Abstract
Visual excitation in rhabdomeric photoreceptors is thought to be mediated by activation of a light-regulated phospholipase C (PLC) and the consequent hydrolysis of phosphatidylinositol bisphosphate. Whereas much attention has been devoted to inositol trisphosphate (IP3) production and intracellular Ca2+ release, little is known about the possible role of the DAG branch in the generation of the light response. We have tested the effect of chemically distinct surrogates of DAG on isolatedLima photoreceptors. Application of the phorbol ester PMA (0.5–10 μm) or the alkaloid (−)-indolactam (20–100 μm) from a holding potential of −50 mV elicited an inward current, several hundred picoamperes in amplitude, accompanied by a pronounced increase in membrane conductance. The stereoisomers 4α-PMA and (+)-indolactam were both inactive, arguing for the specificity of the effects. Elevation of cytosolic Ca2+ by intracellular dialysis accelerated this current, whereas chelerythrine antagonized it, suggesting the involvement of PKC. The reversal potential of the membrane current induced by PKC activators was approximately +10 mV; replacement of extracellular Na with impermeantN-methyl-d-glucamine decreased its amplitude and shifted the reversal potential in the negative direction. Stimulation by PMA and (−)-indolactam was accompanied by a pronounced depression of light responsiveness; conversely, steady illumination reduced the size of the current elicited by these PKC activators. Taken together, these results support the notion that the DAG branch of the PLC cascade, in addition to its suggested participation in visual adaptation, may play a role in the activation of the photoresponse or a component thereof, probably in synergy with IP3-mediated Ca2+ release.
Keywords: visual excitation, PKC, diacylglycerol, rhabdomeric photoreceptors, light-dependent conductance, calcium
A detailed understanding of the mechanisms by which photon absorption elicits a photocurrent in rhabdomeric photoreceptors remains elusive, although substantial evidence points to the critical involvement of a light-regulated phospholipase C (PLC). Central pieces of information were the isolation of a blind Drosophila mutant, norpA, (Pak et al., 1969), together with the demonstration that this gene encodes a PLC expressed in the retina (Bloomquist et al., 1988), and that both PLC activity and light responses are rescued in norpA mutants induced to express the norpA protein (McKay et al., 1995). Light-induced hydrolysis of phosphatidylinositol bisphosphate (PIP2) has been shown in squid (Baer and Saibil, 1988) and Drosophila (Devary et al., 1987), and most of the research on photoexcitation mechanisms has focused on the resulting production of inositol trisphosphate (IP3) (Szuts et al., 1986; Brown et al., 1987) and the consequent release of Ca2+ from internal stores (Brown and Rubin, 1984;Payne et al., 1986b). However, despite the well documented excitatory effects of both IP3 (Brown et al., 1984; Fein et al., 1984) and Ca2+ (Payne et al., 1986a; Shin et al., 1993), this branch of the PLC cascade alone has proved insufficient to fully account for the photoresponse: (1) buffering intracellular calcium changes slows the photocurrent in Limulus, but does not abolish it (Lisman and Brown, 1975; Payne et al., 1986a); (2) low molecular weight heparin, an inhibitor of IP3-induced Ca2+ release, selectively reduces only the early component of the photoresponse (Frank and Fein, 1991; Faddis and Brown, 1993); and (3) recent results indicate that a null mutation for the allegedly sole isoform of IP3 receptor inDrosophila eyes does not adversely affect the light response (Acharya et al., 1997). An additional messenger substance, DAG, is generated by PIP2 hydrolysis; the prime effector of DAG is activation of PKC, which in turn phosphorylates a variety of target proteins. In Drosophila, a null mutation for a photoreceptor-specific PKC (inaC) (Smith et al., 1991) lacks normal deactivation of the light response (Smith et al., 1991; Hardie et al., 1993) and also appears to suffer from a deficit in the light adaptation process (Hardie et al., 1993). To date, however, the possible involvement of PKC stimulation in the activation of any of the membrane conductance mechanisms regulated by light has received comparatively little attention. Nonetheless, some seminal observations in this direction were briefly described by Brown et al. (1991). These authors demonstrated that injection of the DAG surrogates into barnacle photoreceptors caused a transient depolarization; the I–Vrelationship of this response resembles that of the light-induced current. We have used whole-cell clamp recording of membrane current from isolated rhabdomeric photoreceptors of Lima scabra to investigate the consequences of manipulating the DAG branch of the PLC cascade on the membrane conductance, and we examined the interactions with light stimulation and with alterations of the level of intracellular calcium. A preliminary version of this report has been presented in abstract form (Gomez and Nasi, 1997).
MATERIALS AND METHODS
Enzymatically dissociated rhabdomeric photoreceptors fromLima scabra were obtained using the protocols described previously (Nasi, 1991a). After plating in a recording flow chamber mounted on the stage of an inverted microscope (ICM-405, Zeiss), the photoreceptors were continuously superfused (1 ml/min) with artificial seawater (ASW); a system of manifolds permitted the exchange of the superfusate and was used to control the ionic composition of the bath. To apply test substances extracellularly, we relied on a local perfusion technique consisting of a puffer micropipette positioned in the vicinity of the cell that could pressure eject a stream of test solution on activation of a solenoid-operated valve. Previous measurements obtained with a fluorescent dye included in the puffer pipette demonstrated that ∼95% of solution exchange is attained within 200–400 msec, and that the ejection plume completely engulfs the region occupied by the target cell, as visualized with an image-intensified CCD camera (Gomez and Nasi, 1996a). Alternatively, in some experiments, test compounds were administered intracellularly by dialysis via the patch electrode.
Whole-cell patch-clamp recordings were performed as described previously (Nasi, 1991b). All cell manipulations were performed under dim near infrared illumination (>715 nm; Andover). Signals were low-pass-filtered at 100 Hz for chemically activated currents and 1000 Hz for light-evoked currents, using a Bessel 4-pole filter. Records were digitized on-line at a sampling rate of 200 Hz to 3 KHz. Photostimulation was provided by a conventional optical rail delivering small spots of light (∼200 μm in diameter); the maximum effective intensity corresponded to 58 × 1014photons · sec−1 · cm−2at 500 nm, as measured with a calibrated radiometer (United Detector Technology, Hawthorne, CA). Neutral-density filters were interposed to adjust incident light intensity. Voltage- and light-stimuli were applied by a microprocessor-controlled programmable stimulator (Stim 6; Ionoptix, Milton, MA).
Changes in cytosolic Ca2+ were detected using the low-affinity fluorescent indicator calcium green 2 (Ca green 2; Molecular Probes, Eugene, OR). The octapotassium salt of the probe was dissolved in the intracellular solution filling the patch electrode at a concentration of 65–100 μm. Excitation light was provided by a 75 W xenon arc lamp (PTI, South Brunswick, NJ) filtered by a dichroic reflector to reject wavelengths longer than 670 nm (Omega Optical, Brattleboro, VT) and by an interference filter (480 nm, 40 nm bandwidth; Chroma Technology, Brattleboro, VT). The beam was brought to the epi-illumination port of the microscope via a liquid light guide (Oriel, Stratford, CT); the dichroic reflector in the microscope turret had a cutoff wavelength of 505 nm. Emission light collected by a 100×, 1.3 NA oil-immersion objective (Nikon) was filtered sequentially by an additional dichroic to reject λ >610 nm and by a 535 nm interference filter with a 50 nm bandwidth (all supplied by Chroma). An adjustable rectangular mask (Nikon) located at a conjugated image plane was positioned under infrared visualization to restrict the collection of fluorescence emission to the region of interest (usually the light-transducing rhabdomeric lobe of an isolated photoreceptor) to minimize background light. The fluorescence signal was detected by a photomultiplier tube (R4220 PHA; Hammamatsu, Bridgewater, NJ) operated at 800 V in photon-counting mode using a window discriminator and a rate meter (F-100T and PRM-100, respectively; Advanced Research Instruments, Boulder, CO). An analog voltage proportional to the counts accumulated in bins of programmable duration (typically, 10−3 sec) was fed to the analog-to-digital interface of the computer.
Solutions. ASW contained (in mm): 480 NaCl, 10 KCl, 10 CaCl2, 49 MgCl2, 10 HEPES, and 5.5 glucose, pH 7.8 (NaOH).N-methyl-d-glucamine (NMDG) replaced sodium in Na-free seawater, whereas in low-Ca2+ ASW (250 μm) and in nominally Ca-free artificial seawater (0-Ca ASW), calcium was replaced with magnesium on an equimolar basis. A solution lacking Ca2+ and with Mg reduced to 1 mm was also used; in this case, the concentration of NaCl was increased to 567 mm. Phorbol 12-myristate 13-acetate (PMA), 4α-PMA, (+)-indolactam, and chelerythrine were purchased from Alexis Corporation/LC Laboratories (Woburn, MA). (−)-Indolactam V was obtained both from Alexis and from Calbiochem (San Diego, CA). These substances were dissolved in DMSO at 10–50 mm, aliquoted, and kept at −75°C. Synthetic inositol 1,4,5 trisphosphate (IP3) was purchased from Alexis, dissolved in a 10 mm stock solution, aliquoted, and kept at −75°C.
The standard intracellular solution used to fill whole-cell micropipettes contained 200 mm K-glutamate, 100 mm KCl, 22 mm NaCl, 5 mm Mg ATP, 10 mm HEPES, 1 mm EGTA, 100 μm GTP, and 300 mm sucrose, pH 7.3. The internal solution with elevated Ca2+ had a similar composition except that 0.8 mm CaCl2 was added to it, yielding an estimated free calcium concentration of 1 μm, according to the program Chelator (courtesy of Dr. Theo Shoenmakers, University of Nijmegen, The Netherlands).
RESULTS
PKC activators stimulate a membrane conductance
PMA, one of the most potent phorbol ester activators of PKC (for review, see Nishizuka, 1984), was first tested by local superfusion. Figure 1 shows the effect of pressure ejecting PMA at a concentration of 1 μm from a micropipette located ∼50 μm from a cell. The photoreceptor was maintained in darkness and voltage clamped at −50 mV. Repetitive rectangular voltage steps (3 mV peak to peak) were superimposed on the holding potential to monitor changes in membrane resistance. After several seconds of application of PMA, an inward current gradually developed, reaching an amplitude of ∼360 pA. Concomitantly, the size of the current steps elicited by the voltage pulses grew (Fig. 1,insets), indicating a conductance increase from a basal value of 1.6–18.5 nS (an increment of >620%). All cells tested in this manner exhibited a similar response. At a holding potential of −50 mV, the mean amplitude of the inward current was 391 ± 102 pA (±SD;n = 6); the conductance of the membrane increased by 18.2 ± 7.2 nS. The latency of the response, measured as the time required to reach 10% of the maximum inward current (t10%), was 61 ± 32 sec. Little recovery could be observed after interrupting delivery of the PMA, at least within the useful temporal window for recording (usually 20–30 min). This limitation, unfortunately, precluded the possibility of repeated tests on a given cell.
Fig. 1.
Activation of a membrane current by PMA.A, A photoreceptor cell was voltage clamped at −50 mV, with a repetitive 3 mV rectangular pulse superimposed on the holding potential. A puffer pipette containing 1 μm PMA in ASW (final DMSO concentration 0.1%) was positioned ∼50 μm away from the cell. The thick bar indicates pressure ejection. A large inward current was elicited. The change in the current steps (insets) indicates that an increase of membrane conductance occurred. B, Lack of effects of dialysis with normal internal solution (top trace), pressure-application of ASW, ASW containing 5% DMSO, and 1 μm 4α-PMA. In the last trace, no voltage command steps were superimposed on the holding potential; the downward deflections are quantum bumps. Calibration: 1 min. C, Inward current elicited by internal dialysis with 0.5 μmPMA via the patch pipette. A similar inward current, as inA, was elicited, but with a reduced latency.Insets illustrate the increase in conductance during the development of the inward current.
In vitro, PMA has been shown to be effective as a PKC activator at nanomolar concentrations (Nishizuka, 1984); because of the mode of application we used, it is difficult to gauge the amount of PMA reaching its target sites in the interior of the cell. Nevertheless, it appears that the responses shown above were nearly saturating, because similar tests conducted with 10 μm PMA produced only marginally larger currents (average amplitude 536 ± 90 pA;n = 3). This value was not statistically significantly different from that obtained with 1 μm.
The specificity of the effects of PMA were assessed in several ways, as shown in Figure 1B. The top trace in Figure 1B was simply recorded in the dark with a standard intracellular solution immediately after gaining access to the cell interior and confirms that in the absence of additional manipulations the holding current remains stable. To rule out possible confounding effects of the puffer that may stem from the mechanical strain induced by the turbulence during pressure ejection, control cells were tested with a puffer pipette containing ASW; ejection of a stream of solution onto these photoreceptors produced no detectable change in holding current (n = 7) (Fig1B, second trace). Because PMA had to be dissolved initially in DMSO, we also examined possible effects of this solvent. Control measurements were therefore performed by pressure ejecting ASW containing DMSO at concentrations of 1%, 2%, and 5% (n = 2, 2, and 5, respectively) onto cells voltage clamped at −50 mV in the dark. As shown in Figure1B, third trace, the holding current was unaffected by this treatment, although the DMSO concentration was 5- to 10-fold higher than in the test solutions containing PMA (typically <0.5% − 1%). Finally, we conducted similar experiments using the 4α isomer of PMA, which is nearly inert and thus serves as a good negative control. Figure 1B, bottom trace, demonstrates that application of 4α-PMA at 1 μmproduced virtually no change in membrane current (average 21 ± 11 pA; n = 4). We also tested the effect of direct intracellular application of PMA instead of puffing. To this end, PMA was included in the internal solution (0.5–1 μm) and dialyzed through the patch microelectrode. Previous measurements using the same cells demonstrated that small molecules in the pipette-filling solution equilibrate with the cytosolic compartment of the rhabdomeric lobe and in fact reach the sites where light-dependent ion channels are located (Gomez and Nasi, 1996a). Figure 1C shows that shortly after gaining access to the cell interior, a large inward current was elicited; the size of the responses obtained (mean amplitude 277 ± 123 pA; n = 3) was no larger than those produced by extracellular administration, but the time necessary to reach 10% of the final amplitude was significantly shorter (over fivefold briefer) (11.9 ± 6 sec; n = 3), with virtually no latent period. Intracellular application of 1% DMSO without PMA was ineffective.
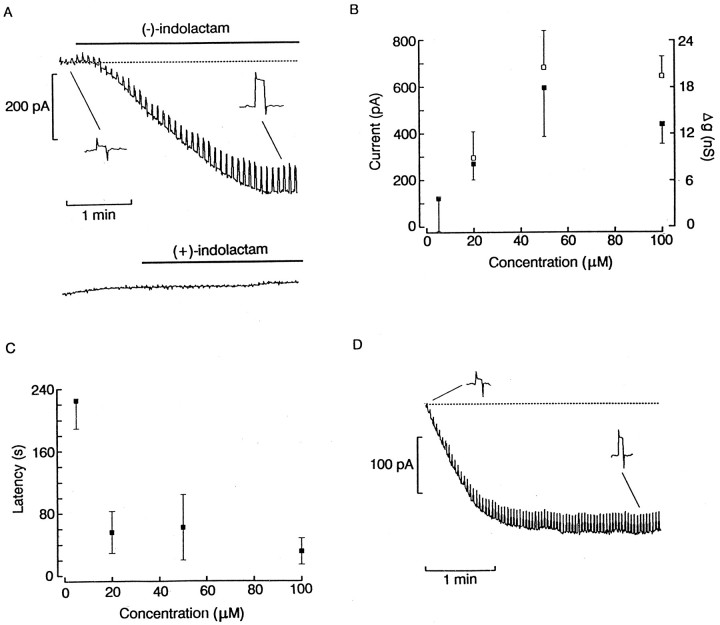
To support the notion that the observed effects of phorbol esters indeed result from an interaction with the DAG branch of the PLC cascade, we examined the effects of a chemically distinct substance that has a similar stimulatory action on PKC. Indolactam V is an alkaloid activator of PKC, presumed to bind at the same site on the enzyme at which phorbol esters act (Heikkilä and Åkerman, 1989). Figure 2A shows an experiment in which (−)-indolactam, the active stereoisomer, was applied in the dark by ejection from a puffer pipette positioned near a photoreceptor cell voltage clamped at −50 mV. The concentration of indolactam was 100 μm; a square wave perturbation of the command voltage (3 mV) was used to monitor changes in membrane conductance. Several seconds after activating the puffer pipette, an inward current developed (peak amplitude ∼400 pA), accompanied by a conspicuous increase in membrane conductance. Similar effects were observed in all seven photoreceptors stimulated with 100 μm (−)-indolactam; the mean amplitude of the response was 415 ± 54 pA, and the observed latency was 31.4 ± 14 sec. To test for specificity of the effects of indolactam, control experiments were performed with the mirror-image molecule (+)-indolactam; this stereoisomer is relatively inert and is ∼100-fold weaker in competing with PMA in binding assays (Fujiki et al., 1984) and in activating PKC-mediated processes (Heikkilä and Åkerman, 1989). Figure 2A, bottom trace, shows that extracellular administration of (+)-indolactam at the same concentration of 100 μm failed to induce any change in membrane current or in membrane conductance (n = 3). We also examined the effect of varying the concentration of (−)-indolactam in the range between 5 and 100 μm. Figure 2B summarizes the obtained data pooled from 22 cells (n = 5–7 in each group). The amplitude of the membrane current and the conductance changes appear to reach saturation at concentrations <50 μm; the latency of the response (t10%) also displayed a marked dose dependency (Fig. 2C), becoming shorter at higher concentrations, which is an effect that probably reflects in part the increased rate of entry of the compound into the cell. Overall, all of the 22 photoreceptors examined under these conditions and stimulated with different concentrations of indolactam at −50 mV responded with an inward current of variable amplitude and, whenever measured, the membrane conductance increased. This compound was also effective when applied intracellularly. As shown in Figure 2D, dialysis of 50 μm (−)-indolactam V evoked inward currents of similar characteristics; the amplitude was not larger than that measured with local superfusion (average 338 ± 235 pA;n = 4; mean increase in membrane conductance 19.6 ± 12 nS), but the latencies were dramatically reduced (t10% 4.0 ± 0.9 sec).
Fig. 2.
Effect of indolactam V on membrane current.A, Local superfusion with 100 μm(−)-indolactam of a rhabdomeric cell held at −50 mV in the dark caused a large inward current accompanied by an increase in membrane conductance. Administration of the stereoisomer (+)-indolactam at the same concentration was ineffective (bottom trace).B, Effects of different concentrations of indolactam on the peak amplitude of the inward current elicited at −50 mV (filled squares) and membrane conductance (open squares). Error bars indicate SD. The response appears to saturate at <50 μm. C, Time required to reach 10% of the maximum amplitude of the current, plotted as a function of concentration. D, Inward current obtained by administering 50 μm (−)-indolactam intracellularly. Notice the dramatic reduction in the latency of the inward current.
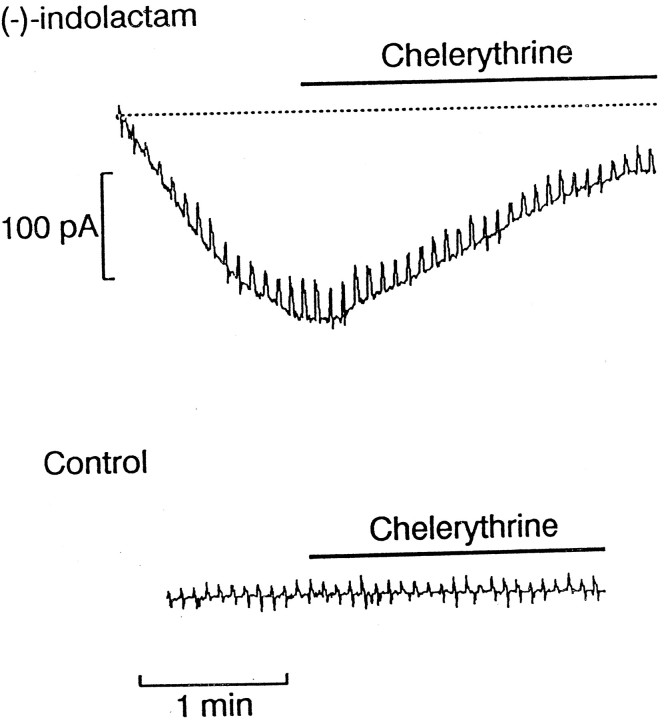
To determine whether the membrane currents observed with administration of these DAG surrogates indeed involved activation of PKC, we examined the effect of treating cells with an inhibitor of PKC on the response to indolactam. Chelerythrine is a highly specific PKC antagonist with half-maximal effectiveness in the submicromolar range and a >100-fold selectivity for PKC over either PKA or Ca-CaM PK (Herbert et al. 1990). To assess the ability of chelerythrine to interfere with the response elicited by PKC activators, we first dialyzed (−)-indolactam internally, causing the development of an inward current atVh −50 mV, and then applied 50 μmchelerythrine with a puffer pipette. As shown in Figure3, pressure ejection of chelerythrine reduced the inward current and caused the membrane conductance to decrease toward the basal level. A similar effect was obtained in three of four photoreceptors tested in this manner. The average reduction in the indolactam-induced inward current was 49 ± 27%. In the absence of indolactam, application of chelerythrine did not affect the holding current (Fig. 3, bottom trace) and appeared not to depress the peak amplitude of the photocurrent (data not shown).
Fig. 3.
DAG surrogates act via PKC activation. To ascertain whether the current induced by administration of indolactam is mediated by its ability to activate PKC, photoreceptors were first stimulated with 50 μm (−)-indolactam V applied by internal dialysis. Once a conspicuous inward current developed at −50 mV, a puffer pipette was used to pressure eject 50 μm of the PKC inhibitor chelerythrine. After several seconds of application of the antagonist, the inward current and the associated increase in membrane conductance were substantially reversed. Bottom trace shows that application of chelerythrine to a control cell dialyzed with the normal internal solution was ineffective.
Interaction of PKC activators with calcium and with light stimulation
Activation of PKC by DAG is profoundly facilitated by the presence of elevated levels of cytosolic calcium (Nishizuka, 1984). We examined the effects of internally dialyzing photoreceptors with a solution containing 1 μm free Ca before external application of PKC activators. Figure4A shows typical traces obtained by puffing 5 μm indolactam in the dark, using either the standard Ca2+-free internal solution (Fig. 4, −) or one containing elevated Ca2+ (Fig.4, +). In the presence of high Ca2+, the response amplitude was significantly enhanced (plateau not shown in Fig. 4), but the most dramatic effect was an acceleration of the action of indolactam: compared with the Ca2+-free intracellular solution, the latency decreased from 225 ± 35 sec (n = 5) to 72 ± 29.7 sec (n = 6). Figure 4B, top, shows the membrane currents from four different cells internally perfused with the high Ca2+ solution and stimulated with a higher concentration of indolactam (50 μm). In this case, the latency was 9.3 ± 10.7 sec (n = 8) compared with 61 ± 36 sec for 0 Cai (n = 7), but the elevated Ca2+ did not increase the steady-state size of the response, probably because of saturation. Control measurements using the high Ca2+ internal solution without indolactam (Fig. 4B, bottom) failed to reveal any development of a net macroscopic current and only induced a progressive increase in membrane noise. A two-factor ANOVA indicated that the effects of internal calcium on response latency were statistically highly significant (p < 0.01), and so was the interaction of indolactam × Ca2+ (p < 0.01). A qualitatively similar though less pronounced effect of Ca2+ was observed with PMA, although the comparison was only done at a single saturating concentration (10 μm): elevated internal Ca2+ decreased the latency by 38% (n = 3 in each condition).
Fig. 4.
Facilitation of the effects of PKC activators by elevation of intracellular Ca2+. A, Recordings were obtained from two different photoreceptor cells internally dialyzed with Ca-free solution (−) and with 1 μm free calcium (+), respectively. The inward current evoked by local application of 5 μm indolactam (top thick bar) had a much more rapid onset and a larger amplitude when an internal solution with elevated calcium was used.B, Responses elicited by 50 μm indolactam in four different cells dialyzed with high Ca2+. In all cases the inward current attained its peak amplitude within 1 min.Bottom traces are from four control cells dialyzed with 1 μm free calcium without indolactam application.C, Mean latency (t10%) of the inward current evoked by the two concentrations of indolactam in the presence or absence of Ca2+ in the internal solution. Error bars indicate the SD.
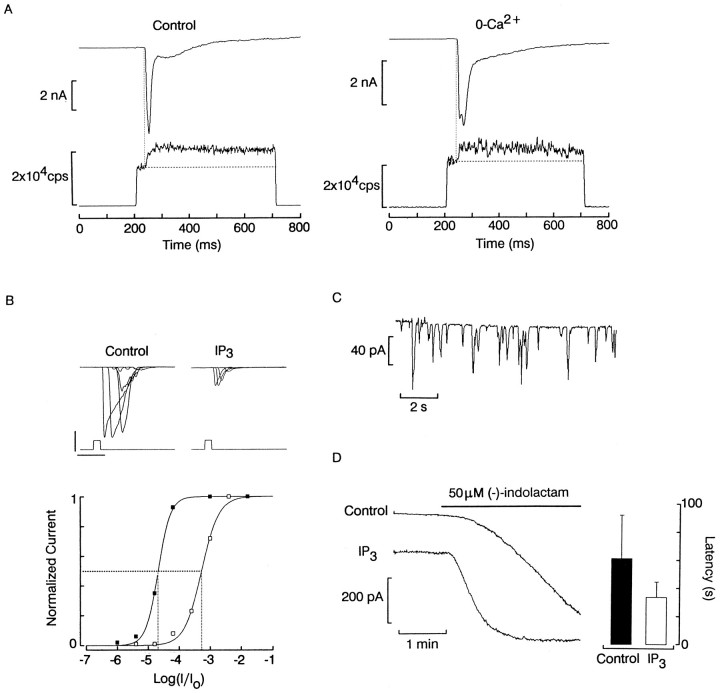
The physiological relevance of the above observation is that in other microvillar photoreceptors a pronounced increase in cytosolic calcium accompanies the light response (Brown and Blinks, 1974). We ascertained that a similar phenomenon occurs in Lima rhabdomeric photoreceptors. Figure 5Ashows the simultaneous recording of membrane current and fluorescence from a 5 × 5 μm window encompassing only the rhabdomeric lobe of an isolated photoreceptor that was loaded with Ca green 2 via the patch electrode and voltage clamped at −50 mV. The optical signal jumped abruptly at the onset of the excitation light, and after a latency of ∼30 msec, a marked increase in fluorescence occurred, coincident with the activation of the saturating photocurrent. Similar results were obtained in four additional cells. Control measurements in which the excitation light was presented under light-adapted conditions (i.e., shortly after another stimulus) failed to evoke a light response, and the fluorescence signal only exhibited a square transition (data not shown). Conversely, the use of Ca-insensitive fluorescent compounds, such as fluorescein, also resulted in a square optical signal, although a robust photocurrent was elicited (n = 3). These observations provide assurance that the kinetics of the Ca green 2 signal recorded in dark-adapted cells indeed reflect a light-induced increase in cytosolic Ca2+, concomitant with the visual excitatory response. Figure 5Bshows a similar recording obtained from a photoreceptor bathed for 8 min in 0-Ca ASW; a similar Ca2+ rise accompanies the light response (n = 3). The same result was also obtained during superfusion with 0 Ca2+/1 mm Mg2+ solution (n = 2). These observations indicate that the photo-induced Ca2+ increase is attributable to, at least in part, release from internal stores.
Fig. 5.
Light-induced Ca2+ release and effects of IP3. A, Left, Simultaneous recording of membrane current (top trace) and fluorescence (bottom trace) in a photoreceptor loaded with 100 μm Ca Green 2 and voltage-clamped at −50 mV. At the onset of the excitation light step (500 msec, 480 nm), the optical signal jumped abruptly to a baseline level, and ∼30 msec later a secondary increase in fluorescence was observed, reflecting the rise in cytosolic calcium. Right, A similar result was obtained in a cell bathed for many minutes in a Ca-free solution.B, Light-intensity series obtained with the standard intracellular solution or one containing 10 μmIP3, respectively; access to the cell interior was through the somatic lobe. The normalized peak amplitude of the photocurrent plotted as a function of light intensity (bottom) reveals a marked desensitization induced by IP3. Calibration: 800 pA, 400 msec. C, Oscillatory inward current evoked by dialysis with IP3directly into the rhabdomeric lobe of a photoreceptor. The recording started immediately on rupturing the patch of membrane to gain access to the cell interior. D, Left, Comparison of the current elicited in the dark by puffer application of 50 μm (−)-indolactam (horizontal bar) in a cell internally dialyzed with the standard intracellular solution and one dialyzed with 10 μm IP3.Right, Histogram comparing the mean latency (t10%) of the indolactam-evoked current in control and IP3-treated photoreceptors (n = 7 and 9, respectively).
The conclusion that the Ca2+ elevation results from intracellular release suggests that, like in other invertebrate photoreceptors, light may mobilize IP3; inLimulus, IP3 rapidly elevates Ca2+, leading to desensitization of the light response, and if injected into the rhabdomeric lobe, it also evokes bursts of inward current (for review, see Payne et al., 1988). Figure5B shows intensity series obtained in a control cell and one dialyzed with 10 μm IP3 into the soma. In the latter, the light response was significantly depressed; the plot in Figure 5B, bottom, shows that sensitivity shifted by ∼1.35 log. A similar reduction of responsiveness was observed in four additional photoreceptors. Internal perfusion through the villous rhabdomeric lobe proved challenging; nevertheless, Figure 5Cshows a successful instance in which bursts of inward current, often exceeding 80 pA in amplitude, occurred as soon as the patch of membrane was ruptured. These results indicate that IP3 is likely to play a similar role in Lima as in other rhabdomeric photoreceptors. We then compared the effect of IP3 on the conductance induced by application of (−)-indolactam. Figure5D shows the membrane current evoked in the dark by puffing 50 μm indolactam in a control cell and in one dialyzed with 10 μm IP3. IP3 significantly shortened the latency of the response to indolactam. Pooling the data from several cells in each condition (Fig. 5D) showed that the effect qualitatively resembles that of 1 μmintracellular Ca2+ (Fig. 4C), although less pronounced.
Although the results presented above indicate that application of PKC activators reliably induces a membrane conductance in rhabdomeric photoreceptor cells, a central question concerns a possible relationship of these effects to the visual excitation process. We took several approaches to examine this issue. To the extent that PKC activators tap an effector mechanism that is also involved in the generation of the photocurrent, one would expect to find an interaction between application of these chemical agents and the effects of photostimulation. We first tested this proposition by comparing the effects of light flashes before and after the induction of a current by either indolactam or PMA. Figure6A shows typical photoresponses obtained under voltage clamp at −50 mV. Under control conditions (Fig. 6A, bottom trace), the peak amplitude of the near-saturating current was several nanoamperes; after comparable responses were repetitively obtained, indolactam (20 μm) was locally applied in the dark, inducing an inward current of ∼500 pA (data not shown). Subsequent presentation of another identical flash only evoked a much reduced photoresponse (Fig.6A, top trace). In all photoreceptors exposed to indolactam under various conditions (n > 20), a marked depression of the light response was observed; the reduction in light sensitivity, measured as the intensity required to elicit a criterion response amplitude, was ∼1.5 log. A comparable effect was also observed in cells stimulated with PMA, either extracellularly (1 μm; n = 4) or by internal dialysis (0.5 μm; n = 3).
Fig. 6.
Interactions between light and PKC activators.A, Depression of the photocurrents by application of (−)-indolactam (50 μm). A photoreceptor cell was voltage clamped at −50 mV and stimulated with 100 msec flashes of light (3.7 × 1014photons · sec−1 · cm−2); subsequently, indolactam was applied by a puffer pipette, evoking a sustained inward current (data not shown). Delivery of another identical flash (top trace) elicited a dramatically reduced photocurrent. B, Effect of steady light on the amplitude and latency of the response to indolactam. The recordings at the top illustrate the responses evoked by 50 μm indolactam (thick lines) in two cells maintained in the dark (left) and two cells continuously illuminated (3.7 × 1014photons · sec−1 · cm−2).Bottom shows the mean steady-state current amplitude (filled bars) and the mean latency (t10%) (open bars) pooled for six cells in each of the two conditions. Error bars indicate SD.
A complementary approach entails comparing the effects of indolactam application in the dark versus during sustained illumination. In the latter case, the light was turned on 2–3 min before the beginning of the recording to ensure a near steady-state of the light response and to minimize any slow drift in membrane current that may result from further light adaptation. Figure 6B, top traces, shows responses elicited by puffing 50 μmindolactam, either in the dark or during steady illumination (in different cells). Figure 6B, bottom, shows average data for several cells tested in each of the two conditions. The response amplitude (Fig. 6B, filled bars) was reduced nearly threefold in the presence of light [from 594 ± 208 pA (n = 7) to 198 ± 89 pA (n = 6); p < 0.02 by the Wilcoxon test] (Hollander and Wolfe, 1973). It is also worth noting that during light the response latency (29 ± 3.3 sec; n = 6) was ∼57% shorter than in darkness (67 ± 41 sec), this difference being statistically significant at p = 0.05. Considering that in rhabdomeric cells photostimulation triggers an elevation of cytosolic Ca2+ (Brown and Blinks, 1974), the observed acceleration is in agreement with the results described above and illustrated in Figure 4 in which photoreceptors were intracellularly dialyzed with solutions containing a high Ca2+ concentration before the administration of PKC activators.
Ionic basis of the current induced by PMA and indolactam
The conduction and selectivity properties of the current evoked by PKC activators were briefly examined. Figure7A, top, illustrates the effect of omitting extracellular sodium: replacement of Na with NMDG resulted in a significantly reduced amplitude of the inward current evoked by application of 50 μm indolactam at Vm = −50 mV. Figure 7A,bottom, summarizes the data obtained from three cells tested in each condition; the average peak current in ASW was 632 ± 32 pA, whereas that in 0 Na was 114 ± 49 pA. As shown in Figure7B, we examined the effect of Na removal on the reversal voltage of the current evoked by PKC activators. Figure 7B,top traces, shows the effect of puffing (−)-indolactam in ASW at two holding voltages (in different cells); at +30 mV a conspicuous outward current was elicited (n = 3), whereas at +10 mV a minute inward current is still detectable. Similar results were obtained with PMA (n = 3: 2 cells tested at +30, 1 at +10 mV). The large size of the outward current at a holding potential only ∼20 mV positive of Vrevsuggests a pronounced outward rectification (compare with the average current at −50 mV shown in Fig. 7A). Figure 7B,bottom, shows that replacement of extracellular sodium with NMDG markedly affected the reversal potential: at 0 mV the current elicited by indolactam is already outwardly directed (n= 3). Testing at −30 mV failed to reveal a consistent response (Fig.7B, bottom, bottom trace), whereas at voltages more negative than −40 mV the response was inward (data not shown; n = 4). Exact determination of the reversal voltage is difficult, because each test must be performed in a different cell so that variability in responsiveness across photoreceptors precludes the use of interpolation. Nevertheless, one can define the range in which responses invert polarity and estimate an approximate shift of Vrev after Na removal on the order of 40 mV. The observation that in Na-free conditions an inward current can be evoked at membrane potentials substantially more positive than EK indicates the participation of other ions; calcium is an obvious candidate. Interestingly, when the extracellular Ca2+ concentration in ASW is reduced to 250 μm, an inward response is evoked by puffing PMA, but its peak amplitude (mean 237 ± 38 pA; n = 3) was significantly less than in normal ASW (p < 0.05, one-sided Wilcoxon rank-sum test).
Fig. 7.
Effect of external sodium removal on the current induced by PKC activators. A, Top, Replacement of Na with NMDG reduced the amplitude of the inward current evoked by application of 50 μm indolactam (thick horizontal lines). Membrane potential: −50 mV. Calibration: 100 pA, 1 min. Bottom, pooled data for several cells stimulated in the presence and absence of Na (n = 3 in each condition). Error bars indicate SD. B,Top, Reversal of the indolactam-evoked current in ASW. The membrane potential was clamped at the levels indicated; the traces were recorded from different cells. Bottom, Shift in the reversal voltage during superfusion with Na-free solution (replaced with NMDG).
DISCUSSION
The present results demonstrate that structurally unrelated DAG surrogates applied to rhabdomeric Lima photoreceptors induce a marked increase in membrane conductance and an ionic current, which, at a holding voltage near the cells’ resting potential, is inwardly directed. Similar excitatory effects are obtained with extracellular local perfusion or with intracellular dialysis of such compounds, but in the latter case the onset of the current is significantly more rapid. The ineffectiveness of a variety of control treatments, including the administration of inert stereoisomers of both indolactam and of PMA, argues for specificity of these effects. The relative potency of PMA and (−)-indolactam V for inducing an increase in membrane conductance in rhabdomeric photoreceptors mirrors their differential effectiveness as activators of protein kinase C inin vitro assays (Heikkilä and Åkerman, 1989). Moreover, the membrane current elicited by indolactam could be partially reversed by administration of chelerithrine, suggesting that the observed effects are indeed mediated by activation of a PKC. Elevation of intracellular calcium, which in vivo acts in synergy with DAG to activate PKC, produced a strong facilitation of the effect of both indolactam and PMA by dramatically shortening the latency of the evoked current. Administration of IP3 also accelerates the onset of the response to indolactam. Because light stimulation leads to an increase in [Ca2+]i in the rhabdomeric lobe, which is attributable to release from internal stores (Fig. 5), the modulatory effect of calcium is likely to be physiologically relevant.
Light responsiveness of the photoreceptors is significantly decreased after stimulation with PKC activators; this effect is in line with the proposition that PKC plays an important role in light adaptation, as suggested from studies with PKC-deficient mutant flies (Hardie et al., 1993). However, the converse effect was also obtained; that is to say, the indolactam-evoked membrane currents are reduced when this substance is applied during illumination. The latter finding suggests that in addition to desensitization, some mutually occlusive action of these two stimulatory treatments exists with respect to activation of membrane ion channels. This observation is compatible with the notion that PKC activators also tap some effector mechanism that is controlled by light stimulation. Several characteristics of the current evoked by PKC activators are indeed remindful of the light response. Its reversal potential (Erev) is several millivolts positive of 0 mV and is displaced in the negative direction after replacing extracellular Na with impermeant NMDG; the magnitude of this shift, a few tens of millivolts, is reminiscent of that of the photocurrent (ΔErev = ∼47 mV; Gomez and Nasi, 1996a), indicating that a sizable portion of the PCK activator-induced current is carried by sodium ions. Furthermore, the large outward currents measured at +30 mV indicate a substantial rectification in the outward direction.
A pertinent question, therefore, concerns a possible scheme that encompasses a role for DAG in the visual excitation process, in addition to the well documented involvement of IP3 and Ca2+. One suggestion is inspired by work conducted by Payne and Fein (1986) in Limulus ventral photoreceptors. These authors examined the leading edge of the photocurrent and demonstrated a marked supralinearity of its rate of rise as a function of the density of impinging photons; this acceleration was antagonized by Ca buffers. They proposed a model in which two parallel cascades are triggered by light, one that controls light-dependent channels and the other that accelerates the rate of the first, and suggested that the accelerating agent could be calcium. Faddis and Brown (1993)examined the effects of intracellular injection of heparin and BAPTA into Limulus ventral photoreceptors and also concluded that visual excitation can proceed in the absence of IP3-induced increases in [Ca2+]i, albeit with a reduction in gain, speed of transduction, and adaptation. It would be tempting to speculate that the DAG branch of the light-triggered cascade could control light-dependent membrane conductance changes, whereas the IP3 branch would modulate the gain and speed of the response by providing the appropriate increase in enzymatic reaction rates via Ca2+.
In principle, the present results are compatible with such a view, whose appeal is obvious. However, at least in its simplest form, this scheme presents shortcomings that deserve closer scrutiny: although the maximum size of the current elicited by PKC activators inLima is substantial (hundreds of picoamperes), it is still considerably smaller than the peak-amplitude of the photocurrent evoked by a flash of saturating intensity (several nanoamperes). One possibility that could account for the quantitative discrepancy in the effectiveness of the two types of stimulation (under the assumption that light and PKC activators indeed converge onto a common target mechanism) is that whereas light is delivered in a nearly instantaneous manner, chemicals applied either by superfusion or by intracellular dialysis require tens of seconds to reach their target sites, and their local concentration rises only gradually. It is conceivable that such slow onset of the stimulus allows for adaptation to set in in such a way that it is not possible to observe the full-blown response. Alternatively, the current activated by DAG surrogates may only be a component of the whole photoresponse. Over the last few years, evidence has accumulated for the existence of multiple light-dependent conductance mechanisms that contribute to the photocurrent in rhabdomeric photoreceptors of several species, includingLima (Nasi, 1991b), Limulus (Deckert et al., 1992), Drosophila (Hardie and Minke, 1992), andHermissenda (Detwiler, 1976). The relative contribution of the various components to the total photocurrent is quite heterogeneous, and in Lima the early transient is typically several-fold larger than the slower “tail,” although the extent of temporal overlap precludes an exact estimate. It is conceivable that PKC activators interact with the photocurrent-generating machinery, triggering the smaller component. This possibility is plausible because if DAG and PKC controlled the main component of the light response, PKC inhibitors would be predicted to exert a strong overall antagonistic effect; by contrast, our initial measurements with chelerythrine failed to consistently show a decrease of the peak amplitude of the light response. This result is also in line with the observation that ininaC mutants the size of the photocurrent does not appear to be systematically lower than in wild type (Smith et al., 1991; Hardie et al., 1993). Another possibility is that the target of PKC activators is the slow light-evoked inward current that we have recently described in Lima (Gomez and Nasi, 1996b); this conductance develops in response to bright lights a few seconds after the termination of the rapid complex photoresponse. Its sluggish time course, requirement for saturating levels of stimulation, and calcium permeability are reminiscent of certain Ca-depletion activated currents found in many cells that use the IP3 signaling pathway (for review, seeFasolato et al., 1994). In other systems, such as Xenopusoocytes and insulin-secreting cell lines, it has in fact been suggested that PKC activation is intimately involved in the control of such ionic mechanisms (Bode and Göke, 1994; Petersen and Berridge, 1994).
For now, positive identification of the conductance elicited by PKC activators in Lima photoreceptors with a specific light-dependent ionic mechanism remains challenging, because their respective reversal voltages differ only by several millivolts, and our measurements presently lack the sensitivity required to make such fine distinctions. Moreover, ionic manipulations are poor tools to help discriminate among relatively unselective channels, and no pharmacological tools are yet available to dissect them. In any case, the present observations are strongly suggestive of an involvement of the diacylglycerol branch of the light-triggered PLC cascade in the control of ion channels and could lead to a clarification of some of the persisting complexities concerning the generation of the light response in rhabdomeric cells.
Footnotes
This work was supported by National Institutes of Health Grant RO1 07559.
Correspondence should be addressed to Dr. Maria del Pilar Gomez, Department of Physiology, Boston University School of Medicine, 80 East Concord Street, Boston, MA, 02118.
REFERENCES
- 1.Acharya JK, Jalink K, Hardy RW, Hartenstein V, Zuker CS. InsP3 receptor is essential for growth and differentiation but not for vision in Drosophila. Neuron. 1997;18:881–887. doi: 10.1016/s0896-6273(00)80328-1. [DOI] [PubMed] [Google Scholar]
- 2.Baer KM, Saibil HR. Light- and GTP-activated hydrolysis of phosphatidylinositol bisphosphate in squid photoreceptor membranes. J Biol Chem. 1988;263:17–20. [PubMed] [Google Scholar]
- 3.Bloomquist BT, Shortridge RD, Schneuwly S, Perdew M, Montell C, Steller H, Rubin G, Pak WL. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988;54:723–733. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- 4.Bode H-P, Göke B. Protein kinase C activates capacitative calcium entry in the insulin secreting cell line RINm5F. FEBS Lett. 1994;339:307–311. doi: 10.1016/0014-5793(94)80436-2. [DOI] [PubMed] [Google Scholar]
- 5.Brown JE, Blinks JR. Changes in intracellular free calcium concentration during illumination of invertebrate photoreceptors. J Gen Physiol. 1974;64:643–665. doi: 10.1085/jgp.64.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown JE, Watkins DC, Malbon CC. Light-induced changes in the content of inositol phosphate in squid (Loligo pealei) retina. Biochem J. 1987;247:293–297. doi: 10.1042/bj2470293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JE, Rubin LJ. A direct demonstration that inositol-trisphosphate induces an increase in intracellular calcium in Limulus photoreceptors. Biochem Biophys Res Commun. 1984;125:1137–1142. doi: 10.1016/0006-291x(84)91402-5. [DOI] [PubMed] [Google Scholar]
- 8.Brown JE, Rubin LJ, Ghalayni AJ, Tarver AP, Irvine RF, Berridge MJ, Anderson RE. Myo-inositol polyphosphate may be a messenger for visual excitation in Limulus photoreceptors. Nature. 1984;311:160–163. doi: 10.1038/311160a0. [DOI] [PubMed] [Google Scholar]
- 9.Brown HM, Burnham J, Smolley J. Diacylglycerol surrogates induce membrane currents in Balanus photoreceptors [abstract]. Biophys J. 1991;59:530. [Google Scholar]
- 10.Deckert A, Nagy K, Helrich CS, Stieve H. Three components in the light-induced current of the Limulus ventral photoreceptor. J Physiol (Lond) 1992;453:69–96. doi: 10.1113/jphysiol.1992.sp019219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Detwiler PB. Multiple light-evoked conductance changes in the photoreceptors of Hermissenda crassicornis. J Physiol (Lond) 1976;256:691–708. doi: 10.1113/jphysiol.1976.sp011346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devary O, Heichal O, Blumenfeld A, Cassel D, Suss E, Barash S, Rubinstein CT, Minke B, Selinger Z. Coupling of photoexcited rhodopsin to inositol phospholipid hydrolysis in fly photoreceptors. Proc Natl Acad Sci USA. 1987;84:6939–6943. doi: 10.1073/pnas.84.19.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faddis MN, Brown JE. Intracellular injection of heparin and polyamines. Effects on phototransduction in Limulus ventral photoreceptors. J Gen Physiol. 1993;101:909–931. doi: 10.1085/jgp.101.6.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fasolato C, Innocenti B, Pozzan T. Receptor-activated Ca2+ influx: how many mechanisms for how many channels? Trends Pharmacol Sci. 1994;15:77–83. doi: 10.1016/0165-6147(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 15.Fein A, Payne R, Corson WD, Berridge MJ, Irvine RF. Photoreceptor excitation and adaptation by inositol 1,4,5-trisphosphate. Nature. 1984;311:157–160. doi: 10.1038/311157a0. [DOI] [PubMed] [Google Scholar]
- 16.Frank TM, Fein A. The role of the inositol phosphate cascade in visual excitation of invertebrate microvillar photoreceptors. J Gen Physiol. 1991;97:697–723. doi: 10.1085/jgp.97.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujiki H, Suganuma M, Nakayasu M, Tahira T, Endo Y, Shudo K, Sugimura T. Structure-activity studies on synthetic analogs (indolactams) of the tumor-promoter teleocidin. Gann. 1984;75:866–870. [PubMed] [Google Scholar]
- 18.Gomez M, Nasi E. Ion permeation through light-activated channels in rhabdomeric photoreceptors: role of divalent cations. J Gen Physiol. 1996a;107:715–730. doi: 10.1085/jgp.107.6.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez M, Nasi E. A slow current induced by light in rhabdomeric photoreceptors: possible relation to Icrac [abstract]. Biophys J. 1996b;70:359. [Google Scholar]
- 20.Gomez M, Nasi E. Excitatory effects of activators of PKC on rhabdomeric photoreceptors[abstract]. Biophys J. 1997;72:92. [Google Scholar]
- 21.Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- 22.Hardie RC, Peretz A, Suss-Toby E, Rom-Glas A, Bishop SA, Selinger Z, Minke B. Protein kinase C is required for light adaptation in Drosophila photoreceptors. Nature. 1993;363:634–637. doi: 10.1038/363634a0. [DOI] [PubMed] [Google Scholar]
- 23.Heikkilä J, Åkerman KEO. (−)-Indolactam V activated protein kinase C and induces changes in muscarinic receptor functions in SH-SY5Y human neuroblastoma cells. Biochem Biophys Res Commun. 1989;162:1207–1213. doi: 10.1016/0006-291x(89)90802-4. [DOI] [PubMed] [Google Scholar]
- 24.Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- 25.Hollander M, Wolfe DA. Nonparametric statistical methods. Wiley; New York: 1973. [Google Scholar]
- 26.Lisman JE, Brown JE. Effects of intracellular injection of calcium buffers on light adaptation in Limulus ventral photoreceptors. J Gen Physiol. 1975;66:489–506. doi: 10.1085/jgp.66.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKay RR, Chen D-M, Miller K, Kim S, Stark WS, Shortridge RD. Phospholipase C rescues visual defect in norpA mutant of Drosophila melanogaster. J Biol Chem. 1995;270:13271–13276. doi: 10.1074/jbc.270.22.13271. [DOI] [PubMed] [Google Scholar]
- 28.Nasi E. Electrophysiological properties of isolated photoreceptors from the eye of Lima scabra. J Gen Physiol. 1991a;97:17–34. doi: 10.1085/jgp.97.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nasi E. Two light-dependent conductances in the membrane of Lima photoreceptor cells. J Gen Physiol. 1991b;97:55–72. doi: 10.1085/jgp.97.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumor promotion. Nature. 1984;308:693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- 31.Pak WL, Grossfield J, White NV. Nonphototactic mutants in a study of vision in Drosophila. Nature. 1969;222:351–354. doi: 10.1038/222351a0. [DOI] [PubMed] [Google Scholar]
- 32.Payne R, Fein A. The initial response of Limulus ventral photoreceptors to bright flashes: released calcium as synergist to excitation. J Gen Physiol. 1986;87:243–269. doi: 10.1085/jgp.87.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Payne R, Corson DW, Fein A. Pressure injection of calcium both excites and adapts Limulus ventral photoreceptors. J Gen Physiol. 1986a;88:107–126. doi: 10.1085/jgp.88.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Payne R, Corson DW, Fein A, Berridge MJ. Excitation and adaptation in Limulus ventral photoreceptors by inositol 1,4,5-trisphosphate result from a rise in intracellular calcium. J Gen Physiol. 1986b;88:127–142. doi: 10.1085/jgp.88.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Payne R, Walz B, Levy S, Fein A. The localization of calcium release by inositol trisphosphate in Limulus photoreceptors and its control by negative feedback. Philo Trans R Soc Lond B Biol Sci. 1988;320:359–379. doi: 10.1098/rstb.1988.0082. [DOI] [PubMed] [Google Scholar]
- 36.Petersen CCH, Berridge MJ. The regulation of capacitative calcium entry by calcium and protein kinase C in Xenopus oocytes. J Biol Chem. 1994;269:32246–32253. [PubMed] [Google Scholar]
- 37.Shin J, Richard EA, Lisman JE. Ca2+ is an obligatory intermediate in the excitation cascade of Limulus photoreceptors. Neuron. 1993;11:845–855. doi: 10.1016/0896-6273(93)90114-7. [DOI] [PubMed] [Google Scholar]
- 38.Smith DP, Ranganathan R, Hardy RW, Marx J, Tsuchida T, Zuker CS. Photoreceptor deactivation and retinal degeneration mediated by a photoreceptor-specific protein kinase C. Science. 1991;254:1478–1484. doi: 10.1126/science.1962207. [DOI] [PubMed] [Google Scholar]
- 39.Szuts EZ, Wood S, Reid MS, Fein A. Light stimulates the rapid formation of inositol trisphosphate in squid retinas. Biochem J. 1986;240:929–932. doi: 10.1042/bj2400929. [DOI] [PMC free article] [PubMed] [Google Scholar]