Two maize nucleus-encoded splicing factors, PPR-SMR1 and Zm-mCSF1, are required for the splicing of most mitochondrial group II introns and subsequent complex I biogenesis, and therefore play important roles in seed development.
Keywords: Group II introns, maize, mitochondria, organelle biogenesis, pentatricopeptide repeat (PPR) proteins, seed development
Abstract
Group II introns are ribozymes that can excise themselves from precursor-RNA transcripts, but plant organellar group II introns have structural deviations that inhibit ribozyme activity. Therefore, splicing of these introns requires the assistance of nuclear- and/or organellar-encoded splicing factors; however, how these splicing factors function remains unclear. In this study, we report the functions and interactions of two splicing factors, PPR-SMR1 and Zm-mCSF1, in intron splicing in maize mitochondria. PPR-SMR1 is a SMR domain-containing pentatricopeptide repeat (PPR) protein and Zm-mCSF1 is a CRM domain-containing protein, and both are targeted to mitochondria. Loss-of-function mutations in each of them severely arrests embryogenesis and endosperm development in maize. Functional analyses indicate that PPR-SMR1 and Zm-mCSF1 are required for the splicing of most mitochondrial group II introns. Among them, nad2-intron 2 and 3, and nad5-intron 1 are PPR-SMR1/Zm-mCSF1-dependent introns. Protein interaction assays suggest that PPR-SMR1 can interact with Zm-mCSF1 through its N-terminus, and that Zm-mCSF1 is self-interacting. Our findings suggest that PPR-SMR1, a novel splicing factor, acts in the splicing of multiple group II introns in maize mitochondria, and the protein–protein interaction between it and Zm-mCSF1 might allow the formation of large macromolecular splicing complexes.
Introduction
Group II introns are catalytic RNAs (ribozymes) that have six structurally conserved domains (I–VI) and are capable of self-splicing from precursor-RNAs. They are also mobile genetic elements via reverse splicing, and are hence considered to be the ancestors of nuclear introns and the spliceosome machinery in eukaryotes (Lambowitz and Zimmerly, 2011). Group II introns are numerous in eubacteria, and are also present in the chloroplast and mitochondrial genomes of plants, fungi, and some animals (Robart et al., 2014). The chemical nature of group II intron splicing involves two transesterification reactions, in which the 5´-splice site is attacked by the 2´-OH of the bulged A-residue in domain VI to generate lariat RNA, followed by a nucleophilic attack by the 3´-OH of the 5´-exon at the 3´-splice site, forming an excised intron lariat and ligated exons (Toor et al., 2008). Some bacterial introns can self-splice in vitro (Ferat and Michel, 1993); however, during the course of evolution plant organellar group II introns have become highly degenerated and lost the capability of self-splicing.
Group II introns are prevalent in organellar genomes of land plants; however, no mitochondrial or chloroplast introns have been reported to have self-splicing activity in vitro (Vogel and Börner, 2002; Bonen, 2008), leading to the notion that these introns have lost this capability. In recent years, protein factors have been found to be required for the splicing of group II introns, and these come from different families and are encoded by both nuclear and organellar genomes. For example, maturase MatR encoded by the mitochondrial genome is required for the splicing of several group II introns (Sultan et al., 2016). Four nucleus-encoded maturases (nMAT1–4) have been shown to reside in mitochondria, with nMAT4 having a possible dual-localization in chloroplasts as well (Keren et al., 2009). Genetic studies in Arabidopsis mitochondria have suggested that nMAT1 is involved in the splicing of three introns, nMAT2 11 introns, and nMAT4 five introns (Keren et al., 2009, 2012; Cohen et al., 2014; Zmudjak et al., 2017). In addition to MATs, different nucleus-encoded RNA-binding proteins have been demonstrated to be required for splicing of mitochondrial group II introns (Brown et al., 2014; Hammani and Giegé, 2014). The CRM/CRS1-YhbY domain protein mCSF1 (a paralog of CAF1) is required for the splicing of more than half of the introns in Arabidopsis mitochondria (Zmudjak et al., 2013). A member of the plant organellar RNA recognition (PORR) domain family WTF9 is essential for the splicing of the introns of rpl2 and ccmF(C) in mitochondria (Francs-Small et al., 2012). The mitochondrial transcription termination factor (mTERF) protein mTERF15 is required for splicing of mitochondrial nad2 intron 3 (Hsu et al., 2014). The DEAD-box RNA helicase PMH2 is required for optimal splicing of multiple mitochondrial group II introns (Köhler et al., 2010; Zmudjak et al., 2017), and the regulator of chromosome condensation (RCC) protein RUG3 is required for efficient splicing of the nad2 introns 2 and 3 (Kühn et al., 2011). Pentatricopeptide repeat (PPR) proteins are a large family of RNA-binding proteins that mediate organellar mRNA processing, including group II intron splicing (Barkan and Small, 2014; Hammani and Giegé, 2014). Many maize PPR proteins are found to be required for splicing of specific mitochondrial pre-mRNAs (Xiu et al., 2016; Cai et al., 2017; Chen et al., 2017; Qi et al., 2017; Ren et al., 2017; Sun et al., 2019).
PPR family proteins are divided into P-, PLS-, and PPR-SMR subfamilies according to their PPR motifs and carboxy-terminal domains (Barkan and Small, 2014). PPR-SMR is a small subfamily containing a small MutS-related (SMR) domain at the C-terminus (Liu et al., 2013a). SMR domains are also found in proteins that serve as DNA or RNA endonucleases for nucleotide cleavage, or that structurally resemble the DNA and RNA binding domains for nucleic acid binding (Fukui and Kuramitsu, 2011). Recently, the SMR domain of one chloroplast-localized PPR-SMR protein, SOT1, was found to have DNA/RNA endonuclease activity in vitro and in vivo (Zhou et al., 2017). In addition, several other chloroplast-localized PPR-SMR proteins have been found to play critical roles in retrograde signaling, chloroplast gene expression, and organelle biogenesis. In maize, two chloroplast-localized PPR-SMR proteins have been functionally characterized: the ATP4 protein promotes translation of the chloroplast atpB/E mRNA (Zoschke et al., 2012), while the PPR53 protein protects mRNAs of rrn23 and ndhA in chloroplasts (Zoschke et al., 2016). In Arabidopsis, four chloroplast-localized PPR-SMR proteins have been functionally characterized: GUN1 physically interacts with plastid ribosomal protein S1 and some of the enzymes in the tetrapyrrole biosynthesis (TPB) pathway to play a role in plastid protein homeostasis (Tadini et al., 2016); SOT1, the homolog of PPR53, binds to and protects the 5´-region of the 23S-4.5S rRNA dicistron (Wu et al., 2016); SVR7, the homolog of ATP4, is associated with FtsH-mediated chloroplast biogenesis (Liu et al., 2010); and pTAC2 is required for the activity of plastid-encoded RNA polymerase (Pfalz et al., 2006). Nevertheless, the functions of several PPR-SMR proteins in mitochondria are still unknown.
In this study, we examined a new PPR-SMR protein, PPR-SMR1, and a CRM domain-containing protein, Zm-mCSF1, in maize. Our results showed that PPR-SMR1 is required for the splicing of nearly 75% of the mitochondrial group II introns, while Zm-mCSF1 is required for the splicing of six introns in mitochondria. We further provide evidence that PPR-SMR1 physically interacts with Zm-mCSF1. Our results imply that PPR-SMR1 and Zm-mCSF1 play important roles in the splicing of many of the group II introns, in mitochondrial functions, and in embryogenesis and endosperm development in maize.
Materials and methods
Plant material
ppr-smr1 and Zm-mcsf1 Mutator-insertion alleles were obtained from the UniformMu population in the maize (Zea mays) inbred W22 genetic background (McCarty et al., 2005). The flanking sequences of Mu insertions in the mutant alleles were confirmed by PCR amplification using gene-specific primers and Mu primers (Tan et al., 2011) and by direct sequencing. Wild-type material refers to normal siblings of the mutants. All the maize plants were grown in an experimental field under natural conditions.
DNA extraction and linkage analysis
Maize leaf genomic DNA was extracted using a urea-phenol-chloroform-based extraction method as described previously (Tan et al., 2011). For linkage analysis, the leaf DNA was extracted from individual plants and the genotype was determined by PCR analysis as described above. Each individual plant was selfed to check the 3:1 segregation of empty pericarp (emp) mutants.
Light microscopy of cytological sections
Immature seeds of wild-types and mutants were dissected from the same ears of self-pollinated heterozygous plants at 9 d after pollination (DAP) and at 13 DAP. Slice samples were prepared as described previously (Liu et al., 2013b). The embedded seeds were sectioned at 8 μm thickness using a Microm HM 315. The sections were stained with Johansen’s Safranin O and observed using a Zeiss Scope A1 microscope.
Subcellular localization of PPR-SMR1 and Zm-mCSF1
The coding sequences of PPR-SMR1 and Zm-mCSF1 were cloned into the entry vector pENTR/D-TOPO (Invitrogen) and then transferred into the pBI221 and pGWB5 vectors by Gateway site-specific recombination, generating the PPR-SMR1::GFP and Zm-mCSF1::GFP fusion genes driven by the Cauliflower mosaic virus 35S promoter (p35S). The constructs pBI221-PPR-SMR1, pBI221-Zm-mCSF1, and the mitochondria reporter gene p35S::F1-ATPase-γ-RFP fusion (Jin et al., 2003) were purified using an EndoFree Maxi Plasmid Kit (TIANGEN). Following this, the plasmids pBI221-PPR-SMR1 and pBI221-Zm-mCSF1 were each transformed into mesophyll protoplasts of Arabidopsis together with the plasmid p35S::F1-ATPase-γ-RFP, as previously described (Yoo et al., 2007). After incubation at 23 °C in the dark for 24–36 h, the GFP and RFP signals were recorded using a confocal laser-scanning microscope (LSM 880, Carl Zeiss). In addition, the pGWB5-PPR-SMR1 and pGWB5-Zm-mCSF1 constructs were each transformed into Agrobacterium tumefaciens strain EHA105. Agrobacterium cells containing each GFP fusion construct were infiltrated into tobacco (Nicotiana tabacum) leaves as described previously (van Herpen et al., 2010). After incubation at 24 °C for 20–26 h, the GFP signal was recorded using a confocal laser-scanning microscope (LSM 700, Carl Zeiss) and mitochondria were stained with MitoTracker Red (Invitrogen) with a working concentration of 100 nM.
RNA extraction, RT-PCR, and qRT-PCR
Total RNA was isolated from ~100 mg of endosperm using an RNeasy® Plant Mini Kit (Qiagen). DNA contamination was removed from RNA samples using an RNase-free DNase I treatment (New England BioLabs, NEB). The removal of DNA contamination was confirmed by PCR amplification without reverse transcription. RNAs were reverse-transcribed into cDNAs using a TransScript® One-Step gDNA Removal and cDNA Synthesis SuperMix Kit (TransGen Biotech, China). For analysis of PPR-SMR1 expression in ppr-smr1 mutant alleles and wild-type seeds, RT-PCR was performed with the primers 64mi-F1 and 63mi-R1 at an annealing temperature of 60 °C for 36 cycles. Similarly, RT-PCR analysis of Zm-mCSF1 expression was performed with the primers CSFL-F1 and CSFL-R1 at an annealing temperature of 60 °C for 32 cycles. The expression of ZmEF1α (GRMZM2G153541) was used to normalize the cDNA levels between different samples. The primers are listed in Supplementary Table S2 at JXB online.
The expression levels of mitochondrial transcripts were analysed as described previously (Xiu et al., 2016; Sun et al., 2019). The intron splicing efficiency between the wild-type and ppr-smr1, and the wild-type and Zm-mcsf1 was compared by RT-PCR and qRT-PCR. The qRT-PCR analysis was performed using FastStart Essential DNA Green Master and a LightCycler® 96 Instrument (Roche). The primers for the mitochondrial transcripts were from our previous study (Liu et al., 2013b) and other primers are listed in Supplementary Table S2.
Western blotting assays
Western blotting was performed according to Sun et al. (2015). Total protein was extracted from 0.3 g of ground frozen endosperm with lysis buffer containing 10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 2 mM EDTA, 0.5% NP-40, and 0.1% protease inhibitor. Loading samples were prepared from total protein for PPR-SMR1 and Zm-mCSF1. Primary antibodies were used against maize Cytc1, ATPase α-subunit (MBL Beijing Biotech), AOX (Xiu et al., 2016), wheat Nad9 (Lamattina et al., 1993), and Arabidopsis Cox2 (Agrisera) .
Yeast two-hybrid assays
Coding sequences of PPR-SMR1 and Zm-mCSF1 were cloned into the bait (pGBKT7) and prey (pGADT7) vectors (Clontech). Different combinations of GAL4 DNA binding domain (BD) and GAL4 activation domain (AD) constructs were co-transformed into the yeast (Saccharomyces cerevisiae) strain Y2HGold (Clontech). The manipulations were performed according to the manufacturer’s instructions.
Protein purification and pull-down analysis
The Zm-mCSF1 coding sequence was cloned into the pMAL-c2x or pGEX4T-1 expression vectors to generate fusions construct with maltose-binding protein (MBP) or glutathione S-transferase (GST) tags. Coding sequences of PPR-SMR1 (containing the 6xHis coding sequence at the C terminus) were cloned into the pMAL-c2x vector to generate fusion constructs with MBP and His tags. The primers are listed in Supplementary Table S2. These constructs were transformed into Rosetta strains of Escherichia coli. After protein expression in the bacterial cells, the MBP-Zm-mCSF1 protein was purified using amylose resin (NEB), GST-Zm-mCSF1 was purified using glutathione agarose beads (GE Healthcare), and MBP-PPR-SMR1-His was purified using Ni-NTA agarose (Qiagen) and amylose resin (NEB) according to the manufacturers’ instructions.
For pull-down assays, equal amounts of the two interacting proteins as well as MBP protein and/or GST protein were mixed, and the mixture was incubated with amylose resin or glutathione agarose beads for 2–3 h at 4 °C. After incubation, the beads were collected by simple centrifugation (8000 g, 10 s) and then washed five times (>60 times bed volume) with a buffer containing 25 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM DTT, and 0.5% Triton X-100. Finally, the bound proteins within 1×SDS loading buffer were released from the beads by heating at 95 °C and subjected to SDS-PAGE. Immunoblotting was used to detect the targeted recombinant proteins with anti-GST (GE Healthcare) and anti-MBP (NEB) antibodies. The primers used to make the constructs are listed in Supplementary Table S2.
Bimolecular fluorescence complementation assays
The coding sequences of PPR-SMR1 and Zm-mCSF1 without stop codons were cloned into pSPYNE-35S and pSPYCE-35S plasmids, respectively, as previously described (Walter et al., 2004). YFPN (aa 1–155) was fused to the C-terminus of PPR-SMR1, and YFPC (aa 156–239) was fused to the C-terminus of Zm-mCSF1. Both resulting plasmids were mixed equally and transformed into mesophyll protoplasts of Arabidopsis as described previously (Yoo et al., 2007). The YFP signal was imaged using a confocal laser-scanning microscope (LSM 880, Carl Zeiss). Mitochondria were labeled using ATPase:RFP marker (Jin et al., 2003).
Phylogenetic analysis and multiple protein sequence alignment
The amino-acid sequences of PPR-SMR1, Zm-mCSF1, and their homologs were identified using BLAST searches in the UniProt database (http://www.uniprot.org). Multiple sequence alignment and construction of the phylogenetic tree (neighbor-joining algorithm with default parameters; bootstrap values were calculated from 1000 iterations) were performed using MEGA7 (Kumar et al., 2016).
Results
PPR-SMR1 is essential for embryo and endosperm development in maize
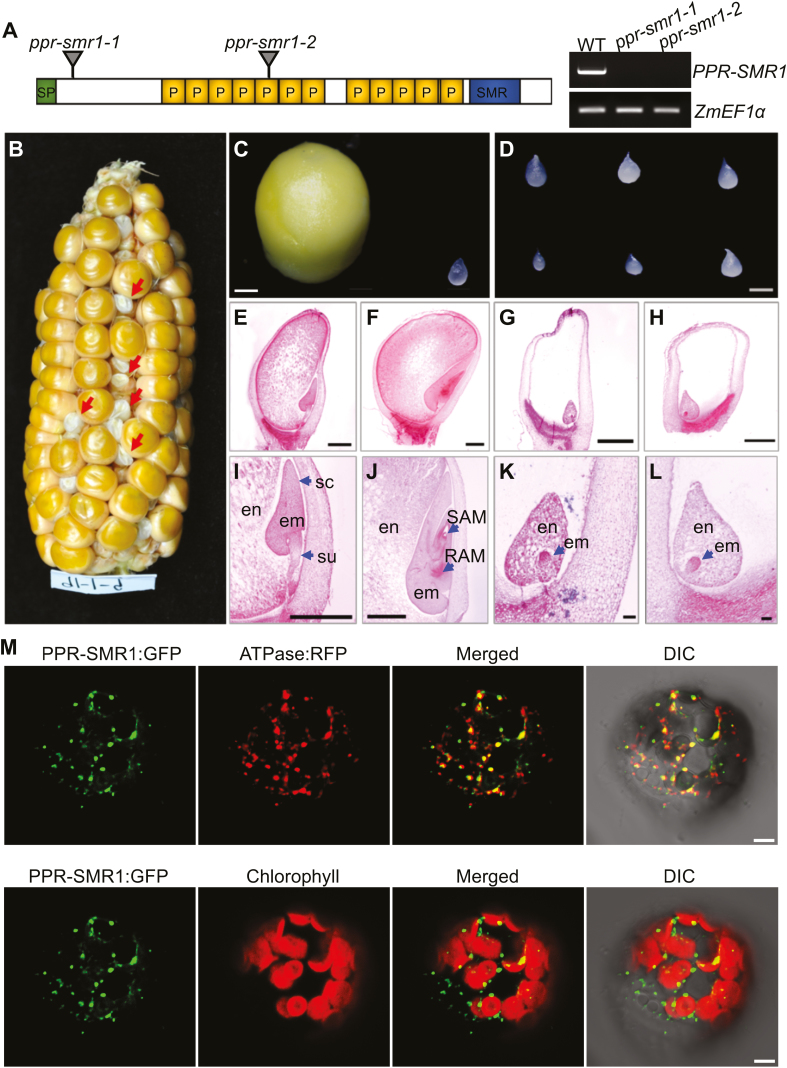
We chose to study maize PPR-SMR1 (GRMZM2G345667; Supplementary Fig. S1) because of its unknown function and predicted mitochondrial localization (Emanuelsson et al., 2000), which is different from previously studied PPR-SMR proteins (Zoschke et al., 2012, 2016; Williams-Carrier et al., 2014; Pfalz et al., 2015). PPR-SMR1 is intronless and encodes a protein with 12 PPR motifs, a C-terminal small MutS-related (SMR) domain, and an unknown N-terminus domain that is highly conserved among maize, rice, and sorghum (Fig. 1A; Supplementary Fig. S1C).
Fig. 1.
Overview of maize PPR-SMR1. (A) Diagram of the PPR-SMR1 protein containing 12 PPR domains, with the positions of the Mutator insertions indicated. Expression of PPR-SMR1 is absent from ppr-smr1 mutants. SP, signaling peptide; P, PPR motif; SMR, small MutS-related. (B) A maize ear showing 3:1 segregation for wild-type (WT) and ppr-smr1-1 mutant kernels (arrows). (C) Dissection of WT (left) and ppr-smr1-1 (right) endosperm at 11 d after pollination (DAP). (D) Mutant endosperm dissected from reciprocal crosses of ppr-smr1-1 and ppr-smr1-2 kernels. (E–L) Comparisons of WT and ppr-smr1-1 kernel development at 9 DAP and 13 DAP. WT kernels at 9 DAP (E, I) and 13 DAP (F, J); ppr-smr1-1 kernels at 9 DAP (G, K) and 13 DAP (H, L). en, endosperm; em, embryo; su, suspensor; sc, scutellum; RAM, root apical meristem; SAM, shoot apical meristem. (M) Localization of the PPR-SMR1 protein. Mesophyll protoplasts from Arabidopsis were transformed with PPR-SMR1::GFP and ATPase::RFP constructs and imaged using confocal microscopy. In the upper images, mitochondria are labeled by fluorescence of ATPase::RFP, and in the lower images, chloroplasts are marked by autofluorescence. GFP, green fluorescent protein; DIC, differential interference contrast. Scale bars are 1 mm in (C–J), 0.1 mm in (K, L), and 5 µm in (M).
Two Mutator insertional mutants were identified from the UniformMu mutagenesis population (McCarty et al., 2005), one with a Mu insertion at 166 bp and the other at 1084 bp downstream of the ATG, named ppr-smr1-1 and ppr-smr1-2, respectively (Fig. 1A). Both alleles were probably null as the insertions were in the N-terminal coding region of PPR-SMR1 and wild-type transcripts were not detected. The selfed ppr-smr1-1 (Fig. 1B) and ppr-smr1-2 heterozygotes segregated emp kernels at a ratio of ~25% (63:260, P<0.05, and 75:270, P<0.05, respectively), indicating that the mutation was recessive. Both alleles were embryo-lethal, so the mutations were kept in heterozygotes. At 11 DAP, the endosperms dissected from mutant kernels were greatly reduced in size when compared with wild-type siblings (Fig. 1C). Linkage analyses indicated that the emp phenotype was tightly linked to the Mu insertions in PPR-SMR1 (Supplementary Fig. S2A, B). In addition, ears from reciprocal crosses between ppr-smr1-1 and ppr-smr1-2 heterozygotes segregated emp kernels, confirming that the emp phenotype was caused by mutations in the PPR-SMR1 gene (Fig. 1D; Supplementary Fig. S2C).
The small and collapsed mature mutant kernels (Fig. 1B) and the extremely small endosperms (Fig. 1C) suggested early embryonic arrest in ppr-smr1 seeds. To verify this, we performed histological examination of the mutant and wild-type kernels from segregating ears. Embryo development in maize is divided into transition, coleoptile, and late-embryogenesis stages (Vernoud et al., 2005). Compared with the wild-type siblings, the embryos of which had long suspensors and scutellum (Fig. 1E, I) at 9 DAP and a developing shoot apical meristem (SAM) and root apical meristem (RAM) at 13 DAP (Fig. 1F, J), the ppr-smr1 embryos were arrested at the transition stage and the endosperms showed extremely small size at 9 DAP (Fig. 1G, K); at 13 DAP, the embryos continued to stay at the transition stage and the endosperms grew slowly and remained small (Fig. 1H, L). These results indicated that both embryo and endosperm development were severely inhibited by the PPR-SMR1 mutation.
PPR-SMR1 is targeted to mitochondria and functions in the splicing of 16 introns
PPR-SMR1 was predicted to contain a mitochondrial-targeting peptide (Emanuelsson et al., 2000). To test the protein localization, we fused PPR-SMR1 (at the N-terminus) with eGFP (at the C-terminus) and transiently expressed the fusion protein in mesophyll protoplasts prepared from Arabidopsis and also in the epidermal cells of tobacco leaves. Green fluorescence signals were detected in spots that merged with mitochondria labeled by the ATPase::RFP marker (Jin et al., 2003) or stained with MitoTracker Red (Fig. 1M, Supplementary Fig. S2E), indicating that PPR-SMR1 was targeted to mitochondria. The non-targeted GFP protein in the negative control was detected in the cytoplasm (Supplementary Fig. S2D).
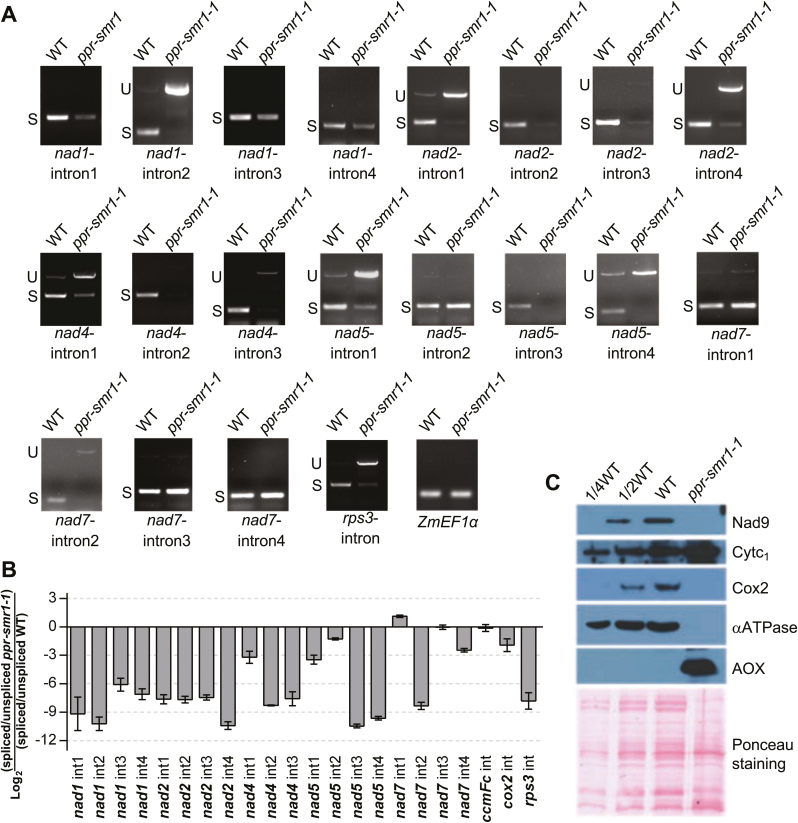
PPR proteins have been implicated in diverse functions of post-transcriptional RNA processing (Barkan and Small, 2014). To determine the molecular function of PPR-SMR1, we compared the transcript levels of 35 mitochondrial genes between the ppr-smr1 mutant kernels and their wild-type siblings. The W22 inbred maize line contains the NB mitochondrial genome (Liu et al., 2013b), and specific primers for the 35 mitochondrial genes were anchored to 5´- and 3´-proximal regions of each gene in order to ensure the analysis of nearly full transcripts. RNAs of ppr-smr1 and their wild-type siblings were extracted from embryos and endosperms at 12 DAP from the same segregating ears. Residual genomic DNA was removed by RNase-free DNase I treatment before reverse-transcription into cDNA. The cDNAs were normalized against maize elongation factor 1 alpha (Lin et al., 2014). The analyses showed that the transcript levels of nad1, nad2, nad4, nad5, and nad7 were dramatically decreased in the ppr-smr1 mutants (Supplementary Fig. S3A). To examine whether this was the result of defective intron splicing, we examined the splicing status of each intron by amplifying the transcript containing the intron and comparing between ppr-smr1 and the wild-type. The maize NB mitochondrial genome encodes 22 group II introns, and primers were designed to anchor on two adjacent exons, as well as one end on exons and the other end on joint introns to detect unspliced introns. RT-PCR analysis revealed that splicing of nad1-introns 1 to 4, nad2-introns 1 to 4, nad4-introns 1 to 3, nad5-introns 1, 3, and 4, nad7-intron 2, and the rps3 single intron was either abolished or significantly reduced in the ppr-smr1 mutant in comparison with the wild-type (Fig. 2A). To quantify the intron splicing efficiency in ppr-smr1, we examined the splicing of 22 mitochondrial group II introns by qRT-PCR (Fig. 2B). Both the RT-PCR and qRT-PCR results indicated that the splicing of 16 group II introns was severely impaired in the ppr-smr1-1 mutant (Supplementary Table S1); the remaining group II introns were spliced normally. These results suggested that PPR-SMR1 is a key splicing factor that is required for the splicing of 16 group II introns in maize mitochondria.
Fig. 2.
PPR-SMR1 is involved in splicing of maize group II introns. (A) Inefficient splicing of group II introns of nad1, nad2, nad4, nad5, and nad7 in the embryos and endosperms of ppr-smr1-1 mutants as determined by reverse-transcription PCR (RT-PCR). S, introns are spliced; U, introns are retained. (B) Quantitative real-time RT-PCR (qRT-PCR) analysis of the 22 intron-containing mitochondrial transcripts in ppr-smr1 mutants. Total RNA was extracted from embryos an endosperms of ppr-smr1 mutants and their wild-type siblings at 12 d after pollination. ZmEF1α was used to normalize the quantifications. Data are means (±SD) of three biological replicates. (C) Immunoblot analysis of core subunits of mitochondrial complexes in ppr-smr1-1 mutants. Immunoblots of embryo and endosperm extract (20 µg protein or the indicated dilutions) were probed with antibodies specific for subunits of Complex I (Nad9), Complex III (Cytochrome c1, Cytc1), Complex IV (Cox2), and ATP synthase (αATPase). AOX, alternative oxidase. Total protein input can be seen on the Ponceau S-stained blots below. (This figure is available in color at JXB online.)
The Nad1, 2, 4, 5, and 7 proteins are components of mitochondrial complex I, which is the entry complex of the oxidative phosphorylation (OXPHOS) electron transfer chain that includes complexes I to V. Defects of intron-splicing in these genes would conceivably lead to deficiencies in the encoded proteins, which may affect the assembly of complexes. To assess the impact of PPR-SMR1 mutation on the expression of complex components, we used immunoblot analyses to examine the protein levels of representative components of each complex, namely NADH dehydrogenase subunit 9 (Nad9) for Complex I, cytochrome c1 (Cytc1) for Complex III, cytochrome oxidase subunit2 (Cox2) for Complex IV, and mitochondrial ATP synthase (ATPase) α subunit for Complex V. In the ppr-smr1 mutants, the loss of PPR-SMR1 function resulted in dramatic reductions of Nad9, Cox2, and the ATPase α subunit, and also caused a moderate increase in the Cytc1 subunit (Fig. 2C). Like the loss-of-function mutations in maize Emp12 and Emp16, nad2 RNA with unspliced introns in emp12 and emp16 mitochondria results in a reduction of Nad9 (Xiu et al., 2016; Sun et al., 2019), and the splicing defects in 15 introns of nad genes in ppr-smr1 mitochondria clearly led to dramatic reductions of Nad9 (Fig. 2C). In addition, in line with the loss of RPS3 function in the maize mppr6 mutant that results in much lower protein levels of Cox2 and Nad9 in mitochondria (Manavski et al., 2012), the rps3 RNA with a retained single intron conceivably encodes a defective RPS3 protein in ppr-smr1 mitochondria, which can also lead to lower protein levels of Cox2 and Nad9 (Fig. 2C). Given the important function of RPS3 in mitochondrial translation, the lower level of organellar-encoded ATPase α subunit could have been caused by the defective RPS3 protein in ppr-smr1 mitochondria. The high level of nuclear-encoded Cytc1 in ppr-smr1 mitochondria might have been triggered by the accumulation of electrons and NADH in ppr-smr1 mitochondria. As a result, the cytochrome pathway of the ppr-smr1 mutant was disrupted, as revealed by the induction of AOX, the expression of which normally cannot be detected in wild type endosperm (Fig. 2C).
Zm-mCSF1 is required for the splicing of six group II introns in mitochondria
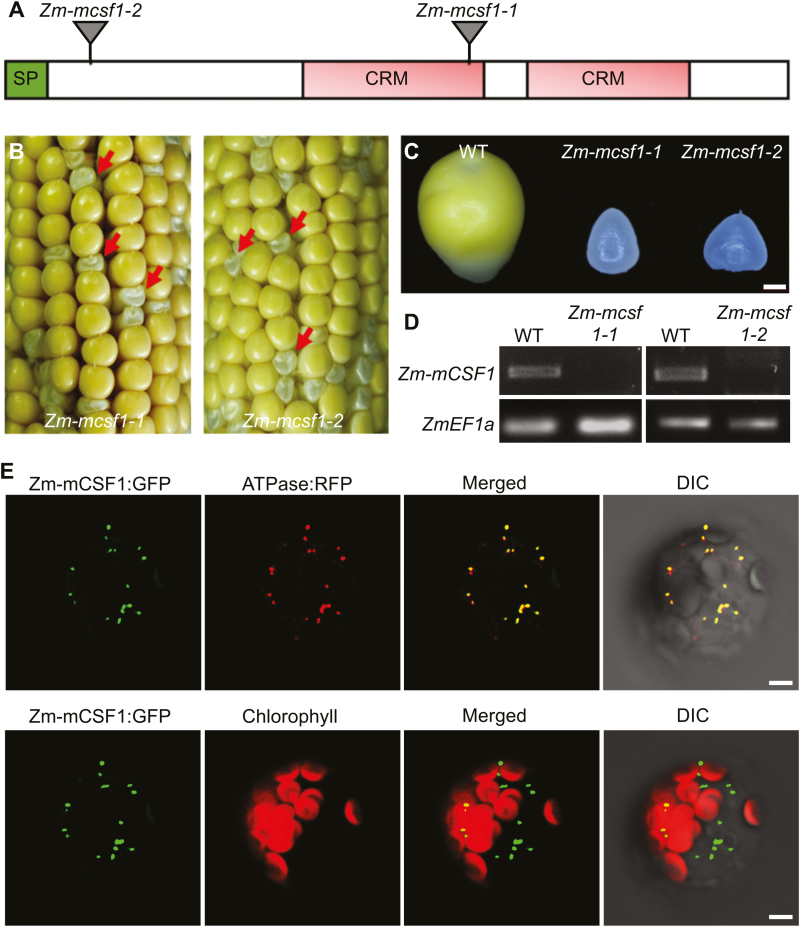
CRM domain-containing proteins are implicated in the splicing of group II introns in plant organelles (Asakura and Barkan, 2007; Barkan et al., 2007). mCSF1, a mitochondrion-targeted CRM domain-containing protein, is required for the splicing of more than half of the mitochondrial introns in Arabidopsis (Zmudjak et al., 2013). Because null mutants of At-mCSF1 are embryo-lethal, which hinders analysis of its function, we sought to study the function of its ortholog in maize by examining its null mutants. The ortholog of At-mCSF1 is GRMZM2G087395, named Zm-mCSF1, which showed 67% similarity to At-mCSF1 (AT4G31010). Zm-mCSF1 contains a predicted mitochondrial targeting sequence, two CRM repeats, and a C-terminal domain with as yet unknown function (Fig. 3A, Supplementary Fig. S4A). To define the molecular functions of Zm-mCSF1 in maize, we isolated two Mutator-insertional mutants, Zm-mcsf1-1 and Zm-mcsf1-2 (McCarty et al., 2005). Both insertions are in the coding region of Zm-mCSF1 (Fig. 3A). The selfed Zm-mcsf1-1 and Zm-mcsf1-2 heterozygotes segregated emp kernels at ratios of ~25% (169:643, P<0.05, and 233:949, P<0.05, respectively; Fig. 3B; Supplementary Fig. S4B), and reciprocal crosses between the two alleles yielded emp kernels as well (Supplementary Fig. S4C). At 15 DAP, the mutant kernels of both alleles were distinctly smaller than their wild-type siblings (Fig. 3B, C). Wild-type Zm-mCSF1 transcripts were not detected in the mutant kernels (Fig. 3D). Taken together, these results indicated that Zm-mCSF1 is the causal gene for the emp phenotype.
Fig. 3.
Overview of Zm-mCSF1. (A) Diagram of the Zm-mCSF1 protein containing two CRS1-YhbY domains (CRM) with the positions of the Mutator insertions in alleles indicated. SP, signaling peptide. (B) Seed phenotype of Zm-mcsf1 mutants. Ears of Zm-mcsf1-1 and Zm-mcsf1-2 were harvested at 15 d after pollination. The arrows indicate homozygous Zm-mcsf1 seed mutants classified as empty pericarp (emp). (C) Comparison of endosperm morphologies of Zm-mcsf1-1 and Zm-mcsf1-2 with the wild-type (WT). (D) Reverse-transcription PCR demonstrating the absence of Zm-mCSF1 mRNA in the Zm-mcsf1 mutants. (E) Localization of the Zm-mCSF1 protein. Mesophyll protoplasts from Arabidopsis were transformed with Zm-mCSF1::GFP and ATPase::RFP constructs and imaged using confocal microscopy. In the upper images, mitochondria are labeled by fluorescence of ATPase::RFP, and in the lower images, chloroplasts are marked by autofluorescence. DIC, differential interference contrast. Scale bars are 1 mm in (C) and 5 µm in (E).
Similar to PPR-SMR1, Zm-mCSF1 protein is predicted to target to mitochondria. Subcellular localization using Zm-mCSF1::GFP fusion showed mitochondrial localization (Fig. 3E; Supplementary Fig. S3C). Hence, PPR-SMR1 and Zm-mCSF1 are nucleus-encoded proteins that are targeted to mitochondria in maize.
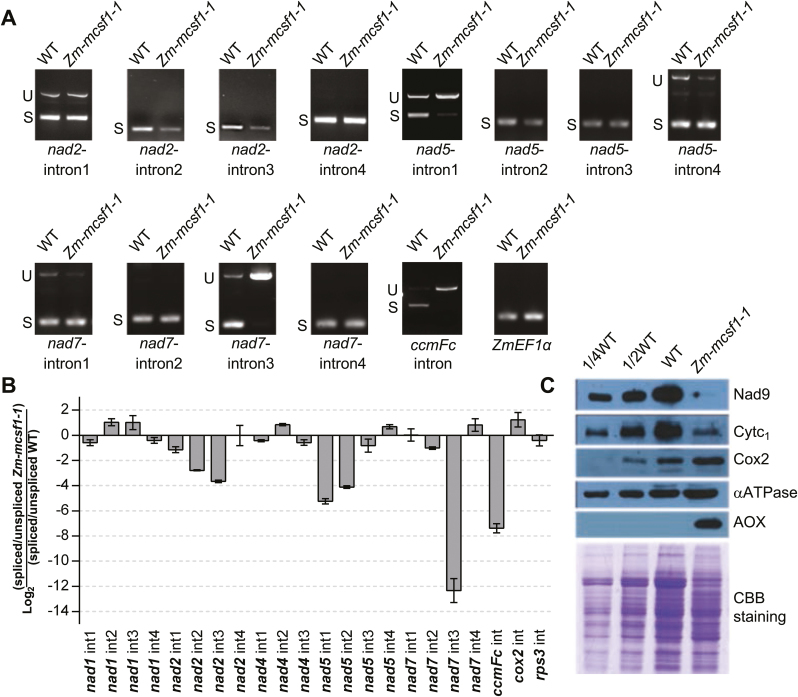
To determine the molecular functions of Zm-mCSF1, we used RT-PCR and qRT-PCR to examine transcripts of mitochondria carrying unspliced introns in Zm-mcsf1 mutants and their wild-type siblings. Both sets of results indicated that splicing of six group II introns was severely impaired in Zm-mcsf1-1, namely nad2-introns 2 and 3, nad5-introns 1 and 2, nad7-intron 3, and the ccmFc intron (Fig. 4A, B, Supplementary Table S1). In contrast, the other mitochondrial group II introns were spliced normally in Zm-mcsf1-1 (Fig. 4B, Supplementary Fig. S3B). In Arabidopsis, knockdown mutants of At-mcsf1 have been reported to have splicing defects in more than half of the mitochondrial group II introns (13 out of 23) (Zmudjak et al., 2013). We found that four of the group II introns were either incompletely spliced (nad2-introns 2 and 3, and nad5-intron 2) or completely unspliced (nad5-intron 1) in the Zm-mcsf1 mutant, suggesting a conserved function of At-mCSF1 and Zm-mCSF1 in the splicing of these group II introns in mitochondria.
Fig. 4.
Zm-mCSF1 is involved in splicing of maize group II introns. (A) Inefficient splicing of group II introns of nad2, nad5, nad7, and ccmFC in the endosperm of Zm-mcsf1-1 mutants by reverse-transcription PCR (RT-PCR). S, introns are spliced; U, introns are retained. (B) qRT-PCR analysis of the 22 intron-containing mitochondrial transcripts in Zm-mcsf1-1 mutants. RNA was extracted from the embryos and endosperms of Zm-mcsf1-1 mutants and their wild-type siblings at 12 d after pollination. ZmEF1α was used to normalize the quantifications. Data are means (±SD) of three biological replicates. (C) Immunoblot analysis of core subunits of mitochondrial complexes in Zm-mcsf1-1 mutants. Immunoblots of embryos and endosperms extract (20 µg protein or the indicated dilutions) were probed with antibodies specific for subunits of Complex I (Nad9), Complex III (Cytochrome c1, Cytc1), Complex IV (Cox2), and ATP synthase (αATPase). AOX, alternative oxidase. Total protein input can be seen in the Coomassie Brilliant Blue (CBB) staining below. (This figure is available in color at JXB online.)
To assess the impact of the Zm-mcsf1 mutation on the expression of mitochondrial complex components, we examined the protein levels of representative components of each complex by immunoblot analyses. In the Zm-mcsf1 mutants, the loss-of-function mutation caused a decrease of Nad9 and Cytc1 subunits, a slight increase of Cox2 and ATPase α subunit, and induction of AOX (Fig. 4C). Like the emp12 (Sun et al., 2019), emp16 (Xiu et al., 2016), and ppr-smr1 (Fig. 2C) mutants, the lower protein level of Nad9 in Zm-mcsf1 mitochondria was caused by defective Nad proteins. However, the decrease of Cytc1 subunit can be attribute to defective CcmFC protein in Zm-mcsf1 mitochondria, as the ccmFc transcripts contained an unspliced intron. The CcmFC protein together with the CcmFN protein forms a functional CcmF protein in plant mitochondria. In the maize emp7 mutant, the abolishment of C-to-U editing at the ccmFN-1553 editing site leads to loss-of-function in the CcmFN protein and a reduction in c-type cytochrome and, as a consequence, the protein level of the Cytc1 subunit decreases (Sun et al., 2015). Accordingly, the loss of function in the CcmFC protein in Zm-mcsf1 mitochondria may also lead to reduction of the Cytc1 subunit. In contrast, there was a slight increase in Cox2 and ATPase α subunit, which might be feedback regulation on the expression of components of complex IV and complex V.
PPR-SMR1 and Zm-mCSF1 physically interact
It has been suggested that several group II intron splicing factors, such as CRM, PPR, APO, and PORR proteins, are present in a high molecular weight complexes in chloroplasts (0.5–1 MDa; Asakura et al., 2008; Hammani and Barkan, 2014; Reifschneider et al., 2016). Our results indicated that splicing of nad2-introns 2 and 3 and nad5-intron 1 was impaired in both ppr-smr1 and Zm-mcsf1 (Figs 2A, B, 4A, B, Supplementary Table S1). Accordingly, it is possible that PPR-SMR1 interacts with Zm-mCSF1 to promote the splicing of these introns from pre-mRNAs in maize mitochondria.
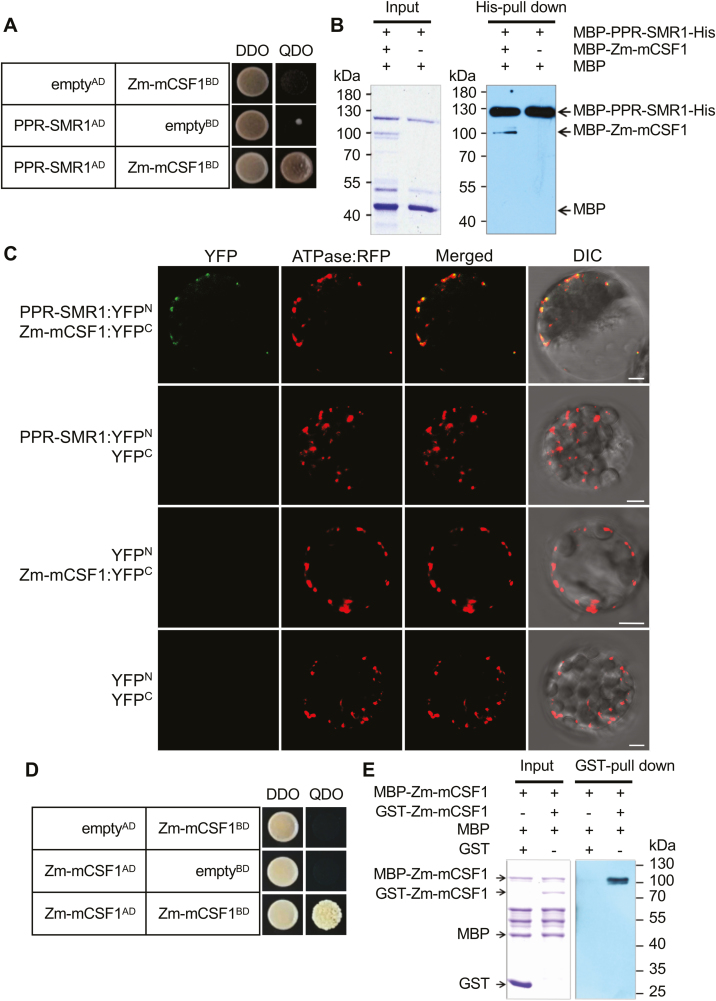
We explored the interaction between PPR-SMR1 and Zm-mCSF1 using yeast two-hybrid (Y2H) assays. The predicted mature PPR-SMR1 (PPR-SMR149-787) interacted with the predicted mature Zm-mCSF1 (Zm-mCSF130-424) (Fig. 5A). To corroborate the Y2H analysis, we examined the interaction by in vitro pull-down assays and bimolecular fluorescence complementation (BiFC) analysis. PPR-SMR1 and Zm-mCSF1 were purified as fusion proteins with MBP, and the interaction between MBP-PPR-SMR1-His and MBP-Zm-mCSF1 was investigated using a His-mediated pull-down assay. Consistent with the observed interactions in the Y2H analysis, MBP-PPR-SMR1-His was able to bind to MBP-Zm-mCSF1 with weak interaction in vitro (Fig. 5B). The MBP protein used as a negative control that was added to the binding reaction between MBP-PPR-SMR1-His and MBP-Zm-mCSF1 failed to be retained in the resin after high-stringency washes (Fig. 5B). In the BiFC assays, YFP was split into N-terminus (YFPN) and C-terminus (YFPC) (Walter et al., 2004), and YFPN was fused to the C-terminus of PPR-SMR1, whilst YFPC was fused to the C-terminus of Zm-mCSF1. The resulting constructs were mixed equally and then transformed into mesophyll protoplasts of Arabidopsis. In mitochondria of protoplasts labeled by ATPase::RFP, we were able to detect YFP fluorescence due to the reconstitution of intact YFP proteins by PPR-SMR1 interacting with Zm-mCSF1 (Fig. 5C). These results suggested that PPR-SMR1 physically interacts with Zm-mCSF1 in vivo. As negative controls, combinations of PPR-SMR1::YFPN and YFPC, YFPN and Zm-mCSF1::YFPC, YFPN and YFPC were transformed into mesophyll protoplasts, but no YFP signals could be detected (Fig. 5C).
Fig. 5.
PPR-SMR1 protein interacts with Zm-mCSF1. (A) Yeast two-hybrid (Y2H) analysis of PPR-SMR1 and Zm-mCSF1 interaction. The Y2HGold strain harboring the indicated bait and prey constructs were spotted on synthetic dropout (SD) medium without Leu and Trp (double-dropout, DDO) and SD without Ade, Leu, Trp, and His (quadruple-dropout, QDO). Yeast cultures on DDO control plates demonstrate the existence of both plasmids. Positive interactions were verified by growth on QDO plates. (B) Recombinant protein MBP-PPR-SMR1-His interacts with MBP-Zm-mCSF1 as determined using in vitro His pull-down assays. (C) Bimolecular fluorescence complementation (BiFC) analysis of the interaction of PPR-SMR1 and Zm-mCSF1 in mesophyll protoplasts of Arabidopsis. Yellow fluorescent protein (YFP) was split into N- and C-terminus, and PPR-SMR1 was fused with the N-terminus of YFP and Zm-mCSF1 was fused with C-terminus of YFP. Scale bars are 5 µm. (D) Zm-mCSF1AD physically interacts with Zm-mCSF1BD in Y2H assays. Yeast Y2HGold was transformed with paired constructs Zm-mCSF1AD and Zm-mCSF1BD, and AD (activating domain) and BD (binding domain) were used as negative controls. (E) Recombinant protein MBP-Zm-mCSF1 interacts with GST-Zm-mCSF1 as determined using in vitro glutathione S-transferase (GST) pull-down assays. ‘+’ and ‘−’ indicate the presence and absence of the corresponding proteins in the reactions, respectively.
Self-interaction of Zm-mCSF1
The Y2H analysis showed Zm-mCSF1 to be a self-interacting protein (Fig. 5D). To confirm this, in vitro pull-down assays were performed. Zm-mCSF1 was purified as fusion proteins with MBP and GST, and the interaction was investigated via GST-mediated pull-down assays (Fig. 5E). MBP and GST proteins were used as negative controls, and could not be found in the elution after washes.
Identification of the interacting domains in the PPR-SMR1/Zm-mCSF1 proteins
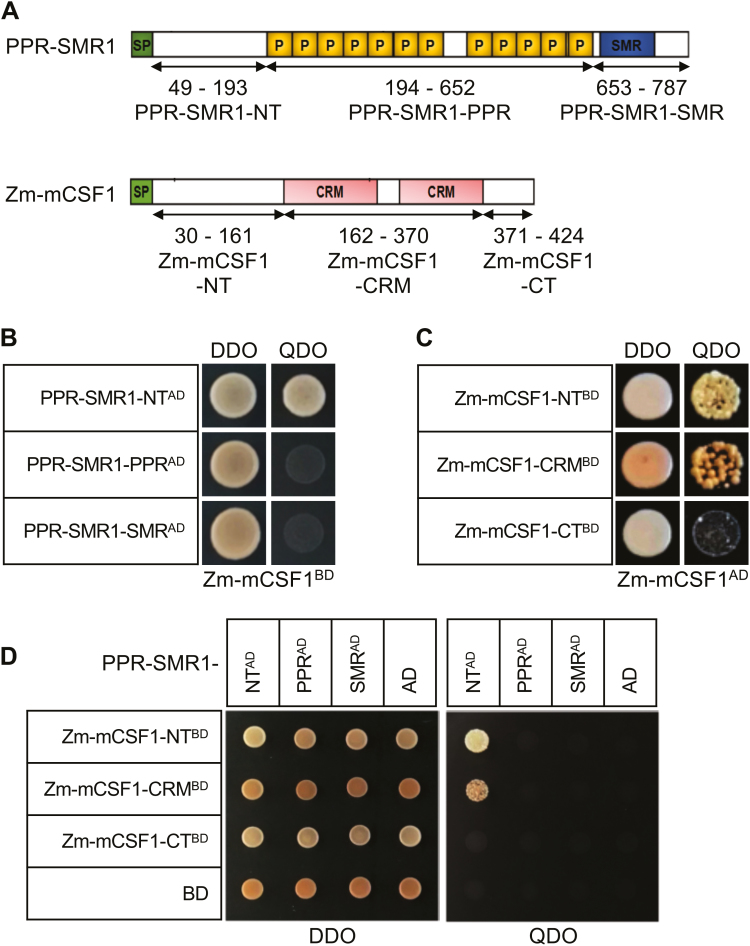
To investigate the regions responsible for the interaction between PPR-SMR1 and Zm-mCSF1, we generated a series of proportional PPR-SMR1 and Zm-mCSF1 cDNAs and cloned them into the prey and bait constructs, respectively (Fig. 6A). The results showed that a construct containing the N terminus (NT, amino acids 49–193) of PPR-SMR1 was sufficient for the interaction with mature Zm-mCSF1 (Fig. 6B), suggesting that the NT region of PPR-SMR1 with an unknown function could be involved in the interaction with Zm-mCSF1. In addition, constructs harboring the N terminus (NT, amino acids 30–161) or the CRM domain (amino acids 162–370) of Zm-mCSF1 were able to interact with the NT segment of PPR-SMR1 (Fig. 6D). However, neither the PPR domain (PPR-SMR1194-652) nor the SMR segment (PPR-SMR1653-787) could interact with the predicted mature Zm-mCSF1 or its split segments in the Y2H analysis (Fig. 6B, D). Zm-mCSF1 was able to interact with itself through the N-terminus (amino acids 30–161) and CRM domain (amino acids 162–370) (Fig. 6C).
Fig. 6.
Protein fragments required for interactions of maize PPR-SMR1 and Zm-mCSF1. (A) Schematic diagram of full-length and split fragment fusions of PPR-SMR1 and Zm-mCSF1 to the GAL4 activating domain (AD) or the GAL4 DNA binding domain (BD), respectively. The PPR-SMR1 protein is split into three fragments: PPR-SMR1-NT (N-terminus, 49–193), PPR-SMR1-PPR (194–652), and PPR-SMR1-SMR (653–787). The Zm-mCSF1 protein is split into three fragments: Zm-mCSF1-NT (N-terminus, 30–161), Zm-mCSF1-CRM (162–370), and Zm-mCSF1-CT (371–424). (B–D) Yeast two-hybrid (Y2H) assays demonstrating the physical interactions. The Y2HGold harboring the indicated bait and prey constructs were spotted on synthetic dropout (SD) medium without Leu and Trp (double-dropout, DDO) and SD without Ade, Leu, Trp, and His (quadruple-dropout, QDO). (B) PPR-SMR1-NTAD physically interacts with Zm-mCSF1BD. (C) Zm-mCSF1-NTBD and Zm-mCSF1-CRMBD interact with Zm-mCSF1AD. (D) PPR-SMR1-NTAD physically interacts with Zm-mCSF1-NTBD and Zm-mCSF1-CRMBD. (This figure is available in color at JXB online.)
Discussion
PPR-SMR1 and Zm-mCSF1 are involving in the splicing of multiple introns in mitochondria
Both the PPR-SMR1 and Zm-mCSF1 proteins were encoded by nuclear genes and targeted to mitochondria (Figs 1M, 3E), and contained mitochondrial signaling peptides as predicted by the TargetP and Predotar tools (Emanuelsson et al., 2000). Using comparative analyses of the mutants and wild-type, we found evidence that loss-of-function mutations in each of these two genes caused defects in the splicing of multiple mitochondrial introns. The Zm-mcsf1 mutants were defective in splicing of nad2 introns 2 and 3, nad5 introns 1 and 2, nad7 intron 3, and the ccmFc intron (Fig. 4A, B). Four of their corresponding introns in Arabidopsis mitochondria required the involvement of mCSF1; however, the number of Zm-mCSF1-dependent introns was smaller than that of Arabidopsis mCSF1. Possible reasons for this may be that the highly degenerated sequences in these introns cannot be recognized by Zm-mCSF1, or that its homolog Zm-mCSF2 could take over and be required for the splicing of those introns. However, the functions of Zm-mCSF2 and Arabidopsis mCSF2 are still unknown due to the lack of loss-of-function mutations. Further efforts are needed to create knockout mutants for Zm-mCSF2 and mCSF2 and to characterize their molecular functions in maize and Arabidopsis, respectively. The ppr-smr1 mutants were defective in splicing of a surprising 16 introns, namely nad1 introns 1 to 4, nad2 introns 1 to 4, nad4 introns 1 to 3, nad5 introns 1, 3, and 4, nad7 intron 2, and the rps3 intron (Fig. 2A, B). The failed splicing of these introns would undoubtedly cause a deficiency of these proteins. Because these Nad proteins are the essential subunits of mitochondrial complex I, which is the entry point of the oxidative phosphorylation (OXPHOS) machinery, the mitochondrial function will be severely impaired. This was manifested in several regards, including the impairment of mitochondrial complex assembly and the altered expression of OXPHOS component proteins (Figs 2C, 4C). Failures in the cytochrome pathway of the OXPHOS machinery also caused up-regulation of AOX protein expression (Figs 2C, 4C), as has been documented previously (Xiu et al., 2016; Sun et al., 2019). Because mitochondria are the sites for the tricarboxylic acid cycle, NADH re-oxidization, reduction of oxygen, and ATP synthesis, they are essential to all cellular activities (Schertl and Braun, 2014). Thus, the dysfunction in mitochondria would be expected to impact seed development, resulting in severely inhibited embryogenesis and endosperm development in the mutants of ppr-smr1 and Zm-mcsf1.
Unlike the P-type PPR proteins that are usually involved in the splicing of one or more introns (Xiu et al., 2016; Cai et al., 2017; Chen et al., 2017; Qi et al., 2017; Ren et al., 2017), each of PPR-SMR1 and Zm-mCSF1 was required for the splicing of multiple introns in maize, particularly PPR-SMR1 that was required for nearly 75% of mitochondrial group II introns. Taken together, PPR-SMR1 and Zm-mCSF1 were required for the splicing of a subset of 19 mitochondrial introns, of which nad2-introns 2 and 3, and nad5 intron 1 were dependent on both PPR-SMR1 and Zm-mCSF1. In addition, those known P-type PPR-dependent introns were also included in this subset of 19 mitochondrial introns. This raises questions as to whether PPR proteins and CRM domain-containing proteins are essential factors for the splicing of all introns in mitochondria, and whether PPR-SMR1 and Zm-mCSF1 are the major players of mitochondrial intron splicing. Since the group II introns in maize mitochondria are large in size (ranging from 792–3425 nt) and complex in structure with trans-introns (Supplementary Table S1), it appears that PPR-SMR1 and Zm-mCSF1 together with other P-type PPR proteins might be involved in the folding of catalytic RNAs (ribozymes) through directly binding to specific sequences within the corresponding introns to block random local RNA base-pairings, or through protein–protein interactions for long-range RNA domain interactions, thus increasing the splicing efficiencies of these introns.
PPR-SMR1 and Zm-mCSF1 may be involved in formation of large and dynamic splicing macromolecules
There is emerging evidence to suggest that large ribonucleoprotein (RNP) complexes related to intron splicing may be present in plant mitochondria (Zmudjak et al., 2017). Moreover, an increasing number of splicing factors have been reported to be required for the splicing of distinct or overlapping mitochondrial introns, including the maturases nMAT1–4 (Keren et al., 2009, 2012; Cohen et al., 2014; Zmudjak et al., 2017), PORR protein WTF9 (Kroeger et al., 2009; Francs-Small et al., 2012; Chettoor et al., 2015), the CRM domain-containing protein mCSF1 (Zmudjak et al., 2013), RUG3 (Kühn et al., 2011), the RNA DEAD-box helicase PMH2 (Köhler et al., 2010; Zmudjak et al., 2017), and several P-type PPR proteins (Xiu et al., 2016; Cai et al., 2017; Chen et al., 2017; Qi et al., 2017; Ren et al., 2017; Sun et al., 2019). However, how these proteins participate in intron splicing is not known. Our results identified a novel central splicing factor PPR-SMR1 in maize and highlighted some of the conserved splicing functions of mCSF1 in plants. Notably, the CRM domain protein Zm-mCSF1 could form a homodimer and physically interact with PPR-SMR1 in Y2H, pull-down, and BiFC assays (Figs 5, 6). Given that many members of PPR proteins, CRM domain proteins, and other families of RNA-binding proteins are splicing factors, and the splicing of some particular introns required the involvement of PPR-SMR1, Zm-mCSF1, and some other P-type PPRs, it suggests that PPR-SMR1 and Zm-mCSF1 might be the core factors for forming large macromolecular complexes to mediate splicing of mitochondrial group II introns, and that these macromolecular complexes could also be able to interact through the interaction between PPR-SMR1 and Zm-mCSF1. However, further efforts need to be made to determine the binding properties for PPR-SMR1 and Zm-mCSF1, and PPR-SMR1 or Zm-mCSF1 should be used as targets to immunoprecipitate the large macromolecular complexes followed by protein mass spectrometry to reveal their members. Currently, we cannot exclude the possibility that these genes might have other activities.
In addition to mitochondria, similar protein complexes may exist to facilitate intron splicing in chloroplasts. Although the event of chloroplast endosymbiosis was 450 million years after that of mitochondria, the mechanism of intron splicing seems to be conserved in both organelles. In maize, chloroplasts have 15 group II introns in the genes rps12, rps16, rpl2, rpl16, ndhA, ndhB, petB, petD, atpF, and six trn genes (Maier et al., 1995). Many PPR proteins are targeted to chloroplasts, and several of them are splicing factors that help to remove distinct introns (Barkan and Small, 2014). In addition, like CRM domain-containing proteins (Till et al., 2001; Ostheimer et al., 2003), the mTERF protein Zm-mTERF4 (Hammani and Barkan, 2014) and the PORR protein WTF1 have been reported to be required for splicing of introns in chloroplasts, and they have been found to be simultaneously present in a 0.55–1 MDa protein complex with potential physical interactions (Hammani and Barkan, 2014). Since the removal of plant organellar group II introns from pre-mRNAs requires the formation of ribozyme structures within introns, this protein complex could function in connecting those RNA domains (I–VI) to form the autocatalytic conformation to excise themselves from exons. In contrast to the nuclear intron splicing machinery that involves small nuclear RNAs (U1, U2, U4/U6, and U5 snRNPs) and ~170 distinct snRNA-associated proteins (Wahl et al., 2009), the splicing of organellar group II introns probably preferentially involves the assistance of nuclear RNA-binding proteins, and evolution has favored nuclear control of organelle gene expression for an unknown reason of fitness.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. PPR-SMR1 and its homologs in Arabidopsis, rice, and sorghum.
Fig. S2. Co-segregation and allelism tests of ppr-smr1 alleles and the emp phenotype.
Fig. S3. Altered expressions of mitochondrial transcripts in ppr-smr1 and Zm-mcsf1 endosperm.
Fig. S4. mCSF1 and mCSF2 of Arabidopsis and maize, and allelism test of Zm-mcsf1 alleles.
Table S1. List of mitochondrial group II introns and their splicing efficiencies in ppr-smr1 and Zm-mcsf1 mutants.
Table S2. Primers used in this study.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Project No. 91735301, 31630053, and 91435201) and the China Postdoctoral Science Foundation (Grant No. 2014M561917). We thank the Maize Genetic Stock Center for providing the maize material and Dr Tsuyoshi Nakagawa (Shimane University, Japan) for providing the pGWB vectors. The authors declare that they have no conflicts of interest related to this work.
Author contributions
ZC and B-CT designed the research; ZC, H-CW, and JS conducted most of the experiments; ZC, FS, MW, CX, and B-CT analysed the data; ZC, H-CW, and B-CT wrote the manuscript.
References
- Asakura Y, Barkan A. 2007. A CRM domain protein functions dually in group I and group II intron splicing in land plant chloroplasts. The Plant Cell 19, 3864–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura Y, Bayraktar OA, Barkan A. 2008. Two CRM protein subfamilies cooperate in the splicing of group IIB introns in chloroplasts. RNA 14, 2319–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A, Klipcan L, Ostersetzer O, Kawamura T, Asakura Y, Watkins KP. 2007. The CRM domain: an RNA binding module derived from an ancient ribosome-associated protein. RNA 13, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A, Small I. 2014. Pentatricopeptide repeat proteins in plants. Annual Review of Plant Biology 65, 415–442. [DOI] [PubMed] [Google Scholar]
- Bonen L. 2008. Cis- and trans-splicing of group II introns in plant mitochondria. Mitochondrion 8, 26–34. [DOI] [PubMed] [Google Scholar]
- Brown GG, Colas des Francs-Small C, Ostersetzer-Biran O. 2014. Group II intron splicing factors in plant mitochondria. Frontiers in Plant Science 5, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M, Li S, Sun F, Sun Q, Zhao H, Ren X, Zhao Y, Tan BC, Zhang Z, Qiu F. 2017. Emp10 encodes a mitochondrial PPR protein that affects the cis-splicing of nad2 intron 1 and seed development in maize. The Plant Journal 91, 132–144. [DOI] [PubMed] [Google Scholar]
- Chen X, Feng F, Qi W, Xu L, Yao D, Wang Q, Song R. 2017. Dek35 encodes a PPR protein that affects cis-splicing of mitochondrial nad4 Intron 1 and seed development in maize. Molecular Plant 10, 427–441. [DOI] [PubMed] [Google Scholar]
- Chettoor AM, Yi G, Gomez E, Hueros G, Meeley RB, Becraft PW. 2015. A putative plant organelle RNA recognition protein gene is essential for maize kernel development. Journal of Integrative Plant Biology 57, 236–246. [DOI] [PubMed] [Google Scholar]
- Cohen S, Zmudjak M, Colas des Francs-Small C, et al. 2014. nMAT4, a maturase factor required for nad1 pre-mRNA processing and maturation, is essential for holocomplex I biogenesis in Arabidopsis mitochondria. The Plant Journal 78, 253–268. [DOI] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G. 2000. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. Journal of Molecular Biology 300, 1005–1016. [DOI] [PubMed] [Google Scholar]
- Ferat JL, Michel F. 1993. Group II self-splicing introns in bacteria. Nature 364, 358–361. [DOI] [PubMed] [Google Scholar]
- Francs-Small CC, Kroeger T, Zmudjak M, Ostersetzer-Biran O, Rahimi N, Small I, Barkan A. 2012. A PORR domain protein required for rpl2 and ccmFC intron splicing and for the biogenesis of c-type cytochromes in Arabidopsis mitochondria. The Plant Journal 69, 996–1005. [DOI] [PubMed] [Google Scholar]
- Fukui K, Kuramitsu S. 2011. Structure and function of the small MutS-related domain. Molecular Biology International 2011, 691735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammani K, Barkan A. 2014. An mTERF domain protein functions in group II intron splicing in maize chloroplasts. Nucleic Acids Research 42, 5033–5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammani K, Giegé P. 2014. RNA metabolism in plant mitochondria. Trends in Plant Science 19, 380–389. [DOI] [PubMed] [Google Scholar]
- Hsu YW, Wang HJ, Hsieh MH, Hsieh HL, Jauh GY. 2014. Arabidopsis mTERF15 is required for mitochondrial nad2 intron 3 splicing and functional complex I activity. PLoS ONE 9, e112360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JB, Bae H, Kim SJ, Jin YH, Goh CH, Kim DH, Lee YJ, Tse YC, Jiang L, Hwang I. 2003. The Arabidopsis dynamin-like proteins ADL1C and ADL1E play a critical role in mitochondrial morphogenesis. The Plant Cell 15, 2357–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren I, Bezawork-Geleta A, Kolton M, Maayan I, Belausov E, Levy M, Mett A, Gidoni D, Shaya F, Ostersetzer-Biran O. 2009. AtnMat2, a nuclear-encoded maturase required for splicing of group-II introns in Arabidopsis mitochondria. RNA 15, 2299–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren I, Tal L, des Francs-Small CC, Araújo WL, Shevtsov S, Shaya F, Fernie AR, Small I, Ostersetzer-Biran O. 2012. nMAT1, a nuclear-encoded maturase involved in the trans-splicing of nad1 intron 1, is essential for mitochondrial complex I assembly and function. The Plant Journal 71, 413–426. [DOI] [PubMed] [Google Scholar]
- Köhler D, Schmidt-Gattung S, Binder S. 2010. The DEAD-box protein PMH2 is required for efficient group II intron splicing in mitochondria of Arabidopsis thaliana. Plant Molecular Biology 72, 459–467. [DOI] [PubMed] [Google Scholar]
- Kroeger TS, Watkins KP, Friso G, van Wijk KJ, Barkan A. 2009. A plant-specific RNA-binding domain revealed through analysis of chloroplast group II intron splicing. Proceedings of the National Academy of Sciences, USA 106, 4537–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn K, Carrie C, Giraud E, Wang Y, Meyer EH, Narsai R, Colas des Francs-Small C, Zhang B, Murcha MW, Whelan J. 2011. The RCC1 family protein RUG3 is required for splicing of nad2 and complex I biogenesis in mitochondria of Arabidopsis thaliana. The Plant Journal 67, 1067–1080. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33, 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamattina L, Gonzalez D, Gualberto J, Grienenberger JM. 1993. Higher plant mitochondria encode an homologue of the nuclear‐encoded 30‐kDa subunit of bovine mitochondrial complex I. The FEBS Journal 217, 831–838. [DOI] [PubMed] [Google Scholar]
- Lambowitz AM, Zimmerly S. 2011. Group II introns: mobile ribozymes that invade DNA. Cold Spring Harbor Perspectives in Biology 3, a003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Zhang C, Lan H, Gao S, Liu H, Liu J, Cao M, Pan G, Rong T, Zhang S. 2014. Validation of potential reference genes for qPCR in maize across abiotic stresses, hormone treatments, and tissue types. PLoS ONE 9, e95445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Melonek J, Boykin LM, Small I, Howell KA. 2013a. PPR-SMRs: ancient proteins with enigmatic functions. RNA Biology 10, 1501–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yu F, Rodermel S. 2010. An Arabidopsis pentatricopeptide repeat protein, SUPPRESSOR OF VARIEGATION7, is required for FtsH-mediated chloroplast biogenesis. Plant Physiology 154, 1588–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Xiu ZH, Meeley R, Tan BC. 2013b. Empty pericarp5 encodes a pentatricopeptide repeat protein that is required for mitochondrial RNA editing and seed development in maize. The Plant Cell 25, 868–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier RM, Neckermann K, Igloi GL, Kössel H. 1995. Complete sequence of the maize chloroplast genome: gene content, hotspots of divergence and fine tuning of genetic information by transcript editing. Journal of Molecular Biology 251, 614–628. [DOI] [PubMed] [Google Scholar]
- Manavski N, Guyon V, Meurer J, Wienand U, Brettschneider R. 2012. An essential pentatricopeptide repeat protein facilitates 5´ maturation and translation initiation of rps3 mRNA in maize mitochondria. The Plant Cell 24, 3087–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty DR, Settles AM, Suzuki M, et al. 2005. Steady-state transposon mutagenesis in inbred maize. The Plant Journal 44, 52–61. [DOI] [PubMed] [Google Scholar]
- Ostheimer GJ, Williams-Carrier R, Belcher S, Osborne E, Gierke J, Barkan A. 2003. Group II intron splicing factors derived by diversification of an ancient RNA-binding domain. The EMBO Journal 22, 3919–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfalz J, Holtzegel U, Barkan A, Weisheit W, Mittag M, Pfannschmidt T. 2015. ZmpTAC12 binds single-stranded nucleic acids and is essential for accumulation of the plastid-encoded polymerase complex in maize. New Phytologist 206, 1024–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfalz J, Liere K, Kandlbinder A, Dietz KJ, Oelmüller R. 2006. pTAC2, -6, and -12 are components of the transcriptionally active plastid chromosome that are required for plastid gene expression. The Plant Cell 18, 176–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W, Yang Y, Feng X, Zhang M, Song R. 2017. Mitochondrial function and maize kernel development requires Dek2, a pentatricopeptide repeat protein involved in nad1 mRNA splicing. Genetics 205, 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifschneider O, Marx C, Jacobs J, Kollipara L, Sickmann A, Wolters D, Kück U. 2016. A ribonucleoprotein supercomplex involved in trans-splicing of organelle group II introns. The Journal of Biological Chemistry 291, 23330–23342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Pan Z, Zhao H, Zhao J, Cai M, Li J, Zhang Z, Qiu F. 2017. EMPTY PERICARP11 serves as a factor for splicing of mitochondrial nad1 intron and is required to ensure proper seed development in maize. Journal of Experimental Botany 68, 4571–4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robart AR, Chan RT, Peters JK, Rajashankar KR, Toor N. 2014. Crystal structure of a eukaryotic group II intron lariat. Nature 514, 193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schertl P, Braun HP. 2014. Respiratory electron transfer pathways in plant mitochondria. Frontiers in Plant Science 5, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan LD, Mileshina D, Grewe F, et al. 2016. The reverse transcriptase/RNA maturase protein MatR is required for the splicing of various group II introns in Brassicaceae mitochondria. The Plant Cell 28, 2805–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Wang X, Bonnard G, Shen Y, Xiu Z, Li X, Gao D, Zhang Z, Tan BC. 2015. Empty pericarp7 encodes a mitochondrial E-subgroup pentatricopeptide repeat protein that is required for ccmFN editing, mitochondrial function and seed development in maize. The Plant Journal 84, 283–295. [DOI] [PubMed] [Google Scholar]
- Sun F, Xiu Z, Jiang R, Liu Y, Zhang X, Yang YZ, Li X, Zhang X, Wang Y, Tan BC. 2019. The mitochondrial pentatricopeptide repeat protein EMP12 is involved in the splicing of three nad2 introns and seed development in maize. Journal of Experimental Botany 70, 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadini L, Pesaresi P, Kleine T, et al. 2016. GUN1 controls accumulation of the plastid ribosomal protein S1 at the protein level and interacts with proteins involved in plastid protein homeostasis. Plant Physiology 170, 1817–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan BC, Chen Z, Shen Y, Zhang Y, Lai J, Sun SS. 2011. Identification of an active new mutator transposable element in maize. Genes Genomes Genetics 1, 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till B, Schmitz-Linneweber C, Williams-Carrier R, Barkan A. 2001. CRS1 is a novel group II intron splicing factor that was derived from a domain of ancient origin. RNA 7, 1227–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toor N, Keating KS, Taylor SD, Pyle AM. 2008. Crystal structure of a self-spliced group II intron. Science 320, 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Herpen TW, Cankar K, Nogueira M, Bosch D, Bouwmeester HJ, Beekwilder J. 2010. Nicotiana benthamiana as a production platform for artemisinin precursors. PLoS ONE 5, e14222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoud V, Hajduch M, Khaled AS, Depege N, Rogowsky PM. 2005. Maize embryogenesis. Maydica 50, 469–483. [Google Scholar]
- Vogel J, Börner T. 2002. Lariat formation and a hydrolytic pathway in plant chloroplast group II intron splicing. The EMBO Journal 21, 3794–3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl MC, Will CL, Lührmann R. 2009. The spliceosome: design principles of a dynamic RNP machine. Cell 136, 701–718. [DOI] [PubMed] [Google Scholar]
- Walter M, Chaban C, Schütze K, et al. 2004. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. The Plant Journal 40, 428–438. [DOI] [PubMed] [Google Scholar]
- Williams-Carrier R, Zoschke R, Belcher S, Pfalz J, Barkan A. 2014. A major role for the plastid-encoded RNA polymerase complex in the expression of plastid transfer RNAs. Plant Physiology 164, 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Liu S, Ruwe H, et al. 2016. SOT1, a pentatricopeptide repeat protein with a small MutS-related domain, is required for correct processing of plastid 23S-4.5S rRNA precursors in Arabidopsis thaliana. The Plant Journal 85, 607–621. [DOI] [PubMed] [Google Scholar]
- Xiu Z, Sun F, Shen Y, Zhang X, Jiang R, Bonnard G, Zhang J, Tan BC. 2016. EMPTY PERICARP16 is required for mitochondrial nad2 intron 4 cis-splicing, complex I assembly and seed development in maize. The Plant Journal 85, 507–519. [DOI] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J. 2007. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nature Protocols 2, 1565–1572. [DOI] [PubMed] [Google Scholar]
- Zhou W, Lu Q, Li Q, Wang L, Ding S, Zhang A, Wen X, Zhang L, Lu C. 2017. PPR-SMR protein SOT1 has RNA endonuclease activity. Proceedings of the National Academy of Sciences, USA 114, E1554–E1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmudjak M, Colas des Francs-Small C, Keren I, Shaya F, Belausov E, Small I, Ostersetzer-Biran O. 2013. mCSF1, a nucleus-encoded CRM protein required for the processing of many mitochondrial introns, is involved in the biogenesis of respiratory complexes I and IV in Arabidopsis. New Phytologist 199, 379–394. [DOI] [PubMed] [Google Scholar]
- Zmudjak M, Shevtsov S, Sultan LD, Keren I, Ostersetzer-Biran O. 2017. Analysis of the roles of the Arabidopsis nMAT2 and PMH2 proteins provided with new insights into the regulation of group II intron splicing in land-plant mitochondria. International Journal of Molecular Sciences 18, 2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoschke R, Kroeger T, Belcher S, Schöttler MA, Barkan A, Schmitz-Linneweber C. 2012. The pentatricopeptide repeat-SMR protein ATP4 promotes translation of the chloroplast atpB/E mRNA. The Plant Journal 72, 547–558. [DOI] [PubMed] [Google Scholar]
- Zoschke R, Watkins KP, Miranda RG, Barkan A. 2016. The PPR-SMR protein PPR53 enhances the stability and translation of specific chloroplast RNAs in maize. The Plant Journal 85, 594–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.