Abstract
Intradendritic recordings in Purkinje cells from a defined area in parasaggital slices of cerebellar lobule HVI, obtained after rabbits were given either paired (classical conditioning) or explicitly unpaired (control) presentations of tone and periorbital electrical stimulation, were used to assess the nature and duration of conditioning-specific changes in Purkinje cell dendritic membrane excitability. We found a strong relationship between the level of conditioning and Purkinje cell dendritic membrane excitability after initial acquisition of the conditioned response. Moreover, conditioning-specific increases in Purkinje cell excitability were still present 1 month after classical conditioning. Although dendritically recorded membrane potential, input resistance, and amplitude of somatic and dendritic spikes were not different in cells from paired or control animals, the size of a potassium channel-mediated transient hyperpolarization was significantly smaller in cells from animals that received classical conditioning. In slices of lobule HVI obtained from naive rabbits, the conditioning-related increases in membrane excitability could be mimicked by application of potassium channel antagonist tetraethylammonium chloride, iberiotoxin, or 4-aminopyridine. However, only 4-aminopyridine was able to reduce the transient hyperpolarization. The pharmacological data suggest a role for potassium channels and, possibly, channels mediating an IA-like current, in learning-specific changes in membrane excitability. The conditioning-specific increase in Purkinje cell dendritic excitability produces an afterhyperpolarization, which is hypothesized to release the cerebellar deep nuclei from inhibition, allowing conditioned responses to be elicited via the red nucleus and accessory abducens motorneurons.
Keywords: rabbit, cerebellum, classical conditioning, nictitating membrane, eyelid, Purkinje cell, slice, dendritic recording, long-term depression
A region of rabbit cerebellar lobule HVI has been identified in which increases in Purkinje cell dendritic excitability can be detected after 3 d of classical conditioning of the rabbit nictitating membrane response (NMR) (Schreurs et al., 1997a). The identified region may correspond to an area of c3 shown to be involved in eyelid responses in the cat and ferret (Hesslow, 1994a,b; Hesslow and Ivarsson, 1994). The purpose of our study was to test the hypothesis that Purkinje cell dendrites in this region of rabbit cerebellar lobule HVI show increases in membrane excitability as a function of the level of conditioning and that increases in excitability are still present 1 month after conditioning. We also wanted to pursue evidence that potassium channels may be involved in these membrane changes in excitability (Schreurs et al., 1997a).
Classical conditioning of rabbit nictitating membrane–eyelid responses involves presentation of a tone conditioned stimulus (CS) followed by air puff to or electrical stimulation around the eye as the unconditioned stimulus (US) (Gormezano et al., 1962, 1983; Schreurs, 1989). Using optimal conditioning parameters, rabbits begin acquiring conditioned responses (CRs) during a single 80-trial session (Scharenberg et al., 1991) and reaching an asymptote of 90% CRs by the third session (Schreurs, 1993; Schreurs et al., 1991, 1997a). Studies of long-term memory of the rabbit NMR (Schreurs, 1993) show that 1 month after training animals can elicit CRs to tone alone at a level of 80% (range, 49–99%).
Extensive lesion and recording data implicate cerebellar deep nuclei and cortex in classical conditioning of the rabbit NMR (McCormick and Thompson, 1984; Yeo et al., 1985a,b; Berthier and Moore 1986; Schreurs et al., 1991, 1997a; Perrett et al., 1993; Thompson and Krupa, 1994;Gruart and Yeo, 1995; Gould and Steinmetz, 1994, 1996; Katz and Steinmetz, 1997). Lesions of lobule HVI and ansiform of cerebellar cortex ipsilateral to the stimulated eye disrupt CRs, and although CRs eventually return, they are considerably lower in frequency and amplitude (Yeo et al., 1985b; Lavond et al., 1987; Lavond and Steinmetz, 1989). Bilateral lesions of lobule HVI abolish or severely impair CRs in trained animals without affecting unconditioned responses and, even more importantly, these lesions prevent relearning of the CR (Gruart and Yeo, 1995; Ivarsson et al., 1997). In vivoextracellular recording in and around lobule HVI during learning suggests that some Purkinje cells show CR-related increases and others show CR-related decreases in simple and/or complex spike activity (Berthier and Moore, 1986; Thompson, 1990; Gould and Steinmetz, 1996;Katz and Steinmetz, 1997). In fact, Berthier and Moore (1986), Gould and Steinmetz (1996), and Katz and Steinmetz (1997) all reported that cells with increased firing rates outnumber cells with decreased firing rates by a ratio of 2:1. Although the location of these cells cannot be precisely determined, the increased firing rates are consistent within vitro findings of increased dendritic excitability in Purkinje cells of lobule HVI after conditioning (Schreurs et al., 1991,1997a).
MATERIALS AND METHODS
Behavior. The subjects were adult male, albino rabbits (Oryctolagus cuniculus) weighing ∼2.0–2.2 kg at the start of training. Rabbits were individually housed, given access to food and water, and maintained on a 12 hr light/dark cycle. Animals were allocated randomly to groups in which they received either paired stimulus presentations (paired) or explicitly unpaired stimulus presentations (unpaired). Rabbits received 1 d of preparation and either 1 or 3 d of stimulus presentation. On adaptation day, the rabbits were prepared for periorbital electrical stimulation and recording of nictitating membrane movement and then adapted to the training chambers for the length of time of subsequent training sessions (80 min). A training session for the paired group consisted of 80 presentations of a 400 msec, 1000 Hz, 82 dB tone CS that coterminated with a 100 msec, 60 Hz, 2 mA electrical pulse US. Paired stimulus presentations were delivered, on average, every 60 sec (range, 50–70 sec). A session for the unpaired group consisted of 80 CS-alone and 80 US-alone presentations, which occurred in an explicitly unpaired manner delivered, on average, every 30 sec (range, 20–40 sec). Stimulus delivery and data collection were accomplished using a Compaq ASYST system previously described (Schreurs and Alkon, 1990) and modeled after systems developed by Gormezano (Gormezano et al., 1962;Gormezano, 1966). A response was scored as a conditioned response if it exceeded a criterion amplitude of 0.5 mm between CS onset and the point of US onset.
Slice preparation. Twenty four hours after 1 d of paired (n = 11) or explicitly unpaired stimulus presentations (n = 11), or after 1 month in the home cage after 3 d of stimulus presentations (paired,n = 16; unpaired, n = 14), rabbits were anesthetized deeply with sodium pentobarbital (30 mg/kg) and decapitated. A rapid craniotomy that removed the occipital bone and mastoid processes allowed the cerebellum and brainstem to be detached, removed, and chilled in 95% O2- and 5% CO2-saturated artificial CSF (ACSF) within ∼70–90 sec. Next, the area surrounding the right HVI lobule (ipsilateral to the side of training; see Fig.1A for details of tissue location) was isolated and attached with cyanoacrylate to an agar block in the cutting chamber. The isolated tissue was then immersed in chilled ACSF, and 400 μm parasagittal slices were cut with a vibrating slicer (Vibratome 1000; Technical Products Inc., St Louis, MO). After this procedure, slices were incubated in saturated ACSF at room temperature for at least 1 hr before being placed in a modified recording chamber in which the ACSF was maintained at 32°C (Schreurs et al., 1991). The ACSF contained (in mm): NaCl 124, KCl 3, MgSO4 1.2, CaCl2 2.1, Na2PO4 1.2, NaHCO2 26, and dextrose 10, and was saturated with a mixture of 95% O2 and 5% CO2, pH 7.4.
Fig. 1.
Learning-specific changes in membrane excitability can be found in a specific zone of lobule HVI. A, Anterior view of the right cerebellum indicating the area from which slices were cut (location of recording sites shown by black rectangle). B, Sample slice depicted so that the rabbit’s left folium of lobule HVI is shown on the left (location of recording sites comprising a “learning zone” shown by gray square). The dotted line represents the division between the granular layer and the molecular layer.
Intradendritic recording. Intracellular recordings from Purkinje cell dendrites were obtained by advancing a glass microelectrode (Leitz micromanipulator, Wetzlar, Germany) through the molecular layer of slices of lobule HVI. The electrodes were targeted at the medial edge of the left folium in the second, third, or fourth slice cut from lobule HVI of the right hemisphere; that is, from the hemisphere ipsilateral to the side of training (Fig.1B; Schreurs et al., 1997a, their Fig. 2). Only stable recordings from Purkinje cell dendrites with membrane potentials lower than −50 mV and input resistances ≥28 MΩ were used (Schreurs et al., 1996, 1997a). Recordings were made until 8 hr after decapitation. Microelectrodes of thick-walled glass (2 mm outer diameter, 1 mm inner diameter; FHC Inc., Bowdoinham, ME) were fabricated on a electrode puller (NE-2; Narishige, Tokyo, Japan), filled with 3 m potassium acetate, and had a DC resistance of 60–120 MΩ. A bridge amplifier (Axoprobe-1A, Axon Instruments, Foster City, CA) was used for all intradendritic recording. The recording electrodes were positioned with the aid of a binocular dissecting microscope (Wild, Switzerland, magnification up to 50×), which permitted visualization of the different cortical layers.
Data measurement and analysis. Data were recorded on videocassette tape using a pulse code modulation videocassette recorder (DX-900, Toshiba) and digitized using pClamp6 or Axoscope software (Axon Instruments). The majority of Purkinje cell dendrites revealed autorhythmic spontaneous activity (Llinas and Sugimori, 1980; Schreurs et al., 1991, 1992). Membrane potential was determined as the potential for somatic activity phase (Schreurs et al., 1991, 1996). Input resistance measures were based on a 0.5 nA, 700 msec hyperpolarizing current step. The current necessary to hyperpolarize the dendrite 20 mV below the somatic spike activity level was determined and applied to the membrane to measure the dendritic spike threshold. The dendritic spike threshold was established by applying current steps starting at −0.5 nA and increasing in 0.2 nA steps to 3.1 nA. Threshold measurements were based on the specific 700 msec current step required to elicit local, dendritic, calcium spikes. Because of the difficulty in passing current >3 nA reliably, only cells that reached a threshold at or below the 3.1 nA step were included in the analysis.
An examination of the subthreshold depolarizing current steps in our previous experiments (Schreurs et al., 1991, their Fig. 2, 1997a, their Fig. 1) suggested a marked reduction in the transient hyperpolarization in cells from animals given 3 d of paired training relative to cells from unpaired control subjects. To examine potential differences in transient hyperpolarization as a function of classical conditioning in the present experiments, the size of transient hyperpolarizations was determined by measuring the difference between the maximum and minimum voltage during the depolarization current step before the occurrence of somatic spikes (see Figs. 2C,4A). In addition, the size of the afterhyperpolarization was assessed by measuring the difference between the baseline voltage before and the minimum voltage after the depolarizing current step used to measure the transient hyperpolarization (see Fig. 4A).
Fig. 2.
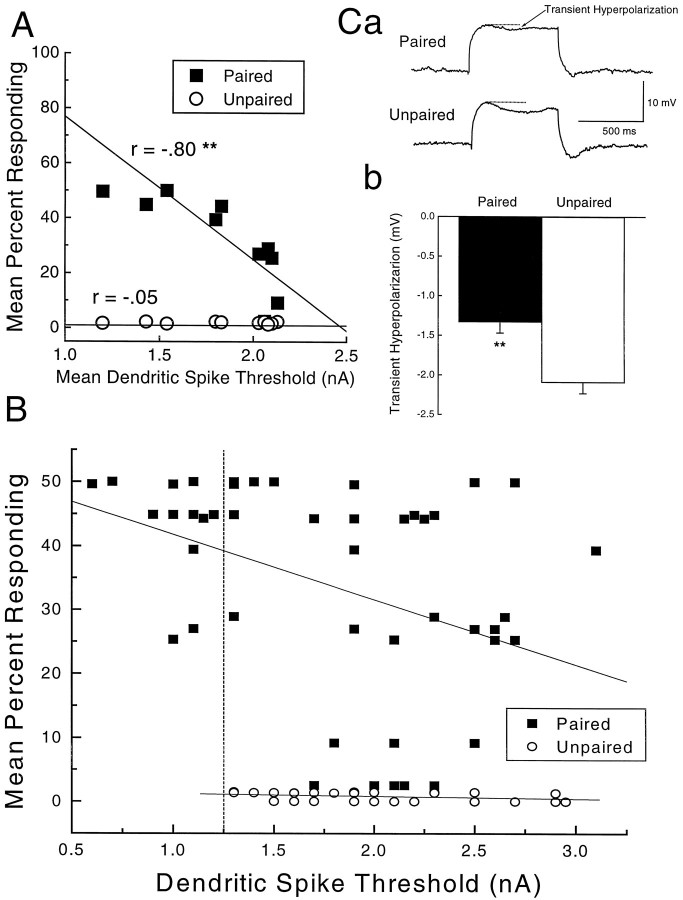
Learning-specific membrane excitability after 1 d of classical conditioning. A, Strong linear relationship between level of conditioning and mean dendritic spike threshold (at least 2 measures per rabbit) for paired rabbits (filled squares, r = −0.80,p < 0.01) relative to unpaired rabbits (open circles, r = −0.05,p > 0.8). B, Scatter- plot of Purkinje cell dendritic spike thresholds from cells obtained in slices from paired and unpaired rabbits in A. Although mean threshold values for paired and unpaired rabbits overlap, the scatterplot shows that to the left of the dotted linethere is a unique group of cells from paired rabbits that have low thresholds. Lines of best fit show a significant correlation for paired rabbits (r = −0.39; p < 0.01) but not for unpaired rabbits (r = −0.27;p > 0.1). C, a, Example of the transient hyperpolarization reduced in a cell from a paired animal (top) but not in a cell from an unpaired animal (bottom). C, b, Mean transient hyperpolarization for all cells from paired animals and all cells from unpaired animals. **p < 0.01.
Fig. 4.
Changes in transient and afterhyperpolarization 1 month after conditioning. A, Example of depolarizing current steps showing smaller transient and afterhyperpolarization in a cell from a paired rabbit than in a cell from an unpaired rabbit.B, Mean transient and afterhyperpolarization for all cells from paired and unpaired animals. ***p < 0.001; **p < 0.01.
Potassium channel pharmacology. The potassium channel antagonist TEA (1–10 mm; Sigma, St Louis, MO), the calcium-dependent potassium channel antagonist iberiotoxin (40–80 nm; Research Biochemicals, Natick, MA), the transient potassium channel IA antagonist 4-AP (50–500 μm; Aldrich, Milwaukee, WI), or the ACSF vehicle was applied to the slice via whole-bath perfusion. Dendritic spike thresholds were determined as the average of at least two threshold measurements taken before a 4 min perfusion of TEA, iberiotoxin, 4-AP, or ACSF and again after the perfusion. Measurement of the transient hyperpolarization and afterhyperpolarization were made from the largest positive current step before the occurrence of somatic spikes.
Statistics.The data in the text and figures are expressed as mean ± SEM. Data were analyzed statistically by ttests or ANOVAs. Differences in frequency distributions were analyzed by χ2 tests.
RESULTS
Purkinje cell dendritic membrane excitability after 1 d of classical conditioning
After 1 d of paired stimulus presentations, rabbits reached a mean level of 36.7% CRs (range, 2.5–82.6% CRs), whereas after 1 d of unpaired stimulus presentations, rabbits showed a mean level of only 1.0% responding to the tone CS (range, 0–3.6%). There was a significant difference between the paired and unpaired groups in the level of responding (F(1,20) = 29.35;p < 0.001).
A total of 95 cells were successfully penetrated and of these, 87 cells met the membrane potential, input resistance, and dendritic spike threshold criteria and were included in the analysis. All of the cells included in the analysis were recorded with the experimenter blind to the level of responding shown by the rabbits. Figure2 details the relationship between percent responding and mean dendritic spike threshold for rabbits with at least two dendritic threshold measurements (Fig.2A), a scatter plot of thresholds for Purkinje cell dendrites for rabbits in Figure 2A (Fig. 2B), a sample depolarizing current step showing the size of the transient hyperpolarization in a cell from a paired rabbit and in a cell from an unpaired rabbit (Fig. 2C, a), and the mean transient hyperpolarization for all cells from paired and unpaired animals (Fig.2C, b).
Inspection of Figure 2A shows a strong linear relationship between percent responding and mean dendritic spike threshold for the paired group. In fact, there was a highly significant correlation between percent responding and mean threshold (r = −0.80; p < 0.01) for paired rabbits, whereas there was only a weak, nonsignificant relationship for unpaired rabbits (r = −0.05; p > 0.8). Although there is some overlap in the distribution of mean threshold values for the two groups, the scatterplot in Figure2B shows that there is a unique group of cells recorded in slices from paired animals that had lower thresholds than any of the cells recorded in slices from unpaired rabbits. An analysis of the relative frequency distribution of thresholds for dendritic spikes between the paired and unpaired groups showed there were significantly more cells with low dendritic spike thresholds in slices taken from rabbits in the paired group than in slices taken from rabbits in the unpaired group (χ2 = 29.79;p < 0.001). Although in the right direction, there was no overall difference in the mean threshold for dendritic spikes between cells from rabbits in the paired group (1.76 ± 0.09 nA) and rabbits in the unpaired group (1.93 ± 0.08 nA;p < 0.09).
A comparison of the depolarizing current step for a cell from a paired and an unpaired animal in Figure 2C, a, shows a clear reduction in the size of the transient hyperpolarization as a result of paired training. Figure 2C, b, shows that the difference in mean transient hyperpolarization between cells from paired (−1.36 ± 0.12 mV) and unpaired animals (−2.09 ± 0.14 mV) was highly significant (p < 0.01). There was no significant difference in the size of the afterhyperpolarization between cells from paired (−1.62 ± 0.11 mV) and cells from unpaired animals (−1.87 ± 0.15 mV; p < 0.08).
The analysis of transient hyperpolarizations suggests that as a result of only 1 d of classical conditioning, changes in potassium channels are already taking place (Hounsgaard and Midtgaard, 1988). Specifically, the transient hyperpolarization in cells from animals that received paired training was lower than in cells from animals that received unpaired stimulus presentations, indicating a reduction in outward potassium currents. These data are consistent with a body of evidence from both vertebrate and invertebrate experiments showing that potassium channels are modified as a consequence of learning (Alkon et al., 1982; Cowan and Siegel, 1986; Sanchez-Andres and Alkon, 1991;Meiri et al., 1997).
Finally, there were no significant differences between cells from the paired group (n = 49) and cells from the unpaired group (n = 38) in membrane potential (−58.5 ± 0.55 vs −58.2 ± 0.62 mV), input resistance (31.2 ± 0.62 vs 30.1 ± 0.48 MΩ), somatic spike amplitude (7 ± 1.03 vs 8.3 ± 1.35 mV), or dendritic spike amplitude (27.1 ± 1.36 vs 26.7 ± 1.95 mV). There was a slight but significant difference in the current required to hyperpolarize the membrane by 20 mV between cells from paired subjects and cells from unpaired subjects (−0.76 ± 0.02 vs −0.84 ± 0.02 nA; p < 0.05).
Purkinje cell dendritic membrane excitability 1 month after classical conditioning
Paired subjects all showed levels of conditioning in excess of 85% conditioned responses by the end of the 3 d of training, whereas unpaired subjects showed only baseline levels of responding (<3%; Schreurs et al., 1991, 1997a). Although the rabbits were not presented with the tone CS before the preparation of slices, previously obtained behavioral data indicated that 1 month after 3 d of paired training, rabbits responded to tone alone presentations at a mean level of 80% CRs and response levels ranged from 49 to 99% CRs (Schreurs, 1993).
A total of 122 cells were successfully penetrated, and 108 of the penetrated cells met the membrane potential, input resistance, and dendritic spike threshold criteria and were included in the analysis. Forty-four of the cells included in the analysis (40%) were recorded with the experimenter blind to the behavioral condition of the animals.
Figure 3 details individual current steps for a Purkinje cell from a paired and unpaired rabbit (Fig.3A) as well as the mean dendritic spike threshold (Fig.3B) and relative frequency distribution of thresholds (Fig.3C) for Purkinje cell dendrites from paired and unpaired rabbits. The individual traces (Fig. 3A) show that, at a current step of 0.5 nA, neither cell reached dendritic spike threshold, but at a current step of 0.7 nA, the cell from the paired animal did reach threshold for dendritic spikes. The column graph (Fig.3B) shows that cells (n = 61) obtained from paired rabbits required a mean current of 1.62 ± 0.05 nA to elicit dendritic spikes, whereas cells (n = 47) obtained from unpaired animals required a significantly higher mean current of 1.82 nA ± 0.04 to elicit the same spikes (p < 0.05). The relative frequency distribution of threshold values (Fig. 3C) suggests that there was a shift to the left (lower thresholds) for cells from paired animals in the lowest bin (0.5–0.9 nA) that contains 15% of the cells from paired rabbits and only 2% from unpaired rabbits. A statistical analysis of the distribution of threshold values yielded a significant difference between the distribution of thresholds for paired and unpaired groups (χ2 = 13.35; p < 0.025).
Fig. 3.
Learning-specific membrane excitability 1 month after 3 d of classical conditioning. A, Sample depolarizing current steps in a Purkinje cell dendrite with a low dendritic spike threshold from a trained rabbit (Paired) 1 month after classical conditioning, showing spike threshold with a 700 msec current pulse of 0.7 nA compared with sample depolarizing current steps in a cell from an unpaired control rabbit (Unpaired) that did not reach spike threshold at a current step of 0.7 nA. B, Mean dendritic spike thresholds showing a significantly lower threshold for cells (n = 61) from paired rabbits than in cells (n = 47) from unpaired rabbits. *p < 0.05. C, Relative frequency distribution shift to the left of Purkinje cell dendritic spike thresholds from cells obtained in slices from paired compared with cells in slices from unpaired rabbits. χ2 < 0.05.
These data suggest that conditioning-specific changes in Purkinje cell dendrite membrane excitability first observed 24 hr after training (Schreurs et al., 1991, 1997a) are still detectable in a learning zone of lobule HVI 1 month after training. This is in contrast to the conditioning-specific changes observed in CA1 and CA3 pyramidal cells of the hippocampus, which are no longer detectable 1 week after training (Moyer et al., 1996; Thompson et al., 1996). In fact, the increase in membrane excitability detected 1 month after conditioning may be the first memory-specific electrophysiological changes that have been detected so long after conditioning.
Figure 4A shows a sample depolarizing current step in a cell from a paired rabbit and in a cell from an unpaired rabbit. Figure 4, B andC, illustrates the mean transient and mean afterhyperpolarizations, respectively, for cells from paired animals and for cells from unpaired animals. A comparison of the depolarizing current step for a cell from a paired rabbit and a cell from an unpaired animal in Figure 4A shows a reduction in the size of the transient hyperpolarization and the afterhyperpolarization as a result of paired training. Figure 4, B andC, shows and statistical analyses confirm that the difference in the mean transient and the mean afterhyperpolarization between cells from paired and unpaired animals was significant (p < 0.001 and p < 0.01, respectively). There was a significant correlation between threshold for dendritic spikes and both transient and afterhyperpolarization for cells from paired animals (r = − 0.56 and −0.36, respectively; p < 0.01) and a weaker correlation between threshold for dendritic spikes and the transient hyperpolarization in cells from unpaired animals (r = −0.31; p < 0.05). These data suggest that the currents underlying the transient and afterhyperpolarizations may comprise one of the currents underlying the dendritic spike threshold measure.
Previous evidence of conditioning-specific changes in potassium channels has been limited to 1–2 weeks after training. For example, reductions in hippocampal CA1 and CA3 cell afterhyperpolarization that are mediated by potassium channel changes have only been observed 1 week after conditioning. These changes in AHPs were observed to return to baseline levels 2 weeks after training (Moyer et al., 1996; Thompson et al., 1996). Similarly, input resistance increases inHermissenda B photoreceptor that were also mediated by potassium channels and were in evidence 6 d after classical conditioning were no longer detectable 2 weeks after training (Matzel et al., 1992). Consequently, the present experiment provides the first evidence of truly long-term changes in potassium channels as a function of classical conditioning.
There were no other significant differences between cells from animals in the paired group (n = 61) and cells from animals in the unpaired group (n = 47) in membrane potential (−58.2 ± 0.54 vs −57.8 ± 0.69 mV), input resistance (29.2 ± 0.7 vs 32 ± 0.73 MΩ), current required to hyperpolarize the membrane by 20 mV (−0.8 ± 0.02 vs −0.8 ± 0.02 nA), somatic spike amplitude (6.7 ± 0.89 vs 7.0 ± 1.10 mV), or dendritic spike amplitude (27.3 ± 1.32 vs 29.7 ± 1.51 mV).
Potassium channel role in Purkinje cell excitability
There is a large body of evidence that suggests that potassium channels, particularly IA and Ca2+-dependent K+, play a major role in physiological changes underlying classical conditioning in a number of preparations (Alkon, 1989; Coulter et al., 1989;Sanchez-Andres and Alkon, 1991; Woody et al., 1991; Schreurs and Alkon, 1992). In the cerebellum, local dendritic calcium spikes are correlated with changes in local internal calcium concentration and controlled by transient outward potassium current (IA) inactivation (Llinas and Sugimori 1980; Midtgaard et al., 1993;Midtgaard 1994, 1995). There is some evidence from slices of rabbit cerebellar cortex to suggest that potassium channels may also play a role in the changes in membrane excitability found after classical conditioning of the rabbit NMR (Schreurs et al., 1997a).
We used the potassium channel blocker TEA, the calcium-dependent potassium channel antagonist iberiotoxin (IBERIO), and the transient potassium channel IA antagonist 4-AP to elucidate the potential role of potassium channels in the changes in excitability, transient hyperpolarization, and afterhyperpolarization of Purkinje cell dendrites after classical conditioning of the rabbit NMR.
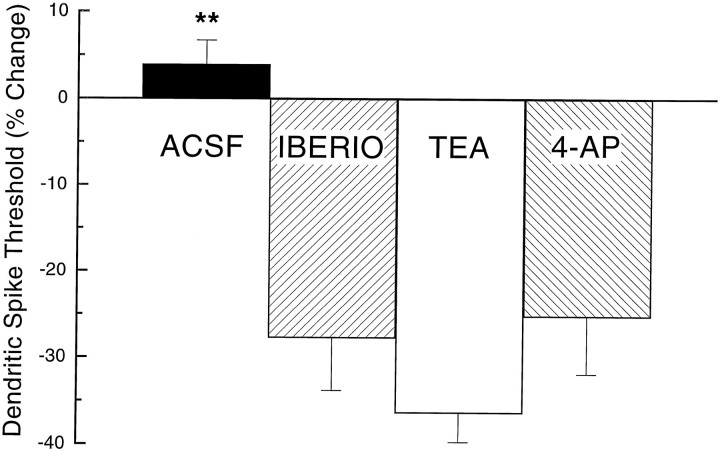
Figure 5 depicts the percent change in dendritic spike threshold in Purkinje cell dendrites in slices from naive rabbits as a function of the perfusion of the ACSF vehicle, iberiotoxin, TEA, or 4-AP. The figure shows a clear and substantial effect of all three potassium channel antagonists on membrane excitability evidenced by a significant decrease in the threshold for dendritic spikes. In particular, the threshold for dendritic spikes decreased dramatically after the perfusion of iberiotoxin (−27.74 ± 6.07%), TEA (−36.37 ± 3.35%), or 4-AP (−25.25 ± 6.62%), whereas the threshold for dendritic spikes changed very little as a result of the ACSF vehicle (3.93 ± 2.74%). Statistical analysis confirmed a significant effect of drug (F(3,40) = 23.24; p < 0.001), which was attributable to the decrease in threshold induced by iberiotoxin, TEA, and 4-AP (p values < 0.01) relative to the ACSF vehicle control. There were no significant differences between the drugs in their ability to increase dendritic excitability.
Fig. 5.
The percent change in dendritic spike threshold in naive cells as a function of the perfusion of the ACSF vehicle, iberiotoxin, TEA, or 4-AP. The figure shows a clear effect of all three potassium channel antagonists on membrane excitability evidenced by a significant decrease in the threshold for dendritic spikes. **p < 0.01.
Figure 6 shows the mean transient (Fig.6A) and afterhyperpolarization (Fig.6B) in naive cells before and after the perfusion of the ACSF vehicle, iberiotoxin, TEA, or 4-AP. The figure shows a clear and substantial effect of 4-AP on both the transient and afterhyperpolarization. In particular, although the transient and afterhyperpolarization of a positive current step changed very little as a result of the ACSF vehicle, iberiotoxin, or TEA perfusion, there was a significant reduction in both the transient (p < 0.01) and afterhyperpolarization (p < 0.05) as a result of 4-AP perfusion.
Fig. 6.
Change in transient and afterhyperpolarization in naive cells as a function of the perfusion of the ACSF vehicle, iberiotoxin, TEA, or 4-AP. A, Mean transient hyperpolarization is reduced significantly by 4-AP; **p < 0.01. B, Mean afterhyperpolarization is also reduced by 4-AP; *p < 0.05.
The antagonist 4-AP is relatively specific for the transientIA-like channel at low concentrations and application produced a 25% reduction in the threshold of the Purkinje cell dendrite. Moreover, only 4-AP was able to reduce the amplitude of the transient and afterhyperpolarizations. AlthoughIA-like channels are usually rapidly inactivating (Rudy, 1988), in the Purkinje cell,IA-like channels are found to inactivate slowly, especially after prolonged depolarization (Midtgaard, 1994). This difference in current inactivation rate may be explained, in part, by the diversity of IA-like potassium channel structure among different cell types (Pongs, 1993).
The effects of 4-AP may have been mediated by another slowly inactivating current, ID (Storm, 1990). There are a number of similarities and differences between theIA-like current in the present experiments with Purkinje cells and the ID current found in hippocampal cells. First, ID is usually effective in hippocampal cells at membrane potentials similar to the potentials at which the transient hyperpolarizations were measured in the present experiment (Storm, 1990). The holding potentials were similar in both cases. Second, like the IA-like current and ID, the transient hyperpolarization in the present experiment demonstrates high sensitivity and selectivity for 4-AP (we saw effects of 4-AP at concentrations as low as 50 μm). Third, inactivation ofID is extremely slow, usually of the order of several seconds (Storm, 1990), whereas in the present experiments the transient hyperpolarization began to recover ∼400–500 msec into the 700 msec depolarizing step and, in many cases, had recovered completely by the end of the depolarizing step, suggesting a faster inactivation. Fourth, ID is sensitive to 4-AP and is thought to be involved in spike repolarization because 4-AP has been shown to broaden spike width in hippocampal cells (Storm, 1990). In contrast, although 4-AP did increase dendritic spike amplitude during dendritic spike threshold measurements (10.0 ± 3.5 mV before 4-AP vs 20.13 ± 6.47 mV after 4-AP; n = 6;p < 0.05), it did not widen dendritic spikes (full width, 3.55 ± 0.73 vs 4.12 ± 1.01 msec; p> 0.7).
DISCUSSION
The principal findings were (1) after 1 d of rabbit classical conditioning, Purkinje cell membrane excitability in an identified area of lobule HVI was related to strength of conditioning; (2) 1 month after 3 d of conditioning, increases in Purkinje cell excitability in this same area were still present; in both cases, excitability was indexed by the minimum current required to elicit dendritic calcium spikes and the amplitude of a transient hyperpolarization; (3) the learning-specific increase in excitability was mimicked in cells from naive animals by blocking potassium channels with TEA, iberiotoxin, or 4-AP; and (4) the learning-specific decrease in transient and afterhyperpolarization was mimicked in naive cells by application of 4-AP, an antagonist of the IA-like potassium current.
The results establish that there are learning-specific changes in membrane excitability of Purkinje cells in a relatively small, circumscribed area of lobule HVI that can be detected in slices 24 hr after just 1 d of training and that similar changes can be detected in the same area 1 month after training. The data extend previous reports of an increase in Purkinje cell membrane excitability in cells from paired animals after training (Schreurs et al., 1991,1997a). Moreover, the changes were found in a specific area of lobule HVI that might be termed a functional “learning zone” (Ito, 1989;Hesslow, 1994a; Chen and Thompson, 1995). Our data support a role for lobule HVI in classical conditioning of the rabbit nictitating membrane–eyelid response (Yeo et al., 1985b,c; Berthier and Moore, 1986; Thompson, 1990; Schreurs et al., 1991; Hesslow, 1994a; Gruart and Yeo, 1995; Gould and Steinmetz, 1996; Schreurs et al., 1997a) and provide further evidence for a cerebellar role in learning and memory (Berthier and Moore, 1986; Thompson, 1986; Leiner et al., 1986, 1989;Supple and Kapp, 1993; LaLonde, 1994; Molchan et al., 1994; Andreasen et al., 1995; Logan and Grafton, 1995; Fiez, 1996; Kleim et al., 1997;Schreurs et al., 1997b).
There are a number of issues raised by conditioning-specific changes in Purkinje cell excitability. First, changes in excitability may be an epiphenomenon and have little to do with conditioning. It has been argued that cerebellar structure and function are sufficiently well understood to conclude that there is no learning-specific cerebellar plasticity and that the cerebellum is only involved in timing and coordination (Welsh and Harvey, 1989; Llinas and Welsh, 1993; Perrett et al., 1993; Bloedel and Bracha, 1995; Anderson and Keifer, 1997). Second, it has been suggested that learning-specific cerebellar plasticity exists but that it takes the form of long-term depression rather than increased excitability (Linden and Connor, 1992; Schreurs and Alkon, 1993). Long-term depression is posited to reduce Purkinje cell excitability, which, in turn, reduces inhibition of deep nuclei allowing CRs to occur (Thompson, 1986; Ito, 1989). We have provided evidence elsewhere suggesting that this may not be correct (Schreurs et al., 1997a). In brief, we found a conditioning-specific increase rather than an expected decrease in Purkinje cell synaptic strength as well as greater difficulty in inducing long-term depression after conditioning.
The third issue raised by a conditioning-specific increase in Purkinje cell excitability is the need for corroboration, and a fourth issue concerns the relevance of increases in excitability for conditioning. Evidence for conditioning-specific increases in excitability comes from the present and previous in vitro experiments (Schreurs et al., 1991, 1997a) and is suggested by in vivo extracellular recordings. Recordings in and around lobule HVI have identified cells with activity correlated with the CS, US, CRs to the CS, and unconditioned responses to the US (Berthier and Moore, 1986; Thompson, 1990; Gould and Steinmetz, 1996; Katz and Steinmetz, 1997). Berthier and Moore (1986) found 59% of Purkinje cells showed increased activity during CRs, whereas only 23% showed decreased activity. These numbers correspond well with studies by Gould and Steinmetz (1996) and Katz and Steinmetz (1997), who found a ratio of 2:1 in the number of Purkinje cells with increased activity during conditioning relative to those with decreased activity. Moreover, Berthier and Moore (1986) recorded from the right gyrus of lobule HVI and their electrode tracks were located along the medial edge of that gyrus. Consequently, althoughBerthier and Moore (1986) did not identify the microzonal location of the in vivo recordings, the locus of Purkinje cells with an increase in activity was similar to the location of the current area and that first described by Schreurs et al. (1997a). A more precise correspondence between the Purkinje cells being studied in vivo and in vitro requires anatomical and functional localization of the recording sites.
The prevailing view of cerebellar output circuitry is that Purkinje cells inhibit the deep nuclei of the cerebellum which, in turn, send excitatory outputs to the red nucleus (Eccles et al., 1967). In the case of a conditioned NMR–eyelid response, CS and US inputs reach both the cerebellar cortex and deep nuclei, and excitation of the red nucleus by the deep nuclei drives motorneurons in accessory abducens and facial motor nuclei responsible for nictitating membrane sweeps and eyelid closure (Thompson, 1986). Accordingly, an increase in Purkinje cell excitability should result in an increase in inhibition of the deep nuclei with a consequent decrease in excitation of cells in the red nucleus and a decrease in elicitation of the CR. The same outcome might result from the 2:1 increase in conditioning-related in vivo activity of Purkinje cells noted above.
There are findings that question these prevailing assumptions about cerebellar structure and function. De Zeeuw and Berrebi (1995) have shown that deep cerebellar neurons receive excitatory as well as inhibitory inputs from Purkinje cells. De Zeeuw and Berrebi (1995) also found that individual Purkinje cells innervate both inhibitory and excitatory deep cerebellar neurons (Chan-Palay, 1977). Consequently, a conditioning-specific increase in Purkinje cell dendrite excitability could produce a CR through selective excitation and/or disinhibition of deep cerebellar nuclei.
Even if the relevant corticonuclear connections associated with the NMR–eyelid were inhibitory (De Zeeuw and Berribe, 1995; Teune et al., 1998), recent information about cerebellar function suggests testable hypotheses about the role Purkinje cell excitability might play in conditioning. First, increased Purkinje cell output could induce long-term depression of inhibitory corticonuclear synapses leading to an increased excitatory cascade to the red nucleus and motorneurons. Evidence for depression of an inhibitory synapse comes from a cerebellar slice experiment in which pairings of depolarizing current and application of the GABAB agonist baclofen resulted in a pairing-specific reduction in the size of the baclofen response (Schreurs et al., 1992). Experiments in hippocampus show that after pairings, GABA synapses can be converted from inhibitory to excitatory (Collin et al., 1995). Second, increased Purkinje cell excitability induces an increased firing of local dendritic calcium spikes that has been shown to result in a pronounced afterhyperpolarization (Midtgaard, 1995). Consequently, after an excitability-induced burst of calcium spikes, the ensuing afterhyperpolarization would silence the Purkinje cell, which, in turn, would allow deep nuclei to excite the red nucleus and the CR could ensue.
Previous studies of neural correlates of classical conditioning have found changes in cellular excitability that last for only several days after conditioning. In the invertebrate Hermissenda, Matzel et al. (1992) found that changes in input resistance after conditioning were detectable up to 6 d after conditioning. Interestingly,Matzel et al. (1992) noted that conditioning-specific changes in input resistance of the Hermissenda B photoreceptor could be reinstated 14 d after original conditioning by renewed training. In rabbit NMR–eyelid conditioning, Moyer et al. (1996) and Thompson et al. (1996) found that changes in the AHP of hippocampal CA1 and CA3 cells returned to baseline levels 7 d after conditioning. Moyer et al. (1996) and Thompson et al. (1996) failed to reinstate conditioning-specific changes in hippocampal cells 14 d after training even if renewed pairings produced asymptotic levels of conditioning. In contrast, the present data provide evidence for neural correlates of learning that persist for 1 month after conditioning in the absence of further training.
The potential role of potassium channels in learning-specific changes in Purkinje cell membrane excitability is consistent with observations of a conditioning-specific role for potassium channels inHermissenda and rabbit hippocampus (Alkon, 1989; Schreurs and Alkon, 1992). In Hermissenda, classical conditioning induces an inactivation of IA and Ca2+-dependent K+ channels resulting in an increase in cell excitability (Alkon, 1984). In rabbit hippocampal CA1 pyramidal cells, classical conditioning induces a reduction in a Ca2+-dependent K+current through the cell membrane (Coulter et al., 1989; Sanchez-Andres and Alkon, 1991). Woody et al. (1991) reported changes in cat motor cortex pyramidal cell IA after pairing of a click with a glabela tap. In the cerebellum, local dendritic calcium spikes are correlated with changes in local internal calcium concentration and controlled by transient outward potassium current (IA) inactivation (Llinas and Sugimori 1980; Midtgaard et al., 1993; Midtgaard, 1995). The present evidence suggests that conditioning-specific increases in dendritic excitability are mediated by changes in IA-like potassium currents.
Footnotes
We thank L. Cochran, A. Grojec, Dr. A. Gruart, and M. A. Hoefler for help in data collection and analysis and Dr. K. T. Blackwell for help with statistical analysis.
Correspondence should be addressed to Bernard G. Schreurs, Laboratory of Adaptive Systems, National Institute of Neurological Disorders and Stroke, Building 36, Room B205, National Institutes of Health, Bethesda, MD 20892.
REFERENCES
- 1.Alkon DL. Calcium-mediated reduction of ionic currents: a biophysical memory trace. Science. 1984;226:1037–1045. doi: 10.1126/science.6093258. [DOI] [PubMed] [Google Scholar]
- 2.Alkon DL. Memory storage and neural systems. Sci Am. 1989;261:42–50. doi: 10.1038/scientificamerican0789-42. [DOI] [PubMed] [Google Scholar]
- 3.Alkon DL, Lederhendler I, Shoukimas JJ. Primary changes of membrane currents during retention of associative learning. Science. 1982;215:693–695. doi: 10.1126/science.7058334. [DOI] [PubMed] [Google Scholar]
- 4.Anderson CW, Keifer J. The cerebellum and red nucleus are not required for in vitro classical conditioning of the turtle abducens nerve response. J Neurosci. 1997;17:9736–9745. doi: 10.1523/JNEUROSCI.17-24-09736.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreasen NC, O’Leary DS, Arndt S, Cizadlo T, Hurtig R, Rezai K, Watkins GL, Boles Ponto LL, Hichiwa RD. Short-term and long-term verbal memory: a positron emission tomography study. Proc Natl Acad Sci USA. 1995;92:5111–5115. doi: 10.1073/pnas.92.11.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berthier NE, Moore JW. Cerebellar Purkinje cell activity related to the classically conditioned nictitating membrane response. Exp Brain Res. 1986;63:341–350. doi: 10.1007/BF00236851. [DOI] [PubMed] [Google Scholar]
- 7.Bloedel JR, Bracha V. On the cerebellum, cutaneomuscular reflexes, movement control and the elusive engrams of memory. Behav Brain Res. 1995;68:1–44. doi: 10.1016/0166-4328(94)00171-b. [DOI] [PubMed] [Google Scholar]
- 8.Chan-Palay V. Cerebellar dentate nucleus. Springer; Berlin: 1977. [Google Scholar]
- 9.Chen C, Thompson RF. Temporal specificity of long-term depression in parallel-Purkinje cell synapses in rat cerebellar slice. Learn Mem. 1995;2:185–198. doi: 10.1101/lm.2.3-4.185. [DOI] [PubMed] [Google Scholar]
- 10.Collin C, Devan WA, Dahl D, Lee CJ, Axelrod J, Alkon DL. Long-term synaptic transformation of hippocampal CA1 gamma-aminobutyric acid synapses and the effect of anandamide. Proc Natl Acad Sci USA. 1995;92:10167–10171. doi: 10.1073/pnas.92.22.10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coulter DA, LoTurco J, Kubato M, Disterhoft JF, Alkon DL. Classical conditioning alters the amplitude and time course of the calcium-dependent after hyperpolarization in rabbit hippocampal pyramidal cells. J Neurophysiol. 1989;61:971–981. doi: 10.1152/jn.1989.61.5.971. [DOI] [PubMed] [Google Scholar]
- 12.Cowan TM, Siegel RW. Drosophila mutations that alter ionic conduction disrupts acquisition and retention of a conditioned odor avoidance response. J Neurogenet. 1986;3:187–201. doi: 10.3109/01677068609106849. [DOI] [PubMed] [Google Scholar]
- 13.De Zeeuw CI, Berrebi AS. Postsynaptic targets of Purkinje cell terminals in the cerebellar and vestibular nuclei of the rat. Eur J Neurosci. 1995;7:2322–2333. doi: 10.1111/j.1460-9568.1995.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 14.Eccles JC, Ito M, Szentagothai J. The cerebellum as a neuronal machine. Springer; Berlin: 1967. [Google Scholar]
- 15.Fiez JA. Cerebellar contribution to cognition. Neuron. 1996;16:3–15. doi: 10.1016/s0896-6273(00)80018-5. [DOI] [PubMed] [Google Scholar]
- 16.Gormezano I. Classical conditioning. In: Sidowski JB, editor. Experimental methods and instrumentation in psychology. McGraw-Hill; New York: 1966. pp. 385–420. [Google Scholar]
- 17.Gormezano I, Schneiderman N, Deaux E, Fuentes I. Nictitating membrane: classical conditioning and extinction in the albino rabbit. Science. 1962;138:33–34. doi: 10.1126/science.138.3536.33. [DOI] [PubMed] [Google Scholar]
- 18.Gormezano I, Kehoe EJ, Marshall BS. Twenty years of classical conditioning research with the rabbit. Prog Psychobiol Physiol Psychol. 1983;10:197–275. [Google Scholar]
- 19.Gould TJ, Steinmetz JE. Multiple-unit activity from rabbit cerebellar cortex and interpositus nucleus during classical discrimination/reversal eyelid conditioning. Brain Res. 1994;652:98–106. doi: 10.1016/0006-8993(94)90322-0. [DOI] [PubMed] [Google Scholar]
- 20.Gould TJ, Steinmetz JE. Changes in rabbit cerebellar cortical and interpositus nucleus activity during acquisition, extinction, and backward classical eyelid conditioning. Neurobiol Learn Mem. 1996;65:17–34. doi: 10.1006/nlme.1996.0003. [DOI] [PubMed] [Google Scholar]
- 21.Gruart A, Yeo CH. Cerebellar cortex and eyeblink conditioning—bilateral regulation of conditioned responses. Exp Brain Res. 1995;104:431–438. doi: 10.1007/BF00231978. [DOI] [PubMed] [Google Scholar]
- 22.Hesslow G. Correspondence between climbing fibre input and motor output in eyeblink-related areas in cat cerebellar cortex. J Physiol (Lond) 1994a;476:229–244. doi: 10.1113/jphysiol.1994.sp020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hesslow G. Inhibition of classically conditioned eyeblink responses by stimulation of the cerbellar cortex in the decerebrate cat. J Physiol (Lond) 1994b;476:245–256. doi: 10.1113/jphysiol.1994.sp020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hesslow G, Ivarsson M. Suppression of cerebellar Purkinje cells during conditioned responses in ferrets. NeuroReport. 1994;4:1127–1130. doi: 10.1097/00001756-199401000-00030. [DOI] [PubMed] [Google Scholar]
- 25.Hounsgaard J, Midtgaard J. Intrinsic determinants of firing pattern in Purkinje cells of the turtle cerebellum in vitro. J Physiol (Lond) 1988;402:731–749. doi: 10.1113/jphysiol.1988.sp017231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito M. Long-term depression. Annu Rev Neurosci. 1989;12:85–102. doi: 10.1146/annurev.ne.12.030189.000505. [DOI] [PubMed] [Google Scholar]
- 27.Ivarsson M, Svensson P, Hesslow G. Bilateral disruption of conditioned responses after unilateral blockade of cerebellar output in the decerrebrate ferret. J Physiol (Lond) 1997;502:189–201. doi: 10.1111/j.1469-7793.1997.189bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz DB, Steinmetz JE. Single-unit evidence for eye-blink conditioning in cerebellar cortex is altered, but not eliminated, by interpositus nucleus lesions. Learn Mem. 1997;3:88–104. doi: 10.1101/lm.4.1.88. [DOI] [PubMed] [Google Scholar]
- 29.Kleim JA, Vij K, Ballard DH, Greenough WTJ. Learning-dependent synaptic modifications in the cerebellar cortex of the adult rat persist for at least four weeks. J Neurosci. 1997;17:717–721. doi: 10.1523/JNEUROSCI.17-02-00717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LaLonde R. Cerebellar contributions to instrumental learning. Neurosci Biobehav Rev. 1994;18:161–170. doi: 10.1016/0149-7634(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 31.Lavond DG, Steinmetz JE. Acquisition of classical conditioning without cerebellar cortex. Behav Brain Res. 1989;33:113–164. doi: 10.1016/s0166-4328(89)80047-6. [DOI] [PubMed] [Google Scholar]
- 32.Lavond DG, Steinmetz JE, Yokaitis MH, Thompson RF. Reacquisition of classical conditioning after removal of cerebellar cortex. Exp Brain Res. 1987;67:569–593. doi: 10.1007/BF00247289. [DOI] [PubMed] [Google Scholar]
- 33.Leiner HC, Leiner AL, Dow RS. Does the cerebellum contribute to mental skills? Behav Neurosci. 1986;100:443–454. doi: 10.1037//0735-7044.100.4.443. [DOI] [PubMed] [Google Scholar]
- 34.Leiner HC, Leiner AL, Dow RS. Reappraising the cerebellum: what does the hindbrain contribute to the forebrain? Behav Neurosci. 1989;103:998–1008. doi: 10.1037//0735-7044.103.5.998. [DOI] [PubMed] [Google Scholar]
- 35.Llinas RR, Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol (Lond) 1980;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Llinas RR, Welsh JP. On the cerebellum and motor learning. Curr Opin Neurobiol. 1993;3:958–965. doi: 10.1016/0959-4388(93)90168-x. [DOI] [PubMed] [Google Scholar]
- 37.Linden DJ, Connor JA. Participation of postsynaptic PKC in cerebellar long-term depression in culture. Science. 1992;254:1656–1659. doi: 10.1126/science.1721243. [DOI] [PubMed] [Google Scholar]
- 38.Logan CG, Grafton ST. Functional anatomy of human eyeblink conditioning determined with regional cerebral glucose metabolism and positron-emission tomography. Proc Natl Acad Sci USA. 1995;92:7500–7504. doi: 10.1073/pnas.92.16.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matzel LD, Collin C, Alkon DL. Biophysical and behavioral correlates of memory storage, degradation, and reactivation. Behav Neurosci. 1992;106:954–963. doi: 10.1037//0735-7044.106.6.954. [DOI] [PubMed] [Google Scholar]
- 40.McCormick DA, Thompson RF. Cerebellum: essential involvement in the classically conditioned eyelid response. Science. 1984;223:296–299. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- 41.Meiri N, Ghelardini C, Tesco G, Galeotti N, Dahl D, Tomsic D, Cavallaro S, Quattrone A, Capaccioli S, Bartolini A, Alkon DL. Reversible antisense inhibition of Shaker-like Kv1.1 potassium channel expression impairs associative memory in mouse and rat. Proc Natl Acad Sci USA. 1997;94:4430–4434. doi: 10.1073/pnas.94.9.4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Midtgaard J. Processing of information from different sources: spatial synaptic integration in the dendrites of vertebrate CNS neurons. Trends Neurosci. 1994;17:166–173. doi: 10.1016/0166-2236(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 43.Midtgaard J. Spatial synaptic integration in Purkinje cell dendrites. J Physiol (Paris) 1995;89:23–32. doi: 10.1016/0928-4257(96)80548-1. [DOI] [PubMed] [Google Scholar]
- 44.Midtgaard J, Lasser-Ross N, Ross WN. Spatial distribution of Ca2+ influx in turtle Purkinje cell dendrites in vitro: role of a transient outward current. J Neurophysiol. 1993;70:2455–2469. doi: 10.1152/jn.1993.70.6.2455. [DOI] [PubMed] [Google Scholar]
- 45.Molchan SE, Sunderland T, McIntosh AR, Herscovitch P, Schreurs BG. A functional anatomical study of associative learning in humans. Proc Natl Acad Sci USA. 1994;91:8122–8126. doi: 10.1073/pnas.91.17.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moyer JR, Jr, Thompson LT, Disterhoft JF. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J Neurosci. 1996;16:5536–5546. doi: 10.1523/JNEUROSCI.16-17-05536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. J Neurosci. 1993;13:1708–1718. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pongs O. Receptor sites for open channel blockers of shaker voltage-gated potassium channels: molecular approaches. J Recept Res. 1993;13:503–512. doi: 10.3109/10799899309073675. [DOI] [PubMed] [Google Scholar]
- 49.Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988;25:729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- 50.Sanchez-Andres JV, Alkon DL. Voltage-clamp analysis of the effect of classical conditioning on the hippocampus. J Neurophysiol. 1991;65:796–807. doi: 10.1152/jn.1991.65.4.796. [DOI] [PubMed] [Google Scholar]
- 51.Scharenberg AM, Olds JL, Schreurs BG, Craig AM, Alkon DL. Protein kinase C redistribution within CA3 stratum oriens during acquisition of NM conditioning in the rabbit. Proc Natl Acad Sci USA. 1991;88:6637–6641. doi: 10.1073/pnas.88.15.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schreurs BG. Classical conditioning of model systems: a behavioral review. Psychobiology. 1989;17:145–155. [Google Scholar]
- 53.Schreurs BG. Long-term memory and extinction of the classically conditioned rabbit nictitating membrane response. Learn Motiv. 1993;24:293–302. [Google Scholar]
- 54.Schreurs BG, Alkon DL. US-US conditioning of the rabbit’s nictitating membrane response: emergence of a conditioned response without alpha conditioning. Psychobiology. 1990;18:312–320. [Google Scholar]
- 55.Schreurs BG, Alkon DL. Memory storage mechanisms, conservation across species. In: Adelman G, Smith BH, editors. Neuroscience year: supplement 2 to the encyclopedia of neuroscience. Birkhauser; Boston: 1992. pp. 99–101. [Google Scholar]
- 56.Schreurs BG, Alkon DL. Rabbit cerebellar slice analysis of long-term depression and its role in classical conditioning. Brain Res. 1993;631:235–240. doi: 10.1016/0006-8993(93)91540-9. [DOI] [PubMed] [Google Scholar]
- 57.Schreurs BG, Sanchez-Andres JV, Alkon DL. Learning-specific differences in Purkinje-cell dendrites of lobule HVI (lobulus simplex): intracellular recording in a rabbit cerebellar slice. Brain Res. 1991;548:18–22. doi: 10.1016/0006-8993(91)91100-f. [DOI] [PubMed] [Google Scholar]
- 58.Schreurs BG, Sanchez-Andres JV, Alkon DL. GABA-induced responses in Purkinje-cell dendrites of the rabbit cerebellar slice. Brain Res. 1992;597:79–87. doi: 10.1016/0006-8993(92)91510-l. [DOI] [PubMed] [Google Scholar]
- 59.Schreurs BG, Oh MM, Alkon DL. Pairing-specific long-term depression of Purkinje cell excitatory postsynaptic potentials results from a classical conditioning procedure in the rabbit cerebellar slice. J Neurophysiol. 1996;75:1051–1060. doi: 10.1152/jn.1996.75.3.1051. [DOI] [PubMed] [Google Scholar]
- 60.Schreurs BG, Tomsic D, Gusev PA, Alkon DL. Dendritic excitability microzones and occluded long-term depression after classical conditioning of the rabbit’s nictitating membrane response. J Neurophysiol. 1997a;77:86–92. doi: 10.1152/jn.1997.77.1.86. [DOI] [PubMed] [Google Scholar]
- 61.Schreurs BG, McIntosh AR, Bahro M, Herscovitch P, Sunderland T, Molchan SE. Lateralization and behavioral correlation of changes in regional cerebral blood flow with classical conditioning of the human eyeblink response. J Neurophysiol. 1997b;77:2153–2163. doi: 10.1152/jn.1997.77.4.2153. [DOI] [PubMed] [Google Scholar]
- 62.Storm J. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- 63.Supple WF, Jr, Kapp BS. The anterior cerebellar vermis: essential involvement in classically conditioned bradycardia in the rabbit. J Neurosci. 1993;13:3705–3711. doi: 10.1523/JNEUROSCI.13-09-03705.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teune TM, Van Der Burg J, De Zeeuw CI, Voogd J, Ruigrok TJH. Single Purkinje cell can innervate multiple classes of projection neurons in cerebellar nuclei of the rat: a light microscopic and ultrastructural triple-tracer study in the rat. J Comp Neurol. 1998;392:164–178. doi: 10.1002/(sici)1096-9861(19980309)392:2<164::aid-cne2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 65.Thompson LT, Moyer JR, Jr, Disterhoft JE. Transient changes in excitability of rabbit CA3 neurons with a time course appropriate to support memory consolidation. J Neurophysiol. 1996;76:1836–1849. doi: 10.1152/jn.1996.76.3.1836. [DOI] [PubMed] [Google Scholar]
- 66.Thompson RF. The neurobiology of learning and memory. Science. 1986;233:941–947. doi: 10.1126/science.3738519. [DOI] [PubMed] [Google Scholar]
- 67.Thompson RF. Neural mechanisms of classical conditioning in mammals. Philos Trans R Soc Lond B Biol Sci. 1990;329:161–170. doi: 10.1098/rstb.1990.0161. [DOI] [PubMed] [Google Scholar]
- 68.Thompson RF, Krupa DJ. Organization of memory traces in the mammalian brain. Annu Rev Neurosci. 1994;17:519–549. doi: 10.1146/annurev.ne.17.030194.002511. [DOI] [PubMed] [Google Scholar]
- 69.Welsh JP, Harvey JA. Cerebellar lesions and the nictitating mem-brane reflex: performance deficits of the conditioned and unconditioned response. J Neurosci. 1989;9:299–311. doi: 10.1523/JNEUROSCI.09-01-00299.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woody CD, Gruen E, Birt D. Changes in membrane currents during Pavlovian conditioning of single cortical neurons. Brain Res. 1991;539:76–84. doi: 10.1016/0006-8993(91)90688-r. [DOI] [PubMed] [Google Scholar]
- 71.Yeo CH, Hardiman MJ, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. I. Lesions of the cerebellar nuclei. Exp Brain Res. 1985a;60:87–98. doi: 10.1007/BF00237022. [DOI] [PubMed] [Google Scholar]
- 72.Yeo CH, Hardiman MJ, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. II. Lesions of the cerebellar cortex. Exp Brain Res. 1985b;60:99–113. doi: 10.1007/BF00237023. [DOI] [PubMed] [Google Scholar]
- 73.Yeo CH, Hardiman MJ, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. IIl. Connections of cerebellar lobule HVI. Exp Brain Res. 1985c;60:114–126. doi: 10.1007/BF00237024. [DOI] [PubMed] [Google Scholar]