Abstract
Mechanisms guiding the first axons from the olfactory placode of the peripheral nervous system (PNS) to the olfactory bulb in the vertebrate CNS are unknown. We analyzed the initial outgrowth of axons from the olfactory placode in zebrafish and found a precocious transient class of pioneer neurons that prefigure the primary olfactory pathway before outgrowth of olfactory sensory axons or expression of olfactory receptor genes. Not only are the pioneers antigenically, morphologically, and spatially distinct from olfactory sensory neurons, they are also developmentally distinct; via fate mapping, we show that they arise from a more anterior region of the lateral neural plate than do the first sensory neurons. After the axons of the sensory neurons grow into the CNS, the pioneer neurons undergo apoptotic cell death. When we ablated the pioneers before axonogenesis, the following sensory axons showed severe misrouting. We propose that the pioneers provide the first necessary connection from the PNS to the CNS and that they establish an axonal scaffold for the later-arriving olfactory sensory neurons.
Keywords: fate map, cell death, olfactory neurons, axon guidance, olfactory receptors, cell lineage
The olfactory organ is a unique cellular population that arises outside the CNS from the ectodermally derived olfactory placode (Farbman, 1992, 1994; Crews and Hunter, 1994). Initially, the olfactory placode is an apparently homogeneous population of cells. As the placode differentiates into the olfactory epithelium, stratified cell types characteristic of the adult epithelium appear, including basal cells, sensory neurons, and support cells. Throughout life, the basal cells generate olfactory sensory neurons that migrate toward the apical surface of the epithelium while extending axons and dendrites (Menco and Farbman, 1985; Farbman, 1994). The cell bodies of the olfactory sensory neurons remain in the epithelium, and their axons grow through the olfactory nerve into the differentiating CNS. Once within the CNS, the axons segregate to form glomeruli, clustered grape-like structures in the olfactory bulb (Shepherd, 1972). The glomerular organization of olfactory sensory afferents is characteristic of both invertebrates and vertebrates (Christensen and Hildebrand, 1987; Farbman, 1992). Within a species, the glomeruli are organized in a stereotyped pattern in the olfactory bulb (Graziadei and Graziadei, 1979; Baier and Korsching, 1994; Byrd and Brunjes, 1995; Rupp et al., 1996).
Little is known about how the axons of the first olfactory sensory neurons navigate to the CNS and initiate patterned connections with the developing olfactory bulb. Dye labeling has shown that individual glomeruli in the olfactory bulb receive inputs from sensory neurons scattered throughout the olfactory epithelium (Riddle and Oakley, 1991). Recent studies indicate that the axons of sensory neurons expressing the same olfactory receptor converge on the same glomerulus (Mombaerts et al., 1996), express receptor transcript in their axon terminals (Ressler et al., 1993; Vassar et al., 1994), and respond to the same small subset of odorants (Zhao et al., 1998). This has led to the suggestion that olfactory receptors may play a role in sensory axon guidance (Singer et al., 1995). These studies did not examine the first connections between the olfactory placode and the telencephalon; thus, whether receptors play a role in the initial connection from the periphery to the bulb is unknown.
Because of their rapid development and the accessibility of early developmental stages, we have used zebrafish to analyze the initial events that connect the axons of the olfactory sensory neurons with the developing bulb. We have discovered a precocious class of neurons, the pioneer neurons, which precede the olfactory sensory neurons, establish initial contact with the telencephalon, and then die. The pioneer neurons are identifiable by their unique location and morphology within the olfactory epithelium, their antigenicity, and their origin from a more anterior region of the neural plate than that which gives rise to the first sensory neurons. By ablating the pioneers, we show that they are necessary to target the incoming axons of the sensory neurons to the developing olfactory bulb. Because the pioneer neurons appear not to express any of the known olfactory receptor genes, it is unlikely that olfactory receptors participate in the initial connection between the olfactory placode and the developing telencephalon.
MATERIALS AND METHODS
Animals. Zebrafish embryos were obtained from natural spawnings of wild-type (AB) fish. Fish were staged by morphology, and times of development are expressed as standard stages (Kimmel et al., 1995) or as hours after fertilization (h) at 28.5°C.
Antibodies and immunocytochemistry. Embryos were fixed overnight at 4°C in 4% paraformaldehyde in 0.1 mphosphate buffer with 0.15 mm CaCl2 and 4% sucrose, pH 7.2. After washes in 0.1 m phosphate buffer and dH2O, embryos were permeabilized in acetone (7 min at −20°C), washed in dH2O followed by 0.1 mphosphate buffer, and then preadsorbed in 2% normal goat serum (NGS) in PBDT [0.1 m phosphate buffer, 2% bovine serum albumen, 1% dimethylsulfoxide (DMSO), and 0.5% Triton X-100]. A mouse monoclonal antibody, zns-2 (Trevarrow et al., 1990), was used (1:300) in PBDT with 2% NGS to label the pioneer neurons. In addition to pioneer neurons in the olfactory placodes, zns-2 also labels neurons in the trigeminal ganglia. Embryos were incubated in this primary antibody overnight at 4°C. They were then rinsed three times over 2 hr and placed in secondary goat anti-mouse antibody (1:200; Sternberger Monoclonals Inc.) or FITC-conjugated goat anti-mouse (1:200; Sigma, St. Louis, MO) in PBDT with 2% NGS overnight at 4°C. FITC-labeled preparations were then mounted in PBS, viewed using a Zeiss (Oberkochen, Germany) confocal microscope, and analyzed with the Voxel View program (Vital Images). Embryos processed for HRP–diaminobenzadine (DAB) coloration were washed five times over 2 hr and incubated in peroxidase-mouse antiperoxidase at 1:500 in PBDT with 2% NGS (Sternberger Monoclonals Inc.) overnight at 4°C. Embryos were then rinsed in 0.1 m phosphate buffer and 1% DMSO three times for 1 hr. The coloration reaction was done using 0.02% DAB in 1:1 dH2O/PBS with 1% DMSO and 0.003% hydrogen peroxide. Fixed embryos were prepared for sectioning by dehydrating in an alcohol series (30, 50, 70, 95, 2 × 100%), embedding in Epon (Westerfield, 1995), and cutting 7.5 μm serial sections, which were then covered in Permount and coverslipped. A variety of antibodies known to label vertebrate olfactory neurons, including neural cell adhesion molecule (NCAM) and olfactory marker protein, were tried with no success. In addition, the antibody reported to mark pioneers in the mouse trigeminal (Stainier and Gilbert, 1990) does not work in zebrafish.
RNA in situ hybridizations. RNA in situ hybridizations were performed using digoxigenin-labeled RNA probes (Thisse et al., 1993) for the olfactory receptors. Probes were generated from zebrafish olfactory receptor cDNAs 2, 4, 9, and 13 (Barth et al., 1996). Based on the number and timing of cells expressing receptor mRNA, the sensitivity of the digoxigenin-labeled probes appeared equal to that of the radio-labeled probes used by Barth et al. (1996). Each preparation was drawn using a camera lucida, and the cells expressing olfactory receptors were scored as being in the basal part of the olfactory organ if they were within two cell diameters (∼20 μm) of the basal surface. Otherwise, they were scored as apical.
Double labeling immuno–RNA in situhybridization. These procedures were combined by first performing immunocytochemistry with DAB coloration using the zns-2 antibody as described above, except that RNAsin (Boehringer Mannheim, Indianapolis, IN) was added to all blocking–incubation solutions. This was followed by RNA in situ hybridization. It should be noted that the quality of the signal when double labeling is dependent on the antibody. The zns-2 antibody shows a decrement in signal after the RNAin situ hybridization, and for some antibodies there is a total loss of signal.
Dye labeling. Olfactory neurons were labeled with the carbocyanine dye DiI (DiIC18(3); Molecular Probes, Eugene, OR) by adding a 0.5% solution of the dye to the embryo medium. The fish were then rinsed three to five times in embryo medium and placed in a clean dish. The live fish were anesthetized (Westerfield, 1995), mounted ventral side up in methyl cellulose, and viewed by fluorescence confocal microscopy. Images were collected on a confocal microscope (Zeiss) and analyzed with the Voxel View program (Vital Images).
Terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL). Embryos were fixed overnight in 4% paraformaldehyde, rinsed several times in PBS, and dehydrated in a graded methanol series. The preparations were then treated with MeOH/H202 (5:1) and rehydrated into 10× Tris-buffered saline (TBS) (1 m Tris base and 1.5m NaCl, pH 7.5), followed by two washes in TBS for 5 min each. After a 30 min digestion in Proteinase-K (1 μg/ml) at 37°C, they were washed three times for 5 min each in 10× TTBST (10× TBS, 2% Triton X-100, and 5% Tween-20) and two times for 5 min each in 5× TTase buffer (125 mm Tris buffer, 1m Na Cacodylate, 1.25 mg/ml BSA, 1% Tween-20, and 1 mm CoCl2). Reaction solution was then added [100 μl of TTase buffer, 0.5 μl of TTase (Boehringer Mannheim), and 0.25 μl of fluorescein dUTP (Boehringer Mannheim)], and the preparations were preincubated on ice for 1 hr, incubated at 37°C for 1 hr, washed two times in TTBST, and washed overnight at 4°C. The preparations were mounted in PBS and viewed with the confocal microscope.
Cell labeling and lineage tracing. Embryos at the four somite stage were embedded in agar with a window cut to expose the lateral edge of the neural plate. These blocks were mounted on a microscope slide and covered with physiological saline. Microelectrodes (∼300 MΩ resistance) were pulled (Sutter Instruments), backfilled with a mixture of Rhodamine dextran dye [2%, molecular weight (MW) 3000; Molecular Probes] and Fluorescein dextran dye (2%, MW 3000; Molecular Probes) in 0.2 m KCl. Cells were visualized with a 40× water immersion lens using a fixed-stage microscope (Zeiss). After penetration of a single cell, dye was passed into the cell by using capacitance compensation to create small voltage changes (Westerfield, 1995). Successful filling of the cell was judged by a rapid check of the Rhodamine fluorescence. Preparations for lineage tracing were allowed to develop to the desired age, and then fluorescent images were collected on a confocal microscope (Zeiss) and reconstructed using the Voxel View and Voxel Math programs (Vital Images).
Ablations. Animals were anesthetized and then rinsed and mounted in agarose blocks ventral side up, exposing the anteroventral aspect of the olfactory placode. At the time when the olfactory placodes were first evident, 18–20 h, the pioneer cells were carefully cut out of the anteroventral part of the placode under a 40× dissecting microscope using glass microneedles. Glass microelectrodes were used because laser ablation of all cells in the 15–20 cell cluster was unreliable, and removal by suction destroys the integrity of the olfactory placode. The cells of the olfactory placode are separate from the developing telencephalon, allowing the removal of the pioneer cells without damaging the telencephalon. The pioneer cells were ablated from a single olfactory placode per animal. The intact side served as a control for assessing both the general health of the embryo after ablation and the quality of the zns-2 labeling (used to score the presence of pioneer neurons). The embryos were fixed at 48 h and labeled with DiI to reveal sensory neuron morphology. Of the 35 total preparations operated on at 18–20 h and analyzed for sensory neuron misrouting, 17 were successfully labeled with zns-2 (Table1). In all experiments, there were some preparations with general growth defects and/or poor DiI labeling; these were not included in the analysis.
Table 1.
Ablation of pioneer neurons results in defects in axon pathfinding of the olfactory sensory neurons
| Misrouted | Normal | |
|---|---|---|
| 20 hr ablations | ||
| No zns-2 labeling | Posterior 83% (5/6); split 17% (1/6) | 0% (0/6) |
| Partial zns-2 labeling | Posterior 0% (0/5); split 100% (5/5) | 0% (0/5) |
| Normal zns-2 labeling | 0% (0/6) | 100% (6/6) |
| 38 hr and 46 hr ablations | 0% (0/38) | 100% (*38/38) |
Thirty-five preparations were analyzed for sensory neuron misrouting by labeling with DiI. Preparations with growth defects and/or poor DiI labeling were excluded from the analysis of both experimental and control groups. 20 hr ablations, A subset of the preparations analyzed with DiI were also analyzed with zns-2 (n=17) and listed here. The misrouting was characterized as “posterior” (Fig. 3B) or “split” (Fig.3C). Numbers indicate the number of preparations showing a given sensory neuron morphology of the total number of preparations with the indicated zns-2 labeling. 38 hr and 46 hr ablations, Ablations done at later times after the first sensory neurons had entered the CNS. *In this group, 5/38 preparations had normal axon projections into the bulb, but within the bulb the terminations appeared disorganized.
RESULTS
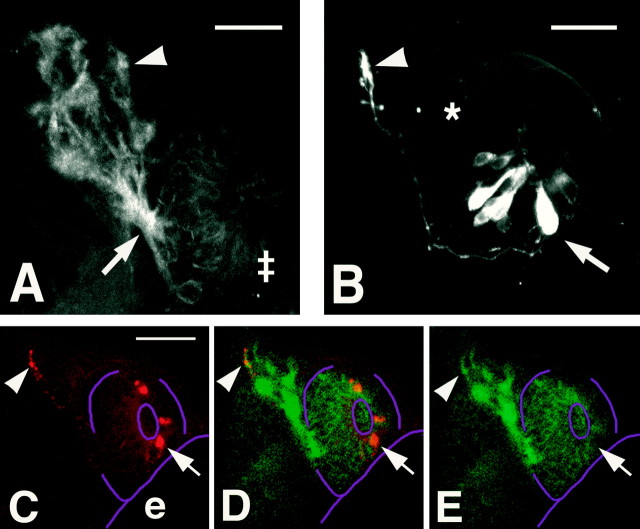
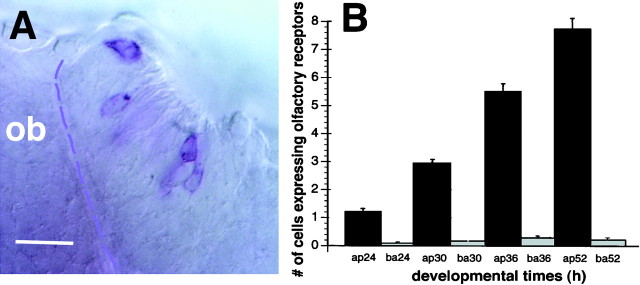
In zebrafish, the olfactory placode first appears at 17 h after fertilization as a thickening of the ectoderm [Fig.1A, H,(A,B)], and this thickening later invaginates to form the naris by 32 h (Whitlock and Westerfield, 1995, 1997). We found that the zns-2 antibody (Trevarrow et al., 1990) recognized neurons whose axons first emerged from the olfactory placode at 24 h. At this time and during the second day of development, there was an average of 11 pioneer neurons (± 2; n = 23 olfactory placodes) labeled in each olfactory placode. These neurons had large round cell bodies (Fig. 1B, arrowhead) and axons that extended along the lateral edge of the telencephalon (Fig.1B, arrow). No dendrites were apparent in live embryos using Nomarski optics or using the antibody (Fig.1B, arrowhead) in fixed preparations. Because these are the first neurons seen with axons emerging from the olfactory placode, thus initiating the connection between the olfactory placode and the developing telencephalon, we identified them as pioneers. The region in which the zns-2-positive axons (Fig.1C,D, black arrows) meet the developing telencephalon expresses emx1 (Fig.1C,E, white arrows), a gene that is expressed in the developing telencephalon (Simeone et al., 1992;Morita et al., 1995). By 30 h, the axons of the pioneer neurons had extended into the developing telencephalon and had started to defasciculate, and by 38 h, axonal condensations of zns-2-positive axons (shown at 38 h in Fig. 1F, white arrow) started to appear in the presumptive olfactory bulb. These axons penetrated the surface of the telencephalon and extended into its most anterior region. By 52 h, distinct condensations of zns-2-positive axons were apparent in the developing bulb (Fig.1G, white arrow, arrowhead;I, arrowhead. These axonal condensations had a very fibrous appearance in both whole mounts (Fig. 1G,arrowhead) and sectioned material (Fig.1I, arrowhead). The zns-2 labeling of cell bodies in the olfactory epithelium was greatly diminished by 42–44 h and was no longer detectable after 48 h, although axonal labeling remained strong.
Fig. 1.
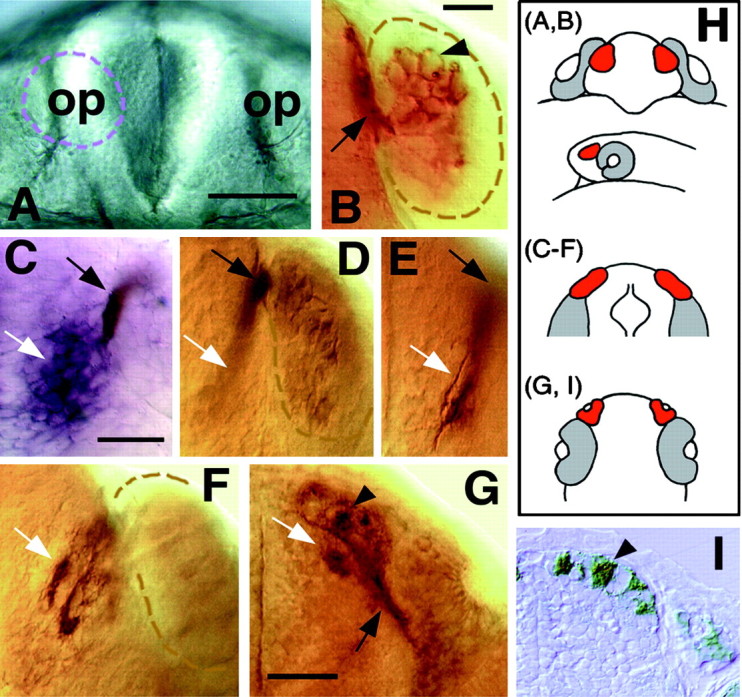
The pioneer neurons initiate the formation of the olfactory nerve and establish glomerular-like structures in the telencephalon. A, B, Frontal views with dorsal to the top. C–F, Dorsal views with anterior to the top. G, Ventral view with dorsal to the top. H, Diagrams labeled with letters corresponding topanels to show orientation. The second diagram is a side view, anterior to the left at a stage equivalent to the frontal view in H (A, B). The olfactory placodes are in red, and the eyes are ingray. I, A transverse section with anterior to the top. A, Frontal view of the head of a 24 h live zebrafish embryo. The olfactory placodes (op) are paired thickenings lying dorsoanteriorly. Thebroken line outlines the placode on theleft. B, Enlargement of a frontal view of a placode labeled with the zns-2 antibody at 24 h. Dendrites are absent on the apical surface (arrowhead), and the axons (arrow) exit basally and grow along the telencephalon. The broken line demarcates the edge of the placode.C, The pioneer axons (brown-blue,black arrow) meet the telencephalon in the region expressing the gene emx1 (purple,white arrow) in a 26 h embryo. D,E, A 30 h preparation showing two focal planes, labeled only with zns-2. The axons exit the placode (black arrows) and defasciculate in the telencephalon (white arrows). The broken line demarcates the posterior edge of the placode. F, At 38 h, the pioneer axons (white arrow) start to form condensations in the telencephalon. G, Axons labeled with zns-2 (black arrow) extend into the developing olfactory bulb in a 52 h embryo. Note the axonal condensations (white arrow) evident in the CNS. H, The developing olfactory placode has moved from a dorsal to a more anterior location in front of the developing eye (see A, B). As a result of this morphogenetic movement, the zns-2-labeled axons are more easily viewed from the ventral side (G,I) as they project anteriorly into the telencephalon. I, A 7.5 μm Epon section through the olfactory organ and bulb at the same developmental stage asG, with the axonal condensations indicated by thearrowhead. Scale bars: A, 40 μm;B, 25 μm; C–F, 35 μm;G, I, 40 μm.
During the second and third days of development, the olfactory nerve became prominent, and the zns-2-labeled axons of the pioneer neurons (Fig. 2A,arrow) formed more complex axonal condensations (Fig.2A, arrowhead) within the bulb. A second wave of neurons, distinct from the pioneers, was visualized by 44 h, after the naris had opened. These later-developing neurons had dendrites, which projected to the naris and, thus, could be labeled by external application of DiI (Fig. 2B). In contrast, the pioneer neurons had no obvious dendrites and were unlabeled by this technique (Fig. 2B, asterisk). Because these later-developing cells had the morphology of sensory neurons and dendrites in contact with the outside world, we identified them as sensory neurons. The first of these later-developing sensory neurons (Fig. 2B, arrow) extended axons into the developing olfactory bulb (Fig. 2B,arrowhead) after the pioneer neurons had formed axonal condensations within the olfactory bulb. Some of the cells in the initial cluster of four to six sensory neurons (Fig.2B) had dendrites but had not extended axons. These neurons lie adjacent to the eye in the apical-lateral region of the olfactory organ (Fig. 2B, arrow), distinct from the position of the pioneer neurons whose cell bodies occupy the basal-medial region (Fig. 2B,asterisk).
Fig. 2.
The axons of the pioneer neurons and first sensory neurons colocalize in the CNS. A, A 44 h embryo labeled with zns-2. The pioneer axons converge to form the olfactory nerve (arrow) and then defasciculate to form axonal condensations in the olfactory bulb (arrowhead).Double cross indicates the region occupied by the (unlabeled) sensory neurons. B, Lateral-oblique view of the nose of a live 50 h fish labeled with DiI. Two cell bodies (arrow) with axons and several without axons are evident lying next to the eye. The axons extend into the developing olfactory bulb (arrowhead). Asterisk marks the region occupied by (unlabeled) pioneer neurons. C–E, The axons of primary olfactory sensory neurons colocalize with the pioneer axons in a single 1.5 μm optical section. Confocal micrographs of ventral view of an olfactory organ (outlined in purple) at 44 h showing sensory neurons labeled with DiI (arrows inC and D, red) and pioneer neurons labeled with zns-2 (D and E,green). The growth cones of the sensory neurons in the differentiating olfactory bulb colocalize (arrowhead) with the axons of the pioneer neurons. e, Eye. Anterior is to the top. This preparation is a ventral view as depicted in Figure 1H, (C–F). Scale bars: A, 30 μm; B, 20 μm;C–E, 40 μm.
We labeled the sensory neurons with DiI (Fig.2C,D, red) and the pioneer neurons with zns-2 in the same preparation (Fig.2D,E, green) and used confocal microscopy to show that the DiI-labeled sensory axons and the zns-2-positive pioneer axons overlap with one another in the telencephalon (Fig. 2C–E, arrowheads). The axons of the sensory neurons are in the proximity of the axons of the pioneer neurons at the exit of the olfactory nerve, and the axons of both cell types are colocalized at their terminations within the presumptive olfactory bulb. These results suggest that, once leaving the olfactory organ, the growth cones of the later developing sensory neurons follow the pioneer axons.
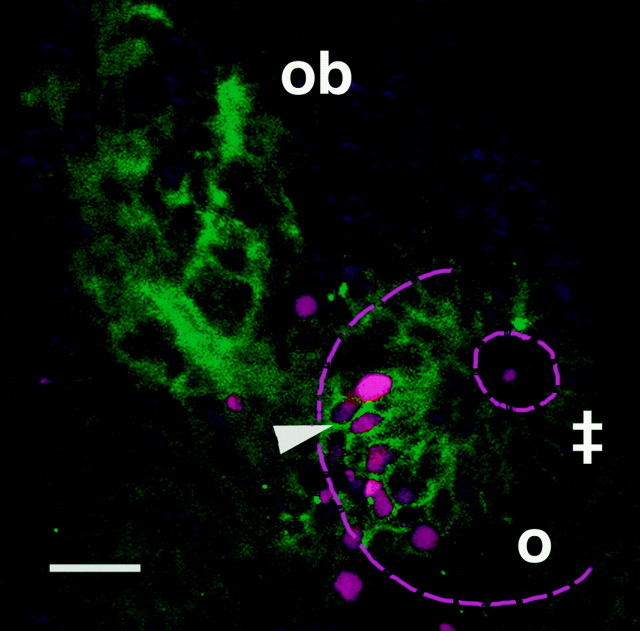
The pioneer neurons are a transient population of cells. We visualized their death using terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) (Gavrieli et al., 1992). Cell death in the developing olfactory organ peaked at ∼48 h and was localized primarily in the basal-medial region (Fig. 3,magenta) occupied by the pioneer neurons. Preparations double labeled for both TUNEL (Fig. 3, magenta) and zns-2 (Fig. 3, green) showed that the majority (80% of the cells;n = 25 animals examined) of the TUNEL-labeled cells within the olfactory organ were also zns-2-positive (Fig. 3,arrowhead) at this time, confirming that the dying cells were the pioneer neurons. Thus, the pioneer neurons undergo apoptosis as the axons of the first sensory neurons reach the differentiating olfactory bulb.
Fig. 3.
The olfactory pioneer neurons die. Preparation labeled for zns-2 (green) and TUNEL (magenta) at 42 h shows pioneer neurons dying at this time. The TUNEL-labeled cells are primarily located in the basal-medial part (arrowhead) of the olfactory organ.o, Olfactory organ; ob, olfactory bulb;double cross indicates the location of the unlabeled sensory neurons. Ventral-oblique view. Serial optical sections from the olfactory organ into the bulb were superimposed. Scale bar, 20 μm.
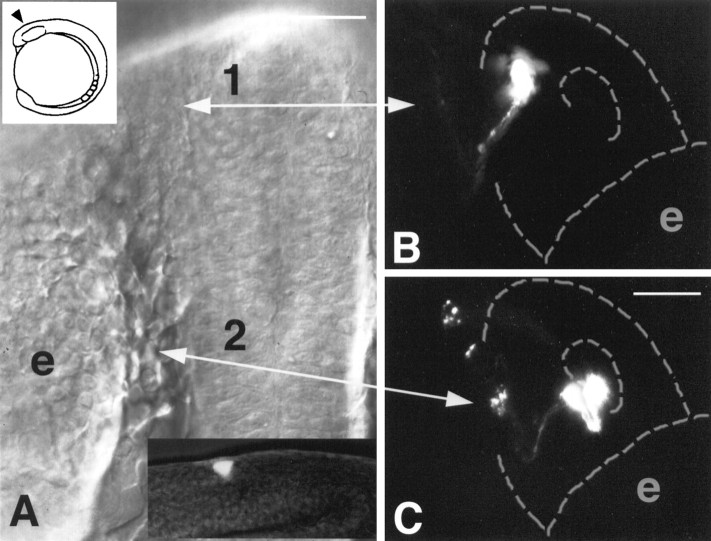
To determine the origins of the pioneer neurons and the olfactory sensory neurons, we labeled single cells (Fig.4, A, bottom inset) at the edge of the anterior neural plate at the four somite stage (∼12 h) (Fig. 4A, top inset) in the domain we know gives rise to the olfactory placode (our unpublished observations). Single cells labeled anteriorly at the lateral edge of the neural plate (Fig. 4A,1) gave rise to clones of cells that included olfactory pioneer neurons (Fig. 4B), identified by their round cell bodies and lack of dendrites, when scored at 36 h. As was observed with zns-2 labeling (Fig. 1B), pioneer cells labeled with lineage tracer dyes did not show dendrites and occupied the position characteristic of pioneer neurons, in the basal-medial region of the olfactory placode. The clones arising in the anterior region (Fig.4A, 1) never contained both pioneer neurons and sensory neurons (n = 13 clones). Moreover, when we labeled cells in this region (Fig. 4A,1) and examined the animals at 48 h, after the wave of cell death, we never observed labeled pioneer cells (n = 22 animals).
Fig. 4.
The pioneer neurons and the olfactory sensory neurons arise from different regions of the neural plate.A, Dorsal view of the neural plate in a live 12 h embryo before the formation of the olfactory placode. Arrows 1and 2 indicate the regions in which single cells were labeled. The location of their progeny is shown in B andC, respectively. Top inset is a lateral view of an embryo, with arrowhead indicating the region shown in the micrograph. Bottom inset is a lateral view of a live embryo with a single cell labeled in position 2 at 12 h, viewed with both bright-field and fluorescence optics.B, Ventral view of live embryo at 36 h, before the onset of olfactory pioneer cell death. Single cells, labeled at the anterior end of the neural plate (A,1), gave rise to pioneer neurons, recognized by their basal-medial location in the developing olfactory organ. C, Ventral view of a live embryo at 48 h. Single cells, labeled farther posterior in the neural plate (A, 2) adjacent to the central region of the developing eye (A, e), gave rise to olfactory sensory neurons lying apically at the posterior edge of the olfactory organ. Anterior is to the top. Scale bars:A, 40 μm; B, C, 20 μm.
In contrast to the fate of lateral anterior cells, cells labeled more posteriorly at the lateral edge of the neural plate, next to the lens placode (Fig. 4A, 2), gave rise exclusively to clones containing early olfactory sensory neurons (Fig.4C) when scored at 48 h. None of these clones contained olfactory pioneer neurons (n = 12). These observations demonstrate that olfactory pioneers and sensory neurons arise from precursors located in different regions of the neural plate, suggesting that they have distinct origins.
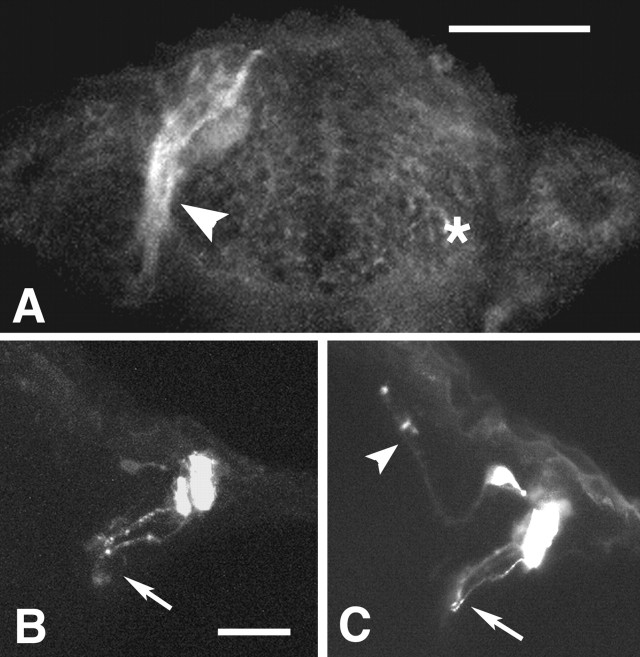
To examine the function of the olfactory pioneers, ablations of the pioneers were conducted in animals at a time just after the formation of a discernible olfactory placode, 18–20 h, yet 4 h before the outgrowth of the axons of the pioneer neurons and ∼1 d before axonal outgrowth of the first olfactory sensory neurons. Animals with ablated pioneers were allowed to develop to 50 h; we then examined the morphology of sensory neurons using DiI and the presence or absence of pioneers using zns-2. Complete ablation of the pioneers, as judged by loss of zns-2 labeling (Fig.5A, compareasterisk, arrowhead), resulted in misrouting of the sensory axons (Fig. 5B,C). The misrouted sensory axons typically extended posteriorly (Fig.5B) toward the anterior or postoptic commissures, where they wandered extensively in the dorsoventral plane, a trajectory never observed in normal embryos. In other embryos with incompletely ablated pioneers, as judged by zns-2 labeling, the sensory axons split between the normal anterior and abnormal posterior directions (Fig.5C). Preparations labeled with both DiI and zns-2 (Table 1) showed that when no pioneers were present sensory axons were misrouted (Fig. 5B, Table 1) and that when at least some pioneer neurons survived the ablation (data not shown) some sensory axons grew in the correct direction (Fig. 5C, Table 1). If the pioneers were ablated at 38 or 46 h, after they had established axonal condensations in the telencephalon and the axons of the sensory neurons had started growing into the CNS, there was no effect on axonal routing. These results strongly suggest that the pioneers are necessary for sensory axon guidance from the olfactory placode to the region of the telencephalon in which the olfactory bulb will develop.
Fig. 5.
Ablation of pioneer neurons results in misrouting of olfactory sensory axons. Ventral views at 50 h with anterior to thetop. A, The absence of zns-2 labeling on the ablated side (right, asterisk) indicates that all the pioneer neurons were removed. Control side (left, arrowhead) shows normal pattern of zns-2 labeling. B, Olfactory sensory neurons labeled with DiI show posterior misrouting in which axons (arrow) grow posteriorly in the absence of pioneer neurons. C, Olfactory sensory neurons labeled with DiI show split misrouting in which axons grow both posteriorly (arrow) and anteriorly (arrowhead) toward telencephalon. Scale bars: A, 80 μm;B, C, 40 μm.
Putative odorant receptor gene transcripts are localized in the cell bodies of sensory neurons (Buck and Axel, 1991) and in sensory axon terminals in the olfactory bulb (Ressler et al., 1993; Vassar et al., 1994). This has led to the suggestion that odorant receptors may play a role in guiding the axons of olfactory sensory neurons in addition to their role in detecting odorants (Singer et al., 1995;Mombaerts et al., 1996). We examined the expression of olfactory receptor messages (Fig.6A) to learn whether or not they are expressed in the pioneer neurons. Of the receptors that have been cloned to date in zebrafish (Barth et al., 1996; Byrd et al., 1996), only four are expressed during the time that olfactory pioneer neurons are alive (Barth et al., 1996). We used a mixture of the four olfactory receptor gene probes and found no expression of receptor RNA in the olfactory placode before 24 h. Receptor expression was first detectable at 24 h in 50% of the animals (n = 46 olfactory organs), and by 30 h all preparations contained at least two to three cells per olfactory organ expressing receptor genes. At 52 h (Fig. 6A), an average of 7.7 cells per olfactory organ (n = 52 olfactory organs) expressed olfactory receptors (Fig. 6B). Throughout the first 2.5 d of development, receptor gene expression remained preferentially located in cells in the apical region of the olfactory organ in which the sensory neurons are located, not in the basal part of the epithelium in which the pioneer neurons lie (Fig.6A,B). These results suggest that the sensory neurons, not the pioneer neurons, express putative odorant receptors and that receptor expression starts after the arrival of the pioneer axons in the telencephalon.
Fig. 6.
Sensory neurons, but not pioneer neurons, express olfactory receptor genes. A, Ventral view of a 52 h olfactory organ showing the combined expression of four olfactory receptor gene transcripts localized in the cytoplasm; axons are unlabeled. Labeled cells (dark blue) are located near the apical surface of the olfactory organ in which the sensory neurons, but not the pioneer neurons, are located. The basal edge of the olfactory organ is demarcated by the broken line. Scale bar, 20 μm. B, Cells expressing olfactory receptors are apically located. Bars represent the average (± SE) number of cells expressing olfactory receptor genes in each olfactory organ as a function of developmental time in the apical (ap) or basal (ba) region. Both olfactory organs from 22 to 28 animals were scored for each time point.
DISCUSSION
Although neurons in the PNS of other vertebrates have been termed “pioneers” based only on their early appearance during development (Stainier and Gilbert, 1990; Gong and Shipley, 1995), we have described here for the first time in vertebrates a class of PNS neurons that are both early born and required for establishing axonal pathways to the CNS. These olfactory pioneers are morphologically and temporally distinct from the olfactory sensory neurons, undergo programmed cell death, are essential to target sensory axons to the developing olfactory bulb, and arise from a distinct region of the neural plate.
The axons of the pioneer neurons form the initial necessary link between the olfactory placode of the PNS and the differentiating telencephalon of the CNS (Fig. 7,green). The pioneer axons grow to the area of the developing telencephalon that expresses emx1 (Fig. 7,stipple). The pattern of olfactory receptor message expression (Fig. 7, orange) correlates with the location of the sensory neurons and not the pioneer neurons. After the arrival of the first sensory neuron axons (Fig. 7,red) within the developing telencephalon, the pioneer neurons undergo apoptotic cell death (Fig. 7,purple).
Fig. 7.
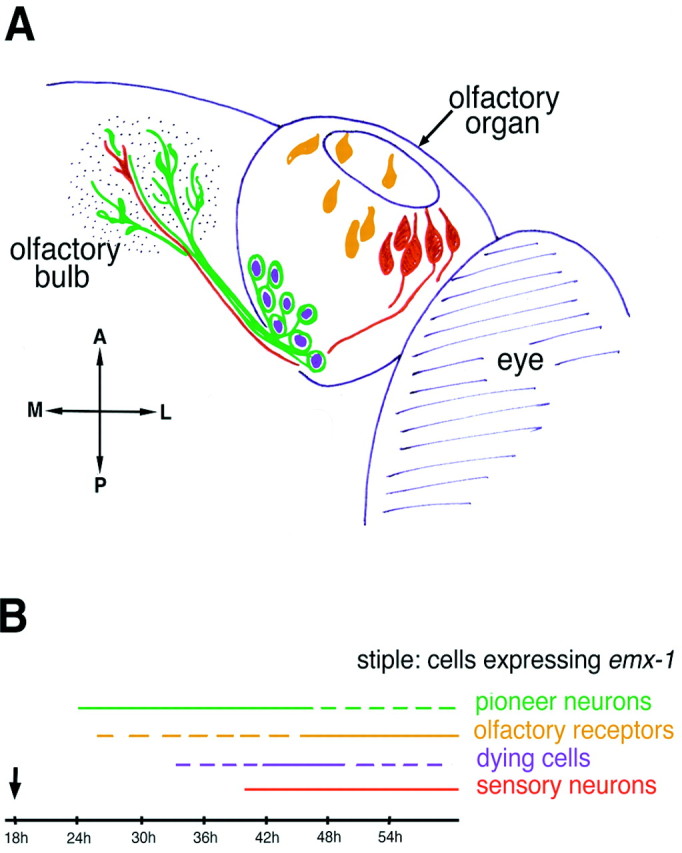
A transient population of pioneer neurons establishes the olfactory pathway. Summary diagram of the developing olfactory system from 24 to 54 h. A, The zns-2-positive pioneer neurons (green) are distinguished by their round dendriteless cell bodies. The stippled area indicates the region of emx1 gene expression in which the pioneer neurons contact the differentiating telencephalon. The sensory neurons (red) extend their axons into the CNS, following the axons of the pioneer neurons. The pioneer neurons become positive for TUNEL (purple), indicating that they die. The cells expressing olfactory receptor mRNAs (orange) are located near the apical surface in the same region that contains the olfactory sensory neurons. B, Time line showing the temporal sequence of developmental events. Thecolors correspond to the coding used inA; the arrow indicates when the olfactory placode becomes apparent with Nomarski optics in the live embryo. Thedashed lines indicate that not all preparations show labeling at the indicated time.
In the embryonic olfactory epithelium, mitotic figures initially appear at the apical rather than basal surface. As the animal develops, the epithelium becomes stratified, and the pattern of cell division shifts basally, with the daughter cells migrating apically as they extend dendrites and axons (Farbman, 1994). The pioneer neurons are located basally early in development, consistent with their being a nondividing population (Fig. 1B,C). Furthermore, it is unlikely that the pioneer neurons later become sensory neurons, because this would require that they sprout dendrites after their axons are in the CNS and then migrate apically, something we have never observed.
Our fate mapping studies support the idea that the pioneer neurons are a developmentally distinct population of neurons by two criteria; clones containing pioneers never contain sensory neurons, and the region of the neural plate giving rise to pioneers is distinct from the region giving rise to the first sensory neurons. The clones resulting from labeling single cells in the anterolateral neural plate, 6 hr before the appearance of the olfactory placode, contain only pioneer neurons. In contrast, cells lying more posteriorly at the edge of the neural plate give rise to the first olfactory sensory neurons (Fig. 4). Thus, our fate map shows that early in development, cells destined to give rise to olfactory pioneer neurons and sensory neurons are lineage-restricted and already occupy distinct positions at the edge of the neural plate. Therefore, these two classes of neurons may be derived from different types of precursor cells.
The first axons to exit the olfactory placode have been described previously by electron microscopic analysis at 20 h (Hansen and Zeiske, 1993), and neurons with axons emanating from the olfactory placode have been identified using horseradish peroxidase labeling at 24 h (Wilson et al., 1990). The axons of the pioneer neurons visualized with zns-2 exit the olfactory placode at the same time (Fig. 1B) as the axons described in previous studies (Wilson et al., 1990; Hansen and Zeiske, 1993), thus supporting their identification as the first axons. In rats, neurons termed pioneers have been described using a GAP-43 antibody (Gong and Shipley, 1995) that recognizes many axon types within the nervous system. In the absence of other information on the identity of these neurons, it thus remains unclear whether they correspond to the mammalian equivalents of the olfactory pioneer neurons described here for the zebrafish. Dynes and Ngai (1998) have recently shown the presence of “unipolar” neurons in the olfactory placode, which may correspond to the pioneers we describe.
When the axons of the zebrafish pioneer neurons leave the olfactory placode, they extend along the surface of the telencephalon and then defasciculate in the region of the presumptive olfactory bulb (Fig. 1). It is currently unknown how these initial axons project to the correct region of the telencephalon. Studies in other systems, such asXenopus (Stout and Graziadei, 1980), suggest that arrival of axons from the olfactory placode helps trigger olfactory bulb development. Our data show that the pioneer axons project specifically to the region of the telencephalon in which cells express theemx1 gene (Fig. 1D). Expression ofemx1 begins before the formation of the olfactory placode (Simeone et al., 1992; Morita et al., 1995) and arrival of the pioneer axons, suggesting that emx1 may help the pioneer axons recognize their target region. The emx1 protein has also been reported to be localized to the axons of olfactory sensory neurons (Briata et al., 1996). These observations are consistent with the hypothesis that borders of regulatory gene expression mediate axon guidance (MacDonald et al., 1994) and that emx1 may regulate expression of instructional cues recognized by the growth cones of the olfactory pioneer neuron axons.
The zebrafish olfactory pioneer neurons provide the initial link between the PNS and the CNS. We have shown that ablation of the pioneer neurons severely disrupts axonal guidance of the sensory neurons, suggesting that these pioneers provide essential guidance cues for pathfinding by later-developing sensory neuron axons (Fig. 5, Table 1). In addition, the pioneer neurons die after they have established the initial pathway to the developing olfactory bulb. Pioneer neurons play a similar required role in several other systems. Within the CNS of mammals, cortical subplate neurons pioneer the connection between the thalamus and the visual cortex, and their ablation results in abnormal axon projections (McConnell et al., 1989). The T1 pioneer neurons in the limb of grasshoppers first establish the path from the PNS to the CNS, and when they are removed, the following sensory neurons fail to reach the CNS (Klose and Bentley, 1989). Furthermore, the zebrafish olfactory pioneer neurons undergo programmed cell death (Fig. 3), like both the T1 (Kutsch and Bentley, 1987) and subplate pioneers (Ghosh et al., 1990).
The olfactory pioneer neurons we have described in zebrafish may correspond to an apparently specialized group of cells previously observed at the base of the developing olfactory placode in mammals. In rats, Lejour (1967) noted that the formation of the olfactory nerve was preceded by an aggregation of placodally derived neuroblast-like cells. This “blastema” appears between the olfactory placode and the telencephalon, and neural fibers of the nasal epithelium and terminal nerve converge on this blastema before contacting the developing forebrain. More recently, Schwanzel-Fukuda et al. (1992) reported a similar blastema in mice that expresses NCAM and precedes the appearance of the olfactory, vomeronasal, and terminal nerves. In zebrafish, the pioneer neurons provide a bridge through which later developing axons pass as they exit the developing placode and extend to the telencephalon. Thus, the pioneers may serve a guidance function analogous to the blastema of mammals in bridging the peripheral and CNS early in development.
The olfactory system of the adult zebrafish is organized similarly to that of other vertebrates (Byrd and Brunjes, 1995; Rupp et al., 1996), with an invariant pattern of glomeruli in the olfactory bulb (Baier and Korsching, 1994). Olfactory sensory axons may affect cell cycle kinetics in the telencephalon (Gong and Shipley, 1995), although how the specific pattern of glomeruli is specified is currently unknown. In insects, in which the formation of glomeruli is much better understood, they appear to arise from an interaction of the incoming sensory axons with the glia of the olfactory bulb (Oland et al., 1990; Tolbert and Sirianni, 1990). When the development of glia, but not the sensory neurons, is blocked, the glomeruli form less distinctly (Oland et al., 1988). Our results show that zebrafish olfactory pioneer neurons provide the first axons from the olfactory placode to the telencephalon and that these pioneer axons form a axonal scaffold in the developing olfactory bulb (Fig. 1). At the developmental times examined here, the number of axonal condensations (15 per bulb) is slightly greater than the number of pioneer neurons, indicating that a pioneer neuron may target axons to more than one axonal condensation. These axonal condensations may be the first olfactory glomeruli, but many more glomeruli must be added later to form the number (80 per bulb) observed in adults (Baier and Korsching, 1994). We currently do not know whether there is a continuous addition of glomeruli or perhaps a metamorphosis-like abrupt addition that leads to the adult glomerular number.
Our results reveal that an axonal scaffold is established by the pioneers and that these pioneers do not appear to express odorant receptors, suggesting that these receptors do not provide the main guidance cue that allows the sensory axons to reach the telencephalon. Likewise, in mice (Mombaerts et al., 1996; Wang et al., 1998), receptor expression does not appear to confer specific target identity on olfactory sensory axons, which supports the hypothesis that other cues, such as cell-surface glycoproteins, guide axons within the CNS (Schwarting et al., 1992a,b; Riddle et al., 1993; Bargman, 1996). However, receptors expressed in later-developing sensory neurons may contribute to regional segregation of afferent axons within the bulb.
Footnotes
This work was supported by grants from the Muscular Dystrophy Association (K.E.W.), National Institutes of Health/National Institute on Deafness and Other Communication Disorders (K.E.W.), National Institutes of Health (M.W.), and the W. M. Keck Foundation. We thank C. Bargman, J. Eisen, J. Ewer, C. Kimmel, and M. Schwanzel-Fukuda for helpful discussions.
Correspondence should be addressed to Kathleen Whitlock, Section of Genetics and Development, 445/449 Biotechnology Building, Cornell University, Ithaca, NY 14853-2703.
REFERENCES
- 1.Baier H, Korsching S. Olfactory glomeruli in the zebrafish form an invariant pattern and are identifiable across animals. J Neurosci. 1994;14:219–230. doi: 10.1523/JNEUROSCI.14-01-00219.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bargmann C. From the nose to the brain. Nature. 1996;384:512–513. doi: 10.1038/384512a0. [DOI] [PubMed] [Google Scholar]
- 3.Barth AL, Justice NJ, Ngai J. Asynchronous onset of odorant receptor expression in the developing zebrafish olfactory system. Neuron. 1996;16:23–34. doi: 10.1016/s0896-6273(00)80020-3. [DOI] [PubMed] [Google Scholar]
- 4.Briata P, Di Blas E, Gulisano M, Mallamaci A, Iannone R, Boncinelli E, Corte G. EMX1 homeoprotein is expressed in cell nuclei of the developing cerebral cortex and in the axons of the olfactory sensory neurons. Mech Dev. 1996;57:169–180. doi: 10.1016/0925-4773(96)00544-8. [DOI] [PubMed] [Google Scholar]
- 5.Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 6.Byrd CA, Brunjes PC. Organization of the olfactory system in the adult zebrafish: histological, immunohistochemical, and quantitative analysis. J Comp Neurol. 1995;358:247–259. doi: 10.1002/cne.903580207. [DOI] [PubMed] [Google Scholar]
- 7.Byrd CA, Jones JT, Quattro JM, Rogers ME, Brunjes PC, Vogt RG. Ontogeny of odorant receptor gene expression in zebrafish, Danio rerio. J Neurobiol. 1996;29:445–458. doi: 10.1002/(SICI)1097-4695(199604)29:4<445::AID-NEU3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Christensen TA, Hildebrand JG. Functions, organization and physiology of the olfactory pathways in the lepidopteran brain. Wiley; New York: 1987. [Google Scholar]
- 9.Crews L, Hunter D. Neurogenesis in the olfactory epithelium. Perspect Dev Neurobiol. 1994;2:151–161. [PubMed] [Google Scholar]
- 10.Dynes JL, Ngai J. Pathfinding of olfactory neuron axons to stereotyped glomerular targets revealed by dynamic imaging in living zebrafish embryos. Neuron. 1998;20:1081–1091. doi: 10.1016/s0896-6273(00)80490-0. [DOI] [PubMed] [Google Scholar]
- 11.Farbman AI. Cell biology of olfaction. Cambridge UP; Canada: 1992. [Google Scholar]
- 12.Farbman AI. Developmental biology of olfactory sensory neurons. Semin Cell Biol. 1994;5:3–10. doi: 10.1006/scel.1994.1002. [DOI] [PubMed] [Google Scholar]
- 13.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh A, Antonini A, McConnell SK, Shatz CJ. Requirement for subplate neurons in the formation of thalamocortical connections. Nature. 1990;347:179–181. doi: 10.1038/347179a0. [DOI] [PubMed] [Google Scholar]
- 15.Gong Q, Shipley MT. Evidence that pioneer olfactory axons regulate telencephalon cell cycle kinetics to induce the formation of the olfactory bulb. Neuron. 1995;14:91–101. doi: 10.1016/0896-6273(95)90243-0. [DOI] [PubMed] [Google Scholar]
- 16.Graziadei PP, Monti Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1979;8:1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- 17.Hansen A, Zeiske E. Development of the olfactory organ in the zebrafish, Brachydanio rerio. J Comp Neurol. 1993;333:289–300. doi: 10.1002/cne.903330213. [DOI] [PubMed] [Google Scholar]
- 18.Kimmel CB, Ballard WW, Kimmel SR, Ullman B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 19.Klose M, Bentley D. Transient pioneer neurons are essential for formation of an embryonic peripheral nerve. Science. 1989;245:982–984. doi: 10.1126/science.2772651. [DOI] [PubMed] [Google Scholar]
- 20.Kutsch W, Bentley D. Programmed death of peripheral pioneer neurons in the grasshopper embryo. Dev Biol. 1987;123:517–525. doi: 10.1016/0012-1606(87)90410-6. [DOI] [PubMed] [Google Scholar]
- 21.Lejour M. Activité de quatre enzymes déphosphorylants au cours de la morphogenèse du palais primaire chez le rat. Arch Biol. 1967;78:389–450. [PubMed] [Google Scholar]
- 22.Macdonald R, Xu Q, Barth KA, Mikkola I, Holder N, Fjose A, Krauss S, Wilson SW. Regulatory gene expression boundaries demarcate sites of neuronal differentiation in the embryonic forebrain. Neuron. 1994;13:1039–1053. doi: 10.1016/0896-6273(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 23.Menco BPM, Farbman AI. Genesis of cilia and microvilli of rat nasal epithelia during pre-natal development I. Olfactory epithelium, qualitative studies. J Cell Sci. 1985;78:283–310. doi: 10.1242/jcs.78.1.283. [DOI] [PubMed] [Google Scholar]
- 24.McConnell SK, Ghosh A, Shatz CJ. Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science. 1989;245:978–982. doi: 10.1126/science.2475909. [DOI] [PubMed] [Google Scholar]
- 25.Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- 26.Morita T, Nitta H, Kiyama Y, Mori H, Mishina M. Differential expression of two zebrafish emx homeoprotein mRNAs in the developing brain. Neurosci Lett. 1995;198:131–134. doi: 10.1016/0304-3940(95)11988-9. [DOI] [PubMed] [Google Scholar]
- 27.Oland LA, Tolbert LP, Mossman KL. Radiation-induced destruction of the glial population during development disrupts the formation of olfactory glomeruli in an insect. J Neurosci. 1988;8:353–367. doi: 10.1523/JNEUROSCI.08-01-00353.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oland LA, Orr G, Tolbert LP. Construction of protoglomerular template by olfactory axons initiates the formation of olfactory glomeruli in the insect brain. J Neurosci. 1990;10:2096–2112. doi: 10.1523/JNEUROSCI.10-07-02096.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ressler KJ, Sullivan SL, Buck LB. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell. 1993;73:597–609. doi: 10.1016/0092-8674(93)90145-g. [DOI] [PubMed] [Google Scholar]
- 30.Riddle DR, Oakley B. Evaluation of projection patterns in the primary olfactory system of rainbow trout. J Neurosci. 1991;11:3752–3762. doi: 10.1523/JNEUROSCI.11-12-03752.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riddle DR, Wong LD, Oakley B. Lectin identification of olfactory receptor neuron subclasses with segregated central projections. J Neurosci. 1993;13:3018–3033. doi: 10.1523/JNEUROSCI.13-07-03018.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rupp B, Wullimann MF, Reichert H. The zebrafish brain: a neuroanatomical comparison with the goldfish. Anat Embryol. 1996;194:187–203. doi: 10.1007/BF00195012. [DOI] [PubMed] [Google Scholar]
- 33.Schwanzel-Fukuda M, Abraham S, Crossin KL, Edelman GM, Pfaff DW. Immunocytochemical demonstration of neural cell adhesion molecule (NCAM) along the migration route of luteinizing hormone-releasing hormone (LHRH) neurons in mice. J Comp Neurol. 1992;321:1–18. doi: 10.1002/cne.903210102. [DOI] [PubMed] [Google Scholar]
- 34.Schwarting GA, Deutsch G, Gattey DM, Crandall JE. Glycoconjugates are stage- and position-specific cell surface molecules in the developing olfactory system. I. The CC1 immunoreactive glycolipid defines a rostrocaudal gradient in the rat vomeronasal system. J Neurobiol. 1992a;23:120–129. doi: 10.1002/neu.480230203. [DOI] [PubMed] [Google Scholar]
- 35.Schwarting GA, Deutsch G, Gattey DM, Crandall JE. Glycoconjugates are stage- and position-specific cell surface molecules in the developing olfactory system. II. Unique carbohydrate antigens are topographic markers for selective projection patterns of olfactory axons. J Neurobiol. 1992b;23:130–142. doi: 10.1002/neu.480230204. [DOI] [PubMed] [Google Scholar]
- 36.Shepherd GM. Synaptic organization of the mammalian olfactory bulb. Physiol Rev. 1972;52:864–917. doi: 10.1152/physrev.1972.52.4.864. [DOI] [PubMed] [Google Scholar]
- 37.Simeone A, Gulisano M, Acampora D, Stornaiuolo A, Rambaldi M, Boncinelli Two vertebrate homeobox genes related to the Drosophila empty spiracles gene are expressed in the embryonic cerebral cortex. EMBO J. 1992;11:2541–2550. doi: 10.1002/j.1460-2075.1992.tb05319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singer MS, Shepherd GM, Greer CA. Olfactory receptors guide axons. Nature. 1995;377:19–20. doi: 10.1038/377019b0. [DOI] [PubMed] [Google Scholar]
- 39.Stainier DYR, Gilbert W. Pioneer neurons in the mouse trigeminal sensory system. Proc Natl Acad Sci USA. 1990;87:923–927. doi: 10.1073/pnas.87.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stout RP, Graziadei PPC. Influence of the olfactory placode on the development of the brain in Xenopus laevis (Daudin). I. Axonal growth and connections of the transplanted placode. Neuroscience. 1980;5:2175–2186. doi: 10.1016/0306-4522(80)90134-7. [DOI] [PubMed] [Google Scholar]
- 41.Thisse C, Thisse B, Schilling TT, Postlethwait JH. Structure of the zebrafish snail 1 gene and its expression in wild-type, spadetail, and no tail. Development. 1993;119:1203–1215. doi: 10.1242/dev.119.4.1203. [DOI] [PubMed] [Google Scholar]
- 42.Tolbert LP, Sirianni PA. Requirement for olfactory axons in the induction and stabilization of olfactory glomeruli in an insect. J Comp Neurol. 1990;298:69–82. doi: 10.1002/cne.902980106. [DOI] [PubMed] [Google Scholar]
- 43.Trevarrow B, Marks DL, Kimmel CB. Organization of hindbrain segments in the zebrafish embryo. Neuron. 1990;4:669–679. doi: 10.1016/0896-6273(90)90194-k. [DOI] [PubMed] [Google Scholar]
- 44.Vassar R, Chao SK, Sitcheran R, Nunez JM, Vosshall LB, Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79:981–991. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 45.Wang F, Nemes A, Mendelsohn M, Axel R. Odorant receptors govern the formation of a precise topographical map. Cell. 1998;93:47–60. doi: 10.1016/s0092-8674(00)81145-9. [DOI] [PubMed] [Google Scholar]
- 46.Westerfield M. The zebrafish book. University of Oregon; Eugene, Oregon: 1995. [Google Scholar]
- 47.Whitlock KE, Westerfield M. Development of olfactory neurons and GnRH containing neuroendocrine cells in the zebrafish olfactory organ. Soc Neurosci Abstr. 1995;21:1999. [Google Scholar]
- 48.Whitlock KE, Westerfield M. A transient class of neurons pioneer the primary olfactory in the developing zebrafish Danio rerio. Chem Senses. 1997;22:822. [Google Scholar]
- 49.Wilson SW, Ross LA, Parrett T, Easter SS. The development of a simple scaffold of axon tracts in the brain of the embryonic zebrafish, Brachydanio rerio. Development. 1990;108:121–145. doi: 10.1242/dev.108.1.121. [DOI] [PubMed] [Google Scholar]
- 50.Zhao H, Ivic L, Otaki JM, Hashimoto M, Mikoshiba K, Firestein S. Functional expression of a mammalian odorant receptor. Science. 1998;279:237–242. doi: 10.1126/science.279.5348.237. [DOI] [PubMed] [Google Scholar]