Abstract
The P2Y2 receptor is a uridine/adenosine triphosphate (UTP/ATP)-sensitive G-protein-linked nucleotide receptor that previously has been reported to stimulate the phosphoinositide signaling pathway. Messenger RNA for this receptor has been detected in brain tissue. We have investigated the coupling of the molecularly defined rat P2Y2 receptor to neuronal N-type Ca2+ channels and to M-type K+channels by heterologous expression in rat superior cervical sympathetic (SCG) neurons. After the injection of P2Y2cRNA, UTP inhibited the currents carried by both types of ion channel. As previously reported [Filippov AK, Webb TE, Barnard EA, Brown DA (1997) Inhibition by heterologously expressed P2Y2nucleotide receptors of N-type calcium currents in rat sympathetic neurones. Br J Pharmacol 121:849–851], UTP inhibited the Ca2+ current (ICa(N)) by up to 64%, with an IC50 of ∼0.5 μm. We now find that UTP also inhibited the K+M current (IK(M)) by up to 61%, with an IC50 of ∼1.5 μm. UTP had no effect on either current in neurons not injected with P2Y2 cRNA. Structure–activity relations for the inhibition ofICa(N) and IK(M)in P2Y2 cRNA-injected neurons were similar, with UTP ≥ ATP > ITP ≫ GTP,UDP. However, coupling to these two channels involved different G-proteins: pretreatment withPertussis toxin (PTX) did not affect UTP-induced inhibition of IK(M) but reduced inhibition of ICa(N) by ∼60% and abolished the voltage-dependent component of this inhibition. In unclamped neurons, UTP greatly facilitated depolarization-induced action potential discharges. Thus, the single P2Y2 receptor can couple to at least two G-proteins to inhibit both Ca2+N and K+M channels with near-equal facility. This implies that the P2Y2 receptor may induce a broad range of effector responses in the nervous system.
Keywords: nucleotide receptors, uridine triphosphate, adenosine triphosphate, sympathetic neurons, calcium currents, potassium currents, M currents
Nucleotides such as ATP play a significant neurotransmitter role in the mammalian nervous system (Burnstock, 1972, 1990; Edwards and Gibb, 1993; Zimmermann, 1994). There are two families of target nucleotide receptors known at the molecular level—ligand-gated P2X receptors and G-protein-coupled P2Y receptors (North and Barnard, 1997).
The P2Y2 receptor is a member of the family of P2Y G-protein-coupled receptors and is sensitive to both ATP and UTP; it was cloned originally from mouse NG108-15 neuroblastoma X glioma hybrid cells (Lustig et al., 1993). In common with other P2Y receptors (Boarder et al., 1995), P2Y2 receptors from different species all couple to the enzyme phospholipase C (PLC), thereby increasing inositol phosphate production and elevating intracellular [Ca2+] (Erb et al., 1993; Lustig et al., 1993;Parr et al., 1994; Rice et al., 1995; Chen et al., 1996; Nicholas et al., 1996).
Messenger RNA for the P2Y2 receptor, which is found in a range of tissues, is also present in the brain (Lustig et al., 1993), so the question arises as to what effect the activation of this receptor might have on neural function. In native NG108-15 cells, UTP inhibits two membrane ionic currents—an M-like K+current (“M-current”) and the voltage-gated Ca2+current (Filippov et al., 1994; Filippov and Brown, 1996). The former was to be expected because other receptors that activate PLC inhibit M-currents (Brown, 1988), but Ca2+ current inhibition was unexpected, especially because it was mediated (in part, at least) by a different G-protein from that responsible for M-current inhibition (Filippov and Brown, 1996). This raised the question whether both effects actually were produced by the same receptor as that previously cloned from these cells or whether two different receptors were responsible. If the former, was this a peculiarity of this particular cell line, or would it also hold for primary neurons?
To address these questions, we have expressed the recombinant rat P2Y2 receptor in primary cultured rat superior cervical sympathetic (SCG) neurons by microinjecting cRNA, in the manner used byIkeda et al. (1995) to express heterologous “metabotropic” glutamate receptors, and then recording the effects of activating these exogenous receptors with UTP on the N-type Ca2+currents (ICa(N); Hirning et al., 1988;Plummer et al., 1989; Regan et al., 1991) and M-type K+ currents (IK(M);Constanti and Brown, 1981) that are present in these neurons. In preliminary experiments (Filippov et al., 1997) we found that the activation of these expressed receptors did indeed inhibitICa(N). In the present experiments we have analyzed this action in more detail and have gone on to test whether the same single receptor also can inhibit the M-type K+ current. We find that it can; the two currents are inhibited by UTP with near-equal potencies and efficacy, although mediated mainly by different G-proteins, and in joint response increase the excitability of these neurons. Thus, the P2Y2 receptor appears to be unusually promiscuous in terms of its coupling to mammalian neuronal G-proteins and ion channels. This has interesting implications for its potential function in nerve cells.
MATERIALS AND METHODS
cRNA preparation. The rat P2Y2 receptor cDNA was obtained from Dr. Zeng-Ping Chen (Department of Neuroscience and Cell Biology, University of Medicine and Dentistry of New Jersey, Robert Wood Johnson Medical School, Piscataway, NJ). A 2 kbEcoRI–XhoI fragment was cloned into the reciprocal sites of pBK/CMV. The wild-type green fluorescent protein (GFP) cDNA, in pBluescript, also was used. Supercoiled plasmid DNA was prepared from both constructs and linearized with XhoI for P2Y2 and with EcoRI for GFP before use as a template for cRNA synthesis. Capped cRNA was transcribed with T3 polymerase (Message Machine, Ambion, Austin, TX), and an aliquot of each was analyzed on a denaturing agarose gel to check its integrity before polyadenylation, using poly(A+) polymerase (Sigma, St. Louis, MO) according to the manufacturer’s recommendations. After extractions in turn with phenol, phenol/chloroform, and chloroform and isopropanol precipitation, the cRNA was stored as an ethanol precipitate at −70°C before use.
Neuron preparation and cRNA injection. Single SCG neurons were dissociated from rats aged 15–19 d, and plated on poly-d-lysine-coated glass coverslips bordered by 2 cm plastic rings as previously described (Marrion et al., 1987). At 4 hr after plating, the neurons were microinjected with an equal mixture of cRNA for the P2Y2 receptor (final pipette concentration of 0.5 or 1.25 μg/μl dissolved in water) and cRNA for GFP (used as a marker for foreign cRNA expression; Marshall et al., 1995) or, for controls, with GFP cRNA alone. cRNA solution (1.2 μl) was loaded into prepulled high-resistance (∼30 MΩ) Pyrex glass pipettes and injected manually into single neurons by the application of gentle pressure to the back of the pipette with a syringe as described previously for injection of antisera (Caulfield et al., 1994). Successful injection usually resulted in a ∼10% increase in cell volume (Ikeda et al., 1995). After injections, cells were incubated for 14–24 hr in a humidified incubator (5% CO2/95% O2) at 37°C. Injected neurons that successfully expressed cRNA were identified as bright fluorescent cells, using an inverted microscope (Diaphot 200, Nikon, Tokyo, Japan) equipped with an epifluorescent N B2E block (Nikon). When required,Pertussis toxin (PTX) at a final concentration of 500 ng/ml was added to the culture media 1–2 hr after neuron injection. Electrophysiological recordings were made 14–24 hr after injection at room temperature (20°C). Some experiments on neuron excitability were made at 34°C.
Ca2+ channel current recording. Currents through voltage-gated Ca2+ channels were recorded by the conventional whole-cell patch-clamp method as described previously (Caulfield et al., 1994). Cells were superfused (20–25 ml/min) with a solution consisting of (in mm) 120 tetraethylammonium chloride, 3 KCl, 1.5 MgCl2, 5 BaCl2 (or 5 CaCl2), 10 HEPES, and 11.1 glucose plus 0.5 μm tetrodotoxin. The pH was adjusted to 7.35 with NaOH. Patch electrodes (2–3 MΩ) were filled with a solution containing (in mm) 110 CsCl, 3 Mg Cl2, 40 HEPES, 3 EGTA, 2 Na2ATP, and 0.5 Na2GTP (pH-adjusted to 7.4 with CsOH). Neurons were voltage-clamped with a discontinuous (“switching”) amplifier (Axoclamp 2B) with a sampling voltage at 6–8 kHz (50% duty cycle). Commands were generated via a Digidata 1200 interface, using pClamp 6 computer software (Axon Instruments, Foster City, CA). Ca2+ channel currents were evoked routinely every 20 sec with a 100 msec depolarizing rectangular test pulse to 0 mV from a holding potential of −90 mV. To obtain current–voltage (I–V) relations, we evoked currents by test pulses in 10 mV increments to +40 mV, starting from the holding potential of −90 mV. Where required, I–V relations were obtained by using 750 msec ramp depolarizations from −90 to + 40 mV (see Docherty et al., 1991). Currents were digitized and stored on a computer for later analysis by pClamp 6 software (Axon Instruments). Ca2+ channel current amplitudes were measured isochronally 10 msec from the onset of the rectangular test pulse (Ikeda et al., 1995), i.e., near to the peak of the control current. To eliminate leak currents, we substituted Co2+ for Ca2+ and Ba2+ in the external solution at the end of each experiment to block all Ca2+ channel currents, and we digitally subtracted the residual current from the corresponding currents in Ca2+ or Ba2+ solution.
M-type K+ current recordings. Whole-cell M-currents (IK(M)) were recorded by the perforated patch-clamp method (Horn and Marty, 1988), as described for the application to SCG neurons by Caulfield et al. (1994). Briefly, patch pipettes (2–4 MΩ) were filled by dipping the tip into a filtered solution containing (in mm) 90 potassium acetate, 20 KCl, 3 MgCl2, 40 HEPES, and 0.1 BAPTA (pH-adjusted to 7.4 by KOH) for 20–60 sec. Then the pipette was back-filled with the same solution containing 0.125 mg/ml amphotericin B as the permeabilizing agent (Rae et al., 1991). Access resistance after permeabilization was 8–15 MΩ. Neurons were superfused (20–25 ml/min) with external modified Krebs’ solution containing (in mm) 120 NaCl, 3 KCl, 1.5 MgCl2, 2.5 CaCl2, 10 HEPES, and 11.1 glucose (pH-adjusted to 7.3 with NaOH). Neurons were voltage-clamped at −20 or −30 mV with a switching amplifier, and M-currents were deactivated with 1 sec hyperpolarizing steps at 5 sec intervals. I–V relationships were obtained by using incremental voltage steps of 10 mV between −10 and −100 mV; currents were measured at the end of each hyperpolarizing step. For dose–response curves, currents were measured at −30 mV from steady-state I–V relations obtained by using a ramp voltage command of 20 sec from −20 to −90 mV. The leak component of current was estimated in both cases by extrapolating a linear fit to theI–V relationship from the negative potential region, where only ohmic currents were observed. All commands, current recordings, and analyses were made with Digidata 1200 interface and pClamp 6 software (Axon Instruments).
Statistical analysis. Data are presented as mean ± SEM as appropriate. Student’s t test (unpaired) was applied to determine statistical significance. The difference was considered significant if p ≤ 0.05. Dose–response curves were determined by using concentrations that were added cumulatively, with 1 min exposure times. Curves were fit (using Origin 4.1 software) to pooled data points to the Hill equation: y = ymax ·xnH/(xnH+ KnH), wherey = the observed percentage of inhibition,ymax = extrapolated maximal percentage of inhibition, x = nucleotide concentration (μm), K = IC50(μm), and nH = the Hill coefficient.
Chemicals. UTP rather than ATP was used as the main agonist throughout to preclude the activation of ATP-sensitive endogenous P2X ligand-gated channels (Cloues et al., 1993). Drugs were applied to the external solution by bath perfusion (bath exchange rate ≤5 sec). Tetrodotoxin was obtained from Calbiochem (La Jolla, CA); uridine 5′-triphosphate (UTP) was from Pharmacia Biotech (Uppsala, Sweden) and from Sigma; ATP, inosine 5′-triphosphate (ITP), guanosine 5′-triphosphate (GTP), acetylcholine chloride, (−)-norepinephrine bitartrate, nifedipine, BAPTA, and amphotericin B were all from Sigma; adenosine 5′-diphosphate (ADP) and uridine 5′-diphosphate (UDP) were from Sigma and Boehringer Mannheim GmbH (Mannheim, Germany); hexokinase was from Boehringer Mannheim GmbH; oxotremorine-M (OxoM) was from Research Biochemicals (Natick, MA); Pertussis toxin (PTX) was from Porton Products (Dorset, UK); CdCl2 (AnalaR grade) was from BDH Chemicals (Poole, UK); BaCl2 and CsCl were from Aldrich (Milwaukee, WI). Nifedipine was prepared as a stock solution (10 mm) in ethanol and protected from light during storage and use.
RESULTS
Ca2+ channel current inhibition
We have reported previously that, in SCG neurons preinjected with 1.25 μg/μl P2Y2 cRNA (together with GFP cRNA), the application of UTP produced a reversible inhibition of the Ca2+ channel current by up to 64.0 ± 0.8%, with an IC50 of 0.50 ± 0.03 μm and that, at 0.5 μg/μl P2Y2 cRNA, UTP inhibited the current by up to 50.2 ± 0.6%, with an IC50 0.90 ± 0.05 μm (Filippov et al., 1997). Because no significant inhibition was detected on applying 100 μm UTP to neurons injected with GFP cRNA alone, this effect could be attributed entirely to the activation of newly expressed P2Y2 receptors and not to the activation of any endogenous UTP-sensitive receptors that might have been present (see below).
Voltage dependence
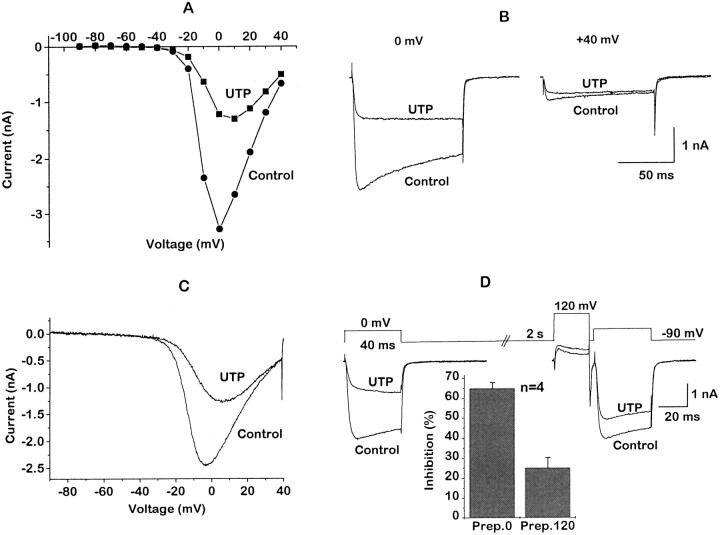
Ca2+ current inhibition in SCG neurons produced by activation of some endogenous receptors (see Hille, 1994) or by heterologously expressed mGluR2 receptors (Ikeda et al., 1995) is voltage-dependent—that is, it is reduced at depolarized commands (or by predepolarization; Grassi and Lux, 1989) and is accompanied by “kinetic slowing” resulting from time-dependent relief of block during the depolarizing command (Bean, 1989). Figure1 shows that the block produced by activating heterologously expressed P2Y2 receptors shares this property. Thus, current– voltage curves constructed by using either stepped (Fig. 1A,B) or ramped (Fig.1C) commands showed a greater inhibition by UTP at negative potentials than at positive potentials (resulting in a positive shift of the current peak). For example, in Figure 1A peak current inhibition was reduced from ∼63% at 0 mV to ∼26% at +40 mV. Also, as shown in Figure 1, B and D, current activation was slowed in the presence of UTP, such that inhibition was less at the end of the 40 msec command than at the beginning. Finally, inhibition was reduced (from 65.0 ± 3.1 to 24.9 ± 5.4%) and the slowing of current activation was abolished when the test command was preceded by a 20 msec depolarizing prepulse to +120 mV (Fig. 1D). Such effects have been interpreted to indicate that the activated G-protein (probably the βγ-subunit;Herlitze et al., 1996; Ikeda, 1996; Delmas et al., 1998a,b) interacts directly with the Ca2+ channel protein to induce a gating shift (Dolphin, 1995; Jones and Elmslie, 1997).
Fig. 1.
Voltage-dependent inhibition of Ca2+ channel currents by UTP in rat SCG neurons expressing heterologous P2Y2 receptors. Neurons were preinjected with 1.25 μg/μl P2Y2 cRNA. Records show leak-subtracted Ca2+ channel currents recorded at room temperature (20°C), using the whole-cell (ruptured patch) variant of the patch-clamp technique with 5 mmBa2+ as the charge carrier (IBa; see Materials and Methods).A, IBa amplitude plotted against membrane voltage (I–V relationship) in the absence (filled circles) and presence (filled squares) of 100 μm UTP. Currents were evoked by 10 mV incrementing test pulses, starting from a holding potential of −90 to +40 mV; amplitudes were measured 10 msec from the onset of the test pulse. B, Superimposed currents from A at 0 and +40 mV test potentials in the absence and presence of UTP. Note that the inhibition is less at +40 than at 0 mV. C, Superimposed currents recorded with 750 msec depolarizing voltage ramps from −90 to + 40 mV (see Materials and Methods) in the absence and presence of 100 μm UTP. Note that the inhibition is less at more positive voltages.D, Superimposed currents (lower traces) recorded with a double-pulse voltage protocol (upper traces) in the absence and presence of 100 μmUTP. The current was recorded first with a 40 msec test pulse to 0 mV; then, after a 2 sec interval, a 25 msec conditioning prepulse to +120 mV was applied, followed 4 msec later by a second 40 msec test pulse to 0 mV. The bar chart at the bottom shows the mean percentage of current inhibition (measured after 10 msec at 0 mV command potential) by 100 μm UTP before (Prep.0) and after (Prep.120) the +120 mV prepulse. Error bars show SEM; n = number of cells. Note that the inhibition is much less after the prepulse. Note also that the prepulse abolished the slowing of the current onset at 0 mV produced by UTP.
Pertussis toxin distinguishes two pathways for P2Y2-mediated Ca2+ channel current inhibition
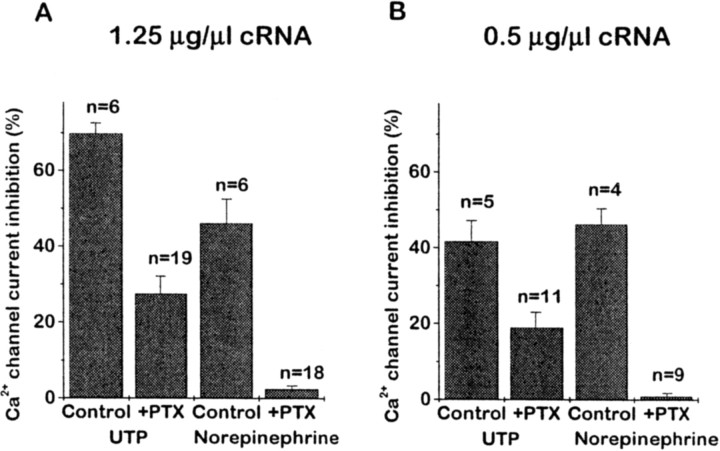
Voltage-dependent Ca2+ current inhibition of the type illustrated in Figure 1 is usually (although not invariably;Ehrlich and Elmslie, 1995) associated with activation by the receptor of a G-protein of the Pertussis toxin-sensitive Gi/Go family (Hille, 1994). We tested whether this applied to expressed P2Y2 receptors by overnight incubation of injected neurons with 0.5 μg/mlPertussis toxin (PTX). In cells preinjected with 1.25 μg/μl P2Y2 cRNA, PTX substantially (∼61%) but incompletely reduced the inhibition produced by 10 μm UTP (Fig. 2A). In contrast, in the same neurons the inhibition produced by 10 μmnorepinephrine (which is mediated primarily by Go;Caulfield et al., 1994; Delmas et al., 1998a) was reduced by >90%, as previously reported (Beech et al., 1992; Chen and Schofield, 1993;Caulfield et al., 1994; Delmas et al., 1998a), thus indicating the effectiveness of the PTX treatment. Nevertheless, because the initial inhibition produced by UTP exceeded that produced by norepinephrine, we were concerned that the substantial component of PTX-resistant inhibition might have resulted from overexpression (and aberrant coupling) of the P2Y2 receptors. This appeared not to be the case, because PTX produced a comparable degree of attenuation (−54%) of the response to UTP in cells preinjected with 0.5 μg/μl P2Y2 cRNA, although the initial inhibition produced by UTP (41.6 ± 5.5%) was now less than that produced by norepinephrine (46.1 ± 4.2%; Fig. 2D).
Fig. 2.
Pertussis toxin (PTX) distinguishes two pathways for P2Y2-mediated Ca2+ channel current inhibition. The bar charts show the mean inhibition ofIBa amplitude by 10 μm UTP and by 10 μm norepinephrine in neurons pretreated with PTX (0.5 μg/ml, overnight; +PTX) and in PTX-untreated neurons (Control). Error bars show SEM; n = number of cells. Neurons were injected with 1.25 μg/μl P2Y2 cRNA (A) or 0.5 μg/μl P2Y2 cRNA (B). Currents were recorded by stepping for 100 msec from −90 to 0 mV and measured 10 msec from the onset of the test pulse. Note that PTX pretreatment completely prevented the effect of norepinephrine, but not that of UTP.
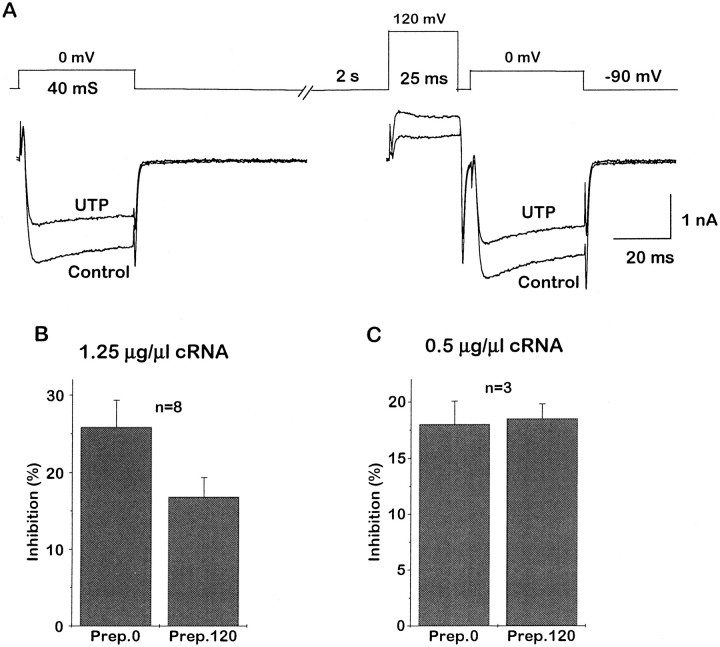
In contrast to the partial antagonism of overall inhibition, PTX pretreatment virtually eliminated the voltage dependence of P2Y2-mediated inhibition; the characteristic slowing of current activation by UTP (compare with Fig. 1) was no longer apparent after PTX treatment (Fig. 3A), and the +120 mV depolarizing prepulse did not reverse the inhibition significantly (Fig. 3B,C). Thus, the PTX-insensitive component of block also appeared to be voltage-insensitive.
Fig. 3.
PTX pretreatment eliminates the voltage dependence of P2Y2-mediated Ca2+ channel current inhibition. Cells were pretreated with 500 ng/ml PTX. Records inA show superimposed IBatraces generated with the same double-pulse voltage protocol as in Figure 1D in the absence and presence of 10 μm UTP. The bar charts show the mean inhibition ofIBa by 10 μm UTP before (Prep.0) and after (Prep.120) a +120 mV prepulse in neurons injected with 1.25 μg/μl P2Y2 cRNA (B) and 0.5 μg/μl P2Y2 cRNA (C). Note that, after PTX treatment, UTP no longer slowed the recorded currents and that the prepulse no longer significantly reduced inhibition (compare with Fig. 1).
One possible explanation for the PTX-resistant voltage-insensitive component of inhibition is that it reflects the inhibition of an L-type current, rather than the N-type current (Hille, 1994). This possibility is enhanced by previous observations that UTP inhibits both L-type and N-type currents in NG108-15 cells (Filippov and Brown, 1996). To check this, we tested the effect of 2–10 μm nifedipine on the response to UTP of PTX-pretreated SCG neurons preinjected with 1.25 μg/μl P2Y2 cRNA. In agreement with previous observations on non-PTX-treated cells (Filippov et al., 1997), nifedipine itself did not produce any significant inhibition of the current (−2.5 ± 1.7%; n = 13), nor did it affect the inhibitory action of UTP (−nifedipine, −29.4 ± 3.5%; +nifedipine, −27.3 ± 4.25%; n = 6). Thus, the residual PTX-insensitive block is not attributable to the inhibition of L-type channels.
Coupling of P2Y2 receptors to M-type K+ currents
M-currents are sustained voltage-gated K+currents that are activated when SCG neurons are depolarized above −70 mV (Constanti and Brown, 1981) (for review, see Brown, 1988). They can be inhibited by activating endogenous M1-muscarinic acetylcholine receptors (Marrion et al., 1989; Bernheim et al., 1992), angiotensin receptors (Shapiro et al., 1994), or bradykinin receptors (Jones et al., 1995)—in all cases via PTX-insensitive G-proteins (probably Gq; see Caulfield et al., 1994; Jones et al., 1995).
There is some evidence for the presence of endogenous P2Y receptors in intact SCGs [Connolly et al. (1993); Boehm et al. (1995) and personal communication; Connolly and Harrison (1995); Von Kugelgen et al. (1997)]. However, we found that UTP did not affect the M-current significantly in the uninjected dissociated neurons that we have used, although some other nucleotides did (see below and Fig. 7A). We therefore have tested whether the activation of heterologously expressed P2Y2 receptors inhibits the M-current in these neurons, and, if so, how this compares with inhibition of Ca2+ current.
Fig. 7.
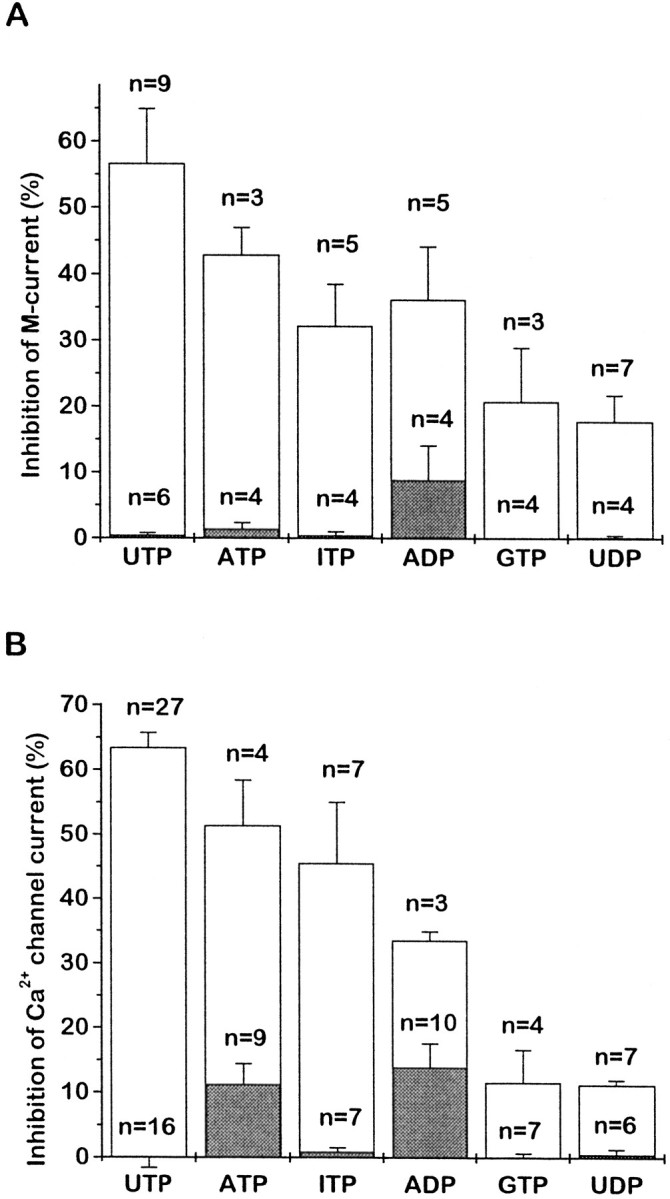
Mean effects of different nucleotides on M-current (A) and Ca2+ channel current (B). Nucleotides were applied at 10 μm. Open bars show the mean percentage of inhibition in neurons preinjected with 1.25 μg/μl P2Y2cRNA; the shaded bars show the effects in control cells (preinjected with GFP cRNA, but without P2Y2 cRNA). Error bars show SEM; n = number of cells. Data for P2Y2 cRNA-injected cells in B were recalculated from Filippov et al. (1997). Inhibition was measured as described in Figures 2 and 6.
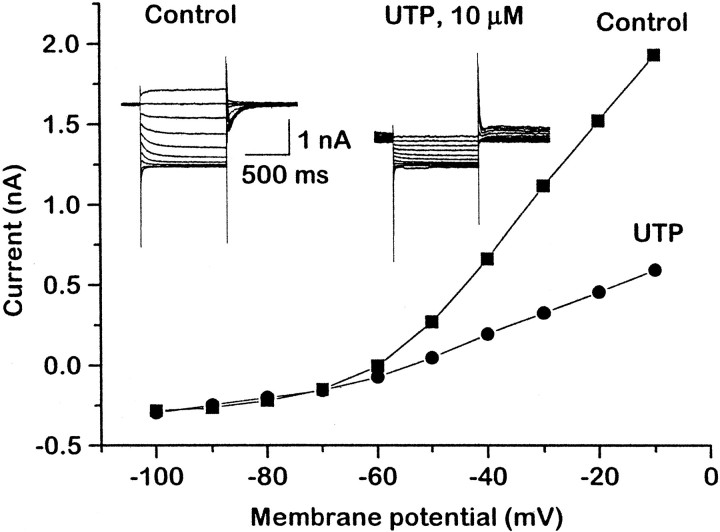
Figure 4 illustrates the effect of UTP on M-currents in cells preinjected with 1.25 μg/μl P2Y2cRNA. The cell was predepolarized to −20 mV to preactivate the M-current and then hyperpolarized in steps of −10 mV for 1 sec each to deactivate the current; deactivation is signaled by the inward tail currents, which reverse at EK (approximately −90 mV). UTP (10 μm) clearly inhibited the M-current; this is indicated by (1) the inward shift in holding current at −20 mV (reflecting the reduction in outward K+ current), (2) reduced current responses to depolarizing steps (reduced conductance) and the loss of M-current deactivation tails, and (3) reduced outward rectification in the current–voltage curve positive to −70 mV, with no change in slope negative to −70 mV (indicating no change in “leak” current).
Fig. 4.
Activation of heterologously expressed P2Y2 receptors inhibits the M-type K+current (M-current; IK(M)) in rat SCG neurons. The neuron was injected 18 hr beforehand with 1.25 μg/μl P2Y2 cRNA. M-current was recorded with a perforated patch electrode by predepolarizing the neuron to −20 mV and then deactivating the current with 1 sec hyperpolarizing steps in increments of 10 mV at 5 sec intervals, as shown in the current records. The graph shows the current amplitude at the end of each 1 sec step measured as change from zero current. Currents were recorded before (filled squares; Control) and after (filled circles) the addition of 10 μm UTP. Note that UTP produced an inward current at the holding potential of −20 mV, reduced the amplitude of the M-current deactivation tail currents during the hyperpolarizing steps, and reduced the outward rectification of the current–voltage curve positive to −70 mV. (The slight outward drift of the holding current in the presence of UTP reflects slow receptor desensitization.)
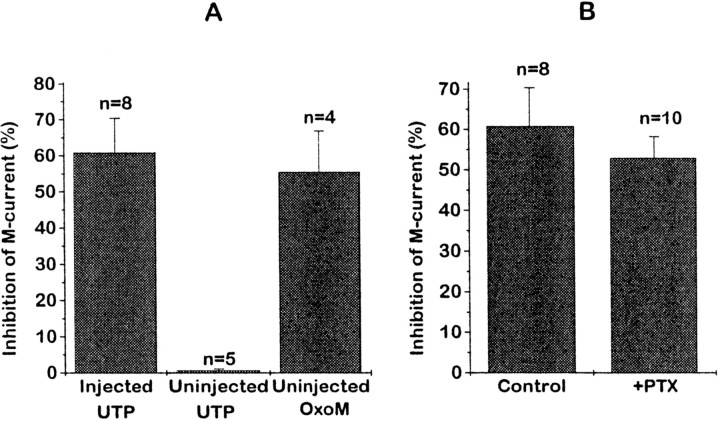
M-current inhibition was quantitated by using voltage ramps to obtain current–voltage curves (see Fig. 6) and then subtracting from the total outward current at −30 mV the extrapolated linear leak currents from potentials negative to −70 mV. UTP (10 μm) inhibited the M-current by 60.8 ± 9.6% (n = 8) in neurons preinjected with 1.25 μg/μl P2Y2 cRNA. As noted above, UTP had no significant effect at 100 μm(0.6 ± 0.4% inhibition) in five noninjected cells (Fig.5A). In four of these same cells the activation of the endogenous muscarinic acetylcholine receptors with 10 μm oxotremorine-M (OxoM) inhibited the current by 55.4 ± 11.4%, in accordance with previous observations (see Caulfield et al., 1994), indicating that the endogenous transduction machinery was intact.
Fig. 6.
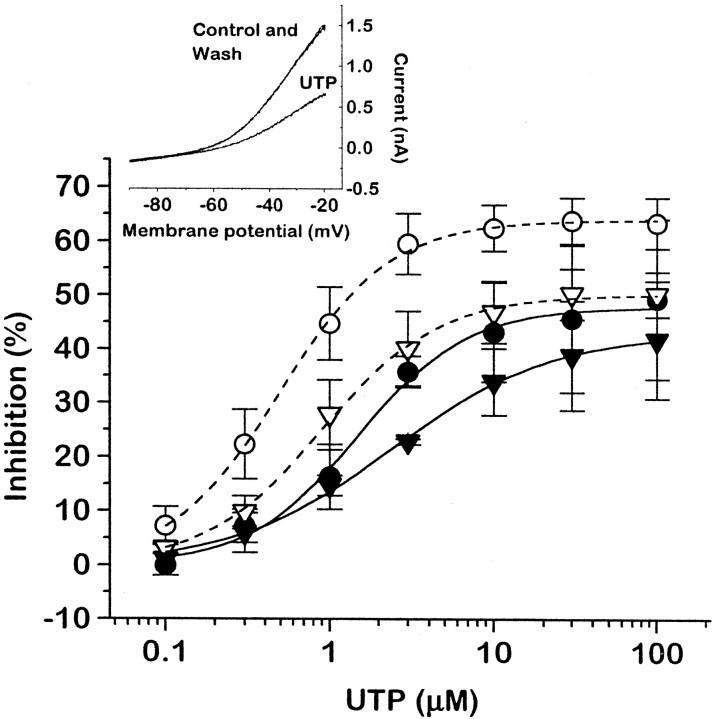
Concentration dependence for UTP inhibition of M-current (solid lines) and N-type Ca2+ channel current (dashed lines) in cells preinjected with 1.25 μg/μl P2Y2 cRNA (circles) or 0.5 μg/μl P2Y2 cRNA (triangles). M-current was recorded by using a voltage ramp protocol (inset) and was measured at −30 mV (see Materials and Methods). The points show the mean ± SEM of measurements in three to four cells; concentrations were added cumulatively, with 1 min exposure times. Curves were fit to pooled data points, using Origin 4.1 software to the Hill equation:y = ymax ·xnH/(xnH+ KnH), wherey = the observed percentage of inhibition,ymax = extrapolated maximal percentage of inhibition, x = nucleotide concentration (μm), K = IC50(μm), and nH = the Hill coefficient. Values of constants (mean ± SEM) for M-current inhibition were ymax = 48.0 ± 1.20%,K = 1.49 ± 0.14 μm,nH = 1.29 ± 0.14 for neurons injected with 1.25 μg/μl P2Y2 cRNA; andymax = 43.2 ± 1.95%,K = 2.41 ± 0.43 μm,nH = 0.88 ± 0.11 for neurons injected with 0.5 μg/μl P2Y2 cRNA. Data for Ca2+ channel current inhibition (dashed lines) are taken from Filippov et al. (1997) and are superimposed for comparison. (Values for constants wereymax = 64.0 ± 0.75%,K = 0.50 ± 0.03 μm,nH = 1.29 ± 0.0714 for neurons injected with 1.25 μg/μl P2Y2 cRNA; andymax = 50.2 ± 0.61%,K = 0.90 ± 0.05 μm,nH = 1.21 ± 0.06 for neurons injected with 0.5 μg/μl P2Y2 cRNA).
Fig. 5.
M-current inhibition by UTP requires heterologous P2Y2 receptor expression (A) and is not prevented by PTX (B). The bar chart inA shows the mean percentage of inhibition of the M-current at −30 mV (see Materials and Methods) by 10 μmUTP in neurons injected with 1.25 μg/μl P2Y2 cRNA (injected) and in uninjected neurons. Current inhibition by 10 μm oxotremorine-M (OxoM) in uninjected neurons is shown for comparison. Error bars show SEM;n = number of cells tested. The bar chart inB shows the mean percentage of inhibition of the M-current by 10 μm UTP at −30 mV in neurons preinjected with P2Y2 cRNA without (Control) or with (+PTX) overnight pretreatment with 0.5 μg/ml PTX.
With the use of increasing concentrations of UTP, mean IC50values and extrapolated maximum inhibitions were 1.49 ± 0.18 μm and 48.0 ± 1.2%, respectively, after 1.25 μg/μl P2Y2 cRNA was injected, and 2.41 ± 0.43 μm and 43.2 ± 1.9% after 0.5 μg/μl P2Y2 cRNA was injected (Fig.6). These IC50 values are approximately three times higher than those determined for N-type Ca2+ current inhibition (Filippov et al., 1997) (superimposed curves in Fig. 6). The lower apparent maximum inhibition with increasing UTP concentrations than those in Figure 5 may reflect some degree of desensitization (apparent as a slow partial recovery of M-current during prolonged application of UTP); the extent to which this affected IC50 estimates is unclear.
In contrast to its effect on Ca2+ current inhibition, PTX produced no significant attenuation of UTP-induced M-current inhibition (Fig. 5B). Also, inhibition of M-current showed no clear voltage dependence in that neither the current–voltage curve nor the kinetics of the deactivation relaxations was altered appreciably in the presence of UTP.
We further tested the effects of several other nucleotides, using a standard concentration of 10 μm (Fig.7A). The approximate order of activity was UTP ≥ ATP > ITP ≫ GTP,UDP. This accords with their relative activities in inhibiting the Ca2+ current in P2Y2-expressing SCG neurons (Fig. 7B) (see Filippov et al., 1997) and also with stimulation of inositol phosphate production or intracellular Ca2+ elevation in other cells expressing cloned P2Y2 receptors (Lustig et al., 1993; Chen et al., 1996;Nicholas et al., 1996) and in NG108-15 cells expressing the endogenous receptor (Lin et al., 1993); the strong activity of ITP and relatively weak activity of UDP (see below) are particularly noteworthy in this respect. The inhibitory activity of ADP was less than that of UTP and appeared to be less than that of ATP, but it was difficult to quantitate because ADP produced some inhibition of both M- and Ca2+ currents in control (GFP cRNA-injected) cells (Fig. 7, shaded columns)—possibly via a low-abundance endogenous P2Y1 receptor. ATP also partly inhibitedIK(M) in control cells; the other nucleotides tested (UTP, UDP, ITP, GTP) did not produce any significant effect on either Ca2+- or M-current in the cells preinjected with GFP cRNA alone. [It should be noted that, because the test concentration of UTP was near-maximal, the relative heights of thebars in Fig. 7 do not provide an accurate index of numerical potency ratios. For example, ADP was ∼50 times less potent than UTP in inhibiting ICa when measured from full dose–response curves (Filippov et al., 1997). Thus the dinucleotides ADP and UDP are likely to be at least one or two orders of magnitude less potent than UTP in inhibiting the M-current. Unfortunately, because of desensitization and slow recovery, it was not possible to construct full dose–response curves for the inhibitory action of the weaker analogs on the M-current.]
To check whether the effects of UDP and ADP in cRNA P2Y2preinjected cells arise from possible contamination of these nucleotides by UTP and ATP (see Nicholas et al., 1996), we performed such tests by using purified UDP and ADP from Boehringer Mannheim (99% pure) immediately after their dissolution in the medium. In addition, we used samples of UDP and ADP pretreated for 1 hr with hexokinase (1 mm UDP or ADP incubated with 10 U/ml hexokinase plus 22 mm glucose at 37°C); this should convert any UTP and ATP that was present to their diphosphates (Nicholas et al., 1996). The hexokinase pretreatment did not significantly reduce the inhibitory effects of 10 μm UDP or ADP, and the pure UDP and ADP produced inhibitions within the range of those observed with the same nucleotides from Sigma.
Effects on excitability
The inhibition of M-type K+ currents and N-type Ca2+ currents through endogenous G-protein-coupled receptors increases the excitability of SCG neurons. This occurs because the M-current itself acts as a “braking” current on action potential discharges (see Brown, 1988), and the entry of Ca2+ through N-type Ca2+ channels opens Ca2+-activated K+ channels and thereby induces a long-lasting afterhyperpolarization (AHP), which further limits subsequent spike activity [see Davies et al. (1996) and references therein]. Thus, the activation of expressed P2Y2 receptors also might be expected to enhance spike activity.
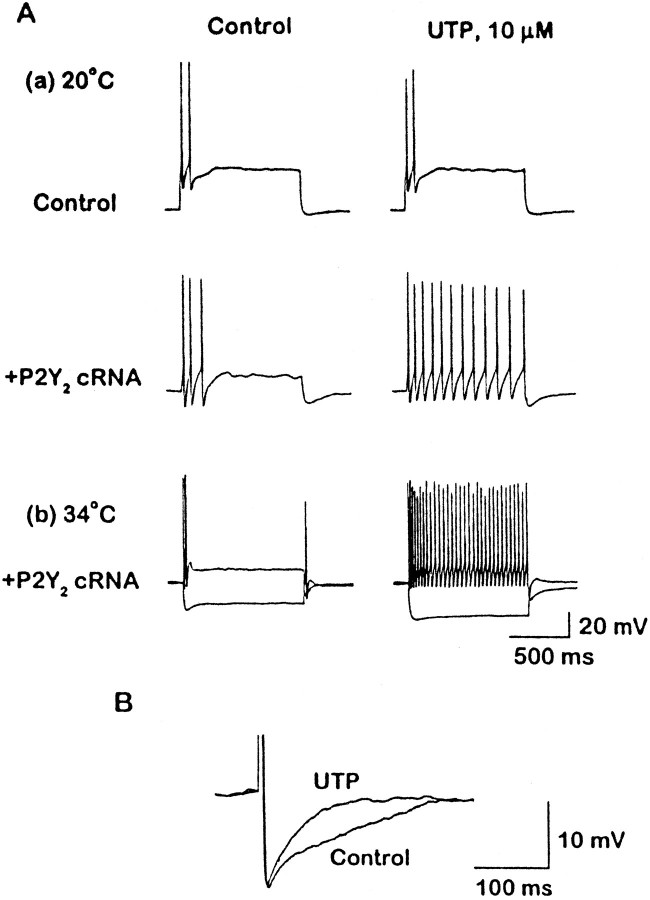
Figure 8 shows that this was the case. In Figure 8A, the cells were challenged with long depolarizing or hyperpolarizing current injections from a preset potential of −60 mV at room temperature, at 20°C (Fig.8Aa), and at 34°C (Fig. 8Ab). The long depolarizing current induced a brief burst of two to three spikes, followed by silence. The application of UTP had no effect on this spike discharge in control cells preinjected with GFP cRNA but greatly prolonged the discharge in cells preinjected with P2Y2cRNA. This effect of UTP was increased dramatically at 34°C (Fig.8Ab); this occurs probably because M-current kinetics are faster at 34°C (Brown, 1988), thereby exerting a faster and more effective “brake” on firing (see Cuevas et al., 1997). UTP also increased the voltage response to hyperpolarizing current pulses (Fig.8Ab), as expected after M-current inhibition (seeAdams et al., 1982). These effects closely resemble the effect of activating endogenous muscarinic receptors in SCG neurons (see Brown and Constanti, 1980).
Fig. 8.
Activation of heterologously expressed P2Y2 receptors enhances repetitive firing (A) and reduces the Ca2+-activated spike afterhyperpolarization (B) in SCG neurons. Aa, Voltage responses to depolarizing current pulses (1 sec) recorded from a control (uninjected) SCG neuron (top panel, 0.4 nA pulse) and from a P2Y2 cRNA (1.25 μg/μl) preinjected neuron (middle panel, 0.1 nA pulse) before and during application of UTP at 20°C. Ab, Voltage responses to depolarizing (0.3 nA) and hyperpolarizing (−0.2 nA) current pulses from P2Y2 cRNA (1.25 μg/μl) preinjected neuron before and during the application of UTP at 34°C. B, Afterhyperpolarization (AHP) that followed an action potential evoked by a brief depolarizing current pulse (1 nA, 1 msec) recorded from a P2Y2 cRNA (1.25 μg/μl) preinjected neuron before and during the application of UTP at 34°C.
Figure 8B shows the effect of stimulating expressed P2Y2 receptors on the Ca2+-activated afterhyperpolarization that follows an action potential: UTP abbreviated the afterhyperpolarization in a manner resembling the effect of stimulating endogenous α2-adrenergic receptors with norepinephrine (Horn and McAfee, 1980), which results from the inhibition of the N-type Ca2+ current (Galvan and Adams, 1982; Schofield, 1990).
DISCUSSION
The principal points emerging from these and the preceding experiments (Filippov et al., 1997) are that the recombinant rat P2Y2 receptor can couple with near-equal facility to two quite different neural ion channels—the N-type voltage-gated Ca2+ channel and the M-type K+channel—when expressed in rat SCG neurons and that this involves the intermediation of at least two different G-proteins. Dual coupling to these particular channels by one species of G-protein-linked receptor is unusual (see below) and suggests that the P2Y2 receptor can have a potentially broader range of effects on neurons than many other neurotransmitter receptors.
This apparent cross-talk is unlikely to be an artifact of receptor overexpression, for two reasons. First, it was preserved after injections of two different amounts of the receptor cRNA, such that, at the lower level (0.5 μg/μl), the maximum response to UTP was less than that obtained on stimulating endogenous adrenergic and muscarinic receptors. Thus, although (in the absence of appropriate antibodies) we have not been able to measure the number of receptors expressed, they are unlikely to be “excessive” in comparison to other endogenous serpentine receptors in these cells. Second, the IC50values for UTP observed in the present experiments (0.5–0.9 μm for Ca2+ current inhibition and 1.5–2.4 μm for M-current inhibition) are not dissimilar to those observed for UTP to elevate intracellular [Ca2+] [1.1 μm, Lustig et al. (1993); ∼1.1 μm, Parr et al. (1994); 0.2 μm, Chen et al. (1996)] or to stimulate inositol phosphate production (∼0.1 μm; Nicholas et al., 1996) when applied to recombinant P2Y2 receptors expressed in other cell lines. (IC50 values likely will vary from one expression system to another, depending on the level of receptor expression, as indicated by the dose–response curves in Fig. 6.) More pertinently, perhaps, the present IC50 values also accord with those that follow the stimulation of the endogenous P2Y2 receptor in NG108-15 cells [∼3 μm for inositol phosphate production (Lin, 1994); 0.8 μm for M-like current inhibition (Filippov et al., 1994); 2.8 μmfor N-type Ca2+ current inhibition (Filippov and Brown, 1996)]. The agreement between the results obtained on stimulating the endogenous receptors in this neural cell line and the exogenously expressed receptors in SCG neurons strongly suggests that the effects we see are likely to be a general phenomenon, applicable in other cells in which the same receptor might be expressed.
These divergent responses start at the level of the G-protein. M-current inhibition was insensitive to PTX; although we have not positively identified the species of PTX-resistant G-protein(s) responsible, previous experiments that used site-directed antibodies on the analogous effects of muscarinic agonists (Caulfield et al., 1994) and bradykinin (Jones et al., 1995) suggest that it is most likely Gq and/or G11 (principally Gq; Haley et al., 1997). In contrast, Ca2+ current inhibition is mediated by at least two G-proteins: a PTX-sensitive G-protein, responsible for the gating shift and for ∼60% of the peak inhibition measured at 0 mV, and a PTX-insensitive G-protein responsible for the residual voltage-insensitive component of inhibition. By analogy with the voltage-dependent effects of norepinephrine, somatostatin, and M4 muscarinic stimulation, the former (PTX-sensitive) G-protein is probably GoA (Caulfield et al., 1994; Delmas et al., 1998a,b), and the gating shift probably results from the interaction of the dissociated free βγ-subunits with the Ca2+ channel protein (see Herlitze et al., 1996;Ikeda, 1996; Delmas et al., 1998a,b). The PTX-insensitive G-protein responsible for the voltage-insensitive component of Ca2+ current inhibition could well be the same as that (Gq?) postulated to cause M-current inhibition, because M1 muscarinic acetylcholine receptor stimulation produces the same dual-effector response via Gq(Delmas et al., 1998b).
In effect, therefore, stimulating the P2Y2 receptor imitates the combined effects of stimulating two separate endogenous muscarinic acetylcholine receptors, the M1 and the M4 receptors (see Hille, 1994). This is summarized in Figure 9; at a minimum, it requires parallel coupling of the P2Y2 receptor to two G-proteins: Gq (leading to inhibition of the M-current and voltage-independent inhibition of the Ca2+ current) and Go (producing a gating shift of the Ca2+ channels).
Fig. 9.
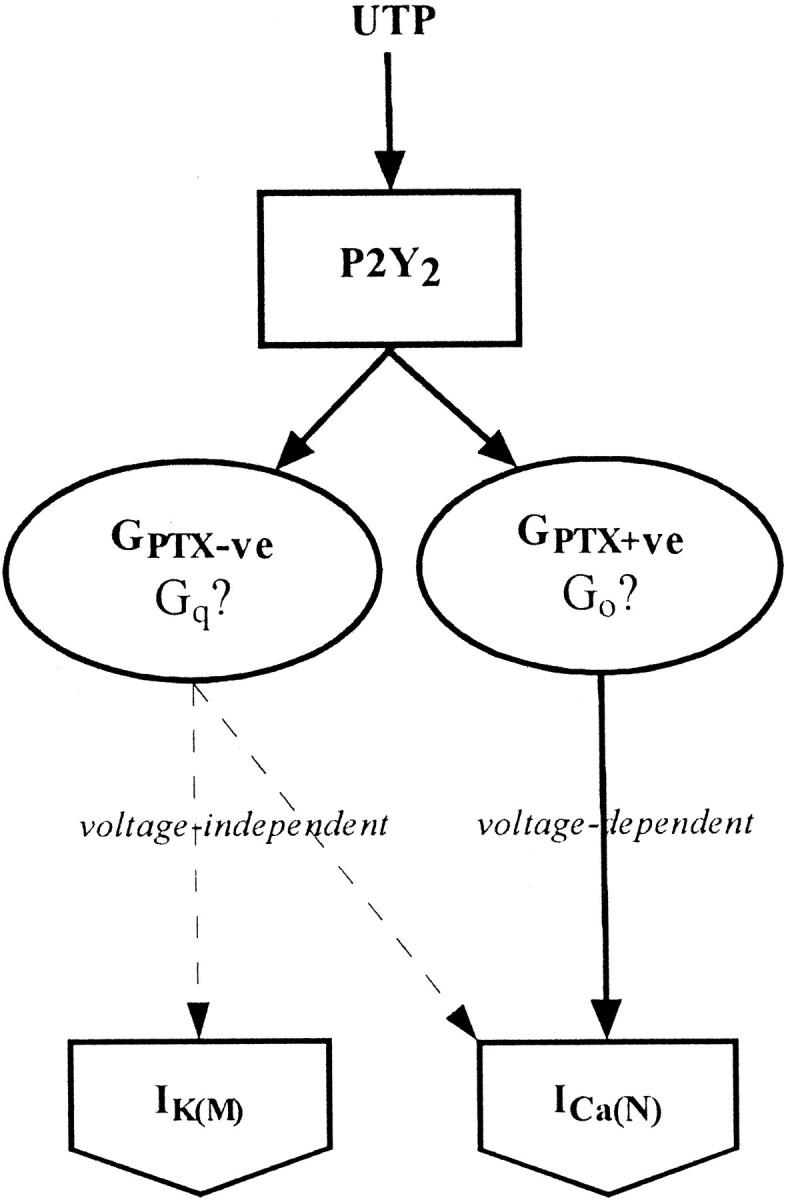
Schematic diagram representing dual coupling of P2Y2 receptor to Ca2+N and K+M channels via two different G-proteins. Solid lines, Direct connections;dashed lines, indirect connections.
There are other instances of “promiscuity” in receptor/G-protein coupling (see Milligan, 1997). This can result in effects on more than one ion channel—for example, the inhibition of Ca2+currents and the activation of inward rectifier (Kir) K+ currents (Surprenant et al., 1992). However, dual coupling to Ca2+N and K+M channels through two G-proteins by a single, defined receptor is more unusual. Thus, although many transmitters can, collectively, modulate Ca2+currents or M-currents in SCG neurons in a manner similar to that produced by P2Y2 receptors (see Hille, 1994), this usually involves the activation of separate receptors, each coupled to a different G-protein. As indicated already, muscarinic acetylcholine agonists can produce the same dual response as UTP, but to do so they have to activate two different molecular species of muscarinic receptor; activation of Go (and the Ca2+ channel gating shift) requires M4receptors, whereas activation of Gq (and consequential voltage-independent inhibition of M-current and Ca2+current) requires M1 receptors (see Beech et al., 1992;Delmas et al., 1998b). This also applies to other heterologous receptors expressed by this method. Thus, there is negligible cross-talk between heterologous mGluR1a and mGluR2 receptors expressed from cRNA injections; the former inhibit M-currents via a PTX-insensitive G-protein with insignificant effect on the Ca2+ current, whereas mGluR2 receptors inhibit Ca2+ currents entirely through a PTX-sensitive G-protein without any effect on M-currents (Ikeda et al., 1995). Likewise, heterologously expressed cannabinoid receptors selectively inhibit Ca2+ currents through a PTX-sensitive G-protein (Pan et al., 1996). The closest analogy is provided by the action of angiotensin, which also couples through two G-proteins (PTX-sensitive and insensitive) to inhibit Ca2+ and M-currents (Shapiro et al., 1994); however, it has not yet been established whether both coupling routes are activated by the same or different molecular species of angiotensin receptor.
Physiological significance
From a functional viewpoint, the net effect of stimulating expressed P2Y2 receptors in SCG neurons is to increase their excitability (see Fig. 8A). This is the expected result of inhibiting the M-current and the N-type Ca2+ current, because reducing the latter will decrease the Ca2+-dependent K+current and abbreviate the spike afterhyperpolarization (as shown in Fig. 8B), and KM andKCa currents act as synergistic braking currents on spike discharge (see Jones and Adams, 1987).
This is essentially a postsynaptic response; it mimics the natural postsynaptic effect of activating the endogenous muscarinic receptors by synaptically released acetylcholine in SCG neurons (see Brown, 1988), so it would provide a mechanism for slow postsynaptic excitation by a nucleotide transmitter. Although no such nucleotide-mediatedsynaptic responses have been reported yet, comparable excitatory effects of exogenous UTP and ATP have been described in, for example, frog sympathetic (Siggins et al., 1977;Adams et al., 1982; Akasu et al., 1983; Lopez and Adams, 1989), frog sensory (Tokimasa and Akasu, 1990), and rat intracardiac (Cuevas et al., 1997) ganglion cells. However, the species of P2Y receptor responsible for these effects has not been determined, nor is there yet any direct evidence for equivalent effects on central neurons.
On the other hand, if endogenous P2Y2 receptors were located presynaptically, then the most likely effect of their stimulation would be to reduce transmitter release via the inhibition of the N-type Ca2+ current, in the same manner as stimulating endogenous presynaptic adrenergic or muscarinic receptors in SCG neurons (Boehm and Huck, 1996; Koh and Hille, 1997). This would provide a mechanism for autoinhibition in nucleotide-releasing nerve terminals. There is some evidence for P2Y-mediated autoinhibition in peripheral sympathetic nerves (Fuder and Muth, 1993) and chromaffin cells (Currie and Fox, 1996); also, P2Y-mediated inhibition of norepinephrine release from isolated brain tissue by exogenous nucleotides has been reported (Von Kugelgen et al., 1994), but the identity of these receptors has not yet been established.
As pointed out in the introductory remarks, there is now direct evidence for the synaptic release of ATP in the brain and the consequent activation of postsynaptic P2X receptors (Edwards and Gibb, 1993). Although there is, as yet, no evidence for the synaptic release of UTP, the latter nucleotide can be released from cells by other mechanisms (Lazarowski et al., 1997). Because mRNA for the P2Y2 receptor is expressed in the brain (Lustig et al., 1993), were this receptor to be activated by either of these endogenously released nucleotides, its dual coupling would provide scope for some unusually divergent effects on neural signaling.
Footnotes
This work was supported by The Wellcome Trust. We thank Brenda Browning and Misbah Malik for tissue culture.
Correspondence should be addressed to Dr. Alexander K. Filippov, Department of Pharmacology, University College London, Gower Street, London WC1E 6BT, United Kingdom.
REFERENCES
- 1.Adams PR, Brown DA, Constanti A. Pharmacological inhibition of the M-current. J Physiol (Lond) 1982;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akasu T, Hirai K, Koketsu K. Modulatory actions of ATP on membrane potentials of bullfrog sympathetic ganglion cells. Brain Res. 1983;258:313–317. doi: 10.1016/0006-8993(83)91157-5. [DOI] [PubMed] [Google Scholar]
- 3.Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- 4.Beech DJ, Bernheim L, Hille B. Pertussis toxin and voltage dependence distinguish multiple pathways modulating calcium channels of rat sympathetic neurons. Neuron. 1992;8:97–106. doi: 10.1016/0896-6273(92)90111-p. [DOI] [PubMed] [Google Scholar]
- 5.Bernheim L, Mathie A, Hille B. Characterization of muscarinic receptor subtypes inhibiting Ca2+ current and M current in rat sympathetic neurons. Proc Natl Acad Sci USA. 1992;89:9544–9548. doi: 10.1073/pnas.89.20.9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boarder MR, Weisman GA, Turner JT, Wilkinson GF. G-protein-coupled P2 purinoceptors: from molecular biology to functional response. Trends Pharmacol Sci. 1995;16:133–139. doi: 10.1016/s0165-6147(00)89001-x. [DOI] [PubMed] [Google Scholar]
- 7.Boehm S, Huck S. Inhibition of N-type calcium channels: the only mechanism by which presynaptic alpha 2-autoreceptors control sympathetic transmitter release. Eur J Neurosci. 1996;8:1924–1931. doi: 10.1111/j.1460-9568.1996.tb01336.x. [DOI] [PubMed] [Google Scholar]
- 8.Boehm S, Huck S, Illes P. UTP- and ATP-triggered transmitter release from rat sympathetic neurons via separate receptors. Br J Pharmacol. 1995;116:2241–2243. doi: 10.1111/j.1476-5381.1995.tb15075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown DA. M-currents. In: Narahashi T, editor. Ion channels, Vol 1. Plenum; New York: 1988. pp. 55–94. [DOI] [PubMed] [Google Scholar]
- 10.Brown DA, Constanti A. Intracellular observations of the effects of muscarinic agonists on rat sympathetic neurones. Br J Pharmacol. 1980;70:593–608. doi: 10.1111/j.1476-5381.1980.tb09778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- 12.Burnstock G. Purinergic mechanisms. Ann NY Acad Sci. 1990;603:1–19. doi: 10.1111/j.1749-6632.1990.tb37657.x. [DOI] [PubMed] [Google Scholar]
- 13.Caulfield MP, Jones S, Vallis Y, Buckley NJ, Kim G-D, Milligan G, Brown DA. Muscarinic M-current inhibition via Gαq/11 and α-adrenoceptor inhibition of Ca2+ current via Gαo in rat sympathetic neurones. J Physiol (Lond) 1994;477:415–422. doi: 10.1113/jphysiol.1994.sp020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C, Schofield GG. Differential neuromodulation of calcium currents by norepinephrine in rat sympathetic neurons. J Neurophysiol. 1993;70:1440–1449. doi: 10.1152/jn.1993.70.4.1440. [DOI] [PubMed] [Google Scholar]
- 15.Chen ZP, Krull N, Xu S, Levy A, Lightman SL. Molecular cloning and functional characterization of a rat pituitary G-protein-coupled adenosine triphosphate (ATP) receptor. Endocrinology. 1996;137:1833–1840. doi: 10.1210/endo.137.5.8612522. [DOI] [PubMed] [Google Scholar]
- 16.Cloues R, Jones S, Brown DA. Zn2+ potentiates ATP-activated currents in rat sympathetic neurons. Pflügers Arch. 1993;424:152–158. doi: 10.1007/BF00374606. [DOI] [PubMed] [Google Scholar]
- 17.Connolly GP, Harrison PJ. Structure–activity relationships of a pyrimidine receptor in the rat isolated superior cervical ganglion. Br J Pharmacol. 1995;116:2764–2770. doi: 10.1111/j.1476-5381.1995.tb17239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connolly GP, Harrison PJ, Stone TW. Action of purine and pyrimidine nucleotides on the rat superior cervical ganglion. Br J Pharmacol. 1993;110:1297–1304. doi: 10.1111/j.1476-5381.1993.tb13959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Constanti A, Brown DA. M-currents in voltage-clamped mammalian sympathetic neurones. Neurosci Lett. 1981;24:289–294. doi: 10.1016/0304-3940(81)90173-7. [DOI] [PubMed] [Google Scholar]
- 20.Cuevas J, Harper AA, Trequattrini C, Adams DJ. Passive and active membrane properties of isolate rat intracardiac neurons: regulation by H- and M-currents. J Neurophysiol. 1997;78:1890–1902. doi: 10.1152/jn.1997.78.4.1890. [DOI] [PubMed] [Google Scholar]
- 21.Currie KP, Fox AP. ATP serves as a negative feedback inhibitor of voltage-gated Ca2+ channel currents in cultured bovine adrenal chromaffin cells. Neuron. 1996;16:1027–1036. doi: 10.1016/s0896-6273(00)80126-9. [DOI] [PubMed] [Google Scholar]
- 22.Davies PJ, Ireland DR, McLachlan EM. Sources of Ca2+ for different Ca2+-activated K+ conductances in neurones of the rat superior cervical ganglion. J Physiol (Lond) 1996;495:353–366. doi: 10.1113/jphysiol.1996.sp021599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delmas P, Brown DA, Dayrell M, Abogadie FC, Caulfield MP, Buckley NJ. On the role of endogenous G-protein βγ subunits in N-type Ca2+ current inhibition by neurotransmitters in rat sympathetic neurones. J Physiol (Lond) 1998a;506:319–329. doi: 10.1111/j.1469-7793.1998.319bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delmas P, Abogadie FC, Dayrell M, Haley JE, Milligan G, Caulfield MP, Brown DA, Buckley NJ. G-proteins and G-protein subunits mediating cholinergic inhibition of N-type calcium currents in sympathetic neurones. Eur J Neurosci. 1998b;10:1654–1666. doi: 10.1046/j.1460-9568.1998.00170.x. [DOI] [PubMed] [Google Scholar]
- 25.Docherty RJ, Robbins J, Brown DA. NG 108-15 cells neuroblastoma X glioma cell line as a model neuronal system. In: Wheal H, Chad J, editors. Cellular Neurobiology: a practical approach. IRL; Oxford: 1991. pp. 75–79. [Google Scholar]
- 26.Dolphin AC. Voltage-dependent calcium channels and their modulation by neurotransmitters and G-proteins. Exp Physiol. 1995;80:1–36. doi: 10.1113/expphysiol.1995.sp003825. [DOI] [PubMed] [Google Scholar]
- 27.Edwards FA, Gibb AJ. ATP—a fast neurotransmitter. FEBS Lett. 1993;325:86–89. doi: 10.1016/0014-5793(93)81419-z. [DOI] [PubMed] [Google Scholar]
- 28.Ehrlich I, Elmslie KS. Neurotransmitters acting via different G-proteins inhibit N-type calcium current by an identical mechanism in rat sympathetic neurons. J Neurophysiol. 1995;74:2251–2257. doi: 10.1152/jn.1995.74.6.2251. [DOI] [PubMed] [Google Scholar]
- 29.Erb L, Lustig KD, Sullivan DM, Turner JT, Weisman GA. Functional expression and photoaffinity labeling of a cloned P2U purinergic receptor. Proc Natl Acad Sci USA. 1993;90:10449–10453. doi: 10.1073/pnas.90.22.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Filippov AK, Brown DA. Activation of nucleotide receptors inhibits high-threshold calcium currents in NG108-15 neuronal hybrid cells. Eur J Neurosci. 1996;8:1149–1155. doi: 10.1111/j.1460-9568.1996.tb01282.x. [DOI] [PubMed] [Google Scholar]
- 31.Filippov AK, Selyanko AA, Robbins J, Brown DA. Activation of nucleotide receptors inhibits M-type K current [IK(M)] in neuroblastoma X glioma hybrid cells. Pflügers Arch. 1994;429:223–230. doi: 10.1007/BF00374316. [DOI] [PubMed] [Google Scholar]
- 32.Filippov AK, Webb TE, Barnard EA, Brown DA. Inhibition by heterologously expressed P2Y2 nucleotide receptors of N-type calcium currents in rat sympathetic neurones. Br J Pharmacol. 1997;121:849–851. doi: 10.1038/sj.bjp.0701270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuder H, Muth U. ATP and endogenous agonists inhibit evoked [3H]-noradrenaline release in rat iris via A1 and P2Y-like purinoceptors. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:352–357. doi: 10.1007/BF00171333. [DOI] [PubMed] [Google Scholar]
- 34.Galvan M, Adams PR. Control of calcium current in rat sympathetic neurones by norepinephrine. Brain Res. 1982;244:135–144. doi: 10.1016/0006-8993(82)90911-8. [DOI] [PubMed] [Google Scholar]
- 35.Grassi F, Lux HD. Voltage-dependent GABA-induced modulation of Ca2+ channels in chick sensory neurons. Neurosci Lett. 1989;105:113–119. doi: 10.1016/0304-3940(89)90021-9. [DOI] [PubMed] [Google Scholar]
- 36.Haley JE, Delmas P, Abogadie FC, Dayrell M, Caulfield MP, Buckley NJ, Brown DA. Muscarinic inhibition of the M-current is mediated by the α subunit of Gq. J Physiol (Lond) 1997;504P:176P. [Google Scholar]
- 37.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein βγ subunits. Nature. 1996;105:113–119. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 38.Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 39.Hirning LD, Fox AP, McLeskey EW, Olivera BM, Thayer SA, Miller RJ, Tsien RW. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988;239:57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- 40.Horn JP, McAfee DA. Alpha-adrenergic inhibition of calcium-dependent potentials in rat sympathetic ganglion cells. J Physiol (Lond) 1980;301:191–204. doi: 10.1113/jphysiol.1980.sp013198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein βγ subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 43.Ikeda SR, Lovinger DM, McCool BA, Lewis DL. Heterologous expression of metabotropic glutamate receptors in adult rat sympathetic neurons: subtype-specific coupling to ion channels. Neuron. 1995;14:1029–1038. doi: 10.1016/0896-6273(95)90341-0. [DOI] [PubMed] [Google Scholar]
- 44.Jones SW, Adams PR. The M-current and other potassium currents of vertebrate neurons. In: Kaczmarek LK, Levitan IB, editors. Neuromodulation. Oxford UP; New York: 1987. pp. 159–186. [Google Scholar]
- 45.Jones SW, Elmslie KS. Transmitter modulation of neuronal calcium channels. J Membr Biol. 1997;155:1–10. doi: 10.1007/s002329900153. [DOI] [PubMed] [Google Scholar]
- 46.Jones SW, Brown DA, Milligan G, Willer E, Buckley NJ, Caulfield MP. Bradykinin excites rat sympathetic neurons by inhibition of M current through a mechanism involving B2 receptors and Gαq/11. Neuron. 1995;14:399–405. doi: 10.1016/0896-6273(95)90295-3. [DOI] [PubMed] [Google Scholar]
- 47.Koh D-S, Hille B. Modulation by neurotransmitters of catecholamine secretion from sympathetic ganglion neurons detected by amperometry. Proc Natl Acad Sci USA. 1997;94:1506–1511. doi: 10.1073/pnas.94.4.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lazarowski ER, Homolya L, Boucher RC, Harden TK. Direct demonstration of mechanically induced release of cellular UTP and its implication for uridine nucleotide receptor activation. J Biol Chem. 1997;272:24348–24354. doi: 10.1074/jbc.272.39.24348. [DOI] [PubMed] [Google Scholar]
- 49.Lin TA, Lustig KD, Sportiello MG, Weisman GA, Sun GY. Signal transduction pathways coupled to a P2U receptor in neuroblastoma X glioma (NG108-15) cells. J Neurochem. 1993;60:1115–1125. doi: 10.1111/j.1471-4159.1993.tb03262.x. [DOI] [PubMed] [Google Scholar]
- 50.Lin W-W. Heterogeneity of nucleotide receptors in NG108-15 neuroblastoma and C6 glioma cells for mediating phosphoinositide turnover. J Neurochem. 1994;62:536–542. doi: 10.1046/j.1471-4159.1994.62020536.x. [DOI] [PubMed] [Google Scholar]
- 51.Lopez HS, Adams PR. A G-protein mediates the inhibition of the voltage-gated potassium M-current by muscarine, LHRH, substance P, and UTP in bullfrog sympathetic neurones. Eur J Neurosci. 1989;1:529–542. doi: 10.1111/j.1460-9568.1989.tb00360.x. [DOI] [PubMed] [Google Scholar]
- 52.Lustig KD, Shiau AK, Brake AJ, Julius D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc Natl Acad Sci USA. 1993;90:5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marrion NV, Smart TG, Brown DA. Membrane currents in adult rat superior cervical ganglia in dissociated tissue culture. Neurosci Lett. 1987;77:55–60. doi: 10.1016/0304-3940(87)90606-9. [DOI] [PubMed] [Google Scholar]
- 54.Marrion NV, Smart TG, Marsh SJ, Brown DA. Muscarinic suppression of the M-current in the rat sympathetic ganglion is mediated by receptors of the M1-subtype. Br J Pharmacol. 1989;98:557–573. doi: 10.1111/j.1476-5381.1989.tb12630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marshall J, Molloy R, Moss GW, Howe JR, Hughes TE. The jellyfish green fluorescent protein: a new tool for studying ion channel expression and function. Neuron. 1995;14:211–215. doi: 10.1016/0896-6273(95)90279-1. [DOI] [PubMed] [Google Scholar]
- 56.Milligan G. Is promiscuity of G-protein interaction an issue in the classification of receptors? Ann NY Acad Sci. 1997;812:126–132. doi: 10.1111/j.1749-6632.1997.tb48152.x. [DOI] [PubMed] [Google Scholar]
- 57.Nicholas RA, Watt WC, Lazarowski ER, Li Q, Harden TK. Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol Pharmacol. 1996;50:224–229. [PubMed] [Google Scholar]
- 58.North RA, Barnard EA. Nucleotide receptors. Curr Opin Neurobiol. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- 59.Pan X, Ikeda SR, Lewis DL. Rat brain cannabinoid receptor modulates N-type Ca2+ channels in a neuronal expression system. Mol Pharmacol. 1996;49:707–714. [PubMed] [Google Scholar]
- 60.Parr CE, Sullivan DM, Paradiso AM, Lazarowski ER, Burch LH, Olsen JC, Erb L, Weisman GA, Boucher RC, Turner JT. Cloning and expression of a human P2U nucleotide receptor, a target for cystic fibrosis. Proc Natl Acad Sci USA. 1994;91:3275–3279. doi: 10.1073/pnas.91.8.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plummer MR, Logothetis DE, Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989;2:1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- 62.Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- 63.Regan LJ, Sah DW, Bean BP. Ca2+ channels in rat central and peripheral neurons: high-threshold current resistant to dihydropyridine blockers and omega-conotoxin. Neuron. 1991;6:269–280. doi: 10.1016/0896-6273(91)90362-4. [DOI] [PubMed] [Google Scholar]
- 64.Rice WR, Burton FM, Fiedeldey DT. Cloning and expression of the alveolar type II cell P2U purinergic receptor. Am J Respir Cell Mol Biol. 1995;12:27–32. doi: 10.1165/ajrcmb.12.1.7811468. [DOI] [PubMed] [Google Scholar]
- 65.Schofield GG. Norepinephrine blocks a calcium current of adult rat sympathetic neurons via an α2 adrenoceptor. Eur J Pharmacol. 1990;180:37–47. doi: 10.1016/0014-2999(90)90590-3. [DOI] [PubMed] [Google Scholar]
- 66.Shapiro MS, Wollmuth LP, Hille B. Angiotensin II inhibits calcium and M-current channels in rat sympathetic neurons via G-proteins. Neuron. 1994;12:1319–1329. doi: 10.1016/0896-6273(94)90447-2. [DOI] [PubMed] [Google Scholar]
- 67.Siggins GR, Gruol D, Padjen A, Formand D. Purine and pyrimidine mononucleotides depolarize neurones of explanted amphibian sympathetic ganglia. Nature. 1977;270:263–265. doi: 10.1038/270263a0. [DOI] [PubMed] [Google Scholar]
- 68.Surprenant A, Horstman DA, Akbarali H, Limbird LE. A point mutation of the α2-adrenoceptor that blocks coupling to potassium but not calcium currents. Science. 1992;257:977–980. doi: 10.1126/science.1354394. [DOI] [PubMed] [Google Scholar]
- 69.Tokimasa T, Akasu T. ATP regulates muscarine-sensitive potassium current in dissociated bullfrog primary afferent neurones. J Physiol (Lond) 1990;426:241–264. doi: 10.1113/jphysiol.1990.sp018136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Von Kugelgen I, Spath L, Starke K. Evidence for P2 purinoceptor-mediated inhibition of noradrenaline release in rat brain. Br J Pharmacol. 1994;113:815–822. doi: 10.1111/j.1476-5381.1994.tb17066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Von Kugelgen I, Norenberg W, Illes P, Schobert A, Starke K. Differences in the mode of stimulation of cultured rat sympathetic neurons between ATP and UDP. Neuroscience. 1997;78:935–941. doi: 10.1016/s0306-4522(96)00691-4. [DOI] [PubMed] [Google Scholar]
- 72.Zimmerman H. Signaling via ATP in the nervous system. Trends Neurosci. 1994;17:420–426. doi: 10.1016/0166-2236(94)90016-7. [DOI] [PubMed] [Google Scholar]