Abstract
Mushroom bodies (MBs) are symmetrically paired neuropils in the insect brain that are of critical importance for associative olfactory learning and memory. In Drosophila melanogaster, the MB intrinsic neurons (Kenyon cells) undergo extensive reorganization at the onset of metamorphosis. A phase of rapid axonal degeneration without cell death is followed by axonal regeneration. This re-elaboration occurs as levels of the steroid hormone 20-hydroxyecdysone (20E) are rising during the pupal stage. Based on the known role of 20E in directing many features of CNS remodeling during insect metamorphosis, we hypothesized that the outgrowth of MB axonal processes is promoted by 20E. Using a GAL4 enhancer trap line (201Y) that drives MB-restricted reporter gene expression, we identified Kenyon cells in primary cultures dissociated from early pupal CNS. Paired cultures derived from single brains isolated before the 20E pupal peak were incubated in medium with or without 20E for 2–4 d. Morphometric analysis demonstrated that MB neurons exposed to 20E had significantly greater total neurite length and branch number compared with that of MB neurons grown without hormone. The relationship between branch number and total neurite length remained constant regardless of hormone treatment in vitro, suggesting that 20E enhances the rate of outgrowth from pupal MB neurons in a proportionate manner and does not selectively increase neuritic branching. These results implicate 20E in enhancing axonal outgrowth of Kenyon cells to support MB remodeling during metamorphosis.
Keywords: steroid hormone, ecdysteroids, metamorphosis, Drosophila melanogaster, polarity, mushroom body, Kenyon cells, neuronal remodeling, neurite outgrowth, cell culture
Steroid hormones play critical roles in orchestrating functional and structural changes of neurons and neuronal circuits throughout development in both vertebrates and invertebrates (for review, see Arnold and Gorski, 1984; Levine et al., 1995). Steroids regulate dendritic and axonal morphology (Kurz et al., 1986; Levine, 1989; VanderHorst and Holstege, 1997), dendritic regression and regrowth (Weeks, 1987; Truman and Reiss, 1988; Schubiger et al., 1998), synaptic density (Gould et al., 1990; Woolley and McEwen, 1992), and cell death (Truman and Schwartz, 1984; Robinow et al., 1993). Many of these hormonal effects have been reproduced on dissociated neurons in vitro (e.g., Levine and Weeks, 1996;Murphy and Segal, 1996). Furthermore, responses to hormone depend on the type and developmental stage of the cells before isolation (Prugh et al., 1992; Streichert et al., 1997).
During insect metamorphosis, the nervous system undergoes extensive reorganization, many features of which are controlled by the ecdysteroid molting hormone 20-hydroxyecdysone (20E) (Levine et al., 1991; Truman, 1996). In the fruit fly Drosophila melanogaster, receptors for this steroid hormone are present in multiple isoforms with complex, dynamic expression patterns in the developing CNS (Truman et al., 1994). 20E activates a genetic regulatory hierarchy, mediated by several hormone-inducible transcription factors (Thummel, 1996), at least one of which is required for metamorphic reorganization of the CNS (Restifo and White, 1991). Downstream targets of some of these transcription factors are known for non-neural tissues (Bayer et al., 1996), and those that shape the responses of the nervous system to 20E are presently being identified (Liu and Restifo, 1998).
We have combined the primary cell culture approach with molecular genetic strategies available in Drosophila to study hormone action underlying nervous system reorganization during metamorphosis. Our initial focus has been on Kenyon cells, the intrinsic neurons of the mushroom bodies (MBs), because they represent the single largest neuronal population whose metamorphic transition in vivo has been described in Drosophila (Technau and Heisenberg, 1982). The MBs command considerable interest because they are essential for higher order sensory integration as well as learning and memory (de Belle, 1995; Davis and Han, 1996).
Unique anatomical features of the MBs have allowed direct examination of neuronal numbers and morphological integrity. Kenyon cell axons project together in a dense parallel array called the peduncle that splits into three lobes (Power, 1943; Yang et al., 1995). Overall MB morphology remains stable throughout metamorphosis. However, the number of axons, counted in cross-sections through the peduncle, plummets dramatically at the onset of metamorphosis, without loss of cell bodies, and then increases as pupal development proceeds (Technau and Heisenberg, 1982). These ultrastructural data indicate that many Kenyon cell axons undergo retraction, followed by regeneration. Because the regrowth occurs as 20E levels are rising (Richards, 1981) and because the MBs express ecdysteroid receptors at this time (Truman et al., 1994), we hypothesized that 20E promotes the regeneration of pupal Kenyon cell axons. In this report, we demonstrate that pupal MB neurons can be identified in dissociated cell culture and that they respond to 20E when grown in vitro.
Parts of this paper have been published previously in abstract form (Kraft et al., 1997).
MATERIALS AND METHODS
Drosophila stocks. Flies were reared at 25°C on standard corn flour–yeast–agar medium (Elgin and Miller, 1978). The P[GAL4] lines 30Y, 72Y, 201Y, c35, c739 (Yang et al., 1995), and c747 (Connolly et al., 1996) were tested for MB-specific GAL4 expression. The reporter line UASG-lacZ (Brand and Perrimon, 1993) exhibits no detectable β-galactosidase (βgal) expression in the CNS (R. Kraft, unpublished observations). GAL4 activity was detected in the progeny of single-pair matings between homozygous P[GAL4] flies and homozygous UASG-lacZ flies using antibodies against βgal, which is distributed throughout the cytoplasm.
Preparation of dissociated neuronal cell cultures. The techniques used to prepare and maintain cultures of dissociatedDrosophila CNS tissue were adapted from those developed forDrosophila larval neurons (Wu et al., 1983) andManduca pupal motor neurons (Prugh et al., 1992). Progeny from 201Y × UASG-lacZ matings were collected as prepupae, sexed, and monitored in a humid 60 mm covered glass Petri dish to determine the time of head eversion, signifying the start of the pupal phase of metamorphosis (Bainbridge and Bownes, 1981). Five hours after head eversion, at a time estimated to be just before the onset of the pupal peak of 20E (Bainbridge and Bownes, 1988), the pupae were surface-sterilized for 1 min in 70% ethanol and rinsed three times in sterile deionized water. The CNS was removed from pupae using watchmaker forceps and immersed in culture medium without antibiotics (modified from O’Dowd, 1995): 1× Schneider’s Insect Medium (Life Technologies, Gaithersburg, MD) with 10% fetal bovine serum (Hyclone, Logan, UT) and 50 μg/ml insulin (Sigma, St. Louis, MO). Brain tissue encompassing the MBs was regionally dissected away from the thoracoabdominal ganglion and optic lobes using minutien pins (Fine Science Tools, Foster City, CA). The isolated tissue was incubated for 1 hr at room temperature in freshly prepared enzyme solution of 0.1 mg/ml collagenase (Worthington, Freehold, NJ) and 0.4 mg/ml dispase (Boehringer Mannheim, Indianapolis, IN) in Rinaldini’s saline: 137 mm NaCl, 2.68 mm KCl, 0.36 mm NaH2PO4, 11.9 mm NaHCO3, and 5.55 mmglucose (Wu et al., 1983).
The tissue was washed by transferring it to 1 ml of culture medium in a 1.5 ml microfuge tube and centrifuging for 1 min at 1000 rpm. Medium (900 μl) was removed and replaced with fresh culture medium, the tissue was again centrifuged for 1 min at 1000 rpm, and 800 μl of medium was removed. To dissociate the cells, we triturated the tissue in the remaining 200 μl of medium by flushing 40 times through a fire-polished, prewetted glass pipette and an additional 40 times through a prewetted 200 μl sterile disposable micropipette tip. The suspension was dispensed in 100 μl aliquots into wells made by cutting an 8 mm hole in a plastic 35 mm culture dish (Corning, Corning, NY) and attaching a gridded glass coverslip (Bellco, Vineland, NJ) to the bottom with Sylgard (Dow Corning, Midland, MI). Before the addition of cells, the dish had been UV-sterilized, and the glass had been coated by exposure to a solution of 167 μg/ml Concanavalin A (Sigma) and 1.67 μg/ml mouse laminin (Collaborative Research, Bedford, MA) for 2 hr at 37°C.
For each hormone treatment experiment, six cultures were prepared from a single brain. Approximately 30 μl of cell suspension was added to enough culture medium in each well to bring the volume to 100 μl. The cells were allowed to settle undisturbed for 2 hr at 25°C. Each dish was flooded with 900 μl of culture medium and sealed with Parafilm (American National Can, Greenwich, CT). Cultures exposed to hormone received 900 μl of medium containing 1 μg/ml 20E (2.1 × 10−6m; Sigma). The concentration of a 20E stock solution prepared in deionized water was determined spectrophotometrically (Rees and Isaac, 1985). No 20E was detected by ELISA in the fetal bovine serum used in the culture medium (R. B. Levine, unpublished observations). To allow an objective analysis, the coding of paired cultures (one dish with and one dish without 20E) was performed by a lab member who was not involved in the subsequent morphometric analyses that were done blind. The culture pairs were allowed to grow for 2 d (48 hr after plating), 3 d (72 hr after plating), or 4 d (96 hr after plating) in a 25°C incubator.
Immunohistochemistry of whole-mount preparations and cell cultures. Larval and pupal CNS were dissected in cold PBS and fixed for 2–4 hr on ice in 4% formaldehyde (Ted Pella, Redding, CA) in 100 mm PIPES, pH 6.6, 1 mm EGTA, 2 mm MgSO4, and 1% Triton X-100 (modified from Sandstrom et al., 1997). After a 10 min wash in PBS, tissue was washed three times for 10 min each in blocking buffer (BB): 50 mm Tris-HCl, pH 7.0, 150 mm NaCl, 0.25% Triton X-100, 0.25% casein (occasionally omitted), 0.02% Na azide, 0.25% bovine serum albumin, and 2% normal goat serum. All washes were performed at room temperature. Tissue was incubated with primary antibodies in BB for 12–18 hr at 4°C and then washed six times for 10 min each in BB. Tissue was incubated with secondary antibodies in BB at room temperature in the dark for 3 hr and then washed six times for 10 min each in BB in darkness. Tissue was briefly rinsed in 0.1m Tris-HCl, pH 8.0, and then mounted in 13.3% polyvinyl alcohol (PVA) with 1.7% DABCO (1,4-diazabicyclo[2,2,2]octane; Sigma) to minimize photobleaching (Banker and Goslin, 1991). Primary antibodies used were either a monoclonal mouse anti-βgal (Promega, Madison, WI) at 1:2000 or a polyclonal rabbit anti-βgal (Cappel, Durham, NC) at 1:5000, first preabsorbed for 16 hr at 4°C by mixing with fixed third instar larval tissue. Secondary antisera used were either Cy3-conjugated goat anti-mouse at 1:500 or Cy3-conjugated goat anti-rabbit at 1:250 (Jackson ImmunoResearch, West Grove, PA). Confocal microscopy and image preparation were performed as described by Liu and Restifo (1998).
Immunohistochemical staining of cells in culture to identify βgal-expressing cells and to simultaneously visualize neuronal membranes used a method modified from that of Vallés and White (1986). This was achieved by double-labeling with anti-βgal in combination with anti-horseradish peroxidase (HRP) (Jan and Jan, 1982) that recognizes Drosophila neuron-specific glycoproteins (Wang et al., 1994; Sun and Salvaterra, 1995). The fixation, washes, and antibody incubations were performed with swirling on a platform rotator. Culture dishes were rinsed twice with 1 ml of cold Ikeda Ringer’s saline (130 mm NaCl, 4.7 mm KCl, 1.8 mm MgCl2, 0.74 mmKH2PO4, and 0.35 mmNa2HPO4) and were fixed for 30 min at 4°C in 4% paraformaldehyde (J. T. Baker Chemical Company, Phillipsburg, NJ) in 0.1 m sodium phosphate buffer, pH 7.2. The dishes were washed three times for 20 min each in 1 ml of PTN (0.1m sodium phosphate buffer, pH 7.2, 0.1% Triton X-100, and 0.1% sodium azide) and were then incubated with primary antibodies in PTN for 12–16 hr, all performed at 4°C. The preabsorbed polyclonal rabbit anti-βgal antiserum was used at 1:5000, and a polyclonal goat anti-HRP antiserum (Sigma) was used at 1:500. Alternatively, the monoclonal mouse anti-βgal antibody was used alone at 1:4000. Dishes were rinsed with PTN and then washed five times for 20 min each in 1 ml of PTN at room temperature. The dishes were incubated with secondary antibodies in PTN at 4°C in the dark for 3 hr, followed by a rinse and five 20 min washes in PTN at room temperature in the dark. A Cy2-conjugated donkey anti-rabbit antiserum (Jackson ImmunoResearch) was used at 1:500, and a Lissamine Rhodamine (LRSC)-conjugated donkey anti-goat antiserum (Jackson ImmunoResearch) was used at 1:250. When the monoclonal mouse anti-βgal antibody was used, Cy3-conjugated donkey anti-mouse antiserum (Jackson ImmunoResearch) was used at 1:500 along with a fluorescein isothiocyanate-conjugated goat anti-HRP antiserum (Cappel) at 1:400. After a rinse with 0.1 mTris-HCl, pH 8.0, 250 μl of the PVA plus DABCO mountant was added, and the culture wells were covered with a glass coverslip.
To detect ecdysone receptors and βgal in cultured neurons, a protocol modified from that of Robinow et al. (1993) was followed. Cultures were briefly rinsed in cold PBS 2 hr after plating and then fixed for 20 min at 4°C with 4% paraformaldehyde in PBS. Dishes were washed three times for 10 min each in PBT (PBS plus 0.3% Triton X-100), followed by a 30 min wash in PBT+NGS (PBT with 10% normal goat serum). All washes were performed at room temperature. Cultures were incubated with primary antibodies in PBT+NGS for 12 hr at 4°C. The preabsorbed polyclonal rabbit anti-βgal antiserum was used at 1:10000, and the mouse monoclonal antibody AD4.4 (generously provided by P. Hurban, Stanford University) against the EcR-B1 ecdysone receptor isoform (Talbot et al., 1993) was used at 1:10. The dishes were washed three times for 10 min each in PBT and once for 30 min in PBT+NGS. The cultures were incubated for 2 hr at room temperature in the dark with secondary antibodies in PBT+NGS. The secondary antisera used were Cy3-conjugated goat anti-rabbit at 1:667 and Cy2-conjugated donkey anti-mouse (Amersham, Arlington Heights, IL) at 1:667. The dishes were then washed two times for 10 min each in PBT and two times for 10 min each in PBS in the dark. They were rinsed in 0.1 mTris-HCl, pH 8.0, and mounted in PVA plus DABCO as described previously. This protocol was also used to simultaneously detect βgal and EcR-B1 in CNS whole-mount preparations, with the exception that the secondary antisera used were Cy2-conjugated donkey anti-rabbit at 1:1000 and Cy3-conjugated goat anti-mouse at 1:1000.
Data acquisition and analysis. Cultures double-labeled for the indirect immunofluorescent detection of βgal and neuronal membranes were observed under epifluorescence illumination with a Nikon Diaphot 300 inverted microscope (Nikon, Melville, NY) using 100× (for 2 d cultures) and 60× (for 3 and 4 d cultures) oil-immersion objectives (numerical aperture, 1.25 and 1.40, respectively). The Cy2 signal was detected with bandpass filter set 41001 from Chroma Technology (Brattleboro, VT). A G-2A longpass filter set (Nikon) was used to visualize the LRSC signal. The anti-βgal and anti-HRP (αHRP) images were photographed with Kodak TMAX 400 Professional Film (Rochester, NY). Black and white photomicrographs (predominantly 8 inches × 10 inches) of cells from each pair of culture dishes were printed by the same individual to ensure consistency.
For experiments 1 and 2, three pairs of dishes, each with a different culture time, were examined. The hormone status of each dish (with or without 20E) was unknown to the individual performing the search. Cultures were systematically screened, starting at the left margin of the well and proceeding to the right in an up-and-down sweep following the alphanumeric-labeled grid. All βgal-positive neurons were noted, but only those for which all neurites appeared amenable to analysis were photographed. Cells were excluded when a branch could not be unambiguously followed because of the overlap of neurites from that cell or from neighboring cells. This was observed more frequently in older cultures in which neuritic outgrowth was most extensive and in rare instances of cell clumping. Each culture was surveyed until ∼50 βgal-positive neurons had been photographed. For experiments 1 and 2, an average of 55 and 86%, respectively, of the surface area of each well was examined. Overall, ∼82% of all cells photographed could subsequently be analyzed, representing ∼55% of all βgal-positive neurons encountered during the survey.
Measurements of total neurite length and branch number per neuron were performed by attaching photomicrographs of each cell to a digitizing tablet (Numonics Model 2210, Lansdale, PA) and tracing the neurites visualized by αHRP staining with a handheld cursor. The data were collected using SigmaScan measurement software (SPSS, Chicago, IL). The individual doing the measurements did not know whether a cell had been grown with or without 20E. A branch was defined as a segment from the cell body or a branch point to a terminus and not as a segment between branch points. The total number of branches by this definition is equivalent to the “degree” or the number of terminal segments for a cell and is related to the number of branch points in that cell (Verwer and van Pelt, 1986; Uylings et al., 1989). Because no assumptions about segment order were made, this definition eliminated any bias that could be introduced by subjective decisions concerning which neurite to continue to follow at a branch point. The total neurite length and branch number for a cell were thus unaffected by the tracing paths chosen.
The length measurement of a branch was initiated at the margin of the cell body or neurite from which it emanated. Very short neurites (less than ∼8 μm) could not be measured reliably because of limitations in the precision of cursor placement at the ends of a measured segment. Furthermore, on Manduca motor neurons in vitrosuch short neurites are transient, actin-based filopodia that rarely contain microtubules ( that stabilize neuritic branches (Matheson and Levine, Ahmad et al., 1993; Smith, 1994). Therefore, those neurites with a measured length <7.8 μm were not included in the length and branch number totals for a cell but were tallied separately. Adoption of this 7.8 μm inclusion threshold minimized within-observer and between-observer measurement discrepancies. In a pilot study in which 46 cells were measured independently by two individuals using this criterion, an average difference in total neurite length of 4% and in branch number of 9% per cell was found. Nonetheless, all measurements within each experiment were performed by the same individual to maximize consistency. A statistical analysis found no consistent significant differences in the number of these short processes between cells grown with or without 20E across experiments, supporting the assumption that they are highly variable.
All cells measured were included in the statistical tests, which were performed using SigmaStat software (SPSS). The nonparametric Mann–Whitney rank sum test was used to assess the effect of hormone treatment on neurite length, branch number, and the relationship between branch number and total neurite length at each time point. The Pearson product moment correlation test was used to determine the correlation between branch number and total neurite length. All statistical analyses were performed at the default α level of 0.05. In addition, branch number was plotted versus total neurite length for each cell, and a least-squares method was used to fit a straight line to the data with SigmaPlot (SPSS).
RESULTS
201Y shows MB-selective expression in the brain during metamorphosis
Drosophila MBs provide an ideal model for testing the role of 20E in metamorphic neuronal reorganization. Two large, densely packed, bilaterally symmetric clusters of MB neurons are located in the dorsal brain and can be identified using reagents specific for several molecular markers (Yang et al., 1995; Schulz et al., 1996). To test the response of pupal MB neurons to 20E in vitro, a reliable, specific marker was required to distinguish them from other neurons in primary dissociated cultures. We screened a number of P[GAL4] enhancer trap lines (Yang et al., 1995) for which MB-specific reporter gene expression in the adult brain had been documented. βGal expression was examined in the late larval CNS of progeny of crosses between the reporter UASG-lacZ and each of six P[GAL4] lines (30Y, 72Y, 201Y, c35, c739, and c747). At this time, the MBs contain ∼2200 Kenyon cells per side (Technau and Heisenberg, 1982; Technau, 1984). In all lines except 201Y, extensive reporter gene expression was seen outside the MB (data not shown). In the brain of 201Y larvae, strong expression of βgal within the MBs was seen (Fig.1A). The only non-MB cells marked by βgal were a small cluster of large neurosecretory cells of the pars intercerebralis, which appear to project to the dorsal vessel, and four unidentified cells lateral to the MB in each brain hemisphere (Fig. 1A).
Fig. 1.
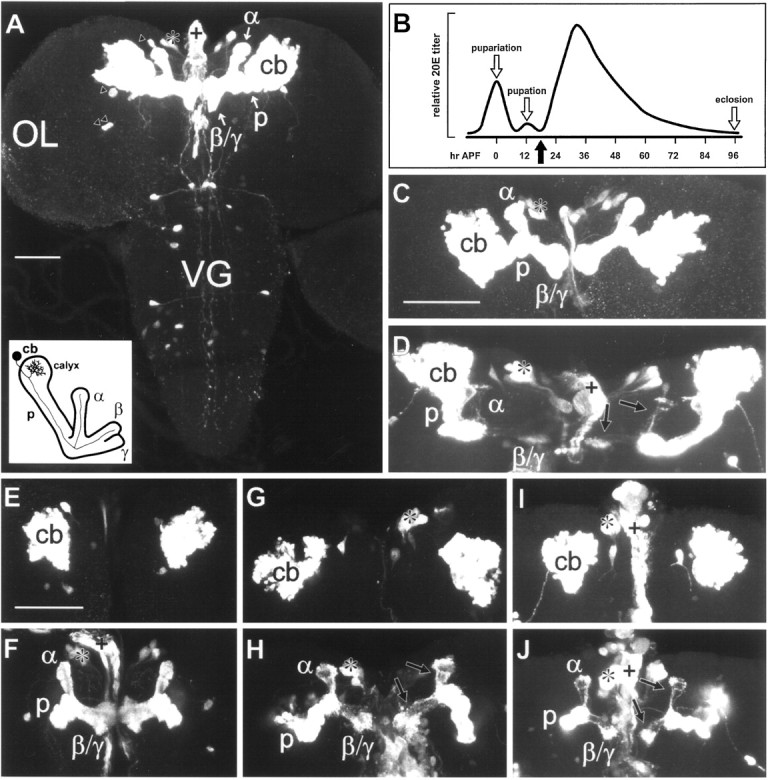
201Y-directed reporter gene expression is restricted predominantly to the MBs during the larval–pupal transition. Confocal images of CNS whole mounts from 201Y × UASG-lacZ larvae, prepupae, and early pupae that were immunostained to detect βgal expression are shown.A, A third instar larval CNS displaying prominent staining within the symmetrically paired MBs, from the cell bodies (cb), through the peduncle (p), and into the dorsally directed α lobe and the medially directed β and γ lobes (α, β, and γ, respectively). Anterior is toward thetop of the figure. Staining is also seen in a few large neurosecretory cells of the pars intercerebralis (*), located medially and anterior to the MBs, and in four additional neurons within each brain hemisphere (triangles). The pars intercerebralis neurons appear to send processes to the dorsal vessel, stained fragments of which are seen along the midline (+). No βgal expression is detected in the optic lobes (OL). Additional neurons are stained in the ventral ganglion (VG), but this region was excluded by dissection when establishing dissociated pupal cultures. Diagram, lower left corner, An oblique anterior view of an MB (adapted from Technau and Heisenberg, 1982; Yang et al., 1995). A single intrinsic neuron is depicted, showing the axonal projection into the lobes and the dendritic arbor within the calyx. B, Relative 20E titer during the larval-to-adult transition in Drosophila (adapted fromRiddiford, 1993; Truman et al., 1994). A prominent ecdysteroid peak is detected at pupariation, marking the onset of metamorphosis. A small peak has been reported at pupation, ∼12 hr (when reared at 25°C) after puparium formation (APF), approximately coinciding with head eversion of the pupa. This is followed by a broad pupal peak of 20E. The adult emerges at eclosion, ∼100 hrAPF. The dissociated cultures used in the experiments were prepared from brain tissue isolated 5–6 hr after head eversion (black arrow). C, Pupariation (seeB), showing a projection of optical sections through the entire CNS. The distribution and intensity of staining are comparable with that seen in the MB of the third larval instar brain.D, An early pupal brain 5 hr after head eversion (seeB, black arrow), showing a projection of optical sections through the entire tissue. Note that staining within the lobes is substantially diminished (arrows).E–J, Pairs of confocal images, depicting projections of optical sections through the cell body regions (E, G,I) and the MB neuropils (F,H, J) for each of three whole-mount preparations representing a developmental series.E, F, Pupariation (see B,C). Note the stained cap and unstained collar of the α lobe and the unstained central cores of the lobes, similar to the pattern in the third instar larva (see A).G, H, Head eversion (pupation), ∼12 hrAPF (see B). Relative to that at pupariation, staining within the lobes is reduced (H,arrows), but cell body staining (G) is similar. I,J, Head eversion plus 5 hr, ∼17 hr APF(see B, D). Staining within the lobes (J, arrows) has diminished further, but cell body staining (I) remains comparable with that seen at pupariation (see E) and pupation (seeG). Scale bars: A, 50 μm;C, D, 50 μm;E–J, 50 μm.
Reporter gene expression throughout the MBs of 201Y animals is extensive but is restricted to subsets of Kenyon cells. In addition, the pattern of βgal staining in the peduncle and lobes appears somewhat different in larvae and adults. In the adult, expression is prominent in central elements of the α and β lobes, as well as throughout the γ lobe (Yang et al., 1995; Connolly et al., 1996). In contrast, the larval MBs reveal expression in outer elements of the lobes surrounding unstained central cores, and the α lobe terminates in a cap demarcated by an unlabeled collar (Fig. 1A; also see Fig. 1F) (Tettamanti et al., 1997). Because of these stage-specific differences and the observation that P[GAL4] lines exhibit dynamic expression patterns during development (Tettamanti et al., 1997), we needed to verify that 201Y-directed reporter gene expression remains MB-selective during the larval-to-pupal transition.
As shown in Figure 1, βgal expression remained highly restricted to the MBs in the brains of 201Y × UASG-lacZprepupae and young pupae, with the exception of the few non-MB cells observed previously in the larva. The extent of 201Y-directed βgal staining in the peduncle and lobes of the MB appeared similar in the third instar larva and the early prepupa (Fig.1A,C,F) but was reduced considerably within the lobes by pupation (Fig.1H) and declined even more 5 hr later (Fig.1D,J). The reduction of labeling intensity in the neuropil is not accompanied by reduced labeling in the cell body region (Fig.1E,G,I). This is consistent with the reduction in axon numbers without concomitant cell death observed by Technau and Heisenberg (1982) early during metamorphosis.
201Y provides a marker for Kenyon cells in dissociated cell cultures
The 201Y CNS whole-mount staining patterns led to the expectation that reporter gene expression would distinguish a discrete population of neurons in heterogeneous primary cultures of which the vast majority would be Kenyon cells. Dissociated cell preparations of CNS tissue from larval and pupal progeny of 201Y × UASG-lacZ matings were cultured for periods of 2 hr to 6 d in medium without 20E (see Materials and Methods). They were then fixed and stained to detect neuronal membranes and βgal distribution. Cells observed 2 hr after plating consisted primarily of a cell body and rarely had processes, indicating that most if not all neurites were lost during tissue dissociation. βGal expression was detected in a subset of cells at this time (see below).
Cells grown for 4 d in culture typically had extensive neuritic trees as visualized by αHRP staining (Fig.2A). The neuronal identity of all cells in culture at this time was confirmed by immunohistochemical staining for ELAV, a neuron-specific nuclear marker (Robinow and White, 1991) (data not shown). Cells expressing βgal could easily be identified within the field of neurons (Fig.2B). Many neurons were not βgal-positive, and their representation reflected the size of the CNS region included in the dissociation (data not shown). As expected, βgal-positive neurons made up the highest fraction of cells when the source material was restricted to the brain hemispheres encompassing the MBs (Fig. 2). Because the adult fly brain has ∼5000 Kenyon cells (Technau and Heisenberg, 1982; Technau, 1984), we estimate that the dissociation and plating protocol yielded a minimum 15–20% recovery of MB neurons. Because GAL4 expression is limited to a subset of Kenyon cells in 201Y animals (Yang et al., 1995) and because pupae have fewer Kenyon cells than adults have (Technau and Heisenberg, 1982), the actual recovery is probably higher.
Fig. 2.
βGal expression is restricted to a subset of neurons in primary cultures derived from 201Y × UASG-lacZ CNS tissue. A, αHRP labeling of neuronal membranes in a fixed 4-d-old culture prepared from a pupal brain isolated 5 hr after head eversion (see Fig.1B) is shown. Four cell bodies and the complete neuritic arbors from three of those neurons are visible.B, βGal is detected in the cell body and neuritic branches of only one of the four neurons shown in A, plus in a branch extending from a neuron outside the field. Scale bar, 20 μm.
Pupal MB neurons express ecdysone receptorsin vitro
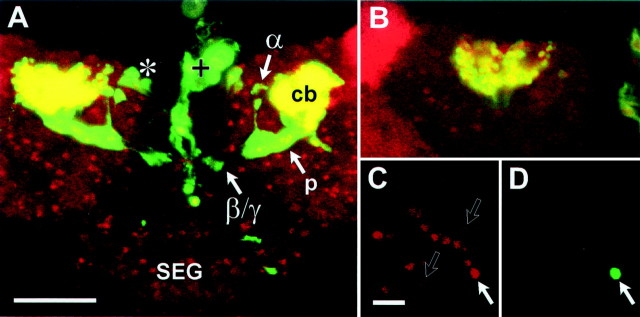
For MB neurons in culture to exhibit a cell-autonomous response to 20E, they must express ecdysone receptors. The concentrations of the various Drosophila ecdysone receptor (EcR) isoforms within the MB neurons during metamorphosis fluctuate in a characteristic pattern (Truman et al., 1994). The EcR-B1 isoform is maximally expressed in the Kenyon cells at pupariation and then decreases but remains detectable for 40 hr after puparium formation. Examination of EcR-B1 expression in brain whole mounts from 201Y × UASG-lacZ animals 5 hr after pupation reveals that MB neuronal cell bodies marked by βgal expression are positive for EcR-B1 expression (Fig.3A,B). To determine whether EcR-B1 is also expressed in MB neurons isolated 5 hr after pupation and dissociated into culture, we plated cells from brain and optic lobes of 201Y × UASG-lacZ animals for 2 hr and then fixed and stained the cells to visualize βgal and EcR-B1. MB neurons identified by βgal expression are invariably positive for EcR-B1, whereas EcR-B1 is also expressed in many, but not all, βgal-negative neurons in the culture (Fig. 3C,D). This is consistent with whole-mount data demonstrating that EcR-B1 is expressed in Kenyon cells as well as in some other neurons of the CNS at this time during metamorphosis (Fig. 3A).
Fig. 3.
βGal-expressing Kenyon cells express ecdysone receptors in vivo and in vitro. Immunocytochemical staining to localize βgal (green) and the ecdysone receptor EcR-B1 isoform (red) in samples from 201Y × UASG-lacZ animals 5 hr after pupation is shown. A, Confocal image showing a projection of optical sections through the entire brain. βGal is evident in the cell body (cb) region, peduncle (p), and lobes (α and β/γ) of the MBs, as well as in cells of the pars intercerebralis (*) and their processes extending to the dorsal vessel (+). Nuclei of cells expressing EcR-B1 are scattered throughout the brain and the subesophageal ganglion (SEG). The region of overlap, representing coexpression of βgal and EcR-B1, isyellow and is localized to the cell body region of the MBs. B, A single optical section through the cell body cluster of the left MB in A, showing the colocalization of βgal and EcR-B1 in individual Kenyon cell somata. At this time during metamorphosis, there is a high level of EcR-B1 expression in cells of the optic lobe, evident by the intense redsignal to the left. C, DThe identical field of cells in a 2 hr culture prepared from the brain and optic lobes of a 201Y × UASG-lacZanimal 5 hr after pupation and immunostained for EcR-B1 (red, C) and βgal (green, D). A single cell (filled arrow), corresponding to an MB neuron, expresses both βgal and EcR-B1. In addition, βgal-negative cells can be seen that are positive for EcR-B1 expression with various levels of intensity (C). There are also cells that appear to be negative for both βgal and EcR-B1 expression (open arrows). Scale bars: A,B, 50 μm; C, D, 10 μm.
αHRP immunostaining faithfully reproduces the neuritic branches of Drosophila neurons in culture
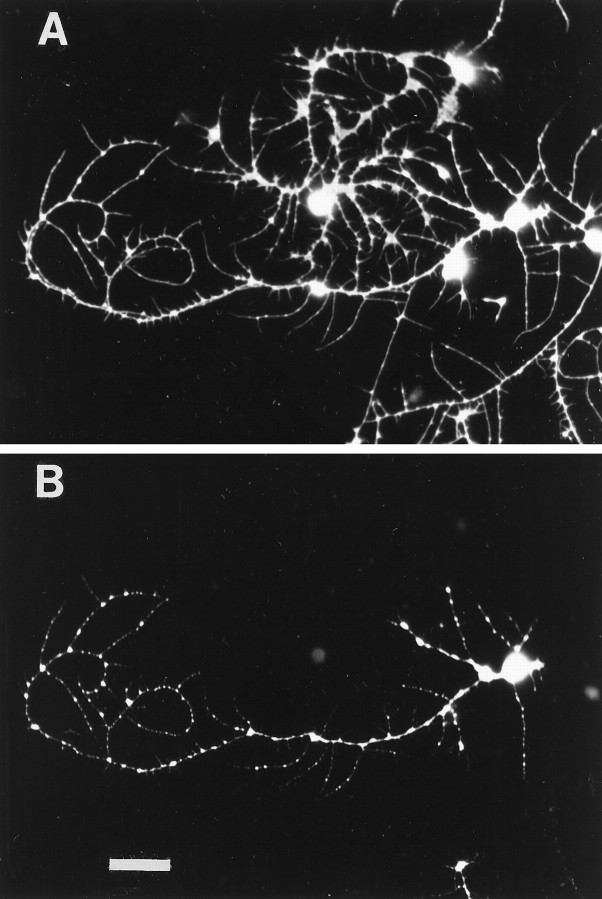
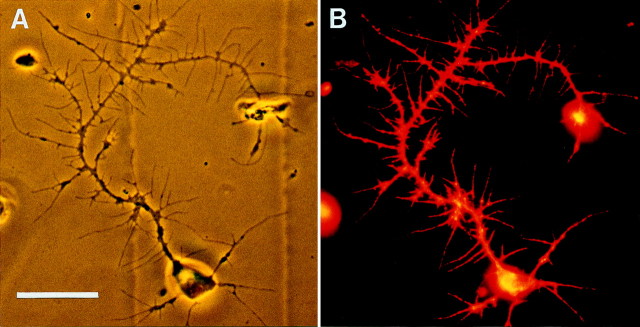
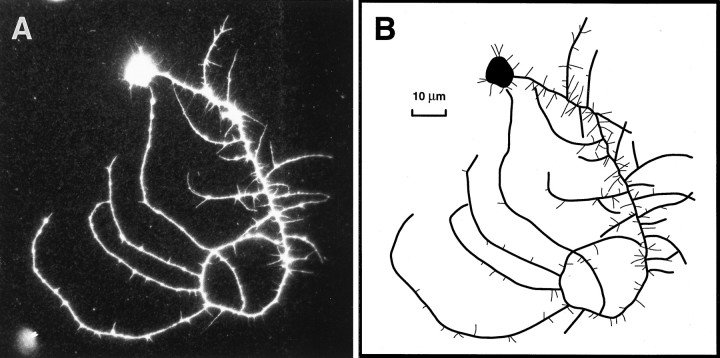
Because the MB neurons were identified after immunostaining for βgal, measurements of branch number and total neurite length were performed on images of fixed cultured cells. To ensure that αHRP immunostaining faithfully represented the living cell in culture, single neurons were photographed before fixation and again after indirect immunofluorescent staining (Fig.4). All the neuritic branches visible on the live cell are reproduced in the fluorescent image, as clearly demonstrated by comparing the phase-contrast image of the live neuron (Fig. 4A) with the same cell fixed and labeled with αHRP primary and LRSC-conjugated secondary antiserum (Fig.4B). Figure 5illustrates an example of how neuronal morphology was interpreted in this study. The cell depicted is an MB neuron cultured for 3 d in the presence of 20E. The thick lines in the diagram (Fig. 5B) represent branches measured from the αHRP-stained image (Fig. 5A) with lengths of 7.8 μm or greater. These branches were included in total branch number and total neurite length measurements for the cell. This Kenyon cell had 25 branches with a total neurite length of 735 μm. The thin lines represent 144 neurites with lengths of <7.8 μm that were not included in the total branch number or total neurite length (see Materials and Methods).
Fig. 4.
αHRP immunostaining of fixedDrosophila neurons in culture faithfully reproduces the neuritic branches of the live cell. A, Phase-contrast image of a live neuron that has grown for 5 d in a dissociated culture prepared from 201Y × UASG-lacZwhole larval CNS. B, αHRP staining (Lissamine Rhodamine, red) of the neuron depicted inA after fixation. Scale bar, 20 μm.
Fig. 5.
A representative example illustrating the morphometric approach used to analyze pupal MB neurons in vitro. A, αHRP immunostaining of a βgal-positive cell identified in a 3 d culture from experiment 1. Such black and white photomicrographs were used to make measurements. B, A line drawing depicting how the neuron in A was interpreted for this study. The thick lines represent neuritic branches with a measured length of 7.8 μm or greater that were included in the total neurite length measurement (735 μm) and branch number tally (25) for this cell. Thethin lines represent branches with measured lengths <7.8 μm, of which there were 144 (see Materials and Methods).
20E enhances total neurite length and branch number of pupal MB neurons in vitro
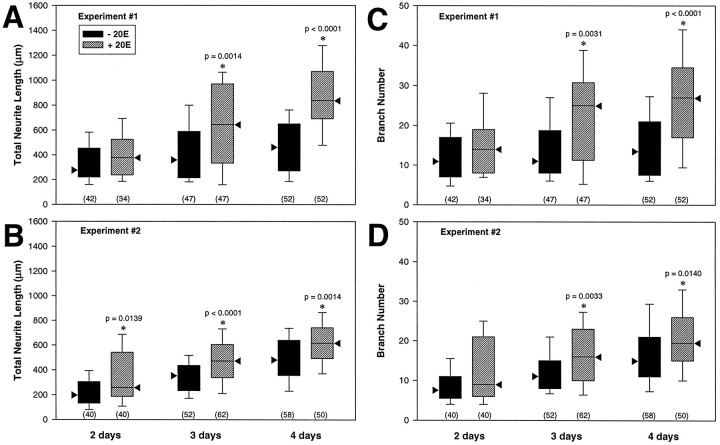
The demonstration that 201Y was a suitable marker for Kenyon cells in culture and that αHRP immunostaining provided an accurate representation of neuronal morphology fulfilled conditions essential to permit the morphometric analysis of the in vitro response of pupal MB neurons to 20E. Two small-scale pilot studies suggested that early pupal Kenyon cells from 201Y × UASG-lacZ brain tissue cultured with 20E for 3 d did exhibit significantly greater total neurite length and branch number compared with that in untreated cells (data not shown). Thus, two comprehensive independent experiments (1, female, and 2, male) were performed to confirm and expand those results. For each experiment, regionally dissected brain tissue from a single animal was isolated 5 hr after pupation (see Fig. 1B) and dissociated into three pairs of culture dishes. When the dishes were flooded with medium 2 hr after plating, one dish from each pair received medium containing 1 μg/ml 20E (2.1 × 10−6m). This concentration of 20E has been shown to elicit a significant response by Manduca motor neurons in culture (Prugh et al., 1992; and is within the physiological range estimated to occur in vivoduring metamorphosis in Drosophila (Matheson and Levine, Fristrom and Fristrom, 1993). Whether hormone was in a dish was unknown to the individual who scanned them for βgal-expressing MB neurons and who later performed the measurements on the photographic images of those cells. The paired dishes were cultured for 2, 3, or 4 d. The results of the two experiments are summarized in Figure6.
Fig. 6.
Drosophila pupal MB neurons grown in culture exhibit significantly greater total neurite length and branch number in response to 20E. The box plots show the distributions for total neurite length (A,B) and branch number (C,D) for cells grown without (black) or with (hatched) 20E for 2, 3, or 4 d in experiments 1 (A, C) and 2 (B,D). The black triangles mark the median values, the boxes represent the 25th and 75th percentile limits, and the bars indicate the 10th and 90th percentile limits. The number of cells included in each data set is shown within parentheses below the plots. The data inA and C represent measurements from the identical cells for experiment 1, as does the data in Band D for experiment 2. Statistically significant differences between treatments on any given day as determined by the Mann–Whitney rank sum test are indicated (*) along with the corresponding p value. For total neurite length, significant differences between treatments were found at 3 and 4 d for experiment 1 (A) and at all three time points for experiment 2 (B). For branch number, significant differences were found between cells grown with and without 20E at 3 and 4 d for both experiments.
Kenyon cells isolated from Drosophila early pupal CNS and cultured in vitro exhibited enhanced process outgrowth when exposed to 20E, manifested by greater total neurite length (Fig.6A,B) and increased branch number (Fig. 6C,D). The difference in total neurite length between cells grown with or without 20E was significant by 3 d for experiment 1, and this difference persisted at 4 d (Fig. 6A). A significant difference in total neurite length between the two treatment groups was evident by 2 d for experiment 2, and this difference persisted at 3 and 4 d (Fig.6B). Similarly, in both experiments branch number was significantly different between cells grown with or without 20E by 3 d in culture, and this difference persisted at 4 d (Fig.6C,D). Nonetheless, 20E was not essential for growth of Kenyon cells in culture, because the mean total neurite length and branch number of untreated cells did increase over time, but to a significantly lesser extent than that for the hormone-treated cells.
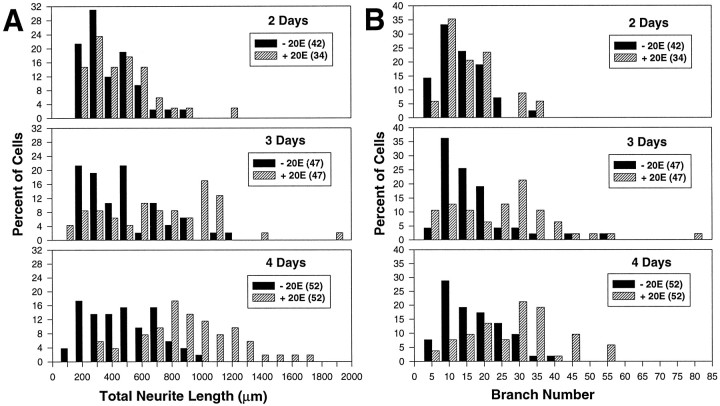
Figure 7 illustrates the changes in neuronal size within Kenyon cell populations over time in vitro in the presence or absence of 20E. The data shown are from experiment 1 and reflect the trend seen in experiment 2. Histograms depicting total neurite length (Fig. 7A) and branch number (Fig. 7B) at 2, 3, and 4 d show that the population distributions representing the two treatment groups overlap at all time points. However, although the treated and untreated populations were similarly distributed at 2 d, they diverged considerably by 4 d. The cell populations cultured without 20E did show increasing size (both length and branch number) over time, but the dishes cultured with 20E had many more large cells, suggesting that 20E enhanced the growth of MB neurons.
Fig. 7.
Histograms depicting the distributions of total neurite length (A) and branch number (B) for pupal MB neurons grown in culture without (black) or with (hatched) 20E at 2, 3, and 4 d (experiment 1). The number of cells included in each group is indicated in parentheses in the legends.A, The percentage of MB neurons for each treatment with total neurite lengths up to and including 100 μm and every 100 μm increment thereafter. The length distributions for untreated and hormone-treated cells are almost identical at 2 d, but by 4 d the distributions have diverged considerably, suggesting an enhanced rate of growth in response to 20E. B, The percentage of MB neurons for each treatment with branch numbers up to and including 5 and every increment of 5 after that. Again, branch numbers for the two cell populations are similarly distributed at 2 d, but by 4 d the distributions have diverged, reflecting enhancement by 20E.
The relationship between branch number and total neurite length remains constant for Kenyon cells in culture regardless of treatment
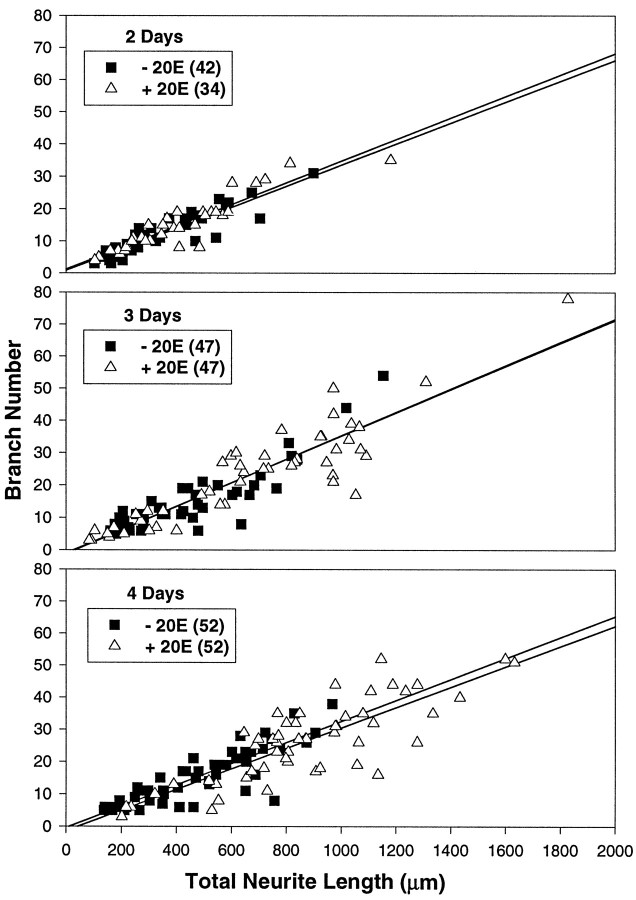
Manduca pupal motor neurons respond to 20E in vitro by increased branching at growth cones (, resulting in increased branch complexity (Matheson and Levine, Prugh et al., 1992). To determine whether 20E also influences branch complexity of culturedDrosophila pupal Kenyon cells, we examined the relationship between branch number and total neurite length of individual cells grown in the presence or absence of 20E. Figure8 depicts the branch number and total neurite length for each cell from experiment 1 for both treatments on each day. The ratios of branch number to total neurite length and total neurite length to branch number did not differ significantly between hormone treatment groups. The median values for these ratios were surprisingly constant regardless of treatment over all 3 d, with branch number per 100 μm ranging from 3.1 to 3.7 and length per branch ranging from 28 to 32 μm for experiment 1. Experiment 2 results were comparable (data not shown). Application of the Pearson product moment correlation test showed that there was a significant positive correlation between branch number and total neurite length, with correlation coefficient values ranging from 0.74 to 0.95.
Fig. 8.
The relationship between branch number and total neurite length of individual pupal MB neurons remains constant over time in culture and is not affected by exposure to 20E. Each cell from experiment 1 was plotted by branch number and total neurite length. Untreated neurons are represented as ▪, and hormone-treated neurons are represented as ▵. The number of cells included in each group is indicated in parentheses in the legends. Straight lines were fit to each set of data points by the least-squares method. The slopes of the lines are 0.033 and 0.034 at 2 d, 0.036 and 0.036 at 3 d, and 0.033 and 0.032 at 4 d for cells grown in the absence and in the presence of 20E, respectively.
The constancy of the relationship between branch number and total length, regardless of time in culture, became particularly apparent when a straight line was fit to the data from each population using the least-squares method (Fig. 8). Furthermore, exposure to 20E seemed to have no effect on this relationship. This was particularly evident for the 4 d cells, in which the two populations had diverged in range but nonetheless were arrayed to yield linear relationships with very similar slopes. This analysis demonstrates that cells with a given total neurite length tend to have similar branch numbers, whether or not they have been exposed to hormone. 20E does not alter the quantitative relationship between branch number and total length, nor does it have an obvious qualitative effect on the shape of MB neuronal arbors. Rather, 20E enhances the overall extent of process outgrowth, increasing total neurite length and branch number proportionately and not selectively influencing the tendency to form new branches. Taken together, the data suggest that 20E increases the rate of MB neurite outgrowth in vitro.
MB neurons display a characteristic morphologyin vitro
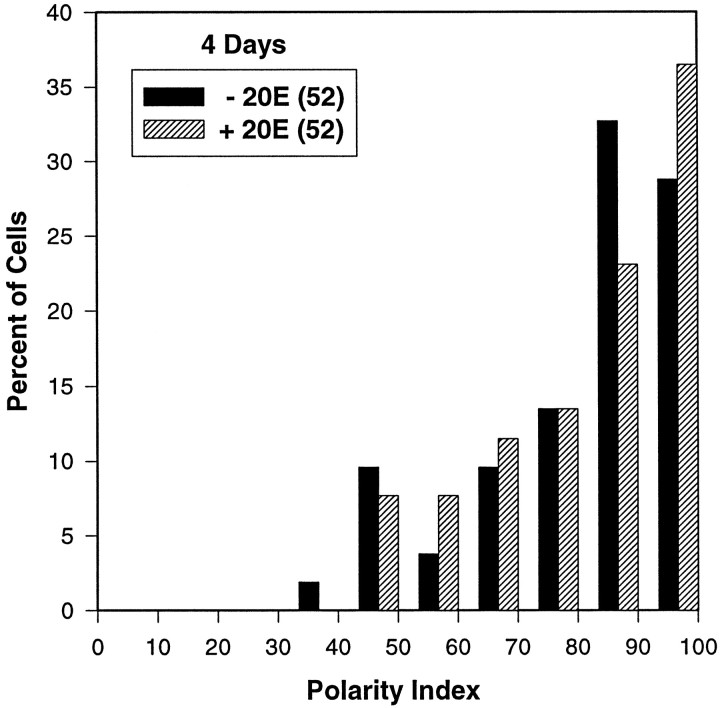
Pupal Kenyon cells growing in vitro exhibited a striking polar morphology, with most if not all branches arising from one primary neurite (see Figs. 2, 5). In contrast, βgal-negative neurons, the majority of which are not Kenyon cells, were much more likely to assume a stellate appearance (Fig. 2A). To quantify this feature, a polarity index, equal to the maximum percentage of total neurite length contributed by a primary neurite and all of its branches, was calculated for each βgal-positive cell that was analyzed. A monopolar neuron would have a polarity index of 100. Figure 9 summarizes the polarity indices for the MB neurons in the 4 d cultures from experiment 1. The majority of Kenyon cells displayed a high degree of polarity, with over 80% of their total neurite length found in a major primary neurite and its associated arbor. There was no significant difference between the polarity indices of cells grown in the presence or absence of 20E, consistent with the conclusion that hormone treatment does not alter qualitative features of MB neuron morphology.
Fig. 9.
The polarity index distributions for pupal MB neurons from the 4 d cultures of experiment 1 demonstrate that the majority of Kenyon cells in vitro display a highly polar morphology, regardless of hormone treatment. The histograms depict the percentage of MB neurons for each treatment (without 20E,black; with 20E, hatched) with polarity index values falling within intervals of 10. The polarity index is equal to the maximum percent of total neurite length in a primary process and all branches arising from it. The number of cells included in each group is indicated in parentheses in the legend.
Four MB neurons representing different points along the polarity index spectrum are shown in Figure 10. A few cells could best be described as stellate, with no obvious polarity and a correspondingly low polarity index (Fig. 10A). Some cells possessed several large primary neurites, with a moderate polarity index (Fig. 10B). Most MB neurons, however, had a high polarity index, with a major dominant primary neurite and one or two additional shorter primary neurites (Fig. 10C), or were monopolar, with all of the total neurite length invested in a single primary neurite and its branches (Fig. 10D). This consistent cell morphology typified the majority of Kenyon cellsin vitro and is remarkably similar to the shape they assume within adult MBs in vivo (Yang et al., 1995), suggesting that MB neurons have an intrinsic propensity to grow in a stereotypical manner to generate a dominant primary neurite.
Fig. 10.
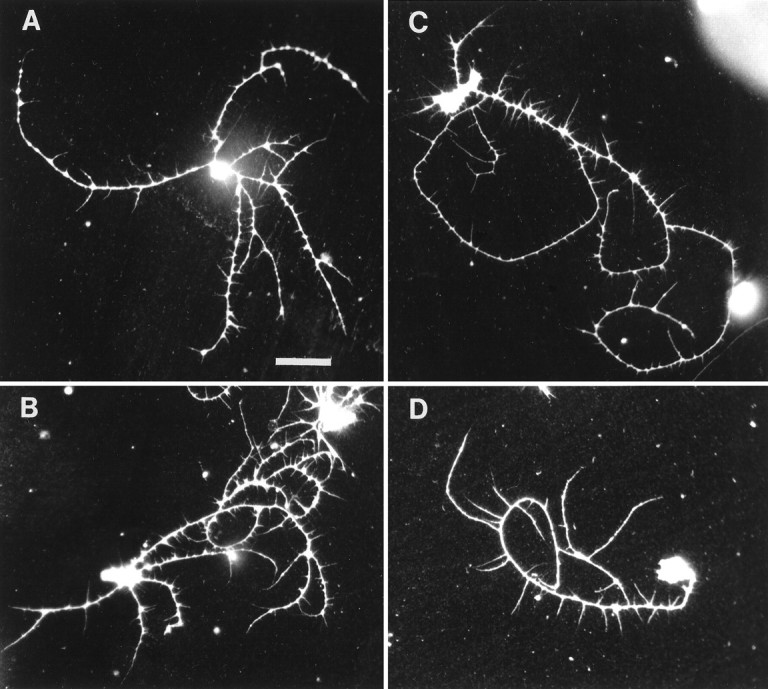
Pupal MB neurons from the 3 d cultures of experiment 1, illustrating the range in morphologies exhibited by Kenyon cells in vitro. A, Polarity index = 49 (note the stellate morphology). B, Polarity index = 67. C, Polarity index = 86.D, Polarity index = 100 (this is a monopolar neuron). Scale bar, 20 μm.
DISCUSSION
Pupal MB neurons respond to 20E in vitro
Several lines of evidence indicate that the MBs undergo considerable reorganization during Drosophila metamorphosis. These include the reduction and subsequent increase in axon number within the peduncle (Technau and Heisenberg, 1982), mutants with MB anatomical phenotypes that become apparent during metamorphosis (Technau and Heisenberg, 1982), and P[GAL4] lines with different MB expression patterns in larvae and adults (Tettamanti et al., 1997). As in other insects (Strausfeld et al., 1995), the MBs of adultDrosophila are essential components of the neural pathway that mediates associative learning (Heisenberg et al., 1985; de Belle and Heisenberg, 1994; Connolly et al., 1996). Experience-dependent neuroanatomical plasticity in the MBs has also been demonstrated during adult life (Technau, 1984; Balling et al., 1987; Heisenberg et al., 1995; Barth and Heisenberg, 1997). Therefore, studying Kenyon cell development during the pupal period, when adult MB organization is initially established, should contribute to understanding the mechanisms that generate the neuronal architecture required for learning and memory.
We have shown that pupal MB neurons exhibited significantly greater total neurite length and branch number when cultured in vitro in the presence of physiological levels of 20E. This response is consistent with our hypothesis of a critical role for 20E in supporting axonal regeneration within the MBs during pupal development in vivo. The available evidence suggests that the growth enhancement we observed was not caused simply by improved neuronal health in the presence of 20E in vitro. First, the MB neurons survive and extend neurites in the absence of 20E, indicating that basal culture conditions are not limiting. Second,in vitro studies in Manduca, performed with similar methodology, demonstrate that the cell culture system can faithfully replicate the cellular specificity of hormonal responses seen in the intact animal. For instance, the response of thoracic motor neurons to 20E depends on their developmental stage both in vivo (Kent and Levine, 1993) and in vitro (Prugh et al., 1992). Similarly, 20E can induce neuron death in vitrowith the same segment specificity (Streichert et al., 1997) that is seen in vivo (Weeks and Ernst-Utzschneider, 1989).
Expression of EcR by the pupal Kenyon cells in vitrosuggests that the response of these dissociated neurons to 20E is mediated via transcriptional regulation of one or more genetic pathways (Richards, 1997) and that this may be occurring in a cell-autonomous manner. However, the presence of numerous EcR-positive generic brain neurons in the cultures allows for the possibility that the influence of 20E on Kenyon cells is indirect, perhaps requiring secreted factors from other neurons. In any case, responsiveness to 20E is unlikely to be unique to MB neurons because many other neurons express EcR bothin vivo and in vitro. The likely targets of 20E action during early pupal development include other larval neurons that are re-elaborating processes (Vallés and White, 1988; Truman, 1990) and newly born imaginal neurons (Ito and Hotta, 1992) that are generating processes de novo.
20E enhances neuronal size but not branch complexity
The enhanced outgrowth exhibited by pupal MB neurons in response to 20E in vitro was proportional, in that the ratio of branch number to total neurite length remained constant regardless of hormone treatment and time in culture. This is consistent with an effect of 20E on the rate of neurite outgrowth. To confirm this interpretation, it will be necessary to identify individual Kenyon cells shortly after plating and then to follow their growth over time. This can be done by using the GAL4-UAS system to direct expression of a suitable marker (Brand, 1995). Such methods could also resolve questions concerning the origin, stability, and fate of short and long branches.
The enhanced growth of pupal MB neurons in response to 20E was more robust in experiment 1, which used female brain tissue, than in experiment 2, which used male tissue. Similar gender differences have been seen in other experiments not presented here (Kraft, unpublished observations). This observation raises the possibility that sexual dimorphism, demonstrated previously for Kenyon cell fiber number (Technau, 1984) and impact on courtship behavior (Ferveur et al., 1995; O’Dell et al., 1995), also exists for the response of MB neurons to 20E during metamorphosis.
Our data indicate that 20E changes the rate, but not the mode, of Kenyon cell growth. Hence, 20E does not affect branch complexity of cultured Drosophila pupal MB neurons. This contrasts withManduca motor neurons, which respond to 20E by increased branching at growth cones (. The difference may reflect cell type-specific, hormone-dependent growth responses that fulfill the distinct requirements of these two neuronal populations during metamorphosis. Whereas Manduca motor neurons elaborate extensive dendritic and axonal arbors to make new synaptic connections (Matheson and Levine, Kent and Levine, 1988, 1993; Consoulas et al., 1996),Drosophila Kenyon cells must extend regenerating axons over a considerable distance to reach synaptic targets in the lobes. We propose that 20E provides a signal for accelerated growth of MB neurons so that they can accomplish this within the 90 hr of pupal development and thereby establish the circuitry necessary for experience-dependent adult behavior.
Recent advances in understanding the control of neuronal growth provide some clues as to the downstream molecular mediators of the 20E effect on pupal Kenyon cells. At a fundamental level, neurite outgrowth and branching require membrane addition, cytoskeletal extension and reorganization, and interaction with the substrate, processes that may be interdependent (Tanaka and Sabry, 1995; Futerman and Banker, 1996;Baas, 1997; Caroni, 1997). For example, recent studies have focused on the role of Rho family GTPases in transducing extracellular signals into a neuritic growth response via reorganization of the actin cytoskeleton (Mackay et al., 1995; Luo et al., 1997; Gallo and Letourneau, 1998; Hall, 1998). We can now test the consequences of disrupting specific signaling pathways by using the GAL4-UAS system (Brand and Perrimon, 1993) to express dominant-negative or constitutively active forms of candidate molecules in Kenyon cells from 201Y animals.
The Kenyon cell culture system
In vitro cell culture systems have proven indispensable for investigating development and plasticity of vertebrate and invertebrate neurons (Beadle et al., 1988; Banker and Goslin, 1991). Use of the P[GAL4] line 201Y has made it possible for us to identify MB neurons in heterogeneous primary cultures from Drosophilapupal brain tissue and to study the effect of 20E on their growth. Furthermore, the majority of pupal Kenyon cells grown for several days in dissociated culture displayed a morphology reminiscent of the natural shape of these cells within the adult MB. This structural fidelity suggests that pupal MB neurons have an endogenous program directing their morphogenetic development, analogous to that proposed for hippocampal pyramidal neurons (Banker and Cowan, 1979; Dotti et al., 1988).
Previous studies of cultured Drosophila neurons of various stages from wild type and mutants allowed morphological and electrophysiological assessment, but without specific identification of the neurons beyond their regional origin (Salvaterra et al., 1987;Byerly and Leung, 1988; Solc and Aldrich, 1988; Wu, 1988; Li and Meinertzhagen, 1995; O’Dowd, 1995; Zhao and Wu, 1997). Primary cultures enriched for MB neurons have been established from brains of other insects (Kreissl and Bicker, 1992; Cayre et al., 1998), but the lack of tools for genetic manipulation limits the versatility of these systems. Acutely dissociated Drosophila MB neurons have been identified using MB-restricted lacZ reporter gene expression in combination with hypotonic loading of a fluorogenic substrate of βgal (Wright and Zhong, 1995; Delgado et al., 1998). However, the long-term growth and survival of these Kenyon cells in vitrois poor (Kraft, unpublished observations). Hence, the method described here represents a significant advance, and one with widespread potential application for the study of Drosophila neuronsin vitro because of the growing number of P[GAL4] lines for identifying neuronal subsets and UAS lines for expression of reporter genes and effector molecules.
Two particularly intriguing questions regarding the MBs can now be addressed using the cell culture system. The first relates to the molecular heterogeneity detected within the MBs ofDrosophila and other insects, which may reflect functionally distinct Kenyon cell populations (Yang et al., 1995; Brotz et al., 1997; Li and Strausfeld, 1997; Tettamanti et al., 1997). In vitro studies can be used to test whether these Kenyon cell subsets differ in their responsiveness to 20E during pupal development. The second pertains to MB structural mutations, mushroom bodies deranged (mbd) and mushroom body defect(mud), that markedly disrupt the adult MB while sparing the larval MB, suggesting aberrant metamorphic reorganization (Technau and Heisenberg, 1982). Growth assessment in dissociated culture should reveal whether mutant Kenyon cells have altered or decreased responsiveness to 20E.
Footnotes
R.K. was supported in part by National Institutes of Health Research Training Grant NS07363. This work was funded by grants to L.L.R. from the John Merck Fund (Program in Developmental Disabilities in Children), the University of Arizona Small Grant Program, and National Institutes of Health (NS28495). We are indebted to M. Anezaki for invaluable assistance in photographic processing and morphometric analysis. The help of K. Della Croce, H. Foster, C. Hedgcock, P. Jansma, R. Luedeman, C. McGonigle, and C. Turner is also appreciated. We thank I. Vilinsky and N. Strausfeld for Drosophilastocks, P. Hurban for antibodies, and M. Vaskova for advice on immunostaining. R. Graf, L. Tolbert, and A. Yool provided thoughtful comments on this manuscript.
Correspondence should be addressed to Dr. Linda L. Restifo, Arizona Research Laboratories, Division of Neurobiology, University of Arizona, P.O. Box 210077, Tucson, AZ 85721-0077.
REFERENCES
- 1.Ahmad FJ, Pienkowski TP, Baas PW. Regional differences in microtubule dynamics in the axon. J Neurosci. 1993;13:856–866. doi: 10.1523/JNEUROSCI.13-02-00856.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- 3.Baas PW. Microtubules and axonal growth. Curr Opin Cell Biol. 1997;9:29–36. doi: 10.1016/s0955-0674(97)80148-2. [DOI] [PubMed] [Google Scholar]
- 4.Bainbridge SP, Bownes M. Staging the metamorphosis of Drosophila melanogaster. J Embryol Exp Morphol. 1981;66:57–80. [PubMed] [Google Scholar]
- 5.Bainbridge SP, Bownes M. Ecdysteroid titers during Drosophila metamorphosis. Insect Biochem. 1988;18:185–197. [Google Scholar]
- 6.Balling A, Technau GM, Heisenberg M. Are the structural changes in adult Drosophila mushroom bodies memory traces? Studies on biochemical learning mutants. J Neurogenet. 1987;4:65–73. [PubMed] [Google Scholar]
- 7.Banker G, Goslin K, editors. Culturing nerve cells. MIT; Cambridge, MA: 1991. [Google Scholar]
- 8.Banker GA, Cowan WM. Further observations on hippocampal neurons in dispersed cell culture. J Comp Neurol. 1979;187:469–494. doi: 10.1002/cne.901870302. [DOI] [PubMed] [Google Scholar]
- 9.Barth M, Heisenberg M. Vision affects mushroom bodies and central complex in Drosophila melanogaster. Learn Mem. 1997;4:219–229. doi: 10.1101/lm.4.2.219. [DOI] [PubMed] [Google Scholar]
- 10.Bayer C, von Kalm L, Fristrom JW. Gene regulation in imaginal disc and salivary gland development during Drosophila metamorphosis. In: Gilbert LI, Tata JR, Atkinson BG, editors. Metamorphosis: postembryonic reprogramming of gene expression in amphibian and insect cells. Academic; San Diego: 1996. pp. 321–361. [Google Scholar]
- 11.Beadle DJ, Lees G, Kater SB, editors. Cell culture approaches to invertebrate neuroscience. Academic; New York: 1988. [Google Scholar]
- 12.Brand A. GFP in Drosophila. Trends Genet. 1995;11:324–325. doi: 10.1016/s0168-9525(00)89091-5. [DOI] [PubMed] [Google Scholar]
- 13.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 14.Brotz TM, Bochenek B, Aronstein K, ffrench-Constant RH, Borst A. γ-Aminobutyric acid receptor distribution in the mushroom bodies of a fly (Calliphora erythrocephala): a functional subdivision of Kenyon cells? J Comp Neurol. 1997;383:42–48. doi: 10.1002/(sici)1096-9861(19970623)383:1<42::aid-cne3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Byerly L, Leung H-T. Ionic currents of Drosophila neurons in embryonic cultures. J Neurosci. 1988;8:4379–4393. doi: 10.1523/JNEUROSCI.08-11-04379.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caroni P. Intrinsic neuronal determinants that promote axonal sprouting and elongation. Bioessays. 1997;19:767–775. doi: 10.1002/bies.950190906. [DOI] [PubMed] [Google Scholar]
- 17.Cayre M, Buckingham SD, Strambi A, Strambi C, Sattelle DB. Adult insect mushroom body neurons in primary culture: cell morphology and characterization of potassium channels. Cell Tissue Res. 1998;291:537–547. doi: 10.1007/s004410051023. [DOI] [PubMed] [Google Scholar]
- 18.Connolly JB, Roberts IJH, Armstrong JD, Kaiser K, Forte M, Tully T, O’Kane CJ. Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science. 1996;274:2104–2107. doi: 10.1126/science.274.5295.2104. [DOI] [PubMed] [Google Scholar]
- 19.Consoulas C, Kent KS, Levine RB. Remodeling of the peripheral processes and presynaptic terminals of leg motoneurons during metamorphosis of the hawkmoth, Manduca sexta. J Comp Neurol. 1996;372:415–434. doi: 10.1002/(SICI)1096-9861(19960826)372:3<415::AID-CNE6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Davis RL, Han K-A. Neuroanatomy: mushrooming mushroom bodies. Curr Biol. 1996;6:146–148. doi: 10.1016/s0960-9822(02)00447-5. [DOI] [PubMed] [Google Scholar]
- 21.de Belle JS. Drosophila mushroom body subdomains: innate or learned representations of odor preference and sexual orientation? Neuron. 1995;15:245–247. doi: 10.1016/0896-6273(95)90029-2. [DOI] [PubMed] [Google Scholar]
- 22.de Belle JS, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- 23.Delgado R, Davis R, Bono MR, Latorre R, Labarca P. Outward currents in Drosophila larval neurons: dunce lacks a maintained outward current component downregulated by cAMP. J Neurosci. 1998;18:1399–1407. doi: 10.1523/JNEUROSCI.18-04-01399.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elgin SCR, Miller DW. Mass rearing of flies and mass production and harvesting of embryos. In: Ashburner M, Wright TRF, editors. The genetics and biology of Drosophila, Vol 2a. Academic; London: 1978. pp. 112–121. [Google Scholar]
- 26.Ferveur J-F, Stortkuhl KF, Stocker RF, Greenspan RJ. Genetic feminization of brain structures and changed sexual orientation in male Drosophila. Science. 1995;267:902–905. doi: 10.1126/science.7846534. [DOI] [PubMed] [Google Scholar]
- 27.Fristrom D, Fristrom JW. The metamorphic development of the adult epidermis. In: Bate M, Martinez Arias A, editors. The development of Drosophila melanogaster. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1993. pp. 843–897. [Google Scholar]
- 28.Futerman AH, Banker GA. The economics of neurite outgrowth—the addition of new membrane to growing axons. Trends Neurosci. 1996;19:144–149. doi: 10.1016/s0166-2236(96)80025-7. [DOI] [PubMed] [Google Scholar]
- 29.Gallo G, Letourneau PC. Axon guidance: GTPases help axons reach their targets. Curr Biol. 1998;8:R80–R82. doi: 10.1016/s0960-9822(98)70051-x. [DOI] [PubMed] [Google Scholar]
- 30.Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 32.Heisenberg M, Borst A, Wagner S, Byers D. Drosophila mushroom body mutants are deficient in olfactory learning. J Neurogenet. 1985;2:1–30. doi: 10.3109/01677068509100140. [DOI] [PubMed] [Google Scholar]
- 33.Heisenberg M, Heusipp M, Wanke C. Structural plasticity in the Drosophila brain. J Neurosci. 1995;15:1951–1960. doi: 10.1523/JNEUROSCI.15-03-01951.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito K, Hotta Y. Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev Biol. 1992;149:134–148. doi: 10.1016/0012-1606(92)90270-q. [DOI] [PubMed] [Google Scholar]
- 35.Jan LY, Jan YN. Antibodies to horseradish peroxidase as specific neuronal markers in Drosophila and in grasshopper embryos. Proc Natl Acad Sci USA. 1982;79:2700–2704. doi: 10.1073/pnas.79.8.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kent KS, Levine RB. Neural control of leg movements in a metamorphic insect: persistence of larval leg motor neurons to innervate the adult legs of Manduca sexta. J Comp Neurol. 1988;276:30–43. doi: 10.1002/cne.902760103. [DOI] [PubMed] [Google Scholar]
- 37.Kent KS, Levine RB. Dendritic reorganization of an identified neuron during metamorphosis of the moth Manduca sexta: the influence of interactions with the periphery. J Neurobiol. 1993;24:1–22. doi: 10.1002/neu.480240102. [DOI] [PubMed] [Google Scholar]
- 38.Kraft R, Levine RB, Restifo LL. Ecdysone enhances the growth of Drosophila pupal mushroom body neurons in cell culture. Soc Neurosci Abstr. 1997;23:60. doi: 10.1523/JNEUROSCI.18-21-08886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kreissl S, Bicker G. Dissociated neurons of the pupal honeybee brain in cell culture. J Neurocytol. 1992;21:545–556. doi: 10.1007/BF01187116. [DOI] [PubMed] [Google Scholar]
- 40.Kurz EM, Sengelaub DR, Arnold AP. Androgens regulate the dendritic length of mammalian motoneurons in adulthood. Science. 1986;232:395–398. doi: 10.1126/science.3961488. [DOI] [PubMed] [Google Scholar]
- 41.Levine RB. Expansion of the central arborization of persistent sensory neurons during insect metamorphosis: the role of the steroid hormone, 20-hydroxyecdysone. J Neurosci. 1989;9:1045–1054. doi: 10.1523/JNEUROSCI.09-03-01045.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levine RB, Weeks JC. Cell culture approaches to understanding the actions of steroid hormones on the insect nervous system. Dev Neurosci. 1996;18:73–86. doi: 10.1159/000111397. [DOI] [PubMed] [Google Scholar]
- 43.Levine RB, Fahrbach SE, Weeks JC. Steroid hormones and the reorganization of the nervous system during insect metamorphosis. Semin Neurosci. 1991;3:437–447. [Google Scholar]
- 44.Levine RB, Morton DB, Restifo LL. Remodeling of the insect nervous system. Curr Opin Neurobiol. 1995;5:28–35. doi: 10.1016/0959-4388(95)80083-2. [DOI] [PubMed] [Google Scholar]
- 45.Li C, Meinertzhagen IA. Conditions for the primary culture of eye imaginal discs from Drosophila melanogaster. J Neurobiol. 1995;28:363–380. doi: 10.1002/neu.480280309. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Strausfeld NJ. Morphology and sensory modality of mushroom body extrinsic neurons in the brain of the cockroach, Periplaneta americana. J Comp Neurol. 1997;387:631–650. [PubMed] [Google Scholar]
- 47.Liu E, Restifo LL. Identification of a Broad Complex-regulated enhancer in the developing visual system of Drosophila. J Neurobiol. 1998;34:253–270. doi: 10.1002/(sici)1097-4695(19980215)34:3<253::aid-neu5>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 48.Luo L, Jan LY, Jan Y-N. Rho family small GTP-binding proteins in growth cone signalling. Curr Opin Neurobiol. 1997;7:81–86. doi: 10.1016/s0959-4388(97)80124-9. [DOI] [PubMed] [Google Scholar]
- 49.Mackay DJG, Nobes CD, Hall A. The Rho’s progress: a potential role during neuritogenesis for the Rho family of GTPases. Trends Neurosci. 1995;18:496–501. doi: 10.1016/0166-2236(95)92773-j. [DOI] [PubMed] [Google Scholar]
- 50.Matheson SF, Levine RB (1999) Steroid hormone enhancement of neurite outgrowth in identified insect motor neurons involves specific effects on growth cone form and function. J Neurobiol, in press. [PubMed]
- 51.Murphy DD, Segal M. Regulation of dendritic spine density in cultured rat hippocampal neurons by steroid hormones. J Neurosci. 1996;16:4059–4068. doi: 10.1523/JNEUROSCI.16-13-04059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Dell KMC, Armstrong JD, Yang MY, Kaiser K. Functional dissection of the Drosophila mushroom bodies by selective feminization of genetically defined subcompartments. Neuron. 1995;15:55–61. doi: 10.1016/0896-6273(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 53.O’Dowd DK. Voltage-gated currents and firing properties of embryonic Drosophila neurons grown in a chemically defined medium. J Neurobiol. 1995;27:113–126. doi: 10.1002/neu.480270111. [DOI] [PubMed] [Google Scholar]
- 54.Power ME. The brain of Drosophila melanogaster. J Morphol. 1943;72:517–559. [Google Scholar]
- 55.Prugh J, Croce KD, Levine RB. Effects of the steroid hormone, 20-hydroxyecdysone, on the growth of neurites by identified insect motoneurons in vitro. Dev Biol. 1992;154:331–347. doi: 10.1016/0012-1606(92)90072-o. [DOI] [PubMed] [Google Scholar]
- 56.Rees HH, Isaac RE. Biosynthesis and metabolism of ecdysteroids and methods of isolation and identification of the free and conjugated compounds. Methods Enzymol. 1985;111:377–410. doi: 10.1016/s0076-6879(85)11024-4. [DOI] [PubMed] [Google Scholar]
- 57.Restifo LL, White K. Mutations in a steroid hormone-regulated gene disrupt the metamorphosis of the central nervous system in Drosophila. Dev Biol. 1991;148:174–194. doi: 10.1016/0012-1606(91)90328-z. [DOI] [PubMed] [Google Scholar]
- 58.Richards G. The radioimmunoassay of ecdysteroid titers in Drosophila melanogaster. Mol Cell Endocrinol. 1981;21:181–197. doi: 10.1016/0303-7207(81)90013-7. [DOI] [PubMed] [Google Scholar]
- 59.Richards G. The ecdysone regulatory cascades in Drosophila. Adv Dev Biol. 1997;5:81–135. [Google Scholar]
- 60.Riddiford LM. Hormones and Drosophila development. In: Bate M, Martinez Arias A, editors. The development of Drosophila melanogaster. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1993. pp. 899–939. [Google Scholar]
- 61.Robinow S, White K. Characterization and spatial distribution of the ELAV protein during Drosophila melanogaster development. J Neurobiol. 1991;22:443–461. doi: 10.1002/neu.480220503. [DOI] [PubMed] [Google Scholar]
- 62.Robinow S, Talbot WS, Hogness DS, Truman JW. Programmed cell death in the Drosophila CNS is ecdysone-regulated and coupled with a specific ecdysone receptor isoform. Development. 1993;119:1251–1259. doi: 10.1242/dev.119.4.1251. [DOI] [PubMed] [Google Scholar]
- 63.Salvaterra PM, Bournais-Vardiabasis N, Nair T, Hou G, Lieu C. In vitro neuronal differentiation of Drosophila embryo cells. J Neurosci. 1987;7:10–22. doi: 10.1523/JNEUROSCI.07-01-00010.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sandstrom DJ, Bayer CA, Fristrom JW, Restifo LL. Broad-Complex transcription factors regulate thoracic muscle attachment in Drosophila. Dev Biol. 1997;181:168–185. doi: 10.1006/dbio.1996.8469. [DOI] [PubMed] [Google Scholar]
- 65.Schubiger M, Wade AA, Carney GE, Truman JW, Bender M. Drosophila EcR-B ecdysone receptor isoforms are required for larval molting and for neuron remodeling during metamorphosis. Development. 1998;125:2053–2062. doi: 10.1242/dev.125.11.2053. [DOI] [PubMed] [Google Scholar]
- 66.Schulz RA, Chromey C, Lu M-F, Zhao B, Olson EN. Expression of the D-MEF2 transcription factor in the Drosophila brain suggests a role in neuronal cell differentiation. Oncogene. 1996;12:1827–1831. [PubMed] [Google Scholar]
- 67.Smith CL. Cytoskeletal movements and substrate interactions during initiation of neurite outgrowth by sympathetic neurons in vitro. J Neurosci. 1994;14:384–398. doi: 10.1523/JNEUROSCI.14-01-00384.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Solc CK, Aldrich RW. Voltage-gated potassium channels in larval CNS neurons of Drosophila. J Neurosci. 1988;8:2556–2570. doi: 10.1523/JNEUROSCI.08-07-02556.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strausfeld NJ, Buschbeck EK, Gomez RS. The arthropod mushroom body: its functional roles, evolutionary enigmas and mistaken identities. In: Breidbach O, Kutsch W, editors. The nervous system of invertebrates: an evolutionary and comparative approach. Birkhäuser; Basel: 1995. pp. 349–381. [Google Scholar]
- 70.Streichert LC, Pierce JT, Nelson JA, Weeks JC. Steroid hormones act directly to trigger segment-specific programmed cell death of identified motoneurons in vitro. Dev Biol. 1997;183:95–107. doi: 10.1006/dbio.1996.8467. [DOI] [PubMed] [Google Scholar]
- 71.Sun B, Salvaterra PM. Characterization of Nervana, a Drosophila melanogaster neuron-specific glycoprotein antigen recognized by anti-horseradish peroxidase antibodies. J Neurochem. 1995;65:434–443. doi: 10.1046/j.1471-4159.1995.65010434.x. [DOI] [PubMed] [Google Scholar]
- 72.Talbot WS, Swyryd EA, Hogness DS. Drosophila tissues with different metamorphic responses to ecdysone express different ecdysone receptor isoforms. Cell. 1993;73:1323–1337. doi: 10.1016/0092-8674(93)90359-x. [DOI] [PubMed] [Google Scholar]
- 73.Tanaka E, Sabry J. Making the connection: cytoskeletal rearrangements during growth cone guidance. Cell. 1995;83:171–176. doi: 10.1016/0092-8674(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 74.Technau G, Heisenberg M. Neural reorganization during metamorphosis of the corpora pedunculata in Drosophila melanogaster. Nature. 1982;295:405–407. doi: 10.1038/295405a0. [DOI] [PubMed] [Google Scholar]
- 75.Technau GM. Fiber number in the mushroom bodies of adult Drosophila melanogaster depends on age, sex and experience. J Neurogenet. 1984;1:113–126. doi: 10.3109/01677068409107077. [DOI] [PubMed] [Google Scholar]
- 76.Tettamanti M, Armstrong JD, Endo K, Yang MY, Furukubo-Tokunaga K, Kaiser K, Reichert H. Early development of the Drosophila mushroom bodies, brain centers for associative learning and memory. Dev Genes Evol. 1997;207:242–252. doi: 10.1007/s004270050112. [DOI] [PubMed] [Google Scholar]
- 77.Thummel CS. Flies on steroids—Drosophila metamorphosis and the mechanisms of steroid hormone action. Trends Genet. 1996;12:306–310. doi: 10.1016/0168-9525(96)10032-9. [DOI] [PubMed] [Google Scholar]
- 78.Truman JW. Metamorphosis of the central nervous system of Drosophila. J Neurobiol. 1990;21:1072–1084. doi: 10.1002/neu.480210711. [DOI] [PubMed] [Google Scholar]
- 79.Truman JW. Steroid receptors and nervous system metamorphosis in insects. Dev Neurosci. 1996;18:87–101. doi: 10.1159/000111398. [DOI] [PubMed] [Google Scholar]
- 80.Truman JW, Reiss SE. Hormonal regulation of the shape of identified motoneurons in the moth Manduca sexta. J Neurosci. 1988;8:765–775. doi: 10.1523/JNEUROSCI.08-03-00765.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Truman JW, Schwartz LM. Steroid regulation of neuronal death in the moth nervous system. J Neurosci. 1984;4:274–280. doi: 10.1523/JNEUROSCI.04-01-00274.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Truman JW, Talbot WS, Fahrbach SE, Hogness DS. Ecdysone receptor expression in the CNS correlates with stage-specific responses to ecdysteroids during Drosophila and Manduca development. Development. 1994;120:219–234. doi: 10.1242/dev.120.1.219. [DOI] [PubMed] [Google Scholar]
- 83.Uylings HBM, van Pelt J, Verwer RWH. Topological analysis of individual neurons. In: Capowski JJ, editor. Computer techniques in neuroanatomy. Plenum; New York: 1989. pp. 215–239. [Google Scholar]
- 84.Vallés AM, White K. Development of serotonin-containing neurons in Drosophila mutants unable to synthesize serotonin. J Neurosci. 1986;6:1482–1491. doi: 10.1523/JNEUROSCI.06-05-01482.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vallés AM, White K. Serotonin-containing neurons in Drosophila melanogaster: development and distribution. J Comp Neurol. 1988;268:414–428. doi: 10.1002/cne.902680310. [DOI] [PubMed] [Google Scholar]
- 86.VanderHorst VGJM, Holstege G. Estrogen induces axonal outgrowth in the nucleus retroambiguus-lumbosacral motoneuronal pathway in the adult female cat. J Neurosci. 1997;17:1122–1136. doi: 10.1523/JNEUROSCI.17-03-01122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Verwer RWH, van Pelt J. Descriptive and comparative analysis of geometrical properties of neuronal tree structures. J Neurosci Methods. 1986;18:179–206. doi: 10.1016/0165-0270(86)90119-6. [DOI] [PubMed] [Google Scholar]
- 88.Wang X, Sun B, Yasuyama K, Salvaterra PM. Biochemical analysis of proteins recognized by anti-HRP antibodies in Drosophila melanogaster: identification and characterization of neuron specific and male specific glycoproteins. Insect Biochem Mol Biol. 1994;24:233–242. doi: 10.1016/0965-1748(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 89.Weeks JC. Time course of hormonal independence for developmental events in neurons and other cell types during insect metamorphosis. Dev Biol. 1987;124:163–176. doi: 10.1016/0012-1606(87)90469-6. [DOI] [PubMed] [Google Scholar]
- 90.Weeks JC, Ernst-Utzschneider K. Respecification of larval proleg motoneurons during metamorphosis of the tobacco hornworm, Manduca sexta: segmental dependence and hormone regulation. J Neurobiol. 1989;20:569–592. doi: 10.1002/neu.480200605. [DOI] [PubMed] [Google Scholar]
- 91.Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wright NJD, Zhong Y. Characterization of K+ currents and the cAMP-dependent modulation in cultured Drosophila mushroom body neurons identified by lacZ expression. J Neurosci. 1995;15:1025–1034. doi: 10.1523/JNEUROSCI.15-02-01025.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu C-F. Neurogenetic studies of Drosophila central nervous system neurons in culture. In: Beadle DJ, Lees G, Kater SB, editors. Cell culture approaches to invertebrate neuroscience. Academic; New York: 1988. pp. 149–187. [Google Scholar]
- 94.Wu C-F, Suzuki N, Poo M-M. Dissociated neurons from normal and mutant Drosophila larval central nervous system in cell culture. J Neurosci. 1983;3:1888–1899. doi: 10.1523/JNEUROSCI.03-09-01888.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang MY, Armstrong JD, Vilinsky I, Strausfeld NJ, Kaiser K. Subdivision of the Drosophila mushroom bodies by enhancer-trap expression patterns. Neuron. 1995;15:45–54. doi: 10.1016/0896-6273(95)90063-2. [DOI] [PubMed] [Google Scholar]
- 96.Zhao ML, Wu C-F. Alterations in frequency coding and activity dependence of excitability in cultured neurons of Drosophila memory mutants. J Neurosci. 1997;17:2187–2199. doi: 10.1523/JNEUROSCI.17-06-02187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]