Abstract
Nitric oxide (NO) production by neurons in the prepositus hypoglossi (PH) nucleus is necessary for the normal performance of eye movements in alert animals. In this study, the mechanism(s) of action of NO in the oculomotor system has been investigated. Spontaneous and vestibularly induced eye movements were recorded in alert cats before and after microinjections in the PH nucleus of drugs affecting the NO–cGMP pathway. The cellular sources and targets of NO were also studied by immunohistochemical detection of neuronal NO synthase (NOS) and NO-sensitive guanylyl cyclase, respectively. Injections of NOS inhibitors produced alterations of eye velocity, but not of eye position, for both spontaneous and vestibularly induced eye movements, suggesting that NO produced by PH neurons is involved in the processing of velocity signals but not in the eye position generation. The effect of neuronal NO is probably exerted on a rich cGMP-producing neuropil dorsal to the nitrergic somas in the PH nucleus. On the other hand, local injections of NO donors or 8-Br-cGMP produced alterations of eye velocity during both spontaneous eye movements and vestibulo-ocular reflex (VOR), as well as changes in eye position generation exclusively during spontaneous eye movements. The target of this additional effect of exogenous NO is probably a well defined group of NO-sensitive cGMP-producing neurons located between the PH and the medial vestibular nuclei. These cells could be involved in the generation of eye position signals during spontaneous eye movements but not during the VOR.
Keywords: eye movements, nitrergic neurons, nitric oxide, oculomotor integrator, prepositus hypoglossi nucleus, soluble guanylyl cyclase
We have reported recently that a balanced production of nitric oxide (NO) in the prepositus hypoglossi (PH) nucleus is necessary for the correct performance of spontaneous eye movements in the alert cat (Moreno-López et al., 1996). An imbalance in the NO concentration between the two PH nuclei, induced by unilateral injections of NO synthase (NOS) inhibitors or NO donors, resulted in nystagmic eye movements whose slow phases were directed toward the side in which NO concentration was higher. In the present work, we have used the cat oculomotor system as a model to investigate the possible mechanisms of action and targets of NO in sensorimotor processing.
The functions of eye movements are to direct the highest visual acuity portion of the retina to the objects of interest and to maintain the stability of the visual targets on the retina, despite displacements of the head or the visual surroundings. To perform these functions, motoneurons in the oculomotor nuclei of the brainstem send two types of commands to the extraocular muscles: a velocity signal and a position signal (Fuchs and Luschei, 1970; Skavenski and Robinson, 1973;Delgado-García et al., 1986; De la Cruz et al., 1990).
Ocular motoneurons are controlled by several premotor structures in which the different types of eye movements are generated. Thus, neurons in the pontine reticular formation encode velocity signals during saccades (Hikosaka et al., 1978; Kaneko et al., 1981; Strassman et al., 1986a,b), whereas neurons in the medial vestibular (MV) nucleus fire at a rate related to head velocity during vestibular stimulation (Baker et al., 1969; Hikosaka et al., 1980; McCrea et al., 1980,1987; Berthoz et al., 1989; Escudero et al., 1992). Robinson (1968, 1975) proposed that position signals for any kind of eye movement result from the temporal integration (in the mathematical sense) of velocity signals, establishing the concept of the neural integrator. Although the sources of position signals are not completely determined, the PH nucleus has been identified as one of the structures responsible for neural integration for horizontal eye movements (López-Barneo et al., 1982; Cheron et al., 1986b; Cannon and Robinson, 1987; Cheron and Godaux, 1987; Delgado-García et al., 1989; Escudero et al., 1992; McFarland and Fuchs, 1992; Mettens et al., 1994; for review, see Fukushima et al., 1992).
The PH nucleus is a long and narrow nucleus located immediately below the floor of the fourth ventricle. A large number of neurons in this nucleus express NOS, as demonstrated by immunocytochemical techniques (Moreno-López et al., 1996). The PH nucleus receives afferents from different premotor structures, such as the MV nuclei, the pontine reticular formation, and the contralateral PH nucleus, and sends efferents to these same structures and to the oculomotor nuclei, including the abducens nucleus (McCrea and Baker, 1985) in which motoneurons and internuclear neurons controlling horizontal eye movements are located.
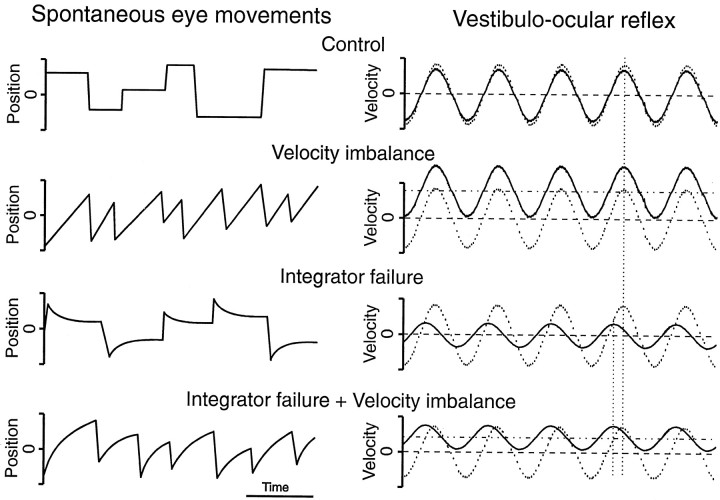
Pharmacological modification of NO concentration in the PH nucleus may affect inputs from premotor structures conveying velocity information; additionally or alternatively, such modification may affect intranuclear neurons involved in the velocity-to-position integration. Depending on the target, velocity imbalance and/or integration deficit will occur, leading to well characterized abnormal eye movements (Cannon and Robinson, 1987; Godaux et al., 1993; Mettens et al., 1994;Pastor et al., 1994; Godaux and Cheron, 1996), which are schematically represented in Figure 1. Each PH nucleus simultaneously receives excitatory and inhibitory signals from the contralateral and ipsilateral MV nuclei, respectively (Baker and Berthoz, 1975). Because MV nucleus neurons projecting to the PH nucleus display a tonic discharge (McCrea et al., 1980; Berthoz et al., 1989), a modification in the transmission of one of these signals would result in a velocity imbalance, as represented in Figure 1, leftand right, for spontaneous eye movements and vestibularly induced eye movements, respectively. On the other hand, a failure in the eye position generation should eliminate or decrease the eye position input to the motoneurons, and the eye movements would reflect the eye velocity commands. The consequence is that, during spontaneous eye movements, the eye is unable to maintain an eccentric position after a saccade and exponentially returns to a central position (Fig.1, left) with a time constant that is inversely proportional to the degree of integrator failure and is finally imposed by the viscoelastic forces acting on the eye in the orbit. During the vestibulo-ocular reflex (VOR), impairment of the velocity-to-position integrator results in a decreased reflex gain and enhanced phase lead, as schematically represented in Figure 1, right.
Fig. 1.
Schematic drawing representing eye movements under normal and altered conditions. Eye position (position) in spontaneous eye movements (left) and eye velocity (velocity) during VOR induction (right) are schematically represented in a control situation and under hypothetical alterations of velocity processing (velocity imbalance), integration (integrator failure), or both. On theright, the solid line represents eye velocity, and the dotted line represents head velocity. Because eyes move in a direction opposite to the head during the reflex response, the head velocity curve has been inverted to facilitate comparison with the eye velocity curve. The horizontal dashed line corresponds to the mean head velocity, which by definition is zero. The horizontal dashed and dotted line represents the mean eye velocity, which is also zero in the absence of velocity imbalance. The vertical dotted lines indicate the head and eye phase peaks.Left, During spontaneous movements, alterations in the processing of velocity signals produce nystagmic eye movements with ramp-like slow phases, whereas a failure of oculomotor integration produces gaze-holding impairment, causing exponential drifts toward a central position. When integration is absent, the drift time constant is estimated to be 0.16 sec (Goldberg, 1980). If both velocity and integration deficits occur at the same time, a nystagmus with curved slow phases will appear. Right, During VOR induction, the velocity imbalance appears as a positive or negative value of the mean slow eye velocity, whereas the integration deficit results in a decreased reflex gain and increased phase lead. A velocity imbalance with gain reduction and enhanced phase lead should be expected when both alterations are present.
In the present study, we have analyzed whether the NO produced by nitrergic neurons in the PH nucleus is involved in the processing of velocity signals, position signals, or both, during either spontaneous eye movements or VOR. We have determined also the possible targets of NO by immunostaining of cGMP, the second messenger that is activated by NO. We found that NO produced by PH neurons participates in the processing of pure velocity signals, probably by interacting with cGMP-containing neuropil within this nucleus. We have also identified a group of NO-sensitive neurons, lateral to the PH nucleus, that may control the generation of position signals during spontaneous eye movements but not during VOR.
MATERIALS AND METHODS
Subjects. Fourteen adult cats (2.5–3.5 kg) of European and Abyssinian strains were obtained from an authorized supplier (IFFA Credo, Arbresle, France) and were used as experimental subjects. Experiments were performed in accordance with the European Union directive 609/86/CEE and with Spanish legislation (RD 233/89) on the use of laboratory animals in acute and chronic experiments.
Immunohistochemistry. Six cats were perfused through the left ventricle with 4% paraformaldehyde in 0.1 m phosphate buffer, pH 7.4. After the brains were removed, the brainstems were post-fixed for 2 hr and then cryoprotected by incubation for 2 d with 30% sucrose at 4°C. Coronal 40 μm sections were obtained with a freezing microtome. To visualize neurons containing NO-sensitive guanylyl cyclase, the procedure described by Southam and Garthwaite (1993) was used with some modifications. Briefly, two of the animals were perfused through the left ventricle with a physiological solution (in mm: 120 NaCl, 2 KCl, 2 CaCl2, 26 NaHCO3, 1.2 KH2PO4, 1.2 MgSO4, and 11 glucose) at 37°C and bubbled with 95% O2 and 5% CO2, containing the NO donor sodium nitroprusside (SNP) (10 mm) and the phosphodiesterase inhibitor 3-isobutylmethylxantine (IBMX) (1 mm), for 5 min at 200 ml/min. Two control animals were treated under the same conditions but in the absence of SNP. Afterward, these cats were perfused with 4% paraformaldehyde, and the tissue was processed as described above.
NOS immunohistochemistry was performed as described previously (Moreno-López et al., 1996), except that a polyclonal antibody raised against a 22.3 kDa protein fragment of human neuronal NOS (Transduction Laboratories, Lexington, KY) was used. Tissue sections were processed according to the avidin–biotin peroxidase complex procedure, using an ABC kit (Vector Laboratories, Burlingame, CA). No immunostaining was observed when the primary antibody was omitted.
To visualize neurons containing NO-sensitive soluble guanylyl cyclase, an antibody raised against a cGMP–paraformaldehyde–bovine thyroglobulin complex (Tanaka et al., 1997), kindly provided by Dr. de Vente (Rijksuniversiteit Limburg, Maastrich, Netherlands), was used. Before fixation, the animal was perfused with SNP to activate soluble guanylyl cyclase. No significant labeling was found when staining was performed under the same conditions in animals perfused with IBMX but without SNP. Details on this staining and the distribution of cGMP-containing neurons in oculomotor nuclei will be given elsewhere (B. Moreno-López, M. Escudero, J. de Vente, and C. Estrada, unpublished data). Drug injection sites were identified in some animals after completion of the recording sessions by injections of either horseradish peroxidase (Boehringer Mannheim, Indianapolis, IN) or biotin dextran amine (Molecular Probes, Eugene, OR) as described previously (Moreno-López et al., 1996).
Physiological experiments. Eight female cats were prepared for chronic recording of eye movements and for microinjection of pharmacological substances into the PH nucleus as described previously (Moreno-López et al., 1996). Briefly, under general anesthesia (Nembutal, 35 mg/kg, i.p.), the cats were implanted bilaterally with Teflon-coated stainless steel coils sutured to the scleral margin of the eye (Fuchs and Robinson, 1966). In the same surgical act, a 4 × 4 mm hole was drilled through the occipital bone to allow access to the posterior brainstem via the cerebellum. Bipolar silver stimulating electrodes were implanted bilaterally on the sixth nerve at its exit from the brainstem (stereotaxic coordinates, lateral 3.5 and posterior 1, according to Berman, 1968). The final location of the stimulating electrode was adjusted to evoke the maximum abducting eye movement with the minimum electrical stimulation (50 μsec, cathodic square pulses of <0.1 mA of current intensity). A head-holding system, consisting of three bolts cemented to the skull perpendicular to the stereotaxic plane, was also implanted. Eye coils and stimulating electrodes were connected to a socket attached to the holding system. Field potential and unitary activity were recorded with glass micropipettes of 2–6 MΩ of electrode resistance. Further details of this type of chronic preparation have been reported elsewhere (Delgado-García et al., 1986; Escudero et al., 1992).
One to 2 weeks later, when there was total recovery from surgery, experiments were performed in the alert cat once every 2–4 d, for 2–3 hr/d, for a maximum of 4–8 weeks. During the experimental sessions, the animal was lightly restrained by elastic bandages, and the head was fixed (21°, nose down) to the recording table by means of the head-holding system. A glass micropipette was advanced through the cerebellum toward either the left or right abducens nucleus, which was identified by the recording of the antidromic field potential induced by electric stimulation of the ipsilateral sixth nerve. The PH nucleus was found in the same parasagittal plane and posterior to the abducens nucleus, just below the floor of the fourth ventricle. All injections were restricted to the rostral third of the PH nucleus. The correct position of the micropipette was confirmed by recording the characteristic firing discharge of PH neurons during spontaneous and vestibularly induced eye movements (Escudero et al., 1992). The horizontal VOR was elicited by sinusoidal rotation around the vertical axis at 0.1 and 1 Hz. The amplitude of the table movement was adjusted to keep maximal angular head velocity constant at 30°/sec for both frequencies. Injections were performed by means of glass micropipettes with tip diameters of 7–8 μm, filled with the corresponding drug dissolved in 0.1 m phosphate buffer, pH 7.4. Air pulses (1 kg/cm2, 1 sec) were applied with an air pressure device connected to the injection micropipette to deliver 40–45 nl/pulse. Spontaneous eye movements were continuously recorded in complete darkness and occasionally in light, with the micropipette in place both before and after injections. Eye movements were calibrated at the beginning of each experimental session by rotating (±10°) the magnetic field frame about both the horizontal and vertical planes. Eye movements during VOR were recorded in the dark both before and after drug injections. Field and unitary electrical activities and head and eye position were stored in an eight-channel video tape recording system and fed into a computer for off-line analysis. Eye and head position signals were sampled at 500 Hz.
Analysis of the data. During spontaneous eye movements in darkness, the alterations induced by drug injections in the PH nucleus consisted of nystagmic eye movements with straight or curved slow phases separated by quick resetting movements. Analysis of the slow phases was performed during the 3 min period of maximum effect for each injection and immediately before vestibular stimulation. Slow phases with duration greater than 0.5 sec were fit separately by the least squares method to linear and exponential equations and were considered to be linear or exponential when >80% of the analyzed phases had a correlation coefficient >0.99 or >0.90, respectively. Because linear and curved slow phases are indicative of two different alterations (velocity imbalance and eye position generation, respectively) that could be simultaneously induced, the time constants of the exponential slow phases were calculated from the first derivative of eye position (eye velocity) to prevent value underestimation attributable to possible concomitant linear phases in the eye position recording.
During VOR, the eye movement response was defined by three parameters: velocity imbalance, reflex gain, and phase lead. The velocity imbalance was measured as the mean slow eye velocity and was expressed in degrees per second. The reflex gain was calculated as the ratio between the peak-to-peak amplitude of slow eye velocity versus the peak-to-peak amplitude of head velocity. To analyze the head and slow eye velocity, a computer program was developed. For each cycle, the sinusoidal function of the head velocity was calculated by fitting a periodic function (trigonometric polynomial) by the least squares method (Batschelet, 1981). This sine wave was adjusted by cursors to the eye velocity signal. The points of the eye velocity signal, which were in the range of ±10% with respect to the reference sine wave, were selected, and the rest, corresponding to the quick phases, were ignored. Parameters of the resulting sine wave were calculated as indicated for head velocity. The phase lead was quantified as the temporal shift between the eye and the head position for each hemicycle and then averaged for each complete cycle. This method avoids the shift produced in the eye position by the velocity imbalance, which is of the same magnitude and opposite sign for each hemicycle. Data from phase shifts were expressed in degrees. Each parameter was measured for at least 10 cycles at 0.1 Hz and 30 cycles at 1 Hz.
Results are presented as mean ± SEM, except for normalized control values and when only two experiments were averaged, in which case, mean ± SD is given. Comparisons within one experiment (for example, when VOR gains were compared in a large number of cycles before and after drug injection) were performed using the Student’st test. Comparisons between two groups of experiments were performed by the nonparametric Mann–Whitney U test. A probability <0.05 was considered significant.
RESULTS
Effect of NOS inhibitors and substrate
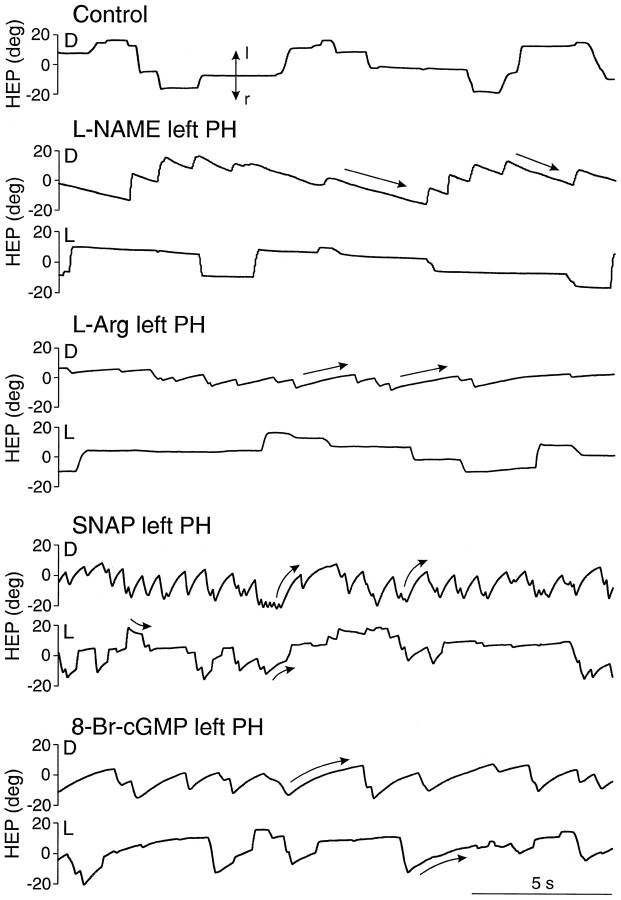
Local inhibition of NOS by injections of eitherl-nitro-arginine methyl ester (l-NAME) (10–50 nmol) (Fig. 2) orN-monomethyl-l-arginine (30–100 nmol) in the PH nucleus induced a nystagmus with slow phases directed contralaterally to the injected site, in agreement with our previously reported results (Moreno-López et al., 1996). When the NOS substrate l-arginine (10–100 nmol) was injected in the PH nucleus, a mild nystagmus toward the ipsilateral side was observed (Fig. 2). In all cases (Fig. 2), the slow phases of the nystagmus were ramp-like with a best fit to a linear equation (r > 0.99). When visual information was presented under light conditions, the nystagmus was considerably reduced, and exponential postsaccadic drifts indicative of a loss of position signal were not observed (Fig. 2). These results indicate that modifications of NO production in the PH nucleus induce a velocity imbalance without apparent changes in the generation of the eye position signal during spontaneous eye movements.
Fig. 2.
Effects of unilateral injections of drugs affecting the NO–cGMP pathway in the PH nucleus of an alert cat during spontaneous eye movements. Recordings of right eye position in the horizontal plane (HEP) under control conditions and after injections of the indicated drugs in the left PH nucleus, in either darkness (D) or light (L). Doses and times after injection were as follows: l-NAME, 50 nmol, 19 (D) and 20 (L) min; l-arginine (L-Arg), 100 nmol, 8 (D) and 11 (L) min; SNAP, 20 nmol, 5 (D) and 4 (L) min; and 8-Br-cGMP, 4 nmol, 6 (D) and 8 (L) min. Eye position is plotted as degrees (deg). Vertical arrows indicate movement direction: l, left; r, right.Straight and curved arrows indicate linear and exponential slow phases, respectively. Slow phases forl-NAME and l-arginine were best fit to a linear equation, whereas slow phases for SNAP and 8-Br-cGMP were best fit to an exponential equation.
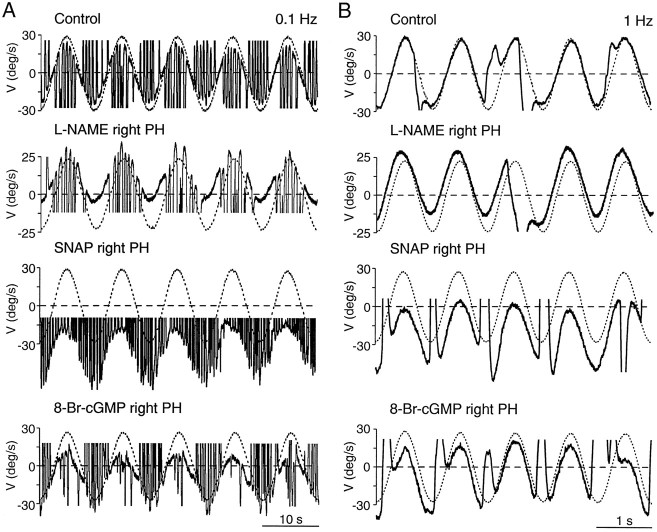
The NOS inhibitors and l-arginine also modified the oculomotor response during VOR at 0.1 and 1 Hz. A typical recording of the effect of l-NAME is shown in Figure3, A and B. Quantification of the data indicated that there was a velocity imbalance (Fig. 4A) without alteration of the reflex gain (Fig. 4B) or the phase lead (Fig. 4C) of the reflex. The velocity imbalance was directed to the contralateral side when PH nucleus NOS was inhibited and toward the ipsilateral side after injection ofl-arginine (Fig. 4A). Therefore, as for spontaneous eye movements, unilateral changes in NOS activity in the PH nucleus resulted in abnormal vestibularly induced eye movements, whose exclusive alteration was a velocity imbalance without modification of the velocity-to-position integrator.
Fig. 3.
Effects of unilateral injections of drugs affecting the NO–cGMP pathway in the PH nucleus of an alert cat during sinusoidal vestibular stimulation in darkness. Representative recordings of eye (solid line) and head (dotted line) velocity (V) during VOR induction by turntable rotation at 0.1 (A) and 1 Hz (B) under control conditions and after injections of the indicated drugs in the PH nucleus. The head velocity curve has been inverted to facilitate comparison with the eye velocity curve. Movement direction as indicated in Figure 2.
Fig. 4.
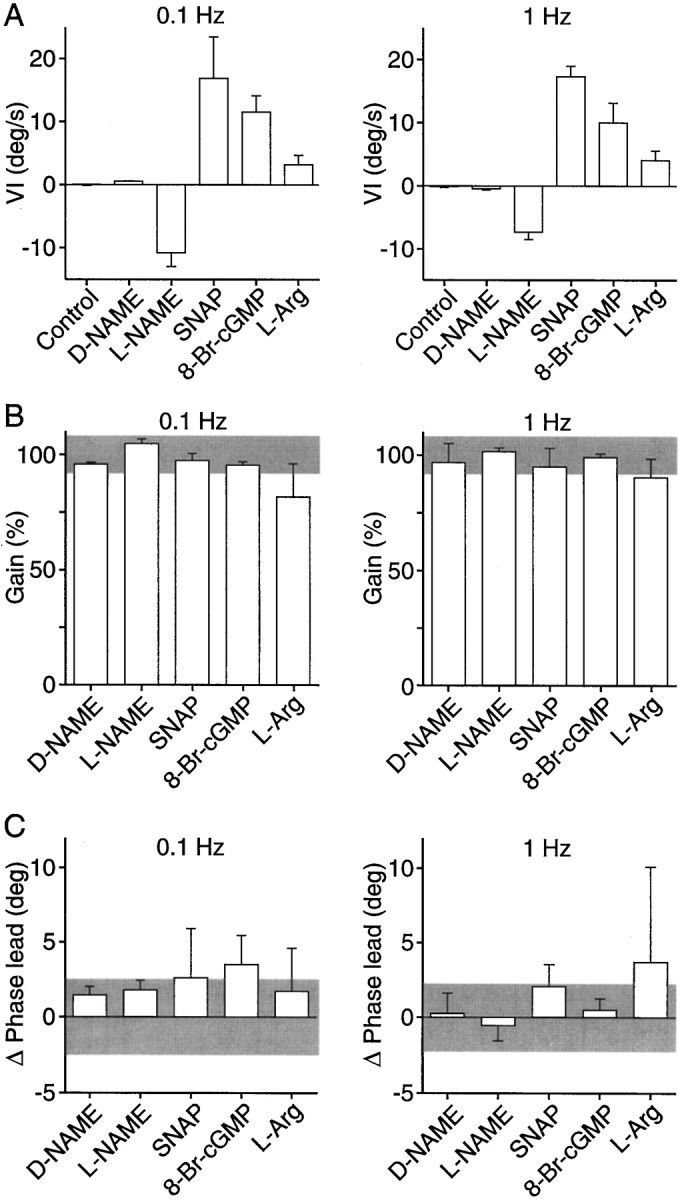
Velocity imbalance, gain, and phase lead of the vestibulo-ocular reflex after local administration in the PH nucleus of drugs affecting the NO–cGMP pathway. A, Velocity imbalance (VI) produced by the different drugs during VOR induction by rotation of the head at 0.1 and 1 Hz. Positive values represent velocity imbalance toward the injected side and negative values toward the contralateral side. B, Gain of the VOR induced by rotation of the head at 0.1 and 1 Hz after injection of the different drugs. Gain is expressed as a percentage of the control value for each experiment. Gray bar, 100% ± SD of control values. C, Changes in phase lead during the VOR induced by rotation of the head at 0.1 and 1 Hz produced by injection of the different drugs. Values are expressed in degrees (deg). Gray bar, 0 ± SD of control values. n = 2 for d-NAME;n = 3 for SNAP and l-arginine;n = 4 for 8-Br-cGMP; and n = 5 for l-NAME.
Effect of NO donors
Unilateral injections of the NO donorsS-nitroso-N-acetylpenicillamine (SNAP) (5–20 nmol) or SNP (5–35 nmol) in the PH nucleus produced nystagmic eye movements with slow phases toward the ipsilateral side as reported previously (Moreno-López et al., 1996). In contrast to the effects of NOS modulators, the slow phases were curved (Fig. 2) and were best fit by an exponential. The mean time constants of the adjusted exponential during the maximum effects of SNAP and SNP were 0.56 ± 0.03 and 0.78 ± 0.23 sec (mean ± SEM;n = 3), respectively. When the nystagmus was attenuated under light conditions, horizontal saccades were followed by centripetal exponential drifts (Fig. 2). These results reveal a velocity imbalance combined with a gaze-holding deficit for horizontal spontaneous eye movements. During VOR, unilateral NO donor injections in the PH nucleus also produced a velocity imbalance (Figs.3A,B, 4A). However, the gain and phase lead of the reflex remained intact (Figs.3A,B,4B,C), indicating no disruption of the velocity-to-position integration during VOR, despite the alterations observed during spontaneous eye movements both before and after the VOR induction period. Table 1shows the mean variation values of gain and phase lead during VOR for individual injections, together with the mean time constant of the postsaccadic drift during spontaneous eye movements measured immediately before vestibular stimulation.
Table 1.
Effect of individual drug injections on VOR gain, phase lead, and postsaccadic drift time constant
| Drug | VOR (0.1 Hz) | VOR (1 Hz) | Spontaneous eye movements | ||
|---|---|---|---|---|---|
| ΔGaina | ΔPhase lead (deg)b | ΔGaina | ΔPhase lead (deg)b | Time constant (sec)c | |
| SNAP | −0.01 | −2.0 | −0.03 | 2.0 | 0.90 ± 0.40 |
| 0.02 | 0.9 | −0.12 | 4.6 | 0.57 ± 0.12 | |
| −0.06 | 8.9* | 0.07 | −0.5 | 0.73 ± 0.26 | |
| 8-Br-cGMP | −0.03 | 7.0 | 0.00 | 2.6 | 1.78 ± 0.44 |
| −0.01 | 6.5 | −0.03 | −0.8 | 1.45 ± 0.76 | |
| −0.03 | 1.1 | 0.03 | −0.1 | 1.70 ± 0.24 | |
| −0.07 | −0.8 | −0.02 | 0.3 | 0.84 ± 0.37 | |
a Values represent changes in VOR gain after drug injection. Absolute gain values in control conditions were 0.83 ± 0.08 for 0.1 Hz and 0.94 ± 0.12 for 1 Hz. n = 7.
b Values represent changes in VOR phase lead after drug injection. Absolute phase lead values in control conditions were 10.2 ± 2.8 for 0.1 Hz and 6.9 ± 2.6 for 1 Hz. n = 7.
c Mean ± SD of time constants calculated immediately before VOR induction.
* p < 0.05 when phase leads before and after injection were compared.
Effect of cGMP analogs
Local injections of the permeant cGMP analog 8-Br-cGMP (4–10 nmol) in the PH nucleus induced a nystagmus whose slow phases, directed ipsilaterally to the injected side, were best fit to an exponential equation with a mean time constant of 0.92 ± 0.10 sec (mean ± SEM; n = 4) during the period of maximum effect. Under light conditions, a loss of eye position signal was also observed (Fig. 2). During VOR, 8-Br-cGMP produced a velocity imbalance (Figs.3A,B, 4A) without alteration of the reflex gain and phase lead (Figs.3A,B,4B,C). Therefore, the cGMP analog behaved exactly like the NO donors, suggesting that the effects of NO on eye velocity during spontaneous movements and VOR and on eye position during spontaneous eye movements were mediated by the activation of soluble guanylyl cyclase.
Localization of neurons containing NOS and NO-sensitive guanylyl cyclase
To find the possible targets of NO, cGMP immunohistochemistry was performed in brainstem sections from cats perfused with SNP before fixation. Using this technique in combination with NOS immunohistochemistry in similar sections, we found that in the PH nucleus NOS was present in a group of densely packed neurons (Fig.5A), whereas cGMP was present in a rich neuropil in the dorsal part of the nucleus (Fig.5B). No cGMP-immunoreactive (cGMP-ir) cell bodies were found in the PH nucleus. In addition, a cluster of NO-sensitive cGMP-ir neuronal cell bodies and neuropil were identified in an intermediate zone between the PH and MV nuclei (Fig.5B,C). The latter structure was at a distance of ∼0.4 mm from the nitrergic neurons in the PH nucleus, suggesting that these cells might be the target for the effects of NO donors and 8-Br-cGMP, which were not observed when NOS activity was modified experimentally.
Fig. 5.
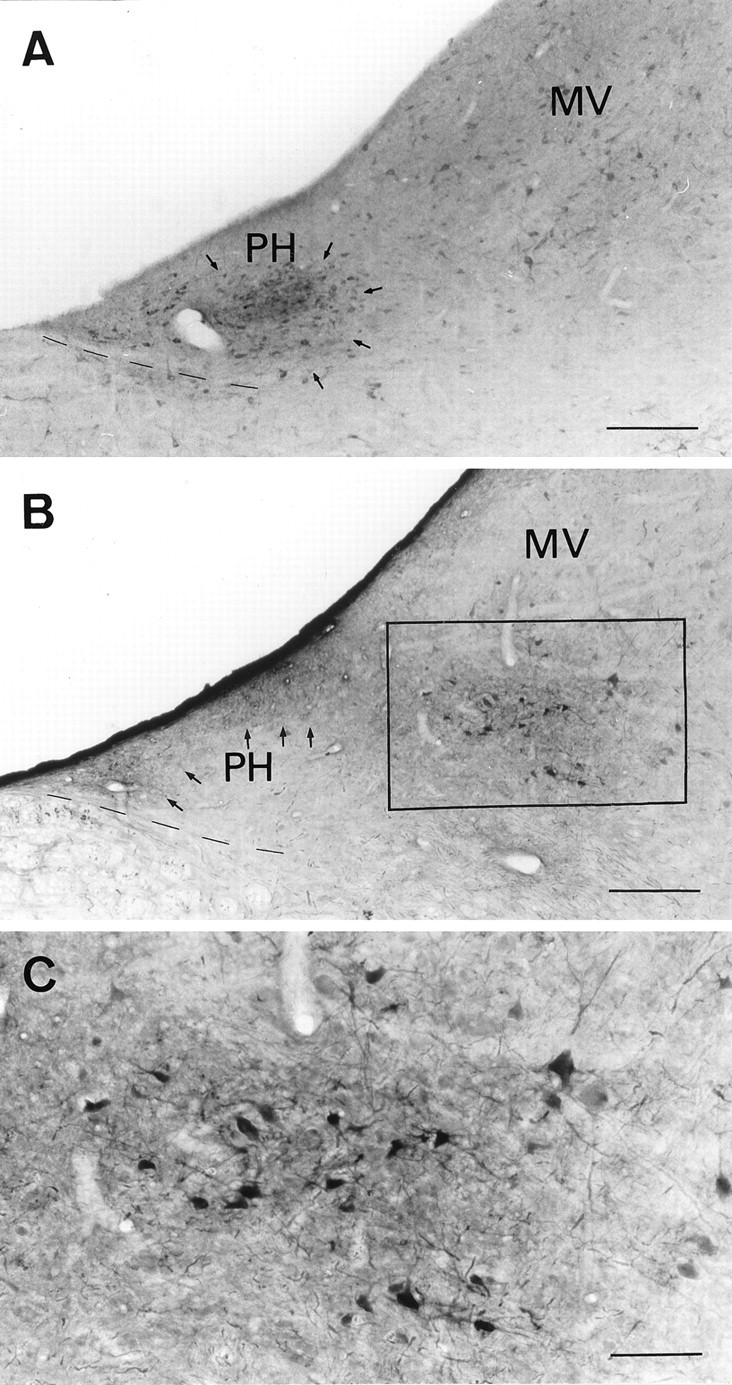
Distribution of nitrergic neurons and neurons containing NO-sensitive guanylyl cyclase in the PH nucleus and adjacent areas. A, Photomicrograph of a coronal brainstem section (posterior 8, according to Berman, 1968) through the PH and MV nuclei stained with a polyclonal antibody recognizing neuronal NOS. A dense group of nitrergic neurons is observed in the PH (arrows). B, Photomicrograph of a coronal brainstem section at a similar rostrocaudal level stained with a polyclonal antibody raised against cGMP. The neuropil is densely stained in the dorsal part of the PH nucleus (arrows), and a cluster of labeled cell bodies (boxed area) appears in an intermediate location between the PH and MV nuclei.C, Higher magnification of the area indicated inB, showing the morphology of NO-sensitive cGMP-producing neurons. Scale bars: A, B, 250 μm;C, 100 μm.
DISCUSSION
In this study, we have shown that NO produced in the PH nucleus participates in the processing of velocity signals in the alert cat, probably by acting on the cGMP-ir neuropil located in the dorsal part of the nucleus. On the other hand, local administration of exogenous NO produced, in addition, an alteration in the velocity-to-position integrator for spontaneous eye movements, suggesting the existence of another NO target, which is not reached by endogenous NO in physiological conditions. A group of neurons, located between the PH and MV nuclei and identified by cGMP immunohistochemistry, might be responsible for the integration deficit caused by exogenous NO.
Modification of NOS activity in the PH nucleus induced a nystagmus during spontaneous eye movements in the dark and a velocity imbalance without alteration of gain or phase lead during VOR. The slow phases of the nystagmic eye movements were ramp-like and, when the animal was returned to conditions of light and the nystagmus was attenuated, no deficits in gaze-holding were observed. All these data indicate that NO produced by PH nitrergic neurons is not involved in the velocity-to-position integration mechanism but only in the processing of pure velocity signals.
With regard to the physiological targets of NO produced by the PH neurons, the absence of cGMP-ir cell bodies in the nucleus rules out a possible effect of NO on intranuclear neurons. Rather, the discovery of an intense cGMP-ir neuropil suggests that NO is acting on nerve fibers or terminals reaching the PH nucleus from other structures. This is in agreement with the proposed role of NO as a retrograde messenger, i.e., NO is being released from the postsynaptic neuron and acting on presynaptic terminals in the CNS (Gally et al., 1990;Schuman and Madison, 1994). The affected terminals cannot originate in the contralateral PH nucleus because of the absence of cGMP-ir cell bodies. According to the functional results, NO may be acting on terminals of vestibular origin, which convey velocity signals and display a basal discharge in the absence of stimulation by head movements. Although the results are not conclusive, this possibility is supported by our recent finding that both the MV and inferior vestibular nuclei contain cGMP-immunostained cell bodies (Moreno-López, Escudero, de Vente, and Estrada, unpublished observations).
Injections of NO donors in the PH nucleus also altered the velocity of spontaneous and vestibularly induced eye movements, but they had an additional effect on the velocity-to-position integration during spontaneous eye movements, as can be deduced from the exponential fit of nystagmic slow phases in darkness and the centripetal drifts that followed saccades under conditions of light. The apparent lack of correspondence between the effects of NOS inhibitors and NO donors can be interpreted by understanding the differences in distribution volumes between endogenous and exogenous NO. After cerebral injections, the diffusion volume of the drugs was probably large enough to include the PH nucleus and some adjacent structures within a few seconds. When NO donors were injected, this entire volume was exposed to NO. However, when an NOS substrate or an NOS substrate analog was injected, they could only affect the activity of NOS, which, within the volume exposed to the injection, is expressed only by the nitrergic cell bodies in the PH nucleus. Activation or inhibition of NOS would modify the endogenous NO production and change NO concentration in a small volume around the nitrergic neurons. This volume can be estimated as a sphere with a radius of ∼100 μm around the NO point source (Wood and Garthwaite, 1994). Therefore, the NO-sensitive structures responsible for the integration deficit caused by NO donors, but not by NOS inhibitors, should lie within the drug diffusion volume but outside the volume of influence of NO produced by PH neurons.
The NO targets responsible for the eye position deficit were probably structures containing soluble guanylyl cyclase, because the same alteration appeared when a cGMP analog was injected. In an attempt to identify the possible targets of NO donors, immunostaining for cGMP was analyzed in brainstem regions close to, but not within, the PH nucleus. This approach revealed the existence of a small area in an intermediate position between the PH and MV nuclei, containing a well defined group of cGMP-ir neurons. This area cannot be defined by cytoarchitectonic criteria using Nissl staining (our unpublished observations), nor has it been described previously in the cat by the use of any other morphological marker. According to its location, this group of cGMP-ir neurons may correspond to the marginal zone nucleus that has been characterized by histological and physiological criteria in monkeys. If the cat marginal zone is the actual target of exogenous NO in our experiments, these neurons should play a role in the integration process during spontaneous eye movements. This is in agreement with the present knowledge on the primate marginal zone, which is considered a component of the saccadic integrator, because virtually all of its neurons are burst-position neurons (McFarland and Fuchs, 1992) projecting to the abducens nucleus (Langer et al., 1986) and to the PH nucleus (Belknap and McCrea, 1988).
It is considered currently that a common integrator is in charge of the different types of eye movements, including the gaze-holding integrator, which maintains eye position after saccades, and the velocity-to-position integrator for the VOR. This hypothesis was initially proposed by Robinson (1975) and has been supported by several experimental findings. Thus, lesions in the PH and/or MV nuclei of cats and monkeys produced simultaneous alterations of integration in the different subsystems tested (Cheron et al., 1986a,b; Cannon and Robinson, 1987). Furthermore, Godaux and Cheron (1996) have reported that in alert cats the eye position sensitivity of PH neurons during intersaccadic fixation was equal to that measured during VOR. However, some pharmacological results suggest that at least some of the subsystems may have separate integrators (Godaux and Laune, 1983;Pastor et al., 1994; Yokota et al., 1994). In our experiments, the use of NO donors, as well as 8-Br-cGMP, revealed an alteration of the integrator for spontaneous eye movements, whereas the VOR gain or phase did not change, indicating that the integrator for VOR remained intact. Hence, the integration processes for at least the two oculomotor subsystems tested occur by different mechanisms, because they can be pharmacologically dissociated.
Our results also suggest that the NO-sensitive cGMP-ir neurons in the cat marginal zone are part of a gaze-holding mechanism specific for saccades. Similarly, small permanent lesions of the primate marginal zone caused by ibotenic acid impaired the gaze-holding in the horizontal plane but not the integration of signals from vestibular sources (Kaneko, 1992, 1997). The lateral location of this area, ∼2 mm from the midline, may explain why Godaux and Cheron (1996), while exploring an area located 1.2–1.6 mm from the midline, did not record neurons with position sensitivity specific for postsaccadic fixations.
Three conclusions can be drawn from the present results: (1) NO produced in the PH nucleus is involved exclusively in the processing of horizontal eye velocity signals, without participation in the integration process occurring in this nucleus; (2) NO action in the PH nucleus is effected by a retrograde action on afferent fibers, probably arriving from the vestibular nuclei; and (3) a cluster of cGMP-ir neurons located in the marginal zone between the PH and the MV nuclei mediates the eye position signal generation during spontaneous, but not vestibularly induced, eye movements, indicating that there is more than one integrator controlling eye movements.
Footnotes
This work was supported by Fondo de Investigación Sanitaria Grants 94/0388 and 97/2054, Comunidad Autonoma de Madrid Grant 08.5/0019/1997, and Dirección General de Investigación Científica y Technológica Grant PB 93–1175. We thank Dr. de Vente for kindly providing the anti-cGMP antibody, Rut González for excellent technical assistance, Dr. Delgado-García for his generous help, and Dr. Elena Galea for critical discussion.
Correspondence should be addressed to Dr. Carmen Estrada, Área de Fisiología, Facultad de Medicina, Universidad de Cádiz, Plaza Fragela s/n, 11003 Cádiz, Spain.
REFERENCES
- 1.Baker R, Berthoz A. Is the prepositus hypoglossi nucleus the source of another vestibulo-ocular pathway? Brain Res. 1975;86:121–127. doi: 10.1016/0006-8993(75)90643-5. [DOI] [PubMed] [Google Scholar]
- 2.Baker R, Mano N, Shimazu H. Postsynaptic potentials in abducens motoneurons induced by vestibular stimulation. Brain Res. 1969;15:577–580. doi: 10.1016/0006-8993(69)90189-9. [DOI] [PubMed] [Google Scholar]
- 3.Batschelet E. Circular statistics in biology. Academic; London: 1981. [Google Scholar]
- 4.Belknap DE, McCrea RA. Anatomical connections of the prepositus and abducens nuclei in the squirrel monkey. J Comp Neurol. 1988;268:13–28. doi: 10.1002/cne.902680103. [DOI] [PubMed] [Google Scholar]
- 5.Berman AL. The brain stem of the cat: a cytoarchitectonic atlas with stereotaxic coordinates. University of Wisconsin; Madison, WI: 1968. [Google Scholar]
- 6.Berthoz A, Droulez J, Vidal PP, Yoshida K. Neural correlates of horizontal VOR cancellation during rapid eye movements in the cat. J Physiol (Lond) 1989;419:717–751. doi: 10.1113/jphysiol.1989.sp017895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon SC, Robinson DA. Loss of the neural integrator of the oculomotor system from brain stem lesions in monkey. J Neurophysiol. 1987;57:1383–1409. doi: 10.1152/jn.1987.57.5.1383. [DOI] [PubMed] [Google Scholar]
- 8.Cheron G, Godaux E. Disabling of the oculomotor neural integrator by kainic acid injections in the prepositus vestibular complex of the cat. J Physiol (Lond) 1987;394:267–290. doi: 10.1113/jphysiol.1987.sp016870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheron G, Gillis P, Godaux E. Lesion in the cat prepositus complex: effects on the optokinetic system. J Physiol (Lond) 1986a;372:95–111. doi: 10.1113/jphysiol.1986.sp015999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheron G, Godaux E, Laune JM, Vanderkelen B. Lesions in the cat prepositus complex: effects on the vestibulo-ocular reflex and saccades. J Physiol (Lond) 1986b;372:75–94. doi: 10.1113/jphysiol.1986.sp015998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De la Cruz RR, Escudero M, Delgado-García JM. Behavior of medial rectus motoneurons in the alert cat. Eur J Neurosci. 1990;1:288–295. doi: 10.1111/j.1460-9568.1989.tb00796.x. [DOI] [PubMed] [Google Scholar]
- 12.Delgado-García JM, Del Pozo F, Baker R. Behavior of neurons in the abducens nucleus of the alert cat. I. Motoneurons. Neuroscience. 1986;17:929–952. doi: 10.1016/0306-4522(86)90072-2. [DOI] [PubMed] [Google Scholar]
- 13.Delgado-García JM, Vidal PP, Gómez C, Berthoz A. A neurophysiological study of prepositus hypoglossi neurons projecting to oculomotor and preoculomotor nuclei in the alert cat. Neuroscience. 1989;29:291–307. doi: 10.1016/0306-4522(89)90058-4. [DOI] [PubMed] [Google Scholar]
- 14.Escudero M, de la Cruz RR, Delgado-García JM. A physiological study of vestibular and prepositus hypoglossi neurones projecting to the abducens nucleus in the alert cat. J Physiol (Lond) 1992;458:539–560. doi: 10.1113/jphysiol.1992.sp019433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuchs AF, Luschei ES. Firing patterns of abducens neurons of alert monkeys in relationship to horizontal eye movement. J Neurophysiol. 1970;33:382–392. doi: 10.1152/jn.1970.33.3.382. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966;21:1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- 17.Fukushima K, Kaneko CRS, Fuchs AF. The neuronal substrate of integration in the oculomotor system. Prog Neurobiol. 1992;39:609–639. doi: 10.1016/0301-0082(92)90016-8. [DOI] [PubMed] [Google Scholar]
- 18.Gally JA, Montague PR, Reeke GN, Edelman GM. The NO hypothesis: possible effects of a short lived, rapidly diffusible signal in the development and function of the nervous system. Proc Natl Acad Sci USA. 1990;87:3547–3551. doi: 10.1073/pnas.87.9.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godaux E, Cheron G. The hypothesis of the uniqueness of the oculomotor neural integrator: direct experimental evidences in the cat. J Physiol (Lond) 1996;492:517–527. doi: 10.1113/jphysiol.1996.sp021326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godaux E, Laune JM. The saccadic system and the vestibulo-ocular reflex in the cat do not share the same integrator. Neurosci Lett. 1983;38:263–268. doi: 10.1016/0304-3940(83)90379-8. [DOI] [PubMed] [Google Scholar]
- 21.Godaux E, Mettens P, Cheron G. Differential effect of injections of kainic acid into the prepositus and the vestibular nuclei of the cat. J Physiol (Lond) 1993;472:459–482. doi: 10.1113/jphysiol.1993.sp019956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldberg J. Activity of abducens nucleus units in the alert cat. PhD thesis. University of California at Berkeley; 1980. [Google Scholar]
- 23.Hikosaka O, Igusa Y, Nakao S, Shimazu H. Direct inhibitory synaptic linkage of pontomedullary reticular burst neurons with abducens motoneurons in the cat. Exp Brain Res. 1978;33:337–352. doi: 10.1007/BF00235558. [DOI] [PubMed] [Google Scholar]
- 24.Hikosaka O, Nakao S, Shimazu H. Postsynaptic inhibition underlying spike suppression of secondary vestibular neurons during quick phases of vestibular nystagmus. Neurosci Lett. 1980;16:21–26. doi: 10.1016/0304-3940(80)90095-6. [DOI] [PubMed] [Google Scholar]
- 25.Kaneko CRS. Effects of ibotenic acid lesions of nucleus prepositus hypoglossi on optokinetic and vestibular eye movements in the alert, trained monkey. Ann NY Acad Sci. 1992;656:408–427. doi: 10.1111/j.1749-6632.1992.tb25225.x. [DOI] [PubMed] [Google Scholar]
- 26.Kaneko CRS. Eye movement deficits after ibotenic acid lesions of the nucleus prepositus hypoglossi in monkeys. I. Saccades and fixation. J Neurophysiol. 1997;78:1753–1768. doi: 10.1152/jn.1997.78.4.1753. [DOI] [PubMed] [Google Scholar]
- 27.Kaneko CRS, Evinger C, Fuchs AF. Role of cat pontine burst neurons in generation of saccadic eye movements. J Neurophysiol. 1981;46:387–408. doi: 10.1152/jn.1981.46.3.387. [DOI] [PubMed] [Google Scholar]
- 28.Langer T, Kaneko CRS, Scudder CA, Fuchs AF. Afferents to the abducens nucleus in the monkey and cat. J Comp Neurol. 1986;245:379–400. doi: 10.1002/cne.902450307. [DOI] [PubMed] [Google Scholar]
- 29.López-Barneo J, Darlot C, Berthoz A, Baker R. Neuronal activity in prepositus nucleus correlated with eye movement in the alert cat. J Neurophysiol. 1982;47:329–352. doi: 10.1152/jn.1982.47.2.329. [DOI] [PubMed] [Google Scholar]
- 30.McCrea RA, Baker R. Anatomical connections of the nucleus prepositus of the cat. J Comp Neurol. 1985;237:377–407. doi: 10.1002/cne.902370308. [DOI] [PubMed] [Google Scholar]
- 31.McCrea RA, Yoshida K, Berthoz A, Baker R. Eye movements related activity and morphology of second order vestibular neurons terminating in the cat abducens nucleus. Exp Brain Res. 1980;40:468–473. doi: 10.1007/BF00236156. [DOI] [PubMed] [Google Scholar]
- 32.McCrea RA, Strassman A, May E, Highstein SM. Anatomical and physiological characteristics of vestibular neurons mediating the horizontal vestibuloocular reflex in the squirrel monkey. J Comp Neurol. 1987;264:547–570. doi: 10.1002/cne.902640408. [DOI] [PubMed] [Google Scholar]
- 33.McFarland JL, Fuchs AF. Discharge patterns in nucleus prepositus hypoglossi and adjacent medial vestibular nucleus during horizontal eye movements in behaving macaques. J Neurophysiol. 1992;68:319–332. doi: 10.1152/jn.1992.68.1.319. [DOI] [PubMed] [Google Scholar]
- 34.Mettens P, Godaux E, Cheron G, Galiana HL. Effect of muscimol microinjection into the prepositus hypoglossi and the medial vestibular nuclei on cat eye movements. J Neurophysiol. 1994;72:785–802. doi: 10.1152/jn.1994.72.2.785. [DOI] [PubMed] [Google Scholar]
- 35.Moreno-López B, Escudero M, Delgado-García JM, Estrada C. Nitric oxide production by brain stem neurons is required for normal performance of eye movements in alert animals. Neuron. 1996;17:739–745. doi: 10.1016/s0896-6273(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 36.Pastor AM, de la Cruz RR, Baker R. Eye position and eye velocity integrators reside in separate brain stem nuclei. Proc Natl Acad Sci USA. 1994;91:807–811. doi: 10.1073/pnas.91.2.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson DA. Eye movement control in primates. Science. 1968;161:1219–1224. doi: 10.1126/science.161.3847.1219. [DOI] [PubMed] [Google Scholar]
- 38.Robinson DA. Oculomotor control signals. In: Lennerstrand G, Bach-y-Rita P, editors. Basic mechanisms of ocular motility and their clinical implications. Pergamon; Oxford: 1975. pp. 337–374. [Google Scholar]
- 39.Schuman EM, Madison DV. Nitric oxide and synaptic function. Annu Rev Neurosci. 1994;17:153–183. doi: 10.1146/annurev.ne.17.030194.001101. [DOI] [PubMed] [Google Scholar]
- 40.Skavenski AA, Robinson DA. Role of abducens neurons in vestibuloocular reflex. J Neurophysiol. 1973;36:724–737. doi: 10.1152/jn.1973.36.4.724. [DOI] [PubMed] [Google Scholar]
- 41.Southam E, Garthwaite J. The nitric oxide-cyclic GMP signalling pathway in the rat brain. Neuropharmacology. 1993;32:1267–1277. doi: 10.1016/0028-3908(93)90021-t. [DOI] [PubMed] [Google Scholar]
- 42.Strassman A, Highstein SM, McCrea RA. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. I. Excitatory burst neurons. J Comp Neurol. 1986a;249:337–357. doi: 10.1002/cne.902490303. [DOI] [PubMed] [Google Scholar]
- 43.Strassman A, Highstein SM, McCrea RA. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. II. Inhibitory burst neurons. J Comp Neurol. 1986b;249:358–380. doi: 10.1002/cne.902490304. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka J, Markerink-van Ittersum M, Steinbusch HWM, de Vente J. Nitric oxide-mediated cGMP synthesis in oligodendrocytes in the developing rat brain. Glia. 1997;19:286–297. [PubMed] [Google Scholar]
- 45.Wood J, Garthwaite J. Models of the diffusional spread of nitric oxide: implications for neural nitric oxide signaling and its pharmacological properties. Neuropharmacology. 1994;33:1235–1244. doi: 10.1016/0028-3908(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 46.Yokota JI, Reisine H, Cohen B. Velocity storage and effects of injection of GABAergic substances in the prepositus hypoglossi nuclei. In: Fuchs AF, Brandt T, Büttner U, Zee D, editors. Contemporary ocular motor and vestibular research: a tribute to David A. Robinson. Georg Thieme Verlag; Stuttgart, Germany: 1994. pp. 453–461. [Google Scholar]