Abstract
Recent data obtained using a classic fear conditioning paradigm showed a dissociation between the retention of associations relative to contextual information (dependent on the hippocampal formation) and the retention of elemental associations (dependent on the amygdala). Furthermore, it was reported that conditioned emotional responses (CERs) could be dissociated from the recollection of the learning experience (declarative memory) in humans and from modifications of the hippocampal–septal excitability in animals. Our aim was to determine whether these two systems (“behavioral expression” system and “factual memory” system) interact by examining the consequences of amygdalar lesions (1) on the modifications of hippocampal–septal excitability and (2) on the behavioral expression of fear (freezing) resulting from an aversive conditioning during reexposure to conditional stimuli (CSs). During conditioning, to modulate the predictive nature of the context and of a discrete stimulus (tone) on the unconditional stimulus (US) occurrence, the phasic discrete CS was paired with the US or randomly distributed with regard to the US. After the lesion, the CER was dramatically reduced during reexposure to the CSs, whatever the type of acquisition. However, the changes in hippocampal–septal excitability persisted but were altered. For controls, a decrease in septal excitability was observed during reexposure to the conditioning context only for the “unpaired group” (predictive context case). Conversely, among lesioned subjects this decrease was observed in the “paired group” (predictive discrete CS case), whereas this decrease was significantly reduced in the unpaired group with respect to the matched control group. The amplitude and the direction of these modifications suggest a differential modulation of hippocampal–septal excitability by the amygdala to amplify the contribution of the more predictive association signaling the occurrence of the aversive event.
Keywords: amygdala central nucleus, lateral septum, hippocampal–septal excitability, elemental versus contextual conditioning, fear conditioning, mice
Two basic forms of mnesic retention can be distinguished in humans: an explicit form (declarative memory), which generally implies conscious and sophisticated information processing, and an implicit form, which is apparent through behavioral or autonomous responses that are often expressed without the explicit consciousness of the subject (Schacter, 1992; Eichenbaum, 1994). It appears that the explicit or “factual memory” form mainly involves the hippocampal formation and related structures, whereas implicit memory, and particularly certain forms of procedural learning involving an emotional component, rather involves the amygdaloid complex (Bechara et al., 1995). Several studies have attempted to elaborate an animal model of this functional dissociation. Thus, data collected in monkeys reveal a specific alteration of emotional reactivity to various stimuli without any memory impairment after amygdalar lesions, whereas a deficit in memory performance for object discriminations, associated with a normal emotional reactivity, is observed after lesions of the hippocampus and associated cortical areas (Zola-Morgan et al., 1991). Therefore, these results provide further evidence for a double dissociation between a “factual memory system,” which would involve the hippocampus (HPC), and a “behavioral memory system,” which would mainly involve the amygdaloid complex.
Concurrent to this dissociation, the HPC may also be involved in the contextual indexation of information (Honey and Good, 1993; Kubie and Ranck, 1984; Phillips and Ledoux, 1994, 1995; McDonald and White, 1995) (a deficit in which would be closely related to the episodic explicit memory deficit), whereas the amygdala seems to play a fundamental role in the acquisition of elemental conditioned associations and in the emotional expression of the whole set of these associations (i.e., both unimodal and, via the hippocampus, polymodal associations) (Phillips and Ledoux, 1992; Ledoux, 1993, 1994; Cahill et al., 1995).
In addition to these data, previous electrophysiological studies have provided evidence for the development of time-locked lateral septum (LS) unit responses to the conditioned stimuli (CSs) as classic conditioning of the eye-blink responses developed (Berger and Thompson, 1978). More recently, it was reported that changes in hippocampal–septal synaptic excitability occur in a test of fear conditioning but with a relative independence between the magnitude and/or the occurrence of these changes and the behavioral expression (freezing) of conditioned fear (Garcia and Jaffard, 1996). Specifically, using measures of field potentials monosynaptically evoked in the LS by fimbrial stimulation (DeFrance et al., 1973, 1976;Garcia and Jaffard, 1992), it was found that reexposure to the conditioning context was associated with a decrease in the amplitude of the N3 component (generated by firing cells in the dorsal aspect of the LS) (Garcia et al., 1997) only when the conditioning context was the sole predictor of foot-shock occurrence.
It emerges from these different results that because the hippocampal–septal axis seems to be particularly involved in the processing of contextual information, the amygdala [especially the central amygdaloid nucleus (CEA)] may be essential for the expression of conditioned emotional response (CER) to both discrete andcontextual stimuli, a hypothesis that is reminiscent, in part, of that which dissociates the role of the hippocampus and cerebellum in Pavlovian eye-blink conditioning (Kim et al., 1995). Thus, to confirm a functional dissociation between these two structures and to describe an eventual interaction between them, we measured retention testing-induced changes in hippocampal–septal excitability in a classic conditioning after lesions to the amygdala.
On the basis of the above-mentioned findings, our working hypothesis was that on the one hand amygdalar lesions would induce a suppression of the CER without abolishing the modifications of hippocampal–septal synaptic excitability (which would be more particularly specific to the encoding of complex contextual associations); on the other hand, amygdalar lesions would differentially alter the modifications of hippocampal–septal excitability as a function of the type of association (elemental or contextual association) established during acquisition. More precisely, because pairing of a phasic (tone) CS with an (shock) unconditioned stimulus (US) during acquisition was reported previously to prevent the decrease in hippocampal–septal excitability induced by reexposure to the context (Garcia and Jaffard, 1996), lesioning the amygdala, which is thought to subserve this elemental CS–US association, would reinstate such a decrease. Conversely, when a CS–US unpairing is provided during acquisition, thus producing in normal mice a decrease in hippocampal–septal excitability during reexposure to the context (i.e., the major predictor to the US), one cannot exclude the possibility that such a decrease would be reduced in amygdala-lesioned mice. In other words, our hypothesis was that in addition to the above-mentioned functions, the amygdala would be involved in processes aimed at amplifying, through a modulation of hippocampal–septal excitability, the contribution of the more predictive association (i.e., elemental or contextual) signaling the occurrence of the US.
MATERIALS AND METHODS
Subjects and surgery
The experiment was performed using 35 young (4–6 months) male mice (C57Bl/6 JI Co, IFFA Credo, Arbresle, France) weighing between 27 and 32 gm. They were housed individually 7 d before the experiment and maintained in an animal room (23°C) with a 12 hr artificial light/dark cycle and with ad libitum access to food and water. Three days before the experiment, they were handled individually for 5 min every morning. All experimental procedures took place during the light portion of the cycle. One group (n = 18) was subjected to a bilateral ibotenic lesion of the CEA: once anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and treated with atropine (0.04 ml of a 1 mg/ml solution), animals were placed in a Kopf stereotaxic apparatus, the cranium was exposed, and two fine burr holes were drilled at the following coordinates: 900 μm posterior to bregma, 2700 μm lateral from midline, and 4600 μm ventral from skull surface. The CEA lesion was made by bilateral ibotenic acid (Sigma, St. Louis, MO) injection (2 μg/0.1 μl phosphate buffer) divided into four successive injections (20 nl followed by 3 × 10 nl) spaced 10 min apart. Injection was performed using a micropipette attached by flexible tubing to a Hamilton microsyringe (1 μl, Poly Labo). For sham animals the same surgery was performed, but the micropipette was inserted just dorsal to the CEA and no injection was performed. All animals were then placed in a Narishige (Tokyo, Japan) stereotaxic apparatus for implantation of electrodes. The stimulating and recording electrodes were each made of two intertwined platinium–iridium wires (90 μm in diameter) that were insulated (except at the tip) and positioned in the fimbria (600 μm posterior to bregma and 600 μm from midline) and LS (900 μm anterior to bregma and 400 μm lateral from midline), respectively, at a location generating a maximum amplitude of the N3 component of the field potential negative complex. The entire miniature (stimulating–recording electrode) system was fixed in place with dental cement. Mice were then allowed to recover in their home cages in the animal room for at least 8 d before recording sessions.
Stimulating and recording general procedures
LS field potentials evoked by single-pulse fimbria stimulation (0.1 msec rectangular biphasic pulses) were recorded through JFET operational amplifiers placed on the head of the mouse. Signals were amplified (gain, 1000), filtered (bandpass, 1–1000 Hz), displayed on an oscilloscope and recorded by a microcomputer for on-line averaging, and stored on disks for off-line analysis. The amplitudes of the N3 field potential were averaged over 20 trials (n = 20 test pulses at 0.2 Hz). Stimulation intensity (50–600 μA) was chosen according to that which produced a response representing ∼80% of the maximal level obtained from the baseline input–output curves.
Apparatus
The behavioral test that was used is a classic aversive conditioning that took place in a Plexiglass box (30 × 24 × 22 cm high). The floor of the conditioning chamber consisted of 60 stainless steel rods (2 mm diameter) spaced 5 mm apart and connected to a shock generator. The four sides of the chamber and the rods of the floor were cleaned with 90% ethanol after each trial. A second chamber with gray plastic walls and with the same dimensions as the first was used for habituation to the electrophysiological recording system and for the auditory cue test.
Procedure
Baseline recording. Baseline recording of LS field potential was established over a 4 d period (one recording session per day). On each session, animals were maintained in their home cages, which were transferred from the animal room to the testing room. Each home cage was placed into the gray plastic chamber to prevent access to visuospatial cues in the room. Each animal was connected to the stimulating–recording system for 10 min.
Preliminary test: elevated plus-maze test. At the end of the adaptation to the laboratory period, animals were subjected to the elevated plus-maze test as an initial anxiety test. The maze is constructed of four arms, two of which are closed by side walls; the other two open arms classically represent an anxiogenic situation for the animals. Each animal was first placed on the central platform and then allowed to explore the maze for 6 min: the greater the percentage of time spent in the closed arms, the higher the anxiety level.
Aversive conditioning. Twenty-four hours after the last baseline recording, mice were randomly divided into four groups: two (CS–US) paired groups (control and lesioned subjects) and two unpaired groups (control and lesioned subjects). Each animal was placed in the conditioning chamber for 4 min. For the paired groups (elemental conditioning), 60 sec after the animals were placed into the chamber a tone (63 dB, 1 kHz) was presented for 15 sec. This tone was presented again 100 sec later. Each tone was terminated with the occurrence of the foot-shock (0.9 mA, 50 Hz, 3 sec). After a final delay of 40 sec, each animal was placed into its home cage. For animals of the unpaired groups (contextual conditioning), two tones and two shocks with the same parameters as those used previously were presented during the acquisition phase but were randomly distributed.
Twenty-four hours later, mice were tested for freezing behavior, used as an index of conditioned fear and defined as the absence of all movement except respiratory-related movements while the animal was in a stereotypical crouching posture (Blanchard and Blanchard, 1969). First, animals in the paired condition were submitted to a verification test of the tone conditioning: they were maintained in their home cage and exposed for 6 min in the gray plastic chamber for the auditory cue test. Three successive recording sessions of behavioral and electrophysiological responses were performed: before (first 2 min), during (next 2 min) and after (last 2 min) tone presentation. Previous results (Garcia and Jaffard, 1996) using the same protocol showed that the amplitude of the N3 component displayed by the “unpaired group” remained stable and without any change during this auditory cue test. Then the animals were returned to the animal room for 1 hr. After this delay, all of the animals (paired and unpaired groups) were reexposed to the conditioning chamber alone (context test), also for 6 min, and three successive 2 min recordings of behavioral and electrophysiological responses were performed. The behavior of the subjects was recorded continuously on video tape for off-line scoring of freezing.
Histology
After completion of the behavioral study, animals were given an overdose of sodium pentobarbital (120 mg/kg) and transcardially perfused with physiological saline, followed by 10% buffered formalin. Brains were post-fixed in formalin–saccharose 30% solution for 1 week, frozen, cut coronally on a sliding microtome into 60 μm sections that were mounted on a gelatin-coated slide, and stained with thionine.
Data analysis
Freezing was calculated as the percentage (±SEM) of the total time spent freezing during every 2 min retention test. The amplitudes of the N3 wave were expressed as the mean percentage (±SEM) of the individual basal values of animals for each group. Statistical analysis of the data was performed using ANOVA (Systat, Evanston, IL).
RESULTS
Histology
A representative CEA lesion is shown in Figure1. Histological analysis revealed that 11 of the 18 lesioned mice had neuronal loss that was confined bilaterally to the CEA. Two animals presented a bilateral lesion that extended to the medial, basolateral, and cortical nuclei of the amygdala, four animals presented only unilateral CEA lesion, and one animal did not present any clear lesion; these seven animals were excluded from the analysis. Only data from subjects that presented a clear bilateral lesion (n = 11) restricted to the CEA were used in the analysis. Among the control animals, five of them were excluded because of dislodgment of an electrode from its implantation site; thus data from 12 CEA-control mice were analyzed.
Fig. 1.
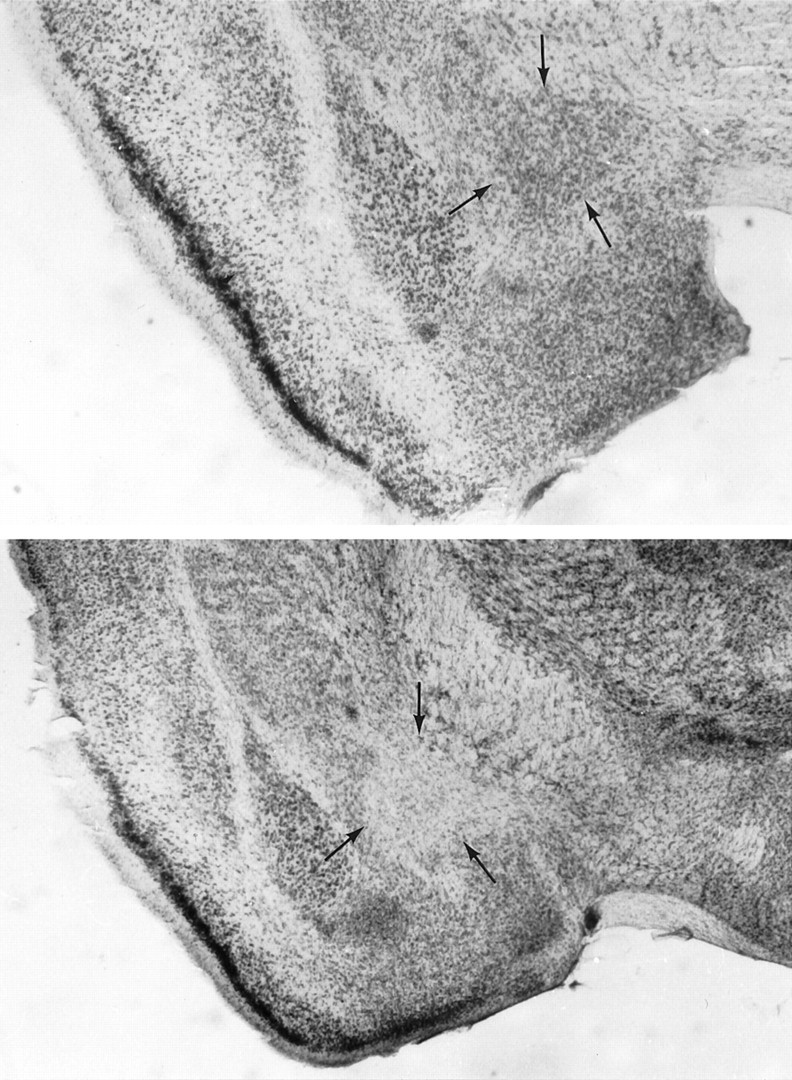
Photomicrographs showing the region of central amygdaloid nucleus (CEA) in a sham brain (top) and in a CEA ibotenic-lesioned brain (bottom). Note neuronal loss at the CEA lesion site and sparing of neurons in control brain.
Context test: behavior
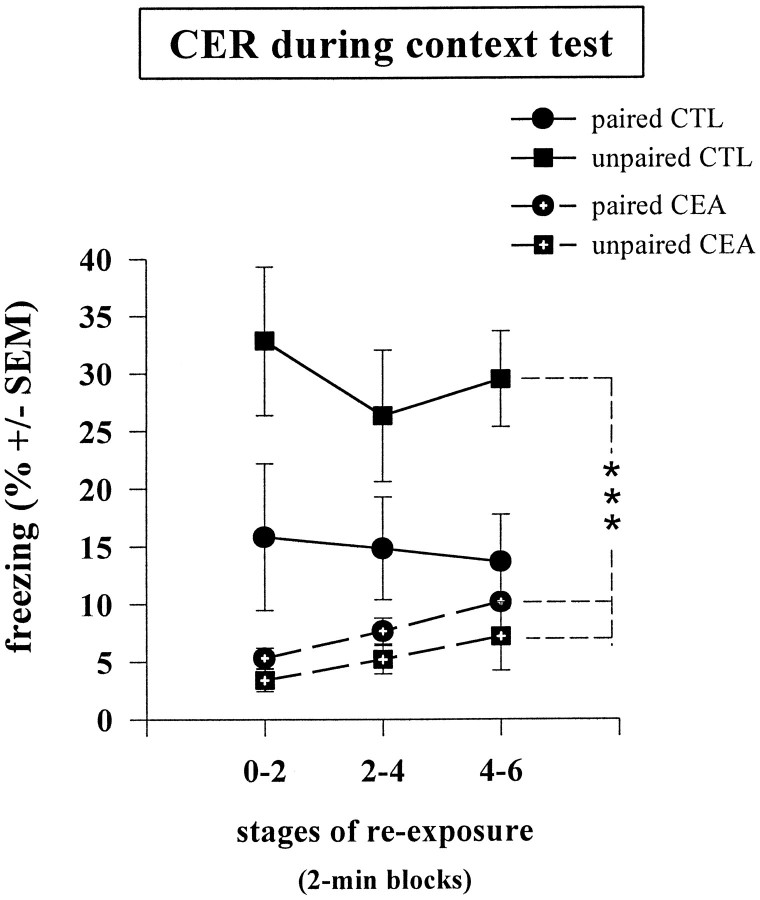
As shown in Figure 2, the magnitude of the freezing response produced by reexposure to the conditioning chamber across the three 2 min blocks differed among the four groups. The more intense freezing was observed in the unpaired control group, whereas virtually no freezing was observed in the two amygdala-lesioned groups. A three-way ANOVA performed on these data with blocks (three levels) as the within-subjects factor and both conditioning condition (paired vs unpaired) and lesion as between-groups factors indicated a significant effect of lesion (F(1,19) = 18.3;p < 0.001) but with a significant lesion × condition interaction (F(1,19) = 4.56;p < 0.05). This is attributable to the fact that in control groups, training using the paired as compared with the unpaired procedure resulted in less, although nonsignificant (F(1,10) = 3.79; p = 0.08), freezing during reexposure to the context, whereas a weak opposite tendency (p = 0.18) was observed in the lesioned groups.
Fig. 2.
Mean percentage freezing (±SEM) scored in control mice [paired CTL (n = 6);unpaired CTL (n = 6)] and lesioned mice [paired CEA (n = 6);unpaired CEA (n = 5)] on successive 2 min block during reexposure to the context; *** statistically significant (effect of lesion, p = 0.001).
Context test: electrophysiology
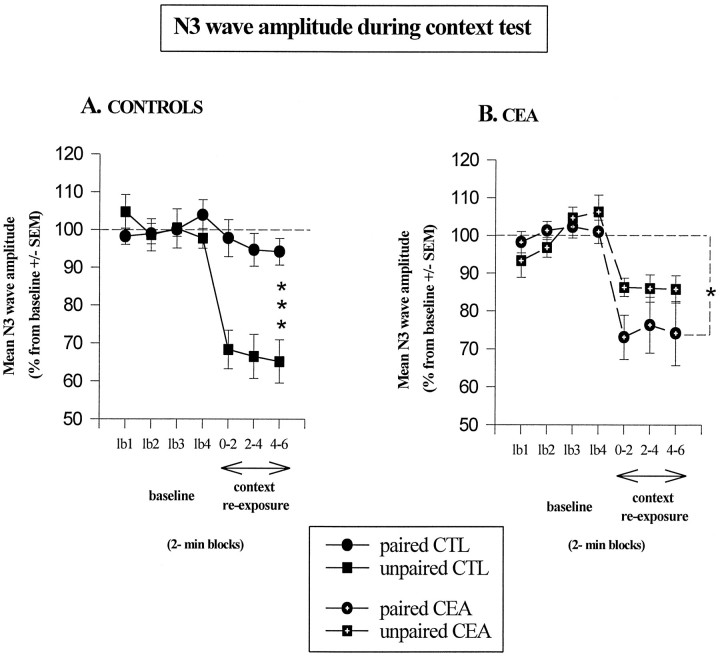
As shown in Figure 3, reexposure to the conditioning chamber was associated, on the whole, with a decrease in the amplitude of N3 with respect to baseline. The magnitude of this decrease was different, however, among the four groups (i.e., control–unpaired > lesioned–paired > lesioned–unpaired > control–paired). As shown in Figure4, in controls the N3 component displayed a significant decrease in amplitude solely for the unpaired group during reexposure to the conditioning context. A three-way ANOVA performed on these data with recording periods (seven levels) as within-subjects factors and both conditioning condition (paired vs unpaired) and lesion as between-groups factors indicated a significant effect of period (F(6,114) = 26.5;p < 0.001), with a significant period × conditioning × lesion interaction (F(6,114) = 8.17; p < 0.001). Thus, in the control group (Fig. 3A), the amplitude of N3 in the unpaired conditioning condition remained significantly below that of the paired condition across the three recording sessions performed during reexposure to the conditioning chamber (F(1,10) = 17.6; p = 0.002), whereas an inverse although nonsignificant tendency was observed in the amygdala-lesioned group (F(1,9) = 2.0;p = 0.19). Specifically, whereas amygdala lesioned animals displayed a significantly greater decrease in the amplitude of N3 than controls in the paired conditioning condition (F(1,10) = 6.7; p = 0.03), a significant attenuation of this decrease was actually observed in the unpaired condition (F(1,9) = 8.4;p = 0.02) [lesion × condition interaction (F(1,19) = 14.4; p < 0.001)].
Fig. 3.
Mean changes in the N3 wave amplitude (±SEM) on successive 2 min blocks during baseline establishment (lb1 → lb4) and during reexposure to the context in control groups (A) and lesioned groups (B); *** statistically significant (paired CTL vs unpaired CTL;p = 0.002); * significantly different from baseline; p < 0.05.
Fig. 4.
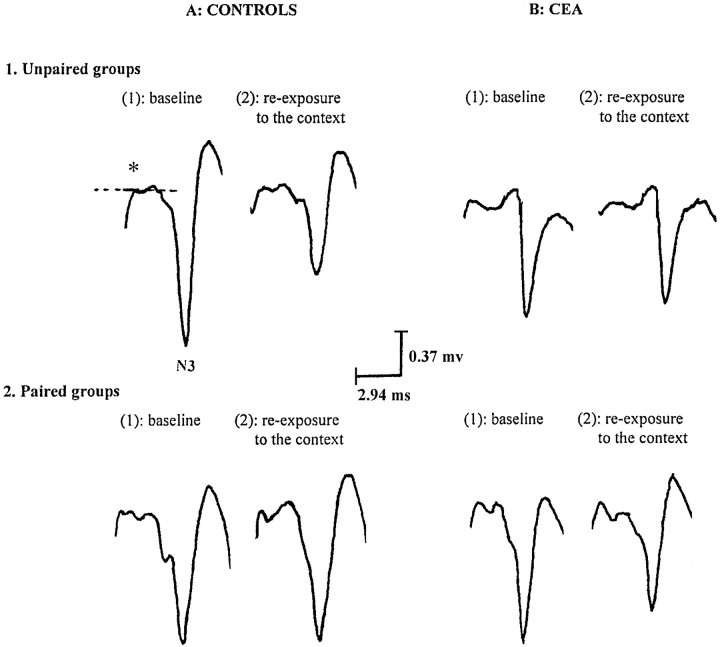
Changes in field potentials recorded from a median subject in each of the independent groups for controls (A), unpaired (1) and paired (2), and for lesioned groups (B: CEA), unpaired (1) and paired (2), during baseline establishment (1) and 24 hr after foot shocks during reexposure to the conditioning context (2). The amplitude of the response was measured from the early peak of positivity (*) to the peak of the N3 component (which displayed significant changes in our studies on fear conditioning) (also see Garcia and Jaffard, 1996;Garcia et al., 1997).
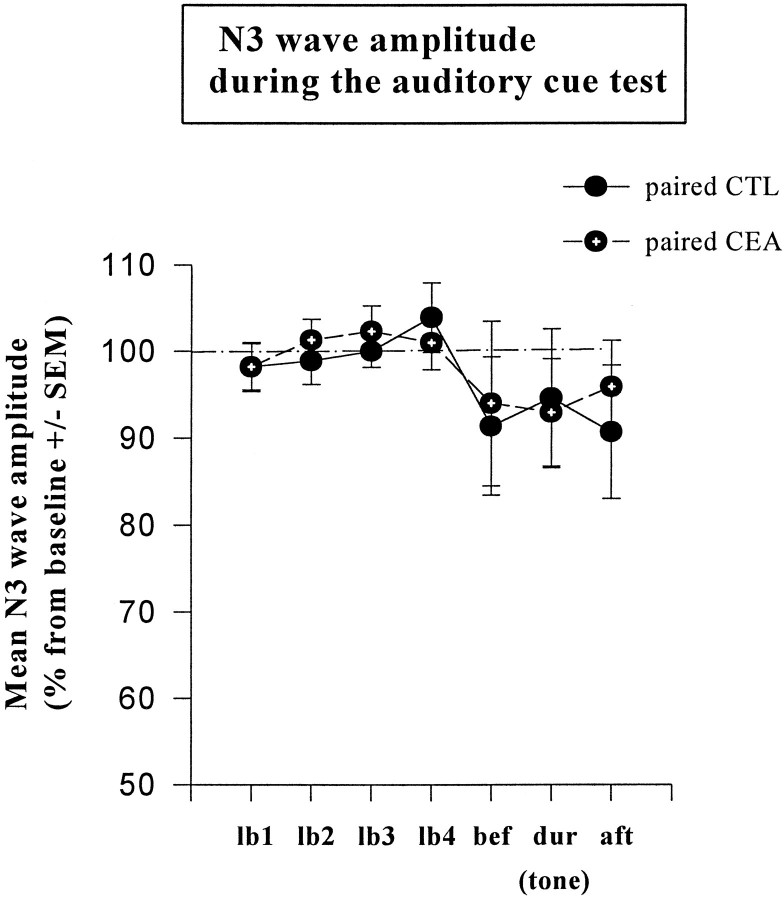
Auditory cue test: behavior and electrophysiology
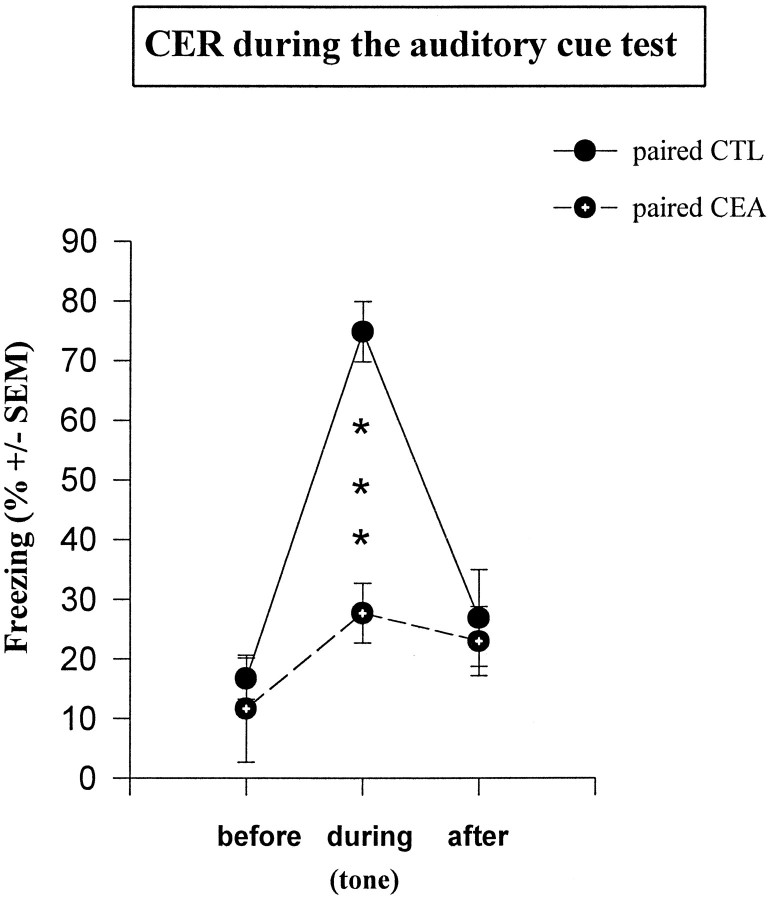
As shown in Figure 5, freezing behavior was specific to tone presentation and was impaired in the amygdala-lesioned group. Specifically, control mice displayed much more freezing during tone presentation (second block) than before (first block) or after (third block) tone presentation (repeated measures:F(2,10) = 35.2; p < 0.001), whereas no significant change was observed in amygdala-lesioned mice (F(2, 10) = 3,38; p > 0.05). Thus, lesioned mice displayed significantly less tone-elicited freezing than controls (F(1,10) = 16.1; p< 0.01). Conversely, tone presentation did not produce any significant change in the amplitude of N3, no matter which group was considered (all F values < 1) (Fig.6).
Fig. 5.
Mean percentage freezing (±SEM) in paired groups on successive 2 min blocks during the auditory cue test; *** statistically significant [control (CTL) versus lesioned (CEA); p = 0.001].
Fig. 6.
Mean changes in N3 wave amplitude (±SEM) in paired groups on successive 2 min blocks during the auditory cue test.
Relationships between behavioral and electrophysiological measures and within behavioral measures
Comparison of animals exhibiting measures of freezing behavior that were roughly matched during electrophysiological recording sessions provided evidence for a dissociation between the magnitude of freezing and alterations of the N3 wave amplitude. In the context test, however, it may be argued that control mice that exhibited the more intense freezing (unpaired group) also displayed the largest decrease in N3, thereby suggesting a direct relationship between the magnitude of conditioned freezing behavior and the magnitude of the decrease in the amplitude of N3. In fact, a regression analysis performed on these data did not provide any evidence for such a correlation (−0.138 < rs < 0.036). Moreover, by using the whole set of data, it can be noted that a significant decrease in N3 may be associated with virtually no freezing (i.e., amygdala-lesioned paired group, context test) (Figs. 2, 3) and that, conversely, intense freezing may be associated with no change in N3 (control group, auditory cue task) (Figs. 5, 6).
Finally, no significant correlation was observed between the level of anxiety as measured in the elevated plus-maze before conditioning and the magnitude of conditioned freezing.
DISCUSSION
The main finding of the present experiment was that ibotenic acid lesion of the CEA, which strongly impairs conditioned freezing behavior to both contextual and discrete (tone) stimuli, alters but does not prevent the concomitant decrease in excitatory hippocampal–septal transmission observed in the control group (unpaired condition). In agreement with previous findings using the same basic procedure in nonlesioned mice (Garcia and Jaffard, 1996), the present data provide additional evidence for a dissociation between the behavioral expression (freezing behavior) of aversive conditioning and the neurophysiological coding (changes in hippocampal–septal synaptic transmission) involved in conditioning. Specifically, in both the previous and present experiments, there was no correlation between these measures, either when examining data for the different groups separately or for all the data.
Previous experiments using either rats (Yadin and Thomas, 1981) or mice (Garcia and Jaffard, 1996; Garcia et al., 1997) as subjects have provided evidence that, as a general rule, unit activity and/or synaptic excitability in the LS decreases during exposure to a conditioned excitator of fear. Importantly, however, results from these experiments also showed that the magnitude of these decreases in hippocampal–septal excitability actually depended on whether a discrete CS was paired with the US during acquisition of fear conditioning. Specifically, as again shown in the control groups of the present experiment, previous pairing of the tone CS with the US totally prevented, with respect to the unpaired group, the decrease in the amplitude of N3 induced by reexposure to the context (Fig.3A). Thus, taken together, these data are not consistent with a general role of fear in the modulation of LS synaptic transmission (or unit activity), because the magnitude of the observed decreases seemed to be dictated by other qualitative properties of the conditioned aversive stimuli. Accordingly, we previously proposed that in the context test, the lack of decrease in N3 displayed by the paired group (with respect to the unpaired group) would be part of a process indicating that the current situation is quite safe until the occurrence of the tone CS. In addition, we provided evidence that this lack of change in hippocampal–septal excitability had no detectable control over overt behavior, because with respect to the unpaired group, animals of the paired group did not exhibit any difference in the amount of conditioned freezing behavior (Garcia and Jaffard, 1996). However, this latter assumption must be viewed with some caution when behavioral and electrophysiological data from the control groups in the context test of the present experiment are considered. Specifically, as shown in Figure 2, animals of the paired group [which displayed no change in the amplitude of N3 (Fig. 3A)] displayed somewhat less, although nonstatistically significant, freezing than animals of the unpaired group (which displayed a decrease in the amplitude of N3). This could mean that in this experiment, partial overshadowing of the context by the tone CS on freezing behavior cannot be totally ruled out. Nevertheless, the total lack of correlation between individual values of the amount of freezing and of the decreases in N3 amplitude (both groups) again strongly suggests a large independence between the two assessed parameters. Whatever the case may be, this observation does not rule out our previous hypothesis according to which the total lack of change in N3 would indicate that the context alone is in no way predictive of the US. If this hypothesis is correct, this lack of predictability should be related to the previously acquired tone CS–US association. Results obtained in the amygdala-lesioned group trained in the paired condition are congruent with this assumption. Indeed, when they were reexposed to the conditioning chamber, these lesioned mice displayed a decrease in the amplitude of N3 with respect to their control group, the magnitude of this decrease being similar to the one observed in the unpaired control group. In other words, they displayed a decrease in N3 as if they had been unable to form the CS–US association, a result that is congruent with data showing that the amygdala is involved in unimodal (i.e., discrete auditory or visual) fear conditioning (Kim and Fanselow, 1992; Phillips and Ledoux, 1992;Kim et al., 1993).
Accordingly, our suggestion is that in normal mice, information about the existence of a CS–US association would imply the existence of an amygdala–septal transmission, which would be either direct or indirect (see below) and the functional impact of which would be a total overshadowing of the previously acquired context–US association as revealed by a lack of change in the hippocampal–septal excitability. In other words, this amygdala–septal transmission, which is closely related to CS–US association, would result in a total loss of predictability of occurrence of the US by context, as if such a process would transform the relatively more predictive CS–US association into an absolutely predictive one. Results obtained in the context test for the unpaired groups globally agree with this speculation. Indeed, in this condition, the effect of amygdala lesions on LS synaptic transmission was reversed with respect to the paired condition. Specifically, in the unpaired condition, amygdala-lesioned subjects displayed significantly less decrease in N3 than controls did, suggesting thereby that in the absence of a consistent CS–US pairing during acquisition, the amygdala in this case would facilitate the decrease in N3 produced by reexposure to the context, thereby giving priority to the more predictive (context–US) association. The fact that conditioned freezing both to the CS and to the context was dramatically altered by CEA lesions does not allow us to determine whether alterations in LS excitability might have a behavioral counterpart. Nevertheless, there are instances in which aversive conditioning to contextual cues has been reported to be spared by amygdala lesions (Selden et al., 1991), presumably because contextual conditioning was assessed using an instrumental (place preference) avoidance response. In this experiment, however, the authors did not find any evidence for enhanced contextual conditioning in amygdala-lesioned subjects that were subjected to simple explicit CS–US pairings on acquisition, as would be expected on the basis of both competitive models of associative learning (Marlin, 1981) and our current hypothesis. It must be noticed, however, that in the experiment by Selden and colleagues (1991), amygdala lesions were aimed at the basolateral region of the amygdala and not at the CEA.
Nevertheless, the interpretation we have just proposed seems congruent with neuroanatomical data showing the existence of projections from the medial amygdala to the LS (Caffé et al., 1987). Moreover, the fact that these projections are vasopressinergic, at least in part, and that vasopressin (VP) has been shown to increase the transmission between fimbria fibers and LS neurons (Van den Hooff et al., 1989; Van den Hooff and Urban, 1990), suggests that an increase in lateral septal VP transmission could be responsible, among other possibilities, for blockade of the decrease in N3 observed in the paired with respect to the unpaired condition (control group) (for further arguments concerning the putative role of VP, see Garcia et al., 1997). A more likely but indirect putative pathway for such a vasopressinergic modulation of hippocampal–septal transmission might involve the bed nucleus of the stria terminalis, which receives fibers from the CEA (Weller and Smith, 1982) and in turn sends vasopressinergic fibers to several forebrain areas, including the LS (De Vries and Buijs, 1983;Woodhams et al., 1983). Whatever the case, our present findings clearly show a differential modulation of hippocampal–septal glutamatergic transmission by either direct or indirect amygdala–septal pathways according to the kind of conditioned association performed (i.e., elemental or contextual). This, in turn, could affect the way the hippocampus processes contextual information (Giovannini et al., 1994;Marighetto et al. 1994), that is, as either the absolutely predictive (unpaired condition) or the absolutely nonpredictive (paired condition) information for the occurrence of the US.
As shown in our previous (Garcia and Jaffard, 1996) and present experiments, aversive conditioning, as expressed by the magnitude of testing-induced freezing behavior, is dissociable from the amplitude of testing-induced alterations in hippocampal–septal synaptic transmission. In addition, we showed here that lesions of the CEA that resulted in a strong impairment of behavioral conditioning nevertheless did not prevent the neurophysiological expression of the previous aversive experience in terms of changes in hippocampal–septal excitability. Our suggestion is that these neurophysiological changes might constitute a form of knowledge about the conditioning situation encountered by our mice the day before, and that would be dissociated from behavioral expression (CER). In a recent experiment conducted in humans, it was shown that patients with bilateral lesion of the amygdala did not acquire conditioned autonomic responses to visual or auditory stimuli but did acquire the declarative facts about which visual or auditory stimuli were paired with the US. In contrast, a patient with selective bilateral damage to the HPC failed to acquire the facts but did acquire the behavioral conditioning (Bechara et al., 1995). It is thus possible that the presently observed sparing of testing-induced alterations in the amplitude of N3 after amygdala lesion reflects a sparing of a hippocampal-dependent equivalent of human declarative knowledge in the mouse.
Within the framework of this rather speculative hypothesis, however, the observation that amygdala lesions clearly interfere with these synaptic changes [which were either enhanced (paired group) or reduced (unpaired group) with respect to controls] would indicate that these two forms of knowledge are not independent but interactive. Specifically, our present data would indicate that the content of hippocampal-dependent representations about relations among various sensory exteroceptive stimuli (Phillips and Ledoux, 1992; Rudy and Sutherland, 1992) would actually be altered if fear conditioning, or the coupling of exteroceptive sensory information with interoceptive information conveying the fear state, is prevented by amygdala lesions.
Footnotes
This study was supported by the Centre National de la Recherche Scientifique. We thank Dr. T. P. Durkin for linguistic corrections of this manuscript and helpful discussions.
Correspondence should be addressed to Aline Desmedt, Laboratoire de Neurosciences Comportementales et Cognitives, Centre National de la Recherche Scientifique Unité de Recherche Associée 339, Université de Bordeaux I, 33405 Talence, France.
REFERENCES
- 1.Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- 2.Berger TW, Thompson RF. Neuronal plasticity in the limbic system during classical conditioning of the rabbit nictitating membrane response. II. Septum and mammillary bodies. Brain Res. 1978;156:293–314. doi: 10.1016/0006-8993(78)90510-3. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard RJ, Blanchard DC. Crouching as a index of fear. J Comp Physiol Psychol. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- 4.Caffé AR, Van Leeuwen FW, Luiten PGM. Vasopressin cells in the medial amygdala of the rat project to the lateral septum and ventral hippocampus. J Comp Neurol. 1987;261:237–252. doi: 10.1002/cne.902610206. [DOI] [PubMed] [Google Scholar]
- 5.Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. The amygdala and emotional memory. Nature. 1995;377:295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- 6.DeFrance JF, Kitai ST, Shimono T. Electrophysiological analysis of the hippocampal-septal projections: II. Functional characteristics. Exp Brain Res. 1973;17:463–476. doi: 10.1007/BF00234862. [DOI] [PubMed] [Google Scholar]
- 7.DeFrance JF, Yoshihara H, Chronister RB. Electrophysiological studies of the septal nuclei: I. The lateral septal region. Exp Neurol. 1976;53:399–419. doi: 10.1016/0014-4886(76)90081-9. [DOI] [PubMed] [Google Scholar]
- 8.De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- 9.Eichenbaum H. The hippocampal system and declarative memory in humans and animals, historical origins. In: Shacter DL, Tulving E, editors. Memory systems. MIT; Cambridge, MA: 1994. pp. 147–201. [Google Scholar]
- 10.Garcia R, Jaffard R. The hippocampo-septal projection in mice: long-term potentiation in the lateral septum. NeuroReport. 1992;3:193–196. doi: 10.1097/00001756-199202000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Garcia R, Jaffard R. Changes in synaptic excitability in the lateral septum associated with contextual and auditory fear conditioning in mice. Eur J Neurosci. 1996;8:809–815. doi: 10.1111/j.1460-9568.1996.tb01266.x. [DOI] [PubMed] [Google Scholar]
- 12.Garcia R, Vouimba RM, Jaffard R. Contextual conditioned fear blocks the induction but not the maintenance of lateral septal LTP in behaving mice. J Neurophysiol. 1997;78:76–81. doi: 10.1152/jn.1997.78.1.76. [DOI] [PubMed] [Google Scholar]
- 13.Giovannini MG, Mutolo D, Bianchi L, Michelassi A, Pepeu G. NMDA receptor antagonists decrease GABA outflow from the septum and increase acetylcholine outflow from the hippocampus: a microdialysis study. J Neurosci. 1994;14:1358–1365. doi: 10.1523/JNEUROSCI.14-03-01358.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honey RC, Good M. Selective hippocampal lesions abolish the contextual specificity of latent inhibition and conditioning. Behav Neurosci. 1993;117:23–33. doi: 10.1037//0735-7044.107.1.23. [DOI] [PubMed] [Google Scholar]
- 15.Kim JJ, Fanselow MS. Modality specific retrograde amnesia of fear. Science. 1992;256:675–676. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 16.Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- 17.Kim JJ, Clarck RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav Neurosci. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- 18.Kubie JL, Ranck JB. Hippocampal neuronal firing, context, and learning. In: Squire LR, Butters N, editors. Neuropsychology of memory. Guilford; New York: 1984. pp. 417–423. [Google Scholar]
- 19.Ledoux JE. Emotional memory systems in the brain. Behav Brain Res. 1993;58:69–79. doi: 10.1016/0166-4328(93)90091-4. [DOI] [PubMed] [Google Scholar]
- 20.Ledoux JE. In search of an emotional system in the brain: leaping from fear to emotion and consciousness. In: Gazzaniga MS, editor. The cognitive neurosciences. MIT; Cambridge, MA: 1994. pp. 1049–1061. [Google Scholar]
- 21.Marighetto A, Micheau J, Jaffard R. Effects of intraseptally injected glutamatergic drugs on hippocampal sodium-dependent high-affinity choline uptake in “naïve” and “trained” mice. Pharmacol Biochem Behav. 1994;49:689–699. doi: 10.1016/0091-3057(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 22.Marlin NA. Contextual associations in trace conditioning. Anim Learn Behav. 1981;9:519–523. [Google Scholar]
- 23.McDonald RJ, White NM. Information acquired by the hippocampus interferes with acquisition of the amygdala-based conditioned-cue preference in the rat. Hippocampus. 1995;5:189–197. doi: 10.1002/hipo.450050305. [DOI] [PubMed] [Google Scholar]
- 24.Phillips RG, Ledoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 25.Phillips RG, Ledoux JE. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn Memory. 1994;1:34–44. [PubMed] [Google Scholar]
- 26.Phillips RG, Ledoux JE. Lesions of the fornix but not the entorhinal or perirhinal cortex interfere with contextual fear conditioning. J Neurosci. 1995;15:5308–5315. doi: 10.1523/JNEUROSCI.15-07-05308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudy JW, Sutherland RJ. Configural and elemental associations and the memory coherence problem. J Cognit Neurosci. 1992;4:208–216. doi: 10.1162/jocn.1992.4.3.208. [DOI] [PubMed] [Google Scholar]
- 28.Schacter DL. Implicit knowledge: new perspective on unconscious processes. Proc Natl Acad Sci USA. 1992;89:11113–11117. doi: 10.1073/pnas.89.23.11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selden NRW, Everitt BJ, Jarrard LE, Robins TW. Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1991;42:335–350. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- 30.Van den Hooff PKC, Urban IJA. Vasopressin facilitates excitatory transmission in slices of the rat dorso-lateral septum. Synapse. 1990;5:201–206. doi: 10.1002/syn.890050305. [DOI] [PubMed] [Google Scholar]
- 31.Van den Hooff PKC, Urban IJA, De Wied D. Vasopressin maintains long-term potentiation in the rat lateral septum slices. Brain Res. 1989;505:181–186. doi: 10.1016/0006-8993(89)91440-6. [DOI] [PubMed] [Google Scholar]
- 32.Weller KL, Smith DA. Afferent connections to the bed nucleus of the stria terminalis. Brain Res. 1982;232:255–270. doi: 10.1016/0006-8993(82)90272-4. [DOI] [PubMed] [Google Scholar]
- 33.Woodhams PL, Roberts GW, Polak JM, Crow TJ. Distribution of neuropeptides in the limbic system of the rat: the bed nucleus of the stria terminalis, septum and preoptic area. Neuroscience. 1983;8:677–703. doi: 10.1016/0306-4522(83)90003-9. [DOI] [PubMed] [Google Scholar]
- 34.Yadin E, Thomas E. Septal correlates of conditioned inhibition and excitation in rats. J Comp Physiol Psychol. 1981;95:331–340. doi: 10.1037/h0077769. [DOI] [PubMed] [Google Scholar]
- 35.Zola-Morgan S, Squire LR, Alvarez-Royo P, Clower RP. Independence of memory functions and emotional behavior: separate contributions of the hippocampal formation and the amygdala. Hippocampus. 1991;1:207–220. doi: 10.1002/hipo.450010208. [DOI] [PubMed] [Google Scholar]