Abstract
Background
Administration of botulinum toxin through intraductal salivary infusion may decrease the risks of percutaneous needle injection and improve delivery to permeate the entire gland parenchyma.
Methods
The safety of intraductal salivary gland infusion was tested with prospective evaluation of two patients using interviews, clinical examination, and pressure measurement during infusion. Retrospective chart review of two subsequently treated patients assessed treatment of a parotid‐cutaneous fistula and sialorrhea.
Results
No complications were identified in the safety study. Pressure changes during infusion supported the concept of botulinum neurotoxin delivery to permeate the gland. Patient‐assessed success was subjectively reported as a reduction in the parotid‐cutaneous output “by 95%” and the sialorrhea “by 90%” at 2‐week follow‐up.
Conclusions
The intraductal route of botulinum toxin delivery to salivary glands was without complication and was effective in two patients treated therapeutically. Pressure measurements during infusion may be helpful to direct treatment.
Level of Evidence
4
Keywords: Botulinum toxin, salivary, intraductal, hypersalivation, sialorrhea
INTRODUCTION
Intraglandular injection of botulinum neurotoxin (BTX) is designed to decrease the cholinergic neural stimulus to salivary glands through the process of “chemical denervation.”1, 2
All salivary gland treatments with BTX had been done without specific designated FDA approval until July 2018. At that time, the FDA announced approval for treatment of chronic sialorrhea with Xeomin (incabotulinum A) intraglandular injection.3 This official approval was specific to the adult population and a dose of 30 units to each parotid gland and 20 units to each submandibular gland.
Despite this limited FDA approval, BTX has been used extensively in the management of salivary disorders associated with a diverse array of diagnoses in pediatric and adult populations.4, 5 The appropriate use of BTX “off‐label” to treat salivary conditions other than sialorrhea is permitted in a general manner by the FDA through use of an “approved drug for an unapproved use” and in a more specific fashion by medical societies through published consensus statements supporting salivary BTX infusion.6, 7, 8, 9
Salivary injection with BTX has been reported as effective in treating not only sialorrhea, sialoceles,10 first bite syndrome,11 and salivary fistulae,12, 13 but also sialadenitis.14, 15, 16 Its use in cosmetic surgery has expanded beyond the more common intramuscular treatment of rhytids to include facial recontouring through parotid injection to induce atrophy,17, 18 and as an adjunct to decreasing submandibular gland size with intraoperative injection to the gland remnant following its partial removal during neck lift surgery.19 Intraoperative direct injection to the parotid gland at the time of open transcervical approach has also been employed during surgical management of other disorders associated with sialadenitis (M. B. Gillespie, personal communication, 2018; R. R. Walvekar, personal communication, 2018).
In addition to the less common direct intraoperative BTX administration to the salivary glands,20 percutaneous needle injection into the salivary parenchyma is more commonly performed using ultrasound to direct needle placement,21 using electromyography to avoid injecting adjacent musculature,22 or by palpation with orientation by anatomic landmarks.16 To date, there have been no reports of intraductal delivery of BTX to the salivary parenchyma.
Intraductal infusion to the salivary glands is performed in the course of conventional sialography. This approach has been shown to have the capacity to deliver contrast agents not only to the salivary acini, but also through the acini with what has been termed “parenchymal clouding”23, 24 (Fig. 1).
Figure 1.
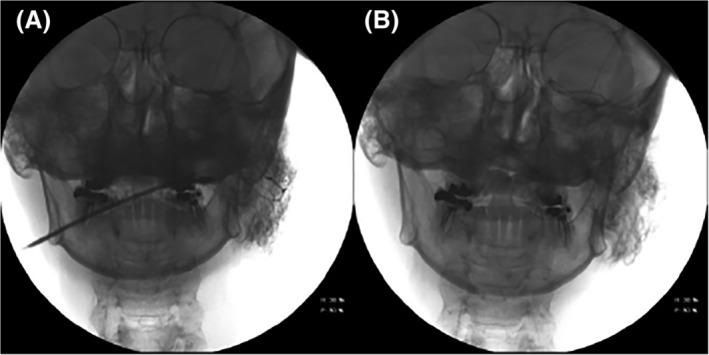
(A) Image of left parotid sialogram with permeation of the acini immediately following insufflation with contrast under pressure and (B) approximately 5 minutes following decannulation and administration of a sialogogue, demonstrating retained contrast and parenchymal clouding.
A murine (rat) study demonstrated that water‐based contrast permeates the acini to extend through the “intercellular spaces between the different parenchyma cells” with “distribution of contrast medium at the basal surface of the various cell types and in the connective tissue.”25 In a 2017 porcine cadaver study, Su et al assessed intraductal infusion of methylene blue and identified it to be evenly delivered throughout the salivary gland.26 These investigators concluded that “intraductal injections might serve as a potential therapeutic procedure in the management of salivary gland disease.”
To ensure the safety of this approach and then to report on its efficacy as applied clinically, we evaluated patients treated with intraductal salivary infusion of BTX. This study employed onabotulinumtoxinA (Botox), which is referred to as BTXA to differentiate it from the more generic abbreviation BTX, which we use to refer to all types of BTX.
MATERIALS AND METHODS
The local institutional review board (IRB) directed and approved an initial prospective study on the safety of the intraductal route of administration for BTXA. All methods were in full accordance with the principles set out by the World Medical Association Declaration of Helsinki. The IRB limited initial study of salivary ductal infusion of BTXA to one gland per patient and 25 units BTXA per gland, with a maximum enrollment of two “study patients” (patient #1 and #2). Three patients with the complaint of hypersalivation, and who were candidates for ultrasound‐guided percutaneous salivary gland injection, were selected from the senior author's practice (convenience sample) and were offered participation in the study. Two patients chose to participate. The study extended from 1/3/2018 to 8/11/2018. For those previously treated with percutaneous BTX injection, a minimal “washout” period of 3 months was required before treatment with intraductal infusion.
Patient #1 complained of sialorrhea presumed to result from poor tolerance of saliva secondary to bilateral hypoglossal nerve paralysis following total laryngectomy performed with extended resection, including bilateral submandibular gland resection for recurrent laryngeal and base of tongue cancer. Bilateral ultrasound‐guided percutaneous parotid injections with 20 units BTXA in 0.8 cc to each parotid gland had been done previously without perceived benefit. Following a washout period of 10 months from the time of his percutaneous injection, he received intraductal insufflation of BTXA to the right parotid gland.
Patient #2 had previously been treated for hypersalivation of unknown cause and reported an initial partial response to the first, but not the second, of two ultrasound‐guided percutaneous salivary gland infusions with 25 units of BTXA delivered to each of the four major glands (100 units total). Following a washout period of 35 months from the most recent of the percutaneous injections, he was enrolled in the safety study with intraductal insufflation of 25 units of BTXA to the left submandibular gland with concurrent measurement of the pressures generated.
Technique
A pre‐enrollment visit included discussion of the study and its requirements as well as completion of a preprocedural questionnaire. Patients with anticipated failure in cannulating the duct orifice or with active inflammation/infection were excluded from consideration. The procedures were performed in an otolaryngology clinic room with a microscope. Patients gave informed consent for the procedure in addition to their written consent to participate in the study.
Patients were asked to arrive well‐hydrated and had begun taking amoxicillin/clavulanate (Augmentin) 875 mg by mouth twice daily for a 3‐day course beginning the morning of the procedure. They were positioned semi‐recumbent with the salivary ducts visualized under a microscope. Topical viscous lidocaine was applied to the oral mucosa. With microscopic guidance, the salivary duct was cannulated with a guide wire (0.015 inch guide wire, COOK Medical, Inc.) over which a 22 gauge angiocatheter was inserted to its hub.
Twenty‐five units in 1 cc of reconstituted BTXA (25 units per cc) were insufflated into one of the major salivary ducts, followed by a “chaser” of up to 6 cc saline, titrated to the subject's reported level of discomfort. For one subject, the pressure of insufflation was measured with an inline disposable pressure transducer (Mirador Biomedical).
To prevent immediate egress of the infusate, the catheter was hubbed flush with the duct orifice under continued inspection for 4 minutes. Immediately following removal of the catheter, intraoral lemon juice or a lemon drop was administered and followed by gland massage with oral rinse. The setup is shown in Figure 2.
Figure 2.
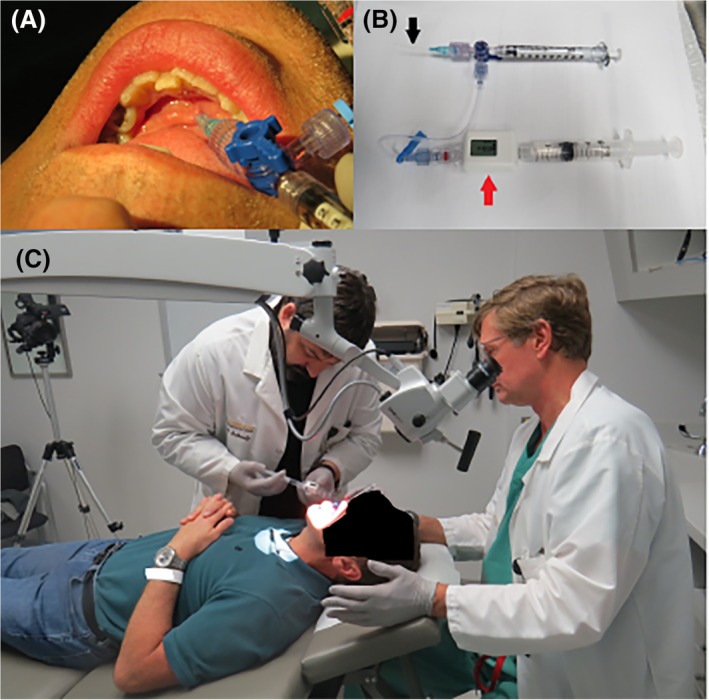
(A) Left submandibular duct cannulation (patient #2) with 22 gauge angiocatheter with three‐way stop cock permitting 1 cc infusion of onabotulinumtoxinA (Botox) (BTXA) followed by saline infusion. (B) Apparatus for infusion with concurrent pressure measurement (patient #2) includes a 1 cc syringe for initial botulinum neurotoxin (BTX) infusion through a 22 gauge angiocatheter (black arrow) attached by IV tubing to a pressure monitor (red arrow) with 5 cc syringe containing saline to provide back pressure after delivery of BTX. (C) Setup (patient #2) for microscopic‐controlled cannulation of the left submandibular duct followed by BTXA insufflation.
The subjects were contacted 1 week after infusion for report of side effects and efficacy, and then returned to clinic in 2 weeks for further analysis of possible side effects including a critical analysis of facial nerve function. The subjects were contacted again in 3 months for the same.
Following closure of this prospective safety study, which included a report to the IRB, two patients were treated therapeutically in a similar manner. Retrospective review of these two patient records fulfilled the exclusion criteria for formal IRB review as “case studies of an innovative therapy with three or fewer patients.” These two therapeutically treated patients are distinguished from initial two “study patients” by being categorized as “clinically treated patients” and numbered patient #3 and patient #4.
Patient # 3 had a parotid cutaneous fistula that persisted over the preceding 14 years despite interventions including surgical closure of the fistula site and botulinum toxin injection to the skin around the fistula for presumed Frey's syndrome. He chose to undergo transductal infusion due to failure of previous ultrasound‐guided percutaneous injection (50 units BTXA) to the parotid parenchyma.
Patient # 4 had sialorrhea associated with Parkinson's disease, and chose treatment with transductal infusion over a percutaneous injection to avoid discontinuing anticoagulation (warfarin) therapy.
Infusion techniques in these clinically treated patients were performed as per the initial study, modified only by the higher dose delivered (50 units BTXA) to the parotid gland with the salivary‐cutaneous fistula (patient #3). Patient #4 received a total of 50 units of BTXA (25 units each) to the submandibular glands followed 4 weeks later by another 50 units (25 units each) to the parotid glands. For these two patients, clinical response was determined by chart review of recorded subjective patient assessment at approximately 2 weeks after the procedure.
RESULTS
Prospective study of single gland infusion with BTXA focused on the safety and tolerance of the procedure with additional assessment of treatment effect. The two patients assessed in this study showed no complications from 25 units of BTXA infused into the right parotid gland (patient #1) and the left submandibular gland (patient #2) (Table 1). Despite the higher volume (total of 7 cc) used to infuse the parotid gland in patient #1, he reported no discomfort with the procedure.
Table 1.
Patient Demographic Information.
| Patient # | Diagnosis | Previous Management | Intraductal Infusion Gland | BTXA (Onabotulinum Toxin A) Dose | Additional Volume of Saline | Complication | Patient‐Reported Benefit |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| Gender | |||||||
| Safety study | |||||||
| 1 72 yr Male |
Sialorrhea Bilateral hypoglossal nerve injury |
Percutaneous BTX injection bilateral parotid 20 units/0.8 cc to each gland (10 mo previously) glycopyrrolate | Right parotid | 25 units/1 cc | 6 cc | None | None |
| 2 50 yr Male |
Sialorrhea Hypersalivation unknown cause |
Percutaneous bilateral SMG and parotid gland BTX injections 100 units total 25 units/1 cc to each gland (35 mo previously) |
Left submandibular | 25 units/1 cc | 3 cc | None | None |
| Clinically treated patients | |||||||
| 3 67 yr Male |
Parotid cutaneous fistula following surgery (done 14 yr previously) | Percutaneous parotid botox injection (4 mo previously); previous botox injection to skin (39 mo and again 37 mo previously); surgical closure of skin tract (13 yr previously) | Right parotid | 50 units/2 cc | 6 cc | None | Problem “95% resolved” |
| 4a 90 yr Male |
Sialorrhea Parkinson's disease Chronic anticoagulation on warfarin |
None recommended to avoid systemic therapy due to comorbidities | Bilateral submandibular | 25 units/1 cc to each gland (total = 50 units) | Left gland 2 cc Right gland 3 cc |
None | None |
| 4b | Same patient later date | Bilateral parotid | 25 units/1 cc to each gland (total = 50 units) | Both glands 5 cc | Dry mouth addressed by drinking more water with eating | Problem “90% resolved” | |
BTX = botulinum neurotoxin; BTXA = onabotulinumtoxinA (Botox); SMG = submandibular gland.
Patient #2 reported that the discomfort experienced in the left submandibular gland as it was expanded in the course of infusion rapidly dissipated by pausing during the otherwise continuous infusion. In this patient, pressure measurements were recorded for each 0.2 cc increment of saline infusion and following each pause during the infusion (Fig. 3). The pauses during infusion were associated with a decrease in the pressure and were followed by an increase in pressure with resumption of the infusion. A peak pressure of 75 mm Hg (following delivery of 2.6 cc) was followed by a rapid decrease to a nadir of 10 mm Hg; final pressure was 15 mm Hg with infusion completed to a volume of 3.0 cc. The final volume instilled (3 cc) was determined by his report of discomfort and desire to not extend the infusion. Follow‐up evaluation permitted a comparison of videos done before and 2 weeks after left submandibular infusion demonstrating marked reduction of salivary flow on the left but not the right gland on massage.
Figure 3.
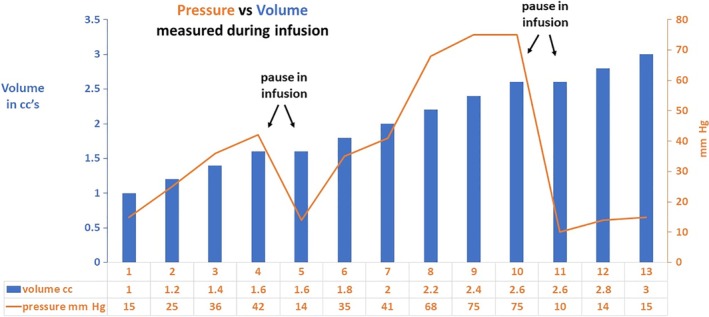
Volume of saline administered in 0.2 cc increments after initial 1 cc of onabotulinumtoxinA (Botox) plotted against pressure measurement (patient #2).
Following completion of the prospective study of the two initial patients, selected patients were offered intraductal BTXA infusion.
Patient #3 was evaluated with a sialogram, which identified the parotid cutaneous fistula with an otherwise normal ductal system without stricture (Fig. 4). Initial treatment with ultrasound‐guided percutaneous intraglandular injection delivered 50 units of BTXA (in 2.0 cc) to the right parotid gland. The patient reported an initial impression of decreased fistula output that was not realized until 1 month after the injection, but still maintained a sufficiently large fistula volume to warrant his request for further treatment. Three and one‐half months after the percutaneous injection, intraductal infusion of to the right parotid gland was performed with BTXA (50 units in 2 cc) followed by 6 cc of saline (total of 8 cc instilled). Two weeks post‐treatment, the patient reported that the result was “95% good” and “will advise if leakage starts up.”
Figure 4.
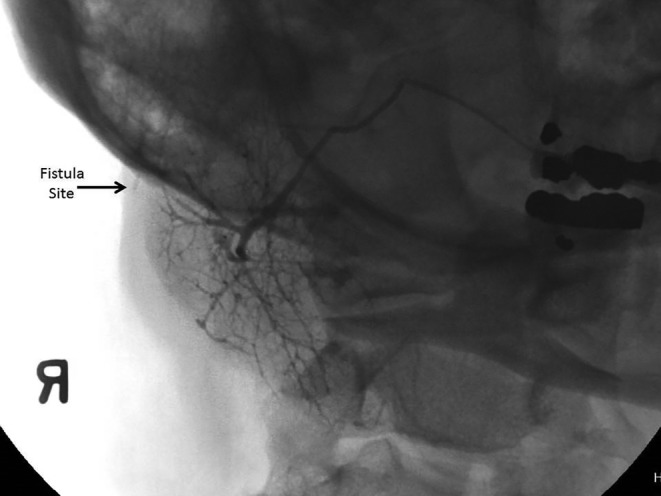
A right parotid sialogram. 6 cc of Isovue 370 contrast agent was instilled, demonstrating fistula extending to the skin surface.
Patient #4 was initially treated with intraductal infusions of 25 units of BTXA to each submandibular gland followed by infusion of saline (2 cc to right, 3 cc to left). Despite the patient's subjective lack of benefit, 6‐week follow‐up examination revealed only scant saliva expressible from the submandibular glands with massage—whereas before the BTXA infusion, robust production of saliva had been identified from all four major salivary glands. Abundant saliva was expressed from both parotid glands at this 6‐week follow‐up, including “gleeking” from the right gland. At this visit, therefore, 25 units of BTXA followed by 5 cc of saline were instilled into each parotid duct.
Follow‐up phone contact with patient #4 on post‐op day 4 following the parotid infusions (7 weeks after the submandibular gland infusions) identified no side effects, that the procedure was not difficult for him, and that he was “90% there” and doing well with respect to improved control of his drooling. He related that the new degree of dryness in his mouth that he attributed to the BTXA infusion now required that he drink more liquids when eating. A follow‐up conversation 2 weeks after the parotid infusion revealed continued success in addressing the sialorrhea, along with the minor annoyance of dry mouth. Patient #4 initiated further follow‐up 2 months after the parotid infusion due to a feeling that the medication was wearing off and that salivation was increasing. Repeat infusion was planned.
DISCUSSION
Intraductal drug delivery to the salivary gland is commonly performed. For example, steroid infusion is often performed at the time of sialendoscopy.27, 28 Successful treatment of salivary swelling by in‐clinic steroid infusion through cannulation without sialendoscopy has also been reported.29 A report by Sun et al identified the value of sialendoscopy‐directed intraductal infusion of a chymotrypsin/gentamicin preparation to address chronic obstructive parotitis.30
In addition to infusion of medications to target ductal abnormalities, drugs have been delivered through the duct to treat abnormalities of the gland parenchyma. Intraductal infusion of methyl violet was employed in China to treat chronic suppurative parotitis in the 1980s. This practice was subsequently supported by Zou et al in 1992, who recommended this treatment for selected patients to induce “atrophy of the parotid with ablation of clinical symptoms.”31
Intraductal BTXA infusion, as we report, delivers the toxin via the ductal system through the parenchyma to the site of cholinergic innervation to the acinar units. The degree to which the neuroeffector site is permeated by this technique of BTXA infusion is likely dependent on the dose administered, the pressure with which it is delivered, and also the structural integrity of the duct and the parenchyma to which it is directed.
The volume of radiocontrast delivered and associated pressures generated in the course of diagnostic sialography have been extensively studied with a large portion of the research published prior to 1980. In 1973, Blair offered an extensive review of salivary infusion techniques and reported the standard volume of radiocontrast delivered ranging from 0.5 cc to 6.0 cc.32 This investigator endeavored to standardize gland infusion through a slow gravity‐delivered “hydrostatic pressure” method to improve upon the variability induced by hand‐injection infusion technique. This “infusion‐to‐the point‐of‐pain” approach still relied on the accepted standard guiding amount of contrast delivered by way of a signal from the patient indicating time to stop the infusion.
In 1975, Zijlstra and ten Bosch expanded on previous study of pressures generated during sialography to identify that larger volumes (7 cc to the parotid and 5 cc to the submandibular gland) could be delivered when administered with lower injection speed and concluded that, when administered by this technique, the injected material was resorbed outside the ductal system.24 They identified that—in a manner similar to our findings—a decrease in pressure normally occurs in the course of infusion presumably due to distribution of the infusion from the duct system into the parenchyma. They supported use of constant hydrostatic pressure measurement as a valuable adjunct to help guide salivary infusion.
In 1990, Lewis et al reported value in pressure monitoring during sialography with high filling pressure as a potential indicator of ductal obstruction or diversion of the infusion cannula outside of the duct, which they termed as being “tissued.”33 Among 296 salivary gland infusions employing aqueous contrast medium, these investigators were able to correlate (P < .001) an elevated filling pressure in 160 of those studied whose sialograms identified ductal obstruction. The pressure device used in our study (Mirador Biomedical Compass inline pressure transducer) is designed to measure compartmental pressures but appears to function as a useful adjunct in monitoring salivary drug infusion, but also to assist in the process of diagnostic sialography.
The safety of the intraductal infusion of BTX is supported not only by our findings, but also by previous extensive study of the diffusion of the drug to possibly affect muscles adjacent the salivary glands. Concern about diffusion of BTX from the parotid gland is mitigated by understanding of the purposeful treatment of injection of the musculature adjacent the parotid gland. It is common practice to inject the masseter muscles to treat hypertrophy and dystonia. In study of 680 patients (2,036 sessions) treated with 20–30 units of onabotulinum toxin for masseter hypertrophy, Peng and Peng reported the most common complications as transient (1 week) muscle weakness with chewing (30%), bruising from the needle placement in a vessel (2.5%), headache (0.58%), and paradoxical masseter muscle bulging (0.49%).34 Petracca et al, studying injection of abobotulinumtoxinA through the percutaneous route to the salivary glands, noted a 1.5% rate of side effects attributable to the injection itself, including pain and bleeding.35 That study also reported rare complications of the intraparenchymal injection, including dysphagia, dysarthria, and aspiration pneumonia. Inadvertent intravascular injection is very rare but has been reported as causing one case of iatrogenic botulism.36
We anticipate that wider experience with the intraductal approach to gland infusion will be associated with a lower complication rate than those studies cited above.
A shortcoming of this study is the retrospective analysis of treatment efficacy limited to only two patients. Their subjectively favorable results coupled with the subjective assessment of decreased salivary production with massage of the submandibular glands in patients #2 and #4 offer support for use of this approach to add to the options available in treating salivary gland disease.
CONCLUSIONS
The intraductal route of botulinum toxin delivery to salivary glands was well tolerated without complication. Pressure measurements during infusion may be helpful to direct treatment. Two patients treated therapeutically by this approach reported benefit in decreasing salivary output.
Editor's Note: This Manuscript was accepted for publication on June 24, 2019.
Conflict of Interest: Henry T. Hoffman: a. COOK Medical: Research consultant and patent b. UpToDate: author c. IoataMotion: Research consultant with patent application.
Funding provided by the University of Iowa Department of Otolaryngology.
BIBLIOGRAPHY
- 1. Kaymak B, Malas FU, Kara M, On AY, Ozcakar L. Comment on Ultrasound guidance for botulinum neurotoxin chemodenervation procedures. Toxins 2017, 10, 18‐Quintessential use of ultrasound guidance for botulinum toxin injections‐muscle innervation zone targeting revisited. Toxins 2018;10(10):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaye R, Blitzer A. Chemodenervation of the larynx. Toxins 2017;9(11):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Approvals of FDA‐regulated products action date 07/03/2018 Xeomin ®(incobotuinumtoxinA) highlights of prescribing information. US Food & Drug Administration; 2018. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125360s073lbl.pdf. Accessed November 22, 2018.
- 4. Chan KH, Liang C, Wilson P, Higgins D, Allen GC. Long‐term safety and efficacy data on botulinum toxin type A: an injection for sialorrhea. JAMA Otolaryngol Head Neck Surg 2013;139(2):134–138. [DOI] [PubMed] [Google Scholar]
- 5. Scully C, Limeres J, Gleeson M, Tomas I, Diz P. Drooling. J Oral Pathol Med 2009;38(4):321–327. [DOI] [PubMed] [Google Scholar]
- 6.Understanding unapproved use of approved drugs “off label”. US Food & Drug Administration; 2018. Available at: https://www.fda.gov/ForPatients/Other/OffLabel/default.htm. Accessed November 22, 2018.
- 7. Frattarelli DA, Galinkin JL, Green TP, et al. Off‐label use of drugs in children. Pediatrics 2014;133(3):563–567. [DOI] [PubMed] [Google Scholar]
- 8. Seppi K, Ray Chaudhuri K, Coelho M, et al. Update on treatments for nonmotor symptoms of Parkinson's disease‐an evidence‐based medicine review. Mov Disord 2019;34(2):180–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bilyk JR, Yen MT, Bradley EA, Wladis EJ, Mawn LA. Chemodenervation for the treatment of facial dystonia: a report by the American Academy of Ophthalmology. Ophthalmology 2018;125(9):1459–1467. [DOI] [PubMed] [Google Scholar]
- 10. Vargas H, Galati LT, Parnes SM. A pilot study evaluating the treatment of postparotidectomy sialoceles with botulinum toxin type A. Arch Otolaryngol Head Neck Surg 2000;126(3):421–424. [DOI] [PubMed] [Google Scholar]
- 11. Lee BJ, Lee JC, Lee YO, Wang SG, Kim HJ. Novel treatment of first bite syndrome using botulinum toxin type A. Head Neck 2009;31(8):989–993. [DOI] [PubMed] [Google Scholar]
- 12. Gillespie MB. Combined parotid techniques. Atlas Oral Maxillofac Surg Clin North Am 2018;26(2):133–143. [DOI] [PubMed] [Google Scholar]
- 13. Laskawi R, Winterhoff J, Kohler S, Kottwitz L, Matthias C. Botulinum toxin treatment of salivary fistulas following parotidectomy: follow‐up results. Oral Maxillofac Surg 2013;17(4):281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ellies M, Gottstein U, Rohrbach‐Volland S, Arglebe C, Laskawi R. Reduction of salivary flow with botulinum toxin: extended report on 33 patients with drooling, salivary fistulas, and sialadenitis. Laryngoscope 2004;114(10):1856–1860. [DOI] [PubMed] [Google Scholar]
- 15. Gillespie MB, Intaphan J, Nguyen SA. Endoscopic‐assisted management of chronic sialadenitis. Head Neck 2011;33(9):1346–1351. [DOI] [PubMed] [Google Scholar]
- 16. Trapeau C, Foletti JM, Collet C, Guyot L, Chossegros C. Clinical efficacy of botulinum toxin in salivary duct stenosis: a preliminary study of six cases. J Stomatol Oral Maxillofac Surg 2017;118(6):349–352. [DOI] [PubMed] [Google Scholar]
- 17. Bae GY, Yune YM, Seo K, Hwang SI. Botulinum toxin injection for salivary gland enlargement evaluated using computed tomographic volumetry. Dermatol Surg 2013;39(9):1404–1407. [DOI] [PubMed] [Google Scholar]
- 18. Wu WT. Botox facial slimming/facial sculpting: the role of botulinum toxin‐A in the treatment of hypertrophic masseteric muscle and parotid enlargement to narrow the lower facial width. Facial Plast Surg Clin North Am 2010;18(1):133–140. [DOI] [PubMed] [Google Scholar]
- 19. Auersvald A, Auersvald LA. Management of the submandibular gland in neck lifts: indications, techniques, pearls, and pitfalls. Clin Plast Surg 2018;45(4):507–525. [DOI] [PubMed] [Google Scholar]
- 20. Hwang J, You YC, Burm JS. Treatment of intractable parotid sialocele occurred after open reduction‐fixation of mandibular subcondylar fracture. Arch Craniofac Surg 2018;19(2):157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoffman HT, Pagedar NA. Ultrasound‐guided salivary gland techniques and interpretations. Atlas Oral Maxillofac Surg Clin North Am 2018;26(2):119–132. [DOI] [PubMed] [Google Scholar]
- 22. Lovato A, Restivo DA, Ottaviano G, Marioni G, Marchese‐Ragona R. Botulinum toxin therapy: functional silencing of salivary disorders. Acta Otorhinolaryngol Ital 2017;37(2):168–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Truong K, Hoffman HT, Policeni B, Maley J. Radiocontrast dye extravasation during sialography. Ann Otol Rhinol Laryngol 2018;127(3):192–199. [DOI] [PubMed] [Google Scholar]
- 24. Zijlstra G, ten Bosch JJ. Sialography with continuous measurement of pressure outside and inside the gland. Int J Oral Surg 1975;4(4):160–167. [DOI] [PubMed] [Google Scholar]
- 25. Qwarnstrom E. Experimental sialography: the effects of retrograde infusion of radiographic contrast media on salivary gland morphology and function. A review article. Oral Surg Oral Med Oral Pathol 1986;62(6):668–682. [DOI] [PubMed] [Google Scholar]
- 26. Su CH, Lee KS, Tseng TM, et al. Intraductal injection as an effective drug delivery route in the management of salivary gland diseases. Eur Arch Otorhinolaryngol 2017;274(1):399–404. [DOI] [PubMed] [Google Scholar]
- 27. Bomeli SR, Schaitkin B, Carrau RL, Walvekar RR. Interventional sialendoscopy for treatment of radioiodine‐induced sialadenitis. Laryngoscope 2009;119(5):864–867. [DOI] [PubMed] [Google Scholar]
- 28. Nahlieli O, Nazarian Y. Sialadenitis following radioiodine therapy ‐ a new diagnostic and treatment modality. Oral Dis 2006;12(5):476–479. [DOI] [PubMed] [Google Scholar]
- 29. Diggelmann HR, Hoffman HT. Intraductal infusion of steroids in patients with Sjogren syndrome to treat painful salivary swelling: report of 2 cases. Ear Nose Throat J 2015;94(6):238–239. [DOI] [PubMed] [Google Scholar]
- 30. Sun HJ, Xiao JQ, Qiao QH, Bao X, Wu CB, Zhou Q. Chymotrypsin with sialendoscopy‐assisted surgery for the treatment of chronic obstructive parotitis. Int J Oral Maxillofac Surg 2017;46(7):877–882. [DOI] [PubMed] [Google Scholar]
- 31. Zou ZJ, Wang SL, Zhu JR, Wu QG, Yu SF. Chronic obstructive parotitis. Report of ninety‐two cases. Oral Surg Oral Med Oral Pathol 1992;73(4):434–440. [DOI] [PubMed] [Google Scholar]
- 32. Blair GS. Hydrostatic sialography. An analysis of a technique. Oral Surg Oral Med Oral Pathol 1973;36(1):116–130. [DOI] [PubMed] [Google Scholar]
- 33. Lewis MA, Lamey PJ, Strang R, Mason WN. Clinical application of computerized continuous‐infusion pressure‐monitored sialography. Dentomaxillofac Radiol 1991;20(2):68–72. [DOI] [PubMed] [Google Scholar]
- 34. Peng HP, Peng JH. Complications of botulinum toxin injection for masseter hypertrophy: incidence rate from 2036 treatments and summary of causes and preventions. J Cosmet Dermatol 2018;17(1):33–38. [DOI] [PubMed] [Google Scholar]
- 35. Petracca M, Guidubaldi A, Ricciardi L, et al. Botulinum toxin A and B in sialorrhea: long‐term data and literature overview. Toxicon 2015;107(pt A):129–140. [DOI] [PubMed] [Google Scholar]
- 36. Reddihough D, Erasmus CE, Johnson H, McKellar GM, Jongerius PH, Cereral Palsy I. Botulinum toxin assessment, intervention and aftercare for paediatric and adult drooling: international consensus statement. Eur J Neurol 2010;17(suppl 2):109–121. [DOI] [PubMed] [Google Scholar]


