Abstract
The opiate antagonist naltrexone suppresses ethanol-reinforced behavior in animals and decreases ethanol intake in humans. However, the mechanisms underlying these actions are not well understood. Experiments were designed to test the hypothesis that naltrexone attenuates the rewarding properties of ethanol by interfering with ethanol-induced stimulation of dopamine activity in the nucleus accumbens (NAcc). Simultaneous measures of the effects of naltrexone on dialysate dopamine levels in the NAcc and on operant responding for oral ethanol were used. Male Wistar rats were trained to self-administer ethanol (10–15%, w/v) in 0.2% (w/v) saccharin during daily 30 min sessions and were surgically prepared for intracranial microdialysis. Experiments began after reliable self-administration was established. Rats were injected with naltrexone (0.25 mg/kg, s.c.) or saline and 10 min later were placed inside the operant chamber for a 20 min waiting period with no ethanol available, followed by 30 min of access to ethanol. A transient rise in dialysate dopamine levels was observed during the waiting period, and this effect was not altered by naltrexone. Ethanol self-administration reliably increased dopamine levels in controls. Naltrexone significantly suppressed ethanol self-administration and prevented ethanol-induced increases in dialysate dopamine levels. Subsequent dose–effect analyses established that the latter effect was not merely a function of reduced ethanol intake but that naltrexone attenuated the efficacy of ethanol to elevate dialysate dopamine levels. These results suggest that suppression of ethanol self-administration by opiate antagonists is the result of interference with dopamine-dependent aspects of ethanol reinforcement, although possible additional effects via nondopaminergic mechanisms cannot be eliminated as a factor in opiate antagonist-induced reduction of ethanol intake.
Keywords: ethanol, naltrexone, reinforcement, dopamine, microdialysis, nucleus accumbens
Opiate antagonists inhibit the self-administration of ethanol in a variety of animal models, suggesting that opioid pathways in the brain participate in the mediation of ethanol-seeking behavior. For example, the nonselective opiate receptor antagonists naloxone, naltrexone, and nalmefene reduce ethanol intake in rats and monkeys (Altshuler et al., 1980; Samson and Doyle, 1985; Froehlich et al., 1990; Weiss et al., 1990; Hubbell et al., 1991; Kornet et al., 1991; Gauvin et al., 1993; Hyytiä and Sinclair, 1993; Davidson and Amit, 1996). μ and δ receptor-selective antagonists have also been reported to suppress ethanol self-administration in rats (Hyytiä, 1993; Krishnan-Sarin et al., 1995; Honkanen et al., 1996). Finally, a growing number of clinical studies suggest that naltrexone is an effective pharmacological adjunct for reducing relapse in human alcoholics (O’Malley et al., 1992; Volpicelli et al., 1992; O’Brien et al., 1996).
The neurochemical mechanisms underlying the attenuation of volitional ethanol intake by opiate receptor antagonists are not well understood but may involve an interaction with dopaminergic neurotransmission. The mesolimbic dopamine pathway that projects from the ventral tegmental area (VTA) to the nucleus accumbens (NAcc) has been implicated as a key site for the reinforcing actions of many drugs of abuse including ethanol (Wise and Rompre, 1989; Koob, 1992; Di Chiara, 1995). Systemic administration of ethanol increases the firing rate of mesolimbic dopamine neurons (Gessa et al., 1985; Diana et al., 1992) and increases extracellular dopamine concentrations in the NAcc as measured by microdialysis (Imperato and Di Chiara, 1986; Yoshimoto et al., 1991;Blanchard et al., 1993; Blomqvist et al., 1993; Rossetti et al., 1993;Kiianmaa et al., 1995; Mocsary and Bradberry, 1996; Yim et al., 1998). More direct evidence of a role of dopamine in ethanol reward comes from findings that oral ethanol self-administration elevates extracellular dopamine in the NAcc (Weiss et al., 1993, 1996), that rats self-administer ethanol directly into the VTA cell body region of mesolimbic dopamine neurons (Gatto et al., 1994), and that pharmacological manipulation of dopamine neurotransmission modifies ethanol-reinforced operant responding and ethanol preference (e.g.,Weiss et al., 1990; Samson et al., 1993; George et al., 1995; Panocka et al., 1995). Both the NAcc and VTA are rich in opioid peptides and receptors (Wamsley et al., 1980; Lewis et al., 1983; Dilts and Kalivas, 1989, 1990). Afferent projections to these areas as well as local interneurons (Khachaturian et al., 1993; de Waele et al., 1995) provide a potential anatomical substrate by which endogenous opioids may modulate the dopaminergic and, ultimately, the rewarding effects of ethanol. In fact, opiate antagonists can blunt ethanol-induced increases in extracellular dopamine in the NAcc. For example, the nonselective opiate antagonist naltrexone has been reported to inhibit the rise in extracellular dopamine concentrations elicited by reverse microdialysis of 5% (v/v) ethanol (Benjamin et al., 1993). Although the pharmacological relevance of local perfusion with such high ethanol concentrations [∼800 mm in Benjamin et al. (1993)] has been questioned (Gonzales et al., 1998), this finding suggests a possible role for opioid receptors in ethanol-induced dopaminergic activation. This possibility is supported further by the finding that reverse transcerebral microdialysis of the selective δ-opioid antagonist naltrindole completely blocked the increases in extracellular dopamine levels in the NAcc induced by systemic ethanol administration (Acquas et al., 1993).
On the basis of these findings, one may hypothesize that interference with ethanol-induced stimulation of dopamine release is one of the mechanisms by which opiate antagonists suppress ethanol self-administration. This possibility remains to be confirmed, however, by the demonstration that reductions in ethanol-reinforced behavior produced by opiate antagonists are predictably coupled to decreases in the efficacy of ethanol to increase extracellular dopamine concentrations in the NAcc. The purpose of the present study was to test this hypothesis by measuring simultaneously the effects of the nonselective opiate receptor antagonist naltrexone on operant responding for oral ethanol and on dialysate dopamine levels in the NAcc of rats and by examining the consequences of naltrexone treatment on the dose–effect function for ethanol-stimulated dialysate dopamine levels.
MATERIALS AND METHODS
Subjects. Male Wistar rats (Charles River Laboratories, Wilmington, MA) weighing between 450 and 550 gm at the time of testing were used. The rats were housed in groups of two or three in a humidity- and temperature (22°C)-controlled environment. The light/dark cycle was 12:12 hr (lights on at 6 A.M. and off at 6 P.M.), and animals had food and water available ad libitum. All procedures were conducted in strict adherence with theNational Institutes of Health Guide for the Care and Use of Laboratory Animals.
Behavioral testing apparatus. Ethanol self-administration training and microdialysis were conducted in standard operant chambers (Coulbourn Instruments, Allentown, PA) modified to accommodate the microdialysis perfusion system as described previously (Weiss et al., 1993). The operant chambers contained a retractable lever that, when activated, delivered 0.1 ml of a solution into a receptacle (volume capacity, 0.15 ml) located 4 cm above the grid floor in the center of the front plate of the chamber. The chambers were enclosed within sound-attenuating, ventilated environmental cubicles (Coulbourn Instruments). Fluid delivery and the recording of behavioral data were controlled by a microcomputer.
Ethanol self-administration training. Rats were trained to orally self-administer an ethanol–saccharin solution in daily 30 min sessions using previously described procedures (Weiss et al., 1993). Briefly, rats were initially placed on a 22 hr water restriction schedule for the first 4 d of training, during which time they were trained to respond to 0.2% (w/v) saccharin on a schedule of continuous reinforcement. After operant responding was acquired successfully, water was made available again ad libitum in the home cage for the remainder of the experiment. On the fourth day of training, ethanol (5%, w/v) was added to the 0.2% saccharin solution. Over a period of 3–4 weeks, the ethanol concentration was gradually increased to 10–15% while the saccharin concentration was unaltered. During this time, a “waiting period” that preceded access to the drinking solution was gradually introduced by placing rats in the operant chamber for increasing amounts of time (1 extra minute per day) before extension of the lever and onset of the session. The final length of the waiting period was 20 min. This procedure was adopted as a precaution because of previous observations that exposure to an environment associated with ethanol availability can produce a transient (15–20 min) rise in dialysate dopamine levels in the NAcc (Weiss et al., 1993). Thus, the waiting period served the purpose of minimizing the effects of possible increases in dialysate dopamine levels induced by environmental stimuli that might otherwise confound the subsequent measurement of the effects of ethanol on accumbal dopamine efflux. Rats remained in the operant chambers for 10 min after the lever was retracted at the end of each 30 min self-administration session. During the self-administration training phase after surgery (see below), the animals were habituated to the microdialysis perfusion and tethering system. This consisted of a stainless steel cannula connector that was attached to a liquid swivel suspended from a balanced lever arm that was positioned over the center of the chamber. During this time, the rats were also habituated to the drug injection procedure by receiving subcutaneous injections of saline on several occasions before being placed in the operant chambers. The final self-administration session involving microdialysis and opiate antagonist drug tests was conducted 7 d after the last training session.
Implantation of guide cannulae. After reliable self-administration of ethanol was established, unilateral stainless steel guide cannulae (Plastics One, Roanoke, VA) were stereotaxically implanted into the rats under halothane anesthesia aimed at the NAcc as described previously (Weiss et al., 1996). Coordinates were (in mm) 1.7 anterior, ±1.4 medial, and −6.1 ventral, relative to bregma (Paxinos and Watson, 1986). The rats were allowed to recover for 7 d before resumption of ethanol self-administration training.
Microdialysis procedures. Microdialysis probes were constructed using the methods of Pettit and Justice (1991). Fused silica tubing (inner diameter, 40 μm) was used as inlet and outlet to a hollow fiber dialysis membrane (outer diameter, 270 μm; molecular weight cutoff, 13,000; Spectrum, Houston, TX) that was sealed at both ends with epoxy. The active dialysis area was 2 mm, which was the distance between the ends of the inlet tubing and the outlet tubing. The probe inlet was connected to a liquid swivel (centered above the cage suspended from the balanced arm) that was connected to a syringe containing artificial CSF (aCSF; NaCl 145 mm, KCl 2.8 mm, MgCl2 1.2 mm, CaCl2 1.2 mm, ascorbate 0.25 mm, and glucose 5.4 mm, pH 7.2–7.4) that was pumped through the system using a pulseless syringe pump (Bioanalytical Systems, West Lafayette, IN). The probe outlet was placed into a 0.25 ml plastic vial for collection of dialysate that was changed manually. Samples were frozen immediately on dry ice and stored at −70°C until analyzed.
On the evening before the experiments, the microdialysis probes perfused with aCSF at a rate of 0.2 μl/min were slowly lowered into place through the guide cannulae while the rats were briefly anesthetized with halothane. After recovery from the anesthesia, the rats remained in the home cage overnight with food and water available. During this time the perfusion flow rate remained at 0.2 μl/min. The next morning the flow rate was increased to 0.5 μl/min, and dialysate samples were collected at 10 min intervals beginning 2 hr after changing the flow rate.
Experimental design and procedures. Dialysate dopamine concentrations were monitored under the following conditions. On the microdialysis day, baseline samples were taken for 1 hr. This was followed by a subcutaneous injection of either the nonselective opiate receptor antagonist naltrexone (0.25 mg/kg) or its vehicle (saline), after which the rats were returned to their home cages. Ten minutes after the naltrexone or vehicle treatment, the animals were placed into the operant chambers. The test session consisted of a 20 min waiting period without access to the lever and drinking solution, a 30 min ethanol self-administration period, and a 10 min period after the session such that the rats remained in the chamber for a total of 60 min/session. Immediately after removal from the operant chambers, tail blood samples were taken for determination of blood alcohol levels in those animals that exhibited significant ethanol intake.
HPLC analysis of dopamine. Dialysate dopamine concentrations were determined by microbore reverse-phase HPLC. Dialysate was injected (3–4.5 μl) onto a Sepstik column (100 × 1 mm; 3 μm ODS; Bioanalytical Systems) using a Valco injector. Mobile phase (19 mm citric acid, 40 mm sodium phosphate, 0.2 mm EDTA, and 0.21 mm 1-decanesulfonic acid containing 19% (v/v) methanol, pH 5.25) was pumped through the column using an ISCO (Lincoln, NE) syringe pump at 25 μl/min. Dopamine was detected using a glassy carbon working electrode controlled by a Model 400 Princeton Applied Research (Princeton, NJ) electrochemical detector. The applied potential was 0.7 V (vs Ag/AgCl). Chromatograms were recorded using a Kipp and Zonen strip-chart recorder.
Blood alcohol determination. In a subset of animals, a tail blood sample was collected after the self-administration session. The blood sample (200 μl) was collected into a 1.5 ml microfuge tube that contained 4 μl of heparin USP (1000 units/ml), and 10 μl of whole blood was immediately transferred into a headspace gas chromatography vial and sealed with a rubber septum. Samples were stored at 4°C until analyzed. A Perkin-Elmer (Emeryville, CA) Sigma 2000 gas chromatograph (stainless steel column, 80/100 Porapak Q5 and 6 feet × 1/8 inch, Supelco) with an HS-100 headspace analyzer was used for ethanol analysis. Nitrogen was used as a carrier gas (flow rate, 30 ml/min). The injector temperature was set to 90°C, and the oven temperature was 190°C. The retention time of ethanol was ∼1.8 min.
Histology. The placement of the microdialysis probes was verified by histological analysis of brains after completion of the experiments. Rats were killed by administration of an overdose of halothane, and their brains were removed and placed in formalin. Fifty micrometer frozen sections were prepared and stained with cresyl violet. In 25 out of the 28 rats, at least 70% of the active dialysis area was located within the NAcc. Two rats were included whose probes extended into the ventral striatal/pallidal area. In one animal the probe location could not be verified because of loss of the sample during slicing.
Statistical analysis. The dopamine concentration data were transformed to percent of basal values and analyzed by a 2 × 12 mixed-factorial ANOVA for differences in dialysate dopamine levels between the saline- and naltrexone-treated animals. Basal dopamine levels were defined as the average of six baseline samples in each animal. There were 7 missing data points out of the 384 collected because of technical problems with sample collection or HPLC. These points were estimated by interpolation, and the degrees of freedom for the ANOVA were corrected for these estimates. Ethanol intake data were analyzed by a 2 × 2 × 6 mixed-factorial ANOVA with two within-subject variables (intake in 5 min bins and day). Differences between individual means (after confirmation of significant interactions in the overall ANOVA) were determined by one-way ANOVAs or planned contrasts using Bonferroni corrections to control for experimentwise error. Relationships between ethanol intake and the maximal percent increase in dialysate dopamine concentration during ethanol self-administration as well as differences between the slopes of the dose–effect functions of the naltrexone versus vehicle groups were analyzed by linear regression (Kenakin, 1997). Significance was accepted when p < 0.05.
RESULTS
Blood ethanol levels after ethanol self-administration
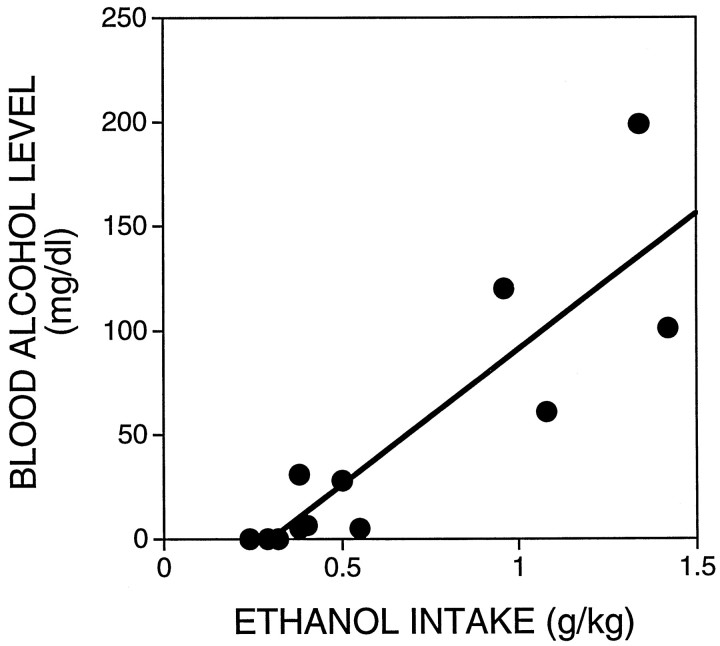
In eight of the control and four of the naltrexone-treated animals, a sample of tail blood was taken after collection of the last dialysate fraction. The time elapsed between cessation of responding for ethanol and blood sampling ranged from 30 to 55 min. Figure1 shows that there was a significant relationship between ethanol intake and blood alcohol level. These results are similar to those obtained previously in male Wistar rats using the alcohol dehydrogenase method for determination of blood alcohol levels (Weiss et al., 1993).
Fig. 1.
Relationship between ethanol intake and blood alcohol levels. Tail blood samples were taken in a subset of rats 30–55 min after termination of ethanol self-administration. Blood alcohol concentrations were determined by gas chromatography and analyzed by linear regression (r = 0.88;p < 0.05).
Naltrexone and ethanol self-administration
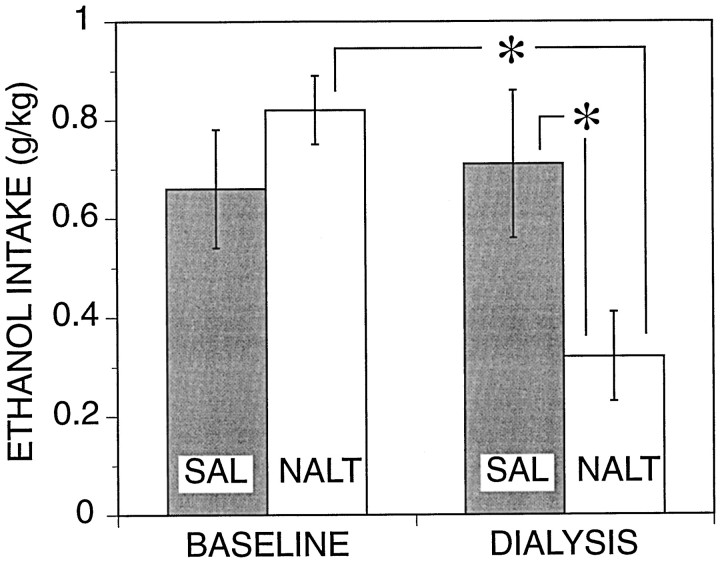
During the last self-administration session (baseline) before the drug and microdialysis test day, the mean responding for ethanol in the subgroups of animals assigned to the saline (control) and naltrexone groups was virtually identical (Fig. 2). Mean ethanol intake in the saline control group remained essentially unaltered on the test day, suggesting that the microdialysis procedure per se did not interfere with ethanol self-administration. In contrast, ethanol consumption was substantially reduced in naltrexone-treated rats compared with both their own baseline levels of intake and ethanol intake in vehicle-treated rats on the test day. The differences in ethanol intake were confirmed by a significant interaction between groups and self-administration session [F(1,27)= 11.2; p < 0.05] and by a significant difference between baseline versus test day for the naltrexone group [F(1,27) = 20.9; p < 0.05;post hoc ANOVA, Bonferroni-corrected]. Additionalpost hoc analyses confirmed that ethanol intake of the naltrexone group was significantly lower than that of the saline group on the test day [F(1,54) = 6.9;p < 0.05].
Fig. 2.
Effects of naltrexone (0.25 mg/kg, s.c.) on ethanol self-administration. Mean (± SEM) ethanol intake during 30 min self-administration sessions is shown for baseline and test (dialysis) days. Shaded and open bars show data from rats assigned to the saline (n = 13;SAL) and naltrexone (n = 16;NALT) groups, respectively. All animals received either saline vehicle or naltrexone injections on the test day, whereas only vehicle was available on the baseline day for both groups. There was no significant main effect of treatment group [F(1,27) = 0.89; p > 0.05], but there was a significant main effect of session [baseline vs microdialysis day; F(1,27) = 7.7;p < 0.05]. Post hoc analyses shown on the figure were performed based on a significant interaction between the main effects as explained in the text; * denotes a significant (p < 0.05) difference from the respective control condition as explained in the text.
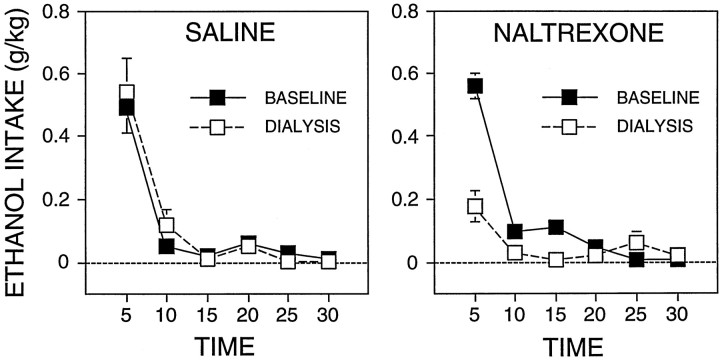
Examination of the distribution of responding over the 30 min self-administration sessions revealed that in the saline group most of the ethanol intake occurred within the first 5 min on both the baseline and test day (Fig. 3). The time course and the total amount of ethanol intake in the naltrexone group during the baseline day was similar to that of the control group. This was reflected in a nonsignificant interaction between group and time course of intake for the baseline day [F(1,54) = 1.1;p > 0.05]. In contrast, on the test day, the naltrexone group showed a significant decrease in ethanol intake during the initial 5 min [F(1,140) = 55;p < 0.05; one-way ANOVA after significant overall interaction, F(1,54) = 19; p < 0.05] without large changes in the pattern of intake during the remainder of the session. The analysis of interactions described above was justified on the basis of a significant three-way interaction for group, session, and the time course of intake within the session [F(5,135) = 10.1; p < 0.05].
Fig. 3.
Distribution of mean (± SEM) ethanol intake over the 30 min self-administration phase during the baseline and test (dialysis) days. The figure represents the same data presented in Figure 2 but shown in 5 min bins.
Effects of ethanol and naltrexone on dialysate dopamine levels
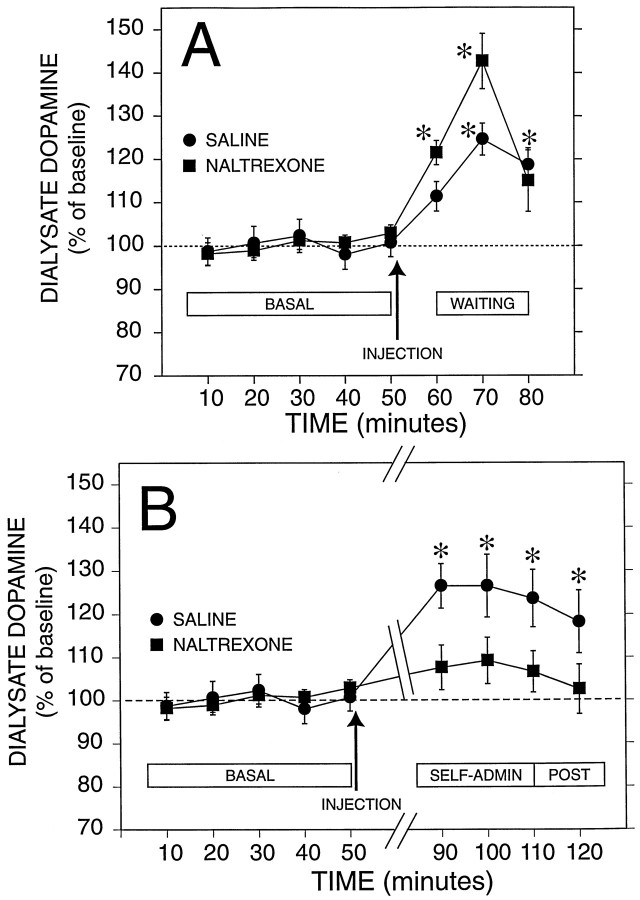
Analysis of the time course of dialysate dopamine concentrations across baseline, injection, waiting, and self-administration periods revealed a significant interaction between time and drug treatment condition [F(11,290) = 1.9; p< 0.05]. There was no significant main effect of drug treatment [F(1,27) = 1.3; p > 0.05], but there was a significant main effect of time [F(11,290) = 11.8; p < 0.05]. Analyses of simple effects and contrasts between means were then performed to determine the source of the significant interaction. Data are shown in Figure 4 as the concentration of dopamine in the dialysate and in Figure5 as the percent of baseline.
Fig. 4.
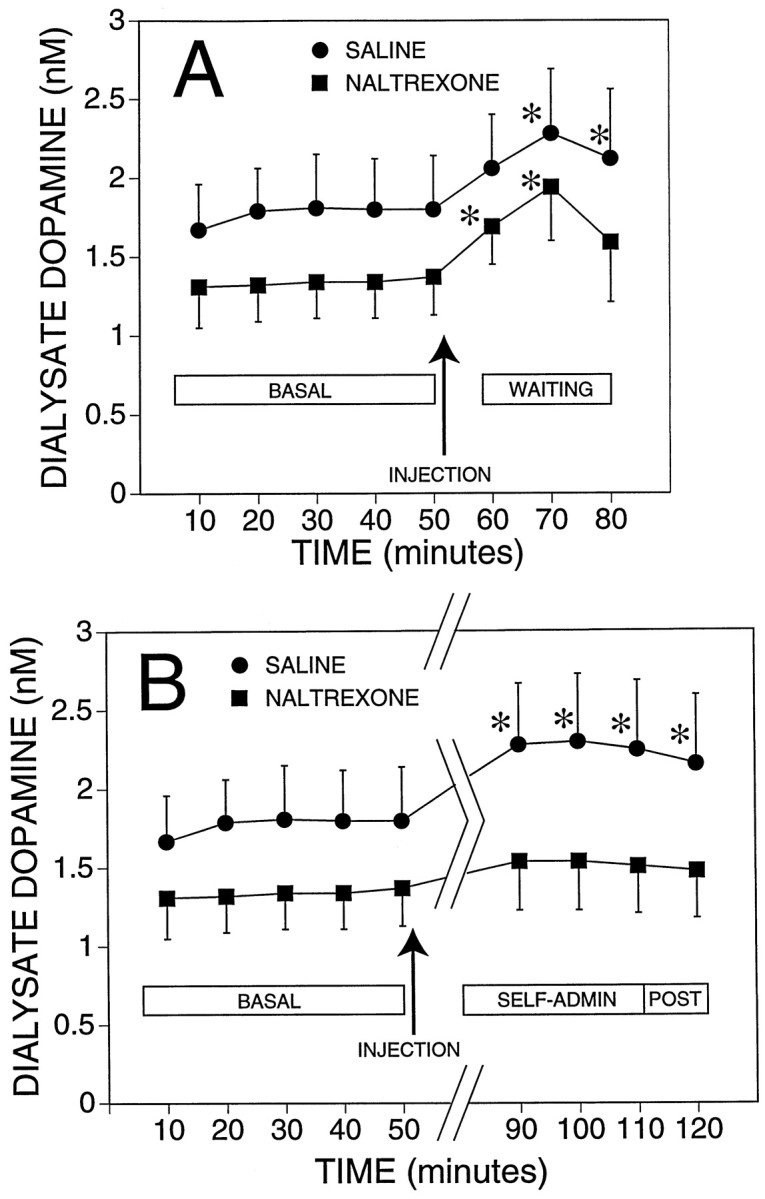
Effect of naltrexone on mean (± SEM) dialysate dopamine concentrations from the nucleus accumbens before, during, and after self-administration of ethanol. Basal (BASAL) represents dialysate dopamine levels while rats were resting in their home cage. The arrows indicate the administration of naltrexone (n = 16) or vehicle (saline;n = 13). A, The effects of the injection and the response during the waiting (WAITING) period during which rats were placed in the operant chambers but did not have access to ethanol. B, The same basal shown inA for comparison with the response during the 30 min self-administration session (SELF-ADMIN) and the 10 min period after the session ended (POST). Data for the waiting period were not shown in this panel to facilitate comparison of basal with the response during self-administration; * indicates a significant difference from the respective pooled basal concentration (p < 0.05, determined by planned contrasts, Bonferroni-corrected, after significant interaction between groups and time in the overall ANOVA).
Fig. 5.
Effect of naltrexone on mean (± SEM) dialysate dopamine response expressed as the percent of basal during ethanol self-administration in the same rats shown in Figure 4. Data are presented as described in the legend to Figure 4 except that concentration values were transformed to the percent of basal for each rat.
Dopamine concentrations in the two groups of rats were stable during the last 50 min of the 1 hr baseline collection period (Figs. 4, 5), and the mean (± SEM) basal dopamine levels in the saline and naltrexone groups were not significantly different from each other (1.77 ± 0.32 and 1.34 ± 0.23 nm, respectively). The samples collected 10 min after the administration of saline or naltrexone showed a slight increase in dopamine concentration compared with the basal level in both groups, although this increase was statistically significant only in the naltrexone group [F(1,182) = 12.7;p < 0.05].
Dialysate dopamine levels transiently increased during the 20 min waiting period (Figs. 4A, 5A) in both groups of rats [saline, F(1,182) = 20.3;p < 0.05; naltrexone, F(1,182)= 36.2; p < 0.05]. In both groups the maximal mean increases in dopamine levels occurred during the first 10 min of this 20 min experimental phase. By the end of the waiting period, dialysate dopamine concentrations in the naltrexone group were no longer significantly different from basal values, whereas dopamine levels in the saline control group remained significantly elevated.
There were clear differences in dialysate dopamine concentrations between the two treatment groups during the ethanol self-administration phase of the experiment. Figures 4B and 5Billustrate that the saline group showed a significant increase in dopamine concentration compared with basal levels during the 30 min session including the last dialysate sample collected 10 min after the session had ended [F(1,182) > 12.4;p < 0.05 for the four contrasts]. Dopamine concentrations in the naltrexone group were not significantly different from basal levels during the self-administration period. Therefore, the major source of the overall interaction between drug treatment conditions and changes in dopamine concentrations over the experimental phases resided in the differences between the effect of ethanol self-administration on dialysate dopamine levels in the naltrexone- versus saline-treated groups.
Inspection of the temporal profile of dialysate dopamine concentrations over the experimental phases (Figs. 4, 5) revealed a biphasic change suggestive of an overlap between the late effects of introduction in the operant chamber (waiting phase) and the early effects of ethanol on dialysate dopamine levels. Specifically, in both groups dopamine levels were transiently elevated during the waiting period. Although this increase in dopamine efflux decreased toward the end of the waiting period, it did not reach baseline levels before the onset of the ethanol self-administration phase. In the saline-treated group, ethanol self-administration maintained dopamine levels significantly above baseline with a second modest increase early during the self-administration phase. In contrast, in the naltrexone group the residual elevation in dopamine levels at the end of the waiting phase was not maintained during the self-administration phase, and dopamine efflux returned to baseline.
Dose–effect relationships between self-administered ethanol and the stimulation of dialysate dopamine levels after naltrexone
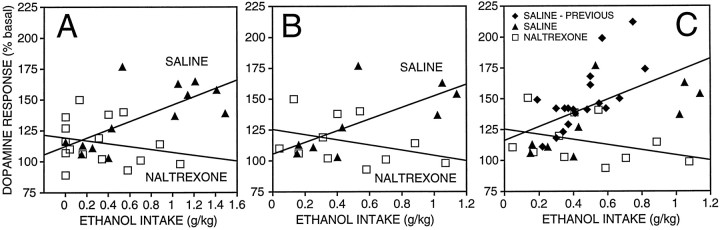
The analyses above revealed significantly lower levels of dopamine in dialysates of ethanol self-administering rats pretreated with naltrexone compared with that in controls. It was important, however, to ascertain that the lower dopamine levels in this group were not merely a function of substantially lower ethanol intake but that naltrexone, in fact, attenuated the efficacy of ethanol to elevate extracellular dopamine concentrations in the NAcc. To accomplish this, dose–response relationships were established between total ethanol intake and the maximal increase in dialysate dopamine concentration (expressed as the percent of basal values) observed during the 30 min self-administration session. As illustrated in the dose–effect plots (Fig. 6A), there was a significant positive relationship between ethanol intake and maximal dopaminergic response in the vehicle control group but not in the naltrexone group [saline, F(1,11) = 10.4;p < 0.05; naltrexone, F(1,14) = 0.7; NS] (i.e., the slope of the dose–effect function of the naltrexone group was not significantly different from zero). The difference between the slopes of the dose–effect functions of the saline and naltrexone-treated rats was verified by linear regression analysis [F(1,25) = 6.9; p < 0.05], confirming that naltrexone significantly reduced the efficacy of ethanol to increase dopamine efflux over the range of doses self-administered by the naltrexone-treated rats.
Fig. 6.
Dose–effect regression lines for increases in dopamine output in response to self-administered ethanol in saline- and naltrexone-treated rats. A, Maximal (percent of basal) increases in dopamine release during the 30 min self-administration session are plotted against total ethanol intake. Data from all rats shown in Figures 2-4 are shown. The saline-treated group showed a significant correlation between ethanol intake and the dopamine response (r = 0.70; p < 0.05), whereas the naltrexone-treated group did not (r = 0.22). B, Only a subset of rats from the control and naltrexone-treated groups, which had a comparable range of ethanol intake, was used for this analysis. The relationship between ethanol intake and the maximal increase in dialysate dopamine levels was significant in the saline-treated controls (r = 0.69; p < 0.05), but there was no significant relationship in naltrexone-treated rats (r = 0.35).C, Comparison of naltrexone effects against a dose–effect function generated from the pooled data of the present control group and a large sample of Wistar rats tested in previous work involving ethanol self-administration under similar conditions (see Results) is shown. The entire set of controls had a correlation coefficient of 0.55 (p < 0.05), and the slope of this regression was significantly different from that of the naltrexone group (p < 0.05).
Because the average ethanol intake in naltrexone-treated rats was substantially lower than that in the saline-treated controls, an additional regression analysis was performed on a restricted sample from both groups to provide comparable distributions of ethanol intake (0 < intake < 1.2 gm/kg). For this purpose, animals of the control group with levels of ethanol intake greater than the maximum intake observed in the naltrexone group, as well as rats that did not drink ethanol (mostly in the naltrexone group), were excluded. This was done to eliminate a potential bias in the data introduced by the nonhomogenous distribution of ethanol intake in the two groups. The dose–effect functions for this more conservative analysis are shown in Figure 6B. The results of this analysis were similar to those obtained with the unrestricted data set in that a significant positive correlation between ethanol intake and dopamine levels [F(1,7) = 6.5; p < 0.05] was obtained in saline-treated controls but not in the naltrexone group [F(1,9) = 1.3; NS]. In addition, the slopes of the two regressions were significantly different from each other [F(1,16) = 6.8; p < 0.05].
To examine the generality of these results, we compared the present ethanol self-administration dose–effect functions with a dose–effect function generated from the data of a pooled large sample of Wistar rats tested in previous work involving ethanol self-administration under near identical conditions with the exception that saccharin had been eliminated from the ethanol drinking solutions (Weiss et al., 1993, 1996; Katner et al., 1996). Thus, this comparison also served the purpose of establishing that there are no differences between the effects of saccharin- versus nonsaccharin-containing ethanol solutions on dialysate dopamine levels. No significant differences in the slope of the dose–effect relationships for the effect of ethanol on dialysate dopamine concentrations were found between the present saline control group and the previously tested sample [F(1,24) = 3.4; p > 0.05], confirming that the increases in extracellular dopamine induced by self-administration of saccharin- versus nonsaccharin-containing ethanol solutions are very similar. The data of the saline group were, therefore, pooled with those of the previously tested animals for comparison with the naltrexone group. The Pearson correlation coefficient for the pooled set of controls was 0.55 [F(1,26) = 11.1; p < 0.05], and the slope of this regression line differed significantly from that of the naltrexone group [F(1,35) = 8.3;p < 0.05; Fig. 6C]. The results of these analyses confirm the reliability of the dose–effect relationship between the amount of self-administered ethanol and the increases in dialysate dopamine concentrations across experiments and, thereby, corroborate the finding that naltrexone diminishes the dopaminergic effects of ethanol.
DISCUSSION
The results support the hypothesis that interference with ethanol-induced stimulation of dopamine release is one of the mechanisms by which opiate antagonists suppress ethanol self-administration. The concurrent measurement of dopamine output in the NAcc and of ethanol-reinforced behavior revealed a direct association between the pharmacological and behavioral effects of naltrexone. Naltrexone significantly reduced the ability of ethanol to increase extracellular dopamine concentrations (as reflected by dialysate dopamine levels) in the NAcc that are induced by self-administration of ethanol, and more importantly, the dose–effect analyses suggest that the inhibition of the response by naltrexone (Figs. 4, 5) was not accounted for simply by reduced ethanol intake. The validity of these dose–effect analyses was corroborated by the excellent correspondence with dose–effect relationships of ethanol-evoked elevations in extracellular dopamine levels in previous work. These findings strongly suggest that naltrexone reduced the efficacy of ethanol to increase extracellular dopamine concentrations in the NAcc over the range of self-administered doses examined and implicate this effect of naltrexone in the suppression of ethanol consumption. The results also extend previous evidence that opiate antagonists can blunt increases in accumbal dialysate dopamine levels after systemic or local administration of ethanol (Acquas et al., 1993;Benjamin et al., 1993) by showing that this action of opiate antagonists is accompanied by a reduction in the reinforcing effects of ethanol.
Before these conclusions can be fully accepted, however, it is necessary to consider two issues. First, dialysate dopamine concentrations that increased during the waiting period had not completely returned to baseline at the beginning of the self-administration phase in the control group. As will be discussed in greater detail below, the dopaminergic activation during the waiting phase may perhaps be related to the presence of alcohol-associated environmental stimuli that predict the availability of ethanol (Vavrousek-Jakuba et al., 1991; Weiss et al., 1993; Katner et al., 1996). Thus, it is possible that the increase in dopamine efflux during the self-administration phase may reflect the additive effects of the direct dopaminergic actions of ethanol plus some residual nonpharmacological dopaminergic activation related to processes operative during the waiting phase. Previous work suggests that the increase in extracellular dopamine during a pre-ethanol waiting period can indeed carry over into the ethanol self-administration phase, although this effect was significant only in a line of genetically selected alcohol-preferring rats (i.e., the Indiana P line) and not in genetically heterogeneous Wistars as used in the present study (Katner et al., 1996). Nonetheless, some contribution of the increase in extracellular dopamine during the waiting period to the elevation of dopamine levels during the self-administration phase cannot be eliminated completely. The question, therefore, is not only whether the effects of naltrexone (i.e., lower dopamine levels during the self-administration phase) are simply the result of lower ethanol intake but also whether naltrexone perhaps reduced the proportion of extracellular dopamine attributable to residual waiting period effects rather than, or in addition to, interfering with ethanol-induced increases in extracellular dopamine. Although this issue cannot be fully resolved on the basis of the present data, the interpretation that naltrexone directly suppresses ethanol-induced increases in extracellular dopamine concentrations is well supported by the literature (Acquas et al., 1993; Benjamin et al., 1993) and, therefore, a more parsimonious account of the data than the interpretation of attenuated carry-over effects from the waiting period into the self-administration phase.
A second issue relevant for the interpretation of the results is that, unlike in similar previous studies, saccharin was retained in the ethanol drinking solution throughout the experiment. It could be argued, therefore, that the presence of the sweetener may have been a factor in the increase in accumbal dopamine output during self-administration. However, self-administration of saccharin solutions, which support rates of operant responding similar to those maintained by ethanol in the present study, do not significantly increase dialysate dopamine concentrations in the NAcc (Weiss et al., 1993; Katner et al., 1996). More importantly, the comparison of the present with previous dose–effect data indicated that the effects of saccharin- versus nonsaccharin-containing ethanol solutions on dialysate dopamine concentrations do not differ and eliminates the possibility that saccharin influenced the results by altering the neurochemical actions of ethanol. It is also unlikely that the naltrexone-induced decrease in self-administration of the drinking solution can be attributed to a reduction in the reinforcing efficacy of saccharin as opposed to ethanol. Opiate antagonists can inhibit behavior maintained by both ethanol and nondrug reinforcers such as saccharin (Cooper, 1980; Ostrowski et al., 1980; Weiss et al., 1990;Gauvin et al., 1993; Krishnan-Sarin et al., 1995). However, mean baseline ethanol intake in the present study was remarkably similar to that in previous work with a nonsaccharin-containing ethanol solution such that the sweetener seems to have added little to the reinforcing efficacy of the drinking solution.
Therefore, the conclusion that the suppressant effects of naltrexone on ethanol self-administration are the result of interference with ethanol-induced increases in extracellular dopamine in the NAcc seems well justified. Interestingly, a dopaminergic link in the suppression of ethanol self-administration by naltrexone was reflected also in the specific behavioral changes induced by the drug. Although the behavioral data were not recorded by means of cumulative response records precluding a more fine-grained behavioral analysis, closer inspection of the distribution of responding in terms of 1 min intervals revealed that naltrexone did not seem to delay the onset of ethanol self-administration (data not shown). Instead, the marked reduction in ethanol intake during the first 5 min of the self-administration phase seemed to be the result of an early termination of responding for ethanol as indicated by the complete suppression of ethanol intake during the subsequent recording intervals. This pattern of effects is similar to that induced by dopamine antagonists that shorten the duration of ethanol drinking bouts and accelerate the offset of ethanol-reinforced responding (Samson and Hodge, 1996). Thus, the specific changes in ethanol-maintained behavior by naltrexone were consistent with the neurochemical data. These observations not only strengthen the conclusion that opiate antagonists suppress ethanol self-administration by interfering with ethanol-induced stimulation of accumbal dopamine activity but also provide further support for the view that dopamine has a central role in ethanol reward.
The present behavioral effects of naltrexone are consistent with several previous reports that a single injection of an opiate antagonist does not affect the initiation of ethanol-seeking behavior but reduces alcohol intake after consumption has begun (Schwarz-Stevens et al., 1992; Hyytiä and Sinclair, 1993). In conjunction with the present neurochemical data, these behavioral observations may provide some insight into the processes that underlie the suppression of ethanol consumption by opiate antagonists. Repeated pairing of opiate antagonist treatment with access to alcohol can lead to a progressive decline in ethanol intake followed by continued suppression of alcohol intake after termination of the chronic drug treatment (Hyytiä, 1993). This pattern of behavioral effects is indicative of extinction of ethanol-reinforced behavior. Thus, it is possible that by inhibiting the pharmacological effects of ethanol on accumbal dopamine activity, opiate antagonists may diminish the rewarding properties of ethanol and, thereby, lead to eventual extinction of ethanol-seeking behavior.
Finally, the inhibition of the effect of ethanol by naltrexone during the self-administration phase also implicates activation of endogenous opioid systems in the dopaminergic effects of ethanol. Opioid peptides are released in response to acute ethanol administration (de Waele and Gianoulakis, 1990, 1993; Gianoulakis, 1990; de Waele et al., 1992,1994), although this has not yet been demonstrated in the context of ethanol self-administration. Nonetheless, the present results support the hypothesis that direct or indirect activation of opioid peptide systems is part of the mechanism by which ethanol increases extracellular dopamine levels in the NAcc and maintains ethanol-reinforced behavior. However, it cannot be eliminated that nondopaminergic mechanisms coupled to opioid receptors may have contributed to the suppression of ethanol intake by naltrexone.
Confirming previous observations (Weiss et al., 1993), a transient rise in dialysate dopamine concentrations was observed during the presession waiting period in both groups of rats. The significance of this dopaminergic activation is, at present, still unclear. It is possible that this effect is a consequence of the presence of incentive motivational stimuli that are predictive of impending availability of ethanol (see also Vavrousek-Jakuba et al., 1991; Katner et al., 1996) and may have an “attentional” function or play a role in the initiation of ethanol-seeking behavior. This phenomenon is not accounted for by effects of nonspecific arousal as a result of handling and transfer of the animals from the home to the test cage because it is not seen under conditions in which no reinforcer is expected (Weiss et al., 1993). Similar “anticipatory” increases in dopamine release have been observed with saccharin and food reinforcers (Weiss et al., 1993; Wilson et al., 1995), and recent data suggest that expectation of a positive reinforcer is associated with a discrete increase in the firing rate of mesolimbic dopamine neurons (Schultz et al., 1997). Thus, stimulation of dopamine neuronal activity and release seems to be associated with the anticipation of reinforcing stimuli in general and is not restricted to drug reinforcers.
Naltrexone seemed to augment the increase in dialysate dopamine concentrations during the early stage of the presession waiting period. The naltrexone effects during the waiting period must be viewed with some caution, however. Although the rise in dopamine during the waiting period was greater in the naltrexone group when the data are examined in terms of the relative (percent of basal) increase in neurotransmitter levels, the actual net increase in dopamine concentrations (in nanomolar) was virtually identical in the two groups, and statistical analysis of the raw (dopamine concentration) data did not reveal significant differences or interactions between the effects of naltrexone and saline treatment during this experimental phase. Therefore, the apparent group differences in the percent of baseline values were attributable to the slightly (but not significantly) lower average baseline dopamine concentrations in the naltrexone versus the control group, which resulted in an inflation of the percent of baseline values after transformation of the data.
The apparent lack of suppression by naltrexone of the increase in dialysate dopamine levels during the waiting period contrasts sharply with the significant attenuation of ethanol-induced dopamine output during the self-administration phase. This is an intriguing observation because it may suggest that activation of accumbal dopamine release in response to anticipation of the drug reinforcer may be mediated by different neural mechanisms than are the dopaminergic effects of ethanol. The interpretation of the naltrexone effects during the waiting period is complicated, however, by pharmacokinetic considerations. Because the present study was designed to investigate the effects of naltrexone on ethanol self-administration and the efficacy of ethanol to increase extracellular dopamine levels, the timing of naltrexone administration (i.e., 30 min pre-ethanol) was scheduled with respect to the onset of the self-administration session. Consequently, at the onset of the waiting period only 10 min had elapsed since naltrexone injections, a time that coincides with the rising phase of the brain and blood levels of the drug (Misra et al., 1976; Wall et al., 1984). Whether or not naltrexone can alter the dopaminergic response to the anticipation of ethanol can, therefore, not be conclusively determined on the basis of the present data. In view of the inhibitory effects of naltrexone on ethanol craving and relapse in human alcoholics (Volpicelli et al., 1995; Jaffe et al., 1996; O’Malley, 1996) and on ethanol-seeking behavior in animal models of relapse (Katner et al., 1999; C. Heyser, K. Mog, and G. Koob, unpublished observations), it will be important to more systematically examine the interactions between this opiate antagonist and the effects of environmental stimuli predictive of ethanol availability.
In summary, the results indicate that both ethanol-reinforced behavior and ethanol-induced elevation of extracellular dopamine in the NAcc are inhibited by acute administration of naltrexone, a nonselective opiate receptor antagonist, at a dose that is selective for opiate receptor blockade in vivo. Analysis of dose–effect functions revealed a significant positive correlation between ethanol intake and increases in accumbal extracellular dopamine levels in saline-treated controls but not in naltrexone-treated rats. Confirming previous observations, a significant rise in dialysate dopamine concentrations was observed during a waiting period that preceded access to ethanol. In contrast to ethanol-induced dopaminergic activation, this effect was not clearly modified by naltrexone under the present experimental conditions. These data support the conclusion that reduction in the efficacy of ethanol to increase extracellular dopamine concentrations in the NAcc contributes to the suppression of ethanol self-administration by naltrexone and, presumably, other opiate antagonists.
Footnotes
This work was supported by the National Institute on Alcohol Abuse and Alcoholism Grants AA10531 (F.W.) and AA00147 (R.A.G.). This manuscript is publication No. 11488NP from The Scripps Research Institute. We wish to thank Dr. Charles O’Brien for inspirational comments and discussion. We also thank Tony Kerr and Brigitte Nadeau for skillful technical assistance with microdialysis and with analytical and behavioral procedures.
Correspondence should be addressed to Dr. Rueben Gonzales, Department of Pharmacology, College of Pharmacy, University of Texas at Austin, Austin, TX 78712.
REFERENCES
- 1.Acquas E, Meloni M, Di Chiara G. Blockade of delta-opioid receptors in the nucleus accumbens prevents ethanol-induced stimulation of dopamine release. Eur J Pharmacol. 1993;230:239–241. doi: 10.1016/0014-2999(93)90809-v. [DOI] [PubMed] [Google Scholar]
- 2.Altshuler HL, Phillips PE, Feinhandler DA. Alteration of ethanol self-administration by naltrexone. Life Sci. 1980;26:679–688. doi: 10.1016/0024-3205(80)90257-x. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin D, Grant ER, Pohorecky LA. Naltrexone reverses ethanol-induced dopamine release in the nucleus accumbens in awake, freely moving rats. Brain Res. 1993;621:137–140. doi: 10.1016/0006-8993(93)90309-b. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard BA, Steindorf S, Wang S, Glick SD. Sex differences in ethanol-induced dopamine release in nucleus accumbens and in ethanol consumption in rats. Alcohol Clin Exp Res. 1993;17:968–973. doi: 10.1111/j.1530-0277.1993.tb05650.x. [DOI] [PubMed] [Google Scholar]
- 5.Blomqvist O, Engel JA, Nissbrandt H, Soderpalm B. The mesolimbic dopamine-activating properties of ethanol are antagonized by mecamylamine. Eur J Pharmacol. 1993;249:207–213. doi: 10.1016/0014-2999(93)90434-j. [DOI] [PubMed] [Google Scholar]
- 6.Cooper SJ. Naloxone: effects on food and water consumption in the non-deprived and deprived rat. Psychopharmacology (Berl) 1980;71:1–6. doi: 10.1007/BF00433244. [DOI] [PubMed] [Google Scholar]
- 7.Davidson D, Amit Z. Effects of naloxone on limited-access ethanol drinking in rats. Alcohol Clin Exp Res. 1996;20:664–669. doi: 10.1111/j.1530-0277.1996.tb01669.x. [DOI] [PubMed] [Google Scholar]
- 8.de Waele JP, Gianoulakis C. Effects of ethanol on the brain beta-endorphin system in inbred strains of mice with variable preference for ethanol solutions: in vitro study. Prog Clin Biol Res. 1990;328:315–318. [PubMed] [Google Scholar]
- 9.de Waele JP, Gianoulakis C. Effects of single and repeated exposures to ethanol on hypothalamic beta-endorphin and CRH release by the C57BL/6 and DBA/2 strains of mice. Neuroendocrinology. 1993;57:700–709. doi: 10.1159/000126428. [DOI] [PubMed] [Google Scholar]
- 10.de Waele JP, Papachristou DN, Gianoulakis C. The alcohol-preferring C57BL/6 mice present an enhanced sensitivity of the hypothalamic beta-endorphin system to ethanol than the alcohol-avoiding DBA/2 mice. J Pharmacol Exp Ther. 1992;261:788–794. [PubMed] [Google Scholar]
- 11.de Waele JP, Kiianmaa K, Gianoulakis C. Spontaneous and ethanol-stimulated in vitro release of beta-endorphin by the hypothalamus of AA and ANA rats. Alcohol Clin Exp Res. 1994;18:1468–1473. doi: 10.1111/j.1530-0277.1994.tb01452.x. [DOI] [PubMed] [Google Scholar]
- 12.de Waele JP, Kiianmaa K, Gianoulakis C. Distribution of the mu and delta opioid binding sites in the brain of the alcohol-preferring AA and alcohol-avoiding ANA lines of rats. J Pharmacol Exp Ther. 1995;275:518–527. [PubMed] [Google Scholar]
- 13.Diana M, Gessa G, Rossetti Z. Lack of tolerance to ethanol-induced stimulation of mesolimbic dopamine system. Alcohol Alcohol. 1992;27:329–333. [PubMed] [Google Scholar]
- 14.Di Chiara G. The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug Alcohol Dep. 1995;38:95–137. doi: 10.1016/0376-8716(95)01118-i. [DOI] [PubMed] [Google Scholar]
- 15.Dilts RP, Kalivas PW. Autoradiographic localization of mu-opioid and neurotensin receptors within the mesolimbic dopamine system. Brain Res. 1989;488:311–327. doi: 10.1016/0006-8993(89)90723-3. [DOI] [PubMed] [Google Scholar]
- 16.Dilts RP, Kalivas PW. Autoradiographic localization of delta opioid receptors within the mesocorticolimbic dopamine system using radioiodinated [2-d-penicillamine, 5-d-penicillamine]enkephalin (125I-DPDPE). Synapse. 1990;6:121–132. doi: 10.1002/syn.890060203. [DOI] [PubMed] [Google Scholar]
- 17.Froehlich JC, Harts J, Lumeng L, Li TK. Naloxone attenuates voluntary ethanol intake in rats selectively bred for high ethanol preference. Pharmacol Biochem Behav. 1990;35:385–390. doi: 10.1016/0091-3057(90)90174-g. [DOI] [PubMed] [Google Scholar]
- 18.Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li T-K. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring (P) rats. Alcohol. 1994;11:557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 19.Gauvin DV, Moore KR, Holloway FA. Do rat strain differences in ethanol consumption reflect differences in ethanol sensitivity or the preparedness to learn? Alcohol. 1993;10:37–43. doi: 10.1016/0741-8329(93)90051-o. [DOI] [PubMed] [Google Scholar]
- 20.George SR, Fan T, Ng GY, Jung SY, O’Dowd BF, Naranjo CA. Low endogenous dopamine function in brain predisposes to high alcohol preference and consumption: reversal by increasing synaptic dopamine. J Pharmacol Exp Ther. 1995;273:373–379. [PubMed] [Google Scholar]
- 21.Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- 22.Gianoulakis C. Characterization of the effects of acute ethanol administration on the release of beta-endorphin peptides by the rat hypothalamus. Eur J Pharmacol. 1990;180:21–29. doi: 10.1016/0014-2999(90)90588-w. [DOI] [PubMed] [Google Scholar]
- 23.Gonzales RA, McNabb J, Ripley TL, Yim HJ, Bungay PM. Quantitative microdialysis of ethanol in rat striatum. Alcohol Clin Exp Res. 1998;22:858–867. [PubMed] [Google Scholar]
- 24.Honkanen A, Vilamo L, Wegelius K, Sarviharju M, Hyytiä P, Korpi ER. Alcohol drinking is reduced by a mu 1–but not by a delta–opioid receptor antagonist in alcohol-preferring rats. Eur J Pharmacol. 1996;304:7–13. doi: 10.1016/0014-2999(96)00118-5. [DOI] [PubMed] [Google Scholar]
- 25.Hubbell CL, Marglin SH, Spitalnic SJ, Abelson ML, Wild KD, Reid LD. Opioidergic, serotonergic, and dopaminergic manipulations and rats’ intake of a sweetened alcoholic beverage. Alcohol. 1991;8:355–367. doi: 10.1016/0741-8329(91)90573-f. [DOI] [PubMed] [Google Scholar]
- 26.Hyytiä P. Involvement of mu-opioid receptors in alcohol drinking by alcohol-preferring AA rats. Pharmacol Biochem Behav. 1993;45:697–701. doi: 10.1016/0091-3057(93)90527-z. [DOI] [PubMed] [Google Scholar]
- 27.Hyytiä P, Sinclair J. Responding for oral ethanol after naloxone treatment by alcohol-preferring AA rats. Alcohol Clin Exp Res. 1993;17:631–636. doi: 10.1111/j.1530-0277.1993.tb00810.x. [DOI] [PubMed] [Google Scholar]
- 28.Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- 29.Jaffe AJ, Rounsaville B, Chang G, Schottenfeld RS, Meyer RE, O’Malley SS. Naltrexone, relapse prevention, and supportive therapy with alcoholics: an analysis of patient treatment matching. J Consult Clin Psychol. 1996;64:1044–1053. doi: 10.1037//0022-006x.64.5.1044. [DOI] [PubMed] [Google Scholar]
- 30.Katner SN, Kerr TM, Weiss F. Ethanol anticipation enhances dopamine efflux in the nucleus accumbens of alcohol-preferring (P) but not Wistar rats. Behav Pharmacol. 1996;7:669–674. [PubMed] [Google Scholar]
- 31.Katner SN, Magalong JG, Weiss F (1999) Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology, in press. [DOI] [PubMed]
- 32.Kenakin T. Pharmacological analysis of drug-receptor interaction. Lippincott-Raven; Philadelphia: 1997. [Google Scholar]
- 33.Khachaturian H, Schaefer MKH, Lewis ME. Anatomy and function of the endogenous opioid systems. In: Akil H, Simon EJ, editors. Opioids I. Springer; New York: 1993. pp. 471–497. [Google Scholar]
- 34.Kiianmaa K, Nurmi M, Nykanen I, Sinclair JD. Effect of ethanol on extracellular dopamine in the nucleus accumbens of alcohol-preferring AA and alcohol-avoiding ANA rats. Pharmacol Biochem Behav. 1995;52:29–34. doi: 10.1016/0091-3057(95)00097-g. [DOI] [PubMed] [Google Scholar]
- 35.Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- 36.Kornet M, Goosen C, Van Ree JM. Effect of naltrexone on alcohol consumption during chronic alcohol drinking and after a period of imposed abstinence in free-choice drinking rhesus monkeys. Psychopharmacology (Berl) 1991;104:367–376. doi: 10.1007/BF02246038. [DOI] [PubMed] [Google Scholar]
- 37.Krishnan-Sarin S, Jing SL, Kurtz DL, Zweifel M, Portoghese PS, Li TK, Froehlich JC. The delta opioid receptor antagonist naltrindole attenuates both alcohol and saccharin intake in rats selectively bred for alcohol preference. Psychopharmacology (Berl) 1995;120:177–185. doi: 10.1007/BF02246191. [DOI] [PubMed] [Google Scholar]
- 38.Lewis ME, Khachaturian H, Watson SJ. Comparative distribution of opiate receptors and three opioid peptide neuronal systems in rhesus monkey central nervous system. Life Sci. 1983;33[Suppl 1]:239–242. doi: 10.1016/0024-3205(83)90487-3. [DOI] [PubMed] [Google Scholar]
- 39.Misra AL, Bloch R, Vardy J, Mule SJ, Verebely K. Disposition of (15,16–3H)naltrexone in the central nervous system of the rat. Drug Metab Dispos. 1976;4:276–280. [PubMed] [Google Scholar]
- 40.Mocsary Z, Bradberry C. Effect of ethanol on extracellular dopamine in nucleus accumbens: comparison between Lewis and Fischer 344 rat strains. Brain Res. 1996;706:194–198. doi: 10.1016/0006-8993(95)01200-1. [DOI] [PubMed] [Google Scholar]
- 41.O’Brien CP, Volpicelli LA, Volpicelli JR. Naltrexone in the treatment of alcoholism: a clinical review. Alcohol. 1996;13:35–39. doi: 10.1016/0741-8329(95)02038-1. [DOI] [PubMed] [Google Scholar]
- 42.O’Malley SS. Opioid antagonists in the treatment of alcohol dependence: clinical efficacy and prevention of relapse. Alcohol Alcohol. 1996;31[Suppl 1]:77–81. [PubMed] [Google Scholar]
- 43.O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- 44.Ostrowski NL, Foley TL, Lind MD, Reid LD. Naloxone reduces fluid intake: effects of water and food deprivation. Pharmacol Biochem Behav. 1980;12:431–435. doi: 10.1016/0091-3057(80)90049-0. [DOI] [PubMed] [Google Scholar]
- 45.Panocka I, Ciccocioppo R, Mosca C, Massi M. Effects of the dopamine D1 receptor antagonist SCH 39166 on the ingestive behaviour of alcohol-preferring rats. Psychopharmacology (Berl) 1995;120:227–235. doi: 10.1007/BF02246198. [DOI] [PubMed] [Google Scholar]
- 46.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; Orlando, Fl: 1986. [DOI] [PubMed] [Google Scholar]
- 47.Pettit HO, Justice JB., Jr . Procedures for microdialysis with smallbore HPLC. In: Robinson T, Justice JB Jr, editors. Techniques in the behavioral and neural sciences. Elsevier; New York: 1991. pp. 117–153. [Google Scholar]
- 48.Rossetti ZL, Hmaidan Y, Diana M, Gessa GL. Lack of tolerance to ethanol-induced dopamine release in the rat ventral striatum. Eur J Pharmacol. 1993;231:203–207. doi: 10.1016/0014-2999(93)90450-v. [DOI] [PubMed] [Google Scholar]
- 49.Samson H, Doyle T. Oral ethanol self-administration in the rat: effect of naloxone. Pharmacol Biochem Behav. 1985;22:91–99. doi: 10.1016/0091-3057(85)90491-5. [DOI] [PubMed] [Google Scholar]
- 50.Samson HH, Hodge CW. Neurobehavioral regulation of ethanol intake (Dietrich RA, Erwin VG, eds), pp 203–226. CRC; New York: 1996. [Google Scholar]
- 51.Samson HH, Hodge CW, Tolliver GA, Haraguchi M. Effect of dopamine agonists and antagonists on ethanol reinforced behavior: the involvement of the nucleus accumbens. Brain Res Bull. 1993;30:133–141. doi: 10.1016/0361-9230(93)90049-h. [DOI] [PubMed] [Google Scholar]
- 52.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 53.Schwarz-Stevens KS, Files FJ, Samson HH. Effects of morphine and naloxone on ethanol- and sucrose-reinforced responding in nondeprived rats. Alcohol Clin Exp Res. 1992;16:822–832. doi: 10.1111/j.1530-0277.1992.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 54.Vavrousek-Jakuba E, Cohen CA, Shoemaker WJ. Ethanol effects on dopamine receptors: in vivo binding following voluntary ethanol (EtOH) intake in rats. In: Naranjo CA, Sellers EM, editors. Novel pharmacological interventions for alcoholism. Springer; New York: 1991. [Google Scholar]
- 55.Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:886–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- 56.Volpicelli JR, Clay KL, Watson NT, O’Brien CP. Naltrexone in the treatment of alcoholism: predicting response to naltrexone. J Clin Psychiatry. 1995;56[Suppl 7]:39–44. [PubMed] [Google Scholar]
- 57.Wall ME, Perez-Reyes M, Brine DR, Cook CE. Naltrexone disposition in man after subcutaneous administration. Drug Metab Dispos. 1984;12:677–682. [PubMed] [Google Scholar]
- 58.Wamsley JK, Young WSD, Kuhar MJ. Anatomical localization of enkephalin immunoreactive sites in rat forebrain. Adv Biochem Psychopharmacol. 1980;22:257–270. [PubMed] [Google Scholar]
- 59.Weiss F, Mitchiner M, Bloom FE, Koob GF. Free-choice responding for ethanol versus water in alcohol preferring (P) and unselected Wistar rats is differentially modified by naloxone, bromocriptine, and methysergide. Psychopharmacology (Berl) 1990;101:178–186. doi: 10.1007/BF02244123. [DOI] [PubMed] [Google Scholar]
- 60.Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- 61.Weiss F, Parsons LH, Schulteis G, Hyytiä P, Lorang MT, Bloom FE, Koob GF. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson C, Nomikos GG, Collu M, Fibiger HC. Dopaminergic correlates of motivated behavior: importance of drive. J Neurosci. 1995;15:5169–5178. doi: 10.1523/JNEUROSCI.15-07-05169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 64.Yim HJ, Schallert T, Randall PK, Gonzales RA. Comparison of local and systemic ethanol effects on extracellular dopamine concentration in rat nucleus accumbens by microdialysis. Alcohol Clin Exp Res. 1998;22:367–374. [PubMed] [Google Scholar]
- 65.Yoshimoto K, McBride W, Lumeng L, Li T. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1991;9:17–22. doi: 10.1016/0741-8329(92)90004-t. [DOI] [PubMed] [Google Scholar]