Abstract
Electrophysiological and behavioral studies point to a role of group I metabotropic glutamate receptors (mGluR1 and mGluR5) in mediating spinal nociceptive responses in rats. However, antagonists with a high degree of specificity for each of these sites are not yet available. We, therefore, examined the effects of antisense deletion of spinal mGluR1 expression in assays of behavioral analgesia and of electrophysiological responses of dorsal horn neurons. Rats treated with an mGluR1antisense oligonucleotide reagent, delivered continuously to the intrathecal space of the lumbar spinal cord, developed marked analgesia as measured by an increase in the latency to tail-flick (55°C) over a period of 4–7 d. This correlated with a selective reduction in mGluR1, but not mGluR5, immunoreactivity in the superficial dorsal horn compared with untreated control rats, in parallel with a significant reduction in the proportion of neurons activated by the mGluR group I agonist 3,5-dihydroxyphenylglycine (DHPG), whereas the proportion of cells excited by the mGluR5 agonist,trans-azetidine-2,4-dicarboxylic acid (t-ADA) remained unaffected. In contrast, rats treated with mGluR1 sense or mismatch probes showed none of these changes compared with untreated, control rats. Furthermore, multireceptive dorsal horn neurons in mGluR1antisense-treated rats were strongly excited by innocuous stimuli to their peripheral receptive fields, but showed severe reductions in their sustained excitatory responses to the selective C-fiber activator mustard oil and in responses to DHPG.
Keywords: metabotropic glutamate receptors, mGluR1, mGluR5, dorsal horn, nociception, antisense oligodeoxynucleotide probe
There is a large body of evidence supporting a role for the excitatory amino acid glutamate in mediating sensory information at the first central synapses in the dorsal horn of the spinal cord. Glutamate receptors in mammalian brain are classified into two functional groups: ionotropic and metabotropic receptors (mGluRs), (Nakanishi, 1992). The metabotropic group of glutamate receptors, comprising eight receptors, is subdivided into three groups, according to their amino acid homology, pharmacological, and signal transduction profiles: group I (mGluRs1/5), group II (mGluRs2/3), and group III (mGluRs4/6/7/8) (Masu et al., 1991; Abe et al., 1992;Nakanishi, 1992; Pin and Duvoisin, 1995). The mGluRs, which couple via G-proteins to several signal transduction pathways, regulate neuronal excitability in the CNS by modulating a variety of ion channels (for review, see Saugstad et al., 1995). Many of the mGluRs have been shown to be present in the spinal cord (Shigemoto et al., 1992, 1993; Ohishi et al., 1993, 1995; Vidnyánszky et al., 1994; Anneser et al., 1995; Romano et al., 1995; Boxall et al., 1996; Petralia et al., 1996;Valerio et al., 1996) where they appear to play a role in mediating nociceptive inputs in the dorsal horn of the spinal cord (Neugebauer et al., 1994; Young et al., 1994, 1995, 1997), as well as in the thalamus (Eaton et al., 1993). In particular (but not exclusively) group I mGluR1/5 receptors have been implicated in nociceptive responses (Young et al., 1994, 1995, 1997; Fisher and Coderre, 1996a,b;Fundytus et al., 1998), and their actions may be mediated, at least in part, by protein kinase C (Young et al., 1995). This role appears to be more prominent after sustained noxious stimuli, such as intraplantar formalin and carrageenan-induced inflammation, where mGluR antagonists prolong the latency of behavioral nociceptive responses (Fisher and Coderre, 1996b; Young et al., 1997) or inhibit the sustained responses of dorsal horn neurons to mustard oil (Young et al., 1997). mGluR1/5 agonists can increase the excitability of dorsal horn neurons (Morisset and Nagy, 1996) and facilitate responses to NMDA and AMPA receptor activation (both of which are likely to participate in processing sustained nociceptive inputs) (Bleakman et al., 1992;Cerne and Randic, 1992; Bond and Lodge, 1995; Jones and Headley, 1995). Similarly, administration of mGluR1/5 agonists appears to interact with an NMDA (and/or AMPA) receptor-dependent mechanism to increase nociceptive behavioral responses to intradermal formalin injection and to noxious sensory stimuli (Coderre and Melzack, 1992;Meller et al., 1993, 1996; Fisher and Coderre, 1996b). In both electrophysiological and behavioral studies, agonists and antagonists with selectivity for mGluR1/5 (as well as neutralizing antibodies) are effective (Young et al., 1995, 1997; Fisher and Coderre 1996a,b; Fundytus et al., 1998), and some of the agents used show partial selectivity for mGluR1 over mGluR5(Hayashi et al., 1994; Sharp et al., 1994; Brabet et al., 1995;Kingston et al., 1995). Nevertheless, because the available pharmacological reagents are not wholly specific, we have taken the alternative strategy of antisense deletion of spinal mGluR1expression to gain an unequivocal assessment of its role in nociceptive processing.
MATERIALS AND METHODS
Animals and evaluation of analgesia. Adult male Wistar rats (weight 280–450 gm, Charles River, Kent, UK) were used. Measurements of tail-flick latency (using a modified Ugo Basile tail-flick unit made in-house) of each rat (30–55°C to the base of the tail) were made for 3 d before, and recommencing 4 d after implantation of an indwelling intrathecal silicone cannula connected to an osmotic minipump for the continuous, quantitative administration of sense, missense, and antisense mGluR1oligonucleotides (see below).
Oligonucleotide probes and implantation of osmotic minipump.The 21-base antisense oligodeoxynucleotides endcapped with phosphorothioate linkages (at the positions marked by *) were designed according to the primary sequence of the rat mGluR1 cDNA (Houamed et al., 1991; Masu et al., 1991). The sequence of the mGluR1 antisense probe was: 5′-G*C*CGGACCATTGTGGCGAAG*A*-3′, targeted around the translation initiation site (nucleotides −11 to +10) and will clearly not differentiate between splice variants in the carboxyl tail region. The complementary mGluR1 sense probe used: 5′-T*C*TTCGCCACAATGGTCCGG*C*-3′, corresponds to the reverse order of nucleotides of the above. The mismatch probe was: 5′-T*C*CGGATCATTGGGGCGACG*A*-3′. None of the oligonucleotide probes shows internal complementarity nor resembles any other known sequences according to the GenBank Database. Custom synthesis, HPLC-purification, and gel filtration were performed by Oswel DNA Service (Southampton, UK), and probes were dissolved and aliquoted in sterile 0.9% saline, pH 7.4, to give 0.25 μg/μl final concentration for infusion, before being stored at −20°C until use.
For continuous infusion into the intrathecal lumbar spinal cord region, each rat was implanted with a minipump with cannula attachment, which was assembled the day before surgery. It consisted of an osmotic minipump [Alza Minipump, model 2001 (Palo Alto, CA); nominal infusion rate, 1 μl/hr) attached to two lengths of sterile cannulae: first, to the pump, a length of ∼1 cm of vinyl cannula (internal diameter 0.76 mm, outer diameter 0.99 mm) and to this was fitted second, a length of ∼6 cm of silicone cannula (internal diameter 0.64 mm, external diameter 1.20 mm) (Degania Silicone). The pump and cannulae were filled with one of the above solutions (or saline control) under sterile conditions, and then the cannulae were joined to the pump, avoiding air bubble formation, before being placed in sterile saline at 37°C overnight.
Surgery was performed under sterile conditions, with Sagatal (Rhone Merieux, Harlow, Essex, UK; 0.06 ml/100 gm, i.p.) anesthesia, followed by a maintenance level of halothane (Zeneca, Cheshire, UK). The minipump was placed intramuscularly at an interscapular site, and the caudally directed cannula was threaded through muscle close to the exposed region of the spinal column. A small area of muscle and vertebral bone was cleared from two dorsal thoracic segments (T10–T12), and the tip of the cannula was placed through a small incision, under the dura, and eased down the dorsal spinal cord by a premeasured distance, to within the region of the lumbar segments L3–L6. After resection of the wound, the rat was then kept for up to 7 d, and its behavior was monitored (see above) before it was either (1) used for an electrophysiological recording experiment or (2) perfused and tissue taken for immunohistochemistry. A further group of rats were assessed behaviorally for 1 week subsequent to a 7 d infusion of antisense, to assess recovery. The position of the tip of the cannula with respect to the level of spinal segment was ascertained at the end of each experiment, and only data from animals with correct cannula placement (L3–L6) were used in analysis. No animal showing abnormal gait or paralysis during the 7 d period was included in the study.
Electrophysiological studies. To assess the physiological effects of the loss of receptor expression after mGluR1antisense treatment, neuronal responsiveness to ionophoretically applied mGluR agonists was investigated. Experiments were performed on 60 rats. Under initial halothane anesthesia, the jugular vein and trachea were cannulated. Intravenous α-chloralose (60 mg/kg) and urethane (1.2 mg/kg) with supplementary doses of α-chloralose (10 mg/ml) were given throughout the experiment as required. Core body temperature was maintained at 37–38°C by means of a thermostatically controlled heated blanket. Animals inspired oxygen-enriched air. The animal was placed in a stereotaxic frame, and the thoracolumbar spinal column was supported using three pairs of swan-necked clamps. A laminectomy was performed at L2–L5, and agar (2% in saline at 37°C) was injected under the most rostral vertebra and over the exposed cord to increase mechanical stability. Above the recording region, a section of the now solidified agar was removed, the dura was removed, and liquid paraffin (37°C) was poured over the exposed cord. Extracellular recordings were made from single neurons in laminae III–V through the center barrel of a seven-barelled glass microelectrode filled with 4 m NaCl (pH 4.0–4.5, tip diameter 4–5 μm, DC resistance 5–8 MΩ). The bandwidth of the recording amplifier was 1 Hz to 7 kHz. The following drugs were ionophoresed from the side barrels of the electrode: group I mGluR agonist: 3,5-dihydroxyphenylglycine (DHPG), 10 mm aqueous, pH 4.5; mGluR5 agonist:trans-azetidine-2,4-dicarboxylic acid (t-ADA), 10 mm aqueous, pH 8.0–8.5; and the AMPA receptor agonist: AMPA, 10 mm aqueous, pH 8.0–8.5. All compounds were obtained from Tocris Cookson, Bristol, UK. Retention currents of 10 nA were used to minimize drug leakage between tests. A remaining barrel contained 1 m NaCl, pH 4.0–4.5, for automatic current balancing, using a Neurophore BH2 ionophoresis system (Medical Systems, Great Neck, NY) and for current controls. The resistance of all side barrels was 20–30 MΩ.
Recordings were made from any multireceptive neuron encountered at a depth from the dorsal surface corresponding to laminae I–IV, as shown in previous studies using electrophoretic deposition of dye (Fleetwood-Walker et al., 1988, 1993). The cutaneous receptive fields of neurons were identified by innocuous brush stimuli and were all on the distal hindlimb. The use of a mechanized rotating fine brush to stimulate hair follicle (Aβ) afferents has been described previously (Fleetwood-Walker et al., 1985) and was qualitatively innocuous to human skin. Further characterization was performed using noxious radiant heat (30–48°C, rise time 5 sec, plateau temperature for 10 sec) and noxious pinch. Approximately 90% of all the cells tested in normal animals also showed sustained responses to mustard oil. The responses of cells to ionophoresed agonists were then explored with drug ejection currents being increased in a stepwise manner, every minute in steps of 10 nA from 5 to 45 nA. The response of neurons to the chemical algogen mustard oil (allyl isothiocyanate; Sigma, Poole, UK; 7.5% in paraffin oil) was observed after being repeatedly applied to the receptive field area (normally ∼2 cm2) every 5 min until sustained activation occurred.
Statistical analysis of the proportion of cells activated by agonists in the different groups of rats (normal, antisense reagent-treated, or sense reagent-treated rats) was performed by Mann–Whitney Utest.
Immunohistochemistry. To directly monitor the loss of receptor protein expression after 5 d of intrathecal treatment with the mGluR1 antisense oligonucleotide probe, immunohistochemical analysis of the lumbar spinal cord was performed. Animals taken for immunohistochemistry were deeply anesthetized with Sagatal (0.12 ml/100 gm, i.p.) and perfused transcardially with 0.1m PBS (containing 3 mm sodium nitrite and 1000 U heparin, pH 7.4) before being perfused with 4% paraformaldehyde/0.1 m PBS. A laminectomy was then performed, and the spinal cord was removed, together with the brain, which were then post-fixed in the same solution for a further 4–5 hr, before being incubated in 25% buffered sucrose overnight (4°C) and then stored in cryoprotectant (30% ethylene glycol and 20% glycerol, in 0.05 m PBS, pH 5.5) at −70°C. Transverse microtome sections (52 μm) were then cut from the frozen tissue, through lumbar segments L3–L6, and suitable sections of brain tissue were used as positive controls for the antibodies used. Tissue sections were stored in cryoprotectant at −20°C, until use. Sections were removed from the cryoprotectant as required, for processing for either mGluR1 or mGluR5 immunoreactivity. Unless otherwise stated, all solutions were made up in 0.1 m PBS, and all incubations were performed at room temperature with gentle agitation. In all steps involving antibodies, the tissue sections were washed twice, for 10 min each, with PBS between succeeding steps. Sections were incubated in 1% hydrogen peroxide (30 min; Sigma) to remove any endogenous peroxidase activity, followed by incubation in normal goat serum (1 hr) to block nonspecific binding. They were then incubated with polyclonal antipeptide antibodies raised to rat mGluR1 (1180–1199) (0.25 μg/ml, 48 hr, 4°C; Chemicon, Temecula, CA) or rat mGluR5 (1152–1171) (1 μg/ml, 48 hr, 4°C; Upstate Biotechnology, Lake Placid, NY) followed by biotinylated goat anti-rabbit IgG antibody (1: 200 in PBS, 1 hr; Vector Laboratories, Peterborough, UK). Sections were then incubated for 90 min with an avidin–biotin complex solution (Vectastain Elite ABC kit, Vector Laboratories). A further wash with PBS was followed by exposure of sections to a solution of 3,3′ diaminobenzidine tetrachloride (DAB; 0.2 mg/ml; Sigma) in the presence of 3% hydrogen peroxide (1 μg/ml) to enable visualization of the receptor protein precipitate. After a final wash in PBS, the sections were mounted onto poly-l-lysine-coated microscope slides, allowed to air dry before dehydration through ascending concentrations of alcohol, and then cleared in xylene (Sigma) and mounted in DePex mountant (BDH). Further immunohistochemical controls consisted of replacing the primary antibodies with nonimmune goat serum, or for preabsorbtion controls, see below. To overcome any potential problems caused by variable development of the DAB reaction, batches of control, sense, mismatch, and antisense-treated spinal sections were processed simultaneously, meaning that direct comparisons between them could be confidently made.
As a preabsorbtion control for specificity of the mGluR1antibody, aliquots were incubated with membranes from COS 7 cells overexpressing the rat mGluR1α receptor from a construct in pcDNA 1 (a gift from S. Nakanishi, Kyoto University, Japan) or with membranes from control COS 7 cells. Transfections were performed using DEAE dextran, and cells were harvested 72 hr later (Lutz et al., 1993). Cells were homogenized, and the crude particulate fraction was washed twice in ice-cold 20 mm HEPES-NaOH, pH 7.2, with phosphatase and peptidase inhibitors. Antibody aliquots were incubated with membranes (16 hr rolling at 4°C) before use (at twice the usual concentration). Preincubation with normal COS 7 cell membranes had no detectable effect, showing staining in normal dorsal horn apparently identical to that with untreated antibody, whereas the mGluR1-expressing membranes caused virtually complete loss of immunostaining (see Fig. 3E).
Fig. 3.
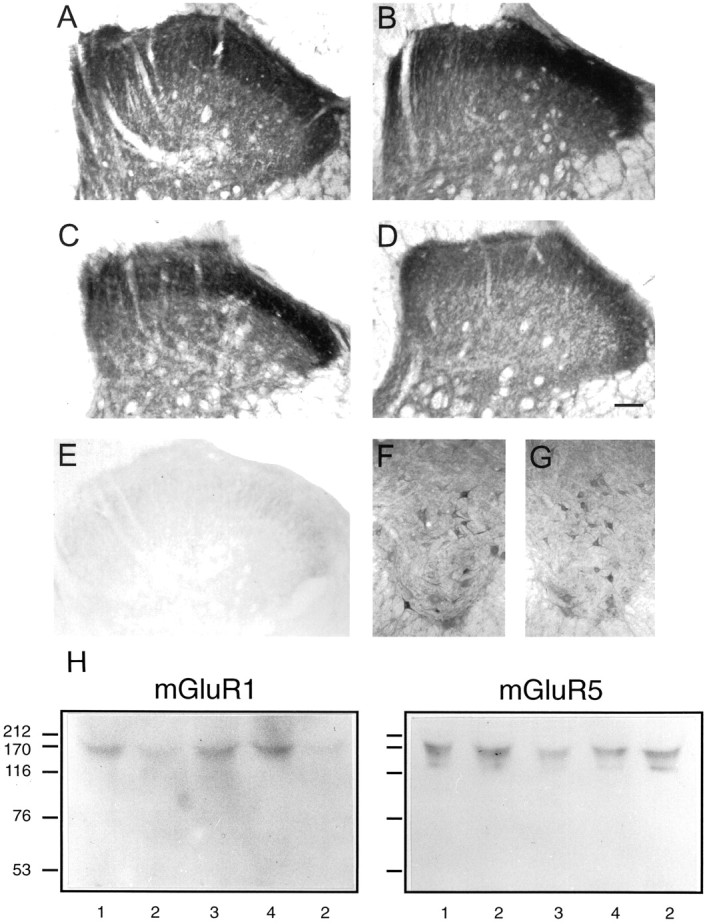
Effects of mGluR1 sense, mismatch, and antisense infusion on mGluR1 and mGluR5immunoreactivity in lumbar spinal cord.A–D show typical representations of mGluR1 immunoreactivity in dorsal horn in control (saline), sense, mismatch, and antisense reagent-treated animals.E shows the virtual lack of immunoreactivity in control dorsal horn when the mGluR1 antibody was preabsorbed with membranes from mGluR1-overexpressing cells.F and G show mGluR1immunoreactivity in ventral horn in control and mGluR1antisense-treated animals. These results are typical of at least five animals in each case. Scale bars, 1.0 mm. H shows immunoblots using mGluR1 and mGluR5 antibodies after gel electrophoresis of lysates from spinal cord segments L3–L6 of (1) control, (2) antisense, (3) sense, and (4) mismatch-treated animals. The running positions of the molecular weight markers are shown in kilodaltons. Results are typical of three separate experiments.
Relative quantification of immunoreactivity was achieved using an Improvision 1.49 image analysis package at 400×. An 80 × 30 μm region of interest (ROI) cursor was aligned consecutively, centered on laminae I, IIouter, IIinner, and III and, in ventral horn, 40 μm diameter ROIs were centered over individual motoneurons. Arbitrary gray scale units (throughout the range 1–200) were assigned to make optimal use of the range for the given sample set. Each section was corrected for the (low) nonspecific background levels recorded from white matter. Individual measurements were performed on 15 or 16 separate sections for each experimental condition.
Gel electrophoresis and immunoblotting. L3–L6 spinal cord samples, which had been frozen at −70°C were thawed into standard Laemmli lysis buffer and denatured at 100°C for 5 min. Proteins were separated by electrophoresis on precast, 20% polyacrylamide minigels (Phast System; Pharmacia, Piscataway, NJ) and transferred to immobilon (Johnson et al., 1993). After blocking with Marvel overnight at 4°C, blots were incubated with the primary mGluR1 (1:200) or mGluR5 (1:300) antibodies. HRP-conjugated donkey anti-rabbit IgG (1:5000) and the Enhanced Chemiluminescence kit (Amersham, Arlington Heights, IL) were used to visualize immunoreactive bands.
RESULTS
The position of the implanted intrathecal cannula was verified 4–7 d after surgery to ensure that only those animals with correct L3–L6 placements were taken for subsequent electrophysiological recording experiments or immunohistochemistry. For the animals included in this study, cannulae were found to lie on the dorsal surface of the spinal cord.
Measurement of behavioral nociceptive responses
Tail-flick latency was measured for 2–3 d before, and also after, surgical implantation of an indwelling intrathecal cannula to the lumbar spinal cord, allowing an intermediate 3 d gap for recovery from surgery.
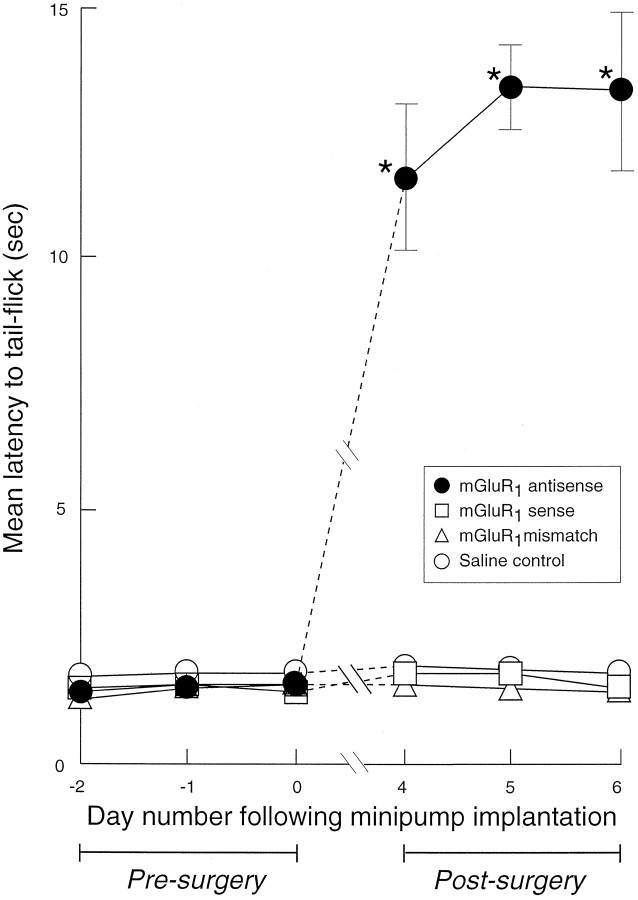
After continuous infusion of the mGluR1 antisense oligonucleotide probe, there was a marked increase in tail-flick latency reaching a peak at 4–7 d after surgery, which was statistically significant compared with presurgery levels (p < 0.05; Mann–Whitney U test,n = 9), saline-infused controls, mismatch-treated, or the sense-treated rats (Fig. 1). In the subsequent 7 d, the tail-flick latency recovered steadily to values approaching presurgery controls, with 36 ± 5%, 78 ± 12%, and 88 ± 4% recovery at days 10, 12, and 14 after surgery, respectively (n = 4). The mGluR1 antisense oligonucleotide-treated animals that were subsequently taken within 7 d after minipump and cannula implantation surgery, for either electrophysiological studies or immunohistochemical investigation, all displayed delayed behavioral nociceptive responses.
Fig. 1.
Effects of mGluR1 sense, mismatch, and antisense oligonucleotide administration on behavioral nociceptive responses (tail-flick latency, in seconds, to noxious heat applied to the base of the tail). Statistically significant increases to tail-flick latency are indicated by * (p < 0.05; Mann–Whitney U test). Attenuation of behavioral nociceptive responses was observed 4–7 d after continuous infusion of the mGluR1 antisense oligonucleotide reagent (•) in saline to the lumbar spinal cord segments L3–L6, compared with saline-infused controls (○), sense (■), or mismatch (▵) oligonucleotide-treated rats with similarly placed intrathecal cannulae. Values are means ± SEM (n = 6–14). Where error bars are not apparent, they fall within the dimensions of the symbol.
Electrophysiological recording experiments
Responses to mGlu receptor agonists
In all experimental rats that were taken for electrophysiological recording experiments, ionophoretic application of the mGluR agonists DHPG or t-ADA was performed at between 5 and 45 nA, at which every minute the ionophoretic current was increased by 10 nA increments until activation was observed, or if none was observed by 45 nA after 2 min, then the drug application was terminated. Activation of cells occurred within the 5 sec to 2 min after the drug had initially been applied. Most cells were activated within 50 sec of either of the drugs being increased to 25 nA.
In normal animals, the mGluR1/5 agonist DHPG, administered in this regimen, caused overt activation of a majority of multireceptive cells (56%), whereas the mGluR5 agonistt-ADA activated a significantly smaller proportion (23%). Table 1 illustrates this, together with corresponding data from antisense, sense, and mismatch-infused animals. A marked and significant reduction in the proportion of cells responding to DHPG was seen after antisense, but not the control reagents. The possibility that nonidentical samples of neurons may have been recorded in control and antisense-treated animals cannot be excluded, but the proportion of cells responding to t-ADA was not altered by any treatment. Furthermore, ionophoretic application of AMPA (10–40 nA) consistently caused very marked and rapid activation of all cells tested, irrespective of whether they had been treated with oligonucleotides or were untreated controls (data not shown).
Table 1.
Effect of mGluR1 antisense treatment on neuronal responses to DHPG and t-ADA
| Treatment | Percentage of cells excited by agonist | |
|---|---|---|
| DHPG | t-ADA | |
| Control (n = 13) | 56 (25/45) | 23 (6/26)* |
| Sense (n = 7) | 53 (16/30) | 21 (8/38) |
| Mismatch (n = 6) | 55 (12/22) | 27 (6/22) |
| Antisense (n = 7) | 20 (13/64)** | 25 (12/49) |
Drugs were ionophoresed at 5–45 nA for 5 sec to 2 min. The numbers of cells responding, of those tested, are shown in parentheses, and the numbers of animals in each case are shown after the respective treatments. (*p < 0.05; **p < 0.01 compared to control response to DHPG in control, sense, and mismatch; χ2 test).
Sensory responses
Although dorsal horn neurons displayed vigorous responses to motor-driven innocuous brush, which was unaffected by mGluR1 sense, mismatch, or antisense oligonucleotide treatments (Table 2), the same neurons showed a greatly reduced ability to respond to noxious chemical stimulation after the mGluR1 antisense oligonucleotide treatment.
Table 2.
Effects of sense, mismatch, and antisense mGluR1 oligonucleotide infusions on different sensory responses of dorsal horn neurons
| Stimulus | Stimulus-evoked response (Hz) | |||
|---|---|---|---|---|
| Normal control | Sense reagent-treated | Mismatch reagent-treated | Antisense reagent-treated | |
| (n = 8) | (n = 7) | (n = 6) | (n = 6) | |
| Innocuous brush | 17.2 ± 2.3 | 22.1 ± 5.7 | 14.5 ± 2.4 | 20.4 ± 5.6 |
| Acute chemical nociception | 30.7 ± 7.2 | 22.7 ± 7.7 | 20.9 ± 3.9 | 1.4 ± 0.5* |
| Sustained activity caused by repeated nociceptive stimuli | 10.1 ± 2.6 | 7.4 ± 1.8 | 7.3 ± 1.6 | 0.5 ± 1.8* |
The sensory responses monitored were the acute neuronal responses to innocuous brush (mean taken over 10 sec) and the chemical algogen mustard oil applied to the peripheral receptive field (mean taken over 10 sec, 20–40 sec after initial application), as well as the sustained sensitized activity caused by repeated application of mustard oil (mean taken over 10 sec, 15–18 min after the first three applications). Basal firing rates of neurons were all within the range 0–1.0 Hz and were no different between treatments. Values are means ± SEM with numbers of neurons in parentheses. *Indicates significantly different from normal, sense, or mismatch reagent controls; p < 0.05, Mann–Whitney U test.
In normal, untreated animals, neuronal activity increased shortly after application of mustard oil to the peripheral receptive field, quickly reaching a peak (at 20–40 sec) and then slowly declining over the next 5 min. This acute chemical nociceptive response (calculated as the mean value over 10 sec, 20–40 sec after initial application) was not significantly altered in sense or mismatch oligonucleotide-treated animals compared with untreated controls, whereas responses from antisense oligonucleotide-treated animals were markedly reduced (p < 0.05; Mann–Whitney U test; Table 2). These observations concur with the changes in acute thermal nociceptive responses (tail-flick) seen in the behavioral experiments. Mechanical nociceptive responses were not investigated quantitatively in the present study.
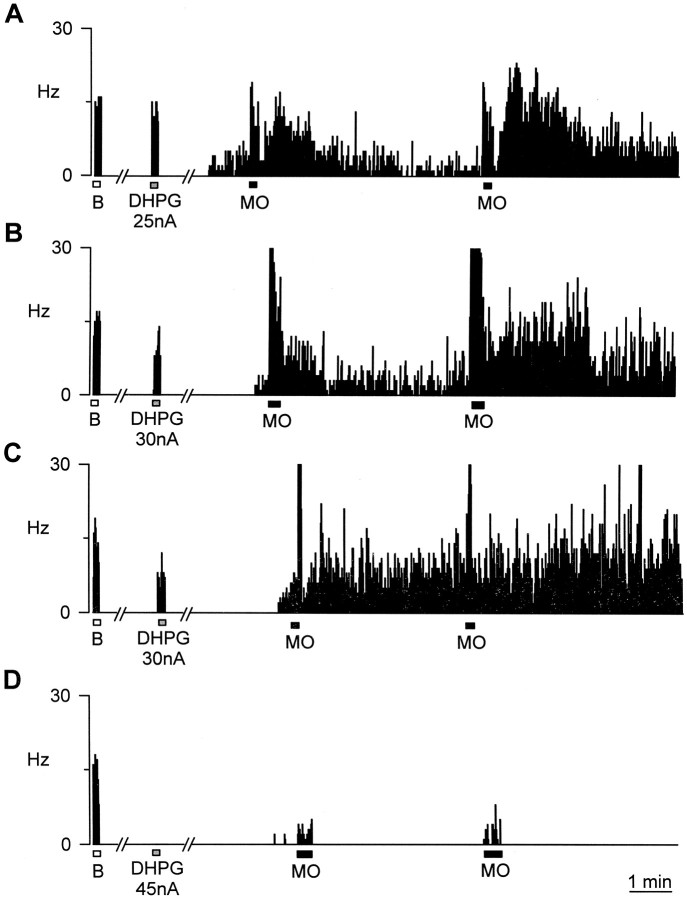
In normal, untreated animals (n = 8), eight of nine cells showed a sustained and incremental excitatory response to repeated, topical application of mustard oil to their peripheral receptive field (up to three applications, every 5 min). The average increase in ongoing activity of these neurons after topical mustard oil application was from 0.3 ± 0.1 Hz before mustard oil to 10.1 ± 2.6 Hz of sustained activity, measured as the mean over 10 sec taken 15–18 min after initial application (Fig.2A). Similarly, in the mGluR1 sense oligonucleotide-treated control animals (n = 5), seven of eight multireceptive cells showed a sustained excitatory response, after three topical mustard oil applications [from 0.2 ± 1.0 Hz before mustard oil application to 7.4 ± 1.8 Hz sustained activity measured 15–18 min after initial application (Fig. 2B)]. Six cells recorded from mismatch-treated animals (n = 6) (Fig.2C) also showed marked ongoing activation as in normals (0.1 ± 0.8 Hz before repeated mustard oil application and 7.3 ± 1.6 Hz afterward). In contrast, in rats treated with the mGluR1 antisense oligonucleotide, which displayed greatly attenuated behavioral nociceptive responses (n = 6), six of six dorsal horn multireceptive cells showed no significant sustained response to repeated topical application of mustard oil to their peripheral receptive field (even with up to five applications); the average increase in ongoing activity being from only 0.1 ± 0.1 Hz before mustard oil, to only 0.5 ± 1.8 Hz (Fig.2D). The sustained mustard oil-induced firing in antisense oligonucleotide-treated animals was significantly less than that in control or sense-treated animals (p < 0.05 by Mann–Whitney U test). All of the cells from which we recorded full sensory responses in normals, sense, and mismatch animals also showed clear responses to DHPG. In the six cells from antisense animals, from which we were able to gain adequate records of both brush and some residual mustard oil response, we found none that responded to DHPG.
Fig. 2.
Ongoing neuronal activity records showing typical excitatory firing responses of dorsal horn neurons to innocuous brush stimuli (■), to ionophoresis of DHPG (░), and to noxious mustard oil stimulation (▪). A shows untreated control,B shows mGluR1 sense oligonucleotide-treated, C shows mGluR1mismatch-treated, and D shows mGluR1antisense-treated animals. All animals were tested for tail-flick responses before electrophysiological recording and were found to conform to the pattern displayed in Figure 1.
Immunohistochemistry and immunoblotting for mGluR1and mGluR5
The specificity of antibody labeling was verified by analyzing the distribution of mGluR1 and mGluR5immunoreactivity in the CNS. In the cerebellar cortex, the molecular and Purkinje layers were strongly immunoreactive for mGluR1, whereas the granular layer was less intensely labeled. The mGluR5 immunoreactivity in the hippocampus was highest in the molecular layer, whereas there were many unlabeled cell bodies in the CA1 and CA3 fields. These observations are entirely consistent with previously published observations (Shigemoto et al., 1992, 1993; Romano et al., 1995).
In lumbar spinal cord of control untreated animals, neural elements strongly labeled for mGluR1 were distributed in laminae I and II of the dorsal horn, to a lesser extent in deeper dorsal horn, and also in the ventral horn around motoneurons (Fig.3A,F). This pattern of immunoreactivity was unchanged in animals treated with the mGluR1 sense or mismatch oligonucleotide probe (Fig.3B,C). In contrast, animals treated with the antisense oligonucleotide for mGluR1, which had displayed attenuated behavioral nociceptive responses, showed a marked decrease in the intensity and distribution in immunoreactive labeling for mGluR1 in the superficial layers of the dorsal horn (Fig. 3D). These losses were routinely observed throughout the extent of L3–L6, after cannula placement at any point within this range, thus giving a minimal estimate of the extent of reagent diffusion. Under the present experimental conditions, the immunoreactivity for mGluR1 that was associated with motoneurons in the ventral horn did not appear to be altered in the mGluR1 antisense oligonucleotide-treated rats (Fig.3G). Antibody specificity was demonstrated by preabsorbtion with membranes from COS 7 cells overexpressing recombinant rat mGluR1α (Fig. 3E). Table3 shows quantification of the mGluR1 immunostaining as gray scale intensity measured across different laminae of the superficial dorsal horn, demonstrating significant reductions in inner (and to a slightly lesser extent, outer) lamina II of antisense-treated animals compared with the other conditions. This loss was confirmed independently in Western blots from polyacrylamide gel electrophoresis of spinal cord lysates (Fig.3H). These showed a single mGluR1-immunoreactive band at ∼160–175 kDa that was clearly depleted in samples from antisense-treated animals compared with others. In contrast, mGluR5 immunoreactivity (which was also concentrated in the superficial dorsal horn) showed no differences in the pattern of staining in animals treated with sense, mismatch, or antisense mGluR1 reagents compared with normals (Table 3). In addition, Western blots showed the main band of mGluR5 immunoreactivity (at ∼160–180 kDa) was unaltered by mGluR1 antisense treatment (Fig.3H).
Table 3.
Image analysis quantification of immunohistochemistry for mGluR1 and mGluR5 in dorsal horn of animals treated with mGluR1 oligonucleotides
| Gray scale intensity (arbitrary units) in excess of dorsal column control | ||||
|---|---|---|---|---|
| Control | mGluR1sense | mGluR1 mismatch | mGluR1 antisense | |
| mGluR1 | ||||
| Lamina I | 96.5 ± 1.1 | 95.4 ± 2.0 | 94.8 ± 1.5 | 86.8 ± 2.9 |
| Lamina II0 | 98.6 ± 0.6 | 97.2 ± 1.6 | 95.3 ± 1.3 | 84.5 ± 3.4*,***,**** |
| Lamina III | 80.3 ± 2.7 | 70.5 ± 5.0 | 75.0 ± 1.7 | 53.1 ± 3.3**,***,***** |
| Lamina III | 23.3 ± 3.0 | 20.3 ± 2.2 | 25.9 ± 2.2 | 16.5 ± 4.8 |
| Ventral horn | 26.3 ± 2.0 | 22.7 ± 1.8 | 28.0 ± 6.3 | 25.7 ± 1.0 |
| mGluR5 | ||||
| Lamina I | 70.6 ± 2.8 | 82.3 ± 2.5 | 77.2 ± 2.5 | 80.4 ± 2.8 |
| Lamina II0 | 66.7 ± 2.8 | 73.5 ± 2.9 | 58.3 ± 3.8 | 71.8 ± 4.5 |
| Lamina III | 43.3 ± 3.3 | 47.4 ± 3.0 | 36.9 ± 2.8 | 43.9 ± 3.7 |
| Lamina III | 17.9 ± 2.5 | 13.7 ± 2.3 | 17.4 ± 3.0 | 13.8 ± 2.4 |
| Ventral horn | 32.0 ± 1.3 | 28.5 ± 2.3 | 26.7 ± 2.7 | 30.4 ± 2.0 |
The statistically significant differences were determined by Mann–Whitney U test (n = 15–16). *p < 0.05, **p < 0.01 compared with control; ***p < 0.05 compared with sense; and ****p < 0.05, *****p < 0.01 compared with mismatch.
DISCUSSION
Various lines of evidence point to a role of mGluRs in spinal somatosensory processing and reflex responses. It appears that mGluRs of group I, II, and III may all have some role to play. The group I/II mGluR agonist, 1S,3R-ACPD (1S,3R-1-amino-1,3-cyclopentane dicarboxylic acid) causes postsynaptic increases in the excitability of dorsal horn neurons (Morisset and Nagy, 1996), and it has been suggested that not only group I, but also group II, mGluRs may contribute to this effect (Bond and Lodge, 1995). In fact, synergistic effects of group I and group II receptor agonists on second messenger production have been described (Schoepp et al., 1996). Group III and group II receptors at presynaptic sites act to inhibit synaptic inputs to ventral horn neurons (Jane et al., 1996) and may also modulate inputs to dorsal horn neurons. Correspondingly, mGluR7 (group III) sites have been identified in primary afferent terminals (Ohishi et al., 1995).
Nevertheless, the present data, together with the other available information from electrophysiological and behavioral studies, suggest strongly that group I mGluRs play a crucial role in physiological nociceptive inputs (Neugebauer et al., 1994; Young et al., 1994, 1995,1997; Fisher and Coderre, 1996a,b; Fundytus et al., 1998). Specifically, our pharmacological and antisense ablation data suggest strongly that a key role is played by mGluR1. This is consistent with our previous evidence from partially selective pharmacological agents that suggested that mGluR1 may play the predominant role (Young et al., 1995, 1997). Both the acute responses to mustard oil (a C-fiber selective activator) and the incremental activity resulting from its repeated application were severely inhibited by mGluR1 ablation (Fig. 2; Table 2), with relative preservation of responses to innocuous brush. It is not possible from the present results to say whether mGluR1ablation has any specific influence on the mechanism of wind-up (increased excitability) per se, because the necessary prerequisite of C-fiber inputs is itself abrogated by the antisense strategy. The behavioral studies (Fig. 1) and previous reports with partially selective mGluR1 antagonists and mGluR1/mGluR5 antisera (Young et al., 1997; Fisher and Coderre, 1996a; Fundytus et al., 1998) are entirely consistent with these observations in showing that prevention of spinal mGluR1 function leads to inhibition of behavioral nociceptive responses.
However, mGluR1 sites are also present in ventral horn, on or around motoneurons (Anneser et al., 1995; Alvarez et al., 1997;Boxall et al., 1998) . Thus, effects of mGluR agents on ventral root potentials evoked by dorsal root stimulation (Boxall et al., 1996) are likely to represent a composite of actions in dorsal and ventral horn. Group I mGluR agonists increase ventral root potentials elicited by ionotropic GluR agonists (Ugolini et al., 1997), whereas intracellular recordings from motoneurons suggest that both postsynaptic facilitatory and presynaptic inhibitory effects are brought about by group I/II mGluR agonists (King and Liu, 1996). Presynaptic effects of group III mGluR agonists are also prominent in motoneuron recordings (Cao et al., 1997). It is clear, therefore, that ventral horn effects of mGluR agents may potentially contribute to, or at least modify, the effects of mGluR manipulations in behavioral analgesia experiments. However, increased latencies or thresholds to nociceptive stimuli were measured in behavioral responses to intrathecally applied mGluR group I antagonists in the absence of any overt signs of motor insufficiency (Fisher and Coderre, 1996a; Young et al., 1997). Similarly, although mGluR1 knock-out mice display a disruption of complex coordination behaviors that may arise from cerebellar dysfunction, they possess well maintained muscle strength and can organize effective goal-oriented swimming behaviors as well as normal animals (Conquet et al., 1994). In the present study, there was no evidence for any deficit in motor coordination, gait, or locomotor activity in the mGluR1 intrathecal antisense-treated animals, corresponding to the lack of change in ventral horn mGluR1immunoreactivity (Fig. 3F,G) after dorsally directed infusion of oligonucleotide. So, although it is not possible to unequivocally exclude a contribution of ventral horn effects to the behavioral results (and reflex indices of function here not tested), it is clear that the effects of mGluR1ablation (focused in the dorsal horn; Fig. 3, Table 3), as defined in the electrophysiological experiments (Table 2, Fig. 2), would alone be quite sufficient to explain the behavioral changes that we observed (Fig. 1) and have similarly been described in mGluR1knock-out mice (Corsi et al., 1996).
In conclusion, the present results demonstrate that the localized antisense ablation of mGluR1 in dorsal horn (without affecting the congener mGluR5) results in a selective abrogation of neuronal responses to noxious stimuli (and perhaps also sensitization) without equivalent changes in non-nociceptive responses. Correspondingly, reflex behavioral responses to noxious thermal stimuli are attenuated in rats with antisense deletion of mGluR1 in lumbar dorsal horn, in the absence of any signs of generalized motor deficit.
Footnotes
This work was supported by Grants 046441 and 039868 from The Wellcome Trust. We thank The Wellcome Animal Research Unit, Royal (Dick) School of Veterinary Studies for facilities, and Colin Warwick for preparation of some of the diagrams.
Correspondence should be addressed to Dr. S. M. Fleetwood-Walker, Department of Preclinical Veterinary Sciences, Royal (Dick) School of Veterinary Studies, University of Edinburgh EH9 1QH, UK.
REFERENCES
- 1.Abe T, Sugihara H, Nawa H, Shigemoto R. Molecular characterisation of a novel metabotropic receptor mGluR5 coupled to inositol phosphate/calcium signal transduction. J Biol Chem. 1992;267:13361–13368. [PubMed] [Google Scholar]
- 2.Alvarez FJ, Dewey DE, Carr PA, Cope CC, Fyffe REW. Down-regulation of metabotropic glutamate receptor 1a in motoneurons after axotomy. NeuroReport. 1997;8:1711–1716. doi: 10.1097/00001756-199705060-00029. [DOI] [PubMed] [Google Scholar]
- 3.Anneser J, Berthele A, Laurie DJ, Sommer B, Tölle TR, Zieglgänsberger W. Differential distribution of metabotropic glutamate receptor mRNA in rat lumbar spinal cord neurons. Eur J Neurosci [Suppl] 1995;8:14. [Google Scholar]
- 4.Bleakman D, Rusin KI, Chard PS, Glaum SR, Miller RJ. Metabotropic glutamate receptors potentiate ionotropic glutamate responses in rat dorsal horn. Mol Pharmacol. 1992;42:192–196. [PubMed] [Google Scholar]
- 5.Bond A, Lodge D. Pharmacology of metabotropic glutamate receptor-mediated enhancement of responses to excitatory and inhibitory amino acids on rat spinal neurons in vivo. Neuropharmacology. 1995;34:1015–1023. doi: 10.1016/0028-3908(95)00046-9. [DOI] [PubMed] [Google Scholar]
- 6.Boxall SJ, Thompson SWN, Dray A, Dickenson AH, Urban L. Metabotropic glutamate receptor activation contributes to nociceptive reflex activity in rat spinal cord in vitro. Neuroscience. 1996;74:13–20. doi: 10.1016/0306-4522(96)00101-7. [DOI] [PubMed] [Google Scholar]
- 7.Boxall SJ, Berthele A, Laurie DJ, Sommer B, Zieglgänsberger W, Urban L, Tölle TR. Enhanced expression of mGluR3 mRNA in the rat spinal cord during ultra-violet irradiation induced peripheral inflammation. Neuroscience. 1998;82:591–602. doi: 10.1016/s0306-4522(97)00246-7. [DOI] [PubMed] [Google Scholar]
- 8.Brabet I, Mary S, Bockaert J, Pin J-P. Phenylglycine derivatives discriminate between mGluR1- and mGluR5-mediated responses. Neuropharmacology. 1995;34:895–903. doi: 10.1016/0028-3908(95)00079-l. [DOI] [PubMed] [Google Scholar]
- 9.Cao CQ, Tse H-W, Jane DE, Evans RH, Headley PM. Antagonism of mGlu receptors and potentiation of EPSCs at rat spinal motoneurones in vitro. Neuropharmacology. 1997;36:313–318. doi: 10.1016/s0028-3908(96)00180-3. [DOI] [PubMed] [Google Scholar]
- 10.Cerne R, Randic M. Modulation of AMPA and NMDA responses in rat spinal dorsal horn neurons by trans-ACPD. Neurosci Lett. 1992;144:180–184. doi: 10.1016/0304-3940(92)90745-s. [DOI] [PubMed] [Google Scholar]
- 11.Coderre TJ, Melzack R. Contribution of excitatory amino acids to central sensitisation and persistent nociception after formalin-induced tissue injury. Neuroscience. 1992;12:3665–3670. doi: 10.1523/JNEUROSCI.12-09-03665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, Franz-Bacon K, Reggiani A, Matarese V, Conde F, Collingridge GL, Crépel F. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372:237–219. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- 13.Corsi M, Quartaroli M, Maraia G, Chiamulera C, Ugolini A, Conquet F, Ratti E, Ferraguti F. PLC-coupled-mGluRs and their possible role in pain. Neuropharmacology. 1996;35:A9. [Google Scholar]
- 14.Eaton SA, Birse EF, Whartion B, Sunter DC, Udvarheyli PM, Watkins JC, Salt TE. Mediation of thalamic sensory responses in vivo by ACPD-activated excitatory amino acid receptors. Eur J Neurosci. 1993;5:186–189. doi: 10.1111/j.1460-9568.1993.tb00484.x. [DOI] [PubMed] [Google Scholar]
- 15.Fisher K, Coderre TJ. The contribution of metabotropic glutamate receptors to formalin-induced nociception. Pain. 1996a;68:255–263. doi: 10.1016/s0304-3959(96)03212-5. [DOI] [PubMed] [Google Scholar]
- 16.Fisher K, Coderre TJ. Comparison of nociceptive effects produced by intrathecal administration of mGluR agonists. NeuroReport. 1996b;7:2743–2747. doi: 10.1097/00001756-199611040-00067. [DOI] [PubMed] [Google Scholar]
- 17.Fleetwood-Walker SM, Mitchell R, Hope PJ, Molony V, Iggo A. An α2 receptor mediates the selective inhibition by noradrenaline of nociceptive responses of identified dorsal horn neurones. Brain Res. 1985;334:243–254. doi: 10.1016/0006-8993(85)90216-1. [DOI] [PubMed] [Google Scholar]
- 18.Fleetwood-Walker SM, Hope PJ, Mitchell R, El-Yassir N, Molony V. The influence of opioid receptor subtypes on the processing of nociceptive inputs in the spinal dorsal horn of the cat. Brain Res. 1988;451:213–226. doi: 10.1016/0006-8993(88)90766-4. [DOI] [PubMed] [Google Scholar]
- 19.Fleetwood-Walker SM, Parker RMC, Munro FE, Young MR, Hope PJ, Mitchell R. Evidence for a role of tachykinin NK2 receptors in mediating brief nociceptive inputs to rat dorsal horn (laminae III-V) neurons. Eur J Pharmacol. 1993;242:173–181. doi: 10.1016/0014-2999(93)90077-u. [DOI] [PubMed] [Google Scholar]
- 20.Fundytus ME, Fisher K, Dray A, Henry JL, Coderre TJ. In vivo antinociceptive activity of anti-rat mGluR1 and mGluR5 antibodies in rats. NeuroReport. 1998;9:731–735. doi: 10.1097/00001756-199803090-00031. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi Y, Sekiyama N, Nakanishi S, Jane DE, Sunter DC, Birse EF, Udvarhelyi PM, Watkins JC. Analysis of agonist and antagonist activities of phenylglycine derivatives for different cloned metabotropic glutamate receptor subtypes. J Neurosci. 1994;14:3370–3377. doi: 10.1523/JNEUROSCI.14-05-03370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houamed KM, Kuijper JL, Gilbert TL, Haldeman BA, O’Hara PJ, Mulvihill ER, Almers W, Hagen FS. Cloning, expression, and gene structure of a G protein-coupled glutamate receptor from rat brain. Science. 1991;252:1318–1321. doi: 10.1126/science.1656524. [DOI] [PubMed] [Google Scholar]
- 23.Jane DE, Thomas NK, Tse H-W, Watkins JC. Potent antagonists at the l-AP4- and (1S,3S)-ACPD-sensitive presynaptic metabotropic glutamate receptors in the neonatal rat spinal cord. Neuropharmacology. 1996;35:1029–1035. doi: 10.1016/s0028-3908(96)00048-2. [DOI] [PubMed] [Google Scholar]
- 24.Johnson MS, MacEwan DJ, Simpson J, Mitchell R. Characterisation of protein kinase C isoforms and enzymic activity from the αT3–1 gonadotrophin-derived cell line. FEBS Lett. 1993;333:67–72. doi: 10.1016/0014-5793(93)80376-6. [DOI] [PubMed] [Google Scholar]
- 25.Jones MW, Headley PM. Interactions between metabotropic and ionotropic glutamate receptor agonists in the rat spinal cord in vitro. Neuropharmacology. 1995;34:1025–1031. doi: 10.1016/0028-3908(95)00055-b. [DOI] [PubMed] [Google Scholar]
- 26.King AE, Liu XH. Dual action of metabotropic glutamate receptor agonists on neuronal excitability and synaptic transmission in spinal ventral horn neurons in vitro. Neuropharmacology. 1996;35:1673–1680. doi: 10.1016/s0028-3908(96)00140-2. [DOI] [PubMed] [Google Scholar]
- 27.Kingston AE, Burnett JP, Mayne NG, Lodge D. Pharmacological analysis of 4-carboxyphenylglycine derivatives: comparison of effects on mGluR1α and mGluR5a subtypes. Neuropharmacology. 1995;34:887–894. doi: 10.1016/0028-3908(95)00069-i. [DOI] [PubMed] [Google Scholar]
- 28.Lutz EM, Mitchell R, Johnson MS, MacEwan D. Functional expression of 5-HT1c receptor cDNA in COS 7 cells and its influence on protein kinase C. FEBS Lett. 1993;316:228–232. doi: 10.1016/0014-5793(93)81298-e. [DOI] [PubMed] [Google Scholar]
- 29.Masu M, Tanabe Y, Tsuchida K, Shigemoto R, Nakanishi S. Sequence and expression of a metabotropic glutamate receptor. Nature. 1991;349:760–765. doi: 10.1038/349760a0. [DOI] [PubMed] [Google Scholar]
- 30.Meller ST, Dykstra C, Gebhart GF. Acute mechanical hyperalgesia in the rat is produced by coactivation of ionotropic AMPA and metabotropic glutamate receptors. NeuroReport. 1993;4:879–882. doi: 10.1097/00001756-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Meller ST, Dykstra C, Gebhart GF. Acute mechanical hyperalgesia in the rat can be produced by coactivation of spinal ionotropic AMPA and metabotropic glutamate receptors, activation of phospholipase A2 and generation of cyclooxygenase products. Prog Brain Res. 1996;110:177–192. doi: 10.1016/s0079-6123(08)62574-1. [DOI] [PubMed] [Google Scholar]
- 32.Morisset V, Nagy F. Modulation of regenerative membrane properties by stimulation of metabotropic glutamate receptors in rat deep dorsal horn neurons. J Neurophysiol. 1996;76:2794–2798. doi: 10.1152/jn.1996.76.4.2794. [DOI] [PubMed] [Google Scholar]
- 33.Nakanishi S. Molecular diversity of glutamate receptors and implications for brain functions. Science. 1992;258:597–609. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- 34.Neugebauer V, Kucke T, Schiable H-G. Requirement of metabotropic glutamate receptors for the generation of inflammation-evoked hyperexcitability in rat spinal cord neurons. Eur J Neurosci. 1994;6:1179–1186. doi: 10.1111/j.1460-9568.1994.tb00616.x. [DOI] [PubMed] [Google Scholar]
- 35.Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridisation study. J Comp Neurol. 1993;335:252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- 36.Ohishi H, Akazawa C, Shigemoto R, Nakanishi S, Mizuno N. Distributions of the mRNAs for l-2-amino-4-phosphonobutyrate-sensitive metabotropic glutamate receptors, mGluR4 and mGluR7, in the rat brain. J Comp Neurol. 1995;360:555–570. doi: 10.1002/cne.903600402. [DOI] [PubMed] [Google Scholar]
- 37.Petralia RS, Wang Y-X, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localisations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- 38.Pin J-P, Duvoisin R. The metabotropic glutamate receptors: structure and function. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- 39.Romano C, Sesma MA, McDonald CT, O’Malley KO, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- 40.Saugstad JA, Segerson TP, Westbrook GL. Modulation of ion channels and synaptic transmission by metabotropic glutamate receptors. In: Wheal H, Thomson A, editors. Excitatory amino acids and synaptic transmission, Ed 2. Academic; New York: 1995. pp. 77–88. [Google Scholar]
- 41.Schoepp DD, Salhoff CR, Wright RA, Johnson BG, Burnett JP, Mayne NG, Belagaje R, Wu S, Monn JA. The novel metabotropic glutamate receptor agonist 2R,4R-ACPD potentiates stimulation of phosphoinositide hydrolysis in the rat hippocampus by 3,5-dihydroxyphenylglycine: evidence for a synergistic interaction between group 1 and group 2 receptors. Neuropharmacology. 1996;35:1661–1672. doi: 10.1016/s0028-3908(96)00121-9. [DOI] [PubMed] [Google Scholar]
- 42.Sharp RL, Mayne NG, Burnett JP. Cyclothiazide differentially modulates human metabotropic glutamate receptors linked to phosphoinositide hydrolysis stimulation in oocytes. Eur J Pharmacol Mol Pharmacol. 1994;269:R5–R7. doi: 10.1016/0922-4106(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 43.Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: an in situ hybridisation study in adult and developing rat. J Comp Neurol. 1992;322:121–135. doi: 10.1002/cne.903220110. [DOI] [PubMed] [Google Scholar]
- 44.Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localisation of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993;163:53–57. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- 45.Ugolini A, Corsi M, Bordi F. Potentiation of NMDA and AMPA responses by Group I mGluR in spinal cord motoneurons. Neuropharmacology. 1997;36:1047–1055. doi: 10.1016/s0028-3908(97)00103-2. [DOI] [PubMed] [Google Scholar]
- 46.Valerio A, Rizzonelli P, Paterlini M, Moretto B, Knoepfel T, Kuhn R, Memo M, Spano PF. mGluR5 immunolocalization in foetal and adult human spinal cord. Neuropharmacology. 1996;35:A33. [Google Scholar]
- 47.Vidnyánszky Z, Hámori J, Négyessy L, Rüegg D, Knöpfel T, Kuhn R, Görcs TC. Cellular and subcellular localisation of the mGluR5a metabotropic glutamate receptor in rat spinal cord. NeuroReport. 1994;6:209–213. doi: 10.1097/00001756-199412300-00053. [DOI] [PubMed] [Google Scholar]
- 48.Young MR, Fleetwood-Walker SM, Mitchell R, Munro FE. Evidence for a role of metabotropic glutamate receptors in sustained nociceptive inputs to rat dorsal horn neurons. Neuropharmacology. 1994;33:141–144. doi: 10.1016/0028-3908(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 49.Young MR, Fleetwood-Walker SM, Mitchell R, Dickinson T. The involvement of metabotropic glutamate receptors and their intracellular signalling pathways in sustained nociceptive transmission in rat dorsal horn neurons. Neuropharmacology. 1995;34:1033–1041. doi: 10.1016/0028-3908(95)00071-d. [DOI] [PubMed] [Google Scholar]
- 50.Young MR, Fleetwood-Walker SM, Dickinson T, Blackburn-Munro G, Sparrow H, Birch PJ, Bountra C. Behavioural and electrophysiological evidence supporting a role for group I metabotropic glutamate receptors in the mediation of nociceptive inputs to the rat spinal cord. Brain Res. 1997;777:161–167. [PubMed] [Google Scholar]