Abstract
Although the genetic link between the ε4 allele of apolipoprotein E (apoE) and Alzheimer’s disease is well established, the isoform-specific activity of apoE underlying this correlation remains unclear. To determine whether apoE influences the neurotoxic actions of β-amyloid (Aβ), we examined the effect of native preparations of apoE3 and E4 on Aβ-induced toxicity in primary cultures of rat hippocampal pyramidal neurons. The source of apoE was conditioned medium from HEK-293 cells stably transfected with human apoE3 or E4 cDNA. ApoE4 (10 μg/ml) alone was toxic to the cultures, whereas apoE3 had no effect. ApoE3 treatment prevented the toxicity induced by 10 μm Aβ(1–40) or Aβ(25–35). The apoE3 protective effect appears to be specific to Aβ-induced toxicity, because apoE3 did not protect against the cytotoxicity produced by NMDA or staurosporine, nor did apoE3 affect the increase in intracellular calcium induced by either NMDA or KCl. ApoE3 had no effect on the toxicity produced by Aβ in the presence of receptor-associated protein, an inhibitor of apoE receptors, particularly the LDL-receptor-related protein. Interaction with apoE receptors may not mediate the toxic actions of apoE4, because receptor-associated protein did not affect apoE4-induced neurotoxicity. Consistent with our previous biochemical experiments, analysis of the culture medium revealed that SDS-stable apoE3:Aβ complex is present in greater abundance than apoE4:Aβ complex. Thus, the protection from Aβ-induced neurotoxicity afforded by apoE3 treatment may result from clearance of the peptide by apoE3:Aβ complex formation and uptake by apoE receptors.
Keywords: apolipoprotein E, β-amyloid, neurotoxicity, primary hippocampal neuron cultures, LDL receptor-related protein, receptor-associated protein, Alzheimer’s disease
Apolipoprotein (apoE), a 35 kDa component of several classes of lipoproteins, acts as a ligand for lipoprotein receptors, thus regulating plasma lipid transport and clearance. In humans, apoE has three major isoforms: E2 (Cys112, Cys158), E3 (Cys112, Arg158), E4 (Arg112, Arg158), products of alleles at a single gene locus. Although not fully understood, apoE also appears to play a role in both normal and pathologic brain function. The ε4 allele is linked genetically to Alzheimer’s disease (AD) (Saunders et al., 1993; Strittmatter et al., 1993). Immunostaining of normal human brain shows apoE in neurons; in AD brain, apoE colocalizes with β-amyloid (Aβ) to senile plaques (Namba et al., 1991; Metzger et al., 1996). In addition, the density of Aβ deposition in cerebral plaques of patients with late-onset AD correlates with the ε4 allele (Rebeck et al., 1993; Schmechel et al., 1993). In vitro, Aβ has been shown to be neurotoxic (Yankner et al., 1990; Pike et al., 1993; Simmons et al., 1994). In spite of this evidence that apoE may interact with Aβ, the isoform-specific role of apoE in the pathogenesis of AD remains unclear. In vitro data suggest that apoE may have an isoform-specific effect both directly on neuronal development and on survival and via modulating the toxic actions of Aβ. Using several different cell culture models, lipid-associated (“native”) apoE3 has been shown previously to increase neurite outgrowth, whereas apoE4 may reduce neurite outgrowth and causes depolymerization of tubulin (Nathan et al., 1994, 1995; Bellosta et al., 1995; Holtzman et al., 1995; Fagan et al., 1996). Purified apoE4, including full length, a 22 kDa thrombin cleavage fragment, and various peptides, has been shown also to be neurotoxic (Marques et al., 1996, 1997; Tolar et al., 1997). In the B12 rat neuronal cell line, apoE exhibited an isoform-specific protective effect (E2 > E3 > E4) on the cytotoxicity induced by oxidative stress, including treatment with Aβ peptides (Miyata and Smith, 1996). In human cortical neurons, apoE4 increased the neurotoxicity of Aβ peptides (Ma et al., 1996). Because the hippocampus is the region of the brain most affected by AD, we studied the effect of apoE3 and E4 on cell viability and Aβ-induced toxicity in primary cultures of rat hippocampal neurons. We have shown previously that Aβ(1–40) and Aβ(25–35) induce neurotoxicity via apoptotic pathways in this culture system (Jordan et al., 1997). Because apoE requires a lipid particle-associated conformation to be biologically active, we used native preparations of apoE3 and E4 (conditioned media), in which the protein is associated with small, dense particles (LaDu et al., 1995a). In biochemical experiments, this native apoE3 formed an SDS-stable complex with Aβ that was 20-fold more abundant than apoE4:Aβ complex (LaDu et al., 1994, 1995b). We hypothesize that this complex formation allows for the clearance of the peptide via apoE receptors. Thus, we predict that apoE3 will protect preferentially against Aβ-induced neurotoxicity in a receptor-mediated process.
MATERIALS AND METHODS
Materials. Aβ(1–40) (Bayer, Wuppertal, Germany), Aβ(25–35) (Sigma, St. Louis, MO), and staurosporine (Sigma) were dissolved in dimethylsulfoxide (DMSO, Sigma). NMDA (Sigma) was dissolved in water. Stock solutions (1000×) for each reagent were prepared and stored at −80°C for no more than 1 month.
Hippocampal cultures. Pyramidal neurons were prepared from the hippocampi of fetal rats at 17 d of gestation (Scholz and Palfrey, 1991). Hippocampi were dissected in Ca2+/Mg2+-free HBSS and incubated in 0.1% trypsin for 15 min. The hippocampi were triturated by aspirating 7–10 times with a normal-bore Pasteur pipette and 7–10 times with a flame-narrowed Pasteur pipette. Cells were plated (2 × 105 cells/ml) in DMEM (Life Technologies, Grand Island, NY) plus 10% horse serum (Life Technologies) on poly-l-lysine (Sigma; 0.5 mg/ml in borate buffer, pH 8.0)-coated 15 mm round glass coverslips (0.2 ml/slip) and allowed to adhere. After 2–4 hr, cells were transferred to 60 mm dishes containing the supporting astrocytes attached to the bottom of the culture dish. Astrocytes were prepared from the cerebral hemispheres of newborn rats (Brorson et al., 1995). Cytosine-β-d-arabinofuranoside (5 μm) was added to each plate 2 d later to inhibit non-neuronal cell proliferation.
Toxicity treatments. Aβ(1–40) or (25–35) was added to the hippocampal pyramidal cultures on day in vitro (DIV)-5 to a final concentration of 10 μm, and cell viability was analyzed 5 d later. Staurosporine was added on DIV-5 to a final concentration of 0.3 μm, and cell viability was analyzed 24 hr later. For NMDA-induced toxicity, DIV-5 cultures were incubated for 20 min in 100 μm NMDA in 2 mmCa2+/Na+ and 10 μmglycine in Mg2+-free saline solution, culture medium was replaced, and cell viability was analyzed 24 hr later. A final concentration of 0.2% DMSO alone, the vehicle in which Aβ and staurosporine were initially dissolved, did not affect the general health of the cultures (data not shown). For fura-2 video imaging, neuronal cultures (DIV-5) were treated with apoE3 or apoE4 (10 μg/ml) for 5 d. On DIV-10, cells were perfused with 50 μmNMDA for 3 min in Mg2+-free saline solution or with 50 mm KCl for 1.5 min. Cells were then washed in balanced salt solution and loaded with 2 μm fura-2 AM, as described below.
ApoE preparations. Serum-free conditioned medium from HEK-293 cells stably transfected with human cDNA encoding apoE3 or E4 was prepared as described (LaDu et al., 1994). Medium was harvested, concentrated, dialyzed (10 kDa cutoff membrane) in PBS, and sterilized by passage through 0.22 μm syringe filters. Samples were diluted serially, run on reducing SDS-PAGE, and visualized by Coomassie blue staining, and the approximate apoE concentration was determined by densitometry (Molecular Dynamics, Sunnyvale, CA) and comparison with a purified apoE standard. ApoE in concentrated medium was 500 μg/ml on average. Control cultures were treated with conditioned medium from HEK cells stably transfected with the neomycin resistance expression vector alone and were not significantly different from untreated cultures (data not shown).
Cell viability. Cell death was analyzed using the fluorescein diacetate/propidium iodide double-staining procedure (Favaron et al., 1988). Living and dead cells were counted on adjacent fields of each coverslip for a total of 300–450 cells. The percentage of neurons surviving was determined on three to four coverslips for each condition and normalized to parallel controls. Each coverslip was treated as a single observation. Cell viability was determined from at least three separate experiments using different batches of both peptide and apoE-conditioned media with n ≥ 10. Results are expressed as means ± SE, and significance was determined by Student’s t test.
Western blot analysis. Media samples were added to 2× nonreducing Laemmli buffer (Laemmli, 1970) (4% SDS, no βME) and frozen at −20°C. Samples were boiled 5 min and electrophoresed on 10–20% SDS-tricine gels, transferred to Immobilon-P membrane (Millipore, Bedford, MA), and probed with antibodies to Aβ (monoclonal antibody 4G8 to amino acids 17–24; Senetec, St. Louis, MO) or apoE antiserum. ApoE antiserum was obtained by immunizing rabbits with apoE purified from human serum. Proteins on Western blots were visualized by enhanced chemiluminescence (Amersham, Arlington Heights, IL).
Fura-2 video imaging. Cells were loaded with 2 μm fura-2 AM using a balanced salt solution (standard buffer) of the following composition (in mm): NaCl 159.0, KCl 5.0, MgSO4 0.4, MgCl2 0.5, KH2PO4 0.64, NaHCO3 3.0, HEPES 20.0, glucose 5.0, Na2HPO4 0.33, CaCl2 2.0, and BSA 0.2% (330 mOsm/kg), pH-adjusted to 7.35. The cells were incubated with fura-2 for 30 min at room temperature to avoid probe compartmentalization and then incubated for an 30 min at room temperature to allow de-esterification of the fura-2 dye. Coverslips were mounted on a coverslip chamber for fluorescence measurements. All measurements were made at room temperature, as described previously (Meucci and Miller, 1996), using standard buffer supplemented with 10 μm glycine. Each cell in the image was analyzed independently for each time point in the captured sequence. All individual cell [Ca2+]itraces shown are representative responses for a given field of cells. For the calibration of fluorescent signals, we used cells loaded with fura-2; Rmax andRmin, ratios at saturating and zero Ca2+, respectively, were obtained by perfusing cells with standard buffer containing 10 mm CaCl2 and 4 μm ionomycin and, subsequently, with a Ca2+-free solution containing 10 mmEGTA. The values of the obtained Rmax andRmin, expressed as gray-level mean, were used to calculate the calibration curve by TARDIS software. The [Ca2+]i was determined according to the equation of Grynkiewicz and associates (1985).
LRP Immunocytochemistry. Cultures (DIV-5) were fixed by incubating with 4% paraformaldehyde in culture medium at 37°C for 15 min. After washing three times in 0.1 m PBS, pH 7.4, cells were incubated for 1 hr in blocking medium (0.1% Tween 20, 4% BSA, in 0.1 m PBS) at room temperature. Incubations were performed overnight at 4°C, using rabbit antisera for rat LDL receptor-related protein (LRP) (1:1000, kindly provided by Dudley Strickland, American Red Cross, Rockville, MD), diluted in blocking medium. Cultures were rinsed three times with blocking medium, incubated with a biotinylated secondary antibody for 1 hr, and then incubated for 30 min with streptavidin conjugated with indocarbocyanine (1:500, Jackson Immunoresearch Lab, Inc, West Grove, PA). Coverslips were mounted using an aqueous mounting solution (10% glycerol in PBS) containing 2.5% (w/v) of 1,4-diazabicyclo[2.2.2.]octane (Sigma). Cell fluorescence was detected using a standard epiillumination fluorescence microscope (Olympus Optical, Tokyo, Japan).
Receptor-associated protein (RAP) treatments. Rat RAP was generously provided by Guojun Bu (Washington University, St. Louis, MO). RAP was generated as a recombinant glutathione S-transferase fusion protein and purified as described previously (Warshawsky et al., 1993). Before use, RAP was dialyzed against cell culture medium, as was a comparable molar concentration of BSA to serve as a control. RAP was added on DIV-5 for a final concentration of 1.0 μm. Additional RAP (0.5 μm) was added on DIV-7 and DIV-9, and cell viability was assayed on DIV-10. The BSA-only control had no effect (data not shown).
RESULTS
ApoE4 is toxic to hippocampal pyramidal neurons
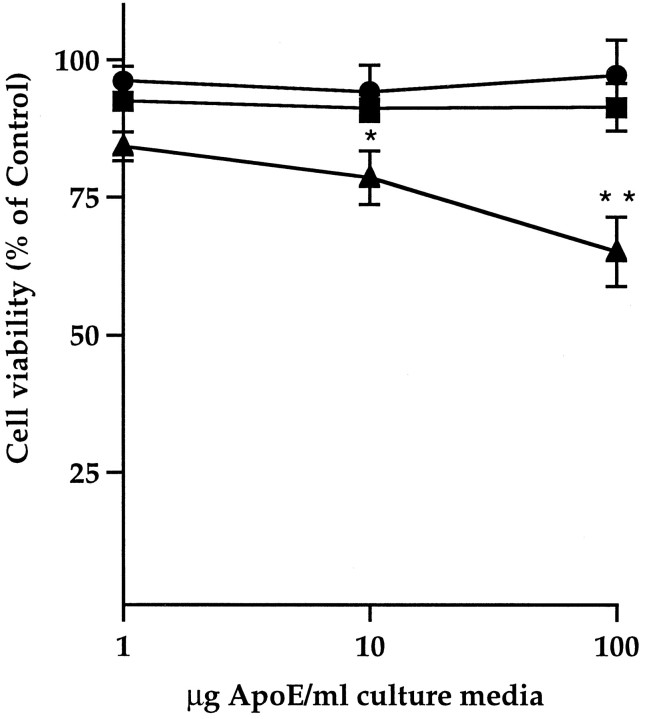
Although the concentration of apoE in the extracellular space of the brain is unknown, the concentration of apoE in normal human CSF is 1.4–2.9 μg/ml, and the plasma concentration is 50 μg/ml (Pitas et al., 1987; Gelman et al., 1988). Primary hippocampal neuronal cultures were treated on DIV-1 with either control or apoE3- or apoE4-conditioned HEK cell medium (final concentration of 1, 10, and 100 μg apoE/ml), and cell viability was assayed after 5 and 10 d by the fluorescein diacetate/propidium iodide double-staining procedure (see Fig. 2). ApoE4 at 10 and 100 μg/ml significantly reduced cell viability in cultures treated for 5 d (data not shown; also, see Fig. 3C,D) and 10 d (Fig.1, also see Fig. 3A), whereas apoE3 and control media had no effect. ApoE4 toxicity resembled the apoptotic cell death induced by Aβ treatment, with propidium iodide labeling of condensed and fragmented nuclei in affected cells (Fig.2C).
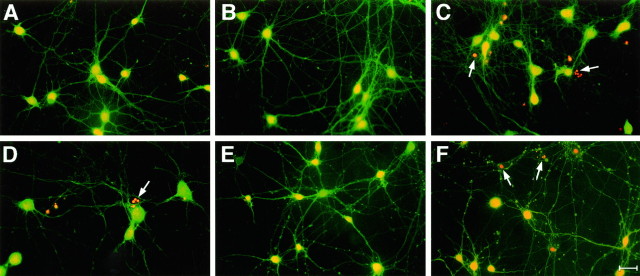
Fig. 2.
Fluorescence staining of cells treated with apoE3 and E4 in the presence and absence of Aβ. Primary rat pyramidal hippocampal cultures (DIV-1) were treated with control medium (A, D), apoE3 (B,E), or apoE4 (C, F) (10 μg apoE/ml). On DIV-5, 10 μm Aβ(1–40) was added to D–F, and cells were stained with fluorescein diacetate/propidium iodide on DIV-10. Source of apoE as described (Fig. 1). Typical micrographs are shown with fluorescein diacetate (yellow/green)- labeled intact cells and propidium iodide (orange)- labeled condensed or fragmented nuclei of compromised cells (arrows in C, D,F). Scale bar, 10 μm.
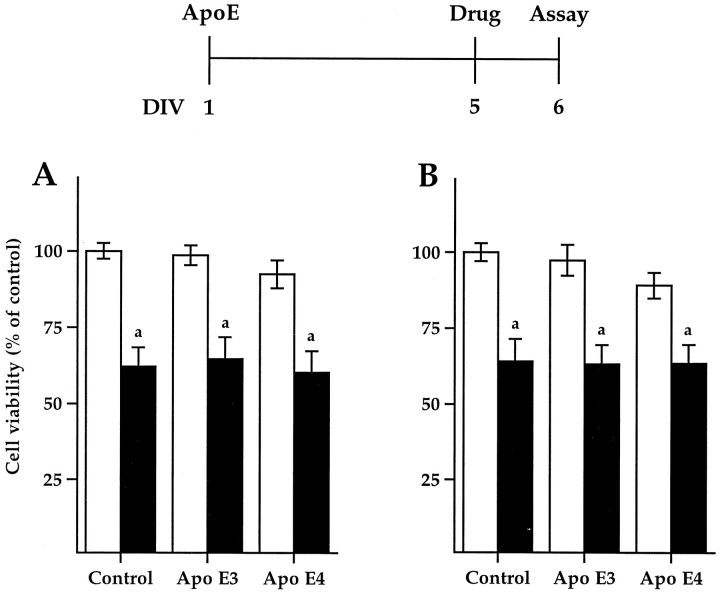
Fig. 3.

ApoE3 protects against Aβ-induced neurotoxicity. Primary rat pyramidal hippocampal cultures were treated on DIV-1 (A, B) or DIV-5 (C,D) with control, apoE3, or E4 (10 μg/ml, white bars). On DIV-5, 10 μm Aβ(1–40) (A, C) or Aβ(25–35) (B,D) was added (shaded bars), 1 hr after apoE treatment to C and D. Cell viability was analyzed on DIV-10 (see time line). Source of apoE as described (Fig. 1). Significance: versus control without Aβ (a1, p < 0.05;a2, p < 0.001); versus control with Aβ (b, p < 0.01).
Fig. 1.
ApoE4 is toxic to primary rat hippocampal neuron cultures. Dose–response. Cultures were treated on DIV-1 with 1, 10, or 100 μg apoE/ml culture medium, and cell viability was assayed on DIV-10. Source of apoE: conditioned medium from HEK cells stably transfected with human cDNA encoding apoE3 (•) or E4 (▴). Control cultures (▪) were treated with conditioned medium from cells stably transfected with the neomycin expression vector alone. Significance: versus control (*p < 0.05; **p < 0.001). Both 10 and 100 μg/ml apoE4 were also significantly neurotoxic after 5 d in culture (p < 0.05 and p < 0.01, respectively; data not shown).
Treatment with apoE3 protects hippocampal pyramidal neurons against β-amyloid-induced death
Aβ(25–35) has been shown previously to be toxic to primary rat hippocampal pyramidal neuron cultures (Loo et al., 1993; Prehn et al., 1996; Jordan et al., 1997). In the current study, 10 μm Aβ(25–35) and Aβ(1–40) treatment of DIV-5 cultures for 5 d produced a significant 30% decrease in cell viability (Fig.3). The involvement of apoptotic pathways in Aβ-induced neuronal toxicity has been shown previously by DNA laddering (Loo et al., 1993), Hoechst 33342 chromatin staining (Jordan et al., 1997), and TUNEL staining (Prehn et al., 1996). In the present study, dying neurons exhibited several of the hallmarks of apoptosis, including cell shrinkage and condensation of the cytoplasm, and chromatin aggregation and fragmentation in situ visualized with propidium iodine staining (Fig. 2D).
To determine the effect of native apoE3 and E4 on Aβ-induced neurotoxicity, we treated DIV-1 cultures with apoE (10 μg/ml) for 5 d before the addition of peptide on DIV-5 and assayed cell viability on DIV-10 (Fig. 3A,B). Only apoE3 protected against the Aβ-induced neurotoxicity. Whereas apoE4 alone was toxic, the toxicity of apoE4 and Aβ was not additive, suggesting that a subset of neurons may be vulnerable to cytotoxic treatments. Because apoE has been reported to affect neuronal development (Holtzman et al., 1995; Nathan et al., 1995; Puttfarcken et al., 1997), DIV-5 cultures were treated with apoE for just 1 hr before the addition of either Aβ(25–35) or Aβ(1–40) (Fig.3C,D). Again, only apoE3 protected against Aβ-induced neurotoxicity.
ApoE3 neuroprotection appears to be specific for β-amyloid-induced death
To determine whether the neuroprotective effect of apoE3 was specific to Aβ-induced toxicity, cultures were treated with staurosporine and NMDA (Fig. 4). Staurosporine is a protein kinase C inhibitor that induces apoptosis in a variety of cultured cells, including neurons (Jordan et al., 1997), whereas NMDA is a glutamate receptor agonist that produces an increase in intracellular calcium, triggering excitotoxic cell death. DIV-1 cultures were treated with either control or apoE3- or apoE4-conditioned HEK cell medium (10 μg apoE/ml) for 5 d before treatment with the toxic agent on DIV-5, and cell viability was assayed after 24 hr. ApoE3 was unable to protect against the 35% decrease in neuronal cell viability produced by both staurosporine (0.3 μm) and NMDA (100 μm) (Fig.4A,B, respectively). In addition, apoE3 had no effect on the increase in intracellular calcium induced by NMDA or KCl (Table 1).
Fig. 4.
ApoE3 does not protect against other neurotoxic stimuli. Primary rat pyramidal hippocampal cultures were treated with apoE3 or E4 (10 μg/ml) alone (white bars) or 5 d before the addition of 0.3 μm staurosporine (A) or 100 μm NMDA (B) (shaded bars), as described in Methods. ApoE was added on DIV-1, drug treatment was on DIV-5, and cell viability was analyzed on DIV-6 (see time line). Source of apoE as described (Fig. 1). Significance: absence versus presence of drug (a, p < 0.001).
Table 1.
ApoE isoforms do not effect Ca2+ homeostasis
| Treatment | NMDA (50 μm) | KCl (50 mm) | n |
|---|---|---|---|
| Control | 642 ± 39 | 464 ± 37 | 45 |
| ApoE3 | 589 ± 29 | 440 ± 83 | 59 |
| ApoE4 | 653 ± 51 | 401 ± 29 | 49 |
Neuronal cultures (DIV-5) were treated with apoE3 or apoE4 (10 μg/ml) for 5 d. The influx of Ca2+ was monitored using fura-2 microfluorimetry (see Methods). Data shown are peaks for [Ca2+]i and are given as nanomolar concentrations. Cells were perfused with 50 μm NMDA for 3 min in Mg2+-free saline solution or with 50 mm KCl for 1.5 min. Data are means ± SEM from neurons from four different cultures. Student’s t test did not yield significance (p < 0.05).
ApoE3:Aβ complex is more abundant than apoE4:Aβ complex in medium of treated neuronal cultures
In previous biochemical studies, native apoE3 formed an SDS-stable complex with Aβ that was 20-fold more abundant than apoE4:Aβ complex (LaDu et al., 1994, 1995b, 1997; Zhou et al., 1996; Yang et al., 1997). To determine whether this complex forms in the medium of neuronal cultures treated with Aβ and apoE, we used SDS-PAGE and Western blot analysis for Aβ and apoE on medium sampled at several time points over the incubation period (Fig.5). Monoclonal antibody 4G8 (Fig.5A) detected an SDS-stable 45 kDa apoE3:Aβ complex in greater abundance than apoE4:Aβ, as well as Aβ monomer (4 kDa), dimer and small aggregates. In addition, a complex with apoE3 dimer and Aβ was also detected. ApoE antiserum detected full-length apoE immunoreactive species corresponding to apoE3 and E4 monomer (35 kDa) and apoE3 dimer even after 10 d of incubation (Fig.5B). ApoE antiserum was unable to detect apoE:Aβ complex at the exposures of the immunoblots possible using this tissue culture medium.
Fig. 5.
ApoE3:Aβ ≫ apoE4:Aβ in the medium of treated neuronal cultures. Western blot analysis of the medium from primary rat pyramidal hippocampal cultures. DIV-5 cultures were treated with 10 μg of apoE/ml of apoE3 (lanes 1-4), apoE4 (lanes 5-8), or control (lanes 8-12) medium 1 hr before the addition of 10 μm Aβ(1–40). Source of apoE as described (Fig. 1). Aliquots of the culture medium were taken after the addition of apoE but before the addition of Aβ (lanes 1,5, 9), 2 hr after the addition of Aβ (lanes 2, 6, 10), on DIV-7 (lanes 3, 7, 11), and on DIV-10 (lanes 4, 8, 12). To visualize SDS-stable complex formation between apoE and Aβ, samples were run in nonreducing Laemmli buffer on 10–20% SDS-tricine gels, transferred to Immobilon-P membrane, and probed for Aβ with 4G8 antibody (A) and for apoE with antiserum (B).
RAP has no effect on apoE4 toxicity but abolishes the protective effect of apoE3 on Aβ-induced toxicity
It has been our working hypothesis that apoE:Aβ complex is cleared by apoE receptors. In vivo, apoE receptors in the brain are localized cell-specifically with neurons immunopositive for LRP (Moestrup et al., 1992; Wolf et al., 1992; Rebeck et al., 1993,1995) and glial cells for the LDL receptor (Pitas et al., 1987; Rebeck et al., 1993). Primary rat hippocampal neurons in vitro are LRP-immunopositive (Fig. 6). Our source of apoE3 and E4 is conditioned medium from HEK-293 cells stably expressing human apoE3 or E4, which is secreted associated with small, dense particles (LaDu et al., 1995a). These particles are taken up by U87 cells, human glioblastoma cells that express abundant LRP (Bu et al., 1994), in a RAP-inhibitable manner (M. J. LaDu and G. Bu, unpublished observations), demonstrating that the apoE in this preparation can serve as a ligand for LRP. RAP was used to inhibit apoE receptor-mediated uptake to determine whether this pathway plays a role in either the toxic actions of apoE4 alone (Fig.7) or the protective effect of apoE3 on Aβ-induced toxicity (Fig. 8). Cultures were treated with RAP on DIV-5 for a final concentration in the cell culture medium of 1.0 μm and additionally with 0.5 μm RAP on DIV-7 and DIV-9. This concentration of RAP is well in excess of the 3.3 nm Kd for LRP (Iadonato et al., 1993) as well as the 250 nm Kd for the LDL receptor (Medh et al., 1995). ApoE4 (10 μg/ml) was added 4 hr after the initial RAP treatment on DIV-5, and cell viability was assayed on DIV-10. ApoE4 treatment produced a 20% decrease in cell viability that was not affected by the addition of RAP (Fig. 7). In contrast, addition of RAP abolished the protective effects of apoE3 on Aβ-induced neurotoxicity. On DIV-5, apoE3 (10 μg/ml) was added 4 hr after the initial RAP treatment and 1 hr before the addition of Aβ (10 μm). Cell viability was assayed on DIV-10. Whereas apoE3 protected against the 25% decrease in cell viability induced by Aβ, RAP inhibited this neuroprotective effect of apoE3 (Fig. 8). In addition, RAP did not effect the neurotoxicity of Aβ in the absence of exogenous apoE, suggesting that in the culture system used here, Aβ is not being cleared by other LRP ligands.
Fig. 6.
Primary rat hippocampal neurons are LRP-immunopositive. Cultures grown on glass coverslips for 5 d were fixed in 4% paraformaldehyde, incubated with LRP antiserum, and visualized using streptavidin conjugated with indocarbocyanine (CY3). Representative photomicrograph is shown. Magnification, 2000×.
Fig. 7.
RAP has no effect on apoE4-induced neurotoxicity. Primary rat pyramidal hippocampal cultures (DIV-5) were treated with 1 μm purified rat RAP 4 hr before the addition of apoE4 (10 μg/ml). Additional RAP (0.5 μm) was added on DIV-7 and DIV-9, and cell viability was assayed on DIV-10 (see time line). Source of apoE as described (Fig. 1). A comparable concentration of BSA was used as a control for RAP and had no effect either alone or with apoE4 (data not shown). Significance: versus control (*p < 0.05).
Fig. 8.
RAP abolishes the protective effect of apoE3 on Aβ-induced neurotoxicity. Primary rat pyramidal hippocampal cultures (DIV-5) were treated with 1 μm RAP 4 hr before the addition of apoE3 (10 μg/ml). Aβ (10 μm) was added 1 hr later. Additional RAP (0.5 μm) was added on DIV-7 and DIV-9, and cell viability was assayed on DIV-10 (see time line). Source of apoE as described (Fig. 1). A comparable concentration of BSA was used as a control for RAP and had no effect alone, with apoE3, or with apoE3 and Aβ (data not shown). Significance: versus control (**p < 0.01).
DISCUSSION
In this paper, we have confirmed the neurotoxicity of the soluble Aβ peptides Aβ(25–35) and Aβ(1–40) and have shown that this neurotoxicity can be attenuated by the presence of apoE3 but not E4 in primary rat hippocampal neuron cultures. The viability of these cells is reduced by apoE4 even in the absence of Aβ. The toxic effects of both the Aβ peptides and apoE4 appear to be mediated by an increase in programmed cell death. The apoE3 protective effect may be specific to Aβ, because apoE3 did not protect the cultures from the neurotoxic effects of either NMDA or staurosporine. Furthermore, we demonstrate that the abrogation of the toxicity of Aβ by apoE3 is dependent on the participation of LRP or related receptors, whereas the toxicity of apoE4 appears to involve a different pathway.
The findings reported in this paper support the hypothesis that apoE3 facilitates the clearance of those Aβ species that are neurotoxic. We demonstrate that apoE3, unlike apoE4, forms readily detectable SDS-stable complex with Aβ(1–40) in the hippocampal cell culture medium under the conditions of these experiments. Although apoE3 almost completely abolishes the toxicity of Aβ, the molar ratio of Aβ and apoE3 in the medium in which the cells are incubated is ∼30:1. The SDS-stable complex between apoE3 and Aβ contains one molecule of Aβ per apoE3, as judged from the size of the complex observed by SDS-PAGE (Fig. 5). Clearly, there is not enough apoE3 present to complex with a major portion of the available Aβ. Therefore, either apoE3 protects against Aβ neurotoxicity by a mechanism that is not mediated by SDS-stable complex formation or only a portion of the Aβ present is actually neurotoxic and these molecules more readily form complex with apoE3. We favor the latter possibility for several reasons. First, the manifestations of Aβ cytotoxicity require prolonged incubation (5 d), during which cytotoxic species of Aβ could be generated. The precise nature of this species is unknown. Possible candidates are fibrillar forms of the peptide (Yankner et al., 1990; Pike et al., 1993; Simmons et al., 1994) or small soluble aggregates (Oda et al., 1995; Roher et al., 1996). Second, the attenuation of Aβ toxicity by apoE3 is reversed in the presence of RAP at concentrations that would block apoE binding to LRP and related receptors, including the LDL receptor. LRP is abundant on the cultured hippocampal neurons used in these experiments. The attenuation of the protective effect of apoE3 by RAP is consistent with the possibility that apoE3 protects against Aβ-induced toxicity by clearing the peptide via this neuronal LRP. RAP has no effect on Aβ-induced toxicity in the absence of exogenous apoE, suggesting that the peptide is not being cleared by other LRP ligands. It is also possible that apoE:Aβ complex is being cleared by astrocytes via the LDL receptor. Of the other candidate apoE receptors for Aβ clearance, gp330 is found only in ependymal cells in the CNS (Zheng et al., 1994), scavenger receptors are present on microglia (Christie et al., 1996; El Khoury et al., 1996), and the receptor for advanced glycation end products, although expressed by neurons, is not thought to be influenced by either apoE or RAP (Yan et al., 1996).
The hypothesis that apoE3 protects against Aβ-induced neurotoxicity by binding and clearing the peptide is also supported by the specificity of the apoE3 protective effect. ApoE3 does not protect against the neurotoxicity of staurosporine, which does not involve a cell surface receptor, or of NMDA, which does not involve an apoE-type receptor. In addition, apoE had no effect on the intracellular Ca2+ spikes induced by either KCl or NMDA. This suggests that apoE3 may be interrupting the Aβ toxicity cascade at a point before the initiation of cellular events and is consistent with a mechanism of action in which extracellular apoE3 interacts physically with the peptide to form a complex that is subsequently cleared by apoE receptors. Miyata and Smith (1996)reported that apoE exhibits an isoform-specific protective effect (E2 > E3 > E4) on the cytotoxicity induced by oxidative stress, including treatment with Aβ peptides. They propose that the protective action of apoE2 and E3 is attributable to the general antioxidant property of apoproteins. Our results do suggest that apoE may interact with Aβ extracellularly. However, because apoE receptors mediate the protective effects of apoE3, the antioxidant effects of apoE would have to occur within the endosomal compartment after the proteins are internalized. Such a mechanism is unknown.
The precise basis for the toxic effects of apoE4 alone remains unclear. It has been shown previously that apoE4 is associated with microtubule depolymerization, suggesting that apoE4 may actively promote neuronal destabilization (Nathan et al., 1995). Whether this contributes to the apoptosis induced by apoE4 in our experiments is not clear. However, the toxicity induced by apoE4 in the current experiments resembles programmed cell death and was not blocked by the addition of RAP. This suggests that apoE4 may signal an apoptotic cascade that is independent of apoE receptor-mediated uptake and subsequent intracellular localization. This is consistent with the neurotoxic effects of both purified full-length and a 22 kDa thrombin cleavage fragment of apoE4, species that have little receptor-binding activity (Marques et al., 1996, 1997). Our observation that apoE receptors do not mediate the neurotoxic actions of apoE4 is in apparent contrast to recent data from Tolar and associates (Tolar et al., 1997). Using chick sympathetic neurons, they report that the toxicity induced by a 22 kDa fragment and various peptides to the receptor-binding domain of apoE4 is abolished by RAP and other agents that disrupt the interaction between apoE and LRP. Although intriguing, the physiologic relevance of this profound toxicity observed with apoE4, toxicity that approached 80% within 24 hr of exposure, to a disease for which symptoms take decades to develop is unclear. In addition, it is not clear how purified apoE4 peptides can serve as ligands for LRP, the preferred substrate for which is apoE-enriched β-VLDL (Kowal et al., 1990).
The effects of Aβ and/or apoE on neural cultures appear to vary depending on the preparation of Aβ and apoE, the duration of exposure, the species and type of neuronal cultures, the developmental status of the neurons, the precise culture conditions, and the specific assay used to quantitate the effects. For example, Aβ can be either neurotrophic or neurotoxic, depending on the physical state and concentration of the peptide, as well as on the maturity of the cells (Whitson et al., 1989; Yankner et al., 1990; Pike et al., 1991, 1993). The effect of the apoE isoforms on neural cultures is also variable. Neurite outgrowth is enhanced by the addition of apoE3 to a variety of culture systems, including primary rat hippocampal neurons and several immortalized CNS cell lines (Holtzman et al., 1995; Nathan et al., 1995; Puttfarcken et al., 1997). However, apoE4 has been observed to inhibit (Bellosta et al., 1995; Nathan et al., 1995), have no effect (Holtzman et al., 1995; Fagan et al., 1996), or even enhance neurite outgrowth (Puttfarcken et al., 1997), apparently depending on the cell type and culture conditions. In addition, as also reported here, apoE4 may be toxic under some conditions (Marques et al., 1996,1997). Thus, the combined effects of apoE and Aβ promise to be no less complicated than their separate actions.
We have reported previously that both apoE3 and E4 are neurotrophic and protect primary rat hippocampal neurons from the toxicity produced by aged Aβ(1–42) (Puttfarcken et al., 1997), a peptide preparation with considerable secondary structure that induces neurotoxicity after as few as 24 hr of exposure (Puttfarcken et al., 1996). In these experiments, because the apoE was added at plating with cells in a minimally supplemented, serum-free medium with no glial feeder layer, we interpreted the results to mean that both apoE isoforms were acting as growth factors in these developing cultures (Puttfarcken et al., 1997). It has also been suggested that apoE modulates the activity of other growth-promoting agents (Mahley, 1988). Indeed, apoE has been shown recently to potentiate the activity of CNTF (Gutman et al., 1997). Thus, the combination and availability of other growth-promoting agents may modulate the neurotrophic actions of apoE, masking these trophic effects in culture models such as the one used here. For the current experiments, we have used primary rat hippocampal cells cultured in the presence of cerebral astrocytes. The glial feeder layer provided basic metabolic support to the neurons, allowing for experiments of longer duration and the possible detection of other apoE functions beside its growth-promoting activity. This longevity is also important when using soluble forms of Aβ, because the peptides manifest their neurotoxicity only after 4–5 d in culture at the concentrations used (current data) (Loo et al., 1993; Prehn et al., 1996; Jordan et al., 1997). The peptide preparation used in this study was dissolved in DMSO immediately before addition to a culture medium already containing apoE3 or E4. Thus, apoE was available during any aging or aggregation process affecting Aβ.
This paper describes an in vitro model in which apoE3 appears to protect against Aβ-induced neurotoxicity by forming an SDS-stable complex with the peptide that is subsequently cleared by apoE receptors. In addition, apoE4 alone induces neurotoxicity via a mechanism that is independent of apoE receptor-mediated uptake. Clearly, additional work is required to confirm and further elucidate both the neuroprotective effects of apoE3 and the neurotoxic actions of apoE4. Recently developed transgenic models expressing the human apoE isoforms in either neurons or glia will facilitate both the in vitro and in vivo study of these issues.
Footnotes
This work was supported in part by Public Health Service Grants DA02121, DA02575, and MH40165; American Health Assistance Foundation Grant 95100; National Institutes of Health Grant 1F32 HL08833-01 (M.J.L.); and a fellowship from the Minister of Education and Science of Spain (J.J). We thank Dudley Strickland for the LRP antibody (American Red Cross, Rockville, MD), Guojun Bu for RAP (Washington University, St. Louis, MO), John Lukens for technical assistance, and Alessandro Fatatis for expertise in Ca2+imaging.
J.J. and M.F.G. contributed equally to this work.
Correspondence should be addressed to Dr. Mary Jo LaDu, Department of Pathology, M/C 6079, University of Chicago, 5841 South Maryland Avenue, Chicago IL 60637.
REFERENCES
- 1.Bellosta S, Nathan BP, Orth M, Dong LM, Mahley RW, Pitas RE. Stable expression and secretion of apolipoproteins E3 and E4 in mouse neuroblastoma cells produces differential effects on neurite outgrowth. J Biol Chem. 1995;270:27063–27071. doi: 10.1074/jbc.270.45.27063. [DOI] [PubMed] [Google Scholar]
- 2.Brorson J, Bindokas V, Iwama T, Marcucilli C, Chisholm J, Miller R. The Ca2+ influx induced by β-amyloid peptide 25–35 in cultured hippocampal neurons results from network excitation. J Neurosci. 1995;15:325–339. doi: 10.1002/neu.480260305. [DOI] [PubMed] [Google Scholar]
- 3.Bu G, Maksymovitch E, Geuze H, Schwartz A. Subcellular localization and endocytic function of low density lipoprotein receptor-related protein in human glioblastoma cells. J Biol Chem. 1994;269:29874–29882. [PubMed] [Google Scholar]
- 4.Christie R, Freeman M, Hyman B. Expression of the macrophage scavenger receptor, a multifunctional lipoprotein receptor, in microglia associated with senile plaques in Alzheimer’s disease. Am J Pathol. 1996;148:399–403. [PMC free article] [PubMed] [Google Scholar]
- 5.El Khoury J, Hickman S, Thomas C, Cao L, Silverstein S, Loike J. Scavenger receptor-mediated adhesion of microglia to β-amyloid fibrils. Nature. 1996;382:716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- 6.Fagan A, Bu G, Sun Y, Daugherty A, Holtzman D. Apolipoprotein E-containing high density lipoprotein promotes neurite outgrowth and is a ligand for the low density lipoprotein receptor-related protein. J Biol Chem. 1996;271:30121–30125. doi: 10.1074/jbc.271.47.30121. [DOI] [PubMed] [Google Scholar]
- 7.Favaron M, Manev H, Alho H, Bertolini M, Ferret B, Guidotti A, Costa E. Gangliosides prevent glutamate and kainate neurotoxicity in primary neuronal cultures of neonatal rat cerebellum and cortex. Proc Natl Acad Sci USA. 1988;85:7351–7355. doi: 10.1073/pnas.85.19.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gelman B, Rifai N, Christenson R, Silverman L. Cerebrospinal fluid and plasma apolipoproteins in patients with multiple sclerosis. Ann Clin Lab Sci. 1988;18:46–52. [PubMed] [Google Scholar]
- 9.Grynkiewicz G, Poeuie M, Tsien RY. A new geneation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 10.Gutman C, Strittmatter W, Weisgraber K, Matthew W. Apolipoprotein E binds to and potentiates the biological activity of ciliary neurotrophic factor. J Neurosci. 1997;17:6114–6121. doi: 10.1523/JNEUROSCI.17-16-06114.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holtzman DM, Pitas RE, Kilbridge J, Nathan B, Mahley RW, Bu G, Schwartz AL. Low density lipoprotein receptor-related protein mediates apolipoprotein E-dependent neurite outgrowth in a central nervous system-derived neuronal cell line. Proc Natl Acad Sci USA. 1995;92:9480–9484. doi: 10.1073/pnas.92.21.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iadonato S, Bu G, Maksymovitch E, Schwartz A. Interaction of a 39 kDa protein with the low-density-lipoprotein-receptor-related protein (LRP) on rat hepatoma cells. Biochem J. 1993;296:867–875. doi: 10.1042/bj2960867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan J, Galindo M, Miller R. Role of calpain- and interleukin-1β converting enzyme-like proteases in the β-amyloid-induced death of rat hippocampal neurons in culture. J Neurochem. 1997;68:1612–1621. doi: 10.1046/j.1471-4159.1997.68041612.x. [DOI] [PubMed] [Google Scholar]
- 14.Kowal R, Herz J, Weisgraber K, Mahley R, Brown M, Goldstein J. Opposing effects of apolipoproteins E and C on lipoprotein binding to low density lipoprotein receptor-related protein. J Biol Chem. 1990;265:10771–10779. [PubMed] [Google Scholar]
- 15.LaDu MJ, Falduto MT, Manelli AM, Reardon CA, Getz GS, Frail DE. Isoform-specific binding of apolipoprotein E to β-amyloid. J Biol Chem. 1994;269:23403–23406. [PubMed] [Google Scholar]
- 16.LaDu MJ, Lukens JR, Pederson TE, Reardon CA, Getz GS, Falduto MT. Particle size distribution of native apolipoprotein E in the presence of β-amyloid peptide. Soc Neurosci Abstr. 1995a;21:5. [Google Scholar]
- 17.LaDu MJ, Pederson TE, Frail DE, Reardon CA, Getz GS, Falduto MT. Purification of apolipoprotein E attenuates isoform-specific binding to β-amyloid. J Biol Chem. 1995b;270:9039–9042. doi: 10.1074/jbc.270.16.9039. [DOI] [PubMed] [Google Scholar]
- 18.LaDu MJ, Lukens JR, Reardon CA, Getz GS. Association of human, rat, and rabbit apolipoprotein E with β-amyloid. J Neurosci Res. 1997;49:9–18. [PubMed] [Google Scholar]
- 19.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Loo D, Copani A, Pike C, Whittemore E, Walencewicz A, Cotman C. Apoptosis is induced by β-amyloid in cultured central nervous system neurons. Proc Natl Acad Sci USA. 1993;90:7951–7955. doi: 10.1073/pnas.90.17.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma JH, Brewer B, Jr, Potter H. Alzheimer Aβ neurotoxicity: promotion by antichymotrypsin, apoE4; inhibition by Aβ-related peptides. Neurobiol Aging. 1996;17:773–780. doi: 10.1016/0197-4580(96)00112-1. [DOI] [PubMed] [Google Scholar]
- 22.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 23.Marques M, Tolar M, Harmony J, Crutcher K. A thrombin cleavage fragment of apolipoprotein E exhibits isoform-specific neurotoxicity. NeuroReport. 1996;7:2529–2532. doi: 10.1097/00001756-199611040-00025. [DOI] [PubMed] [Google Scholar]
- 24.Marques M, Tolar M, Crutcher K. Apolipoprotein E exhibits isoform-specific neurotoxicity. Alzheimer Res. 1997;3:1–6. doi: 10.1097/00001756-199611040-00025. [DOI] [PubMed] [Google Scholar]
- 25.Medh J, Fry G, Bowen S, Pladet M, Strickland D, Chappell D. The 39 kDa receptor-associated protein modulates lipoprotein catabolism by binding to LDL receptors. J Biol Chem. 1995;270:536–540. doi: 10.1074/jbc.270.2.536. [DOI] [PubMed] [Google Scholar]
- 26.Metzger RE, LaDu MJ, Pan JB, Getz GS, Frail DE, Falduto MT. Neurons of the human frontal cortex display apolipoprotein E immunoreactivity: implications for Alzheimer’s disease. J Neuropathol Exp Neurol. 1996;55:372–380. doi: 10.1097/00005072-199603000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Meucci O, Miller RJ. gp120-induced neurotoxicity in hippocampal pyramidal neuron cultures; protective action of TGF-β1. J Neurosci. 1996;16:4080–4088. doi: 10.1523/JNEUROSCI.16-13-04080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyata M, Smith J. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and β-amyloid peptides. Nat Genet. 1996;14:55–61. doi: 10.1038/ng0996-55. [DOI] [PubMed] [Google Scholar]
- 29.Moestrup S, Gliemann J, Pallensen G. Distribution of the α2-macroglobulin receptor/low density lipoprotein receptor-related protein in human tissues. Cell Tissue Res. 1992;269:375–382. doi: 10.1007/BF00353892. [DOI] [PubMed] [Google Scholar]
- 30.Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer’s disease and kuru plaque amyloid in Creutzfeldt–Jakob disease. Brain Res. 1991;541:163–166. doi: 10.1016/0006-8993(91)91092-f. [DOI] [PubMed] [Google Scholar]
- 31.Nathan B, Bellosta S, Sanan D, Weisgraber K, Mahley R, Pitas R. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science. 1994;264:850–852. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- 32.Nathan B, Chang K, Bellosta S, Brisch E, Ge N, Mahley R, Pitas R. The inhibitory effect of apolipoprotein E4 on neurite outgrowth is associated with microtubule depolymerization. J Biol Chem. 1995;270:19791–19799. doi: 10.1074/jbc.270.34.19791. [DOI] [PubMed] [Google Scholar]
- 33.Oda T, Wals P, Osterburg H, Johnson S, Pasinetti G, Morgan T, Rozovsky I, Stine W, Snyder S, Holzman T, Krafft G, Finch C. Clusterin (apoJ) alters the aggregation of amyloid β-peptide (Aβ1–42) and forms slowly sedimenting Aβ complexes that cause oxidative stress. Exp Neurol. 1995;136:22–31. doi: 10.1006/exnr.1995.1080. [DOI] [PubMed] [Google Scholar]
- 34.Pike C, Walencewicz A, Glabe C, Cotman C. Aggregation-related toxicity of synthetic β-amyloid protein in hippocampal cultures. Eur J Pharmacol. 1991;207:367–368. doi: 10.1016/0922-4106(91)90014-9. [DOI] [PubMed] [Google Scholar]
- 35.Pike C, Burdick D, Walencewicz A, Glabe C, Cotman C. Neurodegeneration induced by β-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci. 1993;13:1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pitas R, Boyles J, Lee S, Hui D, Weisgraber K. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B,E(LDL) receptors in the brain. J Biol Chem. 1987;262:14352–14360. [PubMed] [Google Scholar]
- 37.Prehn J, Bindokas V, Jord G, Roos R, Boise L, Thompson C, Krajewski S, Reed J, Miller R. Protective effect of transforming growth factor-β1 on β-amyloid neurotoxicity in rat hippocampal neurons. Mol Pharmacol. 1996;49:319–328. [PubMed] [Google Scholar]
- 38.Puttfarcken PS, Manelli AM, Neilly JT, Frail DE. Inhibition of age-induced β-amyloid neurotoxicity in rat hippocampal cells. Exp Neurol. 1996;138:73–81. doi: 10.1006/exnr.1996.0048. [DOI] [PubMed] [Google Scholar]
- 39.Puttfarcken PS, Manelli AM, Falduto MT, Getz GS, LaDu MJ. Effect of apolipoprotein E on neurite outgrowth and β-amyloid induced toxicity in developing rat primary hippocampal cultures. J Neurochem. 1997;68:760–769. doi: 10.1046/j.1471-4159.1997.68020760.x. [DOI] [PubMed] [Google Scholar]
- 40.Rebeck G, Reiter J, Strickland D, Hyman B. Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron. 1993;11:575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- 41.Rebeck G, Harr S, Strickland D, Hyman B. Multiple, diverse senile plaque-associated proteins are ligands of an apolipoprotein E receptor, the α2-macroglobulin receptor/low-density-lipoprotein receptor-related protein. Ann Neurol. 1995;37:211–217. doi: 10.1002/ana.410370212. [DOI] [PubMed] [Google Scholar]
- 42.Roher A, Chaney M, Kuo Y, Webster S, Stine W, Haverkamp L, Woods A, Cotter R, Tuohy J, Krafft G, Bonnell BS, Emmerling MR. Morphology and toxicity of Aβ-(1–42) dimer derived from neuritic and vascular amyloid deposits of Alzheimer’s disease. J Biol Chem. 1996;271:20631–20635. doi: 10.1074/jbc.271.34.20631. [DOI] [PubMed] [Google Scholar]
- 43.Saunders A, Schmader K, Breitner J, Benson M, Brown W, Goldfarb L, Goldgaber D, Manwaring M, Szymanski M, McCown N, Dole K, Schmechel D, Strittmatter W, Pericak-Vance M, Roses A. Apolipoprotein E e4 allele distributions in late-onset Alzheimer’s disease and in other amyloid-forming diseases. Lancet. 1993;342:710–711. doi: 10.1016/0140-6736(93)91709-u. [DOI] [PubMed] [Google Scholar]
- 44.Schmechel D, Saunders A, Strittmatter W, Crain B, Hulette C, Joo S, Pericak-Vance M, Goldgaber D, Roses A. Increased amyloid β-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scholz W, Palfrey H. Glutamate-stimulated protein phosphorylation in cultured hippocampal pyramidal neurons. J Neurosci. 1991;11:2422–2432. doi: 10.1523/JNEUROSCI.11-08-02422.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simmons LK, May PC, Tomaselli KJ, Rydel RE, Fuson KS, Brigham EF, Wright S, Lieberburg I, Becker GW, Brems DN, Li WY. Secondary structure of amyloid β peptide correlates with neurotoxic activity in vitro. Mol Pharmacol. 1994;45:373–379. [PubMed] [Google Scholar]
- 47.Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tolar M, Marques MA, Harmony JAK, Crutcher KA. Neurotoxicity of the 22 kDa thrombin-cleavage fragment of apolipoprotein E related synthetic peptides is receptor-mediated. J Neurosci. 1997;17:5678–5686. doi: 10.1523/JNEUROSCI.17-15-05678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warshawsky I, Bu G, Schwartz A. Identification of domains on the 39 kDa protein that inhibit the binding of ligands to the low density lipoprotein receptor-related protein. J Biol Chem. 1993;268:22046–22054. [PubMed] [Google Scholar]
- 50.Whitson J, Selkoe D, Cotman C. Amyloid β protein enhances the survival of hippocampal neurons in vitro. Science. 1989;243:1488–1490. doi: 10.1126/science.2928783. [DOI] [PubMed] [Google Scholar]
- 51.Wolf B, Beatriz M, Lopes S, Vanden Berg SR, Gonias S. Characterization and immunohistochemical localization of α2-macroglobulin receptor (low-density lipoprotein receptor-related protein) in human brain. Am J Pathol. 1992;141:37–42. [PMC free article] [PubMed] [Google Scholar]
- 52.Yan S, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt A. RAGE and amyloid-β peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 53.Yang D-S, Smith J, Zhou Z, Gandy A, Martins R. Characterization of the binding of amyloid-β peptide to cell culture-derived native apolipoprotein E2, E3, and E4 isoforms and to isoforms from human plasma. J Neurochem. 1997;68:721–725. doi: 10.1046/j.1471-4159.1997.68020721.x. [DOI] [PubMed] [Google Scholar]
- 54.Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid β protein: reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- 55.Zheng G, Bachinsky D, Stamenkovic I, Strickland D, Brown D, Andres G, McCluskey R. Organ distribution in rats of two members of the low-density lipoprotein receptor gene family, Gp330 and LRP/α2MR, and the receptor-associated protein (RAP). J Histochem Cytochem. 1994;42:531–542. doi: 10.1177/42.4.7510321. [DOI] [PubMed] [Google Scholar]
- 56.Zhou Z, Smith JD, Greengard P, Gandy S. Alzheimer amyloid-β peptide forms denaturant-resistant complex with type E3 but not type E4 isoform of native apolipoprotein E. Mol Med. 1996;2:175–180. [PMC free article] [PubMed] [Google Scholar]