Abstract
The SNARE hypothesis has been proposed to explain both constitutive and regulated vesicular transport in eukaryotic cells, including release of neurotransmitter at synapses. According to this model, a vesicle targeting/docking complex consisting primarily of vesicle- and target-membrane proteins, known as SNAREs, serves as a receptor for the cytosolic N-ethylmaleimide-sensitive fusion protein (NSF). NSF-dependent hydrolysis of ATP disassembles the SNARE complex in a step postulated to initiate membrane fusion. While features of this model remain tenable, recent studies have challenged fundamental aspects of the SNARE hypothesis, indicating that further analysis of these components is needed to fully understand their roles in neurotransmitter release. We have addressed this issue by using the temperature-sensitive Drosophila NSF mutantcomatose (comt) to study the function of NSF in neurotransmitter release in vivo. Synaptic electrophysiology and ultrastructure in comt mutants have recently defined a role for NSF after docking in the priming of synaptic vesicles for fast calcium-triggered fusion. Here we report that an SDS-resistant neural SNARE complex, composed of the SNARE polypeptides syntaxin, n-synaptobrevin, and SNAP-25, accumulates incomt mutants at restrictive temperature. Subcellular fractionation experiments indicate that these SNARE complexes are distributed predominantly in fractions containing plasma membrane and docked synaptic vesicles. Together with the electrophysiological and ultrastructural analyses of comt mutants, these results indicate that NSF functions to disassemble or otherwise rearrange a SNARE complex after vesicle docking and that this rearrangement is required to maintain the readily releasable pool of synaptic vesicles.
Keywords: N-ethylmaleimide-sensitive fusion protein, NSF, comatose, neurotransmitter exocytosis, Drosophila, SNARE complex, synaptic vesicle
Neurons signal to their target cells primarily through the regulated exocytosis of chemical neurotransmitters. Neurotransmitter release involves a discrete series of steps, including formation of neurotransmitter-filled synaptic vesicles, targeting of synaptic vesicles to presynaptic release sites, and calcium-triggered fusion of synaptic vesicles with the nerve terminal plasma membrane. Recent studies of constitutive and regulated secretion have led to the identification of polypeptides that may function in this process, and a model, known as the SNARE hypothesis, proposing functions for these polypeptides (Rothman, 1994; Scheller, 1995; Südhof, 1995). According to this model, synaptic vesicle targeting to and docking at release sites involves the assembly of vesicle- and target-membrane proteins, referred to as v-SNAREs (e.g., synaptobrevin) and t-SNAREs (e.g., SNAP-25 and syntaxin), respectively. This protein complex recruits the soluble NSF attachment proteins (SNAPs) andN-ethylmaleimide-sensitive fusion protein (NSF) to form a pre-fusion complex. NSF-dependent hydrolysis of ATP disassembles the SNARE complex in a step postulated to initiate membrane fusion (Söllner et al., 1993).
Because the SNARE hypothesis was derived largely from analyses of constitutive secretion and in vitro protein binding experiments, recent efforts have been directed at examining the validity of this model in synaptic function. This work has clearly established an important role for SNAREs (Hunt et al., 1994; Broadie et al., 1995; Schulze et al., 1995; Sweeney et al., 1995; O’Connor et al., 1997; Nonet et al., 1998; Deitcher et al., 1998) and SNAPs (DeBello et al., 1995) in neurotransmitter release. However, these studies have also challenged fundamental tenets of the SNARE hypothesis (Hunt et al., 1994; Broadie et al., 1995; O’Connor et al., 1997) and have led to new models by which the SNARE components may function. Despite these advances, the precise functional roles of several key components of the neurosecretory apparatus, including NSF, remain relatively unexplored.
To investigate the role of NSF in neurotransmitter release, we initiated a genetic analysis of NSF function in the model organismDrosophila melanogaster by identifying aDrosophila NSF gene (termed dNSF1) (Ordway et al., 1994) and a pre-existing temperature-sensitive dNSF1 mutant, known ascomatose (comt) (Pallanck et al., 1995).comt was originally identified in a classical genetic screen for recessive mutations causing temperature-sensitive paralysis (Siddiqi and Benzer, 1976), and recent electrophysiological and ultrastructural studies have demonstrated an activity-dependent reduction in neurotransmitter release and a marked accumulation of docked synaptic vesicles at restrictive temperature in comtmutants (Kawasaki et al., 1998). These and other recent studies of NSF function in regulated secretion (Banerjee et al., 1996;Schweizer et al., 1998) lead to the conclusion that NSF serves to prime vesicles for calcium-triggered fusion after docking.
We have investigated the molecular nature of this priming event by characterizing the composition, state of assembly, and subcellular distribution of the SNARE complex in comt mutants. Here we report a marked accumulation of a ternary SNARE complex composed of syntaxin, n-synaptobrevin, and SNAP-25 in comt mutants after exposure to restrictive temperature. Subcellular fractionation experiments indicate that these SNARE complexes accumulate predominantly in plasma membrane fractions. Examination of SNARE complex abundance in comt and other Drosophilaneural signaling mutants indicates that SNARE complex abundance is specifically affected in mutants with synaptic vesicle trafficking defects. Together with other recent work in comt mutants, these results indicate that dNSF1 functions to disassemble or otherwise rearrange the SNARE complex after synaptic vesicle docking in the process of neurotransmitter exocytosis.
MATERIALS AND METHODS
Drosophila strains
Flies were cultured in standard medium at room temperature (22°C). The three comt strains used in this analysis,ST17, ST53, and TP7, each contain single amino acid substitutions in the dNSF1 coding sequence with respect to the wild-type strain, Canton S. The ST17 andST53 mutations have been reported previously (Pallanck et al., 1995), and the TP7 mutation substitutes a serine for a proline at amino acid position 398 of the dNSF1 open reading frame. Transgenic expression of wild-type dNSF1 protein was accomplished using a P-element construct consisting of a dNSF1 cDNA under the control of an hsp70 heat shock promotor (Pallanck et al., 1995). Expression was induced by heat-shocking flies at 38°C once per day for 3 consecutive d (15 min, 30 min, and 30 min). Heat-shocking comtflies that lacked the transgene did not alter the comtphenotypes. With the exception of the membrane fractionation experiments, all of the studies described in this manuscript involved flies aged 3–6 d.
Preparation and analysis of SNARE complexes
Sample preparation, electrophoresis, and Western blotting. After exposure to a given temperature for a given period of time, flies were rapidly frozen in liquid nitrogen. Heads were removed from frozen flies by vortexing and homogenized in SDS sample buffer (Laemmli, 1970). After a brief centrifugation to pellet cuticle, an aliquot of the supernatant was loaded onto a discontinuous SDS-polyacrylamide gel, with the upper three-fourths and lower one-fourth of the separating gel being adjusted to 9 and 15% acrylamide, respectively. An additional aliquot was boiled for 5 min to disrupt SNARE complexes before gel loading. After electrophoresis, gels were electroblotted onto nitrocellulose filters according to manufacturer’s (Bio-Rad, Hercules, CA) directions. Blots were incubated with a monoclonal anti-syntaxin antibody (MAb 8C3) (Wu et al., 1998), with a monoclonal anti-SNAP-25 antibody (MAb CI.71.1) (Otto et al., 1997), or with polyclonal antisera that recognize sequences in the cytoplasmic (NSYB1) or intravesicular domains (NSYB2) of neuronal synaptobrevin (n-synaptobrevin) that are highly divergent in the ubiquitously expressed synaptobrevin isoform (DiAntonio et al., 1993;van de Goor et al., 1995). After blots were stained with antisera, bands were detected by enhanced chemiluminescence (Amersham, Arlington Heights, IL). Quantitation of SNAREs and SNARE complexes was performed using a GS-700 imaging densitometer (Bio-Rad). Standard curves for densitometry were prepared by diluting samples prepared from normal (Canton-S) flies. Each quantitation experiment was performed in triplicate, and only signals falling within the linear range of the standard curves were used. Results of quantitation experiments (see Table 1) are expressed as ratios of SNARE complex to SNARE monomer to exclude gel loading artifacts.
Table 1.
Results of quantitation experiments
| Genotype | SNARE complex/syntaxin (22°C)1-a | SNARE complex/syntaxin (38°C)1-a |
|---|---|---|
| CS | 1 | 1.5 ± 0.7 |
| comtST17 | 2.0 ± 0.9 | 8.1 ± 3.2 |
| comtTP7 | 1.5 ± 1.0 | 11.5 ± 6.8 |
| comtST53 | 1.5 ± 0.2 | 5.8 ± 1.9 |
| shiTS1 | 0.9 ± 0.4 | 0.2 ± 0.04 |
Relative to CS at 22°C. CS, Wild-type control.
Isolation and analysis of the 73 kDa SNARE complex. A fly head lysate, prepared as described above, was subjected to SDS-PAGE. After electrophoresis, the region corresponding to the 73 kDa band (containing polypeptides in the 55–85 kDa size range), and for control purposes the region below the 73 kDa band (40–55 kDa), were excised, boiled 10 min, and each placed separately on top of a second 12% SDS gel. Polypeptides in the gel slices were then electrophoretically separated in these second SDS gels and subjected to Western blot analysis as above.
Subcellular membrane fractionation
Separation of unbound synaptic vesicles from plasma membrane was performed using glycerol velocity sedimentation, essentially as described (van de Goor et al., 1995). Modifications to this published membrane fractionation procedure included use of a 10–30% glycerol gradient for velocity sedimentation experiments, 6 gm of flies per gradient, and an SW 41 Ti ultracentrifuge rotor (Beckman, Fullerton, CA). These modifications were introduced to increase the separation between peak synaptic vesicle- and plasma membrane-containing glycerol gradient fractions. Synaptic vesicle- and plasma membrane-containing gradient fractions were identified by subjecting aliquots of the gradient fractions to SDS-PAGE and Western blotting using antisera to the synaptic vesicle antigens synapsin (SYNORF1) (Klagges et al., 1996), n-synaptobrevin (NSYB1 and NSYB2; see above), and synaptotagmin (DSYT2) (Littleton et al., 1993), and to the 42 kDa plasma membrane antigen [the putative β-subunit of the Na+/K+ ATPase (van de Goor et al., 1995)] recognized by a rabbit anti-horseradish peroxidase antiserum (ICN Pharmaceuticals, Costa Mesa, CA), as described above. Synaptic vesicle- and plasma membrane-containing fractions were combined into separate pools, mixed with an equal volume of 2× SDS-PAGE loading buffer (Laemmli, 1970), aliquoted, and stored frozen at −20°C. Quantitation of SNARE complexes was performed using densitometry as described above.
RESULTS
A Drosophila SNARE complex, composed of syntaxin, n-synaptobrevin, and SNAP-25, is resistant to denaturation by SDS
If NSF is normally required to disassemble a ternary SNARE complex after synaptic vesicle docking, as suggested by the SNARE hypothesis, loss of comt function should lead to increased levels of the ternary complex. To investigate this issue, we sought to develop a method that would allow us to determine the abundance of the ternary SNARE complex in comt and wild-type flies. Previous work in mammals has demonstrated that neural SNARE complexes are resistant to denaturation by SDS, except when boiled, and that de novoassembly of these complexes does not occur in SDS-containing buffer (Hayashi et al., 1994). Thus we reasoned that if DrosophilaSNARE complexes are similarly resistant to SDS, the quantity of SDS-resistant neural SNARE complex recovered from a fly head homogenate should reflect the quantity of SNARE complex present in vivo.
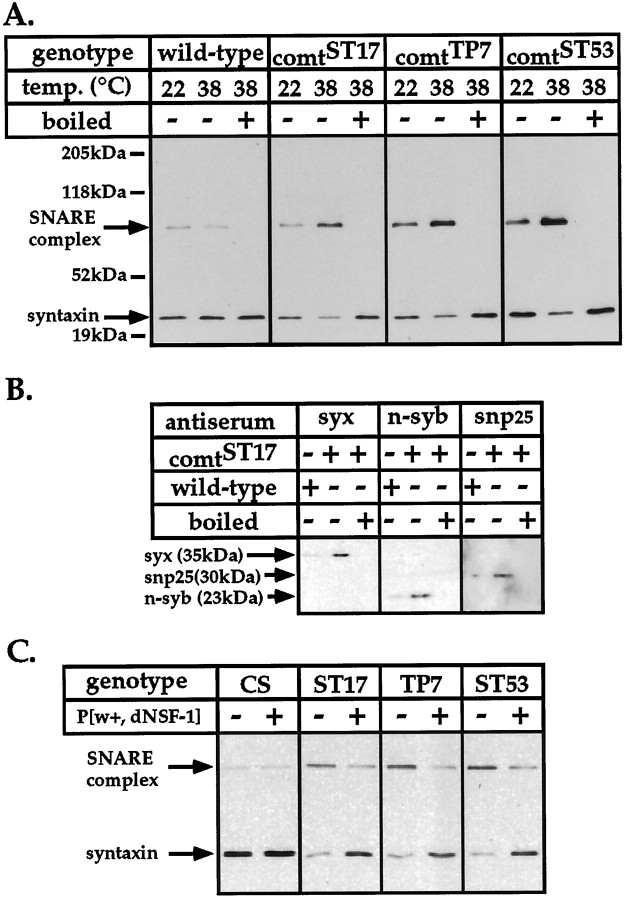
To assay the presence of an SDS-resistant ternary SNARE complex inDrosophila, Western blot analysis was performed on an SDS lysate prepared from wild-type fly heads using monoclonal antibodies to the Drosophila t-SNAREs syntaxin and SNAP-25, and a rabbit polyclonal antiserum to the synaptic vesicle v-SNARE, n-synaptobrevin. In experiments conducted using antisera to syntaxin and n-synaptobrevin, prominent bands corresponding in size to the monomeric forms of these proteins (35 kDa and 23 kDa, respectively) (Schulze et al., 1995; van de Goor et al., 1995) as well as a less abundant, heat-sensitive species of 73 kDa were detected (Fig.1A). Overloading of SDS gels, or prolonged exposures of the immunoblots, resulted in the appearance of additional higher molecular weight bands that might represent higher order SNARE complexes (data not shown). In contrast, the SNAP-25 monoclonal antibody appeared to detect only the monomeric form of SNAP-25 [30 kDa (M. N. Wu, personal communication)], perhaps because the epitope recognized by this antibody was masked in the 73 kDa complex (Fig.1A).
Fig. 1.
Identification of an SDS-resistant neural SNARE complex in Drosophila. A, Western blot analysis of an SDS lysate from wild-type Drosophilaheads, using monoclonal antibodies to syntaxin (syx) and SNAP-25 (snp25) and rabbit polyclonal antisera to the neuronal isoform of synaptobrevin, n-synaptobrevin [(n-syb); results using the NSYB2 antiserum are shown; however, similar results were obtained with the NSYB1 antiserum]. Syntaxin and n-synaptobrevin are detected as monomers of 35 and 23 kDa, respectively, and as part of a higher molecular weight complex (73 kDa) in unboiled samples (−). The 73 kDa complex is disrupted in lysates that were boiled (+) before loading. B, Detection of SNAREs released from the 73 kDa complex by boiling. A head SDS lysate prepared from wild-type flies was electrophoretically separated on an SDS gel, and the 73 kDa region was excised, boiled, and layered onto a second SDS gel. Polypeptides in this gel strip were then subjected to SDS-PAGE and Western blot analysis using the antibodies to syntaxin (syx), n-synaptobrevin (n-syb), and SNAP-25 (snp25) described above. Lanes marked + are samples boiled before loading of the first gel, and those marked − are unboiled samples loaded onto the first gel.
To determine whether the SDS-resistant 73 kDa species does indeed represent a complex composed of syntaxin, n-synaptobrevin, and SNAP-25, the 73 kDa region (containing polypeptides in the 55–85 kDa size range) and for control purposes the region below the 73 kDa band (40–55 kDa) were excised from a gel and boiled to disrupt protein–protein interactions in the putative complex. These gel strips were then layered on top of second SDS gels and subjected to SDS-PAGE and Western blot analysis with antisera to the threeDrosophila SNAREs. Bands corresponding to the expected sizes for monomers of syntaxin, n-synaptobrevin, and SNAP-25 were detected only from gel strips containing the 73 kDa material (Fig.1B; and data not shown), thus establishing the identity of the 73 kDa band.
Excess accumulation of the ternary SNARE complex is observed incomt mutants at restrictive temperature
The abundance of the SDS-resistant 73 kDa ternary SNARE complex was analyzed in three different comt mutants at permissive (22°C) and restrictive (38°C) temperatures (Fig.2, Table 1; and data not shown). For all three comt alleles, SNARE complex abundance relative to monomeric syntaxin and n-synaptobrevin was similar to that seen in wild-type flies at permissive temperature. However, in contrast to wild-type flies, exposure of all threecomt mutants to restrictive temperature resulted in a striking elevation in abundance of the SNARE complex. The increased level of the SNARE complex is paralleled by corresponding decreases of monomeric syntaxin and n-synaptobrevin, indicating that these polypeptides accumulate in a SNARE complex when NSF activity is disrupted in comt mutants. The ratio of 73 kDa SNARE complex to monomeric syntaxin is increased 6- to 11-fold in comtmutants compared with wild-type after a 20 min exposure to restrictive temperature, reflecting both increases in the SNARE complex and decreases in syntaxin monomer. Although this ratio was less severely affected for n-synaptobrevin, similar increases in SNARE complex abundance were detected using antisera to this protein (data not shown).
Fig. 2.
SDS-resistant SNARE complexes accumulate incomt mutants at elevated temperatures. A, Western blot analysis of the 73 kDa SNARE complex fromcomt mutants and wild-type controls (CS) using a syntaxin monoclonal antibody. Flies were maintained at 22°C or exposed to 38°C for 20 min before SNARE complexes were extracted. Samples marked + were boiled before gel loading. Similar results were obtained using antisera to Drosophila n-synaptobrevin.B, SDS lysates prepared from the heads of wild-type flies and comtST17 mutants after exposure to 38°C for 20 min were electrophoretically separated on an SDS gel. After electrophoresis, the 73 kDa region was excised, boiled, and layered onto a second SDS gel, and polypeptides in this gel strip were then subjected to SDS-PAGE and Western blot analysis using antisera to syntaxin (syx), n-synaptobrevin (n-syb) (results using the NSYB1 antiserum are shown), and SNAP-25 (snp25). C, Expression of a dNSF1 transgene rescues SNARE complex accumulation incomt mutants. Western blot analysis of the 73 kDa SNARE complex from three different comt mutant alleles (ST17, TP7, and ST53) and wild-type controls (CS) using a syntaxin monoclonal antibody. Flies with (+) or without (−) the wild-type dNSF1 transgene were exposed to 38°C for 20 min before sample preparation. Similar results were obtained using the n-synaptobrevin antisera.
We also sought to determine whether increased levels of SNAP-25 were recruited into SNARE complexes in comt mutants at restrictive temperature. However, because of the apparent inability of the anti-SNAP-25 antibody to detect SNAP-25 protein in the SNARE complex, we performed Western blot analyses on boiled gel slices containing the 73 kDa SNARE complex, as described above, to compare the levels of incorporation of this protein into SNARE complexes incomt mutants and wild-type flies. To allow direct comparison of the level of recruitment of SNAP-25, syntaxin, and n-synaptobrevin into the SNARE complex in comt mutants with wild-type flies, this analysis was also performed with antisera to syntaxin and n-synaptobrevin. Results from this analysis confirm the increased incorporation of syntaxin and n-synaptobrevin into the SNARE complex incomt mutants at restrictive temperature and demonstrate that SNAP-25 also accumulates in this complex in parallel with these polypeptides (Fig. 2B). Results from this analysis also indicate similar increases in the levels of incorporation of these three polypeptides into the 73 kDa complex in comt mutants at restrictive temperature with respect to wild-type flies. Expression of wild-type dNSF1 protein from a transgene rescues the comtdefect in SNARE complex accumulation but does not alter SNARE complex abundance in normal flies, demonstrating that this phenotype specifically reflects the loss of dNSF1 activity (Fig.2C).
SNARE complex abundance is reduced in a Drosophilasynaptic vesicle recycling mutant and unaffected inDrosophila mutants with altered membrane excitability
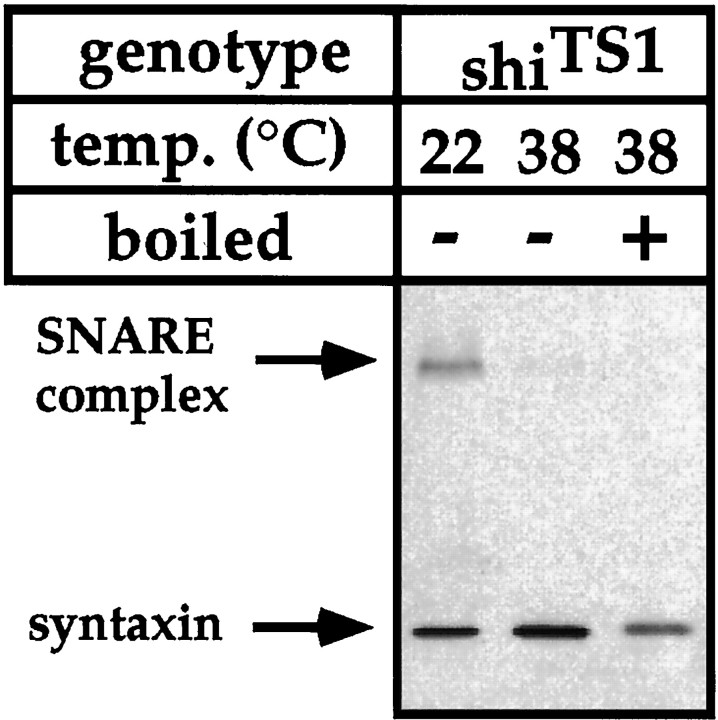
To examine whether the increased abundance of the SDS-resistant ternary SNARE complex observed in comt mutants is a specific feature of the comt phenotype and not a general feature of nervous system dysfunction, we compared SNARE complex abundance in various Drosophila mutants with defects in different aspects of nervous system signaling with SNARE complex abundance in wild-type flies. Specifically, SNARE complex abundance was analyzed in theDrosophila neural signaling mutants Shaker(Sh), Hyperkinetic (Hk), andether-a-go-go (eag) (Wu and Ganetzky, 1992). In addition, SNARE complex abundance was analyzed in theDrosophila temperature-sensitive paralytic mutants no action potential (nap), paralyzed(para), seizure (sei),shibire (shi), and slowpoke(slo), at permissive and restrictive temperatures (Kosaka and Ikeda, 1983; Koenig and Ikeda, 1989; Wu and Ganetzky, 1992; van de Goor et al., 1995). Within this large collection of neural signaling mutants, only shi exhibited an appreciable alteration in the abundance of the ternary SNARE complex (Fig.3; and data not shown). However, in contrast to results observed in analyses of comt mutants, the abundance of the ternary SNARE complex isolated from shimutants is significantly reduced after the shift to restrictive temperature (Fig. 3, Table 1). As was observed for the comtalleles, the ratio of SNARE complex abundance relative to monomeric syntaxin was similar in shi and wild-type flies at permissive temperature.
Fig. 3.
SNARE complex abundance is reduced inshi mutants at restrictive temperature. Western blot analysis of the 73 kDa SNARE complex fromshiTS1 mutants using a syntaxin monoclonal antibody. Flies were maintained at 22°C or exposed to 38°C for 20 min before extracting SNARE complexes. Samples marked + were boiled before gel loading. Similar results were observed using antisera to n-synaptobrevin.
Previous analyses of eag, Hk, nap,para, sei, Sh, and sloindicated that these mutations affect features of membrane excitability (Wu and Ganetzky, 1992), whereas the shi mutation is the only one in this group that specifically affects synaptic vesicle trafficking (Kosaka and Ikeda, 1983; Koenig and Ikeda, 1989; van de Goor et al., 1995). At elevated temperatures, nerve terminals inshi mutants become depleted of synaptic vesicles within several minutes because of a block in the endocytic retrieval of synaptic vesicle membrane (Kosaka and Ikeda, 1983; Koenig and Ikeda, 1989; van de Goor et al., 1995). Thus, the reduction in SNARE complex abundance observed in shi mutants at restrictive temperature is consistent with models for SNARE complex formation occurring during or after synaptic vesicle docking. These results also demonstrate that alterations in SNARE complex abundance are not general features of nervous system dysfunction, but rather are the specific consequences of mutations affecting components that function in synaptic vesicle trafficking.
The SNARE complex colocalizes with plasma membrane markers
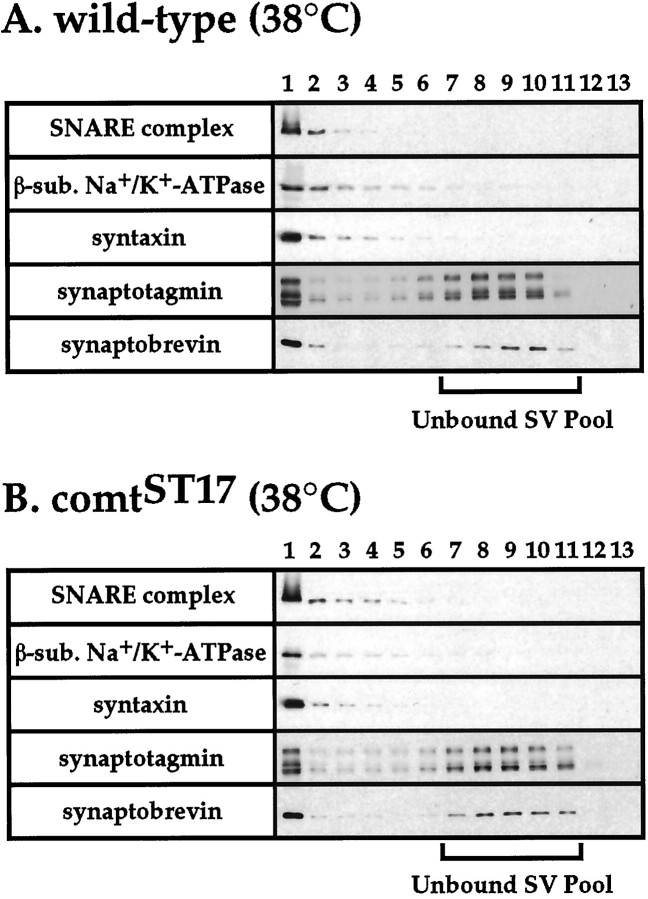
To determine where SNARE complexes accumulate in comtmutants at nonpermissive temperature, we compared the subcellular distribution of SNARE complexes in comt mutants and wild-type flies at permissive and restrictive temperatures. To perform this analysis, postnuclear membrane fractions prepared from head homogenates of comtST17 mutants or wild-type flies were sedimented on a glycerol gradient to separate plasma membrane from synaptic vesicle membrane (van de Goor et al., 1995). After centrifugation, fractions were collected from the bottom of the gradient and subjected to Western blot analysis using an anti-horseradish peroxidase antiserum that recognizes a 42 kDa plasma membrane marker [the putative β-subunit of the Na+/K+ ATPase (van de Goor et al., 1995)], as well as antisera to the synaptic vesicle proteins synapsin, synaptotagmin, and n-synaptobrevin, to identify peak plasma membrane- and synaptic vesicle-containing fractions (Fig.4). In addition, a monoclonal anti-syntaxin antibody was used to identify peak syntaxin- and SNARE complex-containing gradient fractions (Fig. 4).
Fig. 4.
The SNARE complex resides in the plasma membrane. A postnuclear head homogenate from wild-type flies (A) or comtST17mutants (B) was sedimented on a glycerol gradient, and aliquots of the gradient fractions (numbered 1–13 from the bottom to the top of the gradient) were subjected to Western blot analysis to identify peak plasma membrane fractions, peak synaptic vesicle fractions, and SNARE complexes. Western blot analyses were performed using antisera to a 42 kDa plasma membrane marker [the putative β-subunit of the Na+/K+ ATPase, which is recognized by rabbit antisera to horseradish peroxidase (van de Goor et al., 1995)] and to the synaptic vesicle proteins n-synaptobrevin (a mixture of the NSYB1 and NSYB2 antisera), synaptotagmin (DSYT2) (Littleton et al., 1993), and synapsin (data not shown), as well as a monoclonal anti-syntaxin antibody to detect SNARE complexes and monomer syntaxin. Analyses to identify syntaxin- and n-synaptobrevin-containing gradient fractions were performed using samples boiled 5 min to disrupt SNARE complexes. SNARE complex-containing gradient fractions were detected using unboiled samples. No comparison of SNARE complex abundance can be made between A and Bbecause immunoblot exposure times varied in these experiments. Results obtained with the anti-synapsin antiserum were consistent with those obtained with antisera to the other synaptic vesicle antigens analyzed. Results using flies exposed to restrictive temperature (38°C) for 20 min before preparation of head homogenates are shown. Similar results were obtained using flies maintained at permissive temperature (22°C).
In accordance with previous work in Drosophila (van de Goor et al., 1995), peak plasma membrane-containing fractions were found to be distributed at the bottom of the glycerol gradient (heavy membranes), and two peaks of synaptic vesicle antigenicity were observed: a rapidly sedimenting peak colocalizing with heavy membranes and a slowly sedimenting peak (light membranes) (Fig. 4). Previous work (van de Goor et al., 1995) indicates that the light membrane fractions contain unbound synaptic vesicles (attributable to the identical sedimentation rate of material in these fractions with rat brain synaptic vesicles), whereas the rapidly sedimenting peak of synaptic vesicle antigenicity likely represents synaptic vesicle proteins distributed in the plasma membrane as well as synaptic vesicles bound (docked) to plasma membrane. The subcellular distributions of syntaxin and the SNARE complex were also analyzed and found to exhibit sedimentation profiles indistinguishable from the plasma membrane marker used in this analysis (Fig. 4). Quantitative Western blotting of the pooled light membrane fractions (fractions 7–11) and the preceding heavy membrane fractions (fractions 1–6) was used to further document the subcellular distribution of SNARE complexes in comtmutants and wild-type flies at permissive and restrictive temperatures. Results from this analysis indicate that >97% of SNARE complexes sediment with plasma membrane markers in both comt mutants and wild-type flies at permissive and restrictive temperatures.
Although previous work indicates that ternary SNARE complexes can reside within the synaptic vesicle membrane (Otto et al., 1997), we sought to determine whether the low level of SNARE complexes detected in the light membrane fractions from our gradients reside in the synaptic vesicle membrane or, alternatively, represent contamination of the light membrane fractions with plasma membrane-derived SNARE complexes. To facilitate detection of SNARE complexes in the unbound synaptic vesicle pool, large aliquots of glycerol gradient fractions 4–13 were subjected to Western blot analysis using antisera to syntaxin, n-synaptobrevin, and the 42 kDa plasma membrane marker. Results of this analysis reveal a decreasing gradient of SNARE complex, syntaxin, and the 42 kDa plasma membrane marker extending into the unbound synaptic vesicle peak fractions (Fig.5). No second SNARE complex peak, colocalizing with the peak of unbound synaptic vesicles, was detected. Quantitative Western blotting of the light membrane fractions (fractions 7–11) and the preceding heavy membrane fractions (fractions 1–6) using the anti-horseradish peroxidase antiserum indicated that the distribution of the plasma membrane marker recognized by this antiserum closely approximates the distribution of SNARE complexes in these fractions (∼3% resides in the unbound synaptic vesicle pool). Further evidence that SNARE complexes in the unbound synaptic vesicle fractions derive from plasma membrane contamination is provided by the uniform distribution of SNARE complex to monomer syntaxin ratios across the gradient, indicating a single common pool of syntaxin and SNARE complexes (data not shown). In contrast, the ratio of SNARE complex to monomer n-synaptobrevin is increased >25-fold in plasma membrane fractions with respect to the unbound synaptic vesicle fractions in both comt mutants and wild-type flies, indicating that there are two distinct pools of n-synaptobrevin: a plasma membrane pool that is largely in complex with t-SNAREs and an uncomplexed pool associated with unbound synaptic vesicles. Together, these results indicate that most, if not all, of the SNARE complexes present in light membrane fractions arise from contamination with plasma membrane SNARE complexes.
Fig. 5.
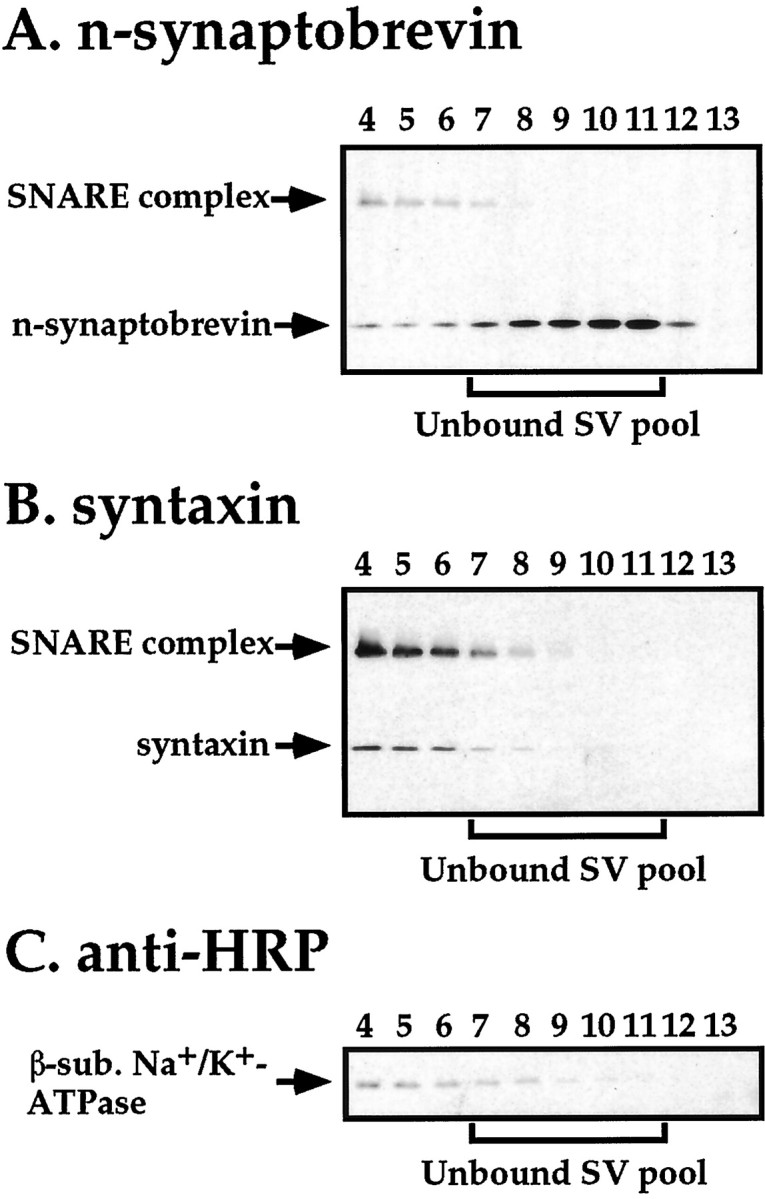
Plasma membrane contamination provides an explanation for the SNARE complexes detected in synaptic vesicle fractions from comt mutants. Aliquots of glycerol gradient fractions 4–13 fromcomtST17 mutants were subject to Western blot analysis using antisera to (A) n-synaptobrevin (a mixture of the NSYB1 and NSYB2 antisera), (B) syntaxin, and (C) horseradish peroxidase (anti-HRP), which recognizes a 42 kDa plasma membrane marker. Results using flies exposed to restrictive temperature (38°C) for 20 min before preparation of head homogenates are shown.
DISCUSSION
Work presented here describes characterization of an SDS-resistantDrosophila neural SNARE complex composed of syntaxin, n-synaptobrevin, and SNAP-25. In wild-type flies the SNARE complex appears to represent a small fraction of the total SNARE protein, and its abundance is not substantially altered as a result of temperature changes or mutations affecting membrane excitability. In contrast, SNARE complex abundance is greatly altered from wild-type levels in the temperature-sensitive paralytic mutants comt andshi during exposure to restrictive temperature. Interestingly, SNARE complex abundance in comt andshi strongly correlates with the state of vesicle docking in these mutants, suggesting that SNARE complexes form during or after synaptic vesicle docking. Furthermore, subcellular fractionation experiments indicate that most, if not all, of the SNARE complex in both comt mutants and wild-type flies colocalizes with fractions containing plasma membrane and docked synaptic vesicles. Although these results are consistent with general features of the SNARE hypothesis, including a role for SNAREs in vesicle docking and an NSF-mediated disassembly of the SNARE complex after docking, they are also compatible with other models.
Studies of the neural SNAREs in Drosophila (Broadie et al., 1995) and at the squid giant synapse (Hunt et al., 1994; O’Connor et al., 1997) indicate that disruption of individual SNARE proteins, either n-synaptobrevin or syntaxin, or disruption of SNARE protein interactions, fails to prevent synaptic vesicle targeting or docking. Furthermore, recent in vitro studies of homotypic vacuole fusion in yeast have provided evidence that the yeast NSF homolog SEC18p is required for docking (Mayer et al., 1996; Mayer and Wickner, 1997). These experiments also indicate that SEC18p is required only before the mixing of donor and acceptor membranes, and thus a role for SEC18p downstream of docking appears unlikely. How can we resolve these results with those obtained in biochemical, ultrastructural, and electrophysiological studies of comt mutants? Although parallel accumulation of docked vesicles and SNARE complexes incomt mutants is consistent with a role for SNAREs in vesicle docking, these results are also compatible with a model in which SNARE complex formation follows morphological vesicle docking. Thus, SNAREs may be dispensable for vesicle targeting and docking (defined by ultrastructural criteria) and instead may function in downstream events, such as bilayer fusion.
Although the conflicting results of molecular studies of yeast vacuole fusion and regulated secretion are currently unresolved, it is possible that these conflicts reflect fundamental differences between homotypic and heterotypic fusion. In the former case, the donor and target membranes presumably contain the same complement of vesicle and target SNAREs. Under these conditions, NSF may be required to disrupt SNARE interactions on the same membrane as a prerequisite to docking (Ungermann et al., 1998). At the synapse, v-SNAREs and t-SNAREs are largely restricted to synaptic vesicles and plasma membrane, respectively, and thus no such requirement for NSF would be expected. An alternative possibility, equally consistent with studies of NSF function in vacuole fusion and the present study of NSF function in neurotransmitter release, is that NSF function is required after synaptic vesicle fusion to disassemble the SNARE complex, thereby reactivating SNAREs for another round of membrane fusion. This model differs in a fundamental way from the SNARE hypothesis in that it proposes a post-fusion role for NSF in the recycling of SNAREs. Recent work showing that SNAREs alone are capable of promoting bilayer fusionin vitro provides further support for such a model (Weber et al., 1998).
A feature that distinguishes these two models of NSF function is the subcellular distribution of SNARE complexes that accumulate under conditions of reduced NSF activity. The SNARE hypothesis predicts that SNARE complexes would accumulate at sites of synaptic vesicle docking under conditions of impaired NSF function. In contrast, a post-fusion role for NSF in SNARE complex disassembly does not place any such constraints on SNARE complex localization because the SNARE complex would be composed of SNAREs residing as neighbors in the same membrane. One possibility raised by the post-fusion model for NSF function is that SNARE complexes accumulating under conditions of reduced NSF activity may become incorporated into recycling synaptic vesicles. However, the subcellular membrane fractionation experiments reported here clearly argue against this possibility. In comt mutants (and wild-type flies), essentially all of the cellular SNARE complexes were found to be distributed with membrane fractions consisting of plasma membrane and docked synaptic vesicles. Although these results do not allow us to distinguish between models of NSF function, they strongly argue against unbound synaptic vesicles as sites of NSF function and provide novel information on SNARE complex distribution in neural tissue.
In summary, data presented here, together with the results of recent physiological and ultrastructural studies of the comt mutant (Kawasaki et al., 1998), clearly establish a role for NSF in the regulated exocytosis of neurotransmitter. The finding that defects in NSF function result in accumulation of docked synaptic vesicles and ternary SNARE complexes in the plasma membrane support a model in which NSF functions after vesicle docking in the disassembly or rearrangement of a neural SNARE complex to maintain the readily releasable pool of synaptic vesicles. Ongoing ultrastructural and biochemical analyses ofcomt mutants should further refine the function of NSF in neurotransmitter release.
Footnotes
This work was supported in part by a grant from the Whitehall Foundation (A98-29) to L.P. We thank Hugo Bellen, Erich Buchner, Ed Giniger, Reinhard Jahn, Regis Kelley, Tom Schwarz, and Konrad Zinsmaier for providing antisera, and Rick Ordway for communication and discussion of unpublished data. Special thanks to Jesse Goldmark for technical assistance in analyses of SNARE complexes.
Correspondence should be addressed Leo Pallanck, Department of Genetics, Box 357360, University of Washington, Health Sciences, J113, 1959 N.E. Pacific Street, Seattle, WA 98195.
REFERENCES
- 1.Banerjee A, Barry VA, DasGupta BR, Martin TFJ. N-Ethylmaleimide-sensitive factor acts at a prefusion ATP-dependent step in Ca2+-activated exocytosis. J Biol Chem. 1996;271:20223–20226. doi: 10.1074/jbc.271.34.20223. [DOI] [PubMed] [Google Scholar]
- 2.Broadie K, Prokop A, Bellen HJ, O’Kane CJ, Schulze KL, Sweeney ST. Syntaxin and synaptobrevin function downstream of vesicle docking in Drosophila. Neuron. 1995;15:663–673. doi: 10.1016/0896-6273(95)90154-x. [DOI] [PubMed] [Google Scholar]
- 3.DeBello WM, O’Connor V, Dresbach T, Whiteheart SW, Wang SS, Schweizer FE, Betz H, Rothman JE, Augustine GJ. SNAP-mediated protein-protein interactions essential for neurotransmitter release. Nature. 1995;373:626–630. doi: 10.1038/373626a0. [DOI] [PubMed] [Google Scholar]
- 4.Deitcher DL, Ueda A, Stewart BA, Burgess RW, Kidokoro Y, Schwarz TL. Distinct requirements for evoked and spontaneous release of neurotransmitter are revealed by mutations in the Drosophila gene neuronal-synaptobrevin. J Neurosci. 1998;18:2028–2039. doi: 10.1523/JNEUROSCI.18-06-02028.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiAntonio A, Burgess RW, Chin AC, Deitcher DL, Scheller RH, Schwarz TL. Identification and characterization of Drosophila genes for synaptic vesicle proteins. J Neurosci. 1993;13:4924–4935. doi: 10.1523/JNEUROSCI.13-11-04924.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayashi T, McMahon H, Yamasaki S, Binz T, Hata Y, Südhof T, Niemann H. Synaptic vesicle membrane fusion complex: action of clostridial neurotoxins on assembly. EMBO J. 1994;13:5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt JM, Bommert K, Charlton MP, Kistner A, Habermann E, Augustine GJ, Betz H. A post-docking role for synaptobrevin in synaptic vesicle fusion. Neuron. 1994;12:1269–1279. doi: 10.1016/0896-6273(94)90443-x. [DOI] [PubMed] [Google Scholar]
- 8.Kawasaki F, Mattiuz AM, Ordway RW (1998) Synaptic physiology and ultrastructure in comatose mutants defines an in vivo role for NSF in neurotransmitter release, in press. [DOI] [PMC free article] [PubMed]
- 9.Klagges BR, Heimbeck G, Godenschwege TA, Hofbauer A, Pflugfelder GO, Reifegerste R, Reisch D, Schaupp M, Buchner S, Buchner E. Invertebrate synapsins: a single gene codes for several isoforms in Drosophila. J Neurosci. 1996;16:3154–3165. doi: 10.1523/JNEUROSCI.16-10-03154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koenig JH, Ikeda K. Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J Neurosci. 1989;9:3844–3860. doi: 10.1523/JNEUROSCI.09-11-03844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosaka T, Ikeda K. Possible temperature-dependent blockage of synaptic vesicle recycling induced by a single gene mutation in Drosophila. J Neurobiol. 1983;14:207–225. doi: 10.1002/neu.480140305. [DOI] [PubMed] [Google Scholar]
- 12.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Littleton JT, Bellen HJ, Perin MS. Expression of synaptotagmin in Drosophila reveals transport and localization of synaptic vesicles to the synapse. Development. 1993;118:1077–1088. doi: 10.1242/dev.118.4.1077. [DOI] [PubMed] [Google Scholar]
- 14.Mayer A, Wickner W. Docking of yeast vacuoles is catalyzed by the Ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF). J Cell Biol. 1997;136:307–317. doi: 10.1083/jcb.136.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of Sec17p (alpha-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 16.Nonet ML, Saifee O, Zhao H, Rand JB, Wei L. Synaptic transmission deficits in Caenorhabditis elegans synaptobrevin mutants. J Neurosci. 1998;18:70–80. doi: 10.1523/JNEUROSCI.18-01-00070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Connor V, Heuss C, De Bello WM, Dresbach T, Charlton MP, Hunt JH, Pellegrini LL, Hodel A, Burger MM, Betz H, Augustine GJ, Schafer T. Disruption of syntaxin-mediated protein interactions blocks neurotransmitter secretion. Proc Natl Acad Sci USA. 1997;94:12186–12191. doi: 10.1073/pnas.94.22.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ordway R, Pallanck L, Ganetzky B. Neurally expressed Drosophila genes encoding homologs of the NSF and SNAP secretory proteins. Proc Natl Acad Sci USA. 1994;91:5715–5719. doi: 10.1073/pnas.91.12.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otto H, Hanson PI, Jahn R. Assembly and disassembly of a ternary complex of synaptobrevin, syntaxin, and SNAP-25 in the membrane of synaptic vesicles. Proc Natl Acad Sci USA. 1997;94:6197–6201. doi: 10.1073/pnas.94.12.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pallanck L, Ordway R, Ganetzky B. A Drosophila NSF mutant. Nature. 1995;376:25. doi: 10.1038/376025a0. [DOI] [PubMed] [Google Scholar]
- 21.Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 22.Scheller RH. Membrane trafficking in the presynaptic nerve terminal. Neuron. 1995;14:893–897. doi: 10.1016/0896-6273(95)90328-3. [DOI] [PubMed] [Google Scholar]
- 23.Schulze KL, Broadie K, Perin MS, Bellen HJ. Genetic and electrophysiological studies of Drosophila syntaxin-1A demonstrate its role in nonneuronal secretion and neurotransmission. Cell. 1995;80:311–320. doi: 10.1016/0092-8674(95)90414-x. [DOI] [PubMed] [Google Scholar]
- 24.Schweizer FE, Dresbach T, DeBello WM, O’Connor V, Augustine GJ, Betz H. Regulation of neurotransmitter release kinetics by NSF. Science. 1998;279:1203–1206. doi: 10.1126/science.279.5354.1203. [DOI] [PubMed] [Google Scholar]
- 25.Siddiqi O, Benzer S. Neurophysiological defects in temperature-sensitive paralytic mutants of Drosophila melanogaster. Proc Natl Acad Sci USA. 1976;73:3253–3257. doi: 10.1073/pnas.73.9.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Söllner T, Whiteheart S, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 27.Südhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 28.Sweeney ST, Broadie K, Keane J, Niemann H, O’Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 29.Ungermann C, Nichols BJ, Pelham HRB, Wickner W. A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion. J Cell Biol. 1998;140:61–69. doi: 10.1083/jcb.140.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van de Goor J, Ramaswami M, Kelly R. Redistribution of synaptic vesicles and their proteins in temperature-sensitive shibire(ts1) mutant Drosophila. Proc Natl Acad Sci USA. 1995;92:5739–5743. doi: 10.1073/pnas.92.12.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Söllner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 32.Wu CF, Ganetzky B. Neurogenetic studies of ion channels in Drosophila. Ion Channels. 1992;3:261–314. doi: 10.1007/978-1-4615-3328-3_9. [DOI] [PubMed] [Google Scholar]
- 33.Wu MN, Littleton JT, Bhat MA, Prokop A, Bellen HJ. ROP, the Drosophila Sec1 homolog, interacts with syntaxin and regulates neurotransmitter release in a dosage-dependent manner. EMBO J. 1998;17:127–139. doi: 10.1093/emboj/17.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]