Abstract
Objectives
The role of the immune system in head and neck squamous cell carcinoma is controversial. The aim of our study was to analyze full blood counts and distribution of T cell subsets in patients affected by laryngeal squamous cell cancer (LSCC) and their association with clinical variables and survival.
Study design
Retrospective study.
Methods
We analyzed the levels of platelets, lymphocytes, and neutrophils, as well as the CD4+, CD8+, and CD3+ T‐cell subpopulations by cytofluorometry in LSCC patients. A cohort of healthy patients was used as control group. The disease‐specific survival (DSS) was considered as survival outcome.
Results
Sixty‐five LSCC patients and 48 controls were enrolled. In LSCC patients, neutrophils were higher than in the healthy group (P < .0001). The neutrophil‐to‐lymphocyte ratio (NLR) and the platelet‐to‐lymphocyte ratio (PLR) were both higher in LSCC patients (P < .0001). In patients treated for recurrent disease, the CD8+/CD3+ ratio was increased (P = .02), while the CD4+/CD8+ (P = .03) and CD4+/CD3+ (P = .04) ratios were lower. In patients with lymph node metastases, leukocytes (P = .03), CD3+ (P = .04), and CD4+ (P = .0098) were all higher. Among Stages III‐IV patients, low lymphocyte and low leukocyte count were associated with worse DSS.
Conclusion
Our data demonstrate that NLR and PLR are significantly increased in LSCC. Lower CD4+/CD8+ and CD3+/CD8+ ratios are related to recurrent disease and a higher level of CD3+ and CD4+ is associated with nodal metastasis.
Level of Evidence
4
Keywords: Laryngeal cancer, hematologic tests, lymphocytes, immune system, outcome
INTRODUCTION
Laryngeal squamous cell cancer (LSCC) represents one of the most common neoplasms among head and neck squamous cell carcinomas (HNSCC) and it is one of a few oncologic diseases in which the 5‐year survival rate has decreased over the past 40 years, from 66% to 63%, although its overall incidence is declining.1, 2 Carcinogenesis is a multifactorial process that has not been fully elucidated, and the role of immune system in the etiopathogenesis and progression of many cancers is a topic of paramount interest, especially in recent years.3 Indeed, these aspects have been thoroughly investigated in many types of solid tumors, allowing a number of immunomodulatory and/or immunostimulating drugs to be actively investigated in clinical practice.4, 5, 6, 7, 8
The immune surveillance theory assigns specific roles to different leukocyte populations: neutrophils, macrophages, and lymphocytes T‐helper 2 (Th2) induce a cytokine environment favoring chronic inflammation, typical of neoplastic diseases, while natural killer cells, CD8+ lymphocytes, and major cytotoxic response factors comprise tumor suppressor populations. However, despite extensive research, reliable systemic inflammatory markers with diagnostic and/or prognostic value are still lacking.
In recent years, there has been rising interest in the use of systemic hematological markers as promising prognosticators in malignancies. For instance, lymphocytopenia, as well as thrombocytosis, have been associated with a higher risk of development of colorectal cancer and mortality.9, 10 The neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR), parameters derived from routine pretreatment full blood count, have been recently investigated in a variety of malignancies in which higher pretreatment ratios have been associated with worse prognosis in terms of mortality and recurrence rates.11, 12, 13
To the best of our knowledge, few investigations have considered this topic in the head and neck literature, with particular emphasis on LSCC.14, 15, 16, 17, 18, 19, 20 Our study aimed to investigate how the immune system might change in patients with LSCC compared to a control group of subjects treated for a nonneoplastic, noninflammatory disease, and to investigate a possible association with clinical‐pathological characteristics and prognosis.
MATERIALS AND METHODS
We enrolled 65 patients treated with curative intent for primary or recurrent LSCC from March 2015 to June 2016 at the Department of Otorhinolaryngology, Head and Neck Surgery, University of Genoa, Italy. Ethical review and approval was not required for this study in accordance with the national and institutional requirements; however, every patient preoperatively signed a consent form for disclosure of privacy in managing personal data for scientific purposes in accordance with the Declaration of Helsinki.
The only inclusion criterion was surgery with curative intent for LSCC. Exclusion criteria were: known distant metastatic disease, previous cancers at other sites, immunological disorders, long‐term steroidal treatments, active concomitant infection or history of chronic inflammatory conditions except for chronic obstructive pulmonary disease, commonly observed in this patient population due to widespread smoking; missing full blood tests or lymphocyte counts. As a control group, we used a cohort of 48 subjects (40 males and 8 females), matched for gender and age, undergoing surgery for nasal septoplasty at the same Department.
The clinical and pathological variables analyzed were: age at diagnosis, gender, smoking habit, status (i.e., first treatment vs. recurrence), site (glottis vs. supraglottis), TNM classification according to the 8th Edition,21 and grade. For survival analysis, we considered the disease‐specific survival (DSS), defined as the time between the date of the surgery and the date of cancer related death or last visit.
Routine full blood tests were obtained at pretreatment workup for all patients (oncologic cohort and control group), and the immunological parameters taken into account were the red blood cells, white blood cells (WBCs), neutrophils, and lymphocyte counts. For the oncologic cohort, quantitative analysis of CD3+, CD4+, and CD8+ T lymphocyte subpopulations was also performed by cytofluorometry (FACS CANTO II Cytometer, Becton Dickinson, San Jose, CA, USA).
The NLR was defined as the absolute neutrophil divided by absolute lymphocyte counts.22 The PLR was considered as the absolute platelet divided by absolute lymphocyte counts.14 We also evaluated the CD4+/CD3+, CD8+/CD3+, and CD4+/CD8+ ratios defined as the ratios of the absolute counts of the respective T‐cell subpopulations.
Statistical Analysis
Categorical data, including patient demographics (age, gender, and smoking habit) and clinical‐pathological characteristics (status, site, T and N categories, staging, and grade) were displayed as frequency counts and percentages. Shapiro–Wilk test of normality was applied to continuous variables (age, immunologic cells counts, NLR, PLR, CD4+/CD3+, CD8+/CD3+, and CD4+/CD8+ ratios). Subsequently, a nonparametric Mann–Whitney test or Fisher's exact test were chosen for group comparisons, as appropriate. As an age of more than 60 years has been proved to be an independent prognostic feature for DSS in HNSCC and LSCC, we selected 60 years of age as the cutoff point.23 Univariate survival analyses for DSS were calculated using the Kaplan–Meier method and the differences in rates were assessed by log rank test. The Cox proportional‐hazards model was applied for multivariable analysis. GraphPad Prism software Version 6.0 (San Diego, CA, USA) and R (version 3.3.1) were used for statistical analysis. In all tests, being tested two‐sided, a P < .05 was considered statistically significant.
RESULTS
Sixty‐five patients (57 males, 8 females; mean age, 68 years; range, 41–83) treated with curative intent for primary or recurrent LSCC were enrolled. Fifty‐one patients (78%) underwent transoral laser microsurgery, 11 (17%) total laryngectomy, and 3 (5%) open partial horizontal laryngectomy; clinical and pathological features are summarized in Table 1. A control group, composed of 48 subjects (40 males and 8 females), matched for gender and age, undergoing surgery for nasal septoplasty at the same Department was identified.
Table 1.
Patient Demographics and Clinical‐Pathological Characteristics.
| Variable | n (%) | |
|---|---|---|
| All | 65 (100) | |
| Age | <60 | 15 (23) |
| ≥60 | 50 (77) | |
| Gender | Female | 8 (12) |
| Male | 57 (88) | |
| Smoke | No | 7 (11) |
| Yes | 58 (89) | |
| Status | First treatment | 52 (80) |
| Recurrence | 13 (20) | |
| Site | Glottis | 55 (85) |
| Supraglottis | 10 (15) | |
| T category | T1‐T2 | 39 (60) |
| T3‐T4 | 26 (40) | |
| N category | N0 | 59 (91) |
| N+ | 6 (9) | |
| Stage | I‐II | 38 (58) |
| III‐IV | 27 (42) | |
| Grading | G1 | 21 (32) |
| G2‐G3 | 44 (68) | |
The oncologic cohort of patients was comparable to the control group according to age (P = .3), gender (P = .59) and presence of comorbidities (P = .85; Table 2). In patients affected by LSCC, the total amounts of leucocytes and neutrophils were significantly higher than in the control group (P = .001 and P < .0001, respectively). The NLR and the PLR were also higher in LSCC patients compared to the healthy group (P < .0001 and P < .0001, respectively) (Table 2 and Fig. 1). Presence of comorbidities was not associated neither with WBC (P = .95), neutrophils (P = .80), lymphocytes (P = .91) or PLT counts (P = .06) nor with NLR (P = .55) or PLR (P = .31).
Table 2.
Comparison of Full Blood Count Parameters in Oncologic Patients and Healthy Controls.
| Variables | Patients N 65 | Controls N 48 | P |
|---|---|---|---|
| Age (years) | 71 (61–77) | 68 (63–73) | .30* |
| Gender (Male) [N (%)] | 57 (88%) | 40 (83%) | .59† |
| Presence of comorbidities [N (%)] | 38 (58%) | 29 (60%) | .85† |
| RBC (106/μL) | 4.8 (4.6–5.1) | 5 (4.65–5.2) | .21* |
| PLT (103/μL) | 216 (174–271) | 216 (186–247) | .88* |
| WBC (103/μL) | 7.3 (5.8–8.5) | 5.9 (5.2–7.23) | .001* |
| Neutrophils (103/μL) | 4.8 (3.7–5.9) | 3.55 (2.8–4.44) | <.0001* |
| Lymphocytes (103/μL) | 1.63 (1.2–2) | 1.79 (1.46–2.03) | .11* |
| NLR | 2.86 (2.04–4.21) | 2.1 (1.64–2.52) | <.0001* |
| PLR | 217 (175–272) | 118 (98–142) | <.0001* |
Median values (I–III quartiles) reported.
P values by Mann–Whitney test.
P values by Fisher's exact test.
RBC = red blood cell; PLT = platelets; WBC = white blood cell.
Figure 1.

Comparison of full blood count parameters in oncologic patients and healthy controls. NLR = neutrophil to lymphocyte ratio; PLR = platelet to lymphocyte ratio.
Among the oncologic cohort, NLR was significantly higher for the supraglottic than the glottic site (P = .046), and PLR was higher in patients younger than 60 years compared to older ones (P = .04). In patients treated for recurrent disease, compared to those previously untreated, the CD8+/CD3+ ratio was significantly increased (P = .02), while the CD4+/CD8+ and CD4+/CD3+ ratios were lower (P = .03 and P = .04, respectively). In patients with lymph node metastases, compared to those without, a significant increase of the WBC (P = .02), CD3+ (P = .04), and CD4+ lymphocytes count (P = .01) was seen (Table 3).
Table 3.
Comparison of Full Blood Count Parameters and T‐Cell Subsets in Subgroups of Patients.
| PLT (*103/μL) | WBC (*103/μL) | Neutrophils (*103/μL) | Lymphocytes (*103/μL) | NLR | PLR | CD3 (*103/μL) | CD4 (*103/μL) | CD8 (*103/μL) | CD4/CD8 | CD4/CD3 | CD8/CD3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | x˜ | P | x˜ | P | x˜ | P | x˜ | P | x˜ | P | x˜ | P | x˜ | P | x˜ | P | x˜ | P | x˜ | P | x˜ | P | x˜ | P | ||
| Age | <60 | 15 (23) | 237 | .04 | 7.30 | .64 | 4.20 | .99 | 1.60 | .41 | 2.42 | .27 | 238 | .04 | 1.50 | .23 | 0.83 | .13 | 0.49 | .79 | 1.89 | .77 | 0.64 | .99 | 0.34 | .53 |
| ≥60 | 50 (77) | 206 | 7.31 | 4.80 | 1.65 | 2.94 | 208 | 1.26 | 0.71 | 0.45 | 1.64 | 0.63 | 0.38 | |||||||||||||
| Status | First treatment | 52 (80) | 217 | .72 | 7.25 | .82 | 4.65 | .51 | 1.63 | .74 | 2.86 | .5 | 218 | .71 | 1.34 | .53 | 0.77 | .18 | 0.42 | .46 | 1.94 | .03 | 0.66 | .04 | 0.34 | .03 |
| Recurrence | 13 (20) | 208 | 7.31 | 4.80 | 1.63 | 2.86 | 210 | 1.32 | 0.71 | 0.54 | 1.40 | 0.59 | 0.42 | |||||||||||||
| Site | GL | 55 (85) | 212 | .46 | 7.31 | .58 | 4.70 | .14 | 1.66 | .31 | 2.70 | .046 | 214 | .43 | 1.34 | .66 | 0.74 | .74 | 0.49 | .61 | 1.67 | .97 | 0.64 | .84 | 0.38 | .96 |
| SGL | 10 (15) | 242 | 7.13 | 5.79 | 1.22 | 3.83 | 244 | 0.97 | 0.65 | 0.35 | 1.80 | 0.62 | 0.35 | |||||||||||||
| T | T0‐T2 | 39 (60) | 212 | .7 | 7.20 | .58 | 4.75 | .9 | 1.66 | 1 | 2.98 | .57 | 214 | .68 | 1.32 | .74 | 0.72 | .51 | 0.45 | .67 | 1.43 | .17 | 0.59 | .22 | 0.40 | .19 |
| T3‐T4 | 26 (40) | 220 | 7.65 | 4.80 | 1.58 | 2.36 | 222 | 1.34 | 0.73 | 0.47 | 2.00 | 0.67 | 0.33 | |||||||||||||
| N category | N0 | 59 (91) | 212 | .23 | 7.26 | .02 | 4.70 | .09 | 1.56 | .07 | 2.86 | .78 | 214 | .22 | 1.32 | .04 | 0.71 | .01 | 0.46 | .66 | 1.66 | .25 | 0.61 | .22 | 0.38 | .32 |
| N+ | 6 (9) | 241 | 9.14 | 5.50 | 2.20 | 2.92 | 243 | 1.76 | 1.15 | 0.42 | 2.44 | 0.69 | 0.29 | |||||||||||||
| Risk factors | No | 44 (68) | 217 | .86 | 7.36 | .98 | 4.73 | .96 | 1.67 | .98 | 3.00 | .67 | 218 | .88 | 1.32 | .96 | 0.78 | .78 | 0.47 | .89 | 1.68 | .73 | 0.65 | .79 | 0.38 | .62 |
| Yes | 21 (32) | 207 | 7.30 | 4.80 | 1.53 | 2.44 | 208 | 1.37 | 0.71 | 0.46 | 1.66 | 0.61 | 0.37 | |||||||||||||
Median (x˜) values reported; P values by Mann–Whitney test.
NLR = neutrophil‐to‐lymphocyte ratio; PLR = platelet‐to‐lymphocyte ratio; PLT = platelets; Risk factors = perineural invasion and/or lymphovascular invasion; WBC = white blood cells.
Comparison between healthy subjects and LSCC patients and within the oncologic cohort itself did not reveal any other significant differences in the levels of immune cells or their ratios (Tables 2 and 3).
The mean DSS was 38.6 months (95% confidence interval: 36.6–40.6); among the entire cohort 11 patients (17%) developed recurrence during follow‐up time, and 5 of them died for disease progression. As all events of death related to the disease were observed among patients in Stages III‐IV, further survival analysis was performed in this subgroup. Among preoperative full blood results, for each continuous variable, the median value was chosen as the cutoff. Low leukocyte and low lymphocyte counts were associated with worse DSS (P = .02 and P = .003, respectively) (Table 4 and Fig. 2); among clinical data, only the presence of risk factors (perineural invasion and/or lymphovascular invasion) was related with a worse prognosis (P = .045). No other significant prognostic variable, among clinical data or blood exams, was found (Table 4). The multivariable analysis confirmed the significantly independent adverse prognostic value of having lower WBC count for DSS (hazard ratio 0.419, P = .045; Table 5), adjusted by lymphocyte count and recurrent disease as a clinically relevant variable.
Table 4.
Univariate Disease‐Specific Survival among Stages III‐IV Patients.
| N (%) | Disease‐Specific Survival | |||
|---|---|---|---|---|
| 2‐y Survival Probability | P | |||
| Age ≥ 60 | No | 6 (22) | 83% | .78 |
| Yes | 21 (78) | 82% | ||
| Gender | Female | 4 (15) | 67% | .54 |
| Male | 23 (85) | 85% | ||
| Status | Primary | 22 (81) | 84% | .81 |
| Recurrence | 5 (19) | 75% | ||
| Type treatment | TLM | 15 (56) | 83% | .73 |
| Open neck | 12 (44) | 82% | ||
| Site | GL | 19 (70) | 87% | .53 |
| SGL | 8 (30) | 71% | ||
| T category | T2‐T3 | 20 (74) | 83% | .69 |
| T4a | 7 (26) | 83% | ||
| N status | N0 | 21 (78) | 80% | .87 |
| N+ | 6 (22) | 83% | ||
| Risk factors | No | 12 (44) | 100% | .045 |
| Yes | 15 (56) | 71% | ||
| PLT | Low | 13 (48) | 84% | .93 |
| High | 14 (52) | 82% | ||
| WBC | Low | 13 (48) | 68% | .02 |
| High | 14 (52) | 100% | ||
| Neutrophils | Low | 12 (44) | 74% | .16 |
| High | 15 (56) | 92% | ||
| Lymphocytes | Low | 13 (48) | 58% | .003 |
| High | 14 (52) | 100% | ||
| NLR | Low | 13 (48) | 83% | .74 |
| High | 14 (52) | 82% | ||
| PLR | Low | 13 (48) | 84% | .93 |
| High | 14 (52) | 82% | ||
| CD3 | Low | 13 (48) | 72% | .12 |
| High | 14 (52) | 92% | ||
| CD4 | Low | 13 (48) | 69% | .07 |
| High | 14 (52) | 93% | ||
| CD8 | Low | 13 (48) | 72% | .12 |
| High | 14 (52) | 92% | ||
| CD4/CD8 | Low | 13 (48) | 74% | .50 |
| High | 14 (52) | 91% | ||
| CD4/CD3 | Low | 13 (48) | 74% | .50 |
| High | 14 (52) | 91% | ||
| CD8/CD3 | Low | 13 (48) | 90% | .66 |
| High | 14 (52) | 76% | ||
Median values, used as cutoff for high or low levels of each variable, were: PLT 222; WBC 8.00; Neutrophils 4.80; Lymphocytes 1.63; NLR 2.42; PLR 224; CD3 1.35; CD4 0.74; CD8 0.49; CD4/CD8 1.94; CD4/CD3 0.66; CD8/CD3 0.32. P values by log‐rank test.
NLR = neutrophil‐to‐lymphocyte ratio; PLR = platelet‐to‐lymphocyte ratio; PLT = platelets; Risk factors = perineural invasion and/or lymphovascular invasion; WBC = white blood cells.
Figure 2.
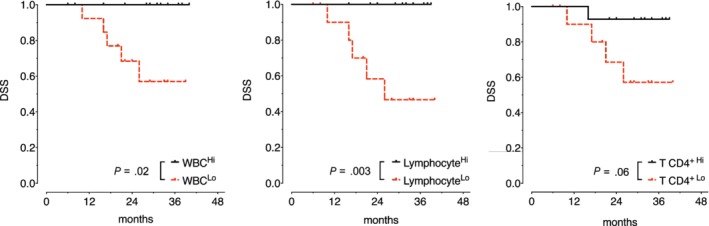
Disease‐specific survival (DSS) estimates (Kaplan–Meier) according to leukocyte (white blood cell), lymphocyte, and CD4+ T cell counts.
Table 5.
Multivariable Analysis for Disease‐Specific Survival among Stages III‐IV Patients.
| B | P | HR | HR (95% CI) | |
|---|---|---|---|---|
| WBC | −0.869 | .045 | 0.419 | 0.42 (0.18–0.98) |
| Lymphocytes | −1.832 | .16 | 0.16 | 0.16 (0.01–2.07) |
| Status (recurrent) | 2.597 | .119 | 13.419 | 13.42 (0.51–352.33) |
B = ß coefficients; CI = confidence interval; HR = hazard ratio; WBC = white blood cell.
DISCUSSION
Already in the nineteenth century, Rudolf Virchow underlined the intimate relationships between inflammation and malignancy based on the presence of leukocytes in neoplastic tissues, while only 30 years ago Dvorak showed how wound healing and tumor stroma formation are linked by many important properties.24, 25 Moreover, malignant cells themselves secrete proinflammatory cytokines. For all these reasons, the persistence of a noxious stimulus induces the presence of lymphocytes, macrophages, and neutrophils in that tissue.26
Chronic inflammation secondary to different causes has been associated with one‐third of all tumors. The tumor itself maintains chronic inflammation, leading to the production of interleukins 4 and 5 (IL 4 and 5) and cytokines associated with Th2 cells, whereas interferon gamma is associated with Th1 responses. Regarding inflammation, tumors represent a paradox: they produce inflammatory cytokines and chemokines, but are infiltrated by leukocytes.27 However, these are associated with a poor capacity to initiate inflammatory reactions at sites other than tumors, and circulating monocytes from cancer patients are notably defective in their ability to respond to cytokines.
Several studies have linked oncological outcomes with circulating neutrophils in a wide variety of tumors.28, 29, 30 In the last decade, a rising number of authors reported the role of both peripheral blood and tumor infiltration neutrophils or lymphocytes in HNSCC,31 with an even more prominent role given to the NLR.13, 15, 32, 33, 34 HNSCCs include a vast variety of tumors in terms of biological behavior and histologies, and not discerning among that multitude may lead to a mixture of results that cause the inherent heterogeneity observed in various subsites in terms of etiologies and survival rates.
Different studies have recently demonstrated that the robust infiltration of HNSCC by CD3+ and CD8+ T cells is a favorable prognostic factor.35 Wolf et al. carried out an elegant study on 40 patients affected by HNSCCs: the patterns and degrees of lymphocyte subpopulation infiltration in tumor parenchyma and stroma did not differ by tumor site, except for LSCC.36 Kum et al. have also shown that NLR may be a useful marker to differentiate SCC patients from those with benign or precancerous lesions, as it is increased in the former.37 Wong et al.38 also showed that patients with Stages III‐IV LSCC have a higher NLR than those with Stages I‐II lesions. Moreover, the prognostic significance of NLR in terms of both overall survival and progression‐free survival was also confirmed by multivariate analysis in a large cohort of 654 patients treated for LSCC.18
Another study evaluated the role of systemic and local inflammation in predicting outcomes in patients with LSCC, finding that markers of systemic and local inflammation, especially PLR and tumor infiltrating lymphocyte density, are reliable prognostic factors.39 The PLR, also correlated with inflammation and malnutrition, has been associated with poorer cancer‐specific survival at both univariate and multivariate analysis in a large cohort (n = 899) by Mao et al.17 Starska et al. studied the function of peripheral CD4+ and CD8+ T cells in LSCC, finding an association between high local stage and the presence of early activation antigens on these cells.19, 20
However, the NLR, PLR, and the simultaneous analysis of the subpopulations of CD3+, CD4+, and CD8+ T cells in peripheral blood samples have never been previously investigated in LSCC, in particular discerning different lymphocyte subgroups in primary or recurrent LSCC. The known bias considering also previously treated patients, enrolled at the time of salvage surgery, was managed including such variable in all analysis and being the investigation of peripheral blood population changes in those patients one of the main objectives of the study. Our results confirm that the levels of circulating leukocytes and neutrophils are higher in LSCC patients compared to healthy subjects. The result about the worse prognostic value of a low level of leukocyte count, also confirmed by the multivariable analysis, is in agreement with the study of Hsueh et al. on a huge cohort of 979 patients affected by LSCC that demonstrated its independent prognostic significance for DSS at multivariate analysis too.16 Moreover, since the CD8+/CD3+, the CD4+/CD3+, and the CD4+/CD8+ ratios are significantly different in patients treated for recurrence compared to untreated ones, we propose to integrate these easy and reproducible tools in the follow‐up of previously treated LSCC patients, as previously it has also been shown a decreasing of lymphocyte count at the time of recurrence in patients treated for head and neck cancer.40
In our series, as previously observed by Drennan et al. in 2013 for laryngeal and oropharyngeal cancers, the levels of CD3+ and CD4+ are higher in patients with positive nodes than in those presenting an N0 neck.41 This finding could be explained by the inflammatory reaction developing in the presence of lymph node metastasis and advanced stages of disease. On the other hand, González et al. found an opposite relationship, but in their study only the relative frequencies of T cell subsets were considered instead of their absolute counts.42 In fact, it is well known that patients with more advanced disease have significant systemic immunological changes, including decreased numbers in activated dendritic cells, an increase in regulatory T cells, depletion in PD‐1 expressing T cells, and poor T cell function.43
CONCLUSION
In conclusion, while other HNSCCs have been investigated more thoroughly, information on systemic inflammatory markers and peripheral T cell subsets is lacking in LSCC. Our preliminary study demonstrates that significant changes in the peripheral immune system could have a role in the development of LSCC or either could be a systemic effect of it. Among patients affected by advanced stages LSCC (Stage III‐IV), the preoperative leukocyte and lymphocyte counts, for their low cost and most of the cases available yet, could be interesting biomarkers for DSS to be further validated in wider analysis.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
Conceptualization: F.M., F.Mi., and G.P.; Data curation: F.M., F.Mi., F.I., M.F., F.Ma., F.Mo., and G.Pa.; Formal analysis: F.Mi. and A.P.; Writing original draft: F.M. and F.Mi.; Supervision ‐ review and editing: C.P. and G.P.
Editor's Note: This Manuscript was accepted for publication on August 10, 2019.
Filippo Marchi and Francesco Missale equally contributed to the manuscript.
Grant Information: None of the authors have any commercial interest in the subject of this study or received any financial or material support for this study.
Presented at the 12th European Laryngological Society Meeting, 16‐19 May, 2018, London, UK, and winner of the “Patrick Bradley Oncology Prize” as the best oral presentation at the Patrick Bradley Oncology Free Paper Session.
BIBLIOGRAPHY
- 1. Steuer CE, El‐Deiry M, Parks JR, Higgins KA, Saba NF. An update on larynx cancer. CA Cancer J Clin 2017;67:31–50. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 3. Varilla V, Atienza J, Dasanu CA. Immune alterations and immunotherapy prospects in head and neck cancer. Expert Opin Biol Ther 2013;13:1241–1256. [DOI] [PubMed] [Google Scholar]
- 4. Clark WH. Tumour progression and the nature of cancer. Br J Cancer 1991;64:631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kawata A, Une Y, Hosokawa M, Uchino J, Kobayashi H. Tumor‐infiltrating lymphocytes and prognosis of hepatocellular carcinoma. Jpn J Clin Oncol 1992;22:256–263. [PubMed] [Google Scholar]
- 6. Nakano O, Sato M, Naito Y, et al. Proliferative activity of intratumoral CD8(+) T‐lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res 2001;61:5132–5136. [PubMed] [Google Scholar]
- 7. Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor‐infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005;102:18538–18543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sharma P, Shen Y, Wen S, et al. CD8 tumor‐infiltrating lymphocytes are predictive of survival in muscle‐invasive urothelial carcinoma. Proc Natl Acad Sci U S A 2007;104:3967–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bang KM, Laing CA. Lymphocytopenia in high cancer risk population: evidence in automobile pattern makers. Cancer Lett 1986;30:311–314. [DOI] [PubMed] [Google Scholar]
- 10. Monreal M, Fernandez‐Llamazares J, Pinol M, et al. Platelet count and survival in patients with colorectal cancer‐‐a preliminary study. Thromb Haemost 1998;79:916–918. [PubMed] [Google Scholar]
- 11. Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation‐based neutrophil‐lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218–230. [DOI] [PubMed] [Google Scholar]
- 12. Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil‐to‐lymphocyte ratio in solid tumors: a systematic review and meta‐analysis. J Natl Cancer Inst 2014;106:dju124. [DOI] [PubMed] [Google Scholar]
- 13. Turri‐Zanoni M, Salzano G, Lambertoni A, et al. Prognostic value of pretreatment peripheral blood markers in paranasal sinus cancer: neutrophil‐to‐lymphocyte and platelet‐to‐lymphocyte ratio. Head Neck 2017;39:730–736. [DOI] [PubMed] [Google Scholar]
- 14. Smith RA, Bosonnet L, Raraty M, et al. Preoperative platelet‐lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg 2009;197:466–472. [DOI] [PubMed] [Google Scholar]
- 15. Rassouli A, Saliba J, Castano R, Hier M, Zeitouni AG. Systemic inflammatory markers as independent prognosticators of head and neck squamous cell carcinoma. Head Neck 2015;37:103–110. [DOI] [PubMed] [Google Scholar]
- 16. Hsueh C, Tao L, Zhang M, et al. The prognostic value of preoperative neutrophils, platelets, lymphocytes, monocytes and calculated ratios in patients with laryngeal squamous cell cancer. Oncotarget 2017;8:60514–60527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mao Y, Fu Y, Gao Y, Yang A, Zhang Q. Platelet‐to‐lymphocyte ratio predicts long‐term survival in laryngeal cancer. Eur Arch Otorhinolaryngol 2018;275:553–559. [DOI] [PubMed] [Google Scholar]
- 18. Du J, Liu J, Zhang X, et al. Pre‐treatment neutrophil‐to‐lymphocyte ratio predicts survival in patients with laryngeal cancer. Oncol Lett 2018;15:1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Starska K, Glowacka E, Kulig A, Lewy‐Trenda I, Brys M, Lewkowicz P. The role of tumor cells in the modification of T lymphocytes activity–the expression of the early CD69+, CD71+ and the late CD25+, CD26+, HLA/DR+ activation markers on T CD4+ and CD8+ cells in squamous cell laryngeal carcinoma. Part I. Folia Histochem Cytobiol 2011;49:579–592. [PubMed] [Google Scholar]
- 20. Starska K, Glowacka E, Kulig A, Lewy‐Trenda I, Brys M, Lewkowicz P. Prognostic value of the immunological phenomena and relationship with clinicopathological characteristics of the tumor‐‐the expression of the early CD69+, CD71+ and the late CD25+, CD26+, HLA/DR+ activation markers on T CD4+ and CD8+ lymphocytes in squamous cell laryngeal carcinoma. Part II. Folia Histochem Cytobiol 2011;49:593–603. [PubMed] [Google Scholar]
- 21. Amin MB, Edge SB. AJCC Cancer Staging Manual. Springer International Publishing, New York, USA; 2017. [Google Scholar]
- 22. Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil‐lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 2005;91:181–184. [DOI] [PubMed] [Google Scholar]
- 23. Yang CC, Su YC, Lin YW, Huang CI, Lee CC. Differential impact of age on survival in head and neck cancer according to classic Cox regression and decision tree analysis. Clin Otolaryngol 2019;44:244–253. [DOI] [PubMed] [Google Scholar]
- 24. Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986;315:1650–1659. [DOI] [PubMed] [Google Scholar]
- 25. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–545. [DOI] [PubMed] [Google Scholar]
- 26. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer‐related inflammation. Nature 2008;454:436–444. [DOI] [PubMed] [Google Scholar]
- 27. Al‐Qahtani D, Anil S, Rajendran R. Tumour infiltrating CD25+ FoxP3+ regulatory T cells (Tregs) relate to tumour grade and stromal inflammation in oral squamous cell carcinoma. J Oral Pathol Med 2011;40:636–642. [DOI] [PubMed] [Google Scholar]
- 28. Teramukai S, Kitano T, Kishida Y, et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non‐small‐cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00‐03. Eur J Cancer 2009;45:1950–1958. [DOI] [PubMed] [Google Scholar]
- 29. Lee YY, Choi CH, Kim HJ, et al. Pretreatment neutrophil:lymphocyte ratio as a prognostic factor in cervical carcinoma. Anticancer Res 2012;32:1555–1561. [PubMed] [Google Scholar]
- 30. Bahig H, Taussky D, Delouya G, et al. Neutrophil count is associated with survival in localized prostate cancer. BMC Cancer 2015;15:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nguyen N, Bellile E, Thomas D, et al. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck 2016;38:1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Salim DK, Mutlu H, Eryilmaz MK, et al. Neutrophil to lymphocyte ratio is an independent prognostic factor in patients with recurrent or metastatic head and neck squamous cell cancer. Mol Clin Oncol 2015;3:839–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tham T, Bardash Y, Herman SW, Costantino PD. Neutrophil‐to‐lymphocyte ratio as a prognostic indicator in head and neck cancer: a systematic review and meta‐analysis. Head Neck 2018;40:2546–2557. [DOI] [PubMed] [Google Scholar]
- 34. Abbate V, Dell'Aversana Orabona G, Salzano G, et al. Pre‐treatment neutrophil‐to‐lymphocyte ratio as a predictor for occult cervical metastasis in early stage (T1‐T2 cN0) squamous cell carcinoma of the oral tongue. Surg Oncol 2018;27:503–507. [DOI] [PubMed] [Google Scholar]
- 35. Balermpas P, Rodel F, Weiss C, Rodel C, Fokas E. Tumor‐infiltrating lymphocytes favor the response to chemoradiotherapy of head and neck cancer. Oncoimmunology 2014;3:e27403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wolf GT, Hudson JL, Peterson KA, Miller HL, McClatchey KD. Lymphocyte subpopulations infiltrating squamous carcinomas of the head and neck: correlations with extent of tumor and prognosis. Otolaryngol Head Neck Surg 1986;95:142–152. [DOI] [PubMed] [Google Scholar]
- 37. Kum RO, Ozcan M, Baklaci D, et al. Elevated neutrophil‐to‐lymphocyte ratio in squamous cell carcinoma of larynx compared to benign and precancerous laryngeal lesions. Asian Pac J Cancer Prev 2014;15:7351–7355. [DOI] [PubMed] [Google Scholar]
- 38. Wong BY, Stafford ND, Green VL, Greenman J. Prognostic value of the neutrophil‐to‐lymphocyte ratio in patients with laryngeal squamous cell carcinoma. Head Neck 2016;38:E1903–E1908. [DOI] [PubMed] [Google Scholar]
- 39. Wang J, Wang S, Song X, et al. The prognostic value of systemic and local inflammation in patients with laryngeal squamous cell carcinoma. Onco Targets Ther 2016;9:7177–7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kuss I, Hathaway B, Ferris RL, Gooding W, Whiteside TL. Decreased absolute counts of T lymphocyte subsets and their relation to disease in squamous cell carcinoma of the head and neck. Clin Cancer Res 2004;10:3755–3762. [DOI] [PubMed] [Google Scholar]
- 41. Drennan S, Stafford ND, Greenman J, Green VL. Increased frequency and suppressive activity of CD127(low/−) regulatory T cells in the peripheral circulation of patients with head and neck squamous cell carcinoma are associated with advanced stage and nodal involvement. Immunology 2013;140:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gonzalez FM, Vargas JA, Gea‐Banacloche JC, et al. Functional and phenotypic analysis of T‐lymphocytes in laryngeal carcinoma. Acta Otolaryngol 1994;114:663–668. [DOI] [PubMed] [Google Scholar]
- 43. Grotz TE, Jakub JW, Mansfield AS, et al. Evidence of Th2 polarization of the sentinel lymph node (SLN) in melanoma. Oncoimmunology 2015;4:e1026504. [DOI] [PMC free article] [PubMed] [Google Scholar]


