Abstract
The novel neuropeptides called hypocretins (orexins) have recently been identified as being localized exclusively in cell bodies in a subregion of the tuberal part of the hypothalamus. The structure of the hypocretins, their accumulation in vesicles of axon terminals, and their excitatory effect on cultured hypothalamic neurons suggest that the hypocretins function in intercellular communication. To characterize these peptides further and to help understand what physiological functions they may serve, we undertook an immunohistochemical study to examine the distribution of preprohypocretin-immunoreactive neurons and fibers in the rat brain. Preprohypocretin-positive neurons were found in the perifornical nucleus and in the dorsal and lateral hypothalamic areas. These cells were distinct from those that express melanin-concentrating hormone. Although they represent a restricted group of cells, their projections were widely distributed in the brain. We observed labeled fibers throughout the hypothalamus. The densest extrahypothalamic projection was found in the locus coeruleus. Fibers were also seen in the septal nuclei, the bed nucleus of the stria terminalis, the paraventricular and reuniens nuclei of the thalamus, the zona incerta, the subthalamic nucleus, the central gray, the substantia nigra, the raphe nuclei, the parabrachial area, the medullary reticular formation, and the nucleus of the solitary tract. Less prominent projections were found in cortical regions, central and anterior amygdaloid nuclei, and the olfactory bulb. These results suggest that hypocretins are likely to have a role in physiological functions in addition to food intake such as regulation of blood pressure, the neuroendocrine system, body temperature, and the sleep–waking cycle.
Keywords: neuropeptide, hypothalamus, immunohistochemistry, blood pressure, feeding, autonomic functions, hypocretin, orexin, melanin-concentrating hormone, neuroendocrine
The hypothalamus is an essential interface between endocrine, autonomic, and somatomotor systems. In mammals, the hypothalamus is a hub of central regulatory centers for autonomic and endocrine homeostatic systems such as cardiovascular, temperature, and abdominal visceral regulation, as well as ingestive behaviors (Swanson, 1987). For some of these systems, particular peptides have been identified as major products of individual nuclei; the localization of vasopressin and oxytocin to the paraventricular and supraoptic nuclei is a prime example. It is likely that other hypothalamic peptides are yet to be identified that may be similarly localized and that may contribute to the physiological functions regulated by the hypothalamus.
To identify novel hypothalamic peptides, an analysis of the mRNAs whose expression is restricted to or enriched in the rat hypothalamus was undertaken using directional tag-PCR subtraction (Gautvik et al., 1996). A nucleotide sequence encoding a 130 residue protein called preprohypocretin (hcrt) was isolated from this hypothalamus-enriched cDNA library (Sutcliffe et al., 1997; de Lecea et al., 1998). In situ hybridization revealed that neurons expressing hcrt mRNA were located exclusively in the tuberal region of the hypothalamus (Gautvik et al., 1996). Sequence analysis indicated that hcrt yields two peptides, hcrt-1 (residues 28–66) and hcrt-2 (residues 69–97). The structure of hcrt, its expression in hypothalamic neurons, and its accumulation in vesicles of axon terminals suggested that the hcrt peptides may have intercellular signaling activity, and synthetic hcrt-2 was excitatory when applied to synaptically coupled rat hypothalamic neurons in vitro (de Lecea et al., 1998).
More recently, screening of high-resolution HPLC fractions on cell lines expressing orphan G-protein–coupled receptors resulted in the isolation of two peptides called orexin A and B (Sakurai et al., 1998) that are identical to hypocretin 1 and 2, respectively. Chemical analyses confirmed the identity of orexin B and hcrt-2, defined the N terminal of orexin A (hcrt-1) as residue 33, and verified that both peptides are amidated at their C terminals. These two peptides activate two distinct G-protein–coupled receptors, OX1 and OX2 (Sakurai et al., 1998).
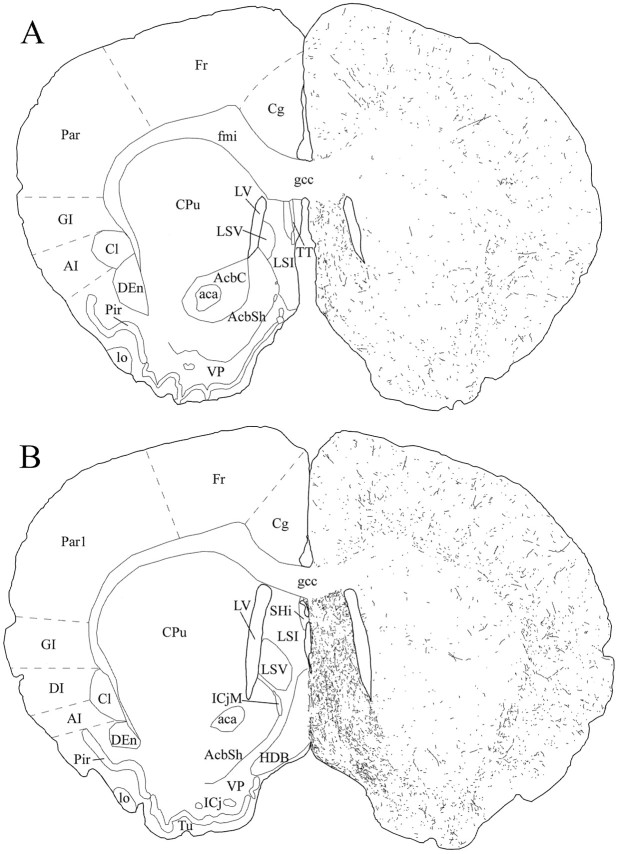
To characterize further these new peptides and to obtain clues about their potential physiological functions, we undertook an immunohistochemical study to examine the distribution of hcrt-immunoreactive neurons and fibers in the brain. Sakurai et al. (1998) reported that intracerebroventricular injection of hcrt stimulates food intake. However, the widespread distribution of hcrt fibers observed in the present study suggests that hcrt is likely to play a role in other physiological functions as well.
Parts of this paper have been published previously in abstract form (Peyron et al., 1997).
MATERIALS AND METHODS
Perfusion, fixation
Adult male Wistar rats were deeply anesthetized with a lethal dose of Nembutal (80 mg/kg) and perfused transcardially with 0.9% saline followed by an ice-cold fixative solution containing 4% paraformaldehyde and 0 or 0.25% glutaraldehyde in 0.1 mphosphate buffer (PB). Brains were removed, post-fixed overnight by immersion in the same fixative without glutaraldehyde, and cryoprotected with 30% sucrose for 2–3 d at 4°C. Brains were rapidly frozen in dry ice and sliced into 20-μm-thick coronal sections on a cryostat (−23°C). Free-floating sections were rinsed several times and stored in 0.1 m PB containing 0.9% NaCl and 0.3% Triton X-100 plus 0.1% sodium azide (PBST-Az) at 4°C until use.
Immunohistochemistry
Immunohistochemical detection of hcrt was done by sequential incubations of free-floating sections in (1) hcrt antiserum raised in rabbit (1:5000 in PBST-Az) for 4 d at 4°C or overnight at room temperature (RT), (2) biotinylated goat anti-rabbit IgG (1:2000 in PBST; Vector Laboratories, Burlingame, CA) for 90 min at RT, and finally (3) avidin–biotin–HRP complex (1:1000 in PBST; Vector Elite Kit; Vectastain) for 90 min at RT. After each incubation, the sections were rinsed twice for 15 min in PBST. The sections were then immersed in 0.05 m Tris-HCl buffer, pH 7.6, containing 0.025% 3,3′-diaminobenzidine-4HCl (DAB; Sigma, St. Louis, MO), 0.6% ammonium nickel (II) sulfate hexahydrate (Nacalai Tesque, Kyoto, Japan), and 0.003% H2O2, for 30 min at RT. The histochemical reaction was stopped by two rinses in PBST-Az. After this procedure, the hcrt staining appeared as black punctuate granules in somata and processes of hcrt neurons (see Fig.1A,C). To visualize the precise location of labeled neurons and fibers in the brain, we counterstained some sections with neutral red (Sigma).
Fig. 1.
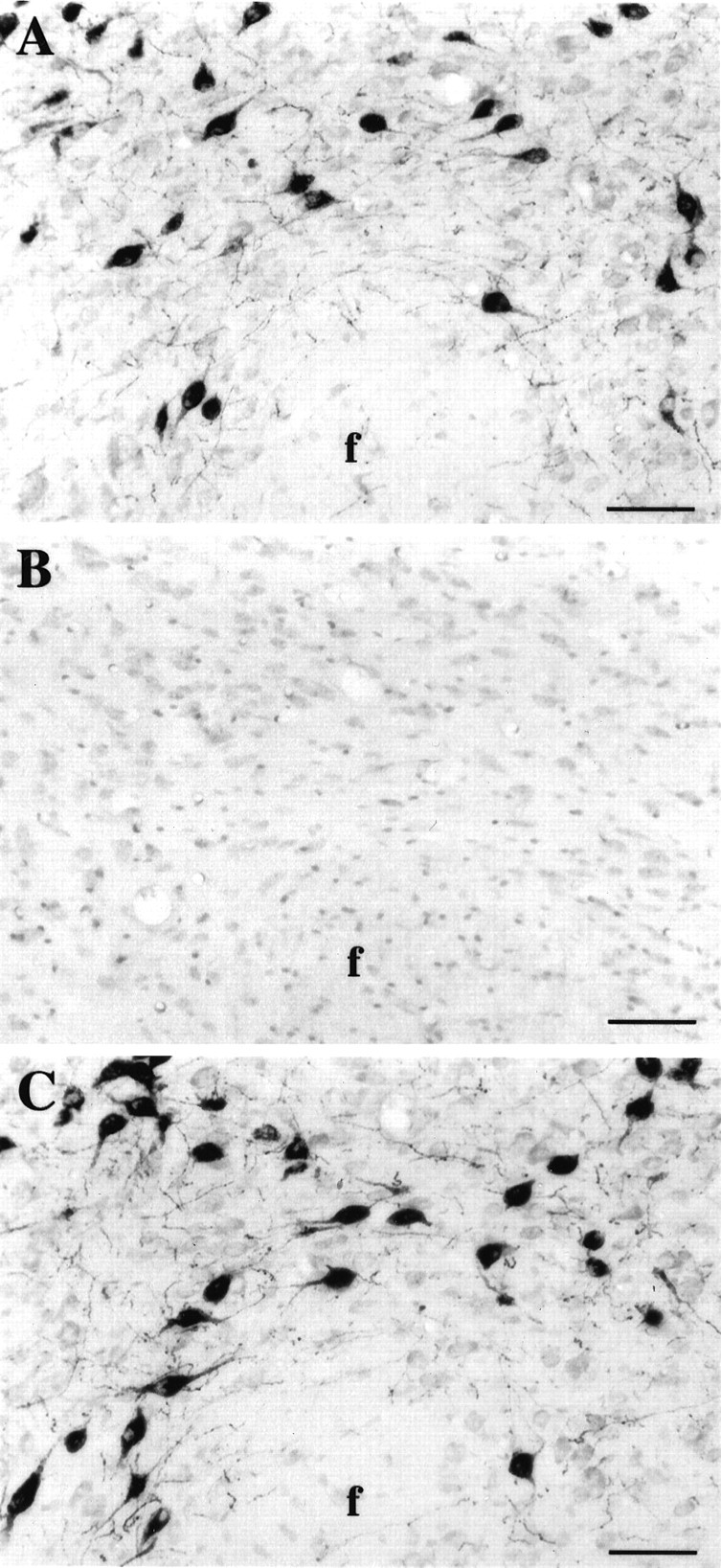
Photomicrographs of adjacent frontal sections counterstained with neutral red at the level of the perifornical nucleus of the hypothalamus. A, Hcrt neurons were labeled using antiserum #2050 against the C-terminal 17 aa portion of the preproprotein. B, Hypocretin immunoreactivity was absent after preincubation of antiserum #2050 with the whole preproprotein, showing that this antibody specifically recognized hcrt.C, Hcrt neurons were labeled with antibody #2123. Identical staining was obtained with both antisera. f, Fornix. Scale bars, 65 μm.
Ultrastructural immunohistochemistry
For electron microscopy sections, brains were fixed by transcardial perfusion with physiological saline followed by 4% paraformaldehyde and 0.2% glutaraldehyde, cut in 50-μm-thick sections, frozen in liquid nitrogen to increase reagent penetration, and immunostained with hcrt antisera as described above but without nickel intensification. After treatment with 1% osmium tetroxide for 45 min, sections were dehydrated in an ascending series of ethanol and embedded in Epon. Ultrathin sections were cut on a Reichert Ultracut microtome and studied in a Jeol 1200 EXII electron microscope at an accelerating voltage of 60,000 V.
Antisera and controls
Antisera were raised in rabbits by immunization with the 17 C-terminal amino acids of hcrt (CPTATATALAPRGGSRV, antiserum #2050) conjugated to keyhole limpet hemocyanin (Sigma) or with the bacterially expressed histidine-tagged preprohypocretin (residues 28–130-amide, antiserum #2123) following the procedures described by Sutcliffe et al. (1983). The antiserum was purified as a polyclonal antibody using the E-Z-SEP kit (Pharmacia, Piscataway, NJ).
Immunohistochemical controls
To confirm the specificity of both antisera for immunohistochemical use, we preincubated each antiserum (1:5000) for 24 hr at 4°C with an excess (10 μg/ml) of five different peptides: the 17 C-terminal amino acid residues of hcrt (113–130-amide, CPTATATALAPRGGSRV), the bacterially expressed preproprotein (residues 28–130-amide), hcrt-2 (residues 69–97-amide, PGPPGLQGRLQRLLQANGNHAAGILTM-NH2), orexin A (residues 33–66-amide, QPLPDCCRQKTCSCRLYELLHGAGNHAAGILTL-NH2), or rat secretin (Peninsula Laboratories, Belmont, CA). Sections were then incubated with those solutions overnight at RT. The immunostaining was performed following the procedure described above.
In situ hybridization procedures
Preparation of hcrt and melanin-concentrating hormone riboprobes.Hcrt cDNA was inserted into the pBCSK+ vector (Stratagene, La Jolla, CA) atNotI and EcoRI sites. Melanin-concentrating hormone (MCH) cDNA was inserted into the pBCSK+ vector at BamHI andEcoRI sites. Both hcrt and MCH plasmids were linearized using EcoRI (Life Technologies, Gaithersburg, MD). Radiolabeled hcrt riboprobes were synthesized by in vitro transcription of a full-length (569 nucleotides) rat hcrt probe using T3 polymerase (Ambion, Austin, TX) and [35S]UTP (DuPont NEN, Boston, MA). For the colorimetric procedure, the hcrt and MCH riboprobes were transcribedin vitro following the standard digoxigenin (DIG)-labeling reaction protocol from Boehringer Mannheim (Indianapolis, IN) using 10× transcription buffer and T3 RNA polymerase (Life Technologies). Riboprobes were then purified by ethanol precipitation and stored at −70°C.
Estimation of DIG-labeled riboprobe yields. The yield of the DIG-labeled hcrt and MCH riboprobes was approximated by comparison with DIG-labeled control RNA (Boehringer Mannheim). Serial dilutions of DIG-labeled control RNA and hcrt and MCH riboprobes were spotted onto a nylon membrane (Nytran Plus; Schleicher & Schuell, Keene, NH). The membrane was then incubated in 0.1 m PBS solution containing 1% Triton X-100 and 4% bovine serum albumin (PBST-BSA) for 15 min at RT, followed by an incubation with a sheep antibody against DIG conjugated with alkaline phosphatase (1:5000 in PBST-BSA; Boehringer Mannheim) for 30 min at RT. The membrane was rinsed in 0.1m PBS for 15 min followed by a 3 min rinse in a development buffer (100 mm Tris buffer, 50 mmMgCl2, and 150 mm NaCl, pH 9). Finally, the reaction was developed with nitroblue tetrazolium (4.5 μl/ml) and 5-bromo-4-chloro-3-indolyl phosphate (3.5 μl/ml) in the development buffer (Bio-Rad, Hercules, CA) for 30 min at RT.
Hybridization. Twenty-five micrometer thick brain sections preserved in a cryoprotectant solution of 0.1 m PBS with 30% glycerol and 30% ethylene glycol were stored at −70°C. After thawing of frozen sections, free-floating sections were washed in 0.1m PBS and then incubated in PBS with 0.5% Triton X-100 for 10 min, deproteinized with 0.1N HCl for 10 min, and acetylated with acetic anhydride (0.25% in 0.1 m triethanolamine hydrochoride, pH 8) for 10 min. The sections were then post-fixed in 4% paraformaldehyde for 10 min. Five minute washes in PBS were done after each of the above steps. The sections were then prehybridized in a solution containing 4× PIPES, 10% (w/v) dextran sulfate, 50% deionized formamide, 5× Denhardt’s, 50 mm DTT, 0.2% (w/v) SDS, 250 mg/ml denatured salmon sperm DNA, and 250 mg/ml yeast tRNA for 3 hr at 55°C. Labeled antisense hcrt or MCH riboprobes (106 cpm/μl for autoradiographic localization; ∼600 ng/ml for colorimetric procedures) were heated at 68°C for 10 min and added to the sections in the prehybridization solution for an overnight incubation at 55°C. The sections were then (1) washed in 2× SSC with 10 mm β-mercaptoethanol (β-ME) for 30 min at RT; (2) digested with 4 μg/ml RNase A in 5× Tris buffer (50 mm Tris-HCl, 5 mm EDTA, and 0.5 mNaCl) at 37°C for 1 hr; (3) rinsed in 1× SSC, 50% formamide, and 5 mm β-ME at 55°C for 2 hr; (4) incubated in 0.2× SSC, 10% formamide, 1 mm β-ME, and 0.1% Sarkosyl at 68°C for 1 hr; and (5) washed three times in PBS at RT.
For autoradiographic localization, sections were mounted on slides, dehydrated in a series of 50–100% ethanol, defatted in 50%/50% ethanol–chloroform followed by 100% ethanol, air dried, and then exposed to Kodak X-Omat AR film for 1–7 d. Sections were subsequently dipped in Ilford K5 liquid photographic emulsion and exposed for 2–4 weeks at 4°C. The resultant autoradiographs were developed in Kodak D-19 and counterstained with Richardson’s blue.
For the colorimetric procedure, sections were washed in PBST-BSA for 2 hr at RT and then were incubated with a sheep anti-DIG-alkaline phosphatase antiserum (1:3000 in PBST-BSA) overnight at RT. For color development, sections were first rinsed twice for 30 min in 0.1m Tris-HCl buffer, pH 8.2, and then immersed in a Tris-HCl buffer containing Fast red (Sigma Fast; Sigma) (see Fig.2A–D) or Vector red (Vector Laboratories) (see Fig.2E,F) as a substrate for alkaline phosphatase for 5 hr at RT. To increase the intensity of the signal, we added 0.3 m NaCl in the Fast red solution as suggested by Chiu et al. (1996). Stained neurons have an homogeneous red color of the cytoplasm with both substrates.
Fig. 2.
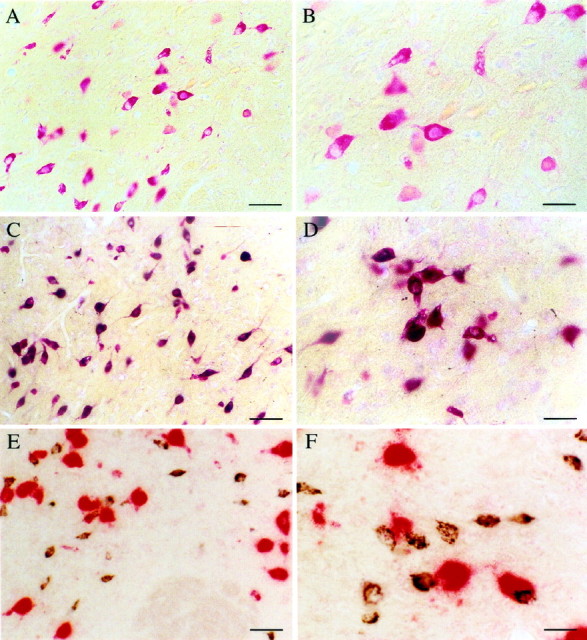
Photomicrographs illustrating hcrt neurons in the perifornical nucleus of the hypothalamus. A, Neurons containing mRNA for hcrt visualized with a homogeneousred coloration of the cytoplasm with an in situ hybridization technique using Fast red as a substrate for alkaline phosphatase. B, Enlargement ofA. C, Photomicrograph showing that all neurons that stained red after in situhybridization (recognizing hcrt mRNA using Fast red) are labeledblack by immunohistochemistry (recognizing the protein with the antiserum #2050) for hcrt. This result indicates that the antiserum #2050 is specific for hcrt. D, High magnification of double-labeled cells after in situhybridization (red) and immunohistochemistry (black). E, Photomicrograph of neurons containing mRNA for the melanin-concentrating hormone (labeled inred by in situ hybridization using Vector red as substrate) and of hcrt neurons (labeled in blackby immunohistochemistry using DAB with nickel as the substrate). Note that no double-labeled cells are present, indicating that MCH and hcrt are found in two distinct populations of neurons. F, High magnification of the MCH (red) and hcrt (black) neurons in the perifornical nucleus. Scale bars:A, C, E, 65 μm;B, D, F, 36 μm.
In situ/immunostaining
After in situ hybridization, the sections were subjected to the same immunohistochemistry procedure as described above but with minor changes. Anti-hcrt (#2050) antiserum was used at a 1:1000 dilution, and successive incubations were done in PBST-BSA. Double-labeled cells were identified by the presence of black granulations over a red coloration of the cytoplasm (see Fig.2C,D). To test whether there was cross-reactivity between the sheep anti-DIG-alkaline phosphatase and the secondary anti-rabbit IgG, we conducted the procedure without the primary antibodies (#2050 and #2123).
Data analysis
The sections were mounted on gelatin-coated glass slides, dried, dehydrated, and coverslipped with Mounting medium (Harleco; Diagnostic Systems, Gibbstown, NJ) or rinsed with water and coverslipped with aqueous mounting medium (Scytek Laboratories, Logan, UT). They were later observed with an Olympus BH-2 microscope. Mapping of neurons and fibers immunoreactive to hcrt in rat brain was done using a Nikon light microscope equipped with a motorized X/Y stage, position encoders, and a video camera connected to a computerized image data analysis system (Neurolucida; MicroBrightField, Colchester, VT). Outlines of sections and major structures were drawn at a low magnification (4×), whereas labeled neurons and fibers were plotted at higher magnification (20–40×). Finally, the drawings were assembled with Adobe Illustrator 7.0 to obtain the figures depicting the distribution of hcrt projections. Counting of hcrt neurons was done bilaterally on 15-μm-thick coronal sections taken every 120 μm (one-eighth of the brain). The number of labeled cells in the brain was estimated using the method of Abercrombie (1946).
Photomicrographs were taken on the Olympus microscope, and the films were scanned with a Kodak slide scanner. To obtain optimal reproduction of the staining, we modified the contrast and luminosity of the crude scans with Adobe Photoshop 4.0. The illustration plates were printed on a Kodak 7700 dye sublimation printer.
RESULTS
Antiserum specificity
We used two antisera, one raised against the C-terminal 17 amino acids of preprohypocretin (#2050) and a second raised against the bacterially expressed preproprotein hcrt (#2123). Competition studies showed that the immunohistochemical staining was absent when antiserum #2050 was preincubated for 24 hr at 4°C with the preproprotein or with the 17 aa portion of hcrt. In contrast, the labeling was still present after preincubation of the antibody with synthetic hcrt-2 or with rat secretin, showing that antiserum #2050 specifically recognizes the 17 aa portion of hcrt but not hcrt-2 or rat secretin (Fig.1). No labeling was obtained after preincubation of #2123 with the preproprotein. However, the labeling was still present after preincubation of #2123 with the 17 aa portion, orexin A, or hcrt-2, indicating that #2123 is a polyclonal antiserum against multiple epitopes of the preproprotein and not just the C-terminal 17 aa portion recognized by #2050. The cell body staining obtained with both antisera (#2050 and #2123) and by autoradiographic (refer to de Lecea et al., 1998) and colorimetric in situhybridization is indistinguishable (Figs. 1,2). The combination of in situhybridization and immunohistochemistry for hcrt showed that neurons that expressed the mRNA coding for hcrt also made the protein (Fig.2C,D).
Because MCH neurons are known to be located in the same tuberal region of the hypothalamus as hcrt cells, we combined in situhybridization for MCH with hcrt immunohistochemistry. In this combination, we did not observe any double-labeled cells, showing that MCH and hcrt were synthesized by distinct neuronal populations and therefore that there was no cross-reactivity between the in situ and immunohistochemistry procedures (Fig.2E,F). Hcrt neurons appeared smaller in size than did MCH cells in Figure 2, E andF. This is likely an artifact because of the different staining procedures used. The granulation of the precipitate obtained with the Vector red substrate is larger than that obtained with Fast red (alkaline phosphatase substrate) or DAB–nickel (substrate of the peroxidase used for immunohistochemistry). Therefore, cells stained with Vector red substrate appeared larger with poorly defined cell borders (Fig. 2E,F). When MCH cells are stained using the other substrates, they do not differ in size in comparison with hcrt cells.
Distribution of hcrt-immunoreactive cell bodies
In situ hybridization and immunohistochemistry against hcrt (mRNA and protein, respectively) showed that hcrt neurons were distributed exclusively in a restricted area of the tuberal region of the hypothalamus (1 mm rostrocaudal) caudal to the paraventricular nucleus of the hypothalamus. On coronal sections, labeled neurons showed a bilaterally symmetric organization. They were observed in the perifornical nucleus, the dorsomedial hypothalamic nucleus, and the dorsal and lateral hypothalamic areas. A few cells were seen in the posterior hypothalamic area and the subincertal nucleus at the junction of the thalamus and the hypothalamus (Fig.3; also see Fig. 8). The labeled neurons were medium in size (25–30 μm in large diameter) and multipolar to fusiform in shape. They typically gave rise to two to three primary dendrites that were either smooth or only very sparsely invested with dendritic spines. Secondary branching was often observed, but third-order divisions were rarely seen.
Fig. 3.

Distribution of hcrt-labeled neurons on frontal sections at three rostrocaudal levels of the tuberal region of the hypothalamus (A to B to C) determined using #2123 antiserum. Sections were counterstained with neutral red (pink staining of all cells). The neutral red staining allowed us to determine the exact location of hcrt neurons in the brain that are localized exclusively in the tuberal region of the hypothalamus ventral to the zona incerta and that extend 1 mm rostrocaudally, beginning caudal to the paraventricular nucleus of the hypothalamus. 3V, 3rd ventricle; Arc, arcuate nucleus; DMH, DM, dorsomedial hypothalamic nucleus; f, fornix; ic, internal capsule; opt, optic tract; SOR, retrochiasmatic part of the supraoptic nucleus; VMH, ventromedial hypothalamic nucleus; ZI, zona incerta. Scale bars, 275 μm.
Fig. 8.
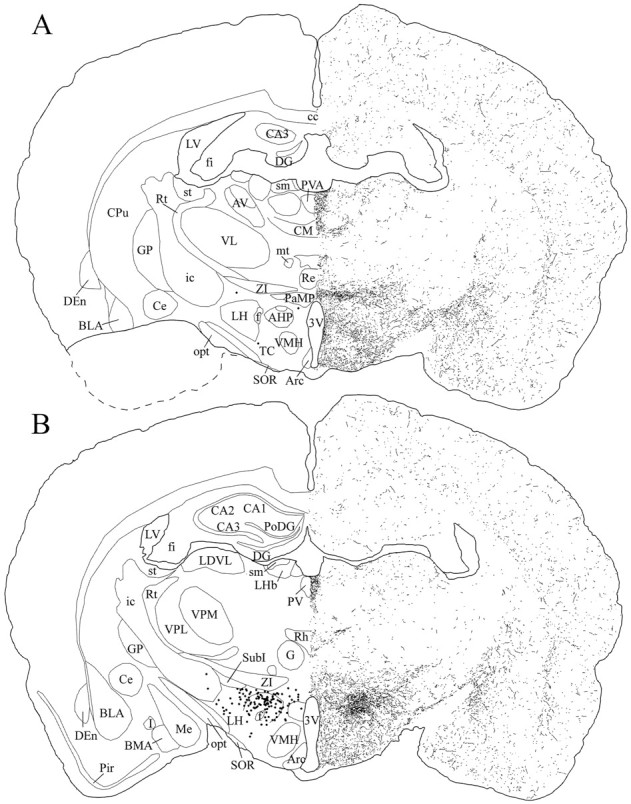
Schematic drawings of 20 μm rostrocaudal coronal sections illustrating the distribution and relative density of hcrt fibers at the level of the tuberal region of the hypothalamus after immunohistochemistry for hcrt using antibody #2050. The position of hcrt cell bodies is indicated as dots in the left hemisphere. 3V, 3rd ventricle; AHP, anterior hypothalamic area, posterior part; Arc, arcuate nucleus; AV, anteroventral thalamic nucleus;BLA, basolateral amygdaloid nucleus, anterior part;BMA, basomedial amygdaloid nucleus, anterior part;CA1–CA3, fields CA1–CA3 of Ammon’s horn;cc, corpus callosum; Ce, central amygdaloid nucleus; CM, central medial thalamic nucleus;CPu, caudate putamen; DEn, dorsal endopiriform nucleus; DG, dentate gyrus;f, fornix; fi, fimbria of the hippocampus; G, gelatinosus thalamic nucleus;GP, globus pallidus; I, intercalated nuclei of the amygdala; ic, internal capsule;LDVL, laterodorsal thalamic nucleus, ventrolateral part;LH, lateral hypothalamic area; LHb, lateral habenular nucleus; LV, lateral ventricle;Me, medial amygdaloid nucleus; mt, mammillothalamic tract; opt, optic tract;PaMP, paraventricular hypothalamic nucleus, medial parvocellular part; Pir, piriform cortex;PoDG, polymorph layer of the dentate gyrus;PV, paraventricular thalamic nucleus;PVA, paraventricular thalamic nucleus, anterior part;Re, reuniens thalamic nucleus; Rh, rhomboid thalamic nucleus; Rt, reticular thalamic nucleus; sm, stria medullaris of the thalamus;SOR, supraoptic nucleus, retrochiasmatic part;st, stria terminalis; SubI, subincertal nucleus; TC, tuber cinereum area; VL, ventrolateral thalamic nucleus; VMH, ventromedial hypothalamic nucleus; VPL, ventral posterolateral thalamic nucleus; VPM, ventral posteromedial thalamic nucleus; ZI, zona incerta.
Using the method of Abercrombie (1946), we found 682.62 ± 11.97 neurons on each side based on four brains stained with each antibody. The mean diameter of labeled cells was ∼25 μm. Therefore, the total number of hcrt cells in the brain is estimated to be: 682 (120/145) × 2 = 1128 cells. The perifornical nucleus contains ∼50% of the hcrt-labeled neurons. Approximately the same number of hcrt neurons was observed by autoradiographic and colorimetric in situ hybridization as by immunohistochemistry.
Electron microscopy
Immunostaining was found in the cytoplasm of cell bodies and proximal dendrites (Fig.4A). In strongly stained cells, peroxidase label was found distributed throughout the cytoplasm. In more lightly stained cells (Fig. 4), the staining was more punctate. With electron microscopy, the light peroxidase immunolabel was found to be restricted to regions of the Golgi apparatus and dense core granules (Fig. 4B,thin arrows). Immunoreactive cells generally had a partially invaginated nucleus and a single large nucleolus and were rich in cytoplasmic organelles (Fig. 4B). Two to three thick dendrites were found in immunoreactive cells, and their proximal region also contained a high density of cytoplasmic organelles.
Fig. 4.
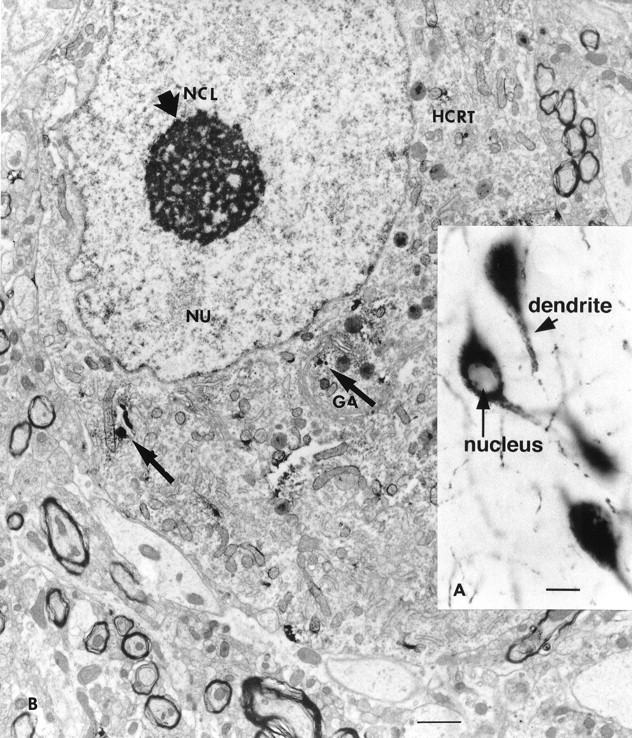
A, Large cells in the lateral hypothalamus are immunoreactive for hypocretin. Staining was found in the cytoplasm and dendrites (short arrow) but not in the nucleus (long arrow). Scale bar, 15 μm.B, Electron microscopic examination of immunoreactive neurons showed punctate staining in the cytoplasm, often associated with dense core granules and parts of the Golgi apparatus (thin arrow). Random organelles near dense core granules sometimes showed peroxidase label, probably because of diffusion during the process of immunocytochemistry. GA, Golgi apparatus; HCRT, hypocretin; NCL, nucleole (thick arrow); NU, nucleus.
Distribution of fibers
Hcrt-IR fibers were distributed throughout the brain as illustrated in Figures5-12. We obtained indistinguishable distribution of fibers with both antibodies (#2050 and #2123). The relative density of fibers observed in different brain regions is reported in Table1. Hcrt-IR fibers were spread throughout the entire hypothalamus (Figs. 7-9). The density of fibers was homogeneous in the tuberal region of the hypothalamus. A high density of fibers was also seen in the other regions of the hypothalamus, although fewer fibers were seen in the medial preoptic nucleus, the anterior part of the ventromedial hypothalamic nucleus, and the paraventricular nucleus (Figs. 7, 8). Hcrt fibers were found around the suprachiasmatic nucleus and the supraoptic nucleus in the anterior hypothalamus (Fig. 7), but few axons were found in these nuclei. Long, thick hcrt fibers with numerous boutons innervated the arcuate nucleus (Fig. 13C) and followed the border of the brain to end laterally in the tuberomammillary nucleus. Hcrt fibers avoided the mammillary bodies and went through the supramammillary nucleus and the posterior hypothalamic area (Fig.9).
Fig. 5.
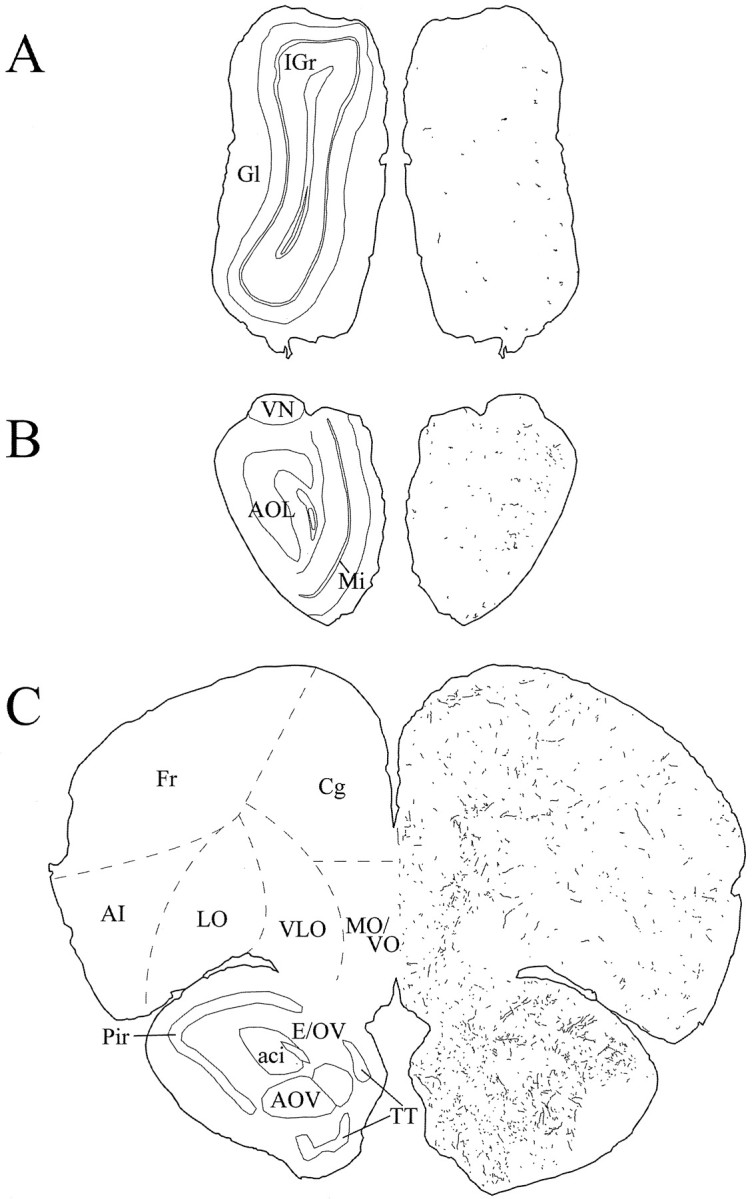
Schematic drawings of 20 μm rostrocaudal coronal sections illustrating the distribution and relative density of hcrt fibers in the prefrontal cortex and the olfactory bulb after immunohistochemistry for hcrt using antibody #2050. aci, Anterior commissure, intrabulbar part;AI, agranular insular cortex; AOL, anterior olfactory nucleus, lateral part; AOV, anterior olfactory nucleus, ventral part; Cg, cingulate cortex;E/OV, ependyma and subependymal layer/olfactory ventricle; Fr, frontal cortex; Gl, glomerular layer of the olfactory bulb; IGr, internal granular layer of the olfactory bulb; LO, lateral orbital cortex; Mi, mitral cell layer of the olfactory bulb; MO/VO, medial/ventral orbital cortex;Pir, piriform cortex; TT, tenia tecta;VLO, ventrolateral orbital cortex; VN, vomeronasal nerve layer.
Fig. 6.
Schematic drawings of 20 μm rostrocaudal coronal sections illustrating the distribution and relative density of hcrt fibers in the telencephalon after immunohistochemistry for hcrt using antibody #2050. aca, Anterior commissure, anterior part;AcbC, accumbens nucleus, core; AcbSh, accumbens nucleus, shell; AI, agranular insular cortex;Cg, cingulate cortex; Cl, claustrum;CPu, caudate putamen; DEn, dorsal endopiriform nucleus; DI, dysgranular insular cortex;fmi, forceps minor of the corpus callosum;Fr, frontal cortex; gcc, genu of the corpus callosum; GI, granular insular cortex;HDB, nucleus of the horizontal limb of the diagonal band; ICj, islands of Calleja; ICjM, islands of Calleja, major island; lo, lateral olfactory tract; LSI, lateral septal nucleus, intermediate part;LSV, lateral septal nucleus, ventral part;LV, lateral ventricle; Par, parietal cortex; Par1, parietal cortex, area 1;Pir, piriform cortex; SHi, septohippocampal nucleus; TT, tenia tecta;Tu, olfactory tubercle; VP, ventral pallidum.
Fig. 7.
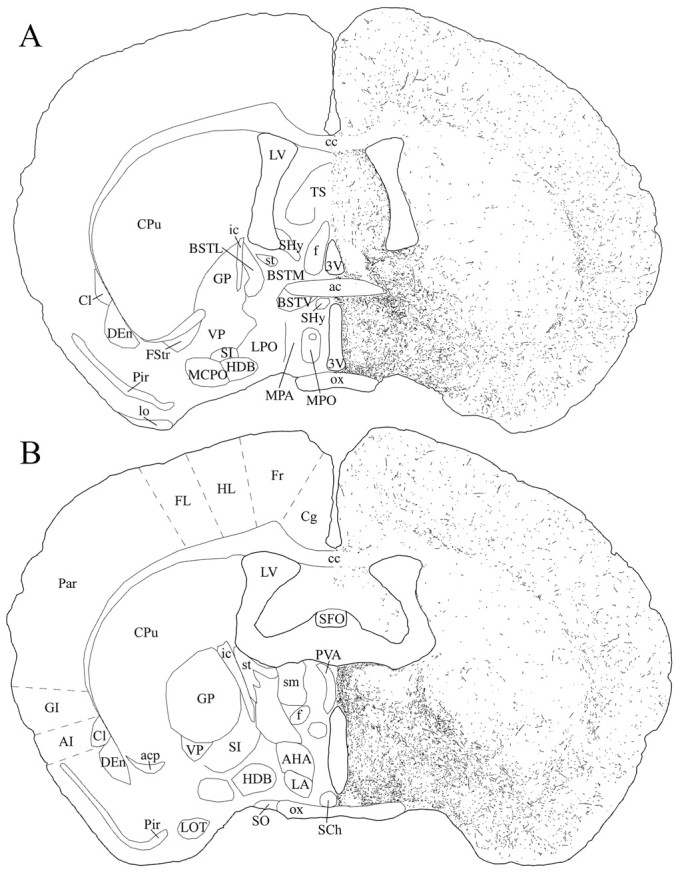
Schematic drawings of 20 μm rostrocaudal coronal sections illustrating the distribution and relative density of hcrt fibers at the level of the preoptic area after immunohistochemistry for hcrt using antibody #2050. 3V, 3rd ventricle;ac, anterior commissure; acp, anterior commissure, posterior part; AHA, anterior hypothalamic area, anterior part; AI, agranular insular cortex;BSTL, bed nucleus of the stria terminalis, lateral division; BSTM, bed nucleus of the stria terminalis, medial division; BSTV, bed nucleus of the stria terminalis, ventral division; cc, corpus callosum;Cg, cingulate cortex; Cl, claustrum; CPu, caudate putamen; DEn, dorsal endopiriform nucleus;f, fornix; FL, forelimb area of the cortex; Fr, frontal cortex; FStr, fundus striati; GI, granular insular cortex; GP, globus pallidus; HDB, nucleus of the horizontal limb of the diagonal band; HL, hindlimb area of the cortex;ic, internal capsule; LA, lateroanterior hypothalamic nucleus; lo, lateral olfactory tract;LOT, nucleus of the lateral olfactory tract;LPO, lateral preoptic area; LV, lateral ventricle; MCPO, magnocellular preoptic nucleus;MPA, medial preoptic area; MPO, medial preoptic nucleus; ox, optic chiasm; Par, parietal cortex; Pir, piriform cortex;PVA, paraventricular thalamic nucleus, anterior part;SCh, suprachiasmatic nucleus; SFO, subfornical organ; SHy, septohypothalamic nucleus;SI, substantia innominata; sm, stria medullaris of the thalamus; SO, supraoptic nucleus;st, stria terminalis; TS, triangular septal nucleus; VP, ventral pallidum.
Fig. 9.
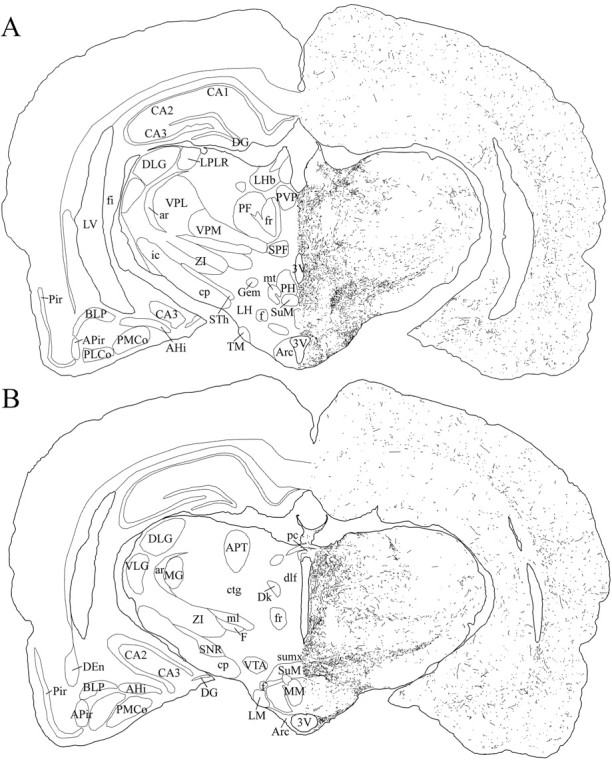
Schematic drawings of 20 μm rostrocaudal coronal sections illustrating the distribution and relative density of hcrt fibers in the caudal part of the hypothalamus after immunohistochemistry for hcrt using antibody #2050. 3V, 3rd ventricle; AHi, amygdalohippocampal area;APir, amygdalopiriform transition area;APT, anterior pretectal nucleus; ar, acoustic stria; Arc, arcuate nucleus;BLP, basolateral amygdaloid nucleus, posterior part;CA1–CA3, fields CA1–CA3 of Ammon’s horn;cp, cerebral peduncle; ctg, central tegmental tract; DEn, dorsal endopiriform nucleus;DG, dentate gyrus; Dk, nucleus Darkschewitsch; dlf, dorsal longitudinal fasciculus; DLG, dorsal lateral geniculate nucleus;f, fornix; F, nucleus of the fields of Forel; fi, fimbria of the hippocampus;fr, fasciculus retroflexus; Gem, gemini hypothalamic nucleus; ic, internal capsule;LH, lateral hypothalamic area; LHb, lateral habenular nucleus; LM, lateral mammillary nucleus; LPLR, lateral posterior thalamic nucleus, laterorostral part; LV, lateral ventricle;MG, medial geniculate nucleus; ml, medial lemniscus; MM, medial mammillary nucleus, medial part;mt, mammillothalamic tract; pc, posterior commissure; PF, parafascicular thalamic nucleus;PH, posterior hypothalamic area; Pir, piriform cortex; PLCo, posterolateral cortical amygdaloid nucleus; PMCo, posteromedial cortical amygdaloid nucleus; PVP, paraventricular thalamic nucleus, posterior part; SNR, substantia nigra, reticular part; SPF, subparafascicular thalamic nucleus;STh, subthalamic nucleus; SuM, supramammillary nucleus; sumx, supramammillary decussation; TM, tuberomammillary nucleus;VLG, ventral lateral geniculate nucleus;VPL, ventral posterolateral thalamic nucleus;VPM, ventral posteromedial thalamic nucleus;VTA, ventral tegmental area; ZI, zona incerta.
Fig. 10.
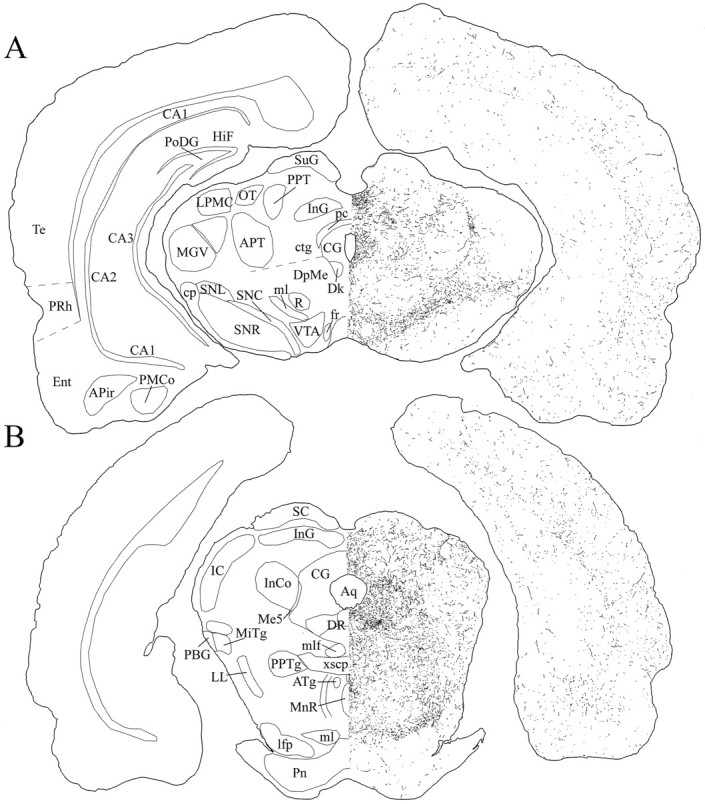
Schematic drawings of 20 μm rostrocaudal coronal sections illustrating the distribution and relative density of hcrt fibers in the midbrain after immunohistochemistry for hcrt using antibody #2050. APir, Amygdalopiriform transition area;APT, anterior pretectal nucleus; Aq, aqueduct; ATg, anterior tegmental nucleus;CA1–CA3, fields CA1–CA3 of Ammon’s horn;CG, central gray; cp, cerebral peduncle;ctg, central tegmental tract; Dk, nucleus Darkschewitsch; DpMe, deep mesencephalic nucleus; DR, dorsal raphe nucleus; Ent, entorhinal cortex; fr, fasciculus retroflexus;HiF, hippocampal fissure; IC, inferior colliculus; InCo, intercollicular nucleus;InG, intermediate gray layer of the superior colliculus;lfp, longitudinal fasciculus of the pons;LL, lateral lemniscus; LPMC, lateral posterior thalamic nucleus, mediocaudal part; Me5, mesencephalic trigeminal nucleus; MGV, medial geniculate nucleus, ventral part; MiTg, microcellular tegmental nucleus; ml, medial lemniscus; mlf, medial longitudinal fasciculus; MnR, median raphe nucleus; OT, nucleus of the optic tract;PBG, parabigeminal nucleus; pc, posterior commissure; PMCo, posteromedial cortical amygdaloid nucleus; Pn, pontine nuclei; PoDG, polymorph layer of the dentate gyrus; PPT, posterior pretectal nucleus; PPTg, pedunculopontine tegmental nucleus; PRh, perirhinal cortex; R, red nucleus; SC, superior colliculus; SNC, substantia nigra, compact part; SNL, substantia nigra, lateral part; SNR, substantia nigra, reticular part;SuG, superficial gray layer of the superior colliculus;Te, temporal cortex; VTA, ventral tegmental area; xscp, decussation of the superior cerebellar peduncle.
Fig. 11.
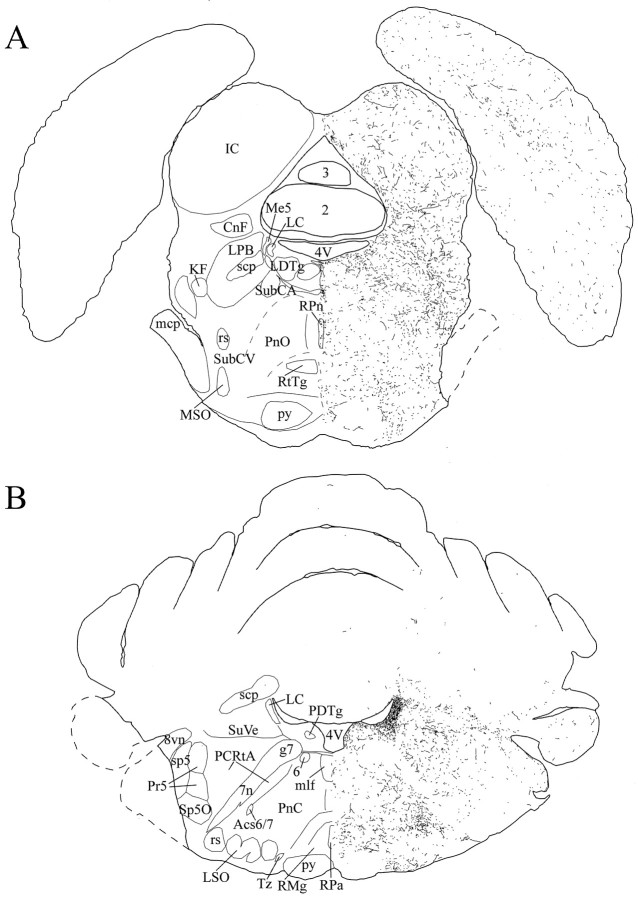
Schematic drawings of 20 μm rostrocaudal coronal sections illustrating the distribution and relative density of hcrt fibers in the pons after immunohistochemistry for hcrt using antibody #2050. 2,3, Cerebellar lobules;4V, 4th ventricle; 6, abducens nucleus;7n, facial nerve or its root; 8vn, vestibular root, vestibulocochlear nerve; Acs6/7, accessory abducens and facial nuclei; CnF, cuneiform nucleus; g7, genu of the facial nerve;IC, inferior colliculus; KF, Kölliker–Fuse nucleus; LC, locus coeruleus;LDTg, laterodorsal tegmental nucleus;LPB, lateral parabrachial nucleus; LSO, lateral superior olive;mcp, middle cerebellar peduncle; Me5, mesencephalic trigeminal nucleus; mlf, medial longitudinal fasciculus; MSO, medial superior olive;PCRtA, parvocellular reticular nucleus, α part;PDTg, posterodorsal tegmental nucleus;PnC, pontine reticular nucleus, caudal part;PnO, pontine reticular nucleus, oral part;Pr5, principal sensory trigeminal nucleus;py, pyramidal tract; RMg, raphe magnus nucleus; RPa, raphe pallidus nucleus;RPn, raphe pontis nucleus; rs, rubrospinal tract; RtTg, reticulotegmental nucleus of the pons; scp, superior cerebellar peduncle;sp5, spinal trigeminal tract; Sp5O, spinal trigeminal nucleus, oral part; SubCA, subcoeruleus nucleus, α part; SubCV, subcoeruleus nucleus, ventral part; SuVe, superior vestibular nucleus; Tz, nucleus of the trapezoid body.
Fig. 12.
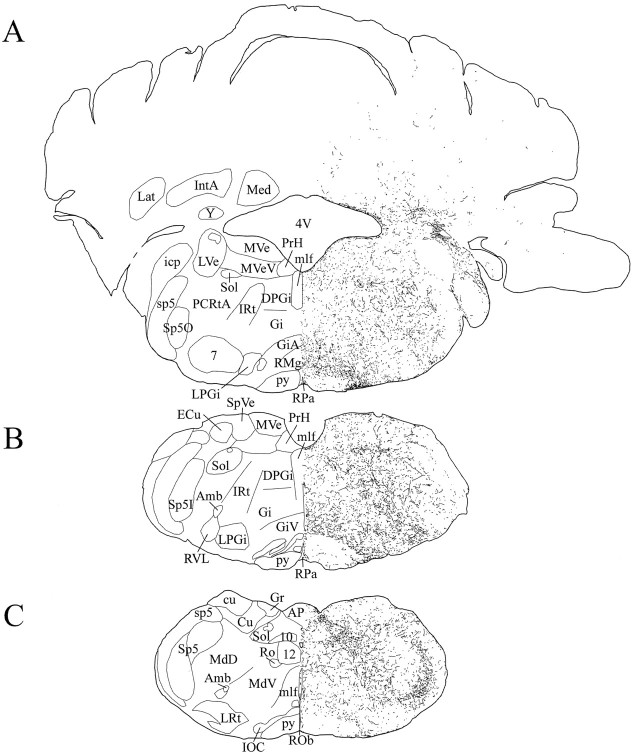
Schematic drawings of 20 μm rostrocaudal coronal sections illustrating the distribution and relative density of hcrt fibers in the medulla after immunohistochemistry for hcrt using antibody #2050. 4V, 4th ventricle; 7, facial nucleus; 10, dorsal motor nucleus of vagus;12, hypoglossal nucleus; Amb, ambiguus nucleus; AP, area postrema;cu, cuneate fasciculus; Cu, cuneate nucleus; DPGi, dorsal paragigantocellular nucleus;ECu, external cuneate nucleus; Gi, gigantocellular reticular nucleus; GiA, gigantocellular reticular nucleus, α part; GiV, gigantocellular reticular nucleus, ventral part; Gr, gracile nucleus;icp, inferior cerebellar peduncle; IntA, interposed cerebellar nucleus, anterior part; IOC, inferior olive, subnucleus C of medial nucleus; IRt, intermediate reticular nucleus; Lat, lateral cerebellar nucleus; LPGi, lateral paragigantocellular nucleus;LRt, lateral reticular nucleus; LVe, lateral vestibular nucleus; MdD, medullary reticular nucleus, dorsal part; MdV, medullary reticular nucleus, ventral part; Med, medial cerebellar nucleus;mlf, medial longitudinal fasciculus; MVe, medial vestibular nucleus; MVeV, medial vestibular nucleus, ventral part; PCRtA, parvocellular reticular nucleus, α part; PrH, prepositus hypoglossal nucleus;py, pyramidal tract; RMg, raphe magnus nucleus; Ro, nucleus of Roller; ROb, raphe obscurus nucleus; RPa, raphe pallidus nucleus;RVL, rostroventrolateral reticular nucleus;Sol, nucleus of the solitary tract; sp5, spinal trigeminal tract; Sp5, spinal trigeminal nucleus;Sp5I, spinal trigeminal nucleus, interpolar part;Sp5O, spinal trigeminal nucleus, oral part;SpVe, spinal vestibular nucleus; Y, nucleus Y.
Table 1.
Relative density of hypocretin-immunoreactive fibers in various regions of the rat brain
| Brain region | Fiber density1-a |
|---|---|
| Cortex | |
| Layers 1–3 | ++ |
| Layer 4 | +(+) |
| Layers 5–6 | ++(+) |
| Olfactory bulb | |
| Main bulb | − |
| Anterior olfactory nuclei | + |
| Endopiriform nucleus | ++ |
| Claustrum | ++ |
| Tenia tecta | + |
| Hippocampus | + |
| Amygdala | |
| Amygdalohippocampal area | + |
| Anterior amygdaloid area | +++ |
| Basolateral nucleus | + |
| Basomedial nucleus | + |
| Cortical nucleus | + |
| Central nucleus | +++ |
| Intercalated nuclei | + |
| Lateral nucleus | + |
| Medial nucleus | ++ |
| Septum | |
| Lateral nucleus | +++ |
| Medial nucleus | +++ |
| Septofimbrial nucleus | ++ |
| Subfornical organ | − |
| Diagonal band of Broca | |
| Horizontal limb | ++ |
| Vertical limb | +++ |
| Bed nucleus of stria terminalis | |
| Lateral nucleus | +++ |
| Medial nucleus | +++ |
| Ventral nucleus | ++++ |
| Posteromedial nucleus | +++ |
| Posterolateral nucleus | ++++ |
| Basal ganglia | |
| Accumbens nucleus, core | + |
| Accumbens nucleus, shell | ++ |
| Caudate putamen | − |
| Globus pallidus | − |
| Fundus of the striatum | +++ |
| Thalamus | |
| Anteroventral thalamic nucleus | − |
| Anterior pretectal nucleus | − |
| Central medial nucleus | ++++ |
| Medial habenular nucleus | − |
| Mediodorsal thalamic nucleus | − |
| Lateral habenular nucleus | +++ |
| Laterodorsal nucleus | + |
| Paraventricular nucleus | ++++ |
| Reuniens nucleus | ++ |
| Rhomboid nucleus | ++ |
| Ventrolateral nucleus | − |
| Ventroposteriomedial nucleus | − |
| Ventroposteriolateral nucleus | − |
| Lateral geniculate nucleus | − |
| Medial geniculate nucleus | − |
| Zona incerta, rostral | ++++ |
| Zona incerta, caudal | ++ |
| Subincertal nucleus | ++++ |
| Subthalamic nucleus | ++++ |
| Subparafascicular thalamic nucleus | ++++ |
| Parafascicular nucleus | ++ |
| Central gray of the thalamus | ++++ |
| Anterior hypothalamus | |
| Medial preoptic area | +++ |
| Medial preoptic nucleus | ++ |
| Lateral preoptic nucleus | +++ |
| Magnocellular preoptic nucleus | +++ |
| Supraoptic nucleus | − |
| Suprachiasmatic nucleus | − |
| Anterior hypothalamic area, anterior part | ++ |
| Lateroanterior nucleus | +++ |
| Substantia innominata | ++++ |
| Ventral pallidum | +++ |
| Posterior hypothalamus | |
| Paraventricular nucleus | +++ |
| Ventromedial nucleus, rostral | ++ |
| Ventromedial nucleus, caudal | ++++ |
| Dorsomedial nucleus | ++++ |
| Dorsal hypothalamic area | ++++ |
| Lateral hypothalamic area | ++++ |
| Tuberum cinereum | ++++ |
| Tuberomammillary nucleus | ++++ |
| Perifornical nucleus | +++++ |
| Posterior hypothalamic area | ++++ |
| Arcuate nucleus | ++++ |
| Submammillary nucleus | ++++ |
| Mammillary bodies | − |
| Pineal gland | − |
| Midbrain | |
| Substantia nigra, compact part | ++++ |
| Substantia nigra, reticular part | − |
| Substantia nigra, lateral part | +++ |
| Ventral tegmental area | +++ |
| Central gray | ++++ |
| Red nucleus | − |
| Mesencephalic reticular formation | ++ |
| Interpeduncular nucleus | +++ |
| Brainstem | |
| Oculomotor nuclei | |
| Abducens nucleus (VI) | − |
| Oculomotor nucleus (III) | − |
| Trochlear motor nucleus (IV) | − |
| Trigeminal motor nucleus (V) | + |
| Facial motor nucleus (VII) | + |
| Motor nucleus of the vagus (X) | + |
| Hypoglossal nucleus (XII) | + |
| Somatosensory nuclei | |
| Superior colliculus | ++ |
| Inferior colliculus | ++ |
| Mesencephalic nucleus | − |
| Parabigeminal nucleus | + |
| Olivary nuclei | − |
| Cochlear nucleus, dorsal | ++ |
| Cochlear nucleus, ventral | − |
| Lateral lemniscus | +++ |
| Trapezoid body | − |
| Vestibular nuclei | ++ |
| Nucleus of the solitary tract | ++++ |
| Area postrema | ++ |
| Parabrachial nucleus | +++ |
| Kölliker-Fuse nucleus | +++ |
| Spinal trigeminal nucleus | − |
| Spinal trigeminal nucleus, gelatinous layer of the caudal part | +++ |
| Central gray nuclei | |
| Nucleus of Darkschwitsch | − |
| Anterior tegmental nucleus | + |
| Ventral tegmental nucleus | + |
| Dorsal tegmental nucleus | − |
| Laterodorsal tegmental nucleus | +++ |
| Barrington’s nucleus | +++ |
| Locus coeruleus | +++++ |
| Periaqueductal gray | ++++ |
| Raphe nuclei | |
| Raphe dorsalis | ++++ |
| Raphe median | +++ |
| Raphe magnus | ++++ |
| Raphe pallidus | ++ |
| Raphe obscurus | ++ |
| Raphe linearis | ++ |
| Raphe pontis | ++ |
| B9 | +++ |
| Reticular formation | |
| Central tegmental field | ++ |
| Pedunculopontine nucleus | +++ |
| Cuneiform nucleus | ++ |
| Parvocellular reticular area (and its α part) | +++ |
| Gigantocellular reticular area | ++ |
| Gigantocellular reticular area, α part | +++ |
| Gigantocellular reticular area, ventral part | ++++ |
| Dorsal paragigantocellular reticular area | ++ |
| Lateral paragigantocellular reticular area | +++ |
| Intermediate reticular field | +++ |
| Lateral reticular nucleus | + |
| Medullary reticular nuclei, dorsal part | ++++ |
| Medullary reticular nuclei, ventral part | ++ |
| Pontine nuclei (oral, ventral, and caudal) | + |
| Other brainstem nuclei | |
| Nucleus prepositus hypoglossi | ++ |
| Rostroventrolateral medulla | +++ |
| Caudoventrolateral medulla | ++ |
| Nucleus subcoeruleus (α, ventral, and dorsal) | ++ |
| Cerebellum (cortex and nuclei) | − |
| Ependyma | ++ |
Rating scale: +++++, greatest fiber density; +, lowest fiber density; and −, insignificant number of fibers.
Fig. 13.
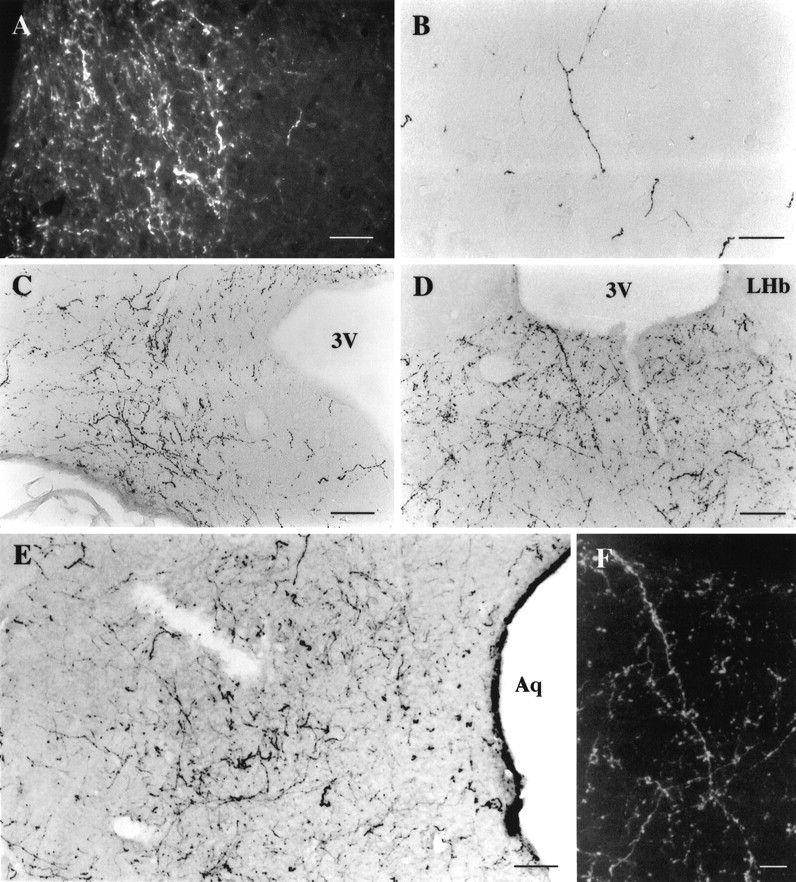
Photomicrographs of hcrt immunoreactive axons in the rat brain using the antiserum #2123. A, Dark-field illustration of thick hcrt fibers (in white) located in the locus coeruleus, lateral to the 4th ventricle. Notice that fibers are restricted to the locus and contain numerous boutons.B, Photomicrograph showing that hcrt-IR fibers were mainly long with varicosities. The density of fibers was relatively low in all cortical areas as shown in this picture of the frontal cortex.C, Illustration of the numerous long and thick fibers seen in the caudal part of the arcuate nucleus. Hcrt fibers contain numerous boutons. D, F, Photomicrographs illustrating one of the main projections for hcrt neurons, the paraventricular nucleus of the thalamus. Fibers were long with numerous varicosities as illustrated in F on a dark-field enlargement of D. E, Photomicrograph showing the high density of varicose fibers located in the lateral periaqueductal gray at the level of the dorsal raphe nucleus. Scale bars: A, 36 μm; B–D, 70 μm; E, 50 μm; F, 25 μm.3V, 3rd ventricle; Aq, Aqueduct;LHb, lateral habenular nucleus.
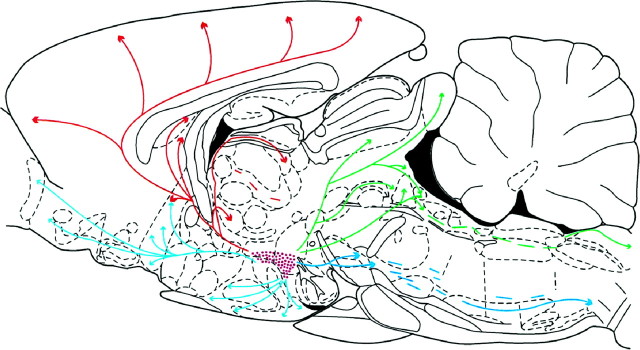
The tracts taken by fibers out of the hypothalamus are subjectively subdivided into four different pathways: dorsal and ventral ascending pathways and dorsal and ventral descending pathways.
Dorsal ascending pathway
Hcrt neurons sent axons through the zona incerta to the paraventricular nucleus of the thalamus (anterior and posterior part) (Fig. 13D,F), the central medial nucleus of the thalamus, and the lateral habenula, avoiding the other thalamic nuclei (see Table 1). Hcrt fibers were also found in the substantia innominata, the bed nucleus of the stria terminalis, the septal nuclei (medial and lateral), and the dorsal anterior nucleus of the olfactory bulb (Fig. 5). Fibers in these nuclei were long and thick with numerous boutons. Hcrt axons going through this pathway avoided the caudate putamen and the globus pallidus and innervated the cortex where fibers were mainly long and thin with varicosities (Fig.13B). Although they were widespread in the cortex, fibers were slightly denser and shorter at the border of the corpus callosum. Axons were also sent more laterally through the zona incerta, the subincertal nucleus of the thalamus, and the subthalamic nucleus following the optic tract to the central, anterior, and medial amygdaloid nuclei (Fig. 8).
Ventral ascending pathway
Hcrt fibers were found in the ventral pallidum, the vertical and horizontal limb of the diagonal band of Broca, the medial part of the accumbens nucleus, and the olfactory bulb. In the olfactory system, fibers were mainly seen in the anterior olfactory nuclei (Fig. 5). A few fibers were observed in the glomerular layer and the internal granular layer, but no fibers were seen in the mitral cell layer (Fig.5).
Dorsal descending pathway
Hcrt fibers were directed up through the mesencephalic central gray to innervate the colliculi and the pontine central gray, particularly the locus coeruleus, the dorsal raphe nucleus, and the laterodorsal tegmental nucleus (Figs. 10, 11). In these nuclei, hcrt fibers were long and thick with numerous boutons. Hcrt fibers also went through the dorsal tegmental area to the pedunculopontine nucleus, the parabrachial nucleus, and the dorsal and α subcoeruleus area (Fig.11). Then, avoiding the vestibular nuclei, they descended to the dorsolateral part of the nucleus of the solitary tract and the parvocellular reticular area and more caudally to the dorsal medullary area and the gelatinous layer of the caudal spinal trigeminal nucleus (Fig. 12). In these last structures, fibers were long and thick with numerous varicosities. Along this pathway, hcrt fibers avoided the geniculate nuclei, the trigeminal motor nucleus, and the spinal trigeminal nuclei.
Ventral descending pathway
Hcrt fibers went through the interpeduncular nucleus, the ventral tegmental area, and the substantia nigra pars compacta (Fig. 10). Hcrt fibers in these nuclei were long with boutons. In the pons and medulla, hcrt fibers were distributed through the raphe nuclei and the reticular formation in the pontis oralis, caudal and ventral, the ventral and α gigantocellular reticular nuclei, and the ventral medullary area (Figs.10-12). Hcrt fibers were particularly dense in the raphe magnus, the lateral paragigantocellular nucleus, and the ventral subcoeruleus area where the A5 noradrenergic cell group is located (Figs. 11, 12). However, fibers avoided several nuclei implicated in motor functions such as the red nucleus, the pontine nucleus, and the facial motor nucleus, as well as auditory structures such as the trapezoid body and the superior and inferior olive nuclei (Figs. 10-12).
DISCUSSION
In the present work, we used two antibodies, one against the 17 aa C-terminal part of preprohypocretin and one against the entire preprohypocretin molecule. With each antiserum, we obtained a strong signal with minimal background. The preabsorption experiments, the consistency of staining with both antibodies, and the agreement within situ hybridization, as well as the double staining of hcrt-positive cells by both in situ hybridization and immunohistochemistry, showed that both antibodies are highly specific to preprohypocretin. The immunostaining pattern of these preprohypocretin antisera was similar to that of an antibody raised against the active peptide, hypocretin-2 (van den Pol et al., 1998), suggesting that the immumoreactive cells and fibers described in the present study represent the distribution pattern of the neuroactive peptide.
We found that hcrt-containing neurons are restricted to a subregion of the hypothalamus. They were located in the perifornical nucleus, the dorsomedial hypothalamic nucleus, and the dorsal and lateral hypothalamic areas. A few cells were seen in the posterior hypothalamic area and the subincertal nucleus. Sakurai et al. (1998) described orexin (hcrt) neurons as a discrete set of cells in the hypothalamic and subthalamic areas such as the zona incerta and the subincertal and the subthalamic nuclei. In our study, we conducted immunohistochemistry with a color-precipitation reaction, and we counterstained the labeled sections with neutral red. Therefore, we were able to identify directly structures containing the labeled neurons and found that hcrt neurons were just ventral to the zona incerta and rostral to the subthalamic nucleus.
We found that ∼50% of the hcrt cells are located in the perifornical nucleus at the tuberal level of the hypothalamus. Some of the hcrt projections observed are similar to those described previously as projections of the perifornical nucleus such as those to the dorsal, dorsomedial, lateral, and posterior hypothalamic areas, the tuberomammillary nuclei, the basal forebrain bundle, the bed nucleus of the stria terminalis, the substantia innominata, the paraventricular nuclei of the thalamus and hypothalamus, the central nucleus of the amygdala, the arcuate nucleus, the central gray, the dorsal and median raphe nuclei, the laterodorsal tegmental nucleus, the locus coeruleus, Barrington’s nucleus, the reticular formation, and the nucleus of the solitary tract (Saper et al., 1976; Allen and Cechetto, 1992, 1993;Valentino et al., 1994; Luppi et al., 1995; Touzani et al., 1996;Peyron et al., 1998). However, we also observed hcrt efferent projections that were not described as projections from the perifornical nucleus such as those to the olfactory bulb, the ventral pallidum, the compact part of the substantia nigra, the ventral tegmental area, and the nucleus raphe magnus. Furthermore, Allen and Cechetto (1992, 1993) report projections from the perifornical nucleus to the mediodorsal thalamic nucleus, the ventral premammillary nucleus, and the dorsal tegmental nucleus that we did not observe; these projections are therefore unlikely to use hcrt as a neurotransmitter and/or neuromodulator. Consequently, the hcrt cell group is a unique system and not simply a subset of the perifornical nucleus.
Although hcrt-containing neurons represent a relatively small number of cells, their projections are widely distributed in the CNS (Fig.14), suggesting that hcrt might be involved in multiple functions as we discuss in the following paragraphs.
Fig. 14.
Schematic summary drawing of pathways taken by hcrt processes that widely innervate rat brain. The sagittal section used is taken from the atlas of Paxinos and Watson (1986).Purple dots: Hypocretin-labeled neurons;red: dorsal ascending pathway; light blue: ventral ascending pathway; green: dorsal descending pathway; dark blue: ventral descending pathway.
Feeding
The perifornical nucleus contains ∼50% of the hcrt-labeled neurons. This nucleus has been shown to be intimately involved in the neural control of food intake (Winn et al., 1984; Stanley et al., 1996) and is the most sensitive brain region for both the orexigenic effect of neuropeptide Y (Stanley et al., 1993) and the suppressive effects of catecholamines on feeding (Leibowitz and Stanley, 1986). Neurons in the perifornical nucleus and the lateral hypothalamic area also respond to internal signals related to the nutritional state of the animal (Himmi et al., 1988). We observed hcrt fibers in nuclei that are known to be involved in the regulation of food intake such as the ventromedial hypothalamic nucleus, the arcuate nucleus, the paraventricular nucleus of the hypothalamus, the parabrachial area, the nucleus of the solitary tract, and the area postrema (Leibowitz and Brown, 1980; Roberts, 1980;Stanley and Leibowitz, 1984; Luiten et al., 1987; Ritter and Stone, 1987; Akabayashi et al., 1994; Ritter et al., 1994; Nishijo and Norgren, 1997; Shimura et al., 1997). Taken together, these data suggest that hcrt might be involved in the regulation of feeding. Indeed, Sakurai et al. (1998) recently showed that orexin A (hcrt-1) and B (hcrt-2) stimulate food intake when injected intracerebroventricularly and that the mRNA accumulates during fasting.
MCH, whose cell bodies are also found in the tuberal region of the hypothalamus (Skofitsch et al., 1985; Nahon et al., 1989; Bittencourt et al., 1992), has also been reported to have potent orexigenic activity (Presse et al., 1996; Qu et al., 1996; Rossi et al., 1997). Based on the absence of colocalization (Fig.2E,F), MCH and hcrt cells are distinct neuronal populations although they are partly intermingled anatomically. MCH and hcrt neurons innervate some of the same brain regions such as the medial septum/diagonal band, the bed nucleus of the stria terminalis, the zona incerta, the entire hypothalamus, the arcuate nucleus, the ventral tegmental area, the periaqueductal gray, the locus coeruleus, and the nucleus of the solitary tract (Bittencourt et al., 1992), suggesting that MCH and hcrt might be involved in the same physiological functions. Håkansson et al. (1998) showed that leptin receptors are located in the perifornical nucleus and the lateral hypothalamic area and are found on MCH neurons as well as other cell types. Whether leptin receptors are also present on hcrt cells remains to be determined.
Blood pressure regulation
Electrical or chemical stimulation of the perifornical nucleus increases blood pressure and heart rate and activates neurons of the lateral paragigantocellular area (Sun and Guyenet, 1986; Allen and Cechetto, 1992). The perifornical nucleus and adjacent lateral hypothalamic area have been identified as the source of neurons responsible for producing cardiovascular responses associated with emotion (Smith et al., 1990). In our study, we found hcrt fibers located in nuclei that are well known to be involved in blood pressure regulation such as the rostral ventrolateral medulla, the lateral paragigantocellular nucleus, the locus coeruleus, the nucleus of the solitary tract, the midbrain periaqueductal gray, the A5 noradrenergic cell group, the parabrachial region, and the area postrema (for review, see Dampney, 1994). Furthermore, the perifornical nucleus receives ascending afferent inputs mainly from the ventromedial central gray, the dorsal raphe nucleus, the laterodorsal tegmental region, and the nucleus of the solitary tract (Allen and Cechetto, 1992). These brainstem structures are known to be closely associated with cardiovascular function (Lindgren, 1961; Reis and Cuénod, 1965;Calaresu and Ciriello, 1980; for review, see Dampney, 1994). Consequently, our results combined with the literature suggest that hcrt could be involved in the regulation of blood pressure.
Neuroendocrine regulation
The arcuate nucleus, highly involved in neuroendocrine regulation, is among the most densely hcrt-innervated areas of the hypothalamus, suggesting that hcrt might also be involved in hormonal regulation. This hypothesis has recently been tested electrophysiologically in hypothalamic slices containing the arcuate nucleus and the median eminence (van del Pol et al., 1998). Application of hcrt increased presynaptic activity of GABAergic cells terminating on electrophysiologically identified neuroendocrine neurons. These data indicate that hcrt modulates the afferent input controlling the neuroendocrine neurons in the arcuate nucleus. Furthermore, the paraventricular nucleus of the hypothalamus (PVN) is innervated by the perifornical nucleus (Allen and Cechetto, 1993; Larsen et al., 1994). The perifornical nucleus, along with the dorsomedial hypothalamic nucleus, provides most of the local excitatory synaptic input to PVN neurons (Boudaba et al., 1997). We have shown previously that hcrt has an excitatory effect on hypothalamic cells in vitro (de Lecea et al., 1998) and, in the present study, that the perifornical input to the PVN includes hcrt axons. These data suggest that hcrt might also modulate hormonal release controlled by PVN neurons.
Thermoregulation
The lateral hypothalamic area, in which we observed hcrt-labeled cells, has been implicated in the behavioral regulation of temperature (Corbett et al., 1988). The lateral hypothalamic area is also involved in heat loss responses, and the neural pathway involved in shivering passes through this region (Hemingway, 1963). The presence of hcrt fibers in the raphe magnus and the subcoeruleus areas, brain regions that also have been implicated in thermoregulation (Werner and Bienck, 1990), suggests that hcrt could modulate the regulation of body temperature.
Sleep–waking cycle
Neurons in the lateral hypothalamic area have been involved in the maintenance of waking state (Vanni-Mercier et al., 1984). Our results showed that hcrt fibers were seen in numerous structures involved in the sleep–waking cycle such as the locus coeruleus, the tuberomammillary nucleus, the pontine reticular formation, the raphe nuclei, the preoptic area, and the laterodorsal tegmental nucleus (for review, see Vertes, 1990; Jones, 1994). The high density of fibers in the locus coeruleus and the tuberomammillary nucleus of the hypothalamus, nuclei containing neurons responsible for the maintenance of the waking state, suggests that hcrt might have an effect on arousal.
Conclusion
The hypothalamus has a major role in regulating various behaviors that contribute to homeostasis such as arousal, feeding, and thermoregulation by integrating external and internal stimuli (Simerly, 1995). As discussed above, hcrt could be involved in the regulation of feeding, blood pressure, hormonal release, temperature, and arousal. Animals encounter situations that require or favor coordinated responses of several autonomic systems. We suggest that the hypocretins could provide such a coordinating signal.
Footnotes
This work was supported by National Institutes of Health Grants AG11084, GM32355, NS33396, and NS34887 and by Air Force Office of Scientific Research, the Army Research Office, and the Fyssen Fondation. We thank Dr. Peter O’Hara for use of his Neurolucida system (MicroBrightField, Colchester, VT) in the analysis of the distribution of hypocretin-immunoreactive fibers. We also thank Drs. Y. Yang for technical assistance with the electron microscopy, Chiaki Fukuhara who made the preprohypocretin used to raise antisera, Masashi Yanagisawa for the gift of orexin A, and Jean-Louis Nahon for the gift of melanin-concentrating hormone cDNA plasmid.
Correspondence should be addressed to Dr. Christelle Peyron, Department of Biological Sciences, Gilbert Hall, 371 Serra Mall, Stanford University, Stanford, CA 94305-5020.
REFERENCES
- 1.Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 2.Akabayashi A, Wahlestedt C, Alexander JT, Leibowitz SF. Specific inhibition of endogenous neuropeptide Y synthesis in arcuate nucleus by antisense oligonucleotides suppresses feeding behavior and insulin secretion. Mol Brain Res. 1994;21:55–61. doi: 10.1016/0169-328x(94)90377-8. [DOI] [PubMed] [Google Scholar]
- 3.Allen GV, Cechetto DF. Functional and anatomical organization of cardiovascular pressor and depressor sites in the lateral hypothalamic area: I. Descending projections. J Comp Neurol. 1992;315:313–332. doi: 10.1002/cne.903150307. [DOI] [PubMed] [Google Scholar]
- 4.Allen GV, Cechetto DF. Functional and anatomical organization of cardiovascular pressor and depressor sites in the lateral hypothalamic area: II. Ascending projections. J Comp Neurol. 1993;330:421–438. doi: 10.1002/cne.903300310. [DOI] [PubMed] [Google Scholar]
- 5.Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- 6.Boudaba C, Schrader LA, Tasker JG. Physiological evidence for local excitatory synaptic circuits in the rat hypothalamus. J Neurophysiol. 1997;77:3396–3400. doi: 10.1152/jn.1997.77.6.3396. [DOI] [PubMed] [Google Scholar]
- 7.Calaresu FR, Ciriello J. Projections to the hypothalamus from buffer nerves and nucleus tractus solitarius in the cat. Am J Physiol. 1980;239:R130–R136. doi: 10.1152/ajpregu.1980.239.1.R130. [DOI] [PubMed] [Google Scholar]
- 8.Chiu K-P, Sullivan T, Bursztajn S. Improved in situ hybridization: color intensity enhancement procedure for the alkaline phosphatase/Fast red system. Biotechniques. 1996;20:964–968. doi: 10.2144/96206bm04. [DOI] [PubMed] [Google Scholar]
- 9.Corbett SW, Kaufman LN, Keesey RE. Thermogenesis after lateral hypothalamic lesions: contributions of brown adipose tissue. Am J Physiol. 1988;255:E708–E715. doi: 10.1152/ajpendo.1988.255.5.E708. [DOI] [PubMed] [Google Scholar]
- 10.Dampney RAL. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 11.de Lecea L, Kilduff TS, Peyron C, Gao X-B, Foye PE, Danielson PE, Fukuhara C, Battenberg ELF, Gautvik VT, Bartlett FS, II, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gautvik KM, de Lecea L, Gautvik VT, Danielson PE, Tranque P, Dopazo A, Bloom FE, Sutcliffe JG. Overview of the most prevalent hypothalamus-specific mRNAs, as identified by directional tag PCR subtraction. Proc Natl Acad Sci USA. 1996;93:8733–8738. doi: 10.1073/pnas.93.16.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Håkansson ML, Brown H, Ghilardi N, Skoda RC, Meister B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci. 1998;18:559–572. doi: 10.1523/JNEUROSCI.18-01-00559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemingway A. Shivering. Physiol Rev. 1963;43:397–422. doi: 10.1152/physrev.1963.43.3.397. [DOI] [PubMed] [Google Scholar]
- 15.Himmi T, Boyer A, Orsini JC. Changes in lateral hypothalamic neuronal activity accompanying hyper- and hypoglycemias. Physiol Behav. 1988;44:347–354. doi: 10.1016/0031-9384(88)90036-4. [DOI] [PubMed] [Google Scholar]
- 16.Jones BE. Basic mechanisms of sleep-waking states. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine, 2nd Edition. Saunders; Philadelphia: 1994. pp. 145–162. [Google Scholar]
- 17.Larsen PJ, Hay-Schmidt A, Mikkelsen JD. Efferent connections from the lateral hypothalamic region and the lateral preoptic area to the hypothalamic paraventricular nucleus of the rat. J Comp Neurol. 1994;342:299–319. doi: 10.1002/cne.903420211. [DOI] [PubMed] [Google Scholar]
- 18.Leibowitz SF, Brown LL. Histochemical and pharmacological analysis of noradrenergic projections to the paraventricular hypothalamus in relation to feeding stimulation. Brain Res. 1980;201:289–314. doi: 10.1016/0006-8993(80)91037-9. [DOI] [PubMed] [Google Scholar]
- 19.Leibowitz SF, Stanley BG. Neurochemical controls of appetite. In: Ritter RC, Ritter S, Barnes CD, editors. Feeding behavior: neural and humoral controls. Academic; Orlando, FL: 1986. pp. 191–234. [Google Scholar]
- 20.Lindgren P. Localization and function of the medullary vasomotor center in intracollicularly decerebrated cats. Circ Res. 1961;9:250–255. doi: 10.1161/01.res.9.2.250. [DOI] [PubMed] [Google Scholar]
- 21.Luiten PGM, ter Horst GJ, Steffens AB. The hypothalamus, intrinsic connections and outflow pathways to the endocrine system in relation to the control of feeding and metabolism. Prog Neurobiol. 1987;28:1–54. doi: 10.1016/0301-0082(87)90004-9. [DOI] [PubMed] [Google Scholar]
- 22.Luppi P-H, Aston-Jones G, Akaoka H, Chouvet G, Jouvet M. Afferent projections to the rat locus coeruleus demonstrated by retrograde and anterograde tracing with cholera-toxin B subunit and Phaseolus vulgaris leucoagglutinin. Neuroscience. 1995;65:119–160. doi: 10.1016/0306-4522(94)00481-j. [DOI] [PubMed] [Google Scholar]
- 23.Nahon JL, Presse F, Bittencourt JC, Sawchenko PE, Vale W. The rat melanin-concentrating hormone messenger ribonucleic acid encodes multiple putative neuropeptides coexpressed in the dorsolateral hypothalamus. Endocrinology. 1989;125:2056–2065. doi: 10.1210/endo-125-4-2056. [DOI] [PubMed] [Google Scholar]
- 24.Nishijo H, Norgren R. Parabrachial neural coding of taste stimuli in awake rats. J Neurophysiol. 1997;78:2254–2268. doi: 10.1152/jn.1997.78.5.2254. [DOI] [PubMed] [Google Scholar]
- 25.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 2nd Edition. Academic; Sydney: 1986. [Google Scholar]
- 26.Peyron C, Tighe DK, Lee BS, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Distribution of immunoreactive neurons and fibers for a hypothalamic neuropeptide precursor related to secretin. Soc Neurosci Abstr. 1997;23:2032. [Google Scholar]
- 27.Peyron C, Petit J-M, Rampon C, Jouvet M, Luppi P-H. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- 28.Presse F, Sorokvsky I, Max JP, Nicolaidis S, Nahon JL. Melanin-concentrating hormone is a potent anorectic peptide regulated by food-deprivation and glucopenia in the rat. Neuroscience. 1996;71:735–745. doi: 10.1016/0306-4522(95)00481-5. [DOI] [PubMed] [Google Scholar]
- 29.Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek J, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 30.Reis DJ, Cuénod M. Central neural regulation of carotid baroreceptor reflexes in the cat. Am J Physiol. 1965;209:1267–1279. doi: 10.1152/ajplegacy.1965.209.6.1267. [DOI] [PubMed] [Google Scholar]
- 31.Ritter S, Stone SL. Area postrema lesions block feeding induced by systemic injections of monosodium glutamate. Physiol Behav. 1987;41:21–24. doi: 10.1016/0031-9384(87)90125-9. [DOI] [PubMed] [Google Scholar]
- 32.Ritter S, Dinh TT, Friedman MI. Induction of Fos-like immunoreactivity (Fos-li) and stimulation of feeding by 2,5-anhydro-d-mannitol (2,5-AM) require the vagus nerve. Brain Res. 1994;646:53–64. doi: 10.1016/0006-8993(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 33.Roberts WW. [14C]Deoxyglucose mapping of first-order projections activated by stimulation of lateral hypothalamic sites eliciting gnawing, eating, and drinking in rats. J Comp Neurol. 1980;194:617–638. doi: 10.1002/cne.901940309. [DOI] [PubMed] [Google Scholar]
- 34.Rossi M, Choi SJ, O’Shea D, Miyoshi T, Ghatei MA, Bloom SR. Melanin-concentrating hormone acutely stimulates feeding, but chronic administration has no effect on body weight. Endocrinology. 1997;138:351–355. doi: 10.1210/endo.138.1.4887. [DOI] [PubMed] [Google Scholar]
- 35.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JRS, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu W-S, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 36.Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976;117:305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- 37.Shimura T, Norgren R, Grigson PS, Norgren R. Brainstem lesions and gustatory function: I. The role of the nucleus of the solitary tract during a brief intake test in rats. Behav Neurosci. 1997;111:155–168. [PubMed] [Google Scholar]
- 38.Simerly RB. Anatomical substrates of hypothalamic integration. In: Paxinos G, editor. The rat nervous system, 2nd Edition. Academic; 1995. pp. 353–376. [Google Scholar]
- 39.Skofitsch G, Jacobowitz DM, Zamir N. Immunohistochemical localization of a melanin concentrating hormone-like peptide in the rat brain. Brain Res Bull. 1985;15:635–645. doi: 10.1016/0361-9230(85)90213-8. [DOI] [PubMed] [Google Scholar]
- 40.Smith OA, DeVito JL, Astley CA. Neurons controlling cardiovascular responses to emotion are located in lateral hypothalamus-perifornical region. Am J Physiol. 1990;259:R943–R954. doi: 10.1152/ajpregu.1990.259.5.R943. [DOI] [PubMed] [Google Scholar]
- 41.Stanley BG, Leibowitz SF. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 1984;35:2635–2642. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- 42.Stanley BG, Magdalin W, Seirafi A, Thomas W, Leibowitz SF. The perifornical area: the major focus of (a) patchily distributed hypothalamic neuropeptide Y sensitive feeding system(s). Brain Res. 1993;604:304–317. doi: 10.1016/0006-8993(93)90382-w. [DOI] [PubMed] [Google Scholar]
- 43.Stanley BG, Willett VL, III, Donias HW, Dee MG, II, Duva MA. Lateral hypothalamic NMDA receptors and glutamate physiological mediators of eating and weight control. Am J Physiol. 1996;270:R443–R449. doi: 10.1152/ajpregu.1996.270.2.R443. [DOI] [PubMed] [Google Scholar]
- 44.Sun MK, Guyenet PG. Hypothalamic glutamatergic input to medullary sympathoexcitatory neurons in rats. Am J Physiol. 1986;251:R798–R810. doi: 10.1152/ajpregu.1986.251.4.R798. [DOI] [PubMed] [Google Scholar]
- 45.Sutcliffe JG, Milner RJ, Shinnick TM, Bloom FE. Identifying the protein products of brain-specific genes with antibodies to chemically synthesized peptides. Cell. 1983;33:671–682. doi: 10.1016/0092-8674(83)90010-7. [DOI] [PubMed] [Google Scholar]
- 46.Sutcliffe JG, Gautvik KM, Kilduff TS, Horn T, Foye PE, Danielson PE, Frankel WN, Bloom FE, de Lecea L. Two novel hypothalamic peptides related to secretin derived from a single neuropeptide precursor. Soc Neurosci Abstr. 1997;23:2032. [Google Scholar]
- 47.Swanson LW. The hypothalamus. In: Björklund A, Hökfelt T, Swanson LW, editors. Handbook of chemical neuroanatomy, Vol 5, Integrated systems of the CNS. Elsevier; Amsterdam: 1987. pp. 1–124. [Google Scholar]
- 48.Touzani K, Taghzouti K, Velley L. Cellular organization of lateral hypothalamic efferents to the central amygdaloid nucleus of the rat. NeuroReport. 1996;7:517–520. doi: 10.1097/00001756-199601310-00034. [DOI] [PubMed] [Google Scholar]
- 49.Valentino RJ, Page ME, Luppi P-H, Zhu Y, Van Bockstaele E, Aston-Jones G. Evidence for widespread afferents to Barrington’s nucleus, a brainstem region rich in corticotropin-releasing hormone neurons. Neuroscience. 1994;62:125–143. doi: 10.1016/0306-4522(94)90320-4. [DOI] [PubMed] [Google Scholar]
- 50.van den Pol AN, Gao X-B, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vanni-Mercier G, Sakai K, Salvert D, Jouvet M. “Waking state specific” neurons in the caudal hypothalamus of the cat. C R Acad Sci Paris. 1984;298:195–200. [PubMed] [Google Scholar]
- 52.Vertes RP. Brainstem mechanisms of slow wave sleep and REM sleep. In: Klemm WR, Vertes RP, editors. Brainstem mechanisms of behavior. Wiley; New York: 1990. pp. 535–581. [Google Scholar]
- 53.Werner J, Bienek A. Loss and restoration of preoptic thermoreactiveness after lesions of the rostral raphe nuclei. Exp Brain Res. 1990;80:429–435. doi: 10.1007/BF00228170. [DOI] [PubMed] [Google Scholar]
- 54.Winn P, Tarbuck A, Dunnett SB. Ibotenic acid lesions of the lateral hypothalamus: comparison with the electric lesion syndrome. Neuroscience. 1984;12:225–240. doi: 10.1016/0306-4522(84)90149-0. [DOI] [PubMed] [Google Scholar]