Abstract
Human papilloma virus (HPV) has been implicated in the development of oropharyngeal squamous cell carcinoma (OPSCC) and is directly attributed to its increasing incidence. The immune microenvironment surrounding HPV‐associated OPSCC tumors is complex and plays a critical role in the carcinogenic process. The neoplastic mechanism includes cells of the innate immunity such as macrophages, and dendritic cells as well as cells of the adaptive immune process such as CD8+ T‐cells. The intricate interactions between these two arms of the immune system allow for a pro‐inflammatory and pro‐tumorigenic environment. Intensive efforts are underway to gain a greater understanding of the mechanisms involved in the immune system's role in tumor development. This study seeks to summarize the current knowledge pertaining to role of the innate and adaptive immune response in HPV‐associated OPSCC.
Level of Evidence
3a
Keywords: Human papilloma virus, HPV‐associated oropharyngeal squamous cell carcinoma, adaptive immunity, innate immunity
INTRODUCTION
The role of the human papillomavirus (HPV) in the development of oropharyngeal squamous cell carcinoma (OPSCC) is increasingly becoming clear. HPV‐associated OPSCC arise mainly in the tonsil and base of tongue and have distinct clinical and pathological features as compared to non‐HPV‐associated tumors. These tumors arise in younger individuals without an extensive alcohol or tobacco history and are often diagnosed in more advanced stage.1 Tumor HPV status is a strong and independent biomarker for prognosis with multiple randomized studies showing improved outcomes in HPV‐associated OPSCC compared to non‐HPV‐associated OPSCC.2, 3, 4, 5 Moreover, p16 positive tumors with disease progression have improved overall survival as compared to p16‐negative tumors. Interestingly, HPV‐associated OPSCC behave unusually with regard to distant metastasis as it tends to occur at longer intervals after completion of radiation, and spreads to multiple organs or unusual sites.6
Salvage surgery in recurrent OPSCC classically had dismal outcomes with recurrence‐free survival around 25%.7, 8 However, recent studies report improved survival in patients with recurrent HPV‐associated OPSCC undergoing salvage therapy. Though HPV‐positive OPSCC is associated with improved survival and response to salvage therapy, the increasing incidence of the disease highlights the importance of developing novel therapies including immunotherapy.
The immune system appears to play a critical role in HPV‐associated OPSCC tumor progression and response to therapy. The immune system is divided into the innate and adaptive response. The innate response plays a crucial role in clearing virus infected cells. The adaptive response comprises the humoral response which is based on B cells and their products as well as the cell‐mediated response based on T‐cell activation. A vigorous T‐cell‐based response is thought to play a major role in HPV‐associated OPSCC tumor progression and outcome.9, 10 Head and neck squamous cell carcinoma cells have developed a myriad of strategies to evade the immune responses including decreased HLA class I expression by tumor cells, tumor‐induced T‐cell apoptosis, galectin‐1 expression by tumor cells, and tumor‐induced senescent T cells with suppressor function.11, 12
Secreted factors from several stromal cells such as fibroblasts, macrophages, dendritic cells (DCs), and natural killer cells are thought to contribute to carcinogenesis (Fig. 1). The role of the immune response in development of HPV‐associated OPSCC is a heavily researched topic and has been found to have significant consequences. Both the innate and adaptive immune responses play a role in tumor progression and suppression. The tumor microenvironment has been shown to be infiltrated by a variety of adaptive immunologic cells including CD3, CD4, and CD8 tumor infiltrating lymphocytes (TIL).13 This study seeks to summarize the current knowledge pertaining to role of the innate and adaptive immune response in HPV‐associated OPSCC.
Figure 1.
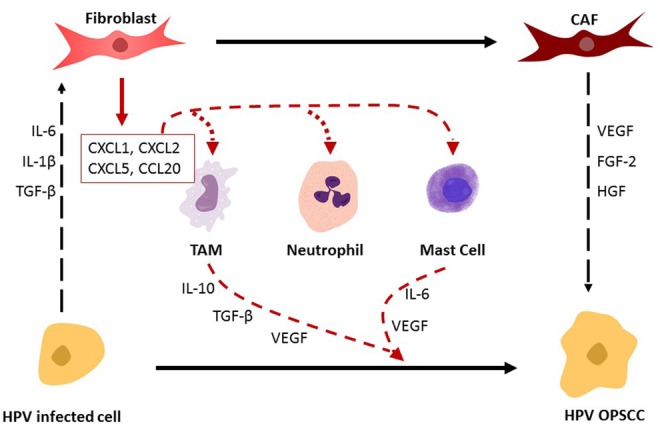
The interaction between stromal cells and innate immune cells and their roles in the carcinogenic conversion of HPV‐infected cells into neoplastic cells (HPV OPSCC). HPV‐infected cells drive conversion of pro‐inflammatory fibroblasts into cancer associated fibroblasts (CAFs) via expression of IL‐6, IL‐1β, and TGFβ. CAFs in‐turn drive transformation of HPV‐infected cells into neoplastic cells and maintain tumor growth, invasion, and progression utilizing various factors such as FGF‐2, HGF, and VEGF. Pro‐inflammatory fibroblasts secrete chemokines, such as CXCL1, CXCL2, CXCL5, and CCL20, which act as chemoattractants for innate immune cells. These innate cells secrete a milieu of factors that play a role in the carcinogenic transformation of HPV infected cells into neoplastic cells.
ROLE OF INNATE IMMUNITY IN HPV + OPSCC
The cells of the innate immune response play a key role in the immune microenvironment of HPV‐associated squamous cell carcinoma. While few studies directly address HPV‐associated OPSCC, the role of the innate immune response in other HPV‐associated tumors is well described. Studies examining the stroma of tumors are particularly interesting and are a growing field in cancer research. The stroma is a foundational scaffolding composed of various cell types, connective tissue, and vasculature that appears to be critical in tumor initiation and neoplastic progression, in multiple HPV‐associated carcinomas including the anogenital tract, skin, cervix, and oropharynx.14, 15
HPV carcinogenesis is highly dependent on stromal cells to induce a pro‐inflammatory environment to support invasion, proliferation, and angiogenesis. In particular, cancer associated fibroblasts (CAFs) appear to play a major role in driving immunosuppression, cell growth, and metastasis in HPV‐associated cancers including OPSCC and cervical.16 CAFs facilitate HPV‐positive cervical cancer cell‐mediated TGFβ1 and matrix metalloproteinase 7 (MMP7) secretion, contributing to tumor progression.17 The mechanisms involved in CAF‐facilitated HPV‐positive HNSCC progression are limited. Through mechanisms that are not entirely clear, normal fibroblasts transition into a pro‐inflammatory state.17 This results in fibroblasts gaining a pro‐inflammatory gene expression signature which includes expression of a variety of factors such as chemokines (CXCL1, CXCL2, CXCL5), interleukins (IL‐1B, IL‐6), and cxyclooxygenase‐2 gene.15 The aforementioned chemokines (C‐X‐C motif ligand chemokines or CXCLs) are ligands for the CXCR2 receptor which act as chemoattractants for neutrophils and macrophages.18 Il‐6 acts to induce expression of chemokine C‐C motif chemokine ligand 20 (CCL20) in stromal fibroblasts which results in infiltration of pro‐inflammatory Th17 cells.19 The resultant effect is to create a pro‐inflammatory and pro‐tumorigenic environment which is critical in the early stages of carcinogenesis. HPV‐infected cells, in addition to keratinocytes attracted to the tumor, secrete a milieu of chemokines and cytokines that induce normal fibroblasts mutation into CAFs. Factors implicated in this conversion include IL‐1β, IL‐6, IL‐8, TGF‐β, CXCL12, and bFGF.17, 19 CAFs then act to drive and maintain transformation of HPV‐positive cells into neoplastic cells resulting in tumor growth, invasion, and progression. These effects are believed to be mediated by several factors including fibroblast growth factor‐2 (FGF‐2),18 HGF, VEGF, and increased matrix metalloprotein expression.20, 21 Thus, it is believed that normal fibroblasts and CAFs play a complementary role in HPV‐related carcinogenesis.
Other important cell types include macrophages. Although there are numerous types of macrophages, in general, two main phenotypes, inflammatory/classic M1 and tumor‐associated macrophages (TAM) M2, have been described pertaining to HPV‐associated cancers.17, 22, 23 TAMs appear to have a role in tumor progression by boosting Th2 response, inhibiting CD4+ and CD8+ T cell responses and secreting VEGF and other proangiogenic factors.15, 17, 21, 24 They have been shown to secrete pro‐tumorigenic and angiogenic factors such as EGF and VEGF as well as anti‐inflammatory cytokines such as IL‐10 and TGFβ.15, 21 Secretion of IL‐10, prostaglandins, TGFβ, and reactive oxygen species all act to prevent T‐cell proliferation and activation thereby inhibiting T‐cell antitumor function which prevents T‐cell‐facilitated adaptive immune response.25 Increasing number of TAM cells in the stroma results in secretion of STAT3 in the tumor microenvironment which also impairs the adaptive response.26, 27 These effects result in promotion of carcinogenesis and metastasis. Although not specific to HPV‐associated OPSCC, TAMs are linked to poor prognosis, progression of disease, locoregional metastasis, and higher grade lesions.28, 29, 30 The M1 phenotype, however, is associated with tumor regression and inducing HPV clearance by favoring a TH1 response.31 TNFα and IFN‐γ from immune cells within the tumor microenvironment promote a change in macrophage polarity towards the M1 phenotype resulting in a pro‐inflammatory environment favoring tumor destruction.26 Multiple studies have shown that HPV actively promotes a TAM phenotype to favor tumor establishment.23, 24, 32 Future studies examining macrophage role in HPV‐associated OPSCC in order to develop therapies targeting macrophage polarization may be beneficial.
DCs are important antigen presenting cells that function as messengers between the innate and adaptive immune response. These cells have numerous receptors on their surface allowing them to interact with all the cells of the immune system which is key for the antiviral immune response. DCs are critical for CD8 T‐cell priming via interactions between CXCR3 on CD8+ T cell and CXCL9, CXCL10, expressed by DC as well as CD27 and CD28 on CD8+ T cells and CD70 and CD80‐86 expressed by DC.26 They also induce differentiation of CD4+ T cells into their antigen specific effector cells.26, 33 In HPV carcinogenesis, multiple studies identified a correlation between neoplastic progression and alteration of stimulatory and inhibitory markers. In particular, the PD‐1/PD‐L1 inhibitory pathway appears to be upregulated in HPV‐associated OPSCC cell lines and in cervical intraepithelial neoplasia, resulting in a loss of function of cytotoxic T lymphocytes allowing cancer and HPV infected cells to avoid immune rejection.31, 34, 35, 36 This pathway has been correlated with reduced survival. Use of antibodies that blocked PD‐1/PD‐L1 signaling resulted in enhanced cytotoxic T lymphocyte function.37 As a result, several clinical trials devoted to developing anti‐PD‐L1 antibodies are ongoing.31, 34 Other interesting therapeutic interventions targeting DCs include the use of vaccines with the hope of activating DCs to induce Th1 and cytotoxic T lymphocyte responses.19
ROLE OF ADAPTIVE IMMUNITY IN HPV‐POSITIVE OPSCC
HPV‐associated OPSCC tumors induce a robust adaptive immune response resulting in infiltration of myriad of important factors. High numbers of specific cells have been identified in the surrounding stromal environment as well as the tumor. The vigorous immune response is thought to play a role in improved outcomes of HPV‐associated OPSCC by suppressing tumor progression.
HPV‐associated OPSCC tumors have been found to express adaptive immune response genes that drive a strong T‐cell based immune response.9, 10 Moreover, the microenvironment of HPV‐associated OPSCC is marked by high expression of pro‐inflammatory chemokines (e.g., CXCL 9, 10, 100) and chemokine receptors (e.g., CXCR3).9 These factors are important in recruitment of TILs, especially CD8+ cytotoxic T cells. Cytotoxic T cells are the principal antitumor effector cells. In general, CD8+ T cells function as a major immune defense against intracellular pathogens and tumor cells. Antigenic peptides presented by MHC class 1 molecules expressed by tumor cells leads to activation of CD8+ T cells.26 Targeted cells are destroyed via secretion of TNFα and IFN‐γ, release of cytotoxic granules containing perforin and granzymes, and apoptosis induced via Fas/FasL interactions.26 High numbers of CD8+ TILs have been linked to improved outcomes in multiple tumor types, especially colorectal cancer.38
Multiple studies have identified massive infiltration of CD8+ TIL in the tumor and stroma of HPV‐associated OPSCC as compared to non‐HPV‐associated tumors.9, 39, 40, 41, 42 High CD8+ TIL in the tumor microenvironment has been found to be an important marker for prognosis. Patients with higher CD8+ TIL have improved overall survival and disease‐free survival.9, 39, 40, 41, 42 HPV‐associated OPSCC tumors with low TIL numbers appear to have similar survival to that of HPV‐negative OPSCC.42 Studies in the head and neck literature, as well as in other cancer sites, have noted reduced numbers of CD8+ T cells in peritumoral areas surrounding metastatic lymph nodes compared to uninvolved lymph nodes.43 This suggests that tumor suppression of cytotoxic T‐cell activity facilitates metastases. It is believed that viral antigens expressed by HPV‐associated OPSCC tumors provoke a robust adaptive immune response resulting in increased infiltration of CD8 + TIL.9, 42 This is supported by the fact that HPV 16‐specific CD8+ T cells have been extracted from the blood of HPV‐positive OPSCC and isolated from tumors.44, 45 More specifically, studies have identified elevated levels of HLA‐A*201 restricted viral E7‐specific T cells in the peripheral blood of patients with HPV+ head and neck tumors.11 However, these same E7‐specific cytotoxic T cells were unable to recognize an HLA‐A*201 expressing HPV+ tumor cell line11, 31 and no studies have yet definitively identified the antigens within the tumor that trigger this host response. Moreover, CD8+ TIL function may have an impact on response to therapy as studies have shown improved disease‐free survival with retained or enhanced CD8+ activity in patients treated with radiotherapy +/− chemotherapy.46 Future interventions directed at increasing immune reactive CD8+ cytotoxic T cells may be beneficial in treatment of HPV‐associated OPSCC.
Other immune cells have been identified in the tumor microenvironment of HPV‐associated OPSCC but their role is unclear. For example, some studies have demonstrated increased infiltration of HPV‐associated OPSCC tumors with CD4+ T helper cells.39 In general, CD4+ cells interact antigens expressed on MHC class II resulting in cytokine secretion and subsequent proliefation and activation of CD8 T cells.26, 47 CD4+ T cells exert their antitumor activities in multiple ways including secretion of IFN‐γ48 and recruitment of effector cells such as macrophages and eosinophils.49 There is also a cooperative interplay with CD8+ T‐cell function via CD4+ T‐cell secretion of IL‐2, modification of antigen presenting cells augmenting tumor antigen presentation to CD8+ T cells, and facilitating trafficking of CD8+ T cells into the tumor.49, 50 In head and neck tumors as a whole, the literature suggests that a high CD4+ TIL count may improve prognosis but more recent studies have found no difference in outcome with increased CD4+ TIL infiltration.39, 51 Some studies have found that a lower tumor infiltrate CD4/CD8 ratio, and more specifically a lower Foxp3/CD8 ratio, were correlated with improved overall and disease‐specific survival independent of HPV status.39, 45 Studies have identified a subpopulation of CD4+ Treg cells that are thought to impair autoimmunity and promote cancer progression. These cells are notable for expressing CD25, CTLA‐4, and CD 36.31 Direct cell to cell contact and production of IL‐10 and TGFβ by these cells results in anergy, apoptosis, and cell cycle arrest of activated T cells resulting in cancer progression.31, 52
In addition, multiple studies have identified significantly higher number of CD3+ TIL In HPV‐associated OPSCC, but this increased infiltration had no impact on patient outcome.9, 53 Natural Killer T cells (NKTs) are also important antitumor cells that secrete a variety of cytokines and induce DC maturation. It is believed that HPV has developed mechanisms to avoid NKTs antitumor effects but more studies are needed.
HPV VIRAL IMMUNOMODULATION IN CARCINOGENESIS
HPV's ability to subvert both the innate and adaptive immune responses to drive chronic infection and increase risk of neoplastic progression is well described in multiple cancers.24, 54 The virus relies on numerous immunomodulatory chemokines and cytokines to foster a suitable environment for tumor growth. For example, HPV oncogenes have been found to block signaling pathways critical to the innate immune response to viral infection. Specifically, inhibition of induction and transcription of CCL20 by HPV E6/E7 during early infection results in lack of recruitment of antigen presenting cells (APCs) to HPV infected epithelium facilitating chronic infection.24, 54, 55, 56 HPV‐transformed cells progressively accumulate and produce IL‐6 which influences tumor‐associated myeloid and inflammatory cell migration and conversion.57 This eventually leads rapid activation of STAT3 in HPV‐infected cells resulting in increased CCL2 production.57 CCL2 is a potent tumor promoting chemokine that is associated with persistent disease and sustained inflammatory microenvironment.24, 57 This all acts to sustain chronic inflammation and create sustained levels of immunosuppressive and tumor promoting local factors that eventually leads to cancer.
IL‐6 is a particularly interesting cytokine pivotal to HPV‐associated carcinogenesis. In particular, IL‐6 overexpression in malignant HPV‐transformed cells is a potent promoter of protumorogenic and immunosuppressive responses.24 Its role in cervical carcinogenesis is better understood and is thought to apply to oropharyngeal cancer progression. IL‐6 promotes the CCL2/CCR2 feedback loop which sustains the inflammatory microenvironment.57 In later stages of carcinogenesis, IL‐6 induces differentiation of fibroblasts into CAFs and increases stromal expression of CCL20 amplifying the chronic inflammatory response.19 IL‐6 acts to disassociate migration receptor CCR7 from CD83 and CD80/86 on DCs preventing expression of CCR7 which results in functionally impaired DCs.24, 58 This results in impaired DC IL‐12 production resulting in upregulated MMP‐9 production and activity. Increased MMP‐9 expression is thought to promote tumor growth and angiogenesis.24 Il‐6 production by tumor cells and subsequent signaling via STAT3 has been found to attract pro‐tumorigenic T‐cells.19 Both IL‐6 and STAT‐3 inhibitors are currently being studied as potential therapeutic agents in cervical cancer. Further studies regarding their role in HPV+ oropharyngeal cancer are warranted.
TGFβ is an important cytokine that is thought to be a potent factor in cancer progression. It has been found to be overexpressed in pre‐neoplastic lesions adjacent to HNSCC suggesting a role in early carcinogensis.59 In the tumor site, it is sequestered in the extracellular matrix bound to TGFβ binding protein and is also secreted by leukocytes, stroma, and tumor cells.60 Extracellular matrix degradation by matrix metalloproteins is thought to play a role in release of TGFβ.26 TGFβ has been implicated in immune dysregulation allowing tumors to outmaneuver host defense systems facilitating tumor growth. In particular, increased expression of cell associated and matrix‐linked TGFβ results in functional inactivation of NK cells and cytotoxic T cells.61 It is also thought to play a key role in recruitment and differentiation of immunosuppressive CD4+ Treg cells.61
SUMMARY
HPV‐associated OPSCC is a distinct entity that is increasing in incidence. The immune system appears to play a significant role in tumor progression. Studies suggest that the cells of the innate immune response play a key role in the immune microenvironment of HPV‐associated squamous cell carcinoma. Stromal cells, macrophages, and DCs all contribute to carcinogenesis and future therapies directed at altering their responses are underway. Multiple studies have examined the role of the adaptive immune response and have noted increased infiltration of CD8+, CD4+, and CD3+ TIL in the tumor microenvironment. High CD8+ TIL infiltration and low CD4/CD8 ratio appears to have prognostic implications with reports of improved survival. The HPV virus utilizes a myriad of immunomodulating cytokines to subvert the host immune response. Further studies are needed to examine therapeutic response in patients with these distinct immunologic subtypes and identify potential targets for de‐escalated therapy.
Editor's Note: This Manuscript was accepted for publication on July 29, 2019.
Funding: This work was supported by the NIH grant CA227838 to SMT and The National Cancer Institute Cancer Center Support Grant to the University of Kansas Cancer Center, P30CA168524.
BIBLIOGRAPHY
- 1. Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus‐positive head and neck squamous cell carcinoma. J Clin Oncol 2015;33:3235–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus‐positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 2008;100:261–269. [DOI] [PubMed] [Google Scholar]
- 4. Fakhry C, Zhang Q, Nguyen‐Tan PF, et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol 2014;32:3365–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sethi S, Ali‐Fehmi R, Franceschi S, et al. Characteristics and survival of head and neck cancer by HPV status: a cancer registry‐based study. Int J Cancer 2012;131:1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang SH, Perez‐Ordonez B, Liu FF, et al. Atypical clinical behavior of p16‐confirmed HPV‐related oropharyngeal squamous cell carcinoma treated with radical radiotherapy. Int J Radiat Oncol Biol Phys 2012;82:276–283. [DOI] [PubMed] [Google Scholar]
- 7. Goodwin WJ Jr. Salvage surgery for patients with recurrent squamous cell carcinoma of the upper aerodigestive tract: when do the ends justify the means? Laryngoscope 2000;110:1–18. [DOI] [PubMed] [Google Scholar]
- 8. Sims JR, Van Abel K, Martin EJ, et al. Management of recurrent and metastatic hpv‐positive oropharyngeal squamous cell carcinoma after transoral robotic surgery. Otolaryngol Head Neck Surg 2017;157:69–76. [DOI] [PubMed] [Google Scholar]
- 9. Jung AC, Guihard S, Krugell S, et al. CD8‐alpha T‐cell infiltration in human papillomavirus‐related oropharyngeal carcinoma correlates with improved patient prognosis. Int J Cancer 2013;132:E26–E36. [DOI] [PubMed] [Google Scholar]
- 10. Thurlow JK, Pena Murillo CL, Hunter KD, et al. Spectral clustering of microarray data elucidates the roles of microenvironment remodeling and immune responses in survival of head and neck squamous cell carcinoma. J Clin Oncol 2010;28:2881–2888. [DOI] [PubMed] [Google Scholar]
- 11. Uppaluri R, Dunn GP, Lewis JS Jr. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in head and neck cancers. Cancer Immun 2008;8:16. [PMC free article] [PubMed] [Google Scholar]
- 12. Young MR. Protective mechanisms of head and neck squamous cell carcinomas from immune assault. Head Neck 2006;28:462–470. [DOI] [PubMed] [Google Scholar]
- 13. Saber CN, Gronhoj Larsen C, Dalianis T, von Buchwald C. Immune cells and prognosis in HPV‐associated oropharyngeal squamous cell carcinomas: review of the literature. Oral Oncol 2016;58:8–13. [DOI] [PubMed] [Google Scholar]
- 14. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013;19:1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spurgeon ME, Lambert PF. Human papillomavirus and the stroma: bidirectional crosstalk during the virus life cycle and carcinogenesis. Viruses 2017;9:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cirri P, Chiarugi P. Cancer‐associated‐fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Rev 2012;31:195–208. [DOI] [PubMed] [Google Scholar]
- 17. Barros MR, Jr. , de Melo CML, Barros M, de Cassia Pereira de Lima R , de Freitas AC, Venuti A. Activities of stromal and immune cells in HPV‐related cancers. J Exp Clin Cancer Res 2018; 37:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chow MT, Luster AD. Chemokines in cancer. Cancer Immunol Res 2014;2:1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walch‐Ruckheim B, Mavrova R, Henning M, et al. Stromal fibroblasts induce CCL20 through IL6/C/EBPbeta to support the recruitment of Th17 cells during cervical cancer progression. Cancer Res 2015;75:5248–5259. [DOI] [PubMed] [Google Scholar]
- 20. Sato T, Sakai T, Noguchi Y, Takita M, Hirakawa S, Ito A. Tumor‐stromal cell contact promotes invasion of human uterine cervical carcinoma cells by augmenting the expression and activation of stromal matrix metalloproteinases. Gynecol Oncol 2004;92:47–56. [DOI] [PubMed] [Google Scholar]
- 21. Woodby B, Scott M, Bodily J. The interaction between human papillomaviruses and the stromal microenvironment. Prog Mol Biol Transl Sci 2016;144:169–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lepique AP, Daghastanli KR, Cuccovia IM, Villa LL. HPV16 tumor associated macrophages suppress antitumor T cell responses. Clin Cancer Res 2009;15:4391–4400. [DOI] [PubMed] [Google Scholar]
- 23. Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol 2010;22:231–237. [DOI] [PubMed] [Google Scholar]
- 24. Smola S. Immunopathogenesis of HPV‐associated cancers and prospects for immunotherapy. Viruses 2017;9:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maimela NR, Liu S, Zhang Y. Fates of CD8+ T cells in tumor microenvironment. Comput Struct Biotechnol J 2019;17:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol 2019;234:8509–8521. [DOI] [PubMed] [Google Scholar]
- 27. Tan B, Shi X, Zhang J, et al. Inhibition of Rspo‐Lgr4 facilitates checkpoint blockade therapy by switching macrophage polarization. Cancer Res 2018;78:4929–4942. [DOI] [PubMed] [Google Scholar]
- 28. Petruzzi MN, Cherubini K, Salum FG, de Figueiredo MA. Role of tumour‐associated macrophages in oral squamous cells carcinoma progression: an update on current knowledge. Diagn Pathol 2017;12:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Swangphon P, Pientong C, Sunthamala N, et al. Correlation of circulating CD64(+)/CD163(+) monocyte ratio and stroma/peri‐tumoral CD163(+) monocyte density with human papillomavirus infected cervical lesion severity. Cancer Microenviron 2017;10:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen XJ, Han LF, Wu XG, et al. Clinical significance of CD163+ and CD68+ tumor‐associated macrophages in High‐risk HPV‐related cervical cancer. J Cancer 2017;8:3868–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ferris RL. Immunology and immunotherapy of head and neck cancer. J Clin Oncol 2015;33:3293–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Deligeoroglou E, Giannouli A, Athanasopoulos N, et al. HPV infection: immunological aspects and their utility in future therapy. Infect Dis Obstet Gynecol 2013;2013:540850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012;12:265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bashaw AA, Leggatt GR, Chandra J, Tuong ZK, Frazer IH. Modulation of antigen presenting cell functions during chronic HPV infection. Papillomavirus Res 2017;4:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zandberg DP, Strome SE. The role of the PD‐L1:PD‐1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol 2014;50:627–632. [DOI] [PubMed] [Google Scholar]
- 36. Song D, Li H, Li H, Dai J. Effect of human papillomavirus infection on the immune system and its role in the course of cervical cancer. Oncol Lett 2015;10:600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen Z, Pang N, Du R, et al. Elevated expression of programmed death‐1 and programmed death ligand‐1 negatively regulates immune response against cervical cancer cells. Mediators Inflamm 2016;2016:6891482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Galon J, Costes A, Sanchez‐Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960–1964. [DOI] [PubMed] [Google Scholar]
- 39. Nordfors C, Grun N, Tertipis N, et al. CD8+ and CD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur J Cancer 2013;49:2522–2530. [DOI] [PubMed] [Google Scholar]
- 40. Oguejiofor K, Hall J, Slater C, et al. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV‐positive oropharyngeal squamous carcinoma. Br J Cancer 2015;113:886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Solomon B, Young RJ, Bressel M, et al. Prognostic significance of PD‐L1(+) and CD8(+) immune cells in HPV(+) oropharyngeal squamous cell carcinoma. Cancer Immunol Res 2018;6:295–304. [DOI] [PubMed] [Google Scholar]
- 42. Ward MJ, Thirdborough SM, Mellows T, et al. Tumour‐infiltrating lymphocytes predict for outcome in HPV‐positive oropharyngeal cancer. Br J Cancer 2014;110:489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pretscher D, Distel LV, Grabenbauer GG, Wittlinger M, Buettner M, Niedobitek G. Distribution of immune cells in head and neck cancer: CD8+ T‐cells and CD20+ B‐cells in metastatic lymph nodes are associated with favourable outcome in patients with oro‐ and hypopharyngeal carcinoma. BMC Cancer 2009;9:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heusinkveld M, Goedemans R, Briet RJ, et al. Systemic and local human papillomavirus 16‐specific T‐cell immunity in patients with head and neck cancer. Int J Cancer 2012;131:E74–E85. [DOI] [PubMed] [Google Scholar]
- 45. Wansom D, Light E, Thomas D, et al. Infiltrating lymphocytes and human papillomavirus‐16‐‐associated oropharyngeal cancer. Laryngoscope 2012;122:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Masterson L, Lechner M, Loewenbein S, et al. CD8(+) T cell response to human papillomavirus 16 E7 is able to predict survival outcome in oropharyngeal cancer. Eur J Cancer 2016;67:141–151. [DOI] [PubMed] [Google Scholar]
- 47. Kershaw MH, Westwood JA, Darcy PK. Gene‐engineered T cells for cancer therapy. Nat Rev Cancer 2013;13:525–541. [DOI] [PubMed] [Google Scholar]
- 48. Mumberg D, Monach PA, Wanderling S, et al. CD4(+) T cells eliminate MHC class II‐negative cancer cells in vivo by indirect effects of IFN‐gamma. Proc Natl Acad Sci U S A 1999;96:8633–8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cho Y, Miyamoto M, Kato K, et al. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res 2003;63:1555–1559. [PubMed] [Google Scholar]
- 50. Marzo AL, Lake RA, Robinson BW, Scott B. T‐cell receptor transgenic analysis of tumor‐specific CD8 and CD4 responses in the eradication of solid tumors. Cancer Res 1999;59:1071–1079. [PubMed] [Google Scholar]
- 51. Badoual C, Hans S, Rodriguez J, et al. Prognostic value of tumor‐infiltrating CD4+ T‐cell subpopulations in head and neck cancers. Clin Cancer Res 2006;12:465–472. [DOI] [PubMed] [Google Scholar]
- 52. Alhamarneh O, Amarnath SM, Stafford ND, Greenman J. Regulatory T cells: what role do they play in antitumor immunity in patients with head and neck cancer? Head Neck 2008;30:251–261. [DOI] [PubMed] [Google Scholar]
- 53. Krupar R, Robold K, Gaag D, et al. Immunologic and metabolic characteristics of HPV‐negative and HPV‐positive head and neck squamous cell carcinomas are strikingly different. Virchows Arch 2014;465:299–312. [DOI] [PubMed] [Google Scholar]
- 54. Schroer N, Pahne J, Walch B, Wickenhauser C, Smola S. Molecular pathobiology of human cervical high‐grade lesions: paracrine STAT3 activation in tumor‐instructed myeloid cells drives local MMP‐9 expression. Cancer Res 2011;71:87–97. [DOI] [PubMed] [Google Scholar]
- 55. Karim R, Meyers C, Backendorf C, et al. Human papillomavirus deregulates the response of a cellular network comprising of chemotactic and proinflammatory genes. PLoS One 2011;6:e17848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sperling T, Oldak M, Walch‐Ruckheim B, et al. Human papillomavirus type 8 interferes with a novel C/EBPbeta‐mediated mechanism of keratinocyte CCL20 chemokine expression and Langerhans cell migration. PLoS Pathog 2012;8:e1002833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Walch‐Ruckheim B, Pahne‐Zeppenfeld J, Fischbach J, et al. STAT3/IRF1 pathway activation sensitizes cervical cancer cells to chemotherapeutic drugs. Cancer Res 2016;76:3872–3883. [DOI] [PubMed] [Google Scholar]
- 58. Pahne‐Zeppenfeld J, Schroer N, Walch‐Ruckheim B, et al. Cervical cancer cell‐derived interleukin‐6 impairs CCR7‐dependent migration of MMP‐9‐expressing dendritic cells. Int J Cancer 2014;134:2061–2073. [DOI] [PubMed] [Google Scholar]
- 59. Lu SL, Reh D, Li AG, et al. Overexpression of transforming growth factor beta1 in head and neck epithelia results in inflammation, angiogenesis, and epithelial hyperproliferation. Cancer Res 2004;64:4405–4410. [DOI] [PubMed] [Google Scholar]
- 60. Wahl SM, Wen J, Moutsopoulos N. TGF‐beta: a mobile purveyor of immune privilege. Immunol Rev 2006;213:213–227. [DOI] [PubMed] [Google Scholar]
- 61. Moutsopoulos NM, Wen J, Wahl SM. TGF‐beta and tumors–an ill‐fated alliance. Curr Opin Immunol 2008;20:234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]


