Abstract
In the mammalian CNS, oligodendrocyte precursor cells (OPCs) express most neurotransmitter receptors, but their function remains unclear. The current studies suggest a physiological role for glutamate (AMPA and/or kainate) receptors in OPC migration. AMPA stimulated αv integrin-mediated OPC migration by increasing both the rate of cell movement and the frequency of Ca2+ transients. A protein complex containing the myelin proteolipid protein (PLP) and αv integrin modulated the AMPA-stimulated migration, and stimulation of OPC AMPA receptors resulted in increased association of the AMPA receptor subunits themselves with the αv integrin/PLP complex. Thus, after AMPA receptor stimulation, an αv integrin/PLP/neurotransmitter receptor protein complex forms that reduces binding to the extracellular matrix and enhances OPC migration. To assess the extent to which PLP was involved in the AMPA-stimulated migration, OPCs from the myelin-deficient (MD) rat, which has a PLP gene mutation, were analyzed. OPCs from the MD rat had a normal basal migration rate, but AMPA did not stimulate the migration of these cells, suggesting that the PLP/αv integrin complex was important for the AMPA-mediated induction. AMPA-induced modulation of OPC migration was abolished by pertussis toxin, although baseline migration was normal. Thus, G-protein-dependent signaling is crucial for AMPA-stimulated migration of OPCs but not for basal OPC migration. Other signaling pathways involved in this AMPA-stimulated OPC migration were also determined. These studies highlight novel signaling determinants of OPC migration and suggest that glutamate could play a pivotal role in regulating integrin-mediated OPC migration.
Keywords: proteolipid protein, AMPA receptor, neurotransmitter receptor, Ca2+ transient, Gi-protein, GluR2, GluR4
Introduction
Oligodendrocyte precursor cells (OPCs) arise in restricted germinal areas of the CNS, and they migrate over considerable distances to the developing white matter, where they differentiate and elaborate myelin sheaths around axons. Because neurotransmitters are an integral part of the chemical environment surrounding OPCs, they have been implicated in mediating some neuron–oligodendrocyte interactions (Barres and Raff, 1993). The fact that neurotransmitter receptors are expressed before the establishment of synaptic networks in early brain development suggests novel biological functions for these receptors, in addition to synaptic transmission (Mattson, 1988; Nguyen et al., 2001; Belachew and Gallo, 2004). OPCs express most neurotransmitter receptors, such as GABA, muscarinic, dopamine, glycine, and AMPA/kainate glutamate receptors. OPCs express several AMPA-specific glutamate receptor subunits: GluR1, GluR2, GluR3, and GluR4 (Yoshioka et al., 1995; Matute et al., 1997). Agonist activation of AMPA receptors on OPCs causes Ca2+ influx via Ca2+-permeable channels, which consist of GluR3 and GluR4 (Itoh et al., 2002). Approximately 30% of OPC AMPA receptors contain the GluR2 subunit, which is edited at the Q/R site, rendering the channel impermeable to Ca2+ (Itoh et al., 2002).
Neurotransmitter receptor expression decreases during oligodendrocyte differentiation, suggesting a specific role in OPCs. In neurons, the coordinated activity of Ca2+ channels and NMDA receptors seems to modulate the amplitude and frequency of intracellular Ca2+ fluctuations, which may provide a major intracellular signal controlling the rate of neuronal cell migration (Komuro and Rakic, 1998). We hypothesized that such molecular events may regulate OPC migration as well.
Recently, we demonstrated that muscarinic acetylcholine receptor agonists induced activation of αv integrin receptors on oligodendrocytes and increased association of αv integrins with the myelin proteolipid protein (PLP) and calreticulin (Gudz et al., 2002). This finding supports the concept that neurotransmitters could regulate integrin-mediated functions in oligodendrocytes, such as proliferation and survival (Barres and Raff, 1999). The most conclusive data on the role of integrin receptors in OPC metabolism come from the group of ffrench-Constant (Milner and ffrench-Constant, 1994), who first established the developmental regulation of several integrin receptors on oligodendrocytes: α6β1, αvβ1, αvβ3, αvβ5, and αvβ8. In particular, this group has demonstrated the importance of α6β1 integrin receptor and its interactions with platelet-derived growth factor receptor α (PDGFαR) for OPC proliferation (Blaschuk et al., 2000; Colognato et al., 2002; Baron et al., 2003).
We used purified OPCs in culture to test the hypothesis that neurotransmitters could regulate integrin-mediated OPC migration. We found that αv integrin-mediated OPC migration was significantly increased in the presence of AMPA. Cell motility was dependent on coordinated activity of AMPA receptor-initiated signaling and intracellular Ca2+ fluctuations. AMPA-induced stimulation of OPC migration was completely abolished by pertussis toxin, indicating the involvement of Gi-proteins. Most intriguingly, activation of AMPA receptors seems to induce the association of GluR2 and GluR4 subunits of glutamate receptors with the αv integrin/PLP complex. These data are the first demonstration of neurotransmitter regulation of OPC migration in vitro and are further support for the existence of a neuron–oligodendrocyte communication network.
Materials and Methods
Materials.
The antibodies used in these studies were directed against the myelin PLP C terminus (AA3 clone; a gift from Dr. S. Pfeiffer, University of Connecticut, Farmington, CT); the myelin PLP extracellular domain (clone 9021, Agmed; Immunodiagnostics, Woburn, MA); β1 integrin (clone P4C10; Chemicon, Temecula, CA); αvβ5 integrin (clone P1F6; Chemicon); αvβ3 integrin (clone LM609; Chemicon); αv integrin (clone P3G8; Chemicon); GluR2 (Chemicon); GluR4 (Chemicon); or PDGFαR antibody (Upstate Biotechnology, Lake Placid, NY). U73343 (1-[6-([17β-3-methoxyestra-1,3-5(10)-trien-17-yl]amino)hexyl]-2,5-pyrroli-dine-dione), U73122 (1-[6-([17β-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl-1H-pyrrole-2,5-dione), bisindolylmaleimide (Bis), pertussis toxin, carbachol, thapsigargin, BAPTA, ruthenium red, and carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP) were obtained from Sigma (St. Louis, MO); glutamate, kainate, NMDA, AMPA, cyclothiazide (CZ), 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide disodium (NBQX), and GYKI52466 [1-(aminophenyl)-4-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine] were obtained from Tocris (Ellisville, MO); H7 [1-(5-isoquinolinesulfonyl)-2-methylpiperazine] and H89 (N-[2-bromocinnamyl(amino)ethyl]-5-isoquinolinesulphonamide) were from Calbiochem (San Diego, CA); KT5720 was from Alomone Labs (Jerusalem, Israel); fibronectin and laminin were from Chemicon; and the Alexa-488 protein labeling kit was from Pierce (Rockford, IL).
OPC cultures.
Mixed glial cultures were generated from 2-d-old wild-type or myelin-deficient (MD) rat pups as described previously (Gudz et al., 2002). Briefly, the cerebra of rat pups were dissected, minced, and digested at 37°C with trypsin/pancreatin (0.06% w/v each) to generate a single-cell suspension. Cells were plated into 75 cm2 flasks and grown in DMEM with 10% fetal bovine serum (FBS) at 37°C and 5% CO2 for 10 d. OPCs were purified from mixed glial cells by a shake-off procedure (McCarthy and de Vellis, 1973). Cells were shaken initially for 1 h at 100 rpm to remove microglia and refed and shaken for 20–22 h at 37°C at 200 rpm. OPCs were collected by centrifugation at 1200 rpm for 5 min, resuspended in DMEM supplemented with N2 medium (Sigma), and centrifuged again to remove FBS. Cells were then plated for specific experiments. To identify pups as MD pups, spinal cord protein from individual pups was analyzed by Western blots to demonstrate the lack of PLP expression in MD cells. All animal protocols were approved by the Cleveland Clinic Foundation Institutional Animal Use and Care Committee.
Migration assay.
To quantify OPC migration, we used a Costar transwell-based assay (Cole Parmer, Vernon Hills, IL). It has advantages over the agarose drop assay, including the ability to monitor cell migration within hours, not days. Thus, there is no impact of proliferation in this assay. Both sides of the transwell membrane (8 μm) were precoated with 10 μg/ml extracellular matrix (ECM) protein overnight. Nonspecific binding sites were blocked with 2% BSA for 1 h. Cells were plated at 80,000 cells/transwell in DMEM/N2 medium and allowed to migrate for 4 h unless stated otherwise. Cells were labeled with calcein-AM (Molecular Probes, Carlsbad, CA) 1 h before the end of the assay. Nonmigrated cells were removed with the cotton swab from the top compartment of the transwell. Calcein fluorescence of cells on the bottom compartment of the transwell was measured in a plate reader at 530 nm (with 480 nm excitation).
Time-lapse measurement of cell movement and intracellular Ca2+ levels.
To monitor the changes in intracellular Ca2+ levels in OPCs, the cells were incubated for 30 min with a cell-permeant, acetoxymethyl ester form of 4 μm Oregon Green 488 BAPTA-1 (Molecular Probes) diluted in the culture medium, which consisted of DMEM supplemented with 1.8 mm glutamine and 24 mm NaHCO3. The cells were subsequently washed three times with the culture medium, and the dye was allowed to de-esterify for an additional 30–60 min in the CO2 incubator. The dishes were transferred into the chamber of a micro-incubator (Medical System, Greenvale, NY) attached to the stage of a confocal microscope (TCS SP; Leica Microsystems, Mannheim Germany). The chamber temperature was kept at 37°C, and the cells were provided with a constant gas flow (95% O2, 5% CO2). OPCs loaded with Oregon Green 488 BAPTA-1 were illuminated with a 488 nm light from an argon laser, and the fluorescence images for Ca2+ measurements (at 530 ± 15 nm) and transmitted images for monitoring cell movement were collected simultaneously every 5 s for up to 2 h.
Immunocytochemistry.
Postnatal day 9 (P9) rat pups were perfused with 4% paraformalaldehyde in PBS, pH 7.6; brains were removed and postfixed in the same fixative overnight at 4°C. Free-floating sections (coronal, at bregma) were incubated for 1.5 h in 10% normal goat serum (NGS; Invitrogen, Grand Island, NY) containing 1% Triton X-100 (v/v). Sections were rinsed and incubated with the avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA). They were incubated overnight at 4°C with PLP antibody (1:1000, AA3 clone) and PDGFαR antibody (1:1000) in PBS, 3% NGS, 1% Triton X-100 (v/v). Sections were washed in PBS and incubated 60 min with biotinylated anti-rat secondary antibody (Vector Laboratories) in 3% NGS in PBS. Sections were then washed with PBS and incubated with Texas Red Avidin D (1:1000; Vector Laboratories) and fluorescein-conjugated rabbit IgG antibody (1:1000; Vector Laboratories) in sodium bicarbonate buffer, pH 8.5, for 30 min. Sections were mounted in Vectashield (Vector Laboratories) and analyzed by a confocal laser scanning microscope (Aristoplan; Leica).
Immunoprecipitation and Western blots.
Cell lysates were made using buffer A: 0.15 m NaCl, 0.05 m Tris, 0.5 mm EDTA, 1% Triton X-100, 0.05% SDS, 5 mm NaF, and 1 mm Na3VO4, pH 7.5, supplemented with Complete Mini Protease Inhibitor Cocktail (Roche Applied Science, Indianapolis, IN). After 1 h on ice, samples were centrifuged at 15,000 × g for 10 min to remove insoluble material. Protein concentrations were determined by the bicinchoninic acid method (Sigma). For immunoprecipitation reactions, lysates (1 mg/ml) were precleared in buffer A supplemented with 2% BSA, pH 7.5, by incubation with appropriate species-specific IgG-conjugated magnetic beads (Dynabeads, Dynal Biotech; Invitrogen, Carlsbad CA) for 1 h. An antibody was then added. After incubation at 4°C overnight with gentle mixing, antibody–antigen complexes were captured with Dynabeads and washed. Immunoprecipitates were eluted by boiling in SDS-sample buffer. Lysates and immunoprecipitates were separated on 8.5–10% SDS-PAGE, blotted to the polyvinylidene difluoride membrane, blocked with 5% nonfat dry milk in TBS-T buffer (10 mm Tris, 150 mm NaCl, and 0.2% Tween 20, pH 8.0) overnight at 4°C, and subsequently probed with the appropriate antibody (αv or AA3). Immunoreactive bands were visualized using the enhanced chemiluminescence kit (ECL-Plus; Amersham Biosciences, Piscataway, NJ).
Fibronectin binding assay.
Fibronectin was conjugated to the Alexa Fluor 488 (Molecular Probes) and purified on the day of the experiment using the Molecular Probes kit according to the manufacturer’s instructions. OPCs were plated in 96-well plates and incubated in the presence of antibodies and/or inhibitors for 30 min at 37°C. Cells were then stimulated with AMPA and CZ for 10 min. (The normally rapid desensitization of AMPA receptors after agonist application is blocked by CZ.) Fibronectin-Alexa Fluor 488 conjugate (10 μg/ml) was added to the wells, and the incubation continued for 30 min. After three washes with PBS, fluorescence at 530 nm (480 nm excitation) was measured. Nonspecific binding of fibronectin to wells filled with medium was subtracted from all measurements.
Statistical analysis.
All assays were performed three or more times. Typically, there were four to six replicates of each treatment in each assay. Data were collected, and the mean value of the treatment groups and SEM were calculated. Data were analyzed for statistically significant differences between groups either by one-way ANOVA or by two-way ANOVAs with post hoc Bonferroni’s test, which adjusts for multiple simultaneous comparisons.
Results
OPC migration was quantified in transwell-based assays. In these studies, transwells were precoated with fibronectin or laminin, which are the ligands of integrin receptors expressed on OPCs, or with collagen, a ligand of integrins absent from OPCs (Plow et al., 2000). First, we examined whether integrin receptors mediate OPC migration on fibronectin or laminin using function-blocking antibodies against integrins. These studies suggested that OPC migration on fibronectin is significantly mediated by αvβ3 integrin, with much less contribution of β1-containing integrins (Fig. 1A). The greater role of αvβ3 integrin, relative to αvβ5 integrin, is consistent with the known abundance of αvβ3 integrin heterodimer at this stage of oligodendrocyte differentiation, relative to αvβ5 integrin (Milner and ffrench-Constant 1994). On the other hand, when migrating on laminin, OPCs did not use αvβ3 or αvβ5 integrins but rather used β1-containing integrins, presumably α6β1. PLP contributed to OPC migration on fibronectin, because an antibody against an extracellular domain of PLP (PLP1) reduced cell migration. An antibody against a PLP intracellular domain (PLP2) was appropriately ineffective.
Figure 1.

OPC migration on fibronectin is mediated by αvβ3 integrin, and PLP and can be modulated by AMPA receptors. A, Transwells were coated with 10 μg/ml fibronectin or laminin. Nonspecific binding sites were blocked with 2% BSA. Wells were treated with antibodies for 30 min before plating the cells. Antibodies against either an extracellular domain (PLP1) or an intracellular domain (PLP2, AA3 antibody) of PLP and antibodies against αvβ3, αvβ5, or β1 integrins (all at 0.01 μg/ml) were used. Calcein-AM (10 μm) was added 1 h before the end of the assay. After 4 h, nonmigrated cells were removed from the top compartment of the transwell with a cotton swab, and the calcein fluorescence of migrated cells on the bottom of the transwell was measured. The data presented are means ± SEM (12 replicates from at least 3 independent experiments). *p < 0.05; **p < 0.01. CON, Control. B, OPC migration was assessed in a 4 h transwell migration assay in the presence of AMPA and/or in the presence of 20 μm GYKI52466, which is an AMPA receptor antagonist. Transwells were precoated with 10 μg/ml fibronectin, laminin, or collagen. Nonspecific binding sites were blocked with 2% BSA. Calcein-AM (10 μm) was added 1 h before the end of the assay. Nonmigrated cells were removed from the top compartment with a cotton swab, and the calcein fluorescence of migrated cells on the bottom of the transwell was measured. The data presented are means ± SEM (12 replicates from at least 3 independent experiments). *p < 0.05; **p < 0.01.
The next study demonstrated that OPC migration was modulated by glutamate receptors (Fig. 1B). In the presence of increasing concentrations of AMPA, starting as low as 50 nm, the number of OPCs migrating on fibronectin or laminin through the transwell was significantly increased. AMPA-induced acceleration of OPC migration was completely abolished by the AMPA receptor antagonist GYKI52466. In contrast, there was no neurotransmitter-induced stimulation of cell migration on collagen, indicating that AMPA-induced intracellular signaling affected only integrin-mediated cell motility. Together, these results suggest that AMPA-initiated intracellular signaling stimulates OPC movement by influencing the activity of an αvβ3 integrin receptor signaling complex that includes PLP.
In addition to AMPA-specific glutamate receptors, OPCs express other neurotransmitter receptors: kainate-specific glutamate receptors (Belachew and Gallo, 2004) and muscarinic acetylcholine receptors (Ragheb et al., 2001). To determine the specificity of the AMPA-induced stimulation of OPC migration, we measured OPC migration in the presence of agonists of these neurotransmitter receptors. This study showed that activation of both AMPA- and kainate-specific glutamate receptors resulted in stimulation of OPC migration on fibronectin (Fig. 2A). In contrast, NMDA had no effect on OPC migration, which is consistent with the inability of NMDA to induce downstream signaling in other studies on these cells (Lui et al., 1997). The stimulation of OPC migration on fibronectin was not limited to the activation of glutamate receptors, because carbachol, an agonist of muscarinic acetylcholine receptors, profoundly increased OPC migration. Consistent with the previous studies using AMPA agonists (Fig. 1B), none of the neurotransmitter receptor agonists modulated OPC migration on collagen.
Figure 2.
Activation of AMPA/kainate and muscarinic receptor results in stimulation of integrin-mediated OPC migration, and PLP is essential for this response. A, OPCs were allowed to migrate in the presence of 100 nm AMPA, 100 nm kainate, 100 nm glutamate, 100 nm carbachol, or 100 nm NMDA for 4 h. Transwells were precoated with 10 μg/ml fibronectin or collagen. Nonspecific binding sites were blocked with 2% BSA. Calcein-AM (10 μm) was added 1 h before the end of the assay. Nonmigrated cells were removed from the top compartment of the transwell with a cotton swab, and the calcein fluorescence of migrated cells on the bottom of the transwell was measured. The data presented are means ± SEM (12 replicates from at least 3 independent experiments). **p < 0.05. B, OPCs derived from wild-type (WT) rats or from the MD rat were allowed to migrate in the presence of 100 nm AMPA, 100 nm glutamate, 100 nm carbachol, or 100 nm NMDA for 4 h. Transwells were precoated with 10 μg/ml fibronectin. Nonspecific binding sites were blocked with 2% BSA. Calcein-AM (10 μm) was added 1 h before the end of the assay. Nonmigrated cells were removed from the top compartment of the transwell with a cotton swab, and the calcein fluorescence of the migrated cells on the bottom of the transwell was measured. The data presented are means ± SEM (12 replicates from at least 3 independent experiments). *p < 0.05.
PLP appeared to play an important role in neurotransmitter-induced stimulation of OPC motility. Because PLP is not typically thought of as a protein in OPCs, we studied whether it was coexpressed in OPCs in vivo. In young rats, some cortical OPCs expressed both PLP and PDGFαR (Fig. 3). We then analyzed the migration of OPCs derived from the MD rat, which has a PLP gene mutation that results in the absence of normal PLP protein expression in MD OPCs. These cells were chosen because there is little or no expression of PLP protein. It must be noted that the protein that is expressed is mutated, and when expressed at high levels, eventually causes death of oligodendrocytes (Jackson and Duncan, 1988). Nevertheless, in these progenitor cells, expression of the mutated PLP protein had no obvious deleterious effect, because the baseline cell motility of the MD OPCs was comparable to that of wild-type cells (Fig. 2B). Thus, low-level expression of mutant PLP in these cells had no negative impact on baseline migration or presumably the health of the progenitor cells. Importantly, activation of glutamate receptors caused increased cell motility in wild-type OPCs, but there was no stimulation of MD OPC motility (Fig. 2B). These data suggest that PLP is crucial for the neurotransmitter-induced stimulation of αv β3 integrin-mediated OPC migration, although its presence or absence has no impact on baseline OPC migration.
Figure 3.
PLP is coexpressed in OPCs in P9 rat cortex. Coronal sections of P9 brain were incubated with PLP antibody and PDGFαR antibody. Cells in the cortical region had the typical bipolar morphology of OPCs and coexpressed PDGFαR (fluorescein) (A) and PLP (Texas Red) (B), as can be seen in the merged image (C). Scale bars, 25 μm.
To examine the molecular mechanisms that control AMPA-induced stimulation of OPC motility, we first focused on Ca2+ signaling. Neuronal migration has been shown to be dependent on NMDA receptor-initiated intracellular Ca2+ fluctuations (Komuro and Rakic, 1998). Additionally, it has been shown that in OPCs, the activation of AMPA receptors results in increased cytosolic Ca2+ levels (Lui et al., 2002). We therefore tested the role of Ca2+ signaling in OPC migration by allowing cells to migrate in the presence of agents disrupting Ca2+ signaling (Fig. 4). BAPTA chelates intracellular Ca2+, resulting in overall decreased cytosolic Ca2+. Thapsigargin inhibits Ca2+ influx into endoplasmic reticulum, ruthenium red blocks mitochondrial Ca2+ transport, and FCCP prevents Ca2+ uptake into mitochondria by de-energization of mitochondria. Thus, these compounds produce increased cytosolic Ca2+ by several mechanisms. Either decreasing intracellular Ca2+ with BAPTA or increasing intracellular Ca2+ with thapsigargin, ruthenium red, or FCCP led to a significant decrease in cell migration (Fig. 4). These results support the concept that OPC migration, as with neuronal migration (Komuro and Rakic, 1998), requires coordinated intracellular Ca2+ signaling and that disruption of Ca2+ signaling in OPCs leads to reduced OPC movement.
Figure 4.
OPC migration is dependent on coordinated activity of intracellular Ca2+ stores. OPCs were allowed to migrate in the presence of the intracellular Ca chelator (20 μm BAPTA-AM), the extracellular Ca2+ chelator (5 mm EGTA), an inhibitor of Ca2+ uptake by endoplasmic reticulum [1 μm thapsigargin (TG)], or inhibitors of mitochondrial Ca2+ uptake [0.5 μm ruthenium red (RR) or 1 μm FCCP] for 4 h. Transwells were precoated with 10 μg/ml fibronectin. Nonspecific binding sites were blocked with 2% BSA. Calcein-AM (10 μm) was added 1 h before the end of the assay. Nonmigrated cells were removed from the top compartment of the transwell with a cotton swab, and the calcein fluorescence of migrated cells on the bottom of the transwell was measured. The data presented are means ± SEM (12 replicates from at least 3 independent experiments). **p < 0.05.
Recently, real-time Ca2+ measurements revealed that Ca2+ transient frequency per se is a major factor controlling the rate of cerebellar granule cell movement (Kumada and Komuro, 2004). Altered migration of cells in a transwell assay could result from selective migration of a subpopulation of cells or altered migration rate for all cells. To distinguish these possibilities and to determine the role of Ca2+ transients in the AMPA-induced stimulation of OPC migration, we used confocal microscopy combined with a Ca2+ indicator dye (Table 1). In untreated control samples, cells migrated at an average rate of 40.5 μm/h with an average frequency of 51.8 Ca2+ transients per hour. In the presence of AMPA, OPCs migrated at a significantly higher rate of 68.2 μm/h with an increased average frequency of 63.4 Ca2+ transients per hour. Thus, AMPA increased the rate of cell movement in the total OPC cell population, along with increasing the frequency of Ca2+ transients. These data suggest that the AMPA-induced acceleration of OPC movement may, at least in part, result from an increase in the frequency of Ca2+ transients.
Table 1.
Cell movement and Ca2+ transients were monitored by confocal microscopy
| Rate of cell movement (μm/h) | Number of Ca2+ transients (per hour) | |
|---|---|---|
| Wild type | ||
| Control | 40.5 ± 4.4 (n = 31) | 51.8 ± 3.3 (n = 31) |
| AMPA (2 μm) | 68.2 ± 5.1* (n = 31) | 63.4 ± 3.5* (n = 31) |
| Mutant type | ||
| Control | 42.5 ± 3.9 (n = 37) | 51.0 ± 2.8 (n = 37) |
| AMPA (2 μm) | 47.2 ± 4.5 (n = 37) | 52.5 ± 3.1 (n = 37) |
| Wild type | ||
| Control | 44.2 ± 3.4 (n = 31) | 48.9 ± 3.8 (n = 31) |
| αv-antagonist | 32.4 ± 2.8* (n = 31) | 51.0 ± 3.2 (n = 31) |
| (40 μg/ml) |
To measure intracellular Ca2+ levels, OPCs were preincubated with 4 μm Oregon Green 488 BAPTA-1. AMPA (2 μm) was added to the medium after 20 min control observations. In the third set of experiments, wild-type OPCs were incubated with 40 μg/ml αv-integrin-blocking antibody. The data presented are means ± SEM.
*p < 0.01.
Because our previous studies demonstrated that PLP was essential for neurotransmitter-induced enhancement of OPC migration, it was important to establish whether this inability to modulate migration in MD cells was upstream or downstream of these alterations in Ca2+ transients. Thus, we analyzed the rate of migration of OPCs isolated from the MD rat and their frequency of Ca2+ transients (Table 1). The mutant OPCs migrated at the rate 42.5 μm/h with an average frequency of 51 Ca2+ transients per hour at baseline, which is comparable to wild-type cells. However, the addition of AMPA had no effect on the rate of cell movement or on the frequency of Ca2+ transients. Thus, the absence of PLP prevents OPCs from responding to AMPA by preventing the increase in the rate of Ca2+ transients, which further shows the essential role of PLP for AMPA-induced acceleration of cell migration.
To assess whether the rate of cell movement was regulated by αv integrin function, cells were incubated in the presence of αv blocking antibody. This reduced the rate of cell movement but had no impact on the frequency of Ca2+ transients (Table 1). Thus, the Ca2+ transients in OPCs were modulated independently of the αv integrin.
We next investigated the involvement of G-proteins in AMPA-induced stimulation of OPC migration. It has been shown that AMPA receptor activation causes Gi-protein activation in cortical neurons by a mechanism independent of its role as a Ca2+ and Na+ channel. Thus, AMPA receptors can operate with both ionotropic and metabotropic activity (Wang et al., 1997). Pertussis toxin, a potent inhibitor of Gi-protein activity, abolished AMPA-induced OPC migration with no effect on the baseline OPC migration rate (Fig. 5). These results indicated that AMPA receptor stimulation triggers Gi-protein activation that leads to increased OPC motility. It has been shown that AMPA can act as a weak agonist to metabotropic glutamate receptors (mGluRs) (Brauner-Osborne et al., 1996). Furthermore, there are mGluRs on OPCs (Luit et al., 2003), and there is evidence that these receptors impact responses to AMPA in OPCs (Kellend and Toms, 2001). It was therefore important to test the possibility that the Gi-protein activation by AMPA arose not from direct AMPA receptor stimulation but through the AMPA-induced activation of mGluRs. However, the mGluR antagonist (S)-MCPG (α-methyl-4-carboxyphenylglycine; 1 mm) had no effect on the AMPA-induced stimulation of OPC migration. In addition, the mGluR agonist (S)-DHPG [(RS)-3,5-dihydroxyphenylglycine; 1–100 μm] also had no effect on OPC motility (data not shown). These data indicate that AMPA-induced stimulation of OPC migration involves the activation of a Gi-protein, which is not coupled to mGluRs on OPCs.
Figure 5.
OPC migration is Gi-protein dependent. OPCs were allowed to migrate in the presence of 100 nm AMPA with or without 50 μm pertussis toxin for various times. Transwells were precoated with 10 μg/ml fibronectin. Nonspecific binding sites were blocked with 2% BSA. Calcein-AM (10 μm) was added 1 h before the end of the assay. Nonmigrated cells were removed from the top compartment of the transwell with a cotton swab, and the calcein fluorescence of migrated cells on the bottom of the transwell was measured. The data presented are means ± SEM (12 replicates from at least 3 independent experiments). **p < 0.05.
Having demonstrated that neurotransmitter-induced stimulation of αv β3-mediated OPC migration also requires PLP, we investigated whether neurotransmitter induces the formation of the PLP/αv integrin complex that we found in carbachol-stimulated OPCs (Gudz et al., 2002). OPCs were allowed to attach to fibronectin-coated plates for 1 h and were stimulated with 100 μm AMPA in the presence of 50 μm CZ, a selective AMPA receptor desensitizer, for various times. There was a time-dependent increase in PLP and αv integrin association that was maximal after a 10 min treatment with an agonist, and it declined somewhat by 20 min (Fig. 6A). Comparable data were obtained when immunoprecipitations were done with PLP antibody (Fig. 6A) or with αv integrin antibody (as seen in Fig. 8). Preincubation of the cells with 10 μm NBQX, a potent AMPA receptor antagonist, reduced the AMPA-induced response at 10 min. Our previous study demonstrated that although OPCs express α6β1 integrin, PLP does not associate with that integrin heterodimer but rather with αv-containing integrins (Gudz et al., 2002). Because αvβ3 integrin receptor is the major αv integrin heterodimer at this stage of oligodendrocyte differentiation (Milner and ffrench-Constant, 1994) and is the active integrin complex that mediates AMPA-stimulated OPC migration (Fig. 1A), PLP seems to associate with αvβ3 integrin after OPC treatment with AMPA receptor agonist.
Figure 6.
AMPA receptor stimulation enhanced PLP association with αv integrin receptor and reduced fibronectin binding to OPCs. A, OPCs were stimulated with AMPA in the presence of CZ. Cells were immunoprecipitated (IP) with PLP antibody (1:50) and analyzed by Western blotting using polyclonal αv antibody (1:100) or an antibody against the intracellular domain of PLP (AA3; 1:100). One sample was incubated with AMPA and CZ for 10 min after a 30 min pretreatment with 10 μm NBQX. B, OPCs plated in 96-well plates were preincubated for 30 min with 10 μm NBQX. Cells were then treated with 2 mm Ca2+ and 100 μm AMPA plus various concentrations of CZ for 10 min. The incubation was continued for another 30 min in the presence of 10 μg of fibronectin-Alexa Fluor 488 conjugate. Cells were washed with PBS, and the fluorescence was measured. The data presented are means ± SEM (12 replicates from 3 independent experiments). Fibronectin (FN) binding significantly decreased in the presence of AMPA and CZ (**p < 0.01, #p < 0.05 compared with untreated control). NBQX treatment abolished the AMPA- and CZ-induced decrease in fibronectin binding compared with cells treated with AMPA plus CZ (*p < 0.05).
Figure 8.
AMPA receptor activation results in increased association of GluR2 and GluR4 with the αv integrin/PLP complex. OPCs were treated with AMPA or muscarinic receptor agonist for 10 min. Cell lysates were immunoprecipitated with αv integrin antibody (clone P3G8; 0.01 μg/ml) or an antibody against the intracellular domain of PLP (clone AA3; 0.01 μg/ml). Immunoprecipitated complexes were loaded into gel in lane 1 (control), lane 2 (cells treated with100 μm AMPA plus 60 μm CZ), and lane 3 (cells treated with the muscarinic acetylcholine receptor agonist, 100 μm carbachol). Western blots were analyzed with anti-PLP (clone AA3; 1:50), polyclonal anti-αv integrin (1:100), polyclonal anti-GluR2 (1:100), or polyclonal anti-GluR4 (1:100) antibody. IP, Immunoprecipitated.
To assess the impact of neurotransmitter on the interaction of the PLP/αvβ3 integrin complex with the ECM, we examined fibronectin binding to OPCs (Fig. 6B). Fibronectin is produced by astrocytes, and it is a major ligand for αv integrins in the CNS (Liesi et al., 1986). AMPA induced a concentration-dependent decrease in Alexa-488–fibronectin binding to OPCs that could be abolished by the AMPA receptor antagonist NBQX. Our data demonstrate that, as with carbachol stimulation, AMPA receptor activation increased the association of PLP with αv integrin, along with reduced integrin receptor binding to ECM ligand.
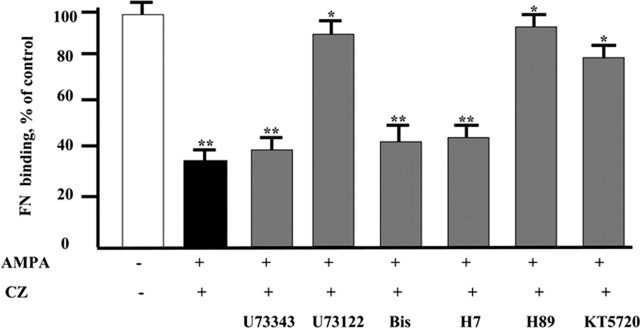
AMPA receptor activation initiates intracellular signaling that might involve phospholipase C (PLC), protein kinase A (PKA), and PKC (Lui et al., 1999). To examine further the signal transduction pathways involved in neurotransmitter-induced modulation of fibronectin binding to OPCs, we treated the cells with selective kinase inhibitors (Fig. 7). The AMPA receptor-induced decrease in fibronectin binding was abolished by PKA inhibitors H89 and KT5720, whereas inhibitors of PKC, H7, and Bis, were ineffective. Inhibition of PLC activity with U73122 also blocked the AMPA/CZ-induced reduction of fibronectin binding to OPCs. These data suggest that PLC and PKA are essential mediators of the AMPA receptor-induced signaling that regulates αv integrin-mediated OPC interaction with the ECM.
Figure 7.
AMPA receptor-induced decrease in fibronectin (FN) binding to OPCs is mediated by PLC and PKA. OPCs were preincubated for 30 min with 20 μm U73122 (PLC inhibitor); 20 μm U73343 (inactive analog of U73122); 80 nm KT5720 and 80 nm H89 (PKA inhibitors); and 5 μm Bis and 5 μm H7(PKC inhibitors). Cells were then treated with 2 mm Ca2+ and 100 μm AMPA plus 60 μm CZ for 10 min, and the incubation continued for an additional 30 min in the presence of 10 μg of fibronectin-Alexa Fluor 488 conjugate. Cells were washed with PBS, and the fluorescence was measured. The data presented are means ± SEM (12 replicates from 3 independent experiments). Fibronectin binding significantly decreased in the presence of AMPA and CZ (**p < 0.01 compared with untreated control). U73122, H89, and KT5720 treatment inhibited the AMPA-induced decrease in fibronectin binding compared with cells treated with AMPA plus CZ (*p < 0.05).
What other proteins are in the αv integrin/PLP complex after AMPA treatment of OPCs? Most interestingly, coimmunoprecipitation studies showed an increased association of GluR2 and GluR4 subunits of AMPA receptor with the αv integrin/PLP complex after agonist stimulation of AMPA receptor (Fig. 8). There was no increased GluR2 or GluR4 association with the complex after stimulation of muscarinic acetylcholine receptor, despite the fact that carbachol also enhances the αv integrin/PLP association and modulates fibronectin binding and OPC migration. Together, these results clearly showed that neurotransmitter receptor stimulation triggers the formation of a multiprotein complex centered at αv integrin receptor and PLP, which includes the neurotransmitter receptor itself. Formation of this complex weakens integrin affinity to ECM ligand, which likely enhances OPC motility.
A model for the overall signaling system described here is proposed in Figure 9. In the absence of AMPA or glutamate, there is only low interaction of PLP, αvβ3 integrin, and AMPA receptor, with strong binding of αvβ3 integrin and fibronectin (Fig. 9A). After activation of the AMPA receptor, there is an increase in the complex containing PLP, αvβ3 integrin, and the AMPA receptor, with signaling coupled to a Gi-protein to increase calcium transients in the cell (Fig. 9B). This, combined with reduced binding to fibronectin, leads to increased migration of OPCs.
Figure 9.

Model for association of PLP with αv integrin and AMPA receptor. A, In the absence of AMPA, little association of PLP with the AMPA receptor or integrins is observed, and αvβ3 integrin associates tightly with fibronectin. B, In contrast, after agonist activation of the AMPA receptor PLP, αvβ3 integrin and the AMPA receptors form a complex. Previous studies (Gudz et al., 2002) demonstrate that PLP binds directly to the C terminus of αv integrin. Signaling from this complex is Gi-protein dependent and is mediated by PLC and PKA. This signaling reduces OPC binding to fibronectin, enhances OPC migration, and is likely mediated through association of signaling and cytoskeletal molecules with the β3 integrin molecule.
Discussion
These studies are unique in establishing a direct connection between neurotransmitter receptor signaling and integrin function modulating OPC migration. Thus, activation of glutamate receptors on OPCs accelerates integrin-mediated OPC motility via mechanisms that involve AMPA receptors. AMPA increased the number of migrated OPCs only when the cells were allowed to migrate on fibronectin or laminin, which are ligands of integrin receptors expressed on OPCs. There was no stimulation of OPC migration when the cells migrated on collagen, which does not bind to the integrins on OPCs. These results extend our previous findings (Gudz et al., 2002) that neurotransmitters are powerful regulators of the integrin receptor function in OPCs. Glutamate receptor agonist stimulated OPC migration mediated both by α6β1 integrin, which binds to laminin, and by αvβ3 integrin, which binds to fibronectin. These observations complement previous studies showing a role of αvβ1 integrin in OPC migration on the ECM derived from astrocytes (Milner et al., 1996). Our data draw attention to possible ligands of αvβ3 integrin in the developing CNS. Several ECM ligands, including fibronectin and vitronectin, have been found within white-matter tracts early in brain development (Neugebauer et al., 1991; Sheppard et al., 1991). Additionally, αvβ3 integrin has been shown to interact with L1, which is a neuronal adhesion molecule expressed in axonal tracts (Ruppert et al., 1995). Based on our cell culture data, it is quite possible that αvβ3 integrin interactions with L1 could provide guidance cues for OPC migration in vivo.
OPCs express AMPA and kainate receptors, which can clearly impact OPC migration. These results suggest that, in vivo, glutamate could serve as a chemoattractant, stimulating the migration of OPCs toward their target destination in the developing brain. It has been well established that glutamate plays a major role regulating cortical neuronal migration in vitro via NMDA receptors (Behar et al., 1999). Also, glutamate has been shown to stimulate neuronal movement in the developing cerebellum by activation of NMDA receptors (Komuro and Rakic, 1993). Our data also support the hypothesis of a widespread role for glutamate as a chemoattractant that provides positional cues for neurons and glial cells in the developing nervous system. These findings provide new evidence suggesting that glutamate receptors may play an early role in the regulation of glial cell migration before the establishment of synaptic networks in early brain development.
Several mechanisms may be involved in the modulation of OPC migration that is mediated by AMPA and other neurotransmitters. The most obvious mechanism seen in these studies is modulation of ECM binding. Thus, neurotransmitter agonists reduce binding of OPCs to fibronectin, an αv integrin ligand. This reduced binding to the ECM likely plays a major role in the enhanced migration of the cells. Fibronectin is produced in the developing brain by both radial glia and neurons. It is initially expressed by radial glia, which align the fibronectin in radial stripes, presumably as part of the guidance role that radial glia provide in the developing brain (Sheppard et al., 1995; Stettler and Galileo, 2004). It is subsequently found in the outer cortical layers, produced primarily by neurons migrating within the cortex (Sheppard et al., 1995). Thus, it is clearly available as an appropriate substrate for OPC migration, likely produced by the same cells that release neurotransmitters.
Another mechanism that is clearly significant in this system is Ca2+ signaling, which is essential for regulation of cell migration. Migration of neuronal progenitors requires coordinated activity of Ca2+ channels, NMDA-type glutamate receptors, and intracellular Ca2+ fluctuations. The combined amplitude and frequency components of Ca2+ fluctuations in the somata of migrating neurons provide the signal that controls the rate of cell migration. Reducing the amplitude or frequency of Ca2+ fluctuations slows the speed of cell movement, whereas increasing these components accelerates migration (Pende et al., 1994). Ca2+ transients are primarily based on coordinated mobilization of intracellular Ca2+ stores. Thus, in cerebellar granule cells, decreasing internal Ca2+ release with thapsigargin or chelating Ca2+ with BAPTA resulted in a significant reduction of Ca2+ transient frequency and a slowdown of granule cell movement (Kumada and Komuro, 2004). The analysis of OPC migration revealed the importance of Ca2+-dependent intracellular signaling and, in particular, of Ca2+ transients in OPCs. Our data demonstrate that disruption of Ca2+ signaling by blocking Ca2+ release from intracellular stores, or Ca2+ uptake by mitochondria, or by chelating cytosolic Ca2+ with BAPTA is accompanied by a reduction in OPC movement. These data suggest that OPC migration could be governed by transient Ca2+ elevations in the cytosolic compartment. Clearly, AMPA treatment of OPCs modulates Ca2+ transient frequency (Table 1). It is well established that activation of AMPA receptors on OPCs leads to increases in the intracellular Ca2+ level attributable to extracellular Ca2+ influx through the channels and Ca2+ release from the intracellular stores (Holzwarth et al., 1994; Pende et al., 1994; Hortzclaw et al., 1995; Lui et al., 1997). The results of our studies show for the first time that an increase in amplitude and frequency of Ca2+ transients is one of the mechanisms underlying AMPA-induced stimulation of OPC migration.
Additionally, AMPA treatment of the cells may modulate migration through recycling of integrins. Recycling integrins has been demonstrated in neutrophils, in response to signaling that alters Ca2+ transient frequency (Lawson and Maxfield, 1995). Neutrophil migration is modulated by αvβ3 adhesion to fibronectin/vitronectin. The relative adhesion of the leading membrane compared with that of the membrane at the rear of the cell is modulated by intracellular Ca2+transients. The density of αvβ3 integrins on the leading edge of the cell is significantly greater than that at the rear of the cell. This differential distribution is maintained by endocytosis of the integrins at the rear of the cell and recycling to the leading edge, and this endocytosis/recycling pathway is dependent on intracellular Ca2+. Thus, the differential distribution of these integrins can be eliminated, and migration can be reduced by altering intracellular Ca2+.
G-protein-coupled second-messenger systems seem to be another essential component involved in the AMPA-induced stimulation of OPC motility. AMPA-induced OPC migration was completely abolished by pertussis toxin, indicating that AMPA activated Gi-protein in OPCs. However, the AMPA-activated G-protein in OPCs was not coupled to a metabotropic receptor. Although not typical of G-protein association with receptors, this is consistent with studies showing AMPA-induced activation of G-protein that was physically associated with GluR1 in neurons (Wang et al., 1997). It also focuses attention on Gi-protein-linked second-messenger pathways in OPCs. Both activation of PLC and accumulation of inositol phosphates have been reported in OPCs exposed to AMPA, which were mediated by G-protein coupled to AMPA receptor, not to mGluR (Lui et al. 1997, 1999). In addition, our data showing PLC dependence of AMPA-induced modulation of integrin ligand binding activity provide further support for the role of PLC in regulation of OPC motility. Detailed analysis of G-protein-dependent second-messenger pathways involved in regulation of AMPA-induced OPC migration is certainly warranted.
PLP is the major integral membrane protein of CNS myelin, and until recently, it was believed to be expressed primarily by differentiated oligodendrocytes. The consensus was that PLP functioned as a structural component of myelin, providing stability and maintaining the compact lamellar structure of myelin. Emerging data demonstrate the importance of PLP expression during early brain development in OPCs and neurons (Timsit et al., 1992; Mallon et al., 2002; Miller et al., 2003), as well as in non-neural cells (Skoff et al., 2004). Moreover, a soma-restricted alternatively spliced isoform of PLP was detected in motor neurons and striated muscle before the onset of myelination (Jacobs et al., 2004). Altogether, the results of these studies raise a possibility of a new biological role for PLP in cell migration, which is independent of myelination. Our data showing that PLP is essential for the neurotransmitter-induced alteration in OPC migration are further support for a critical role of PLP in neuron–oligodendrocyte communication during the early brain development.
Footnotes
T. I. Gudz’s present address: Department of Neuroscience, Medical University of South Carolina, Charleston, SC 29425.
This work was supported by National Institutes of Health Grant NS25304 (W.B.M.) and National Multiple Sclerosis Society Grant PP0944 (T.I.G.). We thank Kapila Navaratne and Cindy Kangas for technical assistance.
References
- Baron W, Decker L, Colognato H, ffrench-Constant C (2003). Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains. Curr Biol 13:1–20. [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC (1993). Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature 361:258–260. [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC (1999). Axonal control of oligodendrocyte development. J Cell Biol 147:1123–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar TN, Scott CA, Greene CL, Wen X, Smith SV, Maric D, Liu Q-Y, Colton CA, Barker JL (1999). Glutamate acting at NMDA receptors stimulates embryonic cortical neuronal migration. J Neurosci 19:449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belachew S, Gallo V (2004). Synaptic and extrasynaptic neurotransmitter receptors in glial precursors’ quest for identity. Glia 48:185–196. [DOI] [PubMed] [Google Scholar]
- Blaschuk KL, Frost EE, ffrench-Constant C (2000). The regulation of proliferation and differentiation in oligodendrocyte progenitor cells by αv integrins. Development 127:1961–1969. [DOI] [PubMed] [Google Scholar]
- Brauner-Osborne H, Slok FA, Skjaerbaek N, Ebert B, Sekiyama N, Nak S, Krogsgaard-Larsen P (1996). A new highly selective metabotropic excitatory amino acid agonist: 2-amino-4-(3-hydroxy-5-methylisoxazol-4-yl)butyric acid. J Med Chem 39:3188–3194. [DOI] [PubMed] [Google Scholar]
- Colognato H, Baron W, Avellana-Adalid V, Baron-Van Evercooren A, Georges-Labouesse E, ffrench-Constant C (2002). CNS integrins switch growth factor signaling to promote target-dependent survival. Nat Cell Biol 4:833–841. [DOI] [PubMed] [Google Scholar]
- Gudz TI, Schneider TE, Haas TA, Macklin WB (2002). Myelin proteolipid protein forms a complex with integrins and may participate in integrin receptor signaling in oligodendrocytes. J Neurosci 22:7398–7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzclaw LA, Gallo V, Russell JT (1995). AMPA receptors shape Ca2+ responses in cortical oligodendrocyte progenitors and CG-4 cells. J Neurosci Res 42:124–130. [DOI] [PubMed] [Google Scholar]
- Holzwarth JA, Gibbons SJ, Brorson JR, Phillipson LH, Miller RJ (1994). Glutamate receptor agonists stimulate diverse calcium responses in different types of cultured rat cortical glial cells. J Neurosci 14:1879–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Beesley J, Itoh A, Cohen AS, Kavanaugh B, Coulter DA, Grinspan JB, Pleasure D (2002). AMPA glutamate receptor-mediated calcium signaling is transiently enhanced during development of oligodendrocytes. J Neurochem 81:390–402. [DOI] [PubMed] [Google Scholar]
- Jackson KF, Duncan ID (1988). Cell kinetics and cell death in the optic nerve of the myelin deficient rat. J Neurocytol 17:657–670. [DOI] [PubMed] [Google Scholar]
- Jacobs EC, Bongazone ER, Campanioni CW, Campanioni AT (2004). Embryonic expression of the soma-restricted products of the myelin proteolipid gene in motor neurons and muscle. J Neurochem Res 29:997–1002. [DOI] [PubMed] [Google Scholar]
- Kelland EE, Toms NJ (2001). Group I metabotropic glutamate receptors limit AMPA receptor-mediated oligodendrocyte progenitor cell death. Eur J Pharmacol 424:R3–R4. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P (1993). Modulation of neuronal migration by NMDA receptors. Science 260:95–97. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P (1998). Orchestration of neuronal migration by activity of ion channels, neurotransmitter receptors, and intracellular Ca2+ fluctuations. J Neurobiol 37:110–130. [PubMed] [Google Scholar]
- Kumada T, Komuro H (2004). Completion of neuronal migration regulated by loss of Ca2+ transients. Proc Natl Acad Sci USA 101:8479–8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson MA, Maxfield FR (1995). Ca2+- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature 377:75–79. [DOI] [PubMed] [Google Scholar]
- Liesi P, Kirkwood T, Vaheri A (1986). Fibronectin is expressed by astrocytes cultured from embryonic and early postnatal rat brain. Exp. Cell Res 163:175–185. [DOI] [PubMed] [Google Scholar]
- Lui H-N, Molina-Holgado E, Almazan G (1997). Glutamate-stimulated production of inositol phosphates is mediated by Ca2+ influx in oligodendrocyte progenitors. Eur J Pharmacol 338:277–287. [DOI] [PubMed] [Google Scholar]
- Lui H-N, Larocca JN, Almazan G (1999). Molecular pathways mediating activation by kainate of mitogen-activated protein kinase in oligodendrocyte progenitors. Mol Brain Res 66:50–61. [DOI] [PubMed] [Google Scholar]
- Lui HN, Giasson BI, Mushinsky WE, Almazan G (2002). AMPA receptor-mediated toxicity in oligodendrocyte progenitors involves free radical generation, and activation of JNK, calpain and caspase 3. J Neurochem 82:398–409. [DOI] [PubMed] [Google Scholar]
- Luit K, Varadi A, Molnar E (2003). Functional metobotropic glutamate receptors are expressed in oligodendrocyte progenitor cells. J Neurochem 84:1452–1464. [DOI] [PubMed] [Google Scholar]
- Mallon BS, Shick HE, Kidd GJ, Macklin WB (2002). Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J Neurosci 22:876–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP (1988). Neurotransmitters in the regulation of neuronal cytoarchitecture. Brain Res Rev 13:179–212. [DOI] [PubMed] [Google Scholar]
- Matute C, Sanchez-Gomez MV, Martinez-Millan L, Miledi R (1997). Glutamate receptor-mediated toxicity in optic nerve oligodendrocytes. Proc Natl Acad Sci USA 94:8830–8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J (1980). Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol 85:890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MJ, Haxhiu MA, Georgiadis P, Gudz TI, Kangas CD, Macklin WB (2003). Proteolipid protein gene mutation induces altered ventilatory response to hypoxia in the myelin-deficient rat. J Neurosci 23:2265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R, ffrench-Constant C (1994). A developmental analysis of oligodendroglial integrins in primary cells: changes in αv-associated β subunits during differentiation. Development 120:3497–3506. [DOI] [PubMed] [Google Scholar]
- Milner R, Edwards G, Streuli C, ffrench-Constant C (1996). A role in migration for the αvβ1 integrin expressed on oligodendrocyte precursors. J Neurosci 16:7240–7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer KM, Emmett CJ, Venstrom KA, Reichardt LF (1991). Vitronectin and thrombospondin promote retinal neurite outgrowth: developmental regulation and role of integrins. Neuron 6:345–358. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Rigo JM, Rocher V, Belachew S, Malgrange B, Rogister B, Leprince P, Moonen G (2001). Neurotransmitters as early signals for central nervous system development. Cell Tissue Res 305:187–202. [DOI] [PubMed] [Google Scholar]
- Pende M, Holtzclaw LA, Curtis JL, Russell JT, Gallo V (1994). Glutamate regulates intracellular calcium and gene expression in oligodendrocyte progenitors through activation of dl-a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Proc Natl Acad Sci USA 91:3215–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow EF, Haas TA, Zhang L, Loftus J, Smith J (2000). Ligand binding to integrins. J Biol Chem 275:21785–21788. [DOI] [PubMed] [Google Scholar]
- Ragheb F, Molina-Halgado E, Cui QL, Khorchid A, Lui HN, Larocca JN, Almazan G (2001). Pharmacological and functional characterization of muscarinic receptor subtypes in developing oligodendrocytes. J Neurochem 77:1396–1406. [DOI] [PubMed] [Google Scholar]
- Ruppert M, Aigner S, Hubbe M, Yagita H, Altevogt P (1995). The L1 adhesion molecule is a cellular ligand for VLA-5. J Cell Biol 131:1881–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard AM, Hamilton SK, Pearlman AL (1991). Changes in the distribution of extracellular matrix components accompany early morphogenetic events in mammalian cortical development. J Neurosci 11:3928–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard AM, Brunstrom JE, Thornton TN, Gerfen RW, Broekelmann TJ, McDonald JA, Pearlman AL (1995). Neuronal production of fibronectin in the cerebral cortex during migration and layer formation is unique to specific cortical domains. Dev Biol 172:504–518. [DOI] [PubMed] [Google Scholar]
- Skoff RP, Bessert DA, Cerghet M, Franclin MJ, Rout UK, Nave KA, Carlock L, Ghandour MS, Armant DR (2004). The myelin proteolipid protein gene modulates apoptosis in neural and non-neural tissues. Cell Death Differ 11:1247–1257. [DOI] [PubMed] [Google Scholar]
- Stettler EM, Galileo DS (2004). Radial glia produce and align the ligand fibronectin during neuronal migration in the developing chick brain. J Comp Neurol 468:441–451. [DOI] [PubMed] [Google Scholar]
- Timsit SG, Bally-Cuif L, Colman DR, Zalc B (1992). DM-20 mRNA is expressed during the embryonic development of the nervous system of the mouse. J Neurochem 58:1172–1175. [DOI] [PubMed] [Google Scholar]
- Wang Y, Small DL, Stanimirovic DB, Morley P, Durkin JP (1997). AMPA receptor-mediated regulation of a Gi-protein in cortical neurons. Nature 389:502–504. [DOI] [PubMed] [Google Scholar]
- Yoshioka A, Hardy M, Younkin DP, Grinspan JB, Stern JL, Pleasure D (1995). Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors mediate excitotoxicity in the oligodedroglial lineage. J Neurochem 64:2442–2448. [DOI] [PubMed] [Google Scholar]