Abstract
Rolling blackout (RBO) is a putative transmembrane lipase required for phospholipase C-dependent phosphatidylinositol 4,5-bisphosphate–diacylglycerol signaling in Drosophila neurons. Conditional temperature-sensitive (TS) rbo mutants display complete, reversible paralysis within minutes, demonstrating that RBO is acutely required for movement. RBO protein is localized predominantly in presynaptic boutons at neuromuscular junction (NMJ) synapses and throughout central synaptic neuropil, and rbo TS mutants display a complete, reversible block of both central and peripheral synaptic transmission within minutes. This phenotype appears limited to adults, because larval NMJs do not manifest the acute blockade. Electron microscopy of adult rbo TS mutant boutons reveals an increase in total synaptic vesicle (SV) content, with a concomitant shrinkage of presynaptic bouton size and an accumulation of docked SVs at presynaptic active zones within minutes. Genetic tests reveal a synergistic interaction between rbo and syntaxin1A TS mutants, suggesting that RBO is required in the mechanism of N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-mediated SV exocytosis, or in a parallel pathway necessary for SV fusion. The rbo TS mutation does not detectably alter SNARE complex assembly, suggesting a downstream requirement in SV fusion. We conclude that RBO plays an essential role in neurotransmitter release, downstream of SV docking, likely mediating SV fusion.
Keywords: Drosophila, temperature-sensitive paralysis, giant fiber circuit, neurotransmission, synapse, membrane lipase, syntaxin
Introduction
Drosophila temperature-sensitive (TS) paralytic mutants have long proven to be critical tools for the discovery and elucidation of the molecular mechanisms of neurotransmission. These mutants have identified numerous classes of proteins acutely required in neuronal signaling, most prominently including ion channels [e.g., paralytic, Na+ channel subunit (Loughney et al., 1989) and cacophony, Ca2+ channel subunit (Kawasaki et al., 2000)] and components of the synaptic vesicle (SV) cycle [e.g., shibire, a dynamin GTPase (van der Bliek and Meyerowitz, 1991) and comatose, N-ethylmaleimide-sensitive fusion (NSF) factor (Ordway et al., 1994)], among others (Littleton et al., 1998; Rao et al., 2001). Such conditional mutants are particularly valuable because they provide the opportunity to study neurotransmission when gene product function is acutely removed and thus bypass any requirement in cell viability or development. Perhaps the most seminal example of this approach has been the isolation and characterization of the shibire TS mutant, which first revealed the essential requirement of dynamin GTPase in SV endocytosis (Koenig et al., 1983; Koenig and Ikeda, 1996; Kidokoro et al., 2004). We report here a new TS paralytic mutant, rolling blackout (rbo), which similarly reveals a novel mechanism essential for SV exocytosis.
Dissection of the molecular mechanisms of the SV cycle particularly benefits from the study of TS mutants, which provide tools to temporally dissect the interlocked pathways of SV exocytosis and endocytosis. The exocytosis pathway can be further subdivided into SV trafficking, tethering, docking, priming, and fusion at the presynaptic active zone (AZ). During the terminal stages of SV exocytosis, the priming step involves activation of NSF attachment protein (SNAP) receptor (SNARE) proteins via UNC-13-mediated opening of syntaxin and the assembly of the SNARE complex, which pulls the SV into close proximity to AZ plasma membrane to make the SV competent for Ca2+-triggered fusion (Jahn and Sudhof, 1999; Lin and Scheller, 2000; Sudhof, 2004). The fusion step itself requires regulated fusion pore opening and expansion. The SNARE complex is sufficient for in vitro liposome fusion (Weber et al., 1998), but physiological SV fusion involves other proteins/lipids acting as facilitory cofactors (Jahn and Sudhof, 1999; Lin and Scheller, 2000; Sudhof, 2004; Tucker et al., 2004). Indeed, a separate fusion machinery may act downstream of SNARE complex assembly. The actions of V0-ATPase, protein phosphatase 1, and Vtc3p, among others, have been mapped subsequent of trans-SNARE (T-SNARE) pairing and may mediate SV fusion downstream of SNAREs (Peters et al., 1999, 2001; Muller et al., 2002; Bayer et al., 2003; Hiesinger et al., 2005). In addition to such putative fusogenic proteins, regulatory lipids such as diacylglycerol (DAG) and phosphatidylinositides [e.g., phosphatidylinositol 4,5-bisphosphate (PIP2)] play critical roles in exocytosis by regulating the trafficking/activation of fusogenic proteins and by directly altering membrane physical properties critical for SV fusion (Goni and Alonso, 1999; Peters et al., 1999; Cremona and De Camilli, 2001; Martin, 2001; Brose and Rosenmund, 2002; Di Paolo et al., 2004; Fratti et al., 2004; Jun et al., 2004; Wenk and De Camilli, 2004; Rohrbough and Broadie, 2005).
We reported previously (Huang et al., 2004) that rbo encodes an integral plasma membrane lipolytic enzyme that has an essential role in phospholipase C (PLC)-dependent PIP2/DAG signaling during Drosophila phototransduction (Hardie, 2003). Here, we show that RBO protein is localized to presynaptic boutons and that conditional rbo mutants are TS paralytic within minutes. At restrictive temperature, rbo TS mutants display a complete, reversible block of synaptic transmission, accumulate docked SVs at the presynaptic active zone, and display a strong synergistic genetic interaction with syntaxin mutants. These data indicate that RBO has an acute, essential role in SV exocytosis and that RBO likely acts in the syntaxin-dependent mechanism of SV priming/fusion or in a parallel pathway. A preliminary description of some of these results has been published (Vijayakrishnan and Broadie, 2006).
Materials and Methods
Genetics
Drosophila stocks were cultured on standard medium and entrained to a 12 h light/dark cycle at 25°C. The wild-type (wt) strain Oregon-R and w1118 were used as controls. rbots1 contains a G487D missense mutation, rbo2 is a complete deletion null allele, rbo3 contains an early stop codon (Q396amber), df(2R)H3D3 is a deficiency with break points: 044D01-04;044F04-05, rbo2/rbo2; rbo-egfp/rbo-egfp is the transgenic rescue stock (Huang et al., 2004). The following TS paralytic mutant stocks were used: cacophony (cacts2) (Dellinger et al., 2000), comatose (comtts17, comts53; kindly provided by Richard Ordway, Pennsylvania State University, University Park, PA), syntaxin1A (syx3–69) (Littleton et al., 1998), and shibire (shibirets1; kindly provided by Barry Ganetzky, University of Wisconsin, Madison, WI).
Behavioral assays
For each genotype, 20 males and 20 females were tested. In each session, animals were transferred into a prewarmed transparent plastic tube and placed at the indicated temperature in a hybridization oven (HO6000V; GeneMate; ISC BioExpress, Kaysville, UT). Animals were observed continuously and the paralysis time point for each individual recorded. Paralysis was defined when an animal was inverted and immobile at the bottom of the tube, lacking any detectable leg movement. Paralyzed animals were transferred to room temperature (RT) to assay recovery time. The recovery time point was recorded when each animal achieved a standing position and could walk when provoked. The veratridine (Sigma, St. Louis, MO) resistance assay was done as described previously (Chandrashekaran, 1993) with slight modification: equal numbers of male and female animals were used, with assays done at 18, 22, and 28°C. Each genotype was introduced separately into vials with a 1.5 cm disk of filter paper containing 100 μg of veratridine in 2% sugar. Animals were counted at 2, 4, 8, and 24 h.
Immunocytochemistry
RBO–enhanced green fluorescent protein (eGFP) imaging was performed on transgenic rescue animals (rbo2/rbo2; rbo-egfp/rbo-egfp), with the expression of RBO–eGFP protein under the control of the endogenous rbo promoter (Huang et al., 2004). For immunocytochemistry, dissected preparations were fixed in 4% paraformaldahyde in PBS at RT for 25 min (larvae) and 45 min (adults). Preparations were incubated in the following primary antibodies for at least 2 h at RT: anti-discs large (DLG; 1:1000; rabbit; from Vivian Budnik, University of Massachusetts Medical School, Worcester, MA), anti-cysteine string protein (CSP; 1:500; mouse; from Konrad Zinsmaier, University of Arizona, Tucson, AZ), anti-synaptotagmin (1:5000; rabbit; from Hugo Bellen, Baylor College of Medicine, Houston, TX), Texas Red-conjugated anti-HRP (1:300; goat; Jackson ImmunoResearch, West Grove, PA), FITC-conjugated anti-HRP (1:500; goat; Jackson ImmunoResearch), and anti-dUNC13 [1:500; rabbit (A2)] (Aravamudan and Broadie, 2003). Preparations were incubated for 1 h at RT with secondary antibodies (1:1000; Invitrogen, San Diego, CA). Images were captured on a Bio-Rad (Hercules, CA) Radiance 2000 or Zeiss (Oberkochen, Germany) LSM 510 Meta confocal microscope.
Electrophysiology
Excitatory junctional potential recording in giant fiber system.
An adult animal anesthetized by CO2 was mounted ventral side down on a glass coverslip with dental soft wax under a dissection microscope. A HCC-100A temperature controller (Dagan, Minneapolis, MN) was used for temperature control. The tip of the temperature probe was embedded in the wax; it took ∼2 min to increase temperature from 25 to 37°C. The output from the temperature controller was transited for simultaneous recording of temperature and electrical signals. A glass reference electrode was inserted into the abdomen, and a glass stimulating electrode was inserted into each eye (Pavlidis and Tanouye, 1995). A glass recording electrode (3 MΩ) was driven through the dorsal thorax cuticle. Intracellular penetration into the muscle was monitored by a sudden potential drop of 40–60 mV. The muscle identity [dorsal longitudinal muscle (DLM) vs tergotrochanteral muscle (TTM)] was determined by electrode placement and verified by recorded latency time. Signals were amplified by IX1 intracellular preamplifier (Dagan) and digitized at 100 kHz by Digidata 1200 or 1322A (Molecular Devices, Union City, CA). Data were collected and analyzed with pClamp software (version 8.0; Molecular Devices). All glass electrodes were filled with 3 m KCl.
Excitatory junctional current recording in DLM muscle cells.
An adult animal was laterally mounted over an opening of a Parafilm tube and secured with soft dental wax so that air was accessible to the trachea. The overlying cuticle and muscles were dissected away to expose one set of DLM cells in Ca2+-free saline consisting of the following (in mm): 128 NaCl, 2 KCl, 4 MgCl2, 5 HEPES, and 36 sucrose. The dissection saline was then replaced with 1.8 mm CaCl2 recording saline, perfused at 0.3–0.4 ml/min. The posterior dorsal mesothoracic nerve carrying the DLM motor neuron (DLMn) axons was cut and sucked into a glass stimulation pipette (Koenig et al., 1983). Two glass microelectrodes (∼10 MΩ) filled with 3 m KCl were inserted in the DLMe (e cell of the DLM) or DLMd (d cell of the DLM) cell. The DLM resting potentials ranged from −70 to −90 mV. Two-electrode voltage clamp was performed using an Axoclamp-2B amplifier (Molecular Devices), with the holding potential at −80mV (Kawasaki et al., 1998) and the voltage-clamp gain over 50. The HCC-100A temperature controller was again used for temperature control, with coincident recording of temperature and electrical signals. Data were analyzed with pClamp software (version 8.0; Molecular Devices).
Recording at the larval neuromuscular junction.
Standard larval neuromuscular junction (NMJ) recording configurations were used (Broadie, 2000). Briefly, recordings were made from muscle 6 in abdominal segments A3–4 of wandering third instar larva using two-electrode voltage-clamp (−60 mV) techniques (Axoclamp 2B amplifier). Intracellular recording electrodes were filled with 3 m KCl and typically had a resistance of ∼10 MΩ. Excitatory junctional currents (EJCs) were evoked by brief stimuli (0.4–1.5 ms) applied to the cut motor nerve using a suction electrode. The recording saline contained the following (in mm): 128 NaCl, 2 KCl, 4 MgCl2, 5 trehalose, 70 sucrose, 5 HEPES, and 1.8 CaCl2, pH 7.1. A probe placed close to the preparation monitored the recording chamber temperature. Data acquisition and analysis were performed using pClamp software (version 8.0; Molecular Devices).
Dye imaging
FM1-43 dye imaging was performed as described previously (Fergestad and Broadie, 2001) with slight modifications. Wandering third instar larvae or adults were dissected in Ca2+-free recording with mutants and controls always prepared in the same chamber to ensure identical processing and imaging conditions (Fergestad and Broadie, 2001). The preparations were exposed to 10 μm FM1-43 (Invitrogen) in 90 mm K+ saline containing 1.8 mm Ca2+ (Fergestad and Broadie, 2001) for 3–5 min to load synaptic terminals, then washed in Ca2+-free recording saline (2 mm K+) for ∼10 min, while the saline was exchanged at least three times. Confocal fluorescence images were acquired with a Zeiss LSM 510 confocal microscope. In each animal, NMJs were imaged, and then samples were transferred to prewarmed (38°C) Ca2+-free recording saline and moved to a 38°C incubator for 15 min. Samples were then treated with prewarmed (38°C) high-K+ saline for 5 min to destain terminals, and confocal imaging was then repeated using identical parameters.
Electron microscopy
Animals at room temperature (22°C) or incubated for 10 min at 37°C were fixed in 2.5% glutaraldeyde overnight. Samples were then washed in PBS for 10 min, transferred to 1% osmium tetroxide in H2O for 1 h, rinsed with double-distilled H2O, and stained en bloc in 2% aqueous uranyl acetate for 1 h. Samples were then dehydrated through a series of graded alcohols and embedded in Araldite. After placing in a vacuum oven for 30 min, animals were placed in fresh Araldite and left to polymerize overnight at 60°C. Ribbons of thin (∼40–50 nm) sections were obtained with a Leica (Nussloch, Germany) Ultracut UCT 54 ultramicrotome and examined on a Phillips CM12 TEM, equipped with a 2 megapixel CCD camera. Digital electron microscopy (EM) images were taken of synaptic boutons from the DLM NMJ. All sections for quantification contained at least one clear presynaptic T-bar, defining an AZ, identified in at least two consecutive sections. Docked vesicles were defined as those within 20 nm from the electron dense presynaptic membrane at the T-bar, as described previously (Aravamudan et al., 1999). Morphometric analysis was performed using the public domain NIH ImageJ software package.
SNARE complex assay
Animals at room temperature (22°C), or incubated for 10 min at 33 or 37°C, were frozen in liquid nitrogen, and heads were immediately collected with a sieve. One hundred fifty milligrams of heads were homogenized in 3 ml of ice-prechilled hypotonic buffer [10 mm MgCl2, 25 mm Tris, pH 7.4, 2.5 mm EGTA, and complete mini-protease inhibitor mixture (Roche Products, Welwyn Garden City, UK)] with a dounce homogenizer. Plasma membrane fractions were collected from the lysed cell suspension by centrifugation at 1000 × g (5 min, 4°C) and subsequent centrifugation of the supernatant at 50,000 × g (30 min, 4°C). Protein extraction was performed by resuspending the final membrane pellet in SDS sample buffer (50 mm Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, 5% 2-mercaptoethanol) for 15 min at 22°C. Total membrane protein concentration was determined by absorbance measurements at 280 nm. Bromophenol blue was added (0.03%), and samples were subjected to SDS-PAGE and Western blot with primary antibody against syntaxin1A (8C3; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), SNAP25 (Leo Pallanck, University of Washington, Seattle, WA), and HRP-conjugated secondary antibodies (Promega, Madison, WI). Signals were visualized by ECL and quantified by LabWorks 4.5 in combination with UVP (Upland, CA) BioImaging systems.
Statistics
Statistical analyses of total SV number, bouton size, and the docked SV number at active zones were done with InStat (GraphPad Software, San Diego, CA); significant differences were determined by the nonparametric Mann–Whitney test. Statistical analyses of all other experiments were done with Microsoft (Redmond, WA) Excel X software; significant changes were determined by an unpaired t test. In all cases, p values <0.05 were considered significant. If not specified, data are represented by mean ± SEM.
Results
RBO is acutely required for movement
At permissive temperatures (≤25°C), rbo TS mutant adult flies appear to act normally and cannot be distinguished from wt based on locomotion or gross behavior. At restrictive temperature (≥37°C), all rbo TS mutant animals are completely paralyzed within minutes, although control animals appear unaffected. At intermediate temperatures, rbo TS mutants show intermediate movement impairment. At 29°C, mutants remain mobile for many hours but become progressively sluggish and die within 1 d, and at 33°C, mutants display reduced movement followed by complete paralysis in ∼30 min. Thus, rbo TS mutants display a progressively temperature-dependent impairment in some mechanism required for movement.
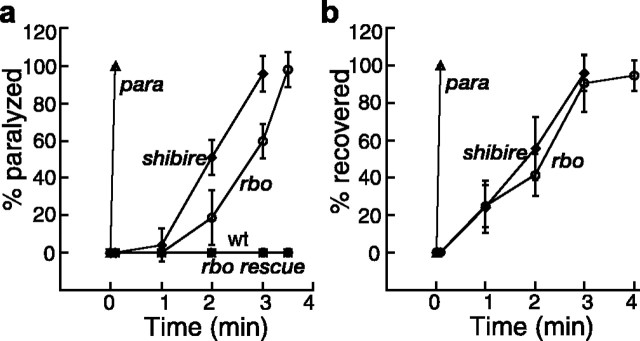
The kinetics of reversible paralysis was quantified in a range of homozygous and trans-heterozygous rbo mutants (rbots1/rbots1, rbots1/rbo2, rbots1/rbo3, rbots1/Df(2R)H3D3; see Materials and Methods). The time course of paralysis and recovery are shown in Figure 1. All rbo mutant allelic combinations display 100% paralysis within 3–4 min when shifted from room temperature (22°C) to 37°C and recover with a similar time course after a return to room temperature (Fig. 1). Control wild-type and rbo/+ flies show no movement impairment under identical conditions. No difference in the kinetics of paralysis or recovery was observed between rbots1 homozygotes and rbots1/rbo2, rbots1/rbo3, rbots1/Df(2R)H3D3 mutants (data not shown). A single copy of a wild-type rbo:egfp transgene fully rescues behavioral paralysis in all of these genotypes (Fig. 1a). Therefore, the temperature-sensitive paralysis can be fully ascribed to the rbots1 mutation.
Figure 1.
TS paralysis and recovery of rbo mutants. a, b, The kinetics of TS paralysis at 37°C (a) and recovery from paralysis at room temperature (22°C) (b). The genotypes are Oregon-R wt, rbots1/rbo2 (rbo), rbots1/rbo2; rbo-egfp/+ (rbo rescue), paralyticts1 (para), and shibirets1 (shibire) homozygous mutants. Error bars represent SD.
To gain insight into possible mechanisms, we compared rbots1 mutants with paralyticts1 [terminates action potentials (APs) (Wu and Ganetzky, 1980)] and shibirets1 [blocks SV endocytosis (Koenig et al., 1983)]. The paralysis and recovery kinetics of rbots1 differs markedly from paralyticts1, in which paralysis/recovery are exceedingly rapid (∼5 s) (Fig. 1). This result suggests that rbo is unlikely to encode a protein directly mediating neuronal excitability, such as an ion channel or channel regulator. Consistent with this conclusion, rbots1 mutants do not show any visible change in death rate after exposure to veratridine for 2, 4, 8, and 12 h at 18, 22, and 28°C (data not shown). Veratridine is a sodium channel-specific neurotoxin that prolongs the activation of voltage-gated Na+ channels (Ritchie, 1979; Suzuki and Wu, 1984; Jackson et al., 1986). Na+ channel mutants and Na+ channel regulator mutants display increased resistance to veratridine at both permissive and restrictive temperatures, including parats, napts, and tipE (Suzuki and Wu, 1984; Jackson et al., 1986). Furthermore, rbots1 mutants do not show genetic interaction with paralyticts1, shaker (IA voltage-gated K+ channel), or ether-a-gogo (a subunit common to different K+ channels) mutants in terms of the kinetics of TS paralysis and recovery (data not shown).
The paralysis kinetics of rbots1 and shibirets1 mutants is quite comparable; rbots1 mutants paralyze slightly more slowly than shibirets1 (Fig. 1a) but recover with an indistinguishable time course (Fig. 1b). The relatively slow paralysis/recovery in shibirets1 is caused by slow depletion/regeneration of the synaptic vesicle pool (Koenig et al., 1983). Similarly, there is an activity-dependent requirement for RBO in phototransduction (Huang et al., 2004), and loss of RBO function likely results in the loss of some resource limiting neurotransmission. Given that RBO is an integral plasma membrane lipase (Huang et al., 2004), one likely possibility is that RBO produces a limiting lipid required for neurotransmission or, alternatively, eliminates a lipid that negatively regulates neurotransmission.
RBO is subcellularly restricted to neuronal synapses
A rbo–egfp fusion transgene was made from genomic DNA including 3.5 kb upstream and 1.5 kb downstream of the rbo coding region. A single copy of this rbo–egfp transgene rescues all rbo mutant phenotypes (Huang et al., 2004). We therefore examined rbo–egfp expression in the rbo null mutant background. Previously, we showed RBO–eGFP protein to be highly enriched in the central synaptic neuropil of embryos, larvae, and adults, in the brain and the thoracic and abdominal ganglia (Huang et al., 2004). To examine RBO expression within individual, identified neurons, we assayed motor neurons and NMJ synapses in both larvae and adults (Fig. 2).
Figure 2.
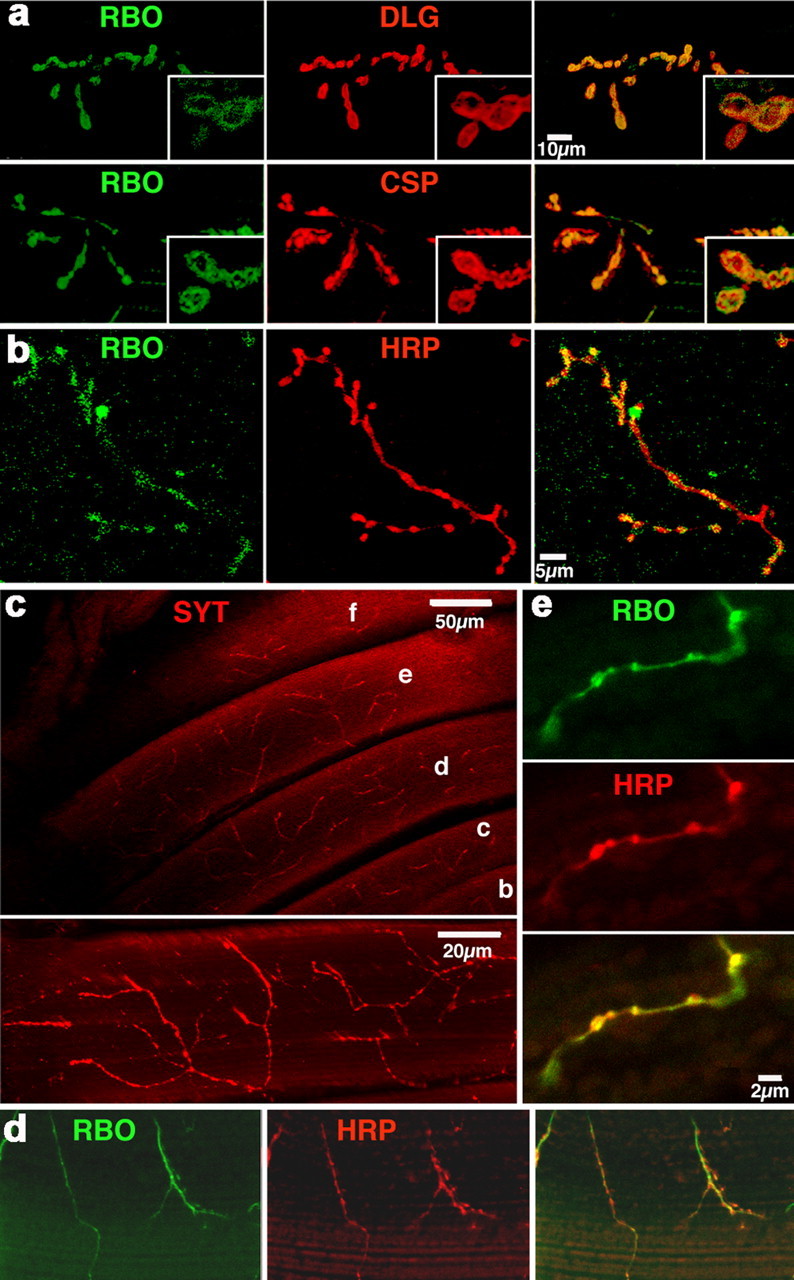
RBO protein is subcellularly restricted to synapses. Confocal imaging of RBO-eGFP in rbo transgenic rescue background (rbo2/rbo2; rbo-egfp/rbo-egfp). a, RBO at wandering third instar larva NMJ synapses. Colocalization of RBO with synaptic markers is shown (DLG, predominantly postsynaptic and CSP, a presynaptic vesicle-associated protein). b, RBO in adult abdominal NMJs. Anti-HRP recognizes the neuronal membrane. c, Innervation pattern of DLM NMJs stained with anti-synaptotagmin. NMJs are deeply embedded and evenly distributed in DLM muscle cells. The bottom is the projection of a single DLM muscle cell. d, e, Lower amplification (d) and higher amplification (e), showing that RBO colocalizes with HRP in DLM NMJs.
RBO protein is undetectable in neuronal soma, present at low levels in axons, and highly enriched specifically within synaptic boutons (Fig. 2). RBO protein is expressed at all examined NMJ synapses innervating the body musculature in both larva (Fig. 2a) and adult stages (Fig. 2b). Double-labeling experiments with either presynaptic (synaptotagmin, CSP, HRP) or postsynaptic (DLG) markers confirms that RBO is specifically subcellularly enriched within synaptic boutons (Fig. 2). The expression pattern is consistent with a predominantly presynaptic localization for RBO, although a low level of muscle postsynaptic localization may also occur. RBO is associated with the plasma membrane throughout the synaptic bouton, without any detectable restriction in smaller membrane domains (Fig. 2a, insets). This expression pattern, together with the rbo TS paralytic phenotype, suggests that RBO functions in synaptic transmission to mediate coordinated movement.
RBO is required for synaptic transmission
To investigate whether the adult conditional rbo paralysis is associated with a failure in synaptic transmission, EJP recordings were made in the adult GF escape circuit (Fig. 3), well characterized in terms of both anatomy and physiology (King and Wyman, 1980; Tanouye and Wyman, 1980; Ikeda and Koenig, 1988; Trimarchi and Murphey, 1997). GF circuit activation involves stimulation of the giant neuron in the brain, AP propagation through the GF into the thoracic ganglion, electrical synapse activation of the peripherally synapsing interneuron (PSI), cholinergic synapse activation of the DLMn, AP propagation along DLMn axon in the peripheral nerve, and finally NMJ glutamatergic transmission at the muscle (Fig. 2c). Records were made by electrically stimulating the paired giant neurons in the brain with extracellular glass electrodes while intracellularly recording output in thoracic DLM muscles (Fig. 3a).
Figure 3.
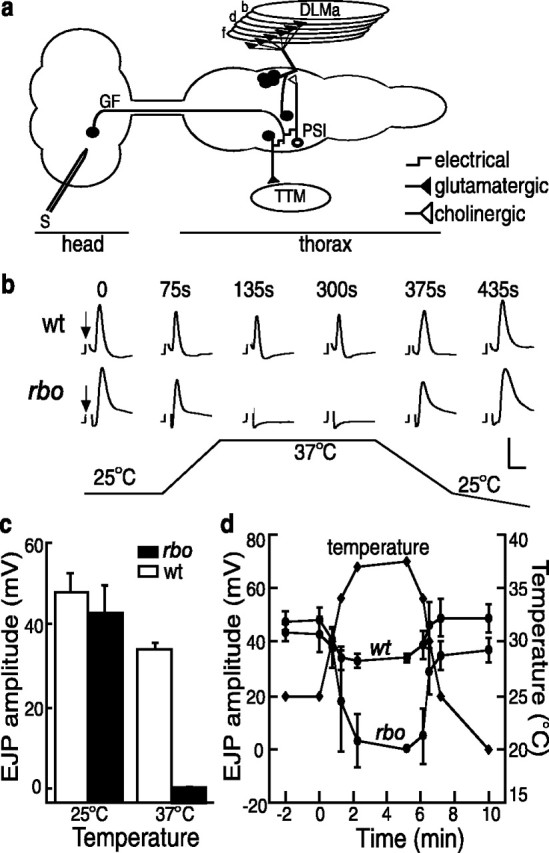
Conditional block of neurotransmission in the giant fiber circuit. a, Schematic diagram of the GF circuit. Note in particular that output onto the TTM requires only electrical synapses before the glutamatergic NMJ, whereas output onto the DLM has a single prerequisite central chemical (cholinergic) synapse between the PSI interneuron and the motor neuron. Stimulation (S) was applied to the brain to activate the GF and in turn activate the motor neurons. b, Representative traces of the temperature-dependent, reversible loss of DLM EJP responses in wt and rbo (rbots1/rbo2) mutants (arrow indicates shock artifact; removed for clarity). The bottom trace shows the concurrently recorded temperature shift (25 to 37°C). c, Quantification of EJP amplitude at 25°C and after 5 min at 37°C. The bars show the mean ± SD. d, Time course of EJP amplitude changes. The recording temperature (right axis) was recorded simultaneously.
At permissive temperature (25°C), evoked EJP amplitudes in rbots1 mutants are indistinguishable from wild type (rbots1/rbo2, 43 ± 6.6 mV, n = 8; wt, 48 ± 4.6 mV, n = 7) (Fig. 3b,c). In contrast, after a shift to 37°C, evoked EJPs in rbots1 mutants are undetectable and remain completely suppressed at 37°C, but then recover after return to 25°C (Fig. 3b–d). In wild type, EJP amplitudes are reduced by ∼25% at 37°C (36 ± 1.8 mV) but persist throughout the entire time course of the temperature shift recording (Fig. 3b–d). These results show that adult conditional paralysis in rbots1 mutants temporally correlates with neural transmission blockade in the adult GF circuit. Interestingly, there is not a similar acute requirement for RBO at the larval NMJ. EJC amplitudes at room temperature (20–22°C) were indistinguishable between rbots1 and control animals (rbots1/rbo2, 185 ± 19 nA, n = 5; wild type, 190 ± 10 nA, n = 5). Incubation of rbots1 wandering third instars at 37°C did not cause significant change in EJC amplitudes. At a stimulation frequency of 0.5 Hz, mean EJC amplitude was 243 ± 18 nA for rbots1/rbo2 (n = 6) and 224 ± 24 nA (n = 6) for control animals, respectively. In all recordings, EJCs could be evoked as long as a stable recording configuration was maintained (>15 min at 37°C), essentially excluding the possibility of a delayed onset of transmission impairment. Similarly, assays of SV cycling with FM1-43 dye loading/unloading at the larval NMJ failed to reveal deficits in rbots1/rbo2 mutants (data not shown). Therefore, the acute requirement for RBO in maintaining neural transmission appears restricted to the adult.
The EJP loss in adult rbots1 mutants could be caused by a defect at any level of GF circuit function, including failure of AP initiation/propagation or a blockade of synaptic transmission at either central or peripheral synapses. To distinguish between these possibilities, we took advantage of the dual DLM and TTM outputs of the GF circuit (Fig. 3a). The DLM circuit contains a single, essential cholinergic chemical synapse between PSI and DLMn, upstream of the glutamatergic DLM NMJ, and thus behaves like a bisynaptic pathway. In contrast, the TTM glutamatergic NMJ is the sole required chemical synapse in the TTM circuit (King and Wyman, 1980): electrical synapses alone are sufficient for transmission to the TTM NMJ, which therefore behaves like a monosynaptic pathway. Any block of AP function would result in an abrupt failure of neural transmission in both outputs of the GF circuit. In contrast, any direct loss of synaptic function would be predicted to differentially block function in the two outputs. If RBO is required only at glutamatergic NMJs, we would expect both DLM and TTM EJPs to be lost in a graded, incremental manner over several minutes, as NMJ synaptic transmission is progressively degraded. If RBO is also required for central transmission, we would predict that a blockade of the weaker cholinergic PSI synapse will lead to a failure of AP initiation in the DLMn, causing a sudden, complete loss of NMJ transmission. Similarly, if a central transmission block occurs, we would predict an abrupt recovery of the DLM EJP once the central transmission recovers versus an incremental improvement of the TTM EJP.
The results of the DLM/TTM comparison strongly indicate that rbo mutants are specifically defective in synaptic transmission in both central and peripheral synapses (Fig. 4). In DLM recordings in rbots1 mutants after shift to restrictive temperature (25 to 37°C), the EJP initially declines, consistent with a progressive weakening of NMJ transmission, but then displays a sudden complete loss (Fig. 4a, middle). In contrast, TTM recording of rbots1 mutants always show a gradual loss of EJP amplitude over several minutes after shift to the restrictive temperature (Fig. 4b, middle) and a similar gradual recovery after return to permissive temperature (Fig. 4b, right). The time to complete EJP loss at restrictive temperature was 4.3 ± 0.5 min (mean ± SD; n = 6), which agrees well with the time to complete TS paralysis (4.5 min). DLM and TTM EJPs in wild-type and transgenic rescue flies (rbots1/rbo2; rbo-egfp/+) persist through the entire recording course (Fig. 4a,b, left).
Figure 4.
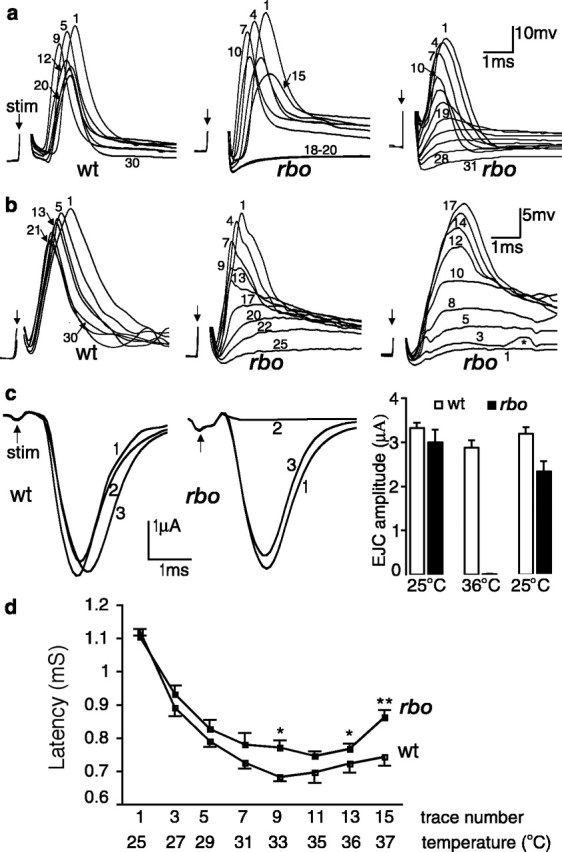
The rbo TS mutation specifically blocks synaptic transmission. a, Representative series of EJP traces from the DLM evoked by 0.1 Hz brain or thoracic stimulation (arrow) during temperature shift from 25 to 37°C. The numbers indicate order of the evoked response. Response amplitudes are reduced slightly in both genotypes during the temperature shift but persist in wt (left) and fail in an abrupt manner in rbo (rbots1/rbo2) mutants when evoked by brain stimulation (middle). However, rbo DLM EJP shows gradual loss when induced by direct-simulating DLM motor neuron (right). b, Representative series of TTM EJP traces from wt and rbo transgenic rescue [(rbots1/rbo2; rbo-egfp/+ (left) and rbo (middle and right)]. The left and middle panels show EJP responses during temperature shift from 25 to 37°C, whereas the right shows the EJP recovery when temperature is returned to 25°C. Note the graded loss of EJP amplitude after shift to restrictive temperature (middle) and the graded recovery of EJP amplitude after shift back to permissive temperature (right). c, Reversible block of EJC in DLM NMJs in rbo mutants. Representative traces at 25°C (1), 36°C (2), and after return to 25°C (3) in both wt (left) and rbo (middle) mutants are shown. d, Quantification of EJC amplitudes; n = 5 for each genotype. e, Quantification of latency of DLM EJPs evoked by brain stimulation in wt and rbo mutants during temperature shift from 25 to 37°C. * and ** indicate significant (p < 0.05) and highly significant (p < 0.01) change, respectively; n = 5 for each genotype. The bars show the mean ± SEM. stim, Stimulation.
These results predict that the DLM should show graded block/recovery if the presumed upstream block in the cholinergic PSI synapse could be bypassed. To test this prediction, we used an extracellular electrode placed in the thoracic ganglion to directly excite the DLM motor neuron axon. As predicted, under this stimulation paradigm, the DLM in rbots1/rbo2 shows graded loss/recovery of EJP amplitude with a time course comparable with the TTM (Fig. 4a, right) (n = 5). Thus, the abrupt loss/recovery of the EJP in the DLM is caused by a central (PSI–DLMn) synaptic defect, superimposed on a simultaneous NMJ transmission blockade. DLM EJPs evoked by brain stimulation require one or more central chemical synaptic relays (Tanouye and Wyman, 1980; Trimarchi and Murphey, 1997; Kawasaki and Ordway, 1999). Impairment of central synaptic transmission results in longer latencies of DLM EJPs (Kawasaki and Ordway, 1999). The presumed block of PSI–DLMn transmission in rbots1 predicts a slowing of AP generation in DLMn and therefore a longer latency of DLM EJP. To test this, we quantified the latencies of DLM EJP in wild type and in rbots1/rbo2. During temperature elevation, both wild-type and mutant DLM EJP latencies first transiently shorten, but then begin to increase at ∼33°C. Above 33–35°C, rbots1/rbo2 latencies are significantly prolonged compared with control (Fig. 4a, left and middle, d).
The EJPs recorded in DLM/TTM are composed of both a synaptically generated postsynaptic potential and a voltage-dependent electrogenic potential (Koenig et al., 1983; Kawasaki and Ordway, 1999). To directly examine synaptic conductance in rbots1 mutants, we performed two-electrode voltage-clamp EJC recordings from DLMe/d cells (Fig. 4d). The cut motor nerve was stimulated via a suction pipette, and muscle cells were clamped at −80 mV. TTX was added to the perfusion solution (0.25 μm) to block action potentials in nerve, allowing direct, passive depolarization of the NMJ terminal. EJCs were evoked by constant 0.1 Hz stimulation, and traces were captured at three time points: (1) the initial responses at 25°C, (2) after 3 min at 36°C, and (3) after return to 25°C (Fig. 4c). At 25°C, EJC amplitude in wild-type and rbots1 mutants were indistinguishable (wt, 3.32 ± 0.12 μA, n = 5; rbots1/rbo2, 3.0 ± 0.29 μA, n = 5) (Fig. 4c). At 36°C, rbots1/rbo2 EJCs are completely abolished, but wild-type EJCs remain robust (2.88 ± 0.16 μA) throughout the entire course of the recording. After a return to 25°C, mutant transmission recovers to 87% of the initial amplitude (2.34 ± 0.23 μA) (Fig. 4c). Together, these results demonstrate that RBO is required in a time course of minutes for both peripheral glutamatergic NMJ and central cholinergic PSI–DLMn synaptic transmission.
RBO is required for synaptic vesicle exocytosis
Our next objective was to determine the mechanistic requirement for RBO in synaptic transmission. We first attempted to perform FM1-43 loading and unloading to assay SV endocytosis and exocytosis, respectively. Unfortunately, we were unsuccessful in stimulating significant dye uptake at the adult DLM/TTM NMJs, presumably because these NMJs are embedded deeply within the muscle fibers (Fig. 2c) and poorly accessible to the applied dye. We therefore directly turned to electron microscopy to perform ultrastructural studies of DLM NMJs of both wild type and rbots1 mutants. EM studies were performed both at permissive room temperature (22°C), in which mutants show no functional defects (Figs. 3, 4), and 10 min after a shift to restrictive temperature (37°C), in which rbots1 mutants lack any detectable neurotransmission (Figs. 3, 4).
At permissive temperature (22°C), the ultrastructure of NMJ terminals in rbots1 mutants and wild-type controls appears essentially indistinguishable (Fig. 5). Both genotypes contain anatomically normal synaptic bouton profiles, active zones, and synaptic vesicles. The synaptic bouton area and the total number of SVs per bouton section are not significantly different (p > 0.05) in the two genotypes (Fig. 5). After a 10 min shift to 37°C, the picture is very different. SV number in rbots1/rbo2 mutants is almost tripled within 10 min (mean SV number per section: 82 ± 16, n = 22 at 22°C vs 241 ± 29, n = 51 at 37°C; p = 0.001) (Fig. 5c,d). At 37°C, SV number in wild type is not changed significantly (Fig. 5d). The conditional increase in SV number is consistent with a block of exocytosis in rbots1 mutants. A similar increase in SV number has been reported in photoreceptor presynaptic terminals in comotase and syntaxin1A TS mutants with a SV exocytosis deficit (Kawasaki et al., 1998; Littleton et al., 1998) and the opposite defect of SV depletion in shibire TS mutant DLM NMJs with a block of SV endocytosis (Koenig et al., 1983). The SV accumulation in rbots1 mutants suggests disruption in the balance between SV fusion and recycling from the plasma membrane, predicting a reduction in the presynaptic terminal membrane area. To test this prediction, NMJ boutons were visualized with anti-synaptotagmin (Fig. 2c) and bouton area quantified with confocal imaging. The temperature shift from 22 to 37°C resulted in a slight reduction of bouton area in rbo mutants (from 0.99 ± 0.02 μm2, n = 224 to 0.92 ± 0.2 μm2, n = 180), but this change was not statistically significant. In EM studies, the largest sections from bouton serial sections containing at least one clearly define synaptic active zone were selected to quantify cross-sectional area. After a 10 min shift to 37°C, the cross-sectional bouton area in rbo mutants was reduced ∼38% (rbots1/rbo2; 4.84 ± 1.37 μm2, n = 22 at 22°C vs 2.98 ± 0.5 μm2, p < 0.05, n = 51). The bouton area of wild-type controls shows no significant change during the same temperature shift. Together, these data support an acute and specific block in SV exocytosis in the absence of RBO function.
Figure 5.
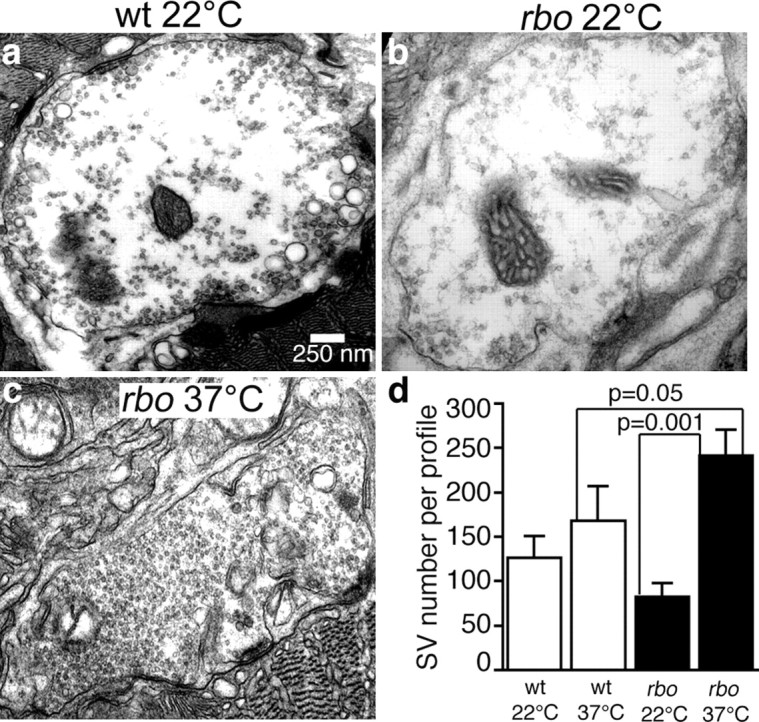
Conditional block of synaptic vesicle exocytosis in rbo mutants. a, Representative electron micrograph of a DLM NMJ synaptic bouton from wt at room temperature (22°C). b, Representative bouton of rbo mutant (rbots1/rbo2) at 22°C. Note that SVs tend to be distributed toward active zones. c, Representative bouton of rbo mutant after a 10 min shift to 37°C. Note dramatic increase in SV density. d, Quantification of SV number per bouton profile. The bars show the mean ± SEM.
To determine the stage of the putative RBO-dependent SV exocytosis block, we next quantified SV density relative to the presynaptic AZ (Fig. 6). AZs in wild-type animals contain one or two SVs in contact (docked) with the electron-dense AZ membrane (Fig. 6a, arrowhead). The rbots1 mutants at permissive temperature (22°C) show a similar profile. In contrast, after 10 min shift to 37°C, there is a significant accumulation of docked SVs in rbots1/rbo2 mutants (SVs per AZ, 4.1 ± 0.1) when compared with both wild type at 37°C (1.8 ± 0.14; p = 0.0001) and with rbots1/rbo2 mutants at 22°C (2.0 ± 0.2; p = 0.0001) (Fig. 6). Typically, all available electron-dense AZ membrane in the mutant appears occupied with docked SVs (Fig. 6a), suggesting that SV docking sites may be nearly or completely saturated. The accumulation of docked SVs is similar to that reported for comatose (DLM NMJs and photoreceptor synapses) and syntaxin (photoreceptor synapses) TS mutants with SV priming or fusion defects (Kawasaki et al., 1998; Littleton et al., 1998). This result further supports the existence of an exocytosis defect in rbots1 mutants and suggests that RBO is required at a postdocking stage of SV exocytosis, during vesicle priming, or fusion stages.
Figure 6.
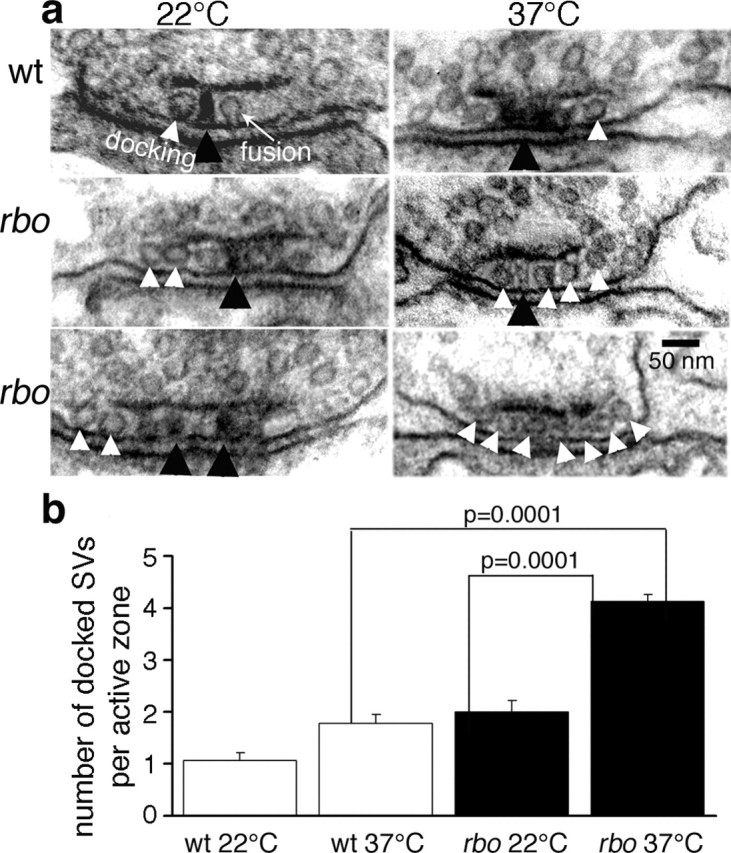
Docked synaptic vesicles arrest at the active zone in rbo mutants. a, Representative electron micrographs of presynaptic AZ in wild type (top) and rbo (rbots1/rbo2) mutants (middle and bottom) at 22°C (left column) and after a 10 min shift to 37°C (right column). The electron-dense AZ T-bar (black arrowheads) and morphologically docked SVs (white arrowheads) are indicated. b, Quantification of the number of docked SVs per AZ. The bars show the mean ± SEM.
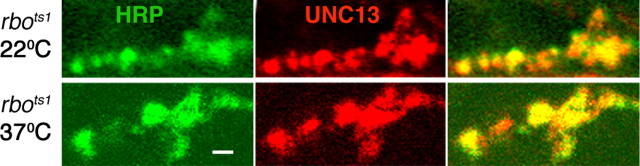
SV priming is dependent on UNC-13, which can be recruited to plasma membrane by binding DAG (Brose and Rosenmund, 2002). We previously reported an acute, activity-dependent depletion of DAG in the brains of rbots1 mutants (Huang et al., 2004) and previously reported a dunc-13 docked SV accumulation phenotype very similar to the rbots1 phenotype reported here (Aravamudan et al., 1999). To test whether the block of SV exocytosis in rbots1 may correlate with mislocalization of dUNC-13, DLM synaptic boutons were stained with a dUNC-13 antibody and confocal imaging used to assay dUNC-13 expression (Fig. 7). The rbots1 mutants at permissive temperature (22°C) showed dUNC-13 expression indistinguishable from wild-type controls. After 10 min shift to 37°C, there was no detectable change in either the expression intensity or the bouton distribution of dUNC-13 (Fig. 7). This negative result might be because of the limited resolution of confocal imaging.
Figure 7.
No detectable alteration of dUNC-13 distribution in rbo mutants. Adult DLM NMJ synaptic boutons double-labeled with anti-HRP (neuronal membrane; green) and an antibody to dUNC-13 (red) are shown. Representative images from rbo (rbots1/rbo2) mutants at permissive temperature (22°C, top) and after a 10 min shift to 37°C (bottom) are shown. Scale bar, 1.0 μm.
RBO interacts with the syntaxin T-SNARE
To investigate the molecular mechanism underlying the RBO requirement in synaptic transmission, we next generated double mutants of rbots1 and other Drosophila TS synaptic mutants. TS mutants tested in combination with rbo included the cacophony presynaptic Ca2+ channel (cacts2) (Dellinger et al., 2000), the syntaxin1A T-SNARE (syx3–69) (Littleton et al., 1998), the comatose NSF ATPase (comtts17, comtts53) (Siddiqi and Benzer, 1976), and the shibire dynamin GTPase (shibirets1) (Grigliatti et al., 1973). To test for genetic interactions, behavioral paralysis was assayed at an intermediate temperature of 33°C, at which rbo single mutants (e.g., rbots1/rbots1, rbots1/rbo2) are only slowly paralyzed over a time course of ∼30 min. For the first 10 min at 33°C, rbo homozygous mutants remain fully upright and mobile (Fig. 8a), thereby providing a sensitized condition in which to assay double homozygotes for accelerated paralysis. Under these conditions, rbo displays no obvious synergistic interaction with cacophony, comatose, or shibire mutants (data not shown). In contrast, there is a strong synergistic interaction between rbots1 and the syx3–69 mutation. To control for genetic background contributions, both rbots1/rbots1; syx3–69/syx3–69 and rbots1/rbo2; syx3–69/syx3–69 mutants were examined. Both types of double mutants showed an indistinguishable synergistic genetic interaction and are pooled together in Figure 8a.
Figure 8.
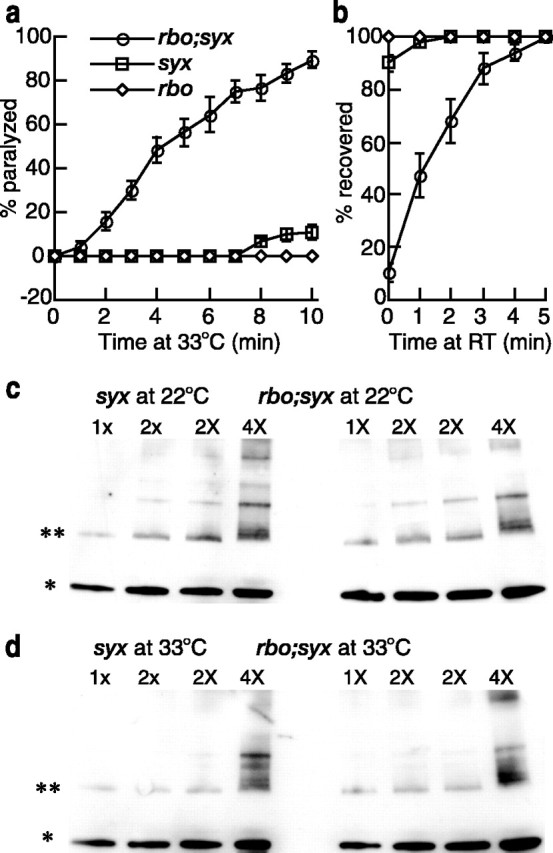
RBO genetically interacts with syntaxin. a, b, The kinetics of TS paralysis at 33°C (a) and recovery from paralysis at room temperature (22°C) (b) in rbo (rbots1/rbo2 and rbots1/rbots1) and syx (syx3–69/syx3–69) single mutants and rbo; syx (rbots1/rbo2; syx3–69/syx3–69) double mutants. The rbo and syx mutants display a strong synergistic interaction. Error bars represent SEM. c, d, Representative anti-syntaxin1A Western blots of SNARE complex abundance in syx single mutants and rbo; syx double mutants. Analyses at room temperature (22°C) (c) and after a 10 min shift to 33°C (d) is shown. 1x, 2x, and 4x indicate the loading amount of total plasma membrane protein determined by absorbance measurements at 280 nm. Equal amount of total membrane protein from syx single mutants and rbo; syx double mutants were loaded. The bands indicated by * and ** represent syntaxin monomer and 7S SNARE complex, respectively.
After 10 min at 33°C, syx3–69 homozygotes display only ∼10% paralysis. Paralyzed syx3–69 flies completely recover to an upright position within 1–2 min after return to room temperature. In contrast, rbots1; syx3–69 double mutants rapidly paralyze at 33°C, with ∼90% of mutants completely paralyzed within 10 min (Fig. 8a), and ∼5 min are required for all paralyzed rbots1; syx3–69 double mutants to regain an upright position after return to room temperature (Fig. 8b). These observations suggest that RBO and syntaxin may act in the same pathway, or parallel pathways, required for neurotransmission.
RBO may act downstream of SNARE complex assembly
The syx3–69 allele is a missense mutation that causes temperature-dependent disruption of syntaxin1A binding to SNAP25 and synaptobrevin, and hence a defect in the formation of SNARE complex (Littleton et al., 1998). Homozygous syx3–69 mutants display reduced SNARE complex formation at room temperature and no detectable SNARE complex formation after 20 min at 38°C (Littleton et al., 1998). Therefore, the synergistic genetic interaction between rbots1 and syx3–69 mutations in behavioral paralysis could be attributable to an additive negative effect on SNARE complex assembly or to a downstream syntaxin-dependent mechanism of SV fusion. To test these possibilities, we assayed the abundance of SNARE complex in syx3–69 mutants and rbots1/rbo2; syx3–69/syx3–69 double mutants at both room temperature and 33°C.
The ternary (7S) SNARE complex, composed of one molecule each of syntaxin, SNAP25, and synaptobrevin, is SDS resistant and migrates at ∼73 kDa in SDS-PAGE (Littleton et al., 1998; Tolar and Pallanck, 1998). Equal amounts of total membrane proteins from brain extracts of both groups of mutants were subjected to SDS-PAGE and Western blot analyses with an antibody against syntaxin1A (8C3) (Fig. 8c,d). The 7S SNARE complex and syntaxin monomer abundance in rbots1/rbo2;syx3–69/syx3–69 were normalized to syx3–69/syx3–69 of the same blot. At room temperature, double mutants contain 1.28 ± 0.15 (n = 10) and 1.16 ± 0.06 (n = 8) of 7S SNARE and syntaxin1A monomers, respectively. After 10 min at 33°C, normalized quantification shows that the double mutants still contain similar relative levels of 7S SNARE complex (1.13 ± 0.10; n = 20) and syntaxin1A monomers (1.23 ± 0.07; n = 19). Thus, no additive negative effect on SNARE complex assembly was detected in double mutants. We also compared SNARE complex abundance in rbots1/rbo2 and wild type (at 22 and 37°C) and did not observe any significant difference between mutants and controls (data not shown). Together, these results suggest that the rbo defect in SV exocytosis likely occurs downstream of SNARE complex assembly.
Discussion
RBO functions at a postdocking step in SV exocytosis
RBO is a predicted integral plasma membrane lipase (Huang et al., 2004). The protein is highly enriched within the nervous system and is subcellularly restricted within central neurons primarily to synaptic domains. At neuromuscular synapses, RBO is predominantly localized to the plasma membrane within presynaptic boutons. The protein may also be present in the postsynaptic compartment, but we are currently unable to resolve this clearly at a confocal level. Conditional rbo mutants paralyze within minutes and display a complete block of synaptic transmission within minutes. This functional block correlates with a sharp increase in SV number within presynaptic boutons and a concomitant shrinkage of presynaptic plasma membrane area. These acute changes appear to arise from the disruption of the balance between SV consumption (exocytosis) and recycling by SV formation (endocytosis). Similar SV accumulation has been reported only in mutants with defective SV fusion, including comatose and syntaxin TS mutants (Kawasaki et al., 1998; Littleton et al., 1998). In rbo TS mutants, docked vesicles accumulate at presynaptic active zones within minutes. This defect is most consistent with a postdocking block of SV priming/fusion. However, because docking may be proportional to overall SV pool size (Weimer et al., 2003), the elevation in SV number might also contribute to the increased number of docked SVs.
Conditional TS paralytic mutations of rbo and syntaxin1A (syx3–69) produce a strong synergistic genetic interaction. Among the pool of TS mutants tested, this interaction appears quite specific to syntaxin. Interactions were not observed between rbo and TS mutant affecting presynaptic Ca2+ influx, SNARE complex disassembly, or SV recycling. The rbo–syx interaction agrees well with the EM characterization indicating a requirement for RBO in postdocking SV exocytosis. The syx3–69 mutants display a temperature-dependent loss of SNARE complexes (Littleton et al., 1998). In rbots1; syx3–69 double mutants, we found no further reduction of SNARE complex assembly. The assay included both trans- and cis-SNARE complexes, making it hard to correlate SNARE complex abundance with functional defects. Nevertheless, the absence of a discernable change in SNARE complex abundance in rbo mutants suggests that RBO is unlikely to function directly in SNARE assembly/disassembly. Together, these data therefore suggest that RBO likely acts either downstream of SNARE complex assembly or in an unknown parallel pathway leading to SV fusion.
The acute requirement for RBO protein appears to be limited to a subset of synapses; larval NMJ synapses do not show the same requirement. One possible explanation may be functional redundancy or differences in synaptic thermal regulation between larval and adult synapses. For unknown reasons, larva NMJ neurotransmission has proven consistently more resistant to disruption by Drosophila TS mutants than the adult (Suzuki et al., 1971; Siddiqi and Benzer, 1976). A second possibility is that RBO may be acutely required at synapses designed to function reliably under conditions of high demand. The central cholinergic and NMJ synapses in the adult Drosophila GF circuit can support 100 Hz synaptic transmission (Tanouye and Wyman, 1980; Engel and Wu, 1992; Allen et al., 2000), far beyond the usage or sustainable range of the larval NMJ.
A role of RBO in SNARE-dependent synaptic vesicle fusion?
It has been well demonstrated that SNARE complex assembly is essential for vesicle priming and can directly mediate membrane fusion (Weber et al., 1998; Jahn and Sudhof, 1999; Lin and Scheller, 2000; Sudhof, 2004). In addition, however, studies of yeast vacuolar homotypic fusion (Peters et al., 1999, 2001; Muller et al., 2002; Bayer et al., 2003) and direct studies of exocytosis of neurotransmitter vesicles including SVs (Ciufo et al., 2005; Hiesinger et al., 2005) suggest that an additional machinery may act downstream of the SNARE complex to mediate fusion. Our data suggest that RBO may similarly act downstream of SNARE complex assembly. We propose that RBO may regulate the function of the SV fusion machinery or may be a novel component of this fusion machinery.
The closest characterized homolog of RBO (42% conserved) is an integral plasma membrane sn-1 DAG lipase (Bisogno et al., 2003; Huang et al., 2004). We reported recently that RBO is essential for PLC-dependent neuronal signaling (Huang et al., 2004). Consistently, after a 10 min shift to 37°C, rbo TS mutants display an accumulation of PIP2 and concomitant reduction of DAG in the brain (Huang et al., 2004). Synaptically localized RBO therefore may regulate the levels of fusogenic lipids (DAG, phosphatidylinositides, polyunsaturated fatty acids) at, or near, AZ fusion sites. These critical lipids may contribute directly to the generation of membrane properties required for SV fusion. Alternatively, these lipids might regulate the activity of lipid-binding fusogenic proteins.
Both lipid partitioning and protein interactions regulate membrane changes to enable fusion. Lipids with compact head groups and space-filling tails, such as PIP2 and DAG, favor the negative membrane curvature required for vesicle fusion, and both PIP2 and DAG directly promote Ca2+-dependent exocytosis (Hay et al., 1995; Mayer et al., 2000; Di Paolo et al., 2004). Lipases including PLC, PLD, and PLA2 are known to promote secretion through the fusogenic effects of their lipid products (Cohen and Brown, 2001; Vitale et al., 2001; Brown et al., 2003; Wei et al., 2003; Staneva et al., 2004). These lipases have been proposed to increase presynaptic release site availability and/or vesicle fusion efficacy. These activities also coordinate the spatial–temporal regulation of numerous synaptic proteins (Wenk and De Camilli, 2004). SV priming is dependent on UNC-13, which binds DAG (Aravamudan et al., 1999). PIP2 and DAG are required for the localized enrichment of SNARES and Rab GTPases at fusion sites (Jun et al., 2004). Other known targets of PIP2 and DAG include synaptotagmin 1 and MUNC18-interacting MINT1,2 (Cremona and De Camilli, 2001; Wenk and De Camilli, 2004). Thus, phosphoinositides may play multiple roles in the formation of the SV fusion domain: directly determining membrane properties, serving as a precursor for other fusogenic lipids (DAG), and serving as anchors/regulators for fusogenic proteins.
Conditional removal of RBO activity in TS mutants causes acute DAG depletion and PIP2 accumulation in the brain (Huang et al., 2004). These changes correlate temporally with the loss of neurotransmission and the arrest of docked SVs at presynaptic AZs. We have yet to develop the technology to determine whether these lipid changes occur locally at the AZ, but the synaptic localization of RBO and the acute requirement of RBO in postdocking SV exocytosis supports this conclusion. Therefore, RBO is proposed to function as a presynaptic phospholipase that modulates the PIP2–DAG pathway to regulate SV fusion. Ongoing studies are aimed at determining the nature of this requirement and how RBO activity is regulated to control SV fusion efficacy and thereby neurotransmission strength.
Footnotes
This work was supported entirely by National Institutes of Health Grant GM54544 to K.B. We are particularly grateful to Sean Speese for imaging assistance and to Emma Rushton, Jeff Rohrbough, and Todd Graham for advice and critical comments. We thank the following for generously providing stocks or antibodies used in this study: Hugo Bellen, Vivian Budnik, Barry Ganetzky, Tom Jongens, Richard Ordway, Leo Pallanck, and Konrad Zinsmaier. As always, we are indebted to the Bloomington Drosophila Stock Center and the Iowa Developmental Studies Hybridoma Bank.
References
- Allen MJ, Shan X, Murphey RK (2000). A role for Drosophila Drac1 in neurite outgrowth and synaptogenesis in the giant fiber system. Mol Cell Neurosci 16:754–765. [DOI] [PubMed] [Google Scholar]
- Aravamudan B, Broadie K (2003). Synaptic Drosophila UNC-13 is regulated by antagonistic G-protein pathways via a proteosome-dependent degradation mechanism. J Neurobiol 54:417–438. [DOI] [PubMed] [Google Scholar]
- Aravamudan B, Fergestad T, Davis WS, Rodesch CK, Broadie K (1999). Drosophila UNC-13 is essential for synaptic transmission. Nat Neurosci 2:965–971. [DOI] [PubMed] [Google Scholar]
- Bayer MJ, Reese C, Buhler S, Peters C, Mayer A (2003). Vacuole membrane fusion: V0 functions after trans-SNARE pairing and is coupled to the Ca2+-releasing channel. J Cell Biol 162:211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P (2003). Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol 163:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadie K (2000). Functional assays of the peripheral and central nervous systems. In: Drosophila protocols (Sullivan WAM, Hawley RS, eds) , pp. 297–312. New York: Cold Spring Harbor Laboratory.
- Brose N, Rosenmund C (2002). Move over protein kinase C, you’ve got company: alternative cellular effectors of diacylglycerol and phorbol esters. J Cell Sci 115:4399–4411. [DOI] [PubMed] [Google Scholar]
- Brown WJ, Chambers K, Doody A (2003). Phospholipase A2 (PLA2) enzymes in membrane trafficking: mediators of membrane shape and function. Traffic 4:214–221. [DOI] [PubMed] [Google Scholar]
- Chandrashekaran S (1993). Mutations at the stm A locus of Drosophila melanogaster confer resistance to the sodium channel neuropoison veratridine. Curr Sci 65:80–82. [Google Scholar]
- Ciufo LF, Barclay JW, Burgoyne RD, Morgan A (2005). Munc18-1 regulates early and late stages of exocytosis via syntaxin-independent protein interactions. Mol Biol Cell 16:470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JS, Brown HA (2001). Phospholipases stimulate secretion in RBL mast cells. Biochemistry 40:6589–6597. [DOI] [PubMed] [Google Scholar]
- Cremona O, De Camilli P (2001). Phosphoinositides in membrane traffic at the synapse. J Cell Sci 114:1041–1052. [DOI] [PubMed] [Google Scholar]
- Dellinger B, Felling R, Ordway RW (2000). Genetic modifiers of the Drosophila NSF mutant, comatose, include a temperature-sensitive paralytic allele of the calcium channel alpha1-subunit gene, cacophony. Genetics 155:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G, Moskowitz HS, Gipson K, Wenk MR, Voronov S, Obayashi M, Flavell R, Fitzsimonds RM, Ryan TA, De Camilli P (2004). Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature 431:415–422. [DOI] [PubMed] [Google Scholar]
- Engel JE, Wu CF (1992). Interactions of membrane excitability mutations affecting potassium and sodium currents in the flight and giant fiber escape systems of Drosophila. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 171:93–104. [DOI] [PubMed] [Google Scholar]
- Fergestad T, Broadie K (2001). Interaction of stoned and synaptotagmin in synaptic vesicle endocytosis. J Neurosci 21:1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratti RA, Jun Y, Merz AJ, Margolis N, Wickner W (2004). Interdependent assembly of specific regulatory lipids and membrane fusion proteins into the vertex ring domain of docked vacuoles. J Cell Biol 167:1087–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goni FM, Alonso A (1999). Structure and functional properties of diacylglycerols in membranes. Prog Lipid Res 38:1–48. [DOI] [PubMed] [Google Scholar]
- Grigliatti TA, Hall L, Rosenbluth R, Suzuki DT (1973). Temperature-sensitive mutations in Drosophila melanogaster. XIV. A selection of immobile adults. Mol Gen Genet 120:107–114. [DOI] [PubMed] [Google Scholar]
- Hardie RC (2003). Regulation of trp channels via lipid second messengers. Annu Rev Physiol 65:735–759. [DOI] [PubMed] [Google Scholar]
- Hay JC, Fisette PL, Jenkins GH, Fukami K, Takenawa T, Anderson RA, Martin TF (1995). ATP-dependent inositide phosphorylation required for Ca2+-activated secretion. Nature 374:173–177. [DOI] [PubMed] [Google Scholar]
- Hiesinger PR, Fayyazuddin A, Mehta SQ, Rosenmund T, Schulze KL, Zhai RG, Verstreken P, Cao Y, Zhou Y, Kunz J, Bellen HJ (2005). The v-ATPase V0 subunit a1 is required for a late step in synaptic vesicle exocytosis in Drosophila. Cell 121:607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang FD, Matthies HJ, Speese SD, Smith MA, Broadie K (2004). Rolling blackout, a newly identified PIP2-DAG pathway lipase required for Drosophila phototransduction. Nat Neurosci 7:1070–1078. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Koenig JH (1988). Morphological identification of the motor neurons innervating the dorsal longitudinal flight muscle of Drosophila melanogaster. J Comp Neurol 273:436–444. [DOI] [PubMed] [Google Scholar]
- Jackson FR, Wilson SD, Hall LM (1986). The tip-E mutation of Drosophila decreases saxitoxin binding and interacts with other mutations affecting nerve membrane excitability. J Neurogenet 3:1–17. [DOI] [PubMed] [Google Scholar]
- Jahn R, Sudhof TC (1999). Membrane fusion and exocytosis. Annu Rev Biochem 68:863–911. [DOI] [PubMed] [Google Scholar]
- Jun Y, Fratti RA, Wickner W (2004). Diacylglycerol and its formation by phospholipase C regulate Rab- and SNARE-dependent yeast vacuole fusion. J Biol Chem 279:53186–53195. [DOI] [PubMed] [Google Scholar]
- Kawasaki F, Ordway RW (1999). The Drosophila NSF protein, dNSF1, plays a similar role at neuromuscular and some central synapses. J Neurophysiol 82:123–130. [DOI] [PubMed] [Google Scholar]
- Kawasaki F, Mattiuz AM, Ordway RW (1998). Synaptic physiology and ultrastructure in comatose mutants define an in vivo role for NSF in neurotransmitter release. J Neurosci 18:10241–10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F, Felling R, Ordway RW (2000). A temperature-sensitive paralytic mutant defines a primary synaptic calcium channel in Drosophila. J Neurosci 20:4885–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y, Kuromi H, Delgado R, Maureira C, Oliva C, Labarca P (2004). Synaptic vesicle pools and plasticity of synaptic transmission at the Drosophila synapse. Brain Res Brain Res Rev 47:18–32. [DOI] [PubMed] [Google Scholar]
- King DG, Wyman RJ (1980). Anatomy of the giant fibre pathway in Drosophila. I. Three thoracic components of the pathway. J Neurocytol 9:753–770. [DOI] [PubMed] [Google Scholar]
- Koenig JH, Ikeda K (1996). Synaptic vesicles have two distinct recycling pathways. J Cell Biol 135:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JH, Saito K, Ikeda K (1983). Reversible control of synaptic transmission in a single gene mutant of Drosophila melanogaster. J Cell Biol 96:1517–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RC, Scheller RH (2000). Mechanisms of synaptic vesicle exocytosis. Annu Rev Cell Dev Biol 16:19–49. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Chapman ER, Kreber R, Garment MB, Carlson SD, Ganetzky B (1998). Temperature-sensitive paralytic mutations demonstrate that synaptic exocytosis requires SNARE complex assembly and disassembly. Neuron 21:401–413. [DOI] [PubMed] [Google Scholar]
- Loughney K, Kreber R, Ganetzky B (1989). Molecular analysis of the para locus, a sodium channel gene in Drosophila. Cell 58:1143–1154. [DOI] [PubMed] [Google Scholar]
- Martin TF (2001). PI(4,5)P(2) regulation of surface membrane traffic. Curr Opin Cell Biol 13:493–499. [DOI] [PubMed] [Google Scholar]
- Mayer A, Scheglmann D, Dove S, Glatz A, Wickner W, Haas A (2000). Phosphatidylinositol 4,5-bisphosphate regulates two steps of homotypic vacuole fusion. Mol Biol Cell 11:807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller O, Bayer MJ, Peters C, Andersen JS, Mann M, Mayer A (2002). The Vtc proteins in vacuole fusion: coupling NSF activity to V(0) trans-complex formation. EMBO J 21:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordway RW, Pallanck L, Ganetzky B (1994). Neurally expressed Drosophila genes encoding homologs of the NSF and SNAP secretory proteins. Proc Natl Acad Sci USA 91:5715–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis P, Tanouye MA (1995). Seizures and failures in the giant fiber pathway of Drosophila bang-sensitive paralytic mutants. J Neurosci 15:5810–5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C, Andrews PD, Stark MJ, Cesaro-Tadic S, Glatz A, Podtelejnikov A, Mann M, Mayer A (1999). Control of the terminal step of intracellular membrane fusion by protein phosphatase 1. Science 285:1084–1087. [DOI] [PubMed] [Google Scholar]
- Peters C, Bayer MJ, Buhler S, Andersen JS, Mann M, Mayer A (2001). Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature 409:581–588. [DOI] [PubMed] [Google Scholar]
- Rao SS, Stewart BA, Rivlin PK, Vilinsky I, Watson BO, Lang C, Boulianne G, Salpeter MM, Deitcher DL (2001). Two distinct effects on neurotransmission in a temperature-sensitive SNAP-25 mutant. EMBO J 20:6761–6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie JM (1979). A pharmacological approach to the structure of sodium channels in myelinated axons. Annu Rev Neurosci 2:341–362. [DOI] [PubMed] [Google Scholar]
- Rohrbough J, Broadie K (2005). Lipid regulation of the synaptic vesicle cycle. Nat Rev Neurosci 6:139–150. [DOI] [PubMed] [Google Scholar]
- Siddiqi O, Benzer S (1976). Neurophysiological defects in temperature-sensitive paralytic mutants of Drosophila melanogaster. Proc Natl Acad Sci USA 73:3253–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staneva G, Angelova MI, Koumanov K (2004). Phospholipase A2 promotes raft budding and fission from giant liposomes. Chem Phys Lipids 129:53–62. [DOI] [PubMed] [Google Scholar]
- Sudhof TC (2004). The synaptic vesicle cycle. Annu Rev Neurosci 27:509–547. [DOI] [PubMed] [Google Scholar]
- Suzuki DT, Grigliatti T, Williamson R (1971). Temperature-sensitive mutations in Drosophila melanogaster. VII. A mutation (para-ts) causing reversible adult paralysis. Proc Natl Acad Sci USA 68:890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Wu CF (1984). Altered sensitivity to sodium channel-specific neurotoxins in cultured neurons from temperature-sensitive paralytic mutants of Drosophila. J Neurogenet 1:225–238. [DOI] [PubMed] [Google Scholar]
- Tanouye MA, Wyman RJ (1980). Motor outputs of giant nerve fiber in Drosophila. J Neurophysiol 44:405–421. [DOI] [PubMed] [Google Scholar]
- Tolar LA, Pallanck L (1998). NSF function in neurotransmitter release involves rearrangement of the SNARE complex downstream of synaptic vesicle docking. J Neurosci 18:10250–10256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi JR, Murphey RK (1997). The shaking-B2 mutation disrupts electrical synapses in a flight circuit in adult Drosophila. J Neurosci 17:4700–4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker WC, Weber T, Chapman ER (2004). Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science 304:435–438. [DOI] [PubMed] [Google Scholar]
- van der Bliek AM, Meyerowitz EM (1991). Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature 351:411–414. [DOI] [PubMed] [Google Scholar]
- Vijayakrishnan N, Broadie KTemperature-sensitive paralytic mutants: insights into the synaptic vesicle cycle. Biochem Soc Trans 34:81–7. [DOI] [PubMed] [Google Scholar]
- Vitale N, Caumont AS, Chasserot-Golaz S, Du G, Wu S, Sciorra VA, Morris AJ, Frohman MA, Bader MF (2001). Phospholipase D1: a key factor for the exocytotic machinery in neuroendocrine cells. EMBO J 20:2424–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE (1998). SNAREpins: minimal machinery for membrane fusion. Cell 92:759–772. [DOI] [PubMed] [Google Scholar]
- Wei S, Ong WY, Thwin MM, Fong CW, Farooqui AA, Gopalakrishnakone P, Hong W (2003). Group IIA secretory phospholipase A2 stimulates exocytosis and neurotransmitter release in pheochromocytoma-12 cells and cultured rat hippocampal neurons. Neuroscience 121:891–898. [DOI] [PubMed] [Google Scholar]
- Weimer RM, Richmond JE, Davis WS, Hadwiger G, Nonet ML, Jorgensen EM (2003). Defects in synaptic vesicle docking in unc-18 mutants. Nat Neurosci 6:1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk MR, De Camilli P (2004). Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proc Natl Acad Sci USA 101:8262–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CF, Ganetzky B (1980). Genetic alteration of nerve membrane excitability in temperature-sensitive paralytic mutants of Drosophila melanogaster. Nature 286:814–816. [DOI] [PubMed] [Google Scholar]