Oscillatory activity is a universal design feature of olfactory systems (Tank et al., 1994), having been demonstrated by Lord Adrian in the olfactory bulb of the hedgehog >60 years ago (Adrian, 1942). Hints as to the computational role of olfactory oscillations have only recently begun to emerge (Stopfer et al., 1997; Teyke and Gelperin, 1999; Nusser et al., 2001) (but see Fletcher et al., 2005). The effort to understand the role of olfactory oscillations is aided by insights from computational models of olfactory networks incorporating realistic dynamics at the cellular and network levels (Linster and Cleland, 2001; Davison et al., 2003; Ermentrout et al., 2004; Sivan and Kopell, 2004; Bazhenov et al., 2005; Galan et al., 2005; Migliore et al., 2005). I will review some of the major issues we face in attempts to understand the computational and behavioral roles of olfactory oscillations (Gelperin, 1999), in the context of more general efforts to understand cortical oscillations in a variety of sensory processing and motor control pathways (Salinas and Sejnowski, 2001; Buzsaki and Draguhn, 2004; Borgers et al., 2005; Mann and Paulsen, 2005; Schnitzler and Gross, 2005).
What is being measured?
Coherent oscillatory activity in olfactory networks is expressed in both periodic transmembrane potential fluctuations (Margrie and Schaefer, 2003; Balu et al., 2004; Hayar et al., 2004a) and local field potential (LFP) measurements (Gelperin and Tank, 1990; Dorries and Kauer, 2000), if permitted by the geometrical and electrotonic properties of the neurons experiencing rhythmic transmembrane events (Hubbard et al., 1969; Harris et al., 2000). A major contributor to the local field potential fluctuations is coherent synaptic activity within a local population of neurons. In the case of the olfactory bulb, the reciprocal synaptic connections between mitral and granule cells give rise to oscillatory local field potentials in the 40–100 Hz (gamma) range mainly because of synaptic currents in granule cells (Neville and Haberly, 2003). It is now clear that the gamma band is composed of two distinct components that predominate in different behavioral states (Kay, 2003). Local field potential oscillations in the beta frequency range (15–40 Hz) result from reciprocal synaptic interactions between the olfactory bulb and the piriform cortex (Neville and Haberly, 2003). The relative prominence of beta and gamma oscillations is dramatically altered during the progress of learning new meanings for an odor cue (Ravel et al., 2003). These studies also make clear the critical importance of measurements made in the awake behaving animal during odor-guided decision making as an essential adjunct to studies in anesthetized animals and olfactory bulb slice preparations.
A comparative perspective
A comparative perspective on olfactory information processing has identified a set of 14 design features and circuit properties found across wide swaths of the phylogenetic tree (Hildebrand and Shepherd, 1997; Gelperin, 1999). These include a critical role for local inhibitory interneurons in shaping network dynamics (Gelperin and Tank, 1990; Lagier et al., 2004; Murphy et al., 2005; Saghatelyan et al., 2005), spontaneous or odor-induced oscillatory activity (Adrian, 1942; Tank et al., 1994; Delaney and Hall, 1996; Nikonov et al., 2002; Lam et al., 2003; Ravel et al., 2003), changes in odor representations attributable to learning in the first stage of central odor processing (Freeman and Schneider, 1982; Kimura et al., 1998; Sandoz et al., 2003; Daly et al., 2004; Martin et al., 2004; Wilson et al., 2004; Kirino et al., 2005), addition of new circuit elements during postpartum odor experience (Mair et al., 1982; Zakharov et al., 1998; Beltz and Sandeman, 2003; Saghatelyan et al., 2005), evidence for spatial segregation of related odor representations (Friedrich and Korsching, 1997; Kimura et al., 1998; Mori et al., 1999; Galizia and Menzel, 2000; Luo and Katz, 2001; Wang et al., 2003; Johnson et al., 2005), temporal evolution of odor representations (Laurent et al., 2001; Lei et al., 2004; Szyszka et al., 2005; Zochowski and Cohen, 2005), role for both chemical and electrical coupling between network elements (Friedman and Strowbridge, 2003; Zhang and Restrepo, 2003; Ermentrout et al., 2004; Christie et al., 2005), control of network dynamics and plasticity by nitric oxide (NO) (Kendrick et al., 1997; Gelperin et al., 2000; Collmann et al., 2004; Fujie et al., 2005; Korneev et al., 2005), synchronization of odor-responsive output neurons (Laurent, 2002; Brody and Hopfield, 2003; Christensen et al., 2003; Friedrich et al., 2004; Hayar et al., 2004b), use of coupled burster neurons to enhance network responses (Ermentrout et al., 2001; Hayar et al., 2004b), continual turnover of receptor cells (Chase and Rieling, 1986; Farbman, 1994), receptor neurons map to glomeruli based on receptor gene expression (Vosshall et al., 2000; Wang et al., 2003; Buck, 2004; Zou et al., 2004), afferents interact with relay neurons in glomeruli (Chase and Tolloczko, 1986; Galizia et al., 1999; Wachowiak et al., 2004), and expression of speed–accuracy tradeoff (Ditzen et al., 2003; Uchida and Mainen, 2003; Abraham et al., 2004; Khan and Sobel, 2004; Friedrich, 2005). This set of features may be incomplete but nevertheless serves to highlight the functional analogies found in a very diverse set of olfactory information processing systems.
The comparative approach reflected above takes seriously the notion that not only olfactory information processing but also human diseases, learning, and other cognitive functions can be fruitfully studied in both mammalian and nonmammalian species (Kazemi-Esfarjani and Benzer, 2002; Barco et al., 2003; Bonini and Fortini, 2003; Greenspan and van Swinderen, 2004). Given this perspective, it is now useful to look at a particular set of molluscan olfactory systems and see in more detail how they illuminate general issues of the functions of olfactory oscillations.
Molluscan model systems
“For many problems there is an animal on which it can be most conveniently studied.” —August Krogh
The terrestrial pulmonate mollusks epitomize Krogh’s principle (Krebs, 1975) for the study of olfactory information processing and in particular the computational role of cellular and network oscillations in olfaction (Gelperin, 1999; Chase, 2002; Kirino et al., 2005). The terrestrial slugs and snails are dominated by olfaction for orientation, nutrition, and reproduction. This macrosmatic lifestyle is reflected in the dedication of most of the neurons in the CNS (∼104) to the processing of olfactory information (Chase, 2000). There are two major olfactory processing structures: the digitate ganglion at the superior tentacle tip right behind the nose (Ito et al., 2000) and the procerebral (PC) lobe in the CNS. The PC lobe receives both first-order input from olfactory receptors and second-order input from the digitate ganglion (Chase and Kamil, 1983; Murakami et al., 2004).
Oscillatory LFPs are recorded from both the digitate ganglion (Ito et al., 2004) and the PC lobe (Gelperin and Tank, 1990; Kawahara et al., 1997; Nikitin and Balaban, 2000). In the PC lobe, activity waves are initiated at the apical pole and propagate to the base at 1.1 mm/s (Delaney et al., 1994; Toda et al., 2000; Watanabe et al., 2004). At a stationary LFP recording site, the recorded oscillation (0.7 Hz in vitro) corresponds to the passing front of the activity wave. Recordings of PC lobe activity in intact behaving slugs implanted with fine-wire electrodes show periods of 0.7 Hz oscillation interspersed with periods of complex multicomponent activity (Cooke and Gelperin, 2001). Optical recordings of the PC lobe wave activity during odor stimulation of the nose show a momentary collapse of the activity wave (Kleinfeld et al., 1994), which is hypothesized to allow nearest-neighbor interactions suppressed by the traveling wave activity (Ermentrout and Kleinfeld, 2001). Activity waves have also been recorded from an in vitro preparation of the ferret thalamus (Kim et al., 1995) and from turtle visual cortex (Prechtl et al., 2000; Robbins and Senseman, 2004).
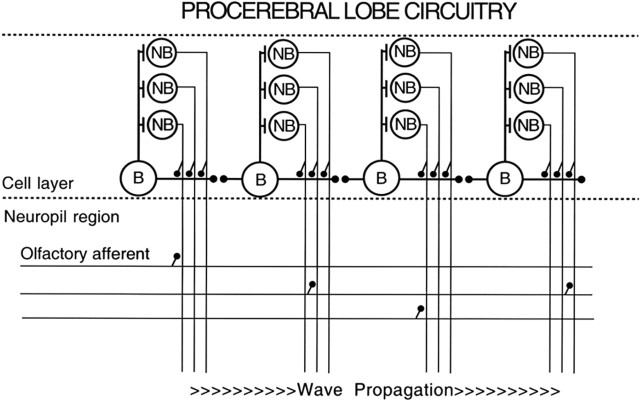
The LFP oscillations and activity wave in the Limax PC lobe arise from intrinsic neuronal properties and network connections among a population of bursting inhibitory interneurons, called B cells (Fig. 1) (Watanabe et al., 1998; Wang et al., 2001). These cells are connected by both chemical and electrical synapses (Ermentrout et al., 2004) and show a gradient of excitability with apical B cells most excitable (higher burst rate), accounting for normal initiation of the activity wave at the apical end in the in vitro preparation (Ermentrout et al., 1998). The gradient of B-cell excitability is attributable to a spatial gradient in the amplitude of chloride-dependent depolarizations in B cells (Watanabe et al., 2003). Activity waves occupy the full width of the PC lobe, most likely attributable to the rapid lateral conduction speed of activity in varicose B cells compared with the apical–basal conduction speed of activity in smooth B cells (Wang et al., 2001). The band-like conduction of activity along the apical–basal axis of the PC lobe is related to the band-like storage of learned odor representations in the PC lobe (Kimura et al., 1998; Teyke et al., 2000). Modification of PC lobe cell properties by learning reflects the general finding that learning affects odor representations in the earliest stages of odor processing (Kay and Laurent, 1999; Wilson et al., 2004).
Figure 1.
Diagram of the intrinsic circuitry of the Limax PC lobe. The intrinsic excitability of the B cells is highest on the left and decreases moving to the right. The B cells are connected by both chemical and electrical synapses. Each B cell connects with and inhibits ∼100 nonbursting (NB) cells, only three of which are shown in the diagram. The processes of the B cells are confined to the cell body layer of the PC lobe, whereas the neurites of the NB cells project into the neuropil layer in which they receive synapses from olfactory afferents and second-order neurons in the digitate ganglion (data not shown). The NB cells also synapse in the neuropil with the neurites of output neurons whose somata are located in the pedal and buccal ganglia (Gelperin and Flores, 1997) and in the metacerebrum (Ratté and Chase, 2000; Shimozono et al., 2001).
The excitability of B cells is modulated by NO (Gelperin, 1994). Suppression of NO synthesis shuts down the LFP oscillation and wave propagation in the PC lobe (Gelperin et al., 2000). Suppression of NO synthesis in the behaving snail (Teyke, 1996) or slug (Sakura et al., 2004) blocks fine odor discrimination or odor learning. These observations of degraded odor processing and odor learning after inhibition of NO synthesis are paralleled by findings in ewes (Kendrick et al., 1997), honeybees (Müller, 1996; Hosler and Smith, 2000), the predatory snail Euglandina (Clifford et al., 2003), mice (Okere et al., 1996), and rats (Samama and Boehm, 1999). The relationship between nitric oxide and synaptic plasticity has been reviewed recently (Susswein et al., 2004).
Computational implications of olfactory oscillations
Recent work has explored the computational utility of synchronous activity in a group of neurons coding stimulus identity, with direct relevance to olfaction (Brody and Hopfield, 2003; Hopfield and Brody, 2004). Part of the mechanism to enhance synchronous activity is a shared membrane potential oscillation (Margrie and Schaefer, 2003), which may result in an oscillation of LFP, depending on geometrical and electrotonic properties of the cells showing the coherent changes in membrane potential. Olfactory interneurons can show odor-elicited synchronous activity not phased to the oscillating LFP (Christensen et al., 2003).
The temporal evolution of activity in the group of synchronously active interneurons can result in distinct temporal patterns within the odor-responsive population (Stopfer and Laurent, 1999; Christensen et al., 2003). As the pattern of activity evolves in time, sparsening of the representation can reduce the overlap of patterns set up by closely related odors (Friedrich et al., 2004; Szyszka et al., 2005). The amplitude and regularity of the LFP oscillation also shows a temporal evolution after odor onset, typically becoming larger and more regular in the first several hundred milliseconds after stimulus onset. There are also history-dependent effects such that a series of closely spaced odor stimuli result in successive epochs of oscillatory responses, with sharper LFP oscillation patterns triggered at shorter latency over the first three odor stimuli (Stopfer and Laurent, 1999). These events provide an opportunity to determine whether the latency for odor identification shows a parallel decrease in latency over the first three odor pulses, as measured by latency to emit a conditioned response (Daly et al., 2004; Yu et al., 2004).
The Limax olfactory system allows the causal link between the LFP oscillation in the PC lobe and odorant-induced behavior to be explored using an in vitro nose–brain preparation (Teyke and Gelperin, 1999; Inoue et al., 2004). Recent work by Inoue et al. (2004) uses the neural substrate of a response to aversive odors, firing of an identified mantle motor neuron, to read out the behavioral decision made by the isolated brain in response to odor stimulation. Previous conditioning of the animal creates an aversive response to a previously attractive odor (Sahley et al., 1981), which survives in the isolated nose–brain preparation (Teyke et al., 2000). Application of an aversively conditioned odor to the superior nose of the in vitro nose–brain preparation causes an increase in LFP oscillation frequency in the PC lobe and firing of the identified mantle motor neuron (Inoue et al., 2004). Directly increasing LFP oscillation frequency in the PC lobe does not activate the mantle motor neuron. It may be that the motor pathway for the aversive mantle reflex is activated in parallel with an increase in PC lobe LFP frequency as learning-related changes are engaged in the PC lobe (Ermentrout et al., 2004). If the naive nose–brain preparation can be trained in vitro while the pharmacology of the PC lobe is selectively manipulated, the role of the PC lobe LFP oscillation and activity wave in odor recognition and odor learning can be directly tested. This possibility is encouraged by the demonstrated learning ability of the isolated Limax lip–brain preparation (Chang and Gelperin, 1980) along with other isolated CNS preparations (Muhlethaler et al., 1993; Kemenes et al., 1997; Mokin and Keifer, 2005).
Multisite network measurements
Tests of computational models of synchronous activity require simultaneous monitoring of activity in dozens or hundreds of olfactory interneurons to provide an adequate sample of neurons responding to a given odor. Arrays of silicon microprobes (Christensen et al., 2000), tetrodes (Egana et al., 2005), and multiple single-unit electrodes (D. Rinberg, A. Koulakov, A. Gelperin, unpublished observations) are beginning to provide simultaneous access to sufficient numbers of mitral cells or projection neurons (insect analog of mitral cells) so that spatial and temporal evolution of odor-responsive cell populations can be analyzed in detail in the awake behaving animal. One or more electrodes in the array are devoted to measuring LFPs so that correlations between responding interneurons can also be indexed to a common field potential (Wang et al., 2003).
Optical recordings in the awake head-fixed animal (Margrie et al., 2002) have the potential to permit a behavioral readout of the results of olfactory processing, whereas voltage- or calcium-sensitive dyes or genetically encoded markers (Bozza et al., 2004) sample large populations of olfactory interneurons. It will be interesting to make these population measurements in animals with substantially reduced olfactory processing networks that spare significant olfactory ability (de Belle and Heisenberg, 1994; Slotnick et al., 2004; Komischke et al., 2005).
Network perturbations to probe causality
The critical need is to test the computational role of synchronous activity and network oscillations using selective perturbations of central olfactory circuits while measuring odor identification and discrimination. The experiments of Stopfer et al. (1997) suggest that pharmacologically induced desynchronization of first-order olfactory interneurons degrades discrimination of similar odors but not dissimilar odors. A similar result was obtained relating oscillations in the Limax PC lobe to odor discriminations, as tested using an in vitro lip–brain preparation (Teyke and Gelperin, 1999). The Limax experiment will be even more informative using a newly developed in vitro nose–brain preparation, also using motor neuron activation to index the attractive or repellent nature of the applied odor (Inoue et al., 2004). Other perturbations used to explore the causal link between olfactory circuit function and odor-guided behavior include lesions of the olfactory bulbs (Slotnick et al., 2004), genetic lesions of specific neurotransmitter receptor subtypes (Nusser et al., 2001) or ionic channel subunits (Fadool et al., 2004) in the olfactory bulb, pharmacological manipulations of the olfactory bulbs during odor-guided behavioral tasks (Ravel et al., 1994; Kendrick et al., 1997), and direct electrical stimulation of the olfactory bulb in awake behaving animals to create “electric odors” (Jirsa and Radil, 1997; Mouly and Gervais, 2002; Roman et al., 2004). Creative use of these and other methods for selective perturbation of olfactory networks during their participation in olfactory computations and the readout of the effects of these perturbations in odor-guided behavior is critical to fully clarify the computational role of olfactory oscillations and synchronous cellular activity during odor processing.
Footnotes
This work was supported by the Army Research Office, the Whitehall Foundation, and National Institutes of Health Grant MH56090.
References
- Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT (2004). Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron 44:865–876. [DOI] [PubMed] [Google Scholar]
- Adrian ED (1942). Olfactory reactions in the brain of the hedgehog. J Physiol (Lond) 100:459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu R, Larimer P, Strowbridge BW (2004). Phasic stimuli evoke precisely timed spikes in intermittently discharging mitral cells. J Neurophysiol 92:743–753. [DOI] [PubMed] [Google Scholar]
- Barco A, Pittenger C, Kandel ER (2003). CREB, memory enhancement and the treatment of memory disorders: promises, pitfalls and prospects. Expert Opin Ther Targets 7:101–114. [DOI] [PubMed] [Google Scholar]
- Bazhenov M, Stopfer M, Sejnowski TJ, Laurent G (2005). Fast odor learning improves reliability of odor responses in the locust antennal lobe. Neuron 46:483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz BS, Sandeman DC (2003). Regulation of life-long neurogenesis in the decapod crustacean brain. Arthropod Structure Dev 32:39–60. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Fortini ME (2003). Human neurodegenerative disease modeling using Drosophila. Annu Rev Neurosci 26:627–656. [DOI] [PubMed] [Google Scholar]
- Borgers C, Epstein S, Kopell NJ (2005). Background gamma rhythmicity and attention in cortical local circuits: a computational study. Proc Natl Acad Sci USA 102:7002–7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza TC, McGann JP, Mombaerts P, Wachowiak M (2004). In vivo imaging of neuronal activity—neurotechnique by targeted expression of a genetically encoded probe in the mouse. Neuron 42:9–21. [DOI] [PubMed] [Google Scholar]
- Brody CD, Hopfield JJ (2003). Simple networks for spike-timing-based computation, with application to olfactory processing. Neuron 37:843–852. [DOI] [PubMed] [Google Scholar]
- Buck LB (2004). Olfactory receptors and odor coding in mammals. Nutr Rev 62:S184–S188. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A (2004). Neuronal oscillations in cortical networks. Science 304:1926–1929. [DOI] [PubMed] [Google Scholar]
- Chang JJ, Gelperin A (1980). Rapid taste-aversion learning by an isolated molluscan CNS. Proc Natl Acad Sci USA 77:6204–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase R (2000). Structure and function in the cerebral ganglion. Microsc Res Tech 49:511–520. [DOI] [PubMed] [Google Scholar]
- Chase R (2002). In: Behavior and its neural control in gastropod molluscs Oxford: Oxford UP.
- Chase R, Kamil R (1983). Neuronal elements in snail tentacles as revealed by HRP backfilling. J Neurobiol 14:29–42. [DOI] [PubMed] [Google Scholar]
- Chase R, Rieling J (1986). Autoradiographic evidence for receptor cell renewal in the olfactory epithelium of a snail. Brain Res 384:232–239. [DOI] [PubMed] [Google Scholar]
- Chase R, Tolloczko B (1986). Synaptic glomeruli in the olfactory system of a snail. Cell Tissue Res 246:567–573. [Google Scholar]
- Christensen TA, Pawlowski VM, Lei H, Hildebrand JG (2000). Multi-unit recordings reveal context-dependent modulation of synchrony in odor-specific neural ensembles. Nat Neurosci 3:927–931. [DOI] [PubMed] [Google Scholar]
- Christensen TA, Lei H, Hildebrand JG (2003). Coordination of central odor representations through transient non-oscillatory synchronization of glomerular output neurons. Proc Natl Acad Sci USA 100:11076–11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Bark C, Hormuzdi SG, Helbig I, Monyer H, Westbrook GL (2005). Connexin36 mediates spike synchrony in olfactory bulb glomeruli. Neuron 46:761–772. [DOI] [PubMed] [Google Scholar]
- Clifford KT, Gross L, Johnson K, Martin KJ, Shaheen N, Harrington MA (2003). Slime-trail tracking in the predatory snail, Euglandina rosea. Behav Neurosci 117:1086–1095. [DOI] [PubMed] [Google Scholar]
- Collmann C, Carlsson MA, Hansson BS, Nighorn A (2004). Odorant-evoked nitric oxide signals in the antennal lobe of Manduca sexta. J Neurosci 24:6070–6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke IRC, Gelperin A (2001). In vivo recordings of spontaneous and odor-modulated dynamics in the Limax olfactory lobe. J Neurobiol 46:126–141. [PubMed] [Google Scholar]
- Daly KC, Christensen TA, Lei H, Smith BH, Hildebrand JG (2004). Learning modulates the ensemble representations for odors in primary olfactory networks. Proc Natl Acad Sci USA 101:10476–10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison AP, Feng JF, Brown D (2003). Dendrodendritic inhibition and simulated odor responses in a detailed olfactory bulb network model. J Neurophysiol 90:1921–1935. [DOI] [PubMed] [Google Scholar]
- de Belle JS, Heisenberg M (1994). Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science 263:692–695. [DOI] [PubMed] [Google Scholar]
- Delaney KR, Hall JB (1996). An in vitro preparation of frog nose and brain for the study of odour-evoked oscillatory activity. J Neurosci Methods 68:193–202. [DOI] [PubMed] [Google Scholar]
- Delaney KR, Gelperin A, Fee MS, Flores JA, Gervais R, Tank DW, Kleinfeld D (1994). Waves and stimulus-modulated dynamics in an oscillating olfactory network. Proc Natl Acad Sci USA 91:669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzen M, Evers JF, Galizia CG (2003). Odor similarity does not influence the time needed for odor processing. Chem Senses 28:781–789. [DOI] [PubMed] [Google Scholar]
- Dorries KM, Kauer JS (2000). Relationship between odor-elicited oscillations in the salamander olfactory epithelium and olfactory bulb. J Neurophysiol 83:754–765. [DOI] [PubMed] [Google Scholar]
- Egana JI, Aylwin ML, Maldonado PE (2005). Odor response properties of neighboring mitral/tufted cells in the rat olfactory bulb. Neuroscience 134:1069–1080. [DOI] [PubMed] [Google Scholar]
- Ermentrout GB, Kleinfeld D (2001). Traveling electrical waves in cortex: insights from phase dynamics and speculation on a computational role. Neuron 29:33–44. [DOI] [PubMed] [Google Scholar]
- Ermentrout B, Flores J, Gelperin A (1998). Minimal model of oscillations and waves in the Limax olfactory lobe with tests of the model’s predictive power. J Neurophysiol 79:2677–2689. [DOI] [PubMed] [Google Scholar]
- Ermentrout B, Wang JW, Flores J, Gelperin A (2001). Model for olfactory discrimination and learning in Limax procerebrum incorporating oscillatory dynamics and wave propagation. J Neurophysiol 85:1444–1452. [DOI] [PubMed] [Google Scholar]
- Ermentrout GB, Wang JW, Flores J, Gelperin A (2004). Model for transition from waves to synchrony in the olfactory lobe of Limax. J Comput Neurosci 17:365–383. [DOI] [PubMed] [Google Scholar]
- Fadool DA, Tucker K, Perkins R, Fasciani G, Thompson RN, Parsons AD, Overton JM, Koni PA, Flavell RA, Kaczmarek LK (2004). Kv1.3 channel gene-targeted deletion produces “Super-Smeller Mice” with altered glomeruli, interacting scaffolding proteins, and biophysics. Neuron 41:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farbman AI (1994). Developmental biology of olfactory sensory neurons. Semin Cell Biol 5:3–10. [DOI] [PubMed] [Google Scholar]
- Fletcher ML, Smith AM, Best AR, Wilson DA (2005). High-frequency oscillations are not necessary for simple olfactory discriminations in young rats. J Neurosci 25:792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WJ, Schneider WS (1982). Changes in spatial patterns of rabbit olfactory EEG with conditioning to odors. Psychophysiology 19:44–56. [DOI] [PubMed] [Google Scholar]
- Friedman D, Strowbridge BW (2003). Both electrical and chemical synapses mediate fast network oscillations in the olfactory bulb. J Neurophysiol 89:2601–2610. [DOI] [PubMed] [Google Scholar]
- Friedrich RW (2006). Mechanisms of odor discrimination: neurophysiological and behavioral approaches. Trends Neurosci 29:40–47. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Korsching SI (1997). Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron 18:737–752. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Habermann CJ, Laurent G (2004). Multiplexing using synchrony in the zebrafish olfactory bulb. Nat Neurosci 7:862–871. [DOI] [PubMed] [Google Scholar]
- Fujie S, Yamamoto T, Murakami J, Hatakeyama D, Shiga H, Suzuki N, Ito E (2005). Nitric oxide synthase and soluble guanylyl cyclase underlying the modulation of electrical oscillations in a central olfactory organ. J Neurobiol 62:14–30. [DOI] [PubMed] [Google Scholar]
- Galan RF, Ermentrout GB, Urban NN (2005). Efficient estimation of phase-resetting curves in real neurons and its significance for neural-network modeling. Phys Rev Lett 94:158101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizia CG, Menzel R (2000). Odour perception in honeybees: coding information in glomerular patterns. Curr Opin Neurobiol 10:504–510. [DOI] [PubMed] [Google Scholar]
- Galizia CG, McIlwrath SL, Menzel R (1999). A digital three-dimensional atlas of the honeybee antennal lobe based on optical sections acquired by confocal microscopy. Cell Tissue Res 295:383–394. [DOI] [PubMed] [Google Scholar]
- Gelperin A (1994). Nitric oxide mediates network oscillations of olfactory interneurons in a terrestrial mollusc. Nature 369:61–63. [DOI] [PubMed] [Google Scholar]
- Gelperin A (1999). Oscillatory dynamics and information processing in olfactory systems. J Exp Biol 202:1855–1864. [DOI] [PubMed] [Google Scholar]
- Gelperin A, Flores J (1997). Vital staining from dye-coated microprobes identifies new olfactory interneurons for optical and electrical recording. J Neurosci Methods 72:97–108. [DOI] [PubMed] [Google Scholar]
- Gelperin A, Tank DW (1990). Odor-modulated collective network oscillations of olfactory interneurons in a terrestrial mollusc. Nature 345:437–440. [DOI] [PubMed] [Google Scholar]
- Gelperin A, Flores J, Raccuia-Behling F, Cooke IRC (2000). Nitric oxide and carbon monoxide modulate oscillations of olfactory interneurons in a terrestrial mollusc. J Neurophysiol 83:116–127. [DOI] [PubMed] [Google Scholar]
- Greenspan RJ, van Swinderen B (2004). Cognitive consonance: complex brain functions in the fruit fly and its relatives. Trends Neurosci 27:707–711. [DOI] [PubMed] [Google Scholar]
- Harris K, Henze DA, Hirase H, Csicsvari J, Buzsaki G (2000). The accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J Neurophysiol 84:401–414. [DOI] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Shipley MT, Ennis M (2004a). Olfactory bulb glomeruli: external tufted cells intrinsically burst at theta frequency and are entrained by patterned olfactory input. J Neurosci 24:1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Ennis M, Shipley MT (2004b). External tufted cells: a major excitatory element that coordinates glomerular activity. J Neurosci 24:6676–6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JG, Shepherd GM (1997). Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci 20:595–631. [DOI] [PubMed] [Google Scholar]
- Hopfield JJ, Brody CD (2004). Learning rules and network repair in spike-timing-based computation networks. Proc Natl Acad Sci USA 101:337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosler JS, Smith BH (2000). Blocking and the detection of odor components in blends. J Exp Biol 203:2797–2806. [DOI] [PubMed] [Google Scholar]
- Hubbard JI, Llinas R, Quastel DMJ (1969). In: Electrophysiological analysis of synaptic transmission London: Arnold.
- Inoue T, Inokuma Y, Watanabe S, Kirino Y (2004). In vitro study of odor-evoked behavior in a terrestrial mollusk. J Neurophysiol 91:372–381. [DOI] [PubMed] [Google Scholar]
- Ito I, Nakamura H, Kimura T, Suzuki H, Sekiguchi T, Kawabata K, Ito E (2000). Neuronal components of the superior and inferior tentacles in the terrestrial slug, Limax marginatus. Neurosci Res 37:191–200. [DOI] [PubMed] [Google Scholar]
- Ito I, Kimura T, Watanabe S, Kirino Y, Ito E (2004). Modulation of two oscillatory networks in the peripheral olfactory system by gamma-aminobutyric acid, glutamate, and acetylcholine in the terrestrial slug Limax marginatus. J Neurobiol 59:304–318. [DOI] [PubMed] [Google Scholar]
- Jirsa R, Radil T (1997). Gamma activity in the piriform cortex and behavioral thresholds for electrical stimulation in the olfactory bulb. Acta Neurobiol Exp (Wars) 57:11–20. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Saber S, Leon M (2005). Effects of functional group position on spatial representations of aliphatic odorants in the rat olfactory bulb. J Comp Neurol 483:192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara S, Toda S, Suzuki Y, Watanabe S, Kirino Y (1997). Comparative study on neural oscillation in the procerebrum of the terrestrial slugs Incilaria bilineata and Limax marginatus. J Exp Biol 200:1851–1861. [DOI] [PubMed] [Google Scholar]
- Kay LM (2003). Two species of gamma oscillations in the olfactory bulb: dependence on behavioral state and synaptic interactions. J Integr Neurosci 2:31–44. [DOI] [PubMed] [Google Scholar]
- Kay LM, Laurent G (1999). Odor- and context-dependent modulation of mitral cell activity in behaving rats. Nat Neurosci 2:1003–1009. [DOI] [PubMed] [Google Scholar]
- Kazemi-Esfarjani P, Benzer S (2002). Suppression of polyglutamine toxicity by a Drosophila homolog of myeloid leukemia factor 1. Hum Mol Genet 11:2657–2672. [DOI] [PubMed] [Google Scholar]
- Kemenes G, Staras K, Benjamin PR (1997). In vitro appetitive classical conditioning of the feeding response in the pond snail Lymnaea stagnalis. J Neurophysiol 78:2351–2362. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Guevara-Guzman R, Zorrilla J, Hinton MR, Broad KD, Mimmack M, Ohkura S (1997). Formation of olfactory memories mediated by nitric oxide. Nature 388:670–674. [DOI] [PubMed] [Google Scholar]
- Khan RM, Sobel N (2004). Neural processing at the speed of smell. Neuron 44:744–747. [DOI] [PubMed] [Google Scholar]
- Kim U, Bal T, McCormick DA (1995). Spindle waves are propagating synchronized oscillations in the ferret LGNd in vitro. J Neurophysiol 74:1301–1323. [DOI] [PubMed] [Google Scholar]
- Kimura T, Suzuki H, Kono E, Sekiguchi T (1998). Mapping of interneurons that contribute to food aversion conditioning in the slug brain. Learn Mem 4:376–388. [DOI] [PubMed] [Google Scholar]
- Kirino Y, Inoue T, Watanabe S (2005). Behavioral determination of odor preference is coded by the oscillation frequency in a collective oscillating network of a terrestrial mollusk. Chem Senses 30:Suppl 1, i154–i155. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Delaney KR, Fee MS, Flores JA, Tank DW, Gelperin A (1994). Dynamics of propagating waves in the olfactory network of a terrestrial mollusc: an electrical and optical study. J Neurophysiol 72:1402–1419. [DOI] [PubMed] [Google Scholar]
- Komischke B, Sandoz JC, Malun D, Giurfa M (2005). Partial unilateral lesions of the mushroom bodies affect olfactory learning in honeybees Apis mellifera. Eur J Neurosci 21:477–485. [DOI] [PubMed] [Google Scholar]
- Korneev SA, Straub V, Kemenes I, Korneeva EI, Ott SR, Benjamin PR, O’Shea M (2005). Timed and targeted differential regulation of nitric oxide synthase (NOS) and anti-NOS genes by reward conditioning leading to long-term memory formation. J Neurosci 25:1188–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs HA (1975). The August Krogh Principle: “For many problems there is an animal on which it can be most conveniently studied.”. J Exp Zool 194:221–226. [DOI] [PubMed] [Google Scholar]
- Lagier S, Carleton A, Lledo P-M (2004). Interplay between local GABAergic interneurons and relay neurons generates γ oscillations in the rat olfactory bulb. J Neurosci 24:4382–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam YW, Cohen LB, Zochowski MR (2003). Odorant specificity of three oscillations and the DC signal in the turtle olfactory bulb. Eur J Neurosci 17:436–446. [DOI] [PubMed] [Google Scholar]
- Laurent G (2002). Olfactory network dynamics and the coding of multidimensional signals. Nat Rev Neurosci 3:884–895. [DOI] [PubMed] [Google Scholar]
- Laurent G, Stopfer M, Friedrich RW, Rabinovich MI, Volkovskii A, Abarbanel HD (2001). Odor encoding as an active, dynamical process: experiments, computation, and theory. Annu Rev Neurosci 24:263–297. [DOI] [PubMed] [Google Scholar]
- Lei H, Christensen TA, Hildebrand JG (2004). Spatial and temporal organization of ensemble representations for different odor classes in the moth antennal lobe. J Neurosci 24:11108–11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Cleland TA (2001). How spike synchronization among olfactory neurons can contribute to sensory discrimination. J Comput Neurosci 10:187–193. [DOI] [PubMed] [Google Scholar]
- Luo M, Katz LC (2001). Response correlation maps of neurons in the mammalian olfactory bulb. Neuron 32:1165–1179. [DOI] [PubMed] [Google Scholar]
- Mair RG, Gellman RL, Gesteland RC (1982). Postnatal proliferation and maturation of olfactory bulb neurons in the rat. Neuroscience 7:3105–3116. [DOI] [PubMed] [Google Scholar]
- Mann EO, Paulsen O (2005). Mechanisms underlying gamma (“40 Hz”) network oscillations in the hippocampus—a mini-review. Prog Biophys Mol Biol 87:67–76. [DOI] [PubMed] [Google Scholar]
- Margrie TW, Schaefer AT (2003). Theta oscillation coupled spike latencies yield computational vigor in a mammalian sensory system. J Physiol (Lond) 546:363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margrie TW, Brecht M, Sakmann B (2002). In vivo, low-resistance, whole-cell recordings from neurons in the anesthetized and awake mammalian brain. Pflügers Arch 444:491–498. [DOI] [PubMed] [Google Scholar]
- Martin C, Gervais R, Hugues E, Messaoudi B, Ravel N (2004). Learning modulation of odor-induced oscillatory responses in the rat olfactory bulb: a correlate of odor recognition. J Neurosci 24:389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore M, Hines ML, Shepherd GM (2005). The role of distal dendritic gap junctions in synchronization of mitral cell axonal output. J Comput Neurosci 18:151–161. [DOI] [PubMed] [Google Scholar]
- Mokin M, Keifer J (2005). Expression of the immediate-early gene-encoded protein Egr-1 (zif268) during in vitro classical conditioning. Learn Mem 12:144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Nagao H, Yoshihara Y (1999). The olfactory bulb: coding and processing of odor molecule information. Science 286:711–715. [DOI] [PubMed] [Google Scholar]
- Mouly AM, Gervais R (2002). Polysynaptic potentiation at different levels of rat olfactory pathways following learning. Learn Mem 9:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlethaler M, de Curtis M, Walton K, Llinas R (1993). The isolated and perfused brain of the guinea-pig in vitro. Eur J Neurosci 5:915–926. [DOI] [PubMed] [Google Scholar]
- Müller U (1996). Inhibition of nitric oxide synthase impairs a distinct form of long-term memory in the honeybee, Apis mellifera. Neuron 16:541–549. [DOI] [PubMed] [Google Scholar]
- Murakami M, Watanabe S, Inoue T, Kirino Y (2004). Odor-evoked responses in the olfactory center neurons in the terrestrial slug. J Neurobiol 58:369–378. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Darcy DP, Isaacson JS (2005). Intraglomerular inhibition: signaling mechanisms of an olfactory microcircuit. Nat Neurosci 8:354–364. [DOI] [PubMed] [Google Scholar]
- Neville KR, Haberly LB (2003). Beta and gamma oscillations in the olfactory system of the urethane-anesthetized rat. J Neurophysiol 90:3921–3930. [DOI] [PubMed] [Google Scholar]
- Nikitin ES, Balaban PM (2000). Optical recording of odor-evoked responses in the olfactory brain of the naive and aversively trained terrestrial snails. Learn Mem 7:422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov AA, Parker JM, Caprio J (2002). Odorant-induced olfactory receptor neural oscillations and their modulation of olfactory bulbar responses in the channel catfish. J Neurosci 22:2352–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Kay LM, Laurent G, Homanics GE, Mody L (2001). Disruption of GABA(A) receptors on GABAergic interneurons leads to increased oscillatory power in the olfactory bulb network. J Neurophysiol 86:2823–2833. [DOI] [PubMed] [Google Scholar]
- Okere CO, Kaba H, Higuchi T (1996). Formation of an olfactory recognition memory in mice: reassessment of the role of nitric oxide. Neuroscience 71:349–354. [DOI] [PubMed] [Google Scholar]
- Prechtl JC, Bullock TH, Kleinfeld D (2000). Direct evidence for local oscillatory current sources and intracortical phase gradients in turtle visual cortex. Proc Natl Acad Sci USA 97:877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratté S, Chase R (2000). Synapse distribution of olfactory interneurons in the procerebrum of the snail Helix aspersa. J Comp Neurol 417:366–384. [PubMed] [Google Scholar]
- Ravel N, Elaagouby A, Gervais R (1994). Scopolamine injection into the olfactory bulb impairs short-term olfactory memory in rats. Behav Neurosci 108:317–324. [DOI] [PubMed] [Google Scholar]
- Ravel N, Chabaud P, Martin C, Gaveau V, Hugues E, Tallon-Baudry C, Bertrand O, Gervais R (2003). Olfactory learning modifies the expression of odour-induced oscillatory responses in the gamma (60–90 Hz) and beta (15–40 Hz) bands in the rat olfactory bulb. Eur J Neurosci 17:350–358. [DOI] [PubMed] [Google Scholar]
- Robbins KA, Senseman DM (2004). Extracting wave structure from biological data with application to responses in turtle visual cortex. J Comput Neurosci 16:267–298. [DOI] [PubMed] [Google Scholar]
- Roman FS, Truchet B, Chaillan FA, Marcheti E, Soumireu-Mourant B (2004). Olfactory associative discrimination: a model for studying modifications of synaptic efficacy in neuronal networks supporting long-term memory. Rev Neurosci 15:1–17. [DOI] [PubMed] [Google Scholar]
- Saghatelyan A, Roux P, Migliore M, Rochefort C, Desmaisons D, Charneau P, Shepherd GM, Lledo PM (2005). Activity-dependent adjustments of the inhibitory network in the olfactory bulb following early postnatal deprivation. Neuron 46:103–116. [DOI] [PubMed] [Google Scholar]
- Sahley C, Gelperin A, Rudy JW (1981). One-trial associative learning modifies food odor preferences of a terrestrial mollusc. Proc Natl Acad Sci USA 78:640–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakura M, Kabetani M, Watanabe S, Kirino Y (2004). Impairment of olfactory discrimination by blockade of nitric oxide activity in the terrestrial slug Limax valentianus. Neurosci Lett 370:257–261. [DOI] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ (2001). Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci 2:539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samama B, Boehm N (1999). Inhibition of nitric oxide synthase impairs early olfactory associative learning in newborn rats. Neurobiol Learn Mem 71:219–231. [DOI] [PubMed] [Google Scholar]
- Sandoz JC, Galizia CG, Menzel R (2003). Side-specific olfactory conditioning leads to more specific odor representation between sides but not within sides in the honeybee antennal lobes. Neuroscience 120:1137–1148. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Gross J (2005). Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci 6:285–296. [DOI] [PubMed] [Google Scholar]
- Shimozono S, Watanabe S, Inoue T, Kirino Y (2001). Identification and characterization of an output neuron from the oscillatory molluscan olfactory network. Brain Res 921:98–105. [DOI] [PubMed] [Google Scholar]
- Sivan E, Kopell N (2004). Mechanism and circuitry for clustering and fine discrimination of odors in insects. Proc Natl Acad Sci USA 101:17861–17866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick B, Cockerham R, Pickett E (2004). Olfaction in olfactory bulbectomized rats. J Neurosci 24:9195–9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopfer M, Laurent G (1999). Short-term memory in olfactory network dynamics. Nature 402:664–668. [DOI] [PubMed] [Google Scholar]
- Stopfer M, Bhagavan S, Smith BH, Laurent G (1997). Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature 390:70–74. [DOI] [PubMed] [Google Scholar]
- Susswein AJ, Katzoff A, Miller N, Hurwitz I (2004). Nitric oxide and memory. The Neuroscientist 10:153–162. [DOI] [PubMed] [Google Scholar]
- Szyszka P, Ditzen M, Galkin A, Galizia CG, Menzel R (2005). Sparsening and temporal sharpening of olfactory representations in the honeybee mushroom bodies. J Neurophysiol 94:3303–3313. [DOI] [PubMed] [Google Scholar]
- Tank DW, Gelperin A, Kleinfeld D (1994). Odors, oscillations, and waves: does it all compute. Science 265:1819–1820. [DOI] [PubMed] [Google Scholar]
- Teyke T (1996). Nitric oxide, but not serotonin, is involved in acquisition of food-attraction conditioning in the snail Helix pomatia. Neurosci Lett 206:29–32. [DOI] [PubMed] [Google Scholar]
- Teyke T, Gelperin A (1999). Olfactory oscillations augment odor discrimination not odor identification by Limax CNS. NeuroReport 10:1061–1068. [DOI] [PubMed] [Google Scholar]
- Teyke T, Wang JW, Gelperin A (2000). Lateralized memory storage and crossed inhibition during odor processing by Limax. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 186:269–278. [DOI] [PubMed] [Google Scholar]
- Toda S, Kawahara S, Kirino Y (2000). Image analysis of olfactory responses in the procerebrum of the terrestrial slug Limax marginatus. J Exp Biol 203:2895–2905. [DOI] [PubMed] [Google Scholar]
- Uchida N, Mainen ZF (2003). Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci 6:1224–1229. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Wong AM, Axel R (2000). An olfactory sensory map in the fly brain. Cell 102:147–159. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Denk W, Friedrich RW (2004). Functional organization of sensory input to the olfactory bulb glomerulus analyzed by two-photon calcium imaging. Proc Natl Acad Sci USA 101:9097–9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, Flores J, Gelperin A, Denk W (2001). Initiation and propagation of calcium-dependent action potentials in a coupled network of olfactory interneurons. J Neurophysiol 85:977–985. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R (2003). Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell 112:271–282. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Kawahara S, Kirino Y (1998). Morphological characterization of the bursting and nonbursting neurones in the olfactory centre of the terrestrial slug Limax marginatus. J Exp Biol 201:925–930. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Inoue T, Kirino Y (2003). Contribution of excitatory chloride conductance in the determination of the direction of traveling waves in an olfactory center. J Neurosci 23:2932–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Shimozono S, Kirino Y (2004). Optical recording of oscillatory neural activities in the molluscan brain. Neurosci Lett 359:147–150. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Best AR, Sullivan RM (2004). Plasticity in the olfactory system: lessons for the neurobiology of memory. The Neuroscientist 10:513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Ponomarev A, Davis RL (2004). Altered representation of the spatial code for odors after olfactory classical conditioning; memory trace formation by synaptic recruitment. Neuron 42:437–449. [DOI] [PubMed] [Google Scholar]
- Zakharov IS, Hayes NL, Ierusalimsky VN, Nowakowski RS, Balaban PM (1998). Postembryonic neuronogenesis in the procerebrum of the terrestrial snail, Helix lucorum. J Neurobiol 35:271–276. [DOI] [PubMed] [Google Scholar]
- Zhang C, Restrepo D (2003). Heterogeneous expression of connexin 36 in the olfactory epithelium and glomerular layer of the olfactory bulb. J Comp Neurol 459:426–439. [DOI] [PubMed] [Google Scholar]
- Zochowski MR, Cohen LB (2005). Oscillations in the olfactory bulb carry information about odorant history. J Neurophysiol 94:2667–2675. [DOI] [PubMed] [Google Scholar]
- Zou DJ, Feinstein PG, Rivers AL, Mathews GA, Kim A, Greer CA, Mombaerts P, Firestein S (2004). Postnatal refinement of peripheral olfactory projections. Science 304:1976–1979. [DOI] [PubMed] [Google Scholar]