Reallocating root biomass from nodal roots to lateral and early-emerging axial roots allows maize to capture more nitrogen under limiting conditions, including by increasing foraging at depth.
Keywords: Adventitious roots, compensation, corn, nitrogen use efficiency, plant economy, shoot-borne roots
Abstract
Simulations indicated that reduced nodal root (NR) number (NRN) was promising for maize breeding, and were partially confirmed by relying on variation in NRN among inbreds. Using maize inbred line B73, experiments were conducted in hydroponics and tall mesocosms containing solid media with treatments involving no NR excision (0% NRE) or excising either 33% or 67% of the NRs as they emerged under high or low levels of nitrogen (N). Reduced NRN was hypothesized to increase elongation of all remaining root classes, N acquisition under low N, and shoot mass. Plants with 67% NRE had 12% and 19% less root mass fraction, 61% and 91% greater lateral to axial root length ratio regardless of N levels, and 61% and 182% greater biomass of embryonic roots under low N, compared with 0% NRE for hydroponics and mesocosms studies, respectively. Under low N in mesocosms, plants with 67% NRE had 52% greater shoot biomass, 450% greater root length at depth, and 232% greater deep-injected 15N content in the shoot relative to 0% NRE. These results reveal the mechanism by which plants with fewer NRs increase N capture and shoot mass by reallocation of biomass to lateral roots, embryonic roots, and first whorl NRs that increases foraging efficiency in solid media.
Introduction
Roots are the major interface between plants and the soil, with key functions to extract nutrients and water that are required for plant productivity, and anchoring the plant (Smith and De Smet, 2012; Meister et al., 2014). Plants have evolved the ability to proliferate roots in response to heterogeneity of resources, belowground competition, and the inevitability of root loss (Lynch, 2018). Plants respond to resource availability by adjusting their relative shoot and root mass allocation to maximize their relative growth rate (Ågren and Franklin, 2003), and generally allocate relatively more biomass to roots if the limiting factor for growth is a soil resource (Poorter et al., 2012). The fraction of newly fixed carbon from photosynthesis allocated to roots can reach 56% in perennial grasses, and the proportion to roots significantly increases under edaphic stress (Lambers et al., 1996; Rachmilevitch et al., 2015). Root phenes are the general elemental units of phenotype (Lynch and Brown, 2012) influencing resource acquisition, while phene states represent the particular value a phene has taken (York et al., 2013). Studies have shown that root phene states that reduce the metabolic cost of soil exploration permit greater root growth, which improves the capture of deep nitrate (Chimungu et al., 2014; Saengwilai et al., 2014b), deep water (Gao and Lynch, 2016), and shallow phosphorus (Strock et al., 2018). ‘Cheap root’ phene states are those that reduce the metabolic burden of the root system with benefits for plant performance, including decreased root diameter, increased allocation to cheaper root classes such as lateral roots and root hairs, reduced root cortical cell area, and reduction in root secondary growth in dicots (Lynch, 2013; Meister et al., 2014; Galindo-Castaneda et al., 2018; Strock et al., 2018).
Maize (Zea mays L.) plays an important role in the global food supply and industrial production, including starch, sweeteners, oil, beverages, glue, industrial alcohol, and fuel ethanol (Ranum et al., 2014). Breeding for optimized maize root system architecture (RSA) is required to obtain maximum grain yield while reducing nutrient leaching and improving drought resistance in high-input agroecosystems by increasing soil resource acquisition efficiency (Mi et al., 2016; Lynch, 2018). The mature maize root system consists of the embryonic root (ER) and post-ER systems. The embryonic primary and seminal roots together with their laterals are important for maize seedling vigor during early development, but the post-embryonic shoot-borne nodal roots (NRs) and their laterals become the dominant root system by mass as the crop matures (Hochholdinger, 2009; Hochholdinger et al., 2018). NRs that are formed belowground are called crown roots, whereas those that are formed aboveground are designated brace roots (Hochholdinger, 2009; Saengwilai et al., 2014b). Though claims are often made that brace roots help prevent lodging (Gu et al., 2017), little evidence exists that directly tests this hypothesis, and some of the evidence shows no effect. Evolution of RSA towards more nitrogen (N)-efficient states corresponds to historical maize yield trends and increased tolerance of N stress in the USA (Hammer et al., 2009; York et al., 2015).
The ‘steep, deep, cheap’ (SCD) ideotype of the maize root system has been proposed for optimal N and water foraging, and consists of root architectural, anatomical, and physiological phene states to increase rooting depth and N and water use efficiency in specific environments (Lynch, 2013; York and Lynch, 2015). Nodal root number (NRN) is an aggregate phene consisting of the number of nodal whorls and the number of roots per whorl (Saengwilai et al., 2014a; York and Lynch, 2015), and flowering time played a key role in shaping NRN via indirect selection during maize domestication (Zhang et al., 2018). The functional–structural plant model SimRoot predicted that reduced NRN could enhance maize growth under low levels of N by increasing growth of lateral roots and N acquisition (York et al., 2013; York, 2014). Field and greenhouse studies later confirmed that maize genotypes with fewer NRs had greater rooting depth and ability to acquire N from deep soil of low N soils (Saengwilai et al., 2014b), and improved water acquisition from drying soil (Gao and Lynch, 2016). However, these studies have relied on intraspecific diversity to create root phene variation, which could be confounded by the multitude of differences that potentially exist among genotypes that were not necessarily measured or reported (Lynch, 2011). In this study, we used root excision to manipulate NRN of maize inbred line B73 grown in growth chamber hydroponics and in greenhouse tall mesocosms to explore the effect of reduced NRN on N acquisition. The objective of this study was using maize plants with the same genetic and phenotypic background to test the hypotheses that reducing NRN under two levels of N supply would (i) increase elongation of ERs and the remaining NRs and their laterals due to reallocation of carbon; (ii) increase shoot biomass in both N conditions due to reallocating carbon to the shoot whether in hydroponics or solid media; and (iii) increase N acquisition in low N environments.
Materials and methods
Growth chamber hydroponics study
Experimental design
A hydroponics nutrient solution experiment was conducted in two growth chambers, arranged as a randomized complete block design replicated five times with a 2×2 factorial arrangement of treatments. The factors were two levels of N supply (high- and low-N conditions), and two levels of NR removal.
Growth conditions
Black plastic pails (height 49.8 cm, top outside diameter 30.2 cm, and bottom outside diameter 25.9 cm, with a volume of 28 liters) with lids were placed in two Conviron E-15 growth chambers (Conviron, Winnipeg, Canada) with a day:night cycle of 14 h:10 h, 28 °C:22 °C, at a flux density at canopy level of ~400 µmol m−2 s−1. Both chambers have the same internal dimensions of 185 cm wide×145 cm high×79 cm deep. Seeds of maize inbred line B73 for the two studies were obtained from Shawn Kaeppler, University of Wisconsin, Madison, WI, USA. Seeds were surface-sterilized in 0.05% NaOCl for 15 min and rinsed three times using deionized (DI) water, then pre-germinated in medium size (0.3–0.5 mm) premium sand placed in darkness at 28 °C for 3 d, and transferred to the growth chamber for 2 d. After that, uniformly germinated seedlings were washed out of the sand, wrapped around the junction between mesocotyl and coleoptiles with L800-D Identi-Plugs foam (Jaece Industries, NY, USA), plugged in 5 cm Gro Pro net pots, and transplanted to a hole with a diameter of 5 cm drilled into the lid with a hole saw. Each pail received one seedling, and the roots were directly submerged in nutrient solution. The nutrient solution for the high N treatment level was composed of (in µM) 190 KH2PO4, 2260 KNO3, 750 CaCl2, 380 MgSO4, 17.29 H3BO3, 2.63 ZnSO4·7H2O, 3.38 MnCl2 ·4H2O, 0.12 CuSO4·5H2O, 0.04 (NH4)6Mo7O24·4H2O, and 300 Fe(III)-EDTA (C10H12N2NaFeO8). For the low N treatment level, KNO3 was reduced to 280 µM, and the difference between potassium supplies was balanced with KCl. The nutrient solution was continuously aerated with an air pump attached to air stones, and solution pH was maintained between 5.9 and 6.1 by additions of KOH or HCl throughout the experiment.
The NRN manipulation treatment levels included: excising two-thirds of all emerged NRs (67% NRE) and no NR excision control (0% NRE). NR removal was started 2 d after transplanting; plants were observed every other day due to the continuous emergence of NRs, and roots were excised as needed to maintain the 67% target. Foam was gently peeled away from the base of the stem to count NR emergence for both root excision and no root excision control treatments, and NRs were then excised close to their corresponding node using a scalpel for root excision treatment only. The targeted 67% NRE was relative to the NRNs of plants without excision.
Sample collection and measurements
One day before harvest, leaf gas exchange of the second youngest fully expanded leaf was measured in the growth chamber with a LI-6800 Portable Photosynthesis System equipped with the Multiphase Flash Fluorometer (LI-COR Inc., Lincoln, NE, USA). Leaf chamber conditions were as follows: fan speed of 10 000 rpm, flow rate of 600 µmol mol−1, overpressure of 0.2 kPa, vapor pressure deficit at the leaf of 1.2 kPa, leaf temperature of 25 °C, saturating irradiance of 1200 µmol m−2 s−1, and reference CO2 at 400 µmol mol−1. CO2 exchange was logged manually using stability criteria of both ΔH2O and ΔCO2 standard deviation limit of 0.1 over a period of 15 s.
Plants were harvested 24 d after transplanting when high-N plants were at the nine-leaf stage and low N plants were at the seven-leaf stage. Shoots were dried at 60 °C for 3 d prior to dry weight determination. After a plant was removed from the nutrient solution, roots were immediately separated into the ER system (including primary, seminal roots, and their laterals) and different whorls of NRs, and each class was further divided into 30 cm sections along the axial roots of that class for an indication of rooting at various depths. In this manuscript, axial root refers to any developmentally first-order root, whether primary, seminal, or nodal, and does not include the respective lateral roots. The number of axial roots per root class was counted for calculation of individual axial root length. All roots for a class and depth sample were blotted using tissue paper to remove excess water, then placed in a 19 ml custom chamber connected to the LI-8100 Automated Soil CO2 Flux System (LI-COR Inc.). The observation duration was 90 s, and the dead band was set at 20 s. All the respiration measurements were performed in an air-conditioned room with temperature maintained at 24 °C. After root respiration measurements, roots from each section were scanned as described below. Following scanning, the roots were dried at 60 °C for 3 d and weighed.
Greenhouse mesocosm study
Experimental design
The mesocosm experiment was conducted in a greenhouse of the Noble Research Institute from 30 July to 10 September 2017, and arranged as a randomized complete block design replicated four times with a 2×3 factorial arrangement of treatments. The factors were two levels of N supply (high- and low-N conditions) and three levels of NRE.
Growth conditions
The mesocosm consisted of a polyvinyl chloride (PVC) pipe (Charlotte, NC, USA) with a diameter of 15.24 cm and height of 152.4 cm, and a flat-bottom PVC cap (IPS Corporation, TN, USA). Each mesocosm was lined with seamless 6 mm heavy duty poly tubing (Uline, WI, USA) to facilitate root sampling at harvest. The growth medium consisted of a mixture (volume based) of 50% medium size (0.3–0.5 mm) premium sand (Quikrete, GA, USA), 40% premium grade vermiculite (Sungro, MA, USA), and 10% perlite (Ambient Minerals, AR, USA). Each mesocosm was filled with 28 liters of the mixture to ensure the same bulk density of the medium. The maize inbred line B73 seeds were obtained from the same source as in the hydroponics experiment. Seeds were surface-sterilized in 0.05% NaOCl for 15 min and rinsed three times using DI water, and were then pre-germinated in rolled germination paper (Anchor Paper, MN, USA) soaked in 0.5 mM CaSO4 and placed in darkness at 28 °C in a germination chamber for 2.5 d. Two days before planting, each mesocosm received 6 liters of nutrient solution. Each mesocosm received one seedling with the seed at a depth of 5 cm. Each plant was watered with 200 ml of nutrient solution every other day. The nutrient solution for the high N treatment was composed of (in µM) 500 KH2PO4, 6000 KNO3, 2000 CaCl2, 1000 MgSO4, 46 H3BO3, 7 ZnSO4·7H2O, 9 MnCl2 ·4H2O, 0.32 CuSO4·5H2O, 0.11 (NH4)6Mo7O24·4H2O, and 77 Fe(III)-EDTA (C10H12N2NaFeO8). For the low N treatment, KNO3 was reduced to 600 µM, and the difference between potassium supply was balanced with KCl. KOH was used to adjust nutrient solutions to pH 6.0. Three NRN manipulation treatments consisted of removing approximately one-third of all emerged NRs (33%), removing two-thirds of all emerged NRs (67%), and no root excision control (0%). NRE was started 6 d after transplanting and continued every other day till the end of the experiment due to the continuous emergence of NRs.
The growth medium permitted root excision without damaging the remaining roots. The approach of NRE was to brush solid medium away from the base of the stem where NRs emerged, use a scalpel to excise the necessary roots near their base, cover the stem again with the medium, and give 200 ml of nutrient solution to each plant in order to allow the medium to settle. The targeted NRE levels of 33% or 67% were based on the calculations made from NRNs of the corresponding plants with excision. The no root excision control (0% NRE) received the same procedure for counting NRN over time, but none was excised.
Sample collection and measurements
The ability of roots to acquire N from deep soil layers was quantified using deep injection of 15NO3− (98% atom) 1 d before harvest. One hole was drilled at 143 cm depth in each mesocosm, and 5 ml of Ca15NO3 solution (0.46 mg 15N ml−1) was injected into the center of each mesocosm using a syringe. The plants were harvested at 42 d after planting. At harvest, the shoot was cut at the stem base and dried at 60 °C for 3 d for biomass determination. The shoot samples were ground using a Labman Automation Robot (Labman Automation, Stokesley, North Yorkshire, UK). The percentages of total N and 15N in shoot tissue were analyzed using a PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20-20 isotope ratio mass spectrometer (Sercon Ltd, Cheshire, UK) at the Stable Isotope Facility of the University of California at Davis (http://stableisotopefacility.ucdavis.edu/).
At harvest, a polyethylene liner in each mesocosm was carefully pulled out and the column of medium and roots placed on a root washing station. Starting from the bottom, the liner was cut open and medium was carefully washed away from roots using a low pressure water hose. After washing, roots were separated into ERs (including primary, seminal roots, and their laterals) and different whorls of NR classes, and the roots of each class were divided into 30 cm sections along the axial roots for quantifying root distribution over depths for each class individually.
Root scanning and image analysis
For both the hydroponics and mesocosm experiments, roots from each class and depth sample were spread in a 5 mm layer of water in transparent plexiglass trays and imaged with a flatbed scanner equipped with a transparency unit (Epson Expression 12000XL, Epson America) at a resolution of 600 dpi. Images were analyzed using WinRhizo software (WinRhizo Pro, Regent Instruments, Quebec, Canada) individually so that diameter thresholds could be adjusted for each image to distinguish axial roots from lateral roots, which would not be possible using batch mode. Root mass fraction was calculated as root dry weight proportion of total plant dry weight. Specific root length was calculated by dividing root length by the corresponding dry weight. The oven-dried root mass and root length quantified using WinRhizo were used to calculate the specific root respiration (SRR) by root dry mass (SRR by root mass; nmol g−1 s−1) and the specific root respiration by root length (SRR by root length; nmol cm−1 s−1), respectively. Individual axial root length was calculated by dividing the total axial root length of each root class by the corresponding number of axial roots.
Statistical analysis
Statistical analyses were conducted using R version 3.5.1 (R Core Team, 2018) through RStudio version 1.1.45 (RStudio, 2016). Prior to data analysis, all the data were checked for normality with Shapiro–Wilk test and log-transformed when necessary. Regardless of data transformation, results presented in tables and figures report non-transformed values. ANOVA was performed across N levels and NRE levels for comparisons between N levels, NRE levels, and their interaction based on the phenes measured at the whole-plant level (not separated by root class or depth). N levels, NRE levels, and their interactions were analyzed as fixed effects in a mixed effects model with block as the random effect. Root distribution data across root classes and rooting depths were analyzed with one-way ANOVA under each N level separately, and with pairwise comparisons of NRE levels under either the same root classes or the same rooting depths, because the focus was on NRE levels and otherwise the models were difficult to interpret and report. Tukey’s test at α=0.05 was used for multiple comparisons among different treatments. The R packages ‘emmeans’ (Lenth, 2018), ‘lme4’ (Bates et al., 2014), and ‘multcompView’ (Graves et al., 2012) were used for statistical analyses, and the R package ‘ggplot2’ (Wickham, 2016) for data visualization.
Results
Growth chamber hydroponics experiment
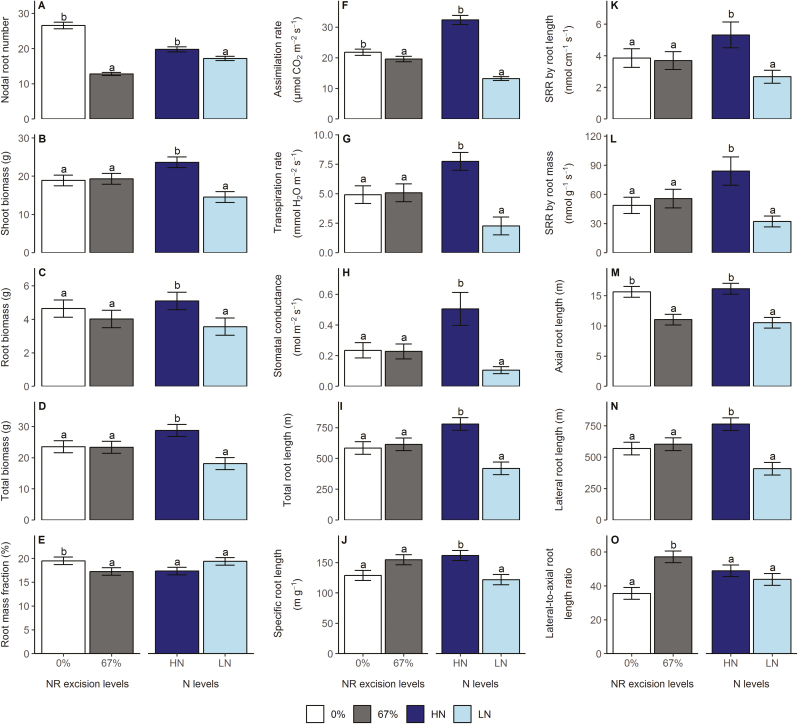
For whole-plant phenes in the hydroponics experiment, the main effects of N treatment and NRE treatment were all significant for NRN, assimilation rate, specific root length, and axial root length. Shoot biomass, root biomass, total biomass, transpiration rate, stomatal conductance, total root length, SRR by root length, SRR by root mass, and lateral root length were only affected by N treatment, and root mass fraction and lateral to axial root length ratio were only affected by NRE treatment (Supplementary Table S1 at JXB online). Low N supply substantially reduced NRN by 13% (Fig. 1A), shoot biomass by 38% (Fig. 1B), root biomass by 30% (Fig. 1C), total biomass by 37% (Fig. 1D), assimilation rate by 59% (Fig. 1F), transpiration rate by 71% (Fig. 1G), stomatal conductance by 79% (Fig. 1H), total root length by 46% (Fig. 1I), specific root length by 25% (Fig. 1J), SRR by root length by 50% (Fig. 1K), SRR by root mass by 62% (Fig. 1L), axial root length by 35% (Fig. 1M), and lateral root length by 46% (Fig. 1N) at 24 days after planting (DAP). An NRE of 67% led to a 52% reduction in NRN (Fig. 1A), 12% reduction in root mass fraction (Fig. 1E), 10% reduction in assimilation rate (Fig. 1F), 29% reduction in axial root length (Fig. 1M), and 61% increase in lateral to axial root length ratio (Fig. 1O). NRE had no effect on NR emergence time (data not shown). No significant interactions between N treatment and NRE treatment were observed for the phenes measured (Supplementary Table S1).
Fig. 1.
Main effects of N treatment and NRE treatment on whole-plant shoot and root phenes in the growth chamber hydroponics study. Each column represents the mean of n=5 for each treatment. Error bars represent SEs, and columns with the same letter under the same main effect were not significantly different at P≤0.05 according to Tukey’s test. SRR specific root respiration.
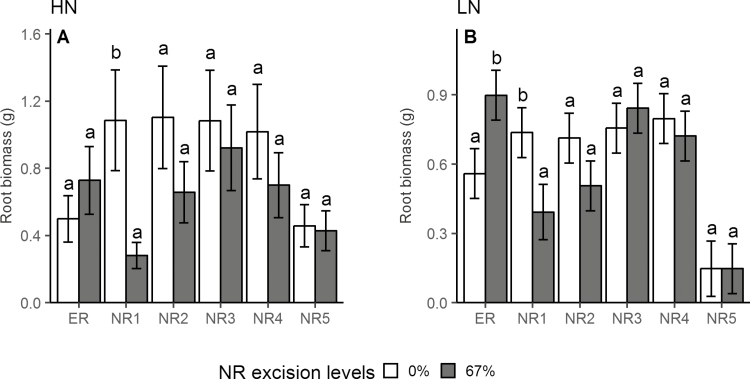
Specific root classes are referred to as ERs and NRs, which are further grouped by whorl with appended numbers referring to order of emergence; therefore NR1 is the first whorl of NR that emerges. Under high-N conditions, 67% NRE reduced root biomass by 74% (Fig. 2A), root respiration by 74% (Supplementary Fig. S1A), root length by 68% (Supplementary Fig. S1B), and axial root length by 72% (Supplementary Fig. S1F) in NR1; and led to a 128% increase (from 1.22 m g−1 to 2.79 m g−1) of specific root length (Supplementary Fig. S1C) and 55% reduction of SRR by root length in NR5 (Supplementary Fig. S1D). A trend of greater root biomass in ER induced by 67% NRE was observed under high-N conditions. Under low-N conditions, 67% NRE increased root biomass by 61% (Fig. 2B) and root respiration by 100% (Supplementary Fig. S1I) in ERs, reduced root biomass by 47% (Fig. 2B) and axial root length by 69% (Supplementary Fig. S1N) in NR1, led to 67% reduction of axial root length (Supplementary Fig. S1N) and 156% increase of lateral to axial root length ratio in NR2 (Supplementary Fig. S1P), increased specific root length by 52% Supplementary Fig. S1K) and lateral to axial root length ratio by 169% (Supplementary Fig. S1P) in NR3, and increased specific root length by 79% (Supplementary Fig. S1K), lateral root length by 222% (Supplementary Fig. S1O), and lateral to axial root length ratio by 267% (Supplementary Fig. S1P) in NR4. An NRE of 67% had no impact on SRR by root mass across all root classes under both N levels (Supplementary Fig. S1E, M).
Fig. 2.
Effect of NRE treatment on root biomass of different root classes under high-N (A) and low-N (B) conditions in growth chamber hydroponics study. Each column represents the mean of n=5 for each treatment. Error bars represent SEs, and columns with the same letter under the same root class were not significantly different at P≤0.05 according to Tukey’s test. ER, embryonic root; NR1, first whorl nodal root; NR2, second whorl nodal root; NR3, third whorl nodal root, NR4, fourth whorl nodal root; NR5, fifth whorl nodal root.
Under high-N conditions, 67% NRE led to a 55% reduction of root biomass (Supplementary Fig. S2A) and a 47% increase of SRR by mass at the depth of 90–120 cm (Supplementary Fig. S2F). Under low-N conditions, 67% NRE reduced root respiration by 40% in 60–90 cm and by 43% in 90–120 cm (Supplementary Fig. S2K), reduced SRR by length by 43% in 60–90 cm, reduced axial root length by 33% in 0–30 cm, by 51% in 30–60 cm, by 62% in 60–90 cm, and by 53% in 90–120 cm (Supplementary Fig. S2P), and led to a 48% increase of specific root length and a 196% increase of lateral to axial root length ratio in 60–90 cm (Supplementary Fig. S2R). NRE had no effect on root length and lateral root length across all depths under both N levels (Supplementary Fig. S2C, L, H, Q). Regardless of NRE levels, SRR by mass increased at the greatest depth under both N levels (Supplementary Fig. S3).
Greenhouse mesocosms experiment
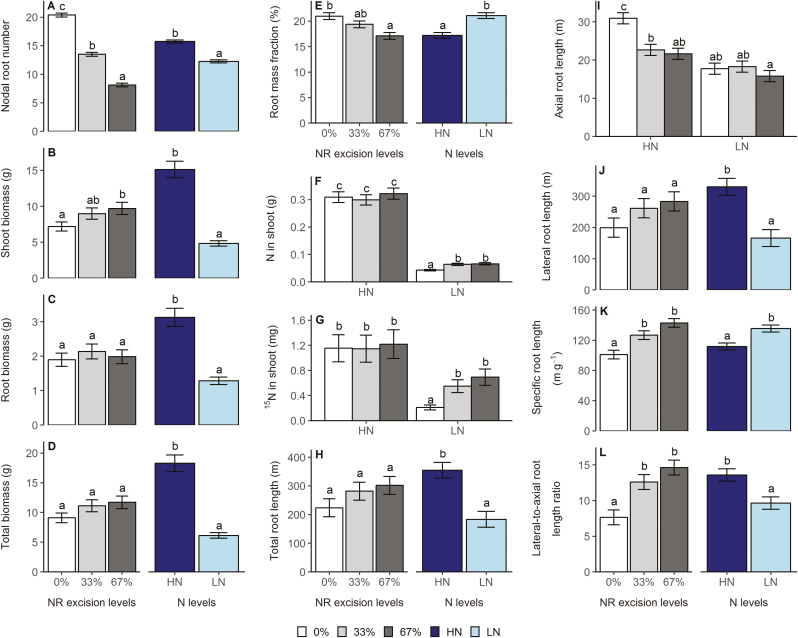
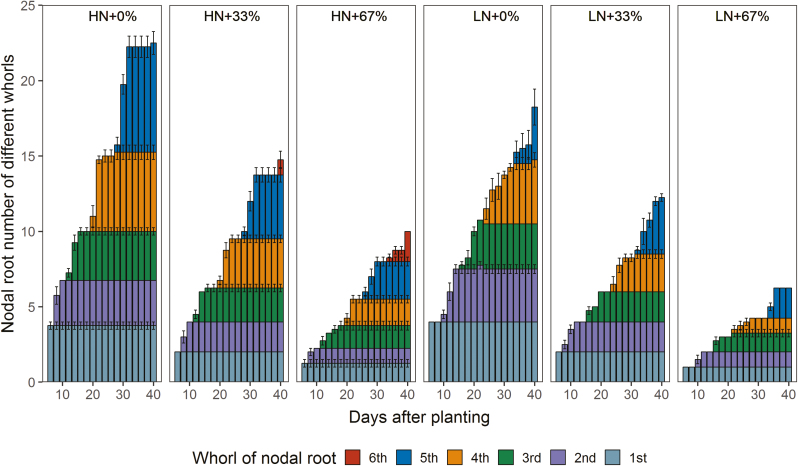
For whole-plant shoot and root phenes in the mesocosms experiment, the main effects of N treatment and NRE treatment were all significant for NRN, shoot biomass, root mass fraction, specific root length, and lateral to axial root length ratio. Root biomass, total biomass, shoot N concentration, shoot 15N concentration, total root length, and lateral root length were only affected by N treatment (Supplementary Table S1). Low N supply reduced NRN by 22% (Fig. 3A), shoot biomass by 68% (Fig. 3B), root biomass by 59% (Fig. 3C), total biomass by 66% (Fig. 3D), shoot N concentration by 43% (Supplementary Fig. S4A), total root length by 48% (Fig. 3H), lateral root length by 50% (Fig. 3J), and lateral to axial root length by 29% (Fig. 3L), and increased root mass fraction by 23% (Fig. 3E), shoot 15N concentration by 101% (Fig. S4B), and specific root length by 21% (Fig. 3K) at 42 DAP. The 33% NRE level decreased NRN by 34% (Fig. 3A), increased specific root length by 25% (Fig. 3K) and lateral to axial root length ratio by 65% (Fig. 3L), and had no impact on shoot biomass (Fig. 3B) and root mass fraction (Fig. 3E) compared with 0% NRE across all N levels. Increasing the NRE level to 67% decreased NRN by 60% (Fig. 3A) and root mass fraction by 19% (Fig. 3E), and increased shoot biomass by 35% (Fig. 3B), specific root length by 41% (Fig. 3K), and lateral to axial root length ratio by 91% (Fig. 3L), as compared with 0% NRE across all N levels. The 67% NRE led to the fifth whorl of NRs emerging 2 d earlier and the sixth whorl of NRs emerging 8 d earlier compared with 0% NRE under a high N level, respectively (Fig. 4). The interactions of N treatment and NRE treatment had significant effects on 15N content in shoot, N content in shoot, and axial root length (Supplementary Table S1). The 33% and 67% NRE levels significantly increased N content in shoot by 48% and 53%, and 15N content in shoot by 162% and 232% under low N, respectively, but this was not observed under high N (Fig. 3F, G). Also, both excision levels significantly decreased axial root length under high N, but this was not observed under low N (Fig. 3I).
Fig. 3.
Main effects of N treatment and NRE treatment (A–E, H, J–L) and their interaction effects (F, G, and I) on whole-plant shoot and root phenes in the greenhouse mesocosms study. Each column represents the mean of n=4 for each treatment. Error bars represent SEs, and columns with the same letter under the same main effect were not significantly different at P≤0.05 according to Tukey’s test.
Fig. 4.
NRN of different whorls with time in the greenhouse mesocosms. Each column with the same color represents the mean of n=4 for each treatment. Error bars represent SEs. HN+0%, 0% NRE under high N; HN+33%, 33% NRE under high N; HN+67%, 67% NRE under high N; LN+0%, 0% NRE under low N; LN+33%, 33% NRE under low N; LN+67%, 67% NRE under low N.
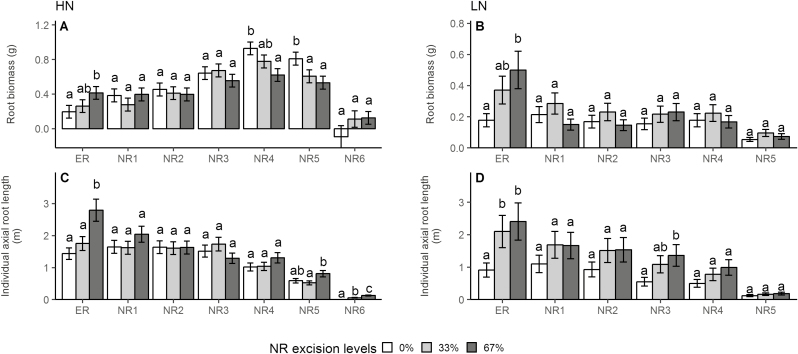
Under high-N conditions, 67% NRE increased root biomass by 112% and individual axial root length by 94% in ERs, decreased root biomass by 33% in NR4 and by 34% in NR5 (Fig. 5A), led to a 109% increase of axial root length in ERs, decreased axial root length by 60% in NR1, 67% in NR2, 63% in NR3, 58% in NR4, and 53% in NR5 (Supplementary Fig. S5B), and increased the lateral to axial root length ratio by 245% in NR2 and 260% in NR5 (Supplementary Fig. S5E). Under low-N conditions, 67% NRE increased root biomass by 182% in ERs (Fig. 5B), increased individual axial root length by 164% in ERs and by 148% in NR3 (Fig. 5D), and increased the lateral to axial root length ratio by 279% in NR4 (Supplementary Fig. S5J). Removal of NRs had no impact on root length and lateral root length across all root classes except NR6 under both N levels (Supplementary Fig. S5A, F, C, H).
Fig. 5.
Effect of NRE treatment on root biomass (A, B) and individual axial root length (C, D) of different root classes under high-N and low-N conditions in the greenhouse mesocosms. Each column represents the mean of n=4 for each treatment. Error bars represent SEs, and columns with the same letter under the same root class were not significantly different at P≤0.05 according to Tukey’s test. ER, embryonic root; NR1, first whorl nodal root; NR2, second whorl nodal root; NR3, third whorl nodal root, NR4, fourth whorl nodal root; NR5, fifth whorl nodal root; NR6, sixth whorl nodal root
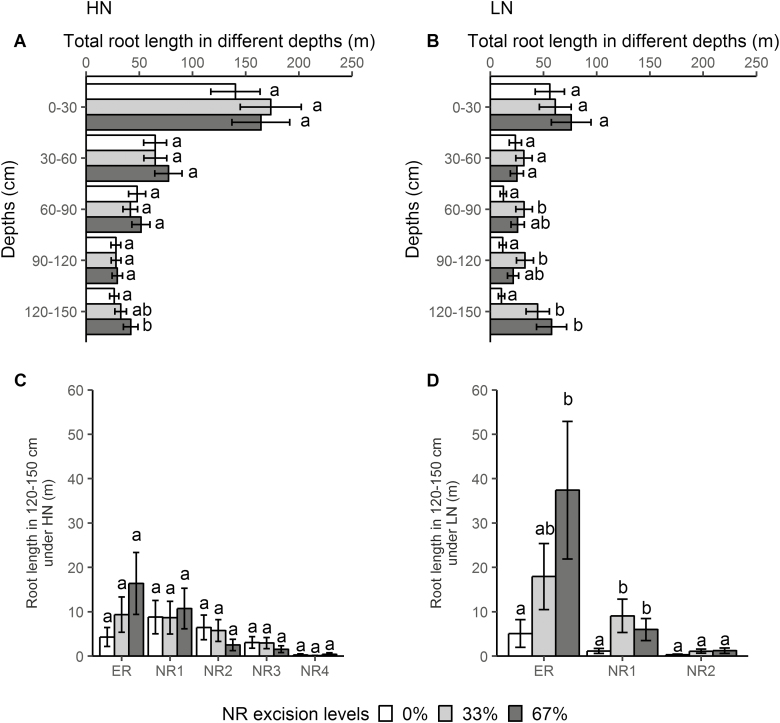
Under high-N conditions, 67% NRE increased root length by 59% (Fig. 6A) and lateral root length by 71% (Supplementary Fig. S6C) in 120–150 cm, decreased axial root length by 48% in 0–30 cm, by 42% in 30–60 cm, by 31% in 60–90 cm, and by 36% in 90–120 cm (Supplementary Fig. S6B), increased specific root length by 48% in 0–30 cm, by 31% in 30–60 cm, and by 33% in 120–150 cm (Supplementary Fig. S6D), and increased lateral to axial root length by 133% in 0–30 cm, by 123% in 30–60 cm, and by 82% in 90–120 cm (Supplementary Fig. S6E). Under low-N conditions, 67% NRE increased root length by 450% (Fig. 6B), lateral root length by 548% (Supplementary Fig. S6H), and root biomass by 228% (Fig. S6F) in 120–150 cm, led to a decrease of axial root length by 52% in 0–30 cm, by 46% in 30–60 cm, and 130% increase in 120–150 cm (Supplementary Fig. S6G), increased specific root length by 53% in 0–30 cm and by 67% in 120–150 cm (Supplementary Fig. S6I), and increased lateral to axial root length by 205% in 0–30 cm, by 114% in 30–60 cm, by 143% in 60–90 cm, and by 178% in 120–150 cm (Supplementary Fig. S6J).
Fig. 6.
Effect of NRE treatment on root length distribution across rooting depths under high-N (A) and low-N (B) conditions, and root length of different root classes at 120–150 cm (C, D) in the greenhouse mesocosms. Each column represents the mean of n=4 for each treatment. Error bars represent SEs, and columns with the same letter under the same depth or root class were not significantly different at P≤0.05 according to Tukey’s test.
ERs and the first four whorls of NRs under high-N conditions, and ERs and the first two whorls of NRs under low-N conditions reached the depth of 120–150 cm at 42 DAP. At a depth of 120–150 cm, 67% NRE increased root length by 630% in ERs and by 690% in NR1 under low-N conditions (Fig. 6D), and a trend of greater root length in ERs induced by 67% NRE was also observed under high-N conditions (Fig. 6C).
Discussion
Reducing the construction and maintenance metabolic costs of roots has been proposed as a strategy for crop breeding to increase N and water use efficiency (Lynch, 2013, 2018). In this study, RSA was manipulated in order to reduce the number of NRs to test hypotheses that this would influence RSA, increase shoot biomass in both high-N and low-N conditions whether in hydroponics or solid media, and increase N uptake in low-N conditions. In hydroponics and mesocosms, 67% NRE led to increased ER mass accumulation and greater overall lateral to axial root ratio, indicating an inherent reallocation of root system carbon to other root sinks. However, contrary to our hypothesis, 67% NRE had no effect on shoot biomass in either N level in hydroponics, but increased shoot biomass by 35% across all N levels in soil mesocosms. A similar result was observed when assessing phosphorus efficiency of two wheat cultivars that differed greatly when grown in hydroponics compared with soil (Hayes et al., 2004). This inconsistency of plant performance is possibly due to the differences in either the physical or chemical characteristics of the growing environment, or root morphology differences induced by these characteristics (Shavrukov et al., 2012). The effect of lateral root branching is especially important when mobility of a nutrient is more limited, and mobility in solid media, whether soil or sand based, is much less than in hydroponics. Remarkably, under low N in mesocosms, 67% NRE allowed the proliferation of 450% more root length at the deepest depth, increased deep-injected 15N content in the shoot by 232%, and increased shoot biomass by 52%, but no effect was observed on total root system biomass. These results indicate that maize has a remarkable ability to maintain the total root system sink strength for carbon that leads to inherent reallocation of mass and length to ERs, first whorl NRs, and lateral roots of all classes. NRE had no effect on shoot biomass in hydroponics, possibly showing that the root system was a stronger sink than the shoot for the excess available carbon. The increased shoot biomass, N content, and 15N content under low N in mesocosms indicates that the inherent modifications to RSA including greater root length of ERs and all class of lateral roots due to NRE leads to greater N uptake in solid media with positive effects on plant productivity. For the first time to our knowledge, a manipulative empirical study has confirmed that reduced NRN enhances N acquisition in maize under N-replete conditions (York et al., 2013; Saengwilai et al., 2014b) and has provided a mechanism through increased allocation to more efficient root classes.
Previous simulations using SimRoot indicated that reduced NRN was a promising target for maize breeding (York et al., 2013; York, 2014), and these simulations were partially confirmed in greenhouse and field studies relying on genotypic variation within and among recombinant inbred line (RIL) populations (Saengwilai et al., 2014b). Many NR-related quantitative trait loci (QTLs) in maize have been reported for dry weight and length of NRs (Burton et al., 2014), and approximately half of the total genetic variation in NRN was derived from QTLs for flowering time (Zhang et al., 2018). Though flowering time is related to NRN variation, the variation in NRN that is not due to flowering time will be more important for breeding. However, the NR-associated QTLs on chromosomes have variation among maize populations, and no genes underlying the QTLs have been identified (Bray and Topp, 2018). Due to the fact that maize RSA is controlled by many genes with generally small effects (Voss-Fels et al., 2018), genes such as rt1 (Jenkins, 1930), rtcs (Hetz et al., 1996), and rtcl (Taramino et al., 2007) in mutants of maize not only regulate NR formation, but also affect the development of other root classes and plant vigor, which means that controlling NRN genetically for physiological experiments is difficult.
Using root excision to manipulate NRN of maize may produce severe changes in metabolic processes, hormone homeostasis, and gas fluxes (Bloom and Caldwell, 1988), possibly like the reduced assimilation rate observed in the hydroponics study. Early changes in hormone levels in the rooting zone induced by root excision (Druege et al., 2016) and possible hormonal regulation of specific transporters to facilitate nutrient acquisition (Ghanem et al., 2011) were hypothesized to partially lead to increased shoot production in all experiments. However, no effect on shoot mass was observed in hydroponics due to NRE; therefore, the fundamental relationship of reduced nodal rooting to shoot mass is probably not due to hormonal differences or increased carbon availability. In fact, NRE eliminates the confounding effects of many phenotypic differences that exist among genotypes, and can increase understanding of adventitious root formation and function (such as NRs), as well as allocation of carbon to the most efficient root classes (Poorter et al., 2012; Steffens and Rasmussen, 2016). NRN in maize has been investigated using simulations (York, 2014), field phenotyping of populations (Trachsel et al., 2011), physiological studies of contrasting lines for NRN (Saengwilai et al., 2014b), and now a manipulative physiological study, which makes this phene aggregate one of the most complete examples of using the functional phenomics pipeline as outlined in York (2019). Therefore, NRN manipulation in large mesocosms using NRE is a valuable tool for better understanding the complex relationships among roots, shoots, and soil resource uptake.
Carbon partitioning is governed by the relative source and sink strengths of all organs (Poorter et al., 2012), and there are trade-offs in carbon allocation among leaf, stem, and root functions (McCarthy and Enquist, 2007). In general, plants should allocate carbon to various organs such that the marginal potential gain of carbon is equal to the marginal carbon allocation (Bloom et al., 1985). Early sixth whorl NR formation was observed at 67% NRE under high N in mesocosms, implying that NR initiation is partially determined by carbon availability. The 67% NRE level significantly decreased root mass faction by 12% in hydroponics (Fig. 1E) and by 19% in mesocosms (Fig. 3E), which suggests that these architectural changes led to more efficient N uptake throughout the experiment, resulting in greater shoot growth in low N. In turn, greater shoot mass may allow greater carbon fixation and subsequent allocation to both roots and shoots, causing positive feedback for plant growth, as predicted by York (2014). In general, no differences in overall root respiration and root system mass were observed due to NRE, indicating a remarkable capacity of maize to maintain total root system sink strength and metabolic burden. For plants supplied with low N in mesocosms, the removal of 67% of NRs increased shoot biomass by 52% as compared with no excision control (Supplementary Table S2), and led to significant increases of root length in the deepest soil layer and an increase of lateral to axial root length ratio across all the rooting depths except 90–120 cm, which could enhance greater soil exploration of heterogeneous supplies of nitrate in soil (Hodge, 2004), and may explain the enhanced plant growth by reducing NRN of plants in mesocosms with low N.
Specific root length is a good indicator of the relative ability to explore soil by roots, largely driven by thinner roots, and is strongly associated with the resource absorption function (Freschet et al., 2015). Removal of 67% of NRs significantly increased specific root length of NR5 under high N and NR3 and NR4 under low N in hydroponics and overall specific root length regardless of N levels in mesocosms, and facilitated a greater lateral to axial root length ratio of NR2, NR3, and NR4 in hydroponics and of NR4 in mesocosms under low N, showing compensatory growth of lateral roots following root excision (Rubio and Lynch, 2007), which indicates enhanced capacity for resource acquisition (Lynch, 2013). Interestingly, this reallocation of NR carbon to lateral roots mimics root plasticity of maize under low N which may reduce NRN while increasing lateral root length (Gaudin et al., 2011). Maize lines with greater lateral root length have been shown to be more efficient at N capture (Zhan and Lynch, 2015). The thinner roots and greater lateral root length may favor root decomposition and build up of soil carbon (Zhang and Wang, 2015), indicating possible ecosystem services beyond maintaining yield at lower N input levels.
SRR by length and SRR by mass of the whole root system under high N were 2.0 and 2.6 times those for roots under low N, which supports that root respiration is positively correlated with plant N concentration (Reich, 2014), and may be related to the changes in root morphological and biochemical phenes (Saglio and Pradet, 1980; Roumet et al., 2016). The reduction in SSR under low N could suggest that it is adaptive in infertile conditions as a ‘cheap root’ phene state. Surprisingly, the deepest roots had greater SRR (mass based) regardless of N levels, possibly due to more axial or lateral root tips per unit of root mass in the deepest layer. Young roots or growing root tips are important for resource acquisition at a high cost of energy consumption, and respiration declines with the aging of the roots (Wells and Eissenstat, 2003). This study indicates that reduced SRR could be an adaptive response to infertile soil conditions, which could possibly be affected by a multitude of root anatomical phenes that were not measured here (Burton et al., 2013). Overall, the results indicate that compensatory growth by ERs, first whole NRs, and lateral roots of all root classes allowed the plant to maintain the total metabolic burden of the root system while increasing soil exploitation under low N, but that further improvements could possibly be made by targeting reduced SRR while increasing allocation to root classes with greater specific root length (cheap root classes).
In conclusion, these results support the hypotheses that decreasing NRN increases the elongation of the remaining primary, seminal roots, NRs, and their laterals, and increases N acquisition under low-N conditions in physical substrate. While compensatory growth of laterals was observed in the hydroponics study after NRE, this did not lead to greater N uptake and shoot biomass, which indicates that excision of NRs did not directly affect shoot biomass production as might be expected due to hormonal changes or direct reallocation of carbon to the shoot. Instead, total root system biomass was essentially maintained and the mass was reallocated to more lateral and deep roots Therefore, our results indicate that by reallocating root mass to less expensive ERs, early emerging NRs, and lateral roots throughout the soil profile, but especially at depth, plants with fewer NRs were able to exploit soil resources more effectively, which led to greater N uptake and shoot growth. This research also highlights the importance of the ER system due to its persistence over the course of the experiment and having the greatest rooting at depth relative to other axial classes in maize near flowering. Therefore, the reduced NRN ideotype is confirmed as a potential target for breeding programs, and a mechanism through greater soil resource uptake by laterals and early-emerging axial roots was revealed.
Data availability
All the data and statistical analysis scripts for R are available in an online public repository (https://doi.org/10.5281/zenodo.2651391).
Supplementary data
Supplementary data are available at JXB online.
Table S1. Summary of ANOVA (F-values and significance levels) of whole-plant shoot and root phenes as influenced by N treatment, NR excision treatment, and their interactions for the two experiments.
Table S2. Mean ±SE of shoot biomass as influenced by NR excision treatment under low N conditions in the greenhouse mesocosms experiment.
Fig. S1. Effect of NR excision treatment on root phenes of different root classes under high and low N levels in the growth chamber hydroponics study.
Fig. S2. Effect of NR excision treatment on root phenes of different rooting depths under high and low N levels in the growth chamber hydroponics study.
Fig. S3. Effects of rooting depths on SRR by root mass under high and low N conditions in the growth chamber hydroponics study.
Fig. S4. Main effects of N treatment and NR excision treatment on shoot N concentration and shoot 15N concentration for the greenhouse mesocosms.
Fig. S5. Effect of NR excision treatment on root phenes of different root classes under high and low N levels in the greenhouse mesocosms study.
Fig. S6. Effect of NR excision treatment on root phenes of different rooting depths under high and low N in the greenhouse mesocosms study.
Acknowledgements
This research was supported by the Noble Research Institute, LLC and the Samuel Roberts Noble Foundation (Cheaper Roots project). We thank Bryce Walker, Anand Seethepalli, and Na Ding for assistance with sampling and root scanning; and David Huhman and Bonnie Watson for their technical assistance in analyzing nitrogen content of samples.
Glossary
Abbreviations
- ER
embryonic root
- NR
nodal root
- NRE
nodal root excision
- NRN
nodal root number
- SRR
specific root respiration
References
- Agren GI, Franklin O. 2003. Root:shoot ratios, optimization and nitrogen productivity. Annals of Botany 92, 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. 2014. lme4: linear mixed-effects models using Eigen and S4. R Package Version 1.1-23. https://cran.r-project.org/web/packages/lme4/index.html [Google Scholar]
- Bloom AJ, Caldwell RM. 1988. Root excision decreases nutrient absorption and gas fluxes. Plant Physiology 87, 794–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ, Chapin FS III, Mooney HA. 1985. Resource limitation in plants—an economic analogy. Annual Review of Ecology and Systematics 16, 363–392. [Google Scholar]
- Bray AL, Topp CN. 2018. The quantitative genetic control of root architecture in maize. Plant & Cell Physiology 59, 1919–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton AL, Brown KM, Lynch JP. 2013. Phenotypic diversity of root anatomical and architectural traits in Zea species. Crop Science 53, 1042–1055. [Google Scholar]
- Burton AL, Johnson JM, Foerster JM, Hirsch CN, Buell CR, Hanlon MT, Kaeppler SM, Brown KM, Lynch JP. 2014. QTL mapping and phenotypic variation for root architectural traits in maize (Zea mays L.). Theoretical and Applied Genetics 127, 2293–2311. [DOI] [PubMed] [Google Scholar]
- Chimungu JG, Brown KM, Lynch JP. 2014. Reduced root cortical cell file number improves drought tolerance in maize. Plant Physiology 166, 1943–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druege U, Franken P, Hajirezaei MR. 2016. Plant hormone homeostasis, signaling, and function during adventitious root formation in cuttings. Frontiers in Plant Science 7, 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freschet GT, Swart EM, Cornelissen JH. 2015. Integrated plant phenotypic responses to contrasting above- and below-ground resources: key roles of specific leaf area and root mass fraction. New Phytologist 206, 1247–1260. [DOI] [PubMed] [Google Scholar]
- Galindo-Castañeda T, Brown KM, Lynch JP. 2018. Reduced root cortical burden improves growth and grain yield under low phosphorus availability in maize. Plant, Cell & Environment 41, 1579–1592. [DOI] [PubMed] [Google Scholar]
- Gao Y, Lynch JP. 2016. Reduced crown root number improves water acquisition under water deficit stress in maize (Zea mays L.). Journal of Experimental Botany 67, 4545–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudin AC, McClymont SA, Holmes BM, Lyons E, Raizada MN. 2011. Novel temporal, fine-scale and growth variation phenotypes in roots of adult-stage maize (Zea mays L.) in response to low nitrogen stress. Plant, Cell & Environment 34, 2122–2137. [DOI] [PubMed] [Google Scholar]
- Ghanem ME, Hichri I, Smigocki AC, Albacete A, Fauconnier ML, Diatloff E, Martinez-Andujar C, Lutts S, Dodd IC, Pérez-Alfocea F. 2011. Root-targeted biotechnology to mediate hormonal signalling and improve crop stress tolerance. Plant Cell Reports 30, 807–823. [DOI] [PubMed] [Google Scholar]
- Graves S, Piepho H-P, Selzer L, Dorai-Raj S. 2012. multcompView: visualizations of paired comparisons. R package version 0.1-5. http://CRAN.R-project.org/package=multcompView. [Google Scholar]
- Gu D, Mei X, Yu T, Sun N, Xu D, Liu C, Cai Y. 2017. QTL identification for brace-root traits of maize in different generations and environments. Crop Science 57, 13–21. [Google Scholar]
- Hammer GL, Dong Z, McLean G, Doherty A, Messina C, Schussler J, Zinselmeier C, Paszkiewicz S, Cooper M. 2009. Can changes in canopy and/or root system architecture explain historical maize yield trends in the U.S. corn belt? Crop Science 49, 299. [Google Scholar]
- Hayes J, Zhu Y-G, Mimura T, Reid R. 2004. An assessment of the usefulness of solution culture in screening for phosphorus efficiency in wheat. Plant and Soil 261, 91–97. [Google Scholar]
- Hetz W, Hochholdinger F, Schwall M, Feix G. 1996. Isolation and characterization of rtcs, a maize mutant deficient in the formation of nodal roots. The Plant Journal 10, 845–857. [Google Scholar]
- Hochholdinger F. 2009. The maize root system: morphology, anatomy, and genetics. In: Bennetzen JL, Hake SC, eds. Handbook of maize: its biology. New York: Springer, 145–160. [Google Scholar]
- Hochholdinger F, Yu P, Marcon C. 2018. Genetic control of root system development in maize. Trends in Plant Science 23, 79–88. [DOI] [PubMed] [Google Scholar]
- Hodge A. 2004. The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytologist 162, 9–24. [Google Scholar]
- Jenkins MT. 1930. Heritable characters of maize. XXXIV. Rootless. Journal of Heredity 21, 79–80. [Google Scholar]
- Lambers H, Atkin OK, Millenaar FF. 1996. Respiratory patterns in roots in relation to their functioning. In: Waisel Y, Eshel A, Beeckman T, Kafkafi U, eds. Plant roots. The hidden half, 3rd edn. Boca Raton, FL: CRC Press, 521–552. [Google Scholar]
- Lenth R. 2018. Emmeans: estimated marginal means, aka least-squares means. R package version 1. https://rdrr.io/cran/emmeans/ [Google Scholar]
- Lynch JP. 2011. Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiology 156, 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2013. Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Annals of Botany 112, 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2018. Rightsizing root phenotypes for drought resistance. Journal of Experimental Botany 69, 3279–3292. [DOI] [PubMed] [Google Scholar]
- Lynch JP, Brown KM. 2012. New roots for agriculture: exploiting the root phenome. Philosophical Transactions of the Royal Society B; Biological Sciences 367, 1598–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MC, Enquist BJ. 2007. Consistency between an allometric approach and optimal partitioning theory in global patterns of plant biomass allocation. Functional Ecology 21, 713–720. [Google Scholar]
- Meister R, Rajani MS, Ruzicka D, Schachtman DP. 2014. Challenges of modifying root traits in crops for agriculture. Trends in Plant Science 19, 779–788. [DOI] [PubMed] [Google Scholar]
- Mi G, Chen F, Yuan L, Zhang F. 2016. Ideotype root system architecture for maize to achieve high yield and resource use efficiency in intensive cropping systems. Advances in Agronomy 139, 73–97. [Google Scholar]
- Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L. 2012. Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytologist 193, 30–50. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rachmilevitch S, Cohen I, Huang B. 2015. Carbon allocation patterns into proteins and lipids associated with superior tolerance of perennial grass to high soil temperature. Crop Science 55, 2262. [Google Scholar]
- Ranum P, Peña-Rosas JP, Garcia-Casal MN. 2014. Global maize production, utilization, and consumption. Annals of the New York Academy of Sciences 1312, 105–112. [DOI] [PubMed] [Google Scholar]
- Reich PB. 2014. The world‐wide ‘fast–slow’ plant economics spectrum: a traits manifesto. Journal of Ecology 102, 275–301. [Google Scholar]
- Roumet C, Birouste M, Picon-Cochard C, Ghestem M, Osman N, Vrignon-Brenas S, Cao KF, Stokes A. 2016. Root structure–function relationships in 74 species: evidence of a root economics spectrum related to carbon economy. New Phytologist 210, 815–826. [DOI] [PubMed] [Google Scholar]
- RStudio. 2016. Integrated development for R. Boston, MA: RStudio, Inc. [Google Scholar]
- Rubio G, Lynch JP. 2007. Compensation among root classes in Phaseolus vulgaris L. Plant and Soil 290, 307–321. [Google Scholar]
- Saengwilai P, Nord EA, Chimungu JG, Brown KM, Lynch JP. 2014a Root cortical aerenchyma enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiology 166, 726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saengwilai P, Tian X, Lynch JP. 2014b Low crown root number enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiology 166, 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio PH, Pradet A. 1980. Soluble sugars, respiration, and energy charge during aging of excised maize root tips. Plant Physiology 66, 516–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavrukov Y, Genc Y, Hayes J. 2012. The use of hydroponics in abiotic stress tolerance research. In: Asao T, ed. Hydroponics—a standard methodology for plant biological researches. IntechOpen. [Google Scholar]
- Smith S, De Smet I. 2012. Root system architecture: insights from Arabidopsis and cereal crops. Philosophical Transactions of the Royal Society B: Biological Sciences 367, 1441–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B, Rasmussen A. 2016. The physiology of adventitious roots. Plant Physiology 170, 603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strock CF, Morrow de la Riva L, Lynch JP. 2018. Reduction in root secondary growth as a strategy for phosphorus acquisition. Plant Physiology 176, 691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taramino G, Sauer M, Stauffer JL Jr, Multani D, Niu X, Sakai H, Hochholdinger F. 2007. The maize (Zea mays L.) RTCS gene encodes a LOB domain protein that is a key regulator of embryonic seminal and post-embryonic shoot-borne root initiation. The Plant Journal 50, 649–659. [DOI] [PubMed] [Google Scholar]
- Trachsel S, Kaeppler SM, Brown KM, Lynch J. 2011. Shovelomics: high throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant and Soil 341, 75–87. [Google Scholar]
- Voss-Fels KP, Snowdon RJ, Hickey LT. 2018. Designer roots for future crops. Trends in Plant Science 23, 957–960. [DOI] [PubMed] [Google Scholar]
- Wells CE, Eissenstat DM. 2003. Beyond the roots of young seedlings: the influence of age and order on fine root physiology. Journal of Plant Growth Regulation 21, 324–334. [Google Scholar]
- Wickham H. 2016. ggplot2: elegant graphics for data analysis. New York: Springer. [Google Scholar]
- York LM. 2014. Integration of root phenes affecting nitrogen acquisition in maize. PhD dissertation, The Pennsylvania State University. [Google Scholar]
- York LM. 2019. Functional phenomics: an emerging field integrating high-throughput phenotyping, physiology, and bioinformatics. Journal of Experimental Botany 70, 379–386. [DOI] [PubMed] [Google Scholar]
- York LM, Galindo-Castañeda T, Schussler JR, Lynch JP. 2015. Evolution of US maize (Zea mays L.) root architectural and anatomical phenes over the past 100 years corresponds to increased tolerance of nitrogen stress. Journal of Experimental Botany 66, 2347–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York LM, Lynch JP. 2015. Intensive field phenotyping of maize (Zea mays L.) root crowns identifies phenes and phene integration associated with plant growth and nitrogen acquisition. Journal of Experimental Botany 66, 5493–5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York LM, Nord EA, Lynch JP. 2013. Integration of root phenes for soil resource acquisition. Frontiers in Plant Science 4, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan A, Lynch JP. 2015. Reduced frequency of lateral root branching improves N capture from low-N soils in maize. Journal of Experimental Botany 66, 2055–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang W. 2015. The decomposition of fine and coarse roots: their global patterns and controlling factors. Scientific Reports 5, 9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhang X, Lin Z, Wang J, Xu M, Lai J, Yu J, Lin Z. 2018. The genetic architecture of nodal root number in maize. The Plant Journal 93, 1032–1044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data and statistical analysis scripts for R are available in an online public repository (https://doi.org/10.5281/zenodo.2651391).