Abstract
LIS-1 is a gene whose hemi-deletion causes the human neuronal migration disorder Miller–Dieker lissencephaly. It encodes a subunit of a brain platelet-activating factor (PAF) acetylhydrolase, an enzyme that inactivates PAF by hydrolyzing the acetyl moiety in the sn2 position of this phospholipid. Because PAF receptor activation has been shown to affect the developing neuronal cytoskeleton, we have hypothesized that a role for PAF in neurodevelopment is that of a modulator of neuroblast movement (a cytoskeletal function) and that an aberrant regulation of PAF could lead to an early arrest in migration. This report examines the effects of the nonhydrolyzable PAF receptor agonist methyl carbamyl PAF (mc-PAF) on the unidirectional in vitro migration of granule cells from cerebellar cell reaggregates on a laminin substrate. Bath treatment with mc-PAF yields a dose-dependent decrease in granule cell migration compared with controls. This effect can be blocked by the simultaneous bath application of BN 52021 andtrans-BTD, PAF receptor-specific antagonists. Although mc-PAF minimally inhibited neurite growth, its primary effect was on somal movement along preextended neurites. These experiments suggest that the stimulation of neuronal PAF receptors could be one crucial step for the regulation of neuroblast migration and that disturbed PAF catabolism during neurodevelopment could contribute to the neuronal migration defects observed in Miller–Dieker lissencephaly.
Keywords: methyl carbamyl platelet-activating factor (mc-PAF), BN 52021, platelet-activating factor (PAF), neuronal migration, Miller–Dieker lissencephaly, LIS-1, PAF acetylhydrolase, PAF receptor
Platelet-activating factor 1-0-alkyl-2-acetyl-sn-glyceryl-3 phosphorylcholine (PAF) is an alkyl-ether phospholipid with probable important roles in the CNS (Kornecki et al., 1984; Birkle et al., 1988;Kornecki and Ehrlich, 1988; Kumar et al., 1988; Del-Cerro et al., 1990;Arai and Lynch, 1992; Clark et al., 1992; Kato et al., 1994). Brain contains enzymes that produce (Bazan et al., 1983; Yue et al., 1990;Baker and Chang, 1993, 1997; Bonoventre and Koroshetz, 1993) and degrade PAF (Hattori et al., 1993). One brain PAF receptor has been characterized (Bito et al., 1992, 1993).
PAF has been implicated in the human neuronal migration disorder Miller–Dieker lissencephaly, a disorder in which neurons are arrested in the migration that forms the cerebral cortical plate and the cerebellar internal granule cell layer (IGL) (Stewart et al., 1975;Jellinger and Rett, 1976; Barth, 1987). In this disorder, the brain has a smooth cortical surface (lissencephaly) caused by a lack of complexity of the outer cortex. Additionally, the brains of these patients manifest a disruption of migration of cerebellar granule cells (Miller, 1963; Stewart et al., 1975). This brain malformation results from a haploinsufficiency of the gene LIS-1 (Ledbetter et al., 1992; Reiner et al., 1993; Mizuguchi et al., 1995; Chong et al., 1997; Ho et al., 1997; Lo Nigro et al., 1997), which encodes a 45 kDa subunit of a brain PAF acetylhydrolase (PAF-AH 1b), an enzyme that converts PAF to the inactive lyso-PAF by removing the acetyl group on the sn2 position of the PAF molecule (Hattori et al., 1993;Hattori et al., 1994). The LIS-1 gene haploinsufficiency in Miller–Dieker lissencephaly could result in defects in PAF catabolism.
Because PAF receptor activation has been shown previously to evoke changes in the neuronal cytoskeleton leading to growth cone collapse and neurite withdrawal, it has been suggested that similar PAF receptor-mediated changes in the neuroblast cytoskeleton could lead to a disruption of neuronal migration (Clark et al., 1995). This possible mechanism was probed using a well established in vitro model of cerebellar granule cell migration from reaggregate cell clusters (Asou et al., 1992). The migration of cerebellar granule cells is well characterized (Hatten and Mason, 1990) and often used for the study of neuronal migration. The neuronal migration from reaggregate clusters, although similar to migration on glia, minimizes the influence of glia on migration because the processes of glial cells contained within the reaggregate clusters do not serve as a migration substrate for the moving granule cells. Furthermore, unlike the in vitromigration on glia, granule cell migration from reaggregate clusters was unidirectional—radially away from the cluster margins. Initially, some neurons extended processes out from the cluster margin on a supportive extracellular matrix. Then granule cells migrated solely away from the margin of the reaggregate cluster along the preextended neuronal processes. They did so by first extending a leading process and then undergoing somal translocation through this leading process. This simple cell reaggregate system allowed for the testing of the effects of PAF receptor agonists and antagonists on large numbers of migrating granule cells and allowed for the quantification of the unidirectional movement of these cells (Edmondson et al., 1987).
MATERIALS AND METHODS
Materials. Minimal essential medium (MEM) and Leibovitz’s L-15 medium for cell cultures were obtained from Life Technologies (Grand Island, NY). Albumin, papain, trypan blue, methanol, and dimethyl sulfoxide (DMSO) were obtained from Sigma (St. Louis, MO). Methyl carbamyl platelet-activating factor 1-0-hexadecyl-2-0-(methyl carbamyl)-sn-glycero-3-phosphorylcholine (mc-PAF) and lyso-PAF (1-0-hexadecyl-sn-glycero-3 phosphorylcholine) were obtained from Cayman Chemical Company (Ann Arbor, MI). BN 52021,trans-2,5-bis-{3,4,5-trimethoxyphenyl}-1,3-dioxolane (trans-BTD), andcis-2,5-bis-{3,4,5-trimethoxyphenyl}-1,3-dioxolane (cis-BTD) were obtained from Biomol (Plymouth Meeting, PA). Laminin, purified from mouse EHS sarcoma, was obtained from Sigma. Glial fibrillary acidic protein (GFAP) antibody was obtained from Dako Antibody (Carpinteria, CA). TUJ-1 antibody [highly reactive with neuron-specific class III β tubulin but not with β tubulin in glia (Lee et al., 1990; Moody et al., 1996)] was obtained from Babco Antibody (Berkeley, CA).
Rat cerebellar granule cell reaggregate cultures. Cerebella were obtained from 8-d-old rats (Harlan Sprague Dawley, Indianapolis, IN) anesthetized with methoxyflurane (Metofane, Pitman-Moore, Mundelein, IL). The cerebellar tissue was digested with papain and then triturated as described previously (Clark et al., 1995). The dissociated cells from a single rat cerebellum were placed in a sterile 50 ml polystyrene centrifuge tube and incubated overnight (without rotation) at 37°C in an atmosphere of 5% C02 in 20 ml of MEM containing 10% horse serum, 10% fetal calf serum, 6 mm glucose, 200 μml-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, 10 μg/ml human transferrin, 10 μg/ml insulin, and 10 ng/ml selenium. The cap on the cell-containing tube was loosened to allow the cells to aerate. This overnight incubation allowed cellular reaggregates to form (Kobayashi et al., 1995). On the next day, 2 ml aliquots of this cell reaggregate suspension were added to 35 × 10 mm tissue culture dishes (Falcon) coated with laminin for 24 hr at 37°C (25 μg/ml, 2 ml per dish). The laminin solution was removed, and the dishes were rinsed once with deionized water immediately before cell plating. The plated cells were returned to the 37°C incubator for 1 hr to allow for the reaggregate cell clusters to adhere to the laminin substrate but not to allow sufficient time for neurite outgrowth, which was slowed by the presence of serum in the media (Liesi, 1992).
Experimental design. Specific concentrations of mc-PAF (0.10, 0.25, 0.50, and 1.00 μm), BN 52021 (50 and 100 μm), cis-BTD (50 μm),trans-BTD (50 μm), lyso-PAF (1 μm), DMSO, and methanol were prepared in serum-free media (identical to the media described above without the horse serum and fetal calf serum). The serum-containing media present in the tissue culture dishes (containing the reaggregate cell clusters) was removed and replaced with 2 ml aliquots of serum-free media containing the experimental compounds (one aliquot of one experimental compound per dish, or a combination of compounds, i.e., mc-PAF and BN 52021, mc-PAF and cis-BTD, and mc-PAF and trans-BTD). Some cells (controls) were treated with serum-free media containing no experimental compound. The cells were returned to the 37°C incubator and removed for observation after 1, 6, 12, and 24 hr. No detectable decrease in the bioactivity of these compounds [as measured by rabbit platelet aggregation (for details, see Clark et al., 1995)] was noted after 24 hr of cell incubation (data not shown).
Measurements of neurite properties and granule cell migration. Reaggregate cell clusters were observed and photographed under a phase contrast microscope (Olympus). After 1 hr of drug incubation, 12 cell clusters (typically each dish contained ∼30 clusters) of similar size (100–120 μm in diameter) were randomly selected from each dish to make measurements. Clusters of similar size (containing approximately the same number of cells) were selected to minimize the possibility that any calculated change in the number of neurites or migrating neurons might be caused by differences in the number of cells in each cluster. Selected cell clusters were also required to be farther than 600 μm away from other clusters or the neurites of other clusters so that the single cell cluster source of neurons and neurites could be clearly identified. After 24 hr of drug incubation, the cells were fixed with 4% paraformaldehyde, and the distribution of granule cells (migrated granule cells) and the lengths of neurite extension beyond the cluster margin were measured. Some experiments were conducted with the examiner blinded to the drug treatment conditions for the cell clusters. All data were statistically analyzed (two-tailed t test) for significance with SigmaStat (version 1.0) for Windows (Jandel Scientific, San Rafael, CA). In other experiments, the number of neurites elaborated from each selected cell cluster (in the various drug conditions), as identified by indirect immunofluorescence with TUJ-1 antibody, was recorded at 1, 3, 6, and 12 hr. Time lapse photography was performed for individual cell clusters using a Bioptechs Δ T culture dish system to maintain a constant temperature of 36°C. Video time lapse images were recorded with a CCD video camera (DAGE-MTI, Michigan City, IN) and Metamorph imaging software version 2.0 for Windows (Universal Imaging Corporation, West Chester, PA). Cell clusters for these experiments were plated (in a similar serum-free media containing a HEPES buffer instead of HCO3) on laminin-coated (in the same manner as described above), 0.5-mm-thick glass-bottomed Δ T dishes that were sealed with a coverslip.
GFAP and TUJ-1 indirect immunofluorescence. Reaggregate cell clusters were cultured on laminin-coated glass coverslips placed in 35 × 10 mm culture dishes. The cells were washed once with PBS and then fixed for 30 min with 4% paraformaldehyde. The cells were blocked and permeabilized for 30 min with 10% goat serum and 0.1% Triton X-100 in PBS. The primary antibody was applied [1:1000 GFAP antibody (polyclonal), 1:500 TUJ-1 antibody (monoclonal)] to the cells for 2 hr followed by three washes with PBS. The cells were again permeabilized for 1 hr with 0.1% Triton X-100 in PBS. The appropriate secondary antibody (fluorescein or rhodamine conjugated, 1:100) was next applied for 2 hr followed by three washes with PBS. The coverslips were then mounted on glass slides with Vectashield (Vector Laboratories, Burlingame, CA) and observed for fluorescence with a Nikon Microphot-FXA microscope equipped with a UV light source.
Trypan blue cell exclusion assay. A 0.2% trypan blue solution was prepared in a saline solution containing (in mm) 150 NaCl, 5 CaCl2, 2.5 KCl, 1 MgCl2, 10 glucose, and 10 HEPES, pH 7.3, 290–310 mOsm. The trypan blue solution was warmed to 37°C and then gently added to the cultured cells. After 30 min of cell incubation at 37°C, the trypan blue solution was removed from the cells, which were subsequently fixed with 4% paraformaldehyde and observed the same day for the incorporation of trypan blue into the cells (indicating cell death).
RESULTS
Within the first hour of plating, cerebellar reaggregate clusters appeared as a sphere of similar cells that adhered to the laminin substrate coating the dish bottom. Minutes after the serum-containing media in the dish was replaced with serum-free media (serum-free media control), cell process extension was observed. In the ensuing 5 hr, more processes extended beyond the margin of the clusters. Many of these processes formed bundles, whereas a few remained as individual, discrete processes. TUJ-1 immunofluorescence confirmed that they were exclusively neurites (Fig. 1). Time-lapse observation of the clusters under the microscope with strict temperature control (36°C) revealed that small bipolar cells migrated unidirectionally away from the cell clusters along these preextended neurites and neurite bundles (Figs. 1, 2; see Fig. 7). The neuronal identity of these cells was confirmed with TUJ-1 staining (Fig. 1). These cells were deemed to be cerebellar granule cells, rather than stellate or basket cells, on the basis of size [granule cells were ∼5–7 μm in diameter (15 μm polarized ends), a well characterized size for granule cells in vivo(Altman, 1972) and in vitro (Gregory et al., 1988) compared with stellate and basket cells, which were 7–12 μm], and shape [granule cells were bipolar, with two long small neurites elaborated in opposite directions, a well characterized feature of granule cellsin vitro (Selak et al., 1985; Rivas and Hatten, 1995) (Figs.1, 2, 3; see Fig. 7) and in vivo (Altman, 1972), whereas stellate and basket cells were elliptical].
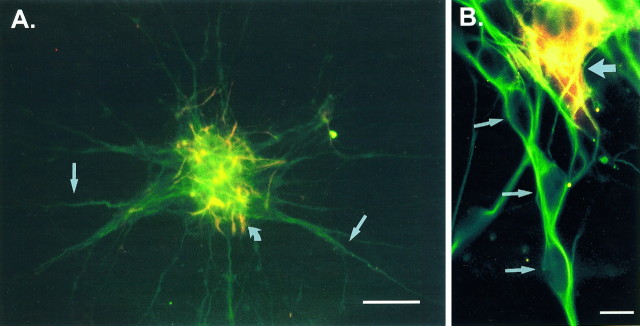
Fig. 1.
Characterization of cellular composition of and granule cell migration from cerebellar reaggregate clusters.A shows an image (40× magnification) of a cerebellar reaggregate cluster immunolabeled with the neuronal cell marker anti-TUJ-1 (neuronal class III β tubulin-specific marker) antibody (green) and with a glial cell marker anti-GFAP antibody (red/yellow; curved arrowindicates a glial cell). Note that the glia present are localized almost exclusively to the reaggregate core, and their processes do not contribute to the processes elaborated from the cluster (green, neuritic processes, straight arrows). Scale bar, 50 μm. B shows three granule cells (small arrows, 100× magnification, immunolabeled in the same manner as in A) migrating away from a reaggregate cluster along a neurite bundle. Note the nearbyred/yellow glial cell (large arrow) that does not contribute any processes as migration substrate for the granule cells. Granule cell migration from the reaggregate clusters occurs along preextended neurites (homotypic migration). Scale bar, 10 μm.
Fig. 2.
Time-lapse characterization of granule cell migration from a cerebellar reaggregate cell cluster. Aand B are images of the same reaggregate cell cluster (in serum-free media at 36°C plated on laminin) taken 1 hr apart. Scale bar, 50 μm. C–E are magnified images taken every 20 min of the reaggregate cell cluster area outlined inA and B. The arrow inC indicates the barely visible soma of a granule cell that is extending a process (indicated by arrowheads) out along another preextended neurite to migrate. This cell can be seen to migrate away from the cluster in D and more so inE. Immediately after images B andE were taken, the serum-free bathing solution was bath-exchanged with one containing 1 μm mc-PAF, and imageF was taken 20 min later. Note the slight rounding of the granule cell. The granule cell has not moved appreciably from its position in E. Other experiments that were performed (Fig. 7) determined that the mc-PAF-treated granule cells migrated again after removal of mc-PAF. Scale bar, 15 μm.
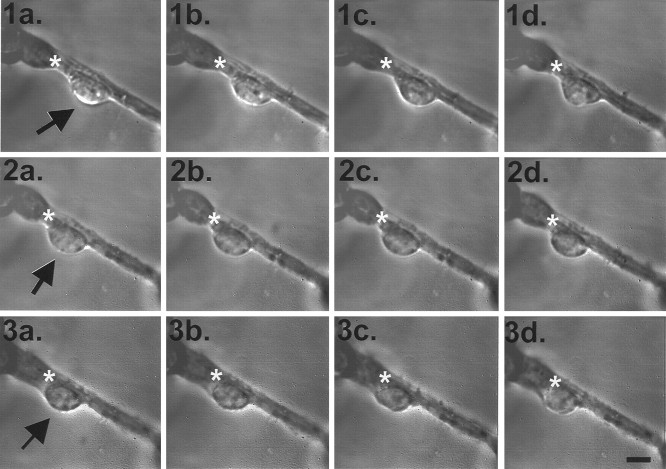
Fig. 7.
mc-PAF disrupts previously initiated granule cell migration observed with time-lapse IR-DIC video microscopy.1a–d, Video time-lapse images taken every 2 min of the same granule cell (arrow) migrating on a bundle of neurites at 36°C in vitro. Immediately after image1d was taken, the bathing solution was exchanged with 1 μm mc-PAF in serum-free media (at 36°C), and image2a was taken 10 sec later. Note the slight rounding of the soma of the cell in 2a–d (images taken every 15 min of the same granule cell in 1 μm mc-PAF conditions) as compared with before mc-PAF exposure in 1a–d. The cell appears to remain stationary for the entire hour. The mc-PAF was then removed with three bath exchanges of serum-free media (at 36°C) immediately after image 2d was taken, and image3a was taken 8 min later. 3a–d images (taken every 8 min) appear to show that the granule cell resumes migration after mc-PAF removal. An asterisk in each image denotes the same fixed location. Scale bar (shown in3d): 5 μm.
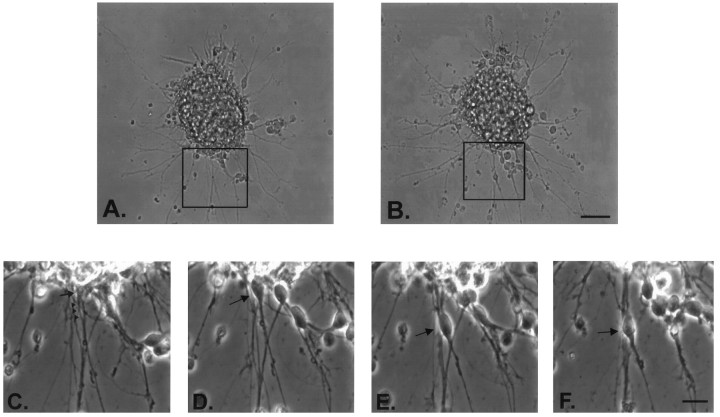
Fig. 3.
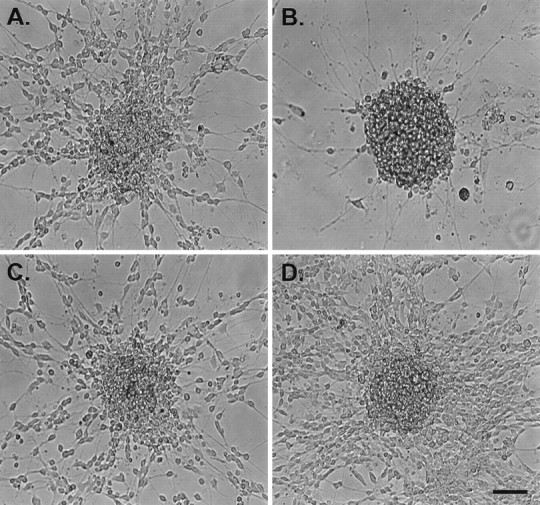
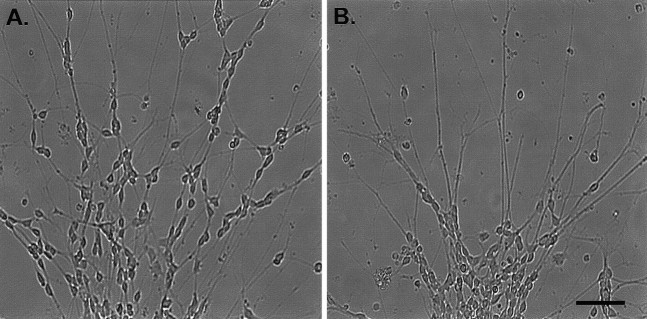
Effects of mc-PAF and BN 52021 on the migration of granule cells from reaggregate cell clusters. The above images show typical cerebellar reaggregate cell clusters plated on a laminin substrate 24 hr after drug incubation. These and all cell clusters used for data collection were selected for similar initial cell number contained in the cluster and for distance away from other clusters or their processes (at least 600 μm) to clearly identify the single cell cluster source of neurons and neurites. A shows a cluster treated with serum-free media alone (control). A substantial number of granule cells have elaborated processes and migrated away from the cluster. B shows a cluster treated with 1 μm mc-PAF in serum-free media. Many neurites can be seen extending from the cluster, but very few cells have migrated.C shows a cluster treated with 1 μm mc-PAF and 50 μm BN 52021 in serum-free media. The disruptive effect of mc-PAF on migration is inhibited. D shows a cluster treated with 100 μm BN 52021 in serum-free media. Many more cells have migrated than in control conditions shown inA. Scale bar, 50 μm.
Migration of granule cells from reaggregate clusters involved the extension of processes along other preextended neurites (TUJ-1 positive, GFAP negative) and then over time, the translocation of their cell bodies outside of the reaggregate cell cluster margin. Higher magnification, time lapse, infrared differential–interference contrast (IR-DIC) video microscopy of these migrating neurons revealed that they migrated by somal translocation through a leading process (see Fig. 7). In addition to granule cells, a small number of basket and stellate cerebellar interneurons (<10% of the total number of cells that migrated) could be seen (Fig. 3A) to have migrated. These cells were not GFAP positive and were not used for granule cell migration calculations.
The reaggregate cluster method used in this study involved neuronal migration along preextended neurites (TUJ-1 positive) elaborated on a defined laminin substrate. GFAP immunofluorescence labeled some cells within the reaggregate cluster (average number of GFAP-positive cells per cluster = 15.1 ± 0.8; n = 12), but none of the processes elaborated from the cluster that served as a neuronal migration substrate (Fig. 1A). Therefore, the homotypic migration of granule cells in this system was confirmed to be similar to that described in other in vitro cerebellar systems such as cerebellar microexplant cultures (Nagata et al., 1990).
Inhibition of granule cell migration by mc-PAF (Fig. 3B)
We examined the long-term effects of PAF receptor activation on neuronal migration using a nonhydrolyzable PAF receptor agonist, mc-PAF, which is not subject to the ubiquitous PAF acetylhydrolases present in CNS tissue in vitro (O’Flaherty et al., 1987;Clark et al., 1995). mc-PAF exposure altered the expected migration of granule cells from the reaggregate cell clusters. Figure 3Billustrates a typical cell cluster treated with 1 μmmc-PAF for 24 hr. This figure reveals a dramatic decrease in the number of granule cells that migrated from the cell cluster compared with the cluster shown in Figure 3A (serum-free media control after 24 hr). mc-PAF caused a significant dose-dependent decrease in the mean total number of granule cells that migrated (78.1 ± 2.7 cells in 500 nm mc-PAF vs 256.6 ± 1.9 cells in control conditions) (Table 1). In addition, the mean distance of migration from the cluster margin measured after 24 hr of mc-PAF incubation also demonstrated a dose-dependent decrease at three mc-PAF concentrations (119.3 ± 0.6 μm in control conditions vs 72.8 ± 0.4 μm in 500 nm mc-PAF). No significant effect was noted at 100 nm mc-PAF (Table1).
Table 1.
mc-PAF effects on measured migration parameters
| Drug condition | Mean total number of migrated cells | Mean distance of migration (μm) | Mean neurite length (μm) | Percentage migration potential fulfilled |
|---|---|---|---|---|
| Control | 256.6 ± 1.9 | 119.3 ± 0.6 | 332.9 ± 1.5 | 35.8 ± 0.2 |
| Methanol drug vehicle | 263.2 ± 2.6 | 118.8 ± 0.6 | ||
| 1 μmlyso-PAF | 255.1 ± 0.7 | 121.7 ± 0.8 | ||
| 1 μmmc-PAF | *45.0 ± 1.5 | *60.1 ± 0.5 | *324.0 ± 1.1 | *18.5 ± 0.1 |
| 1 μm mc-PAF blinded | *46.8 ± 1.9 | *55.5 ± 0.8 | *320.7 ± 1.9 | *14.6 ± 0.7 |
| 500 nm mc-PAF | *78.1 ± 2.7 | *72.8 ± 0.4 | 329.7 ± 1.4 | |
| 250 nmmc-PAF | *132.3 ± 1.7 | *79.4 ± 0.8 | 331.4 ± 1.1 | |
| 100 nm mc-PAF | 254.8 ± 1.7 | 118.7 ± 1.9 | 333.5 ± 0.8 |
mc-PAF caused a significant dose-dependent decrease in the mean total number of cells that migrate (p < 0.001), the mean distance of migration (p < 0.001), and the mean neurite length (p < 0.001) compared with serum-free media controls. The percentage migration potential fulfilled was also significantly less for 1 μmmc-PAF-treated clusters than controls (p < 0.0001; see Results for calculation); 100 nm mc-PAF had no significant effect on cell migration (p = not significant). Methanol (mc-PAF drug vehicle) and lyso-PAF (inactive PAF-like compound) had no significant effect on granule cell migration. Numbers are ± SE. Asterisk indicates values significantly different from the corresponding serum-free media control value.
Experiments in which the investigator was blinded to the drug conditions yielded a mean total number of migrated cells (46.8 ± 1.9) and a mean migration distance (55.5 ± 0.8 μm) for 1 μm mc-PAF-treated cell clusters (n = 12) that were significantly different from blinded serum-free media controls (256.6 ± 1.9 cells and 119.3 ± 0.6 μm mean migration distance) (Table 1) but not from unblinded 1 μmmc-PAF experiments (45.0 ± 1.5 cells migrated and 60.1 ± 0.5 mean migration distance).
Granule cell migration from the cell clusters was not significantly affected by treatment with methanol alone, indicating that the drug vehicle used for mc-PAF was not responsible for the changes in migration. Also, lyso-PAF, which is structurally similar to PAF and mc-PAF but with little PAF receptor activity, did not have any effects on neuronal migration at a concentration of 1 μm(Fig. 4, Table 1). This suggests that mc-PAF effects were receptor-specific. Trypan blue cell exclusion assays revealed no significant differences in the number of dead neurons after 24 hr of 1 μm mc-PAF treatment (2.3 ± 0.1 trypan blue-labeled cells per cell cluster; n = 48) and 24 hr of serum-free media treatment (2.2 ± 0.2 trypan blue-labeled cells per cell cluster; n = 48). Therefore, mc-PAF did not affect neuronal migration by killing cells. Finally, few cells were detached from the laminin substrate in any of the drug conditions used, and no differences could be seen between 1 μm mc-PAF-treated dishes and serum-free media controls.
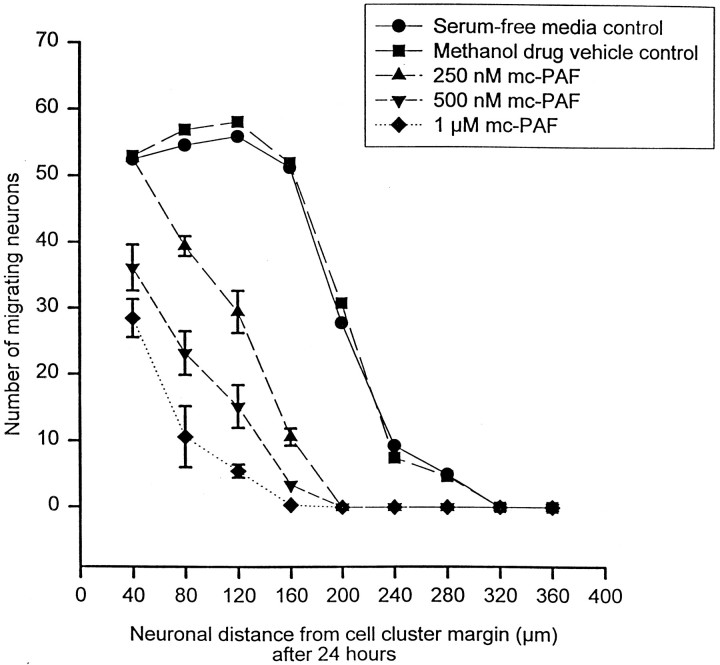
Fig. 4.
mc-PAF inhibits cerebellar granule cell migrationin vitro. The graph illustrates the number of cerebellar granule cells that have migrated and the distance of migration from their reaggregate cell cluster source 24 hr after incubation in serum-free media with 250 nm, 500 nm, and 1 μm mc-PAF, methanol (the drug vehicle for mc-PAF, at the final concentration for 1 μmmc-PAF), or serum-free media control (n = 48 for each condition) at 37°C and 5% CO2. Error bars indicate SE and were removed from several points above for clarity. SEs fromleft to right: serum-free media 2.3, 3.0, 2.6, 1.0, 2.8, 3.1, 3.3, 0.0, 0.0; methanol drug vehicle control 1.4, 1.2, 2.3, 2.0, 4.0, 1.4, 1.3, 0.0, 0.0; 250 nm mc-PAF 3.5, 3.3, 3.2, 2.7, 0.0, 0.0, 0.0, 0.0, 0.0; 500 nm mc-PAF 3.5, 3.3, 3.2, 2.7, 0.0, 0.0, 0.0, 0.0, 0.0; 1 μm mc-PAF, all shown.
Effects of mc-PAF on the migration substrate
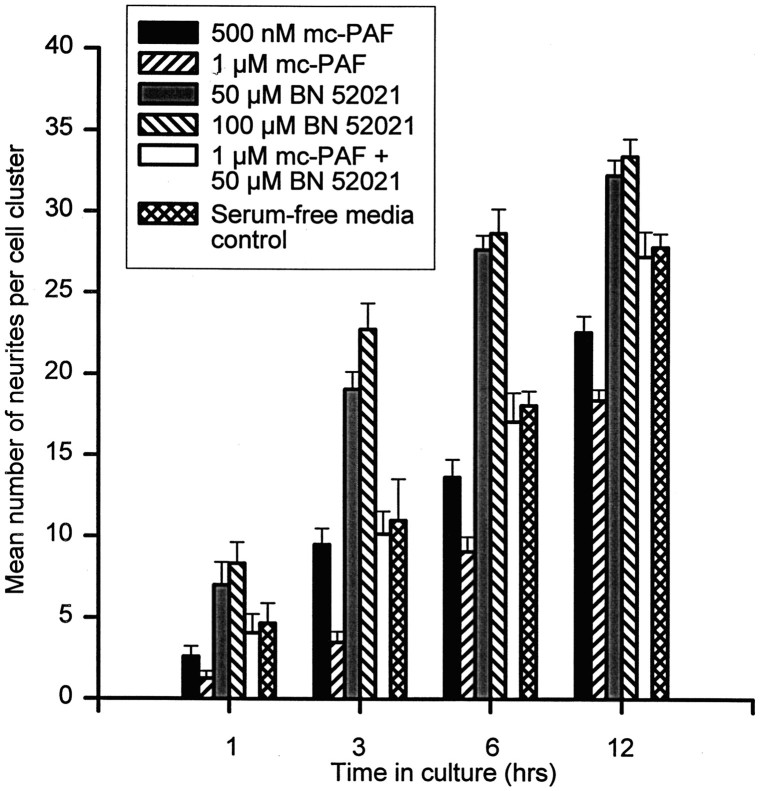
Significant effects of mc-PAF on neurites have been reported (Clark et al., 1995). Therefore, an effect of mc-PAF on the neurite migration substrate in this study could have contributed to the observed inhibition of cell movement. mc-PAF decreased the overall mean number of neurites elaborated (TUJ-1 positive) in a dose-dependent manner during the first 12 hr of cell cluster incubation (Fig.5). The mean number of neurites elaborated from 1 μm mc-PAF-treated clusters was significantly lower than in serum-free media controls (p < 0.001) at all times measured, whereas the mean number of neurites elaborated from 500 nmmc-PAF-treated clusters (Fig. 5) was only significantly different from serum-free media controls after 6 and 12 hr of incubation; 250 nm mc-PAF treatment had no significant effect on neurite number at any time. Measurements of neurite number were not attempted after 12 hr of drug incubation, because after that time larger numbers of migrating neurons in non-mc-PAF conditions tended to obscure individual neurites. However, mc-PAF-treated clusters appeared to have fewer neurites at this time compared with serum-free media-treated controls (Fig. 3A,B).
Fig. 5.
PAF receptor activation causes a dose-dependent decrease in mean neurite number, whereas PAF receptor blockade causes a dose-dependent increase in mean neurite number. Thegraph illustrates the mean number of neurites elaborated from reaggregate granule cell clusters measured 1, 3, 6, and 12 hr after incubation with 500 nm mc-PAF, 1 μmmc-PAF, 50 μm BN 52021, 100 μm BN 52021, 1 μm mc-PAF, and 50 μm BN 52021, or serum-free media control (n = 48 for each condition) at 37°C and 5% CO2. Mean neurite counts were significantly lower than serum-free media control for 500 nm mc-PAF at t = 6 and 12 hr (p < 0.0001). All mean neurite counts for 1 μm mc-PAF were significantly lower than controls (p < 0.0001). Mean neurite counts for 50 and 100 μm BN 52021 were significantly higher than controls (p < 0.0001) att = 3, 6, and 12 hr.
It is also possible that mc-PAF could have altered migration by affecting the fasciculation of neurites. It has been shown in cerebellar microexplants that the disruption of neurite fasciculation with anti-L1 or anti-NCAM antibodies increased the percentage of neurons that migrated away from the explant core (Fischer et al., 1986). In the present study, there was no significant difference in the number of fasciculated TUJ-1-positive neurites of 1 μmmc-PAF or serum-free media treatment (67.5 ± 0.7% of the total number of neurites in mc-PAF clusters were fasciculated,n = 12 clusters, all neurites counted,p = NS; 68.9 ± 0.8% fasciculated neurites in control clusters, n = 12 clusters, all neurites counted). Additionally, no significant differences were found in the mean number of neurites per neurite bundle after 12 hr (3.4 ± 0.7 for 1 μm mc-PAF treated, 3.5 ± 0.2 for serum-free media controls, p = NS; one similarly positioned neurite bundle analyzed per cluster, n = 48 per drug condition). Therefore, no significant effects were observed on the characteristics of the neurite bundles.
At high concentrations, mc-PAF had a small but significant effect on the mean neurite length beyond the cluster margin (Table 1) compared with the mean neurite length in serum-free media controls after 24 hr. Blinded experiments yielded a mean neurite length beyond the cluster margin in 1 μm mc-PAF-treated clusters that was also significantly different from that of serum-free media controls (Table1). However, 100, 250, and 500 nm mc-PAF had no significant effect on the mean neurite length beyond the cluster margin (Table 1) compared with serum-free media controls, although significant effects on migration were noted at 250 and 500 nm mc-PAF. Therefore, mc-PAF effects (at concentrations below 1 μm) on the mean distance of granule cell migration cannot be attributed to shorter neurites.
The decrease in neurite number and to a lesser extent the decrease in mean neurite length (for 1 μm mc-PAF only) limited the amount of migration substrate for the granule cells and therefore could account for some of the decrease in the number of cells that migrated from mc-PAF-treated cell clusters after 24 hr (Figs. 3B, 4, Table 1). However, such a mechanism is insufficient to account for the difference calculated in the mean number of migrating neurons found per neurite bundle after 24 hr (3.1 ± 0.7 for 1 μmmc-PAF treated vs 10.6 ± 0.5 for serum-free media controls;p < 0.001; n = 48 per drug condition). Stated differently, when presented with similar neurite migration substrate, fewer granule cells migrated on this substrate in 1 μm mc-PAF conditions than in control conditions.
To determine whether the difference in mean migration distance induced with 1 μm mc-PAF treatment could be entirely accounted for by effects on neurite length, it was hypothesized that the potential range of somal migration was limited by the length of the preextended neurites elaborated from the cell cluster that the granule cells migrated on. Therefore, a percentage of migration potential fulfilled by each cluster after 24 hr in serum-free media and 1 μm mc-PAF treatment was calculated with the following equation: % of migration potential fulfilled at 24 hr = (mean cell migration distance at 24 hr)/(mean neurite length beyond the cluster margin at 24 hr) × 100. If 1 μm mc-PAF did not affect migration but only decreased the mean neurite length, then a larger percentage of migration fulfillment would be expected. If 1 μm mc-PAF had affected primarily migration along the neurite, then a smaller percentage of migration potential fulfillment was expected. This calculation was independent of the number of neurites elaborated from a cell cluster and the total number of cells that migrated. It revealed very significant differences between the mean percentage for 1 μm mc-PAF-treated clusters (18.5 ± 0.1% fulfillment) and serum-free media-treated control clusters (35.8 ± 0.2% fulfillment; p < 0.0001) (Table 1). Thus, there was a twofold decrease in mean migration distance (Table 1), whereas 1 μm mc-PAF reduced mean neurite length beyond the cluster margin only 2.5%. Therefore, reduced neurite length was not likely to be the main mechanism for 1 μm mc-PAF disruption of migration. Furthermore, it seemed unlikely that under 1 μm mc-PAF conditions a decrease in mean neurite length of 8.9 μm could explain a decrease in mean distance of migration of 59.2 μm.
Dynamic studies of mc-PAF effects on granule cell motility
To determine whether mc-PAF had more direct effects on the somal movement of migration, two different sets of experiments were performed. In the first set of experiments, cell clusters were treated with 1 μm mc-PAF 6 hr after process extension and cell migration had begun. Before mc-PAF addition, some neurons were observed to have migrated 70–80 μm from the cell cluster edge. Eighteen hours after mc-PAF addition (24 hr after serum-free media exchange), the mean length of neurite extension beyond the cluster margin for mc-PAF-treated clusters (330.3 ± 1.6 μm; p = NS) was not significantly different from the serum-free media control (332.9 ± 1.5 μm) (Table 1). Under 1 μm mc-PAF conditions, however, few neurons were found to have migrated beyond 80 μm from the cluster, and the mean distance of migration for these cells (29.8 ± 0.6 μm; p < 0.0001) was significantly different from that of serum-free media controls (119.3 ± 0.6 μm) (Table 1). The percentage of migration potential fulfilled after 24 hr was also substantially different from serum-free media controls (8.0 ± 0.1% for mc-PAF vs 35.8 ± 0.2% for serum-free media controls; p = 0.00001) (Table 1). These values were also significantly different from those determined for 24 hr of 1 μm mc-PAF exposure (Table 1). These differences may have resulted from the obscuring of the cluster margin in the delayed mc-PAF experiments by the relatively large number of cells that began to migrate before mc-PAF addition, or they may have resulted from a retraction of the neurite substrate (Clark et al., 1995). These factors would result in an underestimate of migration parameters. Although the results from the delayed addition of mc-PAF suggested that mc-PAF blocked the further migration of neurons along preextended neurites (Fig. 6), these experiments were not conclusive.
Fig. 6.
mc-PAF disrupts previously initiated granule cell migration. The images of cerebellar granule cells and their processes elaborated from reaggregate clusters plated on laminin demonstrate the effect of adding 1 μm mc-PAF to the clusters 6 hr after plating them in serum-free media. A shows some processes and migrating granule cells from a cell cluster (to illustrate the distribution of migrated cells, the cluster itself is located justbelow the image) after 24 hr of serum-free media incubation at 37°C and 5% CO2. Cells have migrated as far as 300 μm from the cluster source. B shows some processes and migrating granule cells from a similarly positioned cell cluster that was incubated in serum-free media at 37°C and 5% CO2 for 6 hr and then with 1 μm mc-PAF in serum-free media for an additional 18 hr. Cells elaborated neurites and migrated 80 μm from the cell cluster before mc-PAF addition and then no farther after mc-PAF addition. Scale bar, 50 μm.
To better characterize the effects of mc-PAF on previously migrating neurons, time-lapse experiments of migrating granule cells were conducted. Cell clusters plated on laminin coated Δ T dishes (see Materials and Methods) in serum-free media were removed from the incubator after 6 hr, as described above. Granule cell migration was then observed in a controlled temperature environment for 1 hr in serum-free media. After 1 hr, the serum-free media in the dish was bath-exchanged with warmed serum-free media containing 1 μm mc-PAF (n = 9) or no additional drug compound (n = 3). Within 2 min of 1 μmmc-PAF addition, the migrating neurons stopped moving and remained immobile for the entire hour of mc-PAF exposure (n = 9). mc-PAF did not cause any observable morphological change in the neurite migration substrate (n = 9), although slight rounding of the soma of the migrating granule cell was observed within 4 min of mc-PAF addition (Figs. 2, 7). Cell migration usually resumed within 6 min of removal of the mc-PAF and continued uninterrupted for >30 min (n = 9). To avoid adding mc-PAF to migrating neurons coincidentally at a time when they might naturally stop moving (Edmondson et al., 1987; Komuro et al., 1996), the time of mc-PAF application was varied from 8 min, 1 hr, and 1.5 hr after the initial observation of the migrating neuron, and it was determined that the application of 1 μm mc-PAF stopped the movement of the cell regardless of the time that it was applied (n = 9; three migrating neurons per migration time interval).
Inhibition of mc-PAF migration effects with BN 52021 and trans-BTD
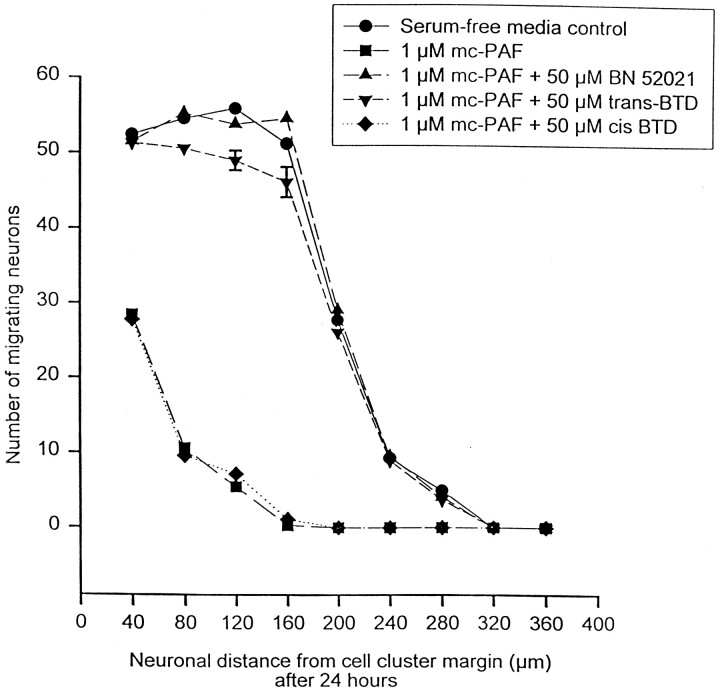
The specific PAF receptor antagonists BN 52021 andtrans-BTD blocked the decrease in migration brought about by 1 μm mc-PAF (n = 48) (Figs.3C, 8). The combination of 1 μm mc-PAF and either one of the antagonists resulted in no significant differences in either the mean total number of cells that migrated (Table 2) or the mean distance of migration as compared with serum-free media controls (Table2). Figure 3C illustrates a typical cell cluster after 24 hr of incubation with 1 μm mc-PAF and 50 μm BN 52021. The extent of neuronal migration from this cluster closely resembled that seen in the serum-free media control rather than that observed in the mc-PAF-treated cell clusters. BN 52021 (50 μm) inhibited the effects of 1 μm mc-PAF on neurite number measured at 1, 3, 6, and 12 hr after drug incubation (p = not significant from serum-free media control) (Fig. 3). These inhibitory effects were not reproduced by adding a combination of DMSO, the drug vehicle used for BN 52021 andtrans-BTD, and 1 μm mc-PAF to cell clusters (Table 2) nor were they reproduced by the combined addition of DMSO and methanol (n = 48; data not shown), which indicates that DMSO was not responsible for blocking the mc-PAF effects. The inhibition of mc-PAF effects on neuronal migration was not reproduced with cis-BTD (Table 2), a stereoisomer oftrans-BTD that is not a PAF receptor antagonist. Finally, blinded experiments with 1 μm mc-PAF and with 50 μm BN 52021 (n = 12) yielded a mean total number of migrated cells, a mean migration distance, and a mean neurite length that were all not significantly different from serum-free media control values (Table 2).
Fig. 8.
PAF receptor antagonists BN 52021 andtrans-BTD, but not cis-BTD (a stereoisomer of trans-BTD that is not a PAF receptor antagonist), inhibit mc-PAF disruption of cerebellar granule cell migration in vitro. The graph illustrates the number of cerebellar granule cells that have migrated and the distance of migration from their reaggregate cell cluster source 24 hr after incubation with serum-free media (control), 1 μmmc-PAF alone, 1 μm mc-PAF with 50 μm BN 52021, 50 μmtrans-BTD, or 50 μmcis-BTD (all in serum-free media;n = 48) at 37°C and 5% CO2. Error bars indicate SE and were removed from several points above for clarity. SEs from left to right: serum-free media and 1 μm mc-PAF (Fig. 2); 1 μm mc-PAF and 50 μm BN 52021 3.1, 2.3, 2.5, 2.4, 1.8, 0.8, 0.9, 0.0, 0.0; 1 μm mc-PAF and 50 μmtrans-BTD 2.8, 1.8, 2.6, 1.6, 2.4, 1.0, 0.7, 0.0, 0.0; 1 μm mc-PAF and 50 μmcis-BTD 3.2, 2.8, 2.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0.
Table 2.
BN 52021 and trans-BTD block the effects of mc-PAF on measured migration parameters
| Drug condition | Mean total number of migrated cells | Mean distance of migration (μm) |
|---|---|---|
| Control | 256.6 ± 1.9 | 119.3 ± 0.6 |
| 1 μm mc-PAF + 50 μm BN 52021 | 258.5 ± 2.0 | 119.9 ± 0.5 |
| 1 μm mc-PAF + 50 μm BN 52021 blinded | 254.3 ± 1.9 | 120.9 ± 0.6 |
| 1 μm PAF + 50 μmtrans-BTD | 236.4 ± 1.6 | 117.8 ± 0.6 |
| 1 μm mc-PAF + 50 μmcis-BTD | *45.9 ± 2.3 | *63.9 ± 0.4 |
| 1 μm mc-PAF + DMSO | *43.2 ± 1.4 | *58.1 ± 0.9 |
BN 52021 and trans-BTD inhibited the effects of mc-PAF on the mean total number of cells that migrated and the mean distance of migration values (p = not significantly different from corresponding serum-free media control values).cis-BTD, a stereoisomer of trans-BTD that is not a PAF receptor antagonist, and DMSO (at the final concentration for 50 μm BN 52021 in serum-free media) both failed to reproduce this inhibition [values significantly different from corresponding serum free-media control values (indicated by asterisk),p < 0.0001; values not significantly different from corresponding 1 μm mc-PAF values, p = not significant]. Numbers are ± SE.
Possible enhancement of migration with BN 52021 and trans-BTD
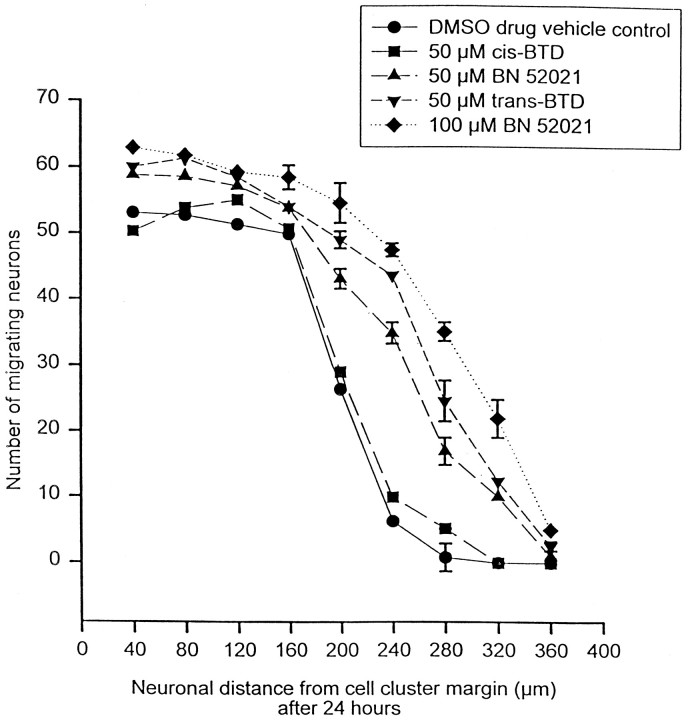
The addition of 50 or 100 μm BN 52021 or 50 μmtrans-BTD alone to the reaggregate cell clusters in some experiments led to a significant increase in the mean total number of granule cells that migrated (407.4 ± 2.3 cells in 100 μm BN 52021 vs 256.6 ± 1.9 cells in control) (Fig. 9, Table3) and their mean distance of migration (159.6 ± 0.7 μm in 100 μm BN 52021 vs 119.3 μm ± 0.6 in control) (Table 3). Figure 3Dillustrates a typical cluster after 24 hr of 100 μm BN 52021 treatment. This image reveals an apparent increase in the number of neurons that migrated from the cluster as compared with the serum-free media control in Figure 3A. The mean neurite lengths for 50 μm BN 52021, 100 μm BN 52021, or 50 μmtrans-BTD-treated clusters after 24 hr were not significantly different from those of serum-free media controls (Table 3). It should be noted, however, that blinded experiments with 100 μm BN 52021 (n = 12) alone failed to yield any significant difference in the mean distance of migration after 24 hr of incubation (Table 3) compared with serum-free media controls, which suggests that BN 52021 may not significantly enhance granule cell migration after all. If these data are included in the above comparison, then a significant effect on the mean distance of migration was still seen (157.6 ± 0.5 μm compared with 119.3 ± 0.6 μm for serum-free media control;p < 0.001). The possible migration-promoting effects of BN 52021 and trans-BTD were not sufficient in magnitude to explain the apparent block of mc-PAF effects in previously described experiments. Finally, 50 μmcis-BTD and DMSO (vehicle for all antagonists) did not appear to have any enhancing affect on migration (Fig. 8, Table 3).
Fig. 9.
BN 52021 and trans-BTD alone but not cis-BTD may enhance cerebellar granule cell migration in vitro. The graph illustrates the number of cerebellar granule cells that have migrated and the distance of migration from their reaggregate cell cluster source 24 hr after incubation with 50 μmcis-BTD, 50 μmtrans-BTD, 50 and 100 μmBN 52021, and DMSO (at the final concentration for 50 μmBN 52021 in serum-free media) all in serum-free media (n = 48; 4 dishes, 12 clusters measured per dish) at 37°C and 5% CO2. Error bars indicate SE and were removed from several points above for clarity. SEs from left to right: DMSO 1.8, 1.4, 2.0, 1.6, 2.0, 1.4, 2.1, 0.0, 0.0; 50 μmcis-BTD 1.3, 2.3, 1.7, 2.5, 1.3, 1.0, 0.8, 0.0, 0.0; 50 μm BN 52021 3.3, 1.3, 1.8, 2.10, 1.50, 1.6, 2.1, 2.9, 0.0; 50 μmtrans-BTD 2.6, 2.3, 1.8, 1.9, 1.3, 2.8, 3.1, 1.2, 0.7; 100 μm BN 52021 2.1, 1.5, 2.7, 1.8, 3.1, 1.0, 1.5, 3.0, 0.3.
Table 3.
BN 52021 and trans-BTD effects on measured migration parameters
| Drug condition | Mean total number of migrated cells | Mean distance of migration (μm) | Mean neurite length (μm) |
|---|---|---|---|
| Control | 256.6 ± 1.9 | 119.3 ± 0.6 | 332.9 ± 1.5 |
| 50 μm BN 52021 | *335.2 ± 1.9 | *143.0 ± 0.6 | 331.3 ± 1.2 |
| 100 μmBN 52021 | *407.4 ± 2.3 | *159.6 ± 0.7 | 333.1 ± 1.1 |
| 100 μm BN 52021 blinded | a258.3 ± 1.3 | a121.1 ± 0.8 | 332.6 ± 1.0 |
| 50 μm trans-BTD | *366.9 ± 1.9 | *150.3 ± 0.6 | 331.8 ± 1.2 |
| 50 μmcis-BTD | 254.7 ± 1.1 | 120.8 ± 0.6 | |
| DMSO | 241.2 ± 1.9 | 121.4 ± 0.8 |
BN 52021 (50 and 100 μm) and 50 μmtrans-BTD significantly increased (p< 0.001) the mean total number of migrating cells and the mean distance of migration compared with serum-free media controls, whereas 50 μmcis-BTD and DMSO (at the final concentration for 50 μm BN 52021 in serum-free media) did not. Surprisingly, these values from blinded experiments with 100 μm BN 52021 were not significantly different from the corresponding serum-free media control values (ap = not significant). BN 52021 andtrans-BTD did not significantly affect the mean neurite length compared with serum-free media-treated controls (p = not significant). Numbers are ± SE. Asterisk indicates values significantly different than the corresponding serum-free media control value.
Cultures treated with 50 and 100 μm BN 52021 also elaborated more neurites per cluster after BN 52021 incubation (Fig. 5) than serum-free media controls at all times measured. This increase in neurite number represented an increase in migration substrate that could have accounted for some of the increase in the number of cells that migrated under these conditions. Because clusters appeared larger in PAF receptor antagonist conditions, an effect of these compounds on granule cell proliferation could not be excluded. However, the effects on migration observed by video microscopy occurred in a short time frame (6–30 min) during which proliferation could not account for the apparent changes in cell position.
DISCUSSION
In the present study, the effects of PAF receptor agonists and antagonists on homotypic or neuron on neurite migration were examined. Although in vivo neuronal migration occurs via neuronal attachment to radial glia in the cerebral cortex (radial migration) or to Bergmann glia in the cerebellum (Fishell and Hatten, 1991; Liesi, 1992), in vivo tangential migration involving the attachment of neurons to other neuronal processes for contact guidance support has also been proposed (O’Rourke et al., 1995, 1997). Additionally, cerebellar granule cells have been shown to migrate predominantly along migration pathways of other neuronal fibers instead of Bergmann glia during the first 8 d of postnatal rat cerebellum development (Hager et al., 1995). In vitro preparations from the neonatal cerebellum including cerebellar reaggregate clusters (this study) and cerebellar microexplants (Nagata et al., 1990) demonstrate this neurite-guided granule cell migration.
In the cerebellar reaggregate system, it was observed that migrating granule cells extended leading processes out along preextended neurites. Soon after this leading process extension, the soma of the granule cells could be seen to move in the direction of leading process extension. This mode of migration appeared to be similar to what occurs in glial-guided migration, in which a migrating cell extends a leading process along a glial substrate before movement of the soma (Rakic, 1990). Therefore, the observed granule cell migration appears to be a process of attachment to a migration substrate, extension of neural processes in the direction of migration, and subsequent movement of the soma in the direction of the leading process.
The observed inhibition of migration in this reaggregate system by the PAF receptor agonist mc-PAF could be caused by an effect on the neurite migration substrate, because this agonist has been shown to produce significant effects on neurites (Clark et al., 1995). Alternatively, because modifications of adhesive substrates [both on glia (Stitt et al., 1990) and on neurites (Linder et al., 1983; Liesi et al., 1992,1995)] have been shown to alter migration, PAF receptor activation could have disturbed the ability of the granule cells to bind and adhere to neuritic processes. Finally, the microtubules and other cytoskeletal components that individual neurons use to migrate could have been affected by mc-PAF, perhaps in a manner similar to the effects of agonist on growth cones and neurites (Clark et al., 1995).
In this study, neuronal PAF receptor activation by mc-PAF interfered with the ability of the neurons to migrate on preextended neurites. Soma of neurons that did attempt to migrate on the neurite substrate in mc-PAF-treated conditions, or on preextended neurites that formed before mc-PAF addition, did not migrate as far from the reaggregate cluster as did controls. Although some effects of mc-PAF on neurite substrate were noted, it seemed unlikely that these effects could explain a larger, more significant effect on migration. These observations are consistent with previous studies, because in those studies neurites were acutely treated with mc-PAF to bring about a rapid neurite withdrawal measured as a change in length (Clark et al., 1995). In the present experiments, neurites were chronically exposed to mc-PAF, and the total neurite length was measured. These conclusions were further supported by video time-lapse experiments that demonstrated that bath application of 1 μm mc-PAF stopped the movement of neurons previously migrating along preextended neurites, with no obvious effects on the neuritic migration substrate. A rapid (∼4 min) shape change was noted in previously migrating granule cells (cell rounding) to accompany the arrest of migration. The rapidity of these observed morphological effects suggested that a downregulation of extracellular cell adhesion molecules or of the recognition sites for these molecules was unlikely. Taken together, these data suggest that PAF receptor activation interferes with the cell motility, perhaps through similar cytoskeletal alterations that lead to growth cone collapse and neurite withdrawal (Clark et al., 1995).
The reported experiments revealed that the effects of mc-PAF were blocked by the PAF receptor antagonists BN 52021 andtrans-BTD, indicating that the phenomenon observed is likely a PAF receptor-mediated one. This conclusion is further supported by the failure of lyso-PAF, a biologically inactive lipid that is structurally similar to PAF and mc-PAF, to have any effects on neuronal migration at the concentrations tested, and the failure ofcis-BTD to block the effects of mc-PAF on migration in the cell reaggregate cluster system. Experiments to determine the effectiveness of these compounds in blocking mc-PAF effects on migration observed in video time-lapse experiments are ongoing.
In some experiments, the application of BN 52021 ortrans-BTD alone to the granule cells appeared to enhance their migration by increasing the number of neuronal processes that formed and by increasing the distance of neuronal migration from the cell clusters. BN 52021 and trans-BTD could have blocked the effects of PAF produced in vitro by the cerebellar granule cells; in vitro PAF production by rat cerebellar granule cells has been described (Yue et al., 1990). Another possibility could have been that both of these antagonists enhanced neuronal survival or neuroblast cell divisions (Gao et al., 1991). The former possibility was deemed unlikely, because trypan blue stains failed to show any differences in the number of dead cells between BN 52021-treated and serum-free media-treated clusters (data not shown). The later possibility could not be excluded; however, in time-lapse video microscopy experiments, cells were under observation for an insufficient time for proliferation to occur. Finally, blinded studies with 100 μm BN 52021 failed to reveal the same enhancement in neuronal migration, but when the data from the blinded studies were included with the data from the unblinded studies, a significant effect on the mean distance of migration was still seen. These results therefore must be interpreted cautiously but may suggest that PAF and PAF receptors have de novo roles in the regulation of migration.
The neuronal PAF receptor mediating the reported effects seems to have led to neuronal cytoskeletal changes that altered cell motility. Motility of the neuroblast is accomplished by changes in the internal cytoskeleton (Gregory et al., 1988). The leading processes of migrating neurons have a microtubular arrangement such that tubulin is added at the end of the microtubular polymer directed toward the migratory trajectory (Rakic et al., 1996). This arrangement makes these processes subject to profound effects from an alteration of microtubules; it has been hypothesized that a microtubule-based mechanism could be responsible for the movement of the soma of migrating neurons (Rakic et al., 1996). Neurite retraction evoked by PAF receptor activation (Clark et al., 1995) and the presently observed cell rounding are likely to involve microtubules.
Although these data support a hypothesis that PAF may normally serve as a migration stop signal, other possibilities should be considered. In the present study, a nonhydrolyzable PAF receptor agonist was applied in a homogenous manner to migrating neuronsin vitro. It is unlikely that PAF would exist in such a manner in vivo. Rather, it may be expressed in some gradient similar to that of many other molecules important in neurodevelopment, such as the lipid retinoic acid (Thaller and Eichele, 1987; Chen et al., 1994). By presenting a nonhydrolyzable PAF receptor agonist (mc-PAF) in a ubiquitous manner, PAF receptors could have been saturated, and this could have effectively removed an important extracellular or intracellular gradient for PAF in the reaggregate system.
Little is known about the in vivo distribution of PAF and PAF receptors in the developing cerebellum or cerebral cortex to support or refute a role of PAF as a modulator of neuronal migration. Cells taken from immature rat cerebella (granule cells from 8-d-old rats) (Yue et al., 1990) or human fetal brains (microglia) (Jaranowska et al., 1995) have been shown to synthesize PAF in vitro.In situ hybridization experiments in the brain for a PAF receptor originally cloned from guinea pig lung (Honda et al., 1991) showed a ubiquitous signal in the rat brain but particularly intense signals in the cerebral cortex, olfactory bulb, pyramidal cell layer of the hippocampus, medial thalamus, hypothalamus, and cerebellar granule cell layer, with developmental expression constant from E18 (Mori et al., 1996). If this PAF receptor were expressed by cerebellar granule cells, one might expect PAF receptor expression in the developing cerebellum to increase substantially, because granule cells undergo dramatic proliferation in the first 2 weeks after birth in the rat. However, the predominant expression of this PAF receptor was found in rat brain microglia. Microglia are not found in significant numbers in our culture system, and recent experiments with Percoll gradient-purified granule cell reaggregate clusters (containing no glia) appear to yield results that are comparable to the experiments described here (unpublished observations). Taken together, these observations suggest that the mechanism of mc-PAF action on cerebellar granule cells in this study is via a different, as yet uncharacterized, neuronal PAF receptor. One possible function of this neuronal PAF receptor could be to transduce a PAF “migration stop signal” as migrating granule cells (from the external granule cell layer) approach the end point of migration in the IGL.
Although highly speculative, it is intriguing to consider that mc-PAF could interact with PAF acetylhydrolase 1B (PAF-AH 1B), a novel G-protein-like complex, to produce changes in the cytoskeleton as theorized recently (Xiang et al., 1995; Ho et al., 1997). In other words, this complex could theoretically serve as a recognition site for PAF that functions as a modulator of the cytoskeleton. The subunits of this complex, including Lis-encoded proteins, are expressed in a pattern that supports a role in neuronal migration (Reiner et al., 1995; Albrecht et al., 1996; Clark et al., 1997).
The data reported here demonstrate that PAF receptor activation inhibits the migration of neurons on preextended neurites. These observations support the hypothesis that PAF receptors, presumably activated in the brain by the lipid messenger PAF, may play a role in the regulation of neuronal migration in vivo. Furthermore, these results suggest that overstimulation of PAF receptors via abnormal levels of PAF in vivo could have dire consequences for the developing brain, consequences that may manifest as neuronal migration disorders such as Miller–Dieker lissencephaly, in which the potential for defects in PAF catabolism exist.
Footnotes
This work was supported by National Institutes of Health (NIH) Grants NS01433 and NS37146 and the Mental Retardation Research Center–NIH Grant HD24064. We thank Dr. M. Elizabeth Ross for suggestions regarding this manuscript, and Robert McNeil for assistance with immunofluorescence experiments.
Correspondence should be addressed to Dr. Gary D. Clark, Department of Pediatrics, Texas Children’s Hospital, 6621 Fannin, MC 3-3311, Houston, TX 77030.
REFERENCES
- 1.Albrecht U, Abu-Issa R, Ratz B, Hattori M, Aoki J, Arai H, Inoue K, Eichele G. Platelet-activating factor acetylhydrolase expression and activity suggest a link between neuronal migration and platelet-activating factor. Dev Biol. 1996;180:579–593. doi: 10.1006/dbio.1996.0330. [DOI] [PubMed] [Google Scholar]
- 2.Altman J. Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972;145:353–398. doi: 10.1002/cne.901450305. [DOI] [PubMed] [Google Scholar]
- 3.Arai A, Lynch G. Antagonist of the platelet-activating factor receptor block long-term potentiation in hippocampal slices. Eur J Neurosci. 1992;4:411–419. doi: 10.1111/j.1460-9568.1992.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 4.Asou H, Miura M, Kobayashi M, Uyemura K, Itoh K. Cell adhesion molecule L1 guides cell migration in primary reaggregation cultures of mouse cerebellar cells. Neurosci Lett. 1992;144:221–224. doi: 10.1016/0304-3940(92)90754-u. [DOI] [PubMed] [Google Scholar]
- 5.Baker RR, Chang HY. The potential for platelet-activating factor synthesis in brain: properties of cholinephosphotransferase and 1-alkyl-sn-glycero-3-phosphate acetyltransferase in microsomal fractions of immature rabbit cerebral cortex. Biochim Biophys Acta. 1993;1170:157–164. doi: 10.1016/0005-2760(93)90066-i. [DOI] [PubMed] [Google Scholar]
- 6.Baker RR, Chang HY. Neuronal nuclear acetyltransferases involved in the synthesis of platelet-activating factor are located in the nuclear envelope and show differential losses in activity. Biochim Biophys Acta. 1997;1345:197–206. doi: 10.1016/s0005-2760(96)00178-6. [DOI] [PubMed] [Google Scholar]
- 7.Barth PG. Disorders of neuronal migration. Can J Neurol Sci. 1987;14:1–16. doi: 10.1017/s031716710002610x. [DOI] [PubMed] [Google Scholar]
- 8.Bazan NG, Rodriguez EBT, Morelli SAL. Free arachidonic acid and membrane lipids in the central nervous system during bicuculline-induced status epilepticus. Adv Neurol. 1983;34:305–309. [PubMed] [Google Scholar]
- 9.Birkle DL, Kurian P, Braquet P, Bazan NG. Platelet-activating factor antagonist BN 52021 decreases accumulation of free polyunsaturated fatty acid in mouse brain during ischemia and electroconvulsive shock. J Neurochem. 1988;51:1900–1905. doi: 10.1111/j.1471-4159.1988.tb01175.x. [DOI] [PubMed] [Google Scholar]
- 10.Bito H, Nakamura M, Honda Z, Izumi T, Iwatsubo T, Seyama Y, Ogura A, Kudo Y, Shimizu T. Platelet-activating factor (PAF) receptor in rat brain: PAF mobilizes intracellular Ca2+ in hippocampal neurons. Neuron. 1992;9:285–294. doi: 10.1016/0896-6273(92)90167-c. [DOI] [PubMed] [Google Scholar]
- 11.Bito H, Kudo Y, Shimizu T. Characterization of platelet-activating factor (PAF) receptor in the rat brain. J Lipid Mediat. 1993;6:169–174. [PubMed] [Google Scholar]
- 12.Bonoventre JV, Koroshetz WJ. Phospholipase A2 (PLA2) activity in gerbil brain: characterization of cytosolic and membrane-associated forms and effects of ischemia and reperfusion on enzymatic activity. J Lipid Mediat. 1993;6:457–471. [PubMed] [Google Scholar]
- 13.Chen YP, Huang L, Solursh M. A concentration gradient of retinoids in the early Xenopus laevis embryo. Dev Biol. 1994;161:70–76. doi: 10.1006/dbio.1994.1008. [DOI] [PubMed] [Google Scholar]
- 14.Chong SS, Pack SD, Roschke AV, Tanigami A, Carrozzo R, Smith ACM, Dobyns WB, Ledbetter DH. A revision of the lissencephaly and Miller–Dieker syndrome critical regions in chromosome 17p13.3. Hum Mol Genet. 1997;6:147–155. doi: 10.1093/hmg/6.2.147. [DOI] [PubMed] [Google Scholar]
- 15.Clark GD, Happel LT, Zorumski CF, Bazan NG. Enhancement of hippocampal excitatory synaptic transmission by platelet-activating factor. Neuron. 1992;9:1211–1216. doi: 10.1016/0896-6273(92)90078-r. [DOI] [PubMed] [Google Scholar]
- 16.Clark GD, McNeil RS, Bix GJ, Swann JW. Platelet-activating factor produces neuronal growth cone collapse. NeuroReport. 1995;6:2569–2575. doi: 10.1097/00001756-199512150-00029. [DOI] [PubMed] [Google Scholar]
- 17.Clark GD, Mizuguchi M, Antalffy B, Barnes J, Armstrong D (1997) Predominant localization of the LISfamily of gene products to Cajal-Retzius cells and ventricular neuroepithelium in the developing human cortex. J Neuropathol Exp Neurol, in press. [DOI] [PubMed]
- 18.Del-Cerro S, Arai A, Lynch G. Inhibition of long-term potentiation by an antagonist of platelet-activating factor receptors. Behav Neural Biol. 1990;54:213–217. doi: 10.1016/0163-1047(90)90595-w. [DOI] [PubMed] [Google Scholar]
- 19.Edmondson JC, Hatten ME. Glial-guided neuron migration in vitro: a high-resolution time-lapse video microscopic study. J Neurosci. 1987;7:1928–1934. doi: 10.1523/JNEUROSCI.07-06-01928.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer G, Kunemund V, Schachner M. Neurite outgrowth patterns in cerebellar microexplant cultures are affected by antibodies to the cell surface glycoprotein L1. J Neurosci. 1986;6:605–612. doi: 10.1523/JNEUROSCI.06-02-00605.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fishell G, Hatten ME. Astrotactin provides a receptor system for CNS neuronal migration. Development. 1991;113:755–765. doi: 10.1242/dev.113.3.755. [DOI] [PubMed] [Google Scholar]
- 22.Gao W-Q, Heintz N, Hatten ME. Cerebellar granule cell neurogenesis is regulated by cell-cell interactions in vitro. Neuron. 1991;6:705–715. doi: 10.1016/0896-6273(91)90168-y. [DOI] [PubMed] [Google Scholar]
- 23.Gregory WA, Edmondson JC, Hatten ME, Mason CA. Cytology and neuron-glial apposition of migrating cerebellar granule cells in vitro. J Neurosci. 1988;8:1728–1738. doi: 10.1523/JNEUROSCI.08-05-01728.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hager G, Dodt HU, Zieglgansberger W, Liesi P. Novel forms of neuronal migration in the rat cerebellum. J Neurosci Res. 1995;40:207–219. doi: 10.1002/jnr.490400209. [DOI] [PubMed] [Google Scholar]
- 25.Hatten M, Mason C. Mechanisms of glial-guided neuronal migration in vitro and in vivo. Experientia. 1990;46:907–915. doi: 10.1007/BF01939383. [DOI] [PubMed] [Google Scholar]
- 26.Hattori M, Arai H, Inoue K. Purification and characterization of bovine brain platelet-activating factor acetylhydrolase. J Biol Chem. 1993;268:18748–18753. [PubMed] [Google Scholar]
- 27.Hattori M, Hideki A, Tsujimoto M, Arai H, Inoue K. Catalytic subunit of bovine brain platelet-activating factor acetylhydrolase is a novel type of serine esterase. J Biol Chem. 1994;269:23150–23155. [PubMed] [Google Scholar]
- 28.Ho YS, Swenson L, Derewenda U, Serre L, Wei Y, Dauter Z, Hattori M, Adachi T, Aoki J, Arai H, Inoue K, Derewenda ZS. Brain acetylhydrolase that inactivates platelet-activating factor is a G-protein-like trimer. Nature. 1997;385:89–93. doi: 10.1038/385089a0. [DOI] [PubMed] [Google Scholar]
- 29.Honda Z, Nakamura M, Miki I, Minami M, Watanabe T, Seyama Y, Okado H, Toh H, Ito K, Miyamoto T, Shimizu T. Cloning by functional expression of platelet-activating factor receptor from guinea-pig lung. Nature. 1991;349:342–346. doi: 10.1038/349342a0. [DOI] [PubMed] [Google Scholar]
- 30.Jaranowska A, Bussolino F, Sogos V, Arese M, Lauro GM, Gremo F. Platelet-activating factor production by human fetal microglia, effect of lipopolysaccharides and tumor necrosis factor-α. Mol Chem Neuropathol. 1995;24:95–106. doi: 10.1007/BF02962136. [DOI] [PubMed] [Google Scholar]
- 31.Jellinger K, Rett A. Agyria-pachygyria (lissencephaly syndrome). Neuropediatrics. 1976;7:6691. doi: 10.1055/s-0028-1091611. [DOI] [PubMed] [Google Scholar]
- 32.Kato K, Clark GD, Bazan NG, Zorumski CF. Platelet-activating factor as a potential retrograde messenger in CA1 hippocampal long-term potentiation. Nature. 1994;367:175–179. doi: 10.1038/367175a0. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi S, Kaoru I, Kensuke H, Inoue HK, Uyemura K, Shirao T. K252a, a potent inhibitor of protein kinases, inhibits the migration of cerebellar granule cells in vitro. Dev Brain Res. 1995;90:122–128. doi: 10.1016/0165-3806(96)83492-4. [DOI] [PubMed] [Google Scholar]
- 34.Komuro H, Rakic P. Intracellular Ca2+ fluctuations modulate the rate of neuronal migration. Neuron. 1996;17:275–285. doi: 10.1016/s0896-6273(00)80159-2. [DOI] [PubMed] [Google Scholar]
- 35.Kornecki E, Ehrlich YH. Neuroregulatory and neuropathological actions of the ether-phospholipid platelet-activating factor. Science. 1988;240:1792–1794. doi: 10.1126/science.3381103. [DOI] [PubMed] [Google Scholar]
- 36.Kornecki E, Ehrlich YH, Lenox RH. Platelet-activating factor-induced aggregation of human platelets specifically inhibited by triazolobenzodiazepines. Science. 1984;226:1454–1456. doi: 10.1126/science.6150550. [DOI] [PubMed] [Google Scholar]
- 37.Kumar R, Harvey S, Kester N, Hanahan D, Olson M. Production and effects of platelet-activating factor in the rat brain. Biochim Biophys Acta. 1988;963:375–383. doi: 10.1016/0005-2760(88)90304-9. [DOI] [PubMed] [Google Scholar]
- 38.Ledbetter SA, Kuwano A, Dobyns WB, Ledbetter DH. Microdeletions of chromosome 17p13 as a cause of isolated lissencephaly. Am J Hum Genet. 1992;50:182–189. [PMC free article] [PubMed] [Google Scholar]
- 39.Lee MK, Tuttle JB, Rebhun LI, Cleveland DW, Frankfurter A. The expression and posttranslational modification of a neuron-specific β-tubulin isotype during chick embryogenesis. Cell Motil Cytoskeleton. 1990;17:118–132. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- 40.Liesi P. Neuronal migration on laminin involves neuronal contact formation followed by nuclear movement inside a preformed process. Exp Neurol. 1992;117:103–113. doi: 10.1016/0014-4886(92)90119-b. [DOI] [PubMed] [Google Scholar]
- 41.Liesi P, Seppala I, Trenkner E. Neuronal migration in cerebellar microcultures is inhibited by antibodies against a neurite outgrowth domain of laminin. J Neurosci Res. 1992;33:170–176. doi: 10.1002/jnr.490330122. [DOI] [PubMed] [Google Scholar]
- 42.Liesi P, Hager G, Dodt HU, Seppala I, Zieglgansberger W. Domain-specific antibodies against the B2 chain of laminin inhibit neuronal migration in the neonatal rat cerebellum. J Neurosci Res. 1995;40:199–206. doi: 10.1002/jnr.490400208. [DOI] [PubMed] [Google Scholar]
- 43.Linder J, Rathjen FG, Schachner M. L1 mono- and polyclonal antibodies modify cell migration in early postnatal mouse cerebellum. Nature. 1983;305:427–430. doi: 10.1038/305427a0. [DOI] [PubMed] [Google Scholar]
- 44.Lo Nigro C, Chong SS, Smith ACM, Dobyns WB, Carrozzo R, Ledbetter DH. Point mutations and an intragenic deletion in LIS1, the lissencephaly causative gene in isolated lissencephaly sequence and Miller–Dieker syndrome. Hum Mol Genet. 1997;6:157–164. doi: 10.1093/hmg/6.2.157. [DOI] [PubMed] [Google Scholar]
- 45.Miller J. Lissencephaly in two siblings. Neurol. 1963;13:841–850. doi: 10.1212/wnl.13.10.841. [DOI] [PubMed] [Google Scholar]
- 46.Mizuguchi M, Takashima S, Kakita A, Yamada M, Ikeda K. Lissencephaly gene product-localization in the central nervous system and loss of immunoreactivity in Miller–Dieker syndrome. Am J Pathol. 1995;147:1142–1151. [PMC free article] [PubMed] [Google Scholar]
- 47.Moody SA, Miller V, Spanos A, Frankfurter U. Developmental expression of a neuron-specific β-tubulin in frog (Xenopus laevis): a marker for growing axons during the embryonic period. J Comp Neurol. 1996;364:219–230. doi: 10.1002/(SICI)1096-9861(19960108)364:2<219::AID-CNE3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 48.Mori M, Aihara M, Kume K, Makoto H, Kohsaka S, Shimizu T. Predominant expression of platelet-activating factor receptor in the rat brain microglia. J Neurosci. 1996;16:3590–3600. doi: 10.1523/JNEUROSCI.16-11-03590.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagata I, Nakatsuji N. Granule cell behavior on laminin in cerebellar microexplant cultures. Dev Brain Res. 1990;52:63–73. doi: 10.1016/0165-3806(90)90222-k. [DOI] [PubMed] [Google Scholar]
- 50.O’Flaherty JT, Redman JF, Jr, Schmitt JT, Ellis M, Surles JR, Marx MH, Piantadosi C, Wykle RL. A biologically potent, non-metabolizable analog of platelet-activating factor. Biochem Biophys Res Commun. 1987;147:18–24. doi: 10.1016/s0006-291x(87)80081-5. [DOI] [PubMed] [Google Scholar]
- 51.O’Rourke NA, Sullivan DP, Kaznowski CE, Jacobs AA, McConnel SK. Tangential migration of neurons in the developing cerebral cortex. Development. 1995;121:2165–2176. doi: 10.1242/dev.121.7.2165. [DOI] [PubMed] [Google Scholar]
- 52.O’Rourke NA, Chenn A, McConnell SK. Postmitotic neurons migrate tangentially in the cortical ventricular zone. Development. 1997;124:997–1005. doi: 10.1242/dev.124.5.997. [DOI] [PubMed] [Google Scholar]
- 53.Rakic P. Principles of neural cell migration. Experientia. 1990;46:882–891. doi: 10.1007/BF01939380. [DOI] [PubMed] [Google Scholar]
- 54.Rakic P, Knyihar–Csillik E, Csillik B. Polarity of microtubule assemblies during neuronal cell migration. Proc Natl Acad Sci USA. 1996;93:9218–9222. doi: 10.1073/pnas.93.17.9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reiner O, Carrozzo R, Shen Y, Wehnert M, Faustinella F, Dobyns WB, Caskey CT, Ledbetter DH. Isolation of a Miller–Dieker lissencephaly gene containing G protein β-subunit-like repeats. Nature. 1993;364:717–721. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- 56.Reiner O, Albrecht U, Gordon M, Chianese KA, Wong C, Gal-Gerber O, Sapir T, Siracusa LD, Buchberg AM, Caskey CT, Eichele G. Lissencephaly gene (LIS1) expression in the CNS suggests a role in neuronal migration. J Neurosci. 1995;15:3730–3738. doi: 10.1523/JNEUROSCI.15-05-03730.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rivas R, Hatten M. Motility and cytoskeletal organization of migrating cerebellar granule neurons. J Neurosci. 1995;15:981–989. doi: 10.1523/JNEUROSCI.15-02-00981.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Selak I, Foidart JM, Moonen G. Laminin promotes cerebellar granule cells migration in vitro and is synthesized by cultured astrocytes. Dev Neurosci. 1985;7:278–285. doi: 10.1159/000112296. [DOI] [PubMed] [Google Scholar]
- 59.Stewart RM, Richman DP, Caviness VSJ. Lissencephaly and pachygyria: an architectonic and topographic analysis. Acta Neuropathol. 1975;31:1–12. doi: 10.1007/BF00696881. [DOI] [PubMed] [Google Scholar]
- 60.Stitt TN, Hatten M (1990) Antibodies that recognize astrotactin block granule neuron binding to astroglia. 5:639–649. [DOI] [PubMed]
- 61.Thaller C, Eichele G. Identification and spatial distribution of retinoids in the developing chick limb bud. Nature. 1987;327:625–628. doi: 10.1038/327625a0. [DOI] [PubMed] [Google Scholar]
- 62.Xiang X, Osmani AH, Osmani SA, Xin M, Morris NR. NudF, a nuclear migration gene in Aspergillus nidulans, is similar to human LIS-1 gene required for neuronal migration. Mol Biol Cell. 1995;6:297–310. doi: 10.1091/mbc.6.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yue TL, Lysko G, Fuerstein G. Production of platelet-activating factor from rat cerebellar granule cells in culture. J Neurochem. 1990;54:1809–1811. doi: 10.1111/j.1471-4159.1990.tb01239.x. [DOI] [PubMed] [Google Scholar]