Abstract
Precisely localized focal stereotaxic electrolytic lesions were made in the corticospinal tract at the level of the first to second cervical segments in the adult rat. This consistently destroyed all central nervous tissue elements (axons, astrocytes, oligodendrocytes, microglia, and microvessels) in a highly circumscribed area.
In a group of these rats immediately after lesioning, a suspension of cultured adult olfactory ensheathing cells was transplanted into the lesion site. Within the first week after transplantation, the cut corticospinal axons (identified by anterograde transport of biotin dextran) extended caudally along the axis of the corticospinal tract as single, fine, minimally branched sprouts that ended in a simple tip, often preceded by a small varicosity. By 3 weeks, the regenerating axons, ensheathed by P0-positive peripheral myelin had accumulated into parallel bundles, which now extended across the full length of the lesioned area and reentered the caudal part of the host corticospinal tract.
The transplants contained two main types of cells: (1) p75-expressing S cells, which later formed typical peripheral one-to-one myelin sheaths around individual ensheathed axons, and (2) fibronectin-expressing A cells, which aggregated into tubular sheaths enclosing bundles of myelinated axons. The point of reentry of the axons into the central nervous territory of the caudal host corticospinal tract was marked by the resumption of oligodendrocytic myelination. Thus the effect of the transplant was to form a “patch” of peripheral-type tissue across which the cut central axons regenerated and then continued to grow along their original central pathway.
Keywords: regeneration, olfactory ensheathing cells, corticospinal tract, white matter, adult spinal cord repair, axon growth, myelinated tracts, transplantation
Transplants of peripheral nerve into the visual system have established that cut axons can reestablish functional connections in the adult CNS (Vidal-Sanz et al., 1987). The transplants recruited axons from the retinal ganglion cells, bypassed their normal retinofugal pathways through the optic nerve, and delivered the regenerating fibers directly to the deafferented terminal field in the tectum. In practice, however, most injuries involve multiple fiber systems. In these situations, repair would require that the regenerating axons reenter their original tracts to be distributed to their correct terminal sites.
Myelinated fiber tracts of the adult CNS have a complex and regular arrangement of three types of glial cells (Suzuki and Raisman, 1992). When a tract is damaged, the cut axons produce local sprouts at the site of injury (Ramon y Cajal, 1928; Li and Raisman, 1995), but even with minimal disturbance to the tract glial framework (Davies et al., 1996), the sprouts did not reenter the distal part of the tract.
To make the damaged tracts favorable for the regeneration of cut adult axons, we (Li and Raisman, 1994, 1997) and others (Xu et al., 1997) have studied transplantation of cultured Schwann cells. Schwann cells integrate into the host tract glial structure (Brook et al., 1993; Li and Raisman, 1997). They greatly increase axon sprouting in lesions of the corticospinal tract (CST), but few sprouts reenter the distal tract (Li and Raisman, 1994). The reluctance of axon sprouts to leave the Schwann cell environment of the transplant and reenter the glial environment of the distal CST resembles the inability of regenerating cut dorsal root fibers to leave the peripheral nerve/Schwann cell environment of the dorsal roots and reenter the glial environment of the dorsal spinal cord (Bignami et al., 1984).
To identify a source of cells that might enable the regenerating axons to reenter the host CST, we therefore sought a situation in which adult axons are normally able to enter the CNS. In the olfactory system, the sensory neurons are replaced throughout adult life, and the newly formed axons continually reenter the CNS (Moulton, 1974; Barber and Raisman, 1978a,b; Graziadei and Montigraziadei, 1979, 1980; Wilson and Raisman, 1981). The entry point of the olfactory axons into the olfactory bulb is associated with special glial cells, known as olfactory ensheathing cells (OECs) (Blanes, 1898; Raisman, 1985;Valverde and Lopez-Mascaraque, 1991; Ramón-Cueto and Nieto-Sampedro, 1992; Barnett et al., 1993). Ramón-Cueto and collaborators (Ramón-Cueto and Nieto-Sampedro, 1994) reported that transplants of cultured OECs mediate the reentry of regenerating dorsal root axons into the dorsal spinal gray matter and that injections of OECs increased axon growth into Schwann cell-filled guidance channels transplanted into the spinal cord (Ramón-Cueto et al., 1998).
In the present study we injected cultured OECs into focal lesions in the rat CST. This experimental paradigm optimizes the opportunity for repair by ensuring that (1) damage is largely restricted to a single tract, (2) the cut axon sprouts come at once into direct contact with the transplanted cells, (3) the distance to be crossed by the regenerating axons is minimized, and (4) the advancing sprouts find themselves in direct contact and alignment with the distal part of their own tract.
Under these highly defined circumstances we observed that OECs induced rapid, aligned growth of cut CST axon sprouts across the lesion and into the caudal CST. In the present paper we describe the unique morphology of these regenerating axons and the cellular arrangements by which the OECs form a bridge conveying them across the lesion and mediating their reentry. In a companion study we have shown that this regeneration can restore lost functions (Li et al., 1997).
MATERIALS AND METHODS
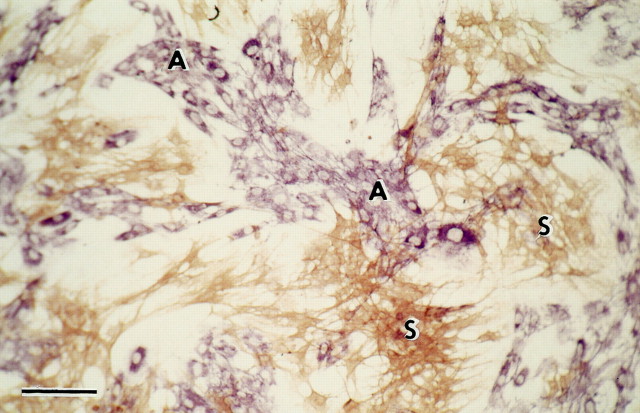
Cell cultures. Syngeneic cells from the outer (nerve fiber and glomerular) layers of adult female AS rat olfactory bulbs were plated out, as in the method of Ramón-Cueto and Nieto-Sampedro (1992), but without purification. Before transplantation, the cells were cultured for 14–17 d in DMEM/F12 Nutrient Mix + 10% fetal calf serum (Life Technologies, Gaithersburg, MD). Immunostaining (Fig. 1) confirmed that after 10–20 d, the cultured cells had segregated into clusters of the two major cell types described by Doucette and Devon (1994),Barnett et al. (1993), and Ramón-Cueto and Nieto-Sampedro (1992): (1) p75LOW AFFINITY NEUROTROPHIN RECEPTOR +, S100+ S cells (named after their resemblance to Schwann cells), and (2) vimentin+ fibronectin (FN)+ A cells (named after a resemblance to astrocytes; but see comment in Discussion). A proportion of both cell types stained with GFAP. At higher power and lower cell density, individual S cells can be seen as elongated, fine spindles, and A cells as more flattened.
Fig. 1.
Clusters of p75-immunoreactive S cells (brown) and fibronectin-immunoreactive A cells (purple). Fifteen days in culture. Scale bar, 100 μm.
Surgical procedures. Details of the procedures are given inLi and Raisman (1994). Briefly, the corticospinal tract was destroyed in 129 adult female rats (200–240 gm body weight) of a locally bred AS strain by a current of 10 μA passed for 8–10 min through a stainless steel electrode inserted stereotaxically on one side between the first and second cervical segments. In 86 rats after lesioning, the electrode was withdrawn, a glass micropipette was inserted into the same position, and 3–5 μl of a suspension containing ∼100,000 cultured OECs was injected into the lesion site. In 73 rats (22 with lesions alone, 51 with lesions and transplanted OECs), the corticospinal axons were anterogradely labeled, at 6–10 d before rats were killed, by injection of biotin dextran (BD) either into the contralateral medullary pyramids or spanning the entire contralateral sensorimotor cortex.
Perfusion and preparation of material. After survivals of 6 d to 3 months (n = 10 at 6 d, 19 at 10 d, 7 at 2 weeks, 21 at 3 weeks, 51 at 4 weeks, 4 at 6 weeks, 3 at 7 weeks, 11 at 9 weeks, 3 at 3 months), (1) 26 operated rats (11 with lesions alone, 15 with lesions and transplanted OECs) and 5 normal rats were perfused with PBS, and immunohistochemical analysis was performed on 10 μm cryostat sections; (2) 73 rats (22 with lesion alone, 51 with lesions and transplanted OECs) were fixed by perfusion with a mixture of 4% paraformaldehyde and 0.15% glutaraldehyde and 0.4% picric acid in 0.1 m phosphate buffer (PB), and 100-μm-thick vibratome sections were used for light microscopic visualization of BD (n = 52), for confocal analysis of the whole transplant region (n = 15), or for electron microscope immunohistochemistry (n = 6); and (3) for electron microscopy, 30 rats (10 with lesions alone, 20 with lesions and transplanted OECs) were fixed with a mixture of 1% paraformaldehyde and 1% glutaraldehyde in PB, and 200-μm-thick vibratome sections taken through the CST were osmicated, dehydrated, and flat-embedded in resin, semithin (1–2 μm) sections were stained with 1% methylene blue and Azur II, and ultrathin sections were stained with uranyl acetate and lead citrate.
Histology. For single antibody application, 10 μm cryostat sections were fixed in acid alcohol or 4% paraformaldehyde in PB and incubated with the primary antibody as in Table1. The secondary antibody (anti-mouse or anti-rabbit as appropriate) was either directly conjugated to HRP (1:100) or biotinylated (1:500) and developed in ABC (1:300; Vector, Burlingame, CA). For simultaneous visualization of p75 and FN or P0 and MOG, the fixed cultures or cryostat sections were incubated with the first primary and then the appropriate secondary antibodies (as in Table 1), visualized by diaminobenzidine (DAB) (brown) or by nickel-glucose oxidase (Ni-GOD) (black) (Li and Raisman, 1995), followed by incubation with the second primary antibody, and the appropriate secondary antibody was visualized with VIP (Vector) (purple).
Table 1.
Antibodies
| Antibody | Source | Type | Fixative | Dilution |
|---|---|---|---|---|
| CaM IIα | Boehringer Mannheim | Mouse monoclonal | AA | 1:500 |
| CC1 | Calbiochem | Mouse monoclonal | 4% para | 1:500 |
| Fibronectin | Life Technologies | Rabbit polyclonal | AA | 1:1000 |
| GFAP | Dako | Rabbit polyclonal | 4% para | 1:100 |
| L1 | Boehringer Mannheim | Mouse monoclonal | 4% para | 1:5 |
| Laminin | Sigma | Rabbit polyclonal | 4% para | 1:500 |
| MOG1-a | Gift | Mouse monoclonal | AA | 1:500 |
| Neurofilament | Sternberger Monoclonals | Mouse monoclonal | AA | 1:1000 |
| OX-42 | Seralab | Mouse monoclonal | 96% alcohol | 1:4000 |
| p75 | Boehringer Mannheim | Mouse monoclonal | AA | 1:10 |
| P01-b | Gift | Rabbit polyclonal | 4% para | 1:3000 |
| S-100 | Dako | Rabbit polyclonal | 4% para | 1:100 |
| Vimentin | ICN Biomedicals | Mouse monoclonal | AA | 1:100 |
AA, Acid alcohol; para, paraformaldehyde.
Gift from Richard Mead, Cardiff University.
Gift from J. J. Archelos, Max Planck Institute, Munich.
For light microscopy, BD was visualized with Ni-GOD. For electron microscopy of BD, the sections were frozen and thawed (Henry et al., 1994) before ABC incubation. For confocal microscopy, the sections were incubated with p75 (1:100) and ABC overnight, washed in PBS, and incubated in biotinylated secondary antibody (1:200) for 1 hr. The BD-labeled axons were visualized with avidin–fluorescein (green), and the transplanted OECs were visualized with avidin–rhodamine (red).
RESULTS
The corticospinal axons were identified by BD anterograde labeling and by selective immunostaining for α calcium/calmodulin-dependent protein kinase II (CaMII) ((Terashima, 1995) (Fig.2A,B). They form a compact, well delineated tract of ∼0.5 mm diameter, located in the ventromedial part of the dorsal columns, ventral to the gracile and cuneate fasciculi. The CST consists of ∼50,000 axons on each side; the large majority are myelinated. In semithin or ultrathin sections or with neurofilament (NF) immunohistochemistry (Fig. 2C), they are distinguished from the larger myelinated ascending sensory axons of the gracile and cuneate fasciculi by their rather uniform diameter (∼1 μm) and by their much higher glial density.
Fig. 2.
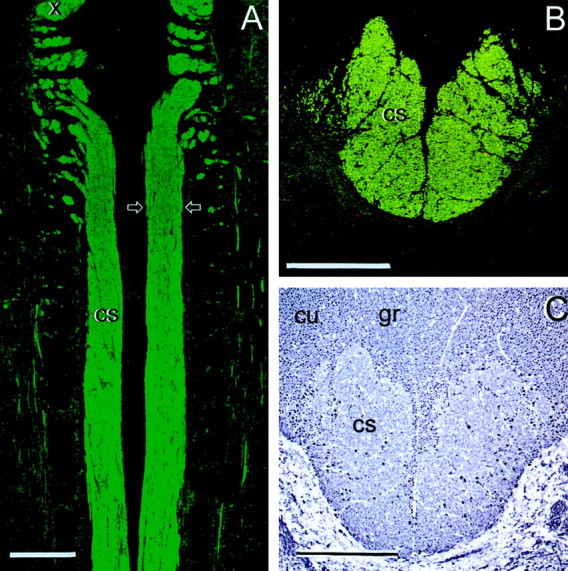
The normal rat corticospinal tract (cs) in horizontal section (A) and cross section (B, C, at the level of the lesions and transplants; arrows in A). A, B, CaMII; C, NF. cu, Cuneate fasciculi; gr, gracile fasciculi; x, fibers leaving the pyramidal decussation. Scale bars, 500 μm.
Lesions
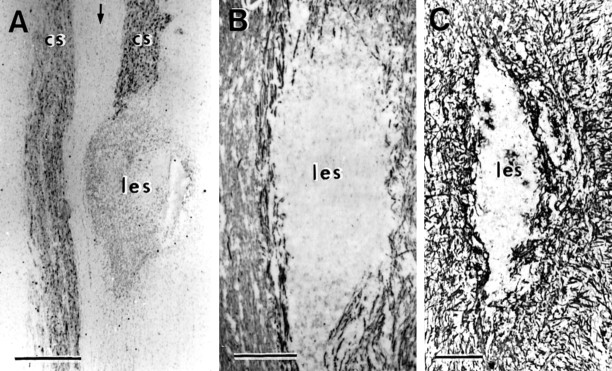
The lesions were largely confined to the CST (Fig.3), and they destroyed an ovoid area of tissue ∼0.5 mm in width and 0.5–1.0 mm in rostrocaudal length. The area of total destruction of the host spinal tissue was clearly demarcated from the myelinated fiber bundles of the adjacent intact spinal tracts with their associated cellular framework of astrocytes, oligodendrocytes, and microglia. Within the lesioned areas, all CNS components were completely eliminated. This was shown by light microscopic immunohistochemistry of CAMII and NF for axons, CC1 for oligodendrocytes (Shuman et al., 1997), OX42 for ramified microglia, and GFAP for astrocytes. Semithin and ultrathin sections confirmed these conclusions and showed that the lesioned area contained only debris and amoeboid macrophages.
Fig. 3.
Horizontal sections through a lesion (les) placed in the CST (cs) on one side to show the complete destruction of axons and glia within the lesioned area. A, CAMII; B, NF; C, GFAP. Arrow indicates midline. Survival times:A, 3 weeks, B, 1 week, C, 6 weeks. Scale bar: A, 500 μm; B, C, 200 μm.
As described in a previous publication (Li and Raisman, 1995), the cut ends of the CST axons arborized in the rostral part of the lesioned area (Fig. 4), but in none of the 69 animals with lesions alone (i.e., with no transplanted OECs) in this or the previous series were there any situations in which we have observed such axons to cross the lesioned area.
Fig. 4.
A, B, Terminal sprouting of cut, BD-labeled axons in lesions of the CST. Survival: 3 weeks. Scale bar, 20 μm.
General morphology of OEC transplants
The transplanted OECs formed a conspicuous, densely hypercellular mass, enclosed within the ovoid, smoothly outlined lesioned area, and elongated along the axis of the host CST. Immunostaining for p75, fibronectin, GFAP, L1, and laminin (Fig.5) clearly shows the position, size, and shape of the mass of transplanted cells. Compared with lesions alone, where a dense astrocytic scar develops in the host CST (Li and Raisman, 1995), there is only a slight upregulation of GFAP in the immediately adjacent host tract astrocytic processes. As in the case of transplants of embryonic central neural tissue (e.g., Lawrence et al., 1984) or cultured peripheral nerve Schwann cells (Brook et al., 1993; Li and Raisman, 1997), the transplanted OECs are highly angiogenic, and from the earliest times they induce a dense plexus of microvessels (clearly shown by laminin immunostaining) (Fig. 5E,F), which contrasts conspicuously with the low vascularity characteristic of the surrounding white matter.
Fig. 5.
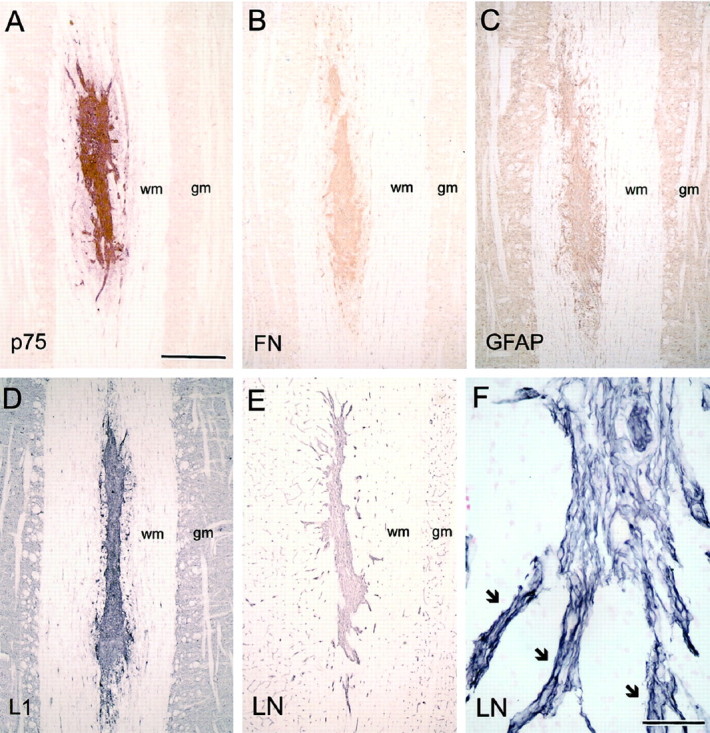
Immunohistochemical characterization of transplanted OECs. A, p75; B, fibronectin (FN); C, GFAP; D, L1; E, F, laminin (LN).gm, Gray matter; wm, white matter.Arrows indicate blood vessels with associated OECs radiating out from the transplant. Survival times: 10–14 d. Scale bars: A–E, 500 μm; F, 50 μm.
Rostral and caudal to the transplants, there is considerable disorganization of the proximal and distal host CST caused by retrograde and orthograde axonal degeneration, fragmentation of myelin, and major invasion of macrophages, and phagocytic activity by microglia, macrophages, and astrocytes. From the earliest times observed, the individual transplanted OECs in the lesioned area become elongated along the tract axis and migrate both rostrally and caudally into the host CST. Later (see Fig. 11) the caudally directed migration becomes very prominent.
Fig. 11.
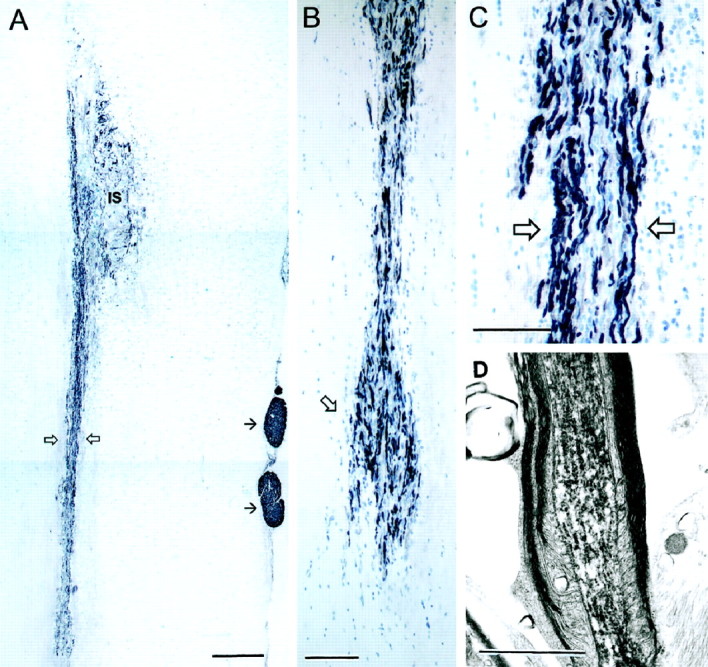
A, Longitudinal section of a transplant of OECs extending from the injection site (IS) for >5 mm caudally (open arrows) in the denervated host CST. Immunohistochemistry for P0 (black) demonstrates the peripherally myelinated internodal segments of the regenerating host CST axons passing through the transplant. Small, single arrows indicate P0 immunostaining of host Schwann cell peripherally myelinated axons in the dorsal roots. B, The caudal end of the cell migration where the columns of transplanted OECs interdigitate with the glial cells rows of the distal host CST and their associated P0 myelinated axons reenter the oligodendrocytic territory of the distal host CST. C, Enlargement (from A) of the migrating OEC column with P0 axons. Blue, Thionin counterstain of cells. D, Electron micrograph of a BD-labeled corticospinal axon with peripheral-type myelin taken from the center of the transplant. Survival: 3 months (A–C), 4 weeks (D). Scale bars: A, 500 μm; B, 200 μm:C, 100 μm: D, 2 μm.
Axon response
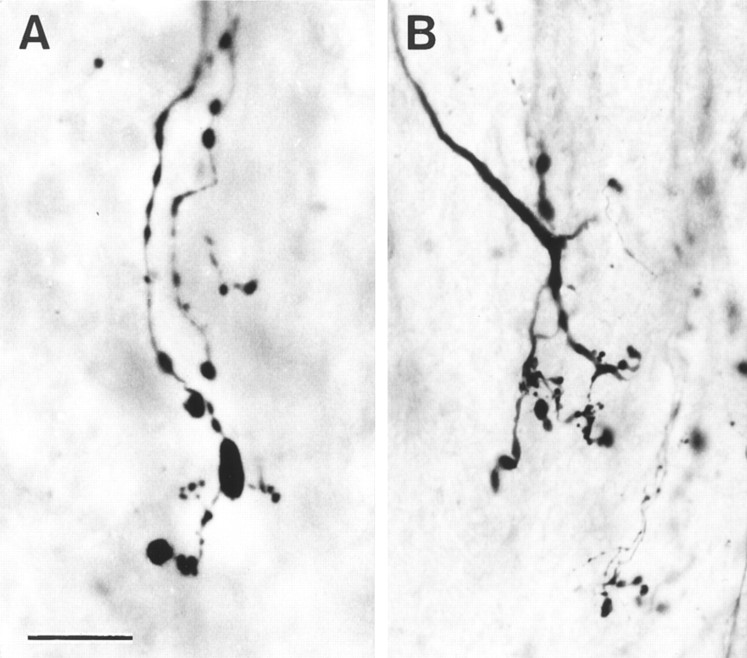
From the earliest times observed (6 d), the BD-labeled regenerating cut corticospinal axons in contact with the transplanted OECs adopt a unique morphology (Fig. 6). The regenerating axons are slightly expanded in diameter as they enter the transplanted area, and they have a moderate number of varicosities. The trajectory of the individual BD-labeled axons is constrained within the longitudinal rostrocaudal axis of the CST. The axons appear almost entirely unbranched, except for occasional spine-like protrusions, and they taper to a diameter of ∼0.1–0.2 μm. Through-focus examination of the full thickness of the 100-μm-thick vibratome sections clearly shows that at earlier survivals (6–10 d), the axons have minute, simple tips (Fig. 6, asterisks), which can be clearly seen to end freely, within the thickness of the block of tissue included in the sections. From as early as 10 d, some regenerating axons have completely traversed the central part of the transplants, and their free tips have already left the caudal end of the mass of transplanted OECs and continued in a straight line, to end at a distance of ≥1 mm into the distal part of the host CST.
Fig. 6.
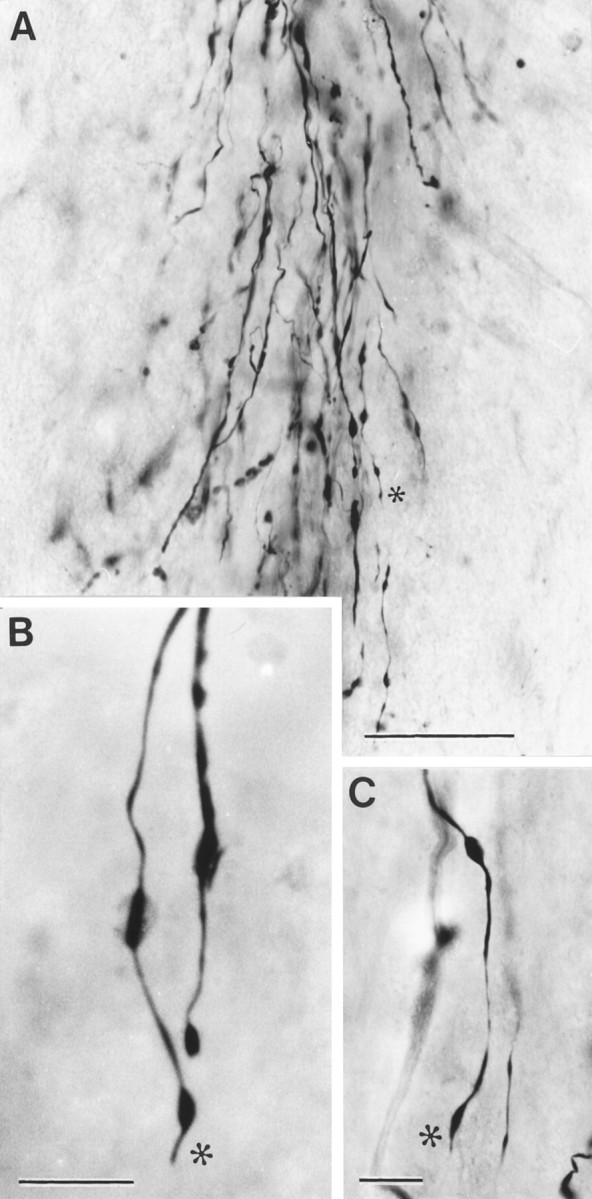
Morphology of regenerating axon sprouts labeled by anterograde transport of BD. A, Bundle of aligned, unbranched regenerating cut CST axons advancing through an OEC transplant. B, C, High-power photographs showing the freely ending tips (*) of regenerating CST axons preceded by small varicosities. Survival time: 10 d. Scale bars: A, 200 μm; B, C, 20 μm.
Electron microscopy of transplanted cell types
S cells
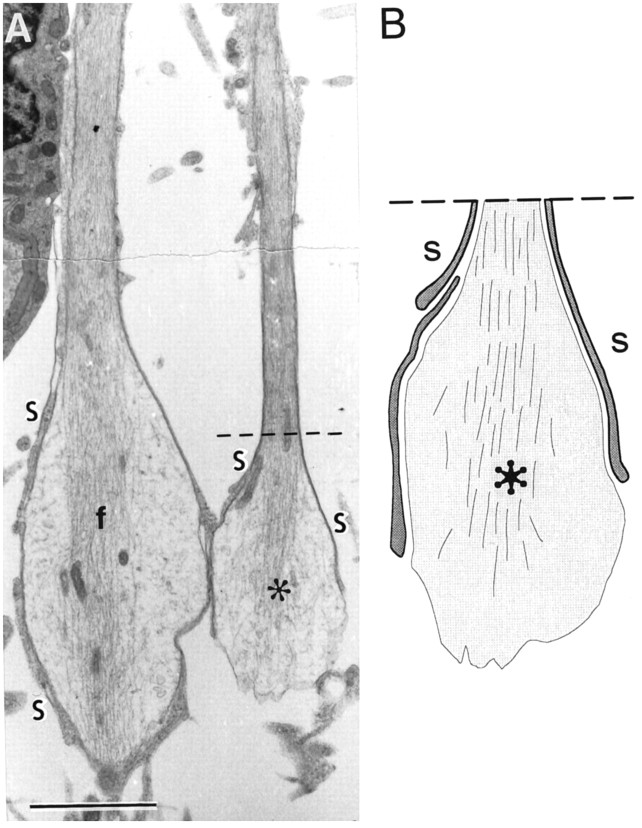
We identify the S cells by their resemblance to the transplanted Schwann cells seen in our previous studies (Li and Raisman, 1994). The S cells (Fig.7A, indicated by S) are solitary and have overall rounded outlines with dark cytoplasm and smooth, rounded dark nuclei with dark areas of heterochromatin. The cell surfaces emit microvillous processes, and from the earliest times observed, S cell processes make direct membrane contact with the axons, forming very thin, single layers, intimately investing the individual axons and their varicosities, and extending all the way to their tips (Fig. 8). Cross sections (Fig.9) show that the single S cell processes can ensheathe multiple, small-diameter axon sprouts (as in developing or unmyelinated peripheral nerve). From as early as 10 d, the abaxonal surfaces of the S cells are covered by a basal lamina (Fig. 9,arrowheads) facing a collagen-containing extracellular space.
Fig. 7.
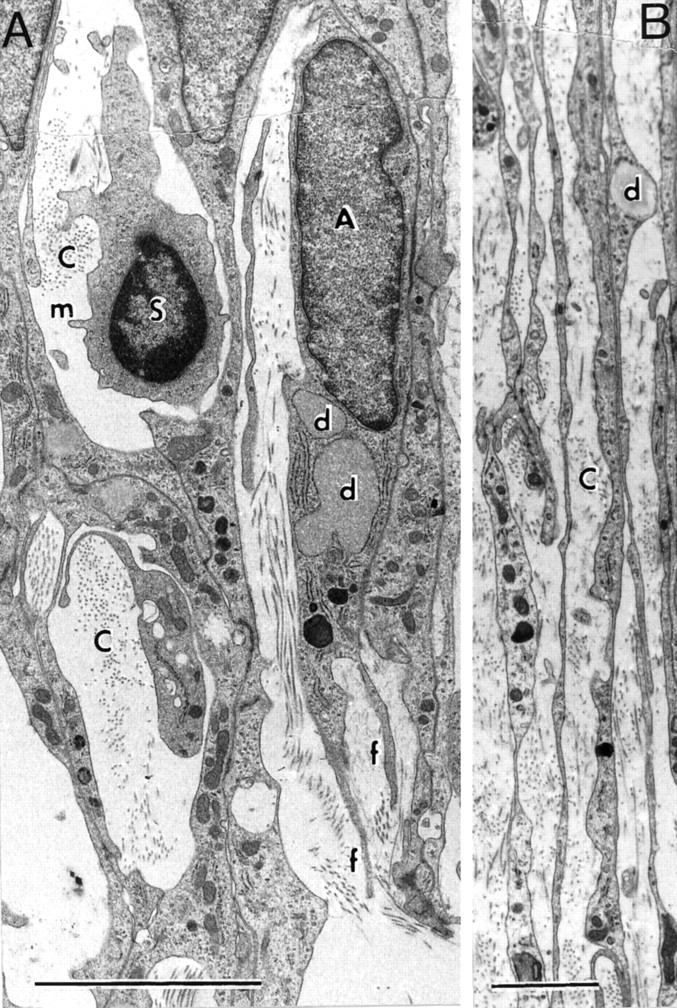
Electron micrographs of longitudinal sections.A, S and A cells; B, fascicle of parallel, longitudinal A cell processes. C, Collagen-containing extracellular space; d, cytoplasmic lipid droplets in A cell processes; f, filopodial extensions of A cells; m, S cell microvillus. Survival: 10 d. Scale bars: A, 5 μm; B, 2 μm.
Fig. 8.
A, Preterminal varicosities of two regenerating axons with central cores of neurofilaments (f) and wrapping by very fine S cells processes (S, drawn enlarged in B). Survival: 10 d. Scale bar, 5 μm.
Fig. 9.
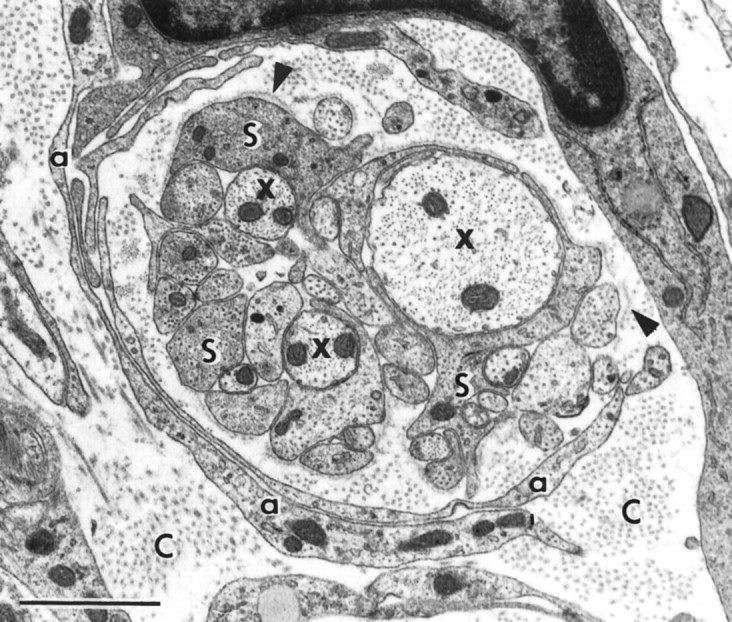
Electron micrograph of a cross section through a bundle of fine regenerating axons (x) enwrapped by S cell processes (S), whose abaxonal surfaces are covered with a basal lamina (arrowheads), in a collagen-containing extracellular space (C), with an outer sheath of slender, curving sheets of loosely apposed A cell processes (a). Survival: 10 d. Scale bar, 1 μm.
A cells
Compared with the S cells, the A cells (Fig. 7A, indicated by A) are larger and more elongated, with paler cytoplasm, containing lipid droplets, and larger, paler nuclei with more irregularly shaped, somewhat rectangular outlines. They lie in the collagen-containing extracellular space and do not contact axons. The A cells aggregate in close contact with each other, with membrane thickenings over much of the contact area. In longitudinal sections, the clusters of A cells and their processes (Fig. 7B) extend in parallel arrays along the rostrocaudal axis of the CST. At the leading edge of the cluster, the A cells are prolonged into two or three long, thin, filopodia-like streamers (Fig. 7A, indicated by f). In cross section, the sheet-like, curving A cell processes (Fig. 9, indicated by a) can be seen to form layered shells, enclosing groups of axons and their associated S cell wrapping.
Longer-term axon and olfactory-ensheathing cell configuration
In 15 rats at 3 weeks to 3 months survival, we prepared confocal pictures of the whole transplant area in consecutive 1 μm steps throughout the entire thickness in each of the five to six members of a continuous series of longitudinal 100-μm-thick vibratome sections through the 10–15 mm longitudinal block of spinal cord containing the transplant (i.e., an aggregate of 500–600 scans from each transplant). We used BD to identify the regenerating corticospinal axons and concomitant p75 to identify the transplanted OECs. This provided a complete visualization of the whole cross-sectional area of each 100-μm-thick section of the transplant region (Fig.10). The regenerating corticospinal axons form parallel bundles confined to the transplant area. They were almost entirely unbranched, in direct alignment with the rostrocaudal axis of the CST, and passed uninterruptedly into the distal host CST caudal to the transplant.
Fig. 10.
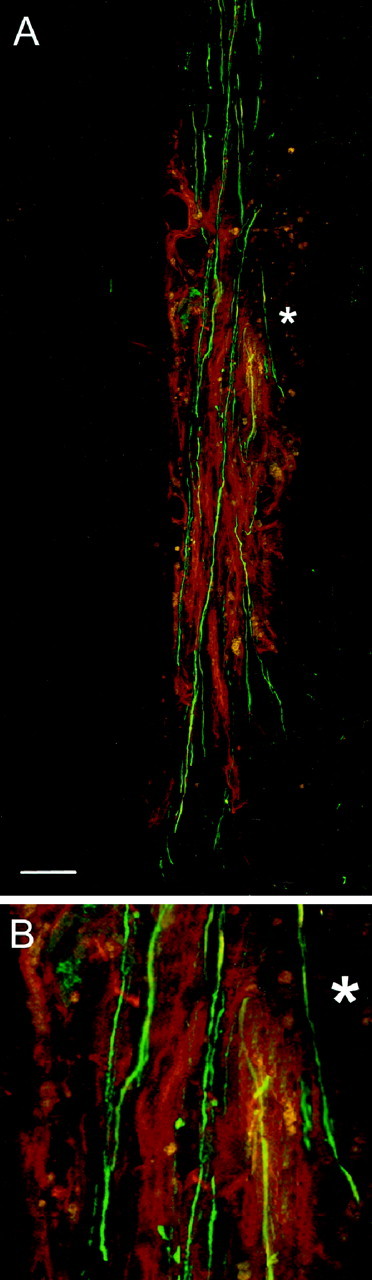
A compilation of 100 sequential 1 μm confocal scans showing the regenerating, BD-labeled corticospinal axons (green fluorescence) passing uninterruptedly, without branching through a transplant of p75-immunoreactive (red fluorescence) OECs in the lesioned CST and reentering the caudal host tract. *, Region enlarged inB. Survival time: 3 weeks. Scale bar, 200 μm.
From 3 weeks onward, the regenerating axons become ensheathed by P0-immunoreactive myelin, which is characteristic of peripheral myelin [as in the dorsal roots (Fig.11A)], but which is completely absent from the normal CST or other spinal tracts. The P0 immunostaining provides a striking overall picture of the regenerating axons passing through the transplanted region and their reentry into the distal host CST. Concomitant with the expression of P0, the expression of p75, which was a conspicuous marker of S cells at earlier times, becomes reduced throughout most of the transplant (although not as much as in the case of Schwann cells when they form myelin in developing or regenerating peripheral nerve (Taniuchi et al., 1988) or after transplantation into the CST (Li and Raisman, 1997). L1 was also greatly downregulated, although still present at low levels throughout the transplants. Fibronectin immunostaining remained as dense as at the earlier times.
Longitudinal semithin and ultrathin sections (Fig.12) show that the axons are traveling through regions that have a structure that closely resembles peripheral nerve and remains devoid of any central glial cell types (astrocytes, oligodendrocytes, or microglia). The S cells no longer enwrap multiple axons but now consistently express the characteristic peripheral-type, one-to-one myelinating relationship with the axons, in which the S cell outer cytoplasmic tongues and all their abaxonal surfaces are closely and uniformly clothed by a basal lamina. Apart from their contact with their individual axons, the S cells do not make contact with other S cells or any other cell type. The S cell myelin is thicker (∼20 turns) and (with our fixative procedure) better-preserved than the central, oligodendrocytic myelin in the adjacent host spinal white matter tracts, and the periodicity of the peripheral myelin is 10% greater than that of the central myelin (Peters et al., 1976; Li and Raisman, 1997). There are frequent nodes and Schmidt-Lantermann clefts. Electron microscopy of the anterogradely transported BD label clearly confirms the corticospinal identity of the peripherally myelinated regenerating axons (Fig. 11D).
Fig. 12.
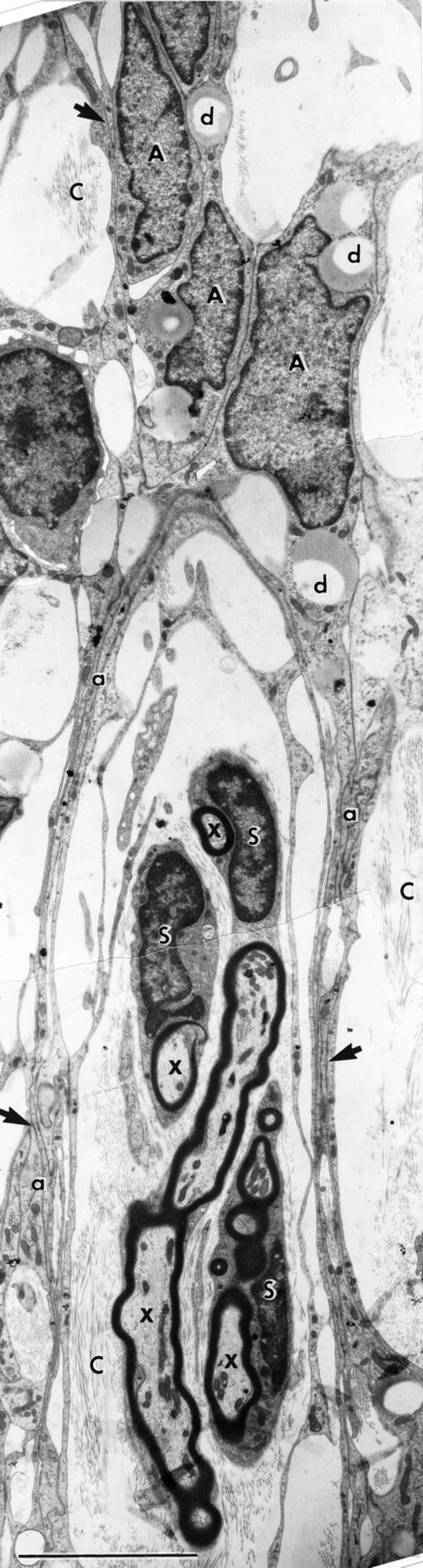
Electron micrograph of a longitudinal section through a group of S cells forming typical peripheral myelin around regenerating axons (x). The processes (a) of the A cells form a perineurial-like sheath. C, Collagen-containing extracellular space;d, cytoplasmic lipid droplets; arrows, regions of intercellular A cell junctions. Survival: 30 d. Scale bar, 5 μm.
As the S cells form myelin, the A cells become compacted into tubular sheaths (Fig. 12) that span the length of the transplants. The sheaths consist of reduplicated, closely apposed thin sheets of cytoplasmic A cell processes (Fig. 12, indicated by a) bound by extensive, complex intercellular junctions (Fig. 12, arrows). As in the case of the perineurial sheaths of peripheral nerve, the A cell sheaths lie in a collagen-containing extracellular space and enclose a territory of ∼5–10 μm in diameter, containing ∼3–10 S cells and their associated axons.
Reentry of the regenerating axons into the CNS
P0 immunostaining shows that the distal part of the host CST becomes selectively infiltrated by a dense mass of transplanted OECs (Fig. 11), extending caudally within the boundaries of the host CST for distances of ∼5 mm at 3–4 weeks and >10 mm at 3 months. At the caudal boundary, there is no appreciable disruption of the alignment of the host glial cells, and the streams of OECs and P0 myelinated axons interdigitate smoothly with the longitudinal interfascicular glial rows (Suzuki and Raisman, 1992) of the distal host CST. After crossing the transplants, the peripherally myelinated regenerating corticospinal axons continue uninterruptedly into the distal part of the host CST, coextensive with the mass of caudally migrating OECs. Combined P0 and MOG immunostaining (Fig.13A,B) shows the transition, at a single internode, from the peripheral myelin of the transplant region (black = P0) to the oligodendrocytic territory of the distal host CST (purple = MOG). In electron micrographs this transition was represented by “mixed” nodes with peripheral myelin rostrally and central myelin caudally (Fig. 13C).
Fig. 13.
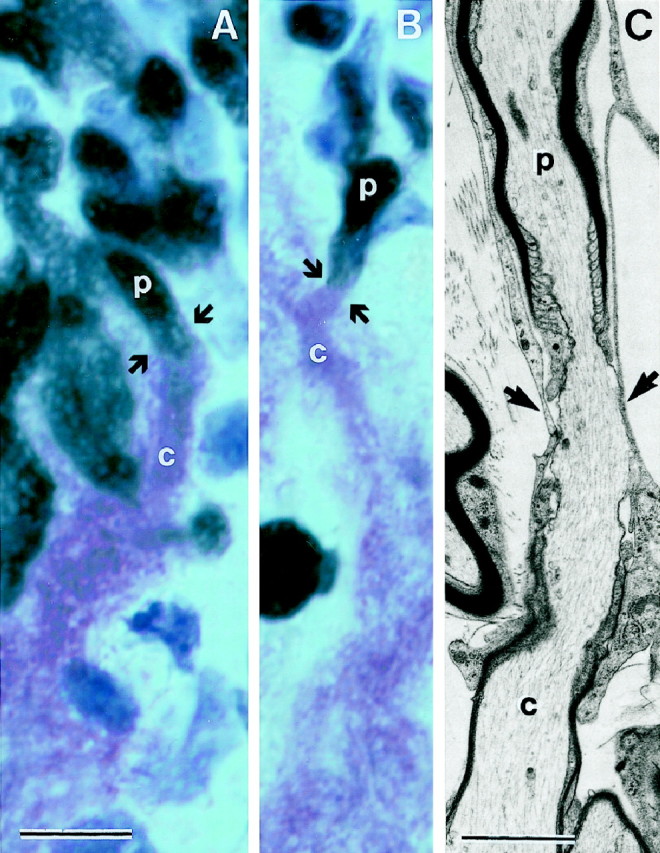
A, B, The junction (arrows) where the regenerating CST axons leave the S cell peripherally myelinated environment (p;black = P0) of the transplant to reenter the distal host CST, where they become centrally myelinated (c;purple = MOG) by the host oligodendrocytes.C, Electron micrograph of a node (arrows) between an internode (top), which is peripherally myelinated (p) by transplanted S cells, and the adjacent caudal internode (bottom), which is centrally myelinated (c) by an oligodendrocyte of the distal host CST. Survival times: 5 weeks (A, B); 4 weeks (C). Scale bars: A, B, 5 μm;C, 2 μm.
DISCUSSION
The axonal response
The tip of a growing axon acts as a sensory structure, exploring the environment and changing shape (Mason and Wang, 1997) in response to the molecular signals that determine advance, collapse, or turning (Fan et al., 1993; Tessier-Lavigne and Goodman, 1996; Mason and Wang, 1997).
The regenerating CST axons induced by OEC transplants have three characteristic morphological features. (1) At shorter survivals, they have simple, unbranched tips, ending freely in the transplants. They resemble the simple tips of developing axons in the optic stalk (Chan et al., 1998, their Fig. 3). We do not see the expanded, hand-like growth cones with filopodia found in tissue culture (Argiro et al., 1984) or at choice points in developing systems (Bovolenta and Mason, 1987). (2) The regenerating axons form parallel fascicles aligned with the long axis of the CST. This is in contrast to the highly tortuous, varicose, branching neuromatous masses and pseudoterminal arborizations found after transplantation of Schwann cells (Li and Raisman, 1994). (3) At longer survivals, the regenerating axons traverse the entire rostrocaudal axis of the transplants and continue uninterruptedly into the denervated host CST.
We believe these to be regenerating axons that have been cut (rather than surviving uncut axons that have been demyelinated and remyelinated by the transplanted OECs) because (1) the lesioning procedure results in a circumscribed area of macrophage-infiltrated debris, totally devoid of central glia and axons (Fig. 3), indicating that all components of CNS tissue have been totally destroyed. In 69 rats (from this and the previous studies) with lesions alone and BD labeling of the CST, the swollen ends of the cut axons produced branching sprays of short sprouts in the rostral edge of the lesions (Fig. 4) (Li and Raisman, 1994, their Fig. 3; Li and Raisman, 1995, their Figs. 3, 4), but we have never seen axon sprouts traversing a lesion without transplanted OECs. (2) At increasing survival times after transplantation of OECs into the lesioned area, we see the free tips of the regenerating cut axons extending progressively into and through the transplants (Figs. 6, 8).
Reentry of the regenerating CST axons into the host CST
Because of the caudal migration of the transplanted OECs, the regenerating axons reenter the distal host CST up to 10 mm caudal to the injection site. After approximately 3 weeks, these axons are myelinated, for their course through the transplants, by peripheral myelin formed by the transplanted S-type OECs. From the internode at the point where they reenter the distal part of the CST, however, they become myelinated by oligodendrocytes. For individual axons the point of reentry is indicated by the presence of a mixed peripheral-to-central myelin node, which can be identified by P0/MOG combined immunohistochemistry or in electron micrographs (Fig. 13). The reformation of central myelin indicates that the axons have left the peripheral environment of the transplant and reentered the oligodendrocytic CNS environment of the host CST.
At present we have not established how far the regenerating CST axons grow or the nature of their terminal distribution. However, it seems likely that they do form effective contacts, because in a functional study we found that OEC-induced regeneration of cut CST fibers across a complete unilateral CST lesion restores a specific directed forepaw reaching function (Li et al., 1997).
The composition and behavior of the transplanted OECs
There are two distinct types of transplanted cells, differing in their phenotype, structure, and behavior. One of the most striking features of the OEC transplants is the “cooperation” between these two cell types. At the earlier stages the A cells form clusters with pioneering filopodia, advancing around the S cell-ensheathed axons. At later stages the S cells myelinate the axons and the A cell clusters coalesce into tubular, perineurial-like structures traversing the whole rostrocaudal length of the transplants (Fig. 14). A cells were named after a supposed resemblance to astrocytes (based on the expression of GFAP) (Barber and Lindsay, 1982; Franceschini and Barnett, 1996). However, both the expression of fibronectin in culture (Fig. 1) and their tissue arrangement after transplantation (Fig. 12), indicate a strong resemblance to fibroblasts.
Fig. 14.
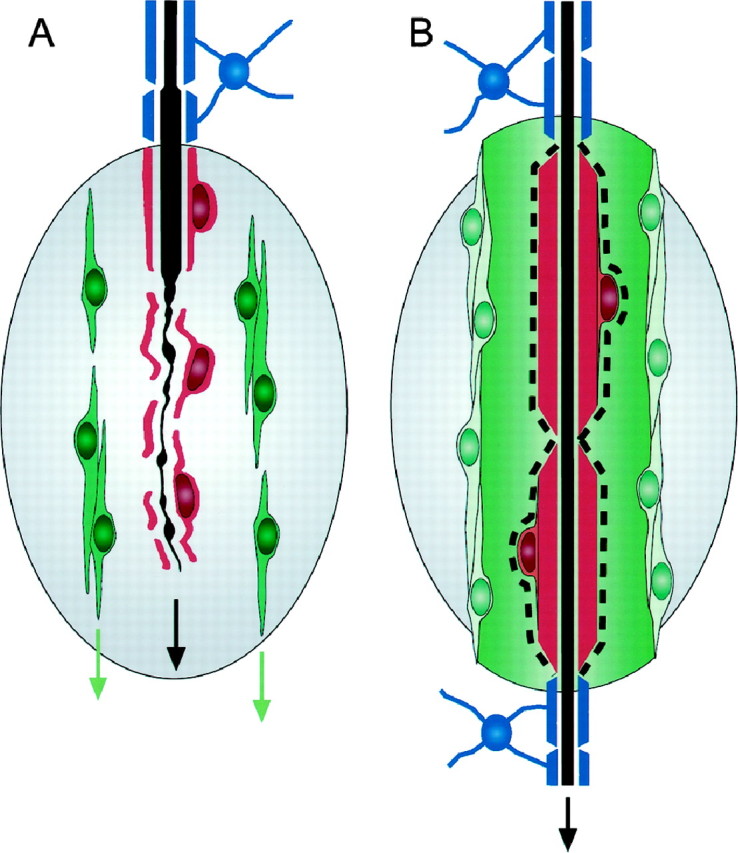
Summary of the cellular changes associated with the advance of the regenerating cut CST axons (black) across lesions (gray) repaired with transplants of OECs (red andgreen) and the reentry of the regenerating axons into the oligodendrocytic territory (blue) of the distal host CST. A, At shorter survivals, the axons advance, intimately clothed by thin sheets of S cell cytoplasm (red), and flanked at a distance by elongated A cells (green) aligned along the rostrocaudal tract axis. B, At longer survivals, the S cells myelinate the regenerating axons with peripheral myelin (red) and are clothed by a basal lamina (dashed line). The A cells form tubular, perineurial-like outer sheaths aligned along the tract axis and bridging the lesion area. Thus, where they leave the rostral host CST (above the transplant), and where they reenter the caudal host CST (below the transplant) the regenerating axons are myelinated by central myelin (blue) formed by the host tract oligodendroglia.
Like transplanted Schwann cells (Li and Raisman, 1997), transplanted OECs suppress scar formation by the host astrocytes. There was no long-term astrocytic hypertrophy or reorganization to form the thick, reduplicated astrocytic scars that are typically found after comparably sized CST lesions without transplants (Li and Raisman, 1995). At the caudal end of the columns of migrating OECs, the longitudinal glial cell alignment of the distal host CST was preserved.
The ability of transplanted OECs to myelinate central axons is all the more striking because the axons with which they are normally associated, in the olfactory system, are totally unmyelinated (Doucette, 1991). Devon and Doucette (1992) demonstrated that OECs myelinate the neurites of dorsal root ganglion cells in culture, and transplanted OECs (Imaizumi et al., 1998) or an OEC cell line (Franklin et al., 1996) is able to remyelinate axons and enhance conduction in a gliotoxic lesion of the spinal cord. In both of these situations, as in the present study, they produce peripheral-type myelin.
In contrast to transplanted Schwann cells (Li and Raisman, 1994, 1997), the greater caudal migration of the OECs (of up to 10 mm) (also seeImaizumi et al., 1998) may be important for maintaining the alignment of the CST axons and presenting the regenerating axon tips to the caudal part of the host tract.
Anton and collaborators have shown the importance of p75 for Schwann cell migration in vitro (Anton et al., 1994). The expression of p75 on transplanted OECs and Schwann cells (Li and Raisman, 1997) may similarly be involved in their migration in vivo. In our previous experiments (Li and Raisman, 1997), p75 had disappeared from the transplanted Schwann cells by 2 months. In the present material a proportion of the transplanted OECs were still expressing p75 at 3 months. This prolonged maintenance of p75 expression may contribute to the enhanced migration of OECs compared with Schwann cells. The transplanted OECs also express high levels of molecules such as laminin (Liesi, 1985) and L1, which may contribute to both the cell migration and the induction of axon growth (Burden-Gulley et al., 1997; Lahrtz et al., 1997).
Relationship to central myelin
There have been previous reports of regeneration of corticospinal axons after intracerebral transplantation of hybridoma cells secreting an antibody against a central myelin-associated molecule (Schnell and Schwab, 1990; Schnell et al., 1994; Bregman et al., 1995). These axons were reported as branched, and their course was deflected by extensive cyst formation at the lesion site, so that they did not reenter the distal CST but descended in abnormal locations in the spinal gray matter. Further work will be needed to elucidate the relationship to the present observations, where we found cyst formation to be minimal and the regenerating axons were unbranched, maintained their original position and alignment, and directly reentered the white matter of the caudal CST, where they became remyelinated by oligodendrocytes.
In view of the evidence that myelin-associated proteins are inhibitory to axon growth (Bandtlow et al., 1990; DeBellard et al., 1996), our observation that the regenerating axons ultimately become myelinated by oligodendrocytes does not necessarily mean that at the time when the regenerating sprouts are reentering the host CST they have made contact with central myelin. As shown by the present electron microscopic study, the newly growing axon sprouts traverse the lesion enwrapped in the cytoplasm of S cells and flanked by migrating A cells and are not in contact with central myelin. On their reentry into the caudal host CST, they may continue to be isolated from any residual myelin by the phagocytic cells, which engulf the degenerating myelin associated with the degenerating distal parts of the cut corticospinal axons and by the membranes of the array of numerous, longitudinal astrocytic processes present in the host tract.
Conclusion and forward look
The conditions of the present experiment were designed to optimize the possibility for repair. The corticospinal fibers form one of the most circumscribed tracts in the spinal cord. The lesion was small, and the reparative cells were injected into it immediately after axotomy. The observed regeneration distance of 10 mm is sufficient for the CST axons to reach the level of the motoneuron pools supplying the forelimb muscles. In a preliminary series (unpublished data) we have obtained functionally effective repair when transplantation was delayed for 5 weeks after the lesion. However, to obtain a comparable result in the larger, more disorganized spinal cord lesions, including much longer term lesions typically encountered in clinical situations in which several different tracts are involved, further interventions will probably be needed to enable the regenerating axons to bridge the greater distances and correctly realign with the appropriate distal tracts (Cheng et al., 1996; Xu et al., 1997).
Footnotes
This project was supported by the British Neurological Research Trust, the International Spinal Research Trust, the Barnwood House Trust, and Smith’s Charity. Dr. Daqing Li provided invaluable consultation and collaboration. We are grateful to Yewande Ajayi, Bernice Watt, and Tammaryn Johnson for their excellent and innovative technical support.
Correspondence should be addressed to Dr. Geoffrey Raisman, Division of Neurobiology, National Institute for Medical Research, The Ridgeway, Mill Hill, London NW7 1AA, UK.
REFERENCES
- 1.Anton ES, Weskamp G, Reichardt LF, Matthew WD. Nerve growth factor and its low-affinity receptor promote Schwann cell migration. Proc Natl Acad Sci USA. 1994;91:2795–2799. doi: 10.1073/pnas.91.7.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Argiro V, Bunge MB, Johnson MI. Correlation between growth cone form and movement and their dependence on neuronal age. J Neurosci. 1984;4:3051–3062. doi: 10.1523/JNEUROSCI.04-12-03051.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandtlow C, Zachleder T, Schwab ME. Oligodendrocytes arrest neurite growth by contact inhibition. J Neurosci. 1990;10:3837–3848. doi: 10.1523/JNEUROSCI.10-12-03837.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber PC, Lindsay RM. Schwann cells of the olfactory nerves contain glial fibrillary acidic protein and resemble astrocytes. Neuroscience. 1982;7:3077–3090. doi: 10.1016/0306-4522(82)90231-7. [DOI] [PubMed] [Google Scholar]
- 5.Barber PC, Raisman G. Cell division in the vomeronasal organ of the adult mouse. Brain Res. 1978a;141:57–66. doi: 10.1016/0006-8993(78)90616-9. [DOI] [PubMed] [Google Scholar]
- 6.Barber PC, Raisman G. Replacement of receptor neurones after section of the vomeronasal nerves in the adult mouse. Brain Res. 1978b;147:297–313. doi: 10.1016/0006-8993(78)90841-7. [DOI] [PubMed] [Google Scholar]
- 7.Barnett SC, Hutchins A-M, Noble M. Purification of olfactory nerve ensheathing cells from the olfactory bulb. Dev Biol. 1993;155:337–350. doi: 10.1006/dbio.1993.1033. [DOI] [PubMed] [Google Scholar]
- 8.Bignami A, Chi NH, Dahl D. Regenerating dorsal roots and the nerve entry zone: an immunofluorescence study with neurofilament and laminin antisera. Exp Neurol. 1984;85:426–436. doi: 10.1016/0014-4886(84)90152-3. [DOI] [PubMed] [Google Scholar]
- 9.Blanes T. Sobre algunes puntos dudosos de la estructura del bulbo olfactorio. Rev Trim Micro. 1898;3:99–127. [Google Scholar]
- 10.Bovolenta P, Mason C. Growth cone morphology varies with position in the developing mouse visual pathway from retina to first targets. J Neurosci. 1987;7:1447–1460. doi: 10.1523/JNEUROSCI.07-05-01447.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, Schwab ME. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Neuron. 1995;15:498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- 12.Brook GA, Lawrence JM, Raisman G. Morphology and migration of cultured Schwann cells transplanted into the fimbria and hippocampus in adult rats. Glia. 1993;9:292–304. doi: 10.1002/glia.440090407. [DOI] [PubMed] [Google Scholar]
- 13.Burden-Gulley SM, Pendergast M, Lemmon V. The role of cell adhesion molecule L1 in axonal extension, growth cone motility, and signal transduction. Cell Tissue Res. 1997;290:415–422. doi: 10.1007/s004410050948. [DOI] [PubMed] [Google Scholar]
- 14.Chan SO, Wong KF, Chung KY, Yung WH. Changes in morphology and behaviour of retinal growth cones before and after crossing the midline of the mouse chiasm: a confocal microcopy study. Eur J Neurosci. 1998;10:2511–2522. doi: 10.1046/j.1460-9568.1998.00257.x. [DOI] [PubMed] [Google Scholar]
- 15.Cheng H, Cao Y, Olson L. Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Sci. 1996;273:510–513. doi: 10.1126/science.273.5274.510. [DOI] [PubMed] [Google Scholar]
- 16.Davies SJA, Field PM, Raisman G. Regeneration of cut adult axons fails even in the presence of continuous aligned glial pathways. Exp Neurol. 1996;142:203–216. doi: 10.1006/exnr.1996.0192. [DOI] [PubMed] [Google Scholar]
- 17.DeBellard ME, Tang S, Mukhopadhyay G, Shen YJ, Filbin MT. Myelin-associated glycoprotein inhibits axonal regeneration from a variety of neurons via interaction with a sialoglycoprotein. Mol Cell Neurosci. 1996;7:89–101. doi: 10.1006/mcne.1996.0007. [DOI] [PubMed] [Google Scholar]
- 18.Devon R, Doucette R. Olfactory ensheathing cells myelinate dorsal root ganglion neurites. Brain Res. 1992;589:175–179. doi: 10.1016/0006-8993(92)91182-e. [DOI] [PubMed] [Google Scholar]
- 19.Doucette JR. PNS-CNS transition zone of the first cranial nerve. J Comp Neurol. 1991;312:451–466. doi: 10.1002/cne.903120311. [DOI] [PubMed] [Google Scholar]
- 20.Doucette R, Devon R. Media that support the growth and differentiation of oligodendrocytes do not induce olfactory ensheathing cells to express a myelinating phenotype. Glia. 1994;10:296–310. doi: 10.1002/glia.440100408. [DOI] [PubMed] [Google Scholar]
- 21.Fan J, Mansfield SG, Redmont T, Gordon-Weeks PR, Raper J. The organization of f-actin and microtubules in growth cones exposed to a brain-derived collapsing factor. J Cell Biol. 1993;121:867–878. doi: 10.1083/jcb.121.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franceschini IA, Barnett SC. Low-affinity NGF-receptor and E-N-CAM expression define two types of olfactory nerve ensheathing cells that share a common lineage. Dev Biol. 1996;173:327–343. doi: 10.1006/dbio.1996.0027. [DOI] [PubMed] [Google Scholar]
- 23.Franklin RJM, Gilson JM, Franceschini IA, Barnett SC. Schwann cell-like myelination after transplantation of an olfactory bulb-ensheathing cell line into areas of demyelination in the adult CNS. Glia. 1996;17:217–224. doi: 10.1002/(SICI)1098-1136(199607)17:3<217::AID-GLIA4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 24.Graziadei PPC, Montigraziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1979;8:1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- 25.Graziadei PPC, Montigraziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. 3. Deafferentation and reinnervation of the olfactory bulb following section of the fila-olfactoria in rat. J Neurocytol. 1980;9:145–162. doi: 10.1007/BF01205155. [DOI] [PubMed] [Google Scholar]
- 26.Henry MA, Westrum LE, Bothwell M, Press S. Electron microscopic localization of nerve growth factor receptor (p75)-immunoreactivity in pars caudalis/medullary dorsal horn of the cat. Brain Res. 1994;642:137–145. doi: 10.1016/0006-8993(94)90915-6. [DOI] [PubMed] [Google Scholar]
- 27.Imaizumi T, Langford KL, Waxman SG, Greer CA, Kocsis JD. Transplanted olfactory ensheathing cells remyelinate and enhance axonal conduction in the demyelinated dorsal columns of the rat spinal cord. J Neurosci. 1998;18:6176–6185. doi: 10.1523/JNEUROSCI.18-16-06176.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lahrtz F, Horstkorte R, Cremer H, Schachner M, Montag D. VASE-encoded peptide modifies NCAM- and L1-mediated neurite outgrowth. J Neurosci Res. 1997;50:62–68. doi: 10.1002/(SICI)1097-4547(19971001)50:1<62::AID-JNR7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence JM, Huang SK, Raisman G. Vascular and astrocytic reactions during establishment of hippocampal transplants in adult host brain. Neuroscience. 1984;12:745–760. doi: 10.1016/0306-4522(84)90167-2. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Raisman G. Schwann cells induce sprouting in motor and sensory axons in the adult rat spinal cord. J Neurosci. 1994;14:4050–4063. doi: 10.1523/JNEUROSCI.14-07-04050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Raisman G. Sprouts from cut corticospinal axons persist in the presence of astrocytic scarring in long-term lesions of the adult rat spinal cord. Exp Neurol. 1995;134:102–111. doi: 10.1006/exnr.1995.1041. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Raisman G. Integration of transplanted cultured Schwann cells into the long myelinated fibre tracts of the adult spinal cord. Exp Neurol. 1997;145:397–411. doi: 10.1006/exnr.1997.6502. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Field PM, Raisman G. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science. 1997;277:2000–2002. doi: 10.1126/science.277.5334.2000. [DOI] [PubMed] [Google Scholar]
- 34.Liesi P. Laminin-immunoreactive glia distinguish regenerative adult CNS systems from non-regenerative ones. EMBO J. 1985;4:2505–2512. doi: 10.1002/j.1460-2075.1985.tb03963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mason CA, Wang LC. Growth cone form is behavior-specific and, consequently, position-specific along the retinal axon pathway. J Neurosci. 1997;17:1086–1100. doi: 10.1523/JNEUROSCI.17-03-01086.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moulton DG. Dynamics of cell populations in the olfactory epithelium. Ann NY Acad Sci. 1974;237:52–61. doi: 10.1111/j.1749-6632.1974.tb49843.x. [DOI] [PubMed] [Google Scholar]
- 37.Peters A, Palay SL, Webster HF. The fine structure of the nervous system: the neurons and supporting cells. W.B. Saunders; Philadelphia: 1976. [Google Scholar]
- 38.Raisman G. Specialized neuroglial arrangement may explain the capacity of vomeronasal axons to reinnervate central neurons. Neuroscience. 1985;14:237–254. doi: 10.1016/0306-4522(85)90176-9. [DOI] [PubMed] [Google Scholar]
- 39.Ramón-Cueto A, Nieto-Sampedro M. Glial cells from adult rat olfactory bulb: immunocytochemical properties of pure cultures of ensheathing cells. Neuroscience. 1992;47:213–220. doi: 10.1016/0306-4522(92)90134-n. [DOI] [PubMed] [Google Scholar]
- 40.Ramón-Cueto A, Nieto-Sampedro M. Regeneration into the spinal cord of transected dorsal root axons is promoted by ensheathing glia transplants. Exp Neurol. 1994;127:232–244. doi: 10.1006/exnr.1994.1099. [DOI] [PubMed] [Google Scholar]
- 41.Ramón-Cueto A, Plant GW, Avila J, Bunge MB. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neurosci. 1998;18:3803–3815. doi: 10.1523/JNEUROSCI.18-10-03803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramon y Cajal S. Degeneration and regeneration of the nervous system. Hafner; New York: 1928. [Google Scholar]
- 43.Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- 44.Schnell L, Schneider R, Kolbeck R, Barde Y-A, Schwab ME. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature. 1994;367:170–173. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- 45.Shuman SL, Bresnahan JC, Beattie MS. Apoptosis of microglia and oligodendrocytes after spinal cord contusion in rats. J Neurosci Res. 1997;50:798–808. doi: 10.1002/(SICI)1097-4547(19971201)50:5<798::AID-JNR16>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki M, Raisman G. The glial framework of central white matter tracts: segmented rows of contiguous interfascicular oligodendrocytes and solitary astrocytes give rise to a continuous meshwork of transverse and longitudinal processes in the adult rat fimbria. Glia. 1992;6:222–235. doi: 10.1002/glia.440060310. [DOI] [PubMed] [Google Scholar]
- 47.Taniuchi M, Clark BH, Schweitzer JB, Johnson EM., Jr Expression of nerve growth factor receptors by Schwann cells of axotomized peripheral nerves: ultrastructural location, suppression by axonal contact, and binding properties. J Neurosci. 1988;8:664–681. doi: 10.1523/JNEUROSCI.08-02-00664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terashima T. Course and collaterals of corticospinal fibers arising from the sensorimotor cortex of the reeler mouse. Dev Neurosci. 1995;17:8–19. doi: 10.1159/000111269. [DOI] [PubMed] [Google Scholar]
- 49.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 50.Valverde F, Lopez-Mascaraque L. Neuroglial arrangements in the olfactory glomeruli of the hedgehog. J Comp Neurol. 1991;307:658–674. doi: 10.1002/cne.903070411. [DOI] [PubMed] [Google Scholar]
- 51.Vidal-Sanz M, Bray GM, Villegas-Pérez MP, Thanos S, Aguayo AJ. Axonal regeneration and synapse formation in the superior colliculus by retinal ganglion cells in the adult rat. J Neurosci. 1987;7:2894–2909. doi: 10.1523/JNEUROSCI.07-09-02894.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson KCP, Raisman G. Estimation of numbers of vomeronasal synapses in the glomerular layer of the accessory olfactory bulb of the mouse at different ages. Brain Res. 1981;205:245–253. doi: 10.1016/0006-8993(81)90336-x. [DOI] [PubMed] [Google Scholar]
- 53.Xu XM, Chen A, Guénard V, Kleitman N, Bunge MB. Bridging Schwann cell transplants promote axonal regeneration from both the rostral and caudal stumps of transected adult rat spinal cord. J Neurocytol. 1997;26:1–16. doi: 10.1023/a:1018557923309. [DOI] [PubMed] [Google Scholar]