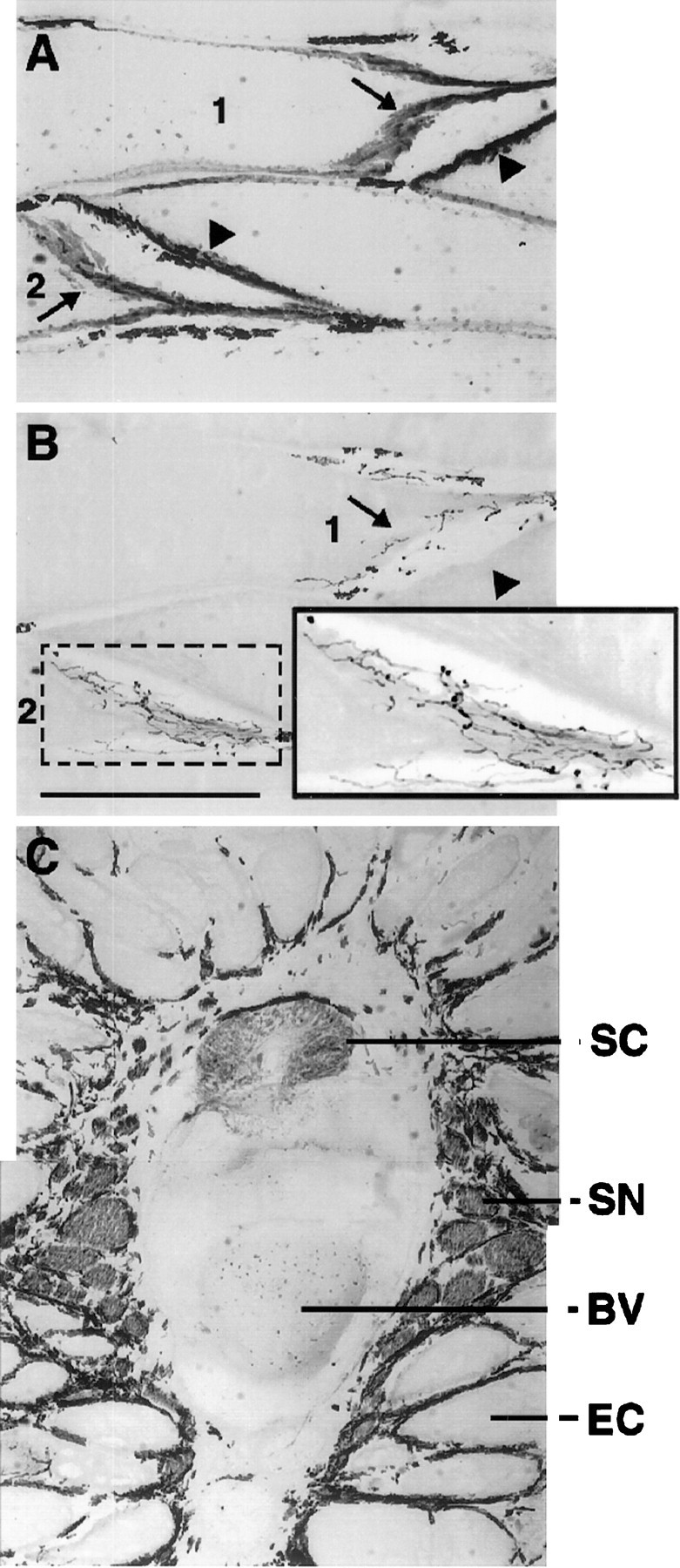
Innervation of electrocytes in 2 week transected fish was similar to that of control. Shown are serial longitudinal sections (12 μm thick) of a tail 2 weeks after spinal transection and immunoreacted with anti-AChR antibody 88b (A) and anti-neurofilament antibody 3A10 (B). The posterior and anterior surfaces of the electrocytes are indicated byarrows and arrowheads, respectively. Antibody 88b labels both the posterior and anterior surfaces of electrocytes, whereas 3A10 labels only axons on the posterior surface, the region innervated by several axons as shown on electrocytes 1 and 2. The posterior region (dotted box) of electrocyte2 is enlarged (solid box) to view clearly the axons labeled by 3A10. C, Part of a cross section (12-μm-thick) of a 2 week transected tail near the caudal end of the anal fin. Immunoreactivity with 3A10 reveals the presence of the spinal cord (SC) and spinal nerves (SN). Dorsal is up; ventral is down.BV, Blood vessel; EC, electrocyte. Scale bar, 500 μm (applies to all three panels).
Electrocytes from 2 week transected fish were labeled with an antibody against keratin (data not shown), a protein expressed in the electrocytes of control fish. In contrast, some electrocytes were found to express the sarcomeric proteins MHC and tropomyosin. Of all of the electrocytes examined after 2 weeks, 35% (n = 100) were immunolabeled by the anti-sarcomeric MHC antibody. In addition, 25% (n = 100) of electrocytes expressing MHC also were labeled with the anti-tropomyosin antibody CH1. Figure5 shows some electrocytes with nerve fibers on their posterior surface (Fig. 5A) that are not immunoreactive with anti-tropomyosin (Fig. 5B) but that have begun to express MHC (Fig. 5C). Interestingly, the expression of MHC in most electrocytes revealed a “patchy” or “cluster”-like immunolabeling pattern (Fig. 5C; see below).
Fig. 5.
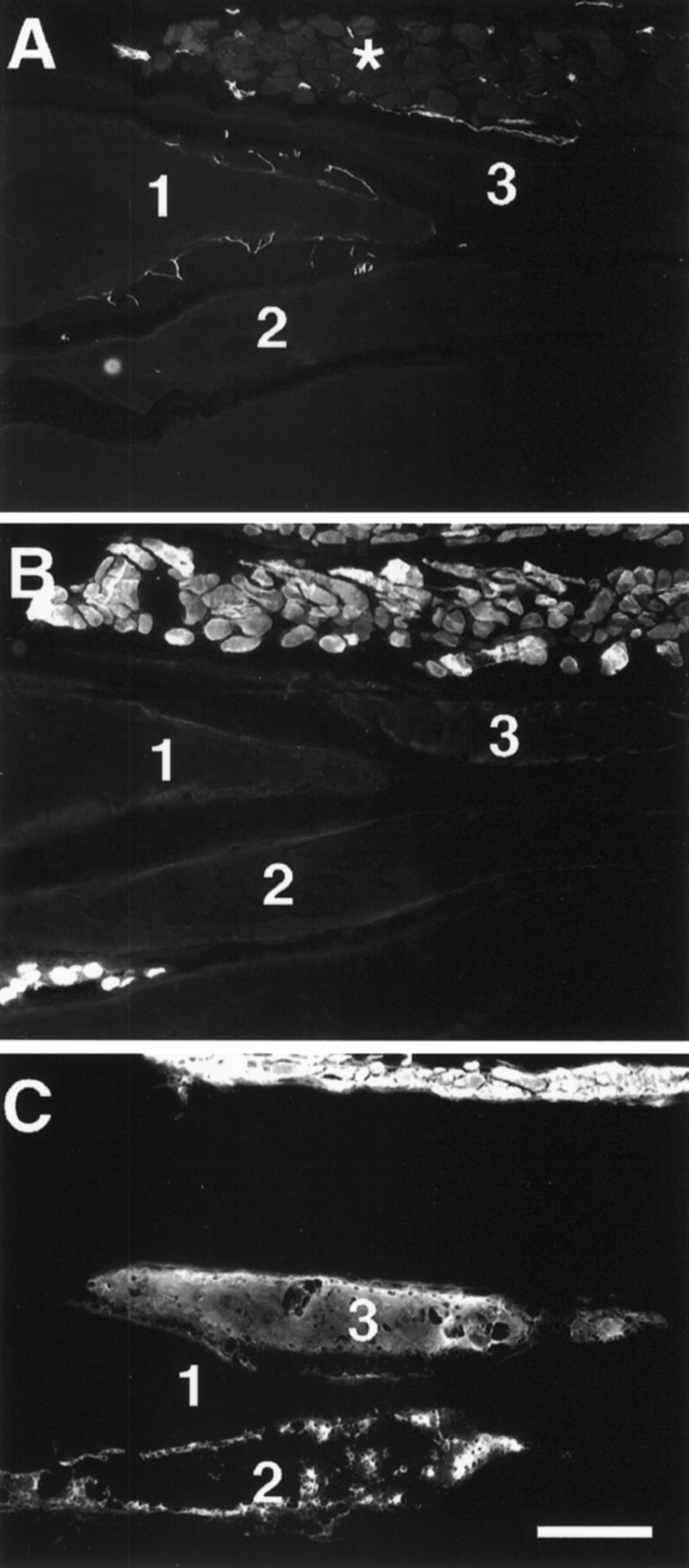
Serial longitudinal sections (12-μm-thick) of a tail from a 2 week transected fish. A, 3A10 labeling shows the presence of nerve fibers on the posterior surface of electrocytes and around the fascicle of muscle fibers (asterisk). All muscle fibers express sarcomeric MHC and tropomyosin, as revealed by immunolabeling with antibodies CH1 (B) and MF20 (C), respectively. In contrast, the electrocytes shown in these panels did not immunoreact with CH1. Electrocytes 1–3 are labeled in each serial section. Note that electrocytes 2 and3, but not 1, were immunolabeled with MF20. MF20 label of electrocyte 2 reveals a patch- or cluster-like pattern, whereas that of electrocyte 3 is more uniform throughout the cytoplasm. Scale bar, 200 μm.
EO phenotype after 5 weeks of inactivity
Even after 5 weeks of electrical inactivity the innervation (Fig.6A) and acetylcholine receptor (AChR) expression pattern (data not shown) of electrocytes remained similar to those of control fish tails. Similarly, all electrocytes maintained their expression of keratin (Fig.6B). However, 5 weeks of electrical inactivity resulted in the expression of both sarcomeric myosin (Fig.6C) and tropomyosin (Fig. 6D) in all (n = 120) of the electrocytes that were analyzed. Keratin expression, on the other hand, was not affected by electrical inactivity.
Fig. 6.
Serial longitudinal sections (12-μm-thick) ofS. macrurus tail after 5 weeks of spinal transection show the presence of axons innervating the posterior surface of electrocytes with 3A10 immunolabel (A). The region within the dotted box is enlarged (solid box) to view the labeled axons more clearly. Expression of keratin in all electrocytes was revealed with AE1 immunoreactivity (B). Electrocytes of 5 week transected fish also expressed MHC and tropomyosin, as seen by the positive labeling with antibodies MF20 (C) and CH1 (D). Note the patchy distribution of the label with MF20 and CH1. The asterisk denotes the same electrocyte in all four serial sections. The dark labelin the extracellular space between electrocytes in C andD corresponds to melanocytes, which always display dark coloration. Scale bar, 500 μm.
Robust expression of sarcomeric proteins in electrocytes after removal of all synaptic input
Fish tails in the 2 and 5 week denervation groups were used for immunohistochemical analysis only if we found no label with the anti-neurofilament antibody 3A10 (Fig.7A) near muscle fibers or near the endplate region of electrocytes (Fig. 7B) and if there was no neural tissue within the vertebral column (Fig.7B).
Fig. 7.
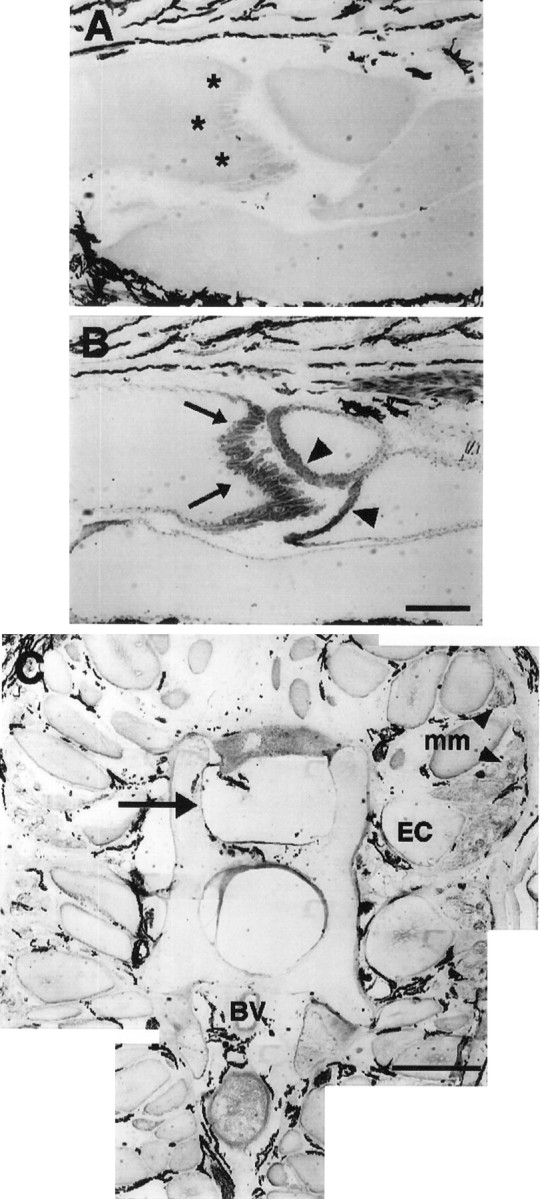
Absence of neural tissue within the region of a tail 2 weeks after denervation. The posterior surface of electrocytes (asterisks) is devoid of innervating axons, as shown by the absence of 3A10 immunolabel (A). Immunolabel of acetylcholine receptors with antibody 88b (B) is revealed in both posterior (arrows) and anterior (arrowheads) surfaces of electrocytes. Melanocytes in the extracellular space around electrocytes always display dark coloration. C, Part of a cross section (12-μm- thick) of a 2 week denervated tail near the caudal end of the anal fin and immunoreacted with 3A10. There is no spinal cord within the vertebral column (arrow) or spinal nerves adjacent to vertebrae (compare with Fig. 4C). Dorsal is up; ventral is down. BV, Blood vessel;EC, electrocyte; mm, muscle fibers (arrowheads in C). Scale bars:A, B, 1 mm; C, 200 μm.
Phenotypic changes 2 weeks after denervation
Two weeks of denervation resulted in the expression of both MHC (Fig. 8A) and tropomyosin (data not shown) in the distal EO. More than one-half of all electrocytes (61%; n = 100) were immunoreactive with anti-MHC. In addition, approximately one-fourth (24%;n = 100) of the MHC-positive electrocytes also were colabeled with anti-tropomyosin. Figure 8 shows electrocytes that expressed MHC (Fig. 8A) but little to no tropomyosin (Fig. 8B). The immunolabel of both anti-sarcomeric antibodies within electrocytes also occurred in a patch-like pattern. In contrast, the labeling of electrocytes with anti-keratin (Fig.8C) or anti-desmin (Fig. 8D) was not altered after 2 weeks of denervation. Furthermore, the staining patterns of keratin and desmin revealed “holes” (regions of negative staining) that appeared to coincide with the patches/clusters of sarcomeric protein immunoreactivity.
Fig. 8.
Serial cross sections (12-μm-thick) of a tail segment that has been denervated for 2 weeks. The electrocyte (EC) shown is labeled with anti-sarcomeric MHC (MF20;A), anti-keratin (AE1; C), and anti-desmin (D76; D), but not with anti-tropomyosin (CH1; B) antibodies. The cytoplasmic labeling of electrocytes with MF20 occurred in a patch-like pattern that was not attributable to cutting or staining artifact, as shown by the cytoplasmic immunolabeling with AE1 (C) or D76 (D). All muscle fibers (mm) immunoreacted with MF20, D76, and CH1, but not with AE1. Thedark label in the extracellular space between electrocytes and muscle fibers corresponds to melanocytes, which always display dark coloration. Scale bar, 100 μm.
Phenotypic changes after 5 weeks of denervation
The absence of neurofilaments in the distal region of fish tails was still evident after 5 weeks of denervation (Fig.9A). Electrocytes continued to express AChRs on their posterior and anterior surfaces (Fig.9B) and keratin (Fig.10A) throughout their cytoplasm. The expression of sarcomeric proteins MHC and tropomyosin was widespread among denervated electrocytes. Specifically, all (n = 185) electrocytes expressed MHC (Fig.10B) and tropomyosin (Fig. 10C).
Fig. 9.
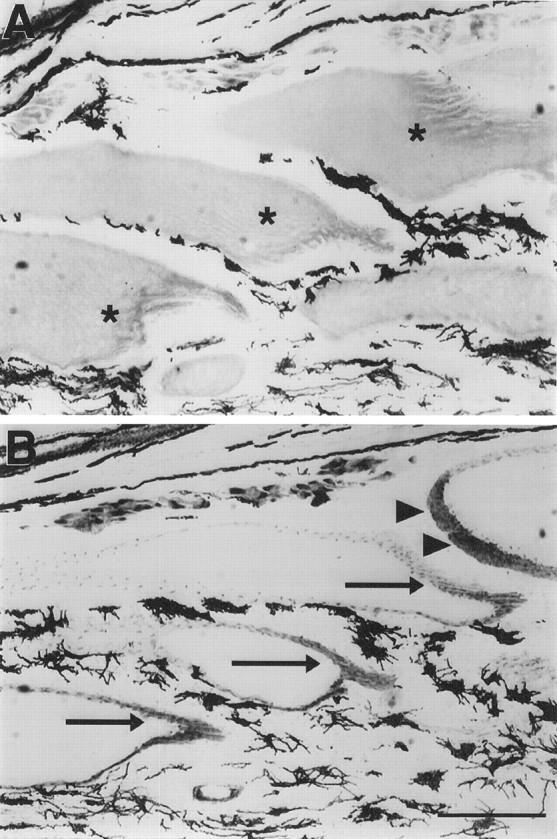
Serial longitudinal sections (12-μm-thick) ofS. macrurus tail after 5 weeks of denervation immunoreacted with anti-neurofilament 3A10 (A) and anti-acetylcholine receptor (B) antibodies. The posterior surface of electrocytes (asterisks) is devoid of innervating axons, as shown by the absence of 3A10 immunolabel (A). Immunolabel of acetylcholine receptors with antibody 88b (B), however, is revealed in both posterior (arrows) and anterior (arrowheads) surfaces of electrocytes. Melanocytes in the extracellular space between electrocytes always display dark coloration. Scale bar, 1 mm.
Fig. 10.
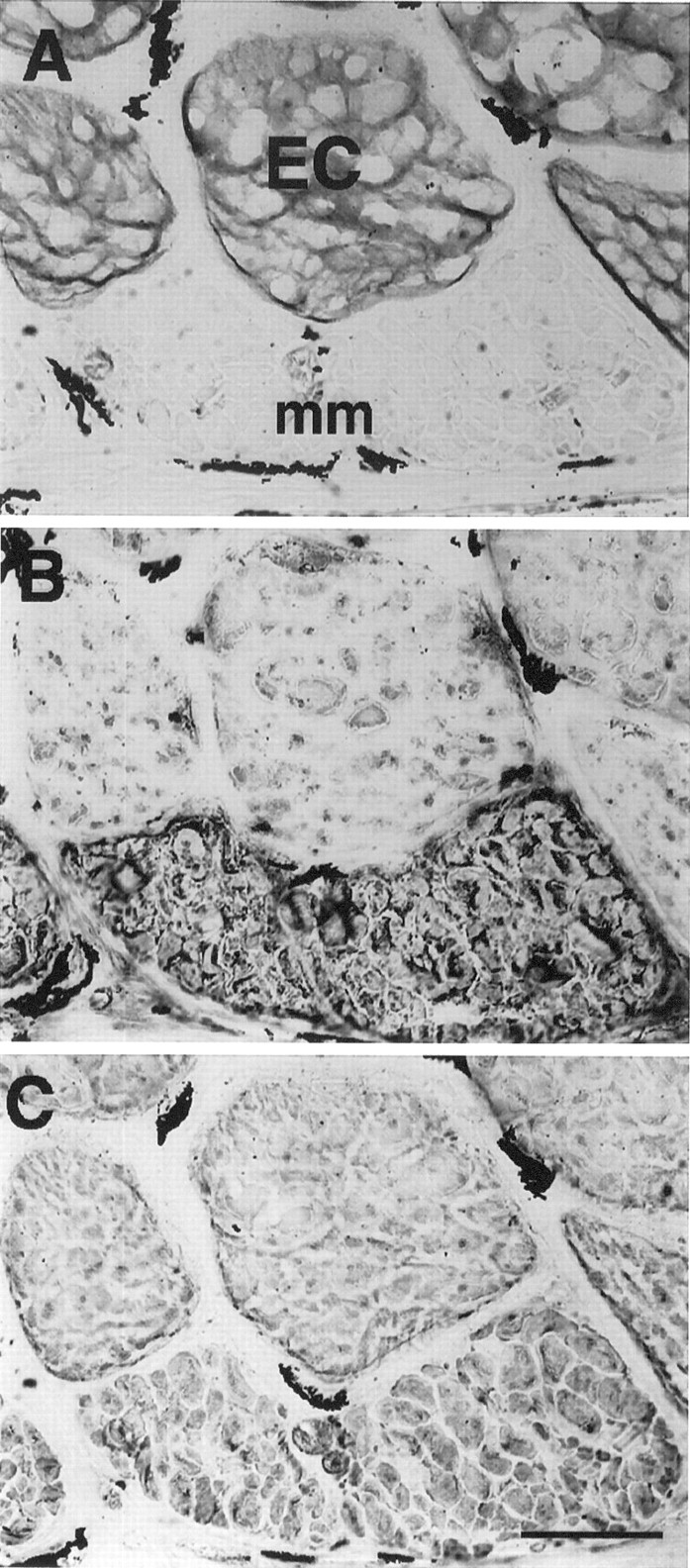
Serial cross sections (12-μm-thick) of a tail segment that has been denervated for 5 weeks and immunoreacted with anti-keratin AE1 (A), anti-sarcomeric MHC MF20 (B), and anti-tropomyosin CH1 (C) antibodies. Electrocytes (EC) express keratin and both sarcomeric proteins. The cytoplasmic labeling of electrocytes with MF20 and CH1 occurred in a patch-like pattern that was not attributable to cutting or staining artifact. Muscle fibers (mm) do not express keratin (A) but do express MHC (B) and tropomyosin (C). Scale bar, 100 μm.
Because MF20 is an antibody that labels all sarcomeric myosin (Bader et al., 1982), whether the expression of MHC in electrocytes after spinal transection or denervation is isoform-specific cannot be ascertained. Thus, to determine further the MHC profile expressed in inactive and denervated electrocytes, we used a battery of antibodies that recognizes different MHC isoforms in mammals and that was used to identify histochemically defined type I and type II fibers in S. macrurus (Unguez and Zakon, 1998). We concentrated on the staining patterns by antibodies BF-F3 and N2.261, which recognize type II and type I muscle fibers in S. macrurus, respectively.
Our results showed that the MHC expressed in electrocytes after either spinal transection (data not shown) or denervation corresponded to an MHC present in type II (Fig.11B), but not type I (Fig. 11C), fibers. On the basis of the finding that electrocytes expressed an MHC found in type II muscle fibers in adult tails, we tested whether other fiber-type specific proteins were upregulated after innervation was altered. Specifically, we used antibody 12-101, which recognizes a sarcoplasmic reticulum-associated protein and labels type II fibers exclusively in adult fish (Unguez and Zakon, 1998). This type II fiber-related protein, however, was not found in electrocytes of fish tails even after 5 weeks of denervation (Fig. 12) or spinal transection (data not shown).
Fig. 11.
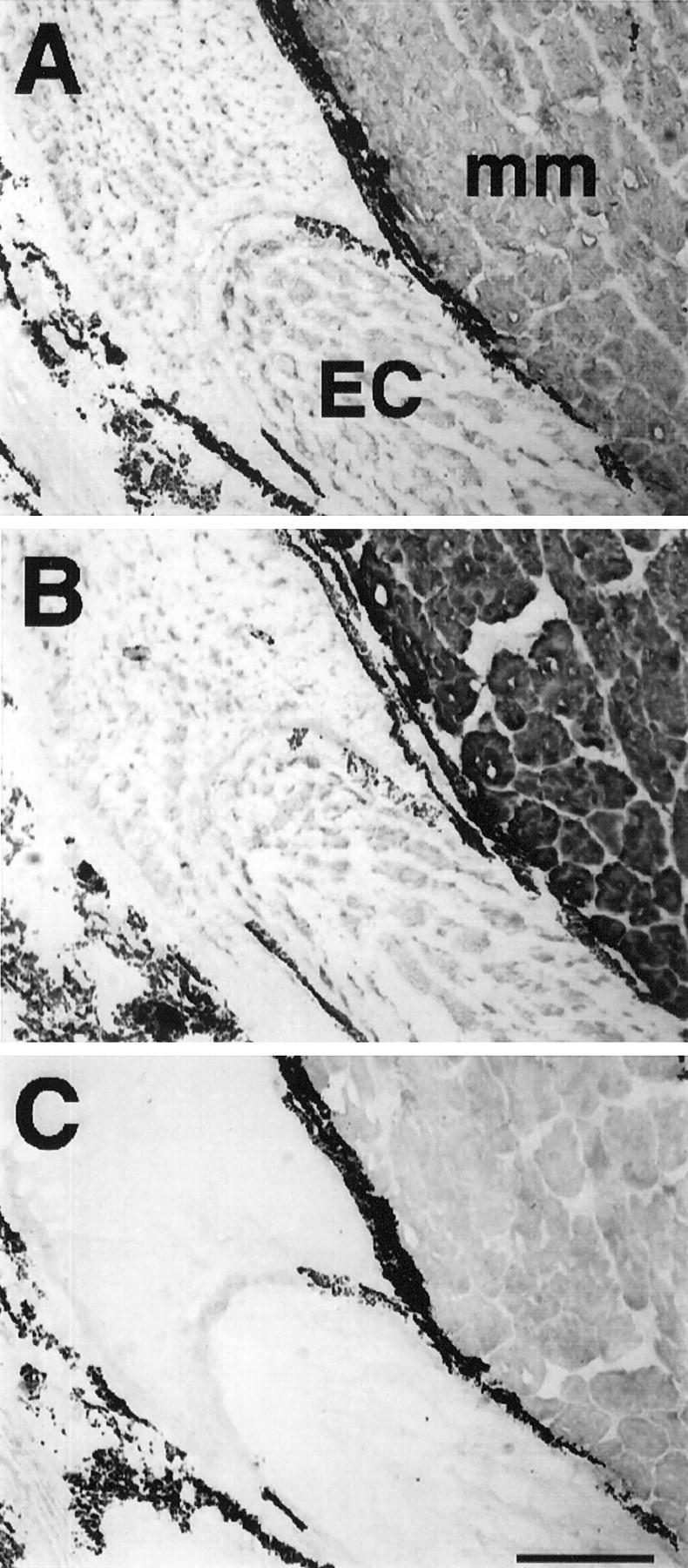
Serial longitudinal sections (12-μm-thick) of a region of a tail 5 weeks after denervation. MF20 (A) labels all sarcomeric MHC in electrocytes (EC) and muscle fibers (mm). The muscle fibers shown adjacent to the electrocytes are those located most centrally (farthest from the epidermis). Electrocytes and adjacent muscle fibers immunoreacted with MF20 (A) and BF-F3 (B), but not with N2.261 (C) antibodies. Note the similar patch-like staining pattern of electrocytes with both MF20 and BF-F3. Thedark label in the extracellular space between electrocytes and muscle fibers corresponds to melanocytes, which always display dark coloration. Scale bar, 400 μm.
Fig. 12.
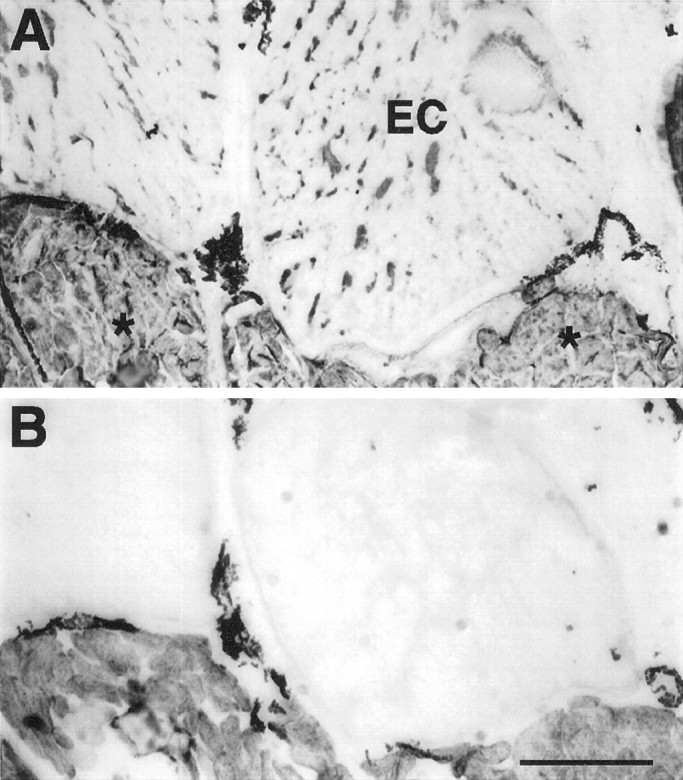
Serial sections of a region of a tail 5 weeks after denervation. Electrocytes (EC) are labeled with MF20 (A), but not with 12-101 (B). In contrast, all centrally located muscle fibers (asterisks) reacted positively with both antibodies. The dark label in the extracellular space between electrocytes and muscle fibers corresponds to melanocytes, which always display dark coloration. Scale bar, 400 μm.
Changes in the biochemical phenotype of electrocytes were accompanied further by the ultrastructural organization of these myofilaments. Electron micrographs of fish tails 5 weeks after denervation revealed organized clusters of myofilaments throughout the cytoplasm of electrocytes (Fig.13A). As shown in Figure13A, these clusters consisted of myofilaments arranged in sarcomeres with well developed Z-lines. The length of a single sarcomere in denervated electrocytes was approximately one-half the length of a sarcomere in muscle fibers from control tails (compare Fig.13A and B). The clusters of myofilaments in sarcomeres are in agreement with the immunohistochemical labeling pattern obtained with MF20 (see Figs. 10A,11A, 12A), CH1 (see Fig.10A) and BF-F3 (see Fig. 11C) antibodies. Furthermore, vesicular structures resembling T-tubules (Fig.13A, arrowheads) also were observed near Z-lines, a finding consistent with observations from the ultrastructure of control muscle fibers (Fig. 13B, arrowheads). The membrane of electrocytes from denervated fish tails also had many convolutions, a structural characteristic not present in the membranes of unoperated (Fig. 13C, asterisks) or sham-operated electrocytes (data not shown). Electrocytes from unoperated (Fig. 13C) and sham-operated control (data not shown) tails did not contain sarcomeres. Instead, the cytoplasm of control electrocytes mainly was devoid of organelles and discernible, organized, filamentous structures (Fig. 13C). Mitochondria (Fig. 13A,C, arrows) and nuclei (Fig. 13A,C, label N) are located peripherally in electrocytes from both denervated and control tails. Muscle fibers also contain many mitochondria (m) in the cell periphery.
Fig. 13.
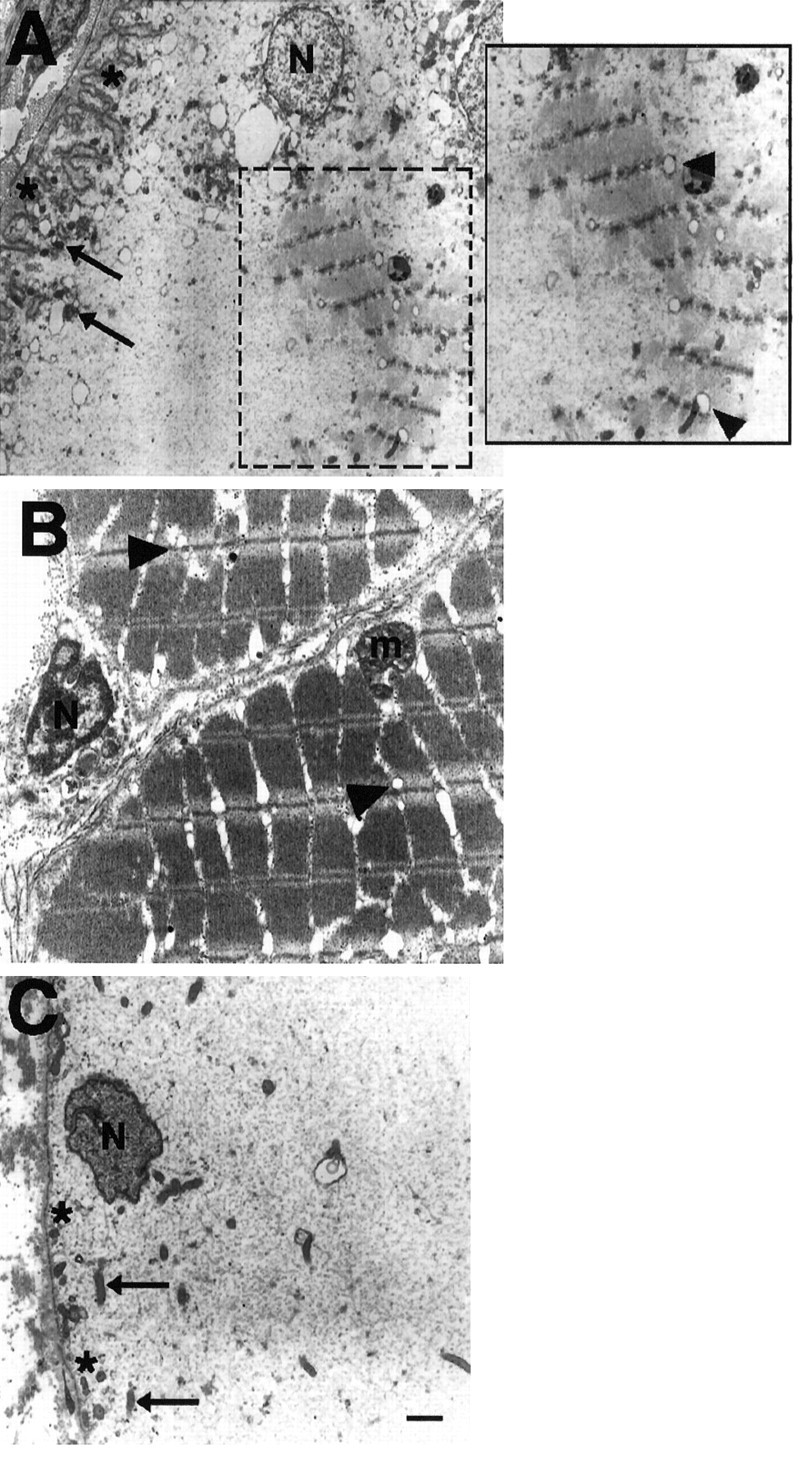
Electron micrographs showing regions from an electrocyte 5 weeks after denervation (A), a muscle fiber (B), and an electrocyte (C) from an unoperated control tail. Note the cluster of myofilaments arranged in sarcomeres within the cytoplasm of a denervated electrocyte (A, boxed region). The boxed region is enlarged for clarity of the structures. Note that T-tubules are evident near the myofilaments and aligned with the Z-lines (arrowheads) as they occur in control muscle fibers. The membrane (asterisks) of denervated electrocytes also had many convolutions, a structural characteristic not present in the membrane of electrocytes from unoperated fish. Electrocytes from control fish tails are devoid of sarcomeres. Mitochondria (arrows) and nuclei (N) are located peripherally in electrocytes from both denervated and control tails. Muscle fibers also contain many mitochondria (m) in the cell periphery. Scale bar, 1 μm.
Together, the immunohistochemical and ultrastructural data reveal that the phenotype of differentiated electrocytes partly reverted to that of a muscle fiber after disruption to its neural input. The phenotypic transformation corresponded not to all fiber types but to that characteristic of type II muscle fibers. Furthermore, similar morphological and biochemical changes occurred in differentiated electrocytes whether electrical activation was eliminated or all synaptic inputs were removed.
Muscle fiber phenotype is unaffected by changes in neural input
The effects of inactivity and denervation on skeletal muscle phenotype also were assessed after 2 and 5 week survival periods. In contrast to the changes that occurred in the biochemical and ultrastructural properties of electrocytes, alterations in the phenotypic properties of muscle fibers were not evident even 5 weeks after either surgical treatment. Muscle fibers maintained their expression of MHC (see Figs. 5C, 10B) and tropomyosin (see Figs. 5C, 10C).
The anti-MHC antibodies used to identify different fiber types in control adult fish (Unguez and Zakon, 1998) revealed no changes from control in the fiber-type populations after either spinal transection or denervation. Distinct fiber-type populations were present, and their original spatial distributions were not altered by either of the two methods that disrupted neural input. For example, as observed in control tails, type I muscle fibers were located peripherally (closest to the epidermis), whereas type II muscle fibers were located centrally (farthest from the epidermis) in spinally transected (data not shown) or denervated regions of the tail (Fig.14). Colabeling with anti-MHC antibodies that label exclusively type I or type II muscle fibers in control tails was not evident among fibers in any spinal transection (data not shown) or denervation (Fig. 14) group. Furthermore, the type II fiber-specific labeling by the sarcoplasmic reticulum-associated protein antibody 12-101 also was restricted to type II fibers (see Fig.12B).
Fig. 14.
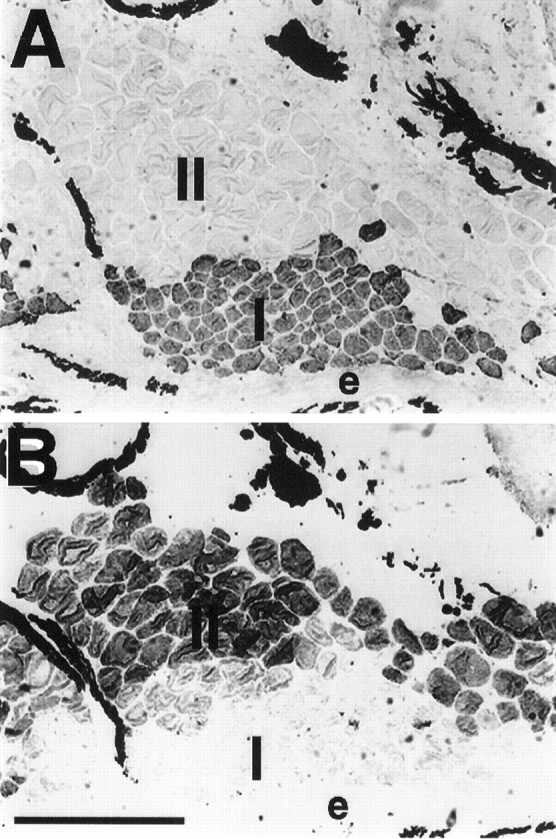
Serial cross sections of a muscle fascicle from a 5 week denervated fish tail that was immunoreacted with N2.261 (A) and BF-F3 (B), MHC antibodies that distinguish type I and type II fibers, respectively. N2.261-positive fibers are located in the periphery (adjacent to the epidermis, e), whereas BF-F3-positive fibers are located more centrally (farther from the epidermis). There was little to no evidence of fibers that immunoreacted with both anti-MHC antibodies. The dark label in the extracellular space between electrocytes and muscle fibers corresponds to melanocytes, which always display dark coloration. Scale bar, 100 μm.
Expression of keratin in muscle fibers was never found (see Fig.10A). In addition, although no quantitative measurements were performed, no obvious changes in the CSA of distinct muscle fiber-type populations were observed. Last, electron micrographs revealed no changes in the muscle fiber ultrastructure after 5 weeks of denervation (data not shown). Therefore, normal phenotypic properties of muscle fibers were maintained in the absence of electrical activity or complete synaptic contact with motoneurons.
DISCUSSION
Our results demonstrate that muscle fibers and electrocytes are influenced differentially by spinal transection or denervation. Whereas distinct muscle fiber populations appeared unaltered, the phenotype of electrocytes partly reverted to a myogenic profile specific of type II muscle fibers. Furthermore, similar morphological and biochemical changes occurred in mature electrocytes after either experimental disruption of their neural input.
Resistance of muscle phenotype to change after neural input alterations
Thus far, few studies have examined the role of innervation on muscle properties in adult fish. In general, these studies have shown a greater independence of muscle properties toward neural control in comparison to muscles of nonpiscine vertebrates. For example, the denervation of muscle fibers of the carp by spinal cord crush resulted in small changes in metabolic markers and contractile protein content (Wittenberger and Coprean, 1977). In the zebrafish (Brachydanio rerio) a similar lesion brought about changes in fiber CSA after 2 weeks, but not in all muscle fiber types (van Asselt et al., 1990). Furthermore, an increase in the number and a change in the distribution of only “intermediate fibers” also were detected (van Raamsdonk et al., 1982). These changes persisted in 10 week denervated zebrafish.
In S. macrurus, denervation of muscle fibers resulted in even fewer profound modifications, because dramatic changes in morphology or MHC-based fiber types were not observed after a 5 week survival period. The present data are in contrast to studies in mammals in which spinal transection or denervation has profound effects on fiber phenotype (Pette and Vrbova, 1985). Our results support fish denervation studies that suggest a greater independence of muscle properties toward neural control in teleosts than in other vertebrates.
Inducible effects by electrical activity versus nonactivity-dependent factors
The strong induction of sarcomeric protein expression clearly demonstrates a regulation of electrocyte phenotype by innervation. The expression of MHC in 35% of electrocytes, with coexpression of tropomyosin in 25%, 2 weeks after spinal transection provides compelling evidence that activation patterns play an instructive role in the maintenance of mature electrocyte phenotype. Furthermore, the effect of spinal transection was not transient, because all electrocytes expressed both sarcomeric proteins after 5 weeks.
The possibility that some electrical activity might remain and continue to influence the EO, however, cannot be excluded from our spinal transection model. Thus, we tested the effect of complete denervation on the electrocyte phenotype. We found a similar time-dependent upregulation of MHC and tropomyosin in the absence of synaptic inputs: (1) expression of MHC in 61% of electrocytes, with coexpression of tropomyosin in 24% after 2 weeks of denervation, and (2) expression of both sarcomeric proteins in all electrocytes after 5 weeks of denervation. Furthermore, similar effects on CSA were observed after both experimental treatments. In summary, the only difference was a greater number of electrocytes expressing MHC 2 weeks after denervation than after spinal transection. The striking similarities observed after either treatment argue for a neuronal influence that is derived primarily, but not completely, from the activation properties of the electromotoneuron. These observations differ from those in studies that use nonpiscine vertebrates in which the effects of denervation on cell phenotype are considerably greater than those of spinal transection (Edgerton et al., 1996).
How neural input might be regulating gene expression is unknown, in large part. Evidence suggests that regulators of transcription play an important role in influencing the differentiation and maintenance of the phenotype of a cell (Ludolph and Konieczny, 1995; Brun et al., 1996; Buckingham and Dexter, 1997). In muscle, myogenic transcription factors have been implied to participate as intracellular mediators through which neural activity regulates gene expression (Eftimie et al., 1991; Witzemann and Sakmann, 1991). The present data provide an excellent basis for future investigations of the identification and function of analogous transcription factors in this system.
Nonactivity-dependent trophic substances also have been identified and shown to have inductive effects primarily on the postsynaptic membrane specialization (Hall and Sanes, 1993). To date, however, nonactivity-dependent trophic factors that affect the contractile protein system of muscle fibers have not been identified. To determine the extent to which the neuronal signal in S. macrurus may represent a “leakage” of neurotransmitter and/or other trophic factor(s), we must rule out the possibility that any neural activity remains after spinal transection. Further experiments (e.g., blocking activity with TTX and/or measuring endplate potentials after spinal transection) would allow us to make such distinctions.
Reversal of the electrocyte phenotype to an earlier developmental stage of its myogenic lineage
The restoration of the myogenic program within mature electrocytes progressed to the formation of nascent sarcomeres, T-tubules, and sarcoplasmic reticulum. Although the length of newly formed sarcomeres was one-half that of sarcomeres in mature muscle, developmental studies of invertebrate (Reedy and Beall, 1993) and vertebrate (Sanger et al., 1986) muscle have shown that nascent sarcomeres and their constituent filaments lengthen during myogenesis. Further investigations would be necessary to determine the temporal sequence of molecular and cellular events in the reconstruction of mature sarcomeres in this system. These studies also would determine whether or not differences in MHC and tropomyosin expression in electrocytes after 2 weeks of denervation versus spinal transection are attributable to differences in transcriptional, translational, or post-translational processes, all processes that are known to influence muscle gene expression (Honda and Epstein, 1990; Cox et al., 1991; Gunning and Hardeman, 1991).
An interesting finding was the expression pattern of these sarcomeric proteins within denervated electrocytes. The patch-like arrangement of MHC and tropomyosin expression corroborated the distribution of well organized sarcomeres throughout the cytoplasm. This expression pattern is intriguing because it replicates the distribution of myofilaments as they disassemble after the fusion of muscle fibers to form electrocytes (Unguez and Zakon, 1998). Disruption of neural input also resulted in the expression of an MHC present in type II fibers. Together, these data intimate that transcription and/or translational mechanisms can be triggered within mature electrocytes that result in the formation of myofibers, specifically, the electrocyte myogenic precursors. The “reversibility” of cell phenotype to that of a less differentiated stage in the electrocyte pathway is similar to the shift that occurs after pharmacological inactivation (Schiaffino et al., 1988) or denervation (Obinata et al., 1984; Schiaffino et al., 1988) of mature muscle fibers in mammals and birds, i.e., a conversion of adult MHC toward an embryonic/fetal-like isoform.
However, not all protein systems that were studied were affected after either spinal transection or denervation. If changes in keratin, 12-101-labeled sarcoplasmic reticulum, or AChR expression occurred, these were undetected by immunohistochemistry. Alternatively, changes may be found after survival periods longer than 5 weeks. Furthermore, these proteins might be less responsive to changes in innervation. Last, it is not clear whether these genes in electrocytes remain active after neural lesion or whether the turnover of their proteins is slower. For example, the absence of the emergence of extrajunctional AChRs after denervation as it occurs in other vertebrates (Hall and Sanes, 1993) has been reported in denervated electrocytes ofElectrophorus (Bourgeois et al., 1973). This discrepancy was explained by a possibly slower turnover of receptor proteins in electric organ (Clementi et al., 1975). Thus, factors independent of innervation play a role in the maintenance of a fully differentiated electrocyte phenotype.
Determinants of EO phenotype in other electric fish species
Our findings are consistent with ultrastructural data showing the appearance of myofibrils in a sarcomeric-like arrangement in electrocytes of adult Torpedo 3 weeks after transection of the nerve branch near its entry into the electric organ (Gautron, 1974). In the latter study, sarcomere formation first was observed 24 d after nerve section. However, no biochemical studies on the time-dependent changes in contractile proteins were performed. To date, no other studies have looked at the effect of innervation on the phenotypic properties of mature EO. Nevertheless, data from this and the present study clearly demonstrate that innervation plays a dominant role in the maintenance of the mature EO phenotype.
As in S. macrurus, electrocytes in Torpedoderive from striated muscle fibers. Such similarities are striking despite the fact that these EOs form from distinct muscles and that both species evolved independently of each other (Darwin, 1859;Bennett, 1971; Bass, 1986). In addition, electrocytes ofTorpedo generate remarkably large voltages and discharge only during self-defense or pursuit of prey, two functions ofTorpedo EO that differ from those of S. macrurusEO (Bennett, 1971). Despite the large phylogenetic distances amongTorpedo, S. macrurus, and other electric fish species, it is feasible that similar regulators and mechanisms underlie the developmental switch from muscle to EO and the phenotypic reversibility of the electrocyte phenotype in the adult.
Footnotes
This research was supported by National Institute of Health Grant R01 NS25513. We are grateful to the Cell Research Institute of the University of Texas at Austin for the use of its electron microscopy facility, Ying Liu for her technical assistance, and Kristina Schlegel for artwork. Antibodies BF-F3 and 88b were generous gifts from Dr. S. Schiaffino, University of Padova, Italy, and Dr. S. Froehner, University of North Carolina, Chapel Hill, respectively. Antibodies 3A10, D76, 12-101, MF20, A4.74, and N2.261 were obtained from the Developmental Studies Hybridoma Bank, John Hopkins University, Baltimore, MD.
Correspondence should be addressed to Dr. Graciela A. Unguez at the above address.
REFERENCES
- 1.Bader D, Masaki T, Fischman DA. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982;95:763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bass AH. Electric organs revisited. In: Bullock TH, editor. Electroreception. Wiley; New York: 1986. pp. 19–100. [Google Scholar]
- 3.Bennett MVL. Electric organs. In: Hoar WS, Randall DJ, editors. Fish physiology, Vol 5. Academic; New York: 1971. pp. 347–491. [Google Scholar]
- 4.Bourgeois JP, Popot JL, Ryter A, Changeaux JP. Consequences of denervation on the distribution of the cholinergic (nicotinic) receptor sites from Electrophorus electricus revealed by high resolution autoradiography. Brain Res. 1973;62:557–563. doi: 10.1016/0006-8993(73)90722-1. [DOI] [PubMed] [Google Scholar]
- 5.Brun RP, Kim JB, Hu E, Altiok S, Spiegelman B. Adipocyte differentiation: a transcriptional regulatory cascade. Curr Opin Cell Biol. 1996;8:826–832. doi: 10.1016/s0955-0674(96)80084-6. [DOI] [PubMed] [Google Scholar]
- 6.Buckingham ME, Dexter TM. Differentiation and gene regulation. From the regulation of growth, differentiation, and death in vitro to the onset and maintenance of differentiation in vivo. Curr Opin Genet Dev. 1997;7:577–581. doi: 10.1016/s0959-437x(97)80002-0. [DOI] [PubMed] [Google Scholar]
- 7.Clementi F, Conti-Tronconi B, Peluchetti D, Morgutti M. Effect of denervation on the organization of the postsynaptic membrane of the electric organ of Torpedo marmorata. Brain Res. 1975;90:133–148. doi: 10.1016/0006-8993(75)90687-3. [DOI] [PubMed] [Google Scholar]
- 8.Cox RD, Weydert A, Barlow D, Buckingham ME. Three linked myosin heavy chain genes clustered within 370 kb of each other show independent transcriptional and post-transcriptional regulation during differentiation of a mouse muscle cell line. Dev Biol. 1991;143:36–43. doi: 10.1016/0012-1606(91)90052-5. [DOI] [PubMed] [Google Scholar]
- 9.Darwin C. The origin of species, pp 178–179. Penguin; New York: 1859. [Google Scholar]
- 10.Edgerton VR, Bodine-Fowler S, Roy RR, Ishihara A, Hodgson JA. Neuromuscular adaptation. In: Rowell LB, Shepherd JT, editors. Handbook of physiology. Exercise: regulation and integration of multiple systems. Oxford UP; New York: 1996. pp. 54–88. [Google Scholar]
- 11.Eftimie R, Brenner HR, Buonanno A. Myogenin and MyoD join a family of skeletal muscle genes regulated by electrical activity. Proc Natl Acad Sci USA. 1991;88:1349–1353. doi: 10.1073/pnas.88.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox GQ, Richardson GP. The developmental morphology of Torpedo marmorata: electric organ—myogenic phase. J Comp Neurol. 1978;179:677–698. doi: 10.1002/cne.901790313. [DOI] [PubMed] [Google Scholar]
- 13.Fox GQ, Richardson GP. The developmental morphology of Torpedo marmorata: electric organ—electrogenic phase. J Comp Neurol. 1979;185:293–316. doi: 10.1002/cne.901850205. [DOI] [PubMed] [Google Scholar]
- 14.Gautron J. Effet de la section du nerf electrique sur la structure des electroplaques de torpille. J Microsc. 1974;21:85–92. [Google Scholar]
- 15.Gunning P, Hardeman E. Multiple mechanisms regulate muscle fiber diversity. FASEB J. 1991;5:3064–3070. doi: 10.1096/fasebj.5.15.1835946. [DOI] [PubMed] [Google Scholar]
- 16.Hall ZW, Sanes JR. Synaptic structure and development: the neuromuscular junction. Cell [Suppl] 1993;10:99–121. doi: 10.1016/s0092-8674(05)80031-5. [DOI] [PubMed] [Google Scholar]
- 17.Honda S, Epstein HF. Modulation of muscle gene expression in Caenorhabditis elegans: differential levels of transcripts, mRNAs, and polypeptides for thick filament proteins during nematode development. Proc Natl Acad Sci USA. 1990;87:876–880. doi: 10.1073/pnas.87.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludolph DC, Konieczny SF. Transcription factor families: muscling in on the myogenic program. FASEB J. 1995;9:1595–1604. doi: 10.1096/fasebj.9.15.8529839. [DOI] [PubMed] [Google Scholar]
- 19.Mills A, Zakon H. Coordination of EOD frequency and pulse duration in a weakly electric wave fish: the influence of androgens. J Comp Physiol. 1987;161:417–430. [Google Scholar]
- 20.Mills A, Zakon HH, Marchaterre MA, Bass AH. Electric organ morphology of Sternopygus macrurus, a wave-type weakly electric fish with a sexually dimorphic EOD. J Neurobiol. 1992;23:420–432. doi: 10.1002/neu.480230712. [DOI] [PubMed] [Google Scholar]
- 21.Obinata T, Saitoh O, Takano-Ohmuro H. Effect of denervation on the isoform transitions of tropomyosin, troponin T, and myosin isoenzyme in chicken breast muscle. J Biochem. 1984;95:585–588. doi: 10.1093/oxfordjournals.jbchem.a134643. [DOI] [PubMed] [Google Scholar]
- 22.Patterson JM, Zakon HH. Differential expression of proteins in muscle and electric organ, a muscle derivative. J Comp Neurol. 1996;370:367–376. doi: 10.1002/(SICI)1096-9861(19960701)370:3<367::AID-CNE7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 23.Patterson JM, Zakon HH. Transdifferentiation of muscle to electric organ: regulation of muscle-specific proteins is independent of patterned nerve activity. Dev Biol. 1997;186:115–126. doi: 10.1006/dbio.1997.8580. [DOI] [PubMed] [Google Scholar]
- 24.Pette D, Vrbova G. Neural control of phenotypic expression in mammalian muscle fibers. Muscle Nerve. 1985;8:676–689. doi: 10.1002/mus.880080810. [DOI] [PubMed] [Google Scholar]
- 25.Reedy MC, Beall C. Ultrastructure of developing flight muscle in Drosophila. I. Assembly of myofibrils. Dev Biol. 1993;160:443–465. doi: 10.1006/dbio.1993.1320. [DOI] [PubMed] [Google Scholar]
- 26.Rome LC, Choi IH, Lutz G, Sosnicki A. The influence of temperature on muscle function in the fast swimming scup. I. Shortening velocity and muscle recruitment during swimming. J Exp Biol. 1992;163:259–279. doi: 10.1242/jeb.163.1.259. [DOI] [PubMed] [Google Scholar]
- 27.Rome LC, Syme DA, Hollingworth S, Lindstedt SL, Baylor SM. The whistle and the rattle: the design of sound-producing muscles. Proc Natl Acad Sci USA. 1996;93:8095–8100. doi: 10.1073/pnas.93.15.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanger JM, Mittal B, Pochapin MB, Sanger JW. Myofibrillogenesis in living cells microinjected with fluorescently labeled α-actinin. J Cell Biol. 1986;102:2053–2066. doi: 10.1083/jcb.102.6.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schiaffino S, Gorza L, Pitton G, Saggin I, Ausoni A, Sartore S, Lomo T. Embryonic and neonatal myosin heavy chain in denervated and paralyzed rat skeletal muscle. Dev Biol. 1988;127:1–11. doi: 10.1016/0012-1606(88)90183-2. [DOI] [PubMed] [Google Scholar]
- 30.Unguez GA, Zakon HH. Phenotypic conversion of distinct muscle fiber populations to electrocytes in a weakly electric fish. J Comp Neurol. 1998;399:20–34. [PubMed] [Google Scholar]
- 31.van Asselt E, van Raamsdonk W, de Graaf F, Smit-Onel MJ, Degenbach PC, Heuts B. Enzyme histochemical profiles of fish spinal motoneurons after cordotomy and axotomy of motor nerves. Brain Res. 1990;531:25–35. doi: 10.1016/0006-8993(90)90754-y. [DOI] [PubMed] [Google Scholar]
- 32.van Raamsdonk W, Pool CW, Heyting C, teKronnie G, Veeken K. Effects of immobilization and partial denervation on the differentiation of muscle fiber types in the zebrafish, Brachydanio rerio. Anat Embryol (Berl) 1982;164:63–74. doi: 10.1007/BF00301879. [DOI] [PubMed] [Google Scholar]
- 33.Wittenberger C, Coprean D. Some effects of denervation upon white and red muscles in carp. Comp Biochem Physiol [A] 1977;56:307–312. [Google Scholar]
- 34.Witzemann V, Sakmann B. Differential regulation of MyoD and myogenin mRNA levels by nerve induced muscle activity. FEBS Lett. 1991;282:259–264. doi: 10.1016/0014-5793(91)80490-t. [DOI] [PubMed] [Google Scholar]