Abstract
During mammalian development, retinal ganglion cell (RGC) axons from nasal retina cross the optic chiasm midline, whereas temporal retina axons do not and grow ipsilaterally, resulting in a projection of part of the visual world onto one side of the brain while the remaining part is represented on the opposite side. Previous studies have shown that RGC axons in GAP-43-deficient mice initially fail to grow from the optic chiasm to form optic tracts and are delayed temporarily in the midline region. Here we show that this delayed RGC axon exit from the chiasm is characterized by abnormal randomized axon routing into the ipsilateral and contralateral optic tracts, leading to duplicated representations of the visual world in both sides of the brain. Within the chiasm, individual contralaterally projecting axons grow in unusual semicircular trajectories, and the normal ipsilateral turning of ventral temporal axons is absent. These effects on both axon populations suggest that GAP-43 does not mediate pathfinding specifically for one or the other axon population but is more consistent with a model in which the initial pathfinding defect at the chiasm/tract transition zone leads to axons backing up into the chiasm, resulting in circular trajectories and eventual random axon exit into one or the other optic tract. Unusual RGC axon trajectories include chiasm midline recrossing similar to abnormal CNS midline recrossing in invertebrate “roundabout” mutants andDrosophila with altered calmodulin function. This resemblance and the fact that GAP-43 also has been proposed to regulate calmodulin availability raise the possibility that calmodulin function is involved in CNS midline axon guidance in both vertebrates and invertebrates.
Keywords: GAP-43, retinal ganglion cell, axon pathfinding, calmodulin, optic chiasm, optic tract, ventral diencephalon midline, growth cone signaling
The X-shaped optic chiasm represents a major midline pathfinding site in the visual system in which retinal ganglion cell (RGC) axons from specific regions of the retina project into the ipsilateral or contralateral optic tract. In mouse the formation of the chiasm occurs in two phases (for review, see Mason and Sretavan, 1997). In the first the chiasm begins to form starting at embryonic day 12–12.5 (E12–E12.5) when the earliest-generated RGC axons from the two eyes enter the ventral diencephalon at the most anterior region of the brain (ventral part of the future hypothalamus). Here, RGC axons from both eyes intersect to grow across the midline, and by E14 the RGC axons have exited the midline region to form the optic tracts within the lateral wall of the diencephalon. Because of the cephalic flexure, axons in the optic tracts run in a ventral to dorsal direction with respect to the head. However, embryologically, the initial portions of the optic tracts should be considered as longitudinal pathways given that they are formed adjacent and parallel to the stripe of Nkx2.2 expression (Marcus et al., 1998), a regulatory gene expressed along the longitudinal neural axis (Price et al., 1992; Barth and Wilson, 1995; Shimamura et al., 1995). Thus the first phase of chiasm development requires RGC axons to cross the midline and then exit the midline region to establish a longitudinal pathway.
Although a nascent X-shaped chiasm is formed by E14, ipsilateral and contralateral axon pathfinding does not begin until a second phase of chiasm development, which takes place between E14 and E16 when RGC axons from more peripheral retinal regions grow into the chiasm. During ipsi–contra pathfinding RGC axons from specific parts of the retina grow along one of two stereotyped axon trajectories (Colello and Guillery, 1990; Godement et al., 1990; Sretavan, 1990). (1) Contralaterally projecting axons, which originate from all retinal regions, grow along fairly smooth trajectories parallel to each other to cross the midline into the opposite optic tract. Axons that cross the midline never turn back to recross a second time and reenter the original side. (2) Ipsilaterally projecting axons, which originate almost exclusively from ventral–temporal retina, approach within 100–200 μm of the midline and then turn away, in some cases at 90–120° angles, to grow into the ipsilateral optic tract. This ipsi–contra pathfinding process gives rise to an adult-like pattern of axon projection at the chiasm by E15–E16. Axons of later-generated RGCs are added continually to the chiasm and optic tracts until approximately birth at E20 [postnatal day 0 (P0)].
Recent studies in mice have shown that the disruption of GAP-43 function interferes with RGC axon growth from the optic chiasm into the optic tract (Strittmatter et al., 1995; Kruger et al., 1998). GAP-43 is a highly abundant peripheral membrane protein found in growth cones. Although interactions with G-proteins have been proposed (Strittmatter et al., 1990), GAP-43 generally is thought to act as a calmodulin binding phosphoprotein whose affinity for calmodulin is regulated via phosphorylation by protein kinase C (PKC) (for review, see Skene, 1990;Benowitz and Routtenberg, 1997). RGC axons in GAP-43-deficient embryos enter the ventral diencephalon on schedule. However, after crossing the midline, they turn at a site ∼450–500 μm lateral to the midline to grow away from the entrance of the optic tract; as a result, an optic tract is not present along the longitudinal axis by E14 (Kruger et al., 1998). Despite this apparent pathfinding defect at the transition zone between the chiasm and the optic tract, it is known that by birth, some RGC axons eventually do enter the diencephalic wall (Strittmatter et al., 1995). It is not known whether this reflects a change with developmental age in the diencephalon environment, in GAP-43 dependence in RGC axons, or in the arrival of later-generated axons that do not require GAP-43 for pathfinding. In addition, although the lack of proper optic tract development is associated with the retinal projections spreading out over a larger territory, thereby giving rise to an enlarged chiasm in older embryos (Strittmatter et al., 1995;Kruger et al., 1998), the manner in which the disruption of GAP-43 function may affect the second phase of chiasm development, namely the formation of the ipsilateral and contralateral retinal projections, has not been determined.
Studies in invertebrates have shed light on pathfinding events at the junction between the midline region and longitudinal axon pathways. Within each CNS segment in Drosophila, ipsilaterally projecting axons do not enter the midline and extend along longitudinal pathways, whereas contralaterally projecting axons cross the midline in the anterior or posterior commissures to join longitudinal pathways on the other side. The growth of ipsilaterally projecting longitudinal axons is affected in flies with mutations in the robo gene (Seeger et al., 1993) and in flies in which Ca2+/calmodulin function is altered (VanBerkum and Goodman, 1995). In these mutants axons fail to extend normally within longitudinal pathways and instead enter the midline region growing in semicircular trajectories to recross the midline, often for multiple times.
Results from the present study using GAP-43 mutant mice described inStrittmatter et al. (1995) and Kruger et al. (1998) show that, after the failure to progress from the chiasm region into the lateral diencephalic wall to form optic tracts, individual RGC axons in GAP-43-deficient embryos grow in highly abnormal trajectories within the chiasm and eventually randomly exit into the ipsilateral or contralateral side. Normally contralaterally projecting axons grow in semicircular paths to recross the chiasm midline and are misrouted into the ipsilateral optic tract. This increase in the number of ipsilaterally projecting axons results in a very unusual visual system in which each retina sends two retinotopically complete representations of itself into the CNS, one within each optic tract. The abnormal midline recrossing of GAP-43-deficient RGC axons has some similarity to the CNS midline axon recrossing seen in Drosophila robo and calmodulin mutants.
MATERIALS AND METHODS
Animals and genotyping. All embryos were obtained from timed pregnant GAP-43 heterozygous females bred with heterozygous males in a colony at University of California San Francisco. The original mouse stock was obtained from Dr. Mark Fishman (Harvard University, Cambridge, MA). In these mice, exon two of GAP-43, which encodes 188 of 226 amino acids of the full-length protein, was removed while exon one encoding the N-terminal 10 amino acids and exon three encoding the C-terminal 28 amino acids remained. We refer to these as GAP-43-deficient or mutant mice. Pregnant mice were anesthetized by intraperitoneal injections of sodium pentobarbital. Embryos were harvested by cesarean section, after which adult animals were euthanized either by pentobarbital overdose and thoracotomy or by exsanguination. Neonatal animals were anesthetized via hypothermia. Genotyping was performed on tail and/or limb tissue DNA, using a PCR-based method as described (Kruger et al., 1998).
DiI labeling. For embryos, the cranium overlying the cortex was dissected away, and the heads with eyes intact were immersion-fixed, using 4% paraformaldehyde in 0.1 mphosphate buffer, pH 7.2 (4% PFA). Neonatal animals were fixed by intracardiac perfusion of the same fixative. DiI labeling was performed after fixation overnight or up to 3 d.
For anterograde labeling of RGC axon trajectories originating from specific quadrants of the retina, tissues overlying the eye, together with the cornea and lens, were removed. After the retina eye cup was blotted dry, small crystals of DiI (D282 Molecular Probes, Eugene, OR) were applied to desired locations such as the ventral–temporal region of the retina by using glass micropipettes under visual observation through a binocular dissecting microscope. Labeled preparations then were incubated in 4% PFA at 37°C for 4–7 d for DiI diffusion along the axons. The appropriate placement of DiI and quadrant of origin of the labeled axons was confirmed by an examination of the retina eye cups or in retinal whole mounts (see below) via fluorescence rhodamine optics.
To determine the distribution of ipsilaterally and contralaterally projecting RGCs in the retinas, we performed retrograde labeling by the placement of DiI crystals in the optic tract region in the diencephalic wall. Tissues covering the ventral diencephalon/optic chiasm region were dissected away to expose the optic tract, chiasm, and proximal parts of the optic nerves. DiI crystals were placed in a row spanning ∼1.5–2 mm along the diencephalic wall to ensure the labeling of the optic tract in case its position on the diencephalon had shifted compared to that in wild-type embryos. (In practice, backfilled RGC axons forming the optic tract were found approximately in the same position along the diencephalic wall as in wild-type embryos.) After DiI labeling, the tissue preparations were incubated in 4% PFA at 37°C for 2–3 d for the tracing of axon trajectories at the chiasm and for 7–10 d for the backfilling of RGC cell bodies in the retinas. The resulting distributions of backfilled ipsilateral and contralateral RGCs in the retinas of wild-type embryos were in the expected pattern, indicating that the backfilling procedure adequately labeled RGCs in all regions of the eye. Furthermore, the conclusions presented here are based on comparisons of the patterns of distribution of ipsilaterally and contralaterally projecting RGCs in wild-type, heterozygous, or homozygous embryos after labeling from one optic tract in each experimental animal. This type of backfilling then was repeated in multiple animals for each genotype, making any systematic sampling bias of either the ipsilaterally or contralaterally projecting RGC population over the other unlikely.
Retinal whole mounts. Retinas containing backfilled RGCs were dissected in situ within the head to remove the cornea and the lens. An orientation cut was made first to define the dorsal pole. Then the retinas were removed, six to eight radial cuts were made into the eye cup to flatten the retina, and the whole mount was placed under a coverslip raised off the glass slide by using two other coverslips as pedestals. Each entire retina whole mount was imaged at 20× magnification as multiple overlapping fields that then were assembled into photo montages.
RESULTS
Optic tracts are formed by RGC axons with abnormal trajectories in the chiasm
Previous studies have demonstrated that the initial RGC axon guidance defect in GAP-43-deficient mice is the inability of RGC axons at E14 to progress from the chiasm into the lateral wall of the diencephalon to form the optic tract (Kruger et al., 1998). A few days later, defects also are seen in the midline region in the form of an enlarged chiasm in which RGC axons are dispersed over a larger than normal area on the ventral surface of the diencephalon (Strittmatter et al., 1995; Kruger et al., 1998). Remarkably, by birth, RGC axons in fact are found in the optic tract (Strittmatter et al., 1995). The origin of these “delayed” optic tract axons is unknown, but they may represent later-generated RGC axons that arrive last at the chiasm and are intrinsically different from earlier RGC axons in that they are not affected by the disruption of GAP-43 function. Alternatively, they may be axons that initially are affected and unable to enter the tract but, as development proceeds, somehow become independent of GAP-43 function for growth into the optic tract.
To distinguish between these two possibilities, we backlabeled axons in GAP-43-deficient mouse embryos that have entered the optic tract with the fluorescent marker DiI and reconstructed their axon trajectories within the optic chiasm region. In E18 heterozygous embryos, like in wild-type embryos, the backlabeling of axons by the placement of DiI crystals into the optic tract (Fig.1A) results in the labeling of both contralaterally projecting axons in the optic nerve on the side opposite to the labeled tract (C) and ipsilaterally projecting RGC axons in the optic nerve on the same side (I). Greater numbers of labeled axons are found in the nerve opposite the labeled tract, indicating that, like in wild-type embryos, the vast majority of RGCs in heterozygous embryos projects across the midline into the contralateral optic tract, whereas a smaller population projects ipsilaterally. The trajectories of contralaterally projecting RGC axons in heterozygotes, like wild-type axons, run parallel to each other in relatively smooth trajectories during their course within the optic chiasm (OC) across the midline.
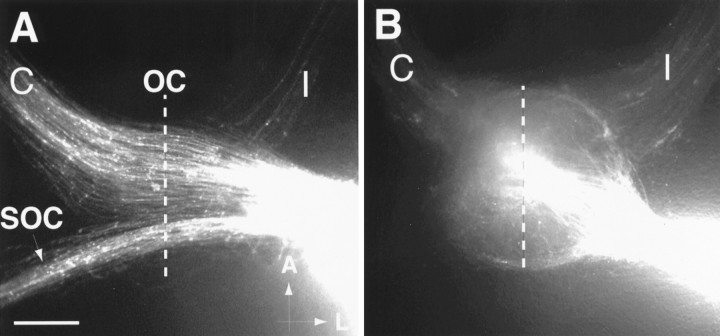
Fig. 1.
Pattern of labeled RGC axons in heterozygous and homozygous GAP-43-deficient embryos after backlabeling from the optic tract. The dotted line is the midline inA and B. A, DiI placed into the optic tract (bright area, rightside of panel) of an E18 heterozygous embryo, like wild-type embryos, retrogradely labels three groups of axons, including contralaterally projecting axons (C) in the opposite optic nerve, ipsilaterally projecting axons (I) in the optic nerve on the same side, and axons of the supraoptic commissure (SOC). As in wild-type embryos, contralaterally projecting axons greatly outnumber ipsilaterally projecting axons. Axon trajectories through the optic chiasm (OC) region are relatively smooth, and axons generally run parallel to each other. Scale bar, 400 μm. A, Anterior; L, lateral.B, Backfilling from the optic tract (bright area, right side of panel) in an E18 GAP-43-deficient embryo resulted in the retrograde labeling of approximately equal numbers of contralaterally (C) and ipsilaterally (I) projecting axons. In addition, axon trajectories at the optic chiasm are grossly disorganized, and axons appear to be massed into a circular structure. No SOC is discernible in E18 GAP-43-deficient embryos after optic tract labeling. Scale bar (as in A), 400 μm.
Backfilling from the optic tract of heterozygous embryos also labels axons in the supra-optic commissure (SOC) (Fig. 1A). SOC axons originate from dorsal regions of the diencephalon and midbrain and project ventrally to cross the midline, forming a commissure posterior to the optic chiasm. After traversing the midline region, these axons again course dorsally to innervate targets on the opposite side (Gitler and Barraclough, 1988; Martinez and Puelles, 1989). Backfilling experiments did not reveal any apparent differences between E18 wild-type and heterozygote embryos in the labeling of RGC and SOC axons.
In GAP-43-deficient embryos, RGC axons can be backfilled from the optic tract consistently, starting at E17–E18 as compared with E13–E14 in wild-type and heterozygous embryos, indicating a delay in RGC axon entry into the optic tract of ∼4 d. RGC axons in mutant embryos form the “delayed” optic tract in approximately the normal location along the lateral diencephalic wall when compared by proximity to landmarks such as the midline and junction of the optic nerve with the diencephalon. In contrast to wild-type and heterozygous embryos, the number of backfilled RGC axons in the contralateral (C) optic nerve of GAP-43-deficient embryos appears to be reduced, whereas the number of axons in the ipsilateral optic nerve (I) appears to be increased (Fig.1B). GAP-43-deficient embryos also appear to lack a SOC, because no backfilled axons could be found at its normal position posterior to the chiasm. In addition to these abnormalities, the trajectories of backfilled RGC axons within the optic chiasm are highly abnormal and circular in appearance in contrast to the more or less straight growth across the midline in the chiasm of wild-type and heterozygous embryos (Fig. 1A). The presence of these abnormal trajectories indicates that RGC axons that grow into the “delayed” optic tracts in GAP-43-deficient embryos initially undertake abnormal trajectories within the chiasm before they finally are able to enter the optic tract.
Abnormal retinal distribution of ipsilaterally and contralaterally projecting RGCs
Given that normally in mouse embryos there is a greater number of contralaterally projecting RGCs as compared with ipsilaterally projecting RGCs, the presence in GAP-43-deficient embryos of altered numbers of axons in the ipsilateral and contralateral optic nerves after retrograde labeling in one optic tract (Fig.1B) suggested that ipsilateral and contralateral axon pathfinding at the chiasm was abnormal in these mice. This was confirmed by comparing the distribution of backfilled RGCs in the retinas of heterozygous and homozygous animals at P0 a few days after the axons begin to enter the optic tracts in GAP-43-deficient animals, and at the time when there is a sufficient number of RGC axons that can be backfilled from the optic tracts to permit an evaluation of ipsilateral and contralateral axon routing at the chiasm.
The distribution of ipsilaterally and contralaterally projecting RGCs in GAP-43 heterozygous embryos is indistinguishable from that in wild-type animals. Contralaterally projecting RGCs are found throughout the entire retina (Fig.2A), whereas ipsilaterally projecting RGCs are more or less restricted to the peripheral parts of the ventral temporal retina (Fig.2B). In contrast, the distribution of contralaterally and ipsilaterally projecting RGCs is highly abnormal in GAP-43-deficient embryos. Both contralaterally and ipsilaterally projecting RGCs are found in all regions of both retinas (Fig.2C,D), where they appear to be evenly distributed in a pattern consistent with individual RGC axons randomly entering either the ipsilateral or contralateral optic tract. Aside from the abnormal distribution of ipsilaterally and contralaterally projecting RGCs, the overall size of the retinas in mutant embryos is equal to that in heterozygous embryos, consistent with the previous findings that eyes in GAP-43-deficient animals are approximately the same size and shape as in wild-type animals and that RGC neurogenesis and differentiation appear to be grossly unaffected in the GAP-43-deficient state (Kruger et al., 1998).
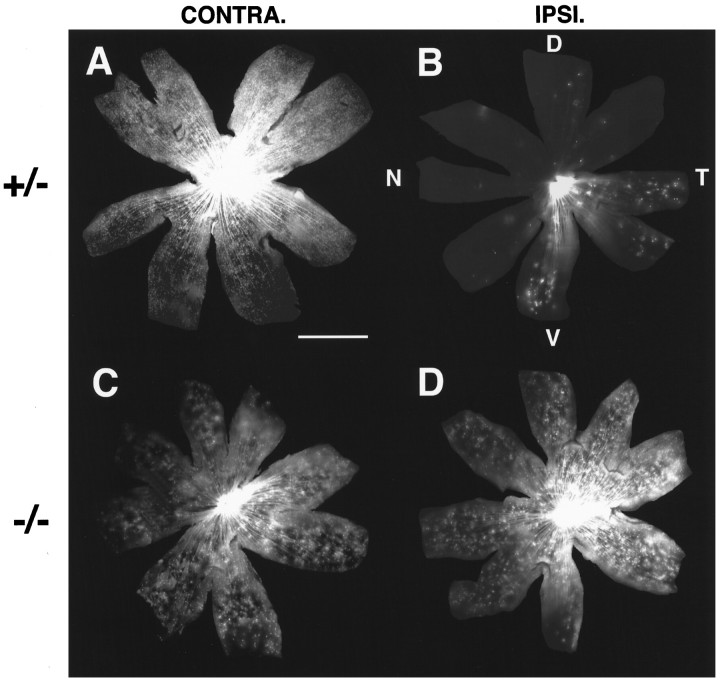
Fig. 2.
Distribution of contralaterally and ipsilaterally projecting RGCs in a heterozygote (A, B) and a GAP-43-deficient (C, D) animal at P0. Scale bar forA–D, 500 μm. D, Dorsal;T, temporal; V, ventral;N, nasal. A, B, Similar to wild-type embryos, contralaterally projecting RGCs (A) in heterozygous animals are found throughout the entire retina on the side opposite to the labeled optic tract. Ipsilaterally projecting RGCs (B) are found almost exclusively in the ventral–temporal portion of the retina on the same side as the labeled optic tract. C,D, In GAP-43-deficient animals, contralaterally projecting RGCs (C) are found throughout the entire extent of the retina on the side opposite to the labeled optic tract. Likewise, in the retina on the same side as the labeled optic tract, ipsilaterally projecting RGCs (D) also are found throughout the entire retina. The overall distributions of ipsilaterally and contralaterally projecting RGCs in the two retinas are indistinguishable.
During development, neurogenesis and differentiation of mouse RGCs occur approximately in a central-to-peripheral gradient (Drager, 1985) such that RGCs in central retina send axons into the chiasm ahead of axons from later-generated RGCs located in more peripheral regions of the retina. The finding in GAP-43-deficient embryos that contralaterally and ipsilaterally projecting RGCs are located in all retinal regions, including central retina, provides additional evidence that axons that are eventually capable of entry into the optic tract likely include early-generated RGC axons that originally fail to grow into the lateral diencephalon.
Randomized chiasm axon routing in GAP-43-deficient embryos
A true randomization of RGC axon pathfinding at the chiasm should result in approximately equal numbers of axons that project into the contralateral and ipsilateral optic tracts. If so, this in turn should be reflected by the fact that, after backfilling from one optic tract, the density of retrogradely labeled RGCs in a given region of the contralateral retina should be approximately the same as the density of retrogradely labeled RGCs in the equivalent region of the ipsilateral retina. In GAP-43-deficient embryos an examination of equivalent midretinal regions in the ventral–nasal quadrant of both the contralateral and ipsilateral retinas (Fig.3B,C) revealed an approximately equal density of retrogradely labeled RGCs (ipsilateral: 22 ± 6.6/300 μm2, n = 3; contralateral: 20 ± 5/300 μm2,n = 3), supporting the notion of a randomization of ipsi–contra pathfinding.
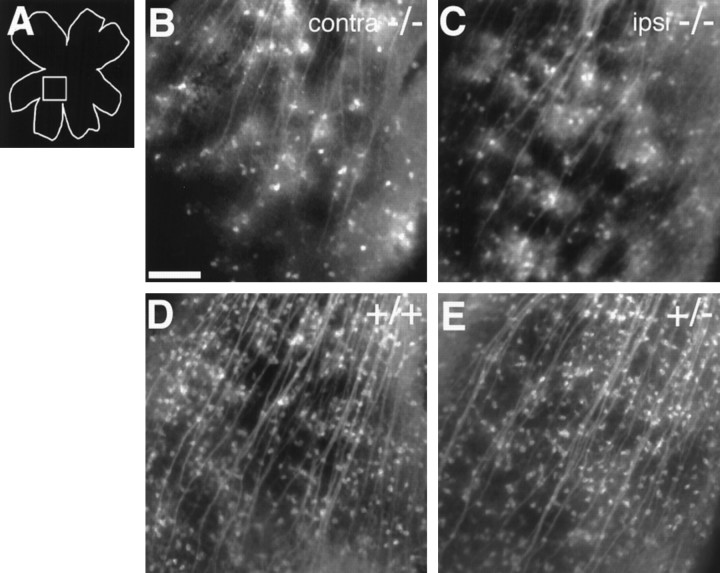
Fig. 3.
Higher magnification views of backlabeled RGCs in the retinas of wild-type, heterozygous, and homozygous GAP-43-deficient littermate pups at P0. Scale bar in B–E, 100 μm.A, Schematic diagram of a retinal whole mount. Thesquare box in the diagram represents the approximate region of the retinas shown in B–E. B,C, DiI backlabeled contralaterally projecting (B) and ipsilaterally projecting (C) RGCs in the retinas of a P0 GAP-43-deficient animal. The density of backfilled RGCs in the two retinas is similar (ipsilateral: 22 ± 6.6/300 μm2,n = 3; contralateral: 20 ± 5/300 μm2, n = 3). Furthermore, both densities are only ∼25% of the density of contralaterally projecting ganglion cells in wild-type (D) or heterozygous (E) animals (see below). D, Contralaterally projecting RGCs in the retina of a wild-type littermate at P0 (density, 82 ± 9/300 μm2;n = 3). E, Contralaterally projecting RGCs in the retina of a heterozygous littermate at P0. The density of contralaterally projecting RGCs in P0 heterozygous pups (80 ± 8/300 μm2; n = 3) is very similar to that of wild-type animals.
Of note, however, is the observation that the density of backfilled RGCs in either the ipsilateral or contralateral retinas of GAP-43-deficient embryos is only ∼25% of that in the contralateral retina of a wild-type (82 ± 9/300 μm2,n = 3; Fig. 3D) or heterozygous (80 ± 8/300 μm2, n = 3; Fig.3E) animal. If we use the density of backfilled RGCs as a rough guide to the number of axons that have grown out of the chiasm into one optic tract, the number of RGC axons able to enter a given optic tract in GAP-43-deficient embryos is <50% of that in a heterozygote or wild-type embryo. This reduction in the number of optic tract axons is unlikely to arise from a reduced number of RGCs in these mutant embryos as compared with wild-type animals, because there are approximately equal numbers cells in the RGC layer in embryos of both genotypes (Kruger et al., 1998). It is more likely that not all RGC axons in GAP-43 mutants make it out of the chiasm into the tract by P0 and that, should these embryos live longer rather than dying soon after birth, a greater number of RGC axons may be found in the optic tracts.
Lack of normal axon turning into the ipsilateral optic tract
The abnormal distributions of ipsilaterally and contralaterally projecting RGCs in the retinas of GAP-43-deficient embryos indicate pathfinding errors at the chiasm. Given that axon trajectories provide a record of axon pathfinding behavior, it was of interest to examine how the two characteristic types of axon trajectories normally present, i.e., the sharp turning of ipsilaterally projecting axons before reaching the midline, and the smooth parallel trajectories of contralaterally projecting axons might be disrupted. In wild-type animals, ipsilaterally projecting RGCs reside in ventral–temporal retina where they are mixed with contralaterally projecting RGCs. As a result, anterograde labeling of the ventral temporal retina normally labels both ipsilaterally and contralaterally projecting axons. Anterograde labeling of ventral–temporal retina in E15–E16 heterozygous animals (Fig.4A), like in wild-type embryos, results in the labeling of both contralaterally and ipsilaterally projecting axons, with ipsilaterally projecting axons turning away from the midline at 90–120° angles at a distance of a 100–200 μm before reaching the midline (Fig.4B).
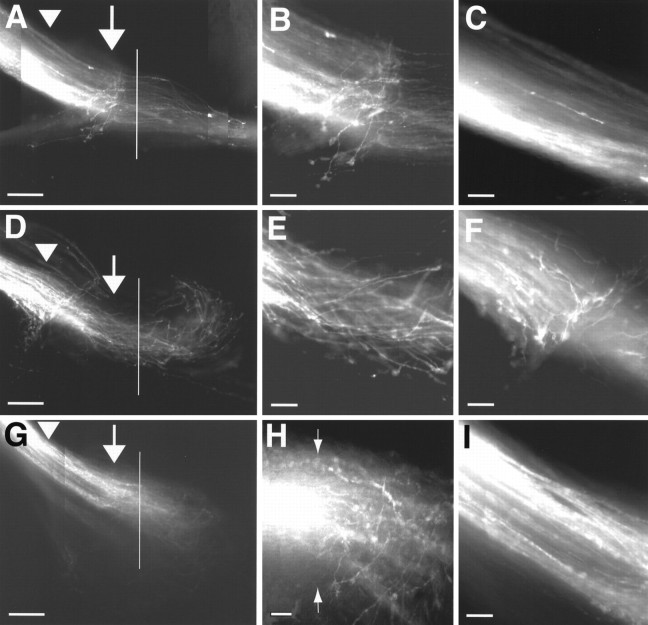
Fig. 4.
Absence of normal ipsilaterally projecting RGC axons at the optic chiasm of GAP-43-deficient mouse embryos. A, D, G, The solid vertical lines depict the location of the midline and were determined under bright-field transillumination of the third ventricle in each specimen.A, Anterior; L, lateral. Scale bars:A, D, G, 200 μm; B–I, 50 μm.A, RGC axons trajectories in the optic nerve and at the ventral diencephalon of an E16 heterozygous embryo after anterograde labeling from ventral–temporal retina. The arrow points to the region 100–200 μm from the midline where ipsilaterally projecting RGC axons normally turn away from the midline to head into the ipsilateral optic tract. The arrowhead marks the approximate site at which RGC axons leave the optic nerve and enter the ventral diencephalon. Note that, as in wild-type embryos, the ventral–temporal retina in heterozygous embryos gives rise to both ipsilaterally and contralaterally projecting RGC axons.B, Higher magnification view of the region marked with an arrow in A. Here, in a region ∼100–200 μm before the midline is reached, ipsilaterally projecting axons in heterozygous embryos, as in wild-type embryos, turn away to grow toward the ipsilateral optic tract. C, Higher magnification view of the region marked by thearrowhead in A. RGC axons at the junction of the optic nerve and the ventral diencephalon run parallel to each other and have smooth trajectories. D, Axon trajectories in the optic nerve and at the ventral diencephalon of an E16 GAP-43-deficient mouse embryo after anterograde labeling from ventral–temporal retina. The arrow points to the location at which ipsilaterally projecting RGC axons in wild-type and heterozygous embryos normally turn away from the midline to grow into the ipsilateral optic tract. The arrowhead marks the junction of the nerve and the ventral diencephalon where a novel population of turning/meandering axons originating from ventral–temporal retina is found. Note that ventral–temporal axons, after crossing the midline, all turn away without entering the contralateral optic tract. E, Higher magnification view of the area marked by the arrow in D. Axons originating from ventral–temporal retina in GAP-43-deficient embryos appear not to turn away from the midline at this site to grow toward the ipsilateral optic tract (compare with B). At this site, however, axons are more tortuous and wavy in their trajectories, compared with axons in wild-type and heterozygous embryos, as they head toward the midline. F, Higher magnification view of the area marked by thearrowhead in D. In ∼30% of GAP-43-deficient embryos a novel population of axons is found at approximately the junction between the optic nerve and the ventral diencephalon, which exhibit 90° turns and meandering trajectories. In wild-type and heterozygous animals, all axons from ventral–temporal retina progress in relatively straight and smooth trajectories through this region (as in C).G, Axon trajectories in the optic nerve and at the ventral diencephalon of an E16 GAP-43-deficient mouse embryo after anterograde labeling from nasal–dorsal retina. Thearrow and arrowhead mark areas equivalent to those marked in A and D.H, Higher magnification view of the midline region inG. RGC axons originating from nasal–dorsal retina display tortuous and disorganized trajectories within the optic chiasm, including axons that appear to grow in a semicircular course after crossing the midline. Arrows mark the position of the midline. I, Higher magnification view of the area marked by the arrowhead in G showing the approximate junction between the optic nerve and the ventral diencephalon. RGC axons from nasal–dorsal retina of GAP-43-deficient embryos at this site grow in smooth trajectories and run parallel to each other. The turning/meandering axons (as shown inF) that originate from ventral–temporal retina in GAP-43-deficient embryos are not apparent.
In contrast, labeling of the ventral–temporal retina in E15–E16 GAP-43-deficient embryos (n = 8; see also Table1) fails to reveal axons that make turns 100–200 μm before the midline to grow into the ipsilateral optic tract (Fig. 4D,E). However, at this location where ipsilateral turns normally are made, ventral–temporal axons differ from wild-type axons by having more wavy trajectories and by not all running in parallel trajectories with each other. (Growth cones pointing away from the midline can be seen on rare occasions, but at these ages the axons do not appear to enter the ipsilateral optic tract.) Anterograde DiI labeling in regions outside of ventral–temporal retina in GAP-43-deficient embryos also fail to reveal the characteristic ipsilaterally projecting axons (Fig.4G), making it unlikely that these ipsilateral axons now originate from a different site in the retina other than the ventral–temporal region. To rule out the possibility that this ipsilateral axon projection simply is delayed in its development, we also performed anterograde labeling in E18 (n = 3) and P0 (n = 3) embryos (Table 1). A normal-appearing ipsilaterally projecting axon population also is not detected at these ages.
Table 1.
Presence of normal ipsilaterally projecting RGC axons from ventral–temporal retina
| Age | Wild-type | Heterozygotes | Homozygotes |
|---|---|---|---|
| E14 | 2 /4 | 2 /4 | 0 /4 |
| E15 | 4 /5 | 2 /4 | 0 /8 |
| E16 | 6 /6 | 4 /4 | 0 /6 |
| E18 | 4 /4 | 2 /2 | 0 /3 |
| P0 | 2 /2 | 3 /3 | 0 /3 |
In wild-type and heterozygous embryos, ventral–temporal retina gives rise to a population of ipsilaterally projecting axons that make the characteristic 90–120° turns 100–200 μm from the midline into the ipsilateral optic. Such axons are not observed in E14–P0 GAP-43-deficient embryos.
Of note, in 30% of E14–E18 GAP-43-deficient embryos after the labeling of the ventral temporal retina, a novel population of axons with sharp turns and meandering trajectories is observed at the approximate junction of the optic nerve and the diencephalon, much earlier in the pathway as compared with the normal turning site of the ipsilaterally projecting axons located within 100–200 μm from the midline (Fig. 4F, Table2). Although their turning and meandering trajectories are quite distinct, these axons after turning do not project into the ipsilateral optic tract. Because ventral–temporal retina normally contains both ipsilaterally and contralaterally projecting RGCs, it is not possible, based simply on the fact that these meandering axons originate within ventral–temporal retina, to conclude that they come from the very same RGCs that normally give rise to the ipsilateral projection seen in normal development. However, meandering axons of this type at the junction of the optic nerve and the ventral diencephalon are not seen after labeling of nasal retinal regions in E15–E18 GAP-43-deficient embryos (n = 8; Fig. 4G,I).
Table 2.
Turning/meandering axons at the junction between the optic nerve and the diencephalon originating from ventral–temporal retina
| Age | Wild-type | Heterozygotes | Homozygotes |
|---|---|---|---|
| E14 | 0 /4 | 0 /4 | 1 /4 |
| E15 | 0 /5 | 0 /4 | 3 /8 |
| E16 | 0 /6 | 0 /4 | 3 /6 |
| E18 | 0 /4 | 0 /2 | 1 /3 |
In ∼30% of GAP-43-deficient embryos, a novel type of turning/meandering axons originating from ventral–temporal retina is present at the approximate junction of the optic nerve and the ventral diencephalon.
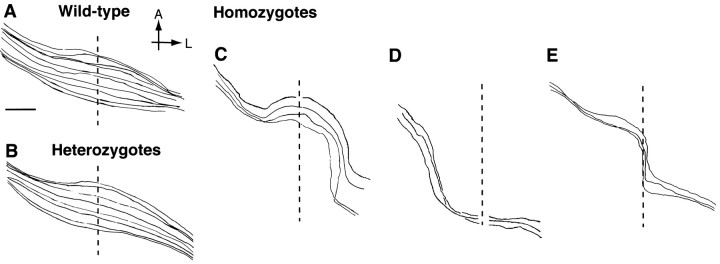
Nasal axons take abnormal routes to project contralaterally
Aside from the characteristic turning of ipsilaterally projecting axons, RGC axons coursing through the chiasm in wild-type and heterozygous embryos have smooth trajectories. In contrast, RGC axons in GAP-43-deficient embryos do not grow in straight smooth trajectories across the midline. RGC axons that grow into the contralateral optic tract exhibit one of three types of trajectories during their course within the chiasm (Fig. 5). The first type of axon trajectory consists of axon growth in a wide arc, giving rise to a trajectory that is semicircular in appearance (Fig.5C). The second type consists of axons that also grow in a wider arc than normal but are found posteriorly (Fig. 5D). These axons appear to form the most posterior edge of the enlarged “circular” optic chiasm seen in GAP-43-deficient embryos (compare with Fig. 1B). The last type consists of axons that, on reaching the physical midline at the chiasm, turn to grow along the midline in a posterior direction before then leaving the midline to grow into the contralateral optic tract (Fig. 5E). RGC axons that grow posteriorly directly along the midline are never observed in wild-type and heterozygous embryos.
Fig. 5.
Trajectories of contralaterally projecting RGC axons within the chiasm region of wild-type, heterozygous, and GAP-43-deficient embryos at E18 visualized after backfilling from the optic tract. The vertical dotted line represents the midline in all cases. The eye of origin is on the upper left in each panel; the optic tract is toward the bottom right. A, Anterior; L, lateral. Scale bars in A–E, 200 μm. A, Contralaterally projecting RGC axons in wild-type embryos grow through the optic chiasm region in generally smooth trajectories parallel to each other. B, The trajectories of contralaterally projecting RGC axons in heterozygous embryos are indistinguishable from those found in wild-type embryos. C, Contralaterally projecting RGC axons in GAP-43-deficient embryos, unlike their counterparts in wild-type or heterozygous embryos, grow along abnormal routes within the chiasm. The three types of axon trajectories seen include those axons that grow in a wide arc tracking along the anterior part of the chiasm. D, A second type of axons grows in a wide arc but tracks along the posterior edge of the chiasm.E, The third type of axons grows posteriorly for a distance directly along the midline before entering the contralateral optic tract.
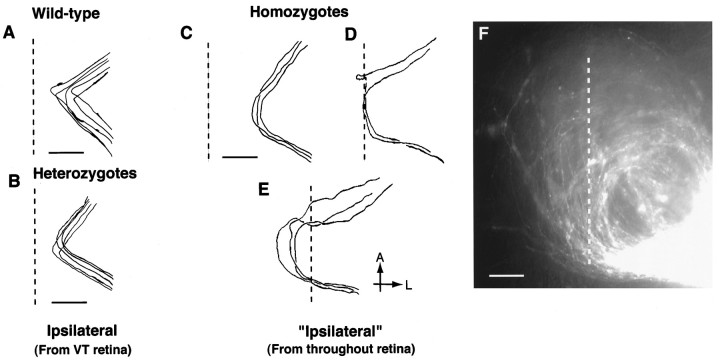
Unusual axon trajectories, including midline recrossing of contralaterally projecting axons into the ipsilateral optic tract
Although the lack of normal ipsilateral axon turning from ventral–temporal retina is highly unusual, it does not explain the randomization of ipsi–contra pathfinding in GAP-43-deficient embryos. The explanation for the overall increase in the number of ipsilaterally projecting axons in these embryos appears to lie with the fact that some RGC axons, which normally grow across the midline and become contralaterally projecting axons, instead take unusual routes within the chiasm and end up growing into the ipsilateral optic tract. In GAP-43-deficient embryos, ipsilateral axons originate from all regions of the retina and belong to three groups, based on the trajectories they take to enter the ipsilateral optic tract (Fig.6). The first group consists of axons that turn into the ipsilateral optic tract at the extreme lateral edge of the chiasm (Fig. 6C). These differ from the normal population of ipsilaterally projecting RGC axons from ventral–temporal retina in that their turns occur at greater distances from the midline (300 vs 100–200 μm) and, instead of a normal acute 90–120° turn, all turns occur in a wider arching trajectory. A second group of axons grows all the way to reach the physical midline at the chiasm and then follows the midline posteriorly until they leave to grow toward the ipsilateral optic tract (Fig. 6D). This group of axons resembles the group of contralaterally projecting axons described above (see Fig. 5E), which also grow posteriorly along the midline of the chiasm. These two groups differ in that, after growing posteriorly along the midline, one group grows into the ipsilateral optic tract whereas the other group grows into the contralateral optic tract.
Fig. 6.
Trajectories of ipsilaterally projecting RGC axons within the chiasm region of wild-type, heterozygous, and GAP-43-deficient embryos at E18 after backfilling from the optic tract. The vertical dotted line represents the midline. The eye of origin is toward the upper left in each panel; the optic tract is toward the bottom right.A, Anterior; L, lateral. Scale bars inA–E, 200 μm. A, Ipsilaterally projecting RGC axons in wild-type embryos execute a characteristic turn at 90–120° angles at a distance of 100–200 μm from the midline to grow away from the midline and enter the ipsilateral optic tract. Ipsilaterally projecting axons originate almost exclusively from ventral–temporal retina. B, Ipsilaterally projecting RGC axons in heterozygous embryos, like their wild-type counterparts, also display a characteristic turn of 90–120° at distances 100–200 μm from the midline to grow away from the midline and enter the ipsilateral optic tract. Like wild-type embryos, ipsilaterally projecting axons in heterozygous animals also originate almost exclusively from ventral–temporal retina. C, Examples of RGC axons in GAP-43-deficient embryos with unusual axon trajectories that project into the ipsilateral optic tract. Unlike ipsilaterally projecting axons in wild-type and heterozygous embryos, these “ipsilateral” axons originate from RGCs located in all retina regions and not just ventral–temporal retina. Three types of ipsilateral axon trajectories were encountered. The first is axons that grow in a wide arc and enter the ipsilateral optic tract along the most lateral edge of the chiasm at 300 μm or more from the midline.D, A second type of RGC axon enters the chiasm, encounters the midline, and runs posteriorly directly along the midline. At the posterior edge of the chiasm these axons leave the midline and grow into the ipsilateral optic tract. E, The third type of RGC axons initially crosses the midline within the anterior part of the chiasm, grows for variable distances, and then recrosses the midline a second time within the posterior part of the chiasm to head toward the ipsilateral optic tract. F, Photograph showing the semicircular trajectories of “ipsilaterally” projecting RGC axons after DiI backlabeling from the optic tract. The vertical dotted line represents the midline. Examples of RGC axons that first cross and then recross the midline a second time are shown. Scale bar, 100 μm.
During normal development and in heterozygous embryos, RGC axons that have crossed the midline never turn around and recross the midline a second time. Thus midline crossing signifies that an axon will grow into the opposite optic tract and normally identifies that particular axon as a contralaterally projecting axon. This definition, however, does not apply to GAP-43-deficient embryos in which a third group of “ipsilaterally” projecting axons are actually contralaterally projecting axons that have crossed the midline initially but then recross the midline a second time to project into the optic tract on the same side of the brain (Fig. 6E,F). Typically, axons in this category initially cross the midline in the anterior part of the chiasm. After growing for variable distances past the midline, they then gradually turn in a wide arc to cross the midline a second time in the posterior part of the chiasm to grow into the ipsilateral optic tract. Axons that cross the midline three or more times have not been observed.
DISCUSSION
The proper routing of visual information in the adult mammalian brain depends on specific RGC axon pathfinding at the optic chiasm during embryonic development. Previous studies have shown that the initial formation of the optic tract is disrupted in mouse embryos deficient in GAP-43 function (Strittmatter et al., 1995; Kruger et al., 1998), reflecting the normal requirement for cell autonomous GAP-43 function in the earliest RGC axons to progress from the optic chiasm into the optic tract (Kruger et al., 1998). Results from the present study demonstrate that, at a later stage in development, RGC axon routing at the chiasm into the ipsilateral and contralateral optic tracts is randomized completely in GAP-43-deficient embryos and is brought about by abnormalities in the routing of both normally ipsilaterally and contralaterally projecting RGC axons. The characteristic ipsilateral turning by RGC axons originating from ventral–temporal retina is lost, RGC axons from all retinal regions grow in unusual semicircular trajectories within the chiasm, and approximately one-half of normally contralaterally projecting axons grow inappropriately into the ipsilateral optic tract, in some instances doing so by turning back and recrossing the midline a second time (see summary Fig. 7). Mutations in RGC axon routing at the chiasm previously have been described and include the reduced ipsilateral projection in albino animals (for review, see Guillery, 1996) and the lack of contralaterally projecting axons in zebrafish (Karlstrom et al., 1996), Pax2 null mouse embryos (Torres et al., 1996), achiasmatic dogs (Williams et al., 1994), and achiasmatic humans (Apkarian et al., 1994). Detour zebrafish mutants have a variable phenotype in which RGC axons from one eye either project ipsilaterally or bilaterally (Karlstrom et al., 1996). The data presented here demonstrate an abnormal visual system in which information from the complete visual world as seen by each eye is sent twice into the CNS, once on each side.
Fig. 7.
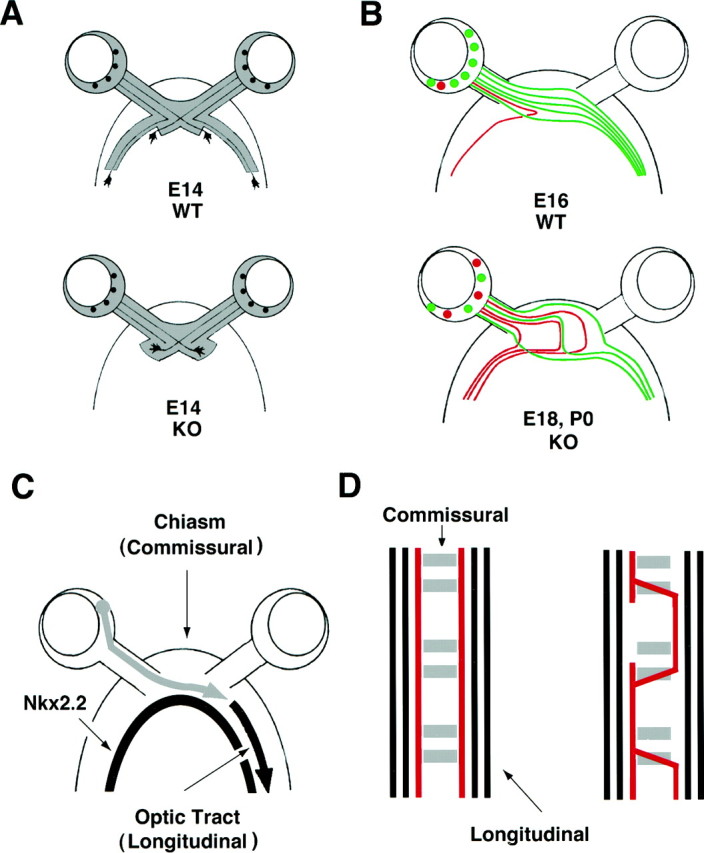
Summary figure. A, By E14 during normal development (top), RGC axons have crossed the midline region and have begun to grow into the lateral wall of the diencephalon, forming the optic tracts. In contrast, RGC axons in GAP-43-deficient embryos (bottom) fail to progress through the transition zone between the chiasm and the optic tract (for details, see Kruger et al., 1998). B, By E16 (top), RGC axons have formed a normal adult-like pattern of routing at the chiasm in which contralaterally projecting RGCs (green) located in all parts of the retina cross the midline in smooth parallel trajectories into the opposite optic tract. Ipsilaterally projecting RGCs (red) are found in ventral–temporal retina and turn away in 90–120° angles ∼100–200 μm from the midline to grow into the ipsilateral optic tract. In GAP-43-deficient embryos (bottom), axon routing at the chiasm appears to be randomized. Contralaterally (green) and ipsilaterally (red) projecting RGCs are intermixed in all regions of the retina. In addition, the normal ipsilateral turns at 90–120° angles 100–200 μm from the midline are lost. RGC axons grow in semicircular trajectories, and ∼50% of all axons project into the ipsilateral optic tract. Of note, unusual trajectories include axons that cross and then recross the midline twice, resembling axons that recross the midline in the Drosophila mutantroundabout and the C.elegans mutant sax3/robo. These trajectories are also reminiscent of axons that cross the midline instead of growing along longitudinal pathways in transgenic flies with disrupted calmodulin function. This diagram illustrates the representative types of RGC axon trajectories observed in GAP-43-deficient embryos. C, Diagram showing the optic tract as a longitudinal pathway that forms adjacent and parallel to the longitudinal stripe of Nkx2.2 expression (only the retinal pathway from one eye is shown). The initial pathfinding defect in the retinal pathway of GAP-43-deficient embryos appears to be an inability of RGC axons to grow from the optic chiasm (a commissural pathway) into the optic tract (a longitudinal pathway).D, Left, Diagram showing the organization of the Drosophila ventral CNS. Commissural axons (gray) cross the midline within the anterior or posterior commissures, whereas longitudinal axons (blackand red) do not. D, Right, Diagram showing the abnormal trajectories of longitudinal axons (red) within the midline region of transgenic flies with disrupted Ca2+/calmodulin function (after VanBerkum and Goodman, 1995). Axons in C. eleganswith mutation in the sax3/robo gene have a similar phenotype (see Discussion for details).
Lack of GAP-43 function differentially affects ventral diencephalon axon pathways
We previously found that the axons of CD44/SSEA-immunopositive neurons situated within the ventral midline region (Sretavan et al., 1994; Marcus and Mason, 1995) normally express GAP-43 (Kruger et al., 1998). The axons of these neurons, however, are not affected in GAP-43-deficient embryos and grow dorsally within the lateral wall of the diencephalon at E10–E11, whereas RGC axons arriving a few days later at E13–E14 are unable to enter the lateral diencephalic wall to form the optic tracts (Kruger et al., 1998). In the present study we find that a third group of axons originating from dorsal diencephalon, which normally projects ventrally to cross the midline posterior to the chiasm as the supra-optic commissure (SOC), also is disrupted in its growth. Given that these axons normally grow from their dorsal origin to the ventral midline, the failure to label SOC axons posterior to the chiasm must mean that they are affected in their growth somewhere within the lateral diencephalic wall. Thus, in the absence of normal GAP-43 function, both RGC axons that project dorsally and SOC axons that project ventrally are disrupted in growth within the lateral diencephalon.
SOC axon growth either may require GAP-43 function directly or else may be affected secondarily if SOC axons normally use RGC axons at the chiasm as guidance cues and are misrouted when RGC axon growth itself at the chiasm is disrupted in GAP-43-deficient embryos. However, SOC axon growth into the lateral diencephalic wall does not always parallel that of RGC axons exactly. At a time when RGC axons in GAP-43-deficient embryos have entered the tract after a 4 d delay, SOC axons still are not found in the supraoptic commissure, consistent with a direct effect of the lack of GAP-43 function on SOC axon pathfinding.
GAP-43 involvement in ipsilateral and contralateral axon pathfinding or indirect effects?
The finding of abnormal ipsilateral and contralateral axon routing at the optic chiasm of GAP-43-deficient embryos raises the possibility that GAP-43 is involved specifically in aspects of intracellular signaling that are required for proper ipsi–contra pathfinding. The lack of the characteristic turning of ipsilaterally projecting RGC axons from ventral–temporal retina in these mutant embryos is consistent with a role of GAP-43 in mediating the response of ipsilaterally projecting RGC axons to the postulated “inhibitory” signal (Wisenmann et al., 1993; Wang et al., 1995), which normally prevents them from crossing the midline region. However, abnormal RGC chiasm pathfinding in GAP-43-deficient embryos not only involves the lack of normal ipsilateral pathfinding at the chiasm. In fact, all RGC axons no longer grow across the midline chiasm region in smooth trajectories parallel to each other but instead exhibit highly unusual semicircular trajectories, including abnormal axon recrossing of the midline. Given these effects on both normally ipsilaterally and contralaterally projecting axons, it seems unlikely that GAP-43 functions specifically to instruct ipsilaterally projecting RGC axons to turn appropriately into the ipsilateral optic tract.
It is more likely that the lack of GAP-43 affects global aspects of axon pathfinding at the chiasm. One possibility is that GAP-43 plays a permissive, but not instructive, role in ipsi–contra pathfinding and is necessary for all RGC axons to grow successfully through the midline region and interact with other, more specific pathfinding cues. RGC axons on entering the ventral diencephalon encounter a change in glial composition (Reese et al., 1994), and in vitro studies suggest that ventral diencephalon tissue contains guidance cues that are inhibitory for RGC axon growth (Wang et al., 1996; Tuttle et al., 1998). One possibility is that proper axon growth through the ventral midline region requires GAP-43 to dampen the inhibitory effects of the local environment on axon growth.
However, a more parsimonious explanation is that the abnormal axon trajectories observed in the ipsi–contra pathfinding phase of chiasm development in GAP-43-deficient embryos results from axons backing up because of the initial pathfinding defect occurring laterally at the chiasm/tract transition zone. This axon “backing up” model is consistent with the initial absence of abnormal axon trajectories in the chiasm at E12–E13 as the earliest-generated RGC axons enter the midline region (Kruger et al., 1998) and with the appearance of semicircular trajectories and the recrossing of the midline only as later-generated RGC axons enter the chiasm. In effect, the abnormal RGC axon growth at the chiasm resembles a Probst bundle, a neuroma-like structure found in acallosal animals, consisting of callosal axons that fail to form the corpus callosum (for examples, see Ozaki and Shimada, 1988). Unlike Probst bundles, which result from the failure of callosal axons to cross the midline, the rounded and enlarged optic chiasm is formed by axons that have crossed the midline but are unable to grow into the lateral diencephalic wall. In this model a guidance defect at the chiasm/tract transition zone causes later-arriving RGC axons to back up and form a neuroma-like structure at the chiasm. Axons in the neuroma grow in semicircular trajectories and eventually randomly exit into the ipsilateral or contralateral optic tract.
Comparison with midline guidance defects in invertebrates
Drosophila roundabout (Seeger et al., 1993) andC. elegans sax3/robo mutants (Zallen et al., 1998) have pathfinding defects in which axons that normally extend along longitudinal pathways grow across the midline instead. Within the ventral midline region the affected axons grow in semicircular trajectories, often meandering from side to side and crossing the midline multiple times (Fig. 7D). It is unknown whether the pathfinding defects represent a failure of the mechanisms that normally prevent longitudinal axons from entering the midline region or a failure in growth along longitudinal pathways that secondarily results in axons crossing the midline. Nevertheless, the mutant axon trajectories in these animals clearly demonstrate that the junction between commissural and longitudinal pathways is a major pathfinding site.
The transition zone between the optic chiasm and the optic tract is a site at the most anterior region of the CNS where RGC axons having crossed the midline region, i.e., commissural axons, then join the optic tracts that embryologically are initiated as longitudinal pathways (Marcus et al., 1998) (see also Fig. 7C). It is somewhere at this junction between commissural and longitudinal pathways that the initial RGC axon pathfinding defect in GAP-43-deficient embryos occurs and is characterized by the failure of RGC axons to progress from the chiasm to form the optic tract (Kruger et al., 1998). In the invertebrate ventral CNS, axon growth through the commissural/longitudinal pathway junction requires a 90° turn in axon trajectory, whereas in the visual system the chiasm and the optic tract are different segments of a continuous pathway and RGC axons appear to transit smoothly from one into the other. Nevertheless, the chiasm/tract transition zone is a site in which differences exist in the degree of RGC axon fasciculation (Colello and Coleman, 1997) and glia cell morphology (Reese et al., 1994), consistent with RGC axons encountering a change in their growth environment at this site.
A comparison of invertebrate robo mutants and GAP-43-deficient mouse embryos shows that they are similar in that an axon guidance defect appears to compromise the ability of axons to extend normally along (robo, sax3/robo) or to enter (GAP-43) a longitudinal pathway. Furthermore, affected axons enter or reenter the midline region by crossing the midline more than once, a pathfinding behavior never seen during normal development. Differences, however, do exist. For instance, the multiple axon recrossings of the midline as affected axons meander from side to side, growing for significant distances within the midline region in robo andsax3/robo mutants (Kidd et al., 1998; Zallen et al., 1998), have not been observed in GAP-43-deficient mouse embryos. GAP-43-deficient RGC axons remain more or less within the confines of the optic chiasm region and do not grow posteriorly along the midline region into the median eminence.
Calmodulin function in axon guidance about the midline
The results in GAP-43 mutant mouse embryos are reminiscent of findings in transgenic flies expressing calmodulin-based protein constructs in subsets of CNS axons designed either to bind and inactivate endogenous calmodulin or to compete with endogenous calmodulin for Ca2+ and substrate binding (VanBerkum and Goodman, 1995). Axon guidance defects in these flies include axon “stalling” at specific locations in stage 14 embryos and the later appearance in stage 16 embryos of axons that abnormally cross the midline instead of extending along longitudinal pathways. These findings parallel the initial inability in E14 GAP-43-deficient mouse embryos of RGC axons to progress from the optic chiasm into the optic tract (Kruger et al., 1998) and the later appearance, beginning at E18, of RGC axons that recross the midline a second time instead of entering the longitudinal optic tract (present study). In addition, although the precise biochemical action of GAP-43 in RGC axons in vivoremains to be determined, in vitro it is known to bind calmodulin (Alexander et al., 1987; Chapman et al., 1991) and release calmodulin when GAP-43 is phosphorylated by PKC (Alexander et al., 1987; Apel et al., 1990; Chapman et al., 1991) and when palmitoylated, GAP-43 is localized to the subplasma membrane region (Skene and Virag, 1989; Zuber et al., 1989). One possibility is that GAP-43 may serve as a growth cone sensor, detecting PKC activation and causing the local release of calmodulin in the region under the growth cone plasma membrane (for review, see Skene, 1990; Benowitz and Routtenberg, 1997). If so, axon guidance around the CNS midline regions in both invertebrates and vertebrates may be conserved and may involve calmodulin function.
Footnotes
This research was supported by the That Man May See Foundation, National Institutes of Health (NIH) Grant EY 10688 (to D.S.), and NIH Grant EY 02162 to University of California San Francisco Department of Ophthalmology. D.S. is the recipient of a Jules and Doris Stein Professorship from Research to Prevent Blindness. We thank Dr. Mark Fishman for the generous gift of GAP-43 mutant mice; Angie Tam, Cindy Lu, and Chris Severin for technical assistance; Suling Wang for help with graphics; and members of the Sretavan Lab for their input on this project.
Correspondence should be addressed to Dr. David Sretavan, Beckman Vision Center, K107, University of California San Francisco, 10 Kirkham Street, San Francisco, CA 94143.
REFERENCES
- 1.Alexander KA, Cimler BM, Meier KE, Storm DR. Regulation of calmodulin binding to P-57: a neurospecific calmodulin binding protein. J Biol Chem. 1987;262:6108–6113. [PubMed] [Google Scholar]
- 2.Apel ED, Byford MF, Au D, Walsh KA, Storm DR. Identification of the protein kinase C phosphorylation site in neuromodulin. Biochemistry. 1990;29:2330–2335. doi: 10.1021/bi00461a017. [DOI] [PubMed] [Google Scholar]
- 3.Apkarian P, Bour L, Barth PG. A unique achiasmatic anomaly detected in non-albinos with misrouted retinal–fugal projections. Eur J Neurosci. 1994;6:501–507. doi: 10.1111/j.1460-9568.1994.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 4.Barth KA, Wilson SW. Expression of zebrafish nk2.2 is influenced by sonic hedgehog/vertebrate hedgehog-1 and demarcates a zone of neuronal differentiation in the embryonic forebrain. Development. 1995;121:1755–1768. doi: 10.1242/dev.121.6.1755. [DOI] [PubMed] [Google Scholar]
- 5.Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- 6.Chapman ER, Au D, Alexander KA, Nicolson TA, Storm DR. Characterization of the calmodulin binding domain of neuromodulin. J Biol Chem. 1991;266:207–213. [PubMed] [Google Scholar]
- 7.Colello RJ, Guillery RW. The early development of retinal ganglion cells with uncrossed axons in the mouse: retinal position and axon course. Development. 1990;108:515–523. doi: 10.1242/dev.108.3.515. [DOI] [PubMed] [Google Scholar]
- 8.Colello SJ, Coleman L. Changing course of growing axons in the optic chiasm of the mouse. J Comp Neurol. 1997;379:495–514. doi: 10.1002/(sici)1096-9861(19970324)379:4<495::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 9.Drager UC. Birthdates of retinal ganglion cells giving rise to the crossed and uncrossed optic projections in the mouse. Proc R Soc Lond [Biol] 1985;224:57–77. doi: 10.1098/rspb.1985.0021. [DOI] [PubMed] [Google Scholar]
- 10.Gitler MS, Barraclough CA. Identification of the hypothalamic site through which locus coeruleus axons decussate to reach and stimulate contralateral LH–RH neurons. Brain Res. 1988;447:205–214. doi: 10.1016/0006-8993(88)91121-3. [DOI] [PubMed] [Google Scholar]
- 11.Godement P, Salaun J, Mason CA. Retinal axon pathfinding in the optic chiasm: divergence of crossed and uncrossed fibers. Neuron. 1990;5:173–186. doi: 10.1016/0896-6273(90)90307-2. [DOI] [PubMed] [Google Scholar]
- 12.Guillery RW. Why do albinos and other hypopigmented mutants lack normal binocular vision, and what else is abnormal in their central visual pathways? Eye. 1996;10:217–221. doi: 10.1038/eye.1996.49. [DOI] [PubMed] [Google Scholar]
- 13.Karlstrom RO, Trowe T, Klostermann S, Baier H, Brand M, Crawford AD, Grunewald B, Haffter P, Hoffman H, Meyer SU. Zebrafish mutations affecting retinotectal axon pathfinding. Development. 1996;123:427–438. doi: 10.1242/dev.123.1.427. [DOI] [PubMed] [Google Scholar]
- 14.Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS. Roundabout controls axon crossing of the CNS midline and defines a novel family of evolutionarily conserved guidance receptors. Cell. 1998;92:205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- 15.Kruger K, Tam AS, Lu C, Sretavan DW. Retinal ganglion cell axon progression from the optic chiasm to initiate optic tract development requires cell autonomous function of GAP-43. J Neurosci. 1998;18:5692–5705. doi: 10.1523/JNEUROSCI.18-15-05692.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcus R, Mason C. The first retinal axon growth in the mouse optic chiasm: axon patterning and the cellular environment. J Neurosci. 1995;15:6389–6402. doi: 10.1523/JNEUROSCI.15-10-06389.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcus RC, Shimamura K, Sretavan D, Lai E, Rubenstein J, Mason C (1998) Domains of regulatory gene expression and the developing optic chiasm: correspondence with retinal axon paths and candidate signaling cells. J Comp Neurol, in press. [PubMed]
- 18.Martinez S, Puelles L. Avian nucleus isthmi ventralis projects to the contralateral optic tectum. Brain Res. 1989;481:181–184. doi: 10.1016/0006-8993(89)90501-5. [DOI] [PubMed] [Google Scholar]
- 19.Mason CA, Sretavan DW. Glia, neurons, and axon pathfinding during optic chiasm development. Curr Opin Neurobiol. 1997;7:647–653. doi: 10.1016/s0959-4388(97)80084-0. [DOI] [PubMed] [Google Scholar]
- 20.Ozaki HS, Shimada M. The fibers which course within Probst’s longitudinal bundle seen in the brain of a congenitally acallosal mouse: a study with horseradish peroxidase technique. Brain Res. 1988;441:5–14. doi: 10.1016/0006-8993(88)91377-7. [DOI] [PubMed] [Google Scholar]
- 21.Price M, Lazzaro D, Pohl T, Mattei M-G, Ruther U, Olivo J-C, Duboule D, DiLauro R. Regional expression of the homeobox gene Nkx-2.2 in the developing mammalian forebrain. Neuron. 1992;8:241–255. doi: 10.1016/0896-6273(92)90291-k. [DOI] [PubMed] [Google Scholar]
- 22.Reese BE, Maynard TM, Hocking DR. Glial domains and axonal reordering in the chiasmatic region of the developing ferret. J Comp Neurol. 1994;349:303–324. doi: 10.1002/cne.903490211. [DOI] [PubMed] [Google Scholar]
- 23.Seeger M, Tear G, Ferres-Marco D, Goodman C. Mutations affecting growth cone guidance in Drosophila: genes necessary for guidance toward or away from the midline. Neuron. 1993;10:409–426. doi: 10.1016/0896-6273(93)90330-t. [DOI] [PubMed] [Google Scholar]
- 24.Shimamura K, Hartigan S, Martinez S, Puelles L, Rubenstein J. Longitudinal organization of the anterior neural plate and neural tube. Development. 1995;121:3923–3933. doi: 10.1242/dev.121.12.3923. [DOI] [PubMed] [Google Scholar]
- 25.Skene JHP. GAP-43 as a “calmodulin sponge” and some implications for calcium signaling in axon terminals. Neurosci Res. 1990;13:S112–S125. doi: 10.1016/0921-8696(90)90040-a. [DOI] [PubMed] [Google Scholar]
- 26.Skene JHP, Virag I. Posttranslational membrane attachment and dynamic fatty acylation of a neuronal growth cone protein, GAP-43. J Cell Biol. 1989;108:613–624. doi: 10.1083/jcb.108.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sretavan DW. Specific routing of retinal ganglion cell axons at the mammalian optic chiasm during embryonic development. J Neurosci. 1990;10:1995–2007. doi: 10.1523/JNEUROSCI.10-06-01995.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sretavan DW, Feng L, Pure E, Reichardt LF. Embryonic neurons of the developing optic chiasm express L1 and CD44 cell surface molecules with opposing effects on retinal axon growth. Neuron. 1994;12:957–975. doi: 10.1016/0896-6273(94)90307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strittmatter SM, Valenzuela D, Kennedy TE, Neer EJ, Fishman MC. Go is a major growth cone protein subject to regulation by GAP-43. Nature. 1990;344:836–841. doi: 10.1038/344836a0. [DOI] [PubMed] [Google Scholar]
- 30.Strittmatter SM, Fankhauser C, Huang PL, Mashimo H, Fishman MC. Neuronal pathfinding is abnormal in mice lacking the neuronal growth cone protein GAP-43. Cell. 1995;80:445–452. doi: 10.1016/0092-8674(95)90495-6. [DOI] [PubMed] [Google Scholar]
- 31.Torres M, Gomez-Pardo E, Gruss P. Pax2 contributes to inner ear patterning and optic nerve trajectory. Development. 1996;122:3381–3391. doi: 10.1242/dev.122.11.3381. [DOI] [PubMed] [Google Scholar]
- 32.Tuttle R, Braisted JE, Richards LJ, O’Leary DDM. Retinal axon guidance by region-specific cues in diencephalon. Development. 1998;125:791–801. doi: 10.1242/dev.125.5.791. [DOI] [PubMed] [Google Scholar]
- 33.VanBerkum MFA, Goodman CS. Targeted disruption of Ca2+/calmodulin signaling in Drosophila growth cones leads to stalls in axon extension and errors in axon guidance. Neuron. 1995;14:43–56. doi: 10.1016/0896-6273(95)90239-2. [DOI] [PubMed] [Google Scholar]
- 34.Wang L-C, Dani J, Godement P, Marcus R, Mason CA. Crossed and uncrossed retinal axons respond differently to cells of the optic chiasm midline in vitro. Neuron. 1995;15:1349–1364. doi: 10.1016/0896-6273(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 35.Wang L-C, Rachel RA, Marcus R, Mason CA. Chemosuppression of retinal axon growth by the mouse optic chiasm. Neuron. 1996;17:849–862. doi: 10.1016/s0896-6273(00)80217-2. [DOI] [PubMed] [Google Scholar]
- 36.Williams RW, Hogan D, Garraghty PE. Target recognition and visual maps in the thalamus of achiasmatic dogs. Nature. 1994;367:637–639. doi: 10.1038/367637a0. [DOI] [PubMed] [Google Scholar]
- 37.Wisenmann A, Thanos S, Boxberg YV, Bonhoeffer F. Differential reaction of crossing and non-crossing rat retinal axons on membrane preparations from the chiasm midline: an in vitro study. Development. 1993;117:725–735. doi: 10.1242/dev.117.2.725. [DOI] [PubMed] [Google Scholar]
- 38.Zallen JA, Yi BA, Bargmann CI. The conserved immunoglobulin superfamily member sax-3/robo directs multiple aspects of axon guidance in C. elegans. Cell. 1998;92:217–227. doi: 10.1016/s0092-8674(00)80916-2. [DOI] [PubMed] [Google Scholar]
- 39.Zuber MX, Strittmatter SM, Fishman MC. A membrane-targeting signal in the amino terminus of the neuronal protein GAP-43. Nature. 1989;341:345–348. doi: 10.1038/341345a0. [DOI] [PubMed] [Google Scholar]