Abstract
Background
Evidence suggests that olfactory impairment (OI) may be a degenerative neurologic complication of diabetes; however, the association is not yet well established. The objective of this work was to systematically review existing literature on the association between diabetes and OI in adults, with meta‐analysis of evaluable studies.
Methods
A literature search encompassing 358 abstracts from the last 75 years in PubMed, EMBASE, and Cochrane was performed. English‐language articles investigating adults with diabetes and OI in comparison to control groups with original data and ≥7 subjects were included. The Newcastle‐Ottawa scale was applied for quality assessment. Two investigators independently reviewed all articles. For meta‐analysis, the odds ratio of OI in diabetes compared with control groups was calculated using the fixed effects model.
Results
The initial search yielded 358 abstracts, from which 21 articles were reviewed and 11 articles (n = 6,747) were included. Studies included were case‐control (64%) or cross‐sectional (36%) with evidence level 3b. On the Newcastle‐Ottawa scale, the mean quality assessment score for case‐control and cross‐sectional studies was 7.4 (maximum of 9) and 7.0 (maximum of 10), respectively. A statistically significant association between diabetes and olfaction compared with controls was found in 6 (55%) of the 11 articles. Four studies were eligible for meta‐analysis, which yielded an overall odds of having OI with diabetes as 1.58 times more likely than in control groups (95% CI [1.16, 2.16]; I 2 = 10.3%).
Conclusions
The reviewed studies support a significant association between diabetes and OI. Further studies are warranted to characterize this association.
Level of Evidence
3a
Keywords: Anosmia, dysosmia, hyposmia, smell disorder, olfactory nerve diseases
INTRODUCTION
Diabetes mellitus (DM) presents an increasingly significant health challenge in the United States, affecting over 30 million adults.1 For individuals with type I (T1D) and type II (T2D) diabetes, microvascular complications, such as peripheral neuropathy and retinopathy, contribute to increased morbidity and health care costs.2 Although visual impairment resulting from microvascular complications in diabetes has been well studied,3 the association between olfactory impairment (OI) and diabetes is not well understood.
Olfaction is an underappreciated sense that is often overlooked in clinical practice in comparison to vision and hearing. However, olfaction plays a critical role in everyday functioning—impacting food intake, safety, survival, and social communication.4 Previous studies have highlighted the importance of olfaction in maintaining health, as OI is associated with decreased quality of life and depressive symptoms.5, 6 Furthermore, OI is an increasingly relevant health concern in an aging population, with estimates of greater than 60% of individuals above 80 years of age experiencing OI.7 Although an epidemiologic study reported 18% prevalence in the general population,8 this is likely an underestimate as many individuals remain undiagnosed and often overestimate their subjective sense of smell.9
Although olfaction is not routinely assessed in the clinical setting, its application in diabetes management may be warranted, as previous studies have suggested an association between OI and diabetes‐related cognitive impairment.10 Moreover, the current availability of low‐cost, validated tools11 for olfactory assessment lends further support for potential utility in clinical practice.
To examine the association between diabetes and OI in adults, we performed a systematic review and meta‐analysis of the existing literature.
METHODS
Information Searches and Sources
A systematic review of published English literature was conducted to investigate the association between diabetes and OI in adults. The systematic review was conducted with adherence to the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) guidelines as shown in Figure 1.12
Figure 1.
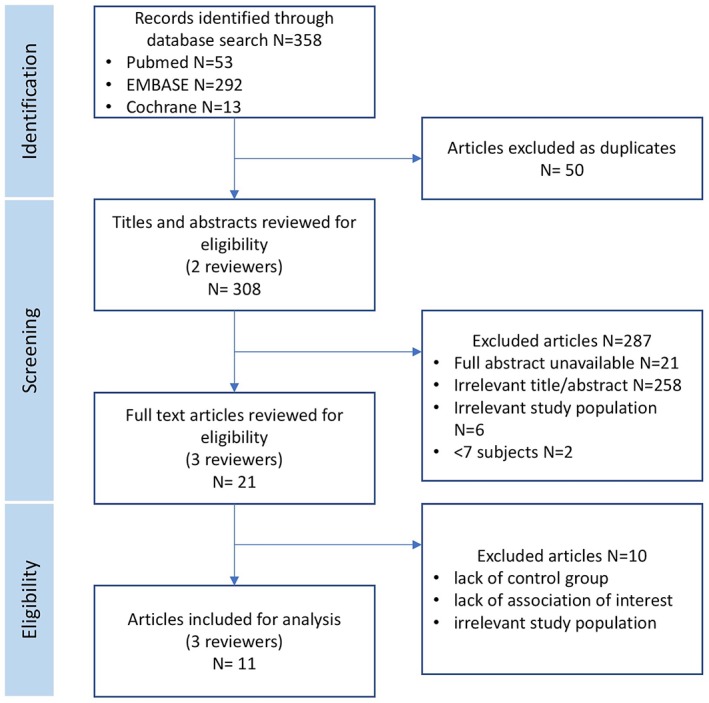
PRISMA flowchart of study selection for systematic review, article review, and selection.
A medical librarian (SS) with expertise in systematic reviews was consulted to develop comprehensive search strategies. PubMed, Cochrane Library, and EMBASE were queried for relevant publications. Date filters were not applied with the intention of generating a broad list of potential studies. A principal electronic search strategy was developed for PubMed and then applied to the other databases. The electronic search incorporated the following Medical Subject Headings (MeSH): olfaction disorders, olfactory nerve diseases, diabetes mellitus, and diabetes insipidus. The following key words were also included: smell disorder, cacosmia, dysosmia, paraosmia, anosmia, and cranial nerve disease (Table 1).
Table 1.
Search Strategy.
| Major MeSH Terms | Major Text Terms |
|---|---|
| Olfaction disorders | Smell disorder |
| Olfactory nerve disease | Cacosmia |
| Diabetes mellitus | Dysosmia |
| Diabetes insipidus | Paraosmia |
| Anosmia | |
| Cranial nerve disease |
MeSH = medical subject headings.
In addition to the electronic search strategy, relevant review articles and references were examined for thorough assessment of the existing literature (Supporting Information Appendix).
Study Selection and Eligibility Criteria
Two investigators (SJK, MJW, or SYL) independently reviewed abstracts and selected studies for inclusion based on prespecified criteria. Where inclusion decisions differed, full articles were discussed to reach consensus. English‐language articles investigating adults with diabetes and OI in comparison to control groups with original data and ≥7 subjects were included. Exclusion criteria were studies with no abstract present; written in a language besides English; not relevant to the study question; duplicate articles; case report/small case series; secondary research (review article, position paper); pediatric population; population with congenital abnormalities (cystic fibrosis, Kallmann syndrome), no outcome of interest; incomplete data; lack of a nondiabetic control group. Accompanying full‐text publications were reviewed by two investigators to confirm that all criteria were met. In cases of disagreement, discussion including a third investigator (SL) was used to reach consensus.
Data Extraction and Quality Assessment
Data were extracted and reviewed independently by two investigators using a predesigned form. Disagreement was addressed through a review of the full‐text article and input from a third investigator (SL). Extracted data included study design, patient demographics, T1D and/or T2D prevalence, method of olfactory testing, method of diabetes diagnosis, estimates of association between prevalent diabetes and OI, and other clinical characteristics. Level of evidence was determined based on published guidelines by the Oxford Centre for Evidence‐Based Medicine, Levels of Evidence (OCEBM Levels of Evidence 2009).13 A modified Newcastle‐Ottawa Assessment Scale14 for assessing nonrandomized studies in systematic reviews was applied for quality assessment. This scale uses the following domains to assess the quality of the study: representativeness and selection of cases and controls, comparability of controls on basis of design/analysis, and ascertainment of exposure/outcome. No studies were excluded based on quality assessment.
Summary Measures and Meta‐Analysis
Articles were categorized based on olfactory test method, diabetes diagnostic criteria, and primary findings (significant association; positive, negative) regarding the relationship between olfaction and diabetes. Meta‐analytic methods that accounted for between‐study heterogeneity were used to estimate pooled effect sizes from the systematic review. Only studies with odds ratios (ORs) describing the odds of OI in participants with diabetes against the odds of OI in the control group were included in the meta‐analysis. Heterogeneity across studies was assessed using the I 2 statistic. If the heterogeneity test was significant, the random effects model was used. If the heterogeneity test was nonsignificant, the fixed effects model was used. Analysis of publication bias was performed using funnel plot techniques in the Egger weighted‐linear regression method. Analyses were performed using Stata 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC).
RESULTS
Search Characteristics
The initial search yielded 358 abstracts (PubMed 53, EMBASE 292, and Cochrane 13), from which 21 articles were reviewed and 11 articles were included. The 11 publications selected in this review included a total of 6,747 subjects. Included articles were case‐control (7, 64%) or cross‐sectional (4, 36%) with evidence level 3b. No randomized controlled trials met inclusion criteria. Study population sizes ranged widely from N = 60–3,151. A summary of all included articles is shown in Table 2.
Table 2.
Articles included in systematic review.
| # | Author | Study Type | Year | N Total (Case, Control) | Population Studied | Age (years)* | Diabetes Subtype | Olfactory Test (Tool, Component) | OI Definition | ENT Exam | Diabetes Diagnosis | Main Findings | Adjustments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Brady et al | Case‐control | 2013 | 70 (51, 19) | Patients from University of Calgary in Canada | Mean, 57.9 (DM), 52.9 (control) | T2D | Sniffin' Sticks TDI, subjective odor ratings (pleasantness, intensity, familiarity) |
No standard definition | No | FSG > 126 mg/dL, OGTT > 200 mg/dL | DM patients showed significant reduction in olfactory function compared with control group (F[6, 63] = 2.68, P = .02) | — |
| 2 | Bramerson et al† | Cross‐sectional | 2004 | 1,387 (not reported) | Swedish population | 20–80+ (stratified) | Not specified | SOIT Identification |
Hyposmia if 10–12, anosmia if <9 on a 16‐point scale | Yes | Self‐report | Risk for anosmia increased with DM (OR = 2.6, 95% CI [1.3–5.5]) | — |
| 3 | Chan et al† | Cross‐sectional | 2017 | 3,151 (657, 2,494) | US nationally representative sample (NHANES) | ≥40 | Not specified | Self‐report 8‐item Pocket Smell Test Identification |
Severe hyposmia or anosmia if <5 correct answers on 8‐item pocket smell test | No | FSG > 126 mg/dL, OGTT > 200 mg/dL, HbA1c ≥ 6.5%, self‐report, use of DM drugs or insulin | Diabetics under insulin treatment showed a higher prevalence of severe hyposmia/anosmia (OR = 1.57, 95% CI [0.89–2.78]) | Age, gender, race, education, smoking, sinonasal symptoms, xerostomia, head injury, hypertension, obesity, cardiovascular disease |
| 4 | Duda‐Sobczak et al† | Case‐control | 2017 | 136 (106, 30) | Patients from Poznan University in Poland | Mean, 35 (DM), 40 (control) | T1D | Sniffin' Sticks Identification |
OI if 0–10 on a 12‐point scale | Yes | Patients recruited from outpatient clinic (HbA1c measured) | No significant difference in hyposmia prevalence in T1D (67.9%) vs. control (53.3%) | — |
| 5 | Gouveri et al | Case‐control | 2014 | 154 (119, 35) | Patients from Democritus University of Thrace in Greece | Mean, 63.6 (DM), 51.5 (control) | T2D | Sniffin' Sticks TDI |
Anosmia if ≤15, hyposmia if 16–34.5 on a 48‐point scale | Yes | Patients recruited from outpatient clinic (plasma glucose, HbA1c measured) | Patients with T2DM had lower TDI scores (29.29 ± 5.24 vs. 34.86 ± 3.72, P < .001) compared to controls | — |
| 6 | Hawkins et al | Cross‐sectional | 2011 | 288 (63, 225) | African Americans living independently in the community | Mean 64.2, range, 55–87 | T2D | BSIT Identification |
No standard definition | No | Self‐report | Patients with and without diabetes performed at near identical levels on BSIT (9.89 ± 1.7 vs. 9.82 ± 2.0, t = 0.26, P = .8) | — |
| 7 | Khil et al† | Cross‐sectional | 2015 | 1,208 (not reported) | Inhabitants of Dortmund, Germany | Mean, 51.9, range, 25–74 | Not specified | Sniffin' Sticks Identification |
OI if <10 on a 12‐point scale | No | Physician diagnosis or blood glucose ≥ 200 mg/dL | No significant relationship between olfaction and diabetes (OR = 1.16, 95% CI [0.69–1.94]) | Age, sex |
| 8 | Le Floch et al | Case‐control | 1993 | 98 (68, 30) | Patients recruited from outpatient clinic in Creteil, France | Mean, 55.6 (T2D insulin), 52.8 (T2D non‐insulin), 36.6, (T1D), 52.5 (control) | T1D, T2D | Smell recognition score Identification |
No standard definition | No | Patients recruited from outpatient clinic (plasma glucose, HbA1c measured) | Smell recognition score was significantly lower in diabetic patients (12.4 ± 0.5 vs. 15.1 ± 0.5, P < .001) | — |
| 9 | Naka et al | Case‐control | 2010 | 105 (76, 29) | Patients recruited from Medical University of Vienna, Austria | Mean, 52.5 (DM), 45.6 (control) | T1D, T2D | Sniffin' Sticks Identification |
No standard definition | Yes | Self‐report (not clearly stated) | Patients with uncomplicated DM showed no clinically significant loss of smell function | — |
| 10 | Seraj et al | Case‐control | 2015 | 60 (30, 30) | Patients recruited from diabetes clinic in Iran | Mean, 47 (DM), 42 (control) | Not specified | Absorbent Perfumer's Paper Strips Threshold |
OI if <median of olfactory threshold in the control group | Yes | Patients recruited from outpatient clinic | Significant difference (P < .01) between the median of olfactory threshold in diabetic patients and control group | — |
| 11 | Yazla et al | Case‐control | 2018 | 90 (60, 30) | Patients recruited from outpatient clinic in Turkey | Mean, 55.4 (T2D, w/o DPN), 60.3 (T2D w/DPN) 55.5 (control) | T2D | Butanol Threshold Test Sniffin’ Sticks Threshold, Identification |
Hyposmic if 7–9, anosmic if ≤6 on 12‐point scale | Yes | Patients recruited from outpatient clinic (HbA1c measured) | Control subjects showed significantly higher Sniffin' sticks and butanol threshold scores than the diabetic patients without DPN (P = .001, P = .009) | — |
Studies varied in how they reported age.
Included in meta‐analysis.
BSIT = brief smell identification test; DM = diabetes mellitus; DPN = diabetic peripheral neuropathy; FSG = fasting serum glucose (mg/dL); OGTT = oral glucose tolerance test (mg/dL); OI = olfactory impairment; OR = odds ratio; SOIT = Scandinavian odor identification test; T1D = type I diabetes; T2D = type II diabetes; TDI = threshold discrimination identification.
Associations by Diabetes Subtype
A statistically significant association between prevalent diabetes (T1D and/or T2D) and olfactory function compared with nondiabetic controls was found in 6 (55%) of the 11 articles. The association between olfactory function and diabetes did not vary by diabetes subtype. Among the six articles that demonstrated a statistically significant association between diabetes and OI, three studies15, 16, 17 specifically evaluated participants with T2D, while two studies18, 19 did not distinguish diabetes subtype, and a single study20 characterized both T1D and T2D patients. Among the five articles that did not find a statistically significant association between diabetes and olfactory function, a single study21 evaluated T1D and T2D, two studies22, 23 did not specify subtype, one study24 examined T1D only, and one study25 examined T2D only. Studies that demonstrated a statistically significant association recruited participants from a wide range of geographies (Canada, Sweden, Greece, France, Iran, and Turkey) with variations in baseline characteristics (Table 2).
Olfactory Testing
Multiple components of olfactory function were assessed including odor threshold, discrimination, identification, pleasantness, intensity, and familiarity. Olfactory threshold testing measures the minimum stimulus required to detect odors, while identification and discrimination, which requires the patient to detect, recognize, and name an odor, reflects higher order processing.26 Among the studies included in this review, odor identification was the most commonly tested component of olfaction (10, 91%). Due to differences in the components of olfaction tested, studies did not align on the scoring system used to quantify OI. Sniffin' Sticks was the most common method for assessment of olfactory function (6, 55%), wherein odors are presented as felt‐tipped pens with multiple choice answer options, with extended versions providing measures for odor threshold, discrimination, and identification. Additional validated measures of olfaction included the Scandinavian Odor Identification Test,18 Brief Smell Identification Test,25 and the butanol threshold test.17
The majority of studies (6, 55%) performed a complete otolaryngologic exam or nasal endoscopy to exclude other factors contributing to OI including, for example, acute or chronic rhinosinusitis, nasal polyps, or septal deviation.16, 17, 18, 19, 21, 24 Among studies that included a complete otolarynglogic exam or nasal endoscopy, four out of six found a significant association between OI and diabetes.16, 17, 18, 19 In addition to olfactory testing, a minority of studies (4, 27%) evaluated other sensory functions including auditory, visual, or gustatory impairment.17, 20, 21, 22 Two studies found that the loss of olfactory and gustatory function is not correlated with the duration of diabetes.17, 21 One study found that diabetes was related to an increased odds of multisensory impairment (OR, 1.75; 95% CI [1.16–2.63]).22
Diabetes Diagnosis
Method of diabetes diagnosis ranged from validated clinical measures to self‐reports and physician diagnoses (Table 2). A minority of studies (3, 27%) used fasting serum glucose measures, oral glucose tolerance tests, or HbA1c measures to verify diabetes diagnosis.15, 22, 23 Otherwise, most studies relied on participant self‐reports (3, 27%)18, 21, 25 or recruited participants directly from outpatient diabetes clinics (5, 46%).16, 17, 19, 20, 24 Among studies that used validated clinical measures to diagnose diabetes, only Brady et al found that DM patients demonstrated a general significant reduction in olfactory function when compared with controls.15 Among studies using participant self‐reports,18, 21, 25 Brämerson et al found that the risk of anosmia increased with DM (OR, 2.6; 95% CI [1.3–5.5]), whereas the other studies did not find a significant association between olfaction and diabetes.21, 25 Interestingly, four out of five studies with participants recruited from outpatient clinics found a significant association between diabetes and olfaction (Table 2).16, 17, 19, 20
Other Findings
In addition to examining the relationship between OI and diabetes, the majority of studies (7, 64%) also investigated the association between diabetic peripheral neuropathy and olfaction. While two studies19, 21 found no significant association, the majority of studies16, 17, 20, 24 found that peripheral neuropathy is associated with lower olfactory scores. Brady et al further reported OI particularly among diabetics with neuropathic pain, suggesting that OI may be partially explained by limited attention and concentration due to pain.15 Additional associations of OI with retinopathy, diabetes duration, treatment (oral, insulin), hypertension, and body mass index (BMI) were explored with results summarized in Table 3.
Table 3.
Additional Findings on the Association Between Olfaction and Other Participant Characteristics.
| Article # | Author | DPN | Retinopathy | Diabetes Duration | Treatment | Hypertension | BMI | Other Sensory Impairment |
|---|---|---|---|---|---|---|---|---|
| 1 | Brady et al | OI partially attributed to NeP, but pain severity not associated with OI | Assessed, but association with olfaction not reported | No association with olfaction | Assessed, but association with olfaction not reported | Assessed, but association with olfaction not reported | — | — |
| 2 | Bramerson et al | — | — | — | — | — | — | — |
| 3 | Chan et al | — | — | No association with olfaction | Among DM participants, significant trend to hyposmia/anosmia for those on aggressive treatment (oral and insulin) compared to those with no drug treatment (OR = 1.33, 95% CI [0.60–2.96] and OR = 2.86, 95% CI [1.28–6.40]; P‐trend .01) | Increased prevalence of hyposmia/anosmia in DM not explained by increased prevalence of hypertension among those on aggressive treatment | Positive association between self‐reported olfactory dysfunction but not objective olfactory dysfunction | — |
| 4 | Duda‐Sobczak et al | Lower olfactory identification scores in neuropathy group (8 points [IQR, 7–9] vs. 10 points [IQR, 9–11]; P = .005) | Lower olfactory identification scores in retinopathy group (9 points [IQR, 8–11] vs. 10 points [IQR, 9–11]; P = .03) | Diabetes duration was an independent predictor of neuropathy and retinopathy | — | — | Negative correlation between olfactory identification score and BMI | — |
| 5 | Gouveri et al | TDI scores lower in the presence of DPN (28.23 ± 4.85 vs. 31.15 ± 5.28, P = .017) | TDI scores lower in the presence of retinopathy (27.63 ± 4.58 vs. 30.65 ± 5.78, P = .047) | No association with olfaction | No association with olfaction | Hypertension associated with lower olfactory scores (29.03 ± 4.92 vs. 33.61 ± 5.54, P < .001) | No association with olfaction | — |
| 6 | Hawkins et al | — | — | — | — | Patients with and without hypertension performed at near identical levels on BSIT (9.88 ± 1.91 vs. 9.75 ± 2.0, t = 0.55, P = .59) | — | — |
| 7 | Khil et al | — | — | — | — | Assessed, but association with olfaction not reported | Assessed, but association with olfaction not reported | Auditory, gustatory, visual impairment assessed. Diabetes was related to an elevated odds of multisensory impairment (OR = 1.75, 95% CI [1.16, 2.63]) |
| 8 | Le Floch et al | SRS was associated with DPN (10.3 ± 1.0 vs. 14.1 ± 0.9, P < .01) | In diabetes patients, SRS was not significantly associated with retinopathy (11.2 ± 1.1 vs. 13.7 ± 0.8) | SRS was associated with diabetes duration (r = 0.27, P < .05) | SRS did not differ significantly in diabetes patients with or without antihypertensive drugs (12.2 ± 0.6 vs. 12.5 ± 0.5) | — | In diabetes patients, BMI (r = 0.03) was not significantly associated with SRS | In diabetes patients, SRS was associated with electrogustometric threshold (r = 0.39, P < .001) |
| 9 | Naka et al | DM patients with microangiopathy exhibited unchanged chemosensory function | DM patients with microangiopathy exhibited unchanged chemosensory function | Diabetes duration did not correlate with the degree of chemosensory function | — | — | Assessed, but association with olfaction not reported | Participants with lower BMI exhibited higher taste sensitivity (r = −0.27, P = .019) |
| 10 | Seraj et al | No association with olfaction | No association with olfaction | No association with olfaction | No association with olfaction | — | — | — |
| 11 | Yazla et al | DPN patients had lower Sniffin' Sticks scores (P < .001) and butanol threshold scores (P < .001) compared to controls | — | No correlation between duration of diabetes and Sniffin' sticks scores and butanol threshold scores | Assessed, but association with olfaction not reported | — | No correlation between BMI and Sniffin' sticks scores and butanol threshold scores | DPN participants had higher sucrose thresholds (P = .002). Gustatory function was better in control subjects compared to DPN participants. |
BMI = body mass index (kg/m2); BSIT = brief smell identification test; DM = diabetes mellitus; DPN = diabetic peripheral neuropathy; IQR = interquartile range; NeP = neuropathic pain; OI = olfactory impairment; OR = odds ratio; SRS = smell recognition score; TDI = threshold, discrimination, and identification.
Meta‐Analysis
Four studies were evaluable for meta‐analysis. We only considered studies with a threshold defining OI that either provided an OR or specified the number of participants with OI in the diabetes and control group from which ORs were computed (Fig. 2). Given that the heterogeneity test was nonsignificant, the fixed effects model was used. Overall, the pooled data demonstrated that the odds of having OI with diabetes was 1.58 times more likely than without diabetes (95% CI [1.16–2.16], I 2 = 10.3%) (Fig. 2). Publication bias was assessed using a standard error funnel plot (Fig. 3). There is symmetry of the effect sizes around the pooled overall effect, suggesting limited publication bias related to this association.
Figure 2.

Forest plot of ORs of olfactory impairment (OI) and diabetes. The square is a measure of effect for each study, and its corresponding horizontal line represents 95% confidence intervals. The blue diamond summarizes the average effect size of the four included studies. *OR was calculated from prevalent OI in diabetes and control group provided in article. OR = odds ratio.
Figure 3.

Precision funnel plot illustrating potential for publication bias. Each circle represents one of the four eligible articles included in the systematic review.
Quality Assessment
The mean modified Newcastle‐Ottawa Quality Assessment scores for case‐control and cross‐sectional studies were 7.4 (maximum of 9) and 7.0 (maximum of 10), respectively (Table 4).
Table 4.
Quality Assessment of Included Articles (Modified Newcastle‐Ottawa Quality Assessment Scale Case‐Control and Cross‐Sectional Studies).
| Article | Selection Grade | Comparability Grade | Exposure/Outcome Grade | Total Score |
|---|---|---|---|---|
| Case‐control | Max 4 | Max 2 | Max 3 | Max 9 |
| Brady et al | 3 | 2 | 3 | 8 |
| Duda‐Sobczak et al | 3 | 2 | 3 | 8 |
| Gouveri et al | 3 | 1 | 7 | |
| Le Floch et al | 4 | 2 | 3 | 9 |
| Naka et al | 2 | 2 | 3 | 7 |
| Seraj et al | 1 | 2 | 3 | 6 |
| Yazla et al | 4 | 0 | 3 | 7 |
| Cross‐sectional | Max 5 | Max 2 | Max 3 | Max 10 |
| Bramerson et al | 3 | 2 | 3 | 8 |
| Chan et al | 2 | 2 | 3 | 7 |
| Hawkins et al | 3 | 1 | 3 | 7 |
| Khil et al | 1 | 2 | 3 | 6 |
DISCUSSION
To our knowledge, this is the first systematic review examining the association between diabetes and OI. The results from the 11 studies included in this review support an association between diabetes and OI, highlighting several mechanisms including olfactory nerve impairment related to neurodeneration15 and microvascular disease.16 In a meta‐analysis of four eligible studies included in this review, the odds of having OI with diabetes were 1.58 times more likely than in the control group, and this was statistically significant. Although causal relationship and pathophysiology is not clearly demonstrated in these studies, the majority of studies found significant associations, pointing to the need for larger prospective studies on this topic.
Although not conducted in a systematic fashion, prior review articles have highlighted the potential utility of olfactory testing for early detection of central diabetic neuropathy and diabetes‐related cognitive impairment.27 In fact, OI has been implicated in Alzheimer's dementia, with support for its use as a predictive marker of cognitive decline.28 Given that validated measures of olfactory testing are readily available as a quick and inexpensive clinical tool, a better understanding of the pathophysiology of OI in diabetes could foreseeably translate into clinical application and patient benefit.
The majority of studies included in this review examined OI in participants with T2D, while several studies did not stratify by diabetes subtype, and others exclusively studied participants with T1D (Table 2). An association between OI and diabetes was found in both subtypes, precluding overarching conclusions on whether the association varied by subtype. The lack of stratification in some studies presents a limitation, as the pathophysiologic differences in subtype may play a role in the potential mechanism or baseline characteristics that explain the association between OI and diabetes. In T2D, hyperglycemia can result in increased cortical thinning of the orbitofrontal cortex, contributing to accelerated cognitive decline.27 Central manifestations of diabetic neuropathy affecting the olfactory nerve has also been proposed as a mechanism.16 Other factors may also contribute to differences in subtype, including the prevalence of comorbidities, medication intake, BMI, and age of participants. Participant characteristics across studies also varied widely in sample size (n = 60–3,151) and population of interest (US population‐based to Iranian diabetic hospital‐based), all likely contributing to the differences in outcome and limited generalizability of findings.
Currently, many validated tools are available for accurate assessment of olfactory function (threshold, discrimination, and identification [TDI]) including Sniffin' Sticks11 and the University of Pennsylvania Smell Identification Test (UPSIT).29 Despite the wide availability of these validated tools, the studies included in this review did not adhere to the same method of assessment. Sniffin' Sticks was the most common tool used in olfactory assessment; however, the definition of OI varied among the studies. While some studies tested all components of OI and calculated TDI scores, others only tested identification (Table 2). If olfactory testing has potential clinical utility in predicting diabetes complications, assessments that include fewer odors with a limited range in scores may not adequately measure the degree of OI among patients in the early stages of olfactory decline where detection may be most useful.
The majority of studies included in this review conducted additional analyses, ranging from the association between OI and diabetes complications, subtype, duration of disease, treatment, hypertension, BMI, and multisensory impairment (Table 3). Among diabetic comorbidities, peripheral neuropathy was the most investigated topic. Interestingly, Brady et al further subcategorized participants with diabetes on the basis of neuropathic pain, since chronic pain can be a potential confounder in olfactory testing due to its influence on attention and concentration.15 Although OI was partially attributed to the presence of neuropathic pain on subcategory assessment, pain severity was not associated with olfactory dysfunction.15 Whether neuropathy contributes to or explains OI is inconclusive at best from current evidence.
This evaluation of the current literature points to a need for large, prospective, high‐quality studies that adhere to a standardized definition of OI to define its association with diabetes and it is time to develop relative to other microvascular complications. Study methodologies should adhere to a single, easily administered method of olfactory assessment that incorporates odor threshold, discrimination, and identification with a unified scoring system that allows for comparison of results across multiple studies. A common language is needed to improve methodolic quality and generalizability in this field.30 Studies would benefit from eligibility criteria including no evidence of structural pathology on nasal endoscopy and objective testing for diabetic staging and comorbidities. To guide future investigation into the underlying mechanism of OI in diabetes, participants should ideally be stratified according to diabetes subtype, presence and severity of microvascular complications, presence of multisensory impairment, and type and duration of treatment.
The current evidence supports an association between OI and diabetes, with a potential link to microvascular complications including peripheral neuropathy. Findings from this systematic review must be considered in light of the heterogeneity in participant characteristics, methodological differences, and study designs outlined above. In addition, we only included articles published in English, which may be a potential source of language and publication bias. As studies included do not examine temporality, we caution against drawing conclusions on the predictive value of OI in diabetes and diabetes microvascular complications until large prospective studies are available.
CONCLUSION
This review supports an association between diabetes and OI. However, all of the studies included in this review were case‐control or cross‐sectional studies, with heterogenous methodologies in selection criteria, method of olfactory assessment, and evaluation of diabetic comorbidities. Further high‐quality studies are needed to confirm the association of OI and diabetes, establish temporality, and elucidate underlying pathologic mechanisms. Expanding the body of literature on this topic may provide support for implementing olfactory evaluation as a low‐cost and widely available clinical tool for early detection of diabetic complications and diabetes management.
Supporting information
Appendix S1: Supplementary Information
Acknowledgments
We thank Stella Seal for assistance with collection of database search results, and Gayane Yenokyan for assistance with the meta‐analysis.
Funding and Conflicts of Interest: Melina Windon (NIH grant 5T32DC000027‐29); Sandra Lin (consultant for Aerin Medical and Redesign Health).
BIBLIOGRAPHY
- 1. CDC . National diabetes statistics report, 2017 estimates of diabetes and its burden in the United States background; 2017. Available at: https://www.cdc.gov/diabetes/pdfs/data/statistics/national‐diabetes‐statistics‐report.pdf. Accessed March 3, 2019.
- 2. Deshpande AD, Harris‐Hayes M, Schootman M. Epidemiology of diabetes and diabetes‐related complications. Phys Ther 2008;88(11):1254–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frank RN. Diabetic retinopathy. N Engl J Med 2004;350(1):48–58. [DOI] [PubMed] [Google Scholar]
- 4. Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life–an updated review. Chem Senses 2014;39(3):185–194. [DOI] [PubMed] [Google Scholar]
- 5. Smeets MAM, Veldhuizen MG, Galle S, et al. Sense of smell disorder and health‐related quality of life. Rehabil Psychol 2009;54(4):404–412. [DOI] [PubMed] [Google Scholar]
- 6. Miwa T, Furukawa M, Tsukatani T, Costanzo RM, DiNardo LJ, Reiter ER. Impact of olfactory impairment on quality of life and disability. Arch Otolaryngol Head Neck Surg 2001;127(5):497–503. [DOI] [PubMed] [Google Scholar]
- 7. Murphy C, Schubert CR, Cruickshanks KJ, Klein BEK, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA 2002;288(18):2307–2312. [DOI] [PubMed] [Google Scholar]
- 8. Vennemann MM, Hummel T, Berger K. The association between smoking and smell and taste impairment in the general population. J Neurol 2008;255(8):1121–1126. [DOI] [PubMed] [Google Scholar]
- 9. Philpott CM, Wolstenholme CR, Goodenough PC, Clark A, Murty GE. Comparison of subjective perception with objective measurement of olfaction. Otolaryngol Head Neck Surg 2006;134(3):488–490. [DOI] [PubMed] [Google Scholar]
- 10. Sanke H, Mita T, Yoshii H, et al. Relationship between olfactory dysfunction and cognitive impairment in elderly patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 2014;106(3):465–473. [DOI] [PubMed] [Google Scholar]
- 11. Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odour identification, odor discrimination and olfactory threshold. Chem Senses 1997;22(1):39–52. [DOI] [PubMed] [Google Scholar]
- 12. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oxford Centre for evidence‐based medicine ‐ levels of evidence (March 2009) ‐ CEBM. Available at: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed July 24, 2018.
- 14. Wells G, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed July 20, 2018.
- 15. Brady S, Lalli P, Midha N, et al. Presence of neuropathic pain may explain poor performances on olfactory testing in diabetes mellitus patients. Chem Senses 2013;38(6):497–507. [DOI] [PubMed] [Google Scholar]
- 16. Gouveri E, Katotomichelakis M, Gouveris H, Danielides V, Maltezos E, Papanas N. Olfactory dysfunction in type 2 diabetes mellitus: an additional manifestation of microvascular disease? Angiology 2014;65(10):869–876. [DOI] [PubMed] [Google Scholar]
- 17. Yazla S, Özmen S, Kıyıcı S, Yıldız D, Haksever M, Gencay S. Evaluation of olfaction and taste function in type 2 diabetic patients with and without peripheral neuropathy. Diabetes Metab Res Rev 2018;34(3):1–6. [DOI] [PubMed] [Google Scholar]
- 18. Brämerson A, Johansson L, Ek L, et al. Prevalence of olfactory dysfunction: the skovde population‐based study. Laryngoscope 2004;114(4):733–737. [DOI] [PubMed] [Google Scholar]
- 19. Seraj JM, Seraj SM, Zakeri H, et al. Olfactory dysfunction in Iranian diabetic patients. Acta Med Iran 2015;53(4):204–206. [PubMed] [Google Scholar]
- 20. Le Floch J, Le Lièvre G, Labroue M, Paul M, Peynegre R, Perlemutter L. Smell dysfunction and related factors in diabetic patients. Diabetes Care 1993;16(6):934–937. [DOI] [PubMed] [Google Scholar]
- 21. Naka A, Riedl M, Luger A, Hummel T, Mueller CA. Clinical significance of smell and taste disorders in patients with diabetes mellitus. Eur Arch Otorhinolaryngol 2010;267(4):547–550. [DOI] [PubMed] [Google Scholar]
- 22. Khil L, Wellmann J, Berger K. Determinants of single and multiple sensory impairments in an urban population. Otolaryngol Head Neck Surg 2015;153(3):364–371. [DOI] [PubMed] [Google Scholar]
- 23. Chan JYK, García‐Esquinas E, Ko OH, Tong MCF, Lin SY. The association between diabetes and olfactory function in adults. Chem Senses 2018;43(1):59–64. [DOI] [PubMed] [Google Scholar]
- 24. Duda‐Sobczak A, Araszkiewicz A, Urbas M, et al. Impaired olfactory function is related to the presence of neuropathy in adults with type 1 diabetes. Diab Vasc Dis Res 2017;14(2):139–143. [DOI] [PubMed] [Google Scholar]
- 25. Hawkins KA, Pearlson GD. Age and gender but not common chronic illnesses predict odor identification in older African Americans. Am J Geriatr Psychiatry 2011;19(9):777–782. [DOI] [PubMed] [Google Scholar]
- 26. Sanders RD, Gillig PM. Cranial nerve I: olfaction. Psychiatry 2009;6(7):30–35. [PMC free article] [PubMed] [Google Scholar]
- 27. Zaghloul H, Pallayova M, Al‐Nuaimi O, Hovis KR, Taheri S. Association between diabetes mellitus and olfactory dysfunction: current perspectives and future directions. Diabet Med 2018;35(1):41–52. [DOI] [PubMed] [Google Scholar]
- 28. Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry 2007;64(7):802–808. [DOI] [PubMed] [Google Scholar]
- 29. Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav 1984;32(3):489–502. [DOI] [PubMed] [Google Scholar]
- 30. Hummel T, Whitcroft KL, Andrews P, et al. Position paper on olfactory dysfunction. Rhinol Suppl 2017;54(26):1–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Information


