Abstract
The role of dendritic morphology in integration and processing of neuronal inputs is still unknown. Models based on passive cable theory suggest that dendrites serve to isolate synapses from one another. Because of decreases in driving force or resistance, two inputs onto the same dendrite would diminish their joint effect, resulting in sublinear summation. When on different dendrites, however, inputs would not interact and therefore would sum linearly. These predictions have not been rigorously tested experimentally. In addition, recent results indicate that dendrites have voltage-sensitive conductances and are not passive cables. To investigate input integration, we characterized the effects of dendritic morphology on the summation of subthreshold excitatory inputs on cultured hippocampal neurons with pyramidal morphologies. We used microiontophoresis of glutamate to systematically position inputs throughout the dendritic tree and tested the summation of two inputs by measuring their individual and joint effects. We find that summation was surprisingly linear regardless of input position. For small inputs, this linearity arose because no significant shunts or changes in driving force occurred and no voltage-dependent channels were opened. Larger inputs also added linearly, but this linearity was caused by balanced action of NMDA and IA potassium conductances. Therefore, active conductances can maintain, paradoxically, a linear input arithmetic. Furthermore, dendritic morphology does not interfere with this linearity, which may be essential for particular neuronal computations.
Keywords: hippocampus, cortex, NMDA, iontophoresis, dendrite, potassium
One of the most striking and beautiful features of neurons is their diverse dendritic morphologies (Ramón y Cajal, 1904). The purpose of these geometrically intricate structures and their role in synaptic integration is still unknown. In addition, the function of the rich variety of voltage-sensitive channels distributed heterogenously throughout the dendritic arbor is also unclear (Johnston et al., 1996; Yuste and Tank, 1996). These morphological and biophysical properties would be expected to significantly influence input integration, providing the neuron with complex response properties.
Theoretical analyses of integration began with the assumption that dendrites can be modeled as passive cables (Rall, 1964; Jack et al., 1975). Passive cable theory predicts that electrically isolated synaptic inputs sum algebraically, whereas synapses that are electrically close are attenuated because of reduction in the driving force of their ions or current shunting caused by a transient decrease of input resistance in the dendrite (Rall, 1995). Although these ideas are widespread, experimental studies of these predictions have been surprisingly scant. Analysis of synaptic potentials from motoneuronsin vivo revealed summed potentials that were less than those expected for independent inputs. This discrepancy was ascribed to sublinear interactions between nearby synapses (Burke, 1967; Kuno and Miyahara, 1969). Nevertheless, in CA1 hippocampal pyramidal neuronsin vitro, separate inputs summated linearly (Langmoen and Andersen, 1983). Finally, iontophoresis at multiple sites on dendrites of turtle spinal cord motoneurons in vitro also showed linear integration (Skydsgaard and Hounsgaard, 1994). In all of these experiments, however, the dendritic morphology and exact position of the inputs was not determined, so the specific effects of morphology on input summation were not explored.
To examine directly how dendritic morphology and active conductances influence synaptic integration, we have studied the summation of simulated subthreshold synaptic inputs in pyramidal hippocampal neurons in culture using focal microiontophoresis of the excitatory neurotransmitter glutamate. Although cultured neurons may differ in particular biophysical properties from in vivo neurons, their rich dendritic morphologies and accessibility make them an ideal model system for studying the effects of dendritic morphology on neuronal integration. As we show, microiontophoresis can be used to exactly position a reproducible excitatory input that resembles endogenous synaptic inputs anywhere on the dendritic tree. Microiontophoresis also allows pharmacological manipulations that would be impossible with stimulated transmitter release.
Here, we examine two questions: (1) do dendritic branches serve to isolate inputs, as predicted by cable theory, and (2) how do active conductances affect input summation? We find that excitatory inputs sum linearly regardless of their position, suggesting that the sublinear scenarios discussed by cable theories do not apply to electrotonically compact cultured neurons and are even less likely to apply to pyramidal neurons in vivo. Also, we find that the linear summation is achieved by a remarkable balance of conductances, canceling each other’s effect.
MATERIALS AND METHODS
Hippocampi from newborn Sprague Dawley rats were dissociated and plated at low density on poly-l-lysine-coated coverglass, without glial support cells, following standard protocols (Goslin and Banker, 1991). Cells from 7- to 14-d-old cultures were used for experiments. Recordings from pyramidal neurons were made using the gigaohm-seal, nystatin perforated-patch technique (Horn and Marty, 1988). Conventional patch pipettes were filled with pipette saline containing (in mm): 10 NaCl, 10 KCl, 10 HEPES, 140 potassium gluconate, and nystatin (Sigma, St. Louis, MO) at a final concentration of 150 μm. Nystatin stock (46 mm; 1 mg/ml in Me2SO) was prepared before each experiment, stored at room temperature in a light-proof container, and used for up to 6 hr after preparation. A conventional gigaohm seal was formed by pressing the pipette gently against the soma of a neuron and providing light suction. Whole-cell recordings developed within 1–5 min of making a seal, with access resistances between 15 and 25 MΩ. The amplifier (Axopatch 1D or Axoclamp 2B, Axon Instruments, Foster City, CA) was then switched to current-clamp mode. In all experiments, potentials were recorded at room temperature, and current was injected if necessary to keep the resting potential at approximately −65 mV, unless otherwise indicated. Voltages were filtered at 1 kHz, digitized, stored, and analyzed using Superscope and an analog-to-digital board (GW Instruments). The recording solution consisted of (in mm): 150 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 100 μm Phaclophen (RBI), pH 7.4. Blockers were bath-applied: AP-5 (100 μm; RBI), tetrodotoxin (TTX) (5 μm; Sigma), NiCl2(1 mm, Sigma), TEA (10 mm, Sigma), and 4-amino-pyridine (4-AP) (5 mm, Sigma). Iontophoresis pipettes were pulled to a fine tip with ∼150 MΩ resistance when filled with 2.5 m NaCl. Pipettes were filled with 250 mm sodium glutamate in Milli-Q water, pH 8.0. Four stimulus isolation units and a Master-8 controller (AMPI, Inc.) were used to provide holding current (∼1–10 nA) and ejection current (∼100 nA) of various durations to the pipettes. Potentials of relatively large amplitude (1–10 mV) and fast rise time (2.2 ± 0.3 mV/msec) were obtained with ejection currents between 0.5 and 5 msec. Action potentials or significantly larger and longer potentials could always be achieved by increasing the ejection current amplitude or duration, indicating that neither the neuron nor the amplifier was saturated by combined iontophoretic events. Each experiment consisted of multiple trials (between 5 and 15) at a given pipette position and amplitude of glutamate response. Throughout this paper, measurements are expressed as the mean ± SEM for a number of experiments unless otherwise indicated.
RESULTS
Microiontophoresis-induced depolarizations resemble EPSPs and can be precisely localized
For all experiments, we used neurons with pyramidal morphologies, i.e., neurons having a pyramidal soma, a large, single apical dendrite that originates from the apex of the soma, and a number of smaller basal dendrites (Fig.1A). The dendritic identity of the processes was established by MAP2 immunocytochemistry (Caceres et al., 1986). Cells with typical pyramidal morphologies were present even in low-density cultures, suggesting that the developmental program that builds pyramidal dendritic trees is cell-autonomous (Banker and Cowan, 1979; K. Zelevinsky, S. Cash, and R. Yuste, unpublished observations). Whole-cell recordings from the somata of the neurons demonstrated an electrophysiology similar to that of CA1 pyramidal neurons of comparable developmental stages, with resting membrane potentials of approximately −65 mV and action potential threshold reached at −55 to −45 mV. Action potentials were 60–125 mV in amplitude and 3–10 msec in duration and occurred in a regular spiking pattern with frequency accommodation (Fig.1B). Finally, many neurons showed afterhyperpolarizations.
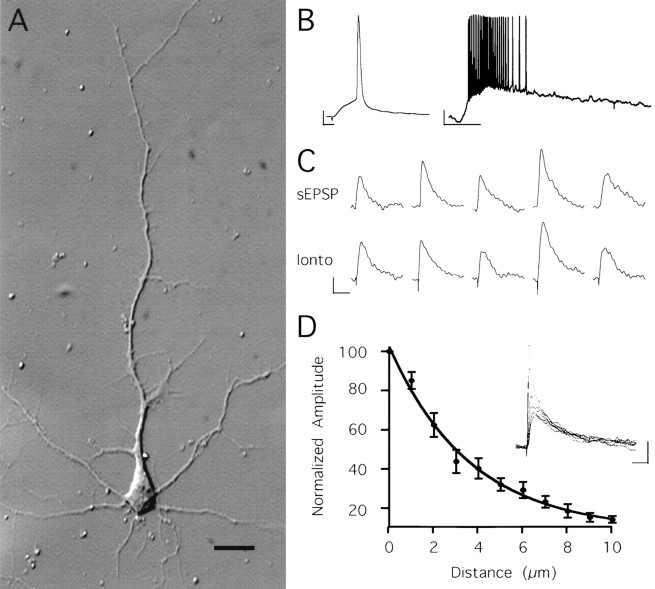
Fig. 1.
Iontophoretic potentials resemble spontaneous EPSPs and are spatially localized. A, Photomicrograph of a 12 d in vitro cultured hippocampal neuron showing a pyramidal morphology, with a single large apical dendrite and several smaller basal dendrites. Scale bar, 40 μm. B, Examples of action potentials elicited from two different neurons using either a short (left) or long (right) glutamate depolarization. Fast, large action potentials that accommodated in frequency with long depolarizations were present in the cultured neurons. Calibration: 10 mV, 10 msec for left; 10 mV, 1 sec for right. C, Spontaneous EPSPs (top set) and iontophoretically induced potentials (bottom set) from five different cells. Note that the two sets of potentials are nearly identical in waveform.D, Peak amplitude of the average of five iontophoretic pulses as the pipette was moved vertically in 1 μm increments away from the point of maximal amplitude. Data from 10 cells was fit with a single exponential. Inset shows the decreases of the responses of a representative cell as the pipette was moved 5 μm from the original location. Note how the response decreasese-fold in ∼5 μm. Calibration for C, D: 5 mV, 30 msec.
Cultured neurons formed excitatory synapses with one another, producing spontaneous EPSPs (sEPSPs) with both NMDA and non-NMDA components. For our experiments, iontophoresis pipettes were positioned and the ejection current amplitude and duration were adjusted so that iontophoretic depolarizations closely resembled these sEPSPs. Rise and decay times of iontophoretic and sEPSPs were not significantly different (0.52 ± 0.25 mV/msec rise and 60 ± 11 msec decay for sEPSPs; 0.47 ± 0.21 mV/msec and 62 ± 8 msec for iontophoresis EPSPs; n = 5 cells) (Fig.1C). Peak amplitudes of sEPSPs ranged from 1 to 15 mV (mean = 3.8 ± 0.6 mV), and iontophoresis potentials used throughout this study were matched to this range.
To verify that the recording and iontophoresis conditions were stable, we characterized the responses to a single pipette by ejecting glutamate twice, with an interval of 5 sec for repeated trials. The peak amplitude of the second depolarization differed from the first by only 0.7% ± 1.7% (mean ± SEM; n = 12 cells), showing that consecutive iontophoretic potentials were identical. We also characterized the spatial localization of our stimulus because a large spread of ejected glutamate could produce overlapping activation of receptors, which would confound true input interactions. We positioned a single pipette to achieve a maximal depolarization and then raised it vertically above the dendrite in 1 μm increments, measuring the potential for five trials at each position (Fig.1C). The amplitude of responses decreased e-fold for every 5 μm movement. Similar results were found when the pipette was moved horizontally away from the dendrite (not shown). In addition, the ejected glutamate was not saturating, because larger potentials than those used for the experiments could always be obtained by increasing the duration or ejection current. Microiontophoresis is therefore a reliable method for excitation of a specific site in the dendritic arbor.
Input summation is linear and independent of position and distance
We first established how two inputs summed using simultaneous iontophoresis at two points on the dendritic tree (Fig.2). While recordings were made from cells at a membrane potential of −65mV, iontophoresis pipettes were positioned at specific dendritic locations. The current from each pipette was adjusted to evoke a subthreshold depolarization even when both were activated simultaneously. Trials in which the combined depolarization triggered an action potential were removed from our analysis, as were trials with large spontaneous activity. The depolarization caused by each input was measured separately and then both were tested together (Fig. 2A). The individual responses were added, and this algebraic summation was compared with the potential evoked by simultaneous excitation from both pipettes (Fig. 2B). As a further control to test whether the two pipettes interacted electrically, we positioned one pipette next to the dendrite and a second pipette 20 μm above it, a location that elicited no response. With this configuration, the depolarization caused by simultaneous activation was the same as that of the first pipette (99.5% ± 3.0%; n = 4 cells). This indicates that the two pipettes act as independent sources of excitation and that there is no significant glutamate spillover.
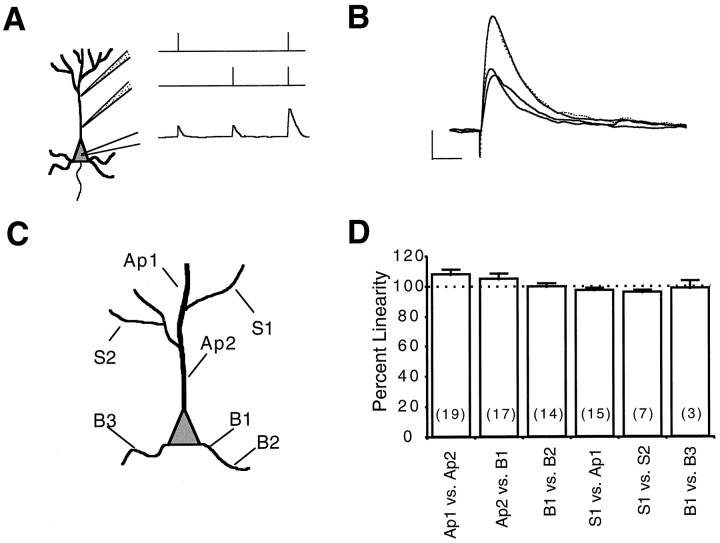
Fig. 2.
Synaptic summation is independent of input position. A, Diagram of the experiment. Two microiontophoresis pipettes were positioned on the dendritic tree of a pyramidal neuron in culture. Glutamate was ejected first from each pipette individually and then from both simultaneously. The algebraic sum of the individual potentials was then compared with the actual potential recorded with simultaneous stimuli. B, Averaged results from a representative cell (5 trials). The twolower lines are the responses from each of the pipettes, the dashed line indicates their algebraic sum, and thetop solid line is the simultaneous response measured. Note the overlap between the expected and actual summed depolarizations, indicating linear summation. Calibration: 2 mV, 25 msec. C, Schematic diagram of a neuron showing pipette locations studied, including apical (Ap), second or higher order branches (S), and basal dendrites (B). D, Histogram of the linearity of the summed responses, measured by the ratio of actual to expected peak amplitudes, for different input configurations. No significant deviations from linearity are observed (ANOVA, p < 0.05; number of experiments in parentheses).
Summation of the peak depolarization of inputs at any two dendritic positions was 102 ± 1.5% of expected (n = 75 pairs of inputs on 40 different cells), a result that is not statistically different from a pure algebraic summation. This linear summation occurred regardless of the specific position of the inputs (Fig. 2C,D), ranging from 96 to 108% for the mean responses. The configurations tested included both inputs on the apical dendrite, on a basal dendrite, on different basal dendrites, on an apical and a basal dendrite, on a second order branch of the apical dendrite and the apical dendrite itself, and on two different second or higher order branches of the apical tree (Fig. 2C). In addition, occasional nonlinearities observed in some experiments did not occur in any particular stimulus configuration. Therefore, the presence of a branching point or the soma between the inputs does not significantly affect the linear summation.
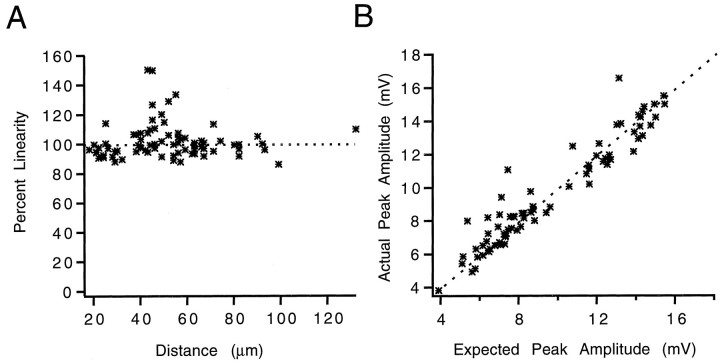
Is dendritic integration affected by the distance between inputs? As predicted by cable models (Rall, 1995), a strong distance dependency would be expected if inputs would interact. Nevertheless, no significant correlation between intra-pipette distance and summation was observed over the range of ∼15–120 μm (Fig.3A), indicating that distance does not significantly affect spatial summation of inputs. We also investigated whether there was a relationship between the amplitude of the stimuli and summation. Such an effect might be expected because of the voltage-dependent dendritic conductances. Nevertheless, the linearity was independent of the amplitude of the stimulus (Fig.3B).
Fig. 3.
Linearity of summation is independent of intrapipette distance and response amplitude. A, Percent linearity of the summation of two inputs in all spatial configurations as a function of distance between the pipettes. Distance was measured as the shortest length along the neuron between the two inputs.Dashed line indicates linearity. No systematic correlation is observed. B, Percent linearity of the summation of two inputs as a function of combined peak amplitude. No significant deviation from linearity is seen. Each pointrepresents a single experiment. More than one experiment may be performed on a given cell in either different positions or amplitudes. These data include 75 different experiments on 40 different cells and are the same data as those plotted in Figure 2.
Linear summation results from balanced activation of NMDA and potassium conductances
We were surprised by the essentially linear summation, because it is well established that dendrites have receptors and voltage-dependent channels, the behavior of which is nonlinear and can be activated by subthreshold EPSPs (Stafstrom et al., 1985; Sutor and Hablitz, 1989;Deisz et al., 1991; Hirsch and Gilbert, 1991; Magee and Johnston, 1995;Stuart and Sakmann, 1995). We dissected the mechanisms underlying the linearity by examining summation of two inputs on the apical dendrite under various pharmacological and electrophysiological conditions (Fig.4). Our logic was first to assess the role of the NMDA receptor in summation, because it is known to produce boosting of synaptic inputs (Jones and Baughman, 1988; Thomson et al., 1988; Artola and Singer, 1990), and then to study the contribution of the voltage-dependent sodium, calcium, and potassium conductances to the process. Because of the voltage-dependency of the activation of the channels, we explored two regimens of excitation: either small (<10 mV) combined potentials near rest or large (>10 mV) potentials approaching spike threshold.
Fig. 4.
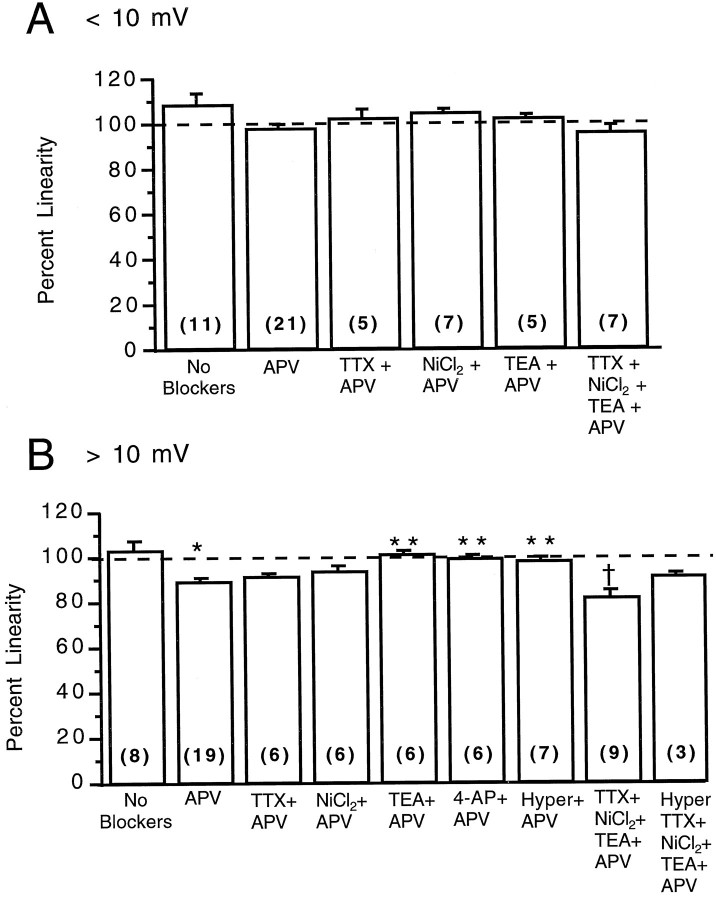
Mechanisms underlying linear summation. Effects of APV, TTX, NiCl2, TEA, 4-AP, and hyperpolarization on the linearity of two inputs on the apical dendrite. A, For combined events <10 mV, none of the manipulations produced a significant change from control conditions or from experiments performed with APV (ANOVA, p < 0.01). This suggests that NMDA, Na+, Ca2+, and K+ conductances do not contribute significantly to the summation. B, For combined events >10 mV, application of APV produced a significance sublinearity compared with controls (*). TEA, 4-AP, and hyperpolarization produced a significant block of the APV effect (**). The presence of all types of blockers revealed a significant sublinearity, compared with theTEA+APV experiments (†, for all cases, ANOVA, p < 0.002). These results suggests that the normal linearity for large amplitude events is produced by a balanced action of NMDA and K+ conductances. Blockade of all conductances reveals a sublinearity that could be attributable to driving force reduction or conductance shunting. Number of experiments in parentheses.
For small depolarizations, linear summation was unaffected by blocking NMDA, sodium, calcium, or potassium channels (Fig.4A). We conclude that small depolarizations do not depolarize the dendrite enough to activate any of these conductances. Also, no significant driving force reduction or shunt must occur, because summation is linear. Thus, the linearity under these conditions is purely a reflection of lack of interaction among inputs.
For larger depolarizations (Fig. 4B), summation was significantly sublinear when NMDA receptors were blocked with APV (91 ± 1%; n = 19 cells). This suggests that, in agreement with previous work (Jones and Baughman, 1988; Thomson et al., 1988; Artola and Singer, 1990), activation of NMDA receptors boosts excitatory inputs. We then explored the mechanisms of the sublinearity revealed under APV by blocking sodium, calcium, and potassium conductances. The selective sodium channel blocker TTX abolished all action potentials but did not produce a significant difference in the sublinearity under APV. Application of the voltage-dependent calcium channel blocker NiCl2 also did not produce a statistically significant difference in the sublinearity. In contrast, blocking of voltage-dependent K+ channels with tetraethylammonium (TEA) removed the sublinearity revealed under APV, making summation linear again (102 ± 1%; n = 6 cells). To investigate which type of potassium channel is responsible, we used 4-AP (5 mm), a blocker specific for the IA subtype of potassium conductance. This also reversed the sublinearity revealed under APV (100 ± 1%; n = 6 cells), suggesting that sublinearity was caused by activation of IA potassium channels. In agreement with this, hyperpolarization of the neuron to −75 mV, which moves the membrane potential away from the activation voltage of the IAconductance, also reversed the sublinearity (99 ± 1%;n = 7 cells). We conclude that summation of large depolarizations is linear because of activation of NMDA and potassium conductances, the effects of which are remarkably balanced.
Why is summation under blocked NMDA receptors and potassium channels still linear? To study whether we could reveal an underlying sublinearity caused by passive cable properties of dendrites, we applied a cocktail of blockers to simultaneously block NMDA, sodium, calcium, and potassium conductances (Fig. 4B). Under these conditions the input resistance increased nearly twofold (738 ± 140 MΩ, n = 7 cells for controls, vs 1311 ± 278 MΩ, n = 5 cells for blockers), making the neuron electrotonically very compact. These experiments were performed with two pipettes on the apical dendrite at least 50 μm and as much as 110 μm apart, a range similar to what was used in all other experiments. In this situation we observed sublinear summation of inputs onto the apical dendrite (83 ± 3%; n = 9 cells), which was not significantly affected by hyperpolarization and may have been caused by reduction in the driving force or shunting of currents. When only NMDA and potassium channel blockers are present, however, this sublinearity is opposed by a boosting produced by sodium and calcium conductances (Stafstrom et al., 1985; Sutor and Hablitz, 1989; Deisz et al., 1991; Hirsch and Gilbert, 1991; Magee and Johnston, 1995; Stuart and Sakmann, 1995). This indicates that unless all conductances are blocked, these cultured neurons do not experience the appropriate regimen of passive electrical properties in which driving force or conductance shunts operate.
DISCUSSION
Dendritic branching does not affect linear summation
Altogether, our results using cultured pyramidal neurons as a model system suggest that the dendritic morphology does not seem to play a major role in neuronal integration of excitatory inputs. Contrary to the classic picture that has emerged from cable theory and is disseminated in current textbooks, we find that two excitatory inputs sum linearly regardless of whether they are on the same dendritic branch or on two different branches. In other words, in cultured cells with pyramidal dendritic morphologies, we do not observe any sublinearities that could be ascribed to a reduction in driving force or shunting, as predicted by cable models.
How relevant are these results from cultured neurons for dendritic integration in vivo? An artifactual lack of interaction could occur if the currents produced by our glutamate iontophoresis on cultured neurons were substantially smaller than normal EPSPs or if the two input positions were too distant to interact. However, currents triggered by our iontophoresis pipettes ranged from approximately 20 to 40 pA, which are larger than EPSCs from CA1 neurons in slices (Bolshakov and Siegelbaum, 1995). Also, the input resistance of our cultured neurons is substantially higher than that of neurons in slices or in vivo, making cultured neurons much more compact electronically. In fact, dendrites in vivo are likely to be electrotonically very long because of constant synaptic bombardment (Bernarder et al., 1991). Our experiments, therefore, maximize the likelihood of nonlinear interactions attributable to driving force reduction or shunting. Yet, even when inputs were located near each other, at distances as close as 15 μm, we did not observe a substantial sublinearity. Therefore, synaptic inputs in vivoshould be more isolated, producing an even more linear synaptic integration. This implies that the regimen in which inputs interact sublinearly because of pure passive cable conditions may not occurin vivo.
Pronounced effects of dendritic morphology on input integration, however, may happen under circumstances that we have not yet tested. For instance, input position may matter for summation of excitatory and inhibitory inputs (Koch et al., 1983). Indeed, The highly specific placement of inhibitory contacts in the dendritic tree (Kisvarday et al., 1987; Buhl et al., 1994) suggests that their integrative interactions may be position-dependent, although this has not been explored experimentally. Another positional effect may arise during interaction of EPSPs with backpropagating action potentials that fail to invade particular branches (Spruston et al., 1995). On the other hand, however, it is also possible that like the branching patterns of trees, dendritic morphologies may have evolved to provide an efficient way of distributing postsynaptic targets in space, and that this branching architecture, intriguing as it may seem, does not play a major role in the electrical properties of the neuron.
Linear summation is achieved by two complementary mechanisms
The second goal of our study is to understand how dendritic active conductances contributed to spatial summation in these cultured neurons. We find that there are two mechanisms underlying the linear summation of simulated EPSPs in cultured neurons. For small inputs, summation was still linear under pharmacological blockade of sodium, calcium, and potassium conductances, indicating that these conductances were not significantly activated by the inputs. Thus a “passive” linearity results from the simple addition of noninteracting voltages. Near resting potential, voltage-dependent channels are not activated substantially, and conductance shunts or driving force reductions are too small to alter linear summation. Potentials initiated at one site may not induce significant depolarization to open channels at a site 20–100 μm away. This is consistent with earlier experiments showing linear integration in which inputs were probably even farther from each other (Langmoen and Andersen, 1983; Skydsgaard and Hounsgaard, 1994).
With larger depolarizations, however, APV revealed a sublinearity that was blocked by TEA and 4-AP. This suggests that in these cultured cells IA potassium channels are activated by large EPSPs and reduce the effect of other simultaneous EPSPs. This reduction, however, is cancelled by the boosting produced by additional activation of NMDA receptors. Although our data were taken from cultured cells, these findings are compatible with known properties of pyramidal neurons in slices, in which NMDA receptors produce EPSP boosting (Thomson et al., 1988) and dendritic IA currents shunt EPSPs (Hoffman et al., 1997). It is quite remarkable that two opposing conductances happen to cancel each other and produce an “active” linear summation. Because voltage-dependent channels are also found throughout the dendritic tree in vivo, it is possible that their actions would also balance each other, as in our experiments. This is reminiscent of the mechanism proposed for dendritic integration by distal apical dendrites of layer 5 cortical neurons, in which a balanced action of calcium and potassium conductances linearizes excitatory inputs (Bernarder et al., 1994). Finally, using a cocktail of blockers (TTX, APV, Ni, and TEA), we uncovered a sublinear summation that is probably caused by the reduction of driving force or resistance predicted by passive cable theory. Indeed, this pharmacological “passification,” together with glutamate iontophoresis, could be used to experimentally test many cable theory predictions in real dendrites with known morphologies.
Computational relevance of linear summation
What might be the function of linear input summation? A linear input arithmetic could provide neurons with a system for keeping an exact count of impinging excitatory inputs, enabling independent processing of multiple channels of information. Indeed, in the visual system, experiments in vivo have found widespread linear summation of inputs (Wandell, 1995), and linear integration may be necessary for both orientation and directional selectivity (Ferster, 1994). In the hippocampus, linear summation would be very useful for an associative memory matrix. Unfortunately, neither the positional effects nor the mechanisms behind linearity in vivo are understood. Our results in cultured neurons imply that the exact position of excitatory inputs in the dendritic tree does not matter for spatial summation, and they suggest two complementary cellular mechanisms to maintain linear summation. This implies that the active dendritic conductances paradoxically may be designed to linearize inputs regardless of their position and thus may counteract the effects of their passive cable structure. Nevertheless, our efforts using an experimentally accessible model system are only a first step in elucidating this issue experimentally, and additional work is clearly necessary. Perhaps the ability of two-photon microscopy to identify the location of individual synaptic inputs in a living cell (Yuste and Denk, 1995) may enable a similar experimental program in brain slices or even in vivo (Denk et al., 1994; Svovoda et al., 1997).
Footnotes
R.Y. is supported by the Sloan, Klingestein, March of Dimes, and EJLB Foundations. We thank Stuart Firestein, Jeanette Kuhn, Ania Majewska, Steven Siegelbaum, and Diana Smetters for comments.
Correspondence should be addressed to Sydney Cash, 1002 Fairchild Building, Department of Biological Sciences, Columbia University, New York, NY 10027.
REFERENCES
- 1.Artola A, Singer W. The involvement of NMDA receptors in induction and maintenance of long-term potentiation in rat visual cortex. Eur J Neurosci. 1990;2:254–269. doi: 10.1111/j.1460-9568.1990.tb00417.x. [DOI] [PubMed] [Google Scholar]
- 2.Banker GA, Cowan WM. Further observations on hippocampal neurons in dispersed cell culture. J Comp Neurol. 1979;187:469–494. doi: 10.1002/cne.901870302. [DOI] [PubMed] [Google Scholar]
- 3.Bernarder O, Douglas RD, Martin KA, Koch C. Synaptic background activity influences spatiotemporal integration in single pyramidal cells. Proc Natl Acad Sci USA. 1991;88:11569–11573. doi: 10.1073/pnas.88.24.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernarder O, Koch C, Douglas RJ. Amplification and linearization of distal synaptic input to cortical pyramidal cells. J Neurophysiol. 1994;72:2743–2753. doi: 10.1152/jn.1994.72.6.2743. [DOI] [PubMed] [Google Scholar]
- 5.Bolshakov VY, Siegelbaum SA. Regulation of hippocampal transmitter release during development and long-term potentiation. Science. 1995;269:1730–1734. doi: 10.1126/science.7569903. [DOI] [PubMed] [Google Scholar]
- 6.Buhl EH, Halasy K, Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994;368:823–828. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- 7.Burke RE. Composite nature of the monosynaptic excitatory postsynaptic potential. J Neurophysiol. 1967;30:1114–1137. doi: 10.1152/jn.1967.30.5.1114. [DOI] [PubMed] [Google Scholar]
- 8.Caceres A, Banker GA, Binder L. Immunocytochemical localization of tubulin and microtubule-associated protein 2 during the development of hippocampal neurons on culture. J Neurosci. 1986;6:714–722. doi: 10.1523/JNEUROSCI.06-03-00714.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deisz RA, Fortin G, Zieglgaensberger W. Voltage-dependence of excitatory postsynaptic potentials of rat neocortical pyramidal neurons. J Neurophysiol. 1991;65:371–382. doi: 10.1152/jn.1991.65.2.371. [DOI] [PubMed] [Google Scholar]
- 10.Denk W, Delaney KR, Gelperin A, Kleinfeld D, Strowbridge BW, Tank DW, Yuste R. Anatomical and functional imaging of neurons using 2-photon laser scanning microscopy. J Neurosci Methods. 1994;54:151–162. doi: 10.1016/0165-0270(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 11.Ferster D. Linearity of synaptic interactions in the assembly of receptive fields in the cat visual cortex. Curr Opin Neurobiol. 1994;4:563–568. doi: 10.1016/0959-4388(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 12.Goslin K, Banker G. Rat hippocampal neurons in low-density culture. In: Banker KGG, editor. Culturing nerve cells. MIT; Cambridge, MA: 1991. pp. 251–282. [Google Scholar]
- 13.Hirsch JA, Gilbert CD. Synaptic physiology of horizontal connections in the cat’s visual cortex. J Neurosci. 1991;11:1800–1809. doi: 10.1523/JNEUROSCI.11-06-01800.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman DA, Magee JC, Colbert CM, Johnston D. Potassium channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- 15.Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jack JJB, Noble D, Tsien RW. Electric current flow in excitable cells. Oxford UP; London: 1975. [Google Scholar]
- 17.Johnston D, Magee JC, Colbert CM, Christie BR. Active properties of neuronal dendrites. Annu Rev Neurosci. 1996;19:165–186. doi: 10.1146/annurev.ne.19.030196.001121. [DOI] [PubMed] [Google Scholar]
- 18.Jones KA, Baughman R. NMDA and non-NMDA receptor components of excitatory synaptic potentials recorded from cells in layer 5 of rat cortex. J Neurosci. 1988;8:3522–3534. doi: 10.1523/JNEUROSCI.08-09-03522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kisvarday ZF, Martin KAC, Friedlander MJ, Somogyi P. Evidence for interlaminar inhibitory circuits in the striate cortex of the cat. J Comp Neurol. 1987;260:1–19. doi: 10.1002/cne.902600102. [DOI] [PubMed] [Google Scholar]
- 20.Koch C, Poggio T, Torre V. Nonlinear interactions in a dendritic tree: localization, timing and role in information processing. Proc Natl Acad Sci USA. 1983;80:2799–2802. doi: 10.1073/pnas.80.9.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuno M, Miyahara JT. Non-linear summation of unit synaptic potentials in spinal motorneurons of the cat. J Physiol (Lond) 1969;201:465–477. doi: 10.1113/jphysiol.1969.sp008767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langmoen IA, Andersen P. Summation of excitatory postsynaptic potentials in hippocampal pyramidal neurons. J Neurophysiol. 1983;50:1320–1329. doi: 10.1152/jn.1983.50.6.1320. [DOI] [PubMed] [Google Scholar]
- 23.Magee JC, Johnston D. Synaptic activation of voltage-gated channels in the dendrites of hippocampal pyramidal neurons. Science. 1995;268:301–304. doi: 10.1126/science.7716525. [DOI] [PubMed] [Google Scholar]
- 24.Rall W. Theoretical significance of dendritic trees for neuronal input-output relations. In: Reiss RF, editor. Neural theory and modeling. Stanford UP; Stanford: 1964. pp. 73–97. [Google Scholar]
- 25.Rall W. The theoretical foundation of dendritic function. MIT; Cambridge, MA: 1995. [Google Scholar]
- 26.Ramón y Cajal S. La Textura del Sistema Nerviosa del Hombre y los Vertebrados. Moya; Madrid: 1904. [Google Scholar]
- 27.Skydsgaard M, Hounsgaard J. Spatial integration of local transmitter responses in motoneurons of the turtle spinal cord in vitro. J Physiol (Lond) 1994;479:233–246. doi: 10.1113/jphysiol.1994.sp020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spruston N, Schiller Y, Stuart G, Sakmann B. Activity-dependent action potential invasion and calcium influx into hippocampal CA1 dendrites. Science. 1995;286:297–300. doi: 10.1126/science.7716524. [DOI] [PubMed] [Google Scholar]
- 29.Stafstrom CE, Schwindt PC, Chabb MC, Crill WE. Properties of persistent sodium and calcium conductances of layer 5 neurons from cat sensorimotor cortex in vivo. J Neurophysiol. 1985;55:153–170. doi: 10.1152/jn.1985.53.1.153. [DOI] [PubMed] [Google Scholar]
- 30.Stuart G, Sakmann B. Amplification of EPSPs by axosomatic sodium channels in neocortical pyramidal neurons. Neuron. 1995;15:1065–1076. doi: 10.1016/0896-6273(95)90095-0. [DOI] [PubMed] [Google Scholar]
- 31.Sutor B, Hablitz JJ. EPSPs in rat neocortical neurons in vitro. II. Involvement of N-methyl-d-aspartate receptors in the generation of EPSPs. J Neurophysiol. 1989;61:621–634. doi: 10.1152/jn.1989.61.3.621. [DOI] [PubMed] [Google Scholar]
- 32.Svovoda K, Denk W, Kleinfeld D, Tank DW. In vivo dendritic calcium dynamics in neocortical pyramidal neurons. Nature. 1997;385:161–165. doi: 10.1038/385161a0. [DOI] [PubMed] [Google Scholar]
- 33.Thomson AM, Girdlestones D, West DC. Voltage-dependent currents prolong single-axon postsynaptic potentials in layer 3 pyramidal neurons in rat neocortical slices. J Neurophysiol. 1988;60:1896–1907. doi: 10.1152/jn.1988.60.6.1896. [DOI] [PubMed] [Google Scholar]
- 34.Wandell BA. Foundations of vision. Sinauer; Sunderland, MA: 1995. [Google Scholar]
- 35.Yuste R, Denk W. Dendritic spines as basic units of synaptic integration. Nature. 1995;375:682–684. doi: 10.1038/375682a0. [DOI] [PubMed] [Google Scholar]
- 36.Yuste R, Tank DW. Dendritic integration in mammalian neurons, a century after Cajal. Neuron. 1996;16:701–716. doi: 10.1016/s0896-6273(00)80091-4. [DOI] [PubMed] [Google Scholar]