Abstract
Zic genes encode zinc finger proteins, the expression of which is highly restricted to cerebellar granule cells and their precursors. These genes are homologs of theDrosophila pair-rule gene odd-paired. To clarify the role of the Zic1 gene, we have generated mice deficient in Zic1. Homozygous mice showed remarkable ataxia during postnatal development. Nearly all of the mice died within 1 month. Their cerebella were hypoplastic and missing a lobule in the anterior lobe. A bromodeoxyuridine labeling study indicated a reduction both in the proliferating cell fraction in the external germinal layer (EGL), from 14 d postcoitum, and in forward movement of the EGL. These findings suggest thatZic1 may determine the cerebellar folial pattern principally via regulation of cell proliferation in the EGL.
Keywords: Zic1, transcription factor, cerebellum, granule cell, cerebellar foliation, ataxia, gene targeting
Determination of the molecular mechanisms of cerebellar development remains one of the great challenges of developmental neurobiology (Altman and Bayer, 1996). Several genes have been shown to play a role in the patterning of this region (for review, see Hatten and Heintz, 1995; Hatten et al., 1997;Herrup and Kuemerle, 1997), and gene disruption studies in mice have identified several genes that are involved in midbrain–hindbrain patterning (McMahon and Bradley, 1990; Thomas and Capecchi, 1990;Joyner et al., 1991; Wurst et al., 1994; Urbanek et al., 1997).Zic1 was identified as a gene encoding a zinc finger protein that was expressed in and highly restricted to the cerebellar granule cell lineage (Aruga et al., 1994; Yokota et al., 1996). Genes that are structurally related to Zic1 are also expressed in cerebella (Aruga et al., 1996a,b). The amino acid sequence and genomic organization of these genes suggest that they are the vertebrate homologs of the Drosophila pair-rule geneodd-paired (opa) (Benedyk et al., 1994; Cimbora et al., 1995; Aruga et al., 1996a). The opa mutation causes severe pair-rule type segmentation abnormalities and impairment in the timing of activation of the segment polarity genes winglessand engrailed (Benedyk et al., 1994). The vertebrate homologs of these genes, En1, En2, andWnt-1, were shown to be involved in vertebrate neural pattern formation (McMahon and Bradley, 1990; Thomas and Capecchi, 1990; Joyner et al., 1991; Wurst et al., 1994).
Furthermore, the zinc finger domain of the Zic protein is homologous to those of Gli (Kinzler et al., 1988) and Opa and is capable of binding to the Gli protein target sequence (Aruga et al., 1994). The Gli proteins are vertebrate homologs of the Drosophila cubitus interruptus (Orenic et al., 1990) and have been shown to play essential roles in body pattern formation (Hui et al., 1994; Mo et al., 1997).
In the mouse, three related genes, Zic1, Zic2, and Zic3, are expressed in partially overlapping sites (Nagai et al., 1997). Of these, the Zic1 transcript is the most abundant throughout development, particularly in postnatal cerebella (Aruga et al., 1996a). Zic1 was first detected in the neuroectoderm during formation. Later, expression is restricted to the dorsal neural tube and certain portions of somites and their derivatives. Within the cerebella, expression was detected in the entire cerebellar anlage as early as 12 d postcoitum [embryonic day 12 (E12)], at which time the external germinal layer (EGL) had not yet formed. Thereafter, expression became restricted to the EGL, which contains granule cell precursors and granule cells.
These findings led us to hypothesize that Zic1 plays significant roles in vertebrate development, particularly in cerebellar development. In the present study, we disrupted the Zic1gene by homologous recombination in embryonic stem (ES) cells. Mice homozygous for the mutated Zic1 gene showed abnormal behavior. Dysgenesis of the CNS, including the cerebellum, accounted for these abnormalities. Our findings suggest that Zic1plays essential roles in cerebellar development.
MATERIALS AND METHODS
Construction of targeting vector. The λ phage clones containing the mouse Zic1 gene were obtained from a 129/Sv mouse genomic library (Stratagene, La Jolla, CA). The genomic structure was described previously (Aruga et al., 1996a). The 5′ flanking region and first exon comprising an 8 kbEcoRI–SalI fragment and a 2.4 kbBglII–XhoI fragment containing the first intron to the third exon were subcloned into Bluescript vectors. AnXhoI–SalI fragment containing a neogene driven by the phosphoglycerate kinase (PGK) promoter (Rudnicki et al., 1992) or an XhoI–SalI fragment containing the diphtheria toxin A gene driven by the MC1 promoter (Yagi et al., 1993) was inserted into the XhoI site of a plasmid. TheSalI–NotI fragments from this plasmid were inserted into a NotI–SalI-digested plasmid. The resultant targeting vector was linearized at a NotI site at the 5′ end of the 5′ homologous fragment before electroporation into ES cells.
Electroporation and generation of germ line-transmitting mice. J1 ES cells (Li et al., 1992) were electroporated with 20 μg of linearized targeting vector DNA using a Bio-Rad (Hercules, CA) Gene Pulser. The electroporated cells were then cultured on feeder cells, which had been prepared from G418-resistant primary embryonic fibroblasts as described previously (Rudnicki et al., 1992) and selected for resistance to G418 (175 μg/ml) over a 6–11 d period. Selected clones were cultured for an additional 7 d; the clones were then frozen, and their genomic DNA was isolated for Southern blot analysis.
Homologous recombinants, identified by Southern blot analysis using the 3′ external probe, were expanded for injection and DNA isolation. Southern blot analyses of 126 ES cell clones correctly identified one targeted clone. The correctly targeted clone was confirmed by Southern blot, using several restriction enzymes, and then expanded. Mutant ES cells were trypsinized, centrifuged, and resuspended in ES medium. ES cells were injected into blastocoel cavities of E3.5 blastocysts from C57BL/6J mice. Injected blastocysts were surgically transferred into the uteri of pseudopregnant ICR recipients at postcoitum day 2.5. Male chimeras with extensive ES cell contributions to their coats were bred with C57BL/6J female mice, and germ line transmission of the dominant agouti coat color marker was observed. Germ line transmissions of the Zic1−allele were screened by applying Southern blot analysis, and heterozygous F1 animals were intercrossed. Genotyping of embryos and postnatal animals was performed by Southern blot or PCR with the Zic1com primer (5′-TCGAACAGAAAGGACTCAAGAAAGTCCCTG-3′), the PGK1 primer (5′-GCTAAAGCGCATGCTCCAGACTGCCTTG-3′), and the Zic1w primer (5′-CGCGTTCAGAGAACCTCAAGATCCACAA-3′), which correspond specifically to the mutated allele (Zic1com and PGK1) and wild-type (Zic1com and Zic1w)Zic1 allele. PCR consisted of 30 cycles at 94°C for 1 min and 70°C for 2 min. The initial heterozygotes were mated with C57BL/B6 or C3H/HeN, and their heterozygous offspring (C57BL/B6, N2; C3H/HeN, N1) were subsequently back-crossed with C57BL/B6 or C3H/HeN until the N5 or N4 generation, respectively. The homozygotes (Zic1−/−) were obtained by double heterozygous breeding of mice within both genetic backgrounds. There was no change in phenotype among different generations until C57BL/6J N5 or C3H/HeN N4 with regard to abnormalities in the cerebella and axial skeleton. The majority of results presented herein were obtained from C3H/HeN N1 and N2 except for the pictures in Figure 1A–H (C57BL/6J N2). The mice were maintained by the Division of Experimental Animal Research at the Institute of Physical and Chemical Research (RIKEN).
Fig. 1.
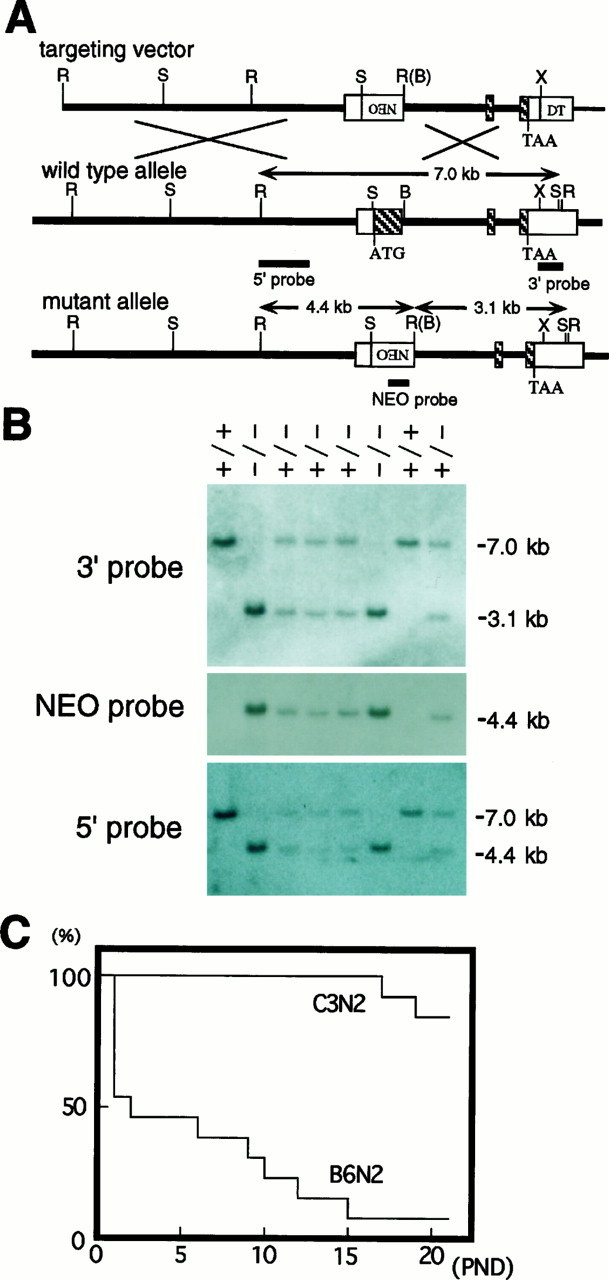
Mouse Zic1 gene, targeting construct, mutated Zic1 gene, and verification of the targeted insertion. A, The wild-type mouseZic1 gene (middle) consists of three exons (Aruga et al., 1996a). The first contains the initiator methionine and three of five C2H2 type zinc finger motifs. The targeting vector (top) contains 8 and 2.4 kb regions homologous to the Zic1 gene and a neomycin resistance gene, respectively, driven by the PGK promoter (NEO). The diphtheria toxin A fragment gene driven by the MC1 promoter (DT) (Yagi et al., 1993) was inserted in the 3′ end of the Zic1 gene to eliminate nonspecific integration. In a properly mutated allele (bottom), the protein coding region and splicing donor site of the mouseZic1 gene exon 1 has been replaced by the neomycin resistance gene expression unit. B,EcoRI-digested genomic DNA samples extracted from a litter were analyzed by Southern blotting using three probes (5′, 3′, NEO). The band size and the corresponding fragments are indicated A and B, respectively. B, BglII; R, EcoRI; S,SalI; X, XhoI. C, Viability of Zic1−/− mice. Survival rates of 13Zic1−/− mice with the C57BL N2 background and 13 with the C3H N2 background were determined up to postnatal day (PND) 21. Pups in each group were derived from at least five litters. There were no deaths among Zic1+/+ andZic1+/− littermates during the experimental period.
In situ hybridization. In situ hybridizations were performed essentially as described by Aruga et al. (1994). Briefly, the postnatal animals were perfused transcardially with 4% paraformaldehyde. The dissected tissue was immersed in the same fixative for 2–12 hr. The tissues were then immersed in a 20% sucrose solution and embedded in OCT compound. Sections of 10 μm in thickness were prepared using a cryostat. Immunohistochemistry was performed as described elsewhere (Aruga et al., 1994).
Histology and bromodeoxyuridine labeling. The fixation, paraffin embedding, sectioning, and hematoxylin–eosin staining were performed as described previously (Aruga et al., 1994). The folial patterns were examined by preparing sagittal or coronal serial sections through entire cerebella. Bromodeoxyuridine (BrdU) labeling was performed as described by Yuasa et al. (1991). Briefly, the pregnant mice were injected with BrdU (150 mg/kg, i.p.). The mice were killed 5 hr after injection by decapitation. The brains of embryos were immersed in 95% ethanol and 5% acetic acid for 12 hr at room temperature. The samples were dehydrated and embedded in paraffin. Paraffin sections (8 μm) were deparaffinized and immersed in 0.1N HCl for 15 min at 4°C and then in a solution consisting of 50% formamide and 0.01× PBS for 5 min at 80°C. The sections were then reacted with anti-BrdU antibody (Beckton Dickinson, Mountain View, CA; 1:50) overnight at 4°C. The bound antibody was detected histochemically as described (Aruga et al., 1994). At a minimum, three pairs of Zic1+/+ andZic1−/− mice derived from two independent litters were analyzed at each developmental stage.
Morphometric analyses. The cell number counting and the perimeter measurements were performed on a Macintosh computer using an NIH Image program (developed at the National Institutes of Health, Bethesda, MD; available on the Internet athttp://rsb.info.nih.gov/nih-image/). Four sets of Zic1+/+,Zic1+/−, and Zic1−/− mice derived from four independent litters were used. The midsagittal sections and sections through the cerebellar hemisphere, which were prepared from P21–P27 cerebella (5 μm), were stained with hematoxylin–eosin. Four sections per mouse were analyzed.
Electron microscopy. Three pairs of Zic1+/+ andZic1−/− mice (P17) derived from three independent litters were perfused under deep sodium pentobarbital anesthesia with Ringer’s solution, followed by a mixture of 2% paraformaldehyde and 2% glutaraldehyde in 0.1 m phosphate buffer, pH 7.3. The cerebella were removed and cut into parasagittal sections with razor blades. Sections through the cerebellar midline were chosen and immersed in the same fixative at 4°C overnight. The sections were osmificated, dehydrated through a graded ethanol series, and embedded in Epon 812 (TAAB). Thin sections containing the cerebellar cortex facing the primary fissure were cut on an ultramicrotome, stained with uranyl acetate and lead citrate, and examined on a Hitachi H-7000 electron microscope.
Twenty electron micrographs were taken randomly from the middle layer of the cerebellar molecular layer in each mouse at an original magnification of 6000×. The number of synaptic profiles was counted for each electron micrograph printed at a final magnification of 16,500×. The neuropil area, which contains no blood vessels, cell bodies, or dendritic shafts thicker than 2 μm in caliber, was measured following a method described previously by Kano et al. (1997). The mean synaptic number/100 μm2 of neuropil area was calculated for each mouse.
RESULTS
Targeted disruption of Zic1 and generation of mutant mice
A targeting vector was constructed to replace a portion of the first exon of the Zic1 gene with a neomycin resistance gene, resulting in homologous recombination and deletion of an initiator methionine and three of five zinc finger motifs (Fig.1A). Electroporated ES cells (J1 cell) were selected for G418 resistance, and DNAs extracted from the drug-resistant colonies were analyzed by Southern blotting. Correctly targeted ES cells were injected into C57BL/6J host blastocysts to generate chimeric mice. Heterozygotes were obtained by cross-breeding of the chimeras with C57BL/6J mice. Heterozygotes (Zic1+/−) were fertile and had no apparent abnormalities.
Viability and behavioral abnormalities of Zic1mutant mice
Examination of the embryonic genotypes generated by double heterozygous breeding showed no distortion of Mendelian inheritance (Zic1+/+, 26%; Zic1+/−, 51%; andZic−/−, 24%; n = 95 at E18.5), indicating that Zic1−/− mice did not die during embryonic development. However, ∼50% of the Zic1−/− animals generated by C57BL/F2 Zic1+/− mating died within 1 d after birth, and most homozygous mutants died within 3 weeks (Fig.1C). In contrast, the survival of Zic1−/− mice with the C3H genetic background was better than that of mice with the C57BL background. The C3H Zic1−/− mice occasionally survived as long as 8 weeks. Therefore, most of the analyses on cerebella were done on C3H N1 and N2 (See Materials and Methods). The neonatal death potentially may not be associated with the cerebellar abnormality described below, and the cause of death is currently under investigation.
The Zic1−/− mice were significantly smaller than littermates after the P2, and their weights were one-third to one-half of those of littermates. The stomachs of homozygotes nearly always contained less milk than those of littermates, based on observations made through their thin body walls. Therefore, the growth retardation appeared to be attributable, in part, to the poor suckling. Further examinations are required to elucidate the mechanism of this growth retardation.
The behavioral abnormality manifests in Zic1−/− mice after P2. They often lie on their backs, and their forelimbs are extended anteriorly with irregular motions (Fig.2A). At P10,Zic1+/+ and Zic1+/− pups were able to turn over easily within a few seconds when they were placed on their backs. In contrast, it took the Zic1−/− mice more than 1 min to turn over, and, in some cases, mice were completely unable to recover their posture.
Fig. 2.
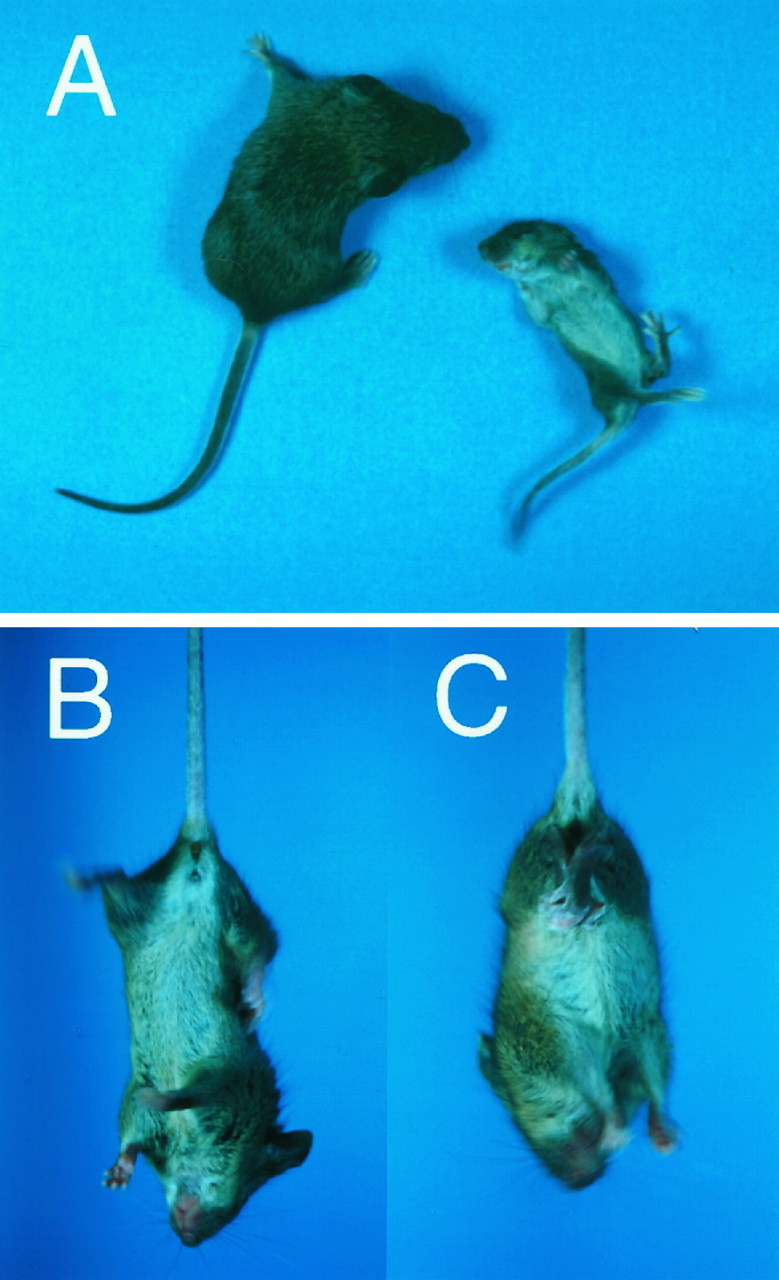
Abnormal behavior was observed in theZic1−/− mice. A,Zic1−/− mice (P10) (right) often lay on their backs in infancy, whereas littermates (left) did not. The mouse distorted its body in an attempt to turn over but was unable to do so. When the mice were suspended by their tails, theZic1−/− mice (P14) often crossed or clasped their hindlimbs (C), whereas the normal mice usually extended and shook their hindlimbs (B).
The gait of 2-week-old Zic1−/− mice was also severely disturbed, as they often dragged their hindlimbs behind them.The Zic1−/− mice were generally unable to walk in a straight line, and most fell to one side. When the mice were lifted by the tail, typically, their hindlimbs were clasped, whereas the wild-type mice extended their legs (Fig.2B,C). Thus, the loss of coordinated movements involving all four limbs was peculiar to Zic−/− mice during their development. Two weeks after birth, the ataxia became more severe, and some mice showed tonic convulsions.
In addition to the behavioral phenotypes, the thoracic regions of theZic1−/− mice were variably kyphotic, concomitant with marked cervical flexion. These findings may be attributable to abnormal patterning of the developing sclerotome in which Zic1 is also strongly expressed. The phenotypes will be described in detail elsewhere.
Cerebellar abnormalities
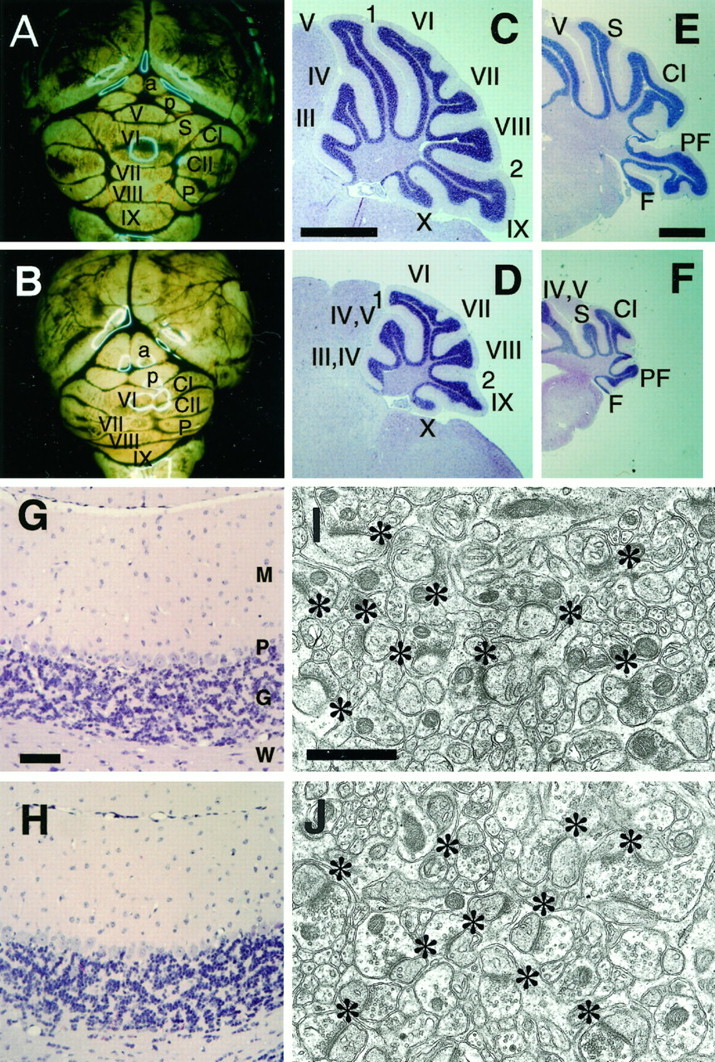
In addition to the highly restricted Zic1 expression in the CNS, the abnormal behaviors of Zic1−/− mice suggested alteration in the CNS. We therefore examined the morphological features of the CNS in homozygous mice. Macroscopically, the most remarkable change found in the CNS was hypoplasia of the cerebellum (100% in 53Zic1−/− pups after birth) (Fig. 3A,B). In addition to reduction of the cerebella, the cerebellar fissures and folia were also deformed in Zic1−/− mice. In almost all cases, a folia in the anterior lobe was completely missing, and the crus and paraflocculus lobule were hypoplastic (Fig. 3C–F). However, foliation pattern abnormalities were not confined to specific portions of the cerebella, because all of the fissures appeared to be shallow. We found no overt abnormalities in the Zic1+/− cerebella by macroscopic observation. In contrast to the abnormal foliation, the layer organization of Zic1−/− cerebella was grossly normal (Fig. 3G,H). The Purkinje cell and granule cell layers were equally clearly observed in the cerebella ofZic1−/−, Zic1+/+, and Zic1+/− mice.
To define the effect of the Zic1 mutation on gross cerebellar organizations more clearly, morphometric analyses were performed on both midsagittal sections and parasagittal sections through cerebellar hemispheres of P21–P27 cerebella, in which the inward migration of the granule cells is nearly complete. When the areas of the granule cell and molecular layers and the length of the Purkinje cell layer were compared, the abnormalities inZic1+/− and Zic1−/− cerebella became apparent. Although the Zic1+/− cerebella were significantly smaller than Zic1+/+ cerebella with respect to all three parameters, the extent of reduction was not as severe as that inZic1−/− cerebella, in which the values were approximately half those in Zic1+/+ cerebella (Fig.4A,B).
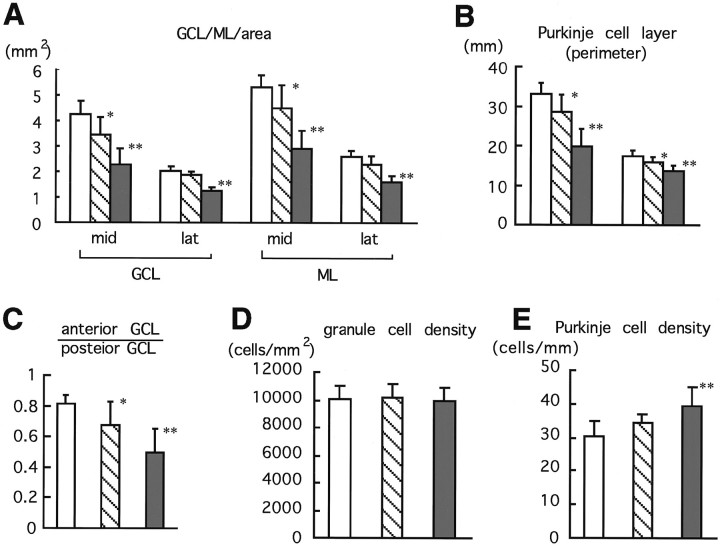
Fig. 4.
Morphometric analyses of midsagittal sections of mature cerebella. A, Areas of the granular cell layer (GCL) and molecular layer (ML).B, Perimeter of the Purkinje cell layer in both midsagittal (mid) and cerebellar hemisphere (lat) regions. C, Ratios of anterior GCL area (rostral to primary fissure) to posterior GCL area (caudal to primary fissure). Granule cell density (D) and Purkinje cell number per millimeter (E) in midsagittal regions were measured in Zic1+/+ (open bar), Zic1+/− (hatched bar), and Zic1−/− (shaded bar) cerebella. Error bars indicate SEM. *p < 0.01; **p < 0.001.
Because a folium rostral to the primary fissure was absent in theZic1−/− cerebella, we compared the granule cell layer (GCL) areas anterior and posterior to the primary fissure (Fig.4C). As expected, the ratio (anterior GCL/posterior GCL) was reduced, markedly in homozygotes and mildly in heterozygotes. In contrast to the severe reduction in granule cell layer area, granule cell density was not changed (Fig. 4D), suggesting that the total number of granule cells was decreased because of a reduction in the size of the granule cell layer. On the other hand, Purkinje cell density was greater in the Zic1−/− cerebella (Fig. 4E). This may suggest that the total Purkinje cell number is not as severely reduced as that of granule cells.
We next assessed whether cells in the mature Zic1−/− cerebella had undergone normal differentiation. Hematoxylin–eosin (HE) sections were almost indistinguishable from those of a normal specimen, the exception being the more slender Purkinje cell body in theZic1−/− compared with Zic1+/+ cerebella. Then, we performed in situ hybridization and immunohistochemical staining to examine the expression of cell type-specific markers. Molecular markers for granule cells Zic2 (Aruga et al., 1996a; Nagai et al., 1997) and En2 (Davis and Joyner, 1988) (Fig. 5), which encode transcription factors located in the granule cells and markers for Purkinje cells, type 1 inositol trisphosphate receptor (Furuichi et al., 1989), and cGMP-dependent protein kinase (Schlichter et al., 1980) were normally expressed in the Zic1−/− cerebella (data not shown).
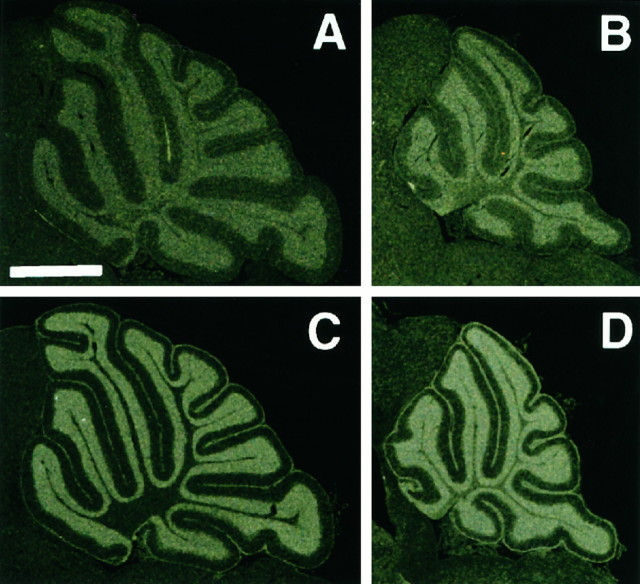
Fig. 5.
In situ hybridization of tissues from Zic1+/+ (A, C) orZic1−/− (B, D) cerebella. In situ hybridization using En2 (A, B) and Zic2 (C, D) probes showed the expression of these genes to be essentially unchanged in theZic1−/− cerebella. The cerebella were taken from C3H N1 mice. Scale bar, 1 mm.
When the mutant cerebella were examined electron microscopically (Fig. 3I,J), it became clear that normal synapses were present, that the number of synapses between the parallel fibers of granule and Purkinje cells was not changed (Zic1+/+, 23.12 ± 3.38; Zic1−/−, 22.58 ± 3.81 synapses/100 μm2) and that granule cells and Purkinje cells were morphologically indistinguishable inZic1+/+ and Zic1−/− mice. Thus, taken together with the normal expression of several cerebellar markers, we assumed that differentiation and neural circuit formation were fundamentally normal in Zic1−/− cerebella.
Abnormalities of developing cerebella
To understand the basis of abnormalities found inZic1−/− cerebella, we next examined the process of cerebellar development. Before E17.5, the Zic1−/− brains were apparently indistinguishable from those of Zic1+/− orZic1+/+ animals. Abnormalities were first recognized in the cerebella of E17.5 mice (Fig.6A,D). At this stage, there was a lucent area in the immature Purkinje cell layer, which may correspond to the most recently localized Purkinje cells on the caudal end of the prospective lobule VI (Altman and Bayer, 1996) (Fig.6A,D, closed arrowheads). This site was more anterior in the Zic1−/− cerebella than in the Zic1+/+ cerebella. The migrating granule cells and the site of the primary fissure were more anteriorly located in E18.5 cerebella (Fig.6B,E, open arrowheads). These results indicate that the anterior cerebellar lobe was more severely reduced than the posterior lobes, an observation consistent with the morphometric analyses of mature cerebella. The sites where primary fissures appeared in Zic1−/− cerebella were always rostral to those ofZic1+/+. These abnormalities in the developing cerebella were consistently observed irrespective of the genetic background (C57BL or C3H).
Fig. 6.
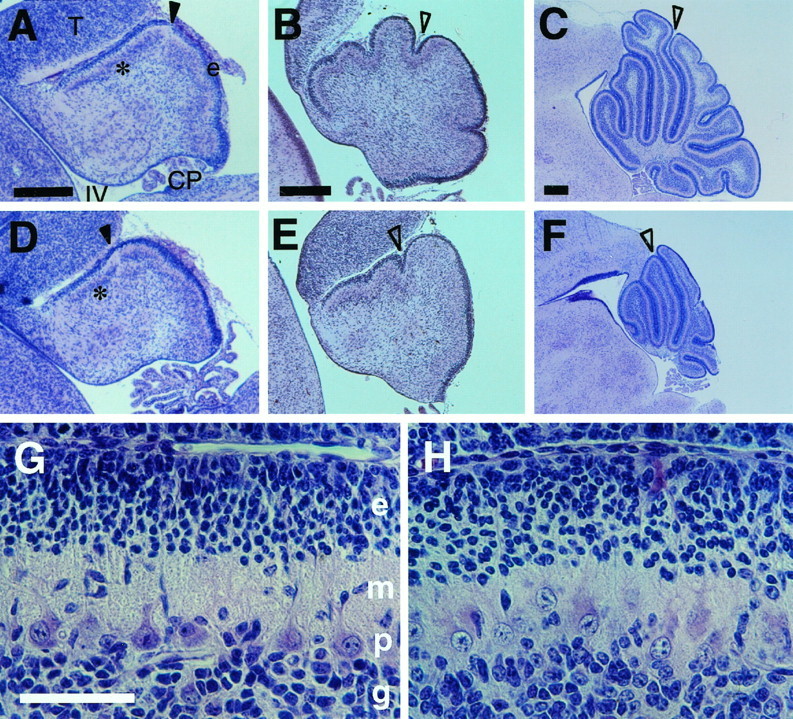
Phenotypes of the developing cerebella. A series of sagittal sections through the midsagittal planes of theZic1+/+ (A–C, G) orZic1−/− (D–F, H) cerebella were stained with hematoxylin–eosin. E17.5 (A, D), E18.5 (B, E), and P5 (C, F) cerebella were examined. CP, Choroid plexus; T, tectum; IV, fourth ventricle; e, external germinal layer; closed arrowheads, lucent portions indicating the cessation of the immature Purkinje cell layer (asterisk); open arrowheads, primary fissures. G, H, Higher magnifications ofC and F, respectively. e, External germinal layer; g, granule cell layer;m, molecular layer; p, Purkinje cell layer. Note that the Purkinje cells of Zic1−/− cerebella are morphologically immature at this time point. All panels indicate the C3H N1 cerebella. Scale bars: A–C, 200 μm; G, 100 μm.
During postnatal development, folial pattern abnormalities and reduced cerebellar mass became increasingly obvious. Poor development of all folia was observed in the Zic1−/− P5 cerebella (Fig.6C,F). Purkinje cells were not aligned in a monolayer, and the apical cytoplasms were hypertrophic and rectangular (Fig. 6G,H). These features are consistent with those of immature Purkinje cells (Altman, 1972). There may thus be a delay in the maturation of cerebella in these animals.
The driving force behind cerebellar foliation has been thought to be the existence of an expanding germinal cell layer on the surface of the cerebellum. We initially performed terminal deoxynucleotidyl transferase-mediated dUTP–biotin nick end-labeling (TUNEL) staining to compare the frequency of apoptotic cell death from E14 to E17 and at P7 when cell death peaked (Wood et al., 1993). However, we found no changes (data not shown). In addition, there were very few differences in the number of pyknotic cells at all stages examined. Because the cerebellar abnormality manifests as early as E18.5, cell death probably contributes less to the Zic1−/− cerebellar phenotype.
We therefore considered whether the proliferative capacity of the EGL is altered in the Zic1−/− cerebella. To answer this question, the proliferating cell number in the cerebellar anlage was examined by BrdU labeling (Fig. 7). We counted the BrdU-labeled cells in an entire EGL. As expected, there was a significant reduction of BrdU-labeled cell number in the EGL region. This reduction was apparent from the beginning of EGL forma- tion (E14.5) to birth (Fig. 7A). We also measured the extent of the EGL in the anterior to posterior direction during the same period (Fig. 7B). The Zic1+/+ EGL was longer than that of Zic1−/−, starting from E15.5, with the difference gradually becoming larger. When we divided the BrdU-labeled cell number by the EGL length, it became clear that there was still a consistent reduction of BrdU-labeled cell number inZic1−/− cerebella (p < 0.001, paired t test) (Fig. 7C). These results indicate that cell proliferation activity is lower in the Zic1−/− EGL than in the Zic1+/+ EGL from E14.5 through E18.5.
Fig. 7.
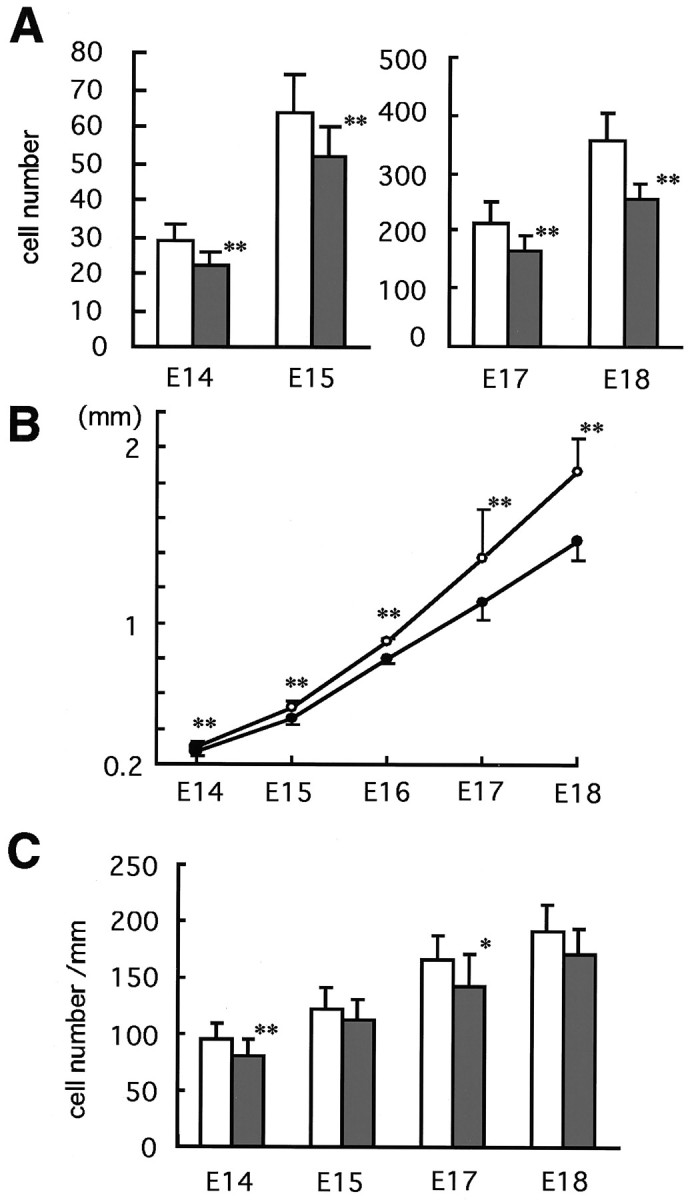
BrdU labeling study of developing cerebella.A, BrdU-labeled cells in the EGL, on sections of the cerebellar sections anlage, were counted. B, Change in EGL length in the developing cerebella. Rostral to caudal lengths of the EGL were measured at each developmental stage. In Aand B, all data for Zic1−/− are significantly different from those for Zic1+/+ cerebella (p < 0.001, t test).C, BrdU-labeled cells are compared in a lengthwise portion. Open and closed bars(circles) indicate the Zic1+/+ andZic1−/− cerebella, respectively. From E14 through E18, the data for Zic1−/− cerebella are significantly different from those for Zic1+/+ cerebella (p < 0.001, paired t test). The data are presented as means. Error bars indicate SEM. *p < 0.01; **p < 0.001.
The reduction in BrdU-labeled cells in the E14.5 cerebellar anlage was the earliest sign of the cerebellar abnormality in Zic1−/− mice, although Zic1+/+ and Zic1−/− cerebella were indistinguishable at this time point in HE sections. These findings suggest that Zic1 may promote cell proliferation in the EGL in wild-type cerebella. However, the difference might be traced back to an earlier stage. For example, there may be different numbers of initial progenitors or different proliferation start times. Further studies will address these points.
DISCUSSION
Role of the Zic1 gene in cerebellar granule cell development
The present study showed that one role of the Zic1 gene is regulation of cerebellar development. Zic1 may regulate granule cell proliferation throughout periods of germinal cell expansion in the EGL.
EGL cells only give rise to cerebellar granule cells (Gao and Hatten, 1994; Zhang and Goldman, 1996). Embryonic precursor cells from the rhombic lip are specific to cerebellar granule neurons (Alder et al., 1996). Therefore, cells in the EGL may represent the single source of granule cells. As a consequence, the number of granule cells is determined by the extent of cellular proliferation in the EGL as well as by cell death in later stages (Wood et al., 1993). The involvement of cell death in reducing the cell number in Zic1−/− mice is probably minimal, because the abnormal pattern was already defined at birth, and TUNEL staining showed that apoptotic cell death occurred equally in Zic1−/− and Zic1+/+ mice at E17 and P7. The role of Zic1 in cerebellar development therefore probably involves promotion of cellular proliferation of granule cell precursors.
In our previous study, we showed that Zic1 is persistently expressed during granule cell development (Aruga et al., 1994).Zic1 expression in the rhombic lip, EGL, and mature granule cells is abundant and highly restricted. Although other members of theZic family are also expressed in the adult cerebellum, their levels of expression are considerably lower than that ofZic1 (Aruga et al., 1996a,b). The phenotypes observed in this study appear to correspond closely with the persistent expression of Zic1 in the granule cell precursor, and withZic1 being the predominantly expressed member of theZic family. However, the role of the Zic1 gene in mature cerebella is not clear as yet, given that we found no evidence of abnormalities in granule cell differentiation during synapse formation or in marker expression. Further study, including physiological characterization, is necessary to resolve this issue.
Human Zic1 is also expressed in the cerebellar granule cell lineage in a highly restricted manner (Yokota et al., 1996). In addition, among various human tumors, the gene is predominantly expressed in medulloblastoma. Therefore, the expression ofZic1 is compatible with cells in a proliferative state, supporting the observations made in this study. It would be interesting to examine whether Zic1 is structurally altered in medulloblastoma.
Role of the Zic1 gene in cerebellar patterning
Zic1−/− cerebella lacked a lobule rostral to the primary fissure. This abnormality may be relevant to the behavioral abnormalities, because afferents from the hindlimbs, trunk, and forelimbs have abundant terminations in the anterior lobe (Oscarsson, 1973), in which mass reduction was most severe in theZic1−/− mice. Abnormal foliation was consistently observed in the Zic1−/− cerebella, suggesting that Zic1has an essential role in determining the cerebellar foliation pattern. Therefore, we speculated on how Zic1 may be involved in cerebellar patterning.
First, the phenotypes may be produced by the reduction of forward movement of granule cell precursors. The forward movement of the granule cell precursors may in part be driven by cell proliferation in the EGL. The majority of forward-moving cells have a rounded or irregular shape, rather than the elongated spindle shape of streaming cells, with the long axes of the cells oriented in the direction of dispersal, as is typical of actively migrating cells (Altman and Bayer, 1996). Therefore, insufficient cell proliferation in the EGL might account, in part, for the abnormal foliation pattern. Consistent with this, we showed the forward extension of the EGL and that cell proliferation is reduced in Zic1−/− cerebella.
Second, foliation of the cerebella may result from the postnatal expansion of the cells in the EGL and differences in germinative activities between areas where fissures will form and areas destined to be folial convexities (Mares and Lodin, 1970). Destruction of proliferating cells in the rat EGL by x-ray irradiation from birth disturbs the formation of folial patterns (Altman and Anderson, 1972). In addition, EGL agenesis leads to the loss of foliation inMath1 mutant mice (N. Ben-Arie and H. Zoghbi, personal communication). These findings indicate that the germinative activity of the EGL is essential in cerebellar foliation.
However, the expression of Zic1 in the EGL is uniform, based on in situ hybridization results. We must therefore consider a third possibility; that Zic1 may cooperate with other spatially restricted factors. The present studies, as well as analyses of development of En2−/− hemisphere foliation (Millen et al., 1994), indicate that the basic plan of foliation is determined before birth, as early as E17.5. Similarly, Millen et al. (1995)reported that homologs of the Drosophila segment polarity genes En2, En1, Wnt-7B, Pax-2, and Gli are expressed in perinatal cerebella in spatially restricted manners. In addition, several genes are differentially expressed along the anterior to posterior axis in developing cerebella (Herrup and Kuemerle, 1997). It is possible that Zic1 interacts with a spatially restricted gene to define a uniform folial pattern.
A combination of these processes is also possible. First, a spatially restricted factor may define the sites of major fissures, possibly from the caudal end of the cerebellar anlage. Then, Zic1 defines the anterior extent of the EGL. Finally, differential germinative activity in the EGL results in the formation of folia.
Comparisons with other genetic cerebellar folial abnormalities
The abnormal foliation observed in this study is clearly different from other genetically defined abnormal cerebellar foliation patterns, such as Wnt1, Swaying, meander tail, leaner, andcerebellar foliation pattern. (Herrup and Wilczynski, 1982;McMahon and Bradley, 1990; Neumann et al., 1990; Thomas and Capecchi, 1990; Fletcher et al., 1991; Thomas et al., 1991). However, the abnormal foliation patterns in En2 mutant mice (Joyner et al., 1991) are somewhat similar to that of Zic1−/− mice. In En2−/− cerebella, reductions in the extents of crus I and crus II folia and the paraflocculus lobule were observed, as inZic1 homozygotes (Joyner et al., 1991; Millen et al., 1994).En2 is also expressed in granule cells and their precursors (Davis and Joyner, 1988). These findings suggest a possible genetic interaction between Zic1 and En2 as is the case in their Drosophila homologs odd-paired andengrailed (Benedyk et al., 1994). Although in situ hybridization showed that the expression of En2 in the adult was not changed in this manner, it would be interesting to analyze further the pattern of En2 gene expression inZic1−/− embryos as well as the cerebellar phenotypes ofZic1 and En2 compound mutants.
Possible interactions with other Zic genes
The abnormalities found in the Zic1−/− mice are grossly restricted in later development of the CNS and somite derivatives. Both tissues express Zic1 (Aruga et al., 1994;Nagai et al., 1997). However, even in cerebella, Zic2 andZic3 are expressed in addition to the Zic1 gene (Aruga et al., 1996a). As shown in this study, Zic2, which is the most similar Zic family gene to Zic1, is still expressed in mature granule cells as well as in the developing granule cell lineage of Zic1−/− mice (J. Aruga, unpublished data), indicating that the expression of Zic2 is independent of that of Zic1. The role of the Zicfamily in cerebellar development may be more clearly defined by analyses of compound Zic family mutants.
Although Zic1 expression begins at an earlier stage of development, i.e., almost simultaneous with neuroectoderm formation (Nagai et al., 1997), no abnormalities were observed in theZic1 mutants at this stage. We found that aXenopus homolog of the Zic gene has a role in neuroectoderm formation (Nakata et al., 1997). The absence of overt abnormalities in early stage Zic1−/− embryos may be explained by functional redundancy among members of the Zicfamily. The expression patterns of Zic1, Zic2, and Zic3 overlap considerably during gestation as well (Nagai et al., 1997). Furthermore, the amino acid sequence is highly conserved among Zic family members (Aruga et al., 1996a). Therefore, it is possible that, to some extent, Zic genes have similar roles.
Besides the interaction with other Zic genes, the relationships between Zic1 and other genes, such as those of the Gli, Pax, and En families, await further clarification. The production of compound mutants involving mutations of these genes might further clarify the role of theZic1 gene in mammalian development.
Footnotes
This work was supported by special coordination funds for promoting science and technology, Grants from the Japanese Ministry of Education, Science, and Culture to J.A. and K.M., a Core Research for Evolutional Science and Technology grant from the of Japan Science and Technology Cooperation to J.A., and Japanese Brain Science Foundation funds awarded to J.A. We thank Drs. Hidehiro Mizusawa, Shigeki Yuasa, Toshio Terashima, Katsunori Nakata, Toshiyuki Nakagawa, Hitoshi Niwa, Ken-ichi Yamamura, and Masahiko Watanabe for their helpful advice, Drs. Nissim Ben-Arie and Huda Zoghbi for sharing their results before publication, Dr. Paul Greengard for providing one of the antibodies, Dr. Takeshi Yagi for plasmids, and Ms. Qing Nie, Naoko Fujimoto, Yoko Nishi, Noriko Sugae, and Megumi Yakuwa for technical assistance.
Correspondence should be addressed to Jun Aruga, Molecular Neurobiology Laboratory, Tsukuba Life Science Center, Institute of Physical and Chemical Research (RIKEN), 3-1-1 Koyadai, Tsukuba, Ibaraki 305, Japan.
REFERENCES
- 1.Alder J, Cho NK, Hatten ME. Embryonic precursor cells from the rhombic lip are specified to a cerebellar granule neuron identity. Neuron. 1996;17:389–399. doi: 10.1016/s0896-6273(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 2.Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972;145:399–463. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- 3.Altman J, Anderson WJ. Experimental reorganization of the cerebellar cortex: I. Morphological effects of elimination of all micro neurons with prolonged x-irradiation started at birth. J Comp Neurol. 1972;146:355–406. doi: 10.1002/cne.901460305. [DOI] [PubMed] [Google Scholar]
- 4.Altman J, Bayer SA. Development of the cerebellar system. CRC; New York: 1996. [Google Scholar]
- 5.Aruga J, Yokota N, Hashimoto M, Furuichi T, Fukuda M, Mikoshiba K. A novel zinc finger protein, Zic, is involved in neurogenesis, especially in the cell lineage of cerebellar granule cells. J Neurochem. 1994;63:1880–1890. doi: 10.1046/j.1471-4159.1994.63051880.x. [DOI] [PubMed] [Google Scholar]
- 6.Aruga J, Nagai T, Tokuyama T, Hayashizaki Y, Okazaki Y, Chapman VM, Mikoshiba K. The mouse Zic gene family: homologues of Drosophila pair-rule gene odd-paired. J Biol Chem. 1996a;271:1043–1047. doi: 10.1074/jbc.271.2.1043. [DOI] [PubMed] [Google Scholar]
- 7.Aruga J, Yozu A, Hayashizaki Y, Okazaki Y, Chapman VM, Mikoshiba K. Identification and characterization of Zic4, a new member of Zic gene family. Gene. 1996b;171:291–294. doi: 10.1016/0378-1119(96)00111-4. [DOI] [PubMed] [Google Scholar]
- 8.Benedyk MJ, Mullen JR, DiNardo S. odd-paired: a zinc finger pair-rule protein required for the timely activation of engrailed and wingless in Drosophila embryos. Genes Dev. 1994;8:105–117. doi: 10.1101/gad.8.1.105. [DOI] [PubMed] [Google Scholar]
- 9.Cimbora DM, Sakonju S. Drosophila midgut morphogenesis requires the function of segmentation gene odd-paired. Dev Biol. 1995;169:580–595. doi: 10.1006/dbio.1995.1171. [DOI] [PubMed] [Google Scholar]
- 10.Davis CA, Joyner AL. Expression patterns of the homeobox containing genes En-1 and En-2 and the proto-oncogene diverge during mouse development. Genes Dev. 1988;2:1736–1744. doi: 10.1101/gad.2.12b.1736. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher C, Norman DJ, Heintz N. Genetic mapping of meander tail, a mouse mutation affecting cerebellar development. Genomics. 1991;9:647–655. doi: 10.1016/0888-7543(91)90358-l. [DOI] [PubMed] [Google Scholar]
- 12.Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda N, Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989;342:32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- 13.Gao WQ, Hatten ME. Immortalizing oncogenes subvert the establishment of granule cell identity in developing cerebellum. Development. 1994;120:1059–1070. doi: 10.1242/dev.120.5.1059. [DOI] [PubMed] [Google Scholar]
- 14.Hatten ME, Heintz N. Mechanisms of neural patterning and specification in the developing cerebellum. Annu Rev Neurosci. 1995;18:385–408. doi: 10.1146/annurev.ne.18.030195.002125. [DOI] [PubMed] [Google Scholar]
- 15.Hatten ME, Alder J, Zimmerman K, Heintz N. Genes involved in cerebellar cell specification and differentiation. Curr Opin Neurobiol. 1997;7:40–47. doi: 10.1016/s0959-4388(97)80118-3. [DOI] [PubMed] [Google Scholar]
- 16.Herrup K, Kuemerle B. The compartmentalization of the cerebellum. Annu Rev Neurosci. 1997;20:61–90. doi: 10.1146/annurev.neuro.20.1.61. [DOI] [PubMed] [Google Scholar]
- 17.Herrup K, Wilczynski SL. Cerebellar cell degeneration in the leaner mutant mouse. Neuroscience. 1982;7:2185–2196. doi: 10.1016/0306-4522(82)90129-4. [DOI] [PubMed] [Google Scholar]
- 18.Hui C-c, Slusarski D, Platt KA, Holmgren R, Joyner AL. Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm- and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev Biol. 1994;162:402–413. doi: 10.1006/dbio.1994.1097. [DOI] [PubMed] [Google Scholar]
- 19.Inouye M, Oda S. Strain-specific variations in the folial pattern of the mouse cerebellum. J Comp Neurol. 1980;190:357–362. doi: 10.1002/cne.901900209. [DOI] [PubMed] [Google Scholar]
- 20.Joyner AL, Herrup K, Auerbach BA, Davis CA, Rossant J. Subtle cerebellar phenotype in mice homozygous for a targeted deletion of the En-2 homeobox. Science. 1991;251:1239–1243. doi: 10.1126/science.1672471. [DOI] [PubMed] [Google Scholar]
- 21.Kano M, Hashimoto K, Kurihara H, Watanabe M, Inoue Y, Aiba A, Tonegawa S. Persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking mGluR1. Neuron. 1997;18:71–79. doi: 10.1016/s0896-6273(01)80047-7. [DOI] [PubMed] [Google Scholar]
- 22.Kinzler KW, Ruppert JM, Bigner SH, Vogelstein B. The GLI gene is a member of the Kruppel family of zinc finger proteins. Nature. 1988;332:371–374. doi: 10.1038/332371a0. [DOI] [PubMed] [Google Scholar]
- 23.Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferasegene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 24.Mares V, Lodin Z. The cellular kinetics of the developing mouse cerebellum. II. The function of the external granuler layer in the process of gyrification. Brain Res. 1970;23:343–352. doi: 10.1016/0006-8993(70)90061-2. [DOI] [PubMed] [Google Scholar]
- 25.McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- 26.Millen KJ, Wurst W, Herrup K, Joyner AL. Abnormal embryonic cerebellar development and patterning of postnatal foliation in two mouse Engrailed-2 mutants. Development. 1994;120:695–706. doi: 10.1242/dev.120.3.695. [DOI] [PubMed] [Google Scholar]
- 27.Millen KJ, Hui C-c, Joyner AL. A role for En-2 and other murine homologues of Drosophila segment polarity genes in regulating positional information in the developing cerebellum. Development. 1995;121:3935–3945. doi: 10.1242/dev.121.12.3935. [DOI] [PubMed] [Google Scholar]
- 28.Mo R, Freer AM, Zinyk DL, Crackower MA, Michaud J, Heng HH-Q, Chik KW, Shi X-M, Tsui L-C, Cheng SH, Joyner AL, Hui C-c. Specific and Redundant function of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development. 1997;124:113–123. doi: 10.1242/dev.124.1.113. [DOI] [PubMed] [Google Scholar]
- 29.Nagai T, Aruga J, Takada S, Gunther T, Sporle R, Schughart K, Mikoshiba K. The expression of the mouse Zic1, Zic2 and Zic3 gene suggests an essential role for Zic gene in body pattern formation. Dev Biol. 1997;182:299–313. doi: 10.1006/dbio.1996.8449. [DOI] [PubMed] [Google Scholar]
- 30.Nakata K, Nagai T, Aruga J, Mikoshiba K. Xenopus Zic3, a primary regulator both in neural and neural crest development. Proc Natl Acad Sci USA. 1997;94:11980–11985. doi: 10.1073/pnas.94.22.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neumann PE, Mueller GG, Sidman RL. Identification and mapping of a mouse gene influencing cerebellar folial pattern. Brain Res. 1990;524:85–89. doi: 10.1016/0006-8993(90)90495-w. [DOI] [PubMed] [Google Scholar]
- 32.Orenic TV, Slusarski DC, Kroll KL, Holmgren RA. Cloning and characterization of the segment polarity gene cubitus interruptus Dominant of Drosophila. Genes Dev. 1990;4:1053–1067. doi: 10.1101/gad.4.6.1053. [DOI] [PubMed] [Google Scholar]
- 33.Oscarsson O. Functional organization of spinocerebellar paths. In: Iggo A, editor. Handbook of sensory physiology, Vol 2, Somatosensory system. Springer; Berlin: 1973. pp. 340–380. [Google Scholar]
- 34.Rudnicki MA, Braun T, Hinuma S, Jaenisch R. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell. 1992;71:383–390. doi: 10.1016/0092-8674(92)90508-a. [DOI] [PubMed] [Google Scholar]
- 35.Schlichter DJ, Detre JA, Aswad DW, Chehrazi B, Greengard P. Localization of cyclic GMP-dependent protein kinase and substrate in mammalian cerebellum. Proc Natl Acad Sci USA. 1980;77:5537–5541. doi: 10.1073/pnas.77.9.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas KR, Capecchi MR. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature. 1990;346:847–850. doi: 10.1038/346847a0. [DOI] [PubMed] [Google Scholar]
- 37.Thomas KR, Musci TS, Neumann PE, Capecchi MR. Swaying is a mutant allele of the proto-oncogene Wnt-1. Cell. 1991;67:969–976. doi: 10.1016/0092-8674(91)90369-a. [DOI] [PubMed] [Google Scholar]
- 38.Urbanek P, Fetka I, Meisler MH, Busslinger M. Cooperation of Pax2 and Pax5 in midbrain and cerebellum development. Proc Natl Acad Sci USA. 1997;94:5703–5708. doi: 10.1073/pnas.94.11.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wood KA, Dipasquale B, Youle RJ. In situ labeling of granule cells for apoptosis-associated DNA fragmentation reveals different mechanisms of cell loss in developing cerebellum. Neuron. 1993;11:621–632. doi: 10.1016/0896-6273(93)90074-2. [DOI] [PubMed] [Google Scholar]
- 40.Wurst W, Auerbach AB, Joyner AL. Multiple developmental defects in Engrailed-1 mutant mice: an early mid-hindbrain deletion and patterning defects in forelimbs and sternum. Development. 1994;120:2065–2075. doi: 10.1242/dev.120.7.2065. [DOI] [PubMed] [Google Scholar]
- 41.Yagi T, Nada S, Watanabe N, Tamemoto H, Kohmura N, Ikawa Y, Aizawa S. A novel negative selection for homologous recombinants using diphtheria toxin A fragment gene. Anal Biochem. 1993;214:77–86. doi: 10.1006/abio.1993.1459. [DOI] [PubMed] [Google Scholar]
- 42.Yokota N, Aruga J, Takai S, Yamada K, Hamazaki M, Iwase T, Mikoshiba K. Predominant expression of human Zic in cerebellar granule cell lineage and medulloblastoma. Cancer Res. 1996;56:377–383. [PubMed] [Google Scholar]
- 43.Yuasa S, Kawamura K, Ono K, Yamakuni T, Takahashi Y. Development and migration of Purkinje cells in the mouse cerebellar primordium. Anat Embryol. 1991;184:195–212. doi: 10.1007/BF01673256. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L, Goldman JE. Developmental fates and migratory pathways of dividing progenitors in the postnatal rat cerebellum. J Comp Neurol. 1996;370:536–550. doi: 10.1002/(SICI)1096-9861(19960708)370:4<536::AID-CNE9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]