Abstract
The suprachiasmatic nucleus (SCN) is a central pacemaker in mammals, driving many endogenous circadian rhythms. An important pacemaker target is the regulation of a hormonal message for darkness, the circadian rhythm in melatonin synthesis. The endogenous clock within the SCN is synchronized to environmental light/dark cycles by photic information conveyed via the retinohypothalamic tract (RHT) and by the nocturnal melatonin signal that acts within a feedback loop. We investigated how melatonin intersects with the temporally gated resetting actions of two RHT transmitters, pituitary adenylate cyclase-activating polypeptide (PACAP) and glutamate. We analyzed immunocytochemically the inducible phosphorylation of the transcription factor Ca2+/cAMP response element-binding protein (CREB) in the SCN of a melatonin-proficient (C3H) and a melatonin-deficient (C57BL) mouse strain. In vivo, light-induced phase shifts in locomotor activity were consistently accompanied by CREB phosphorylation in the SCN of both strains. However, in the middle of subjective nighttime, light induced larger phase delays in C57BL than in C3H mice. In vitro, PACAP and glutamate induced CREB phosphorylation in the SCN of both mouse strains, with PACAP being more effective during late subjective daytime and glutamate being more effective during subjective nighttime. Melatonin suppressed PACAP- but not glutamate-induced phosphorylation of CREB. The distinct temporal domains during which glutamate and PACAP induce CREB phosphorylation imply that during the light/dark transition the SCN switches sensitivity between these two RHT transmitters. Because these temporal domains are not different between C3H and C57BL mice, the sensitivity windows are set independently of the rhythmic melatonin signal.
Keywords: suprachiasmatic nucleus, circadian, phase shifts, mice, brain slice, CREB (Ca2+/cAMP response element-binding protein), glutamate, PACAP (pituitary adenylate cyclase-activating polypeptide), melatonin
Many biological rhythms persist in the absence of environmental cues (zeitgebers) with a circadian period of ∼24 hr. In mammals, important circadian rhythms are driven by an oscillator in the hypothalamic suprachiasmatic nucleus (SCN) (Klein et al., 1991). The principal cue entraining the endogenous rhythm in SCN activity to ambient light/dark conditions is photic information conveyed by the retinohypothalamic tract (RHT).
Mechanisms of cellular processing required to integrate environmental stimuli into the endogenous genetic program of circadian timing in the SCN to pace biological rhythms and anticipate cyclic changes in the light/dark regimen are not known. The sensitivity of the SCN to the resetting actions of neurochemical factors implicated in entrainment is restricted to discrete temporal windows. These windows, overlapping with equally discrete molecular gates for distinct signaling pathways, are notably out of phase with each other. During daytime, phase shifts can be induced by activation of the cAMP-signaling pathway (Prosser and Gillette, 1989), whereas the prevailing stimulus for phase shifts during nighttime results from activation of the Ca2+- and/or the cGMP-signaling pathway(s) (Prosser et al., 1989; Ding et al., 1998). In the rodent RHT, glutamate signals “light” to the pacemaker during nighttime (Castel et al., 1993;Ding et al., 1994, 1997). Conversely, the pituitary adenylate cyclase-activating polypeptide (PACAP) makes the SCN sense “darkness” during daytime, by elevating the intracellular cAMP concentration (Hannibal et al., 1997; Kopp et al., 1997). At dusk and possibly also at dawn, a sensitivity window exists for the pineal hormone melatonin to affect clock activity (Redman et al., 1983;Cassone et al., 1987; Stehle et al., 1989; McArthur et al., 1991). Thus, melatonin, the hormonal message for darkness, may fine-tune circadian timing via interference with other pathways, thereby defining SCN sensitivity to resetting cues. Because phase shifts in SCN activity require protein synthesis (Zhang et al., 1996) and induce DNA-binding proteins (Kornhauser et al., 1990; Rusak et al., 1990;Ginty et al., 1993; Stehle et al., 1996), transcription is part of pacemaker adjustment. Therefore, a molecular interface for neuronal (RHT neurotransmission) and/or endocrine (melatonin) cues must be able to serve different signaling pathways, to react fast, and to convey external stimuli rapidly to the transcriptional machinery necessary for consolidation or even initiation of phase shifts. There is increasing evidence that the transcription factor Ca2+/cAMP response element-binding protein (CREB) may act as an integrator involved in resetting circadian rhythms (Ginty et al., 1993; McNulty et al., 1998).
To gain further insights into the temporal gating of the SCN to resetting cues and to examine the potential impact of melatonin, we compared oscillator properties of C3H mice with a rhythmic melatonin synthesis, with those of C57BL mice with an undetectable melatonin synthesis in vivo (Ebihara et al., 1986; Goto et al., 1989). Using both in vivo and in vitro approaches, we found that phase-shifting stimuli converge within the SCN at a molecular level with the phosphorylation of CREB. Pacemaker-resetting cues act on CREB at different temporal sensitivity windows that are set intrinsically, independently of the endogenous melatonin signal.
MATERIALS AND METHODS
Animals and in vivo studies. All animal experimentation reported in this manuscript was conducted in accordance with the Policy on the Use of Animals in Neuroscience Research and the Policy on Ethics as approved by the Society for Neuroscience. Male C3H (substrains HeN or J) and C57BL/6 mice aged 6–10 weeks (Charles River Wiga, Sulzfeld, Germany) were housed in individual cages equipped with running wheels and with constant room temperature and food and water available ad libitum. Because no differences between the two C3H substrains were evident in any of the experiments conducted, these animals are further referred to as C3H mice. Wheel-running activity was recorded continuously and analyzed by a computer system (Viglen Contender PC, Alberton, UK) running Dataquest IV software (Data Sciences, Frankfurt, Germany). The mice were entrained to a photoperiod of 12 hr of light (250 lux):12 hr of darkness (dim red light < 15 lux) for at least 1 week before the experiments. Subsequently, animals were transferred into constant dim red light (DD), and free-running activity rhythms were monitored for 16 d consecutively. Activity onset was defined as circadian time 12 (CT12). The light pulses were delivered by moving the animals from the DD environment to a monochromatic light source with an intensity of 1000 lux (15 min) in a neighboring room during the projected subjective day (CT06 and CT10) or subjective night (CT14 and CT18). Control experiments were conducted by moving the mice without light exposure. To examine resetting responses, we exposed animals to between one and three light pulses at different circadian times, with at least 5 d between pulses to allow for consolidation of phase shifts. Phase shifts were determined by measuring the phase difference between eye-fitted lines through successive daily activity onsets, using observers blind to treatment. Negative values represent stimulus-induced phase delays; positive values represent phase advances.
To investigate in vivo the light-induced phosphorylation of CREB in the SCN, we anesthetized animals after light or sham exposure with sodium pentobarbital (1.6 gm/kg of body weight, i.p.) and transcardially perfused the animals with 4% paraformaldehyde (PFA) in PBS with heparin (2000 U/mouse) as an additive. Brains were removed, post-fixed for 2 hr in 4% PFA, and then cryoprotected with 20% sucrose in PBS overnight. Coronal hypothalamic sections (40 μm for immunocytochemistry; 100 μm for confocal laser microscopy) were cut on a freezing microtome and collected into PBS for immediate free-floating immunocytochemistry (see below).
In vitro studies. Mice kept under standard 12:12 hr light/dark conditions were decapitated, brains were removed quickly, and coronal hypothalamic slices (400 μm thick) containing the paired SCN were cut at 4°C using a vibratome. To avoid phase shifting of the circadian clock by brain slicing (Gillette, 1986), we prepared slices at least 2 hr before the onset of the dark phase. Stimulations of slices were conducted subsequently at zeitgeber time 06 (ZT06), ZT10, ZT14, and ZT18, with ZT12 designated as the onset of the donor’s dark phase. After preincubation for at least 2 hr in artificial CSF [aCSF (in mm), NaCl 145; KCl 5; CaCl2 1.8; MgCl2 0.8; HEPES 10; and glucose 10; pH 7.35; at 37°C], slices were stimulated with glutamate [100 μm; a dose according to Shirakawa and Moore (1994)] or PACAP [100 nm; a dose according to Hannibal et al. (1997)] for 15 min. To investigate the impact of melatonin on PACAPergic and glutamatergic effects, we added melatonin [1 nm; a dose according to McNulty et al. (1994)] alone for 30 min to aCSF or for 15 min before and during glutamate or PACAP application. Unstimulated slices served as controls. Subsequently, slices were fixed with 4% PFA for 12–16 hr, cryoprotected (20% sucrose in PBS), and sectioned on a cryostat. Sections (14 μm) were mounted on gelatin-coated slides and stored at −20°C until immunocytochemistry was performed.
Immunocytochemistry. Immunoreaction (IR) for the phosphorylated form of CREB (pCREB) in the SCN was visualized with a standard avidin–biotin labeling method, with diaminobenzidine as the chromogen as described (Sumova et al., 1994; Tamotsu et al., 1995). Primary polyclonal antibodies against CREB, phosphorylated at the residue Ser133 (pCREB; New England Biolabs, Beverly, MA; Upstate Biotechnology, Lake Placid, NY), were used at dilutions of 1:1000 and 1:500, respectively.
For double-label immunofluorescence, free-floating sections were incubated with the rabbit anti-pCREB antibody (1:100; New England Biolabs) and a monoclonal anti-VIP antibody (1:200; Bio Trend, Köln, Germany). Subsequently, the sections were incubated for 1 hr at room temperature in a mixture of goat anti-rabbit Cy3 (1:500; Jackson ImmunoResearch, West Grove, PA) and goat anti-mouse fluorescein isothiocyanate (FITC; 1:500; Sigma, St. Louis, MO). The buffer for all incubation steps was PBS with 1% BSA and 0.5% Triton X-100; intermediate washing steps were done in PBS. Sections were mounted on gelatin-coated slides and coverslipped with fluorescent mounting medium (Dako, Carpinteria, CA).
Confocal laser microscopy. A Zeiss LSM 510 confocal imaging system equipped with a monochromatic argon laser light source (wavelength, 458 and 488 nm) was attached to an inverse Axiovert 100 Zeiss (Göttingen, Germany) microscope. The 488 nm line of this laser was used to excite the FITC fluorophore, the beam passing through a dichroic beam splitter (FT 488) and an emission bandpass filter (BP 505–530). A second helium–neon laser was used to emit monochromatic light at 543 nm. This laser line was applied to excite the Cy3 fluorophore, using a dichroic beam splitter FT 543 and an emission bandpass filter BP 580–615. Immunofluorescence images of both channels were stored for further analysis as digitized images with an eight bit resolution (1024 × 1024 pixels).
Analysis of pCREB-IR. Initially, all sections of the SCN immunostained for pCREB were inspected routinely with a Zeiss microscope (Axioplan; 100×) equipped with a video camera that was connected to a computerized image analysis system (VIDAS, Kontron, Germany). Semiquantitative analyses were performed as described (Pfeffer et al., 1998). Briefly, the images were digitized; background staining was used to define the lower threshold. Within the area of the SCN all cell nuclei showing a pCREB-IR exceeding the threshold were marked. Because pCREB-stained cell nuclei were homogeneously distributed throughout the rostrocaudal extent of the SCN, three sections of the intermediate aspect of the SCN were chosen at random for further analysis. Nuclei with a pCREB-IR were counted in a blind manner, and the mean numbers (± SEM) of stained nuclei within SCN boundaries were used for statistical analysis.
Statistical analysis. Statistical analysis of the in vivo and the in vitro experiments was performed using Graph Pad Prism (Graph Pad, San Diego, CA). Significant differences between groups were determined with a one-way ANOVA, followed by Tukey’s post hoc test. Data are presented as the mean ± SEM. Values were considered significantly different withp < 0.05.
Materials. Drugs and chemicals were obtained from the following sources: PACAP, melatonin, and forskolin were from Calbiochem (Lucerne, Switzerland); all others drugs and chemicals were purchased from Sigma.
RESULTS
Effects of light on locomotor activity in mice kept under DD
The period length of the locomotor activity rhythm in constant darkness was not significantly different between C57BL (23.77 ± 0.11 hr; n = 16) and C3H (23.65 ± 0.07 hr;n = 16) mice. Exposure to a light pulse during subjective day (CT06, for C3H, n = 9; for C57BL,n = 6; CT10, for C3H, n = 5; for C57BL,n = 6) or sham exposure in DD (controls, for C3H,n = 10; for C57BL, n = 8) had no significant effects on the phase of locomotor activity rhythms (Figs.1,2A). Light pulses applied during subjective night (CT14 and CT18) resulted in stable phase delays in both strains of mice (Figs. 1, 2A); light exposure of animals 2 hr after activity onset (CT14) was most effective to induce phase delays in C57BL (−1.78 ± 0.18 hr;n = 7; p < 0.01 vs controls) and C3H (−1.52 ± 0.2 hr; n = 6; p < 0.01 vs controls) animals. C57BL mice showed almost similar phase delays at CT18 (−1.71 ± 0.5 hr; n = 6;p < 0.01 vs controls) and at CT14, whereas in C3H mice phase delays were significantly smaller at CT18 (−0.78 ± 0.16 hr; n = 6; p < 0.05 vs controls and vs CT14) compared with that at CT14. The light-induced phase delays at CT18 were significantly larger (p < 0.01) in C57BL mice than in C3H mice (Fig. 2A).
Fig. 1.
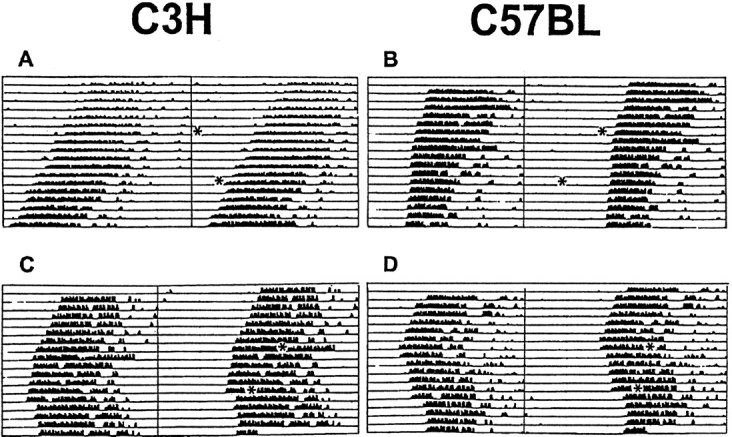
Light-induced effects on locomotor activity of C3H and C57BL mice. Animals were kept under constant darkness (dim red light). Representative double-plotted actograms of animal wheel-running activity are shown with asterisks indicating the time of light pulses (15 min; 1000 lux). Light presented during subjective day (CT06 and CT10) had little or no effect in C3H (A) and C57BL (B) mice, whereas light applied during subjective night (CT14 and CT18) resulted in stable phase delays in C3H (C) and C57BL (D) mice.
Fig. 2.
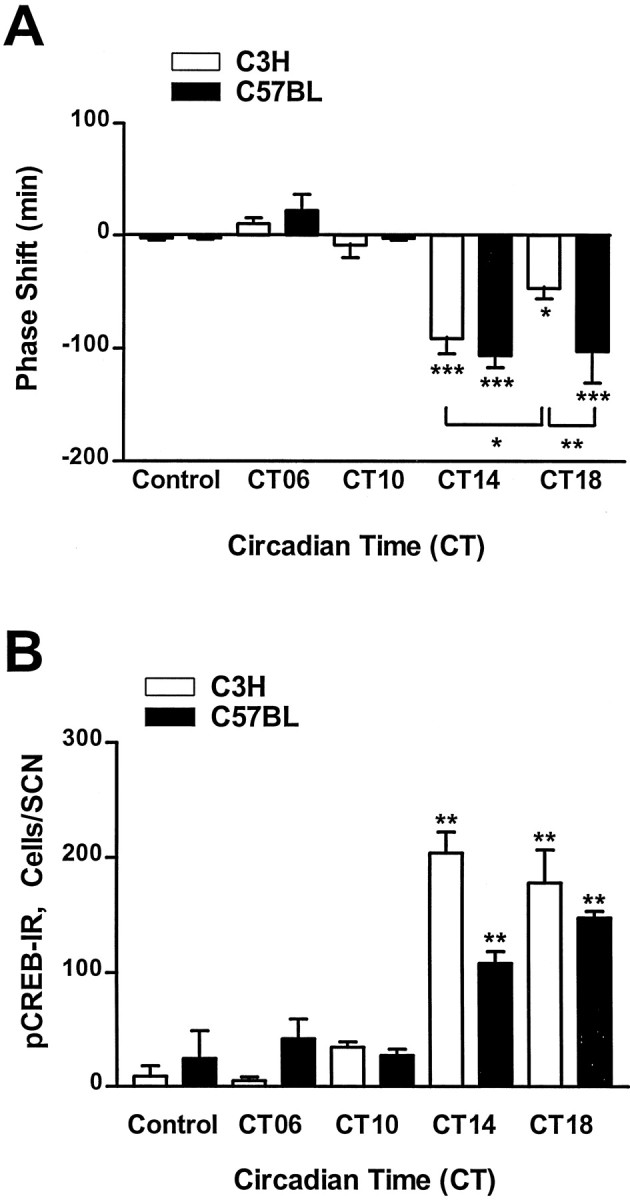
Comparison of light-induced effects on locomotor activity (A) and phosphorylation of CREB (B) in the mouse SCN. C3H and C57BL mice were kept in constant darkness (dim red light), and brief light pulses (15 min; 1000 lux) were delivered at the times indicated. Control animals were handled but not exposed to light. A, Light-induced phase shifts in locomotor activity were analyzed from recorded actograms. Negative values represent phase delays; positive values represent phase advances. Note that the light exposure at CT18 induced significantly smaller phase delays in C3H mice compared with C57BL mice. B, Light-induced pCREB-IR (see also Fig. 3) was quantified in cryostat-cut serial brain sections of the hypothalamic region containing the SCN. Each data point represents the mean ± SEM of 5–16 (A) or 3–9 (B) experiments. *p < 0.05; **p < 0.01; ***p < 0.001.
Induction of pCREB-IR in the SCN after light exposure
Light pulses applied during subjective day (CT06, for C3H,n = 5; for C57BL, n = 3; CT10, for C3H,n = 3; for C57BL, n = 4) or handling the animals in DD at any of the time points investigated (for both strains, n = 4) did not induce pCREB-IR. In both mouse strains a light pulse during subjective night (CT14 and CT18, for both strains, n = 3 for all groups) induced a robust pCREB-IR within the SCN (Figs. 2B,3, 4). In the ventrolateral part of the SCN, double staining for cell nuclei showing a pCREB-IR with perikarya showing a VIP-IR could be observed. In addition, some SCN cells showed only pCREB-IR, whereas others showed only VIP-IR (Fig. 4). Thus, because the pCREB-IR overlaps only partially with the VIP-IR that marks cells receiving a direct retinal input (Tanaka et al., 1993), the phosphorylation of CREB may contribute to the integration of photic information with pacemaker adjustment.
Fig. 3.
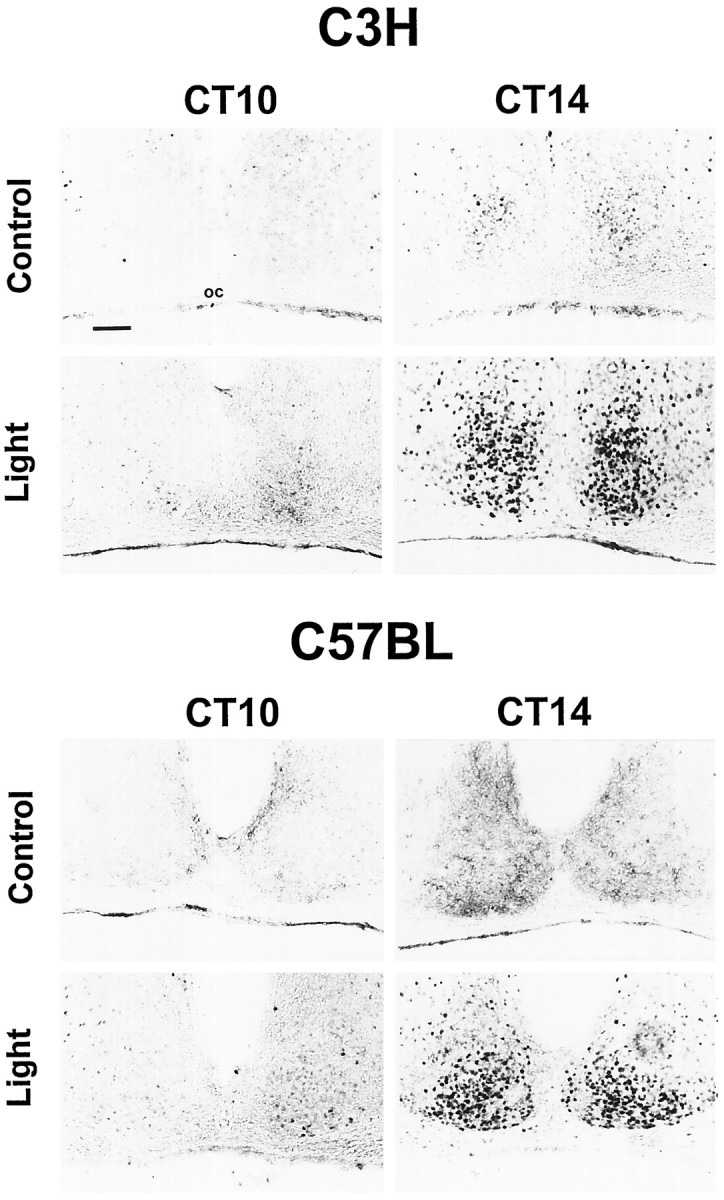
Immunocytochemical demonstration of a light-induced CREB phosphorylation in mouse SCN. Representative coronal sections through the hypothalamic region containing the SCN of C3H and C57BL mice are shown. Nuclear pCREB-IR in the SCN of mice under free-running conditions was induced when a brief light pulse (Light) was delivered 2 hr after activity onset (CT14) or in the middle of the subjective night (CT18; data not shown). Light stimulation had no effect when given 2 hr before activity onset (CT10) or in the middle of the subjective day (CT06; data not shown). Control animals not exposed to the light stimulus (Control) showed only a weak basal pCREB-IR within the SCN. Scale bar, 50 μm. oc, Optic chiasm.
Fig. 4.
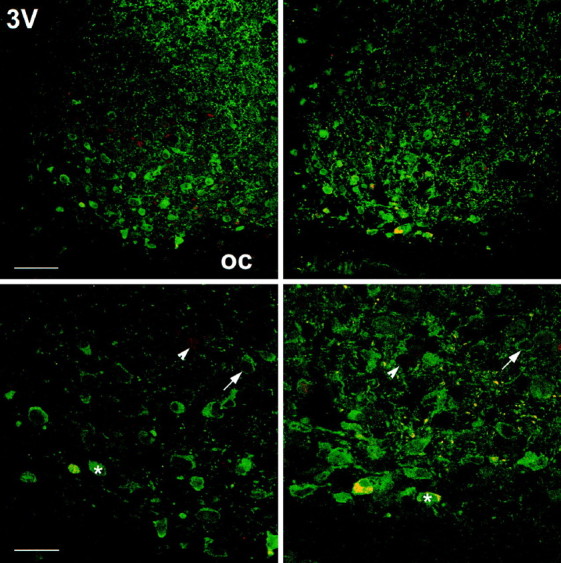
Distribution of pCREB-IR and VIP-IR in the mouse SCN. C3H (left) and C57BL (right) mice, kept under standard light/dark conditions, were exposed to bright white light (10 min) at ZT14. Double-label immunocytochemistry using a confocal laser-scanning microscope shows the spatial distribution of light-induced pCREB-IR nuclei (red) and VIP-IR cells (green). Several cells show both nuclear pCREB-IR and cytoplasmic VIP-IR (arrows); some cells show either a nuclear pCREB-IR (arrowheads) or a cytoplasmic VIP-IR (asterisks) only. Scale bars: upper, 50 μm; lower, 25 μm. oc, Optic chiasm;3V, third ventricle.
PACAP- and glutamate-induced pCREB-IR in the SCNin vitro
There was no significant difference in the levels of basal pCREB-IR in untreated slices of both mouse strains, at any of the zeitgeber times investigated (controls, for both strains,n = 16). Induction of pCREB-IR by the two agonists was phase- dependent, but with contrasting windows of sensitivity. Glutamate stimulated CREB phosphorylation at ZT14 and ZT18 in the SCN of C3H (ZT14, n = 5; p < 0.001 vs controls; ZT18, n = 5; p < 0.05 vs controls) and C57BL (ZT14, n = 4; p < 0.001 vs controls; ZT18, n = 8; p < 0.05 vs controls) mice. Glutamate application at ZT06 (for C3H,n = 5; for C57BL, n = 7) or ZT10 (for C3H, n = 6; for C57BL, n = 7) did not induce a significant pCREB-IR in either strain (Fig.5). PACAP application at ZT06 (for C3H,n = 4; for C57BL, n = 3), at ZT14 (for both strains, n = 4), or at ZT18 (for both strains,n = 3) did not induce a pCREB-IR in the SCN of either mouse strain. In contrast, at ZT10 PACAP treatment evoked a robust pCREB-IR in the SCN of C3H and C57BL mice (for both strains,n = 5; p < 0.001 vs controls; Fig. 5). The PACAP- or glutamate-induced pCREB-IR did not differ between C3H and C57BL mice at any of the time points investigated. In both strains the pCREB-IR was found predominantly in the ventrolateral region of the SCN (see Fig. 7).
Fig. 5.
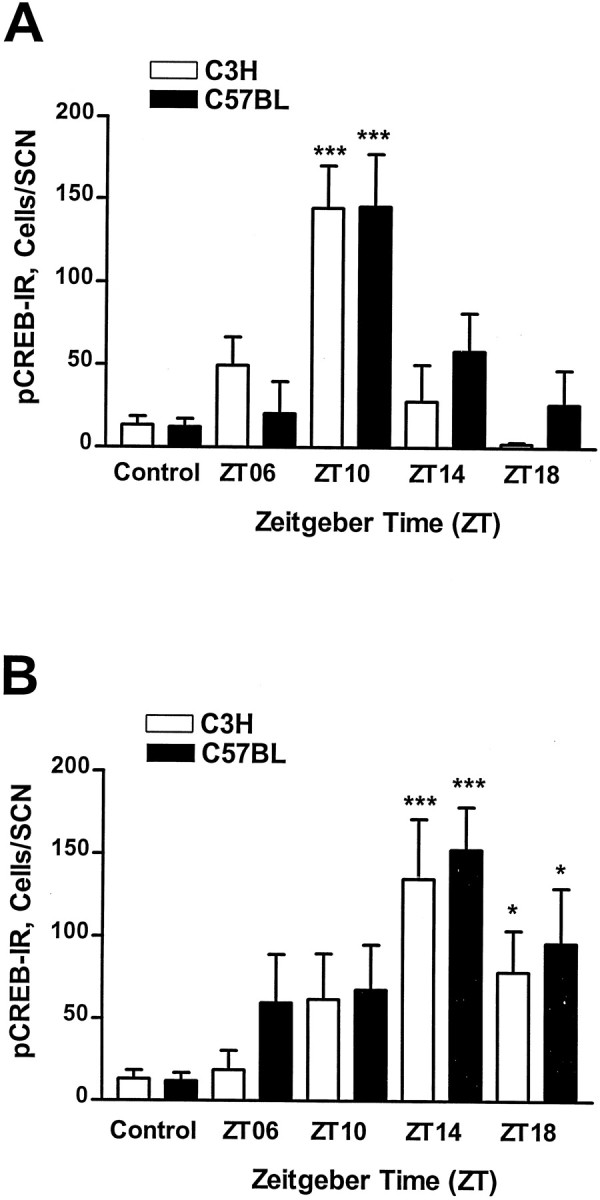
Semiquantitative analysis of the pCREB-IR induced by PACAP (A) or glutamate (B) in mouse SCN brain slices. A, PACAP application (100 nm) evoked a robust pCREB-IR in the SCN of C3H and C57BL mice when applied at ZT10 but had no effect at ZT14, ZT18, or ZT06. B, Glutamate application (100 μm) induced pCREB-IR in the SCN of C3H and C57BL mice at ZT14 and ZT18 but had only slight effects at ZT06 and ZT10. Untreated slices (controls) showed a very low basal pCREB-IR (see also Fig. 7). Each data point represents the mean ± SEM of four to nine animals. Asterisks indicate significantly different values of stimulated slices compared with controls; *p < 0.05; ***p < 0.001.
Fig. 7.
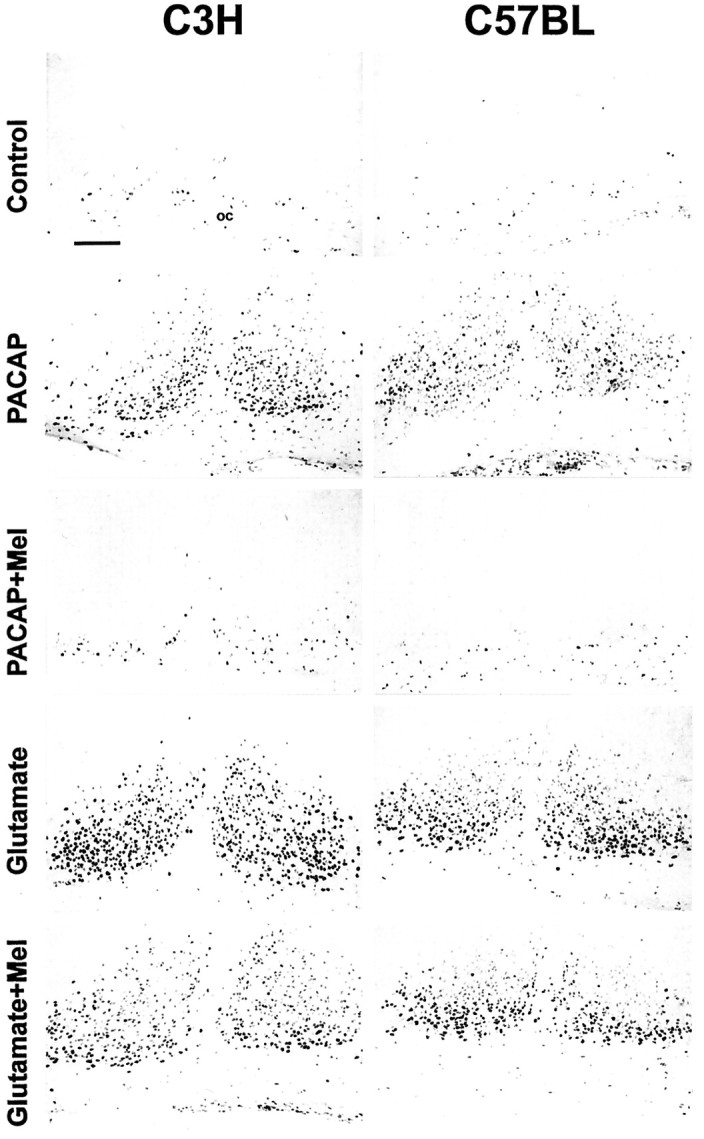
Effects of melatonin on the PACAP- or the glutamate-induced pCREB-IR. Untreated control slices (Control) showed no basal pCREB-IR in the SCN of both mouse strains after 2 hr in culture (ZT10) or after 6 hr in culture (ZT14; data not shown). PACAP application (PACAP) at ZT10 induced a nuclear pCREB-IR in the SCN of C3H and C57BL mice. The PACAP effect was suppressed when slices were preincubated with melatonin (PACAP + Mel). Glutamatergic stimulation (Glutamate) of slices at ZT14 evoked a nuclear pCREB-IR in the SCN of both mouse strains. Glutamate-effects were not affected by melatonin (Glutamate + Mel). In both mouse strains melatonin alone did not induce a pCREB-IR in the SCN (data not shown). Scale bar, 50 μm.oc, Optic chiasm.
Effects of melatonin on PACAP- and glutamate-induced CREB phosphorylation
Melatonin was applied to SCN slices at ZT10 and ZT14, because at these time points a maximal pCREB-IR was induced by PACAP and glutamate, respectively. Although melatonin (1 nm) alone was without effect (data not shown), it significantly inhibited the PACAP-induced CREB phosphorylation in C3H (n = 4;p < 0.001) and C57BL (n = 5;p < 0.001). In contrast, the glutamate-evoked pCREB-IR remained unaltered in both mouse strains when slices were incubated with melatonin before and during drug treatment (for C3H,n = 5; for C57BL, n = 4) (Figs.6, 7).
Fig. 6.
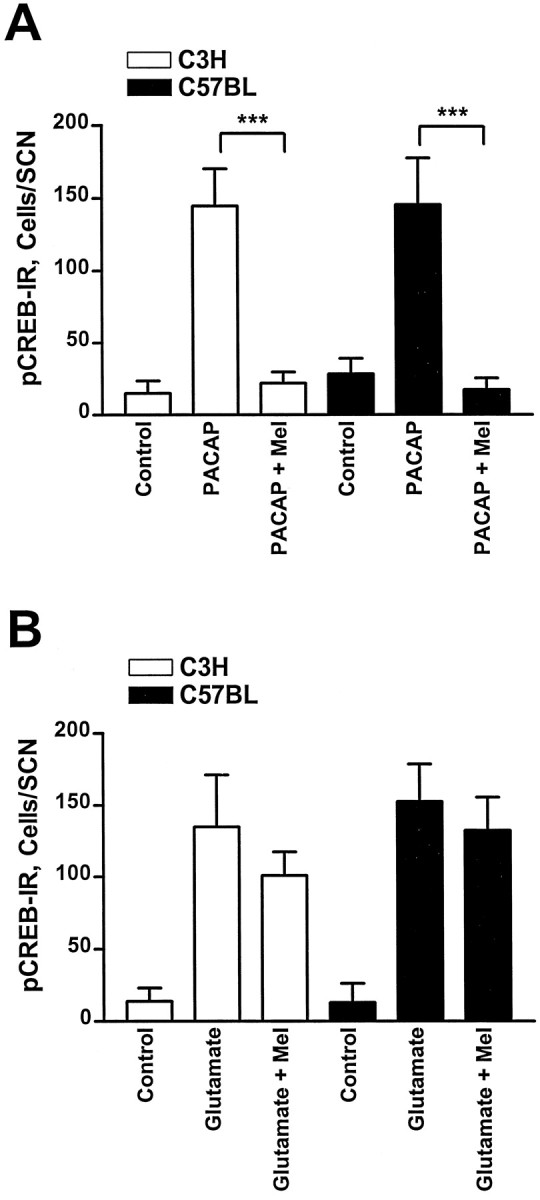
Semiquantitative analysis of the effects of melatonin on the PACAP- or the glutamate-induced pCREB-IR.A, A preceding incubation of SCN brain slices with melatonin (Mel; 1 nm) prevented the induction of a pCREB-IR by PACAP at ZT10 in both mouse strains.B, The glutamate-induced pCREB-IR in the SCN of both mouse strains at ZT14 was unaltered when slices were preincubated with melatonin. Each data point represents the mean ± SEM of four to eight animals; ***p < 0.001.
DISCUSSION
Adaptation of the circadian pacemaker in the mammalian SCN to changing environmental light/dark cycles is fundamental for survival. Our analyses of signaling events in the mouse SCN highlight a major role for the transcription factor CREB as a molecular interface between various resetting cues accessing the circadian clock. In particular, we demonstrate that light and two important transmitters of the RHT, PACAP and glutamate, activate CREB in the SCN by phosphorylation during discrete time windows that match with the corresponding temporal domains for these signals to induce phase shifts in vivo(for review, see Gillette, 1996). The comparative analyses of melatonin-deficient and -proficient mice show that sensitivity windows for resetting cues are determined predominantly by cell-autonomous mechanisms within the SCN rather than by a phasic melatonin signal.
The principal natural stimulus for the phase adjustment of the circadian oscillator is light (for review, see Meijer, 1991). The light-induced phase delays in locomotor activity of both mouse strains are consistent with previous reports (Schwartz and Zimmerman, 1990;Benloucif and Dubocovich, 1996). Exogenous melatonin was reported to attenuate photically induced phase delays in C3H mice at CT14 and CT18 (Dubocovich et al., 1996). Because C3H mice show an elevated melatonin synthesis only during the second half of the night (Goto et al., 1989), the endogenous hormone effect on light-induced phase shifts should not be detectable at CT14. Indeed, phase delays are attenuated at CT18 as compared with CT14. Although this interpretation awaits further experimentation, it is supported by consistently large phase delays in melatonin-deficient C57BL mice at CT14 and at CT18 (Ebihara et al., 1986; Goto et al., 1989). In both mouse strains, resetting light pulses were always associated with CREB phosphorylation in the SCN, a molecular link originally observed in the Syrian hamster (Ginty et al., 1993). Notably, the dynamic profile of the pCREB-IR was not different between the two mouse strains and, thus, independent of the size of the phase delays. These results imply that endogenous melatonin may modulate the magnitude of light-induced phase shifts, but not the acute cellular responses of the SCN to light, including the induction of pCREB. These observations profile a feedback function for the pineal hormone, with melatonin potentially setting the gain of SCN sensitivity to resetting stimuli on a diurnal and a seasonal basis.
Glutamate, a principal transmitter of the RHT (for review, see Ebling, 1996), induced CREB phosphorylation in the SCN probably via activation of the Ca2+/calmodulin-signaling pathway. In both mouse strains glutamate-induced CREB phosphorylation was observed during subjective night but not during subjective daytime. This observation extends the results of a previous study in rat, which compared the effects of glutamate at CT07 and CT20 (Ding et al., 1997). The temporal window of sensitivity to glutamate coincides with the period of glutamate-induced phase shifts in rat SCN explants (Ding et al., 1994). Notably, CREB phosphorylation is a consistent cellular response to resetting glutamatergic cues, regardless of the direction of the phase response, because it can be induced at early subjective night (this study) when glutamate induces maximal phase delays (Ding et al., 1994) and at CT20 (Ding et al., 1997) when glutamate induces phase advances (Ding et al., 1994).
PACAP is an RHT transmitter that stimulates cAMP production in the SCN via the PACAP-R1 receptor (Hannibal et al., 1997). Because PACAP levels in the rat SCN are reduced by light (Fukuhara et al., 1997), any release of PACAP by RHT fibers would make the SCN “sense darkness” and allow the pacemaker to readjust rapidly to ambient lighting conditions. In our hands PACAP induced a maximal CREB phosphorylation at ZT10, and the effect was not different between the two mouse strains. Therefore, as for glutamate and light, the gate of sensitivity is set independently of any rhythmic melatonin signal from the pineal gland. The sensitivity window for PACAP overlaps temporally with phase advances induced either by dark pulses in vivo(Ellis et al., 1982) or by application of cAMP analogs in vitro (Prosser and Gillette, 1989). The phasic induction of pCREB-IR by PACAP described here for mice conforms to studies in rat showing that the resetting action of this peptide is restricted to subjective daytime (Hannibal et al., 1997). However, Hannibal et al. investigated the resetting action of PACAP at ZT06 and not at ZT10, a time point when we observed maximal induction of pCREB in mice. All data support the concept that phosphorylation of CREB mediates PACAP-mediated retinal signaling to the clock.
Our investigations with the two RHT transmitters imply a switch in SCN sensitivity between PACAP (daytime), activating the cAMP/adenylate cyclase-signaling pathway (Gillette, 1996), and glutamate (nighttime), stimulating the Ca2+/calmodulin-signaling pathway (Ebling, 1996). This switch occurs endogenously around the light/dark transition and is set independently of a phasic melatonin signal, because it is present in C3H and also in C57BL mice. All these findings support the notion that transcription is part of the mechanism for adjustment of oscillator timing.
Exogenous melatonin applied around dusk is also a potent resetting cue in rats (Armstrong et al., 1986), in Siberian hamsters (, and in some strains of mouse (Duffield et al., Benloucif and Dubocovich, 1996). In the current study, melatonin reversed PACAP-induced phosphorylation of CREB when applied at ZT10 in both C3H and C57BL mice. This conforms to an autoradiographic demonstration of melatonin-binding sites in the SCN of both strains (Siuciak et al., 1990). Characterization of the two melatonin receptor subtypes expressed in the mouse SCN, the Mel1a (Roca et al., 1996) and the Mel1b receptor (Liu et al., 1997), has shown that both are coupled to inhibition of adenylate cyclase activity (for review, see Reppert et al., 1996). It is therefore likely that in the SCN of both mouse strains, molecular cross talk exists between melatonin- and PACAP-regulated signaling at the level of cAMP accumulation but not between melatonin and the glutamate-activated Ca2+-dependent pathway. The insensitivity of this signal transduction pathway toward melatonin may secure the responsiveness of the SCN to light-induced phase shifts at night. This is consistent with a recent report that melatonin cannot affect glutamatergic induction of pCREB-IR in the SCN of neonatal Syrian hamsters (McNulty et al., 1998).
Our data suggest that CREB serves in the mouse SCN as a molecular interface to translate a gated transmitter preference for adjustment of the phase of the pacemaker. This convergence of multiple and separately inducible signaling pathways onto CREB phosphorylation is well known (Montminy et al., 1990; Dash et al., 1991; Sheng et al., 1991). In the mouse SCN such convergence seems to be achieved via a rapid and efficient but restricted intracellular molecular cross talk and may affect and adjust clockwork transcription. The evidence supporting this idea can be derived from observations that in the SCN of both mouse strains, CREB phosphorylation is induced within a few minutes by (1) light stimuli that reset the SCN pacemaker, (2) glutamatergic receptor activation, and (3) PACAPergic receptor activation. Importantly, (4) melatonin interferes selectively with the PACAP-induced CREB phosphorylation in both mouse strains. The case for granting CREB a central role for the integration of photic information into the clockwork can also be inferred from known molecular details of this transcription factor (Ginty et al., 1993; McNulty et al., 1994, 1998;Tamotsu et al., 1995; Kako et al., 1996). It should be noted, however, that our data do not allow us to eliminate a possible parallel processing of clock resetting and CREB phosphorylation, both affected by PACAP and glutamate signaling (Gillette, 1996).
The very rapid stimulus-induced CREB phosphorylation in the rodent SCN allows this event to intersect with clock mechanisms at the earliest time point possible. CREB phosphorylation induced by photic stimulation at nighttime occurs before the transcriptional induction of immediate early genes that is associated with light-induced phase shifts in clock function (Wollnik et al., 1995; for review, seeHastings, 1997) and before the rapid elevation of mPerI andmPerII mRNA levels, the putative mouse ortholog of theDrosophila clock gene period (Shearman et al., 1997; Zylka et al., 1998). As CREB phosphorylation is known to be a key step in coupling shortterm neuronal stimuli to long-term intracellular responses (Yamamoto et al., 1988; Montminy et al., 1990), the light-induced CREB phosphorylation in rodent SCN may be the molecular initiator to reset the phase of circadian behavioral cycles. It may be envisioned that pCREB induction affects clock genes likemPer to cause phase delays during the early night by retarding the spontaneous decline in SCN activity and to cause phase advances during late night by activating a precocious increase in the molecular oscillation, based on mPer.
Footnotes
This study was supported by grants from the Deutsche Forschungsgemeinschaft (H.-W.K. and J.H.S.), the Wellcome Trust (G.E.D. and M.H.H.), and the European Federation of Experimental Morphology (C.v.G.). We thank H. Wicht for advice with image analysis, H. Meissl for helpful discussion, and I. Schneider-Hüther for technical support.
Parts of this paper have been presented at the 1998 Meeting of the Anatomical Society, Greifswald, Germany, and appeared in the meeting proceedings.
Correspondence should be addressed to Dr. Jörg H. Stehle, Dr. Senckenbergische Anatomie, Anatomisches Institut II, Johann Wolfgang Goethe-Universität, Theodor-Stern-Kai 7, D-60590 Frankfurt, Germany.
REFERENCES
- 1.Armstrong SM, Cassone VM, Chesworth MJ, Redman JR, Short RV. Synchronization of mammalian circadian rhythms by melatonin. J Neural Transm. 1986;21:375–394. [PubMed] [Google Scholar]
- 2.Benloucif S, Dubocovich ML. Melatonin and light induce phase shifts of circadian activity rhythms in the C3H/HeN mouse. J Biol Rhythms. 1996;11:113–125. doi: 10.1177/074873049601100204. [DOI] [PubMed] [Google Scholar]
- 3.Cassone VM, Roberts MH, Moore RY. Melatonin inhibits metabolic activity in the rat suprachiasmatic nuclei. Neurosci Lett. 1987;81:29–34. doi: 10.1016/0304-3940(87)90335-1. [DOI] [PubMed] [Google Scholar]
- 4.Castel M, Belenky M, Cohen S, Ottersen OP, Storm-Mathisen J. Glutamate-like immunoreactivity in retinal terminals of the mouse suprachiasmatic nucleus. Eur J Neurosci. 1993;5:368–381. doi: 10.1111/j.1460-9568.1993.tb00504.x. [DOI] [PubMed] [Google Scholar]
- 5.Dash PK, Karl KA, Colicos MA, Prywes R, Kandel ER. cAMP response element-binding protein is activated by Ca2+/calmodulin- as well as cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1991;88:5061–5065. doi: 10.1073/pnas.88.11.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding JM, Chen D, Weber ET, Faiman LE, Rea MA, Gillette MU. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science. 1994;266:1713–1717. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- 7.Ding JM, Faiman LE, Hurst WJ, Kuriashkina LR, Gillette MU. Resetting the biological clock: mediation of nocturnal CREB phosphorylation via light, glutamate, and nitric oxide. J Neurosci. 1997;17:667–675. doi: 10.1523/JNEUROSCI.17-02-00667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding JM, Buchanan GF, Tischkau SA, Chen D, Kuriashkina L, Faiman LE, Alster JM, McPherson PS, Campbell KP, Gillette MU. A neuronal ryanodine receptor mediates light-induced phase delays of the circadian clock. Nature. 1998;394:381–384. doi: 10.1038/28639. [DOI] [PubMed] [Google Scholar]
- 9.Dubocovich ML, Benloucif S, Masana MI. Melatonin receptors in the suprachiasmatic nucleus. Behav Brain Res. 1996;73:141–147. doi: 10.1016/0166-4328(96)00086-1. [DOI] [PubMed] [Google Scholar]
- 10.Duffield GE, Hastings MH, Ebling FJP (1999) Investigations into the regulation of the circadian system by dopamine and melatonin in the adult Siberian hamster (Phodopus sungorus). J Neuroendocrinol, in press. [DOI] [PubMed]
- 11.Ebihara S, Marks T, Hudson DJ, Menaker M. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science. 1986;231:491–493. doi: 10.1126/science.3941912. [DOI] [PubMed] [Google Scholar]
- 12.Ebling FJP. The role of glutamate in the photic regulation of the suprachiasmatic nucleus. Prog Neurobiol. 1996;50:109–132. doi: 10.1016/s0301-0082(96)00032-9. [DOI] [PubMed] [Google Scholar]
- 13.Ellis GB, McKleen RE, Turek FW. Dark pulses affect the circadian rhythm of activity in hamsters kept in constant light. Am J Physiol. 1982;242:44–50. doi: 10.1152/ajpregu.1982.242.1.R44. [DOI] [PubMed] [Google Scholar]
- 14.Fukuhara C, Suzuki N, Matsumoto Y, Nakayama Y, Aoki K, Tsujimoto G, Inouye SIT, Masuo Y. Day-night variation of pituitary adenylate cyclase-activating polypeptide (PACAP) level in the rat suprachiasmatic nucleus. Neurosci Lett. 1997;229:49–52. doi: 10.1016/s0304-3940(97)00415-1. [DOI] [PubMed] [Google Scholar]
- 15.Gillette MU. The suprachiasmatic nuclei: circadian phase-shifts induced at the time of hypothalamic slice preparation are preserved in vitro. Brain Res. 1986;379:176–181. doi: 10.1016/0006-8993(86)90273-8. [DOI] [PubMed] [Google Scholar]
- 16.Gillette MU. Regulation of entrainment pathways by the suprachiasmatic circadian clock: sensitivities to second messengers. In: Buijs RM, Kalsbeek A, Romijn HJ, Pennartz CMA, Mirmiran M, editors. Progress in brain research, Vol III, Hypothalamic integration of circadian rhythms. Elsevier; Amsterdam: 1996. pp. 121–132. [DOI] [PubMed] [Google Scholar]
- 17.Ginty DD, Kornhauser JM, Thompson MA, Bading H, Mayo KE, Takahashi JS, Greenberg ME. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science. 1993;260:238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- 18.Goto M, Oshima I, Tomita T, Ebihara S. Melatonin content of the pineal glands in different mouse strains. J Pineal Res. 1989;7:195–204. doi: 10.1111/j.1600-079x.1989.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 19.Hannibal J, Ding JM, Chen D, Fahrenkrug J, Larsen PJ, Gillette MU, Mikkelsen JD. Pituitary adenylate cyclase-activating peptide (PACAP) in the retinohypothalamic tract: a potential daytime regulator of the biological clock. J Neurosci. 1997;17:2637–2644. doi: 10.1523/JNEUROSCI.17-07-02637.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hastings MH. Central clocking. Trends Neurosci. 1997;20:459–463. doi: 10.1016/s0166-2236(97)01087-4. [DOI] [PubMed] [Google Scholar]
- 21.Kako K, Wakamatsu H, Ishida N. c-fos CRE-binding activity of CREB/ATF family in the SCN is regulated by light but not a circadian clock. Neurosci Lett. 1996;216:159–162. doi: 10.1016/0304-3940(96)13018-4. [DOI] [PubMed] [Google Scholar]
- 22.Klein DC, Moore RY, Reppert SM. Suprachiasmatic nucleus: the mind’s clock (Klein DC, Moore RH, Reppert SM, eds), pp 1–467. Oxford UP; New York: 1991. [Google Scholar]
- 23.Kopp M, Meissl H, Korf HW. The pituitary adenylate cyclase-activating polypeptide-induced phosphorylation of the transcription factor CREB (cAMP response element binding protein) in the rat suprachiasmatic nucleus is inhibited by melatonin. Neurosci Lett. 1997;227:145–148. doi: 10.1016/s0304-3940(97)00312-1. [DOI] [PubMed] [Google Scholar]
- 24.Kornhauser JM, Nelson DE, Mayo KE, Takahashi JS. Photic and circadian regulation of c-fos gene expression in the hamster suprachiasmatic nucleus. Neuron. 1990;5:127–134. doi: 10.1016/0896-6273(90)90303-w. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK, Reppert SM. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19:91–102. doi: 10.1016/s0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 26.McArthur AJ, Gillette MU, Prosser RA. Melatonin directly resets the rat suprachiasmatic circadian clock in vitro. Brain Res. 1991;565:158–161. doi: 10.1016/0006-8993(91)91748-p. [DOI] [PubMed] [Google Scholar]
- 27.McNulty S, Ross AW, Barrett B, Hastings MH, Morgan PJ. Melatonin regulates the phosphorylation of CREB in ovine pars tuberalis. J Neuroendocrinol. 1994;6:523–532. doi: 10.1111/j.1365-2826.1994.tb00615.x. [DOI] [PubMed] [Google Scholar]
- 28.McNulty S, Schurov IL, Sloper PJ, Hastings MH. Stimuli which entrain the circadian clock of the neonatal Syrian hamster in vivo regulate the phosphorylation of the transcription factor CREB in the suprachiasmatic nucleus in vitro. Eur J Neurosci. 1998;10:1063–1072. doi: 10.1046/j.1460-9568.1998.00114.x. [DOI] [PubMed] [Google Scholar]
- 29.Meijer JH. Integration of visual information by the suprachiasmatic nucleus. In: Klein DC, Moore RH, Reppert SM, editors. Suprachiasmatic nucleus: the mind’s clock. Oxford UP; New York: 1991. pp. 107–119. [Google Scholar]
- 30.Montminy MR, Gonzalez GA, Yamamoto KK. Regulation of cAMP-inducible genes by CREB. Trends Neurosci. 1990;13:184–188. doi: 10.1016/0166-2236(90)90045-c. [DOI] [PubMed] [Google Scholar]
- 31.Pfeffer M, Kühn R, Krug L, Korf HW, Stehle JH. Rhythmic variation in β1-adrenergic receptor mRNA levels in rat pineal gland: circadian and developmental regulation. Eur J Neurosci. 1998;10:2896–2904. doi: 10.1111/j.1460-9568.1998.00309.x. [DOI] [PubMed] [Google Scholar]
- 32.Prosser RA, Gillette MU. The mammalian circadian clock in the suprachiasmatic nuclei is reset in vitro by cAMP. J Neurosci. 1989;9:1073–1081. doi: 10.1523/JNEUROSCI.09-03-01073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prosser RA, McArthur AJ, Gillette MU. cGMP induces phase shifts of a mammalian circadian pacemaker at night, in antiphase to cAMP effects. Proc Natl Acad Sci USA. 1989;86:6812–6815. doi: 10.1073/pnas.86.17.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redman J, Armstrong SM, Ng KT. Free-running activity rhythms in the rat: entrainment by melatonin. Science. 1983;219:1089–1091. doi: 10.1126/science.6823571. [DOI] [PubMed] [Google Scholar]
- 35.Reppert SM, Weaver DR, Godson C. Melatonin receptors step into the light: cloning and classification of subtypes. Trends Pharmacol Sci. 1996;17:100–102. doi: 10.1016/0165-6147(96)10005-5. [DOI] [PubMed] [Google Scholar]
- 36.Roca AL, Godson C, Weaver DR, Reppert SM. Structure, characterization, and expression of the gene encoding the mouse Mel1a receptor. Endocrinology. 1996;137:3469–3477. doi: 10.1210/endo.137.8.8754776. [DOI] [PubMed] [Google Scholar]
- 37.Rusak B, Robertson HA, Wisden W, Hunt SP. Light pulses that shift rhythms induce gene expression in the suprachiasmatic nucleus. Science. 1990;248:1237–1240. doi: 10.1126/science.2112267. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz WJ, Zimmerman P. Circadian timekeeping in BALB/c and C57BL/6 inbred mouse strains. J Neurosci. 1990;10:3685–3694. doi: 10.1523/JNEUROSCI.10-11-03685.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shearman LP, Zylka MJ, Weaver DR, Kolakowski LF, Reppert SM. Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron. 1997;19:1261–1269. doi: 10.1016/s0896-6273(00)80417-1. [DOI] [PubMed] [Google Scholar]
- 40.Sheng M, Thompson MA, Greenberg ME. CREB: a Ca2+-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- 41.Shirakawa T, Moore RY. Glutamate shifts the phase of the circadian neuronal firing rhythm in the rat suprachiasmatic nucleus. Neurosci Lett. 1994;178:47–50. doi: 10.1016/0304-3940(94)90286-0. [DOI] [PubMed] [Google Scholar]
- 42.Siuciak JA, Fang JM, Dubocovich ML. Autoradiographic localization of 2-[125I]iodomelatonin binding sites in the brains of C3H/HeN and C57BL/6J strains of mice. Eur J Pharmacol. 1990;180:387–390. doi: 10.1016/0014-2999(90)90328-4. [DOI] [PubMed] [Google Scholar]
- 43.Stehle JH, Vanecek J, Vollrath L. Effects of melatonin on spontaneous electrical activity of neurons in rat suprachiasmatic nuclei: an in vitro iontophoretic study. J Neural Transm. 1989;78:173–177. doi: 10.1007/BF01252503. [DOI] [PubMed] [Google Scholar]
- 44.Stehle JH, Pfeffer M, Kühn R, Korf H-W. Light-induced expression of transcription factor ICER (inducible cAMP early repressor) in rat suprachiasmatic nucleus is phase-restricted. Neurosci Lett. 1996;217:169–172. [PubMed] [Google Scholar]
- 45.Sumova A, Ebling FJP, Maywood ES, Herbert J, Hastings MH. Non-photic circadian entrainment in the Syrian hamster is not associated with phosphorylation of the transcriptional regulator CREB within the suprachiasmatic nucleus, but is associated with adrenocortical activation. Neuroendocrinology. 1994;59:579–589. doi: 10.1159/000126708. [DOI] [PubMed] [Google Scholar]
- 46.Tamotsu S, Schomerus C, Stehle JH, Roseboom PH, Korf HW. Norepinephrine-induced phosphorylation of the transcription factor CREB in isolated rat pinealocytes: an immunocytochemical study. Cell Tissue Res. 1995;282:219–226. doi: 10.1007/BF00319113. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka M, Ichitani Y, Okamura H, Tanaka Y, Ibata Y. The direct retinal projection to VIP neuronal elements in the rat SCN. Brain Res Bull. 1993;31:637–640. doi: 10.1016/0361-9230(93)90134-w. [DOI] [PubMed] [Google Scholar]
- 48.Wollnik F, Brysch W, Uhlmann E, Gillardon F, Bravo R, Zimmermann M, Schlingensiepen KH, Herdegen T. Block of c-Fos and JunB expression by antisense oligonucleotides inhibits light-induced phase shifts of the mammalian circadian clock. Eur J Neurosci. 1995;7:388–393. doi: 10.1111/j.1460-9568.1995.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto KK, Gonzalez GA, Biggs WH, III, Montminy MR. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988;334:494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Takahashi JS, Turek FW. Critical period for cycloheximide blockade of light-induced phase advances of the circadian locomotor activity rhythm in golden hamsters. Brain Res. 1996;740:285–290. doi: 10.1016/s0006-8993(96)00900-6. [DOI] [PubMed] [Google Scholar]
- 51.Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside the brain. Neuron. 1998;20:1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]


