Abstract
Objectives
To describe and investigate facial nerve displacement in deep lobe parotid lesions in children and to determine clinical and radiographic predictors of abnormal facial nerve position.
Methods
Retrospective case review of children who underwent total parotidectomy for deep lobe parotid lesions at a tertiary care center between January 2014 and December 2017. Aberrant facial nerve trajectory was defined as ascension of the nerve at an angle of 45° or greater. Elongation was defined as the main trunk >2 cm in length. Patient demographics, radiographic, pathologic results, postoperative nerve weakness, and intraoperative nerve findings were collected. Wilcoxon rank‐sum test and Fisher's exact test were used to assess the associations between variables of interest and facial nerve position.
Results
A total of 20 patients were included. The mean age was 7.7 ± 5 years. The most common pathologies were lymphatic malformation, pleomorphic adenoma, and first branchial cleft cyst. Twelve out of twenty (60.0%) patients had abnormal intraoperative facial nerve position. There was no significant difference in distribution of pathologies between those with or without an abnormal intraoperative nerve position (P = .41). Neither radiographic lesion size nor distance between the lesion and proximal portion of the facial nerve (mastoid tip) were associated with abnormal facial nerve position intraoperatively.
Conclusion
Pediatric deep lobe parotid lesions can displace the facial nerve and distort its anatomy in a posterior lateral direction, in approximately 60% of patients. Statistical analysis of increased numbers of patients to further define predictors of aberrant nerve course is warranted.
Level of Evidence
4.
Keywords: Parotid, salivary, facial nerve, deep lobe parotid, pediatric
INTRODUCTION
Much has been written about displacement of the intracranial portion of the facial nerve as it pertains to neurotologic surgery,1 but little to nothing has been reported about aberrancy of the nerve in parotid surgery. Extensive review of the literature revealed several studies that have discussed the indications and surgical approach to deep lobe parotid lesions2, 3, 4, 5; however, there was no discourse regarding abnormal facial nerve position due to deep lobe lesions. There is also a paucity of literature on deep lobe parotid lesions specific to the pediatric population.6, 7, 8, 9
The purpose of this article is to describe and investigate facial nerve displacement in parotid surgery in children through retrospective case review, photo documentation, illustration, and a 30 year experience with parotid surgery. We sought to identify if lesion size, lesion pathology, or other radiographic parameters would be predictive of an abnormal facial nerve position.
MATERIALS AND METHODS
Study Design and Subjects
This study was conducted at the Ann & Robert H. Lurie Children's Hospital of Chicago, a tertiary care hospital located in Chicago, USA and was approved by its institutional review board. Children less than 18 years of age who underwent total parotidectomy with facial nerve dissection (lesion present deep to facial nerve) from January 2014 through December 2017 for parotid masses, branchial anomalies, or vascular anomalies primarily confined to the parotid gland were included in the study. Patients with incomplete records, unavailable radiographic data, or incomplete follow‐up on facial nerve function were excluded. To gain data from high volume surgeons, only cases from our pediatric head and neck surgeons (JM, JCR, and DRJ) were included. Deep lobe parotid lesions were defined as entities that are either neoplastic or congenital in nature and through inherent growth or accumulation of fluid and/or debris cause expansion of the deep lobe. Previous normative data have established a well‐accepted reference standard length of the main trunk of the facial nerve of 15–20 mm, which runs anteriorly in the horizontal plane bisecting the parotid gland.10 We acknowledge that this is mainly based on adult data; however, this is the only normative data that exist regarding accepted length and angle of the facial nerve. We thus defined increased trajectory as ascension of the facial nerve at an angle of 45° or greater. Elongation was defined as the main trunk being >2 cm in length. These two measurements were done intraoperatively in a prospective fashion, as the surgeons report these two measures in all operative reports of parotidectomy surgery. Measurements were done with calipers from the stylomastoid foramen to the pes anserinus. Facial nerve function was followed up beyond the 30‐day postoperative period in patients with early postoperative weakness (defined as within 30 days). Data from the patient case series were then retrospectively examined, including demographic information (age, gender, and ethnicity), radiographic data, pathologic results, postoperative facial nerve weakness, and if the intraoperative position of the facial nerve was normal or abnormal. Radiologic data included the greatest measurement for each of three dimensions in centimeters (anterior‐posterior [AP], superior‐inferior [SI], and medial‐lateral [ML]), the estimated volume in cubic centimeters as determined by AP × SI × ML, and the distance from the mastoid tip and stylomastoid foramen to the closest aspect of the lesion. The distance from the mastoid tip and stylomastoid foramen to the lesion was chosen to best explore the relationship of the lesion to the proximal portion of the facial nerve trunk, hypothesizing that the intraoperative position of the nerve would be more abnormal in lesions closer to the proximal facial nerve. All patients had preoperative computed tomography and/or magnetic resonance imaging (MRI). Each radiographic study was analyzed by a pediatric radiologist (AI) and by a pediatric otolaryngologist (DRJ or JM). All 20 patients were included in the descriptive statistical analysis. For the analysis of radiographic measurements and the presence/absence of postoperative facial nerve paresis, 19 patients were included instead of 20 due to unavailable data.
Statistical Analysis
Descriptive statistics are reported for all variables of interest for both the overall sample, and also by nerve position status. Mean ± SD, median (interquartile range), and minimum/maximum values are reported for continuous variables, whereas frequencies and percentages are reported for all categorical variables. Due to small sample size and non‐normality assessed via histograms and Shapiro‐Wilk tests for normality, the Wilcoxon rank‐sum test was used to assess the associations between continuous variables of interest and facial nerve position status. Furthermore, due to small sample size and cell counts, Fisher's exact test was used to assess the associations between categorical variables of interest and facial nerve position status. A two‐sided alpha level of .05 was used in all tests as there were no adjustments made for multiple tests. All analyses were completed using SAS version 9.4 (Cary, NC).
RESULTS
A total of 20 patients were included in the final analysis. Eight (40.0%) patients were male and 12 (60.0%) were female. The mean age of the patients was 7.7 ± 5.5 (min–max; 0.2–16.8) years. The frequencies of pathological findings are reported in Table 1, with lymphatic malformation, pleomorphic adenoma, and first branchial cleft cyst representing 14/20 lesions (70%). Twelve out of twenty (60.0%) patients had an abnormal intraoperative facial nerve position, as determined from description in the operative note. There was no significant difference in distribution of pathologies between those with or without an abnormal intraoperative position of the facial nerve (Pearson χ 2, P = .41; Table 1). The mean radiographic lesion size in patients with an abnormal intraoperative facial nerve position was 4.2 ± 1.3 cm (SI), 2.9 ± 1.4 cm (ML), and 3.4 ± 1.2 cm (AP), respectively. The mean radiographic lesion size in patients with a normal intraoperative facial nerve position was 3.4 ± 1.1 cm (SI), 2.7 ± 0.8 cm (ML), and 2.8 ± 0.9 cm (AP), respectively. There was no significant difference between the SI (P = .29), ML (P = .65), and AP (P = .28) measurements between groups (Table 2).
Table 1.
Postoperative Pathology and Rate of Nerve Paresis in Patients Presenting with Deep Lobe of Parotid Lesions (n = 20).
| Intraoperative Facial Nerve Position | P Value* | |||
|---|---|---|---|---|
| Abnormal | Normal | |||
| Pathology | n (%) | n (%) | n (%) | |
| First branchial cleft cyst | 4 (20.0) | 2 (16.7) | 2 (25) | .41 |
| Lymphatic malformation | 6 (30.0) | 2 (16.7) | 4 (50) | |
| Veno‐lymphatic malformation | 2 (10.0) | 2 (16.7) | 0 (0) | |
| Venous malformation | 1 (5.0) | 0 (0) | 1 (12.5) | |
| Mammary analogue secretory carcinoma | 1 (5.0) | 1 (8.3) | 0 (0) | |
| Pleomorphic adenoma | 4 (20.0) | 3 (25) | 1 (12.5) | |
| Sclerosing polycystic adenosis | 1 (5.0) | 1 (8.3) | 0 (0) | |
| Sialolipoma | 1 (5.0) | 1 (8.3) | 0 (0) | |
| Nerve paresis (Yes) | 7 (35) | 2 (25) | 5 (42) | .36 |
P value is calculated by Pearson's correlation between patients with or without abnormal position of nerve.
Table 2.
Radiographic Findings in Patients with Normal and Abnormal Intraoperative Facial Nerve Position (n = 19).
| Measurements | Intraoperative Facial Nerve Position | P Value | |
|---|---|---|---|
| Abnormal (n = 12) | Normal (n = 7) | ||
| SI (cm) | 4.0 ± 1.3 | 3.4 ± 1.1 | .28 |
| ML (cm) | 2.9 ± 1.4 | 2.7 ± 0.7 | .65 |
| AP (cm) | 3.4 ± 1.1 | 2.8 ± 0.9 | .28 |
| Volume (cm3) | 51.1 ± 14.6 | 28.9 ± 6.9 | .28 |
| Mastoid tip (cm) | 5.25 ± 6.57 | 9.75 ± 7.50 | .21 |
| Stylomastoid foramen (cm) | 7.92 ± 5.87 | 14.13 ± 8.39 | .13 |
AP = anterior‐posterior; ML = medial‐lateral; SI = superior‐inferior.
We further tested whether the distance from the mastoid tip and the stylomastoid foramen (as surrogates for the most proximal portion of the facial nerve) to the lesion was predictive of an abnormal position of the facial nerve intraoperatively. The mean distances from the mastoid tip and the stylomastoid foramen to the nearest portion of the lesion in those with abnormal facial nerve position were 5.25 ± 6.57 cm and 7.92 ± 5.87 cm, respectively. Alternatively, the mean distances from the mastoid tip and the stylomastoid foramen to the nearest portion of the lesion in those with normal facial nerve position were 9.75 ± 7.50 cm and 14.13 ± 8.39 cm, respectively. Although the group with abnormal facial nerve position intraoperatively had a shorter mean distance between the lesion and the proximal portion of the facial nerve, the difference between groups was not statistically significant (P = .21, P = .13) (Table 2).
Similarly, the association analysis found no association of age, gender, ethnicity, or postoperative facial nerve paresis with the intraoperative facial nerve position. The rate of early postoperative facial paresis was 7/19 cases (36.8%). Persistent paresis occurred in 2/19 (10.5%) cases at last follow‐up (4 months and 7 years).
DISCUSSION
Parotidectomy in children is a relatively uncommon procedure at most centers.6, 7, 8 More than 80% of Parotid surgeries are performed for benign inflammatory disease or masses of the parotid gland.11 At our institution, we perform a high volume of parotidectomy in children. It is recognized that the most notable morbid complication of parotidectomy is facial nerve paresis or paralysis. Although some studies have aimed to determine factors associated with temporary and/or permanent facial nerve dysfunction after parotid surgery,2, 12 no authors included a discussion of aberrant facial nerve anatomy that can result from deep lobe lesions.
The premise of this article is to present a previously undescribed surgical reality whereby the facial nerve anatomy is distorted by deep lobe lesions of the parotid gland. We have observed that lesions involving the deep lobe of the parotid gland can significantly displace the main trunk of the facial nerve and its branches in a posterior lateral direction. It also has the effect of elongating the main trunk of the facial nerve. This places the main trunk of the nerve in a vertical rather than a horizontal trajectory and places its branches in a superficial and almost subcutaneous location (Fig. 1).
Figure 1.
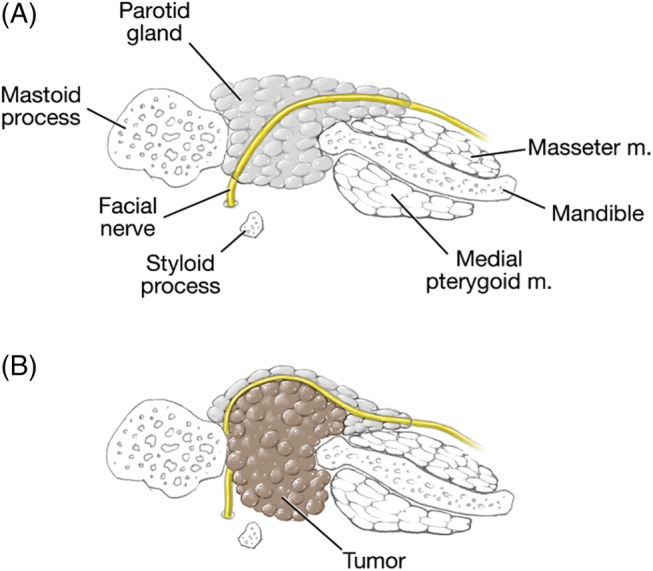
Drawings in the axial plane demonstrating the course of the facial nerve as it traverses the deep lobe of the parotid. (A) As it appears in normal anatomy and (B) displacement by deep lobe lesion.
The facial nerve exits the temporal bone through the stylomastoid foramen. It then takes a horizontal course between an envelope formed by the stylohyoid muscle (medially), and the posterior belly of the digastric muscle (laterally). It then enters the parotid gland as the main trunk of the facial nerve dividing the gland into superficial and deep lobes. In the proximal 20% of the parotid, it divides into superior and lower divisions at the pes anserinus.11, 13 Topographical analyses have found that several structures can serve as reliable surgical landmarks for identification and preservation of the nerve including the tragal pointer, posterior belly of digastric muscle, and tympanomastoid suture.13, 14 With respect to deep lobe lesions, the authors correctly point out that greater facial nerve dissection is required, with a resultant higher incidence of temporary paresis.2, 3, 4, 5, 8, 12 De Ru et al devised a radiologic technique to help determine whether a lesion was located in the superficial or deep lobe of parotid, which may help to inform the surgical approach and extent of nerve dissection required.14 However, the possibility of the facial nerve having an abnormal course which may limit the use of these anatomic and radiologic techniques was not discussed in any of these studies. It has been the authors' observation that deep lobe lesions can significantly alter the course of the main trunk of the facial nerve as well as elongate it. These lesions, when significant, displace the course of the nerve from a horizontal trajectory to one that approaches a vertical plane, almost in a juxtaposition to the auricular cartilage. This has the effect of laterally displacing the distal portion of the nerve and pes anserinus to a more superficial, almost subcutaneous location (Fig. 2). This displacement phenomenon can also be compounded by the splaying and pressure atrophy of the superficial lobe of the parotid gland by the enlarging mass in the deep lobe (Fig. 2). This distortion makes reliance on the classic landmarks to identify the main trunk of the facial nerve, namely the auricular cartilage, posterior belly of the digastric muscle, and tympanomastoid suture less accurate. Awareness by the surgeon of this anatomic variant can alter the surgical approach and decrease dependence on the facial nerve monitor to preserve the facial nerve's integrity.
Figure 2.
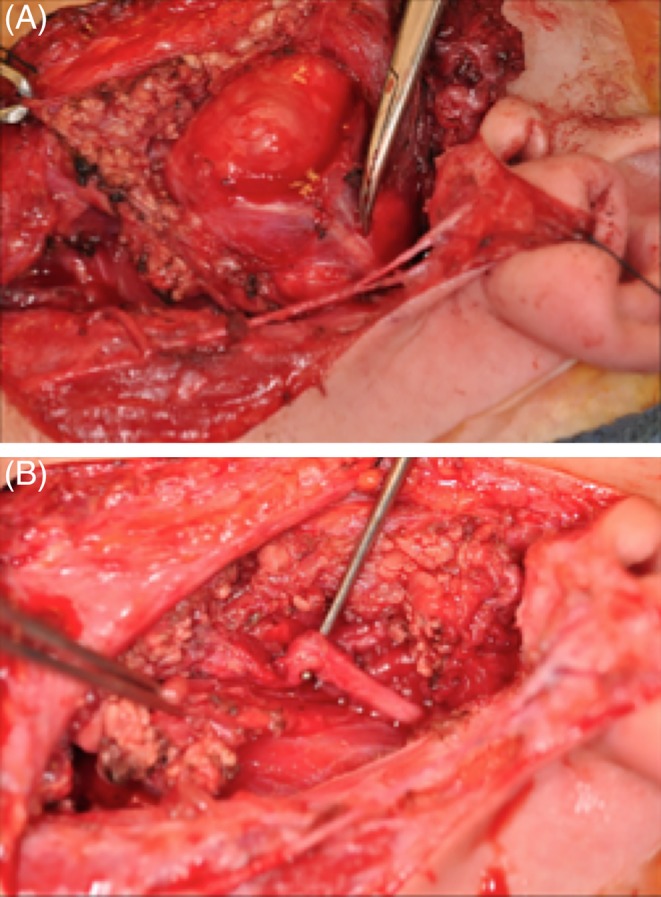
(A) Intraoperative photo of facial nerve: note the elongation of the facial nerve, its vertical trajectory, superficial location, as well as displacement of the pes anserinus. (B) Intraoperative photo of facial nerve after tumor resection. The redundancy of the facial nerve is evident.
In our review and case study, our data indicated that 60% of the patients operated with deep lobe lesions had an abnormal position of the facial nerve. Although we report this anatomic condition and recognize that operating surgeons should be aware of it, we have not been able to establish a correlate as regards to pathology or lesion dimension in our statistical analysis. We ascribe these results to inadequate numbers in our cohort. The lack of increased facial nerve injury can be secondary to inadequate sample size and also to the fact that our operating team perform a high volume of parotid surgery and have been aware of this anatomic challenge for many years. Further endeavors to study this abnormality should include acquisition and cataloging of increased number of patients to provide more relevant predictive factors as to the position of the facial nerve. Frey syndrome, recurrence rates, and other long‐term outcome measures were not included as they were beyond the scope of this study, but would augment future studies. Due to the rarity of deep lobe parotid lesions in children, this may require interinstitutional cooperation and sharing of data.
There are inherent problems in any retrospective case study. There were no controlled measurements or angles of trajectory taken of the facial nerve as the reported findings were observed intraoperatively and in real time. The use of calipers and other measuring devices in this type of clinical setting can be subjective when comparing different surgeons. The optimal method to assess these values would be through the direct evaluation of images of the facial nerve. Extracranial imaging of the nerve is not well documented or performed. Chu et al have reported excellent imaging of the extracranial portion of the facial nerve by using high‐resolution MRI based on a micro surface coil15; however, these techniques are not readily available at most institutions. When these modalities are refined and in more general use, the findings reported here can be more accurately defined and assessed.
CONCLUSION
Deep lobe lesions of the parotid gland in the pediatric population can displace the course of the facial nerve and distort its anatomy in a posterior lateral direction, in approximately 60% of patients. Statistical analysis of increased numbers of patients to further define the nuances peculiar to these lesions is warranted. Advances in MRI imaging and facial nerve mapping may assist the operating surgeon in the approach to this problem.
Editor's Note: This Manuscript was accepted for publication 02 August 2019.
Funding: None.
Conflicts of Interest: None.
BIBLIOGRAPHY
- 1. Palabiyik FB, Hacikurt K, Yazici Z. Facial nerve anomalies in paediatric cochlear implant candidates: radiological evaluation. J Laryngol Otol 2017;131(1):26–31. [DOI] [PubMed] [Google Scholar]
- 2. Gaillard C, Périé S, Susini B, St Guily JL. Facial nerve dysfunction after parotidectomy: the role of local factors. Laryngoscope 2005;115(2):287–291. [DOI] [PubMed] [Google Scholar]
- 3. Vaiman M, Abuita R, Jabarin B. Selective deep lobe parotid surgery for benign tumors. Acta Otolaryngol 2015;135(12):1319–1322. [DOI] [PubMed] [Google Scholar]
- 4. Ulku CH, Uyar Y, Unaldi D. Management of lipomas arising from deep lobe of the parotid gland. Auris Nasus Larynx 2005;32(1):49–53. [DOI] [PubMed] [Google Scholar]
- 5. Olsen KD, Moore EJ. Deep lobe parotidectomy: clinical rationale in the management of primary and metastatic cancer. Eur Arch Otorhinolaryngol 2014;271(5):1181–1185. [DOI] [PubMed] [Google Scholar]
- 6. Lee DH, Yoon TM, Lee JK, Lim SC. Clinical features of pediatric parotid tumors: 10‐year experience of a single institute. Acta Otolaryngol 2013;133(11):1213–1218. [DOI] [PubMed] [Google Scholar]
- 7. Liu B, Liu JY, Zhang WF, Jia J. Pediatric parotid tumors: clinical review of 24 cases in a Chinese population. Int J Pediatr Otorhinolaryngol 2012;76(7):1007–1011. [DOI] [PubMed] [Google Scholar]
- 8. Carter JM, Rastatter JC, Bhushan B, Maddalozzo J. Thirty‐day perioperative outcomes in pediatric parotidectomy. JAMA Otolaryngol Head Neck Surg 2016;142(8):758–762. [DOI] [PubMed] [Google Scholar]
- 9. Hinson D, Poteet P, Bower C. Duplicated facial nerve trunk with a first branchial cleft cyst. Laryngoscope 2014;124(3):662–664. [DOI] [PubMed] [Google Scholar]
- 10. May M, Schaitkin B. The Facial Nerve. 2nd ed New York, NY: Thieme; 2000. [Google Scholar]
- 11. Gandolfi MM, Slattery W. Parotid gland tumors and the facial nerve. Otolaryngol Clin North Am 2016;49(2):425–434. [DOI] [PubMed] [Google Scholar]
- 12. Ikoma R, Ishitoya J, Sakuma Y, et al. Temporary facial nerve dysfunction after parotidectomy correlates with tumor location. Auris Nasus Larynx 2014;41(5):479–484. [DOI] [PubMed] [Google Scholar]
- 13. Cannon CR, Replogle WH, Schenk MP. Facial nerve in parotidectomy: a topographical analysis. Laryngoscope 2004;114(11):2034–2037. [DOI] [PubMed] [Google Scholar]
- 14. De Ru JA, Bleys RL, van Benthem PP, Hordijk GJ. Preoperative determination of the location of parotid gland tumors by analysis of the position of the facial nerve. J Oral Maxillofac Surg 2001;59(5):525–528. [DOI] [PubMed] [Google Scholar]
- 15. Chu J, Zhou Z, Hong G, et al. High‐resolution MRI of the intraparotid facial nerve based on a microsurface coil and a 3D reversed fast imaging with steady‐state precession DWI sequence at 3T. Am J Neuroradiol 2013;34(8):1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]


