Abstract
Basic fibroblast growth factor (FGF-2) influences the differentiation and survival of retinal photoreceptors in vivo and in vitro, but it is not known whether it acts directly on photoreceptor FGF receptors or indirectly through activation of surrounding cells. To clarify the effects of FGF-2 on photoreceptor survival, we developed a purified photoreceptor culture system. The outer nuclear layers of postnatal day 5–15 rat retinas were isolated by vibratome sectioning, and the photoreceptor fractions obtained were enzymatically dissociated. Photoreceptors were maintained in monolayer culture for 1 week in a chemically defined medium. Immunocytochemical labeling showed that >99.5% of cells were photoreceptors, and glial contamination represented ∼0.2%. Photoreceptors from postnatal day 5–9 retinas survived for at least 24 hr in vitro, whereas cells from postnatal day 10–15 retinas died rapidly. Subsequent studies performed with postnatal day 5 photoreceptors showed that their survival was increased in a dose-dependent manner after the addition of FGF-2. In control cultures, 36% of originally seeded photoreceptors were alive after 5 din vitro, and in the presence of 20 ng/ml FGF-2 this number was doubled to 62%. This increase was not caused by proliferation of photoreceptor precursors. Denaturing or blocking FGF-2 prevented enhancement of survival. Conversely, only 25.5% of photoreceptors survived in the presence of epidermal growth factor (EGF). FGF- and EGF-receptor mRNA and proteins were detected in purified photoreceptors in vitro, and addition of FGF-2 or EGF led to tyrosine phosphorylation of photoreceptor proteins. These data support a direct mechanism of action for FGF-2 stimulation of photoreceptor survival.
Keywords: photoreceptors, cell culture, basic fibroblast growth factor, epidermal growth factor, survival, immunocytochemistry
Photoreceptors (PRs) of the retina are highly specialized neurons that transduce light stimuli to membrane potential changes signaled to second-order neurons. These cells are essential for normal vision, but they degenerate in a number of conditions, including genetic diseases (Portera-Cailliau et al., 1994), environmental insults such as light damage (LaVail et al., 1992), and as a result of normal aging (Gao and Hollyfield, 1992a; Curcio et al., 1993). PR rescue or neuroprotection are topics of great current interest that are necessary for formulating therapeutic approaches.
Neurotrophic factors are essential for the development, maintenance, and survival of CNS neurons (Barde, 1989; Oppenheim, 1991). The presence and effects in the retina of many trophic factors have been described [e.g., brain-derived growth factor (Johnson et al., 1986); epidermal growth factor (EGF) (Anchan et al., 1991); and ciliary neurotrophic factor (Fuhrmann et al., 1995)]. Basic fibroblast growth factor (or FGF-2) belongs to a large family of polypeptide growth factors exerting effects on neural tissue (Wagner, 1991) that act through binding to specific membrane-bound tyrosine kinase receptors (Partanen et al., 1992). In the retina, FGF-2 has been localized within the PR by immunocytochemistry (Gao and Hollyfield, 1992b) and byin situ hybridization (Noji et al., 1990). FGF-2 binds to low- and high-affinity binding sites within the eye in vivo(Jeanny et al., 1987; Fayein et al., 1990) and is bound and released from PR rod outer segments (Plouët et al., 1988; Mascarelli et al., 1989). High-affinity FGF receptors (FGFRs) have been localized throughout the developing and adult retina (Heuer et al., 1990; Tcheng et al., 1994). In vivo studies on animal models of PR degeneration suggest a survival-promoting effect of this factor on PRs, because it has been shown to delay PR degeneration in the Royal College of Surgeons rat, a model of inherited retinal dystrophy (Faktorovich et al., 1990), and in a rat model of light damage (LaVail et al., 1992). Cell culture studies demonstrated that FGF-2 induces the differentiation of PRs in a dose- and age-dependent manner (Hicks and Courtois, 1992), but no evidence of its putative effect on their survival has been reported.
The in vitro models used for studies of the effects of growth factors and other molecules on PR cell biology have consisted of mixed cultures containing many types of retinal neurons and glia (Watanabe and Raff, 1990; Hicks and Courtois, 1992; Lillien and Cepko, 1992; Jing et al., 1996). Such approaches cannot distinguish between effects caused by direct activation of growth factor receptors located on PRs or those elicited indirectly by stimulation of the other cell types present. We examined the question of whether specific direct survival-promoting effects of FGF-2 could be demonstrated in PRs through the use of an original culture model consisting of purified postmitotic rat PRs. We demonstrate that purified PRs possess both FGFRs and EGFRs, that these receptors are activated by their respective ligands, and that FGF-2 increases transiently PR survival whereas EGF promotes their degeneration.
MATERIALS AND METHODS
Materials. DMEM, CO2-independent DMEM (CIM), and fetal bovine serum were purchased from Life Technologies (Grand Island, NY). Desoxyribonuclease type I, gelatin, poly-d-lysine, laminin, bovine serum albumin (BSA), suramin, tyrphostin 23, insulin-transferrin-selenium pre-mix, monoclonal anti-vimentin (clone V9), secondary antibodies, and all other reagents used for culture medium were from Sigma (St. Louis, MO). Papain was from Worthington (Freehold, NJ). Recombinant human FGF-2 was from Pharma Biotechnologie (Hannover, Germany). EGF (receptor grade) was from Chemicon International (Temecula, CA). Monoclonal anti-FGFR type 1 (R1) (ab6) was a generous gift from Dr. A. Baird (The Whittier Institute, Scripps Memorial Hospital, La Jolla, CA). Polyclonal anti-arrestin was a generous gift from Dr. I. Gery (National Institutes of Health, Bethesda, MD). Polyclonal anti-recoverin was a generous gift from Dr. A. Dizhoor (University of Washington, Seattle, WA). Monoclonal anti-EGFR was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal anti-mouse IgG Bodipy FL and streptavidin Texas Red were from Interchim (Montluçon, France). Monoclonal anti-bovine FGF-2 type I and monoclonal anti-phosphotyrosine (4G10) were from Upstate Biotechnology (Lake Placid, NY). Peroxidase-conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). Kaleidoscope prestained standards were from Bio-Rad Laboratories (Hercules, CA). Tissue culture plastic ware was from Nunc (Roskilde, Denmark). Live/Dead Kit (L-3224) was from Molecular Probes Europe BV (Leiden, The Netherlands). PCR primers were from Life Technologies (Paris, France), and reagents used for RT-PCR were from Promega (Lyon, France) and Eurobio (Les Ulis, France).
Tissue collection. Animals used in these studies were cared for and handled according to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Postnatal day 5–15 Wistar rats were used for these experiments. They were anesthetized by CO2 inhalation, killed rapidly by cervical dislocation, and enucleated.
Photoreceptor isolation. PRs were isolated from the rest of the retina using a mechanical technique originally developed for retinal transplantation (Silverman and Hughes, 1989) and modified by us (Dreyfus et al., 1996; Fontaine et al., 1998) to allow the preparation of purified PR cultures. The retina was carefully removed from the eye in chilled CIM plus antibiotics [penicillin (10 U/ml), streptomycin (10 μg/ml)] at 4°C, the vitreous was detached, and the tissue was put on a glass slide in a drop of CIM. The retina was then flattened carefully with four radial cuts, mounted PR surface down on a gelatin block (20% in CIM), and attached to it by gently expulsing warmed gelatin (42°C, 4% in CIM) between the retina and the gelatin block. Excess 4% gelatin was aspirated, and the entire preparation was cooled at 4°C with ice-cold CIM. Preliminary studies determined the appropriate depth to cut (150–200 μm depth) from the vitreal surface to obtain a PR cell layer uncontaminated with other retinal cells (see Fig. 1). To ensure PR purity, the tissue slice bordering the outer plexiform layer was eliminated systematically (see Fig.1A), and any retinas that were mounted improperly were not processed further. For each sample, verification of cell purity was performed by microscopic observation of small sections taken in the center and periphery of the PR layer. A final cut of 250–300 μm undercut the PR layer still fastened to the gelatin block. To separate the PR from the gelatin, fractions were incubated in Ringer’s solution consisting of (in mm): NaCl 125.4, KCl 3.6, MgCl2 1.2, NaHCO3 22.6, NaH2PO4 0.1, Na2HPO40.4, Na2SO4 1.2, glucose 10, without Ca2+, plus EDTA (2.5 mm), at 37°C for 10 min.
Fig. 1.
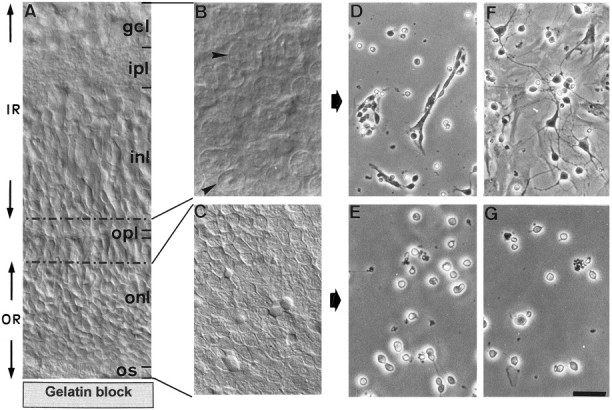
Photographs showing the successive steps of PR culture preparation. Rat retinas (5 d old) (a transverse section showing the different cell and fiber layers is shown inA) were flat-mounted onto the gelatin block with the ganglion cell layer (gcl) uppermost and sectioned along a horizontal plane using a vibratome. An initial cut of 150 μm permitted isolation of the inner retina (IR), composed of the gcl, inner plexiform layer (ipl), and the majority of the inner nuclear layer (inl). A second cut of 30–40 μm containing the remaining inl, outer plexiform layer (opl), and a fraction of the outer nuclear layer (onl) was eliminated (corresponding to the area delimited by dotted lines in A) to ensure purity of the final fraction. A final cut of 200 μm containing only the outer retina (OR) composed of the onl and PR outer segments (os) was then made. B andC demonstrate the microscopical aspect of the IR and OR horizontal slices, respectively. In IR, neuronal cell bodies of different sizes are visible (B, arrows). OR contains only regularly sized PR (C). Cell cultures were obtained after enzymatic digestion and cell dissociation of IR and OR (D–G). Cultures are shown after 1 d (D, E) and 5 d (F, G)in vitro. IR-derived cultures contained multipolar neurons and glial cells (D), the latter of which proliferated to form a monolayer after 5 d in vitro(F). On this glial monolayer, neurons extended long neuritic processes. In contrast, OR-derived cultures were composed of small round cells isolated or present in small groups, sometimes exhibiting small thin neurites (E). After 5 d in vitro, the number of PRs had decreased, but there was no glial proliferation (G). Scale bars:A, 20 μm; B, C, 12.5 μm;D–G, 30 μm.
Purified photoreceptor cultures. After three washes in Ringer’s solution, the PR layer was incubated in 500 μl of papain (0.1 mg/ml Ringer’s solution) (preactivated for 30 min in a solution containing 1.1 mm EDTA, 0.067 mmβ-mercaptoethanol, and 5.5 mm cysteine) at 37°C for 20 min, pH 6.2. The digestion was stopped with 500 μl of DMEM supplemented with 10% fetal bovine serum; then 50 μl of desoxyribonuclease 1 (DNase1)/BSA (0.1 mg/ml) was added, and the cells were dissociated after a final incubation at 37°C for 5 min. After centrifugation at 800 rpm for 15 min, the cells were resuspended in 1 ml serum supplemented medium and counted in trypan blue (1:1) on a hemocytometer. For experiments on cell survival and immunocytochemistry, PRs were seeded into 24-multiwell dishes on glass coverslips pretreated with poly-d-lysine (2 μg/cm2 during 30 min) and laminin (2 μg/cm2 overnight) at a density of 105 cells/cm2. For experiments on biochemical and molecular biological analyses of growth factors, PRs were seeded at higher density (106PR/cm2) and seeded into 35 mm Petri dishes coated with poly-d-lysine.
Verification of the preparation purity. To verify the purity of our preparations, cells were labeled with specific antibodies. Cells were fixed with 4% paraformaldehyde for 15 min at room temperature, permeabilized with Triton X-100 (0.1% in PBS) for 5 min, and saturated with 1% BSA in PBS for 15 min. Double-label immunocytochemistry was performed using as primary antibodies polyclonal anti-arrestin, polyclonal anti-recoverin, monoclonal anti-vimentin, and monoclonal anti-opsin Rho-4D2 (Hicks and Barnstable, 1987). All antibodies were used at a final dilution of 10 μg/ml and incubated with cells for 2 hr. Polyclonal antibodies were followed by anti-rabbit IgG biotin conjugate (10 μg/ml, 1 hr) and streptavidin Texas Red (10 μg/ml, 1 hr). Monoclonal antibodies were followed by anti-mouse IgG Bodipy FL (10 μg/ml, 1 hr). Cells were examined using a Nikon Optiphot 2 photomicroscope equipped with differential contrast interference and fluorescence optics.
To estimate the degree of Müller glial cell (MGC) contamination in our cultures, MGC numbers were determined through immunocytochemistry and Western blotting (see below).
Growth factor assays. After 24 hr in serum-supplemented DMEM, PR culture medium was removed, and cells were rinsed twice gently with DMEM that was immediately replaced by a chemically defined medium (CDM): DMEM supplemented with insulin–transferrin–selenium pre-mix (5 ng/ml), sodium pyruvate (1 mm), putrescine (100 μm), progesterone (64 nm), prostaglandin F2α (210 nm), triiodo-l-thyronine (31 nm), hydrocortisone (5.5 μm), taurine (3 mm), cytidine 5′-diphosphoethanolamine (2.9 μm), cytidine 5′-diphosphocholine (5.2 μm), and antibiotics, and supplemented or not with growth factors EGF (1–50 ng/ml) and FGF-2 (1–50 ng/ml). Growth factors (20 μl) were added again to the culture medium after 72 and 120 hr. The same volume of CDM was added at the same time in control wells. In additional trials, inactivated FGF-2 (heated to 60°C for 5 min), suramin (50 μm), or tyrphostin-23 (10 μm) were added to some wells. At different times (3, 5, and 7 d), the viability of PRs was tested by the Live/Dead assay (Vaughan et al., 1995) used at concentrations of 1 μm for both calcein AM and ethidium homodimer-1. This highly sensitive cytotoxicity assay is based on the hydrolysis by live cells of membrane-permeable calcein AM by nonspecific esterases to membrane-impermeable fluorescent calcein. Green fluorescence is hence a reliable indicator of cellular esterase activity and intact membranes. Ethidium homodimer-1 is incorporated exclusively into nuclei of dead or dying cells. For each coverslip, images of 25 fields (observed using 20× objectives) were recorded using Visiolab 1000 image analysis software (Biocom, Lyon, France), and cells were counted. For each treatment in each experiment, two coverslips were counted, and the experiments were performed at least three times. Any experiments in which glial numbers exceeded 0.2% of the total cells in control cultures were excluded from survival assays.
RT-PCR. After 24 hr in vitro, PRs were harvested from Petri dishes by scraping, frozen in liquid nitrogen, and conserved at −70°C. Total RNA was isolated by the acid guanidium thiocyanate–phenol–chloroform method (Chomczynski and Sacchi, 1987) and digested with DNase I to ensure removal of possible DNA contamination. RNA quality was verified by agarose gel electrophoresis, and concentration was determined. RT was performed as described byKendall and Latchman (1996) using 1 μg RNA. PCR amplifications were made on 4 μl of cDNA in a MJ-Research thermal cycler model PTC-100 with 200 μm dNTP, 10 mm Tris-HCl, pH 8.8, 50 mm KCl, 1.5 mm MgCl2, and 2 U Taq DNA polymerase. The sequence of each primer and the length of the amplified products are given in Table1. The total number of cycles was 35, and each cycle consisted of a heat-denaturation step at 94°C for 30 sec, annealing of primers for 30 sec at 58°C, and polymerization at 72°C for 30 sec.
Table 1.
Forward and reverse primer sequences used for RT-PCR
| mRNA species | Nucleotide sequence | Product length (bp) |
|---|---|---|
| Rat arrestin 3′-primer | 5′-AATAGCCAATGTGGTTCTCTACTC-3′ | 464 |
| Rat arrestin 5′-primer | 5′-CATCCTCATCTTTCTTCCCTTC-3′ | |
| Mouse EGFR 3′-primer | 5′-TTGACCAAAATCATCTGTGCCC-3′ | 401 |
| Mouse EGFR 5′-primer | 5′-CCAATGCCTATGCCATTACAAAC-3′ | |
| Rat FGFR1 3′-primer | 5′-ACAGACAACACCAAACCAAACC-3′ | 299 |
| Rat FGFR1 5′-primer | 5′-TTAATGCTCCCATACTCGTTCTC-3′ | |
| Rat rhodopsin 3′-primer | 5′-ATTCACCACCACCCTCTACACC-3′ | 395 |
| Rat rhodopsin 5′-primer | 5′-AGAAGAAGATGACGATCATGGG-3′ |
Protein extraction and Western blotting. For immunodetection of vimentin, purified cultures of postnatal day 5 (3 d in vitro) and freshly obtained vibratome sections of outer and inner postnatal day 5 retina were rinsed with PBS and collected on ice in lysis buffer (20 mm Tris-HCl, pH 7.4, 150 mmNaCl, 1% Nonidet P-40, 1 mm EDTA, 1 mm NaF, 1 mm Na3VO4) containing a mixture of protease inhibitors. For anti-phosphotyrosine and EGFR and FGFR1 immunoblots, high-density PR cultures (prepared from postnatal day 5 rat retinas) were washed once with PBS and stimulated with EGF (100 ng/ml after 24 hr in vitro) and FGF-2 (100 ng/ml after 72 hr in vitro) for 30 sec to 5 min. Cells were preincubated for 10 min with a phosphatase inhibitor mixture (PBS containing 1 mm Na3VO4, 0.1 mm NaF, 0.1 mm EDTA) before the addition of growth factor. The reaction was stopped with liquid nitrogen, and cells were lysed as above. Lysates (10 μg total protein/lane for phosphotyrosine antibody, 30 μg/lane for all other antibodies) were separated by electrophoresis on 7.5% polyacrylamide minigels and transferred to nitrocellulose membranes. For probing with the anti-phosphotyrosine antibody, the membranes were blocked with 1% dry milk and 3% BSA in PBS and 0.2% Tween-20 overnight at 4°C. For all other antibodies, membranes were blocked with 3% dry milk and 1% BSA in PBS and 0.1% Tween-20 for 1 hr at room temperature. Membranes were incubated with a 1:1000 dilution of appropriate primary antibodies: anti-FGFR1 overnight at 4°C and anti-EGFR, anti-phosphotyrosine, or anti-vimentin each for 1 hr at room temperature. Bound primary antibody was detected using peroxidase-conjugated goat anti-mouse or goat anti-rabbit secondary antibodies (1:15,000 dilution). Immunoreactive bands were visualized using a Pierce super signal substrate kit according to the manufacturers instructions. Molecular masses were determined by comparison with prestained molecular mass markers.
Statistics. Data were compared using the parametric Peritz’ F test according to Harper (1984), accepting significance values of p < 0.05, 0.01, and 0.001.
RESULTS
Purified photoreceptors can survive in vitro in the absence of glial cells and serum
Figure 1 shows representative fields of cultures obtained after digestion, dissociation, and seeding of the outer and inner layers separated by the vibratome (Fig.1B,C). Cultures of the inner layer were composed of neurons and glial cells (Fig. 1D), the latter of which had proliferated after 5 d in vitro (Fig.1F). In contrast, cultures of the outer layer were composed uniquely of small rounded cells, no glial cells being observed at 1 d (Fig. 1E) and 5 d in vitro (Fig. 1G).
Purified photoreceptor survival in vitro depends on age
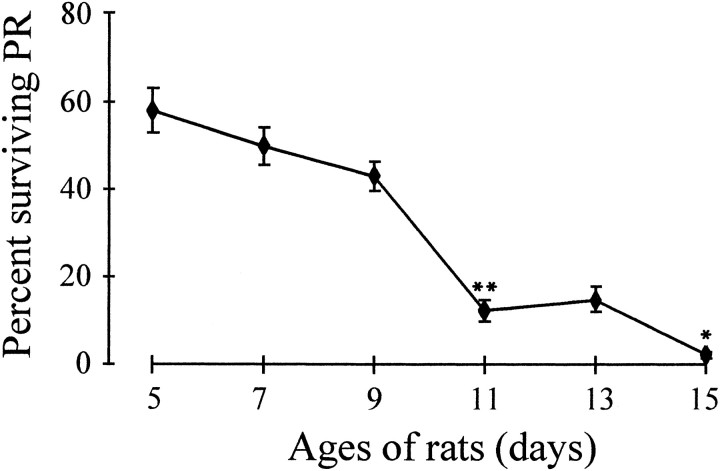
Retinas dissected from rats of different postnatal ages were examined to see whether survival of purified PRs in vitrowas age-dependent. Cultures were prepared from retinas of rats between postnatal days 5 and 15, and after 24 hr in vitro cell survival was monitored by the Live/Dead assay. Figure2 shows that PR survival under these conditions is dependent on age. With donor ages of 5 and 7 d, >50% of PRs were still alive 24 hr after seeding, whereas cultures from 9- to 15-d-old rats showed a sharp decrease in the number of surviving PRs, which became nearly zero. For all further studies, PR cultures were prepared from postnatal day 5 rat retina, corresponding to an age when almost all PRs are differentiated and the retinal layers are sufficiently formed to be separable.
Fig. 2.
PR survival in vitro depends on donor age. PR cultures were prepared from postnatal day 5, 7, 9, 11, 13, and 15 retinas of rats. PRs were counted after 24 hr and compared with the total number of seeded cells. PR survival was higher when retinas were taken at 5 d (58% live PRs). Between 9 and 11 d, PR survival decreased dramatically (from 43 to 12%) to become nearly zero (2.5%) at 15 d. Error bars are SEM (n = 3). *p < 0.01; **p < 0.001.
Verification of culture purity
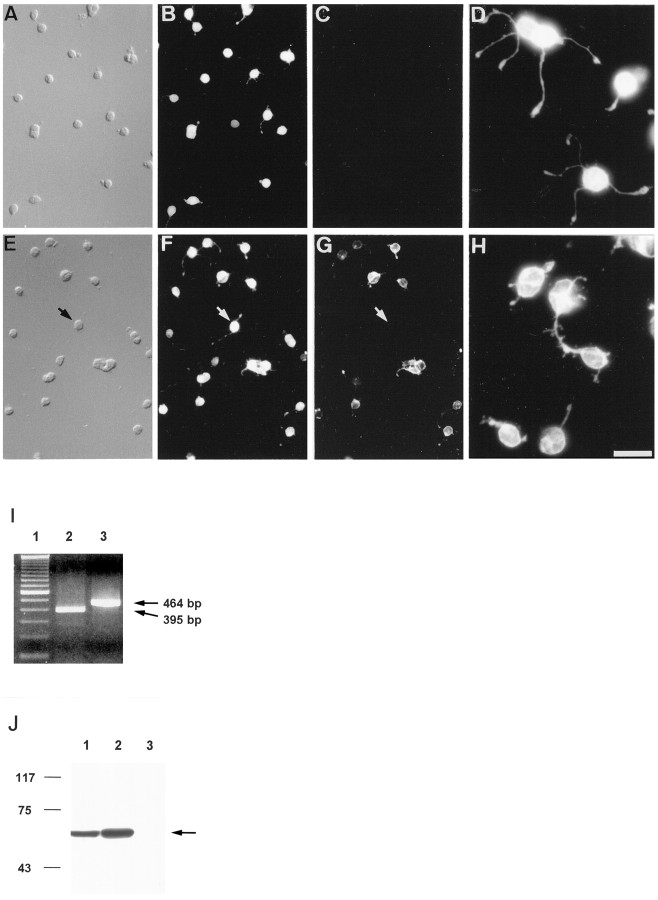
To verify the PR purity of our cultures, cells were labeled by immunocytochemistry with specific antibodies for PRs and MGCs (Fig.3A–H), expression of rhodopsin and arrestin mRNA was verified by RT-PCR (Fig.3I), and vimentin expression was examined in culture lysates by Western blotting (Fig. 3J). Figure3A–C,E–G shows double-labeling of representative fields of PR cultures after 1 d in vitro. Cells were labeled with anti-recoverin (Fig. 3B) or anti-arrestin (Fig.3F), two antibodies that are specific for rod and cone PRs in the rat retina [although recoverin is also reported to label bipolar cells (Euler and Wässle, 1995)], and with anti-vimentin specific for MGC (Fig. 3C) or Rho-4D2 specific for rod PRs (Fig. 3G). These results show that >99.5% of cells observed by Nomarski optics (Fig. 3A,E) were PRs (Fig.3B,F). In addition, almost all cells exhibited a patchy nucleus characteristic of differentiated rod PRs. In Figure 3, comparison between F and G shows that many PRs were unlabeled with anti-rhodopsin antibody. We did not establish whether these cells were cone or rod PRs that did not yet express rhodopsin. Figure 3C shows the absence of MGC in PR culture. Higher magnification of PRs labeled with anti-recoverin (Fig.3D) or Rho-4D2 (Fig. 3H) showed that PR cell bodies were of similar size but displayed different numbers and length of neurites.
Fig. 3.
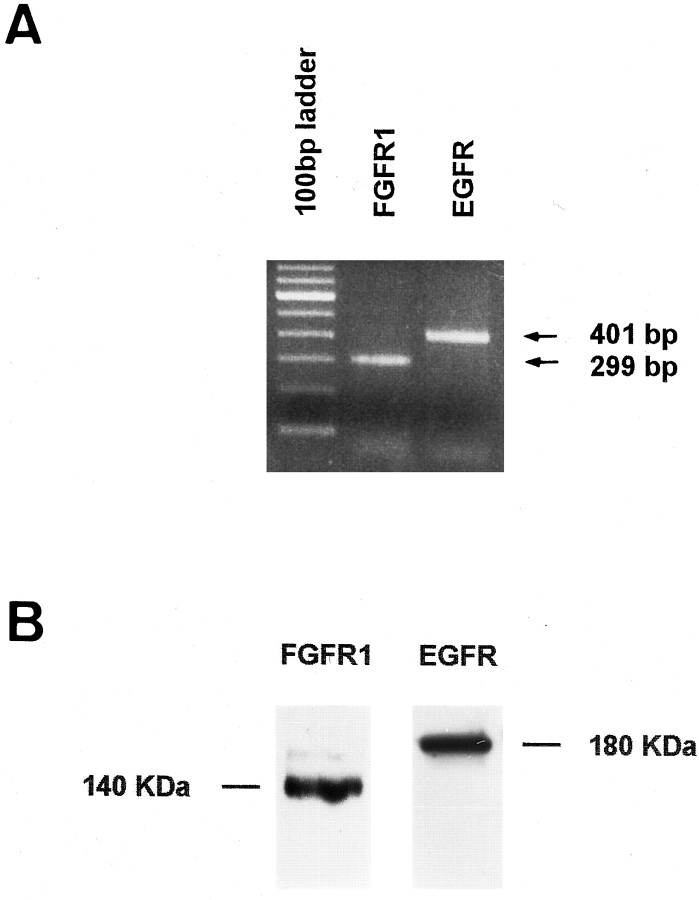
Immunocytochemical and molecular characterization of PR cultures after 24 hr in vitro.A–C and E–G show double-immunolabeling of PR cultures with antibodies specific for recoverin (B), vimentin (C), arrestin (F), and rhodopsin (G). All the cells were positive for recoverin and arrestin, indicating that they were PRs. Rhodopsin-immunopositive PRs represented only 30% of total PRs at this time (the arrow in E–Gindicates an arrestin-immunopositive, opsin-immunonegative PR). Vimentin-immunopositive cells were absent. D andH represent higher magnifications of recoverin- and rhodopsin-immunopositive PRs, showing small neurites. Scale bars:A–C, E–G, 25 μm; D, H, 10 μm.I, Expression analysis of rhodopsin (lane 2) and arrestin (lane 3) mRNA in PR cultures was examined by RT-PCR. Lane 1, 100 bp DNA ladder.J, A comparative analysis of vimentin expression in OR (lane 1), IR (lane 2), and PR cultures (lane 3) was performed by Western blot. Although a single band of the expected molecular weight for vimentin was observed in OR and IR preparations (arrow), no signal could be detected in PR cultures.
The expression of rhodopsin and arrestin mRNA (Fig.3I) confirmed the existence of living PRs. When immunoblots of freshly obtained vibratome sections of outer and inner retina were probed with anti-vimentin antibody, a single prominent 58 kDa band was visible in both cases (the former corresponding to apical extensions of MGC bodies in the outer layer and the latter to MGC bodies and basal extensions in the inner layer) (Fig. 3J,lanes 1 and 2). In contrast, no band was detectable in immunoblots prepared from PR cultures (Fig.3J, lane 3), confirming their purity.
Exogenous FGF-2 increases survival of PRs and EGF accelerates their degeneration
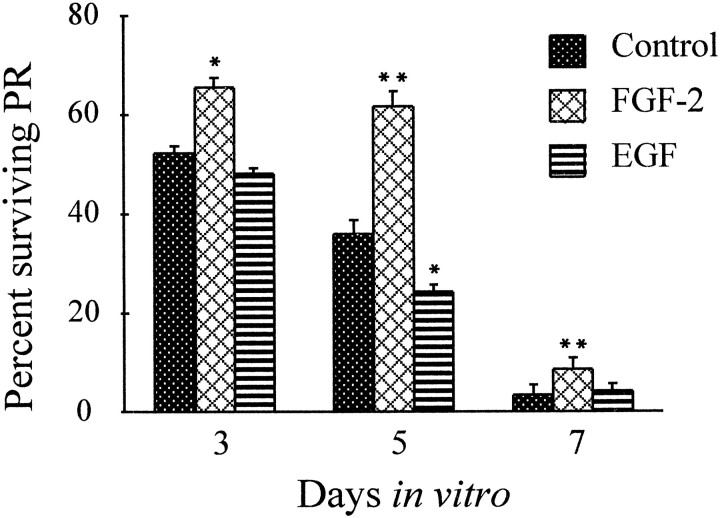
To quantify the effects of FGF-2 and EGF on PR survival, coverslips of control and treated PRs were incubated after 1, 3, 5, and 7 d in vitro with the Live/Dead assay kit for 15 min at 37°C. Live and dead PRs are represented in Figure4. For quantification of PR survival, only live PRs were counted, and results were expressed in percentage of surviving PRs compared with the number of PRs after 1 d in vitro. Significantly more PRs survived in the presence of 20 ng/ml FGF-2 than nontreated or PRs treated with 10 ng/ml EGF. After 3 din vitro, 65.5% of PRs treated with FGF-2 were still alive compared with 52.1% for the control and 48.5% for EGF-treated PRs. After 5 d in vitro, this difference was highly statistically significant: 61.7% FGF-2-treated PRs were alive versus 35.8% for the control, and only 25.5% for PRs treated with EGF. At this time, EGF had accelerated the degeneration of PRs, with 30% more PRs (statistically significant) being dead compared with controls (Fig.5). By 7 d all PRs had undergone degeneration to reach 3.3%, 6.4%, and 2.9% for control, FGF-2-, and EGF-treated PRs, respectively.
Fig. 4.
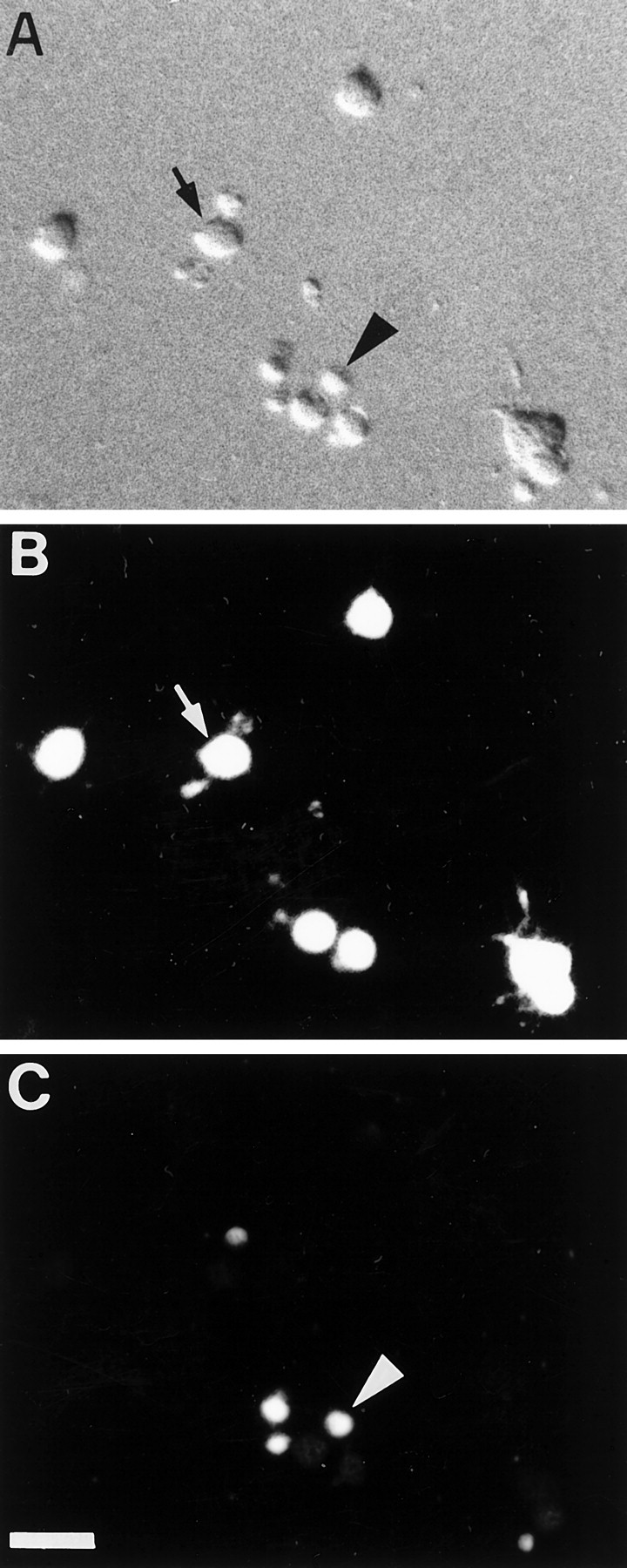
PR stained by the Live/Dead assay.A, Nomarski image; live (B) and dead (C) cells and nuclei within the same microscopic field. “Live” (arrow) and “dead” (arrowhead) cell labeling was mutually exclusive. Scale bar, 10 μm.
Fig. 5.
PR survival in vitro is modulated by FGF-2 and EGF. Relative percentages of surviving PR isolated from 5-d-old rat retinas were calculated by comparing the number of live PRs at a given time with the number after 24 hr in vitro. In the control after 3 d in vitro, 52.1% represents 24,000 live PRs per coverslip. After 24 hr in vitro, PR culture serum-supplemented medium was replaced with CDM with or without FGF-2 (20 ng/ml) or EGF (10 ng/ml), and aliquots of growth factors or the same volume of CDM were added again after 3 and 5 d. FGF-2-treated PR survived significantly better than EGF-treated or nontreated PRs, particularly after 5 d in vitro. EGF-treated PRs degenerated faster than control PRs. Between 5 and 7 d in vitro, PRs degenerated rapidly in both treated and nontreated wells. Error bars are SEM (n= 8). *p < 0.01; **p < 0.001.
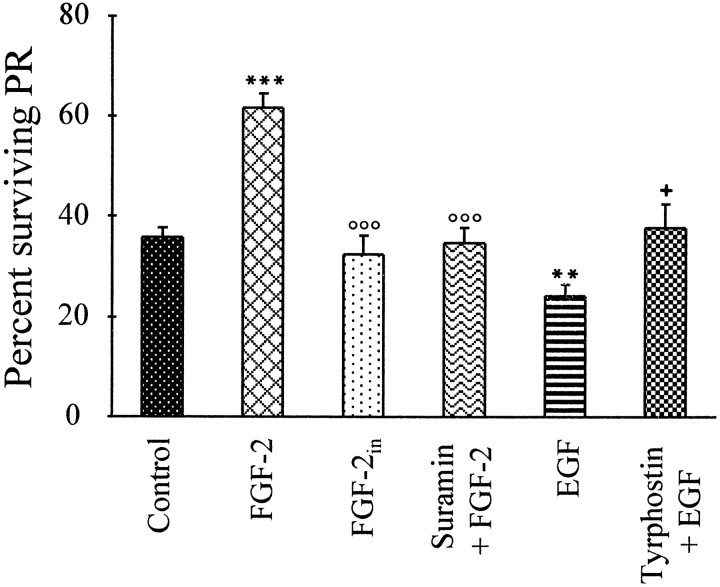
To determine whether the effects of FGF-2 and EGF were dose-dependent, similar trials were conducted using increasing concentrations (1–50 ng/ml) of the two factors. As in Figure 5, estimates of PR survival were made after 3, 5, and 7 d in vitro. Because maximal effects were observed at 5 d in vitro for 20 ng/ml FGF-2, this time point is illustrated in Figure6. Concentrations of 1 and 5 ng/ml FGF-2 had no effect on PR survival, and 10 ng/ml led to a statistically significant increase. The maximal stimulation of PR survival by FGF-2 was obtained at 20 ng/ml, whereas 50 ng/ml was without effect (Fig.6A). For EGF, 10 and 20 ng/ml reduced PR survival; other concentrations led to numbers similar to those of control cultures (Fig. 6B). To further test for the specificity of these effects, heat-denatured FGF-2 was also added to some cultures and had no effect on PR survival at 5 d (Fig.7). Furthermore, the survival-promoting effects of 20 ng/ml FGF-2 at 5 d were neutralized by the simultaneous addition of suramin, and the survival-inhibiting effects of 10 ng/ml EGF were neutralized by the simultaneous addition of the specific EGFR blocker tyrphostin-23 (Fig. 7). Addition of either suramin or tyrphostin-23 alone was without effect (data not shown).
Fig. 6.
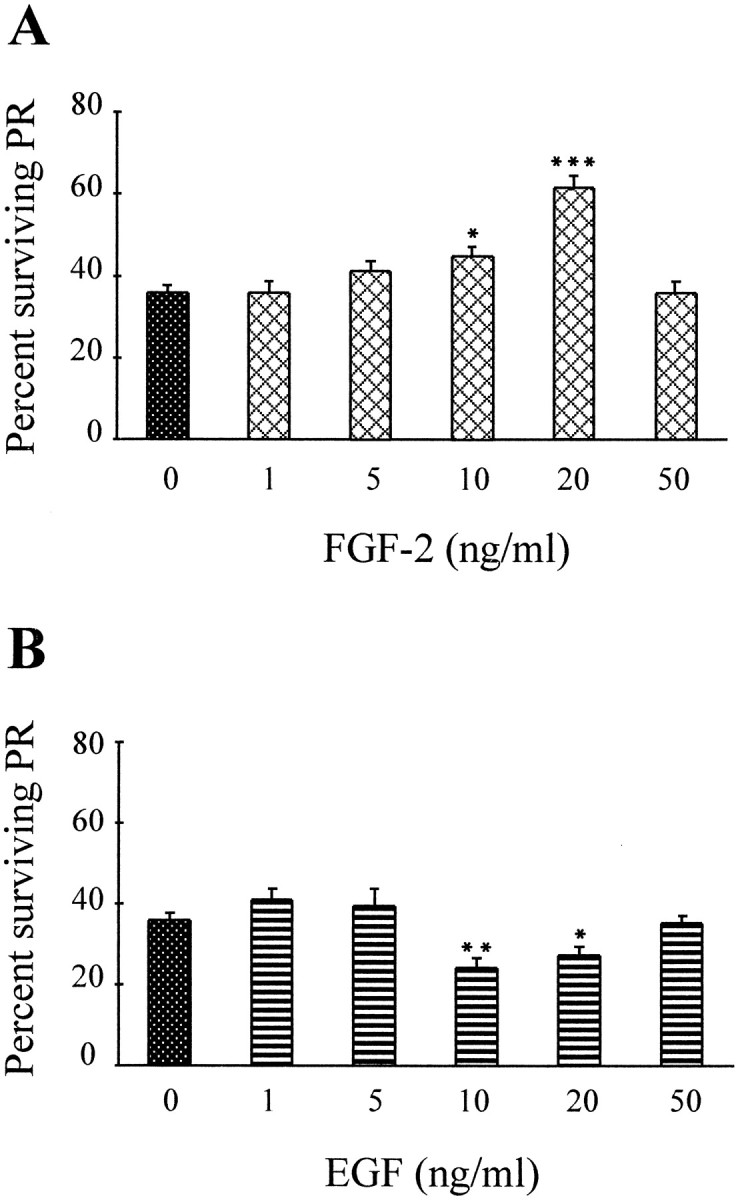
FGF-2 and EGF effects on PR survival are concentration-dependent. Determination of relative percentages of surviving PRs and treatments were performed as in Figure 5.A, Increasing doses of FGF-2 (1–50 ng/ml) led to increasing survival of PRs up to 20 ng/ml (maximum), whereas further increases in concentration did not stimulate survival (50 ng/ml).B, Increasing doses of EGF (1–50 ng/ml) led to decreasing survival of PRs (maximal at 10 ng/ml). As with FGF-2, higher doses had no effect on PR survival (50 ng/ml). Error bars are SEM (n = 4). *p < 0.05; **p < 0.01; ***p < 0.001.
Fig. 7.
Survival-promoting effects of FGF-2 depend on normal biological activity. Determination of relative percentages of surviving PRs and treatments were performed as in Figure 5. Treatments consisted of CDM alone (controls), FGF-2 (20 ng/ml), heat-inactivated (FGF-2in) FGF-2 (20 ng/ml), suramin (50 μm) plus FGF-2 (20 ng/ml), EGF (10 ng/ml), and tyrphostin-23 (10 μm) plus EGF (10 ng/ml). Although FGF-2 led to increased survival and EGF led to decreased survival as above, inactivated FGF-2 was without effect, suramin addition suppressed the effects of FGF-2, and tyrphostin suppressed the effects of EGF, after 5 d in vitro. Error bars are SEM (n = 4). Statistical treatments:asterisks above FGF-2 and EGF are with respect to controls: **p < 0.01, ***p < 0.001. Small circles aboveFGF-2in and suramin + FGF-2 are with respect to FGF-2 alone: °°°p < 0.001. The small plus sign above Tyrphostin + EGF is with respect to EGF alone: p < 0.05. Suramin and tyrphostin when tested alone did not influence survival (data not shown).
To ensure that increased PR numbers in the presence of FGF-2 were not attributable to the proliferation of PR precursors or MGC in cultures, 5-bromodeoxyuridine incorporation and MGC numbers were determined. Immunocytochemical detection of incorporated 5-bromodeoxyuridine showed that no proliferating PRs were present (whereas rare MGCs were stained; data not shown). Percentages of MGCs compared with PRs ranged from 0.1 to 0.41%, corresponding to the control at 3 d (minimum) and EGF treatment at 7 d (maximum), respectively (Table2).
Table 2.
The proportion of MGC in low-density PR culture does not change significantly over the in vitro period
| Days in vitro | Percentages of Müller glial cells | ||
|---|---|---|---|
| Control | FGF-2 | EGF | |
| 3 | 0 | 0.06 | 0.05 |
| 5 | 0.06 | 0.15 | 0.23 |
| 7 | 0.04 | 0.25 | 0.41 |
After 3, 5, and 7 d in vitro, cells immunolabeled with anti-vimentin antibody were counted on 25 fields on each coverslip from four separate experiments with a 40× fluorescence objective. The relative percentages of MGCs in PR cultures were determined by comparing the number of MGCs at each time with the number of living PRs after 24 hr in vitro. In absolute numbers, 0.15% MGCs represent 69 MGCs for 28,400 live FGF-2-treated PRs at 5 din vitro. These data show that the proportion of MGCs did not increase significantly over the period of cell culture, even in the presence of FGF-2 or EGF in the medium.
FGF and EGF receptors are expressed by PRsin vitro
To determine whether the effects of FGF-2 and EGF on PR survival depended on the existence of their respective receptors, we investigated their presence in PR cultures. By RT-PCR, FGFR1 and EGFR mRNA expression were found in PR cultures (Fig.8A), and their respective protein products were detected by Western blot using specific antibodies (Fig. 8B). To examine FGFR and EGFR activation, PR cultures were incubated separately for different times with each factor, and the samples were assayed using anti-phosphotyrosine antibody. FGF-2-induced tyrosine phosphorylation could not be detected at 24 hr in vitro. However, after 3 d in vitro, FGF-2 induced time-dependent phosphorylation of five major bands and two minor ones. Major bands were detectable with apparent molecular masses of ∼140, 120, a closely spaced doublet of 105 and 95, 74, and 65 kDa; minor bands were visible at ∼180 and 155 kDa. Increased tyrosine phosphorylation compared with phosphatase inhibitor mixture-treated control cultures was detectable in all of these bands, and it varied with incubation time. The most prominent band corresponded to the expected molecular mass for FGFRs (140 kDa), and phosphotyrosine incorporation had already increased by 30 sec, becoming maximal by 2 min and less intense by 5 min (Fig. 9A). The kinetics of tyrosine phosphorylation of the 95/105 doublet and 74 and 65 kDa bands lagged behind that of FGFRs, with phosphorylation increasing steadily over the duration of the incubation. Phosphotyrosine antibody staining of PR cultures also revealed brighter labeling of FGF-2-treated cells compared with nonstimulated controls (data not shown). EGF stimulation of PR cultures after 24 hr in vitro resulted in time-dependent prominent tyrosine phosphorylation of a single band corresponding to the expected molecular mass of EGFR (180 kDa) (Fig.9B). Maximum phosphorylation was obtained after 5 min of EGF stimulation; it remained intense at 10 min.
Fig. 8.
FGFR1 and EGFR mRNA and protein are present in PR cultures after 3 d in vitro. The expression of mRNA (A) and proteins (B) of FGFR1 and EGFR were analyzed by RT-PCR and Western blot, respectively.
Fig. 9.
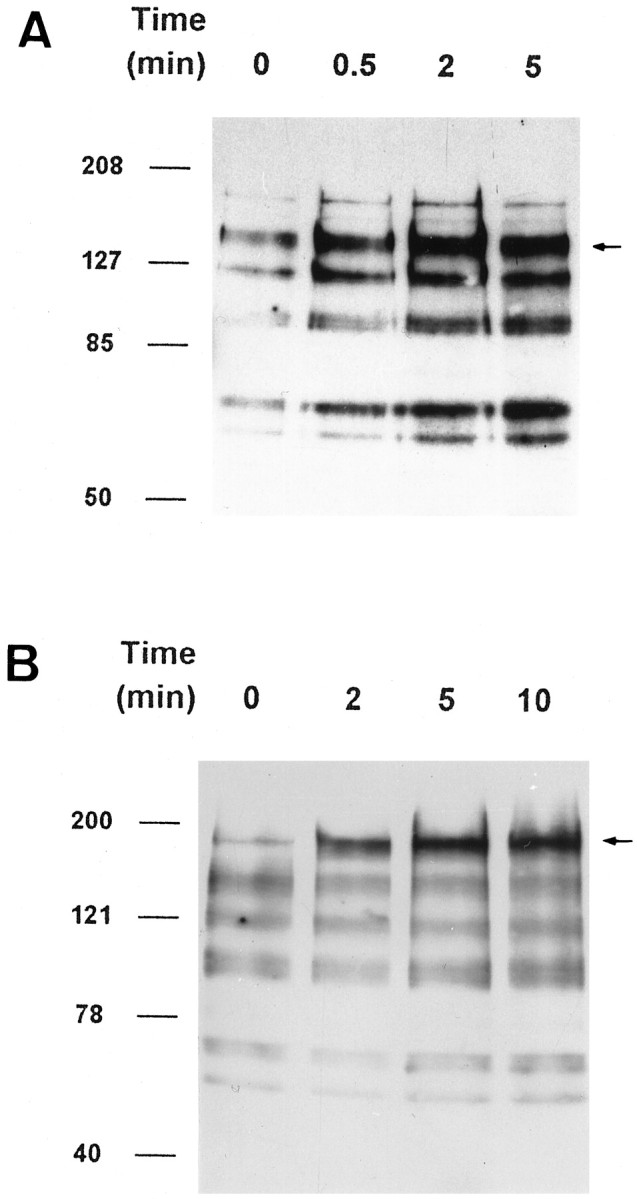
FGFR and EGFR phosphorylation in PR cultures. Representative immunoblots using anti-phosphotyrosine antibody of PR either nonstimulated (0) or stimulated for 0.5, 2, and 5 min with FGF-2 (100 ng/ml after 3 d in vitro) (A) or 2, 5, and 10 min with EGF (100 ng/ml after 24 hr in vitro) (B). FGF-2 addition led to time-dependent phosphorylation of the putative FGFR (140 kDa, arrow), as well as four other lower molecular mass bands. FGFR tyrosine phosphorylation was maximal by 2 min, whereas maximal phosphorylation of second messenger proteins lagged behind (A). Notice the increasing phosphorylation of a single band corresponding to the putative EGFRs, saturating at 5 min (B, arrow). See Results for additional details.
DISCUSSION
In this work, we demonstrate the isolation and maintenance for 1 week under defined culture conditions of a purified neuronal population of CNS origin. It is observed that these cells express at least two different tyrosine kinase receptors and that binding to these receptors by their natural ligands leads to dose-dependent activation of distinct signaling cascades with opposing effects on survival. Exogenous FGF-2 acts directly on FGFRs in postmitotic PRs to increase their survivalin vitro, whereas the activation of EGFRs by EGF promotes their degeneration.
The postmitotic retina, because of its laminated architecture in which different cell types are restricted to different layers, is amenable to mechanical fractionation that permits the isolation of pure PR samples. Such purification has permitted the analysis of gangliosides within the developing (Fontaine et al., 1998) and adult (Dreyfus et al., 1996) PR layer. In the present study, these preparations were composed of >99% postmitotic PRs as identified by the absence of cell division and the expression of PR-specific proteins in vitro. Opsin was synthesized in only a subpopulation of PRs, but the donor age chosen for most studies (postnatal day 5) still contains many immature PRs in peripheral retina. In addition, a small percentage (rat PRs are estimated to be 97% rod pure) of such cells may represent cones. Sensitive cytotoxicity assays showed that many PRs were able to survive for several days in a completely serum-free medium with no added growth factors. The temporary survival of postmitotic PR in defined medium was dependent on the age of the donor. Although 50–60% survival was observed between 5 and 9 d after birth, this capacity declined sharply by 11 d. It may be that PR–MGC interactions become progressively more important for PR survival, because maturation of both types proceeds in parallel during this period (Young, 1985).
Although the beneficial effects of FGF-2 on neuronal development, differentiation, and survival within the retina and multiple regions of the brain have been known for many years, it has been difficult to attribute such effects to direct activation of FGFRs present at the surface of target neurons. Because of the widespread distribution of FGFRs in neurons, glia, and non-neural cells within the brain (Heuer et al., 1990; Wanaka et al., 1990) and retina (Heuer et al., 1990; Tcheng et al., 1994), any experimental model containing multiple cell types would be expected to exhibit multiple responses. Even in vitro it has proven difficult to completely eliminate accessory cells, and indeed in some cases neuronal survival induced by FGF-2 seems to depend on glia (Engele and Bohn, 1991). In the present study FGF-2 was clearly able to stimulate the survival of pure PR under completely defined culture conditions. The effect was dose-dependent and could be blocked by heat inactivation of FGF-2 (Gospodarowicz and Cheng, 1986) or with the polyanionic sulfated compound suramin, a heparin-like molecule known to inhibit FGF-2 actions in other model systems (Mascarelli et al., 1991). Excessive concentrations of FGF-2 did not stimulate survival. Such phenomena have been reported previously (Hicks and Courtois, 1992) and have been attributed to downregulation of FGFRs. The likelihood of glial cells mediating the response could be excluded because their numbers represented <0.2% after 5 d in vitro, at which time survival effects were maximal. Furthermore, glial numbers were actually maximal in EGF-treated cultures in which PR survival was the lowest. This does not mean that the secondary neurons, Müller glia, and pigmented epithelium surrounding the PRs do not participate normally in FGF regulation within the retina. All of these cell types express FGF-2 and FGFRs (Sternfeld et al., 1989; Noji et al., 1990; Bugra and Hicks, 1997; Cao et al., 1997) and represent potential sources of this growth factor in vivo. Intravitreal injections of FGF-2 rescue PR in rat models of PR degeneration (Faktorovich et al., 1990, 1992), Müller glia upregulate endogenous FGF-2 expression in response to exogenous FGF-2 application (Cao et al., 1997), and targeted ablation of pigmented epithelium early in development leads to malformation or loss of the neural retina (Raymond and Jackson, 1995). These accessory cells are thus clearly vital for continued PR functioning.
Considerable attention has been focused on possible neurotrophic effects of FGF-2 in the retina, and experimental evidence supports the putative survival role of this factor for PR cells in vivo. Subretinal injection of FGF-2 has been shown to delay PR degeneration in the Royal College of Surgeons rat (Faktorovich et al., 1990) and the Fischer 344 rat, which exhibit an age-related peripheral retinopathy (Lin et al., 1997). Systemic administration of α2-adrenergic agonists upregulates FGF-2 expression in PRs and protects them from subsequent phototoxic insults (Wen et al., 1996). Similarly, injection of FGF-2 into the vitreous of rats before exposure to constant light decreases PR cell death (LaVail et al., 1992). FGF-2 also protects partially the retina from pressure-induced ischemia (Unoki and LaVail, 1994; Zhang et al., 1994). Another group demonstrated the importance of FGF signaling in PR survival by using a transgenic mouse model in which PR FGFRs were inactivated, leading to late onset PR degeneration (Campochiaro et al., 1996). In vitro, FGF-2 was shown to enhance the proliferation of embryonic retinal cells in culture (Lillien and Cepko, 1992), to stimulate outgrowth of retinal ganglion cell processes (Bahr et al., 1989) and PR differentiation (Hicks and Courtois, 1992), and to protect retinal neuronal death induced by excitotoxicity (Heidinger et al., 1997). On the other hand, FGF-2 has been reported to have no effect on PR survival in inherited retinal degeneration or phototoxicity in the mouse (LaVail et al., 1998) and to actually induce apoptosis in cultured chick PR (Yokoyama et al., 1997). Thus although much data demonstrate the survival effects of FGF-2 on in vivo retinal models, this has not been shown clearly in vitro.
We show in the present study for the first time stimulation of tyrosine phosphorylation in purified living PR after FGF-2 addition. The 140 kDa band probably represents FGFR1, because anti-FGFR1 antibody labeled a band near this weight in the present work and in other studies using the same antibody (Hanneken et al., 1995). FGFRs corresponding to this mass have been identified also in hippocampal neurons (Walicke et al., 1989). The 120 kDa protein may constitute an alternatively glycosylated form of the same receptor (Feige and Baird, 1988) or another FGFR (Partanen et al., 1992). Both FGFR1 and FGFR2 have been described in the retina (Tcheng et al., 1994). Definitive identification of the proteins activated in the intracellular cascade constitutes our priority for further studies, but molecular weights correspond to such commonly recruited second messenger proteins as syp/SHPTP-2 (∼65 kDa) (Feng and Pawson, 1994). Interestingly the profile of FGF-2-induced intracellular signaling is very different from that observed for EGF treatment of the same cells, and also compared with FGF treatment of purified Müller glia (Meuillet et al., 1996), indicating cell type-specific differences in the FGF pathway.
Several studies have shown the effects of EGF on the proliferation of CNS and retinal precursor cells in vitro (Anchan et al., 1991; Mytilineou et al., 1992; Mahanthappa and Schwarting, 1993; Kelley et al., 1995). The neurotrophic effects of EGF in retina, like those of many other factors, have been demonstrated essentially during development, when EGF has been reported to suppress rod PR differentiation (Lillien, 1995; Ahmad et al., 1998). In these studies, EGF was never shown to induce cell degeneration. Although EGF and its receptors are highly expressed in the rat retina during the early postnatal period (Anchan et al., 1991; Powers and Planck, 1997), including in PRs at postnatal day 5 (data not shown), nothing has been reported on EGF effects on postnatal retinal cells in culture. Here, activation of EGFR in PRs was observed clearly by phosphotyrosine immunodetection, providing evidence that EGF promotes postmitotic PR degeneration in vitro. Further evidence comes from suppression of the effect using the EGFR blocker tyrphostin (Dvir et al., 1991). Although EGF itself is downregulated in differentiated retina, TGF-α is still expressed and could activate such EGFR. The function of such abundant EGFR in postmitotic PR remains to be determined.
Between 5 and 7 d in vitro, PRs degenerate rapidly even in the presence of FGF-2. This observation is in agreement with that of Politi and Adler (1988), who have shown a massive degeneration of PR in mixed retinal cultures after 1 week. It may be speculated that as for other cells, PRs require continuous exogenous signals in addition to FGF-2 to stimulate their prolonged survival, and that such signaling molecules were absent from the medium. The nature of these signals is being investigated currently, but several candidate molecules have been implicated in PR differentiation and survival in mixed retinal cultures [FGF-1 (Hicks and Courtois, 1988), taurine (Altshuler et al., 1993), retinoic acid (Kelley et al., 1994), ciliary neurotrophic factor (Fuhrmann et al., 1995), glial-derived neurotrophic factor (Jing et al., 1996; Ezzeddine et al., 1997), and leukemia inhibitory factor (Neophytou et al., 1997), as well as currently unidentified factors (Watanabe and Raff, 1990; Sheedlo et al., 1995;Layer et al., 1997)].
Footnotes
This work was supported by Fédération des Aveugles et Handicapés Visuels de France, IPSEN Pharmaceuticals, Fondation de l’Avenir, and Mutuelle Générale de l’Education Nationale-Institut National de la Santé et de la Recherche Médicale. V.F. was assisted by grants from Retina France, ADRET-Alsace, and the Fondation pour la Recherche Médicale. N.K. was supported by grants from the Fondation Entente Franco-Allemande and Pro Retina Deutschland. We thank Dr. Serge Picaud for helpful comments with this manuscript.
Correspondence should be addressed to Valérie Fontaine, Laboratoire de Physiopathologie Rétinienne, Médicale A, Centre Hospitalier et Universitaire de Strasbourg, BP426, 67091 Strasbourg Cedex, France.
REFERENCES
- 1.Ahmad I, Dooley CM, Afiat S. Involvement of Mash-1 in EGF-mediated regulation of differentiation in the vertebrate retina. Dev Biol. 1998;194:86–98. doi: 10.1006/dbio.1997.8809. [DOI] [PubMed] [Google Scholar]
- 2.Altshuler D, LoTurco JJR, Cepko CL. Taurine promotes the differentiation of a vertebrate retinal cell type in vitro. Development. 1993;119:1317–1328. doi: 10.1242/dev.119.4.1317. [DOI] [PubMed] [Google Scholar]
- 3.Anchan RM, Reh TA, Angello J, Balliet A, Walker M. EGF and TGF-α stimulate retinal neuroepithelial cell proliferation in vitro. Neuron. 1991;6:923–936. doi: 10.1016/0896-6273(91)90233-p. [DOI] [PubMed] [Google Scholar]
- 4.Bahr M, Vanselow J, Thanos S. Ability of adult rat ganglion cells to regrow axons in vitro can be influenced by fibroblast growth factor and gangliosides. Neurosci Lett. 1989;96:197–201. doi: 10.1016/0304-3940(89)90057-8. [DOI] [PubMed] [Google Scholar]
- 5.Barde YA. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- 6.Bugra K, Hicks D. Acidic and basic fibroblast growth factor messenger RNA and protein show increased expression in adult compared to developing normal and dystrophic rat retina. J Mol Neurosci. 1997;9:13–25. doi: 10.1007/BF02789391. [DOI] [PubMed] [Google Scholar]
- 7.Campochiaro PA, Chang M, Ohsato M, Vinores SA, Nie Z, Hjelmeland L, Mansukhani A, Basilico C, Zack DJ. Retinal degeneration in transgenic mice with photoreceptor specific expression of a dominant-negative fibroblast growth factor receptor. J Neurosci. 1996;16:1679–1688. doi: 10.1523/JNEUROSCI.16-05-01679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao W, Wen R, Li F, Cheng T, Steinberg RH. Induction of basic fibroblast growth factor mRNA by basic fibroblast growth factor in Müller cells. Invest Ophthalmol Vis Sci. 1997;38:1358–1366. [PubMed] [Google Scholar]
- 9.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thicyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 10.Curcio CA, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci. 1993;34:3278–3296. [PubMed] [Google Scholar]
- 11.Dreyfus H, Guérold B, Fontaine V, Sahel J, Hicks D. Simplified ganglioside composition of photoreceptors compared to other retinal neurons. Invest Ophthalmol Vis Sci. 1996;37:574–585. [PubMed] [Google Scholar]
- 12.Dvir A, Milner Y, Chomsky O, Gilon C, Gazit A, Levitski A. The inhibition of EGF-dependent proliferation of keratinocytes by tyrphostin tyrosine kinase blockers. J Cell Biol. 1991;113:857–865. doi: 10.1083/jcb.113.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engele J, Bohn MC. The neurotrophic effects of fibroblast growth factors on dopaminergic neurons in vitro are mediated by mesencephalic glia. J Neurosci. 1991;11:3070–3078. doi: 10.1523/JNEUROSCI.11-10-03070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Euler T, Wässle H. Immunocytochemical identification of cone bipolar cells in the rat retina. J Comp Neurol. 1995;361:461–478. doi: 10.1002/cne.903610310. [DOI] [PubMed] [Google Scholar]
- 15.Ezzeddine ZD, Yang X, DeChiara T, Yancopoulos G, Cepko CL. Postmitotic cells fated to become rod photoreceptors can be respecified by CNTF treatment of the retina. Development. 1997;124:1055–1067. doi: 10.1242/dev.124.5.1055. [DOI] [PubMed] [Google Scholar]
- 16.Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM. Photoreceptor degeneration in inherited retinal dystrophy delayed by fibroblast growth factor. Nature. 1990;347:83–86. doi: 10.1038/347083a0. [DOI] [PubMed] [Google Scholar]
- 17.Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM. Basic fibroblast growth factor and local injury protect photoreceptors from light damage in the rat. J Neurosci. 1992;12:3554–3567. doi: 10.1523/JNEUROSCI.12-09-03554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fayein N, Courtois Y, Jeanny J-C. Ontogeny of basic fibroblast growth factor binding sites in mouse ocular tissues. Exp Cell Res. 1990;188:75–88. doi: 10.1016/0014-4827(90)90280-n. [DOI] [PubMed] [Google Scholar]
- 19.Feige J-J, Baird A. Glycosylation of the basic fibroblast growth factor receptor. J Biol Chem. 1988;263:14023–14029. [PubMed] [Google Scholar]
- 20.Feng G, Pawson T. Phosphotyrosine phosphatases with SH2 domains: regulators of signal transduction. Trends Genet. 1994;10:54–58. doi: 10.1016/0168-9525(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 21.Fontaine V, Hicks D, Dreyfus H. Changes in ganglioside composition of photoreceptors during postnatal maturation of the rat retina. Glycobiology. 1998;8:183–190. doi: 10.1093/glycob/8.2.183. [DOI] [PubMed] [Google Scholar]
- 22.Fuhrmann S, Kirsch M, Hofmann HD. Ciliary neurotrophic factor promotes chick photoreceptor development in vitro. Development. 1995;121:2695–2706. doi: 10.1242/dev.121.8.2695. [DOI] [PubMed] [Google Scholar]
- 23.Gao H, Hollyfield JG. Aging of the human retina: differential loss of neurons and retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1992a;33:1–17. [PubMed] [Google Scholar]
- 24.Gao H, Hollyfield JG. Basic fibroblast growth factor (bFGF) immunolocalization in the rodent outer retina demonstrated with an anti-rodent bFGF antibody. Brain Res. 1992b;585:355–360. doi: 10.1016/0006-8993(92)91236-8. [DOI] [PubMed] [Google Scholar]
- 25.Gospodarowicz D, Cheng J. Heparin protects basic and acidic FGF from inactivation. J Cell Physiol. 1986;128:475–484. doi: 10.1002/jcp.1041280317. [DOI] [PubMed] [Google Scholar]
- 26.Hanneken A, Maher PA, Baird A. High affinity immunoreactive FGF receptors in the extracellular matrix of vascular endothelial cells: implications for the modulation of FGF-2. J Cell Biol. 1995;128:1221–1228. doi: 10.1083/jcb.128.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harper JF. Peritz’ F test. Basic program of a robust multiple comparison test for statistical analysis of all differences among group means. Comput Biol Med. 1984;14:437–445. doi: 10.1016/0010-4825(84)90044-1. [DOI] [PubMed] [Google Scholar]
- 28.Heidinger V, Hicks D, Sahel J, Dreyfus H. Peptide growth factors but not ganglioside protect against excitotoxicity in rat retinal neurons in vitro. Brain Res. 1997;767:279–288. doi: 10.1016/s0006-8993(97)00605-7. [DOI] [PubMed] [Google Scholar]
- 29.Heuer JG, von Bartheld CS, Kinoshita Y, Evers PC, Bothwell M. Alternating phases of FGF receptor and NGF receptor expression in the developing chicken nervous system. Neuron. 1990;5:283–296. doi: 10.1016/0896-6273(90)90165-c. [DOI] [PubMed] [Google Scholar]
- 30.Hicks D, Barnstable CJ. Different rhodopsin monoclonal antibodies reveal different binding patterns on developing and adult rat retina. J Histochem Cytochem. 1987;35:1317–1328. doi: 10.1177/35.11.3655327. [DOI] [PubMed] [Google Scholar]
- 31.Hicks D, Courtois Y. Acidic fibroblast growth factor stimulates opsin levels in retinal photoreceptor cells in vitro. FEBS Lett. 1988;234:475–479. doi: 10.1016/0014-5793(88)80141-8. [DOI] [PubMed] [Google Scholar]
- 32.Hicks D, Courtois Y. Fibroblast growth factor stimulates photoreceptor differentiation in vitro. J Neurosci. 1992;12:2022–2033. doi: 10.1523/JNEUROSCI.12-06-02022.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeanny J-C, Fayein N, Moenner M, Chevallier B, Barritault DJ, Courtois Y. Specific fixation of bovine brain and retinal acidic and basic fibroblast growth factors to mouse embryonic eye basement membranes. Exp Eye Res. 1987;171:63–75. doi: 10.1016/0014-4827(87)90251-5. [DOI] [PubMed] [Google Scholar]
- 34.Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, Louis J-C, Hu S, Altrock BW, Fox GM. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-α, a novel receptor for GDNF. Cell. 1996;85:1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- 35.Johnson JE, Barde Y, Schwab M, Thoenen H. Brain-derived neurotrophic factor supports the survival of cultured rat retinal ganglion cells. J Neurosci. 1986;6:3031–3038. doi: 10.1523/JNEUROSCI.06-10-03031.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelley MW, Turner JK, Reh TA. Retinoic acid promotes differentiation of photoreceptors in vitro. Development. 1994;120:2091–2102. doi: 10.1242/dev.120.8.2091. [DOI] [PubMed] [Google Scholar]
- 37.Kelley MW, Turner JK, Reh TA. Regulation of proliferation and photoreceptor differentiation in fetal human retinal cell cultures. Invest Ophthalmol Vis Sci. 1995;36:1280–1289. [PubMed] [Google Scholar]
- 38.Kendall G, Latchman DS. Polymerase chain reaction for RNA analysis. Methods: a companion to methods in enzymology. 1996;10:279–282. doi: 10.1006/meth.1996.0103. [DOI] [PubMed] [Google Scholar]
- 39.LaVail MM, Unoki K, Yasumura D, Matthes MT, Yancopoulos GD, Steinberg RH. Multiple growth factors, cytokines and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci USA. 1992;89:11249–11253. doi: 10.1073/pnas.89.23.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LaVail MM, Yasumura D, Matthes MT, Lau-Villacorta C, Unoki K, Sung C-H, Steinberg RH. Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophthalmol Vis Sci. 1998;39:592–602. [PubMed] [Google Scholar]
- 41.Layer PG, Rothermel A, Hering H, Wolf B, deGrip WJ, Hicks D, Willbold E. Pigmented epithelium sustains cell proliferation and decreases expression of opsins and acetylcholinesterase in reaggregated chicken retinospheroids. Eur J Neurosci. 1997;9:1795–1803. doi: 10.1111/j.1460-9568.1997.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 42.Lillien L. Changes in retinal cell fate induced by overexpression of EGF receptor. Nature. 1995;377:158–161. doi: 10.1038/377158a0. [DOI] [PubMed] [Google Scholar]
- 43.Lillien L, Cepko C. Control of proliferation in the retina: temporal changes in responsiveness to FGF and TGFα. Development. 1992;115:253–266. doi: 10.1242/dev.115.1.253. [DOI] [PubMed] [Google Scholar]
- 44.Lin N, Fan W, Sheedlo HJ, Turner JE. Basic fibroblast growth factor treatment delays age-related photoreceptor degeneration in Fischer 344 rats. Exp Eye Res. 1997;64:239–248. doi: 10.1006/exer.1996.0208. [DOI] [PubMed] [Google Scholar]
- 45.Mahanthappa NK, Schwarting GA. Peptide growth factor control of olfactory neurogenesis and neuron survival in vitro: roles of EGF and TGF-βs. Neuron. 1993;10:293–305. doi: 10.1016/0896-6273(93)90319-m. [DOI] [PubMed] [Google Scholar]
- 46.Mascarelli F, Raulais D, Courtois Y. Fibroblast growth factor phosphorylation and receptors in rod outer segments. EMBO J. 1989;8:2265–2273. doi: 10.1002/j.1460-2075.1989.tb08351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mascarelli F, Tassin J, Courtois Y. Effects of FGFs on adult bovine Müller cells: proliferation, binding, internalization. Growth Factors. 1991;4:81–95. doi: 10.3109/08977199109000260. [DOI] [PubMed] [Google Scholar]
- 48.Meuillet E, Cremel G, Dreyfus H, Hicks D. Differential modulation of basic fibroblast and epidermal growth factor receptor activation by ganglioside GM3 in cultured retinal Müller glia. Glia. 1996;17:206–216. doi: 10.1002/(SICI)1098-1136(199607)17:3<206::AID-GLIA3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 49.Mytilineou C, Park TH, Shen J. Epidermal growth factor-induced survival and proliferation of neuronal precursor cells from embryonic rat mesencephalon. Neurosci Lett. 1992;135:62–66. doi: 10.1016/0304-3940(92)90136-u. [DOI] [PubMed] [Google Scholar]
- 50.Neophytou C, Vernallis AB, Smith A, Raff MC. Muller-cell-derived leukaemia inhibitory factor arrests rod photoreceptor differentiation at a postmitotic pre-rod stage of development. Development. 1997;124:2345–2354. doi: 10.1242/dev.124.12.2345. [DOI] [PubMed] [Google Scholar]
- 51.Noji S, Matsuo T, Koyama E, Yamaai T, Nohno T, Matsuo N, Tanigushi S. Expression pattern of acidic and basic fibroblast growth factor genes in adult rat eyes. Biochem Biophys Res Commun. 1990;168:343–349. doi: 10.1016/0006-291x(90)91714-4. [DOI] [PubMed] [Google Scholar]
- 52.Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 53.Partanen J, Vainikka S, Korhonen J, Armstrong E, Alitalo K. Diverse receptors for fibroblast growth factor. Growth Factor Res. 1992;4:69–83. doi: 10.1016/0955-2235(92)90005-3. [DOI] [PubMed] [Google Scholar]
- 54.Plouët J, Mascarelli F, Loret M, Faure J-P, Courtois Y. Regulation of eye-derived growth factor binding to membranes by light, ATP or GTP in photoreceptor outer segments. EMBO J. 1988;7:373–376. doi: 10.1002/j.1460-2075.1988.tb02823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Politi L, Adler R. Selective failure of long-term survival of isolated photoreceptors from both homozygous and heterozygous rd (retinal degeneration) mice. Exp Eye Res. 1988;47:269–282. doi: 10.1016/0014-4835(88)90010-3. [DOI] [PubMed] [Google Scholar]
- 56.Portera-Cailliau C, Sung C-H, Nathans J, Adler R. Apoptotic photoreceptor cell death in mouse models of retinitis pigmentosa. Proc Natl Acad Sci USA. 1994;91:974–978. doi: 10.1073/pnas.91.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Powers MR, Planck SR. Immunolocalization of transforming growth factor-alpha and its receptor in the normal and hyperoxia-exposed neonatal rat retina. Curr Eye Res. 1997;16:177–182. doi: 10.1076/ceyr.16.3.177.15406. [DOI] [PubMed] [Google Scholar]
- 58.Raymond SM, Jackson IJ. The retinal pigmented epithelium is required for development and maintenance of the mouse retina. Curr Biol. 1995;5:1286–1295. doi: 10.1016/s0960-9822(95)00255-7. [DOI] [PubMed] [Google Scholar]
- 59.Sheedlo HJ, Li L, Fan W, Turner JE. Retinal pigment epithelial cell support of photoreceptor survival in vitro. In Vitro Cell Dev Biol Anim. 1995;31:330–333. doi: 10.1007/BF02634278. [DOI] [PubMed] [Google Scholar]
- 60.Silverman MS, Hughes SE. Transplantation of photoreceptors to light damaged retina. Invest Ophthalmol Vis Sci. 1989;30:1684–1690. [PubMed] [Google Scholar]
- 61.Sternfeld MD, Robertson JE, Shipley GD, Tsai J, Rosenbaum JT. Cultured human retinal pigment epithelial cells express basic fibroblast growth factor and its receptor. Curr Eye Res. 1989;8:1029–1037. doi: 10.3109/02713688908997395. [DOI] [PubMed] [Google Scholar]
- 62.Tcheng M, Fuhrmann G, Hartmann M-P, Courtois Y, Jeanny J-C. Spatial and temporal expression patterns of FGF receptor genes type 1 and 2 in the developing chick retina. Exp Eye Res. 1994;58:351–358. doi: 10.1006/exer.1994.1025. [DOI] [PubMed] [Google Scholar]
- 63.Unoki K, LaVail MM. Protection of the rat retina from ischemic injury by brain-derived neurotrophic factor, ciliary neurotrophic factor, and basic fibroblast growth factor. Invest Ophthalmol Vis Sci. 1994;35:907–915. [PubMed] [Google Scholar]
- 64.Vaughan PJ, Pike CJ, Cotman CW, Cunningham DD. Thrombin receptor activation protects neurons and astrocytes from cell death produced by environmental insults. J Neurosci. 1995;15:5389–5401. doi: 10.1523/JNEUROSCI.15-07-05389.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wagner JA. The fibroblast growth factors: an emerging family of neural growth factors. Curr Topics Microbiol Immunol. 1991;165:95–118. doi: 10.1007/978-3-642-75747-1_6. [DOI] [PubMed] [Google Scholar]
- 66.Walicke PA, Feige J-J, Baird A. Characterization of the neuronal receptor for basic fibroblast growth factor and comparison to receptors on mesenchymal cells. J Biol Chem. 1989;264:4120–4126. [PubMed] [Google Scholar]
- 67.Wanaka A, Johnson EM, Jr, Milbrandt J. Localization of FGF receptor mRNA in the adult rat central nervous system by in situ hybridization. Neuron. 1990;5:267–281. doi: 10.1016/0896-6273(90)90164-b. [DOI] [PubMed] [Google Scholar]
- 68.Watanabe T, Raff MC. Rod photoreceptor development in vitro: intrinsic properties of proliferating neuroepithelial cells change as development proceeds in the rat retina. Neuron. 1990;4:461–467. doi: 10.1016/0896-6273(90)90058-n. [DOI] [PubMed] [Google Scholar]
- 69.Wen R, Cheng T, Li Y, Cao W, Steinberg RH. α2-adrenergic agonists induce basic fibroblast growth factor expression in photoreceptors in vivo and ameliorate light damage. J Neurosci. 1996;16:5986–5992. doi: 10.1523/JNEUROSCI.16-19-05986.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yokoyama Y, Ozawa S, Seyama, Namiki H, Hayashi Y, Kaji K, Shirama K, Shioda M, Kano K. Enhancement of apoptosis in developing chick neural retina cells by basic fibroblast growth factor. J Neurochem. 1997;68:2212–2215. doi: 10.1046/j.1471-4159.1997.68052212.x. [DOI] [PubMed] [Google Scholar]
- 71.Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- 72.Zhang C, Takahashi K, Lam TT, Tso MOM. Effects of basic fibroblast growth factor in retinal ischemia. Invest Ophthalmol Vis Sci. 1994;35:3163–3168. [PubMed] [Google Scholar]