Abstract
There is often little correspondence between human and animal examples of nondeclarative memory. The serial reaction time task (SRT) is a sequence learning example of human nondeclarative memory that may be suitable for development as an animal model. The SRT is believed to be impaired by basal ganglia, not limbic system damage, but there is uncertainty whether limbic system pathology does in fact leave the SRT unimpaired. We therefore developed a new rat model that closely approximated the human SRT, using intracranial self-stimulation to promote rapid continuous responding to four adjacent nose pokes in a single test session. Intact rats that experienced repeated sequences demonstrated robust interference effects when switched to a random sequence of cued responses (at 4-, 8-, and 12-sequence lengths), unlike intact controls that experienced the random sequences only. The interference effect in the human task is a key measure for nondeclarative sequence learning. Rats with dorsal caudate lesions that experienced massed sequence repetitions showed an interference effect at the four-sequence length only. By contrast, rats with dorsal hippocampal lesions showed an interference effect at all sequence lengths. This new rat SRT model clarifies the basal ganglia–limbic system dichotomy suggested by human work.
Keywords: declarative memory, nondeclarative memory, procedural memory, multiple-memory systems, sequence learning, caudate, hippocampus, lesion, rat, serial reaction time task, intracranial self-stimulation
Introduction
Declarative and nondeclarative systems characterize two separate memory domains. In animals, deficits associated with hippocampal system damage are considered measures of declarative memory, whereas changes specific to disruption in nonhippocampal pathways are implicated in nondeclarative memory (Eichenbaum and Cohen, 2001). For example, hippocampal lesions disrupt spatial working memory, whereas caudate lesions disrupt stimulus–response learning (Packard et al., 1989). Unfortunately, presumed animal nondeclarative memory tasks are vastly different from parallel human tasks or are prone to declarative memory strategies in humans (Broadbent et al., 2002). Here, we describe an animal model that bears close resemblance to the human serial reaction time task (SRT), a perceptuomotor skill example of nondeclarative memory for a repeating sequence of stimuli.
Few animal studies have addressed sequence learning phenomena. In rats, both caudate and hippocampal lesions disrupt acquisition of serially presented rewards of varying magnitude (Compton, 2001). Cortical ablation produces a deficit in the performance of serially ordered grooming behavior (Berridge and Wishaw, 1992). Striatal lesions in rats impair the acquisition of a repeating sequence of radial-arm maze visits (DeCoteau and Kesner, 2000). Protocols more directly comparable with the human SRT, however, have produced unclear findings. Nixon and Passingham (2000) reported acquisition deficits on a four-trial SRT in three monkeys with cerebellar lesions, but there was no interference condition or any evidence from controls. The interference effect, the disruption when conditions switch from repeating to random sequences, provides more convincing evidence than acquisition changes of specific SRT learning. For example, Proyck et al. (2000) found an interference effect after training with a four-trial sequence in one intact monkey, but a second monkey displayed a reaction time (RT) decrease when switched to random sequences.
Individual studies with small groups of amnesic patients or Alzheimer's disease (AD) patients in the early stages of dementia reported no impairment in the SRT interference effect (Knopman and Nissen, 1987; Nissen and Bullemer, 1987; Ferraro et al., 1993; Reber and Squire, 1994, 1998). Conversely, Parkinson's disease (PD) and Huntington's disease (HD) patients often show marked SRT deficits (Knopman and Nissen, 1991; Westwater et al., 1998; Sommer et al., 1999). A meta-analysis of this literature, however, revealed that memory-disordered patients are actually associated with a moderate deficit [Cohen's d = 0.68; 95% confidence interval (CI) 0.45–0.95], although this effect is less severe than that found in basal ganglia disorders (d = 1.14; CI 0.93–1.35) (Christie and Dalrymple-Alford, 2002). The problem with human disorders is that specific neuropathology is unlikely. PD and HD patients show neurodegeneration that may include medial temporal lobe structures (Braak and Braak, 2000; Rosas et al., 2002); early AD patients and many amnesic patients have pathology beyond the hippocampal system (Kopelman, 2002). Our rat model therefore addressed the effects of specific basal ganglia and hippocampal system damage on an animal analog of the human SRT. Intracranial self-stimulation (ICSS) was used to provide immediate reward and encouraged rapid, uninterrupted responding in a single session. SRT effects were examined in control, caudate, and hippocampal rats using short (4-trial), medium (8-trial), and long (12-trial) sequences.
Materials and Methods
Animals
Ninety PVG hooded rats, aged ∼120 d old at surgery, were housed individually with food and water available ad libitum. Testing occurred during the lights-off period of a reversed 12 hr light/dark cycle (lights on at 6 P.M.).
Lesions and ICSS implant surgery
Rats received atropine sulfate (0.18 mg/kg, i.p.) 20 min before sodium pentobarbital anesthesia (100 mg/kg, i.p.). Lesion rats received bilateral damage to either the dorsal caudate or the dorsal hippocampus before ICSS electrode implantation in a single surgery. Nonlesion rats received ICSS electrode implants only. Using flat skull, the coordinates for caudate lesions were (in mm): anteroposterior (AP) +0.3 (bregma), mediolateral (ML) ±3.2, dorsoventral (DV) –4.5 (dura). Dorsal hippocampal lesions were made at AP –3.0, ML ±2.0, DV –3.3 and at AP –3.7, ML ±2.0, DV –3.4. Radiofrequency lesions (RFG-4; Radionics Inc., Burlington, MA) were made with electrode tip (0.7 mm) temperatures maintained for 1 min at 60°C (caudate) and at 56° and 57°C (respective hippocampal sites). Bilateral ICSS electrodes (MS305/1; Plastics One, Roanoke, VA) were implanted into the medial forebrain bundle at the level of the lateral hypothalamus (AP –2.6, ML ±1.8, DV –8.6). Electrodes were prepared for implantation by being cut to length (12 mm) with the exposed tips (∼0.5 mm) separated slightly (∼1 mm).
Apparatus
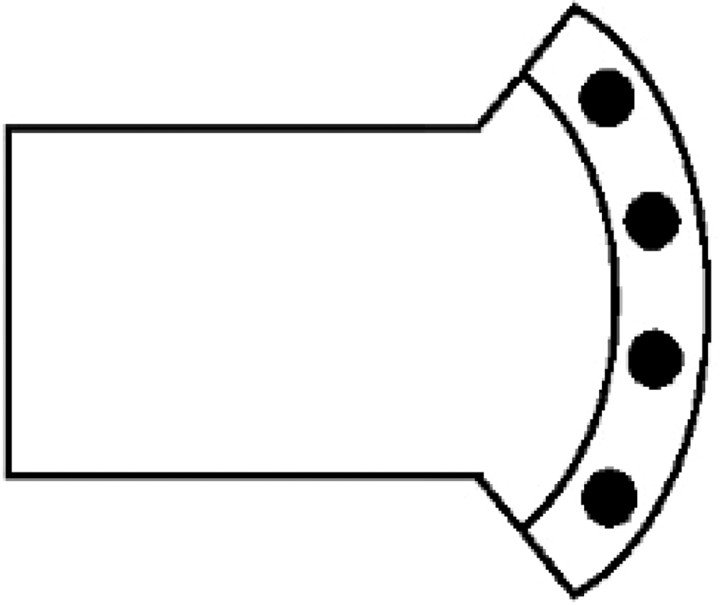
SRT performance was examined in a novel ICSS-SRT chamber that provided four stimulus–response positions similar to the human counter-part (Fig. 1). The chamber had a rectangular aluminum body, 20 cm long by 15 cm wide by 15 cm high, with one end open to a slightly wider (27 cm) semicircular area that had a small ledge (5 cm wide and 5 cm high) sloped at an angle of 15° between the floor and the end wall. The ledge held four equally spaced nose-poke recesses (3 cm diameter, 7 cm apart from center to center) that were easily accessible to a rat at the center of the semicircular area. An ICSS cable ran from a two-channel commutator (Plastics One; model SL2C) fastened to an aluminum arm that projected to a point 40 cm directly above the center of the chamber. The overhead ICSS commutator arm also held an infrared camera for behavioral observation. A sine wave stimulator (Lafayette Instruments Co., Lafayette, IN; model 82408) was used to generate ICSS. The stimulator delivered from 0.01 to 10 μa bipolar stimulation with a 0.5 sec train duration at a fixed pulse frequency of 50 Hz. Actual stimulation strength varied across individual rats (see below). Nose-poke lights, ICSS stimulation, and response measures were controlled and recorded by Med Associates Win-MPC operant software and interface.
Figure 1.
Top-down schematic of the ICSS-SRT chamber.
Procedure
ICSS rate-response function and pretraining. Before testing in the ICSS-SRT chamber, rats were trained to discriminate lit (ICSS reward) and unlit (no reward) nose-poke recesses in a gray training box (40 cm wide, 50 cm long, 30 cm high) that had two central 3.5-cm-diameter recesses in the floor, 6.5 cm apart center to center. After habituation to the training box, nose-poke rate-response functions were generated using systematically varying current values. Nose poke in either illuminated recess produced stimulation. After using an initial current value sufficient to produce nose poking via one electrode (alternate side used as a backup), this level was incremented or decremented by 0.005 or 0.01 μa as appropriate, and response rates were averaged to generate the appropriate function. The current value at which an individual rat responded at two-thirds of its maximal response rate was used for that rat throughout the remainder of the study. Importantly, then, this procedure enabled us to equate the motivational value of reinforcement across rats, which is impractical with food reward.
In the ICSS-SRT chamber, all four nose-poke recesses were initially lit, and a response to any of them produced stimulation. In the next session, each nose-poke light was extinguished immediately as soon as a response was made, and any subsequent nose poke was unrewarded until all four nose pokes were sampled, at which point the process was repeated. Once the rat was responding efficiently, nose-poke recesses were lit individually in a random manner for a short, 100 trial sequence, with each correct nose poke reinforced individually, before moving on to SRT itself the next day.
Experimental design and SRT testing. Each sequence-length condition was tested within a single session, because preliminary work with a fourtrial repeating sequence indicated that a single session of massed trials produced more robust interference effects than multiple sessions. The first session trained rats in a SRT4 procedure in which there were 645 repetitions of a sequence of four light positions (2580 trials) before being switched, uncued, to 60 random “sequences” of the four light positions (240 trials). The second session used a similar number of total trials but a SRT8 procedure with repetition of a sequence of eight positions of the four nose-poke lights before 30 random sequences of eight light positions. The third session trained rats in an SRT12, with repetition of a sequence of 12 light positions before random presentation of the 4 lights for 20 random sequences of 12 light positions. These training regimens were used for the “control-repeating” sham lesion group (n = 11; no lesions), “caudate-repeating” lesion group (n = 12), and “hippocampal-repeating” lesion group (n = 15). In addition, a “control-random only” sham lesion group (n = 12; no lesions) only ever experienced random sequences throughout each 2820 trial session. Data for one rat in the control-random only group were lost from the SRT8 condition because of computer failure. Only five rats in the control-random only group and six rats in the control-repeating condition were run in the SRT12 condition. Reaction time was measured from the onset of nose-poke illumination until a successful response. All reaction-time data were log transformed, per Knopman and Nissen (1987). In addition, nose pokes at any of the three unlit holes during a trial were recorded as error responses.
The 4-trial repeating sequence of light positions was 3–1-4–2; the 8-trial sequence was 2–4-2–1-4–3-1–3; and the 12-trial sequence was 4–1-3–2-4–3-2–4-1–2-1–3. An important methodological feature was that random sequences were generated as per Reed and Johnson (1994) to ensure that all frequency information was identical between random and repeating sequences. Thus frequency information such as transition probability and “reversals” (a stimulus position repeating immediately after an intervening stimulus position, e.g., 1–3-1) did not differ between the random and repeating sequences. This ensured that the only difference between the sequence structures of the repeating and random conditions was the presence or absence of the repeating sequence. For example, in the case of transition probabilities, the probability in the SRT12 sequence that stimulus position one immediately follows stimulus position four was 66%. Although this transition probability was maintained in each set of 12 trials in the random sequences used in the SRT12 condition, the order of transitions within which each set of 12 random trials occurred was not maintained. Sets of random sequences were combined per Fellows (1967) to create a unique 480 trial block of random sequences for each sequence length, and the block examined to ensure it contained no unintended sequence blocks identical to the repeating sequence. The random sequence set was scrutinized, and any reversals included in the repeating sequence and any four-trial, or greater, fragments of the repeating sequence were removed. A 480 trial block of random trials was generated for each sequence length and used as the basis for any sequence to random transition within that sequence length condition. Each subject used half of the 480 trial random block, and the start position within the block varied randomly for each subject in each session.
Histology. Rats were killed with an overdose of sodium pentobarbitone and perfused with 4% formalin, and the brain was transferred to a longterm sucrose solution. Sections (50 μm) were cut on a cryostat, and relevant sections through a given target region were stained with cresyl violet.
Results
Histology
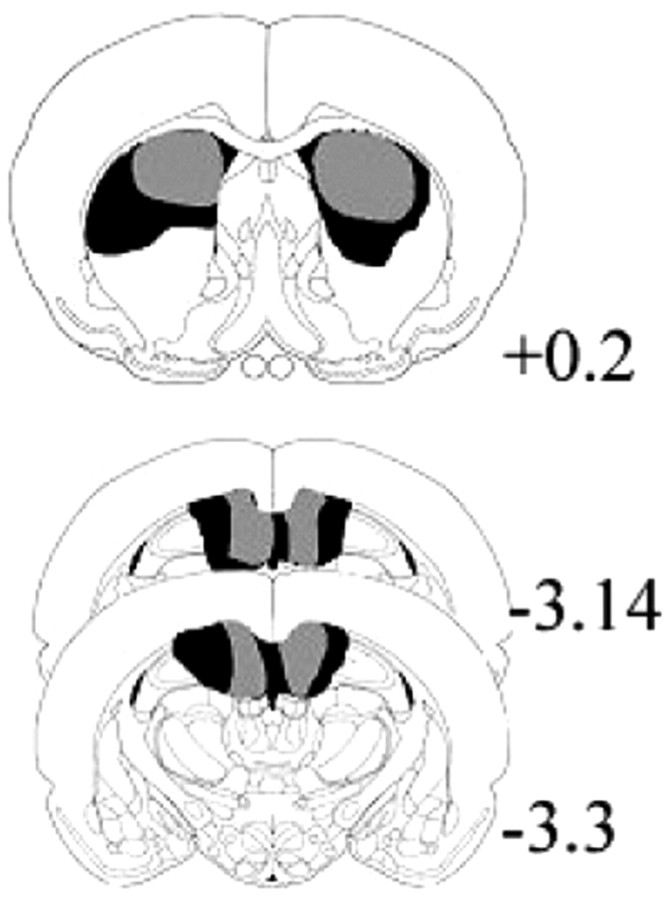
The minimum and maximum extent of the radiofrequency lesions are shown in Figure 2. Rats in the dorsal caudate group exhibited appropriately located damage with almost no extra-caudate damage. Hippocampal lesions were restricted within the dorsal hippocampal formation, particularly the dentate gyrus, CA1 region, dorsal subicular complex, and dorsal fornix; in addition, there was generally bilateral damage to the overlying white matter, including the cingulum bundle, and slight damage to the underlying thalamus. No association was evident between extent of lesion and SRT behavior in either lesion group. Regardless of group, all rats had ICSS electrode tips located within the boundaries of the medial forebrain bundle at the level of the lateral hypothalamus.
Figure 2.
Reconstruction of minimum (gray areas) and maximum (dark areas) radiofrequency lesions of the caudate lesions (A) and the hippocampal lesions (B). Values show distance from bregma [adapted with permission from Paxinos and Watson (1986)].
SRT performance
Acquisition was evaluated by examining performance across blocks of 120 trials each. The first 60 trials of each session were regarded as a preliminary warm-up and were not included in the analyses. Thus there were 21 blocks of 30 sequences per block for the SRT4 condition, 21 blocks of 15 sequences per block for SRT8, and 21 blocks of 10 sequences per block for SRT12. Each measure, RT, and errors were analyzed for each SRT condition using a multiple ANOVA (group by block) with block as a repeated measure. As shown in Figure 3, a block main effect indicating an overall improvement in performance was evident for both RT and errors in each SRT condition, with generally weaker changes across blocks as rats progressed over the sequence lengths (RT and errors, respectively, for SRT4: F(20,60) = 55.72, p < 0.001 and F(20,60) = 42.09, p < 0.001; SRT8: (F(20,60) = 21.53, p < 0.001 and F(20,60) = 17.59, p < 0.001; SRT12: F(20,60) = 11.92, p < 0.001 and F(30,60) = 13.91, p < 0.001). Note that RT in particular was more variable in the random-only controls than in other groups. The caudate rats were numerically slower than other rats in the SRT8 and SRT12 conditions, and hippocampal rats made fewer errors in the SRT4 condition, but there were no main effects of group when the data were collapsed across all acquisition blocks for either measure at any sequence length, other than for error rate in the SRT4 condition (F(3,60) = 2.96; p < 0.05). This result was attributable to the lower error rates of the hippocampal lesion group (Fig. 3B). Overall, there were indications from the acquisition data that all groups showed improved performance across training in each condition, with initially poor performance at the start of any new day of training. Performance during acquisition is, however, a mixture of changes in general factors such as sensorimotor skills and attention, in addition to any specific sequence learning. To maximize the chances of detecting sequence learning differences during acquisition, we compared groups across the last three trial blocks for each condition. Regardless of apparent differences in Figure 3, however, the groups did not differ statistically, even at the end of training in each SRT condition. Disruption revealed by the interference effect provides specific evidence of the influence of acquisition of the sequence itself, because the only change is the predictability of the sequence of stimuli. As in the classic human studies, interference effects were analyzed by comparing behavior from the 10 preceding sequence repetitions with that of the 10 sequence repetitions immediately after the switch to random sequences, which also equates the number of sequences across the various sequence lengths. Pilot work with the SRT4 procedure in intact rats confirmed that these trials immediately after switch produced the clearest interference effects, before nonspecific factors presumably again influenced performance. Difference scores were generated and analyzed by single-sample t test to determine whether individual groups displayed a significant change in behavior relative to their performance before switch. One-way ANOVAs were used to analyze these difference scores between groups.
Figure 3.
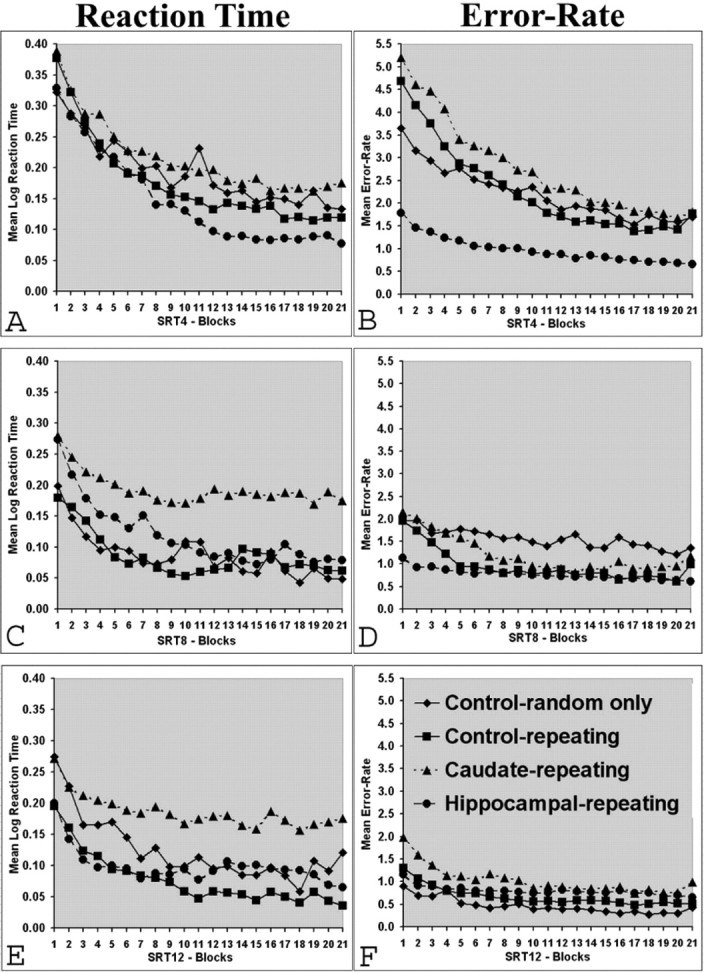
Acquisition performance: mean reaction time and error rate for each sequence length condition. Diamonds indicate control-random only; squares indicate control-repeating; triangles indicate caudate-repeating; circles indicate hippocampal-repeating.
The interference effects in terms of RT and error rate for each sequence length are shown in Figure 4. For the SRT4 condition (Fig. 4A), all groups demonstrated a significant deterioration in RT performance when switched from the four-trial repeating sequence to the random sequence (control-random only, t(11) = 2.73, p < 0.05; control-repeating, t(10) = 2.23, p < 0.05; caudate-repeating, t(11) = 2.24, p < 0.05; and hippocampal-repeating, t(14) = 4.7, p < 0.001). It seems likely that the apparent RT interference effect for the control-random only group, albeit relatively weak, was caused by their marked preexisting variation in RT performance. Both the control-repeating and the hippocampal-repeating rats also demonstrated significant error-rate interference effects in the SRT4 condition (t(10) = 2.9 p < 0.025, and t(14) = 3.37, p < 0.01, respectively), whereas the caudate-repeating group just failed to reach significance on this measure (t(11) = 2.19, p = 0.051) (Fig. 4B). Error rate interference effects were not evident in the control-random only group (t(11) = –1.08; NS). The between-groups ANOVA just failed to show a significant overall difference for the RT measure (F(3,46) = 2.48; p = 0.07), whereas the between-group analysis for the error-rate measure was significant (F(3,46) = 3.0; p < 0.05). Post hoc tests indicated that the control-random only group was significantly different from the hippocampal-repeating group for the RT measure, and different from all other groups in the error-rate measure (all p values <0.025).
Figure 4.
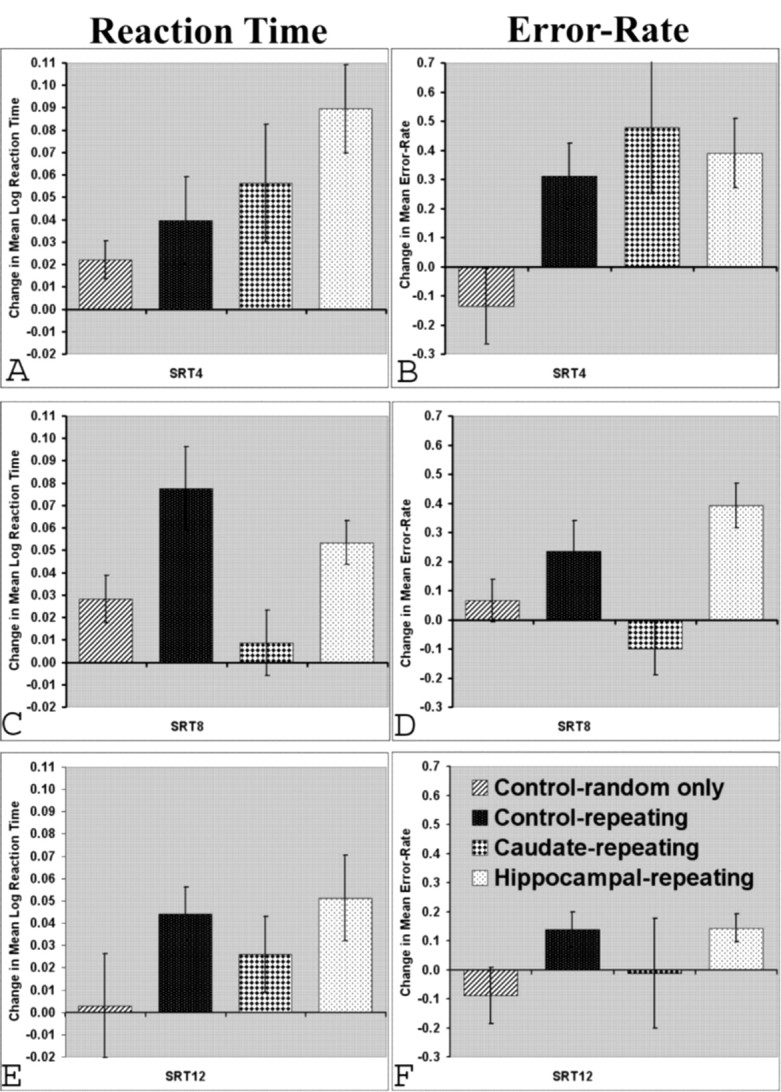
Interference effect (difference) scores for each sequence length condition. Difference scores were generated by comparing the 10 sequences immediately after sequence switch with the 10 sequences immediately before the sequence switch. Positive scores indicate a deterioration in performance. Error bars represent ±1 SEM.
The SRT8 analyses (Fig. 4C,D) revealed that the control-repeating group and the hippocampal-repeating group again demonstrated significant interference effects for both measures (RT: control-repeating, t(10) = 4.34, p < 0.0025, and hippocampal-repeating, t(14) = 5.6, p < 0.001; Errors: control-repeating, t(10) = 2.35, p < 0.005, and hippocampal-repeating, t(14) = 5.37, p < 0.001) (Fig. 4C,D). The caudate-repeating group showed no SRT8 interference effect on either measure (RT: t(11) = 0.62, NS; Errors: t(11) = –1.14, NS). The control-random only group showed no interference effect for the error rate analysis (t(10) = 0.932; NS), but again probably because of earlier variation displayed an apparent interference effect in terms of the RT measure for the SRT8 condition (t(10) = 2.8; p < 0.05). The between-groups ANOVA for SRT8 was significant for both measures (RT: F(3,45) = 5.16, p < 0.01; Errors: F(3,45) = 6.84, p < 0.001). Post hoc tests confirmed differences between caudate-repeating animals and both control-repeating and hippocampal-repeating groups for both measures (all p values <0.02). There was also a significant difference between the control-random only group and hippocampal animals for the error rate measure (p < 0.05).
Interference effects for the SRT12 condition were generally weaker (Fig. 4E,F); however, both control-repeating and the hippocampal-repeating groups again demonstrated an interference effect when switched from the repeating to the random sequences (RTs: control-repeating, t(5) = 4.12, p < 0.01; hippocampal-repeating, t(14) = 2.75, p < 0.025; Errors: control-repeating, t(5) = 2.71, p < 0.05, hippocampal-repeating, t(14) = 3.13, p < 0.01). By contrast, both the caudate-repeating and control-random only groups failed to demonstrate any interference effects for the SRT12 condition. The ANOVAs detected no overall group main effect, but pairwise differences were evident between the control-random only group and both the control-repeating and hippocampal-repeating groups on the error-rate measure.
Discussion
We tested the ability of rats to perform an animal analog of the human SRT task. Interference effects were examined in control, caudate, and hippocampal rats after they had been trained on repetitions of short (4-trial), medium (8-trial), and long (12-trial) sequences of stimuli, each followed by a within-session switch to random sequences. Disruption to ongoing responding, in terms of both reaction time and errors, provided evidence of acquisition specific to these repeating sequences in the control and hippocampal groups at all sequence lengths. In contrast, rats with caudate lesions showed evidence of learning the short (4-trial) sequence but were unable to learn the medium (8-trial) or long (12-trial) sequence.
The new rat SRT shares many key similarities with the human SRT. Our use of four stimulus–response locations is analogous to the sensorimotor aspects of the human paradigm and, like the human task, enabled us to use varying sequence lengths. Similarly, the rats performed massed trials of a repeating sequence before being switched, within session, to a random sequence, which provides evidence of critical interference effects. Rats performed the task rapidly and seamlessly, with minimal pause for behavioral reward (<1 sec). Following Reed and Johnson (1994), we provided an equal stimulus probability and equated other statistical aspects of frequency information between the repeating and random sequences. To equate the number of sequences across the various sequence lengths, interference effects were generated from the 10 preceding sequence repetitions and the 10 sequence repetitions immediately after the switch to random sequences, in a format similar to human SRT studies.
Our inclusion of random-only controls also validates this new rat SRT. These intact rats, which experienced only random sequences, learned to respond faster and made fewer errors within sessions. The overall pattern of results, however, confirmed that clear interference effects were evident only in rats trained on repeating sequences. Performance during acquisition is confounded by nonspecific variables, such as general sensorimotor skills and attentional factors, rather than or in addition to sequence learning. We interpret the fact that the random-only controls did not show consistently worse “acquisition” than the controls that were trained with repeating sequences as a further indication that acquisition data in SRT tasks are problematic. Human studies have also found no difference in RT performance during acquisition between random-only and repeating-sequence conditions (Stadler, 1992; Reed and Johnson, 1994; Jackson et al., 1995). Conversely, weak acquisition improvements, for example in AD or amnesic patients, is not necessarily associated with markedly poor or absent sequence learning, as reflected by robust interference measures in these subjects (Knopman and Nissen, 1987; Nissen and Bullemer, 1987; Ferraro et al., 1993; Reber and Squire, 1994, 1998). The random-only controls in the current study, however, did display more variable reaction time performance during acquisition. This RT variability led to some weak indications of RT changes in the randomonly group at the point of switch for the repeating-sequence groups, but these behavioral controls showed no corresponding changes in error rate, unlike the control-repeating group.
It was also clear that rats with dorsal caudate lesions showed behavioral improvements during each acquisition phase and thus were capable of performing the underlying requirements of the task. As mentioned, these rats showed evidence of acquisition of the short-sequence SRT. Hence the caudate impairment at longer sequences was not caused by any general sensorimotor or attentional impairment or an inability to learn simple stimulus–reward associations. The failure by caudate rats at the longer sequences indicates that their generally impaired SRT is dependent on the complexity of the task. Our rat SRT findings clarify the neural basis of sequence learning examples of nondeclarative memory in that they strongly imply that it is specifically dorsal caudate dysfunction, not hippocampal system injury, that provides a major contribution to sequence learning impairments in basal ganglia disorders. Furthermore, the SRT impairments in early AD and in amnesic cases that we identified in our metaanalysis (Christie and Dalrymple-Alford, 2002) are thus probably related to damage outside the hippocampus. One consequence of our evidence is that basal ganglia patients may perform better when trained on an SRT with shorter sequences, perhaps using eye movements or verbal responses to minimize declarative strategies, which suggests a new assay for screening early cases and monitor disease progression.
An interesting previous rat SRT model used an eight-arm radial maze procedure (DeCoteau and Kesner, 2000). Single food-rewarded maze arms were opened sequentially and the order of opened maze doors constituted the repeating sequence that rats were expected to learn “implicitly.” A noteworthy declarative memory counterpart, in which the rat had to deliberately orientate toward the appropriate target door (“declare intent”) before it opened, was also used. Rats with hippocampal lesions were impaired in the declarative version of the task but not the procedural version. The converse was true of rats with medial caudate lesions. Rats with lateral caudate lesions were not impaired in either task. The latter evidence suggests a similar dissociation within the caudate for our SRT paradigm, but other evidence from medial and lateral caudate lesions supports a dorsal–ventral striatal axis rather than a medial–lateral one for response memory (Adams et al., 2001).
The DeCoteau and Kesner (2000) procedure, however, has several potentially important disparities in comparison with the human and rat SRT. In the maze task, rats performed only two discrete repetitions of the sequence per day over several weeks, in comparison with the 40–100 continuous sequences that humans perform within a single session and the 215–645 repetitions that our rats performed within a single session. The failure of their rats with medial caudate lesions to show any behavioral change during acquisition of the nondeclarative repetitions means that for caudate rats the decisive interference effect cannot be evaluated in the maze version. Rats in all of our groups showed clear improvement before the interference test. Sequence conditions were also switched between, rather than within, sessions in the maze task. A within-session switch is a far more sensitive and better indication of sequence learning, because it avoids nonspecific increments in reaction times. DeCoteau and Kesner (2000) used a 5-trial repeating sequence, whereas human SRT studies typically use either a 10- or 12-trial sequence, with four options, similar to our rat SRT conditions. The need for rats to stop and feed in a maze introduces regular pauses into the animal's behavior, unlike the continuous responding typical of the human, and our rat, SRT procedure. Human SRT studies have demonstrated that interrupting stimulus presentation (typically during “dual-stimulus” SRT protocols) reduces a subject's ability to learn the repeating sequence by forcing them to parse the sequence into nonmeaningful chunks (Stadler and Neely, 1997).
It is to be expected that there will be some differences between how humans and rats perform an SRT. Even neurological patients make few errors (typically <5%), whereas our intact rats typically made far more errors. Rats tended to “sweep” their noses past intermediate nose pokes when moving to the target, and such semi-aborted responses were often recorded as errors. Regardless of the dissimilarity with human SRT performance, the modest basal error rate in rats has proven useful as a sensitive measure of SRT performance. The use of ICSS to reward behavior, irrelevant to the human SRT, enabled us to equate reward salience between animals and across sessions, which is impossible with food reward. Furthermore, ICSS encouraged fast and continuous responding without satiation (Gallistel, 1964), which allowed the use of a substantial number of trials within a single session. The longest time by a rat to complete a 2820 trial session was ∼90 min. Thus the use of ICSS conferred several advantages for an animal SRT.
It is of value to have an animal model of a human implicit memory task for which there is already reasonable evidence in humans that it reflects a motor skill–habit learning that is unlikely to be affected by declarative memory. Currently, most animal studies typically dissociate declarative memory from “another” independent memory system. This independent memory system is labeled “nondeclarative” primarily by virtue of being insensitive to hippocampal system damage (Packard and White, 1991; Packard and McGaugh, 1992; McDonald and White, 1994); however, many animal nondeclarative memory tasks often fail to make good conceptual or behavioral contact with human non-declarative memory tasks (Rovee-Collier et al., 2001). For example, Hood et al. (1999) suggested that amnesics are impaired on concurrent discrimination tasks because nonamnesics use declarative memory strategies, yet concurrent discrimination is used as an example of nondeclarative memory in animals (Broadbent et al., 2002). The rat SRT provides a better opportunity for demonstrating nondeclarative memory in animals and dissociation across neural substrates. The degree of anatomical and behavioral similarities between humans and animals in SRT performance–impairment suggests that an ICSS animal SRT will be useful for addressing theoretical and empirical questions involving memory systems and those syndromes that disrupt them.
Footnotes
This work was supported by the Neurological Foundation (New Zealand) and the Psychology Department, University of Canterbury.
Correspondence should be addressed to Dr. Michael Christie, Center for Aging, Genetics, and Neurodegeneration, 114 16th Street, Charlestown Naval Yards, Charlestown, MA 02129. E-mail: mchristi@helix.mgh.harvard.edu.
DOI:10.1523/JNEUROSCI.3340-03.2004
Copyright © 2004 Society for Neuroscience 0270-6474/04/241034-06$15.00/0
References
- Adams S, Kesner R, Ragozzino ME (2001) Role of the medial and lateral caudate-putamen in mediating an auditory conditional response association. Neurobiol Learn Memory 76: 106–116. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Whishaw IQ (1992) Cortex, striatum and cerebellum: control of serial order in a grooming sequence. Exp Brain Res 90: 275–290. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E (2000) Pathoanatomy of Parkinson's disease. J Neurol 247 [Suppl 2]: II/3-II/10. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Clark RE, Zola S, Squire LR (2002) The medial temporal lobe and memory. In: Neuropsychology of Memory, Ed 3 (Squire LR, Schacter DL, eds), pp 3–23. New York: Guilford.
- Christie MA, Dalrymple-Alford JC (2002) The serial reaction time (SRT) task: meta-analysis, development of a direct animal analogue of this human non-declarative memory task, and contributions of both human and animal SRT tasks to the multiple memory systems theory. FENS 1: A008.5. [Google Scholar]
- Compton DM (2001) Are memories for stimulus-stimulus associations or stimulus-response associations responsible for serial-pattern learning in rats? Physiol Behav 72: 643–652. [DOI] [PubMed] [Google Scholar]
- DeCoteau WE, Kesner RP (2000) A double dissociation between the rat hippocampus and medial caudoputamen in processing two forms of knowledge. Behav Neurosci 114: 1096–1108. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ (2001) From conditioning to conscious recollection: memory systems of the brain. Oxford psychology series, no. 35. London: Oxford UP.
- Fellows BJ (1967) Chance stimulus sequences for discrimination tasks. Psychol Bull 67: 87–92. [DOI] [PubMed] [Google Scholar]
- Ferraro FR, Balota DD, Connor LT (1993) Implicit memory and the formation of new associations in nondementing Parkinson's disease individuals and individuals with senile dementia of the Alzheimer type: a serial reaction time (SRT) investigation. Brain Cognition 21: 163–180. [DOI] [PubMed] [Google Scholar]
- Gallistel CR (1964) Electrical self-stimulation and its theoretical implications. Psychol Bull 61: 23–34. [DOI] [PubMed] [Google Scholar]
- Hood KL, Postle BR, Corkin S (1999) An evaluation of the concurrent discrimination task as a measure of habit learning: performance of amnesic subjects. Neuropsycholgia 37: 1375–1386. [DOI] [PubMed] [Google Scholar]
- Jackson GM, Jackson SR, Harrison J, Henderson L, Kennard C (1995) Serial reaction time learning and Parkinson's disease: evidence for a procedural learning deficit. Neuropsychologia 33: 577–593. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Baxter MG (2001) Multiple brain-memory systems: the whole does not equal the sum of its parts. Trends Neurosci 24: 324–330. [DOI] [PubMed] [Google Scholar]
- Knopman D, Nissen MJ (1987) Implicit learning in patients with probable Alzheimer's disease. Neurology 37: 784–788. [DOI] [PubMed] [Google Scholar]
- Knopman D, Nissen MJ (1991) Procedural learning is impaired in Huntington's disease: evidence from the serial reaction time task. Neuropsychologia 29: 245–254. [DOI] [PubMed] [Google Scholar]
- Kopelman MD (2002) Disorders of memory. Brain 125: 2152–2190. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM (1994) Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol 61: 560–570. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P (1987) Attentional requirements of learning: evidence from performance measures. Cognit Psychol 19: 1–32. [Google Scholar]
- Nixon PD, Passingham RE (2000) The cerebellum and cognition: cerebellar lesions impair sequence learning but not conditional visuomotor learning in monkeys. Neuropsychologia 38: 1054–1072. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL (1992) Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: further evidence for multiple memory systems. Behav Neurosci 106: 439–446. [DOI] [PubMed] [Google Scholar]
- Packard MG, White NM (1991) Dissociation of hippocampus and caudate nucleus memory systems by posttraining intracerebral injection of dopamine agonists. Behav Neurosci 105: 295–306. [DOI] [PubMed] [Google Scholar]
- Packard MG, Hirsch R, White NM (1989) Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. J Neurosci 9: 1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. San Diego: Academic.
- Proyck E, Dominey PF, Amiez C, Joseph JP (2000) The effects of sequence structure and reward schedule on serial reaction time learning in the monkey. Cognit Brain Res 9: 239–248. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Squire LR (1994) Parallel brain systems for learning with and without awareness. Learn Memory 1: 217–229. [PubMed] [Google Scholar]
- Reber PJ, Squire LR (1998) Encapsulation of implicit and explicit memory in sequence learning. J Cognit Neurosci 10: 248–263. [DOI] [PubMed] [Google Scholar]
- Reed J, Johnson P (1994) Assessing implicit learning with indirect tests: determining what is learned about sequence structure. J Exp Psychol Learn Mem Cogn 20: 585–594. [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B (2002) Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology 58: 695–701. [DOI] [PubMed] [Google Scholar]
- Rovee-Collier C, Hayne H, Columbo M (2001) The development of implicit and explicit memory. Advances in consciousness research. Amsterdam: John Benjamins.
- Sommer M, Grafman J, Clark K, Hallett M (1999) Learning in Parkinson's disease: eyeblink conditioning, declarative learning and procedural learning. J Neurol Neurosurg Psychiatry 67: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler MA (1992) Statistical structure and implicit serial learning. J Exp Psychol 18: 318–327. [Google Scholar]
- Stadler MA, Neely CB (1997) Effects of sequence length and structure on implicit serial learning. Psychol Res 60: 14–23. [Google Scholar]
- Westwater H, McDowall J, Siegert R, Mossman S, Abernathy D (1998) Implicit learning in Parkinson's disease: evidence from a verbal version of the serial reaction time task. J Clin Experimental Neuropsychol 20: 413–418. [DOI] [PubMed] [Google Scholar]