Abstract
The effect of hypoxia (3–4 min of 95% N2, 5% CO2) on thalamocortical (TC) neurons was investigated using the whole-cell patch-clamp technique in rat dorsal lateral geniculate nucleus slices kept submerged at 32°C. The predominant feature of the response of TC neurons to hypoxia was an increase in input conductance (ΔGN = 117 ± 15%, n = 33) that was accompanied by an inward shift in baseline holding current (IBH) at −65 and −57 mV (ΔIBH = −45 ± 6 pA,n = 18, and −25 ± 8 pA,n = 33, respectively) but not at −40 mV. The hypoxia-induced increase in GN (as well as the shift in IBH) was abolished by procedures that are known to blockIh, i.e., bath application of 4-(N-ethyl-N-phenylamino)-1,2-dimethyl-6-(methylamino)-pyrimidinium chloride (100–300 μm) (ΔGN= 5 ± 13%, n = 11) and CsCl (2–3 mm) (ΔGN = 16 ± 16%,n = 5), or low [Na+]o(ΔGN = 10 ± 10%,n = 5), whereas bath application of BaCl2 (0.1–2.0 mm) had no significant effect (ΔGN = 128 ± 14%,n = 8). The hypoxic response was also abolished in low [Ca+2]o(ΔGN = 25 ± 16%, ΔIBH = −6 ± 8 pA,n = 13), but was unaffected by recording with electrodes containing EGTA (10 mm), BAPTA (10–30 mm), Cs+, or Cl−, as well as in the presence of external tetraethylammonium and 4-aminopyridine. Furthermore, preincubation of the slices with botulinum toxin A (100 nm), which is known to reduce Ca2+-dependent transmitter release, blocked the hypoxic response (ΔGN = −3 ± 15%, ΔIBH = 10 ± 5 pA,n = 4).
We suggest that a positive shift in the voltage-dependence ofIh and a change in its activation kinetics, which transforms it into a fast activating current, may be responsible for the hypoxia-induced changes in GN andIBH, probably via an increase in Ca+2-dependent transmitter release.
Keywords: hypoxia, Ih, inward rectification, cesium, ZD 7288, dorsal lateral geniculate nucleus, botulinum toxin, transmitter release
A common feature of many mammalian neurons is their sensitivity to oxygen deprivation, although different types of neurons, even within the same brain region, show large variations in their sensitivity to hypoxia/ischemia (Hochachka et al., 1993; Krnjevic, 1993; Martin et al., 1997). Brief periods of hypoxia, for instance, cause a total, but apparently fully reversible, loss of synaptic transmission in the hippocampus (Leblond and Krnjevic, 1989;Krnjevic, 1993), which may be responsible for the rapid disruption of higher brain functions in the absence of oxygen. The prominent feature of the hypoxic response in hippocampal neurons is an increase in input conductance (GN) associated with an outward shift in baseline holding current (IBH). This effect is believed to be caused mainly by an enhanced K+ conductance that may serve as a protective mechanism to decrease or delay excitotoxicity (Fujiwara et al., 1987; Krnjevic and Leblond, 1989; Leblond and Krnjevic, 1989). On the other hand, brainstem neurons, which subserve autonomic functions, are slightly depolarized in response to hypoxia, so that they maintain cardiovascular functions during oxygen deprivation (Haddad and Donelly, 1990; Cowan and Martin, 1992; Haddad and Jiang, 1993; Nolan and Waldrop, 1996).
The thalamus shows a marked sensitivity to ischemic insults (Szelies et al., 1991; Steinke et al., 1992), and in situ immunohistochemical studies have revealed that both ventral and dorsal thalamic nuclei (including the ventral posterolateral, the ventral posteromedial, and the medial and dorsal lateral geniculate nucleus) are highly sensitive to ischemic challenges. Indeed, these nuclei, together with the primary sensory cortex and the basal ganglia, appear to be part of a system-preferential, topographically organized brain injury that contributes to a selective vulnerability to ischemia, particularly in the newborn (Martin et al., 1997). Although these thalamic nuclei have recently been the subject of many electrophysiological studies because of their central role in various physiological functions and in a number of neurological disorders (Jones, 1985; Steriade and Llinas, 1988; Steriade et al., 1993;Williams et al., 1996, 1997; McCormick and Bal, 1997; Turner et al., 1997), little is known about the electrical behavior of single thalamocortical (TC) neurons in response to hypoxia.
In the present experiments, we have investigated the effects of brief periods of hypoxia on TC neurons of the dorsal lateral geniculate nucleus (dLGN) maintained in slices using the whole-cell patch-clamp technique. Our results suggest that activation of the hyperpolarization-activated inward current,Ih, brought about by a positive shift in its voltage-dependence and changes in its kinetics, is the major factor responsible for the response of TC neurons to hypoxia.
Preliminary reports of some of these results have been published previously (Crunelli and Erdemli, 1997; Erdemli and Crunelli, 1998).
MATERIALS AND METHODS
Slice preparation and recording solutions. For the preparation of dLGN slices, rats (Wistar, 100–150 gm) were decapitated under full anesthesia with halothane. The brain was quickly removed and placed in an ice-cold oxygenated saline (Williams et al., 1996, 1997). A block of brain tissue containing the thalamus was dissected out, and 400-μm-thick dLGN slices were cut in a plane parallel to the optic tract using a vibroslice (Campden Instruments). Slices were then kept for at least 1 hr at room temperature in the standard artificial CSF (ACSF) containing (in mm): NaCl 134, KCl 2, KH2PO4 1.25, Mg2SO4 1, CaCl2 2, NaHCO3 16, and glucose 10, and were aerated continuously with carbogen (95% O2, 5% CO2, pH 7.3).
Before the start of the electrical recording, a slice was transferred to a submerged chamber, where both the ACSF and the aerating gas were warmed to 32 ± 1°C. The patch electrodes (2.5–3 μm tip diameter) were prepared from 1.5 mm outer diameter borosilicate glass (Clark Electromedical Instruments, Pangbourne, UK) and filled with solution containing (in mm): KMeSO4 118, KCl 18, HEPES 10, EGTA 1 or 10, CaCl2 0.1 or 1, Mg-ATP 2, Na-GTP, 0.3, and NaCl 8. When it was necessary, KMeSO4 was replaced by KCl and in some other experiments KMeSO4 and KCl were replaced by cesium gluconate and CsCl, respectively. In a few experiments, CsF (118 mm) was used instead of cesium gluconate, and because the results obtained with these two internal solutions were similar, data were pooled together. In some experiments EGTA was replaced with BAPTA (10 or 30 mm). The pH was always adjusted to 7.2 with KOH or CsOH. The osmolarity of the internal solution (measured with a micro-osmometer, Viescor Inc.) was kept in the range of 310–320 mOsm by reducing [KMeSO4] as needed.
To minimize the indirect effects of synaptic transmission, slices were always perfused with ACSF containing kynurenic acid (KYN, 1 mm), picrotoxin (PIC, 100 μm), and tetrodotoxin (1 μm) (Ben-Ari, 1990a). In some experiments PIC was replaced by (−)-bicuculline methiodide (30 μm), and dl-2-amino-5-phosphonopentoic acid (100 μm) (Tocris Neuramine) and 6-cyano-7-nitroquinoxaline-2,3-dione (20 μm) (Tocris Neuramine) were used instead of KYN. In recordings with Cs+-filled electrodes, tetraethylammonium chloride (TEA, 10–20 mm) was also added to the perfusion medium. For low Na+ (16 or 100 mm) solution, Na+ was replaced either withN-methyl-d-glucamine (134 mm, tittered with HCl to pH 7.3) or TEA (50 mm), and Ca2+ currents were blocked by a low Ca2+ (0.5 mm) to high Mg2+ (8–10 mm) solution containing CdCl2 (300 μm) and NiCl2 (1 mm) (Crunelli et al., 1989; Guyon and Leresche, 1995). The following agents were also tested by bath application: 4-aminopyridine (4-AP, 0.1 mm), botulinum toxin A (100 nm, from a stock solution in 0.2 m NaCl2 and 0.05m sodium acetate), BaCl2 (0.1–2 mm), CsCl (2–3 mm), 4-(N-ethyl-N-phenylamino)-1,2-dimethyl-6-(methylamino)-pyrimidinium chloride) (ZD 7288, Zeneca, UK) (100–300 μm). Chemicals were purchased from Sigma (Poole, UK), except where indicated.
Patch recording and data analysis. The patch electrodes had an initial resistance of 2–3 MΩ, and data from whole-cell recordings where the electrode series resistance increased to values >13 MΩ were discarded. All recordings were done “blind,” and the criteria used to identify TC neurons included the presence of a relatively large inward rectification, low-threshold Ca2+ potentials, and strong outward rectification (Williams et al., 1996). In some experiments, 0.5–1% biocytin was included in the intracellular patch electrode solution. At the end of these recording sessions, the slices were immediately fixed and then processed as described previously (Williams et al., 1996).
The electrical signals were amplified by an Axopatch 1D (Axon Instruments, Foster City, CA), and the data were analyzed with pClamp (v6.1, Axon Instruments). Cells were clamped near resting membrane potential (−60 mV), and voltage-dependent currents were elicited by 1-sec-long hyperpolarizing and depolarizing voltage steps. Current–voltage (I–V) relationships were constructed using the instantaneous current evoked by hyperpolarizing voltage steps, and the steady-state current was evoked by depolarizing voltage steps. The GN was obtained from the slope of the first-order regression lines fitted to the linear portion of the I–V plots, in the region negative to the holding potential.
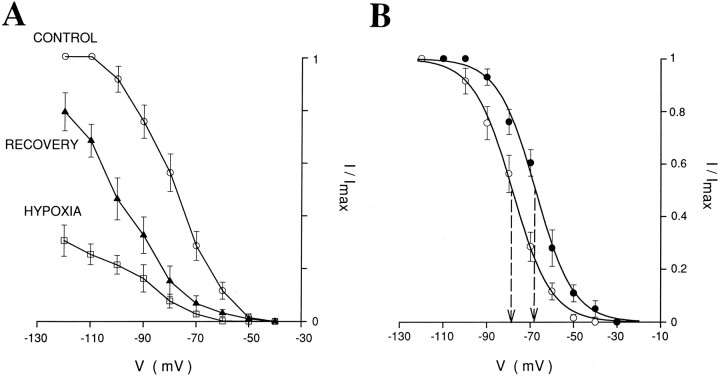
The activation curve of Ih was constructed from the amplitude of the inward relaxations calculated by subtracting the instantaneous current from the steady-state current elicited by hyperpolarizing voltage steps. Ih tail currents were not used for this purpose, because the contributions of the low-threshold Ca2+ current could not be eliminated because of sensitivity of the hypoxic response to extracellular Ca2+ (see Results). For the results presented in Figure 4, the hypoxic current Idif (i.e., the instantaneous current recorded during hypoxia minus the instantaneous current recorded in control conditions) andIh were normalized to their respective maximal amplitude and plotted against the step potential. The resulting data were fitted with the Boltzmann equation of the form y = 1/[1 + e(V1/2 − Vm)/k], whereVm is the membrane potential,V1/2 is the membrane potential at whichIdif or Ih is half-activated, and k is the slope factor.
Fig. 4.
Effects of hypoxia on inward relaxations (Ih) and voltage-dependence ofIh and Idif.A, Normalized amplitude of the inward relaxations (Ih) shows the reversible depression produced by hypoxia in eight neurons recorded with Cs+-filled electrodes in the presence of extracellular BaCl2 (1–2 mm), TEA (10–20 mm), and 4-AP (0.1 mm). Error bars represent SEM. The three data sets were normalized usingImax of the control data. B, Normalized activation curve of Ih measured in normoxic conditions (○) and normalized amplitude ofIdif (•) constructed from the same eight neurons as in A. Error bars and curves represent SEM and the Boltzmann curves, respectively (for details, see Materials and Methods). Dashed lines point to theV1/2 of the two curves. Note the similarity in slope and the 10 mV difference in V1/2between the two curves.
The effects of hypoxia were examined by using the method described byLeblond and Krnjevic (1989). The periods of exposure to ACSF saturated with 95% N2, 5% CO2 were 3–4 min long, i.e., 1–2 min longer than in the experiments by Leblond and Krnjevic (1989), in which slices were directly exposed to the aerating gases. The longer exposure used in the present study was to ensure that a major decrease in oxygen was indeed achieved in the submerged slices. Data were collected after the first 2 min of oxygen deprivation.
All quantitative data in the text and figures are expressed as mean ± SEM, and their significance was assessed by Student’st test. For unpaired differences with unequal population variances, the significance was estimated using thed-statistic and Fisher–Behrens distribution (Campbell, 1989).
RESULTS
The effect of hypoxia were studied in a total of 106 TC neurons. At a holding potential (VH) of −57.7 ± 0.8 mV, the initial resting GNand the IBH of these 106 neurons were 5.8 ± 0.4 nS and 27 ± 11 pA, respectively.
Effects of hypoxia
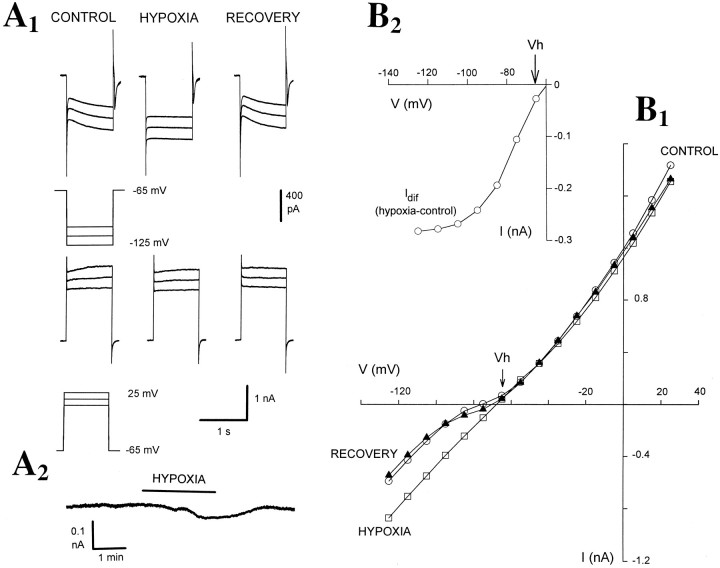
In the 33 neurons that were recorded with electrodes containing KMeSO4 and clamped at approximately −57 mV, a brief period of hypoxia caused a marked and consistent increase inGN (ΔGN = 117 ± 15%) (n = 33) and a small but significant change inIBH (ΔIBH = −25 ± 8 pA; p < 0.02 from the controlIBH=33 ± 16 pA; n = 33) (Fig. 1A,B, Table1). These effects were invariably accompanied by a marked increase in the instantaneous current (101 ± 17%, n = 21), and a small increase in steady-state current (21 ± 6%, n = 21) evoked by hyperpolarizing voltage steps, resulting in a substantial reduction (77 ± 4%, n = 21) of the amplitude of the inward relaxations (Fig. 1A) (control amplitude: 294 ± 12 pA, n = 29; measured at −120 mV) attributable to activation of Ih (McCormick and Pape, 1990a;Soltesz et al., 1991; Pape, 1996). The inward shift inIBH was more prominent and consistent in 18 of these neurons clamped at −65 mV (ΔIBH = −45 ± 6 pA; p < 0.001) (Table 1). On the other hand, 13 hypoxic tests performed while the neurons were held at −40 mV did not cause any significant change in IBH(ΔIBH = 5 ± 25 pA; n = 9), although the increase in GN(ΔGN = 111 ± 23%; n = 9) and the decrease in the inward relaxations (83 ± 12%;n = 9) were still present. The reversal potential of the hypoxia-evoked current(s), obtained from the point of intersection of the instantaneous I–V plots in control and during hypoxia, was −53.7 ± 3.2 mV (n = 25). This value was not different from the reversal potential measured from the intersection of the regression lines fitted to the linear portion of the I–V plots (−53.2 ± 4.7 mV;n = 25).
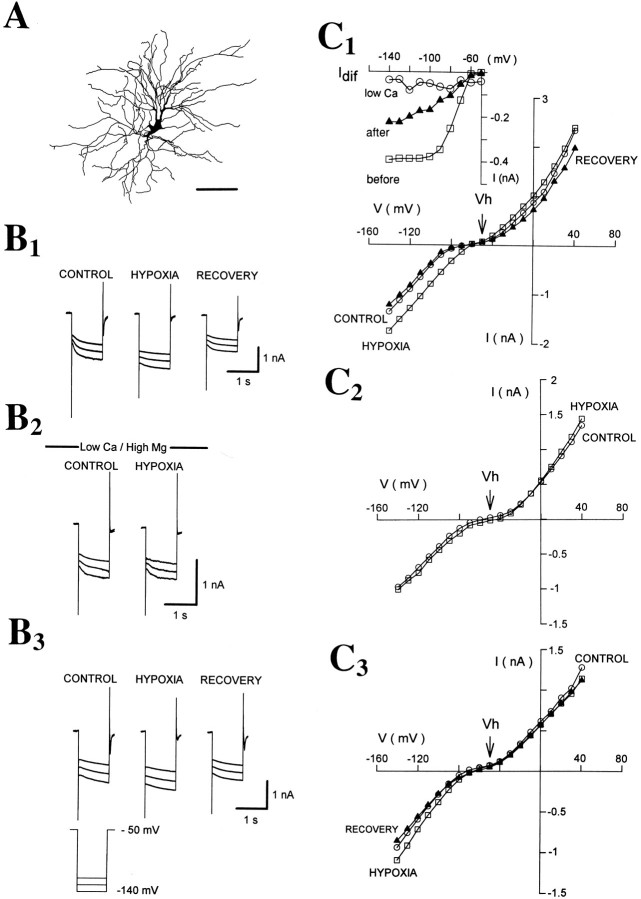
Fig. 1.
Hypoxia causes an increase inGN, an inward shift inIBH, and a decrease in the amplitude of inward relations in TC neurons. A1, Voltage-clamp traces obtained with an electrode containing KMeSO4 show the inward and outward currents evoked by depolarizing and hyperpolarizing voltage steps before (CONTROL), during (HYPOXIA), and 5 min after hypoxia (RECOVERY). Note the marked increase in instantaneous current and the small increase in steady-state current evoked by the hyperpolarizing voltage steps during hypoxia. A2, Continuous trace shows the inward current activated during hypoxia. B1,I–V plot obtained from the same neuron as in A1 shows the substantial increase inGN during hypoxia. In this and otherI–V plots in the following figures,open circles, open squares, andclosed triangles represent data obtained before, during, and after hypoxia, respectively. B2, Plot of the difference current Idif (i.e., the instantaneous current measured during hypoxia minus the instantaneous current measured in control conditions) from the data shown in B1. In this and the following figures,Vh indicates the holding potential (for further details, see Materials and Methods).
Table 1.
Effects of hypoxia on the membrane properties of thalamocortical neurons
| Electrode content | n | GN(control) (nS) | GN (hypoxia) (nS) | ΔGN(%) | ΔIBH (pA) |
|---|---|---|---|---|---|
| KMeSO4 | 33 | 5.1 ± 0.4 | 9.5 ± 0.9* | 117 ± 15* | −45 ± 6a,* |
| KCl | 6 | 5.7 ± 1.4 | 8.6 ± 2.5** | 104 ± 41** | −57 ± 7* |
| Cs (fluoride or gluconate) | 20 | 4.2 ± 0.6 | 6.9 ± 0.8* | 91 ± 22* | −57 ± 20** |
| EGTA (10 mm) | 11 | 3.8 ± 0.5 | 7.2 ± 1.5** | 97 ± 29** | −35 ± 17*** |
| BAPTA (10–30 mm) | 13 | 8.7 ± 0.6 | 16.8 ± 1.2* | 99 ± 16* | −70 ± 20** |
n, Number of cells; GN, input conductance; IBH, baseline holding current.
ΔIBH for KMeSO4-containing electrodes calculated only on 18 neurons clamped at −65 mV.
*p < 0.001; **p < 0.01; ***p < 0.05.
The effect of hypoxia was reversible: 4–5 min after readmission of oxygen, GN,IBH, and the amplitude of the inward relaxations were not significantly different from the corresponding control values (9 ± 17%, n = 27; 13 ± 10%, n = 27; and −13 ± 11%, n= 21, respectively) (Figs. 1A,B,2, 3; see Figs. 5-8). In addition, the hypoxic challenge could be repeated at intervals of 10–15 min without any obvious long-lasting effect. Thus, in contrast to previous observations in other neuronal types (Reid et al., 1984; Leblond and Krnjevic, 1989), TC neurons responded to hypoxia in a very consistent and reproducible manner.
Fig. 2.
The hypoxic response of TC neurons is not blocked by intracellular Cs+. Whole-cell recording with a patch electrode containing cesium gluconate. A, Examples of currents evoked by hyperpolarizing voltage steps before (CONTROL), during (HYPOXIA), and 4 min after hypoxia (RECOVERY). B,I–V plot from the same neuron as inA shows a marked increase inGN during hypoxia. C, Plot ofIdif from the data shown inB.
Fig. 3.
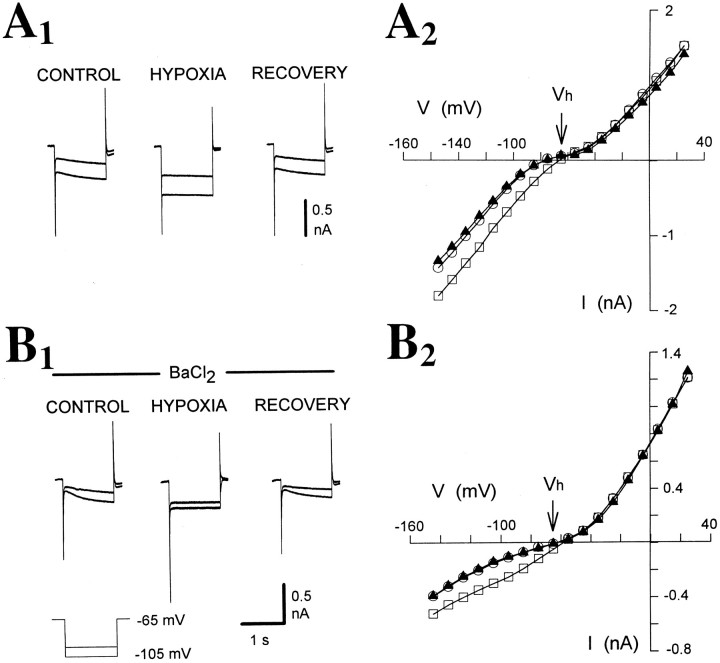
Bath application of BaCl2 does not affect the hypoxic response. A1, B1, Traces recorded before, during, and 4 min after hypoxia in the absence (A1) and presence (B1) of BaCl2 (1 mm). Note the substantial decrease in instantaneous current during perfusion with BaCl2, whereas the hypoxia-induced increase inGN and the block of inward relaxations remains in the presence of BaCl2.A2, B2,I–V plots recorded before (A2) and during (B2) BaCl2 application (data from same neuron as inA1 and B1, respectively).
Fig. 5.
ZD 7288 blocks the hypoxic response of TC neurons.A, Examples of currents recorded in control, during, and 3 min after hypoxia in the absence (top row) and presence (bottom row) of ZD 7288 (300 μm).B, I–V plots (from the same neuron as in A) obtained in the absence (B1) and presence (B2) of ZD 7288. C, Idif measured before (○) and during (□) bath application of ZD 7288 (from the data shown in B1 and B2, respectively).
Fig. 6.
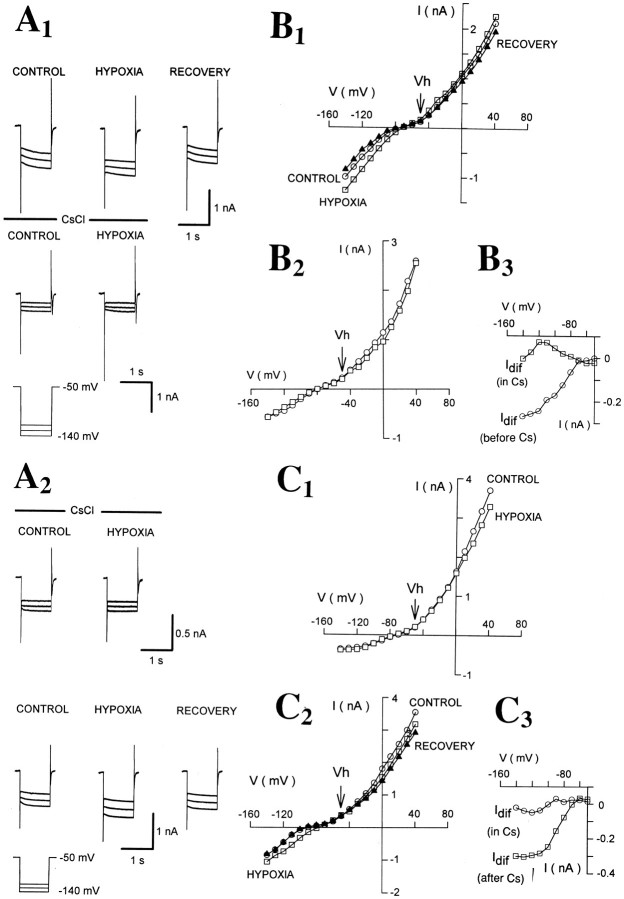
Bath application of CsCl reversibly abolishes the hypoxic response. A1, Examples of currents evoked by hyperpolarizing voltage steps in the absence (top row) and presence (bottom row) of CsCl (3 mm). A2, Traces from another TC neuron recorded in the presence (top row) and after 15 min washout (bottom row) of CsCl (3 mm).B1, B2,I–V plots measured in the absence (B1) and presence (B2) of CsCl from the same neuron as in A.B3, Idif recorded in the absence (○) and presence (□) of CsCl (from the data shown inB1 and B2, respectively).C1, C2,I–V plots in the presence (C1) and after 15 min washout (C2) of CsCl (3 mm) from the same neuron as in A2. C3,Idif measured in the presence (○) and after 15 min washout of CsCl (□) (from the data shown inC1 and C2, respectively).
Fig. 7.
Ca2+ dependence of the hypoxic response. A, Camera lucida reconstruction of the neuron (from which all data in this figure were obtained) shows the characteristic morphology of a dLGN TC neuron. Whole-cell recording with a biocitin-containing patch electrode obtained in standard ACSF (B1, C1), then in solution containing low Ca2+ (0.5 mm), high Mg2+ (8 mm), NiCl2 (1 mm), and CdCl2 (300 μm) (B2, C2), and during washout with standard ACSF (B3, C3). In all cases, examples of currents recorded before, during, and 4 min after hypoxia are shown. C1, C2, C3,I–V plots obtained from the data shown in B1, B2, and B3. Note the marked depression of the hypoxic response in low Ca2+/high Mg2+solution. Inset graph in C1 showsIdif measured before (□), during (○), and after (▴) perfusion with the solution containing low Ca2+ (from the data shown inC1, C2, and C3, respectively).
Fig. 8.
Ca2+ chelators fail to produce any significant effect on the hypoxic response.A1, A2, Pen recorder traces from two TC neurons recorded with electrodes containing EGTA (10 mm) (A1) or BAPTA (30 mm) (A2). The pen recorder speed was accelerated (time calibrations in brackets inA2) during the hyperpolarizing voltage steps (from −65 to −85 mV) to visualize the changes in instantaneous and steady-state current elicited during hypoxia.B1, B2,I–V plots obtained from the two other TC neurons show the effect of hypoxia recorded with electrodes containing EGTA (10 mm) and BAPTA, respectively.
In contrast to what is usually observed in hippocampal cells (Krnjevic and Leblond, 1989; Leblond and Krnjevic, 1989), in TC neurons hypoxia did not produce any significant effect on voltage-activated whole-cell outward currents (IOUT) (ΔIOUT = −27 ± 49 pA; from the controlIOUT = 1482 ± 165 pA, measured at 30 mV;n = 29) (Fig.1A1,B1).
Intracellular Cl− or Cs+does not affect the hypoxia-induced changes inGN, IBH, and inward relaxations
In six neurons clamped at −65 mV, Cl− was used as the main anion in the electrode solution (KCl, 136 mm), and the effect of hypoxia on GN(ΔGN = 104 ± 41%),IBH (ΔIBH = −57 ± 7 pA), and the inward relaxations (71 ± 11%) was not different from the one observed when recording with KMeSO4-filled electrodes (Table 1). In these six cells the reversal potential of the hypoxia-induced current(s) was −53.9 ± 6.4 mV.
In 20 neurons recorded with electrodes containing cesium gluconate (n = 10) or CsF (n = 10), hypoxia still produced a marked increase in GN(ΔGN = 91 ± 22%; p < 0.001), an inward shift in IBH(ΔIBH = −57 ± 20 pA; p< 0.05 from IBH = 95 ± 42 pA), and a block of the inward relaxations (84 ± 10%) (Fig.2A,B, Table 1). However, the reversal potential of the hypoxia-evoked current(s) recorded with Cs+-filled electrodes was shifted to a more depolarized potential (−38.6 ± 4.7 mV), a value similar to the reversal potential of Ih in TC neurons (McCormick and Pape, 1990a,b; Soltesz et al., 1991; Pape, 1996).
As is clearly shown in Figure 2B, the high-threshold Ca2+ currents of TC neurons were not depressed during a 3- to 4-min-long hypoxic challenge (ΔICa = −5 ± 22%, measured at −10 mV;n = 13). This is in contrast to hippocampal neurons where high threshold Ca2+ currents are depressed in the first 3 min of oxygen deprivation after a transient initial potentiation (Krnjevic and Leblond, 1989).
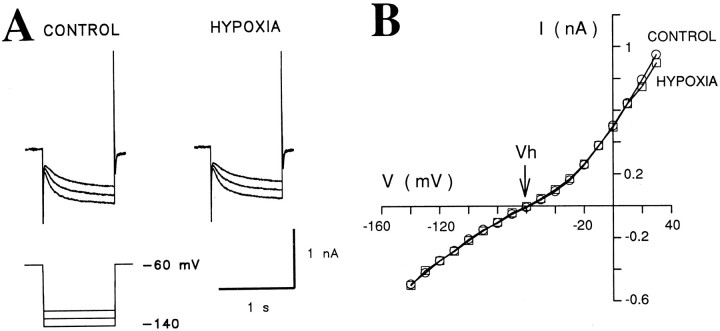
Effect of Ba2+
To eliminate a possible contribution by the fast inward rectifier present in TC neurons (Williams et al., 1997), we tested the effect of BaCl2 on the hypoxic response of TC neurons. Bath application of BaCl2 (0.1–2 mm) failed to produce any significant effect on the hypoxia-induced changes inGN and IBH(ΔGN = 128 ± 14%, ΔIBH = −36 ± 9 pA in BaCl2, compared with ΔGN = 123 ± 11%, ΔIBH = −33 ± 10 pA in control conditions in the same eight neurons) (Fig. 3, Table2). In two of these cells after 20 min perfusion with BaCl2 containing saline, the concomitant application of ZD 7288 (100 μm) abolished the hypoxic response (data not shown).
Table 2.
Pharmacological properties of the hypoxic response of thalamocortical neurons
| Contents of bath application | n | GN (control) (nS) | GN (hypoxia) (nS) | ΔGN(%) | ΔIBH (pA) |
|---|---|---|---|---|---|
| ZD 7288 (0.1–0.3 mm) | 11 | 4.5 ± 0.4 | 5.0 ± 0.8 | 5 ± 13 | −5 ± 8 |
| CsCl (2–3 mm) | 5 | 5.6 ± 1.2 | 6.2 ± 2.8 | 16 ± 16 | 8 ± 7 |
| Low Na+ (16 mm)2-a | 5 | 5.7 ± 1.04 | 6.0 ± 1.8 | 10 ± 10 | −14 ± 19 |
| BaCl2 (0.1–2 mm) | 8 | 3.0 ± 0.9 | 6.7 ± 1.8* | 128 ± 14* | −36 ± 9** |
| Low Ca2+ (0.5 mmCa2+)2-b | 13 | 3.3 ± 0.4 | 4.3 ± 1.1 | 25 ± 16 | −6 ± 8 |
| Botulinium toxin (100 nm)2-c | 4 | 5.2 ± 0.6 | 5.0 ± 1.3 | −3 ± 15 | 10 ± 5 |
n, Number of cells; GN, input conductance; IBH, baseline holding current.
Na+ was replaced withN-methyl-d-glucamine.
In presence of Mg2+ (8–10 mm), NiCl2 (1 mm), and CdCl2 (300 μm).
Slices were preincubated for 2–5 hr.
*p < 0.01; **p < 0.05.
Voltage-dependence of the Idif
In eight neurons clamped at −40 mV and recorded with Cs+-filled electrodes in the presence of external BaCl2 (1–2 mm), TEA (10 mm), and 4-AP (0.1 mm), we looked at the reversible effect of hypoxia on the amplitude of the inward relaxations evoked by hyperpolarizing voltage steps (Fig.4A) and compared it with Idif (i.e., the difference in the instantaneous current measured in control and during hypoxia).Idif had a threshold for activation of approximately −45 mV, reached a maximum at −95 mV, and had aV1/2 of −77.6 ± 2.3 mV and a kof 8.7 ± 0.9 (n = 8) (Fig. 4B,closed circles). In agreement with previous studies (McCormick and Pape, 1990a,b; Pape, 1996; Soltesz et al., 1991), the corresponding values for Ih wereV1/2 = −88.4 ± 2.1 and k= 8.8 ± 0.8 (n = 8) (Fig. 4B,open circles). There was, therefore, a striking similarity between the k of Idif and that ofIh (Fig. 4B), whereas theV1/2 of Idif was 10 mV more depolarized than that of Ih.
Block of Ih depresses the hypoxic response
Because of the similarity in the voltage-dependence and reversal potential of Ih andIdif, we tested the effects of ZD 7288, a selective blocker of Ih (Harris and Constanti, 1995; Williams et al., 1997) in 11 TC neurons, where in control conditions hypoxia had produced the usual increase inGN (ΔGN = 109 ± 18%) and inward shift in IBH(ΔIBH = −26 ± 6 pA). Bath application of ZD 7288 (100–300 μm) blockedIh and significantly depressed the hypoxic changes in GN and IBH (by 88 ± 6 and 92 ± 9%, respectively) (Fig.5, Table 2). Because the action of ZD 7288 on Ih is irreversible (Harris and Constanti, 1995; Williams et al., 1997), no attempt was made to wash out the effect of ZD 7288 on the hypoxic response.
As a further test, five neurons were perfused with a solution containing 2–3 mm CsCl, a reversible blocker ofIh (McCormick and Pape, 1990a; Soltesz et al., 1991). In these conditions, Ih was blocked but hypoxia failed to produce an increase in GN(ΔGN = 16 ± 16%; n = 5), in contrast to the consistent GN increase seen in the same neurons during hypoxic tests in the absence of extracellular CsCl (ΔGN = 113 ± 19%;p < 0.01) (Fig. 6, Table2). In two of these neurons that could be reliably clamped for a sufficient period of time, the effect of hypoxia recovered after 15 min washout of CsCl (ΔGN = 67 and 82%) (Fig.6).
It is well known that Ih is carried by Na+ and K+ ions (McCormick and Pape, 1990a; Soltesz et al., 1991). In our experiments, we reduced the [Na+]o to 16 mm by replacing NaCl with N-methyl-d-glucamine (134 mm). Under these conditions, Ih was blocked and the hypoxia-induced changes in GNand IBH were abolished (ΔGN = 10 ± 10%, ΔIBH = −14 ± 19 pA; p> 0.05 for both; n = 5), compared with a ΔGN = 99 ± 16% (p < 0.01) and a ΔIBH= −26 ± 8 pA (p < 0.05) in control conditions. In the one neuron that could be clamped for a period sufficiently long to allow the re-establishment of the control [Na+]o, the hypoxic response resumed (ΔGN = 73%) (data not shown).
Sensitivity of the hypoxic response to [Ca2+]o
Because metabolic arrest soon leads to a rise in cytosolic Ca2+ (Hansen, 1985; Kaplin et al., 1996), it has been suggested that Ca2+ could trigger the hypoxic changes in membrane properties (Krnjevic, 1993; Belousov et al., 1995).
In our experiments we tested the contribution of Ca2+-mediated changes to the hypoxic response of TC neurons by decreasing the [Ca2+]o to 0.5 mm and increasing the [Mg2+]o to 8–10 mm and concomitant bath application of the voltage-activated Ca2+-channel blockers NiCl2 (1 mm) and CdCl2 (300 μm). In another five neurons the [Mg2+]o was increased to 8–10 mm, whereas the [Ca2+]o was left unchanged (2 mm). In all of these cells (n = 20), hypoxia failed to produce any significant changes inGN and IBH(ΔGN = 17 ± 13% and ΔIBH = 6 ± 8 pA) (Fig.7, Table 2). In three of these neurons, the hypoxic response recovered 15–20 min after returning to the control solution (ΔGN = 90 ± 27%, ΔIBH = −26 ± 5 pA) (Fig. 7).
Internally applied Ca2+ chelators
An increase of the EGTA concentration (to 10 mm) in the internal solution had no effect on the hypoxia-induced changes inGN and IBH because 21 hypoxic tests in 11 neurons produced a 97 ± 29% increase inGN, associated with an inward shift of −35 ± 17 pA in IBH and a 89 ± 10% reduction of the amplitude of inward relaxations (Fig.8, Table 1). Because of the fast and pH-independent Ca2+-chelating ability, we also tested BAPTA in 13 cells. BAPTA (10 mm in five cells, 30 mm in eight cells) failed to prevent the hypoxia-induced increase in GN (ΔGN = 99 ± 16%; n = 13), the inward shift inIBH (ΔIBH = −70 ± 20 pA; n = 13), and the depression of inward relaxations (81 ± 14%; n = 13) (Fig. 8, Table1). On the other hand, neurons recorded with BAPTA, but not with 10 mm EGTA, showed a significantly greaterGN in control conditions compared with neurons recorded with the standard 1 mm EGTA in the intracellular solution (BAPTA, GN = 8.7 ± 0.6 nS,n = 13, p < 0.01 compared with 1 mm EGTA; 10 mm EGTA, GN= 3.8 ± 0.5 nS, n = 11) (Table 1).
Inhibition of transmitter release
In four slices, the Ca2+-dependent release of transmitters was blocked by preincubation (2–5 hr) with botulinum toxin A (100 nm) (Sanchez-Prieto et al., 1987). In four neurons, one in each of the four slices, hypoxia failed to produce any significant effect on GN(ΔGN = −3 ± 15%),IBH (ΔIBH = −10 ± 6 pA), or the amplitude of the inward relaxations (12 ± 8% increase) (Fig. 9B, Table 2). Note that preincubation of the slices had no effect on the amplitude of the inward relaxations (332 ± 53 pA; n = 4) (Fig.9).
Fig. 9.
Lack of hypoxic response in TC neurons preincubated with botulinum toxin A. A, Whole-cell recording with an electrode containing KMeSO4 shows the absence of any effect of hypoxia in a TC neuron from a slice preincubated with botulinum toxin A (100 nm) for 4 hr.B, I–V plot from the same neuron as in A.
Block of transmitter receptors
Because the sensitivity to botulinum toxin of the hypoxic response of TC neurons suggested an involvement of presynaptic mechanisms, we further assessed this hypothesis by testing the effect of antagonists for transmitter receptors whose activation is known to affectIh in TC neurons. Combined application of propanol (30 μm), cimetidine (50 μm), and methysergide (30 μm) produced a significant reduction in the effect of hypoxia on GN (58 ± 17%),IBH (48 ± 2%), andIh (45 ± 9%) in three TC neurons (each in a different slice).
DISCUSSION
The main findings of this study in TC neurons of the rat dLGN are that (1) brief periods of hypoxia cause an inward shift inIBH, accompanied by an increase inGN and a decrease in the amplitude of the inward relaxations elicited by hyperpolarizing voltage steps, and (2) these hypoxia-induced changes are Ca2+-dependent and abolished by selective blockers of Ih and by botulinum toxin but are unaffected by high concentrations of internally applied Ca2+ chelators. The simplest explanation of these results is that hypoxia causes a 10 mV positive shift in the voltage-dependence of Ih together with a change in its kinetics that transforms Ih into a fast activating current. An increase in Ca2+-dependent release of transmitters may underlie some of these effects.
Mechanism of the hypoxia-induced changes in GN and IBH
K+ and Cl− channels
An increase in GN caused by the activation of K+ currents during hypoxia by either a depletion of intracellular ATP (Ben-Ari, 1990b; Fujimura et al., 1997) or an increase in [Ca2+]i (Belousov et al., 1995) has been shown in hippocampal slices. In our experiments, however, no significant change in either voltage-activated outward currents (in the range from −40 to 30 mV) orIBH (at potentials more than or equal to −40 mV) was observed during hypoxia. In addition, internally applied Cs+, which has been widely used as a powerful tool for eliminating various K+ currents (Gay and Stanfield, 1977; Cook, 1988; Halliwell, 1990), together with bath applications of TEA, BaCl2, and 4-AP, failed to affect the hypoxia-induced changes in GN,IBH, and inward relaxations. The lack of effect of BaCl2 also indicates that the hypoxia-induced depression of the inward relaxations was not secondary to a K+ current activation as shown in substantia nigra, zona compacta neurons (Watts et al., 1996) and eliminates a possible contribution of a leak conductance (Hagiwara et al., 1978; Cook, 1988;Halliwell, 1990) and the fast inward rectifier (Williams et al., 1997) to the hypoxic response of TC neurons.
The reversal potential of the hypoxia-induced current, however, was shifted from −57 to −37 mV when recording with Cs+-filled electrodes, suggesting some contribution by current(s) with a reversal potential less than −57 mV. In contrast to previous results in the hippocampus (Zhang and Krnjevic, 1993;Belousov et al., 1995), the lack of any difference in the effect of hypoxia measured with either MeSO−4-, Cl−-, F−-, or gluconate-filled electrodes excluded any significant contribution of Cl− currents to the hypoxic response. Together these results indicates that current or currents with a reversal potential less than −57 mV, possibly carried by K+and blocked by internal Cs+, contribute to the hypoxic response of TC neurons. No further attempt was made in this study to identify this current(s).
Ih channels
A consistent block of the hypoxic response was observed with three different manipulations that are known to abolishIh in TC neurons: bath applications of ZD 7288 (Williams et al., 1997) and of CsCl (McCormick and Pape, 1990a; Soltesz et al., 1991) and a decrease in [Na+]o(McCormick and Pape, 1990a). The reversibility of the block produced by the last two procedures suggests that this abolishment of the hypoxic response is attributable to a selective block ofIh and not to a rundown of the hypoxic response, as has been observed in the hippocampus (Zhang and Krnjevic, 1993). Although extracellular Cs+ has been shown to depress the M-current (Coggan et al., 1994), which may also have been enhanced by a hypoxia-induced rise in [Ca2+]i(Yu et al., 1994), there is no study that indicates a similar effect of ZD 7288 and low [Na+]o on this voltage-activated K+ current.
Thus, the pharmacological sensitivity and the reversal potential (measured with Cs+-filled electrodes) ofIdif are identical to those ofIh, suggesting that the former current may represent Ih that has undergone a 10 mV positive shift in its voltage-dependence caused by hypoxia. This interpretation accounts for the hypoxia-induced inward change inIBH (observed at more depolarized potentials than the normal activation range of Ih) and for the increase in steady-state current. During hypoxia, therefore, Ih might be activated and not blocked, as the depression of the inward relaxations might indicate. In line with this suggestion, our explanation for the substantial increase in instantaneous current and the resulting depression of the inward relaxations is that hypoxia somehow changes the kinetics ofIh, transforming it into a fast activating current. Under these conditions, Ihchannels would open within a few milliseconds of the start of hyperpolarizing voltage steps, explaining the large increase in instantaneous current (as well as in GN) and the depression of inward relaxations. Clearly, this scenario represents the most parsimonious explanation of the present results, but we cannot exclude the alternative possibility that in TC neurons hypoxia blocks Ih and concomitantly activates a novel, very fast activating current that has a voltage-dependence similar to, and pharmacological properties and an ionic permeability identical to, those of Ih.
What activates Ih during hypoxia?
Ca2+-dependence of the hypoxic response
The hypoxic response of TC neurons was found to be highly sensitive to [Ca2+]o, although there is no direct evidence from TC neurons, measurements of Ca2+ influx or [Ca2+]i in other brain regions have shown that hypoxia can elicit an increase in cytosolic Ca2+ (Hansen, 1985; Kass and Lipton, 1986; Dubinsky and Rothman, 1991; Kaplin et al., 1996). This increased [Ca2+]i may play a role both presynaptically by increasing transmitter release and postsynaptically by directly activating Ih (Hagiwara and Irisawa, 1989; Ingram and Williams, 1996) or the other current(s) responsible for the hypoxia-mediated effects in TC neurons.
In our experiments, we did not observe any significant effect of hypoxia on high voltage-activated Ca2+ currents. In addition, the lack of action of high concentrations of internally applied Ca2+ chelators does not support a postsynaptic origin of the Ca2+-dependence of the hypoxic response, although the effectiveness of these chelators would be somewhat limited if the hypoxia-activated channels were locally regulated by Ca2+ released from internal stores close to the surface membrane or if Ca2+ would enter the neuron via channels located in close proximity to the hypoxia-activated channels. The increase in restingGN observed when using BAPTA (cf. Zhang et al., 1995) may indicate that a K+ conductance, which is normally suppressed by the normal [Ca2+]i, is activated when BAPTA lowers [Ca2+]i below a critical level, as is the case for r-eag type of gK(Stansfeld et al., 1996).
The lack of action of internally applied Ca2+chelators and the block by botulinum toxin, an agent known to inhibit Ca2+-dependent transmitter release (Sanchez-Prieto et al., 1987), suggest that the Ca2+-dependence of the hypoxic response of TC neurons is likely to have a presynaptic origin, i.e., during hypoxia the Ca2+-dependent release of transmitters may increase, therefore leading indirectly to some of the observed effects of hypoxia. In ischemic conditions, (i.e., lack of oxygen and glucose) Ca2+-independent release of transmitters is increased because of reverse operation of the uptake system, secondary to a reduced Na+ electrochemical gradient (Kauppinen et al., 1988; Nicholls and Attwell, 1990;Szatkowski and Attwell, 1994). Depletion of the [ATP]i, which is essential for the Na+/K+ exchanger, is the major factor responsible for a reduced Na+ electrochemical gradient. On the other hand, oxygen deprivation alone, i.e., not accompanied by hypoglycemia, causes only very minor changes in [ATP]i (Madl and Burgesser, 1993), indicating that the Ca2+-dependent transmitter release may be upregulated during brief periods of hypoxia.
Might transmitters activate Ihduring hypoxia?
The results of the above experiments indicate that an increase in transmitter release might underlie some of the effects of hypoxia in TC neurons. Interestingly, several neurotransmitters, including noradrenaline, serotonin (McCormick and Pape, 1990b; Soltesz et al., 1991), histamine (McCormick and Williamson, 1991), and nitric oxide (Pape and Mager, 1992; for review, see Pape, 1996) are known to increase Ih in TC neurons by eliciting a positive shift in its steady-state activation curve. This action gives rise to an inward current at potentials around −60 mV and an increase in steady-state current elicited by hyperpolarizing voltage steps (McCormick and Pape, 1990b; Soltesz et al., 1991; Pape, 1996). The size of this inward current and the magnitude of this increase in steady-state current are comparable to the shift inIBH and to the increase in steady-state current, respectively, evoked during hypoxia. It is reasonable, therefore, to suggest that a hypoxia-mediated release of one or more of these transmitters may be responsible for some of the effects elicited by hypoxia in TC neurons. Indeed, strong support for this hypothesis is provided by the finding that a combined block of noradrenaline (β), serotonin, and histamine (H2) receptors produced a substantial reduction of the hypoxic response.
Although an increase in the activation rate ofIh in the presence of these transmitters has been reported (McCormick and Pape, 1990a,b; McCormick and Williamson, 1991), none of them, applied alone, has been shown to be able to produce as large a change in the kinetics properties ofIh as to transform it into a very fast activating (i.e., instantaneous) current during hypoxia. Interestingly, each of these transmitters increases the instantaneous current slightly (compare Fig. 5, A and C, in McCormick and Pape, 1990b; and Fig. 6, A and B, in McCormick and Williamson, 1991), and forskolin, which mimics the effects of noradrenaline and serotonin on Ih in TC neurons (Pape, 1996), appears to be able to transform IQof sympathetic neurons into an instantaneous current (D. A. Brown, personal communication). Nevertheless, several possibilities for the large, hypoxia-mediated change in Ih kinetics remain to be elucidated: (1) a synergistic action of known thalamic transmitters, possibly involving both cAMP and cGMP; (2) the action of some unknown transmitter(s) released during hypoxia; and (3) the additional action of hypoxia-mediated postsynaptic effects, such as changes in intracellular pH or cell swelling.
Pathophysiological role of Ihactivation during hypoxia
It has been shown that hypoxia/ischemia causes highly organized, system-preferential, topographic encephalopathy and targets regions that play a pivotal role in sensory integration. Injury mediated by oxygen deprivation is found preferentially in the somatosensory cortex, the basal ganglia (including putamen and subthalamic nucleus), the ventral thalamus, the medial and dorsal LGN, and the tectal nuclei (Martin et al., 1997). The hypoxia-mediated inward current and increase in conductance observed in this study will affect the amplitude and kinetics of synaptic potentials generated in, and as a consequence the output of, TC neurons. These effects may be carried forward from the thalamus to the cortex via corticothalamic connections (Jones, 1985;Salt et al., 1995;) and then to the striatum and the subthalamus via corticosubthalamic projections (Fujimoto and Kita, 1993; Bevan et al., 1995; Jones et al., 1977), explaining the topographic cascade of transneuronal injury in brain areas involved in sensorimotor integration.
Footnotes
This work was supported by the Wellcome Trust (Grant 37089). We thank Bob Jones for photography and Tim Gould for technical assistance with some experiments.
Correspondence should be addressed to V. Crunelli, Physiology Unit, School of Molecular and Medical Biosciences, University of Wales Cardiff, Museum Avenue, Cardiff, CF1 3US, UK.
REFERENCES
- 1.Belousov AB, Godfraind J-M, Krnjevic K. Internal Ca2+ stores involved in anoxic responses of rat hippocampal neurones. J Physiol (Lond) 1995;486:547–556. doi: 10.1113/jphysiol.1995.sp020833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Ari Y. Galanine and glibenclamide modulate the anoxic release of glutamate in rat CA3 hippocampal neurons. Eur J Neurosci. 1990a;2:62–68. doi: 10.1111/j.1460-9568.1990.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Ari Y. Hippocampal potassium ATP channels and anoxia: presynaptic, postsynaptic or both. Trend Neurosci. 1990b;13:409–410. doi: 10.1016/0166-2236(90)90121-p. [DOI] [PubMed] [Google Scholar]
- 4.Bevan MD, Francis CM, Bolam JP. The glutamate-enriched cortical and thalamic input to neurons in the subthalamic nucleus of the rat: convergence with GABA-positive terminals. J Comp Neurol. 1995;361:491–511. doi: 10.1002/cne.903610312. [DOI] [PubMed] [Google Scholar]
- 5.Campbell RC. Statistics for biologists. Cambridge UP; Cambridge, UK: 1989. [Google Scholar]
- 6.Coggan JS, Purnyn SL, Knoper SR, Kreulen DL. Muscarinic inhibition of two potassium currents in guinea-pig prevertebral neurons: differentiation by extracellular cesium. Neuroscience. 1994;59:349–361. doi: 10.1016/0306-4522(94)90601-7. [DOI] [PubMed] [Google Scholar]
- 7.Cook NS. The pharmacology of potassium channels and their therapeutic potential. Trends Pharmacol Sci. 1988;9:21–28. doi: 10.1016/0165-6147(88)90238-6. [DOI] [PubMed] [Google Scholar]
- 8.Cowan AI, Martin RL. Ionic basis of membrane potential changes induced by anoxia in rat dorsal vagal motoneurones. J Physiol (Lond) 1992;455:89–109. doi: 10.1113/jphysiol.1992.sp019292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crunelli V, Erdemli G. Pharmacological properties of the response of thalamocortical neurones to brief hypoxia. Soc Neurosci Abstr. 1997;23:2078. [Google Scholar]
- 10.Crunelli V, Lightowler S, Pollard CE. A T-type Ca2+ current underlies low-threshold Ca2+ potentials in cells of the cat and rat lateral geniculate nucleus. J Physiol (Lond) 1989;413:543–561. doi: 10.1113/jphysiol.1989.sp017668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubinsky JM, Rothman SM. Intracellular calcium concentrations during “chemical hypoxia” and excitotoxic neuronal injury. J Neurosci. 1991;11:2545–2551. doi: 10.1523/JNEUROSCI.11-08-02545.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erdemli G, Crunelli V. Hypoxia activates Ih in rat thalamocortical neurones in vitro. J Physiol (Lond) 1998;506:150P. [Google Scholar]
- 13.Fujimoto K, Kita H. Response characteristics of subthalamic neurons to the stimulation of the sensorimotor cortex in the rat brain. Brain Res. 1993;609:185–192. doi: 10.1016/0006-8993(93)90872-k. [DOI] [PubMed] [Google Scholar]
- 14.Fujimura N, Tanaka E, Yamamoto S, Shigemori M, Higashi H. Contribution of ATP-sensitive potassium channel to hypoxic hyperpolarization in rat hippocampal CA1 neurons in vitro. J Neurophysiol. 1997;77:378–385. doi: 10.1152/jn.1997.77.1.378. [DOI] [PubMed] [Google Scholar]
- 15.Fujiwara N, Higashi H, Shimoji K, Yoshimura M. Effects of hypoxia on rat hippocampal neurones in vitro. J Physiol (Lond) 1987;384:131–151. doi: 10.1113/jphysiol.1987.sp016447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gay LA, Stanfield PR. Cs+ causes a voltage-dependent block of inward K currents in resting skeletal muscle fibers. Nature. 1977;267:169–170. doi: 10.1038/267169a0. [DOI] [PubMed] [Google Scholar]
- 17.Guyon A, Leresche N. Modulation by different GABAB receptor types of voltage-activated calcium currents in rat thalamocortical neurones. J Physiol (Lond) 1995;485:29–42. doi: 10.1113/jphysiol.1995.sp020710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haddad GG, Donelly DF. O2 deprivation induces a major depolarization in rain stem neurons in the adult but not in the neonatal rat. J Physiol (Lond) 1990;429:411–428. doi: 10.1113/jphysiol.1990.sp018265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haddad GG, Jiang C. O2 deprivation in the central nervous system: on mechanisms and injury. Prog Neurobiol. 1993;40:277–318. doi: 10.1016/0301-0082(93)90014-j. [DOI] [PubMed] [Google Scholar]
- 20.Hagiwara N, Irisawa H. Modulation by intracellular Ca2+ of the hyperpolarization-activated inward current in rabbit single sino-atrial node cells. J Physiol (Lond) 1989;409:121–141. doi: 10.1113/jphysiol.1989.sp017488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagiwara S, Miyazaki W, Mood W, Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol (Lond) 1978;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halliwell JV. K+ channels in the central nervous system. In: Cook NS, editor. Potassium channels. Wiley; Chichester, England: 1990. pp. 348–381. [Google Scholar]
- 23.Hansen AJ. Effect of anoxia on ion distribution in the brain. Pharmacol Rev. 1985;65:101–148. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- 24.Harris NC, Constanti A. Mechanisms of block by ZD 7288 of the hyperpolarization-activated inward rectifying current in guinea pig substantia nigra neurons in vitro. J Neurophysiol. 1995;74:2366–2378. doi: 10.1152/jn.1995.74.6.2366. [DOI] [PubMed] [Google Scholar]
- 25.Hochachka PW, Lutz PL, Sick T, Rosenthal M, van der Thillart G. Surviving hypoxia. CRC; Boca Raton, FL: 1993. [Google Scholar]
- 26.Ingram L, Williams JT. Modulation of the hyperpolarization-activated current (IH) by cyclic nucleotides in guinea-pig primary afferent neurons. J Physiol (Lond) 1996;492:97–106. doi: 10.1113/jphysiol.1996.sp021292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones EG. The thalamus. Plenum; New York: 1985. [Google Scholar]
- 28.Jones EG, Coulter JD, Burton H, Porter R. Cells of origin and terminal distribution of cortical fibers arising in the sensory-motor cortex of monkeys. J Comp Neurol. 1977;173:53–80. doi: 10.1002/cne.901730105. [DOI] [PubMed] [Google Scholar]
- 29.Kaplin AI, Snyder SH, Linden DJ. Reduced nicotinamide adenine dinucleotide-selective stimulation of inositol 1,4,5-triphosphate receptors mediates hypoxic mobilization of calcium. J Neurosci. 1996;16:2001–2011. doi: 10.1523/JNEUROSCI.16-06-02002.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kass IS, Lipton P. Calcium and long-term transmission damage following hypoxia in dentate gyrus and CA1 regions of the rat hippocampal slice. J Physiol (Lond) 1986;378:313–334. doi: 10.1113/jphysiol.1986.sp016221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kauppinen RA, McMahon HT, Nicholls DG. Ca2+-dependent and Ca2+-independent glutamate release, energy status and cytosolic free Ca2+ concentration in isolated nerve terminals following metabolic inhibition: possible relevance to hypoglycemia and anoxia. Neuroscience. 1988;27:175–182. doi: 10.1016/0306-4522(88)90228-x. [DOI] [PubMed] [Google Scholar]
- 32.Krnjevic K. Membrane current activation and inactivation during hypoxia in hippocampal neurons. In: Hochachka PW, Lutz PL, Sick T, Rosenthal M, van der Thillart G, editors. Surviving hypoxia. CRC; Boca Raton, FL: 1993. pp. 365–387. [Google Scholar]
- 33.Krnjevic K, Leblond L. Changes in membrane currents of hippocampal neurons evoked by brief hypoxia. J Neurophysiol. 1989;62:15–30. doi: 10.1152/jn.1989.62.1.15. [DOI] [PubMed] [Google Scholar]
- 34.Leblond J, Krnjevic K. Hypoxic changes in hippocampal neurones. J Neurophysiol. 1989;62:1–14. doi: 10.1152/jn.1989.62.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Madl JE, Burgesser K. Adenosine triphosphate depletion reverses sodium-dependent, neuronal uptake of glutamate in rat hippocampal slices. J Neurosci. 1993;13:4429–4444. doi: 10.1523/JNEUROSCI.13-10-04429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin LJ, Brambrink A, Koehler RC, Traystman RJ. Primary sensory and forebrain motor systems in the new-born brain are preferentially damaged by hypoxia-ischemia. J Comp Neurol. 1997;377:262–285. doi: 10.1002/(sici)1096-9861(19970113)377:2<262::aid-cne8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 37.McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 38.McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol (Lond) 1990a;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCormick DA, Pape HC. Noradrenergic and seratonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J Physiol (Lond) 1990b;431:319–342. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCormick DA, Williamson A. Modulation of neuronal firing mode in cat and guinea pig LGNd by histamine: possible cellular mechanisms of histaminergic control of arousal. J Neurosci. 1991;11:3188–3199. doi: 10.1523/JNEUROSCI.11-10-03188.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicholls D, Attwell D. The release and uptake of excitatory amino acids. Trends Pharmacol Sci. 1990;11:462–468. doi: 10.1016/0165-6147(90)90129-v. [DOI] [PubMed] [Google Scholar]
- 42.Nolan PC, Waldrop TG. Ventrolateral medullary neurons show age-dependent depolarization to hypoxia in-vitro. Dev Brain Res. 1996;91:111–120. doi: 10.1016/0165-3806(95)00166-2. [DOI] [PubMed] [Google Scholar]
- 43.Pape H-C. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- 44.Pape H-C, Mager R. Nitric oxide controls oscillatory activity in thalamocortical neurons. Neuron. 1992;9:441–448. doi: 10.1016/0896-6273(92)90182-d. [DOI] [PubMed] [Google Scholar]
- 45.Reid KH, Schurr A, Tseng MT, Edmonds HLJR. Resistance to hypoxia in the rat hippocampal slice. Brain Res. 1984;302:387–391. doi: 10.1016/0006-8993(84)90255-5. [DOI] [PubMed] [Google Scholar]
- 46.Salt TE, Meier CL, Seno N, Krucker T, Herrling PL. Thalamocortical and corticocortical excitatory postsynaptic potentials mediated by excitatory amino acid receptors in the cat motor cortex in vivo. Neuroscience. 1995;64:433–442. doi: 10.1016/0306-4522(94)00357-b. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez-Prieto J, Sihra TS, Evans D, Ashton A, Dolly JO, Nicholls DG. Botulinum toxin A blocks glutamate exocytosis from guinea-pig cerebral cortical synaptosomes. Eur J Biochem. 1987;165:675–681. doi: 10.1111/j.1432-1033.1987.tb11494.x. [DOI] [PubMed] [Google Scholar]
- 48.Soltesz I, Lightowler S, Leresche N, Jassik-Gerschenfeld D, Pollard CE, Crunelli V. Two inward currents and the transformation of low-frequency oscillations of rat and cat thalamo-cortical cells. J Physiol (Lond) 1991;441:175–197. doi: 10.1113/jphysiol.1991.sp018745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stansfeld CE, Roper J, Ludwig J, Weseloh RM, Marsh SJ, Brown DA, Pongs O. Elevation of intracellular calcium by muscarinic receptor activation induces a block of voltage-activated rat ether-à-go-go channel in a stable transfected cell line. Proc Natl Acad Sci USA. 1996;93:9910–9914. doi: 10.1073/pnas.93.18.9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steinke W, Sacco RL, Mohr JP, Foulkes MA, Tatemichi TK, Wolf PA, Price TR, Hier DB. Thalamic stroke, presentation and prognosis of infarcts and hemorrhages. Arch Neurol. 1992;49:703–710. doi: 10.1001/archneur.1992.00530310045011. [DOI] [PubMed] [Google Scholar]
- 51.Steriade M, Llinas R. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68:649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- 52.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 53.Szatkowski M, Attwell D. Triggering and execution of neuronal death in brain ischemia: two types of glutamate release by different mechanisms. Trend Neurosci. 1994;17:359–365. doi: 10.1016/0166-2236(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 54.Szelies B, Herholz K, Pawlik G, Karbe H, Hebold I, Heiss WD. Widespread functional effects of discrete thalamic infarction. Arch Neurol. 1991;48:178–182. doi: 10.1001/archneur.1991.00530140072019. [DOI] [PubMed] [Google Scholar]
- 55.Turner JP, Anderson CM, Williams SR, Crunelli V. Morphology and membrane properties of neurones in the cat ventrobasal thalamus in vitro. J Physiol (Lond) 1997;505:707–726. doi: 10.1111/j.1469-7793.1997.707ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watts AE, Williams JT, Henderson G. Baclofen inhibition of the hyperpolarization-activated cation current, Ih, in rat substantia nigra zona compacta neurons may be secondary to potassium current activation. J Neurophysiol. 1996;76:2262–2270. doi: 10.1152/jn.1996.76.4.2262. [DOI] [PubMed] [Google Scholar]
- 57.Williams SR, Turner JP, Anderson CM, Crunelli V. Electrophysiological and morphological properties of interneurones in the rat dorsal lateral geniculate nucleus in vitro. J Physiol (Lond) 1996;490:129–147. doi: 10.1113/jphysiol.1996.sp021131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams SR, Turner JP, Hughes SW, Crunelli V. On the nature of anomalous rectification in thalamocortical neurones of the cat ventrobasal thalamus in vitro. J Physiol (Lond) 1997;505:727–747. doi: 10.1111/j.1469-7793.1997.727ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu SP, O’Malley MD, Adams PR. Regulation of M current by intracellular calcium in bullfrog sympathetic ganglion neurones. J Neurosci. 1994;14:3487–3499. doi: 10.1523/JNEUROSCI.14-06-03487.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J, Krnjevic K. Whole-cell recording of anoxic effects on hippocampal neuron in slices. J Neurophysiol. 1993;69:118–127. doi: 10.1152/jn.1993.69.1.118. [DOI] [PubMed] [Google Scholar]
- 61.Zhang L, Pennefather P, Velumian A, Tymianski M, Harlton M, Carlen PL. Potentiation of a slow Ca2+-dependent K+ current by intracellular Ca2+ chelators in hippocampal CA1 neurones of rat brain slices. J Neurophysiol. 1995;74:2225–2241. doi: 10.1152/jn.1995.74.6.2225. [DOI] [PubMed] [Google Scholar]