Abstract
The neurological mouse mutant strain reeler displays abnormal laminar organization of several brain structures as a consequence of a defect in cell migration during neurodevelopment. This phenotype is a result of the disruption of reelin, a gene encoding a protein that has several structural characteristics of extracellular matrix proteins. To understand the molecular basis of the action of Reelin on neuronal migration, we constructed a full-lengthreelin clone and used it to direct Reelin expression. Here, we demonstrate that Reelin is a secreted glycoprotein and that a highly charged C-terminal region is essential for secretion. In addition, we demonstrate that an amino acid sequence present in the N-terminal region of Reelin contains an epitope that is recognized by the CR-50 monoclonal antibody. CR-50 was raised against an antigen expressed in normal mouse brain that is absent in reelermice. The interaction of CR-50 with its epitope leads to the disruption of neural cell aggregation in vitro. Here, we used CR-50 to precipitate Reelin from reticulocyte extracts programmed withreelin mRNA, from cells transfected withreelin clones, and from cerebellar explants. Thereelin gene product seems to function as an instructive signal in the regulation of neuronal migration.
Keywords: reeler, cerebral cortex, cerebellum, extracellular matrix, glycosylation, mutant mice, neuronal migration
reeler (rl) is a mouse autosomal recessive mutation that affects the organization of the developing brain (Caviness and Rakic, 1978; Goffinet, 1984; Rakic and Caviness, 1995). The most striking aspects of the reelerphenotype are the abnormal positioning of neurons throughout the cerebral cortex, the cerebellum, and the hippocampus and the aberrant orientation of cell bodies and fibers. In the cerebral cortex ofreeler mice, neurons generated early in development, normally destined to form the subplate, occupy ectopic positions in superficial cortical layers. Neurons generated at later stages, destined to form the cortical plate, migrate radially but fail to bypass previously generated neurons. Thus, the pattern of neocortical histogenesis in reeler mice seems to be inverted. Thereeler cerebellum is considerably smaller than normal, and it lacks foliation and a Purkinje cell layer (Mariani et al., 1977;Mikoshiba et al., 1980; Goffinet et al., 1984). Neurons positioned abnormally also are encountered in the hippocampus (Caviness, 1973;Stanfield and Cowan, 1979). Although neurogenesis and neuronal connectivity primarily are preserved, brain function is impaired inreeler mice, and they exhibit severe motor defects, including tremors, ataxia, and difficulty in balance and locomotion (Falconer, 1951).
Neuroanatomical analysis suggested that the gene affected by thereeler mutation is critical for the appropriate migration of postmitotic neurons (Goffinet, 1984; Caviness et al., 1988). The recent cloning of reelin, the gene mutated in reelermice (D’Arcangelo et al., 1995), has provided new insights into the molecular mechanisms underlying the reeler defects (Goffinet, 1995; Rakic and Caviness, 1995; Rugarli and Ballabio, 1995).reelin encodes a protein of 3461 amino acids with several features of extracellular molecules, including a cleavable signal peptide, several potential glycosylation sites, and EGF-like repeats.
In two strains of reeler, rl (Falconer, 1951) andrltg (Miao et al., 1994), no reelinmRNA was detected (D’Arcangelo et al., 1995). The defect inrl originates from a 150 kb deletion in thereelin gene (Bar et al., 1995; D’Arcangelo et al., 1995), whereas in rltg it arises from an intragenic deletion of reelin sequences associated with the insertion of a transgene (Miao et al., 1994; D’Arcangelo et al., 1995). In a third strain, rlorl (Guenet, 1981), a small deletion in reelin was described that affects the 3′ end of the coding sequence (Curran et al., 1995; Hirotsune et al., 1995). This deletion results from exon skipping as a consequence of the transposition of an active L1 sequence into the gene (Takahara et al., 1996).
In situ hybridization studies revealed thatreelin is expressed in a complex pattern during neurodevelopment (D’Arcangelo et al., 1995). In the embryonic cerebral cortex, reelin mRNA is expressed specifically by a subset of neurons in the most superficial layer, the Cajal–Retzius cells, which are among the first neurons to differentiate in the brain (Marin-Padilla and Marin-Padilla, 1982; Derer and Derer, 1990;D’Arcangelo et al., 1995; Hirotsune et al., 1995). In the cerebellum,reelin mRNA is expressed very highly in the granule cell layers (D’Arcangelo et al., 1995).
The distribution of reelin mRNA is very similar to that recently reported for an epitope recognized by the CR-50 monoclonal antibody. Generated by immunizing reeler mice with normal brain homogenate, this antibody interferes with the pattern of aggregation of neocortical cells in vitro (Ogawa et al., 1995). In the present study, we generated full-length and truncatedreelin cDNA clones to investigate the biochemical properties of Reelin and its relationship to the CR-50 epitope.
MATERIALS AND METHODS
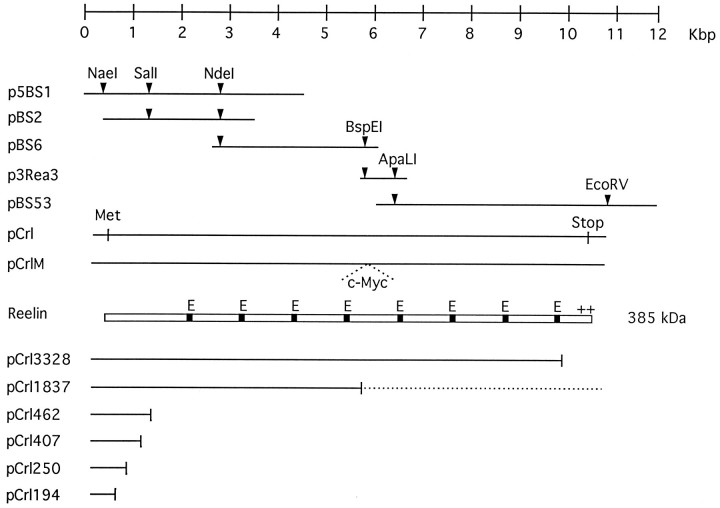
Construction of reelin clones. The entirereelin open reading frame was assembled by fusing five overlapping cDNA clones isolated previously (D’Arcangelo et al., 1995) and subcloning them into the vector pcDNA3 (Invitrogen, San Diego, CA). This vector allows mammalian expression from the human cytomegalovirus (CMV) promoter or in vitro transcription from the T7 promoter. The following reelin fragments were used: 1.2 kbNaeI–SalI from p5′BS1, 1.3 kbSalI–NdeI from pBS2, 3.1 kbNdeI–BspEI from pBS6, 770 bpBspEI–ApaLI from p3Rea3, and 4.2 kbApaLI-EcoRV from pBS53. The final clone (pCrl) contains the entire reelin open reading frame (10,383 bp) plus 95 bp of sequence 5′ to the initiator methionine codon and 82 bp of 3′ untranslated sequence (reelin cDNA nucleotides 188–10,748). To produce an epitope-tagged version of Reelin (pCrlM), we annealed oligonucleotides encoding the human c-Myc epitope 9E10 (QKLISEEDLN) flanked by BspEI restriction site sequences and inserted them, in frame, into the unique BspEI site present in pCrl. To generate a truncated pCrl3328 clone lacking the C terminus, we eliminated a 500 bp XhoI fragment from pCrl. Other expression constructs contain reelin cDNA nucleotides 188–865 (pCrl194), 188–1029 (pCrl250), 188–1505 (pCrl407), and 188–1664 (pCrl462) cloned into pcDNA3. pCrl1837 is similar to pCrlM except for the orientation of the c-Myc epitope in the BspEI site, which is inverted, thus creating a stop codon immediately after nucleotide 5789. The nucleotide sequence of reelin has been deposited in GenBank; the accession number is U24703.
In vitro transcription/translation. Expression plasmids were transcribed, and the resulting RNA was translated in vitro via a reticulocyte lysate transcription/translation system according to the manufacturer’s instructions (TnT, Promega, Madison, WI). DNA (2 μg) was transcribed in a 50 μl reaction mix with T7 RNA polymerase. Translation was performed in the presence of 40 μCi of [35S]methionine or a mix of [35S]methionine and [35S]cysteine in the absence of unlabeled methionine. One microliter of the reaction mix was diluted in 100 μl of SDS sample buffer and subjected to SDS-PAGE. The remainder of the reaction mix was diluted in RIPA buffer (50 mm Tris, pH 8, 150 mm NaCl, 1% Nonidet P40, 0.5% Na deoxycholate, and 0.1% SDS) for immunoprecipitation analysis.
COS cell transfection. COS-7 cells (4 × 105) were plated in 60 mm dishes in the presence of 5 ml of DMEM (BioWhittaker, Walkersville, MD) supplemented with 10% fetal bovine serum (Life Technologies, Gaithersburg, MD) and incubated overnight. On the next day, the medium was replaced with Opti-MEM (Life Technologies), and the cells were transfected with the LipofectAMINE reagent (Life Technologies) according to the manufacturer’s instructions. After 5–7 hr of transfection in 2 ml of Opti-MEM, 2 ml of DMEM containing 20% fetal bovine serum was added, and the cultures were incubated overnight. On the next day, the medium was replaced with fresh DMEM containing 10% serum. After one additional day, cells were examined by immunofluorescence analysis, or they were prepared for immunoprecipitation analysis as described below.
Cerebellar primary cultures. The cerebellum was removed by dissection from postnatal day 5–8 (P5–P8) normal or reelerB6C3Fe mice, which were generated as the progeny of a rlheterozygous cross (Jackson Laboratory, Bar Harbor, ME). Then the tissue was chopped finely with a razor blade and triturated in a plastic pipette. Cells were resuspended in basal Eagle’s medium supplemented as described (Fischer, 1982), with 5% horse serum (HyClone, Logan, UT). Explants or dispersed cells (2 × 105 cells/cm2) were plated in tissue culture dishes that had been precoated with 20 μg/ml poly-l-lysine. The cultures were switched to serum-free medium 1 d after plating and incubated for 1–4 d. Extensive neurite outgrowth during this period was indicative of healthy cultures.
Immunoprecipitation. Reelin was produced in vitrousing rabbit reticulocyte lysates in the presence of [35S]methionine or a mix of [35S]methionine and [35S]cysteine (Amersham, Arlington Heights, IL, or New England Nuclear, Boston, MA) in the absence of unlabeled methionine. The transcription/translation reaction was diluted in 1 ml of RIPA buffer supplemented with the protease inhibitors leupeptin (20 μg/ml), aprotinin (0.1 mg/ml), and phenylmethylsulfonyl fluoride (PMSF; 2 mm). Aliquots of the diluted mixture were used for immunoprecipitation. To prepare radiolabeled Reelin expressed in COS cells, we incubated cultures overnight in DMEM lacking methionine and cysteine, supplemented with 1% fetal bovine serum and 300 μCi/ml of a mix of [35S]methionine and [35S]cysteine. Reelin expressed in cerebellar cultures was labeled overnight with the [35S] labeling mix in basal Eagle’s medium depleted of methionine (Cellgro) and supplemented as previously described (Fischer, 1982). For immunoprecipitation analysis, culture medium was collected from COS or cerebellar cells and diluted 1:1 with 2× RIPA buffer. Cell extracts were prepared in RIPA buffer supplemented with protease inhibitors as described above. Both supernatants and lysates were precleared by centrifugation at 1500 rpm in a refrigerated Eppendorf microfuge for 10 min.
Anti-Myc monoclonal antibody 9E10 (a gift of G. I. Evan, Imperial Cancer Research Fund, London, UK, and M. J. Bishop, University of California, San Francisco, CA, or obtained commercially, Babco, Richmond, CA), CR-50 ascites (Ogawa et al., 1995), rabbit antisera anti-Rlp3 raised against amino acids 1144–1163 of Reelin, or affinity-purified rabbit antibody anti-rp5 raised against amino acids 3443–3461 of Reelin were added to the samples and incubated at 4°C for 2 hr to overnight. Immobilized G-protein agarose beads (Immunopure plus, Pierce, Rockford, IL) were added to precipitate the antibodies, and the samples were incubated for an additional 30 min. Immunoprecipitates were collected by centrifugation and washed three times with RIPA buffer. Samples were resuspended in 20 μl of SDS sample buffer, boiled, and loaded onto SDS polyacrylamide gels (4–12% gradient gels, Novex, San Diego, CA, or 4% Duracryl, Oxford Glycosystems, Abingdon, UK).
Glycosylation analysis. Immunoprecipitates were resuspended in the appropriate glycosidase buffer and digested as follows. For peptide-N-glycosidase F (PNGaseF) digestion, samples were resuspended in 2× PNGaseF buffer (100 mm potassium phosphate, pH 7, 0.4% SDS, and 2% β-mercaptoethanol) and boiled for 5 min. The concentration of Nonidet P-40 was adjusted so that it exceeded SDS by sevenfold in a 20 μl reaction mix. The sample was divided into two aliquots, and PNGaseF (0.2 U, Boehringer Mannheim, Indianapolis, IN) was added to one tube. For neuraminidase digestion, samples were resuspended in 2× neuraminidase buffer (200 mm sodium acetate, pH 5.5, 0.5% SDS, and 0.7% β-mercaptoethanol) and boiled for 5 min. The concentration of Nonidet P-40 was adjusted so that it exceeded SDS by sevenfold in a 20 μl reaction mix. The sample was divided and neuraminidase fromClostridium perfringens (1 μl of NeuA, Worthington, Freehold, NJ) was added to one tube. For O-glycosidase, samples were resuspended in 50 mm sodium phosphate, pH 5, and O-glycosidase DS (1 mU, Glyko, Novato, CA) was added to one sample. For chondroitinase ABC digestion, samples were resuspended in 20 mm Tris HCl and 40 mm sodium acetate, pH 8; chondroitinase ABC (10 mU, Boehringer Mannheim) was added to one sample. All samples were incubated for 1 hr at 37°C. After incubation, an equal volume of 2× SDS sample buffer was added to each, and the samples were boiled and loaded onto 4% SDS-polyacrylamide.
Immunofluorescence. COS cells were transfected and plated on glass slide chambers (Nunc, Naperville, IL). Two days after transfection, cells were rinsed with PBS, fixed with 2% paraformaldehyde, and permeabilized with 1% Triton X-100. Primary mouse monoclonal antibodies 9E10 (anti-Myc) or CR-50 ascites were diluted 1:200 in PBS containing 2.5% normal horse serum (Vector Laboratories, Burlingame, CA). Cells were incubated with the primary antibody for 1–2 hr at room temperature and then washed and incubated for 30 min with the secondary antibody, FITC-labeled rabbit anti-mouse (Dako, Carpenteria, CA), diluted 1:80 as described above. Cells were rinsed twice with PBS before mounting on coverslips with fluorescence mounting medium (Vectashield, Vector Laboratories). The staining was analyzed under a microscope with phase-contrast and fluorescence settings (BX60, Olympus Optical, Tokyo, Japan).
RESULTS
Generation of reelin clones
To facilitate the investigation of Reelin function, we assembled a full-length reelin construct by the consecutive cloning of five contiguous cDNAs, as described in Materials and Methods. The full-length construct contains 95 bp of sequence 5′ to the initiator methionine codon and 82 bp of 3′ untranslated sequence. The open reading frame of 10,383 bp is sufficient to encode a protein of 3461 amino acids. After cleavage of the signal peptide, the molecular mass of Reelin was predicted to be ∼385 kDa. The full-length open reading frame was placed under the control of the CMV promoter for expression in mammalian cells (pCrl, Fig. 1). This vector also contains a promoter sequence for the T7 RNA polymerase, which allows transcription in vitro. To facilitate detection of the recombinant protein, we inserted a short DNA sequence encoding a human c-Myc epitope in frame in the fourth Reelin repeat, as illustrated (pCrlM, Fig. 1).
Fig. 1.
Assembly of full-length and truncatedreelin cDNAs. Five overlapping plasmids (lines) encoding portions of the reelincDNA (p5BS1, pBS2, pBS6, p3Rea3, and pBS53) were digested with the indicated restriction enzymes and cloned consecutively into pcDNA3 to generate a full-length clone that contains the entire open reading frame under the control of the mammalian CMV and the bacteriophage T7 promoters (pCrl). The initiator methionine codon (Met) and the stop codon (Stop) are indicated. After signal peptide cleavage, the full-length protein (box) is predicted to be 385 kDa in size. EGF-like repeats (E, black boxes) and a highly positively charged region in the C terminus (++) are indicated. A double-stranded oligonucleotide encoding a c-Myc epitope, 9E10 (c-Myc), was cloned in frame into the uniqueBspEI restriction site of pCrl to generate the pCrlM construct. Other expression constructs (lines) encoding truncated Reelin proteins in pcDNA3 are shown below the protein diagram. The numbers contained in the expression construct names refer to the last encoded amino acid of Reelin. The pCrl1837 contains an inverted c-Myc oligonucleotide at theBspEI restriction site, which puts the C-terminal half of the protein out of frame (dotted line).
Several truncated clones expressing portions of the Reelin sequence also were generated. These contain truncations at amino acid 194 (pCrl194), 250 (pCrl250), 407 (pCrl407), 462 (pCrl462), 1837 (pCrl1837), and 3328 (pCrl3328), as illustrated in Figure 1.
The CR-50 monoclonal antibody recognizes Reelin
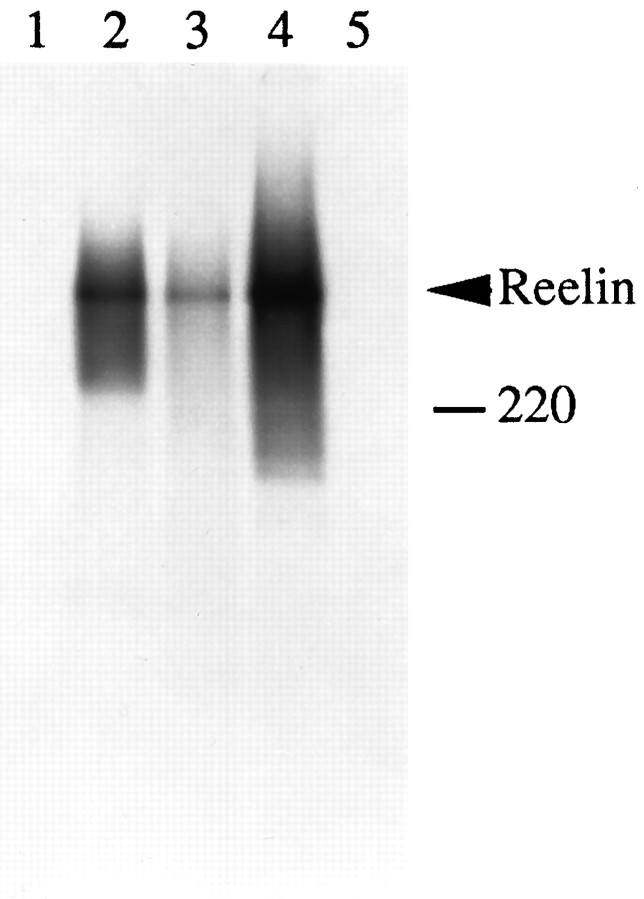
The staining pattern of CR-50, a monoclonal alloantibody raised inreeler mice against normal brain homogenate (Ogawa et al., 1995), is very similar to the distribution of reelin mRNA. Furthermore, this antibody interferes with the pattern of aggregation of neocortical cells in vitro (Ogawa et al., 1995). To determine whether CR-50 recognizes Reelin, we expressed the full-lengthreelin construct containing a c-Myc epitope (pCrlM) in vitro, using a coupled transcription/translation system. Reelin was detected as a single polypeptide of ∼385 kDa on SDS-polyacrylamide gels. It was immunoprecipitated by CR-50, as well as by antibodies specific for the c-Myc epitope, and by antibodies raised against amino acids 1144–1163 of Reelin (Rlp3; Fig. 2,lanes 2–4). In contrast, no Reelin was precipitated in the absence of antibodies or by preimmune sera (Fig. 2, lanes 1, 5). This suggests that the epitope recognized by CR-50 is encoded by the primary amino acid sequence of Reelin, because many post-translational modifications, such as glycosylation, do not occur in the reticulocyte lysate.
Fig. 2.
Expression of full-length Reelin in vitro. A plasmid encoding full-length Myc-tagged Reelin (pCrlM) was transcribed in vitro by the T7 RNA polymerase and translated by using a rabbit reticulocyte lysate. The [35S]-labeled final product was analyzed on a 4–12% SDS-polyacrylamide gel. Aliquots of the reaction were treated with no antibody (lane 1), monoclonal antibody against c-Myc (9E10, lane 2), monoclonal antibody CR-50 (lane 3), polyclonal anti-Reelin peptide Rlp3 (lane 4), or preimmune serum (lane 5). The immunoprecipitated Reelin protein is ∼385 kDa.
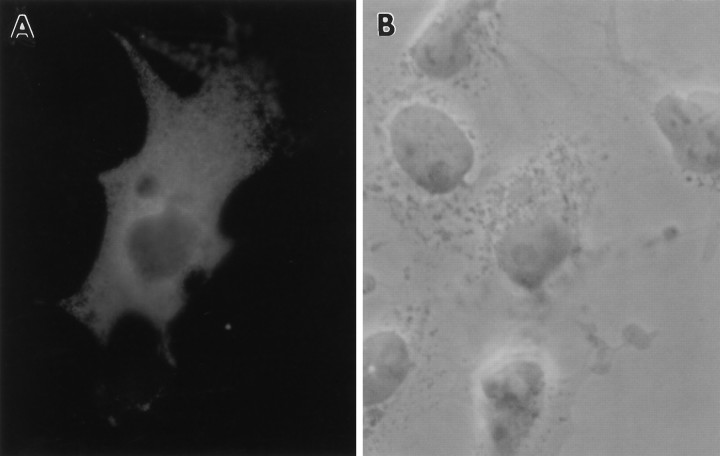
Immunocytochemistry was used to confirm the finding that CR-50 recognizes Reelin. CR-50 was used previously to demonstrate the presence of a reeler-related antigen in brain sections and in cultured neurons (Ogawa et al., 1995; Miyata et al., 1996). COS cells were transfected with or without pCrlM, fixed, and incubated with CR-50, as described in Materials and Methods. After incubation with FITC-labeled secondary antibody, positive cells were visualized under a fluorescent microscope. Approximately 5% of the cells transfected with pCrl revealed a strong intracellular signal (Fig. 3), whereas no signal was detected in cells transfected in the absence of pCrl (data not shown), demonstrating the specificity of CR-50 for Reelin. The CR-50 staining pattern, which was particularly intense near the nucleus, was similar to that obtained with the anti-Myc antibody (data not shown).
Fig. 3.
Intracellular localization of Reelin in COS cells. After transfection with pCrlM encoding full-length Reelin under the control of the CMV promoter, COS cells were fixed, permeabilized, and incubated for 1 hr with the primary CR-50 antibody and for an additional 30 min with FITC-tagged anti-mouse secondary antibody.A, Fluorescent image of a cell expressing Reelin (80× magnification). B, Phase-contrast image of the same cell field.
CR-50 recognizes the N terminus of Reelin
To map the CR-50 epitope on Reelin, we used a series of truncatedreelin expression clones (Fig. 1) in an in vitrotranscription/translation assay. Each construct encoded a truncated Reelin protein of the expected size (Fig.4A), which was subjected to immunoprecipitation with the CR-50 antibody. As for full-length Reelin (Fig. 2, lane 3), the CR-50 antibody was able, specifically, to immunoprecipitate truncated proteins containing amino acids 1–1837 (pCrl1837), 1–462 (pCrl462), and 1–407 (pCrl407), but not proteins containing only amino acids 1–194 (pCrl194) or 1–250 (pCrl250) (Fig.4B). No appreciable signal was detected when a control IgG antibody used for immunoprecipitation (data not shown). These data demonstrate that the CR-50 epitope is located between Reelin amino acids 251 and 407.
Fig. 4.
CR-50 epitope mapping. Plasmids encoding truncated Reelin proteins were transcribed in vitro by the T7 RNA polymerase and translated by using a rabbit reticulocyte lysate in the presence of [35S]methionine and [35S]cysteine. A, The products of pCrl1837 (206 kDa, lane 1), pCrl462 (51 kDa, lane 2), pCrl407 (46 kDa, lane 3), pCrl250 (27.5 kDa,lane 4), and pCrl194 (22 kDa, lane 5) were analyzed on a 4–12% SDS-polyacrylamide gel.B, The same in vitro translated proteins as in A were subjected to immunoprecipitation with the monoclonal antibody CR-50 and analyzed on a 4–12% SDS-polyacrylamide gel. Because all truncated proteins except those encoded by pCrl194 and pCrl250 are recognized by CR-50, the epitope is located between amino acids 251 and 407 of Reelin.
Reelin is a secreted glycoprotein
Analysis of the phenotype of reeler mice led to the proposal that they lack a signaling molecule required for the regulation of cellular interactions during neuronal migration. This was confirmed and extended by studies using chimeric reeler and normal mice, which demonstrated that at least some Purkinje cells fromreeler mice are able to differentiate properly when exposed to a normal cerebellar environment (Terashima et al., 1986). One interpretation of these data is that the reeler gene encodes a protein that provides an extrinsic signal for migrating neurons. This is consistent with the predicted amino acid sequence of Reelin, which indicates the presence of a cleavable signal peptide at the N terminus. This feature, together with the sequence similarity to F-spondin and the presence of EGF-like repeats, suggests that Reelin is a secreted protein. To investigate this possibility formally, we looked for the presence of secreted Reelin in medium from COS cells expressing pCrlM and in medium from cerebellar explants.
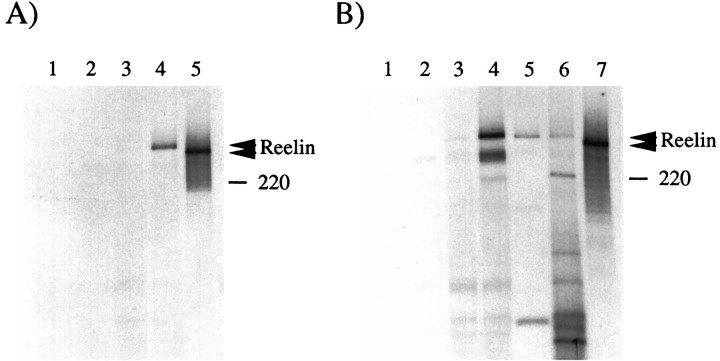
COS cell cultures were labeled overnight with a mixture of [35S]methionine and [35S]cysteine, as described in Materials and Methods. Media were collected and processed for immunoprecipitation with antibodies directed against the c-Myc epitope (Fig. 5A) and CR-50 (Fig.5B). A c-Myc antibody specifically precipitated Reelin (∼400 kDa) from the medium obtained from COS cells transfected with pCrlM (Fig. 5A, lanes 3, 4), but not from that obtained from control cultures (Fig. 5A, lanes 1, 2). Similarly, CR-50 specifically precipitated Reelin from the medium of transfected COS cells (Fig. 5B, lanes 1–4). Other protein bands were detected occasionally in immunoprecipitation assays; however, these represent either cross-reacting cellular proteins or degradation products of Reelin. To analyze the expression of endogenous Reelin, we prepared cultures from developing cerebelli dissected at P5–P8. After 1–4 d in culture, cells were labeled biosynthetically with [35S]methionine and [35S]cysteine. Reelin (∼400 kDa) was clearly detected both in CR-50 immunoprecipitates from the culture medium and from cell extracts of cultured cerebellum (Fig. 5B,lanes 5, 6). Additional bands may correspond to Reelin proteolytic fragments or may result from antibody cross-reactivity. The size of Reelin produced by cerebellar cells was similar to that of the protein synthesized in COS cells. Immunoprecipitated Reelin produced by COS or cerebellar cells migrated at a higher apparent molecular weight than that expressed in vitro in reticulocyte extracts, which is predicted to be ∼385 kDa (Fig. 5A,B, lanes 5, 7, respectively). This suggests that Reelin undergoes post-translational modification in intact cells. These data confirm the finding that CR-50 recognizes Reelin specifically, and they demonstrate that Reelin is a secreted protein that is post-translationally modified.
Fig. 5.
Reelin is modified and secreted by COS or cerebellar cells. A, COS cells (lanes 1–4) were transfected with (lanes 3, 4) or without (lanes 1, 2) pCrlM. The [35S]-labeled supernatant was subjected to immunoprecipitation with no antibody (lanes 1, 3) or with anti-Myc antibody 9E10 (lanes 2, 4). A specific band of ∼400 kDa (top arrowhead) was detected only in the sample containing both pCrlM and anti-Myc. The predicted 385 kDa in vitro translated [35S]-labeled product of pCrlM was immunoprecipitated with the anti-Myc antibody (lane 5). The protein produced in vitro (bottom arrowhead) seems to have a lower molecular mass than that obtained in COS cells. B, The supernatant from COS cells transfected with no DNA (lanes 1, 2) or pCrlM (lanes 3, 4), cerebellar cell supernatant (lane 5) or cerebellar cell lysates (lane 6), and in vitro translation reaction of pCrlM (lane 7) were labeled with [35S], as described in Materials and Methods, and subjected to immunoprecipitation with the CR-50 antibody. All immunoprecipitates from transfected COS or cerebellar cells migrated at a higher apparent molecular weight (top arrowhead) than the in vitro translated product (bottom arrowhead). The position of a 220 kDa molecular weight marker is indicated.
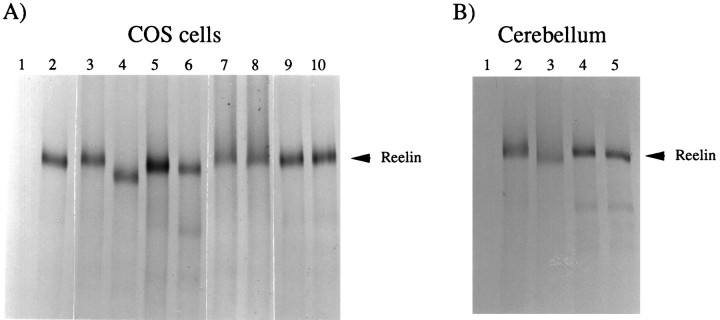
So that the nature of the post-translational modifications of Reelin that result in an apparent increase in molecular weight might be investigated, CR-50 immunoprecipitates from transfected COS cell medium and from medium removed from cerebellar explants were digested with a series of glycosidases, and the products were analyzed by SDS-PAGE. CR-50 immunoprecipitated Reelin from the medium of COS cells transfected with pCrlM (Fig. 6A, lanes 2–10), but not from the medium obtained from mock-transfected cells (Fig. 6A, lane 1). After digestion with PNGaseF, an enzyme that specifically cleaves asparagine-linked N-glycans, the apparent molecular mass of Reelin was reduced (Fig.6A, compare lanes 3 and4). Digestion with O-glycosidase, an enzyme that cleaves O-linked carbohydrate chains attached to serine and threonine residues, resulted in a modest decrease in the apparent mass of Reelin (Fig. 6A, lanes 5, 6). No appreciable effect was observed after digestion with neuraminidase A (Fig. 6A, lanes 7,8), which cleaves sialic acid groups, or with chondroitinase ABC (Fig. 6A, lanes 9,10), which cleaves chondroitin sulfate and dermatan sulfate side chains from proteoglycans. CR-50 also precipitated the endogenous Reelin from the medium of cerebellum explants obtained from normal mice (Fig. 6B, lanes 2–5), but not from explants obtained from reeler mice (Fig. 6B, lane 1). Treatment with PNGaseF (Fig. 6B, lane 3) and, to a lesser extent, O-glycosidase (Fig. 6B,lane 5) resulted in an apparent size reduction, whereas neuraminidase A (Fig. 6B, lane 4) had no effect. These data indicate that Reelin is subject to N-linked and, to a lesser extent, to O-linked glycosylation.
Fig. 6.
Reelin is glycosylated. A, COS cells were transfected with no DNA (lane 1) or with pCrlM (lanes 2–10). Cells were labeled with [35S], and Reelin was immunoprecipitated from the supernatant with the CR-50 antibody. Immunocomplexes were resuspended in gel loading buffer (lanes 1, 2) or in the appropriate glycosidase buffer and incubated with (lane 4) or without (lane 3) PNGaseF, with (lane 6) or without (lane 5)O-glycosidase, with (lane 8) or without (lane 7) neuraminidase A, and with (lane 10) or without (lane 9) chondroitinase ABC. After digestion, gel loading buffer was added, and the samples were analyzed on a 4% SDS polyacrylamide gel. B, Cerebellar cells were obtained from postnatal day 7 reeler(lane 1) or normal (lanes 2–5) mice. Cells were labeled with [35S], and Reelin was immunoprecipitated from the supernatant with the CR-50 antibody. Immunocomplexes were resuspended in gel loading buffer (lanes 1, 2) or in the appropriate glycosidase buffer and digested with PNGaseF (lane 3), neuraminidase A (lane 4), or O-glycosidase (lane 5). Samples were analyzed on a 4% SDS polyacrylamide gel.
The C terminus of Reelin is required for secretion
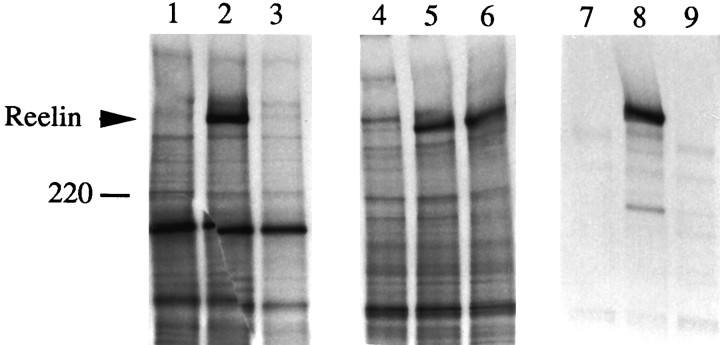
Several independent alleles of the reeler mutation have been described. In two strains, rl (Falconer, 1951) andrltg (Miao et al., 1994), the mutation is a consequence of a relatively large deletion of reelin, which results in a complete loss of expression (Bar et al., 1995;D’Arcangelo et al., 1995). In contrast, mice of therlorl strain (Guenet, 1981) have a small deletion of a 220 bp exon near the 3′ end of reelin, which results in a frame shift in the C terminus of Reelin (Curran et al., 1995; Hirotsune et al., 1995; Takahara et al., 1996). The predicted protein product of the rlorl gene lacks 205 C-terminal amino acids and contains 70 novel amino acids from an out-of-frame region. Reverse transcription-polymerase chain reaction (RT-PCR) analysis indicated that reelin mRNA is present inrlorl mice (Hirotsune et al., 1995), suggesting that the protein also might be present. We noted that the region of Reelin mutated in rlorl contains a short C-terminal region with a very high positive charge that might be required for the function of Reelin. Therefore, we created a truncated version of reelin (pCrl3328) lacking a region encoding 133 C-terminal amino acids, which includes this highly charged domain. We confirmed the truncation in the product of this construct with affinity-purified anti-rp5 antibodies directed against C-terminal amino acids 3443–3461 of Reelin. As expected, the anti-rp5 antibodies precipitated Reelin from extracts of COS cells transfected with full-length Reelin (pCrlM), but not from cells transfected with pCrl3328 or from untransfected cells (Fig. 7,lanes 1–3). Bands smaller than 400 kDa are attributable to antibody cross-reactivity. Furthermore, we confirmed that COS cells express high levels of pCrl3328 by immunofluorescence analysis with the CR-50 antibody, which recognizes an N-terminal epitope (data not shown). When the CR-50 antibody was used in the immunoprecipitation assay, both full-length and truncated Reelin were precipitated from extracts of transfected cells (Fig. 7, lanes 4–6). CR-50 also precipitated full-length Reelin from transfected cell supernatants; however, it failed to precipitate the truncated protein (Fig. 7, lanes 7–9). These data demonstrate that the highly charged C terminus of Reelin is required for secretion. Because this region is missing in rlorl, we suggest that thereeler phenotype is a consequence of the absence of extracellular Reelin.
Fig. 7.
The C terminus of Reelin is required for secretion. COS cells were transfected with no DNA (lanes 1, 4, 7), pCrlM (lanes 2, 5, 8), or pCrl3328 (lanes 3, 6, 9) and labeled with [35S]. Lysates (lanes 1–6) or supernatants (lanes 7–9) were subjected to immunoprecipitation with anti-rp5 (lanes 1–3) or with CR-50 antibody (lanes 4–9). The anti-rp5 antibody, directed against the C terminus of Reelin, recognized the full-length product of pCrl, but not the truncated product of pCrl3328. CR-50 immunoprecipitated both proteins from the cell lysate, but it only precipitated the full-length protein from the cell supernatant. The migration of a 220 kDa molecular weight marker is indicated.
DISCUSSION
The data presented here demonstrate that Reelin is a secreted protein that contains the CR-50 epitope. Thus, the neurodevelopmental defect in reeler mice is a consequence of the loss of an extracellular signal that is required for the regulation of neuronal migration. This is clearly the case for the rland rltg mice, because no reelin mRNA is expressed in these strains (D’Arcangelo et al., 1995). However,reelin mRNA was reported to be present inrlorl mice. The rlorlstrain has a deletion in the reelin gene that introduces an out-of-frame deletion in the C terminus (Curran et al., 1995; Hirotsune et al., 1995). Interestingly, when we introduced a similar mutation into Reelin, the protein was no longer secreted (Fig. 7), indicating that the C-terminal region is essential for maturation and export. Thus, the rlorl strain also may be missing extracellular Reelin because of a failure in protein transport.
The demonstration that the CR-50 epitope is encompassed within thereelin coding sequence allows interpretation of the effects of the CR-50 monoclonal antibody in terms of the function of Reelin. CR-50 recognizes an antigen in normal brain that is absent inreeler (Ogawa et al., 1995). The antigen distribution mainly parallels reelin mRNA expression in the developing cerebral, cerebellar, and hippocampal cortices. In addition, CR-50 applied extracellularly recognizes cell types, such as Purkinje cells in the cerebellum, that do not express reelin mRNA (Miyata et al., 1996a). This could reflect the interaction of extracellular Reelin with a receptor located on the surface of Purkinje cells. CR-50 alters the aggregation pattern of cortical cell cultures in vitro(Ogawa et al., 1995), the organization of the Purkinje cell plate in cerebellar explants (Miyata et al., 1996b), and the formation of hippocampal layering in vivo (Nakajima et al., 1996). These effects closely resemble the phenotype of reeler mice in which Reelin is absent. Our demonstration that CR-50 recognizes a Reelin peptide epitope indicates that the antibody interferes with Reelin activity directly.
In cerebral, cerebellar, and hippocampal cortices, neuronal cells located superficially, near the pia, synthesize and secrete Reelin from the earliest stages of development. Other neuronal cell types, located in deeper positions, may become responsive to the Reelin extracellular signal by virtue of the presence of an as yet unidentified cell surface receptor. These target cells migrate from the ventricular layer toward more superficial layers along radial fibers. The termination of their migration, and therefore their ultimate location, is determined by the Reelin signal. However, the exact outcome of the interaction with Reelin with its receptor is likely to vary according to the cell type.
At early stages of mouse cerebral cortex development (embryonic day 10–12), a preplate composed of Cajal–Retzius and subplate cells is present. The Cajal–Retzius cells are neurons destined to reach the most superficial layer (layer I or marginal zone), where they fully differentiate into horizontal cells (Marin-Padilla and Marin-Padilla, 1982; Derer and Derer, 1990). The subplate neurons are destined to reside in the deepest layer of the cortical plate (layer VIB; Shatz et al., 1988). In normal brain, the Cajal–Retzius cells produce and secrete Reelin into the preplate extracellular environment. The preplate then splits into two components, the marginal zone and the subplate layer, and the cortical plate develops between them (embryonic day 13–16) (Marin-Padilla, 1978). Reelin remains closely associated with the surface of Cajal–Retzius cell bodies and processes (Ogawa et al., 1995), some of which may descend into the cortical plate that is developing underneath (Marin-Padilla, 1978). In the reelerbrain, Cajal–Retzius cells do not produce extracellular Reelin, the preplate does not split, and the cortical plate develops ectopically underneath subplate neurons. Because the failure of the preplate to split is the earliest event associated with the reelerphenotype (Goffinet, 1979), it is possible that subplate neurons represent target cells that are repelled by Reelin. This interpretation is consistent with the observation that dissociated cells from thereeler cortex are more adhesive than normal, particularly the cells that are born early (embryonic day 10–12; Hoffarth et al., 1995). The separation of subplate cell bodies from the Cajal–Retzius cell layer may be a consequence of an active repulsion event associated with cell body or dendrite retraction, or it could result from maturation-dependent translocation of subplate cell bodies, a consequence of apical dendrites anchoring to the Reelin-rich marginal zone (Marin-Padilla, 1988). Alternatively, Reelin might preferentially attract cortical plate neurons to migrate past the subplate toward the marginal layer. This hypothesis requires either that Reelin must be able to diffuse into the developing cortical plate or that Cajal–Retzius processes must come in direct contact with cortical plate neurons. Given the solubility of Reelin demonstrated in the present study and the observation that some Cajal–Retzius cell processes descend into the cortical plate (Marin-Padilla, 1978), this latter hypothesis is also viable.
In the developing hippocampus, Cajal–Retzius-like cells in the marginal zone express Reelin (data not shown), which orchestrates the laminar organization of the hippocampus proper and the dentate gyrus. These cells, like the neocortical Cajal–Retzius cells, are generated very early during development and occupy a superficial layer (Soriano et al., 1994). However, a more detailed analysis of the early cell interactions involved in hippocampus formation is required to formulate a hypothesis on the mode of action of Reelin in this as well as other structures affected by the reeler mutation.
In the embryonic cerebellum, Reelin is produced by neuronal precursors in the nuclear transitory zone that are destined to give rise to cells of the deep nuclei and by postmitotic neurons in the deepest part of the external granular layer and in the internal granular layer (D’Arcangelo et al., 1995; Ogawa et al., 1995; Miyata et al., 1996a). No expression was detected in Bergmann glia or in Purkinje cells. In the normal cerebellum, Purkinje cells form a plate underneath the external granule layer, and they develop complex dendritic trees in the molecular layer. Reelin-positive postmitotic granule neurons migrate inwardly, crossing the molecular layer and the Purkinje cell body layer to form the internal granule layer, where they lose Reelin extracellular immunoreactivity (Miyata et al., 1996a). Inreeler mice, Purkinje cells do not form a cortical plate, but they remain ectopically located in the deep cerebellar nuclear mass. Because Purkinje cells accumulate Reelin on the extracellular membrane (Miyata et al., 1996a), it is conceivable that they express a receptor that mediates an increased adhesion with Reelin-producing granule cells. Recent functional studies demonstrate that the CR-50 antibody interferes with the formation of the Purkinje cell layer (Miyata et al., 1996b). Thus, it is conceivable that a common Reelin-dependent mechanism underlies the formation of both the cortical plate in the cerebral cortex and the Purkinje cell layer in the cerebellar cortex.
A general consideration from all the reported studies is that Reelin is expressed by specific neuronal populations to help other neurons determine their correct positioning in developing laminar structures. So far, Reelin expression or binding activity has not been described in radial fibers despite the fact that the orientation and the end feet of radial fibers are abnormal in the reeler brain (Derer, 1979). Recent findings suggest that Cajal–Retzius cells produce a factor that affects radial fiber development (E. Soriano, personal communication). Thus, it is possible that Reelin may affect the formation of the radial glia scaffold indirectly. It is to be hoped that some of the questions concerning the development of laminar organization in brain structures will be resolved by the identification and characterization of proteins that interact with Reelin, including potential cell surface receptors and possibly other extracellular signaling molecules.
Footnotes
This work was supported in part by National Institutes of Health Cancer Center Support CORE Grant P30CA21765, the American Lebanese Syrian Associated Charities, National Research Service Award NSO9698 from National Institute of Neurological Disorders and Stroke (G.D.), the Science and Technology Agency of the Japanese Government, and the Ministry of Education, Science, and Culture of Japan. We thank M. Sheldon, D. Eberhart, R. Homayouni, and J. Morgan for critically reading this manuscript and the members of the animal facilities of St. Jude Children’s Research Hospital and the Institute of Physical and Chemical Research for their help to maintain animals.
Correspondence should be addressed to Dr. Tom Curran, Department of Developmental Neurobiology, St. Jude Children’s Research Hospital, 332 North Lauderdale, Memphis, TN 38105.
REFERENCES
- 1.Bar I, Lambert De Rouvroit C, Royaux I, Krizman DB, Dernoncourt C, Ruelle D, Beckers MC, Goffinet AM. A YAC contig containing the reeler locus with preliminary characterization of candidate gene fragments. Genomics. 1995;26:543–549. doi: 10.1016/0888-7543(95)80173-j. [DOI] [PubMed] [Google Scholar]
- 2.Caviness VSJ. Time of origin in the hippocampus and dentate gyrus of normal and reeler mice: an autoradiographic analysis. J Comp Neurol. 1973;151:113–120. doi: 10.1002/cne.901510203. [DOI] [PubMed] [Google Scholar]
- 3.Caviness VSJ, Rakic P. Mechanisms of cortical development: a view from mutations in mice. Annu Rev Neurosci. 1978;1:297–326. doi: 10.1146/annurev.ne.01.030178.001501. [DOI] [PubMed] [Google Scholar]
- 4.Caviness VSJ, Crandall JE, Edwards MA. The reeler malformation. Implications for neocortical histogenesis. In: Peters A, Jones EG, editors. Cerebral cortex, Vol 7, Development and maturation of cerebral cortex. Plenum; New York: 1988. pp. 59–89. [Google Scholar]
- 5.Curran T, D’Arcangelo G, Goffinet AM. reeler gene discrepancies. Nat Genet. 1995;11:12–13. doi: 10.1038/ng0995-12a. [DOI] [PubMed] [Google Scholar]
- 6.D’Arcangelo G, Miao GG, Chen S-C, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 7.Derer P. Evidence for the occurrence of early modifications in the “glia limitans” layer of the neocortex of the reeler mouse. Neurosci Lett. 1979;13:195–202. doi: 10.1016/0304-3940(79)90041-7. [DOI] [PubMed] [Google Scholar]
- 8.Derer P, Derer M. Cajal-Retzius cell ontogenesis and death in mouse brain visualized by HRP and electron microscopy. Neuroscience. 1990;36:839–856. doi: 10.1016/0306-4522(90)90027-2. [DOI] [PubMed] [Google Scholar]
- 9.Falconer DS. Two new mutants trembler and reeler, with neurological actions in the house mouse. J Genet. 1951;50:192–201. doi: 10.1007/BF02996215. [DOI] [PubMed] [Google Scholar]
- 10.Fischer G. Cultivation of mouse cerebellar cells in serum free, hormonally defined media: survival of neurons. Neurosci Lett. 1982;28:325–329. doi: 10.1016/0304-3940(82)90079-9. [DOI] [PubMed] [Google Scholar]
- 11.Goffinet AM. An early developmental defect in the cerebral cortex of the reeler mouse. Anat Embryol (Berl) 1979;157:205–216. doi: 10.1007/BF00305160. [DOI] [PubMed] [Google Scholar]
- 12.Goffinet AM. Events governing organization of postmigratory neurons: studies on brain development in normal and reeler mice. Brain Res. 1984;319:261–296. doi: 10.1016/0165-0173(84)90013-4. [DOI] [PubMed] [Google Scholar]
- 13.Goffinet AM. A real gene for reeler. Nature. 1995;374:675–676. doi: 10.1038/374675a0. [DOI] [PubMed] [Google Scholar]
- 14.Goffinet AM, So KF, Yamamoto M, Edwards M, Caviness VSJ. Architectonic and hodological organization of the cerebellum in reeler mutant mice. Brain Res. 1984;318:263–276. doi: 10.1016/0165-3806(84)90031-2. [DOI] [PubMed] [Google Scholar]
- 15.Guenet J-L (1981) A new allele of reeler. Mouse News Lett 41.
- 16.Hirotsune S, Takahara T, Sasaki N, Hirose K, Yoshiki A, Ohashi T, Kusakabe M, Murakami Y, Muramatsu M, Watanabe S, Nakao K, Katsuki M, Hayashizaki Y. The reeler gene encodes a protein with an EGF-like motif expressed by pioneer neurons. Nat Genet. 1995;10:77–83. doi: 10.1038/ng0595-77. [DOI] [PubMed] [Google Scholar]
- 17.Hoffarth RM, Johnston JG, Krushel LA, van der Kooy D. The mouse mutation reeler causes increased adhesion within a subpopulation of early postmitotic cortical neurons. J Neurosci. 1995;15:4838–4850. doi: 10.1523/JNEUROSCI.15-07-04838.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mariani J, Crepel F, Mikoshiba K, Changeux JP, Sotelo C. Anatomical, physiological, and biochemical studies of the cerebellum from reeler mutant mouse. Philos Trans R Soc Lond [Biol] 1977;281:1–28. doi: 10.1098/rstb.1977.0121. [DOI] [PubMed] [Google Scholar]
- 19.Marin-Padilla M. Dual origin of the mammalian neocortex and evolution of the cortical plate. Anat Embryol (Berl) 1978;152:109–126. doi: 10.1007/BF00315920. [DOI] [PubMed] [Google Scholar]
- 20.Marin-Padilla M. Early ontogenesis of the human cerebral cortex. In: Peters A, Jones EG, editors. Cerebral cortex, Vol 7, Development and maturation of cerebral cortex. Plenum; New York: 1988. pp. 1–34. [Google Scholar]
- 21.Marin-Padilla M, Marin-Padilla TM. Origin, prenatal development, and structural organization of layer I of the human cerebral (motor) cortex. A Golgi study. Anat Embryol (Berl) 1982;164:164–206. doi: 10.1007/BF00318504. [DOI] [PubMed] [Google Scholar]
- 22.Miao GG, Smeyne RJ, D’Arcangelo G, Copeland NG, Jenkins NA, Morgan JI, Curran T. Isolation of an allele of reeler by insertional mutagenesis. Proc Natl Acad Sci USA. 1994;91:11050–11054. doi: 10.1073/pnas.91.23.11050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mikoshiba K, Nagaike K, Kosaka S, Takamatsu K, Aoki E, Tsukada Y. Developmental studies on the cerebellum from reeler mutant mice in vivo and in vitro. Dev Biol. 1980;79:64–80. doi: 10.1016/0012-1606(80)90073-1. [DOI] [PubMed] [Google Scholar]
- 24.Miyata T, Nakajima K, Aruga J, Takahashi S, Ikenaka K, Mikoshiba K, Ogawa M. Distribution of the reeler gene-related antigen in the developing cerebellum: an immunohistochemical study with an allogenic antibody CR-50 on normal and reeler mice. J Comp Neurol. 1996a;372:215–228. doi: 10.1002/(SICI)1096-9861(19960819)372:2<215::AID-CNE5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Miyata T, Nakajima K, Mikoshiba K, Ogawa M. CR-50 antigen, a reeler gene-related molecule, is responsible for the arrangement of Purkinje cells. Soc Neurosci Abstr. 1996b;22:288. [Google Scholar]
- 26.Nakajima K, Mikoshiba K, Miyata T, Kudo C, Ogawa M. Disruption of hippocampal development in vivo by a CR-50 monoclonal antibody, which recognizes Cajal-Retzius neurons. Soc Neurosci Abstr. 1996;22:288. [Google Scholar]
- 27.Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, Yamamoto H, Mikoshiba K. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- 28.Rakic P, Caviness VSJ. Cortical development: view from neurological mutants two decades later. Neuron. 1995;14:1101–1104. doi: 10.1016/0896-6273(95)90258-9. [DOI] [PubMed] [Google Scholar]
- 29.Rugarli EI, Ballabio A. Reelin: a novel extracellular matrix protein involved in brain lamination. BioEssays. 1995;17:832–834. doi: 10.1002/bies.950171003. [DOI] [PubMed] [Google Scholar]
- 30.Shatz CJ, Chun JJM, Luskin MB. The role of the subplate in the development of the mammalian telencephalon. In: Peters A, Jones EG, editors. Cerebral cortex, Vol 7, Development and maturation of cerebral cortex. Plenum; New York: 1988. pp. 35–58. [Google Scholar]
- 31.Soriano E, Del Rio JA, Martinez A, Super H. Organization of the embryonic and early postnatal murine hippocampus. I. Immunocytochemical characterization of neuronal populations in the subplate and marginal zone. J Comp Neurol. 1994;342:571–595. doi: 10.1002/cne.903420406. [DOI] [PubMed] [Google Scholar]
- 32.Stanfield BB, Cowan WM. The development of the hippocampus and dentate gyrus in normal and reeler mice. J Comp Neurol. 1979;185:461–484. doi: 10.1002/cne.901850303. [DOI] [PubMed] [Google Scholar]
- 33.Takahara T, Ohsumi T, Kuromitsu J, Shibata K, Sasaki N, Okazaki Y, Shibata H, Sato S, Yoshiki A, Kusakabe M, Muramatsu M, Ueki M, Okuda K, Hayashizaki Y. Dysfunction of the Orleans reeler gene arising from exon skipping due to transposition of a full-length copy of an active L1 sequence into the skipped exon. Hum Mol Genet. 1996;5:989–993. doi: 10.1093/hmg/5.7.989. [DOI] [PubMed] [Google Scholar]
- 34.Terashima T, Inoue K, Inoue Y, Yokoyama M, Mikoshiba K. Observations on the cerebellum of normal-reeler mutant mouse chimera. J Comp Neurol. 1986;252:264–278. doi: 10.1002/cne.902520209. [DOI] [PubMed] [Google Scholar]