Abstract
This article comments on:
Guo H, York LM. 2019. Maize with fewer nodal roots allocates mass to more lateral and deep roots that improve nitrogen uptake and shoot growth. Journal of Experimental Botany70, 5299–5309.
Yang JT, Schneider HM, Brown KM, Lynch JP. 2019. Genotypic variation and nitrogen stress effects on root anatomy in maize are node-specific. Journal of Experimental Botany70, 5311–5325.
Keywords: Maize, nitrogen acquisition, root system architecture, nodal roots, nitrogen use efficiency
Two new articles find that nodal root phenes are key traits for high nitrogen use efficiency and discovering variation amongst inbred and hybrid lines that will enable the genetic mechanisms underpinning this phenotypic relationship to be investigated. Using nodal root excision, Guo and York (2019) show that reduced nodal root number results in greater root length throughout the vertical profile and especially at depth, demonstrating how root system architecture modifications can facilitate nitrogen and water foraging. In a complementary study, Yang et al. (2019) show that the cellular anatomy of nodal roots varies among nodes but also depending on nitrogen use efficiency and the degree of nitrogen limitation.
The ability of cereal crops to efficiently take up, use or transport nitrogen from the soil affects overall yield. Maize is one of the main crops produced and consumed globally, and thus its production is expected to increase from the current >1 billion tons by 16% by 2027, especially in developing countries. Due to its importance for animal production, human consumption and other industrial uses, a key aim for maize breeding is to develop high nitrogen use efficiency (NUE) genotypes that produce superior grain yields per unit of N uptake, in low soil N conditions (Presterl, 2002).
Challenges in measuring root system architecture (RSA)
Most studies on maize nutrient acquisition have utilised immature plants due to the difficulties of phenotyping large root systems. However, nodal roots are the main drivers of nutrient acquisition in mature maize and thus we must consider all RSA characteristics, often termed ‘phenes’ (York et al., 2013), that could lead to more efficient soil exploration. For maize, the ‘steep, deep and cheap’ ideotype (Lynch, 2013) and phene states such as fewer nodal roots (Saengwilai et al., 2014b), reduced investment in cortex formation or an increase in aerenchyma (Saengwilai et al., 2014a) have been found to improve NUE.
Nodal root excision enhances embryonic root elongation and nitrogen acquisition
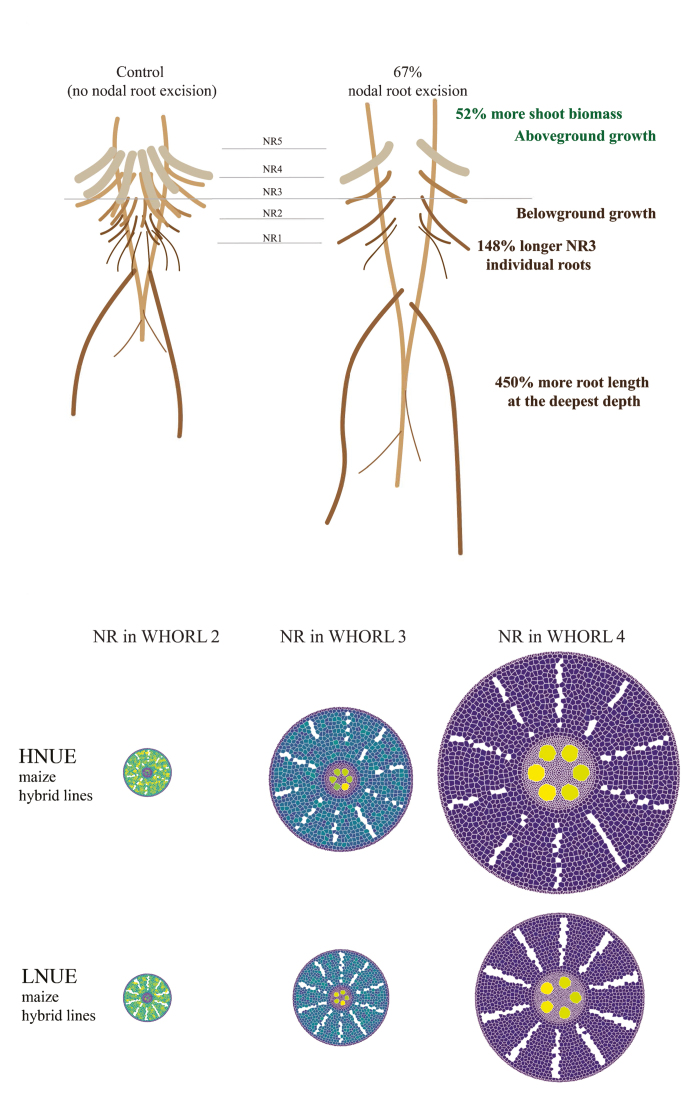
Previous simulation modelling predicted that decreased nodal root number (NRN) could enhance maize growth under low N (LN) levels (York et al., 2013) and these hypotheses have been partially confirmed using recombinant inbred lines (RILs) (Saengwilai et al., 2014a). Guo and York (2019) excised different proportions of nodal roots in the maize genotype B73. When grown hydroponically, excising two thirds of NR slightly decreased root biomass and N assimilation rate under both high and low levels of N supply. The effects were generally most pronounced in earlier emerging whorls; for NR1, excision decreased root biomass by 74% and 47% under high N (HN; 2.26 mM KNO3) and LN (0.28 mM KNO3) conditions, respectively, after 24 days. Although embryonic root (ER) biomass increased by 61% in LN conditions, shoot biomass or shoot N content was not affected. Thus, the plant re-allocated resources within the root system to maintain N homeostasis and carbon balance. When plants were grown in deep (154 cm) sand/vermiculite/perlite mesocosms for 42 days, the same root excision only decreased NR biomass at HN (6 mM KNO3), not LN (0.6mM KNO3). ER biomass increased by 112% and 182% in HN and LN conditions, respectively, and shoot biomass increased by 35% at both HN and LN. At LN, maximum root depth increased 450% and there was a 232% greater uptake of deep injected 15N in the shoot. The hydroponic and mesocosm studies both indicate that the maize root system has a remarkable ability to maintain carbon demand by allocating more mass to lateral and deep roots when fewer nodal roots are present. However, N uptake and shoot mass were only improved in low nitrogen mesocosms. This indicates that the mechanism for enhanced growth with reduced nodal rooting is increased foraging throughout the soil, and especially at depth, that can sustain greater leaf growth, nitrogen homeostasis, and total photosynthesis. Greater N acquisition is not limited to genotypes with fewer NRs, suggesting that maize can be bred for lower NRN and higher N-acquisition. However, since excising fewer nodal roots (only a third) had no significant effects, genotypes with wide variation in NRN are likely to be most fruitful for study.
Genotypes with higher nitrogen use efficiency have thicker nodal roots
Studying the consequences of reduced NRN on uptake of water and other nutrients is important; maize genotypes with many crown roots acquire more P from the topsoil (Sun et al., 2018), for example. Following the hypothesis that variability in root traits determines nitrogen uptake efficiency, especially at low N (Hawkesford, 2014), Yang et al. (2019) used laser ablation tomography to characterise root anatomical phenes across genotypes that vary in NRN. Specifically under low N conditions, nodal roots of high NUE (HNUE) genotypes had 14% larger root diameter, 22% greater total metaxylem vessel conductance and 54% less aerenchyma compared to low NUE (LNUE) genotypes. These differences occurred without variance in shoot biomass, highlighting the importance of NR traits in determining NUE. When analysing a range of hybrid and inbred lines, root diameter-related phenes such as cortex, stele and total metaxylem as well as the number of cortical cell files and metaxylem vessels increased in each successive node, explaining most of the observed variation in root anatomical phenes amongst genotypes (Yang et al., 2019). These findings are supported by earlier work that measured a large number of root architectural traits across nodes and similarly found a huge influence of nodal position (York and Lynch, 2015). An important question is whether growth patterns, nutrient/water uptake and low input stress responses differ in inbreds and hybrids, since hybrid lines grown at LN have drastically reduced biomass compared to inbred lines, even though they perform well under HN. HNUE lines typically have fewer nodal roots per whorl (Saengwilai et al., 2014b). Variation in nodal root architecture across nodes could therefore reflect plant nutritional needs.
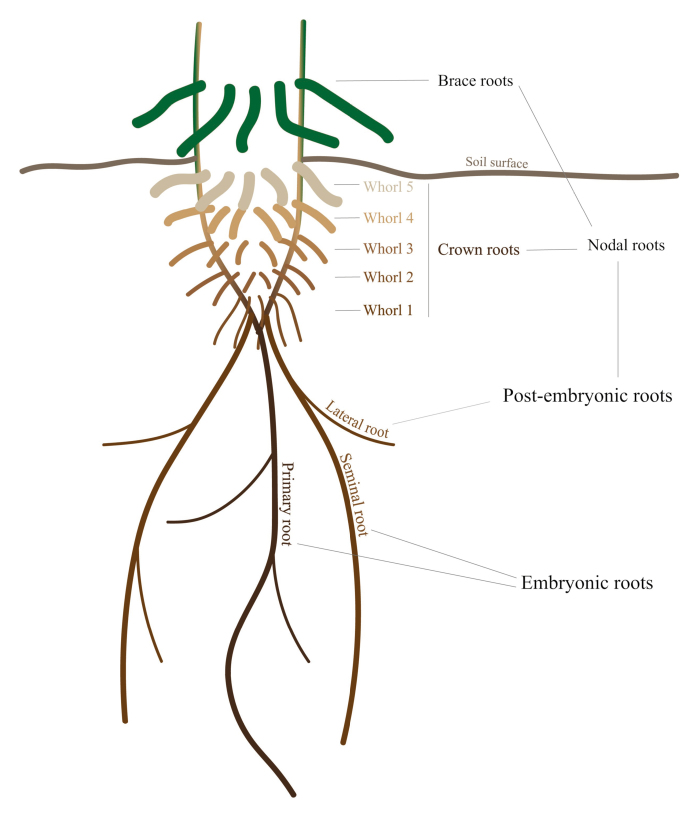
Box 1. The mature maize root system.
Maize RSA consists of primary and seminal embryonic roots as well as post-embryonic shoot-borne nodal and lateral roots (Hochholdinger and Tuberosa, 2009). Lateral roots emerge from the pericycle of all root types. Nodal roots develop from discrete spatially separated nodes (or whorls) and individual roots in each node form a circle surrounding the primary stem/root. Nodal roots are termed brace or crown roots if they emerge above-ground or below-ground, respectively. A mature maize plant typically develops up to six crown root whorls below-ground and up to three brace root nodes above-ground. Roots from nodes 1–6 emerge successively, with roots from nodes 1-2-3 emerging at early stages of development and roots from nodes 4-5-6 developing later when the plant has a higher nutrient and water demand. The term ‘axial’ or ‘axile’ can refer to either the primary root, the seminal root, or a nodal (crown or brace) root.
Box 2. Nodal influence on maize nitrogen dynamics.
Taken together, Guo and York (2019) and Yang et al. (2019) find that fewer and thicker nodal roots provide a better nitrogen use efficiency (NUE) strategy. Upper panel summarises the experimental approach of Guo and York (2019). Nodal root excision may lead to reallocation of carbon resources towards the embryonic root system, resulting in formation of deeper roots that capture deep nitrogen more effectively. In both hydroponic and solid mesocosm conditions there was reallocation of mass to form more lateral roots and greater axial root length, although nodal number-related root architecture and yield effects differed. Moreover, effects on the shoot were only found in mesocosms, suggesting an influence of water, air and nitrogen depletion zones. Lower panel summarises the model-based approach of Yang et al. (2019). Digital root sections were generated using GRANAR (https://plantmodelling.shinyapps.io/CrossSim/, Heymans et al., 2019 [preprint]). High NUE genotypes, compared to low NUE genotypes, had thicker nodal roots with less aerenchyma. These differences were greater in mature whorls and likely affect water and stress responses.
Reshaping the root for enhanced NUE: implications for water uptake?
The results described here reveal much greater architectural complexity in how the root system responds to edaphic changes. Both Guo and York (2019), and Yang et al. (2019) highlight how important it is to consider the responses of individual phenes of root system architecture, since root parameters are often under individual genetic control as well as being differentially responsive to stress (Walker et al., 2017). How these changes affect water acquisition should be investigated in experiments that factorially manipulate soil N and water availability.
In comparable experiments to those described above that instead imposed low and high soil water availability, crown root excision limited leaf area and transpiration of young well-watered plants (Cambre & Dodd, Unpublished). In contrast, genotypes with fewer crown roots maintained water uptake by proliferating more roots at depth in field trials (Gao and Lynch, 2016). Decreased lateral root branching improves water acquisition under water deficit (Zhan et al., 2015), while roots exposed to long-term flooding develop more aerenchyma as an adaptation response (Steffens and Rasmussen, 2016). Cellular characterisation of longer embryonic and nodal roots is required to determine if they are thicker and have more aerenchyma when grown in substrate mesocosms, as might be expected. Enhanced brace root development is associated with increased drought tolerance in maize (Santosh and Carena, 2016) and greater plant stability to resist lodging (Gaudin et al., 2011). Carbon allocation trade-offs among different root classes also seem important in optimising plant responses within multi-stress environments. For example, enhanced P uptake efficiency was found under reduced water availability in root systems with decreased NR thickness and increased secondary root branching (De Bauw et al., 2019).
As a greater diversity of substrates for plant growth have been developed and imaging and phenomics technologies been improved (Brackin et al., 2017; Morris et al., 2017; York, 2019), we are able to gain a detailed understanding of root phenes that are relevant to plant-soil environment situations. Both Guo and York (2019) and Yang et al. (2019) have highlighted the central importance of studying plant physiology in enabling us to understand agriculturally important plant-environment interactions.
References
- Brackin R, Atkinson BS, Sturrock CJ, Rasmussen A. 2017. Roots-eye view: using microdialysis and microct to non-destructively map root nutrient depletion and accumulation zones. Plant, Cell and Environment 40, 3135–3142. [DOI] [PubMed] [Google Scholar]
- De Bauw P, Vandamme E, Lupembe A, Mwakasege L, Senthilkumar K, Merckx R. 2019. Architectural root responses of rice to reduced water availability can overcome phosphorus stress. Agronomy 9, 11. [DOI] [PubMed] [Google Scholar]
- Gao Y, Lynch JP. 2016. Reduced crown root number improves water acquisition under water deficit stress in maize (Zea mays L.). Journal of Experimental Botany 67, 4545–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, York LM. 2019. Maize with fewer nodal roots allocates mass to more lateral and deep roots that improve nitrogen uptake and shoot growth. Journal of Experimental Botany 70, 5297–5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudin AC, McClymont SA, Holmes BM, Lyons E, Raizada MN. 2011. Novel temporal, fine-scale and growth variation phenotypes in roots of adult-stage maize (Zea mays L.) in response to low nitrogen stress. Plant, Cell and Environment 34, 2122–2137. [DOI] [PubMed] [Google Scholar]
- Hawkesford MJ. 2014. Reducing the reliance on nitrogen fertilizer for wheat production. Journal of Cereal Science 59, 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymans A, Couvreur V, La Rue T, Paez-Garcia A, Lobet G. 2019. GRANAR a new computational tool to better understand the functional importance of root anatomy. bioRxiv doi:10.1101/645036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochholdinger F, Tuberosa R. 2009. Genetic and genomic dissection of maize root development and architecture. Current Opinion in Plant Biology 12, 172–177. [DOI] [PubMed] [Google Scholar]
- Lynch JP. 2013. Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Annals of Botany 112, 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris EC, Griffiths M, Golebiowska A, et al. 2017. Shaping 3D root system architecture. Current Biology 27, R919–R930. [DOI] [PubMed] [Google Scholar]
- Presterl T, Groh S, Landbeck M, Seitz G, Schmidt W, Geiger HH. 2002. Nitrogen uptake and utilization efficiency of European maize hybrids developed under conditions of low and high nitrogen input. Plant Breeding 121, 480–486. [Google Scholar]
- Saengwilai P, Nord EA, Chimungu JG, Brown KM, Lynch JP. 2014a. Root cortical aerenchyma enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiology 166, 726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saengwilai P, Tian X, Lynch JP. 2014b. Low crown root number enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiology 166, 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santosh S, Carena MJ. 2016. Brace: a method for high throughput maize phenotyping of root traits for short-season drought tolerance. Crop Science 56, 2996–3004. [Google Scholar]
- Steffens B, Rasmussen A. 2016. The physiology of adventitious roots. Plant Physiology 170, 603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Gao Y, Lynch JP. 2018. Large crown root number improves topsoil foraging and phosphorus acquisition. Plant Physiology 177, 90–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L, Boddington C, Jenkins D, et al. 2017. Changes in gene expression in space and time orchestrate environmentally mediated shaping of root architecture. The Plant Cell 29, 2393–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JT, Schneider HM, Brown KM, Lynch JP. 2019. Genotypic variation and nitrogen stress effects on root anatomy in maize are node-specific. Journal of Experimental Botany 70, 5309–5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York LM. 2019. Functional phenomics: an emerging field integrating high-throughput phenotyping, physiology, and bioinformatics. Journal of Experimental Botany 70, 379–386. [DOI] [PubMed] [Google Scholar]
- York LM, Lynch JP. 2015. Intensive field phenotyping of maize (Zea mays L.) root crowns identifies phenes and phene integration associated with plant growth and nitrogen acquisition. Journal of Experimental Botany 66, 5493–5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York LM, Nord EA, Lynch JP. 2013. Integration of root phenes for soil resource acquisition. Frontiers in Plant Science 4, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan A, Schneider H, Lynch JP. 2015. Reduced lateral root branching density improves drought tolerance in maize. Plant Physiology 168, 1603–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]