Abstract
In the present study, we have characterized properties of steroid withdrawal using a pseudopregnant rat model. This paradigm results in increased production of endogenous progesterone from ovarian sources and as such is a useful physiological model. “Withdrawal” from progesterone induced by ovariectomy on day 12 of pseudopregnancy resulted in increased anxiety, as determined by a decrease in open arm entries on the elevated plus maze compared to control rats and pseudopregnant animals not undergoing withdrawal. Similar findings were obtained 24 hr after administration of a 5α-reductase blocker to a pseudopregnant animal, suggesting that it is the GABAA-modulatory 3α-OH-5α-pregnan-20-one (3α,5α-THP) that produces anxiogenic withdrawal symptoms. Twenty-four hours after steroid withdrawal, the time constant for decay of GABAA-gated current was also reduced sixfold, assessed using whole- cell patch-clamp procedures on pyramidal neurons acutely dissociated from CA1 hippocampus. Thus, 3α,5α-THP withdrawal results in a marked decrease in total GABAA current, a possible mechanism for its anxiogenic, proconvulsant sequelae. In addition, 3α,5α-THP withdrawal resulted in insensitivity to the normally potentiating effect of the benzodiazepine lorazepam (LZM) on GABAA-gated Cl− current. This withdrawal profile is similar to that reported for other GABAA-modulatory drugs such as the benzodiazepines (BDZs), barbiturates, and ethanol. These changes were also associated with significant two and threefold increases in both the mRNA and protein for the α4 subunit of the GABAA receptor, respectively, in hippocampus. The pseudopregnancy paradigm may be a useful model for periods of endogenous 3α,5α-THP withdrawal such as premenstrual syndrome and postpartum or postmenopausal dysphoria, when increased emotional lability and BDZ insensitivity have been reported.
Keywords: GABAA receptor, rat, allopregnanolone, progesterone, α-4 subunit, decay time constant, elevated plus maze, kinetics, GABAA-gated current
We have previously reported (Gallo and Smith, 1993; Costa et al., 1995; Smith et al., 1998) that the endogenous steroid progesterone produces withdrawal symptoms via its GABAA-modulatory metabolite 3α-OH-5α-pregnan-20-one (3α,5α-THP). Upon abrupt discontinuation after chronic administration of progesterone via injection or implant, increased anxiety (Gallo and Smith, 1993) and proconvulsant (Smith et al., 1998) effects are observed. Anxiogenic behavior has been demonstrated after progesterone withdrawal using light/dark transition (Gallo and Smith, 1993) and the defensive burying paradigm (Gallo and Smith, 1993), as well as the elevated plus maze (Moran et al., 1996). This anxiogenic withdrawal property could be prevented by previous administration of a 3α-hydroxysteroid oxidoreductase blocker indomethacin (Gallo and Smith, 1993), suggesting that it is in fact the GABAA-modulatory 3α,5α-THP that produces these withdrawal signs. In a similar manner, proconvulsant effects of progesterone withdrawal have been observed (Smith et al., 1998) as assessed by seizure activity measures: severity, frequency, latency, and duration after injection of convulsant drugs, picrotoxin, or a β-carboline benzodiazepine (BDZ) inverse agonist. These withdrawal properties of the GABAA-modulatory 3α,5α-THP are similar to those reported for other positive GABAAmodulators, such as the BDZs, barbiturates, and ethanol (Finley and Nolan, 1989; File, 1990; Kokka et al., 1993).
The GABAA-modulatory 3α,5α-THP metabolite enhances GABAA-gated chloride current in hippocampus and spinal cord, as demonstrated in vitro (Majewska et al., 1986;Twyman and Macdonald, 1992), and GABAA-mediated inhibition of cerebellar Purkinje cells assessed in vivo after systemic administration of progesterone (Smith et al., 1987a,b; Smith, 1989,1994). Acutely, in vitro application of 3α,5α-THP increases binding of the BDZ flunitrazepam to hippocampal and cortical membranes (Majewska et al., 1986). Chronically administered in cell cultures at high doses, 3α,5α-THP can prevent modulation of GABAA-gated current by BDZs and barbiturates (Roca et al., 1990; Yu and Ticku, 1995). These findings suggest a striking similarity between 3α,5α-THP and other GABAA-modulatory drugs.
The purpose of the present study was to use a physiological model for producing sustained elevations in circulating levels of progesterone and 3α,5α-THP before steroid withdrawal to characterize anxiogenic and cross-tolerance withdrawal properties of endogenous neuroactive steroids. The advantage of the pseudopregnant model is that endogenous progesterone production by the ovaries is both within physiological levels and is episodic (Kim and Greenwald, 1986; Robinson et al., 1981), a condition that may alter the potency of tolerance development and withdrawal properties, as has been shown for the BDZs (Gallagher et al., 1986). Withdrawal from 3α,5α-THP can be induced either by ovariectomy or by injection of a 5α-reductase blocker.
In the present study we tested the hypothesis that steroid withdrawal produces increased anxiety and changes in sensitivity to BDZ modulation of GABAA-gated current, assessed electrophysiologically and behaviorally. In addition, alterations in the kinetics of GABAA-gated current, as well as potential changes in GABAA receptor subunits relevant for the observed electrophysiological findings, were investigated after steroid withdrawal from elevated, circulating progesterone and 3α,5α-THP levels produced by pseudopregnancy. This paradigm may be a useful and a more physiological rat model of both premenstrual and postpartum conditions than use of injection or implanted capsule.
MATERIALS AND METHODS
Subjects
Female Long–Evans rats (Harlan Sprague Dawley, Indianapolis, IN), 26 d old on arrival, were housed in groups of three under a reverse light/dark cycle (14/10 hr light/dark). Food and water were available ad libitum in an environmentally controlled animal facility. Rats were treated and tested during the dark part of the circadian cycle and were handled only during these times as well. The stage of the estrous cycle was evaluated by microscopic evaluation of the vaginal lavage, as described (Smith et al., 1987b).
Hormone and drug administration
Pseudopregnancy. Pregnant mare serum gonadotropin (Sigma, St. Louis, MO) dissolved in saline was injected intraperitoneally (20 IU/0.2 ml) on postnatal day 27 (Robinson et al., 1981; Kim and Greenwald, 1986). Human chorionic gonadotropin (Sigma) was injected (10 IU/0.2 ml) on postnatal day 29, which was also considered day 0 of pseudopregnancy. All ovariectomies were conducted under halothane anesthesia.
Progesterone withdrawal: pseudopregnancy. On day 11 of pseudopregnancy, the rats were ovariectomized, and ovaries were weighed. Increased ovarian weight was correlated with elevated serum 3α,5α-THP levels (r = 0.788) and was considered, for our purposes, as evidence of increased progesterone release. (Ovarian weight was increased in 90% of the cases.) In intact, nonovariectomized rats, serum progesterone and CNS 3α,5α-THP levels are well correlated (Corpechot et al., 1993). These rats were tested on day 12, 24 hr after withdrawal from elevated hormone levels associated with pseudopregnancy (pseudopregnant wd). Results were compared with controls, with rats ovariectomized on day 26, and with intact pseudopregnant animals (day 12).
MK-906 or indomethacin: pseudopregnancy. In some pseudopregnant groups, rats were given MK-906 (50 mg/kg in oil, i.p.; Merck, Darmstadt, Germany) on day 11 of pseudopregnancy to inhibit the metabolism of progesterone. Testing on the elevated plus maze occurred on day 12, as described below, to test the hypothesis that the withdrawal properties of progesterone were attributable to its GABAA-modulatory metabolite, as we have previously shown (Gallo and Smith, 1993; Costa et al., 1995; Smith et al., 1998).
Hormone levels. Serum and brain tissue from various pseudopregnant wd, pseudopregnant, and control groups was collected. Concentration of the progesterone metabolite 3α,5α-THP was determined by RIA (Purdy et al., 1990; Frye et al., 1998) following a methanol extraction.
Behavioral experiments
Variable speed treadmill locomotion test. Tests were conducted on a variable speed treadmill (Smith and Chapin, 1996) in a small room lit by an incandescent lamp. The performance of the rats on the treadmill was recorded with a Beta Max recorder. Each rat was habituated to the treadmill for 5 min before testing. Rats were scored for 5 min on their ability to maintain position while locomoting on a treadmill with speed randomly varied between 4–11 cm/sec. Frequencies of (1) position slip (slipping back or “riding” the treadmill) and (2) motor error (bumping into the back of the treadmill) were determined before and 20 min after an injection of lorazepam (LZM; 0.75 mg/kg, i.p.; Sanofi Co.). A significant increase in either or both of these parameters was considered a sedative response to the BDZ, which produces decreased locomotor activity (File, 1981).
Elevated plus maze test. Tests on this apparatus were conducted in a room with fluorescent lighting and low to minimal noise. The plus maze consists of two open arms, 50 × 10 cm, and two enclosed arms, 50 × 10 × 40 cm, with an open roof, arranged so that the two open arms and the two closed arms are opposite each another. The maze is elevated to a height of 50 cm. On the day of testing, rats were brought to the room housing the maze. Each rat was placed at the center of the maze facing an open arm. The frequency and duration of open and closed arm entries were recorded for 5 min by an observer seated nearby to the maze. An entry into an arm was noted when the rat placed both forelimbs into the arm. The maze was wiped clean with 70% ethanol after testing each rat. Anxiogenic effects of progesterone withdrawal were assessed based on the frequency of open arm entries versus closed entries as well as the time spent in the open arms (Pellow and File, 1985; Bitran and Dowd, 1996; Moran et al., 1996). Decreased time spent on the open arms and a low frequency of open arm entries relative to control animals is consistent with an increase in anxious behavior (Pellow and File, 1985).
Electrophysiology
Pyramidal neurons were acutely isolated from CA1 hippocampus 24 hr after ovariectomy or injection of MK-906 (progesterone and/or 3α,5α-THP withdrawal, respectively). Tissue was digested at 32°C for 50–60 min under a pure oxygen atmosphere in PIPES-buffered saline containing (in mm): NaCl 120, KCl 5, CaCl2 1, MgCl2 1, d-glucose 25, PIPES 20, and trypsin (type XI, Sigma), 0.8 mg/ml, pH 7.0. After a 1 hr enzyme-free incubation at room temperature, cells were isolated by trituration in 1 ml of 20 mm HEPES-buffered DMEM. The cell suspension was then plated onto a 35 mm Petri dish, and the cells were allowed to settle 7–10 min before the DMEM was replaced with recording medium.
GABAA-activated current was recorded at room temperature (20–25°C) in a bath solution containing (in mm): NaCl 120, CsCl 5, CaCl2 2, MgCl2 1, TEA Cl 15, 4-aminopyridine 5, HEPES 10, and glucose 25, pH 7.4, 320 mOsm/kg H2O. The pipette solution contained (in mm):N-methyl-d-glucamine chloride 120, Cs4 BAPTA 5, and Mg ATP 5. The ATP regeneration system Tris phosphocreatinine (20 mm) and creatine kinase were added to minimize GABA rundown.
GABAA-gated Cl− currents (10 μm GABA) were recorded in these cells using whole-cell patch-clamp techniques at a holding potential of −50 mV with a Medical Systems Axopatch-1D amplifier. GABAA-evoked currents were filtered at 10 kHz (−3 dB, eight-pole low-pass Bessel filter) and digitally sampled at 500 μsec per point using the pClamp 5.51 software package (Axon Instruments, Foster City, CA). Drug delivery was accomplished via a solenoid-activated rapid superfusion system positioned within 50 μm of the cell that released drugs for 20 msec at 1–3 min intervals to prevent GABA rundown. Peak GABAA-gated current was calculated as GABA potentiation (or inhibition) for all drug concentrations as (IGABA drug − IGABA control)/(I GABA control). Drugs included LZM, N-methyl-β-carboline-3-carboxamide (β-CC), RO15-1788, and 3α,5α-THP (all drugs except RO15-1788 from Research Biochemicals, Natick, MA).
Kinetics of GABAA-gated current.GABAA-gated currents (10 μm GABA) were recorded from acutely isolated CA1 hippocampal pyramidal cells (5–10 msec application) using whole-cell patch-clamp techniques at a holding potential of −50 mV, as described above. Analysis of current kinetics was accomplished with the Origin software package (Microcal Software Inc.) using a pCLAMP transfer module; time constants for rate of deactivation were determined using a monoexponential decay function and linear curve fitting. SE was determined using χ2analysis. [This fit was accepted when the sum of squared errors (R2 value) was <0.95]. Current time course was approximated from the peak current to 90% of recovery.
Western blot analysis
Rats were killed, brains were removed, and hippocampus was dissected and homogenized, as described (Towbin et al., 1979; Smith et al., 1998). Hippocampal membranes electrophoresed onto 9% SDS polyacrylamide gels were transferred to a polyvinyl difluoride membrane (Bio-Rad, Hercules, CA). The α4 subunit of the GABAAreceptor was detected with a rabbit antibody (characterized by Smith et al., 1998) against a peptide [amino acids (aa) 517–523 with an N-terminal cysteine] of rat α4 as a 67 kDa band (Kern and Sieghart, 1994; Genosys, TX). The γ2 subunit of the GABAA receptor was detected with an antibody (characterized by Smith et al., 1998) against a peptide (aa 316–352) of rat γ2 as a 48 kDa band (Mossier et al., 1994; Genosys). Negative controls included the use of preimmune serum (data not shown), as well as blockade with α4 or γ2 peptide (Genosys). Membranes were incubated for 1–2 hr at room temperature with a 1:5000 dilution of the antibody, followed by a 1:5000 dilution of horseradish peroxidase-conjugated donkey anti-rabbit IgG (Amersham, Arlington Heights, IL). Immunoreactive band densities from the gel for α4 and γ2 subunits were quantified using a CCD camera (Umax Scanner) and one-Dscan software, which uses multiple Gaussian fits to achieve accurate band density profiles. Results were standardized to a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control protein (36 kDa band).
Semiquantitative reverse transcriptase polymerase chain reaction
Oligonucleotide primers (Tyndale et al., 1994) for α4 (503 bp) and γ2 (311 bp) subunits of the GABAA receptor (Operon Inc.) were used with a control primer, GAPDH (657 bp). Total RNA was isolated using Tri reagent and a chloroform extraction and was followed by reverse transcription (SuperScript II RNase RT, Life Technologies, Gaithersburg, MD). cDNA containing identical amounts of total RNA was subjected to PCR amplification at 23–30 cycles (Lau et al., 1993) using two different concentrations of cDNA (25 or 50 ng). Amplified cDNA (20–40 μl) was removed for 3.6% NuSieve agarose gel electrophoresis (FMC Bioproducts, Rockland, ME). Ethidium bromide-stained DNA bands (single band per PCR product) were calibrated against a molecular weight marker, and band density was quantified as described above, standardized to the GAPDH control (Lau et al., 1993). Values were averaged from both cDNA concentrations at the lower cycle number known to be in the linear range.
Statistical analysis
Differences between groups were assessed using the Student’st test (two groups) or standard ANOVA and Student–Newman–Keuls (>2 groups) post hocprocedures. In cases where the data did not fit a normal distribution (>2 groups), the nonparametric procedure, Kruskal–Wallis one-way ANOVA on ranks, was implemented to determine differences between experimental and control groups. Significance was determined whenp < 0.05.
RESULTS
3α,5α-THP levels during pseudopregnancy
CNS levels of 3α,5α-THP assessed on day 12 of pseudopregnancy (Table 1) were increased to 13.20 ± 0.48 ng/gm, significantly above control values taken from a rat ovariectomized on postnatal day 26 (1.75 ± 0.06 ng/gm). Ovariectomy on day 11 of pseudopregnancy reduced 3α,5α-THP levels to 3.37 ± 0.7 ng/gm, suggesting that the increase in circulating levels of this steroid is from an ovarian source. Injection of the 5α-reductase blocker MK-906 (50 mg/kg) also significantly reduced 3α,5α-THP levels to 4.84 ± 0.59 ng/gm. These results suggest that induction of pseudopregnancy, followed by ovariectomy or injection of MK-906, is a valid method to produce sustained increased levels of 3α,5α-THP before withdrawal.
Table 1.
Levels of 3α,5α-THP
| CNS(ng/gm) | Serum (ng/ml) | |
|---|---|---|
| Pseudopregnancy, day 10 | 40 ± 6.2* | |
| Pseudopregnancy, day 12 | 13.20 ± 0.48* | 28 ± 3.5* |
| Pseudopregnancy + MK-906 | 4.84 ± 0.59 | 9 ± 2 |
| Pseudopregnancy and ovx, day 11 | 3.37 ± 0.70 | 3.8 ± 0.5 |
| Control | 1.75 ± 0.06 | 11 ± 2.8 |
CNS and serum blood levels of 3α,5α-THP are elevated during pseudopregnancy as demonstrated by RIA results. Ovariectomy or injection of a 5α-reductase blocker (MK-906) on day 11 of pseudopregnancy significantly lowered levels of 3α,5α-THP, assessed on day 12, below those measured on days 10 and 12 of pseudopregnancy, ovx, Ovariectomized. (n = 4–6 animals per group, assessed in triplicate).
*p < 0.05 versus MK-906, ovx, and control.
Progesterone withdrawal and the elevated plus maze
Pseudopregnant animals, ovariectomized on day 11 and tested on day 12 (24 hr progesterone withdrawal, pseudopregnant wd), displayed a decreased amount of time in the open arm of the elevated plus maze (Table 2; 18 ± 2 sec) compared with ovariectomized controls (42 ± 3.5 sec) and pseudopregnant animals (39.8 ± 4.2 sec). In a similar manner, injection of pseudopregnant rats with the 5α-reductase blocker MK-906 on day 11 to produce 3α,5α-THP withdrawal also decreased the open arm time when rats were tested on day 12 of pseudopregnancy (9.8 ± 3.2 sec).
Table 2.
Elevated plus maze
| Open arm entries | Closed arm entries | |||
|---|---|---|---|---|
| Duration (in sec) | Frequency | Duration (in sec) | Frequency | |
| Pseudopregnant WD | 18 ± 2* | 8.8 ± 1.4* | 282.8 ± 10 | 6.2 ± 0.7 |
| Pseudopregnant + MK-906 | 9.8 ± 3.2* | 7.8 ± 2.1* | 290 ± 12.6 | 6.9 ± 0.9 |
| Pseudopregnant | 39.8 ± 4.2 | 15.6 ± 3.3 | 259 ± 11 | 10.3 ± 2.4 |
| Control | 42 ± 4.5 | 14 ± 1.7 | 255.7 ± 13.5 | 9.3 ± 1.6 |
Progesterone withdrawal decreases open arm entries on the elevated plus maze. Withdrawal from elevated progesterone and 3α,5α-THP levels following ovx on day 11 of pseudopregnancy (pseudopregnant WD) resulted in a decrease in open arm time (duration) and a decrease in open arm entries (frequency) on the elevated plus maze, compared to pseudopregnant (pseudopregnant) and control animals. Blockade of 3α,5α-THP formation with MK-906 (pseudopregnant + MK-906) also reduced both open arm time and the open arm entries, in a manner similar to ovx values. (“Closed arm entries” include center square entries.) (Pseudopregnant WD, n = 30; pseudopregnant + MK-906, n = 13; pseudopregnant,n = 8; control, n = 36).
*p < 0.05 versus PP, CON.
In addition, progesterone withdrawal after ovariectomy resulted in a significant decrease (p < 0.05) in the number of open arm entries compared with both pseudopregnant and control groups (Table 2, 8.8 ± 1.4 pseudopregnant progesterone wd vs 15.6 ± 3.2 pseudopregnant; control, 14 ± 1.7). MK-906-treated animals also displayed a decreased number of open arm entries (7.8 ± 2.1; p < 0.01) compared with controls and pseudopregnant rats. These results suggest that withdrawal from the 5α-reduced metabolites of progesterone increase anxiety-like behavior in the elevated plus maze paradigm.
GABAA-gated current: progesterone withdrawal
Analysis of the kinetics of GABAA-gated current recorded using whole- cell patch-clamp procedures in acutely isolated CA1 hippocampal pyramidal neurons was accomplished using a single order exponential decay function. The decay time for recovery of GABAA-gated current to control baseline levels was reduced by a mean 84 ± 9.1% (Fig. 1, Table3; τ = 978.7 ± 114 msec, pseudopregnant wd vs τ = 6043.7 ± 1247.3 msec, control;p < 0.001) after hormone withdrawal in pseudopregnant rats. Two days after steroid withdrawal the decay time was partially recovered (τ = 3727.8 ± 411 msec) to control values. In contrast, the decay time constant for GABAA-gated current was not significantly different from control values when assessed in pseudopregnant rats not undergoing withdrawal (τ = 5879 ± 980 msec, pseudopregnant). The GABA EC50 for peak GABAA-gated current was unchanged after progesterone withdrawal, as was the reversal potential for GABA-gated current, compared to control values (data not shown). The observed decrease in decay time constant would effectively reduce the total GABAA-gated current after progesterone withdrawal in pseudopregnant rats.
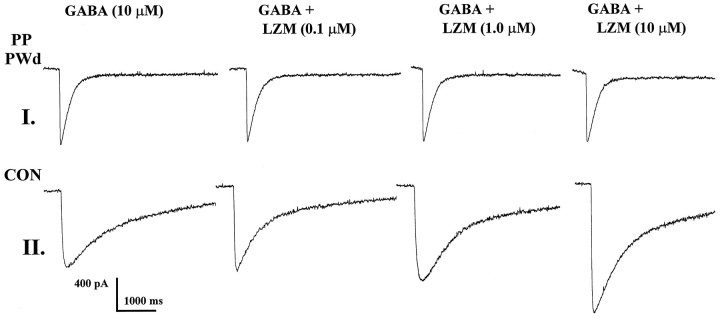
Fig. 1.
GABAA-gated current and lorazepam potentiation: progesterone withdrawal. GABAA (10 μm) current traces before and during application of lorazepam (LZM, 0.1–10 μm) depict potent enhancement of GABAA-gated current under control conditions (CON). In contrast, after progesterone withdrawal in pseudopregnant animals (PP PWd), LZM was ineffective in altering GABAA-gated current.
Table 3.
τ-Decay time constant for GABA-gated current (msec)
| Control | 6043.7 ± 1247.3 |
| Pseudopregnant, 1 d wd | 978.7 ± 114* |
| Pseudopregnant, 2 d wd | 3898.6 ± 498** |
| Pseudopregnant | 5897 ± 1003 |
The decay time for GABA current (τ) is reduced 84% (p < 0.005) 1 d after progesterone withdrawal. No difference in τ is noted for pseudopregnant rats not undergoing withdrawal. Time constants were estimated with a first order exponential decay function using Origin software (Microcal, Inc.). These results are representative of those observed in 15–16 cells per 6 rats per group.
*p < 0.005 versus control, pseudopregnant.
**p < 0.05 versus control, pseudopregnant.
Benzodiazepine agonists, antagonists, and inverse agonists: progesterone withdrawal
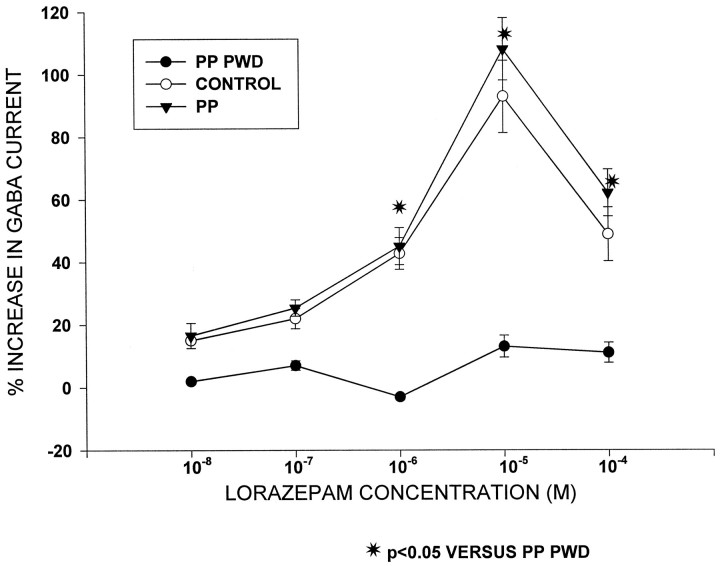
Twenty-four hours after ovariectomy on day 11 of pseudopregnancy (progesterone withdrawal), LZM was ineffective in potentiating GABAA-gated current at any dose tested (10−8–10−4m; Figs. 1, 2). In contrast, under control conditions, LZM potentiated GABAA-gated current by 20 ± 0.4 to 102 ± 11.2% across the same concentration range. However, LZM potentiation of GABAA current was similar to control values when assessed on day 12 of pseudopregnancy in rats not undergoing withdrawal: 16.5 ± 4 to 108 ± 10% potentiation.
Fig. 2.
Progesterone withdrawal and LZM potentiation of GABAA-gated current: summary diagram. This graph depicts no significant enhancement of GABAA-gated current by LZM at any dose after progesterone withdrawal in pseudopregnant animals (PP PWD) compared to control and pseudopregnant animals not undergoing withdrawal (PP). In contrast, LZM significantly potentiated GABAA-gated current by 40–100% at the highest doses tested (10−6–10−4m).n = 15–16 cells per group.
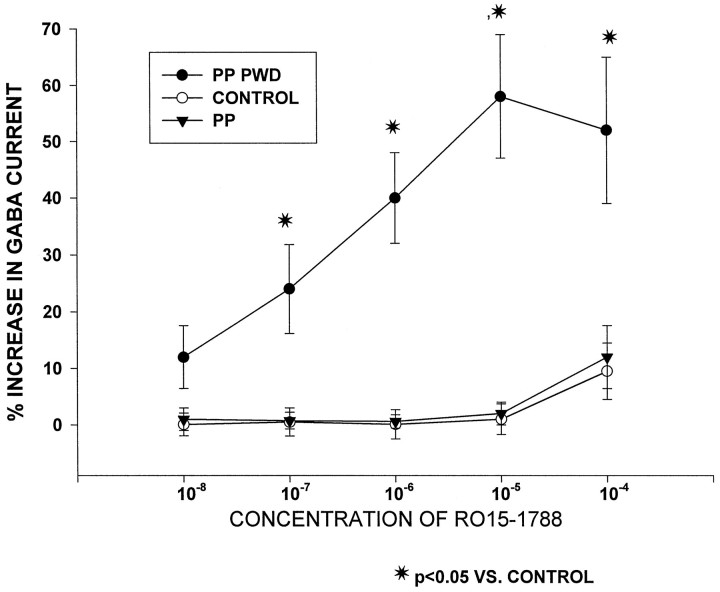
Under control and pseudopregnant conditions, the benzodiazepine antagonist RO15-1788 produced only minimal effects on GABAA-gated current at the highest dose tested (Fig.3, 8 ± 4.3% at 10−4m). In contrast, after progesterone withdrawal, significant enhancement of GABAA-gated current by RO15-1788 was observed, from 10.6 ± 5.1% to 58 ± 11.2% across the 10−8–10−4 concentration range. The actions of this antagonist were converted to those of an agonist.
Fig. 3.
Progesterone withdrawal converts a benzodiazepine antagonist into an agonist. Graph depicts significant enhancement of GABAA-gated current by the benzodiazepine antagonist RO15-1788 after progesterone withdrawal in pseudopregnant animals (PP PWD). In contrast, under control and pseudopregnant (PP) conditions, RO15-1788 did not significantly alter GABAA-gated current. n = 10–12 cells per group.
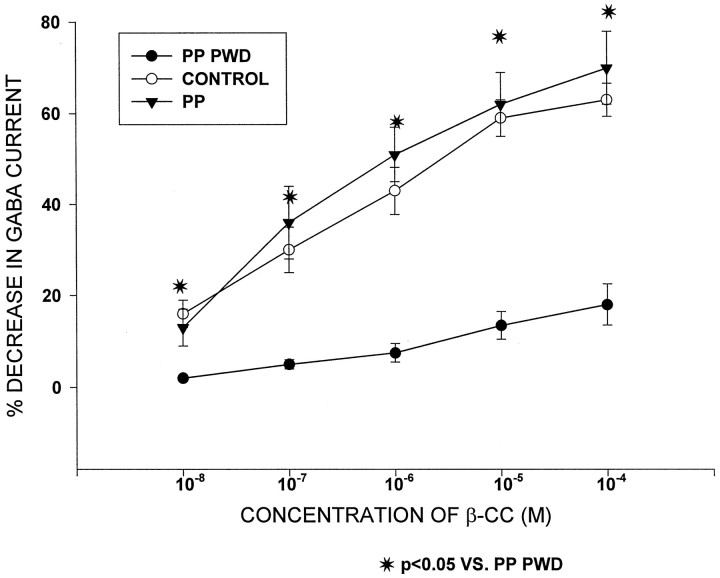
The benzodiazepine inverse agonist, β-CC (10−8–10−4m) decreased GABAA-gated current by 18.2 ± 2 to 62.1 ± 3% under control conditions (Fig.4). Similar decreases in GABAA-gated current were observed after β-CC administration (13 ± 4 to 70 ± 8) to hippocampal pyramidal neurons from pseudopregnant rats not undergoing withdrawal. However, after progesterone withdrawal, the ability of β-CC to decrease GABAA-gated current was significantly reduced (p < 0.05); at this time, inhibition of GABAA-gated current by 2.3 ± 0.2 to 12.5 ± 3.5% was observed across the 10−8–10−4 concentration range of β-CC.
Fig. 4.
Progesterone withdrawal decreases the efficacy of a benzodiazepine inverse agonist. After progesterone withdrawal (PP PWD), the inverse agonist β-CC was ineffective in decreasing GABAA-gated current. Under control and pseudopregnant (PP) conditions, however, this drug was able to inhibit GABA current by 20–60%. n = 10–12 cells per group.
3α,5α-THP potentiation of GABAA-gated current: progesterone withdrawal
Progesterone withdrawal by ovariectomy on day 11 of pseudopregnancy resulted in an almost complete inability of the GABAA-modulatory 3α,5α-THP metabolite (10 nm) to potentiate GABAA-gated current (Table4). Under control conditions, this metabolite increased GABAA-gated current by 81 ± 17.3%. In contrast, after progesterone withdrawal, 3α,5α-THP enhanced GABAA-gated current by 11.2 ± 5.2%, an insignificant level of potentiation.
Table 4.
Percent increase in GABA-gated current by 3α,5α-THP (10 nM)
| Control | 81 ± 17.3* |
| Pseudopregnant, 1 d wd | 11.2 ± 5.2 |
Progesterone withdrawal abolishes the modulatory effect of 3α,5α-THP on GABA current. After progesterone withdrawal (Pseudopregnant 1 d wd), 10 nM 3α,5α-THP was ineffective in significantly altering GABA current. In contrast, under control conditions, this neurosteroid enhanced GABA current up to 80%.n = 20 cells per group.
*p < 0.05 versus 1 d wd.
3α,5α-THP withdrawal and the α4 subunit of the GABAA receptor
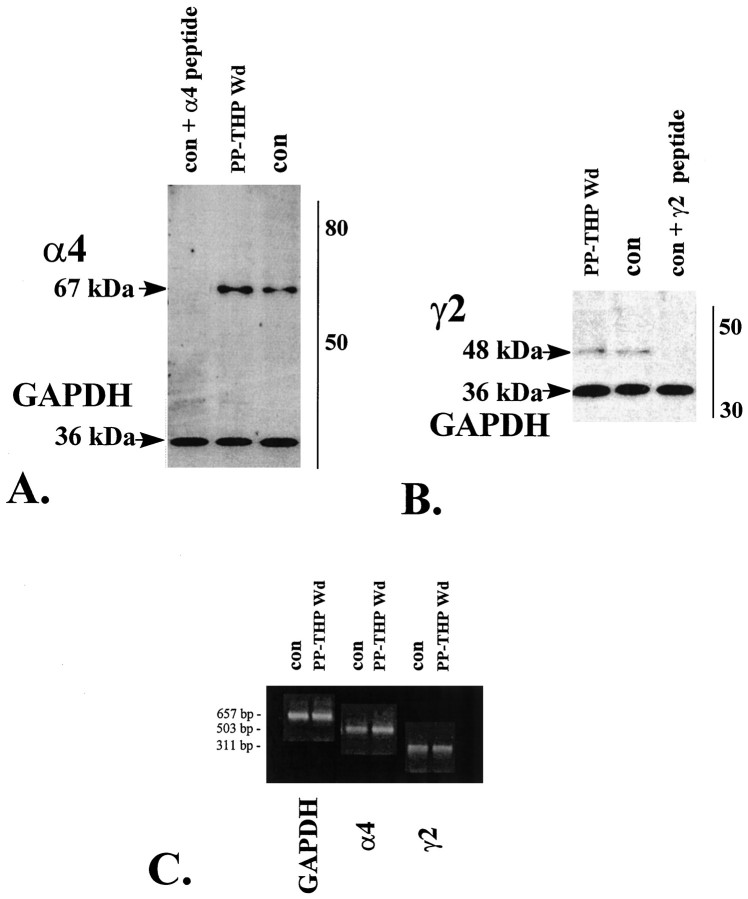
Because the pharmacological spectrum of BDZ effects as well as the change in GABAA-gated current decay constant can both be explained by alterations in the α4 subunit of the GABAAreceptor, we tested whether α4 subunit levels were increased after 3α,5α-THP withdrawal (induced by MK-906) in a pseudopregnant animal. Western blot analysis (Fig. 5) of α4 band densities revealed a 205.3 ± 12.2% increase in α4 protein levels after 3α,5α-THP withdrawal (pseudopregnant THP wd) compared to ovariectomized control values (p < 0.01) after standardization to GAPDH, an invariant protein. In contrast, no significant change in γ2 subunit levels was observed.
Fig. 5.
3α,5α-THP withdrawal is associated with increases in both mRNA and protein for the α4 subunit of the GABAA receptor. Western blot analysis of α4 (A) and γ2 (B) subunit protein reveals significant threefold increases in α4 (67 kDa band), but not γ2 (48 kDa band) or GAPDH (36 kDa band) levels after 3α,5α-THP withdrawal in a pseudopregnant rat (PP-THP Wd) compared to control (con,p < 0.01). In both cases, staining of the α4 and γ2 band could be prevented by previous addition of the appropriate peptide (con + α4 or γ2 peptide).C, Semiquantitative PCR procedures reveal twofold increases in α4 mRNA after 3α,5α-THP withdrawal (PP-THP Wd) compared to control (con,p < 0.05). In contrast, no change in GAPDH or γ2 mRNA levels were noted. These results are representative of those from six rats; three replications at two different cycle numbers and concentrations.
Assessment of α4 mRNA levels after 3α,5α-THP withdrawal with semiquantitative reverse transcriptase polymerase chain reaction (RT-PCR) procedures (Fig. 5) revealed a 154.2 ± 9.8% increase in α4 mRNA after 3α,5α-THP withdrawal (pseudopregnant THP wd) compared to control values (p < 0.05). No change in GAPDH or γ2 mRNA levels was observed across hormone state.
The sedative potency of LZM: progesterone withdrawal
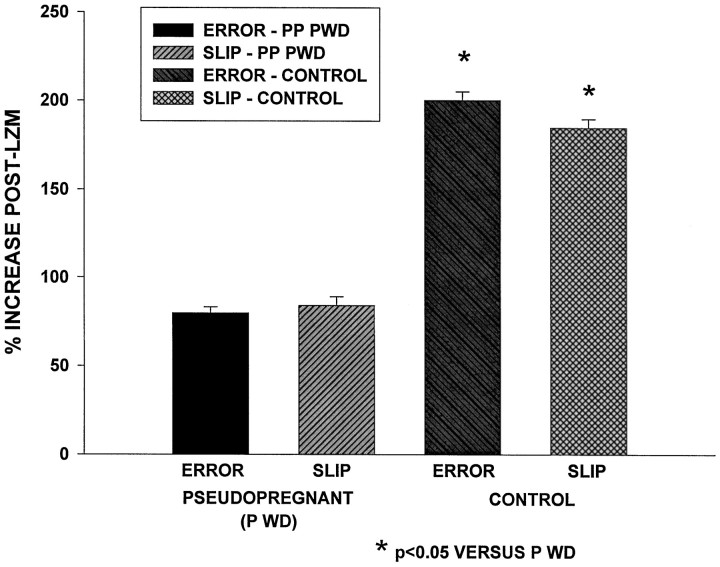
The sedative potency of LZM was tested using a variable speed treadmill paradigm (Fig. 6). Under control conditions, an increase in motor error and position slip is seen by 20 min after an intraperitoneal injection of LZM (0.75 mg/kg; “post-LZM increase”). Although LZM was able to significantly increase both error and slip parameters compared to pre-LZM values (p < 0.05) after progesterone withdrawal (data not shown), the percent post-LZM increase was significantly (p < 0.05) reduced (7.2 and 10.3%, respectively, for error and slip frequencies, progesterone withdrawal) compared to control values (15.5 and 20.6%, respectively; Fig. 6). These results suggest that the sedative potency of LZM is reduced by 50% after progesterone withdrawal, compared to its effect under control conditions.
Fig. 6.
Progesterone withdrawal reduces the sedative effect of LZM in a variable speed treadmill locomotion task. Increases in ERROR (bumping into the rear of the treadmill) andSLIP (slipping back on the treadmill) are observed under control conditions after administration of 0.75 mg/kg LZM (% INCREASE POST-LZM) compared to predrug values when rats are run on a treadmill with varying speed changes. After progesterone withdrawal (PSEUDOPREGNANT P WD) the post-LZM increases in ERROR and SLIP were significantly reduced compared with control conditions, although the sedative effect of LZM was not abolished.n = 10 animals per group.
DISCUSSION
The results from the present study demonstrate that withdrawal from elevated levels of circulating steroids using a pseudopregnancy paradigm result in a withdrawal syndrome characterized by increased anxiety and cross-tolerance to the benzodiazepines. A previous study from this laboratory (Smith et al., 1998) demonstrated that progesterone withdrawal also results in increased seizure susceptibility. These withdrawal effects were shown to be attributable to abrupt decreases in circulating levels of the GABAA-modulatory steroid 3α,5α-THP and were closely correlated with subsequent increases in CNS levels of the α4 subunit of the GABAA receptor. As such, this neuroactive steroid withdrawal syndrome is strikingly similar to withdrawal properties of other GABAA-modulatory agents, such as the BDZs, barbiturates, and ethanol (File, 1981; Finley and Nolan, 1989; Kokka et al., 1993).
The fact that both ovariectomy and administration of the 5α-reductase blocker MK-906 were effective in achieving similar anxiogenic withdrawal effects suggests several conclusions: (1) the greatest source of active steroid pool in this model is the ovary; and (2) a 5α-reduced metabolite of progesterone, 3α,5α-THP, is the active steroid that triggers withdrawal. These conclusions are not surprising in view of the fact that this metabolite is a potent positive modulator of the GABAA receptor, as demonstrated in whole-cell patch clamp (Majewska et al., 1986), single channel (Twyman and Macdonald, 1992) and in vivo iontophoresis (Smith et al., 1987a,b) studies. The 3α,5α-THP metabolite increases the duration of opening of the Cl channel in a manner similar to the barbiturates (Twyman and Macdonald, 1992) and at high doses can increase the frequency of opening of the Cl channel in a manner similar to the BDZs (Twyman and Macdonald, 1992). Behaviorally, acute administration of this steroid directly or indirectly as a result of conversion from systemically administered progesterone produces anxiolytic (Fernandez-Guasti and Picazo, 1990; Bitran et al., 1991a,b, 1993; Brot et al., 1997) and anticonvulsant (Belelli et al., 1989; Devaud et al., 1995; Frye, 1995) effects similar to the GABAA-modulatory BDZs and barbiturates.
The proconvulsant (Smith et al., 1998) anxiogenic withdrawal properties of 3α,5α-THP suggest that increases in neuronal excitability result from neurosteroid withdrawal. In general, drugs that are anxiogenic and proconvulsant increase neuronal excitability by a number of mechanisms, including decreased GABAA response (Zeng et al., 1995), as well as the development of silent GABAA synapses (Poisbeau et al., 1997) in hippocampus. In the present study, total GABAA-gated current was decreased in response to a single 20 msec GABA pulse after progesterone and 3α,5α-THP withdrawal. This was attributable to a GABAA-gated current with a markedly faster decay time, compared with control. Thus, on the whole circuit level, such an effect would be expected to result in a decrease in inhibitory tone with anxiogenic proconvulsant sequelae possible.
In the present study, both electrophysiological and behavioral results suggest that steroid withdrawal during pseudopregnancy produces cross-tolerance with the benzodiazepines. The BDZ insensitivity observed here was maximal 24 hr after steroid withdrawal and was not only a result of chronic progesterone treatment. We have previously reported (Costa et al., 1995; Smith et al., 1998) that withdrawal from progesterone, administered as continuous release subcutaneous implants, results in BDZ insensitivity, assessed using whole-cell patch-clamp techniques on acutely isolated pyramidal neurons from CA1 hippocampus. This cross-tolerance effect of progesterone withdrawal was again shown to be attributable to 3α,5α-THP (Costa et al., 1995; Smith et al., 1998) and could also be blocked by picrotoxin administration (Costa et al., 1995), suggesting an action at the level of the GABAAreceptor. A similar BDZ insensitivity has been noted after chronic treatment and/or withdrawal from BDZs, barbiturates, and ethanol (Buck and Harris, 1990; Roca et al., 1990).
BDZ insensitivity is observed in women with premenstrual syndrome (PMS) (Sundstrom et al., 1997), suggesting clinical relevance of these findings. Altered response to BDZs is also observed across the estrous cycle or after ovariectomy (Fernandez-Guasti and Picazo, 1990; Bitran et al., 1991b; Wilson, 1992; Bitran and Dowd, 1996). Additional evidence for cross-tolerance of BDZs with neuroactive steroids is provided by behavioral studies, which demonstrate that 3α,5α-THP can substitute for several BDZs in a drug discrimination task (Ator et al., 1993). Other studies have demonstrated that exposure to elevated levels of 3α,5α-THP in vitro results in cross-tolerance to the BDZs, as assessed by electrophysiological and biochemical indices (Yu and Ticku, 1995).
In contrast to these similarities, chronic ethanol treatment (Buck and Harris, 1990) produces certain effects that are opposite to those observed after progesterone withdrawal in the present study: increased response to the β-carboline inverse agonist and conversion of the BDZ antagonist RO15-1788 to an inverse agonist. However, progesterone withdrawal produced a decreased response to a β-carboline and transformed RO15-1788 to an agonist. The reason for the difference in results is not known, but may be related to one of the many effects of ethanol on other neurotransmitter systems that could then alter GABAA and BDZ function. Alternatively, additional GABAA subunit changes may result from chronic ethanol exposure (Devaud et al., 1997).
The mechanism underlying the withdrawal effects of 3α,5α-THP observed in the present study may be related to increases in the α4 subunit of the GABAA receptor. Threefold increases in levels of the α4 subunit were detected by Western blot analysis in the present study by 24 hr after steroid withdrawal using the pseudopregnant model. In addition, significant increases in α4 mRNA were also observed after 3α,5α-THP withdrawal, suggesting that one mechanism for the increase in α4 protein is an effect on transcription. The pharmacological profile presented here, i.e., insensitivity to a BDZ agonist, decreased response to a BDZ inverse agonist, and conversion of a BDZ antagonist into an agonist is identical to that reported for oocytes transfected with the α4 subunit of the GABAA receptor (Wisden et al., 1991;Knoflach et al., 1996; Wafford et al., 1996). Our previous studies (Smith et al., 1998) have also demonstrated that increases in both mRNA and protein content of the α4 subunit are observed by 8–24 hr after withdrawal from progesterone, administered as subcutaneous implants, for an intermittent 3 week, multiple-withdrawal paradigm.
More importantly, we have also demonstrated (Smith et al., 1998) that suppression of the α4 subunit by previous administration of α4 antisense, but not missense, oligonucleotides intraventricularly prevents several of the steroid withdrawal properties observed, including (1) the faster decay time for GABAA-gated current, thus resulting in increased total GABAA-gated current and decreased neuronal excitability; (2) the BDZ insensitivity, observed both behaviorally and electrophysiologically; (3) the alterations in BDZ antagonist effects; and (4) the increased seizure susceptibility. The sum of these findings suggests that specific increases in the α4 subunit of the GABAA receptor may account for the observed withdrawal effects of progesterone and 3α,5α-THP observed in the present study. Similar increases in α4 mRNA and protein have been described after chronic exposure or withdrawal from BDZs and ethanol, respectively (Devaud et al., 1997;Holt et al., 1996; Mahmoudi et al., 1997). Other studies have also demonstrated altered GABAA-gated current kinetics in association with altered α subunit composition (Lavoie et al., 1997).
In the present study, steroid withdrawal using the pseudopregnancy model resulted in tolerance to the GABAA-modulatory actions of 3α,5α-THP. This type of desensitization has been reported after chronic exposure of cultured cells to high doses of the neurosteroid (Friedman et al., 1993) and across the estrous cycle (Finn and Gee, 1993), but it is not explained by the observed increase in the α4 subunit after steroid withdrawal here. Oocytes transfected with GABAA receptors containing the α4 subunit do not exhibit a decrease in response to modulation of GABA-gated current by 3α,5α-THP (Wafford et al., 1996). Other possible mechanisms include (1) altered levels of δ or ε subunits, both of which have been reported to confer insensitivity to the GABAA-modulatory actions of this neuroactive steroid (Zhu et al., 1996; Davies et al., 1997), and (2) alteration in phosphorylation state, also reported to result in differential response to GABAA-modulatory agents (Gyenes et al., 1994).
The use of a pseudopregnancy model here provided several advantages over use of single or multiple injection or chronic implant, as reported earlier: (1) it is a more physiological paradigm than the other procedures, because progesterone is produced by an endogenous, ovarian source; (2) the time course of progesterone exposure, with progesterone elevated from day 4 to 12, is similar to progesterone exposure during the luteal phase of the menstrual cycle (Dennerstein et al., 1985; Rapkin et al., 1997), thus making this a useful model for PMS and postpartum conditions; (3) not only are progesterone and 3α,5α-THP released, but a number of other steroids are also naturally released by the ovary and corpus luteum, also making this a better in vivo model (Robinson et al., 1981; Kim and Greenwald, 1986); (4) release of progesterone under physiological states, such as estrus, pregnancy, and pseudopregnancy is somewhat episodic (Robinson et al., 1981; Kim and Greenwald, 1986), a phenomenon that could not easily be replicated by artificial means of administration. The fact that hormone release is episodic in this model may explain why a single withdrawal cycle was effective in the present study in triggering withdrawal properties, whereas in earlier studies, multiple withdrawal cycles were required (Costa et al., 1995; Smith et al., 1998). Intermittent exposure, as would occur with episodic release, has been shown to be effective in enhancing withdrawal properties of ethanol (Kokka et al., 1993) and the BDZs (Gallagher et al., 1986).
The behavioral data in this study do not reveal as potent a BDZ insensitivity as the electrophysiological data. This apparent discrepancy may relate to the CNS sites involved. The hippocampus is valid as a site for spatial memory (Wilson and McNaughton, 1993) and thus, relevant for the variable speed treadmill task reported here. However, the sedative effect measured behaviorally may be related to multiple sites of action throughout the brain.
More importantly, the hippocampus is one relevant limbic structure for generation of anxiety, as noted by a number of studies indicating that anxiogenic and anxiolytic effects of BDZ compounds are observed after local application to the dorsal hippocampus (Huttunen et al., 1986;Katoaka et al., 1991; Talaenko, 1993). Especially relevant to the present study is the recent finding that local hippocampal administration of 3α,5α-THP produces anxiolytic effects (Bitran et al., 1997). Other limbic structures, notably the amygdala, are also well known for their role in anxiety (Britton et al., 1996).
In conclusion, withdrawal from 3α,5α-THP using the pseudopregnancy model results in an anxiogenic withdrawal syndrome, characterized by marked decreases in total GABAA-gated current and cross-tolerance to the BDZs. These effects were correlated with significant increases in hippocampal levels of the α4 subunit of the GABAA receptor, a possible mechanism for the observed neurosteroid withdrawal effects. Use of the pseudopregnant model here may have application for increased emotional lability and altered BDZ response reported for the premenstrual and postpartum periods (Dennerstein et al., 1985; Sundstrom et al., 1997). In addition, increased CNS levels of 3α,5α-THP have been noted during periods of chronic stress in male rats (Purdy et al., 1991), suggesting that 3α,5α-THP withdrawal may also arise in association with stressful episodes in both sexes.
Footnotes
This work was supported by National Institutes of Health Grant DA-09618 to S. S. Smith. The MK-906 was a generous gift from Merck and Company. We are grateful to R. S. Markowitz for helpful technical assistance. Correspondence should be addressed to S. S. Smith, Department of Neurobiology and Anatomy, Allegheny University of the Health Sciences, EPPI Division, 3200 Henry Avenue, Philadelphia, PA 19129.
REFERENCES
- 1.Ator NA, Grant KA, Purdy RS, Paul SM, Griffiths RR. Drug discrimination analysis of endogenous neuroactive steroids in rats. Eur J Pharmacol. 1993;241:237–244. doi: 10.1016/0014-2999(93)90208-y. [DOI] [PubMed] [Google Scholar]
- 2.Belelli D, Bolger MB, Gee KW. Anticonvulsant profile of the progesterone metabolite 5α-pregnan-3α-20-one. Eur J Pharmacol. 1989;166:125–129. doi: 10.1016/0014-2999(89)90077-0. [DOI] [PubMed] [Google Scholar]
- 3.Bitran D, Dowd JA. Ovarian steroids modify the behavioral and neurochemical responses of the central benzodiazepine receptor. Psychopharmacology. 1996;125:65–73. doi: 10.1007/BF02247394. [DOI] [PubMed] [Google Scholar]
- 4.Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3α-hydroxy-5α[β]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991a;561:157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- 5.Bitran D, Hilvers RJ, Kellogg CK. Ovarian endocrine status modulates the anxiolytic potency of diazepam and the efficacy of γ-aminobutyric acid-benzodiazepine receptor-mediated chloride ion transport. Behav Neurosci. 1991b;105:653–662. doi: 10.1037//0735-7044.105.5.653. [DOI] [PubMed] [Google Scholar]
- 6.Bitran D, Purdy RH, Kellogg CK. The anxiolytic effect of progesterone is associated with increases in cortical allopregnanolone and GABAA receptor function. Pharmacol Biochem Behav. 1993;45:423–428. doi: 10.1016/0091-3057(93)90260-z. [DOI] [PubMed] [Google Scholar]
- 7.Bitran D, Dugan M, Renda P, Solano S, Dowd S, Kilbansky D, Ellis R, Rausch E. Behavior in animal models of anxiety after intracranial microinjection of pregnanolone in hippocampus, lateral septum and basolateral amygdala. Soc Neurosci Abstr. 1997;23:1844. [Google Scholar]
- 8.Britton KT, Akwa Y, Purdy RH, Koob GR. The amygdala mediates the anxiolytic-like activity of the neurosteroid allopregnanolone. Soc Neurosci Abstr. 1996;22:1292. [Google Scholar]
- 9.Brot MD, Akwa Y, Purdy RH, Koob GF, Britton KT. The anxiolytic-like effects of the neurosteroid allopregnanolone: interactions with GABA(A) receptors. Eur J Pharmacol. 1997;325:1–7. doi: 10.1016/s0014-2999(97)00096-4. [DOI] [PubMed] [Google Scholar]
- 10.Buck KJ, Harris RA. Benzodiazepine agonist and inverse agonist actions of GABAA receptor-operated chloride channels II: chronic effects of ethanol. J Pharmacol Exp Ther. 1990;253:713–719. [PubMed] [Google Scholar]
- 11.Corpechot C, Young J, Calvel M, Wehrey C, Veltz JN, Touter G, Mouren M, Prasad VV, Banner C, Sjovall J, Baulieu EE, Robel P. Neurosteroids: 3α-hydroxy-5α-pregnan-20-one and its precursors in the brain plasma and steroidogenic glands of male and female rats. Endocrinology. 1993;133:1003–1009. doi: 10.1210/endo.133.3.8365352. [DOI] [PubMed] [Google Scholar]
- 12.Costa A-MN, Spence KT, Smith SS, ffrench-Mullen Withdrawal from the endogenous steroid progesterone results in GABAA currents insensitive to benzodiazepine modulation in rats CA1 hippocampus. J Neurophysiol. 1995;74:464–469. doi: 10.1152/jn.1995.74.1.464. [DOI] [PubMed] [Google Scholar]
- 13.Davies PA, Hanna MC, Hales TG, Kirkness EF. Insensitivity to anaesthetic agents conferred by a class of GABAA receptor subunit. Nature. 1997;385:820–823. doi: 10.1038/385820a0. [DOI] [PubMed] [Google Scholar]
- 14.Dennerstein L, Spencer-Gardner C, Gotts G, Brown JB, Smith MA, Burrows GD. Progesterone and premenstrual syndrome: a double blind crossover trial. Br Med J. 1985;290:1617–1621. doi: 10.1136/bmj.290.6482.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devaud LL, Purdy RH, Morrow AL. The neurosteroid 3α-hydroxy-5α-pregnan-20-one protects against bicuculline-induced seizures during ethanol withdrawal in rats. Alcohol Clin Exp Res. 1995;19:350–355. doi: 10.1111/j.1530-0277.1995.tb01514.x. [DOI] [PubMed] [Google Scholar]
- 16.Devaud LL, Fritschy J-M, Sieghart W, Morrow AL. Bidirectional alterations of GABAA receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J Neurochem. 1997;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Guasti A, Picazo O. The actions of diazepam and serotonergic anxiolytics vary according to the gender and the estrous cycle phase. Pharmacol Biochem Behav. 1990;37:77–81. doi: 10.1016/0091-3057(90)90044-i. [DOI] [PubMed] [Google Scholar]
- 18.File SE. Rapid development of tolerance to the sedative effects of lorazepam and triazolam in rats. Psychopharmacology. 1981;73:240–245. doi: 10.1007/BF00422410. [DOI] [PubMed] [Google Scholar]
- 19.File SE. The history of benzodiazepine dependence: a review of animal studies. Neurosci Biobehav Rev. 1990;14:135–146. doi: 10.1016/s0149-7634(05)80214-3. [DOI] [PubMed] [Google Scholar]
- 20.Finley PR, Nolan PE. Precipitation of benzodiazepine withdrawal following sudden discontinuation of midazolam. DICP Ann Pharmacother. 1989;23:151–152. doi: 10.1177/106002808902300209. [DOI] [PubMed] [Google Scholar]
- 21.Finn DA, Gee KW. The influence of estrous cycle on neurosteroid potency at the GABAA receptor complex. J Pharmacol Exp Ther. 1993;265:1374–1379. [PubMed] [Google Scholar]
- 22.Friedman L, Gibbs TT, Farb DH. GABAA receptor regulation: chronic treatment with pregnanolone uncouples allosteric interactions between steroid and benzodiazepine recognition sites. Mol Pharmacol. 1993;44:191–197. [PubMed] [Google Scholar]
- 23.Frye CA. The neurosteroid 3 α 5 α-THP has antiseizure and possible neuroprotective effects in an animal model of epilepsy. Brain Res. 1995;696:113–120. doi: 10.1016/0006-8993(95)00793-p. [DOI] [PubMed] [Google Scholar]
- 24.Frye CA, Scalise TJ, Bayon LE. Finasteride blocks the reduction in ictal activity produced by exogenous estrous cyclicity. J Neuroendocrinol. 1998;10:291–296. doi: 10.1046/j.1365-2826.1998.00202.x. [DOI] [PubMed] [Google Scholar]
- 25.Gallagher DW, Heninger K, Heninger G. Periodic benzodiazepine antagonist administration prevents benzodiazepine withdrawal symptoms in primates. Eur J Pharmacol. 1986;132:31–38. doi: 10.1016/0014-2999(86)90005-1. [DOI] [PubMed] [Google Scholar]
- 26.Gallo MA, Smith SS. Progesterone withdrawal decreases latency to and increases duration of electrified prod burial: a possible rat model of PMS anxiety. Pharmacol Biochem Behav. 1993;46:897–904. doi: 10.1016/0091-3057(93)90219-j. [DOI] [PubMed] [Google Scholar]
- 27.Gyenes M, Wang Q, Gibbs TT, Farb DH. Phosphorylation factors control neurotransmitter and neuromodulator actions at the gamma-aminobutyric acid type A receptor. Mol Pharmacol. 1994;46:542–549. [PubMed] [Google Scholar]
- 28.Holt RA, Bateson AN, Martin IL. Chronic treatment with diazepam or abecarnil differentially affects the expression of GABAA receptor subunit mRNAs in the rat cortex. Neuropharmacology. 1996;35:1457–1463. doi: 10.1016/s0028-3908(96)00064-0. [DOI] [PubMed] [Google Scholar]
- 29.Huttunen P, Myers RD. Tetrahydro-β-cargoline micro-injected into the hippocampus induces an anxiety-like state in the rat. Pharmacol Biochem Behav. 1986;24:1733–1738. doi: 10.1016/0091-3057(86)90513-7. [DOI] [PubMed] [Google Scholar]
- 30.Kataoka Y, Shibata K, Miyazaki A, Inoue Y, Tominaga K, Koizumi S, Ueki S, Niwa M. Involvement of the dorsal hippocampus in mediation of the antianxiety action of tandospirone, a 5-hyrdoxytryptamine1A agonistic anxiolytic. Neuropharmacology. 1991;30:475–480. doi: 10.1016/0028-3908(91)90009-z. [DOI] [PubMed] [Google Scholar]
- 31.Kern W, Sieghart W. Polyclonal antibodies directed against an epitope specific for the α4-subunit of GABAA receptors identify a 67 kDa protein in rat brain membranes. J Neurochem. 1994;62:764–769. doi: 10.1046/j.1471-4159.1994.62020764.x. [DOI] [PubMed] [Google Scholar]
- 32.Kim I, Greenwald GS. Steroidogenic effects of lipoproteins and 25-hydroxycholesterol on luteal and ovarian cells: a comparison of two pseudopregnant rat models. Proc Natl Acad Sci USA. 1986;181:1230–1240. doi: 10.3181/00379727-181-42248. [DOI] [PubMed] [Google Scholar]
- 33.Knoflach F, Benke D, Wang Y, Scheurer L, Luddens H, Hamilton BJ, Carter DB, Mohler H, Benson JA. Pharmacological modulation of the diazepam-insensitive recombinant gamma-aminobutyric acid (A) receptors α4-β2-γ2 and α6-β2-γ2. Mol Pharmacol. 1996;50:1253–1261. [PubMed] [Google Scholar]
- 34.Kokka N, Sapp DW, Taylor AM, Olsen RW. The kindling model of alcohol dependence: similar persistent reduction in seizure threshold to pentylenetetrazol in animals receiving chronic ethanol or chronic pentylenetetrazol. Alcoholism. 1993;17:525–531. doi: 10.1111/j.1530-0277.1993.tb00793.x. [DOI] [PubMed] [Google Scholar]
- 35.Lau EC, Li A-Q, Santos V, Slavkin HC. Messenger RNA phenotyping for semi-quantitative comparison of glucorticoid receptor transcript levels in the developing embryonic mouse palate. J Steroid Biochem. 1993;46:751–758. doi: 10.1016/0960-0760(93)90315-n. [DOI] [PubMed] [Google Scholar]
- 36.Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. Activation and deactivation rates of recombinant GABAA receptor channels are dependent on α-subunit isoform. Biophys J. 1997;73:1–9. doi: 10.1016/S0006-3495(97)78280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahmoudi M, Kang MH, Tillakaratne N, Tobin AJ, Olsen RW. Chronic intermittent ethanol treatment in rats increases GABA(A) receptor α4-subunit expression: possible relevance to alcohol dependence. J Neurochem. 1997;68:2485–2492. doi: 10.1046/j.1471-4159.1997.68062485.x. [DOI] [PubMed] [Google Scholar]
- 38.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 39.Moran MH, Goldberg M, Wieland SJ, Bitran D, Smith SS. Progesterone withdrawal produces benzodiazepine insensitivity: a behavioral study. Soc Neurosci Abstr. 1996;22:1292. [Google Scholar]
- 40.Mossier B, Togel M, Fuchs K, Sieghart WJ. Immunoaffinity purification of GABAA receptors containing γ1 subunits: evidence for the presence of a single type of γ subunit in GABAA receptors. Biol Chem. 1994;269:25777–25782. [PubMed] [Google Scholar]
- 41.Pellow S, File SE. The effects of putative anxiogenic compounds (FG 7142 CGS 8216 and Ro15-1788) on the rat corticosterone response. Physiol Behav. 1985;35:587–590. doi: 10.1016/0031-9384(85)90145-3. [DOI] [PubMed] [Google Scholar]
- 42.Poisbeau P, Williams SR, Mody I. Silent GABAA synapses during flurazepam withdrawal are region-specific in the hippocampal formation. J Neurosci. 1997;17:3467–3475. doi: 10.1523/JNEUROSCI.17-10-03467.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purdy RH, Moore PH, Rao PN, Hagino M, Yamaguchi T, Schmidt P, Rubinow DR, Morrow AL, Paul SM. Radioimmunoassay of 3α-hydroxy-5α-pregnane-20-one in rat and human plasma. Steroids. 1990;55:290–296. doi: 10.1016/0039-128x(90)90031-6. [DOI] [PubMed] [Google Scholar]
- 44.Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rapkin AJ, Morgan M, Goldman L, Brann DW, Simone D, Mahesh BB. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90:709–714. doi: 10.1016/S0029-7844(97)00417-1. [DOI] [PubMed] [Google Scholar]
- 46.Robinson J, Merry BJ, Lightfoot ME, Hall AK. Dynamics of progesterone metabolism in the pseudopregnant rat. J Endocrinol. 1981;90:359–366. doi: 10.1677/joe.0.0900359. [DOI] [PubMed] [Google Scholar]
- 47.Roca DJ, Friedman L, Schiller GD, Rozenberg I, Gibbs TT, Farb DH. γ-aminobutyric acid receptor regulation in culture: altered allosteric interactions following prolonged exposure to benzodiazepines barbiturates and methylxanthines. Mol Pharmacol. 1990;37:710–719. [PubMed] [Google Scholar]
- 48.Smith SS. Progesterone enhances inhibitory responses of cerebellar Purkinje cells mediated by the GABAA receptor subtype. Brain Res Bull. 1989;23:317–322. doi: 10.1016/0361-9230(89)90215-3. [DOI] [PubMed] [Google Scholar]
- 49.Smith SS. Female sex steroid hormones: from receptors to networks to performance—actions on the sensorimotor system. Prog Neurobiol. 1994;44:55–86. doi: 10.1016/0301-0082(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 50.Smith SS, Chapin JK. The estrous cycle and the olivo-cerebellar circuit I Contrast enhancement of sensorimotor-correlated cerebellar discharge. Exp Brain Res. 1996;111:371–384. doi: 10.1007/BF00228726. [DOI] [PubMed] [Google Scholar]
- 51.Smith SS, Waterhouse BD, Chapin JK, Woodward DJ. Progesterone alters GABA and glutamate responsiveness: a possible mechanism for its anxiolytic effect. Brain Res. 1987a;400:353–359. doi: 10.1016/0006-8993(87)90634-2. [DOI] [PubMed] [Google Scholar]
- 52.Smith SS, Waterhouse BD, Woodward DJ. Locally applied progesterone metabolites alter neuronal responsiveness in the cerebellum. Brain Res Bull. 1987b;18:739–747. doi: 10.1016/0361-9230(87)90209-7. [DOI] [PubMed] [Google Scholar]
- 53.Smith SS, Gong QH, Hsu F-C, Markowitz RS, ffrench-Mullen JMH, Li X. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- 54.Sundstrom I, Ashbrook D, Backstrom T. Reduced benzodiazepine sensitivity in patients with premenstrual syndrome: a pilot study. Psychoneuroendocrinology. 1997;22:25–38. doi: 10.1016/s0306-4530(96)00035-2. [DOI] [PubMed] [Google Scholar]
- 55.Talaenko AN. The neurochemical profile of the dorsal hippocampus and the antiaversive effects of anxiolytics in different models of anxiety in rats. J Nerv Ment Dis. 1993;43:621–626. [PubMed] [Google Scholar]
- 56.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Twyman RE, MacDonald RL. Neurosteroid regulation of GABAA receptor-single channel kinetic properties of mouse spinal cord neurons in culture. J Physiol (Lond) 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tyndale RF, Hales TG, Olsen RW, Tobin AJ. Distinctive patterns of GABAA receptor subunit mRNAs in 13 cell lines. J Neurosci. 1994;14:5417–5428. doi: 10.1523/JNEUROSCI.14-09-05417.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human γ-aminobutyric acid a receptors containing the α4 subunit. Mol Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- 60.Wilson MA. Influences of gender gonadectomy and estrous cycle of GABA/BZ receptors and benzodiazepine responses in rats. Brain Res Bull. 1992;29:165–172. doi: 10.1016/0361-9230(92)90022-p. [DOI] [PubMed] [Google Scholar]
- 61.Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- 62.Wisden W, Herb A, Wieland H, Keinanen K, Luddens H, Seeburg PH. Cloning pharmacological characteristics and expression pattern of the rat GABAA receptor α4 subunit. FEBS Lett. 1991;289:227–230. doi: 10.1016/0014-5793(91)81076-k. [DOI] [PubMed] [Google Scholar]
- 63.Yu R, Ticku MK. Chronic neurosteroid treatment produces functional heterologous uncoupling at the gamma-aminobutyric acid type A/benzodiazepine receptor complex in mammalian cortical neurons. Mol Pharmacol. 1995;47:603–610. [PubMed] [Google Scholar]
- 64.Zeng X, Xie XH, Tietz EI. Reduction of GABA-mediated inhibitory postsynaptic potentials in hippocampal CA1 pyramidal neurons following oral flurazepam administration. Neuroscience. 1995;66:87–99. doi: 10.1016/0306-4522(94)00558-m. [DOI] [PubMed] [Google Scholar]
- 65.Zhu WJ, Wang JF, Krueger KE, Vicini S. Delta subunit inhibits neurosteroid modulation of GABAA receptors. J Neurosci. 1996;16:6648–6656. doi: 10.1523/JNEUROSCI.16-21-06648.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]