Abstract
Monoclonal antibody Cat-315 recognizes a chondroitin sulfate proteoglycan (CSPG) expressed on the surface of subsets of neurons in many areas of the mammalian CNS (Lander et al., 1997). The cell type-specific expression exhibited by the Cat-315 CSPG and other perineuronal net CSPGs imparts a distinct molecular surface identity to a neuron (Celio and Blumcke, 1994; Lander et al., 1997). The cell type(s) producing these surface-associated proteins and yielding this cellular diversity has remained in question. The expression of the Cat-315 CSPG in primary rat cortical cultures has permitted an examination of the cellular source of the Cat-315 antigen, as well as a determination of its spatial relationship to the neuronal surface.
Live-cell labeling of primary neuronal cultures demonstrates that the Cat-315 CSPG is on the extracellular surface of neurons. Furthermore, extraction experiments demonstrate that the Cat-315 CSPG lacks a transmembrane domain and that the entire molecule is extracellular and, therefore, can be considered a constituent of brain extracellular matrix. Several lines of evidence indicate that neurons with cell surface staining produce the Cat-315 CSPG. First, neurons with cell surface staining also show intracellular Cat-315 immunoreactivity. Second, β-xyloside or monensin, reagents that inhibit the synthesis and transport of CSPGs, increase intracellular Cat-315 immunoreactivity within neurons that express cell surface Cat-315 immunoreactivity. Third, double labeling with Cat-315 and a polyclonal antibody for the Golgi complex demonstrates a precise colocalization of the intracellular Cat-315 immunoreactivity with the Golgi. Together, these observations demonstrate that neurons contribute to the extracellular matrix of brain and that the Cat-315 CSPG is produced by the neurons that carry Cat-315 cell surface immunoreactivity.
Keywords: perineuronal net, brain extracellular matrix, neuronal subsets, rat cortex, primary neuronal cultures, glycosaminoglycan
Although some components of the extracellular matrix (ECM) of brain are expressed throughout both gray and white matter, other ECM constituents are found in extremely restricted patterns, in association with the surface of subsets of neurons. These perineuronal nets, which are likely to represent the neuronal extracellular matrix, are composed of glycoproteins, the glycosaminoglycan hyaluronan, and an increasingly complex array of proteoglycans, principally of the chondroitin sulfate class (Celio and Blumcke, 1994; Lander and Hockfield, 1997). A number of reagents, including monoclonal antibodies and lectins, reveal the perineuronal nets; in addition, histochemical-staining techniques indicate that perineuronal nets surround most, if not all, neurons within the brain (for review, see Hockfield, 1990; Celio and Blumcke, 1994).
Although the molecular composition of the perineuronal nets is not yet known in great detail, it is clear from a number of studies that the constituents of these nets are highly heterogeneous and that different neuronal subsets can be distinguished by the complement of chondroitin sulfate proteoglycans (CSPGs) that their nets contain (Hockfield and McKay, 1983; Fujita et al., 1989; Watanabe et al., 1989; Bertolotto et al., 1990, 1991, 1996; Hockfield et al., 1990; Lander et al., 1997). Brain proteoglycans exhibit heterogeneity in core protein composition (Oohira et al., 1988; Gowda et al., 1989; Herndon and Lander, 1990;Hockfield et al., 1990; Lander et al., 1997) and in the patterns of glycosylation or sulfation; further heterogeneity is seen among proteoglycans with the same core protein, which can differ through developmentally regulated alternative splicing or proteolytic processing (for review, see Hardingham and Fosang, 1992; Margolis and Margolis, 1993; Oohira et al., 1994a). The diverse components of individual perineuronal nets could regulate the extracellular microenvironment surrounding each neuron and subserve cell type-specific functions.
Despite over a decade of work on the identification and characterization of neuronal cell surface CSPGs, the cellular source of most of these proteins remains uncertain. We have approached this issue by using the monoclonal antibody Cat-315 in a series of experiments on primary neuronal cultures. Cat-315 was shown previously to recognize a perineuronal CSPG found in association with specific subsets of neurons in intact cat (Lander et al., 1997) and rat (C. Lander and S. Hockfield, unpublished observations) cortex. Here, we show that the Cat-315 antibody also recognizes a CSPG in primary neuronal culture and demonstrate that the Cat-315 antigen expressed in culture shares many properties with the Cat-315 antigen characterized in vivo. The in vitro system has permitted us to determine that the entire Cat-315 molecule, and not just the Cat-315 epitope, is extracellular and, therefore, is a constituent of the ECM. We also demonstrate that the Cat-315 CSPG is produced by neurons, providing important evidence that neurons contribute to the ECM of brain and that the cell type-specific association exhibited by neuronal cell surface CSPGs may be determined by cell type-specific gene expression.
MATERIALS AND METHODS
Cell culture. Cerebral cortices from embryonic day 16 (E16) Sprague Dawley rats were dissected free of meninges, washed in calcium- and magnesium-free Dulbecco’s PBS (DPBS; GIBCO, Grand Island, NY), and digested with trypsin (0.05% in DPBS plus 0.53 mmEDTA) at 37°C for 20 min. The resulting cell suspension was washed in medium [50% DMEM, 25% HBSS, 0.38% HEPES, 0.5% glucose, 8% fetal bovine serum (FBS), and 20 ng/ml 2.5 S NGF] (Redmond et al., 1997) and triturated with a fire-polished pasteur pipette. Dissociated cells were pelleted by centrifugation at 1000 × g for 5 min; the resulting supernatant was discarded, and the cell pellet was resuspended in fresh medium. The cell suspension was plated at a density of 100,000 cells/well in 24-well plates containing polyornithine- (PORN; 0.01 mg/ml) and laminin (2 μg/ml)-coated glass coverslips. For cell homogenates, cells were plated at a density of 500,000 cells/well.
For drug treatment of cultures, E16 or neonatal (P0) cultures were plated onto PORN- and laminin-coated coverslips at a density of 100,000 cells/well and maintained in serum-containing medium for a range of culture days before the addition of drug. For monensin (Calbiochem, La Jolla, CA), P0 cultures were maintained for 7 d and then incubated in monensin-containing medium (1 × 10−6m) for 8 additional hours. For β-xyloside (Sigma, St. Louis, MO), E16 cultures were plated for 3 d and then maintained in β-xyloside-containing medium (1.5 μm) for 10 additional days.
For astrocyte cultures, cerebral cortices from P0 rats were dissected free of meninges and dissociated, as described above. Dissociated cells were plated onto poly-l-lysine (0.1 mg/ml; Sigma)-coated tissue culture flasks in DMEM plus 10% FBS. Twenty-four hours after plating, cultures were shaken to remove neurons and oligodendrocytes (Smith et al., 1990). Cell homogenates and culture supernatants were collected after 7 d in culture.
Immunocytochemistry. Cultures plated onto PORN- and laminin-coated coverslips were fixed in 4% paraformaldehyde for 10 min and then rinsed extensively in phosphate buffer. Coverslips were incubated in permeabilization medium (DMEM, 0.2% Triton X-100, 0.01% lycine, and 0.01% glycine) for 1.5 hr and then rinsed in phosphate buffer immediately before antibody staining.
Coverslips were incubated overnight at 4°C in the monoclonal antibody Cat-315 (an IgM), followed by 2 hr in Texas Red-conjugated goat anti-mouse IgM (Southern Biotechnology, Birmingham, AL). For double-labeling experiments, coverslips were incubated overnight in a rabbit polyclonal antibody raised against rat liver Golgi membranes (Louvard et al., 1982; a generous gift from Pietro deCamilli), an IgG monoclonal antibody to class III β-tubulin (TuJ1; Lee et al., 1990a; a generous gift from Anthony Frankfurter), rabbit polyclonal or IgG monoclonal anti-glial fibrillary acidic protein (GFAP; Sigma), or the IgG monoclonal antibody Rat-401, which recognizes the intermediate filament protein nestin (Hockfield and McKay, 1985), followed by 2 hr in FITC-conjugated goat anti-mouse IgG (for TuJ1, GFAP, and nestin incubations) or FITC-conjugated goat anti-rabbit (for Golgi and GFAP polyclonal incubations) secondary antibody (Southern Biotechnology). Cultures were rinsed extensively in phosphate buffer and then incubated overnight in Cat-315, followed by a 2 hr incubation with Texas Red-conjugated goat anti-mouse IgM secondary antibody. Control experiments showed no cross-reactivity between the subclass-specific secondary antibodies and the inappropriate first antibodies. Immunocytochemical labeling was visualized and photographed using a Nikon fluorescence microscope. Cellular localization was also analyzed using a Bio-Rad confocal microscope.
For live-cell staining, unfixed, unpermeabilized cultures were rinsed in DMEM without serum and then incubated in Cat-315 (without sodium azide) for 30 min. Cultures were again rinsed in DMEM and then incubated for 30 min with Texas Red-conjugated goat anti-mouse IgM secondary antibody. Cultures were rinsed in DMEM and then fixed for 10 min in 4% paraformaldehyde.
Cell and tissue homogenates. Postnatal day 1 (P1) rat brains were homogenized in DPBS (5 ml/gm of tissue) containing a cocktail of protease inhibitors (5 μg/ml leupeptin, 5 mm aminocaproic acid, and 5 mmN-ethylmaleamide dissolved in 5 mm sodium phosphate buffer; 1 mmphenylmethylsulfonyl fluoride and 5 μg/ml leupeptin dissolved in DMSO). For cell homogenates, 100 μl of DPBS (plus protease inhibitors) was added to each well, and cells were collected by scraping cultures with a pipette tip.
Immunoprecipitation. Cat-315 was adsorbed to goat anti-mouse IgM agarose beads (Sigma) by mixing overnight at 4°C. Beads were washed and then mixed overnight at 4°C with conditioned medium from E16 cortical cultures that had been maintained on PORN- and laminin-coated coverslips for a variety of incubation periods (1–21 d). Antigens were eluted by boiling in SDS-PAGE sample buffer with β-mercaptoethanol or by digestion with bovine testicular hyaluronidase (Wydase; see below).
Enzymes. Enzymes used were as follows: bovine testicular hyaluronidase, which has both hyaluronidase and chondroitinase activity (Wydase; 75 U/ml; Wyeth-Ayerst, Philadelphia, PA), chondroitinase ABC (0.25 U/ml; ICN Biomedicals, Cleveland, OH), and phosphatidylinositol phospholipase C (PI-PLC; 5mU/ml; Boehringer Mannheim, Indianapolis, IN). For each, samples were incubated overnight at 37°C in the presence of enzyme and protease inhibitors and were analyzed by Western blotting. For PI-PLC digestion, 0.16% Triton X-100 (w/v) was added during enzyme incubation.
Determination of mechanism of membrane association. For analysis of the mechanism of association of the antigen with the particulate fraction, cell homogenates were separated by centrifugation (30,000 × g for 1 hr) into pellet and supernatant fractions. The pellet was rehomogenized in DPBS with protease inhibitors, centrifuged at 30,000 × g for 1 hr, and then resuspended in PBS (with protease inhibitors) twice. Aliquots of this suspension were incubated in PBS, 0.1 mNa2CO3 buffer at pH 11, 1% Triton X-100, or PI-PLC (as described above). After digestion, samples were again centrifuged, and supernatant and pellet fractions were analyzed by Western blotting.
Western blot analysis. Samples were combined with gel-loading buffer (20 mm Tris-HCl, pH 6.8, 3% SDS, 10% glycerol, and 0.01% bromphenol blue) and β-mercaptoethanol, boiled for 5 min, and electrophoresed on 3–8% acrylamide gradient gels in 50 mm Tris base, 0.38 m glycine, and 0.2% SDS. Proteins were electrophoretically transferred to nitrocellulose overnight at 100 mA in 25 mm Tris, 0.192 mglycine, 0.1% SDS, and 20% methanol. Blots were blocked in 5% nonfat dry milk in TBS for 1 hr, washed, and incubated with primary antibody containing 0.5% Triton X-100 overnight. Blots were washed and then incubated with alkaline phosphatase-conjugated goat anti-mouse IgM secondary antibody (Cappel, West Chester, PA) for Cat-315 (diluted in DMEM plus 5% FCS and 0.5% Triton) for 2 hr. Immunoreactive bands were visualized with nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Sigma).
RESULTS
Monoclonal antibody Cat-315 identifies neuronsin vitro
Monoclonal antibody Cat-315 was one product of an immunization strategy designed to generate antibodies that recognize neuronal cell surface-associated CSPGs (Lander et al., 1997). Cat-315 recognizes a CSPG that is distributed along the surface of cell bodies and proximal dendrites of a subset of neurons in many areas of the mammalian CNS. Although many neuronal cell surface CSPGs have now been identified, the cellular source of most of these cell type-specific proteins, as well as their mechanism of association with the cell surface, has not been resolved. Here, we have used neuronal primary cultures to address these two issues.
One property of the Cat-315 antigen is an association with the perimeter of cortical neurons in tissue sections. We first asked whether Cat-315 also stains cortical neurons in vitro. Primary cultures of dissociated cerebral cortices from E16 rats were maintained in either serum-containing or serum-free medium for a range of time periods [1, 3, 7, 14, and 21 culture days (CD)]; cultures from each time point were fixed, permeabilized, and then stained with Cat-315 (Fig. 1). Cat-315 labeled cells with the morphological appearance of neurons at all time points and from cultures maintained with or without serum. Surface-associated staining along cell bodies and neurites was observed at the earliest time point examined (1 CD; Fig.1A,C). After longer periods in culture (Fig. 1B,D), Cat-315 immunoreactivity increased, with extensive labeling of cell bodies and processes. In addition, at all culture time points, intracellular Cat-315 immunoreactivity was frequently observed in cells that also displayed cell surface-associated labeling (Fig.1A,C, arrows). This suggested that the cells with cell surface-associated staining may be the site of the synthesis of the Cat-315 antigen (addressed in more detail below).
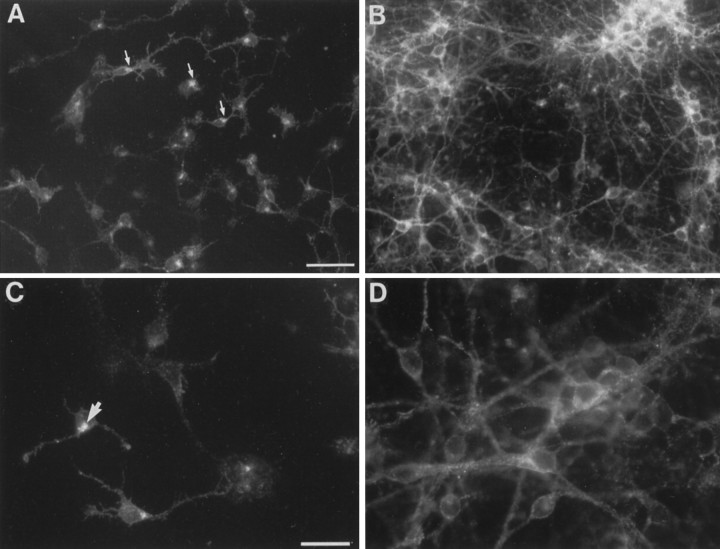
Fig. 1.
Monoclonal antibody Cat-315 recognizes cells with the morphological appearance of neurons in primary cultures of rat cortex. A, C, Low (A) and high (C) magnification photomicrographs of cultures from E16 rats were maintained in culture for 1 d (E16 plus 1 CD) and then fixed and stained with Cat-315. Cat-315 immunoreactivity is detected in association with cell surfaces. In addition to the surface-associated staining, immunoreactivity is also seen in an intracellular compartment (arrows). B, D, Low (B) and high (D) magnification photomicrographs of E16 plus 7 CD cultures stained with Cat-315 demonstrate that, after longer periods in culture, cell surface immunoreactivity increases, extensively labeling cells and their processes. Scale bars: A, B, 50 μm;C, D, 25 μm.
In the intact brain, Cat-315 immunoreactivity is associated with neurons (Lander et al., 1997). To determine the cell type labeled by Cat-315 in vitro, we double labeled cultures with Cat-315 and cell type-specific markers (Fig. 2). Nestin, recognized by monoclonal antibody Rat-401, is an intermediate filament protein expressed by neural progenitor cells and immature astrocytes, including radial glial cells (Hockfield and McKay, 1985). There was no overlap between the set of cells labeled by Cat-315 (Fig.2A) and the set that was positive for Rat-401 (Fig.2B). GFAP is expressed by astrocytes, and in our cultures, GFAP identified cells with a flat morphology. There was no overlap between the set of cells labeled by Cat-315 (Fig.2C) and the set labeled by antibodies to GFAP (Fig.2D). Together, these two results indicate that Cat-315 does not label precursor cells or differentiated astrocytes. Class III neuron-specific β-tubulin is a cytoskeletal protein, identified by the monoclonal antibody TuJ1, that is expressed by neurons during or directly after their final mitotic division (Lee et al., 1990a,b). In marked contrast to the results with GFAP and nestin, all Cat-315-positive cells (Fig. 2E) also expressed class III β-tubulin (Fig. 2F), demonstrating that Cat-315 selectively labels neurons. Importantly, although all Cat-315 cells expressed class III β-tubulin, not all cells that were class III β-tubulin-positive were labeled by Cat-315 (Fig.2E,F). At all culture time points examined and in the presence or absence of serum, the Cat-315-positive cells represented a subset of the class III β-tubulin-positive cells. This observation is consistent with our observations in intact rat (unpublished observations) and cat (Lander et al., 1997) cerebral cortex, in which Cat-315 labels only a subset of cortical neurons. All of the double label studies taken together demonstrate that in vitro, as in vivo, Cat-315 recognizes a subset of cortical neurons.
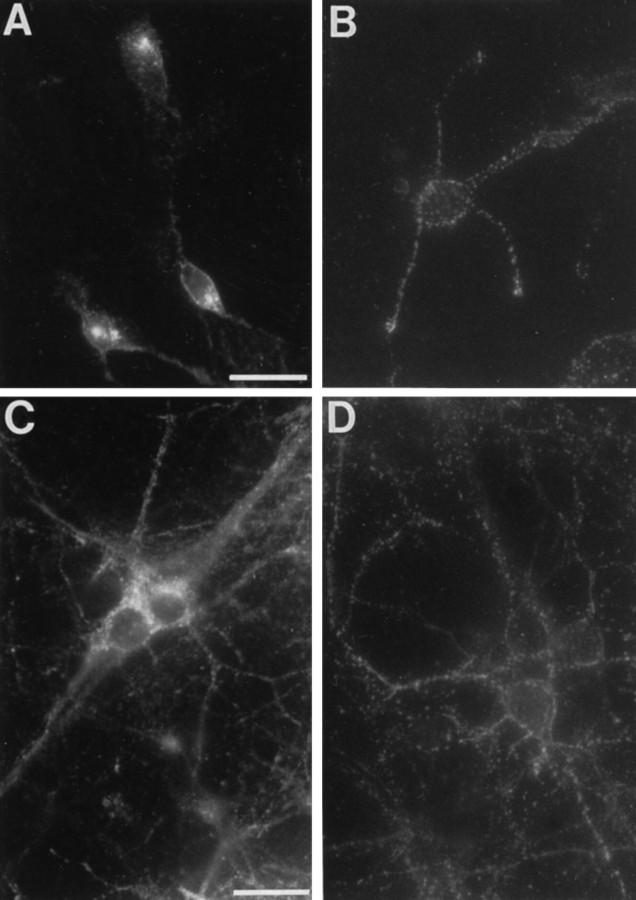
Fig. 2.

Cell surface Cat-315 immunoreactivity is associated with neurons and not with non-neuronal cells. Double label immunofluorescence experiments with Cat-315 and cell type-specific markers demonstrate that Cat-315 labels a subset of cortical neurons in culture. A, B, An E16 plus 3 CD culture double labeled with Cat-315 (A) and Rat-401 (B) demonstrates that the cells labeled by Cat-315 are not labeled by Rat-401, and vice versa. There is no overlap in the cells stained with these two antibodies. C,D, An E16 plus 7 CD culture double labeled with Cat-315 (C) and GFAP (D) demonstrates that the cells labeled by Cat-315 are negative for GFAP. Again, there is no overlap in the cells stained with these two antibodies. E, F, An E16 plus 7 CD culture double labeled with Cat-315 (E) and an antibody to the neuron-specific TuJ1 (F) demonstrates that all cells labeled by Cat-315 are also labeled by TuJ1; Cat-315 therefore labels neurons. Although all Cat-315-positive cells are TuJ1-positive (arrows), not all TuJ1-positive cells are Cat-315-positive (asterisks). This shows that Cat-315 labels only a subset of neurons. Scale bars:A–D, 50 μm; E–F, 25 μm.
Cat-315 recognizes a CSPG
Monoclonal antibody Cat-315 identifies a CSPG that migrates as a high molecular weight, polydisperse band on Western blots of guanidine extracts of cat visual cortex (Lander et al., 1997) and rat cortex (unpublished observations). We next asked whether the antigen recognized by Cat-315 in vitro was biochemically related to the antigen identified in vivo.
Conditioned medium and cell homogenates from primary cortical cultures (E16 plus 7 CD) as well as cortical tissue from animals of an equivalent age (P1) were analyzed by Western blotting. Conditioned medium (CM) was collected from cortical cultures and immunoprecipitated with goat anti-mouse IgM agarose beads preadsorbed to Cat-315 (an IgM). Adherent cells from cortical cultures (cell homogenates) and P1 rat cortices were homogenized in PBS (plus protease inhibitors) and, along with the CM immunoprecipitates, subjected to enzymatic digestion before Western blot analysis (Fig. 3).
Fig. 3.
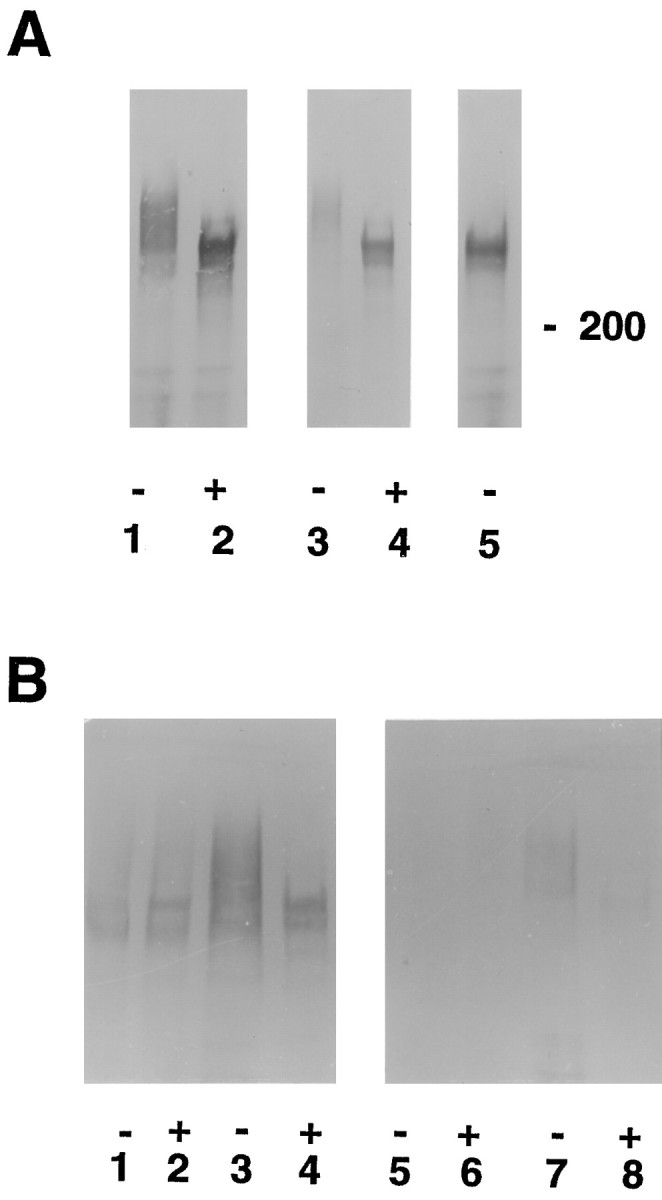
Cat-315 recognizes a CSPG of the same apparent molecular weight in vivo and in vitro, which is primarily produced by neurons. A, Cat-315 immunoprecipitates of conditioned medium from mixed cultures (lanes 1, 2) and cortical homogenates from postnatal day 1 rats (lanes 3,4) incubated in the presence (+) (lanes 2, 4) or absence (-) (lanes 1, 3, 5) of an enzyme that removes chondroitin sulfate glycosaminoglycans were Western blotted and probed with Cat-315. Removal of chondroitin sulfate glycosaminoglycans produces an increase in the mobility of the antigen recognized by Cat-315 (lanes 2, 4), indicating that Cat-315 recognizes a CSPG. The CSPG detected by Cat-315 fromin vivo and in vitro preparations has the same apparent molecular weight both before and after the removal of chondroitin sulfate, suggesting that Cat-315 recognizes the same CSPG in both preparations. For cortical homogenates, each lane was loaded with 25 μg of protein. The deglycosylating enzyme did not produce a shift in mobility of neurofilament (data not shown), which lacks chondroitin sulfate chains, indicating that the increase in mobility on Western blots of the Cat-315 antigen is specifically caused by the removal of glycosaminoglycans and not by proteolysis. Lane 5, To test the efficacy of β-xyloside in prohibiting the addition of glycosaminoglycans, we maintained E16 plus 3 CD cultures in β-xyloside-containing medium for an additional 10 d. Cat-315 immunoprecipitates from conditioned medium from β-xyloside-treated cultures contain an immunoreactive band that migrates to the same molecular weight as the Cat-315 antigen from which chondroitin sulfates have been enzymatically removed. This shows that β-xyloside effectively inhibited glycosaminoglycan addition. B, Cell homogenates (lanes 1, 2,5, 6) and Cat-315 immunoprecipitates of conditioned medium (lanes 3,4, 7, 8) from mixed (lanes 1–4) and pure astrocyte (lanes 5–8) cultures incubated in the presence (+) (lanes 2, 4, 6, 8) or absence (-) (lanes 1, 3,5, 7) of bovine testicular hyaluronidase (which has chondroitinase activity) were Western blotted and probed with Cat-315. Cat-315 immunoreactivity was detected in both cell homogenates and conditioned medium from mixed cultures. Cell homogenates from pure astrocyte cultures were devoid of Cat-315 immunoreactivity; however, trace amounts of Cat-315 immunoreactivity were detected in conditioned medium from these cultures. For cell culture homogenates, each lane was loaded at 50 μg of protein per lane (compare with cortical homogenates in A loaded at 25 μg per lane).
In all preparations, Cat-315 recognized a polydisperse band at ∼680 kDa (Fig. 3A, lanes 1,3,B, lanes 1,3,7). Digestion with either of two enzymes that remove chondroitin sulfate glycosaminoglycans, bovine testicular hyaluronidase (Fig. 3) or chondroitinase ABC, produced an apparent tightening of the immunoreactive band and a shift in mobility to ∼580 kDa (Fig.3A, lanes 2,4, B,lanes 2,4,8). The Cat-315 antigens in both in vitro and in vivopreparations comigrated on Western blots both before (Fig.3A, lanes 1,3, B,lanes 1,3,7) and after (Fig. 3A, lanes 2,4,B, lanes 2,4,8) removal of chondroitin sulfate. Cat-315 immunoreactivity was not detected in serum-containing medium that was not conditioned by cell cultures (data not shown). This experiment demonstrates that in vitro, as in vivo, Cat-315 recognizes a CSPG and also provides evidence that the in vivo antigen is likely to be the same as, or closely related to, the in vitroantigen.
The Cat-315 epitope is extracellular
We reported previously that Cat-315 immunoreactivity is associated with neuronal cell surfaces (Lander et al., 1997). To determine whether the cell surface-associated staining observed with Cat-315 in culture was localized to the intracellular or extracellular surface of neurons, we stained unfixed and unpermeabilized cultures with Cat-315 (Fig.4). In the absence of fixation or permeabilization, Cat-315 immunoreactivity was still detected in association with neuronal cell surfaces at all culture time points (Fig. 4B,D). The surface-associated staining in live-cell labeling experiments showed a distribution very similar to that observed in cultures that had been fixed and permeabilized before staining (Fig.4A,C). In addition to the surface-associated immunoreactivity, Cat-315-positive neurons in fixed and permeabilized preparations showed intracellular immunoreactivity, which was not observed after live labeling (addressed further below). The surface-associated immunoreactivity appeared somewhat less in nonpermeabilized cells as compared with that in fixed and permeabilized cells. This difference may be attributable to the shorter antibody incubation times for the live labeling experiments (30 min each in primary and secondary antibodies) compared with the times for the labeling of fixed, permeabilized cells (48 hr in primary antibody and 2 hr in secondary antibody). The persistence of surface-associated immunoreactivity on live, unpermeabilized cells indicates that the Cat-315 epitope, and possibly the entire Cat-315 molecule, is localized to the extracellular surface of the neuronal membrane.
Fig. 4.
The Cat-315 epitope is extracellular. E16 plus 1 CD (A, B) and E16 plus 7 CD (C, D) cultures were fixed and permeabilized before immunostaining (A,C) or reacted with antibody Cat-315 before fixation (B, D). The cell surface-associated immunoreactivity seen in fixed and permeabilized cultures is retained when cultures are stained without fixation or permeabilization, whereas the intracellular staining is not seen in the unfixed, unpermeabilized cultures. This demonstrates that the surface-associated Cat-315 epitope is extracellular. Scale bars: A–D, 25 μm.
As shown in Figure 3, the Cat-315 antigen is found both secreted into the culture medium and in association with neuronal surfaces. To determine the mechanism of association of the Cat-315 antigen with the cell surface, we subjected the particulate fraction of cell culture homogenates to extraction with various reagents. Cell homogenates were separated into supernatant and particulate (PBS insoluble fraction containing cell membranes, extracellular matrix, and cytoskeletal elements) fractions by centrifugation, and the particulate fraction was washed three times by resuspension in PBS. Aliquots of the final particulate fraction were resuspended in PBS and incubated with either PBS, 1% Triton X-100, 0.1 m Na2C03buffer at pH 11, or the enzyme PI-PLC. The resulting digests were again separated into supernatant and pellet fractions and were analyzed by Western blotting (Fig. 5). PBS (Fig. 5,lanes 1,2) did not release Cat-315 immunoreactivity from the pellet fraction into the supernatant fraction. Although Cat-315 was also not released into the supernatant by either Triton X-100 (Fig. 5, lanes 3,4) or PI-PLC (data not shown), treatment with Na2C03 (Fig. 5, lanes 5,6) resulted in a partial release of Cat-315 immunoreactivity into the supernatant from the particulate fraction. The inability to extract the Cat-315 antigen from the particulate fraction with Triton X-100, although not eliminating the possibility that some Cat-315 molecules may contain a transmembrane domain, does indicate that such an anchor is unlikely to exclusively localize the Cat-315 antigen to the neuronal surface. The partial release of the Cat-315 antigen from the particulate fraction with high pH (pH 11) carbonate buffer demonstrates that at least a proportion of this CSPG exists as a peripheral membrane protein, as would be expected for an ECM protein that is first secreted into the medium and subsequently bound to the neuronal surface via interactions with other surface macromolecules. Together with the ability of the Cat-315 antibody to bind to the surface of live, unpermeabilized cells, these data provide evidence that at least a proportion of the Cat-315 antigen is entirely extracellular and therefore part of the extracellular matrix of brain.
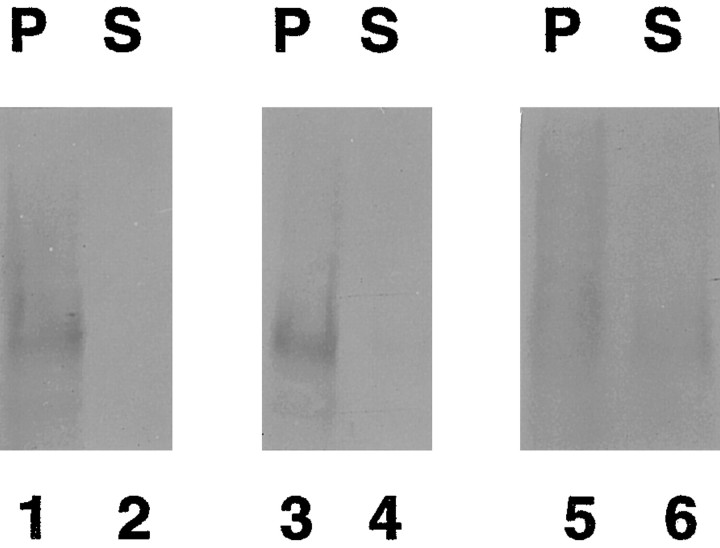
Fig. 5.
The Cat-315 CSPG is a constituent of the extracellular matrix. Cell homogenates were separated into particulate and soluble fractions by centrifugation. The particulate fraction was rinsed several times by resuspension in PBS followed by centrifugation. The washed particulate fraction was subjected to extraction with various reagents and then separated again into supernatant and particulate fractions. These final supernatant and particulate fractions were Western blotted and probed with Cat-315; eachlane contains 25 μg of protein. Neither PBS (lanes 1, 2) nor Triton X-100 (lanes 3, 4) released Cat-315 immunoreactivity from the particulate into the soluble fraction. Na2CO3 (lanes 5,6) partially released Cat-315 immunoreactivity from the particulate to the soluble fraction. Together with the ability of Cat-315 to stain the surfaces of unfixed, unpermeabilized cells, these data indicate that at least a portion of the Cat-315 CSPG is entirely extracellular and peripherally attached to the neuronal surface and thus is a constituent of the extracellular matrix.P, Particulate fraction; S, soluble fraction.
The Cat-315 CSPG is produced by neurons
Although many neuronal cell surface CSPGs have been described, the extracellular location of these proteins has made definitive determination of their cellular sites of synthesis problematic. Three experimental observations indicate that neurons are the site of synthesis of the Cat-315 CSPG. First, as described above, substantial amounts of Cat-315 immunoreactivity were detected in the supernatant and cell homogenates from mixed neuronal cultures (Fig. 3). In contrast, no immunoreactivity was detected in cell homogenates from pure astrocyte cultures (Fig. 3B, lanes 5,6). Only trace amounts of immunoreactivity were observed in Cat-315 immunoprecipitates of astrocyte conditioned medium (Fig. 3B, lanes 7,8).
Second, in fixed and permeabilized cultures, neurons with surface-associated Cat-315 immunoreactivity also frequently exhibited intracellular immunoreactivity (see Figs.1A,C, 4A). The intracellular Cat-315 staining had a perinuclear distribution, suggesting an association with the Golgi complex, the site of glycosaminoglycan addition to proteoglycans. To determine the subcellular localization of the intracellular Cat-315 immunoreactivity, we double labeled fixed and permeabilized cortical cultures with Cat-315 and an antibody raised against rat liver Golgi fractions (Louvard et al., 1982). Confocal microscopy (Fig.6) showed Cat-315 immunoreactivity distributed over the neuronal cell surface (in green) and anti-Golgi immunoreactivity in intracellular compartments (inred). The intracellular Cat-315 immunoreactivity showed a perinuclear distribution and appeared yellow, because of its precise colocalization with immunoreactivity for the anti-Golgi antibody.
Fig. 6.
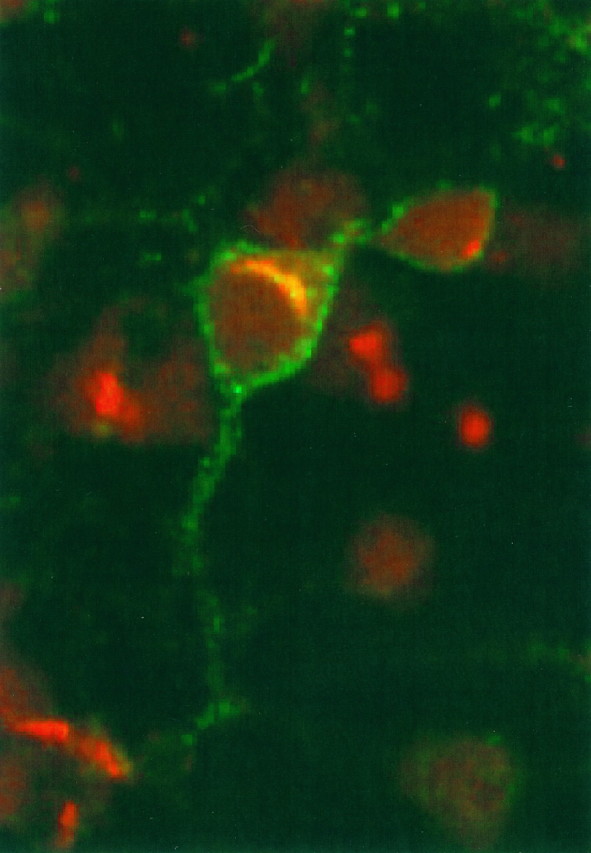
Double label, confocal microscopy demonstrates that intracellular Cat-315 immunoreactivity localizes to the Golgi complex. E16 plus 3 CD cultures were fixed, permeabilized, and then double labeled with Cat-315 (green) and a polyclonal antibody that recognizes the Golgi apparatus (red). Cat-315 immunoreactivity is distributed along the neuronal cell surface (green surface staining). The intracellular Cat-315 signal appears yellow, because of signals from both antibodies. In every case, intracellular Cat-315 immunoreactivity precisely colocalizes with immunoreactivity for the anti-Golgi antibody (yellow). However, there are many cells with Golgi labeling but without Cat-315 labeling (red). The figure shows a 2-μm-thick confocal image, in which antibodies were visualized with FITC- or Texas Red-conjugated, species-specific secondary antibodies.
The third approach to determine the site of synthesis of the Cat-315 antigen was based on the demonstration that the Cat-315 antigen is a CSPG. Monensin and β-xyloside interfere with the synthesis and/or secretion of CSPGs. In the presence of monensin, proteoglycan core protein continues to be produced but is retarded within the Golgi complex; in addition, glycosaminoglycan chain addition and sulfation are inhibited (Nishimoto et al., 1982). β-Xyloside serves as an exogenous substrate for glycosaminoglycan addition; in the presence of β-xyloside, the core protein of proteoglycans continues to be produced, but the addition of the chondroitin sulfate chains is inhibited (Schwartz, 1977). Cortical cultures were treated with monensin (Fig.7A,B) or β-xyloside (Fig. 7C,D) and analyzed immunobiochemically (Fig. 3A, lane 5) and immunohistochemically (Fig. 7). On Western blots, Cat-315 immunoprecipitates from conditioned medium of β-xyloside-treated cultures showed an increase in the mobility of the Cat-315 antigen equivalent to that seen in material treated with enzymes to remove chondroitin sulfate glycosaminoglycans, indicating the inhibition of glycosaminoglycan addition. Immunohistochemical analysis of treated cultures showed an increase in the amount of intracellular Cat-315 immunoreactivity and a marked decrease in cell surface-associated Cat-315 immunoreactivity, especially of neurites (Fig.7B,D). Double labeling with TuJ1 (Fig. 7A′,B′) showed that immunoreactivity for Cat-315 was markedly decreased on neurites (Fig.7B,B′) compared with control cultures (Fig. 7A,A′). In cultures treated with β-xyloside (Fig. 7D), there was a pronounced reduction in the surface-associated staining of neurites and a pronounced increase in the intracellular pool of Cat-315 immunoreactive material. Although treatment with either β-xyloside or monensin reduced the staining of neurites, all cells that showed intracellular Cat-315 immunoreactivity still showed some cell surface immunoreactivity. Taken together, these observations demonstrate that the Cat-315 CSPG is synthesized by the neurons that carry cell surface Cat-315 immunoreactivity.
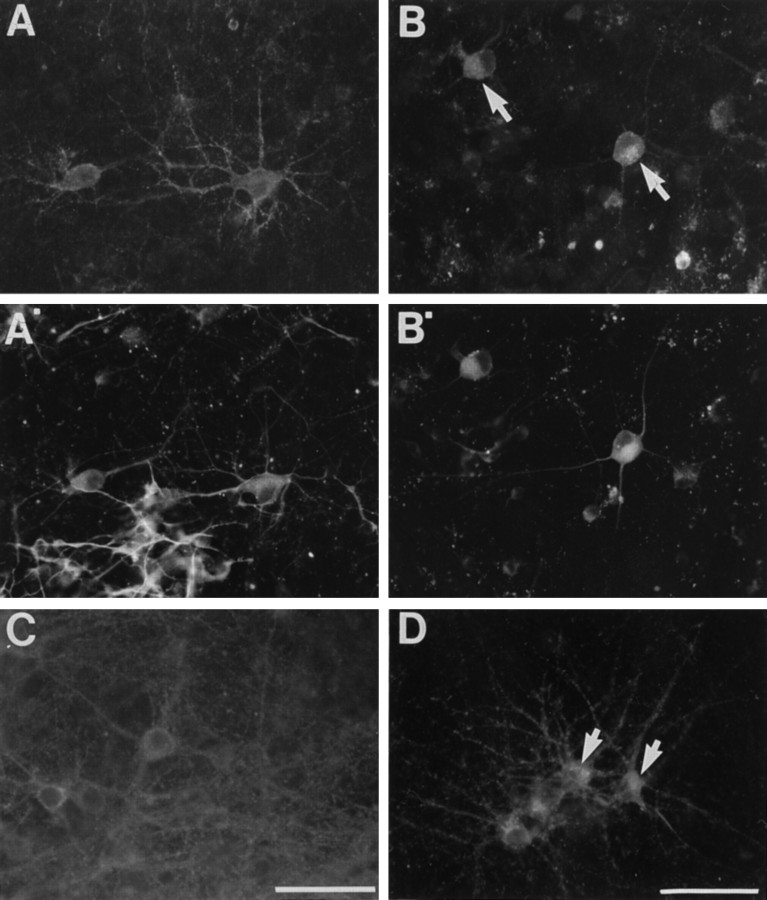
Fig. 7.
Treatment of cortical cultures with reagents that inhibit the synthesis and/or secretion of CSPGs leads to a retention of the Cat-315 CSPG in neurons with cell surface Cat-315 immunoreactivity. Primary cortical cultures from P0 rats were maintained for 7 d in serum-containing medium followed by an additional 8 hr without (A, A′) or with (B,B′) monensin and then fixed, permeabilized, and double labeled with Cat-315 and TuJ1. Cultures from E16 rats were maintained for 3 d in serum-containing medium and then maintained for an additional 10 d with (D) or without (C) β-xyloside. A, P0 cultures at 7 d plus 8 hr in control medium show robust Cat-315 surface-associated staining. A′, The same culture visualized for TuJ1 shows that TuJ1-immunoreactive processes are Cat-315-positive. B, P0 cultures at 7 d plus 8 hr in monensin show a marked increase in intracellular Cat-315 immunoreactivity (arrows) and a reduction of Cat-315 labeling of neurites. B′, TuJ1 immunroeactivity shows that TuJ1-labeled neurites lack surface-associated Cat-315 staining.C, Three-day-old E16 cultures maintained for an additional 10 d in control medium show Cat-315 labeling of neuron cell bodies, against a dense background of Cat-315-positive neurites.D, After 10 d of β-xyloside treatment, there is a marked decrease in Cat-315 labeling of neurites and a marked increase in intracellular Cat-315 immunoreactivity (arrows). These experiments provide evidence that the Cat-315 CSPG is synthesized by the neurons that carry cell surface Cat-315 immunoreactivity. Scale bars: A–D, 50 μm.
DISCUSSION
Here we have used monoclonal antibody Cat-315 to identify a CSPG in primary cortical cultures that exhibits many of the properties of the Cat-315 antigen characterized in vivo. Both in vivo and in vitro, Cat-315 immunoreactivity is found in association with the extracellular surface of a subset of cortical neurons, and the CSPGs recognized by Cat-315 in cortical culture and in cortical homogenates comigrate on Western blots before, as well as after, the removal of chondroitin sulfate glycosaminoglycans. These observations indicate that the Cat-315 antibody is likely to recognize the same (or a closely related) antigen in both preparations. We further demonstrate that neurons synthesize the Cat-315 CSPG, providing one important possible mechanism for the cell type specificity exhibited by neuronal cell surface CSPGs.
Neurons contribute to the extracellular matrix of brain
Because the brain lacks many of the features that define ECMs in other tissues, until relatively recently the existence of an ECM surrounding the neurons and glia of the brain was debated (Sanes, 1989). However, histochemical techniques that fix as well as stain ECM components, along with the generation of antibodies that identify specific components of the matrix, have permitted the demonstration of molecules characteristic of non-CNS ECMs as well as novel molecules that are unique to the brain matrix (Sanes, 1989; for review, seeHockfield, 1990). One of the more unusual features of the ECM of brain, in comparison with other, non-neural matrices, is its heterogeneous composition. Some constituents of brain ECM, in addition to outlining neuronal surfaces, are expressed more or less ubiquitously throughout the brain. These molecules include the glycoproteins tenascin (Celio and Chiquet-Ehrismann, 1993) and versican (Bignami et al., 1992, 1993) and the glycosaminoglycan hyaluronan (Bignami and Asher, 1992; Bignami et al., 1993). Other components of brain ECM are expressed in extremely restricted patterns, exclusively in association with the surface of subsets of neurons. The molecules comprising these perineuronal nets may, then, represent the ECM of neurons.
Since the first description of the perineuronal net, the cellular origin of this structure has been the subject of considerable debate. Immunohistochemical experiments with lectins and antibodies recognizing CSPGs at both light and electron microscopic levels reveal reaction product interposed between glial processes and neuronal surfaces (Hockfield and McKay, 1983; Nakagawa et al., 1986; Zaremba et al., 1989; Bertolotto et al., 1991; Bruckner et al., 1993; Schweizer et al., 1993). However, because these reagents could bind extracellular epitopes present on molecules with transmembrane domains, immunoreactive constituents have been interpreted by various investigators as being localized to glial end feet, the neuronal surface, or the extracellular space in between (for review, see Lafarga et al., 1984; Celio and Blumcke, 1994; Blumcke et al., 1995). Held [1902, in Celio and Blumcke (1994)] first proposed that the perineuronal net consists of a syncytium of glial processes, and until quite recently, the glial nature of this structure was widely accepted (Brauer et al., 1982, 1984; Lafarga et al., 1984; Schweizer et al., 1993). However, two recent studies examining the relationship between lectin-positive perineuronal nets and astrocytic processes indicate that glial processes and perineuronal nets represent distinct structures that are often, but not always, in register (Blumcke et al., 1995; Derouiche et al., 1996). In addition, each astrocyte can impinge on many perineuronal nets; conversely, each perineuronal net may be contacted by many astrocytes (Blumcke et al., 1995; Derouiche et al., 1996). Consistent with this, several of the “ubiquitously”-expressed components of perineuronal nets, including tenascin, hyaluronan, and versican, have been demonstrated to be produced by glia (Grumet et al., 1985; Asher and Bignami, 1991;LeBaron, 1996).
A second class of constituents of the perineuronal nets, principally CSPGs, is expressed on the surfaces of specific subsets of neurons. The particular complement of CSPGs in a perineuronal net imparts a unique molecular surface identity to each neuronal subset. Although several mammalian CNS proteoglycans have been demonstrated to be produced by neurons (Hoffman et al., 1988; Engel et al., 1996), few of the genes encoding the perineuronal CSPGs recognized by lectins and monoclonal antibodies have been identified. Therefore, despite the demonstration of an association of these CSPGs with the extracellular surface of neurons by electron microscopy (Hockfield and McKay, 1983; Bertolotto et al., 1991) or live-cell labeling in vivo (Zaremba et al., 1989), the cellular source of neuronal cell surface CSPGs has remained in question. Several observations suggested that these neuron-specific components of the ECM might be produced by the neurons themselves. First, lectins that recognize N-acetylgalactosamine, the amino sugar present in chondroitin sulfate disaccharides (Hardingham and Fosang, 1992), outline subpopulations of neurons in various areas of the CNS (Nakagawa et al., 1986; Kosaka and Heizmann, 1989; Naegele and Katz, 1990; Hartig et al., 1992, 1994; Luth et al., 1992; Bruckner et al., 1993; Schweizer et al., 1993; Seeger et al., 1994; Koppe et al., 1997). Lectin binding sites also have been detected in association with the Golgi complex of neurons, consistent with neuronal production of the respective glycoconjugates (Nakagawa et al., 1986; Streit et al., 1986; Schweizer et al., 1993). However, because lectins recognize multiple glycoproteins, as evidenced by the multiple immunoreactive bands that they identify on Western blots (Naegele and Katz, 1990;Schweizer et al., 1993), whether or not the Golgi-associated immunoreactivity corresponds to the same protein or proteins localized by the lectins on the neuronal cell surfaces remains unresolved. Second, phosphacan/6B4 proteoglycan, a CSPG identified by the 6B4 monoclonal antibody, has been reported by one group to be detected on the surface of subsets of neurons and to be produced predominantly by neurons in primary culture (Oohira et al., 1994b; Maeda et al., 1995). However, another group has reported that phosphacan is associated with and produced by glia (Engel et al., 1996; Meyer-Puttlitz et al., 1996). Neuronal production of perineuronal CSPGs would provide a biological basis for the cell type-specific expression exhibited by these molecules.
In the culture system used here, the appearance of Cat-315 reaction product within neurons as well as on their extracellular surfaces is consistent with the synthesis of the Cat-315 CSPG by neurons. However, although Cat-315 did not label non-neuronal cells, the intracellular antigen might reflect production of the CSPG by other cell types and subsequent endocytosis by neurons. Three lines of evidence indicate that neurons are the most likely cellular source of the Cat-315 CSPG. First, although high levels of antigen were detected in mixed neuronal cultures, we never observed a Cat-315-positive cell double labeled with an astrocytic marker, and almost undetectable levels were detected in pure astrocyte cultures, demonstrating that astrocytes alone are not the major source of the Cat-315 CSPG. Second, because the Cat-315 antibody recognizes a protein epitope (Lander et al., 1997), it should be possible to visualize the antigen soon after translation of its protein core. A series of post-translational modifications, including glycosaminoglycan addition, occurs during the intracellular transport of proteoglycan core proteins from the endoplasmic reticulum through the Golgi complex (Alberts et al., 1994). If the Cat-315 CSPG is produced by neurons, it should, therefore, be detectable within the Golgi complex as well as in association with the neuronal surface. Here we have demonstrated a precise colocalization of the intraneuronal, perinuclear Cat-315 immunoreactivity with a marker for the Golgi complex. Third, treatment of primary cultures with reagents that lead to a retention of the proteoglycan core proteins within the endoplasmic reticulum or Golgi led to an increase in the intracellular, perinuclear Cat-315 immunoreactivity exclusively in neurons with cell surface Cat-315 immunoreactivity. Other investigators have used similar methodologies for determining which cell type(s) produces extracellular matrix molecules; for example, treatment of cortical cultures with brefeldin A, another reagent that inhibits protein transport, reveals neuronal production of fibronectin (Sheppard et al., 1995). Together, these data provide convincing evidence that the Cat-315 antigen is produced by neurons.
To our knowledge, the Cat-315 CSPG is the first mammalian perineuronal CSPG shown definitively to be produced by neurons. Based solely on apparent molecular mass, the Cat-315 CSPG is not likely to be identical to neurocan (Rauch et al., 1992; Oohira et al., 1994b), BEHAB/brevican (Jaworski et al., 1994; Yamada et al., 1994), versican (LeBaron, 1996), NG2 (Stallcup et al., 1983), or any of the CSPGs identified by Herndon and Lander (1990). Several CSPGs do fall within a molecular weight range similar to that determined for the Cat-315 CSPG, such as the DSD-1 proteoglycan (Faissner et al., 1994). However, although the DSD-1 proteoglycan associates with subsets of neurons in the adult cerebral cortex, antibodies recognizing this CSPG associate with glial, but not neuronal, surfaces in cerebellar cultures (Faissner et al., 1994;Wintergerst et al., 1996). As discussed above, the cellular source of phosphacan, another large CSPG, has been debated. When the gene for the Cat-315 CSPG is cloned, it will be possible to determine the relationship between this and other neural CSPGs.
Different subsets of neurons express different complements of cell surface CSPGs, suggesting that CSPGs may variably regulate the extracellular microenvironment surrounding the neurons with which they associate. Some of the more ubiquitously expressed components of perineuronal nets, such as tenascin, versican, and hyaluronan, have been shown to be produced by glia. The results presented here provide evidence that neurons contribute to the extracellular matrix of brain. They also suggest that the distinct molecular surface identity imparted by CSPGs is regulated at the level of cell type-specific protein expression.
Footnotes
This work was supported by National Institutes of Health Grant EY06511. We thank Dr. Michael Tiemeyer for valuable discussions throughout the course of this work and Gian Carlo Ochoa for assistance with anti-Golgi immunohistochemistry.
Correspondence should be addressed to Dr. Susan Hockfield, Section of Neurobiology, Yale University, 333 Cedar Street, SHM C-405, New Haven, CT 06520-8001.
Dr. Lander’s present address: The Rockefeller University, 1230 York Avenue, New York, NY 10021.
REFERENCES
- 1.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. The extracellular matrix of animals. In: Robertson M, editor. Molecular biology of the cell, 3rd Edition. Garland; New York: 1994. pp. 971–995. [Google Scholar]
- 2.Asher R, Bignami A. Localization of hyaluronate in primary glial cultures derived from newborn rat brain. Exp Cell Res. 1991;195:401–411. doi: 10.1016/0014-4827(91)90390-g. [DOI] [PubMed] [Google Scholar]
- 3.Bertolotto A, Rocca G, Schiffer D. Chondroitin 4-sulfate proteoglycan forms an extracellular network in human and rat central nervous system. J Neurol Sci. 1990;100:113–123. doi: 10.1016/0022-510x(90)90021-e. [DOI] [PubMed] [Google Scholar]
- 4.Bertolotto A, Rocca G, Canavese G, Migheli A, Schiffer D. Chondroitin sulfate proteoglycan surrounds a subset of human and rat CNS neurons. J Neurosci Res. 1991;29:225–234. doi: 10.1002/jnr.490290213. [DOI] [PubMed] [Google Scholar]
- 5.Bertolotto A, Manzardo E, Guglielmone R. Immunohistochemical mapping of perineuronal nets containing chondroitin unsulfate proteoglycan in the rat central nervous system. Cell Tissue Res. 1996;283:283–295. doi: 10.1007/s004410050538. [DOI] [PubMed] [Google Scholar]
- 6.Bignami A, Asher R. Some observations on the localization of hyaluronic acid in adult, newborn and embryonal rat brain. Int J Dev Neurosci. 1992;10:45–57. doi: 10.1016/0736-5748(92)90006-l. [DOI] [PubMed] [Google Scholar]
- 7.Bignami A, Asher R, Perides G, Rahemtulla F. The extracellular matrix of cerebral gray matter: Golgi’s pericellular net and Nissl’s nervosen grau revisited. Int J Dev Neurosci. 1992;10:291–299. doi: 10.1016/0736-5748(92)90018-u. [DOI] [PubMed] [Google Scholar]
- 8.Bignami A, Hosley M, Dahl D. Hyaluronic acid and hyaluronic acid-binding proteins in brain extracellular matrix. Anat Embryol (Berl) 1993;188:419–433. doi: 10.1007/BF00190136. [DOI] [PubMed] [Google Scholar]
- 9.Blumcke I, Eggli P, Celio MR. Relationships between astrocytic processes and “perineuronal nets” in rat neocortex. Glia. 1995;15:131–140. doi: 10.1002/glia.440150205. [DOI] [PubMed] [Google Scholar]
- 10.Brauer K, Werner L, Leibnitz L. Perineuronal nets of glia. J Hirnforsch. 1982;23:701–708. [PubMed] [Google Scholar]
- 11.Brauer K, Bruckner G, Leibnitz L, Werner L. Structural and cytochemical features of perineuronal glial nets in the rat brain. Acta Histochem. 1984;74:53–60. doi: 10.1016/S0065-1281(84)80026-4. [DOI] [PubMed] [Google Scholar]
- 12.Bruckner G, Brauer K, Hartig W, Wolff JR, Rickmann MJ, Derouiche A, Delpech B, Girard N, Oertel WH, Reichenback A. Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia. 1993;8:183–200. doi: 10.1002/glia.440080306. [DOI] [PubMed] [Google Scholar]
- 13.Celio MR, Blumcke I. Perineuronal nets–a specialized form of extracellular matrix in the adult nervous system. Brain Res Rev. 1994;19:128–145. doi: 10.1016/0165-0173(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 14.Celio MR, Chiquet-Ehrismann R. “Perineuronal nets” around cortical interneurons expressing parvalbumin are rich in tenascin. Neurosci Lett. 1993;162:137–140. doi: 10.1016/0304-3940(93)90579-a. [DOI] [PubMed] [Google Scholar]
- 15.Derouiche A, Hartig W, Brauer K, Bruckner G. Spatial relationship of lectin-labeled extracellular matrix and glutamine synthetase-immunoreactive astrocytes in rat cortical forebrain regions. J Anat. 1996;189:363–372. [PMC free article] [PubMed] [Google Scholar]
- 16.Engel M, Maurel P, Margolis RU, Margolis RK. Chondroitin sulfate proteoglycans in the developing central nervous system. I. Cellular sites of synthesis of neurocan and phosphacan. J Comp Neurol. 1996;366:34–43. doi: 10.1002/(SICI)1096-9861(19960226)366:1<34::AID-CNE3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 17.Faissner A, Clement A, Lochter A, Streit A, Mandl C, Schachner M. Isolation of a neural chondroitin sulfate proteoglycan with neurite outgrowth promoting properties. J Cell Biol. 1994;126:783–799. doi: 10.1083/jcb.126.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujita SC, Toda Y, Murakami F, Hayashi M, Matsamura M. Glycosaminoglycan-related epitopes surround different subsets of mammalian central nervous system neurons. Neurosci Res. 1989;7:117–130. doi: 10.1016/0168-0102(89)90052-7. [DOI] [PubMed] [Google Scholar]
- 19.Gowda DC, Margolis RU, Margolis RK. Presence of the HNK-1 epitope on poly (N-acetyllactosaminyl) oligosaccharides and identification of multiple core proteins in the chondroitin sulfate proteoglycans of brain. Biochemistry. 1989;28:4468–4474. doi: 10.1021/bi00436a052. [DOI] [PubMed] [Google Scholar]
- 20.Grumet M, Hofmann S, Crossin KL, Edelman G. Cytotactin, an extracellular matrix protein of neural and non-neural tissue that mediates glia–neuron interaction. Proc Natl Acad Sci USA. 1985;82:8075–8079. doi: 10.1073/pnas.82.23.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardingham TE, Fosang AJ. Proteoglycans: many forms and many functions. FASEB J. 1992;6:861–870. [PubMed] [Google Scholar]
- 22.Hartig W, Brauer K, Bruckner G. Wisteria floribunda agglutinin-labeled nets surround parvalbumin-containing neurons. NeuroReport. 1992;3:869–872. doi: 10.1097/00001756-199210000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Hartig W, Brauer K, Bigl V, Bruckner G. Chondroitin sulfate proteoglycan-immunoreactivity of lectin-labeled perineuronal nets around parvalbumin-containing neurons. Brain Res. 1994;635:307–311. doi: 10.1016/0006-8993(94)91452-4. [DOI] [PubMed] [Google Scholar]
- 24.Herndon ME, Lander AD. A diverse set of developmentally regulated proteoglycans is expressed in the rat central nervous system. Neuron. 1990;4:949–961. doi: 10.1016/0896-6273(90)90148-9. [DOI] [PubMed] [Google Scholar]
- 25.Hockfield S. Proteoglycans in neural development. Semin Dev Biol. 1990;1:55–63. [Google Scholar]
- 26.Hockfield S, McKay RDG. A surface antigen expressed by a subset of neurons in the vertebrate central nervous system. Proc Natl Acad Sci USA. 1983;80:5758–5761. doi: 10.1073/pnas.80.18.5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hockfield S, Kalb RG, Zaremba S, Fryer H. Expression of neural proteoglycans correlates with the acquisition of mature neuronal properties in the mammalian brain. Cold Spring Harb Symp Quant Biol. 1990;55:505–513. doi: 10.1101/sqb.1990.055.01.049. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman S, Crossin KL, Edelman GM. Molecular forms, binding functions, and developmental expression patterns of cytotactin and cytotactin-binding proteoglycan, an interactive pair of extracellular matrix molecules. J Cell Biol. 1988;106:519–532. doi: 10.1083/jcb.106.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaworski DM, Kelly GM, Hockfield S. BEHAB, a new member of the proteoglycan tandem repeat family of hyaluronan-binding proteins that is restricted to the brain. J Cell Biol. 1994;125:495–509. doi: 10.1083/jcb.125.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koppe G, Bruckner G, Brauer K, Hartig W, Bigl V. Developmental patterns of proteoglycan-containing extracellular matrix in perineuronal nets and neuropil of the postnatal rat brain. Cell Tissue Res. 1997;288:33–41. doi: 10.1007/s004410050790. [DOI] [PubMed] [Google Scholar]
- 32.Kosaka T, Heizmann CW. Selective staining of a population of parvalbumin-containing GABA-ergic neurons in the rat cerebral cortex by lectins with specific affinity for terminal N-acetylgalactosamine. Brain Res. 1989;483:158–163. doi: 10.1016/0006-8993(89)90048-6. [DOI] [PubMed] [Google Scholar]
- 33.Lafarga M, Berciano MT, Blanco M. The perineuronal net in the fastigial nucleus of the rat cerebellum. Anat Embryol (Berl) 1984;170:79–85. doi: 10.1007/BF00319461. [DOI] [PubMed] [Google Scholar]
- 34. Lander C, Hockfield S. The extracellular matrix of the peripheral and central nervous systems. Encyclopedia of neuroscience Adelman G, Smith B. 1998. Elsevier; Amsterdam, in press. [Google Scholar]
- 35.Lander C, Kind P, Maleski M, Hockfield S. A family of activity-dependent neuronal cell-surface chondroitin sulfate proteoglycans in cat visual cortex. J Neurosci. 1997;17:1928–1939. doi: 10.1523/JNEUROSCI.17-06-01928.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LeBaron RG. Versican. Perspect Dev Neurobiol. 1996;3:261–271. [PubMed] [Google Scholar]
- 37.Lee MK, Rebhun LI, Frankfurter A. Post-translational modification of class II beta-tubulin. Proc Natl Acad Sci USA. 1990a;87:7195–7199. doi: 10.1073/pnas.87.18.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee MK, Tuttle JB, Rebhun LI, Cleveland DW, Frankfurter A. The expression and posttranslational modification of a neuron-specific beta-tubulin isotype during chick embryogenesis. Cell Motil Cytoskeleton. 1990b;17:118–132. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- 39.Louvard D, Reggio H, Warren G. Antibodies to the Golgi complex and the rough endoplasmic reticulum. J Cell Biol. 1982;92:92–107. doi: 10.1083/jcb.92.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luth H-J, Fischer J, Celio MR. Soybean lectin binding neurons in the visual cortex of the rat contain parvalbumin and are covered by glial nets. J Neurocytol. 1992;21:211–221. doi: 10.1007/BF01194979. [DOI] [PubMed] [Google Scholar]
- 41.Maeda NN, Hamanaka H, Oohira A, Noda A. Purification, characterization and developmental expression of a brain-specific chondroitin sulfate proteoglycan, 6B4 proteoglycan/phosphacan. Neuroscience. 1995;67:23–35. doi: 10.1016/0306-4522(94)00069-h. [DOI] [PubMed] [Google Scholar]
- 42.Margolis RK, Margolis RU. Nervous tissue proteoglycans. Experientia. 1993;49:429–446. doi: 10.1007/BF01923587. [DOI] [PubMed] [Google Scholar]
- 43.Meyer-Puttlitz B, Junker E, Margolis RU, Margolis RK. Chondroitin sulfate proteoglycans in the developing central nervous system. II. Immunocytochemical localization of neurocan and phosphacan. J Comp Neurol. 1996;366:44–54. doi: 10.1002/(SICI)1096-9861(19960226)366:1<44::AID-CNE4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 44.Naegele JR, Katz LC. Cell surface molecules containing N-acetylgalactosamine are associated with basket cells and neurogliaform cells in cat visual cortex. J Neurosci. 1990;10:550–557. doi: 10.1523/JNEUROSCI.10-02-00540.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakagawa F, Schulte BA, Spicer SS. Selective cytochemical demonstration of glycoconjugate-containing terminal N-acetylgalactosamine on some brain neurons. J Comp Neurol. 1986;243:280–290. doi: 10.1002/cne.902430210. [DOI] [PubMed] [Google Scholar]
- 46.Nishimoto SK, Kajiwara T, Tanzer ML. Proteoglycan core protein is accumulated in cultured chondrocytes in the presence of the ionophore monensin. J Biol Chem. 1982;257:10558–10561. [PubMed] [Google Scholar]
- 47.Oohira A, Matsui F, Matsuda M, Takida Y, Kuboki Y. Occurrence of three distinct molecular species of chondroitin sulfate proteoglycan in the developing rat brain. J Biol Chem. 1988;263:10240–10246. [PubMed] [Google Scholar]
- 48.Oohira A, Katoh-Semba R, Watanabe E, Matsui F. Brain development and multiple molecular species of proteoglycan. Neurosci Res. 1994a;20:195–207. doi: 10.1016/0168-0102(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 49.Oohira A, Matsui F, Watanabe E, Kushima Y, Maeda N. Developmentally regulated expression of a brain specific species of chondroitin sulfate proteoglycan, neurocan, identified with a monoclonal antibody 1G2 in the rat cerebrum. Neuroscience. 1994b;60:145–157. doi: 10.1016/0306-4522(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 50.Rauch U, Karthikeyan L, Maurel P, Margolis RU, Margolis RK. Cloning and primary structure of neurocan, a developmentally regulated, aggregating chondroitin sulfate proteoglycan of brain. J Biol Chem. 1992;267:19536–19547. [PubMed] [Google Scholar]
- 51.Redmond L, Xie H, Ziskind-Conhaim L, Hockfield S. Cues intrinsic to the spinal cord determine the pattern and timing of primary afferent growth. Dev Biol. 1997;182:205–218. doi: 10.1006/dbio.1996.8488. [DOI] [PubMed] [Google Scholar]
- 52.Sanes JR. Extracellular matrix molecules that influence neural development. Annu Rev Neurosci. 1989;12:491–516. doi: 10.1146/annurev.ne.12.030189.002423. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz NB. Regulation of chondroitin sulfate synthesis. Effect of beta-xylosides on synthesis of chondroitin sulfate proteoglycan, chondroitin sulfate chains, and core protein. J Biol Chem. 1977;252:6316–6321. [PubMed] [Google Scholar]
- 54.Schweizer M, Streit WJ, Muller CM. Postnatal development and localization of an N-acetylgalactosamine containing glycoconjugate associated with nonpyramidal neurons in cat visual cortex. J Comp Neurol. 1993;329:313–327. doi: 10.1002/cne.903290303. [DOI] [PubMed] [Google Scholar]
- 55.Seeger G, Brauer K, Hartig W, Bruckner G. Mapping of perineuronal nets in the rat brain stained by colloidal iron hydroxide histochemistry and lectin cytochemistry. Neuroscience. 1994;58:371–388. doi: 10.1016/0306-4522(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 56.Sheppard AM, Brunstrom JE, Thornton TN, Gerfen RW, Broekelmann TJ, McDonald JA, Pearlman AL. Neuronal production of fibronectin in the cerebral cortex during migration and layer formation is unique to specific cortical domains. Dev Biol. 1995;172:504–518. doi: 10.1006/dbio.1995.8034. [DOI] [PubMed] [Google Scholar]
- 57.Smith GM, Rutishauser U, Silver J, Miller RH. Maturation of astrocytes in vitro alters the extent and molecular basis of neurite outgrowth. Dev Biol. 1990;138:377–390. doi: 10.1016/0012-1606(90)90204-v. [DOI] [PubMed] [Google Scholar]
- 58.Stallcup WB, Beasley L, Levine JM. Cell surface molecules that characterize different stages in the development of cerebellar interneurons. Cold Spring Harb Symp Quant Biol. 1983;48:761–774. doi: 10.1101/sqb.1983.048.01.078. [DOI] [PubMed] [Google Scholar]
- 59.Streit WJ, Schulte BA, Balentine D, Spicer SS. Evidence for glycoconjugate in nociceptive primary sensory neurons and its origin from the Golgi complex. Brain Res. 1986;377:1–17. doi: 10.1016/0006-8993(86)91185-6. [DOI] [PubMed] [Google Scholar]
- 60.Watanabe E, Fujita SC, Murakami F, Hayashi M, Matsumura M. A monoclonal antibody identifies a novel epitope surrounding a subpopulation of the mammalian central neurons. Neuroscience. 1989;29:645–657. doi: 10.1016/0306-4522(89)90137-1. [DOI] [PubMed] [Google Scholar]
- 61.Wintergerst ES, Faissner A, Celio MR. The proteoglycan DSD-1-PG occurs in perineuronal nets around parvalbumin-immunoreactive interneurons of the rat cerebral cortex. Int J Dev Neurosci. 1996;14:249–255. doi: 10.1016/0736-5748(96)00011-1. [DOI] [PubMed] [Google Scholar]
- 62.Yamada H, Watanabe K, Shimonaka M, Yamaguchi Y. Molecular cloning of brevican, a novel brain proteoglycan of the aggrecan/versican family. J Biol Chem. 1994;267:12149–12161. [PubMed] [Google Scholar]
- 63.Zaremba S, Guimaraes A, Kalb RG, Hockfield S. Characterization of an activity-dependent, neuronal surface proteoglycan identified with monoclonal antibody Cat-301. Neuron. 1989;2:1207–1219. doi: 10.1016/0896-6273(89)90305-x. [DOI] [PubMed] [Google Scholar]