Abstract
One of the most abundant nicotinic receptors in the nervous system is a species that contains the α7 gene product, rapidly desensitizes, and binds α-bungarotoxin with great affinity. The receptor has a high relative permeability to calcium and performs a variety of functions including presynaptic modulation of transmitter release and postsynaptic generation of synaptic currents. Fast excitatory transmission in mammalian intracardiac ganglia is mediated primarily by nicotinic receptors, and although intracardiac ganglion neurons express the α7 gene, no toxin-sensitive response has been detected previously in them. We report here that whole-cell patch-clamp recordings from freshly dissociated intracardiac ganglion neurons reveal a nicotinic response that desensitizes slowly and is blocked by α-bungarotoxin in a rapidly reversible manner. The only rat gene previously thought capable of forming such receptors was α9, but no evidence suggests that the α9 gene is expressed in neurons. We find that reverse transcription (RT)–PCR detects α7 but not α9 mRNA in the ganglia. In addition, the pharmacology of the nicotinic response is typical of α7-containing receptors but differs in several respects from that expected for α9. Binding experiments with immunotethered receptors identifies a ganglionic species that contains the α7 gene product. Moreover, intracellular perfusion of the cells with an anti-α7 monoclonal antibody specifically reduces the amplitude of the toxin-sensitive response. The results indicate that α7-containing receptors are responsible for the slowly desensitizing, toxin-reversible response and suggest that the receptors are modified in cell-specific ways to influence their functional properties.
Keywords: nicotinic, receptors, acetylcholine, intracardiac ganglion, neuronal, α7, α-bungarotoxin, patch clamp
The nicotinic acetylcholine receptor (AChR) α7 gene is widely expressed in the nervous system and accounts for the most abundant species of neuronal AChR in both chick and rat (Marks et al., 1986; Couturier et al., 1990; Schoepfer et al., 1990;Anand et al., 1993b; Chen and Patrick, 1997; Conroy and Berg, 1998). When heterologously expressed in Xenopus oocytes, α7 protein assembles into homopentameric ligand-gated ion channels that are cation-selective, rapidly desensitize, and bind α-bungarotoxin (αBgt) with high affinity (Couturier et al., 1990; Bertrand et al., 1993; Seguela et al., 1993). Responses from native AChRs containing the α7 gene product (α7-AChRs) have been reported in several systems and have always been found to be similar to those of the homopentamer in oocytes; namely, they rapidly desensitize and are blocked by αBgt in a long-lasting manner (Zorumski et al., 1992; Alkondon and Albuquerque, 1993; Zhang et al., 1994).
Native α7-AChRs are likely to serve a number of physiological roles. Recent evidence indicates they can act presynaptically to modulate neurotransmitter release (McGehee et al., 1995; Gray et al., 1996;Coggan et al., 1997) and can function at extra- or perisynaptic sites on neurons to generate synaptic currents as well (Zhang et al., 1996; Ullian et al., 1997). Genetic studies have linked the receptors to a form of schizophrenia (Freedman et al., 1997). Cell culture analysis has suggested the receptors may be important for early developmental events because they can be found on growing neurites (Pugh and Berg, 1994; Fu and Liu, 1997). This diversity of function raises the question of whether the properties of α7-AChRs vary with cellular location to accommodate site-specific job requirements.
Most puzzling has been the repeated finding of αBgt binding on neurons with no apparent αBgt-sensitive response (Duggan et al., 1976; Carbonetto et al., 1978; Betz, 1981; Lipton et al., 1987; Sucher et al., 1990; Zhang and Feltz, 1990; Sargent and Garrett, 1995). This has frequently been the finding with mammalian autonomic neurons (Nurse and O’Lague, 1975; Brown and Fumagalli, 1977; Ascher et al., 1979;Mandelzys et al., 1995). Other than α7, the only known genes that produce αBgt-binding receptors are the muscle α1 and either the α9 in mammals or the α8 in chick. Neither the α1 nor the α9 genes are expressed in neurons (Elgoyhen et al., 1994; Karlin and Akabas, 1996). Although the chick α8 is expressed in neurons, it either coassembles with α7 subunits to produce heteromers or self-assembles to produce α8-containing homomers (Schoepfer et al., 1990; Anand et al., 1993a), and both are capable of αBgt-sensitive responses when expressed in oocytes (Gerzanich et al., 1994).
An interesting system to explore the nature of α7-AChR responses is provided by mammalian intracardiac ganglia. The ganglia mediate efferent parasympathetic input to the heart and are thought to exert local regulation over cardiac function by integrating information from efferent and afferent pathways of both parasympathetic and sympathetic origin (Moravec and Moravec, 1987; Gagliardi et al., 1988). Extrinsic and intrinsic innervation of the ganglia is predominantly cholinergic, with activation of AChRs resulting in fast excitatory transmission (Seabrook et al., 1990). Rat intracardiac ganglion neurons apparently express multiple AChR subtypes, and the combination of subtypes expressed varies among cells (Poth et al., 1997). Although many of the neurons express the α7 gene (Poth et al., 1997), no αBgt-sensitive responses have been detected previously in the cells (Selyanko and Skok, 1992).
We have used whole-cell patch-clamp recording, together with rapid application of agonist, to examine the nicotinic ACh responses of dissociated rat intracardiac ganglion neurons. The neurons display a slowly desensitizing response that is blocked by αBgt in a rapidly reversible manner. Pharmacological analysis, reverse transcription (RT)–PCR, immunoprecipitation, and intracellular dialysis with subunit-specific monoclonal antibodies (mAbs) are each consistent with the conclusion that α7-AChRs produce the response. The implication is that α7-AChRs can be modified or regulated to display different properties in different environments. If α7-AChRs in intracardiac ganglion neurons retain the feature of having a high relative permeability to calcium, their ability to sustain long-duration currents in this case is likely to empower them with a major role in ganglionic signaling and regulation of cardiac function.
MATERIALS AND METHODS
Tissue preparation. Neurons from neonatal rat intracardiac ganglia were isolated and maintained as described previously (Cuevas and Adams, 1994). Briefly, to obtain intracardiac ganglion neurons, we killed postnatal day 3 (P3)–P7 rats by decapitation. The hearts were excised and placed in a saline solution containing (in mm): 140 NaCl, 3 KCl, 2.5 CaCl2, 0.6 MgCl2, 7.7 glucose, and 10 histidine, pH 7.2 with NaOH. The atria were separated and incubated in saline solution containing collagenase (1 mg/ml; Type 1A; Worthington, Freehold, NJ) at 37°C for 60 min. After enzymatic treatment, clusters of ganglia were dissected from the epicardial ganglion plexus and dispersed by titration in a high glucose culture medium (DMEM; Life Technologies, Gaithersburg, MD) with 10% fetal calf serum, 100 units/ml penicillin, and 0.1 mg/ml streptomycin. The dissociated neurons were then plated on glass coverslips coated with laminin, incubated at 37°C under a 95% air/5% CO2atmosphere, and examined 36–72 hr later.
Chick ciliary ganglion neurons were dissociated from 14–15 d embryos as described previously (Margiotta and Gurantz, 1989). Briefly, the ganglia were dissected from the embryo, incubated with 1 mg/ml collagenase for 30 min at 37°C, and transferred to culture medium made up of Eagle’s Minimal Essential Medium (Life Technologies) supplemented with 3% (v/v) embryonic eye extract (Nishi and Berg, 1981). The cells were dispersed by trituration, plated on a substratum of poly-d-lysine in 35 mm Costar culture dishes, and examined 1–3 hr later.
Electrophysiological recordings. Neurons plated on glass coverslips were transferred to a recording chamber (volume, 0.5 ml) mounted on an inverted phase-contrast microscope (magnification, 400×) that allowed isolated cells to be identified. Membrane currents in intracardiac neurons were studied under voltage-clamp mode using the whole-cell patch-clamp technique (Hamill et al., 1981). Electrical access was achieved conventionally by rupturing the membrane under the patch pipette or via the use of the perforated-patch method (Horn and Marty, 1988). Patch electrodes were pulled from thin-walled [outer diameter (o.d.), 1.5 mm] borosilicate glass (Drummond Scientific, Broomall, PA) using a Sutter Instruments P-87 pipette puller (Novato, CA) and had resistances of 1–1.5 MΩ. For conventional (dialyzing) whole-cell experiments, the intracellular solution contained (in mm): 140 CsCl, 10 glucose, 2 EGTA, and 10 HEPES, pH 7.2 with CsOH. In some conventional whole-cell experiments, cells were dialyzed (≥10 min) with patch pipette solutions containing subunit-specific anti-AChR mAbs; the specificities of the mAbs have been described previously [see references in Vernallis et al. (1993);Conroy and Berg (1995)]. The intracellular solution in perforated-patch experiments contained (in mm): 75 K2SO4, 55 KCl, 5 MgSO4, 360 μg/ml amphoterecin B, 0.6% DMSO, and 10 HEPES, pH 7.2 with N-methyl-d-glucamine. The procedures for achieving electrical access with amphoterecin B were identical to those described previously (Cuevas et al., 1997) and resulted in series resistance ≤ 3 MΩ after compensation (50%).
Membrane currents were amplified and filtered (5 kHz) using an Axopatch 200A (Axon Instruments, Foster City, CA) patch-clamp amplifier, digitized with a Digidata 1200B (Axon Instruments), and acquired (20 kHz) using Clampex 6 (Axon Instruments) on a pentium/133 MHz computer. Peak amplitude and kinetics of agonist-evoked currents were analyzed using Clampfit 6 (Axon Instruments).
The external solution for whole-cell recordings was physiological saline solution containing (in mm): 140 NaCl, 3 KCl, 2.5 CaCl2, 1.2 MgCl2, 7.7 glucose, and 10 HEPES, pH 7.2 with NaOH. Agonists and antagonists were applied via a rapid application system as reported previously (Zhang et al., 1994). Briefly, control and drug-containing solutions were delivered onto the cell soma from a linear array of glass tubes (inner diameter, 250 μm; o.d., 350 μm; Polymicro Technologies, Phoenix, AZ). Flow of solution through the individual tubes was induced by gravity feed and regulated by solenoid valves (General Valve, Fairfield, NJ). Movement of the tube array was mediated by a piezoelectric bimorph connected to a voltage generator (Burleigh, Fishers, NY). The rate of solution change expected to be observed by the cell was determined by recording the liquid junction potential change from an open patch pipette and was < 5 msec.
Solid-phase immunoprecipitations. Solid-phase immunoprecipitation assays were conducted as described previously (Conroy and Berg, 1995). Atria from neonatal rats (4 and 14 d old) were dissected as described above, and the medial region containing the pulmonary veins and the superior and inferior vena cava was isolated. These segments were homogenized in 2% (w/v) Triton X-100 extraction buffer and incubated at 4°C for 1 hr. Extracts were then centrifuged at 17,000 × g for 20 min, and the supernatant fraction was collected. Rat brain extracts were prepared from whole brains of neonatal rats (20 d old) using methods described previously (Conroy et al., 1992). Aliquots were incubated overnight at 4°C in microtiter wells precoated with anti-α7 mAb 319 to immunotether AChRs containing the α7 gene product. Receptor binding was quantified with125I-αBgt. Nonspecific binding was determined by including either 1 μm unlabeled αBgt or 1 mm nicotine in the binding reaction with125I-αBgt and was subtracted from total binding to obtain specific binding. In some experiments the anti-β2 mAb 270 and the anti-α8 mAb 308 were used as immunotethering antibodies [for mAb specificities, see references in Vernallis et al. (1993); Conroy and Berg (1995)].
Epibatidine binding was determined using a filter binding assay (Conroy and Berg, 1995). Protein extracts (25 μl aliquots) were incubated in 2 nm [3H]epibatidine for 2 hr at room temperature. The reactions were then diluted with 4 ml of wash buffer [0.05% (w/v) Triton X-100 in 10 mm Tris, pH 7.5], and the solution was immediately filtered through Whatman GF/B filters (Maidstone, UK) presoaked for 1 hr in 0.5% polyethyleneimine. Filters were rinsed twice more with wash buffer and then counted by liquid scintillation (Ecoscint H; National Diagnostics, Atlanta, GA). Nonspecific binding was determined by including 1 mmnicotine in the binding reaction with [3H]epibatidine.
RT–PCR. The use of RT–PCR for detection of AChR gene expression in cultured neurons from rat intracardiac ganglia has been described previously (Poth et al., 1997). Briefly, RNA was extracted (RNeasy; Qiagen, Hilden, Germany) either from a dish of cultured intracardiac neurons containing ∼100 neurons plus a number of other cell types (e.g., cardiac myocytes, Schwann cells, and fibroblasts) or from intact intracardiac ganglia and associated tissue (same as in culture preparations). RNA was reverse-transcribed in a 20 μl reaction volume using a Life Technologies SuperScript Preamplification System kit. Negative controls including an RT reaction without reverse transcriptase and a PCR reaction with only water were conducted to eliminate the possibility of false positives because of contaminating cDNA. Primers for α7 and α9 transcripts were identical to those used previously [α7 (Poth et al., 1997); α9 (Elgoyhen et al., 1994)]: α7(forward)-GGAGTGAAGAATGTTCGTTTTCCAGATGG, α7(reverse)-CCCTGGCTCTGCTGGTATTCTTGC, α9(forward)-CTAATGGTGGCAGAGATCATGCCA, and α9(reverse)-TATGATCAAGACGGTCATGACAAACACCA. They yielded product sizes of 476 and 573 bp, respectively. PCR reactions were conducted using the Life Technologies SuperScript Preamplification System kit, and the cycling parameters were five cycles of 94°C for 45 sec, 55°C for 1 min, and 72°C for 1.5 min. This was followed by 30 cycles of 94°C for 45 sec, 57°C for 1 min, and 72°C for 1.5 min.
Restriction digestion. A restriction digestion strategy was used to confirm the identity of PCR reaction products as being those expected for amplification of specific cDNAs. The two α7 primer products were gel-purified and digested with HaeII andBanII. Each of these endonucleases targeted a different exon found in the region amplified by the primers used. Digestion of the 476 bp fragment with both enzymes produces digestion products of 321, 78, and 77 bp. Sequence analysis of cloned PCR products was performed commercially (Retrogen, San Diego, CA).
Reagents and statistical analysis. All chemicals used were of analytical grade. Acetylcholine chloride (ACh), cytisine chloride, atropine sulfate, nicotine, and mecamylamine chloride were purchased from Sigma (St. Louis, MO). αBgt was purchased from Biotoxins (St. Cloud, FL) and radioiodinated using chloramine T to a specific activity of 0.3–0.7 × 1018 cpm/mol. [3H]Epibatidine (56.5 Ci/mmol) was a gift from DuPont NEN (Boston, MA), and unlabeled epibatidine was purchased from Research Biochemicals (Natick, MA). Several mAbs were generously supplied by Dr. Jon Lindstrom (University of Pennsylvania, Philadelphia, PA).
Data are presented as the mean ± SD unless otherwise stated and were compared using paired or unpaired t tests as appropriate.
RESULTS
αBgt-sensitive ACh-evoked currents
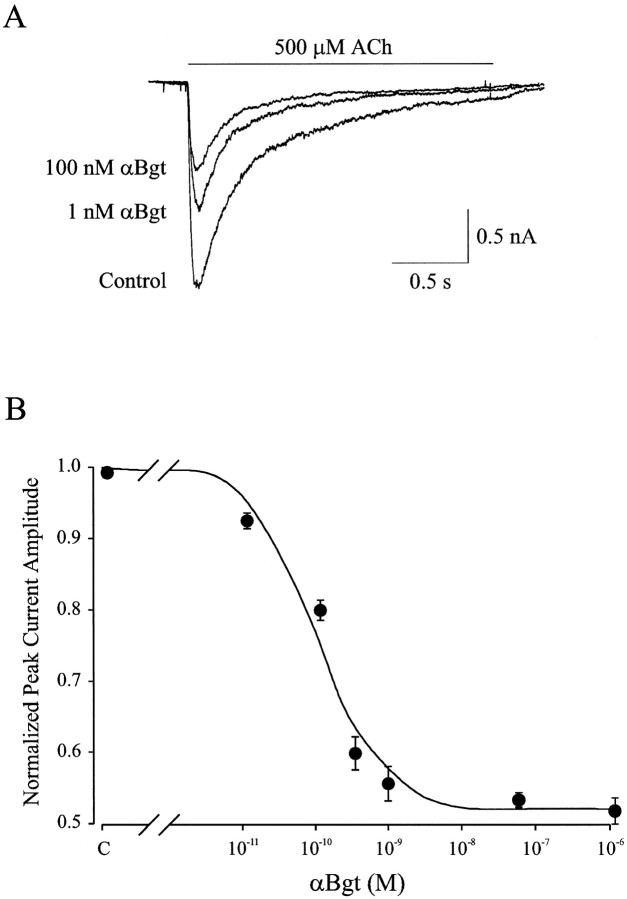
Whole-cell patch-clamp techniques were used to record ACh-induced currents from dissociated rat intracardiac ganglion neurons maintained 1–3 d in culture. Rapid focal application of agonist was used to minimize loss of response because of receptor desensitization. Figure1A shows a representative membrane current response evoked by 500 μmACh from a neuron electrically accessed with the amphoterecin B perforated-patch method and voltage clamped at −60 mV. ACh elicited a transient inward current that desensitized during the 2 sec exposure to agonist. After a 10 min application of 100 nm αBgt, the ACh-evoked current decreased by >40% in amplitude (Fig.1A). Mean values of 2.3 ± 0.3 and 1.3 ± 0.3 nA (n = 5 cells) were obtained for the peak response in the absence and presence, respectively, of 100 nm αBgt. The αBgt-induced decrement was statistically significant (p < 0.02). Similar values for the peak ACh-induced currents plus and minus αBgt were observed when cells were electrically accessed using the conventional patch-clamp (dialyzing) whole-cell recording configuration: 1.6 ± 0.2 and 2.5 ± 0.2 nA, respectively (n = 4 cells).
Fig. 1.
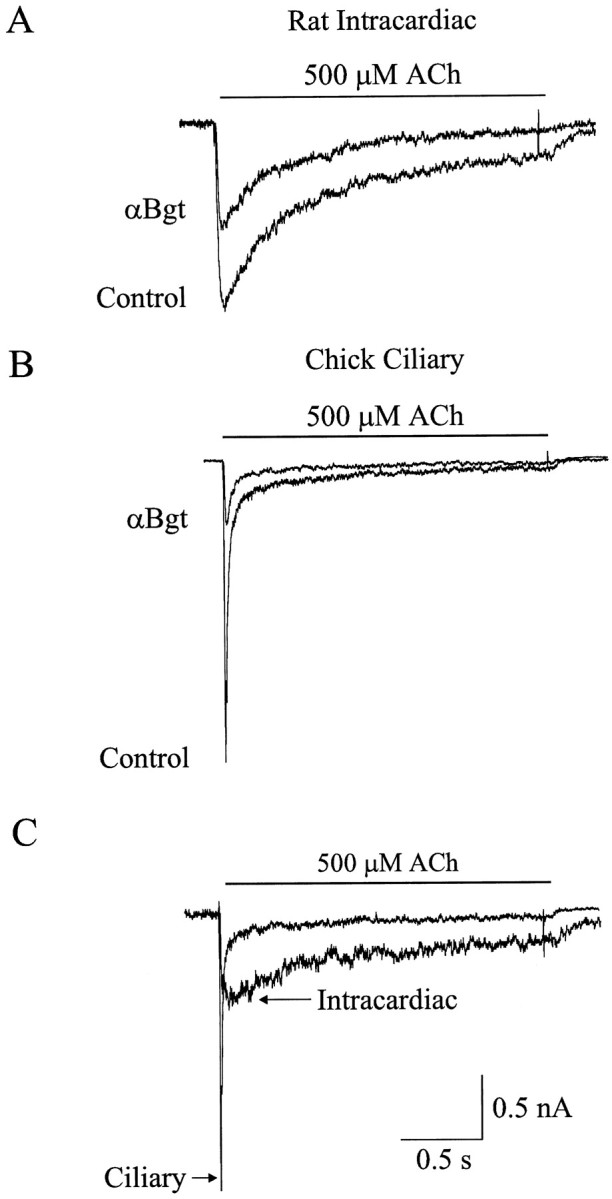
αBgt blocks a slowly desensitizing ACh-evoked current in rat intracardiac neurons. A,B, Whole-cell currents evoked by rapid focal application of 500 μm ACh to the soma of an isolated rat intracardiac ganglion neuron (A) and an isolated chick ciliary ganglion neuron (B), each voltage clamped at −60 mV, in the absence (Control) and presence of 100 nm αBgt. C, Net αBgt-sensitive ACh-evoked current, determined by subtracting the current induced by ACh in the presence of 100 nm αBgt from that recorded in the absence of the toxin for the experiments shown in Aand B.
For comparison, ACh-induced currents were also recorded from dissociated chick ciliary ganglion neurons before and after application of 100 nm αBgt (Fig. 1B). As with intracardiac neurons, the cells were electrically accessed with the perforated-patch method and were voltage clamped at −60 mV. The time course of the αBgt-sensitive current in both cell types was determined by subtracting the ACh-evoked current recorded in the presence of αBgt from that recorded in its absence in the same cell (Fig. 1C). The αBgt-sensitive response in rat intracardiac neurons decays much more slowly than does the response in ciliary ganglion neurons. The decay phase of the αBgt-sensitive response in intracardiac neurons was best fit by the sum of two exponential functions with decay half-times of 170 ± 20 and 930 ± 70 msec (n = 4 cells).
Concentration dependence and reversibility of αBgt blockade
The concentration dependence of the αBgt blockade was examined by comparing the peak amplitude of the ACh-induced current before and after application of αBgt at a range of concentrations (10 pm to 1 μm). Figure2A shows a set of currents evoked in this manner from a single neuron voltage clamped at −60 mV. Approximately 80% of the neurons (32 out of 41 cells) revealed an αBgt-sensitive ACh response. The peak amplitude of ACh-evoked currents in the presence of αBgt was normalized to the maximum response from the same neuron in the absence of αBgt, and the results from a number of cells were compiled to generate a plot of mean peak amplitude versus αBgt concentration (Fig. 2B). A fit of the data using a single-site adsorption isotherm indicates half-maximal inhibition (IC50) at 120 pmand a maximal inhibition of 47 ± 2% at 1 μm αBgt (n = 7).
Fig. 2.
Dose-dependent inhibition of nicotinic ACh-evoked currents by αBgt. A, Whole-cell currents evoked from a single neuron by focal application of 500 μm ACh in the absence (Control) and presence of αBgt (1 and 100 nm). The holding potential was −60 mV.B, ACh-evoked whole-cell current amplitude at −60 mV normalized to values obtained from the same cells in the absence of αBgt and plotted as a function of αBgt concentration. Data points represent mean ± SEM (n = 7–12 determinants; 25 neurons). The curve represents a best fit to the data using a single-site adsorption isotherm with half-maximal inhibition at 120 pm αBgt and a maximal inhibition of 47%.
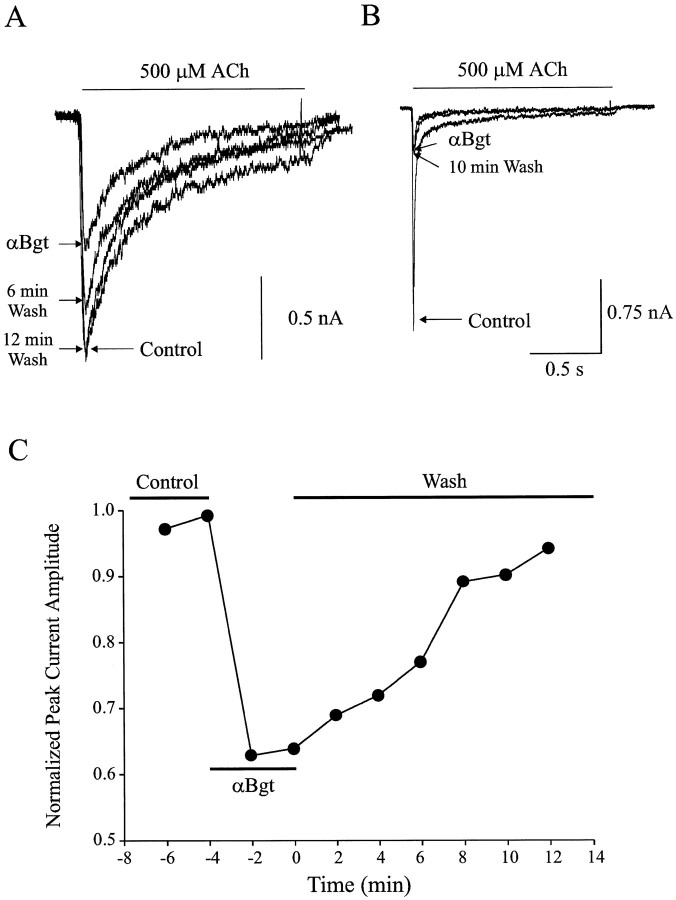
The duration of αBgt blockade was determined by comparing the peak amplitude of ACh-evoked currents in the same neuron before, during, and after exposure to 100 nm αBgt. Results from individual intracardiac ganglion neurons suggest the response largely recovers after only a few minutes of rinsing to remove toxin (Fig.3A). For comparison, similar experiments were performed on chick ciliary ganglion neurons, which have been reported previously to show no reversibility of the αBgt blockade over short times (Zhang et al., 1994). A 10 min wash under the same conditions used for the intracardiac neurons allows no recovery of the αBgt-sensitive component in ciliary ganglion neurons (Fig.3B) The peak amplitudes of the ACh-evoked currents obtained from individual intracardiac ganglion neurons throughout the procedure were normalized to the maximum response obtained from the same neuron before application of αBgt application. Compiling such results from a number of neurons indicates that the onset of blockade in 100 nm αBgt occurs within 2 min but quickly reverses (Fig. 3C). After 10 min of rinsing to remove the toxin, the ACh-evoked currents had recovered to 87 ± 5% of control values (n = 6).
Fig. 3.
Reversibility of αBgt-induced blockade.A, B, A family of currents evoked by 500 μm ACh recorded from a single rat intracardiac ganglion neuron (A) and a single embryonic day 14 chick ciliary ganglion neuron (B) at −60 mV in the absence (Control) and presence of 100 nm αBgt and after washout of toxin for the indicated time periods. C, ACh-evoked whole-cell current amplitudes before application (Control), during application (αBgt), and after removal (Wash) of 100 nm αBgt. Values have been normalized to the maximal response from the same cell and plotted as a function of time.
AChR gene transcripts: α7 versus α9
The quick reversibility of the αBgt blockade and the slow desensitization of the αBgt-sensitive response in rat intracardiac ganglion were unexpected. Although many of the neurons express the α7 gene (Poth et al., 1997), α7-AChRs in other systems generate rapidly desensitizing currents that are essentially irreversibly blocked by αBgt (Zorumski et al., 1992; Alkondon and Albuquerque, 1993; Zhang et al., 1994; Blumenthal et al., 1997). The only candidates for AChRs in rat that produce slowly desensitizing currents that are reversibly blocked by αBgt are those composed of the α9 gene product (Elgoyhen et al., 1994). The α9 gene appears to be expressed exclusively in non-neuronal cells (Elgoyhen et al., 1994), but it seemed prudent to test whether α9 transcripts could be detected in intracardiac ganglia. This was done using RT–PCR.
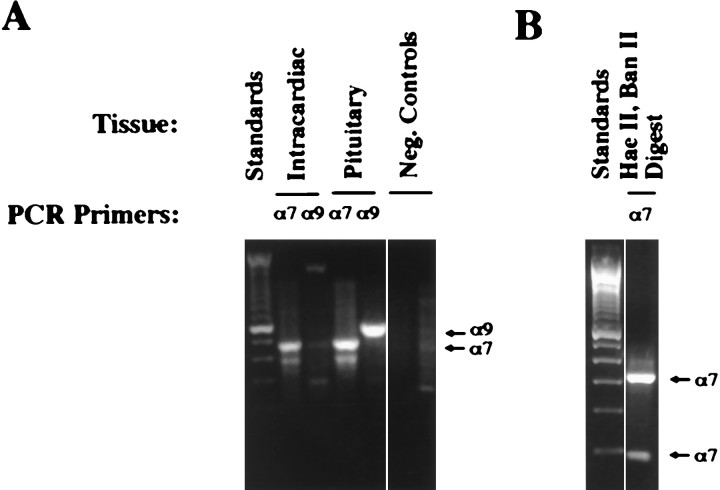
Total RNA was extracted from cultures of rat intracardiac neurons, reverse-transcribed, and amplified by PCR for α7 and α9 transcripts (Fig. 4A). The primers were designed to span introns so that false positives resulting from genomic DNA contamination could be distinguished. Reactions with α7 primers generated a band having the expected size for the α7 product (476 bp) from intracardiac ganglion RNA. No band of the size expected for the α9 product (573 bp) was generated from intracardiac ganglion RNA when α9 primers were used. Positive controls for the RT–PCR were performed with RNA extracted from neonatal rat pituitary ganglia because both α7 and α9 mRNAs are known to be present in this case (Elgoyhen et al., 1994). Both α7 and α9 products were generated using the appropriate primers (Fig. 4A). Two sets of negative controls were conducted; these include omission of reverse transcriptase from the RT incubation and substitution of water for template in the PCR incubation (Fig. 4A). Restriction digestion (Lambolez et al., 1992) provided additional evidence that the major PCR product obtained with the α7 primers (∼476 bp) originated from the α7 transcript (Fig. 4B). The digestion yielded products of the predicted sizes (321, 77, and 78 bp). Sequence analysis confirmed that the major PCR product had the expected α7 sequence; the smaller product of ∼390 bp (Fig. 4A) was not α7 in origin (data not shown).
Fig. 4.
RT–PCR analysis of α7 and α9 gene expression in rat intracardiac ganglion neurons. A, RT–PCR products. Primers/template RNA: lane 1 (on theleft), 100 bp standards; lane 2, α7/intracardiac ganglion; lane 3, α9/intracardiac ganglion; lane 4, α7/rat pituitary; lane 5, α9/rat pituitary; lane 6, α7 + α9/water; lane 7, α7 + α9/rat intracardiac without RT. The expected product sizes are 476 bp for α7 and 573 bp for α9.B, Restriction digest of the RT–PCR product amplified from intracardiac ganglion RNA with α7 primers. Lane 1(on the left), 100 bp standards; lane 2,HaeII and BanII digest. Expected product sizes are 321, 78, and 77 bp for α7 transcripts. Neg., Negative.
Pharmacology of αBgt-sensitive currents
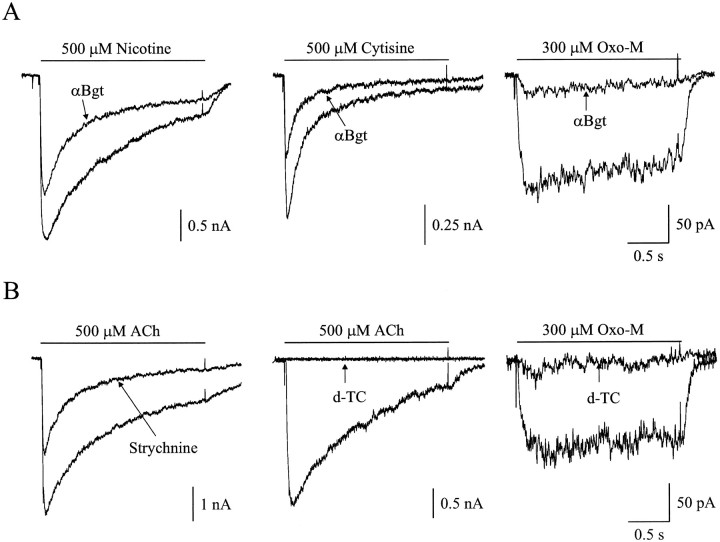
The pharmacology of αBgt-sensitive ACh responses in dissociated intracardiac ganglion neurons was examined to determine whether the currents had the properties expected for α7-AChRs. Although ACh activates both α7- and α9-AChRs, cytisine and nicotine activate only the former. Cytisine fails to activate α9-AChRs, whereas nicotine blocks such receptors (Elgoyhen et al., 1994). The M1 muscarinic agonist oxotremorine M (Oxo-M) activates both classes of receptors, whereas the glycinergic antagonist strychnine and the nicotinic antagonist d-tubocurarine block both.
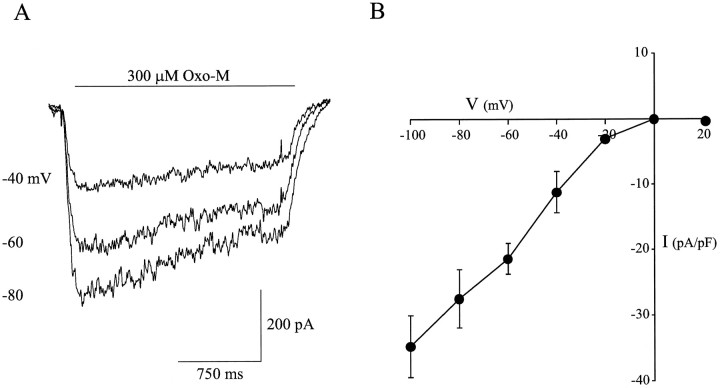
Figure 5A shows representative membrane currents evoked by focal application of 500 μmnicotine, 500 μm cytisine, and 300 μm Oxo-M in the presence and absence of 100 nm αBgt. With nicotine and cytisine as agonists, αBgt blocked 37 ± 3% (n = 3) and 41 ± 3 (n = 3) of the current, respectively. These values are similar to those obtained with ACh as agonist and are consistent with the αBgt-sensitive response being the product of α7-AChRs. With Oxo-M as agonist, αBgt blocked 87 ± 5% (n = 2) of the current. Strychnine blocked 43 ± 6% (n = 3) of the ACh-evoked current, a value comparable with the fractional response blocked by αBgt (Fig. 5B). The broad spectrum nicotinic antagonistd-tubocurarine at 100 μm blocked the ACh-evoked responses as well as those induced by Oxo-M (Fig.5B). The current–voltage relationship of the αBgt-sensitive currents induced by Oxo-M is that expected for cation-selective neuronal AChRs, namely, a linear relationship at negative potentials, a reversal potential near 0 mV, and marked inward rectification visible at positive membrane potentials (Fig.6). The results support the conclusion that the slowly desensitizing, αBgt-sensitive currents obtained from intracardiac ganglion neurons are produced by activation of α7-AChRs.
Fig. 5.
Pharmacology of αBgt-sensitive currents in intracardiac ganglion neurons. A, Currents evoked by rapid focal application of 500 μm nicotine (left), 500 μm cytisine (middle), and 300 μm Oxo-M (right) in the absence and presence of αBgt.B, ACh-evoked (left andmiddle) and Oxo-M-evoked (right) responses before and after treatment with 100 nm strychnine or 100 μmd-tubocurarine (d-TC).
Fig. 6.
Voltage dependence of peak Oxo-M-evoked current amplitude. A, A family of currents evoked by 300 μm Oxo-M in the presence of 100 nm atropine, recorded from a neuron held at the indicated membrane potentials.B, Current–voltage relationship for currents induced by 300 μm Oxo-M. Points represent mean ± SEM for three neurons each.
Quantification of α7-AChRs in rat intracardiac ganglion extracts
The number of α7-AChRs present in intracardiac ganglia was measured with a solid-phase immunoprecipitation assay. Extracts were prepared from P4 rat intracardiac ganglia and incubated with the anti-α7 mAb 319 to immunotether α7-AChRs. Bound receptors were quantified with 125I-αBgt. A single round yielded nearly 5 fmol of binding sites per heart equivalent of intracardiac ganglia (Fig. 7A). A second round yielded an additional 0.5 fmol, indicating that most of the α7-AChRs had been collected in the first pass. The recovered extracts were then incubated with [3H]epibatidine and filtered to collect and quantify other classes of neuronal AChRs (Gerzanich et al., 1995; Houghtling et al., 1995; Conroy and Berg, 1998). This procedure yielded ∼2–3 fmol per P4 heart equivalent of intracardiac ganglia (Fig. 7A). No toxin binding was detected in the solid-phase assay when either the anti-α8 mAb 308 or the anti-β2 mAb 270 was used to immunotether AChRs, nor was any [3H]epibatidine binding detected in the assay when the anti-α7 mAb 319 was used to immunotether AChRs (data not shown).
Fig. 7.
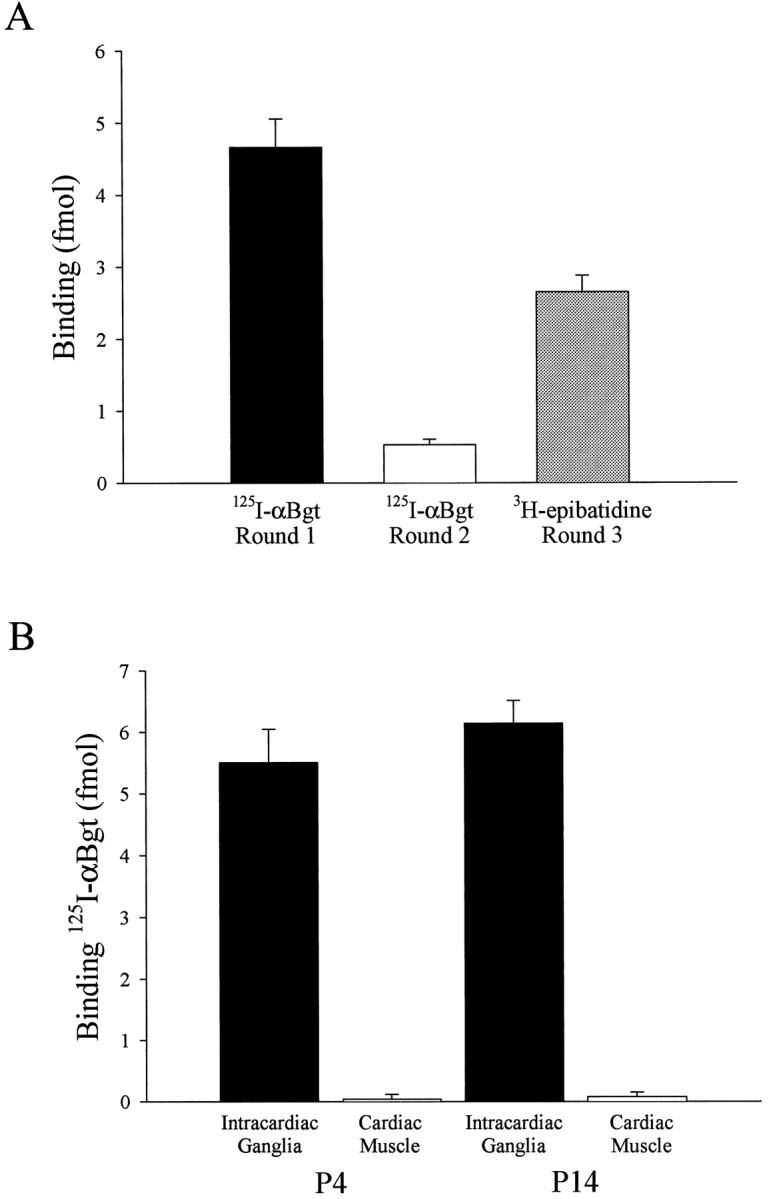
Quantification of α7-AChRs using a solid-phase immunoprecipitation assay. A, Solubilized intracardiac ganglion α7-AChRs were immunotethered with the anti-α7 mAb 319 in two sequential rounds (Rounds 1 and 2) and then quantified with 125I-αBgt; the recovered supernatant fractions were then assayed for [3H]epibatidine-binding receptors using a polyethyleneimine filter assay (Round 3).Bars represent the mean (± SEM) amount of binding per single heart equivalent of intracardiac ganglia for 11 (black bar), 8 (white bar), or 3 (shaded bar) determinations. B, 125I-αBgt binding sites in solubilized tissue samples (Rounds 1and 2 summed) are shown. Bars represent the mean (± SEM) amount of binding per single heart equivalent of intracardiac ganglia (black bars) or an ∼10-fold excess by weight of cardiac muscle tissue (white bars), a small portion of which might have contaminated the ganglia samples. Eight determinations were done for P4 samples, and three determinations were performed for P14 samples.
The numbers of α7-AChRs per heart equivalent of ganglia did not change dramatically during early postnatal development. Values obtained with P14 ganglia were not significantly greater than those obtained at P4 (Fig. 7B). The binding was specific for the ganglia because a large excess of tissue that might have contaminated ganglia preparations (namely, small segments of atria, ventricles, thymus gland, aorta, and lungs) failed to display significant binding on their own (Fig. 7B).
Selective blockade of αBgt-sensitive currents by anti-α7 mAbs
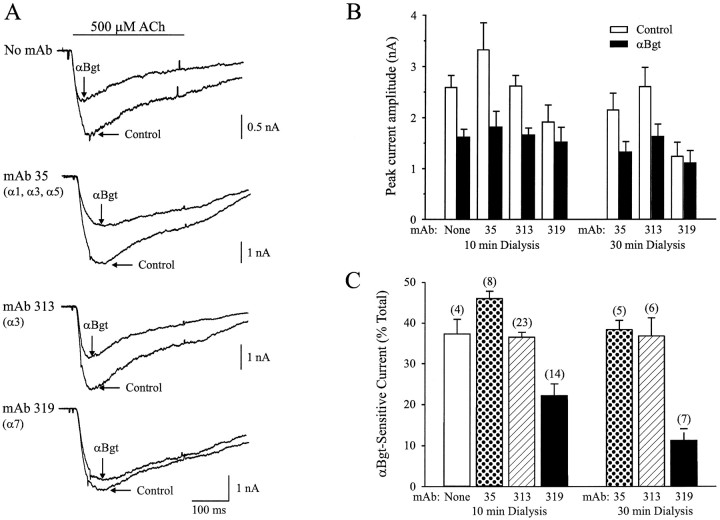
To obtain further evidence that the αBgt-sensitive currents in intracardiac ganglion neurons are mediated by activation of α7-AChRs, we dialyzed cells intracellularly with subunit-specific mAbs. The hope was that mAbs specific for intracellular epitopes on particular AChR subunits might bind to those sites on native receptors in situ and inhibit receptor function either by occluding the channel from the inside or by preventing allosteric or regulatory changes required for channel opening.
Intracellular dialysis of neurons via a conventional patch pipette for periods up to 30 min with mAb 35, which recognizes extracellular epitopes on the α1, α3, and α5 but not α7 subunits, had no statistically significant effect either on the total ACh-evoked current or on that portion that could be blocked by αBgt (Fig.8). Similarly, intracellular dialysis with mAb 313, which is specific for an intracellular epitope on α3 subunits, had no effect on either component of the response. In contrast, intracellular dialysis for even 10 min with mAb 319, which is specific for an intracellular epitope on α7 subunits, produced a significant and specific decrement in the αBgt-sensitive portion of the ACh-evoked response (Fig. 8). Dialysis with mAb 319 for periods up to 30 min produced an even greater selective reduction in the toxin-blockable response. Thus only 11 ± 2% (mean ± SEM) of the whole-cell ACh response was αBgt-sensitive at 30 min, whereas 22 ± 3% was αBgt-sensitive at 10 min (p= 0.01 for 10 vs 30 min values). Both components comprising the decay phase of the αBgt-sensitive response were affected. No preferential rundown of the αBgt-sensitive response occurred under these conditions, as can be seen by comparing the 10 and 30 min values for cells receiving the control mAbs 35 and 313 (Fig. 8). The results provide strong evidence for the conclusion that α7-AChRs are responsible for the slowly desensitizing ACh-evoked responses that are reversibly blocked by αBgt.
Fig. 8.
Specific blockade of αBgt-sensitive ACh-evoked currents by intracellular dialysis with an anti-α7 mAb.A, Whole-cell currents evoked by focal application of 500 μm ACh onto an intracardiac ganglion neuron in the absence (Control) and presence of 50 nm αBgt after a 10 min intracellular dialysis of the cell with either no mAb, the anti-α1/α3/α5 mAb 35, the anti-α3 mAb 313, or the anti-α7 mAb 319 via the patch pipette. B, Peak currents evoked by 500 μm ACh (white bars) and ACh plus 50 nm αBgt (black bars) from neurons dialyzed with the indicated mAbs. Currents were recorded after a 10 and a 30 min dialysis. C, The αBgt-sensitive portion of the ACh-evoked current expressed as a percent of the peak whole-cell ACh response current for neurons after dialysis for either 10 or 30 min with the indicated mAbs. Only the anti-α7 mAb affected the whole cell response, and it selectively reduced the αBgt-sensitive portion of it. Barsrepresent the mean ± SEM of the number of neurons indicated inparentheses; the same number of neurons was tested inB and C.
DISCUSSION
The results presented here provide the first demonstration of ACh-evoked currents in the mammalian peripheral nervous system attributable to α7-AChRs. The currents are blocked by nanomolar concentrations of αBgt, but, unexpectedly, the blockade is rapidly reversible and the currents affected are slow to desensitize. In all previous cases in which native α7-AChRs have been activated by rapid application of agonist, the resulting currents were found to desensitize rapidly and to remain inhibited long after unbound αBgt had been removed. In addition to chick ciliary ganglion neurons (Zhang et al., 1994), examples include rat hippocampal neurons in culture (Zorumski et al., 1992; Alkondon and Albuquerque, 1993) and the rat pheochromocytoma cell line PC12 (Blumenthal et al., 1997). Clearly some populations of rat α7-AChRs are capable of rapid desensitization and long-lasting αBgt blockade. The rapidly reversible blockade described here may explain why no αBgt-sensitive currents have been reported previously for mammalian autonomic neurons.
The α9 gene is known to produce AChRs that slowly desensitize and reversibly bind αBgt, but all evidence to date indicates the gene is not expressed in neurons (Elgoyhen et al., 1994). RT–PCR analysis in the present experiments failed to detect α9 transcripts in rat intracardiac ganglion RNA, although the positive controls with rat pituitary RNA proved successful. Moreover, the pharmacology of the αBgt-sensitive response in intracardiac ganglion neurons was consistent with that of α7-AChRs rather than that of α9-AChRs, and immunoprecipitation experiments confirmed the presence of α7-AChRs in intracardiac ganglion extracts.
The number of α7-AChRs detected by 125I-αBgt binding in P4 rat intracardiac ganglia (∼5 fmol per heart equivalent) was substantially lower than the ∼30 fmol per ganglion observed for P4 rat superior cervical ganglia in the same assay (A. Roth and J. Cuevas, unpublished results) or the ∼20 fmol per ganglion for chick ciliary ganglia at a comparable stage of development (Chiappinelli and Giacobini, 1978; Smith et al., 1983). Intracardiac ganglia, however, contain only ∼4 × 103 neurons in aggregate per heart equivalent, whereas rat superior cervical ganglia contain ∼3 × 104 (Paxinos, 1995), and chick ciliary ganglia contain ∼3 × 103 (Landmesser and Pilar, 1974). When the amount of 125I-αBgt binding is normalized for the number of neurons estimated to be present, the number of sites is comparable for the two mammalian sources (∼1 fmol per 103 neurons) but is lower than that found in chick ciliary ganglia (∼7 fmol per 103neurons).
One of the strongest lines of evidence indicating that the αBgt-sensitive responses arise from α7-AChRs was provided by the intracellular dialysis experiments with subunit-specific mAbs. The fact that anti-α7 mAbs selectively reduced the αBgt-sensitive ACh-evoked current while other mAbs had no effect on either the αBgt-sensitive or -resistant components of the response clearly implicates α7-AChRs in producing the toxin-sensitive portion of the response. One might have expected the anti-α3 mAb 313 to reduce the αBgt-resistant response because it almost certainly arises from α3-containing AChRs (Poth et al., 1997). It is not known, however, whether mAb 313 recognizes rat α3 protein as it does chick α3; many subunit-specific anti-AChR mAbs do not cross-react between chick and rat proteins (W. Conroy and D. Berg, unpublished results). Some, but not all, anti-AChR mAbs previously tested on receptors reconstituted in artificial membranes were able to influence single channel properties (Blatt et al., 1986). If intracellular dialysis with appropriate subunit-specific mAbs proves to be widely applicable for selectively targeting receptor subtypes in situ, it may prove powerful for correlating individual receptor species with unique functional responses.
The intracellular mechanism by which the mAbs block receptor function is of considerable interest. Possibly the antibodies simply occlude the ion channel from the cytoplasmic side. Alternatively, the antibodies may prevent a conformational change required for channel opening. Extensive evidence suggests muscle AChRs undergo such conformational changes (Karlin and Akabas, 1996), and a recent report indicates that the large cytoplasmic loop influences channel function (Milone et al., 1998). Another possibility is that the mAbs may prevent an intermolecular interaction such as receptor phosphorylation or linkage to a cytoskeletal element required to optimize receptor functionality. Future experiments will explore these possibilities.
The finding that rat intracardiac ganglion α7-AChRs behave quite differently from rat hippocampal and PC12 α7-AChRs invites speculation. It is possible that the rat α7 gene product can combine with other as yet unidentified subunits to produce heteromeric receptors with different properties. The rat α7 gene product can coassemble with muscle AChR subunits under some conditions when coexpressed in Xenopus oocytes (Helekar et al., 1994), but it has not been shown to do so with any of the known AChR gene productsin vivo. No new rat AChR gene products have been identified since α9, and α9 is non-neuronal with a very limited pattern of expression. Nonetheless, if an AChR gene were primarily confined to expression in subpopulations of autonomic neurons, it could well have escaped detection. Similar considerations apply to hypotheses based on α7 splice variants generating receptors with different properties.
A different kind of explanation is that the functional differences among rat α7-AChR populations may be produced by cell-specific or location-specific regulatory interactions. The diverse functions currently attributed to α7-AChRs (McGehee et al., 1995; Gray et al., 1996; Zhang et al., 1996; Coggan et al., 1997; Fu and Liu, 1997; Ullian et al., 1997) could certainly necessitate complex regulatory options. Perhaps the most likely mechanism is one using some form of second messenger-mediated receptor phosphorylation (Huganir and Greengard, 1990). Alternatively, receptor interactions with the cytoskeleton and associated molecules could alter receptor function, as suggested above. Intracellular dialysis of neurons via the patch pipette should provide a means for manipulating the cytoplasmic milieu and testing several of these hypotheses.
A final issue is the significance of α7-AChRs for signaling through intracardiac ganglia. Approximately one-half of the intracardiac ganglion neurons sampled by single-cell RT–PCR were shown to have an α7 transcript (Poth et al., 1997), and, in approximate agreement, ∼80% of the neurons tested here displayed αBgt-sensitive ACh responses. It is not known which neuronal subpopulations in the ganglia such cells comprise, but they are likely to include the large principal neurons that innervate cardiac muscle directly.
If intracardiac ganglion α7-AChRs retain the high relative calcium permeability observed for α7-AChRs elsewhere (Bertrand et al., 1993; Seguela et al., 1993), their resistance to desensitization could enable them to have a major impact on calcium-dependent events in the neurons. Changes in intracellular calcium, for example, modulate the firing patterns of intracardiac ganglion neurons (Allen and Burnstock, 1987). Such changes in neuronal firing properties may be a mechanism by which integration of neuronal signals and coding of information occur in mammalian intracardiac ganglia (Cuevas et al., 1997). A portion of the receptors may be destined for presynaptic sites on the cells. In this case a slow rate of desensitization coupled with a high relative calcium permeability could have a major effect on modulation of transmitter release by the receptors, a role advanced previously for them in other systems (McGehee et al., 1995; Gray et al., 1996; Coggan et al., 1997). Either of these effects could enable α7-AChRs to be a major contributor to the neural circuit that exerts local control over cardiac function.
Footnotes
This work was supported by the National Institutes of Health Grants NS 12601 and 35469 and by TRDRP Grant RT65–0050. J.C. is a University of California President’s Fellow. We thank Dr. Jon Lindstrom (University of Pennsylvania, Philadelphia, PA) for generously supplying monoclonal antibodies.
Correspondence should be addressed to Dr. Darwin K. Berg, Department of Biology, 0357, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0357.
Dr. Cuevas’s present address: Department of Pharmacology and Therapeutics, University of South Florida College of Medicine, Tampa, FL 33612-4799.
REFERENCES
- 1.Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther. 1993;265:1455–1473. [PubMed] [Google Scholar]
- 2.Allen TGJ, Burnstock G. Intracellular studies of the electrophysiological properties of cultured intracardiac neurones of the guinea-pig. J Physiol (Lond) 1987;388:349–366. doi: 10.1113/jphysiol.1987.sp016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anand R, Peng X, Ballesta J, Lindstrom J. Pharmacological characterization of α-bungarotoxin-sensitive acetylcholine receptors immunoisolated from chick retina: contrasting properties of α7 and α8 subunit-containing subtypes. Mol Pharmacol. 1993a;44:1046–1050. [PubMed] [Google Scholar]
- 4.Anand R, Peng X, Lindstrom J. Homomeric and native α7 acetylcholine receptors exhibit remarkably similar but non-identical pharmacological properties, suggesting that the native receptor is a heteromeric protein complex. FEBS Lett. 1993b;327:241–246. doi: 10.1016/0014-5793(93)80177-v. [DOI] [PubMed] [Google Scholar]
- 5.Ascher P, Large WA, Rang HP. Studies on the mechanism of action of acetylcholine antagonists on rat parasympathetic ganglion cells. J Physiol (Lond) 1979;295:139–170. doi: 10.1113/jphysiol.1979.sp012958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertrand D, Galzi JL, Devillers-Thiéry A, Bertrand S, Changeux JP. Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal α7 nicotinic receptor. Proc Natl Acad Sci USA. 1993;90:6971–6975. doi: 10.1073/pnas.90.15.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Betz H. Characterization of the α-bungarotoxin receptor in chick embryo retina. Eur J Biochem. 1981;117:131–139. doi: 10.1111/j.1432-1033.1981.tb06311.x. [DOI] [PubMed] [Google Scholar]
- 8.Blatt Y, Montal MS, Lindstrom JM, Montal M. Monoclonal antibodies specific to the β and γ subunits of the Torpedo acetylcholine receptor inhibit single-channel activity. J Neurosci. 1986;6:481–486. doi: 10.1523/JNEUROSCI.06-02-00481.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blumenthal EM, Conroy WG, Romano SJ, Kassner PD, Berg DK. Detection of functional nicotinic receptors blocked by α-bungarotoxin on PC12 cells and dependence of their expression on posttranslational events. J Neurosci. 1997;17:6094–6104. doi: 10.1523/JNEUROSCI.17-16-06094.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown DA, Fumagalli L. Dissociation of α-bungarotoxin binding and receptor block in the rat superior cervical ganglion. Brain Res. 1977;129:165–168. [Google Scholar]
- 11.Carbonetto SR, Fambrough DM, Muller KJ. Non-equivalence of α-bungarotoxin receptors and acetylcholine receptors in chick sympathetic neurons. Proc Natl Acad Sci USA. 1978;75:1016–1020. doi: 10.1073/pnas.75.2.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen D, Patrick JW. The α-bungarotoxin-binding nicotinic acetylcholine receptor from rat brain contains only the α7 subunit. J Biol Chem. 1997;272:24024–24029. doi: 10.1074/jbc.272.38.24024. [DOI] [PubMed] [Google Scholar]
- 13.Chiappinelli VA, Giacobini E. Time course of appearance of α-bungarotoxin binding sites during development of chick ciliary ganglion and iris. Neurochem Res. 1978;3:465–478. doi: 10.1007/BF00966328. [DOI] [PubMed] [Google Scholar]
- 14.Coggan JS, Paysan J, Conroy WG, Berg DK. Direct recording of nicotinic responses in presynaptic nerve terminals. J Neurosci. 1997;17:5798–5806. doi: 10.1523/JNEUROSCI.17-15-05798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conroy WG, Berg DK. Neurons can maintain multiple classes of nicotinic acetylcholine receptors distinguished by different subunit compositions. J Biol Chem. 1995;270:4424–4431. doi: 10.1074/jbc.270.9.4424. [DOI] [PubMed] [Google Scholar]
- 16.Conroy WG, Berg DK. Nicotinic receptor subtypes in the developing chick brain: appearance of a species containing the α4, β2, and α5 gene products. Mol Pharmacol. 1998;53:392–401. doi: 10.1124/mol.53.3.392. [DOI] [PubMed] [Google Scholar]
- 17.Conroy WG, Vernallis AB, Berg DK. The α5 gene product assembles with multiple acetylcholine receptor subunits to form distinctive receptor subtypes in brain. Neuron. 1992;9:679–691. doi: 10.1016/0896-6273(92)90031-8. [DOI] [PubMed] [Google Scholar]
- 18.Couturier S, Bertrand D, Matter J-M, Hernandez M-C, Bertrand S, Millar N, Valera S, Barkas T, Ballivet M. A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homo-oligomeric channel blocked by α-Btx. Neuron. 1990;5:847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- 19.Cuevas J, Adams DJ. Local anaesthetic blockade of neuronal nicotinic ACh receptor channels in rat parasympathetic ganglion cells. Br J Pharmacol. 1994;111:663–672. doi: 10.1111/j.1476-5381.1994.tb14789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuevas J, Harper AA, Trequattrini C, Adams DJ. Passive and active membrane properties of isolated rat intracardiac neurons: regulation by H- and M-currents. J Neurophysiol. 1997;78:1890–1902. doi: 10.1152/jn.1997.78.4.1890. [DOI] [PubMed] [Google Scholar]
- 21.Duggan AW, Hall JG, Lee CY. Alpha-bungarotoxin, cobra neurotoxin and excitation of Renshaw cells by acetylcholine. Brain Res. 1976;107:166–170. doi: 10.1016/0006-8993(76)90106-2. [DOI] [PubMed] [Google Scholar]
- 22.Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. α9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 1994;79:705–715. doi: 10.1016/0092-8674(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 23.Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerly W. Linkage of a neurophysiological deficit in schizophrenia to chromosome 15 locus. Proc Natl Acad Sci USA. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu W-M, Liu J-J. Regulation of acetylcholine release by presynaptic nicotinic receptors at developing neuromuscular synapses. Mol Pharmacol. 1997;51:390–398. [PubMed] [Google Scholar]
- 25.Gagliardi M, Randall WC, Bieger D, Wurster RD, Hopkins DA, Armour JA. Activity of in vivo canine cardiac plexus neurons. Am J Physiol. 1988;255:H789–H800. doi: 10.1152/ajpheart.1988.255.4.H789. [DOI] [PubMed] [Google Scholar]
- 26.Gerzanich V, Anand R, Lindstrom J. Homomers of α8 and α7 subunits of nicotinic receptors exhibit similar channel but contrasting binding site properties. Mol Pharmacol. 1994;45:212–220. [PubMed] [Google Scholar]
- 27.Gerzanich V, Peng X, Wang F, Wells G, Anand R, Fletcher S, Lindstrom J. Comparative pharmacology of epibatidine: a potent agonist for neuronal nicotinic acetylcholine receptors. Mol Pharmacol. 1995;48:774–782. [PubMed] [Google Scholar]
- 28.Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- 29.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recordings from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 30.Helekar SA, Char D, Neff S, Patrick J. Prolyl isomerase requirement for the expression of functional homo-oligomeric ligand-gated ion channels. Neuron. 1994;12:179–189. doi: 10.1016/0896-6273(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 31.Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houghtling RA, Davila-Garcia MI, Kellar KJ. Characterization of (±)-[3H]epibatidine binding to nicotinic cholinergic receptors in rat and human brain. Mol Pharmacol. 1995;48:280–287. [PubMed] [Google Scholar]
- 33.Huganir RL, Greengard P. Regulation of neurotransmitter receptor desensitization by protein phosphorylation. Neuron. 1990;5:555–567. doi: 10.1016/0896-6273(90)90211-w. [DOI] [PubMed] [Google Scholar]
- 34.Karlin A, Akabas M. Toward a structural basis for the function of the nicotinic acetylcholine receptors and their cousins. Neuron. 1996;15:1231–1244. doi: 10.1016/0896-6273(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 35.Lambolez B, Audinat A, Bochet P, Crepel F, Rossier J. AMPA receptor subunits expressed by single Purkinje cells. Neuron. 1992;9:247–258. doi: 10.1016/0896-6273(92)90164-9. [DOI] [PubMed] [Google Scholar]
- 36.Landmesser L, Pilar G. Synaptic transmission and cell death during normal ganglionic development. J Physiol (Lond) 1974;241:737–749. doi: 10.1113/jphysiol.1974.sp010681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lipton SA, Aizenman E, Loring RH. Neural nicotinic acetylcholine responses in solitary mammalian retinal ganglion cells. Pflügers Arch. 1987;410:37–43. doi: 10.1007/BF00581893. [DOI] [PubMed] [Google Scholar]
- 38.Mandelzys A, De Koninck P, Cooper E. Agonist and toxin sensitivities of ACh-evoked currents on neurons expressing multiple nicotinic ACh receptor subunits. J Neurophysiol. 1995;74:1212–1221. doi: 10.1152/jn.1995.74.3.1212. [DOI] [PubMed] [Google Scholar]
- 39.Margiotta JF, Gurantz D. Changes in the number, function, and regulation of nicotinic acetylcholine receptors during neuronal development. Dev Biol. 1989;135:326–339. doi: 10.1016/0012-1606(89)90183-8. [DOI] [PubMed] [Google Scholar]
- 40.Marks MJ, Stitzel JA, Romm E, Wehner JM, Collins AC. Nicotinic binding sites in rat and mouse brain: comparison of acetylcholine, nicotine, and α-bungarotoxin. Mol Pharmacol. 1986;30:427–436. [PubMed] [Google Scholar]
- 41.McGehee D, Heath M, Gelber S, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1697. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- 42.Milone M, Wang H-L, Ohno K, Prince R, Fukudome T, Shen X-M, Brengman JM, Griggs RC, Sine SM, Engel AG. Mode switching kinetics produced by a naturally occurring mutation in the cytoplasmic loop of the human acetylcholine receptor ε subunit. Neuron. 1998;20:575–588. doi: 10.1016/s0896-6273(00)80996-4. [DOI] [PubMed] [Google Scholar]
- 43.Moravec J, Moravec M. Intrinsic nerve plexus of mammalian heart: morphological basis of cardiac rhythmical activity? Intl Rev Cytol. 1987;106:89–147. doi: 10.1016/s0074-7696(08)61711-8. [DOI] [PubMed] [Google Scholar]
- 44.Nishi R, Berg DK. Two components from eye tissue that differentially stimulate the growth and development of CG neurons in cell culture. J Neurosci. 1981;1:505–513. doi: 10.1523/JNEUROSCI.01-05-00505.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nurse CA, O’Lague PH. Formation of cholinergic synapses between dissociated sympathetic neurons and skeletal myotubes of the rat in cell culture. Proc Natl Acad Sci USA. 1975;72:1955–1959. doi: 10.1073/pnas.72.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paxinos G. The rat nervous system, 2nd Edition. Academic; San Diego: 1995. [Google Scholar]
- 47.Poth K, Nutter TJ, Cuevas J, Parker MJ, Adams DJ, Luetje CW. Heterogeneity of nicotinic receptor class and subunit mRNA expression among individual parasympathetic neurones from rat intracardiac ganglia. J Neurosci. 1997;17:586–596. doi: 10.1523/JNEUROSCI.17-02-00586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pugh PC, Berg DK. Neuronal acetylcholine receptors that bind α-bungarotoxin mediate neurite retraction in a calcium-dependent manner. J Neurosci. 1994;14:889–896. doi: 10.1523/JNEUROSCI.14-02-00889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sargent PB, Garrett EN. The characterization of α-bungarotoxin receptors on the surface of parasympathetic neurons in the frog heart. Brain Res. 1995;680:99–107. doi: 10.1016/0006-8993(95)00250-t. [DOI] [PubMed] [Google Scholar]
- 50.Schoepfer R, Conroy WG, Whiting P, Gore M, Lindstrom J. Brain α-bungarotoxin binding protein cDNAs and mAbs reveal subtypes of this branch of the ligand-gated ion channel gene superfamily. Neuron. 1990;5:35–48. doi: 10.1016/0896-6273(90)90031-a. [DOI] [PubMed] [Google Scholar]
- 51.Seabrook GR, Fieber LA, Adams DJ. Neurotransmission in neonatal rat cardiac ganglion in situ. Am J Physiol. 1990;259:H997–H1005. doi: 10.1152/ajpheart.1990.259.4.H997. [DOI] [PubMed] [Google Scholar]
- 52.Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain α7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Selyanko AA, Skok VI. Acetylcholine receptors in rat cardiac neurones. J Auton Nerv Syst. 1992;40:33–48. doi: 10.1016/0165-1838(92)90223-4. [DOI] [PubMed] [Google Scholar]
- 54.Smith MA, Margiotta JF, Berg DK. Differential regulation of acetylcholine sensitivity and α-bungarotoxin-binding sites on ciliary ganglion neurons in cell culture. J Neurosci. 1983;3:2395–2402. doi: 10.1523/JNEUROSCI.03-11-02395.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sucher NJ, Cheng TPO, Lipton SA. Neural nicotinic acetylcholine responses in sensory neurons from postnatal rat. Brain Res. 1990;533:248–254. doi: 10.1016/0006-8993(90)91346-i. [DOI] [PubMed] [Google Scholar]
- 56.Ullian EM, McIntosh JM, Sargent PB. Rapid synaptic transmission in the avian CG is mediated by two distinct classes of nicotinic receptors. J Neurosci. 1997;17:7210–7219. doi: 10.1523/JNEUROSCI.17-19-07210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vernallis AB, Conroy WG, Berg DK. Neurons assemble acetylcholine receptors with as many as three kinds of subunits while maintaining subunit segregation among receptor subtypes. Neuron. 1993;10:451–464. doi: 10.1016/0896-6273(93)90333-m. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Z-w, Feltz P. Nicotinic acetylcholine receptors in porcine hypophyseal intermediate lobe cells. J Physiol (Lond) 1990;422:83–101. doi: 10.1113/jphysiol.1990.sp017974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Z-w, Vijayaraghavan S, Berg DK. Neuronal acetylcholine receptors that bind α-bungarotoxin with high affinity function as ligand-gated ion channels. Neuron. 1994;12:167–177. doi: 10.1016/0896-6273(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Z-w, Coggan JS, Berg DK. Synaptic currents generated by neuronal acetylcholine receptors sensitive to α-bungarotoxin. Neuron. 1996;17:1231–1240. doi: 10.1016/s0896-6273(00)80253-6. [DOI] [PubMed] [Google Scholar]
- 61.Zorumski CF, Thio LL, Isenberg KE, Clifford DB. Nicotinic acetylcholine currents in cultured postnatal rat hippocampal neurons. Mol Pharmacol. 1992;41:931–936. [PubMed] [Google Scholar]