Abstract
We examined the role of hippocampal galanin in an animal model of status epilepticus (SE). Control rats showed abundant galanin-immunoreactive (Gal-IR) fibers in the dentate hilus, whereas no Gal-IR neurons were observed. Three hours after the onset of self-sustaining SE (SSSE), induced either by intermittent stimulation of the perforant path for 30 min (PPS) or by injection of lithium and pilocarpine, Gal-IR fibers disappeared in the hilus and remained absent for up to 1 week afterward. Twelve hours after the induction of SE by PPS or 3 hr after pilocarpine administration, Gal-IR neurons appeared in the hilus; these neurons increased in number after 1 d and gradually declined 3 and 7 d later. Galanin concentration in the hippocampus, measured by ELISA, significantly decreased on the plateau of SSSE and increased 24 hr after PPS. Galanin (0.05 nmol) injected into the hilus prevented the induction of SSSE, and 0.5 nmol of galanin stopped established SSSE. These effects were attenuated by galanin receptor antagonists (M35 > M40 ≥ M15). 2-Ala-galanin (5 nmol), a putative agonist of galanin type 2 receptors, prevented but was unable to stop SSSE. M35 facilitated the development of SSSE when given before PPS. We suggest that hippocampal galanin acts as an endogenous anticonvulsant via galanin receptors. SE-induced galanin depletion in the hippocampus may contribute to the maintenance of seizure activity, whereas the increase of galanin concentration and the appearance of galanin-immunoreactive neurons may favor the cessation of SSSE. The seizure-protecting action of galanin SSSE opens new perspectives in the treatment of SE.
Keywords: status epilepticus, hippocampus, galanin, immunocytochemistry, galanin receptor ligands, anticonvulsant effects
Galanin, a bioactive peptide containing 29 or 30 amino acid residues, is widely distributed throughout the CNS (Skofitsch and Jacobowitz, 1985, 1986;Melander et al., 1986a–d; Bartfai et al., 1993; Merchenthaler et al., 1993), where it coexists with classical neurotransmitters (Melander et al., 1985, 1986b,c; Fisone et al., 1987; Bartfai et al., 1988; Senut et al., 1989) and often inhibits their release (Kask et al., 1995). Three subtypes of galanin receptors (GalR) that have been cloned, GalR1 (Habert-Ortoli et al., 1994; Burgevin et al., 1995), GalR2 (Howard et al., 1997; Smith et al., 1997; Wang et al., 1997a), and GalR3 (Wang et al., 1997b), belong to a superfamily of G-protein-linked receptors. Activation of galanin receptors results in the opening of K+ channels, in the inhibition of cAMP synthesis (GalR1 and GalR2) (Chen et al., 1992; Karelson et al., 1995; Wang et al., 1997a), and probably in the activation of phospholipase C (GalR2) (Smith et al., 1997).
Galanin is abundant in several brain structures, including septum in which it coexists with acetylcholine and locus coeruleus in which it is colocalized with norepinephrine. The axons of the neurons located in these structures project diffusely to the hippocampus (Melander et al., 1985; Lamour et al., 1989; Senut et al., 1989; Cortes et al., 1990; Merchenthaler et al., 1993) where the density of galanin receptors is high (Melander et al., 1986a,d; Skofitsch et al., 1986; Servin et al., 1987; Fisone et al., 1989a,b). Sparse hippocampal galanin-immunoreactive (Gal-IR) neurons and those expressing preprogalanin mRNA are revealed after intrahippocampal administration of colchicine (Skofitsch and Jacobowitz, 1985; Cortes et al., 1990). Physiological studies suggest that the action of galanin in the hippocampus is predominantly inhibitory. Thus, galanin inhibited EPSP in pyramidal neurons of CA1 (Dutar et al., 1989) and inhibited long-term potentiation at Schaffer collateral–CA1 synapses in hippocampal slices (Sakurai et al., 1996). Galanin decreased the release of excitatory amino acids in hippocampal slices under both normal and ischemic conditions (Ben-Ari and Lazdunski, 1989; Zini et al., 1993a,b). The mentioned physiological properties of galanin suggest that it may have modulatory effects on seizures. However, although the role of galanin in memory function (Mastropaolo et al., 1988; Sundström et al., 1988) in the regulation of the secretion of hypothalamopituitary hormones (Bartfai et al., 1993) is well established, its involvement in seizure mechanisms has received little attention (Mazarati et al., 1992; Chepurnov et al., 1997). In the present report, we show that status epilepticus (SE) induces distinct time-dependent changes in hippocampal galanin. We also provide evidence of a strong, receptor-mediated anticonvulsant effect of intrahippocampal galanin application and of the facilitation of SE resulting from the blockade of hippocampal galanin receptors.
MATERIALS AND METHODS
Animals and surgery. The experiments were performed on male Wistar rats, 8–10 weeks old (Simonsen Labs, Gilroy, CA). Animals were kept at room temperature with a 12 hr artificial dark/light cycle and access to standard diet and tap waterad libitum. All experiments were performed according to the protocol approved by the Animal Care Committee of Sepulveda Veterans Administration Medical Center.
Under ketamine (60 mg/kg) and xylazine (15 mg/kg) anesthesia, animals were implanted with a bipolar stimulating electrode into the angular bundle of the perforant path (4.5 mm left of and 0.5 mm posterior to lambda) and with a bipolar recording electrode combined with a guide cannula (internal diameter, 0.6 mm) into the granule cell layer of the ipsilateral dentate gyrus (2.2 mm left of and 3.5 mm anterior to lambda). The depth of both electrodes was optimized by finding the population spike of maximal amplitude evoked from the dentate gyrus by the stimulation of the perforant path (single square-wave monophasic stimuli; 20 V; 0.1 msec). To standardize the conditions of perforant path stimulation (PPS) and of the site of electrographic recording, we used only those animals in which population spike amplitude was ≥2 mV. Some animals were additionally injected during surgery with colchicine (100 μg in 10 μl) into the medial septum (MS) (0.8 mm anterior to bregma and 8.3 mm deep from the cortical surface) by means of a Hamilton microsyringe to block axonal transport of galanin (Skofitsch and Jacobowitz, 1985). These animals were not used for the induction of SE but were killed 3 d after the surgery for immunostaining.
Induction and analysis of SE. To induce self-sustaining SE (SSSE), animals were stimulated in the awake state for 30 min with 10 sec, 20 Hz trains (1 msec square wave; 20 V) delivered every minute, together with 2 Hz continuous stimulation (Mazarati et al., 1998). Some animals underwent 7 min of PPS using the same parameters. Another protocol for the induction of SE used intraperitoneal administration of LiCl (3 meq/kg) followed 18 hr later by intraperitoneal injection of pilocarpine HCl (60 mg/kg) (Sankar et al., 1997). Electrographic activity from the dentate gyrus was monitored and recorded during PPS and for 24 hr after the end of PPS using the Monitor 8.1 computer program (Stellate Systems). The software was configured for automatic detection and saving of seizures and spikes. EEG was analyzed off-line using the same software. After the end of PPS, EEGs were recorded for 10 min to verify the presence of SSSE, before any treatment was initiated. The duration of SSSE was determined as the time between the end of PPS and the last paroxysmal event (seizure or spike). The severity of behavioral seizures was evaluated using the Racine (1972)scale. Control animals underwent surgery and received sham PPS or injection of 0.9% NaCl instead of pilocarpine.
Studies of galanin-like immunoreactivity. Three, 12, or 24 hr or 3 or 7 d after the end of PPS or pilocarpine administration, animals were deeply anesthetized with sodium pentobarbital (60 mg/kg) and perfused through the ascending aorta with 200 ml of 0.1 m sodium PBS, pH 7.4, followed by 0.1m sodium PBS containing 4% paraformaldehyde. The brains were post-fixed in the same solution for 4 hr and placed in Tris-PBS and 30% sucrose, pH 7.4 (4°C), for 24 hr and then serially sectioned in 40-μm-thick coronal sections on a cryotome. The sections were blocked in Tris-PBS containing 10% normal goat serum (NGS) and 0.3% Triton X-100 for 1 hr, incubated in the rabbit anti-galanin antiserum (Peninsula Laboratories) at a concentration of 1:5000 overnight at room temperature, washed with Tris-PBS containing 1% NGS and 0.3% Triton X-100 for 30 min at room temperature, incubated in biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA) for 1 hr at room temperature, and washed with Tris-PBS for 30 min and an avidin–biotin–peroxidase complex (Vicastain; Vector Laboratories) for 1 hr. To identify the immunoreaction product, we visualized the horseradish peroxidase with diaminobenzidine and glucose oxidase, with nickel intensification. The specificity of the anti-galanin antiserum was confirmed by the absence of staining in the hippocampus when the primary antiserum was replaced with nonimmune serum and by the absence of labeling when the primary antiserum was omitted.
Gal-IR was quantified by a blinded investigator in the hilus and in the granule cell layer of the dentate gyrus, in CA3 and in MS. For the quantification of Gal-IR fibers, the sections were captured with a 10× objective and a Sony DKC 5000 camera (Tokyo, Japan), converted to digital images, and analyzed by means of the Image-Pro Plus software. The optical density of Gal-IR fibers was measured by manually outlining the region of the hilus and CA3. Background values were obtained from the stratum radiatum of CA1. The optical density was measured in five sections from each brain and was expressed as absolute values, with higher numbers corresponding to a higher density of Gal-IR. The number of Gal-IR neurons in five sections from each brain was counted on an Olympus AX-70 microscope with a 10× objective.
Galanin concentration in the hippocampus. The hippocampal galanin concentration was examined by means of competitive ELISA. Six or twenty-four hours after PPS, animals were deeply anesthetized with methoxyflurane. The brains were quickly removed; the whole hippocampi were dissected on ice, homogenized in 2 macetic acid containing 10 μg/ml aprotinin (Sigma, St. Louis, MO), and centrifuged for 15 min at 4°C. The supernatant was stored at −70°C. For the preparation of conjugate, 200 μg of rat galanin was coupled to 2 mg of bovine serum albumin with glutaraldehyde (both from Sigma) (Folkesson and Terenius, 1985). The conjugate was dialyzed for 2 d at 4°C with three changes of PBS, diluted to 0.4 mg of protein per milliliter, brought to 0.02% in sodium azide, and coated to the 96 well plate. For ELISA, the samples and the standards (eight concentrations from 800 to 0.05 ng of galanin/ml of PBS) were added in 12.5 μl of PBS/bovine serum albumin with 87.5 μl of galanin antibodies at 1:50,000 (Chemicon, Temecula, CA) to the conjugate-coated plates. The plates were incubated overnight at 4°C, repeatedly rinsed with PBS, and incubated with peroxidase-labeled goat anti-rabbit IgG at 1:1500 (Sigma) for 2 hr at room temperature. This was followed by addition of 100 μl of substrate solution (O-phenylenediamine HCl with H2O2; both from Sigma). The reaction was stopped in 30 min with 100 μl of 2.5 mH2SO4. The results were analyzed on an ELISA plate reader (Titertek) at 490 nm.
Administration of galanin receptor ligands. The following ligands of galanin receptors were used: rat galanin (0.01, 0.05, and 0.5 nmol), which is a mixed GalR1/GalR2 agonist; the putative GalR2 agonist 2-Ala-Galanin (0.5 and 5 nmol); and the antagonists M15, M35, and M40 (0.5 and 5 nmol) (Bartfai et al., 1992; McDonald et al., 1997). Galanin and M15 were purchased from American Peptide Company. Other peptides were synthesized on an ABI peptide synthesizer using the t-Boc strategy of solid-phase peptide synthesis according to the protocol described previously (Langel et al., 1992). The purity of the peptides was >99% as demonstrated by HPLC on an analytical nucleosil 120–3 C18 reverse-phase HPLC column (0.4 × 10 cm). The molecular masses of the peptides were determined with a Plasma Desorption Mass Spectrometer (Bioion 20; Applied Biosystems, Foster City, CA), and the calculated values were obtained in each case. The peptide solutions were prepared extempore by dissolving in 0.9% NaCl and were injected, in a volume of 0.5 μl, into the hilus of freely moving rats by a Hamilton microsyringe connected to the guide cannula for 5 min. Peptides were injected either 10 min before the beginning or 10 min after the end of PPS. Control animals were treated with 0.9% NaCl.
Statistics. Data were analyzed by one-way ANOVA followed by Newman–Keuls test or by Kruskal–Wallis test followed by Mann–Whitney test, where appropriate; p < 0.05 was accepted to be statistically significant.
RESULTS
Gal-IR in control animals
In controls, a fine dense network of Gal-IR fibers was observed in all hippocampal areas. No Gal-IR neurons were present (Figs.1A,2). MS was characterized by a high degree of immunostaining of Gal-IR fibers. In the animals injected with colchicine into the MS, the density of galanin-positive fibers in both the hilus and area CA3 was significantly lower than that in controls. The decline in optical density was more pronounced in CA3 than in the hilus (6 and 26% of control values, respectively). No Gal-IR neurons were observed in the hippocampus in colchicine-treated animals (Figs.1B, 2).
Fig. 1.
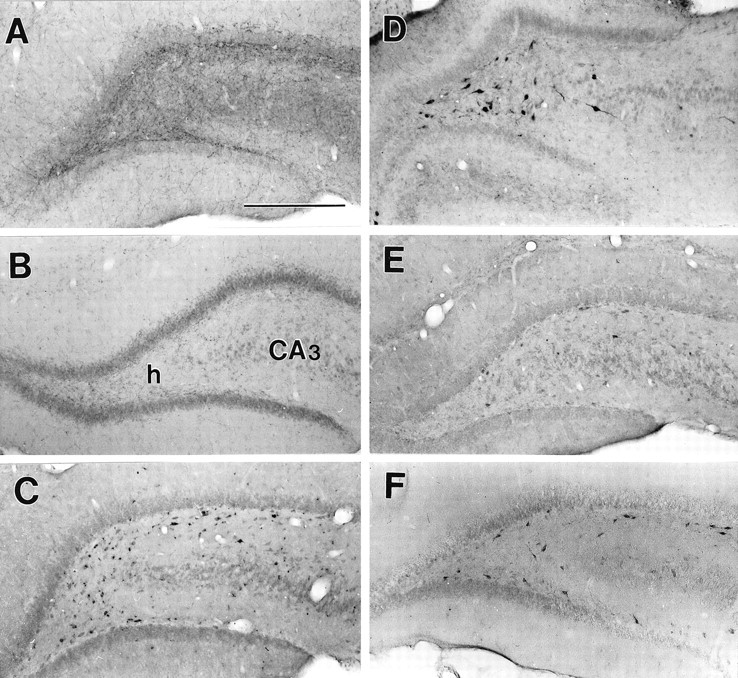
SE-induced changes in galanin-like immunoreactivity in the hippocampus. A, Control. Abundant galanin-immunoreactive fibers are present in the hilus and CA3. B, Three days after injection of colchicine into the medial septum. No Gal-IR fibers are visualized. h, Hilus. C, Twelve hours after PPS. D, Twenty-four hours after PPS. E, Seven days after PPS.F, Three hours after the induction of Li-pilocarpine SE. Note the absence of Gal-IR fibers and the presence of Gal-IR neurons. Scale bar, 200 μm.
Fig. 2.
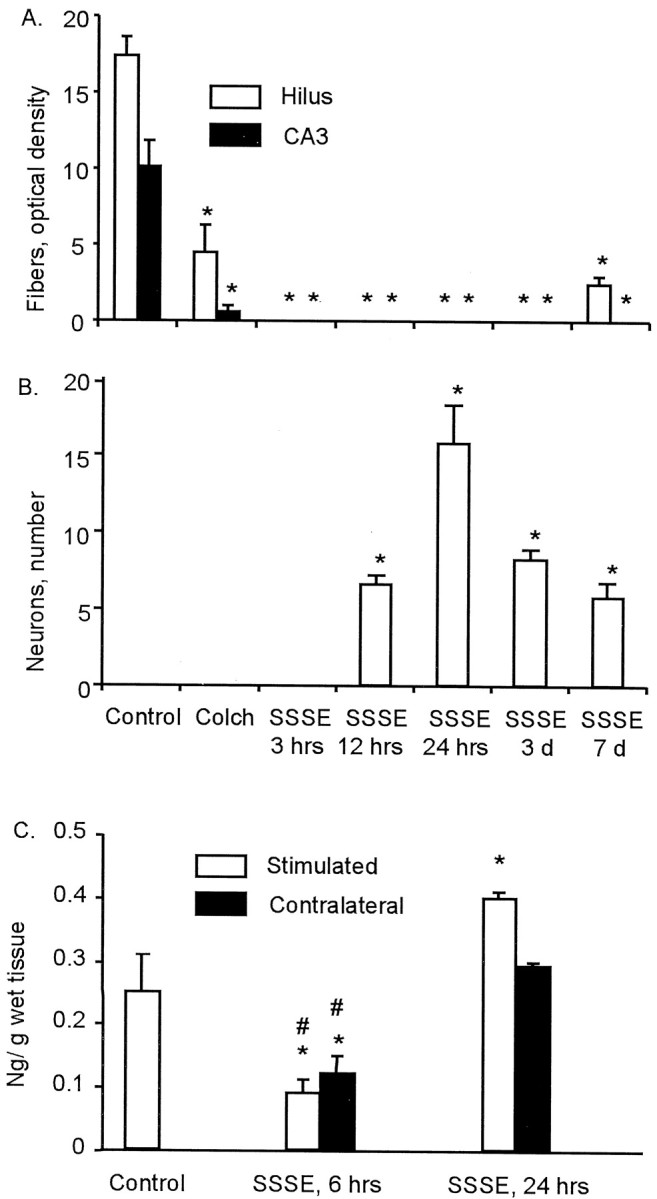
Changes in galanin-like immunoreactivity and galanin content in the hippocampus induced by self-sustaining SE.A, B, Optical density of Gal-IR fibers (A) and the number of Gal-IR neurons per section (B) in the hilus (open bars) and CA3 (black bars). Colch, Colchicine. Time is hours and days after SSSE, which was induced by 30 min of PPS. Because there were no differences between stimulated and contralateral sides, data from both hippocampi were pooled. C, Galanin content in the hippocampus, measured by ELISA 6 and 24 hr after PPS. Data for the left and the right hippocampi were pooled together in the control. Error bars indicate SD. *p < 0.05 versus controls; #p < 0.05 versus 24 hr (one-way ANOVA plus Newman–Keuls test). Controls included four animals, and all other groups had three animals.
Description of SE
Thirty minutes of PPS induced behavioral and electrographic seizures in all animals, starting after 5–10 min of stimulation. The seizures were initially facial myoclonus (stage 1), which progressed through the head and forelimb clonic seizures (stage 3) to rearing (stage 4) or rearing and falling (stage 5). Stage 4–5 convulsions were accompanied by high-frequency, high-amplitude paroxysmal activity in the dentate gyrus (Fig. 3A), which recurred at intervals of 3–30 min and was recognized as seizures by the software. Stage 4–5 seizures alternated with stage 1–3 behavior, accompanied by spike-and-wave complexes in the EEG. Behavioral and electrographic seizure activity (stage 1–5) was observed for 6–18 hr after the end of PPS in different animals (Fig.3A). In the Li-pilocarpine model, seizures started 10–15 min after injection of pilocarpine. Repetitive stage 3–5 seizures were observed for 4–8 hr after their onset.
Fig. 3.
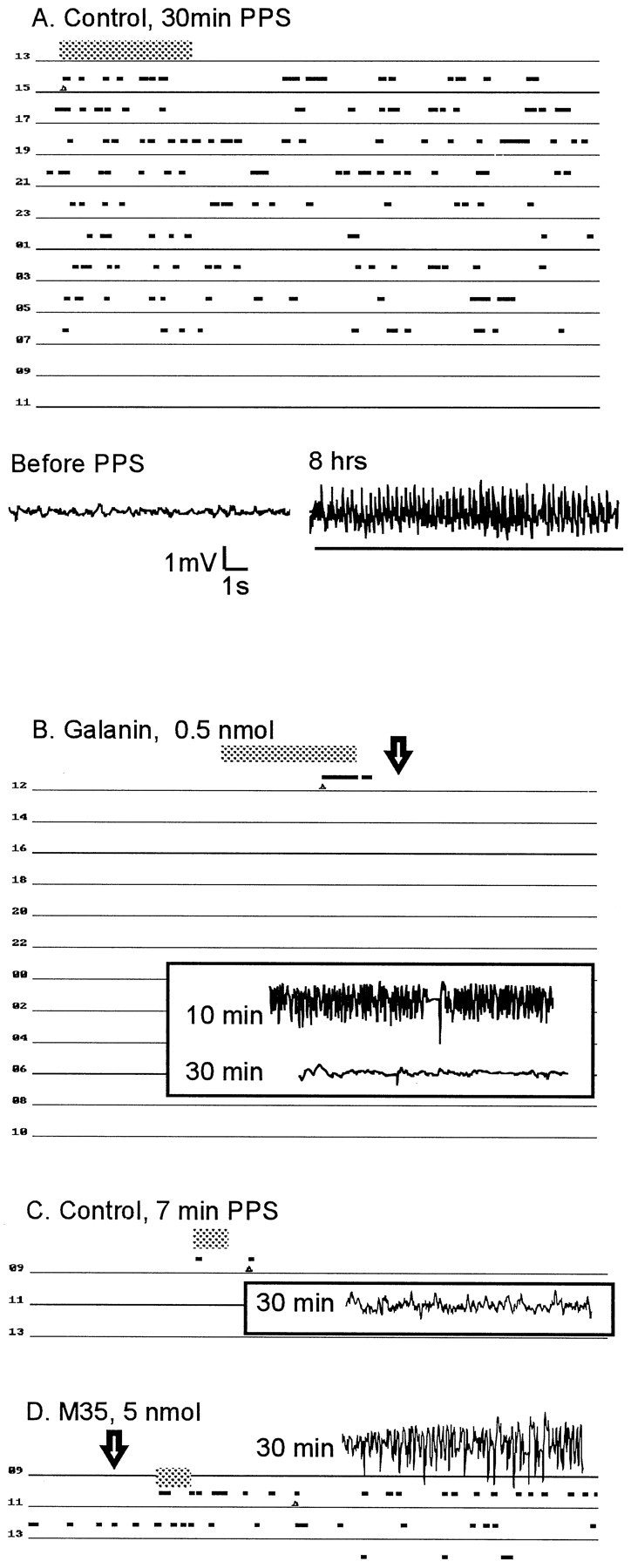
Electrographic activity during SSSE in control and galanin ligand-treated animals. A, Representative time course of SSSE. Top, Eachline represents 2 hr of monitoring. The duration of PPS is indicated by the large horizontal gray bar. Each electrographic event recognized as a seizure (usually corresponding to stage 4–5 behavioral convulsions) is indicated by a small horizontal black bar. Seizures recurred for 18 hr in this animal. Bottom, Sample EEG indicates electrographic activity before and 8 hr after PPS. The event recognized as a seizure by the software is underlined. Between the seizures, frequent spikes occurred (accompanied by stage 1–3 behavioral convulsions). B, Representative tracing from a rat injected with intrahippocampal galanin 10 min after the end of PPS (arrow). Note the presence of seizures (as indicated inA) during PPS and between the end of PPS and galanin administration and their disappearance after galanin injection. Sample electrographic recordings show seizure activity 10 min after the end of PPS (just before galanin injection) and the absence of seizure activity 30 min after administration of galanin. C, Recording during and after 7 min of PPS in a control animal. Only a single seizure occurred several minutes after PPS. Sample EEG taken 30 min after PPS shows no paroxysmal activity. D, The effects of pretreatment with M35 on the ability of the animal to establish SSSE. In contrast to control animals, this rat developed self-sustaining seizures, which lasted for 3 hr.
Changes in hippocampal Gal-IR and galanin concentration during and after SE
In the animals killed 3 hr after the end of PPS, during steady-state SSSE, Gal-IR fibers were absent in all hippocampal areas (Fig. 2). Gal-IR fibers were still absent 12 hr after PPS, as well as 1 and 3 d later (Figs. 1C,D,2A). One week after SSSE, some Gal-IR fibers reappeared in the hilus, although their density was still significantly lower than that in control animals (Figs. 1E, 2). Starting from 12 hr after PPS, Gal-IR neuronal bodies, located mostly in the hilus on the border of the granule cell layer, appeared (Fig.1C). Twenty-four hours after PPS, the number of galanin-positive neurons further increased, and they were distributed evenly on the border of the dentate granule cell layer and in the middle of the hilus (Fig. 1D). Three and 7 d after PPS, the number of Gal-IR neurons gradually decreased (Figs.1E, 2B). No Gal-IR neurons were observed in the dentate granule cell layer and in CA3. These changes occurred on both the stimulated and the contralateral sides. After SE induced by Li-pilocarpine, the decline in hippocampal fibers was similar to that observed after SSSE. However, Gal-IR neurons in the hilus appeared as early as 3 hr after pilocarpine administration (Fig.1F) and were present at 1, 3, and 7 d. In both models of SE, no significant changes in Gal-IR were seen in MS.
Six hours after PPS, on the plateau of SSSE, galanin concentration in both stimulated and contralateral hippocampi was significantly lower than that in controls. On the next day after PPS, galanin content returned to the initial level in the contralateral hippocampus, whereas in the stimulated hippocampus it significantly exceeded the concentration in control animals (Fig. 2C).
Effects of galanin receptor ligands on SSSE
Perihilar injection of 0.05 nmol (n = 5) and 0.5 nmol (n = 6) of galanin before 30 min of PPS significantly shortened the duration of self-sustaining seizures (Fig.4A). After PPS, stage 4 seizures continued for only a few minutes, and spikes were observed for another 10–20 min, after which no electrographic or behavioral seizure was seen (Fig. 3B). 2-Ala-galanin did not affect the course of SSSE, when given before PPS in a dose of 0.5 nmol (n= 4). A dose of 5 nmol (n = 4) significantly decreased the duration of self-sustaining seizures, which lasted for 2–4 hr (Fig. 4A).
Fig. 4.
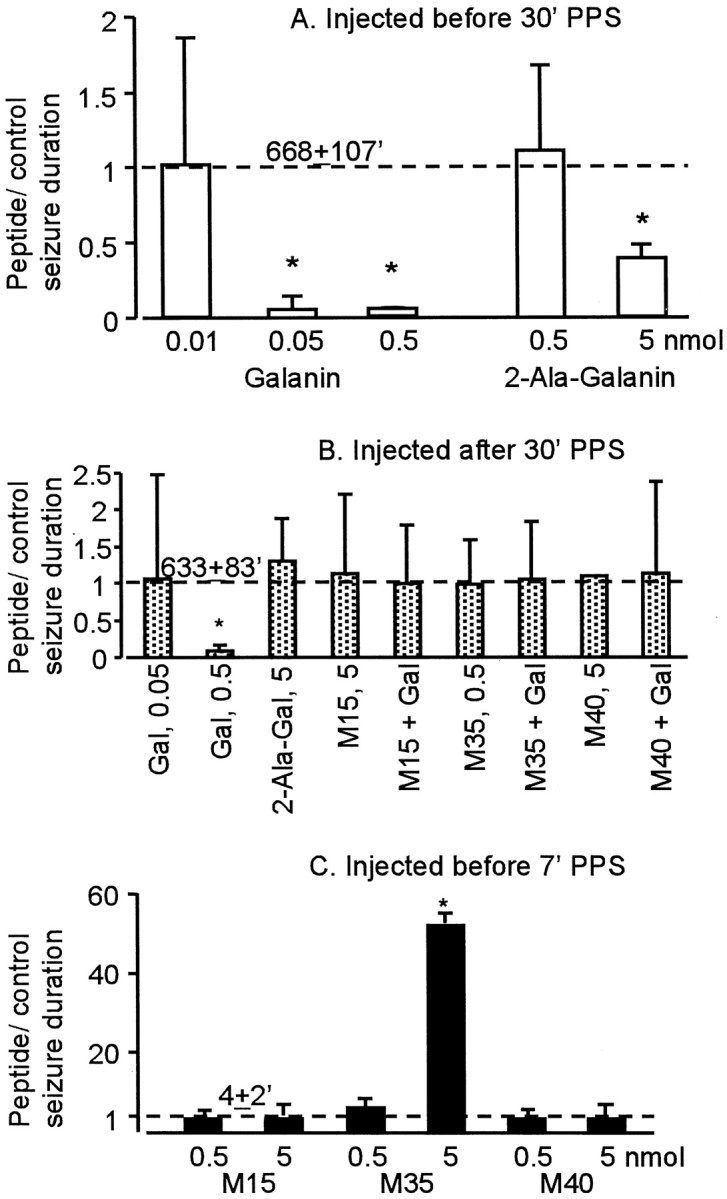
The effects of galanin receptor ligands on SSSE.A, Effects of peptides injected before 30 min of PPS.B, Effects of peptides injected after the end of PPS. Galanin (Gal), but not 2-Ala-galanin (2-Ala-Gal), stopped established SSSE when administered after PPS. These effects were abolished by all three galanin receptor antagonists. C, Effects of galanin receptor antagonists injected before 7 min of PPS. M35, but not two other galanin receptor antagonists, favored the establishment of SSSE when given before 7 min of PPS. Error bars indicate SD of the mean ratio of SSSE duration in the peptide-treated to control animals in which SSSE duration is presented as 1 and indicated by thedashed line. Absolute values (mean + SD; in minutes) of SSSE duration in control rats are indicated above thedashed line. *p < 0.05 versus control (Kruskal–Wallis test followed by Mann–Whitney post hoc test).
In the next set of the experiments, we injected galanin 10 min after the end of PPS, a time sufficient to document that seizures are self-sustaining (Mazarati et al., 1998). Perihilar administration of 0.5 nmol (n = 5) but not 0.05 nmol (n = 3) of galanin stopped self-sustaining seizures within 25 min (Fig.4B). 2-Ala-galanin in a dose that prevented the establishment of SSSE (5 nmol) did not stop previously established SSSE (n = 3). The seizure-blocking effects of galanin were canceled by its coadministration with any of the three galanin receptor antagonists, given in doses that did not alter seizures (Fig. 4,n = 4 in each group)
Because galanin displayed anticonvulsant effects, we examined the consequences of administration of galanin receptor antagonists before our experiments. In this set of experiments, the animals received PPS for 7 min after the administration of placebos or galanin antagonists. As shown earlier (Mazarati et al., 1998), 7 min of PPS never induced SSSE in control animals (n = 4) (Fig.3C). No SSSE was observed in the rats pretreated with either M15 or M40 (Fig. 4C, 0.5 and 5 nmol; n = 4 in each group). However, animals (n = 4) that received 5 nmol of M35 developed SSSE and continued to seize for 180–250 min after PPS (Figs. 3D, 4C).
DISCUSSION
Our studies show that both SSSE induced by 30 min of PPS and SE induced by Li-pilocarpine were accompanied by time-dependent changes of galanin stores in the hilus and CA3. Gal-IR, which was confined to fibers in the hippocampi of normal animals, dramatically declined after colchicine administration into the MS, confirming that the bulk of those fibers are the axons of MS neurons (Lamour et al., 1989). The rest of the fibers come from different sources, such as locus coeruleus (Xu et al., 1998) and hypothalamus (Skofitsch and Jacobowitz, 1985). SE was accompanied by the rapid disappearance of galanin from hilar and CA3 fibers, which lasted for at least 1 week. The mechanism of this disappearance is the subject of further studies. However, considering the importance of MS neurons as a source of galanin delivered to the hilus and the functional anatomy of the septohippocampal complex, we can suggest a sequence of events that may underlie these changes. The glutamatergic pathway originating from the hippocampus provides excitation for GABAergic neurons in the lateral septum (LS) (Malthe-Sorenssen et al., 1980; Stevens and Cotman, 1986). Those LS neurons in turn inhibit galanin-containing MS neurons (McLennan and Miller, 1974). The neurons from the MS project back to the hippocampus. There is also a less abundant direct glutamatergic projection from the hippocampus to the MS (Gaykema et al., 1991). Therefore, galanin-containing neurons in the MS experience a dual hippocampal feedback, inhibitory via the hippocampo–lateral septum–MS loop and excitatory via the hippocampo–MS projection. With continuous hippocampal activation, the first circuit may decrease the outflow of galanin to the hippocampus. The second circuit might initially have the opposite effect, but because SE lasts, the increased release of galanin because of continuous firing of MS axons and synapses may exhaust their galanin stores. Either tonic inhibition of galanin-containing MS neurons via the lateral septum or galanin fatigue via the hippocampo–MS projection could result in the disappearance of galanin fibers in the hippocampus. Finally, the inhibition of polypeptide synthesis during SE (Dwyer and Wasterlain, 1984) and the reported inhibition of axonal transport by seizures (Divac et al., 1984) may also play a role in inhibiting the replenishment of presynaptic hippocampal galanin stores.
Another change induced by SE was the expression of galanin by hilar neurons, which were completely devoid of Gal-IR in the control rats. This phenomenon probably was not a compensatory response to the depletion of galanin from the septohippocampal fibers. First, the depletion of galanin from hilar fibers in colchicine-treated animals was not accompanied by its appearance in hilar neurons. Second, in SE, no Gal-IR neurons were seen in CA3, although galanin fibers projecting to that area also lost their immunoreactivity. These data suggest that the observed changes specifically relate to the effects of SE on the hilus. Alternatively, galanin might be expressed in neurons that are degenerating as a result of excitotoxic damage. However, SSSE-induced neuronal injury spreads far beyond the hilus of the dentate gyrus, to other hippocampal as well as extrahippocampal areas (Mazarati et al., 1998), and therefore the distribution of Gal-IR neurons and that of neuronal injury are quite different. The earlier appearance of Gal-IR neurons in Li-pilocarpine SE compared with SSSE may reflect differences in seizure intensity in these two models. Li-pilocarpine SE was characterized by continuous severe stage 4–5 seizures, whereas during SSSE, such convulsions alternated with less severe (stage 1–3) seizures. Thus, the earlier expression of Gal-IR seems to correlate with greater seizure severity. The nature of Gal-IR neurons appearing as a result of SE is uncertain and is a matter of future studies. The fact that these cells appear initially at the edge of the granule cell layer suggests that at least some of these neurons may be basket cells. However, later Gal-IR neurons are distributed evenly throughout the hilus and thus may belong to other subpopulations of hilar interneurons.
Changes in hippocampal Gal-IR correlated with galanin content. On the plateau of SSSE, when galanin disappeared from hippocampal fibers, peptide concentration went down in both stimulated and contralateral hippocampi, suggesting that the decrease of Gal-IR during seizures reflects profound depletion of hippocampal galanin. Recovery of galanin concentration soon after SSSE may reflect the accumulation of galanin in hippocampal neurons, evidenced by the appearance of galanin-immunopositive neurons at this time point.
Changes resembling those observed in our study were described earlier for neuropeptide Y (NPY), which appeared de novo in mossy fibers (Marksteiner et al., 1990; Lurton et al., 1996; Vezzani et al., 1996), and for the GAD67 isoform of glutamic acid decarboxylase (Schwarzer and Sperk, 1995), which appeared in dentate granule cells. Although the significance of such changes is uncertain, they may represent endogenous adaptive mechanisms that counteract seizure activity. Both GABA (the product of GAD) and NPY (Klapstein and Colmers, 1997; Sperk and Herzog, 1997), as well as galanin (Dutar et al., 1989; Sakurai et al., 1996), may reduce hippocampal excitability under most circumstances, and their increased expression may function as a braking mechanism on SE.
The significance of the changes in hippocampal galanin in the course of SE was confirmed in pharmacological experiments. We found that galanin, a natural nonselective agonist for all types of galanin receptors (Habert-Ortoli et al., 1994; Burgevin et al., 1995; Howard et al., 1997; Smith et al., 1997; Wang et al., 1997a,b), had a potent seizure-protecting effect when injected into the dentate gyrus. Galanin was not only able to prevent the initiation of SSSE but, when given after PPS, it effectively and irreversibly aborted the maintenance phase of established SSSE. This effect seems even more spectacular, considering that the standard anticonvulsant drug diazepam failed to abort the maintenance of SSSE, as was shown earlier (Mazarati and Wasterlain, 1997). The anticonvulsant effect of galanin was mediated via galanin receptors, because it was abolished by coinjection of any of the three galanin receptor antagonists in doses that correspond to their affinity for GalR1 receptors: M35 equimolar to galanin, and M15 or M40 in a 10-fold excess dose (Smith et al., 1997). Moreover, M35, the most potent of the three and a preferential antagonist of GalR1 (Smith et al., 1997), facilitated the development of SSSE when administered before PPS. The mechanism by which exogenously applied galanin counteracts seizures more likely includes presynaptic inhibition of glutamate release via opening of ATP-dependent K+ channels, the effect that has been reported earlier (Ben-Ari and Lazdunski, 1989; Zini et al., 1993a,b). It is worth mentioning that the compounds acting as K+channel openers are considered a promising class of anticonvulsants (Meldrum, 1997).
The evidence from this study suggests that both GalR1 and GalR2 receptors may play a role in SSSE but that they may affect different phases of its pathophysiology to different degrees. Both M35, which has a higher affinity for GalR1, and M15 and M40, which show some preference for GalR2 (Smith et al., 1997), blocked the effects of galanin on the maintenance of SSSE, suggesting that the maintenance phase depends on both receptor types. It has been reported (Smith et al., 1997) that modifications of the galanin sequence in position 2 may reduce its affinity for GalR1 receptors more severely than that for GalR2. 2-Ala-galanin, a putative GalR2 agonist, showed much weaker seizure-preventing effect than did galanin in spite of its 10-fold higher dose and the greater abundance of GalR2 compared with GalR1 in the hippocampus (Gustafson et al., 1996; Xu et al., 1998). This suggests that the role of GalR2 receptors in the endogenous anticonvulsant effects of galanin in the initiation phase of SSSE may be relatively weak. On the other hand, the effects of M35 suggest an important role of GalR1 in the initiation phase of SSSE. Further pharmacological analysis using more selective ligands is needed to clarify the role of GalR1 and GalR2 in the seizing hippocampus.
Most of the studies on the effects of galanin in the hippocampus have concentrated on the ventral rather than the dorsal hippocampus. However, the latter, including the hilus, receives abundant innervation from MS galanin-containing neurons (Lamour et al., 1989). Our data indicate the importance of galanin in the dorsal hippocampus in the control of hippocampal excitability.
In conclusion, our data suggest that hippocampal galanin is dramatically altered by SE. Depletion of galanin from the hippocampus soon after the onset of SE observed in both immunocytochemical and immunobiochemical studies may reflect the failure of normal functioning of the hippocampal galanin system and may contribute to the establishment of a vicious cycle, which favors self-maintenance of seizure activity. On the other hand, increased galanin concentration in the hippocampus soon after SSSE, which probably reflects de novo or increased peptide synthesis in hippocampal neurons, may be a compensatory response to prolonged ictal activity and depletion of galanin from septal efferents. The high efficacy of exogenously applied galanin in protecting the animals from SE, together with recent progress in the development of potent specific and stable ligands for galanin receptors (Pooga et al., 1998), opens an intriguing opportunity for the development of novel drugs for the treatment of SE.
Footnotes
C.G.W. is supported by National Institute of Neurological Disorders and Stroke (NINDS) Grant NS 11315 and by the research service of Veteran Health Administration. R.S. is supported by NINDS Grant NS 01792. U.L. is supported by a grant from the Swedish Research Council. U.S. is supported by a stipend from the Swedish Institute.
Correspondence should be addressed to Dr. Andrey M. Mazarati, Department of Neurology, University of California, Los Angeles, School of Medicine, Veterans Administration Medical Center (111N1), 16111 Plummer Street, Sepulveda, CA 91343-2099.
REFERENCES
- 1.Bartfai T, Bertorelli R, Consolo S, Diaz-Arnesto L, Fisone G, Hökfelt T, Iverfeldt K, Palazzi E, Ogren SO. Acute and chronic studies on functional aspects of coexistence. J Physiol (Paris) 1988;83:126–132. [PubMed] [Google Scholar]
- 2.Bartfai T, Bedecs K, Land T, Langel Ü, Bertorelli R, Girotti P, Consolo S, Xu XJ, Wiesenfeld-Hallin Z, Nilsson S, Pieribone VA, Hökfelt T. M-15: high-affinity chimeric peptide that blocks the neuronal actions of galanin in the hippocampus, locus coeruleus, and spinal cord. Proc Natl Acad Sci USA. 1991;88:10961–10965. doi: 10.1073/pnas.88.23.10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartfai T, Fisone G, Langel Ü. Galanin and galanin antagonists: molecular and biochemical perspectives. Trends Pharmacol Sci. 1992;13:312–317. doi: 10.1016/0165-6147(92)90098-q. [DOI] [PubMed] [Google Scholar]
- 4.Bartfai T, Hökfelt T, Langel Ü. Galanin–a neuro-endocrine peptide. Crit Rev Neurobiol. 1993;7:229–274. [PubMed] [Google Scholar]
- 5.Ben-Ari Y, Lazdunski M. Galanin protects hippocampal neurons from the functional effects of anoxia. Eur J Pharmacol. 1989;165:331–332. doi: 10.1016/0014-2999(89)90732-2. [DOI] [PubMed] [Google Scholar]
- 6.Burgevin MC, Loquet I, Quarteronet D, Habert-Ortoli E. Cloning, pharmacological characterization, and anatomical distribution of a rat cDNA encoding for a galanin receptor. J Mol Neurosci. 1995;6:33–41. doi: 10.1007/BF02736757. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Laburthe M, Amiranoff B. Galanin inhibits adenylate cyclase of rat brain membranes. Peptides. 1992;13:339–341. doi: 10.1016/0196-9781(92)90118-m. [DOI] [PubMed] [Google Scholar]
- 8.Chepurnov SA, Chepurnova NE, Abbasova KR, Smirnova MP. The neuropeptide galanin and the seizure reactions of the developing brain. Uspekhi Fiziol Nauk. 1997;28:3–20. [PubMed] [Google Scholar]
- 9.Cortes R, Ceccatelli S, Schalling M, Hökfelt T. Differential effects of intracerebroventricular colchicine administration on the expression of mRNAs for neuropeptides and neurotransmitter enzymes, with special emphasis on galanin: an in situ hybridization study. Synapse. 1990;6:369–391. doi: 10.1002/syn.890060410. [DOI] [PubMed] [Google Scholar]
- 10.Divac I, Petrovic-Minic B, Mogensen J. Focal cortical seizures prevent HRP and HRP-WGA labeling only in neurons bidirectionally connected to the cortex. Brain Res. 1984;311:189–193. doi: 10.1016/0006-8993(84)91417-3. [DOI] [PubMed] [Google Scholar]
- 11.Dutar P, Lamour Y, Nicoll RA. Galanin blocks the slow cholinergic EPSP in CA1 pyramidal neurons from ventral hippocampus. Eur J Pharmacol. 1989;164:355–360. doi: 10.1016/0014-2999(89)90477-9. [DOI] [PubMed] [Google Scholar]
- 12.Dwyer BE, Wasterlain CG. Selective focal inhibition of brain protein synthesis during generalized bicuculline seizures in newborn marmoset monkeys. Brain Res. 1984;308:109–121. doi: 10.1016/0006-8993(84)90922-3. [DOI] [PubMed] [Google Scholar]
- 13.Fisone G, Wu CF, Consolo S, Nordstrom O, Brynne N, Bartfai T, Melander T, Hökfelt T. Galanin inhibits acetylcholine release in the ventral hippocampus of the rat: histochemical, autoradiographic, in vivo, and in vitro studies. Proc Natl Acad Sci USA. 1987;84:7339–7343. doi: 10.1073/pnas.84.20.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisone G, Berthold M, Bedecs K, Unden A, Bartfai T, Bertorelli R, Consolo S, Crawley J, Martin B, Nilsson S, Hökfelt T. N-terminal galanin-(1–16) fragment is an agonist at the hippocampal galanin receptor. Proc Natl Acad Sci USA. 1989a;86:9588–9591. doi: 10.1073/pnas.86.23.9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisone G, Langel Ü, Carlquist M, Bergman T, Consolo S, Hökfelt T, Unden A, Andell S, Bartfai T. Galanin receptor and its ligands in the rat hippocampus. Eur J Biochem. 1989b;181:269–276. doi: 10.1111/j.1432-1033.1989.tb14721.x. [DOI] [PubMed] [Google Scholar]
- 16.Folkesson R, Terenius L. Enzyme-linked immunosorbent assay of substance P and its metabolite SP1–7. A comparison with RIA. J Neurosci Methods. 1985;14:169–176. doi: 10.1016/0165-0270(85)90032-9. [DOI] [PubMed] [Google Scholar]
- 17.Gaykema RPA, Van der Kuil J, Hersh LB, Luiten PGM. Patterns of direct projections from the hippocampus to the medial septum-diagonal band complex: anterograde tracing with Phaseolus vulgaris leuagglutinin combined with immunohistochemistry of choline acetyltransferase. Neuroscience. 1991;43:349–360. doi: 10.1016/0306-4522(91)90299-4. [DOI] [PubMed] [Google Scholar]
- 18.Gustafson EL, Smith KE, Durkin MM, Gerald C, Branchek TA. Distribution of a rat galanin receptor mRNA in rat brain. NeuroReport. 1996;7:953–957. doi: 10.1097/00001756-199603220-00025. [DOI] [PubMed] [Google Scholar]
- 19.Habert-Ortoli E, Amiranoff B, Loquet I, Laburthe M, Mayaux J-F. Molecular cloning of a functional human galanin receptor. Proc Natl Acad Sci USA. 1994;91:9780–9783. doi: 10.1073/pnas.91.21.9780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard AD, Tan C, Shiao L, Palyha OC, McKee KK, Weinberg DH, Feighner SD, Cascieri MA, Smith RG, Van Der Ploeg LHT, Sullivan KA. Molecular cloning and characterization of a new receptor for galanin. FEBS Lett. 1997;405:285–290. doi: 10.1016/s0014-5793(97)00196-8. [DOI] [PubMed] [Google Scholar]
- 21.Karelson E, Laasik J, Sillard R. Regulation of adenylate cyclase by galanin, neuropeptide Y, secretin and vasoactive intestinal polypeptide in rat frontal cortex, hippocampus and hypothalamus. Neuropeptides. 1995;28:21–28. doi: 10.1016/0143-4179(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 22.Kask K, Langel Ü, Bartfai T. Galanin–a neuropeptide with inhibitory actions. Cell Mol Neurobiol. 1995;15:653–673. doi: 10.1007/BF02071130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klapstein GJ, Colmers WF. Neuropeptide Y suppresses epileptiform activity in rat hippocampus in vitro. J Neurophysiol. 1997;78:1651–1661. doi: 10.1152/jn.1997.78.3.1651. [DOI] [PubMed] [Google Scholar]
- 24.Lamour Y, Senut MC, Dutar P, Bassant MH. Neuropeptides and septo-hippocampal neurons: electrophysiological effects and distributions of immunoreactivity. Peptides. 1989;9:1351–1359. doi: 10.1016/0196-9781(88)90202-1. [DOI] [PubMed] [Google Scholar]
- 25.Langel Ü, Land T, Bartfai T. Design of chimeric peptide ligands to galanin receptors and substance P receptors. Int J Pept Protein Res. 1992;39:516–522. doi: 10.1111/j.1399-3011.1992.tb00282.x. [DOI] [PubMed] [Google Scholar]
- 26.Lurton D, Coussemacq M, Barrow P, Sundstrom LE, Rougier A. Widespread ectopic neuropeptide-Y immunoreactivity in contralateral mossy fibres after a unilateral intrahippocampal kainic acid injection in the rat. Neurosci Lett. 1996;213:181–184. doi: 10.1016/0304-3940(96)12854-8. [DOI] [PubMed] [Google Scholar]
- 27.Malthe-Sorenssen D, Odden E, Walaas I. Selective destruction by kainic acid of neurons innervated by putative glutamatergic afferents in septu, and nucleus of diagonal band. Brain Res. 1980;182:461–465. doi: 10.1016/0006-8993(80)91204-4. [DOI] [PubMed] [Google Scholar]
- 28.Marksteiner J, Ortler M, Bellmann R, Sperk G. Neuropeptide Y biosynthesis is markedly induced in mossy fibers during temporal lobe epilepsy of the rat. Neurosci Lett. 1990;112:143–148. doi: 10.1016/0304-3940(90)90193-d. [DOI] [PubMed] [Google Scholar]
- 29.Mastropaolo J, Nadi NS, Ostrowski NL, Crawely JN. Galanin antagonizes acetylcholine on memory task in basal forebrain lesioned rats. Proc Natl Acad Sci USA. 1988;85:9841–9845. doi: 10.1073/pnas.85.24.9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazarati AM, Wasterlain CG. Pharmacological treatment of self-sustaining status epilepticus. Soc Neurosci Abstr. 1997;23:1687. [Google Scholar]
- 31.Mazarati AM, Halaszi E, Telegdy G. Anticonvulsive effects of galanin administered into the central nervous system upon the picrotoxin-kindled seizure syndrome in rats. Brain Res. 1992;589:164–166. doi: 10.1016/0006-8993(92)91179-i. [DOI] [PubMed] [Google Scholar]
- 32.Mazarati AM, Wasterlain CG, Sankar R, Shin D. Self-sustaining status epilepticus after brief electrical stimulation of the perforant path. Brain Res. 1998;801:251–253. doi: 10.1016/s0006-8993(98)00606-4. [DOI] [PubMed] [Google Scholar]
- 33.McDonald MP, Wenk GL, Crawley JN. Analysis of galanin and the galanin antagonist M40 on delayed non-matching-to-position performance in rats lesioned with the cholinergic immunotoxin 192 IgG-saporin. Behav Neurosci. 1997;111:552–563. doi: 10.1037//0735-7044.111.3.552. [DOI] [PubMed] [Google Scholar]
- 34.McLennan H, Miller JJ. The hippocampal control of neuronal discharges in the septum of the rat. J Physiol (Lond) 1974;237:607–624. doi: 10.1113/jphysiol.1974.sp010500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melander T, Staines WA, Hökfelt T, Rokaeus A, Eckenstein F, Salvaterra PM, Wainer BH. Galanin-like immunoreactivity in cholinergic neurons of the septum-basal forebrain complex projecting to the hippocampus of the rat. Brain Res. 1985;360:130–138. doi: 10.1016/0006-8993(85)91228-4. [DOI] [PubMed] [Google Scholar]
- 36.Melander T, Hökfelt T, Nilsson S, Brodin E. Visualization of galanin binding sites in the rat central nervous system. Eur J Pharmacol. 1986a;124:381–382. doi: 10.1016/0014-2999(86)90247-5. [DOI] [PubMed] [Google Scholar]
- 37.Melander T, Hökfelt T, Rokaeus A. Distribution of galaninlike immunoreactivity in the rat central nervous system. J Comp Neurol. 1986b;248:475–517. doi: 10.1002/cne.902480404. [DOI] [PubMed] [Google Scholar]
- 38.Melander T, Hökfelt T, Rokaeus A, Cuello AC, Oertel WH, Verhofstad A, Goldstein M. Coexistence of galanin-like immunoreactivity with catecholamines, 5-hydroxytryptamine, GABA, and neuropeptides in the rat CNS. J Neurosci. 1986c;6:3640–3654. doi: 10.1523/JNEUROSCI.06-12-03640.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melander T, Staines WA, Rokaeus A. Galanin-like immunoreactivity in hippocampal afferents in the rat, with special reference to cholinergic and noradrenergic inputs. Neuroscience. 1986d;19:223–240. doi: 10.1016/0306-4522(86)90017-5. [DOI] [PubMed] [Google Scholar]
- 40.Meldrum B. Identification and preclinical testing of novel antiepileptic compounds. Epilepsia. 1997;38(Suppl 9):S7–S15. doi: 10.1111/j.1528-1157.1997.tb05204.x. [DOI] [PubMed] [Google Scholar]
- 41.Merchenthaler T, Lopez FJ, Negro-Vilar A. Anatomy and physiology of central galanin-containing pathways. Prog Neurobiol. 1993;40:711–769. doi: 10.1016/0301-0082(93)90012-h. [DOI] [PubMed] [Google Scholar]
- 42.Pooga M, Jureus A, Razaei K, Hasanvan H, Saar K, Kask K, Kjellen P, Land T, Halonen J, Maeorg U, Uri A, Solyom S, Bartfai T, Langel Ü. Novel galanin receptor ligands. J Peptide Res. 1998;51:65–74. doi: 10.1111/j.1399-3011.1998.tb00418.x. [DOI] [PubMed] [Google Scholar]
- 43.Racine RJ. Modification of seizure activity by electrical stimulation. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 44.Sakurai E, Maeda T, Kaneko S, Akaike A, Satoh M. Galanin inhibits long-term potentiation at Schaffer collateral-CA1 synapses in guinea-pig hippocampal slices. Neurosci Lett. 1996;212:21–24. doi: 10.1016/0304-3940(96)12772-5. [DOI] [PubMed] [Google Scholar]
- 45.Sankar R, Shin D, Wasterlain CG. Serum neuron-specific enolase is a marker for neuronal damage following status epilepticus in the rat. Epilepsy Res. 1997;28:129–136. doi: 10.1016/s0920-1211(97)00040-5. [DOI] [PubMed] [Google Scholar]
- 46.Schwarzer C, Sperk G. Hippocampal granule cells express glutamic acid decarboxylase-67 after limbic seizures in the rat. Neuroscience. 1995;69:705–709. doi: 10.1016/0306-4522(95)00348-m. [DOI] [PubMed] [Google Scholar]
- 47.Senut MC, Menetrey D, Lamour Y. Cholinergic and peptidergic projections from the medial septum and the nucleus of the diagonal band of Broca to dorsal hippocampus, cingulate cortex and olfactory bulb: a combined wheatgerm agglutinin-apohorseradish peroxidase-gold immunohistochemical study. Neuroscience. 1989;30:385–403. doi: 10.1016/0306-4522(89)90260-1. [DOI] [PubMed] [Google Scholar]
- 48.Servin AL, Amiranoff B, Rouyer-Fessard C, Tatemoto K, Laburthe M. Identification and molecular characterization of galanin receptor sites in rat brain. Biochem Biophys Res Commun. 1987;144:298–306. doi: 10.1016/s0006-291x(87)80510-7. [DOI] [PubMed] [Google Scholar]
- 49.Skofitsch G, Jacobowitz DM. Immunohistochemical mapping of galanin-like neurons in the rat central nervous system. Peptides. 1985;6:509–546. doi: 10.1016/0196-9781(85)90118-4. [DOI] [PubMed] [Google Scholar]
- 50.Skofitsch G, Jacobowitz DM. Quantitative distribution of galanin-like immunoreactivity in the rat central nervous system. Peptides. 1986;7:609–613. doi: 10.1016/0196-9781(86)90035-5. [DOI] [PubMed] [Google Scholar]
- 51.Skofitsch G, Sills MA, Jacobowitz DM. Autoradiographic distribution of 125I-galanin binding sites in the rat central nervous system. Peptides. 1986;7:1029–1042. doi: 10.1016/0196-9781(86)90133-6. [DOI] [PubMed] [Google Scholar]
- 52.Smith KE, Forray C, Walker MW, Jones KA, Tamm JA, Bard J, Branchek TA, Linemeyer DL, Gerald C. Expression cloning of a rat hypothalamic galanin receptor coupled to phosphoinositide turnover. J Biol Chem. 1997;272:24612–24616. doi: 10.1074/jbc.272.39.24612. [DOI] [PubMed] [Google Scholar]
- 53.Sperk G, Herzog H. Anticonvulsant action of neuropeptide Y. Neuropeptide Y may act as an endogenous anticonvulsant through Y5 receptors suggesting a new target for antiepileptic drugs. Nat Med. 1997;3:728–729. doi: 10.1038/nm0797-728. [DOI] [PubMed] [Google Scholar]
- 54.Stevens DR, Cotman CW. Excitatory amino acid antagonists depress transmission in hippocampal projections to the lateral septum. Brain Res. 1986;382:437–440. doi: 10.1016/0006-8993(86)91359-4. [DOI] [PubMed] [Google Scholar]
- 55.Sundström E, Archer T, Melander T, Hökfelt T. Galanin impairs acquisition but not retrieval of spatial memory in rat studies in the Morris swim maze. Neurosci Lett. 1988;88:331–335. doi: 10.1016/0304-3940(88)90233-9. [DOI] [PubMed] [Google Scholar]
- 56.Vezzani A, Schwarzer C, Lothman EW, Williamson J, Sperk G. Functional changes in somatostatin and neuropeptide Y containing neurons in the rat hippocampus in chronic models of limbic seizures. Epilepsy Res. 1996;26:267–279. doi: 10.1016/s0920-1211(96)00059-9. [DOI] [PubMed] [Google Scholar]
- 57.Wang S, Hashemi T, He C, Strader C, Bayne M. Molecular cloning and pharmacological characterization of a new galanin receptor subtype. Mol Pharmacol. 1997a;52:337–343. doi: 10.1124/mol.52.3.337. [DOI] [PubMed] [Google Scholar]
- 58.Wang S, He C, Hashemi T, Bayne M. Cloning and expressional characterization of a novel galanin receptor. Identification of different pharmacophores within galanin for the three galanin receptor subtypes. J Biol Chem. 1997b;272:31949–31952. doi: 10.1074/jbc.272.51.31949. [DOI] [PubMed] [Google Scholar]
- 59.Xu ZQ, Shi TJ, Hökfelt T. Galanin/GMAP- and NPY-like immunoreactivities in locus coeruleus and noradrenergic nerve terminals in the hippocampal formation and cortex with notes on the galanin-R1 and -R2 receptors. J Comp Neurol. 1998;392:227–251. doi: 10.1002/(sici)1096-9861(19980309)392:2<227::aid-cne6>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 60.Zini S, Roisin M, Langel Ü, Bartfai T, Ben-Ari Y. Galanin reduces release of endogenous excitatory amino acids in the rat hippocampus. Eur J Pharmacol. 1993a;245:1–7. doi: 10.1016/0922-4106(93)90162-3. [DOI] [PubMed] [Google Scholar]
- 61.Zini S, Roisin MP, Armengaud C, Ben-Ari Y. Effects of potassium channel modulators on the release of glutamate induced by ischaemic-like conditions in rat hippocampal slices. Neurosci Lett. 1993b;153:202–205. doi: 10.1016/0304-3940(93)90322-c. [DOI] [PubMed] [Google Scholar]


