Abstract
Numerous studies of learning and memory in Aplysiahave focused on primary mechanosensory neurons innervating the siphon and having their somata in the left E (LE) cluster of the abdominal ganglion. Although systematic analyses have been made of the responses of these LE cells to mechanical stimulation of the tightly pinned siphon, little is known about corresponding responses when the siphon is unrestrained. The present study demonstrates that LE mechanosensory thresholds in the freely moving siphon are much higher than in the pinned siphon. Light tactile stimuli adequate to activate central neurons and reflexive siphon movements often fail to activate the LE cells when the siphon is unrestrained. Because the LE cells display increasing discharge to increasing pressures, with maximal activation by crushing or tearing stimuli that cause tissue injury, they satisfy accepted definitions of nociceptor. Indeed, they show similarities to vertebrate Aδ nociceptors, including a property apparently unique (among primary afferents) to nociceptors—sensitization by noxious stimulation of their receptive field. Either pinching or pinning the siphon decreases LE cell mechanosensory threshold and enhances soma excitability. Such stimuli reduce effective tissue compliance and cause neuromodulation that enhances sensory responsiveness. These results, and recent descriptions of predatory attacks on Aplysia, suggest that LE sensory neurons are tuned to grasping and crushing stimuli that threaten or produce bodily harm. LE cell sensitization has effects, resembling hyperalgesia and allodynia, that compensate for loss of sensory function during injury and help protect against subsequent threats.
Keywords: nociceptor, mechanoafferent, sensitization, hyperalgesia, allodynia, alarm
Many investigations of learning and memory mechanisms have used mechanosensory neurons having somata in the left E (LE) cluster of the abdominal ganglion of Aplysia (for review, see Carew and Sahley, 1986; Byrne et al., 1993; Walters, 1994;Krasne and Glanzman, 1995). These cells, the first sensory neurons to be identified in Aplysia (Castellucci et al., 1970; Byrne et al., 1974), innervate the animal’s siphon and mantle shelf (Fig.1). Byrne and colleagues (1974, 1978a,b), using a feedback-regulated electromechanical stimulator for precise control of force and displacement, systematically characterized LE cell responses to mechanical stimulation of their receptive fields (RFs). For this device to deliver controlled stimuli, it was necessary to tightly pin out the siphon over a firm substrate so that the soft tissue did not passively move away from the probe during sustained force application. The authors found the LE cells had low mechanical thresholds and slowly adapting sensory responses, which they likened to those of low-threshold, type I mechanoreceptors in mammals—neurons that do not encode noxious stimuli (Burgess and Perl, 1973) .
Fig. 1.
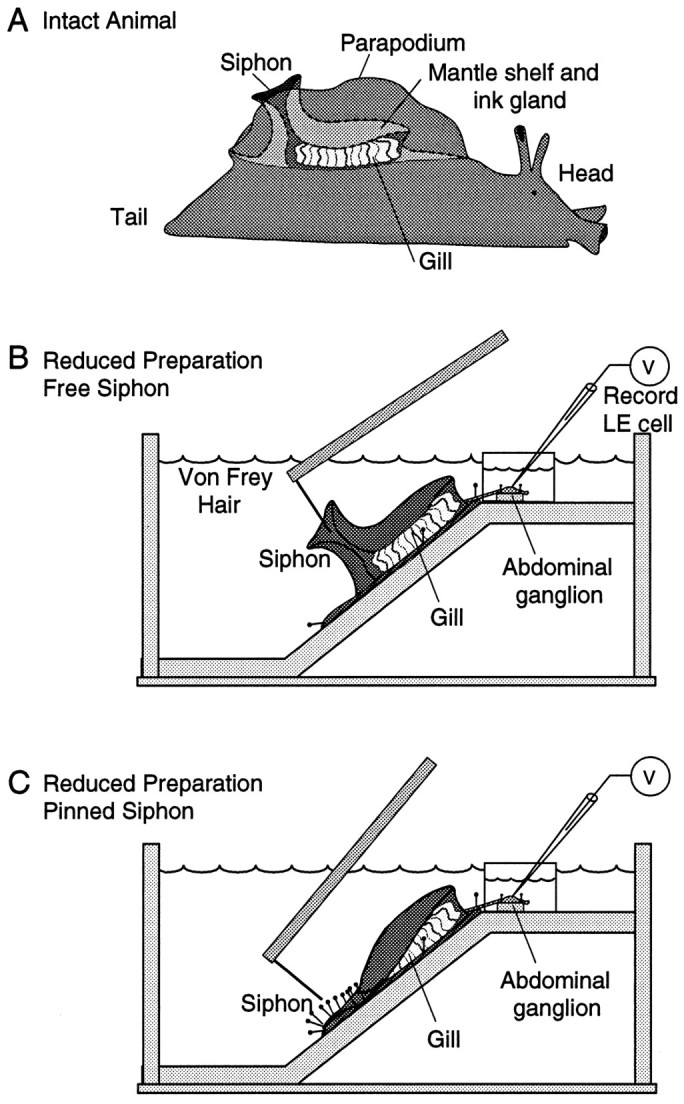
Preparations used to examine response properties of LE sensory neurons. A, Diagram of intact animal showing the mantle shelf and siphon within the mantle cavity (bounded by a parapodium on each side). Most of the siphon is normally hidden by the two parapodia. B, Reduced preparation allowing free movement of the siphon. Restraining pins were restricted to the floor of the mantle cavity and denervated bases of the parapodia, which are not innervated by the LE cluster. Turgor of the siphon and mantle organs was maintained by perfusion through a cannula in the genital artery (data not shown). Intracellular recordings were made from LE cell somata in the abdominal ganglion, placed in a Vaseline-sealed inner well. Hand-held von Frey hairs were applied to the inside and outside surfaces of the siphon. C, Reduced preparation after the siphon was tightly pinned to the substrate.
Little response saturation in the LE cells was seen by Byrne et al. (1974, 1978a) during application to the siphon of light to moderate punctate pressure (up to 17 g/mm2). This observation, along with the absence of information about how the LE cells respond to damaging mechanical stimuli in either pinned or unrestrained preparations, left open the possibility that these neurons have nociceptive functions. Supporting this possibility are several considerations. First, the range of forces sufficient to activate some nociceptors in mammals overlaps the activation range of several other types of mechanoreceptors; nociceptors are distinguished by being activated maximally by noxious stimuli, whereas other mechanoreceptors show a decrease in firing frequency at noxious levels (Sherrington, 1947; Burgess and Perl, 1973; Kumazawa, 1990; Light, 1992). Second, a nociceptor’s sensitivity is matched to mechanical properties of the surrounding tissue. Thus, nociceptors innervating the delicate siphon, like those in the mammalian cornea (Belmonte and Giraldez, 1981), should have low mechanical thresholds. Third, the LE cells appear indistinguishable in physiology and pharmacology from apparently homologous mechanosensory neurons in the ventrocaudal (VC) clusters of the pleural ganglia from the same animal (Walters et al., 1983a,b;Brunet et al., 1991; Wright and Kirschman, 1995). The VC sensory neurons are wide-dynamic-range nociceptors (Walters et al., 1983a; Clatworthy and Walters, 1993a). Finally, the VC cells become more sensitive after noxious stimulation of their RF (Clatworthy and Walters, 1993a). Such sensitization of primary mechanoreceptors appears to be unique to nociceptors (Light, 1992).
These considerations led us to examine the responses of LE sensory neurons to noxious mechanical stimuli. We find that LE neurons are optimally activated and sensitized by noxious stimuli, indicating a nociceptive function important for understanding the rich plasticity of these neurons. Some of these results have been reported in abstract form (Illich and Walters, 1995).
MATERIALS AND METHODS
Aplysia californica (120–250 gm) were supplied by Alacrity Marine Biological Services (Redondo Beach, CA) and theAplysia Mariculture Facility (University of Miami). Animals were housed individually within aquaria containing artificial seawater (ASW) (Instant Ocean, Burlington, NC) at 15–18°C for 1–5 d. Constant body weight was maintained by a diet of dried seaweed laver.
To record intracellularly from LE sensory neuron somata during siphon stimulation, it was necessary to use a surgically reduced preparation. Intact Aplysia (Fig. 1A) were first anesthetized by injection of isotonic MgCl2 (∼50% of their volume), which produces a general reduction of synaptic transmission and an increase in action potential threshold of peripheral axons and central neuronal somata (J. Gunstream, X. Liao, and E. Walters, unpublished observations). A reduced mantle organ–CNS preparation (Fig. 1B) was then set up as described byHickie and Walters (1995). Briefly, a perfusion line (0.30 mm inner diameter and 0.64 mm outer diameter) was inserted into the genital artery and attached to the overlying mantle muscle with four 6–0 silk sutures. The siphon, mantle shelf, gill, and base of each parapodium were excised together, mounted dorsal side up on the slanted floor (45°) of a SYLGARD-covered (Dow Corning, Corning, NY) Plexiglass chamber and secured by pins inserted into the denervated parapodia (Fig. 1B). The chamber was rinsed and perfused continuously through the genital artery with filtered ASW containing (in mm): 460 NaCl, 10 KCl, 11 CaCl2, 55 MgCl2, and 10 Tris, pH 7.5. In this preparation, the siphon has normal turgor and displays natural, unrestricted movements (Illich et al., 1994; Hickie and Walters, 1995). In some experiments, the perimeter of the siphon was tightly pinned down, leaving the inner surface of the siphon exposed (Fig. 1C), similar to how it was pinned out in previous studies (Byrne et al., 1974, 1978a). One difference, however, is that we pinned the siphon over a thin layer of tissue making up the floor of the mantle cavity, whereas Byrne et al. pinned it directly to a firm SYLGARD substrate.
Responses of LE cells to several different mechanical stimuli were investigated. A calibrated von Frey hair (Stoelting) exerting a bending pressure of 62 g/mm2 was initially used to locate and crudely map the RF on the siphon while recording from the LE cell soma (see below). This was the softest von Frey hair that reliably activated most LE cells in the freely moving siphon. For ease of comparison with previous studies in Aplysia and with some studies in mammals (see Light, 1992), we present our threshold pressures as g/mm2, where g indicates the weight exerted by a mass of 1 gm. It should be noted that nociceptor activation may depend not only on pressure but on the dimensions of the probe (Billy and Walters, 1989; Garell et al., 1996) and that activation by fine filaments may depend more on total force than pressure. To permit interpretation of our results in terms of the force corresponding to each pressure, we list the bending force, diameter, and pressure exerted by each von Frey hair in Table1. To reduce the effects of repeated stimulation, a formal determination of threshold (see Fig. 3) was conducted with a series of increasingly stiff von Frey hairs exerting 4, 6, 15, 25, 35, 62 g/mm2 to the RF, followed by pinch with toothed forceps (2 mm tip). Each stimulus lasted ∼0.5 sec, and successive stimuli were separated by 20–40 sec. Although each stimulus in the series was directed to the center of the RF, no attempt was made to stimulate exactly the same spot each time. In some experiments, the ascending series of von Frey hair stimuli was terminated when the sensory neuron was activated. Mechanical threshold was defined as the lowest pressure von Frey hair that activated the cell when the von Frey hair bent. In a few cases, a vertical cut (5–10 mm) was made in the tip of the siphon with sharp scissors. In some experiments, mechanical threshold was determined before and 5–60 min after noxious stimulation of the RF with three to five pinches (1 sec each, delivered at 5 sec intervals). In other experiments, a calibrated water jet (70–100 msec, 40–90 psi) through an 8 mm (inner diameter) nozzle positioned 5 mm from the skin was ejected from a Picospritzer (General Valve). Similar methods were used to determine electrophysiological thresholds before and 10–120 min after the siphon was tightly pinned to the substrate. In some experiments, no anesthesia was used during the pinning of the siphon, as per the procedure of Byrne et al. (1974). In others, partial anesthesia during pinning was provided by perfusing the siphon through the genital artery with a 1:1 mixture of ASW and isotonic MgCl2 solution and washing it out with ASW at least 30 min before testing. Full blockade and return of normal siphon movements occurred within 15 min of perfusing each solution through the artery.
Table 1.
Properties of von Frey hairs used to test siphon sensory responses
| Bending pressure | Bending force | Diameter | |
|---|---|---|---|
| (g/mm2) | (bars) | (mN) | (mm) |
| 1.6 | 0.15 | 0.06 | 0.070 |
| 2.5 | 0.24 | 0.1 | 0.072 |
| 4 | 0.4 | 0.3 | 0.10 |
| 6 | 0.6 | 0.6 | 0.11 |
| 15 | 1.5 | 1.4 | 0.11 |
| 25 | 2.4 | 2.8 | 0.12 |
| 35 | 3.4 | 6.1 | 0.15 |
| 40 | 3.9 | 7.8 | 0.16 |
| 44 | 4.3 | 9.8 | 0.17 |
| 55 | 5.4 | 21 | 0.22 |
| 62 | 6.1 | 43 | 0.30 |
| 73 | 7.2 | 54 | 0.31 |
| 75 | 7.6 | 63 | 0.33 |
Fig. 3.
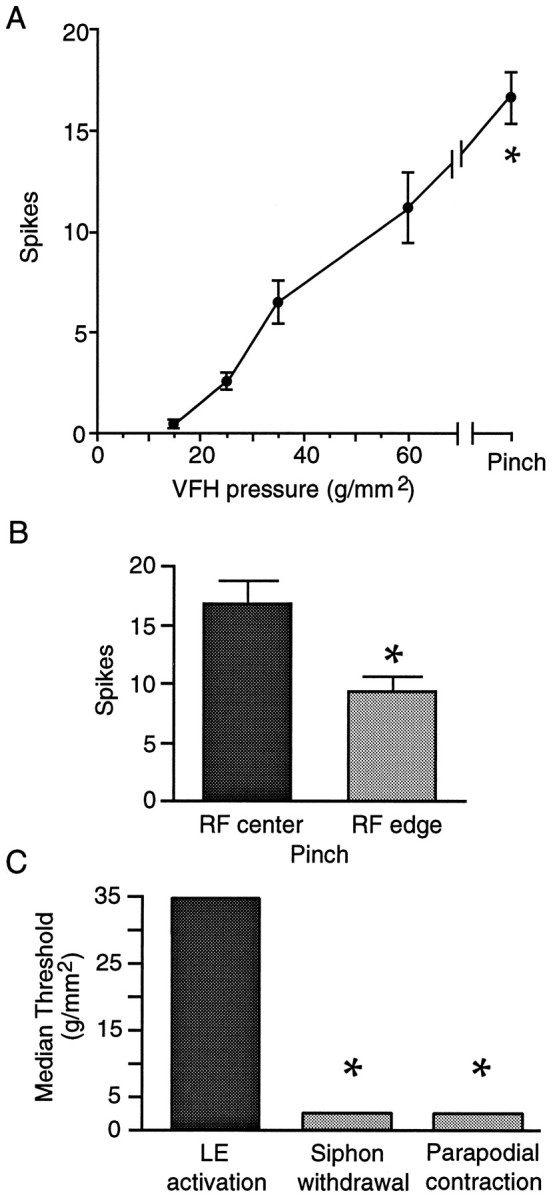
LE cells are tuned to noxious stimuli.A, Mean number of spikes evoked by increasingly stiff von Frey hairs and forceps pinch applied sequentially to the center of the RF while recording from each cell (16 cells in 12 preparations). When more than one cell was tested in a preparation, the values were averaged, yielding a single data point per preparation per stimulus for statistical analysis. Asterisk indicates significantly greater response to pinch than all other stimuli (p < 0.001 in each case). See Table 1 for bending force corresponding to each bending pressure. B, Larger response evoked by pinching the center than the margin of the RF (p < 0.001). C, Higher threshold of LE cells to stimulation of the unrestrained siphon than siphon or parapodial reflex responses to siphon stimulation in the intact animal (p < 0.05 in each case).
Intracellular recordings from the somata of LE sensory neurons and several unidentified neurons near the LE region were made with glass microelectrodes filled with 3 m potassium acetate (electrode resistance 15–25 MΩ). Soma excitability of the LE cells was assessed with a standard protocol that measured both spike threshold and repetitive firing properties (Walters et al., 1991;Clatworthy and Walters, 1994). Spike threshold was first determined with an ascending series of 20 msec depolarizing pulses delivered at 1–2 sec intervals. Repetitive firing was examined by counting spikes during a 1 sec depolarizing pulse. To separate changes in spike accommodation from changes in spike threshold, the current used in the 1 sec pulse was normalized to 2.5 times the current required to reach threshold during the 20 msec pulse.
Behavioral responses of the siphon in the intact animal and reduced preparation were scored by the tester and, in many cases, by a second observer, using criteria developed by Walters and Erickson (1986) andHickie (1994). To distinguish passive movements of the siphon caused by pushing by the probe from active responses, all stimuli were brief (0.5 sec or less), and the scoring did not begin until at least 0.5 sec after termination of the stimulus.
Statistical comparisons of spike number were made with repeated, one-way ANOVA, coupled with Newman–Keuls post hoc tests. In some experiments, paired t tests were performed. Because thresholds were measured on a discrete scale with unequal intervals, central tendency was expressed as the median, and differences were evaluated with nonparametric Wilcoxon or Mann–Whitney Utests. A probability <0.05 was considered significant.
RESULTS
LE neurons encode noxious and threatening mechanical stimuli
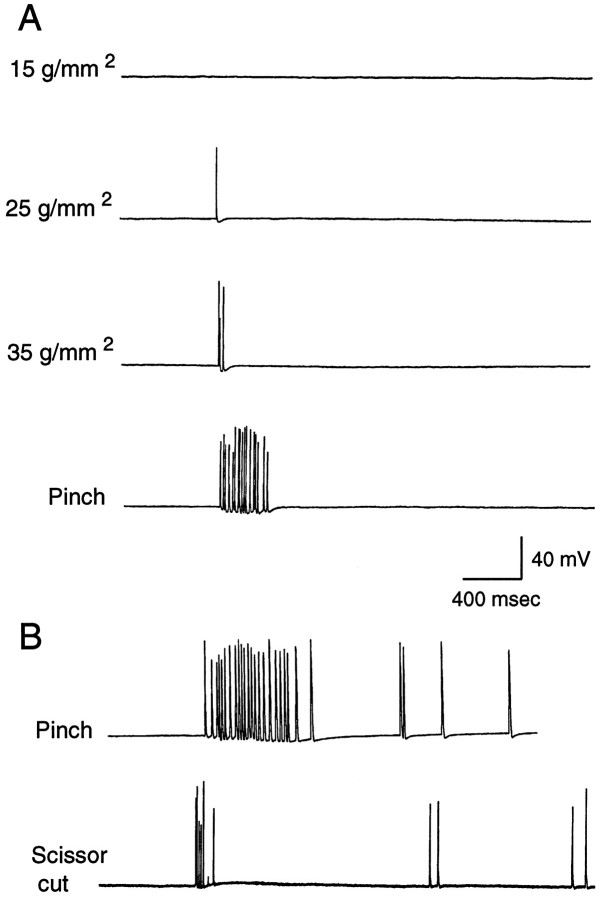
Previous studies of the response properties of LE sensory neurons examined only a limited range of mechanical stimulus intensities and primarily used a pinned siphon preparation displaying mechanical and physiological properties that differ from those of the unrestrained siphon (see below). We have examined sensory responses during mechanical stimulation of the freely moving siphon. A preparation was used (Fig. 1B) that exhibits normal turgor and displays siphon reflexes that are largely indistinguishable from those in the intact animal [however, siphon movements mediated by mantle contraction rather than siphon contraction are absent in this preparation (Hickie, 1994)]. Most mechanical stimuli were delivered with calibrated von Frey hairs, which in a previous study of LE cell responses in the pinned siphon yielded mechanosensory thresholds similar to those obtained with a feedback-controlled electromechanical stimulator (Byrne et al., 1974). Figure2A shows examples of responses evoked by sequential application of increasingly stiff von Frey hairs to the center of an LE cell’s RF on the external surface of the siphon. No action potentials were evoked by von Frey hairs exerting bending pressures below 15 g/mm2. Increasing pressures caused a graded increase in the number of evoked action potentials (spikes). An additional increase occurred in response to a brief, intense pinch of the siphon (estimated pressure >200g/mm2), which left obvious marks on the skin. Intense pinch or a quick scissors cut to the tip of the siphon, but not von Frey hair stimulation, sometimes evoked delayed afterdischarge that persisted for up to 15 sec (Fig. 2B). Group data for the responses to von Frey hairs and pinch are summarized in Figure3. An ANOVA showed that different stimulus intensities evoked significantly different numbers of spikes (F1,44 = 50.2, p < 0.00001), whereas post hoc comparisons confirmed that strong pinch produced the largest responses (p < 0.001 for each comparison). Pinching stimuli applied inside but near the edge of the RF evoked significantly fewer action potentials than did the same stimuli applied near the center of the RF (Fig. 3B;p < 0.001). Interestingly, in separate experiments in which the RF was not mapped initially with a stiff von Frey hair, the median threshold was 60 g/mm2 (n= 12 cells in 6 preparations). Because many of these responses were probably evoked off the center of the RF, the higher threshold in this study suggests that LE cell thresholds are lower at the RF center than at the edge (and/or that previous mapping sensitizes the RF) (see below).
Fig. 2.
Typical examples of graded responses of LE cells to innocuous and noxious stimuli. A, Responses of an LE cell (0, 1, 2, and 17 spikes) to increasingly intense mechanical stimuli delivered with von Frey hairs and, finally, forceps to its RF. Spike amplitudes in this and all other illustrations are variably attenuated because of the limited sampling rate of the data acquisition system. All actual spike amplitudes were >75 mV on the oscilloscope.B, Responses of LE cells to noxious stimuli. One LE cell showed an intense immediate response to pinch of 23 spikes, followed by an afterdischarge of 4 spikes. Another LE cell responded to a quick, shallow cut of the siphon tip with a brief immediate response of seven spikes, of which one was partially blocked (see Clatworthy and Walters, 1993b), and a very delayed, irregular discharge of four spikes.
Rapid, sharp vertical cuts to the siphon evoked a mean of 9.5 spikes (5 cells in 3 preparations, 8 cuts). In most cases, strong pinch to the same preparation before or after the cut evoked a larger number of spikes, suggesting that crushing is a more effective stimulus than cutting. Grasping and briefly tearing the siphon with two pairs of forceps produced the most dramatic response observed (52 spikes). We were also curious to know whether the LE cells would be activated by rapidly stroking von Frey hairs or paint brush bristles over the surface of the unrestrained siphon in a flicking motion like that used previously as a weak tactile stimulus for the classical conditioning of siphon responses (Carew et al., 1981). In eight of eight cells tested from five preparations, relatively stiff bristles and von Frey hairs (up to 0.3 mm diameter) that evoked spikes in the LE cells by application of punctate pressure failed to activate the LE cells when “flicked” sideways across the surface of the siphon. Interestingly, in one preparation, the flicking stimulus did become effective after pinch of the RF, suggesting that local noxious stimulation can sensitize LE responses to light tactile stimuli (see below).
To estimate the amount of force needed to damage siphon tissue, we examined the isolated siphon through a dissecting microscope during application of calibrated von Frey hairs. The siphon was relaxed by previous injection with isotonic MgCl2 solution. To keep the small area of stimulated tissue (0.1–0.3 mm diameter) within the focal plane of the microscope during stimulation, the siphon was placed against the chamber floor and loosely secured with a few pins distant from the site of testing. Under these conditions, perforation of the skin was first observed at 25–35 g/mm2, although these pressures did not always cause obvious damage. No damage was seen after sideways stroking or flicking by von Frey hairs in this range of stiffness. Von Frey hairs exerting 44g/mm2 or more almost always left holes when pushed into the surface of the thin siphon tissue. When von Frey hairs exerting as little as 25 g/mm2 were pressed repeatedly into the same spot, holes soon appeared and became progressively deeper and wider, penetrating into the muscle beneath the skin. These results confirm that siphon tissue is quite delicate and indicate that the force needed to damage the siphon (at least when relaxed) is not much greater than the mechanical threshold of the LE cells when the siphon is unrestrained.
Thresholds for LE cell activation exceed behavioral and afferent thresholds
The median threshold for activation of LE neurons by application of von Frey hairs to the center of RFs in the freely moving siphon was 35 g/mm2, with a range of 15–60g/mm2 (Fig. 3C; n = 16 cells in 12 animals). In LE cells tested by application of von Frey hairs to randomly selected points on the RF (i.e., without previous mapping of the RF), the median threshold for LE cell activation was 60g/mm2, with a range of 15–75g/mm2 (n = 12 cells in 6 preparations). Both sets of thresholds were considerably higher than the 2.5 g/mm2 threshold (range, 1–4g/mm2) for evoking behavioral responses of the siphon and parapodia by identical application of von Frey hairs to the siphon of intact, freely moving animals (Fig. 3C;n = 6 animals; p < 0.01 in each case). Examination of siphon response thresholds in reduced siphon–mantle preparations (n = 11) yielded a median threshold of 4g/mm2, which was significantly lower than the LE thresholds measured in the RF center (p < 0.01). It should be noted that most of the reflexive withdrawal of the siphon in response to weak siphon stimulation is attributable to movement of the underlying mantle rather than to contraction of the siphon itself (Hickie, 1994), and our dissection eliminates mantle movements. Thus, in the reduced preparation, siphon responses to weak siphon stimulation (in contrast to siphon responses to other inputs) differ qualitatively from the movements of the siphon seen in the intact animal.
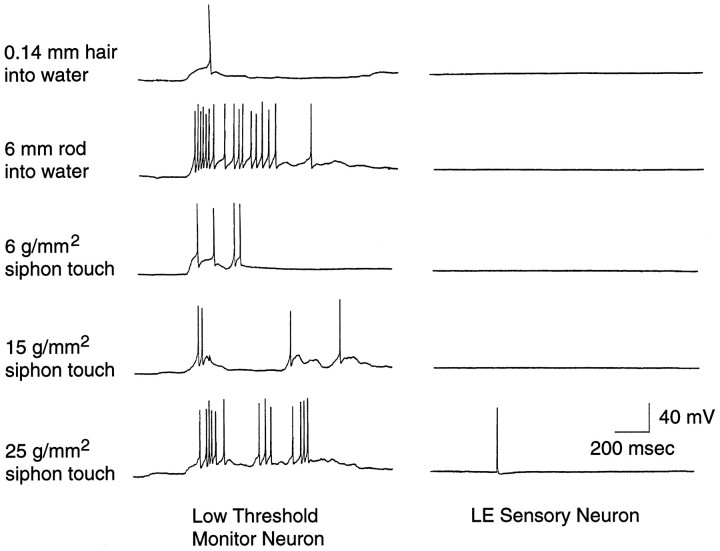
It might be argued that in the intact animal, the LE cells could be responsible for carrying information about very weak siphon stimuli to the CNS but that some aspect of the reduced preparation raises their thresholds. If so, the absence of LE activation during weak siphon responses in the reduced preparation might have occurred because the observed siphon responses were mediated entirely by the peripheral nervous system. To verify that in our reduced preparation, sensory information about very weak mechanical stimuli does reach the CNS, we recorded from various neurons in the abdominal ganglion. In every preparation tested (n = 4), we found presumptive interneurons of undetermined function near the LE cells on the ventral surface of the ganglion that responded reliably to near-field water disturbances and to very light touch. Each of these low-threshold monitor neurons (n = 5) showed repeatable responses to various stimuli too weak to activate concurrently recorded LE cells, including near-field disturbances produced by insertion of a von Frey hair through the water surface. Figure 4 illustrates the difference in responsiveness of an LE cell and one such interneuron both to soft von Frey hairs pressed into the siphon and to gentle disturbance of the water surface with a von Frey filament and a rod 4–5 cm from the siphon. Because these interneurons were used merely as a monitor of afferent input to the CNS, we did not attempt to identify or characterize them further. In all of our recordings from LE cells during von Frey hair application (>100 cells), we have never observed an LE cell fire in response to movement of the filament through the water surface, and similar observations have been made by Byrne et al. (1978b) and Cohen et al. (1991). Activation of the monitor neurons and weak excitation of some motor neurons (Byrne et al., 1978b; Cohen et al., 1991) by these very weak mechanical stimuli in the consistent absence of LE cell activation indicates that at least one other population of mechanosensory neurons conveys information to the CNS about low-pressure stimuli impinging on the siphon.
Fig. 4.
LE cells do not respond to very weak mechanical stimuli that activate central neurons. The left column shows responses of a presumptive interneuron in the abdominal ganglion. The right column shows simultaneous recordings from an LE cell. The top two rows show responses to insertion of a hair and rod into the water several centimeters from the siphon. The bottom three rows show responses to application of von Frey hairs to the center of the LE cell’s RF.
Although the results illustrated in Figures 2 and 3 show that the LE sensory neurons are not activated by pressing soft von Frey hairs into the freely moving siphon, the possibility remained that weak pressure applied to a larger surface area might activate the LE cells. This possibility was of particular interest, because many behavioral studies have used water jets (relatively weak mechanical stimuli affecting a large surface area) to evoke siphon and gill withdrawal (Carew et al., 1972; Pinsker et al., 1973; Frost et al., 1985). In addition, a previous study reported that LE cells can be activated by water jet stimuli as light as 1 g/mm2 delivered to the pinned siphon and 2.5 g/mm2 delivered to the unpinned siphon (Byrne et al., 1978a). In our freely moving siphon preparation, we found that delivery of progressively more intense, 100 msec water jets exerting pressures of 0.1–10g/mm2 failed to evoke spikes in any of the LE cells tested (n = 8 cells in 6 animals). Differences between our results and those of Byrne et al. might be attributable to the longer (800 msec) oscillating stimulus delivered by their Water Pik (Teledyne) or by differences in the preparation. In particular, we stimulated a continuously perfused siphon in its normal orientation, so that the tissue moved freely away from the applied stimulus. The unpinned siphons stimulated by Byrne et al. (1978a) were unperfused, which would reduce compliance. Moreover, they lay on a substrate that might resist movement of the siphon away from the stimulus (depending on the angle of the water jet), which could increase the effectiveness of the applied force.
LE neurons are sensitized by pinning the siphon
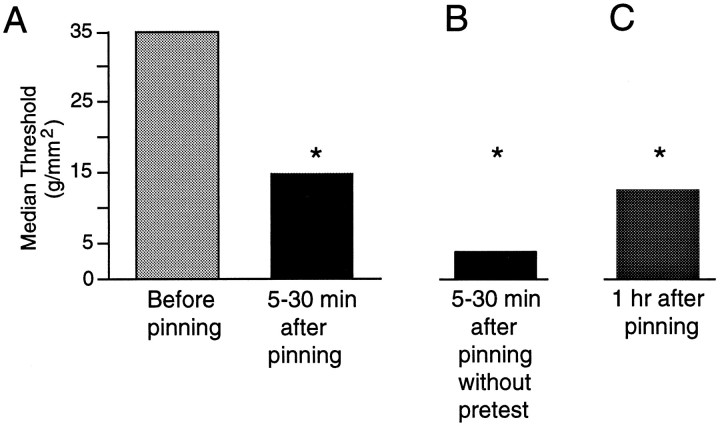
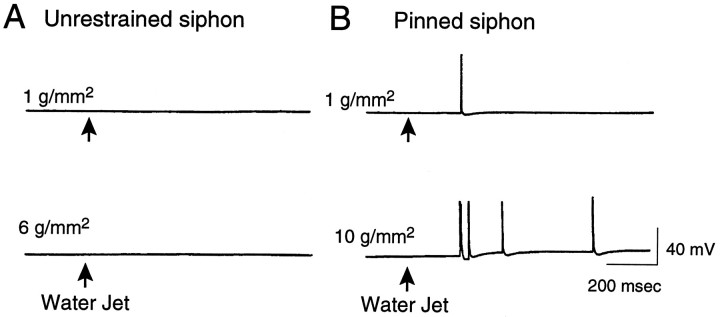
The median mechanical threshold we observed for LE cells (35g/mm2) in the freely moving siphon preparation was considerably higher than the average threshold of 1.3g/mm2 first reported for these cells in a pinned siphon preparation (Byrne et al., 1974). To see whether this difference is attributable at least in part to greater sensitivity of the LE cells to mechanical stimulation in the pinned preparation, we tested one subset of LE cells in the freely moving siphon with von Frey hairs and then tested another subset from the same LE cluster after the entire border of the siphon had been tightly pinned, as described by Byrne et al. (1974) (Fig. 1C). Mechanosensory threshold was significantly decreased 5–30 min after the completion of pinning (Fig.5A, p < 0.02,n = 13 cells in 7 preparations). The somewhat lower thresholds in the pinned siphon found by Byrne et al. (1974) were probably attributable to the tighter pinning and the lack of cushioning from underlying tissue in their more reduced preparation. We asked whether the thresholds would be even lower after pinning in the absence of prepinning tests, which might cause activity-dependent adaptation and elevation of mechanosensory threshold (Clatworthy and Walters, 1993b). Indeed, thresholds tested after pinning in preparations that had not been tested before pinning were significantly lower than corresponding thresholds tested after pinning in separate animals (Fig.5B; p < 0.05, n = 12 cells in 9 preparations), indicating that pretests tend to elevate threshold and that the decrease in threshold consistently seen after pinning is attributable to pinning rather than to the pretests. The decrease in threshold after pinning lasted at least 1 hr (Fig. 5C;p < 0.05, n = 7 cells in 4 preparations). In two of these preparations, the pinning was done while the siphon was anesthetized by perfusion with a 1:1 solution of isotonic MgCl2 and ASW. Thresholds found in these preparations (median = 6 g/mm2) were at least as low as those pinned in ASW alone (15g/mm2) (see below). As illustrated in Figure6, the decrease in threshold after the siphon was pinned out sometimes permitted activation of LE cells by 1–10g/mm2 water jet stimuli that were subthreshold when applied to the freely moving siphon. This was seen in four of six cells in six experiments.
Fig. 5.
Mechanosensory threshold of LE cells is reduced by pinning the siphon. A, Thresholds measured in different, randomly selected LE cells within the same cluster before and 5–30 min after pinning (p < 0.02). B, Thresholds measured 5–30 min after pinning in the absence of any siphon pretests (p < 0.05 compared with 5–30 min thresholds determined in separate preparations; a random subset of the cells tested in A). C, Thresholds tested 1 hr after pinning were significantly lower than median pretest thresholds (35 g/mm2) (p < 0.05). In two of the four preparations, pinning was performed while the siphon was perfused with a 1:1 solution of isotonic MgCl2 and ASW, but little difference was found between thresholds in these preparations (6g/mm2) and those in which the siphon was perfused with ASW alone (15 g/mm2).
Fig. 6.
Pinning the siphon facilitates LE cell responses to water jet stimuli. A, Example of an LE cell showing no response to stimulation of the freely moving siphon by 100 msec water jets exerting pressures of 1 and 6g/mm2. No responses to water jets exerting up to 10 g/mm2 were observed in any cells tested (n = 8 cells in 6 animals).B, Example of an LE cell activated during stimulation of the pinned siphon by water jets exerting 1 and 10g/mm2.
Mechanical and physiological factors may contribute to sensitization after pinning
One factor that could contribute to the increase in mechanosensitivity after pinning is a large decrease in tissue compliance. Tightly pinning the siphon greatly reduces the force that is dissipated in passive movement away from a mechanical stimulus, increasing the amount transmitted to the sensory receptors. In the freely moving siphon, we found that even our softest von Frey hair (1.6g/mm2) caused small movements of the delicate siphon tip. The thicker tissue at the base of the siphon was less compliant but yielded noticeably when pressed with most von Frey hairs. As each von Frey hair was pressed into the siphon, a position was usually reached (sometimes after the siphon tip had moved several centimeters) where the tissue yielded no farther and the hair began to bend. These observations indicate that the rate of von Frey hair force application is much higher in the pinned preparation, but the maximal force acting on the receptors reaches about the same steady-state level in the freely moving siphon preparation. The two preparations might also differ, however, in how the effective stimulus force is divided between compressive and tensile components. Indirect, preliminary evidence for a mechanical contribution to the increase in mechanosensitivity after pinning was obtained in experiments in which the pinning was performed with the siphon anesthetized by intra-arterial perfusion with isotonic MgCl2. Despite the probable reduction of local sensitizing neuromodulator release during the pinning procedure, mechanical threshold 1 hr after washout of the MgCl2 (6 g/mm2; n = 4 cells in 2 preparations) was not higher than that observed when the pinning was conducted without anesthesia (15g/mm2) (see above). It should be noted, however, that sensitizing neuromodulators might have been released between the washout of the MgCl2 and the 1 hr test.
A second factor that could contribute to the greater sensitivity of the LE cells in the pinned siphon preparation is physiological modulation. Two sources of noxious stimulation might have sensitized peripheral branches of the LE cells in previous experiments. One was the dissection procedure, which was performed entirely without anesthesia in one study (Byrne et al., 1974). In another, after the initial dissection, elevated Mg2+ saline was briefly superfused over the CNS and siphon during pinning (Byrne et al., 1978a). Although this would block central sensitizing effects, access of such solutions to peripheral synapses is very slow (Byrne et al., 1974), unlike access during perfusion through the vasculature (Walters et al., 1983a) (E. Walters, unpublished observations). The other potential source of noxious stimulation was the presence of restraining pins in many LE RFs, which might provide continuing noxious stimulation.
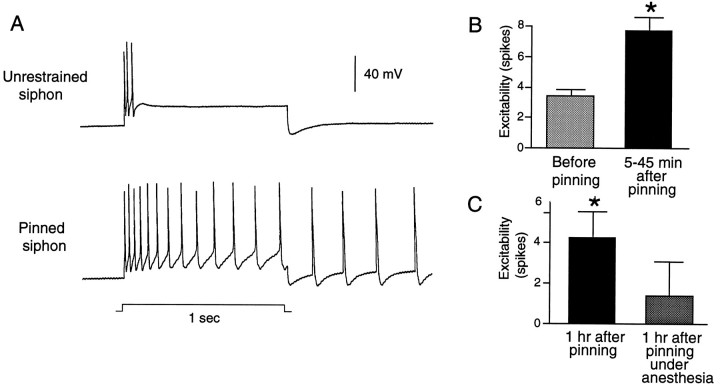
We examined the possibility that pinning the siphon triggers widespread physiological changes in the LE cells by testing the electrical excitability of the centrally located LE cell soma. In the similar VC sensory neurons of the pleural ganglia, noxious stimulation delivered to the RF is associated with peripheral sensitization and central hyperexcitability, which probably depend on both activation of the sensory neuron and modulation by extrinsic chemical signals (Walters, 1987b, 1994; Clatworthy and Walters, 1993a). Central hyperexcitability of the VC cells is expressed as a decrease in both spike threshold and accommodation in the centrally located soma as well as the occurrence of afterdischarge generated in or near the soma. Figure7A illustrates central hyperexcitability in LE neurons triggered by pinning the siphon. In the freely moving siphon preparation, an LE cell displays a typical, rapidly accommodating response to a 1 sec depolarizing pulse delivered to the soma. In contrast, after the siphon had been pinned, another LE cell in the same ganglion responded with more spikes during the depolarizing pulse and with an afterdischarge. Significant soma hyperexcitability was found 5–45 min (p < 0.01, n = 32 cells in 6 preparations) and 1 hr or more (p < 0.01, n = 30 cells in 8 preparations) after pinning (Fig. 7B,C). Interestingly, no significant hyperexcitability was observed at 1 hr if insertion of the pins was performed while transmitter release and spike activity in the siphon were transiently reduced by perfusion with a 1:1 solution of isotonic MgCl2 and ASW (Fig. 7C;n = 18 cells in 5 preparations). This suggests that either a critical level of afferent activity or release of peripheral neuromodulators during pinning is important for inducing central soma hyperexcitability that lasts 1 hr or more.
Fig. 7.
Pinning the siphon increases the excitability of LE cell somata in the abdominal ganglion. A, Examples of different LE cells from the same cluster tested for excitability before and ∼15 min after pinning the siphon. Excitability was tested by injecting into the soma a 1 sec depolarizing pulse at 2.5 times the threshold current for initiating a spike with a 20 msec pulse. Note that the cell tested after pinning showed an immediate discharge of 13 spikes and an afterdischarge of 4 spikes. B, Mean number of spikes evoked in different subsets of LE cells before and 5–45 min after pinning (p < 0.01). C, Hyperexcitability relative to baseline observed 1 hr after pinning is blocked by perfusing the siphon with a 1:1 solution of isotonic and ASW during pinning. Left, Hyperexcitability at least 1 hr (60–90 min) after pinning with ASW perfusion of the siphon (p < 0.01). Right, Lack of significant hyperexcitability at least 1 hr after pinning with MgCl2 perfusion. Values are means of the average difference in spike number (postpinning–prepinning) in each preparation.
LE neurons are sensitized by pinching the freely moving siphon
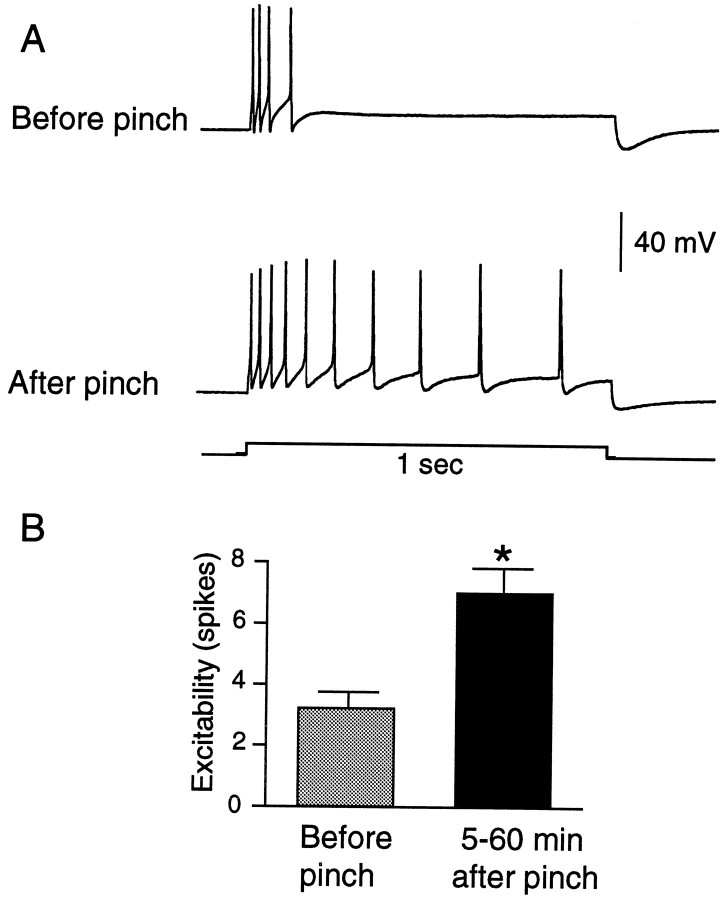
To see whether sensitization of the LE cells would occur under more natural conditions, we examined responses to von Frey hairs before and after three to five sharp pinches to the cell’s RF in the freely moving siphon preparation. Figure8A illustrates the ability of siphon pinch to decrease mechanosensory threshold and to increase the number of spikes evoked by an adequate mechanical stimulus. In a quantitative study, we focused on the change in threshold, using a protocol in which we tested up to two LE cells in each animal having nonoverlapping RFs. To minimize possible sensitization or adaptation from application of the von Frey hair test stimuli, we stopped each test sequence as soon as the LE cell showed any activation. Mechanosensory threshold of the LE cells was significantly decreased by pinch (Fig.8B; p < 0.05, n = 18 cells in 7 preparations). Similar to the effects of pinning, pinching the siphon not only sensitized mechanosensory responses, it also produced hyperexcitability of the LE soma, as shown by increased discharge to depolarizing test pulses delivered to the soma 5–60 min after the pinch (Fig. 9; p < 0.01,n = 26 cells in 8 preparations). No tendency was seen for hyperexcitability to decay during this period. The mean change in spike number was 2.7 for cells tested 5–30 min after pinch (n = 9) and 4.5 for cells tested 31–60 min after pinch (n = 4).
Fig. 8.
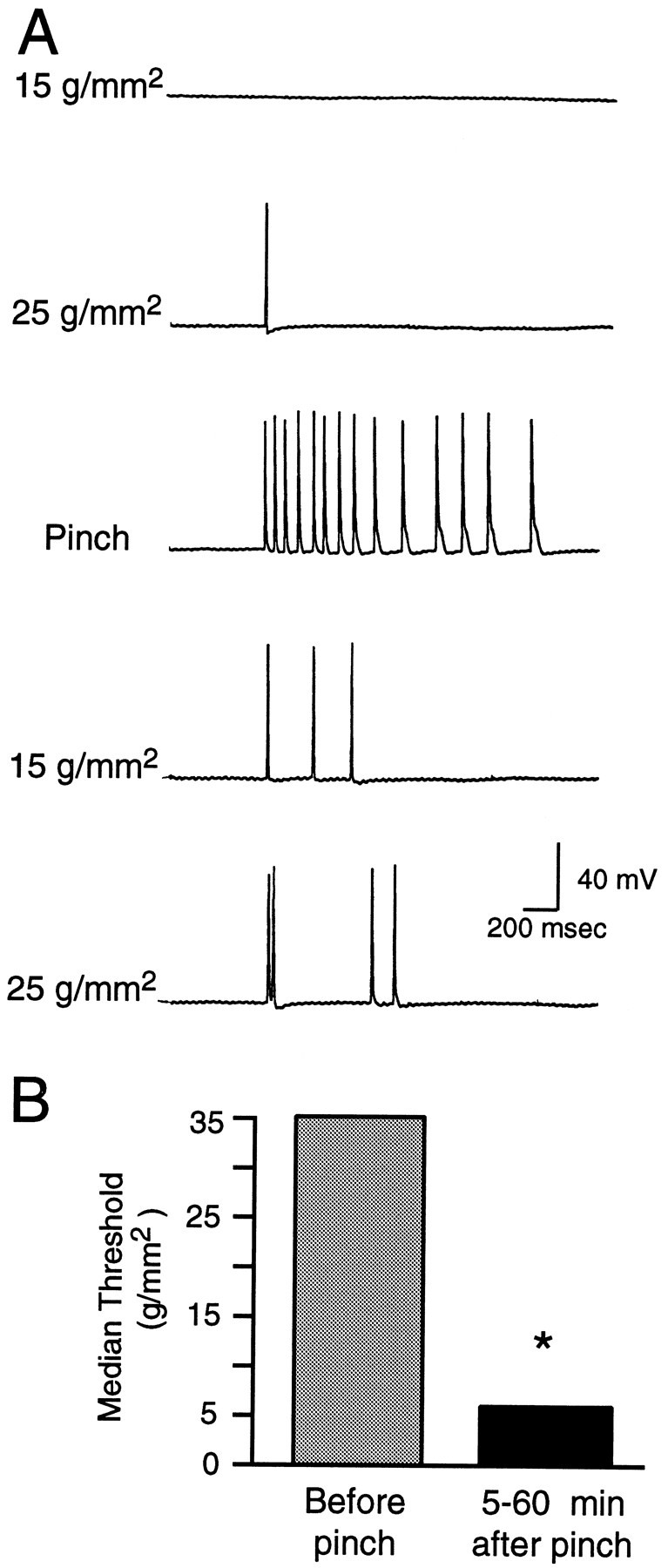
Siphon pinch sensitizes the responses of LE cells to subsequent siphon stimulation. A, Example of change in LE cell response to two von Frey hairs 5 min after three pinches (only 1 pinch response is shown). Note the decrease in threshold and increase in spike number evoked by each von Frey hair after pinch.B, Depression of median threshold 5–60 min after pinch (p < 0.05).
Fig. 9.
Pinching the siphon increases the excitability of LE cell somata. A, Example of an LE cell tested for excitability before and 15 min after four brief pinches to the siphon.B, Mean number of spikes evoked in the same LE cells before and 5–60 min after pinching (p < 0.01).
DISCUSSION
Light tactile stimuli often fail to activate LE cells
Because of their low mechanical thresholds in a tightly pinned preparation, Byrne et al. (1974) suggested the LE cells are analogous to low-threshold mechanoreceptors in mammals. However, our results in a freely moving siphon preparation extend various suggestions of a lack of LE cell activation by light tactile stimuli (Byrne et al., 1978b;Cohen et al., 1991; Wright et al., 1991; Hawkins and Frost, 1995) (see also Hickie et al., 1995, 1996). We found that LE cells are not activated by weak von Frey hair, water jet, or near-field vibratory stimuli applied to the unrestrained siphon, even though these same stimuli reliably evoke behavioral and neural responses. Some of these stimuli exert pressures far lower than the 1–15g/mm2 we and Byrne et al. (1974) found necessary for activating LE cells in pinned siphon preparations. Moreover, we found that substantially greater forces (15–35g/mm2) are needed to activate LE cells in the perfused, unrestrained siphon. This indicates that information about the large range of pressures <15 g/mm2 that evoke neural and behavioral responses is normally conveyed by unidentified sensory populations. One possibility is mechanosensory neurons with peripheral somata (Xin et al., 1995). Although the 15–35g/mm2 thresholds for activating LE cells in the unrestrained siphon are relatively high, they are somewhat lower than the level at which clear tissue damage occurs. Thus in this range, the weak responses of LE cells may encode information about innocuous stimuli. As discussed below, this range can be altered dramatically by pinning or pinching the siphon.
Implications of LE response properties for Aplysialearning studies
Light tactile stimulation has often been used as a test or conditioned stimulus (CS) in Aplysia learning studies, with the assumption that such stimuli activate LE neurons. Our failure to observe activation of LE cells by filaments applied to the unrestrained siphon with a flicking motion suggests that the light flicks used as a CS in some studies (Carew et al., 1981, 1983) did not activate the LE cells. We do not yet know, however, whether neuromodulation triggered by distant noxious stimuli might increase the chances of LE activation during prolonged training. It should also be noted that the electric shocks used as CS in the same studies do activate the LE cells as well as other afferents (P. Illich and E. Walters, unpublished observations). Direct extrapolation of results from the present study to patterns of sensory activation expected in others is difficult because of differences or uncertainties among published studies in (1) stimulus properties (Picospritzer vs Water Pik), (2) biomechanical properties of the siphon (e.g., in younger vs older animals), and (3) delivery of hand-held stimuli (e.g., in angle of application of water jets or filaments). Thus, it is unclear whether LE cells would have been activated by the water jets applied to unrestrained siphons in many studies of habituation and sensitization (Carew et al., 1972;Pinsker et al., 1973; Frost et al., 1985). Our findings emphasize the need for verifying that cells assumed to be involved in learning are actually activated during training, as has been done in some of the more recent studies of activity-dependent learning inAplysia (Walters, 1987b; Colebrook and Lukowiak, 1988;Hawkins and Frost, 1996). Moreover, they suggest that unidentified sensory populations may be important for learning involving light tactile stimulation of the siphon. This possibility is supported by evidence that synaptic potentials in gill motor neurons from unidentified low-threshold siphon afferents display plasticity quite similar to that of the LE cell connections (R.D. Hawkins, personal communication). Together, these observations suggest that the behavioral significance of some of the rich plasticity of the LE cells still needs to be explicitly tested.
LE cells have nociceptive functions
The LE cells show a graded increase in activity as stimulus force is increased into the noxious range, displaying maximal activation by crushing or tearing stimuli that clearly injure skin and body wall. LE cells thus meet standard definitions of nociceptor (Sherrington, 1947;Burgess and Perl, 1973; Light, 1992). Pinning the siphon causes a significant reduction in the mechanosensory thresholds of LE cells. Similarly, mammalian nociceptors show much lower mechanical thresholds in in vitro preparations in which the skin is mounted against a firm substrate (Kress et al., 1992). Part of this reduction is probably attributable to the decrease in effective compliance enhancing the rate that force reaches the receptors (cf. Kress et al., 1992). A mechanical contribution is supported by the persistence of depressed thresholds 1 hr after pinning (Fig. 5C), even when the pinning is performed under anesthesia that largely prevents hyperexcitability of the soma at the same test point (Fig.7C). The softness of the siphon under natural conditions and the dependence of LE cell threshold on tissue compliance suggest that these mechanosensory neurons are tuned to stimuli that minimize effective compliance, in particular, pinching, biting, and sharp grasping stimuli that prevent passive recoil of the tissue. This suggestion accords with evidence that Aplysia are subject to attacks by predators (lobsters and crabs) that pinch, bite, and grasp with sharp appendages (Pennings, 1990; Walters et al., 1993).
LE cell properties resemble those of myelinated nociceptors in mammals
Wide dynamic range and sensitivity to both noxious and innocuous mechanical forces also occur in mammalian nociceptors (Treede et al., 1990; Light, 1992; Cooper et al., 1993; Leem et al., 1993). For example, while responding maximally to noxious pressures of 100–1000g/mm2, some myelinated and unmyelinated cutaneous nociceptors in mammals respond to punctate stimuli exerting as little as 10 g/mm2. This is only two to three times the level necessary to activate most low-threshold mechanoreceptors in mammals, which generally show a decrease rather than an increase in response as stimuli reach noxious intensities (Light, 1992). Although von Frey hairs exerting 10–25g/mm2 do not normally produce pain when applied to human skin, stimulation in some regions of the body can yield pricking sensations in this range (Light, 1992; Meyer et al., 1994). The sensitivity of a nociceptor is usually matched to the toughness of the tissue it innervates; e.g., corneal nociceptor thresholds are <10% of cutaneous nociceptor thresholds (Belmonte and Giraldez, 1981;Tanelian and Beuerman, 1984), and the thresholds of cutaneous nociceptors in thick-skinned pigs are higher than those in thin-skinned rodents (Lynn et al., 1995). Because siphon tissue is thin and delicate, nociceptor thresholds should be relatively low. This is especially true if a major function of the LE cells, like myelinated Aδ nociceptors in mammals (Lynn, 1984), is to provide early warning of incipient injury. LE and VC sensory neurons in Aplysiashare a number of similarities with Aδ nociceptors, including the relatively high frequency of their peak responses (>30 vs <20 Hz for C polymodal nociceptors), relatively large RFs, and lack of activation by most chemical activators of C polymodal nociceptors (Burgess and Perl, 1973; Byrne et al., 1974; Walters et al., 1983a; Light, 1992;Clatworthy and Walters, 1993a; Leem et al., 1993) (P. Illich, M. Dulin, A. Billy, and E. Walters, unpublished observations). Sensitization of LE and VC cells also resembles that of Aδ nociceptors, which sometimes display marked decreases in mechanosensory threshold after noxious mechanical stimulation (Reeh et al., 1987; Meyer et al., 1991;Light, 1992). A major role for LE and VC cells in warningAplysia of the onset of injury is supported by their strong synaptic connections to neurons mediating rapid defensive responses (Walters et al., 1983a; Cleary et al., 1995; Frost and Kandel, 1995). Nociceptors have not yet been found in Aplysia that respond to chemical or thermal stimuli or that display sustained background firing. An invertebrate nociceptor with these properties is the leech N cell, which has revealed several similarities to C-polymodal nociceptors (Pastor et al., 1996).
Functional implications of nociceptive plasticity of LE cells
Support for a nociceptive role of LE cells comes from their marked sensitization by noxious stimulation. Primary afferent sensitization after noxious stimulation appears to be unique to nociceptors; indeed, low-threshold mechanoreceptors usually become less sensitive after tissue-damaging stimuli (Light, 1992). Such sensitization probably has compensatory and protective functions (Walters, 1991,1994). Compensatory sensitization of surviving branches should be useful in partially damaged LE and VC (as well as Aδ) nociceptors, which have relatively large RFs subserved by multiple axonal branches, some of which might be spared during injury. Enhanced sensitivity of surviving branches and neighboring nociceptors should help compensate for loss of nociceptor function in damaged branches. Peripheral sensitization may even make the region around an injury more sensitive than normal, thus helping to protect against predators and parasites attracted to a wound (Walters, 1994). Widely studied mechanisms by which central regions of these cells show enhanced excitability and transmitter release (Byrne et al., 1993; Byrne and Kandel, 1996) should also contribute to these compensatory and protective functions, as should downstream changes in interneurons (Billy and Walters, 1989;Walters, 1991, 1994; Clatworthy and Walters, 1993; Cleary et al., 1995). Various activity-dependent and injury-dependent increases in the signaling effectiveness of VC and LE cells appear important for storing persistent, site-specific memories of noxious unconditioned stimuli (Walters, 1987b; Walters and Ambron, 1995). Enhanced nociceptor responses to noxious stimuli produce the functional equivalent of hyperalgesia (Walters, 1987a). Decreased threshold, permitting a nociceptor to respond to normally ineffective stimuli, yields the functional equivalent of allodynia, promoting rapid withdrawal at the first contact with potential threats.
Footnotes
This work was supported by National Institute of Mental Health (NIMH) Grant MH 38726 (E.T.W.) and NIMH Fellowship MH 10727 (P.A.I.). We thank J. H. Byrne, A. L. Clatworthy, R. D. Hawkins, C. Hickie, and E. R. Kandel for helpful comments.
Correspondence should be addressed to Dr. Edgar T. Walters, Department of Integrative Biology and Pharmacology, University of Texas-Houston Medical School, P.O. Box 20708, Houston, TX 77225.
REFERENCES
- 1.Belmonte C, Giraldez F. Responses of cat corneal sensory receptors to mechanical and thermal stimulation. J Physiol (Lond) 1981;321:355–368. doi: 10.1113/jphysiol.1981.sp013989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billy AJ, Walters ET. Long-term expansion and sensitization of mechanosensory receptive fields in Aplysia support an activity-dependent model of whole-cell sensory plasticity. J Neurosci. 1989;9:1254–1262. doi: 10.1523/JNEUROSCI.09-04-01254.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunet JF, Shapiro E, Foster SA, Kandel ER, Iino Y. Identification of a peptide specific for Aplysia sensory neurons by PCR-based differential screening. Science. 1991;252:856–859. doi: 10.1126/science.1840700. [DOI] [PubMed] [Google Scholar]
- 4.Burgess PR, Perl ER. Cutaneous mechanoceptors and nociceptors. In: Iggo A, editor. Handbook of sensory physiology, somatosensory system. Springer; Berlin: 1973. pp. 29–78. [Google Scholar]
- 5.Byrne JH, Kandel ER. Presynaptic facilitation revisited: state and time dependence. J Neurosci. 1996;16:425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne JH, Castellucci V, Kandel ER. Receptive fields and response properties of mechanoreceptor neurons innervating siphon skin and mantle shelf in Aplysia. J Neurophysiol. 1974;37:1041–1064. doi: 10.1152/jn.1974.37.5.1041. [DOI] [PubMed] [Google Scholar]
- 7.Byrne JH, Castellucci VF, Carew TJ, Kandel ER. Stimulus-response relations and stability of mechanoreceptor and motor neurons mediating defensive gill-withdrawal reflex in Aplysia. J Neurophysiol. 1978a;41:402–417. doi: 10.1152/jn.1978.41.2.402. [DOI] [PubMed] [Google Scholar]
- 8.Byrne JH, Castellucci VF, Kandel ER. Contribution of individual mechanoreceptor sensory neurons to defensive gill-withdrawal reflex in Aplysia. J Neurophysiol. 1978b;41:418–431. doi: 10.1152/jn.1978.41.2.418. [DOI] [PubMed] [Google Scholar]
- 9.Byrne JH, Zwartjes R, Homayouni R, Critz SD, Eskin A. Roles of second messenger pathways in neuronal plasticity and in learning and memory. Insights gained from Aplysia. Adv Second Messenger Phosphoprotein Res. 1993;27:47–108. [PubMed] [Google Scholar]
- 10.Carew TJ, Sahley CL. Invertebrate learning and memory: from behavior to molecules. Annu Rev Neurosci. 1986;9:435–487. doi: 10.1146/annurev.ne.09.030186.002251. [DOI] [PubMed] [Google Scholar]
- 11.Carew TJ, Pinsker HM, Kandel ER. Long-term habituation of a defensive withdrawal reflex in Aplysia. Science. 1972;175:451–454. doi: 10.1126/science.175.4020.451. [DOI] [PubMed] [Google Scholar]
- 12.Carew TJ, Walters ET, Kandel ER. Classical conditioning in a simple withdrawal reflex in Aplysia californica. J Neurosci. 1981;1:1426–1437. doi: 10.1523/JNEUROSCI.01-12-01426.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carew TJ, Hawkins RD, Kandel ER. Differential classical conditioning of a defensive withdrawal reflex in Aplysia californica. Science. 1983;219:397–400. doi: 10.1126/science.6681571. [DOI] [PubMed] [Google Scholar]
- 14.Castellucci V, Pinsker H, Kupfermann I, Kandel ER. Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167:1745–1748. doi: 10.1126/science.167.3926.1745. [DOI] [PubMed] [Google Scholar]
- 15.Clatworthy AL, Walters ET. Rapid amplification and facilitation of mechanosensory discharge in Aplysia by noxious stimulation. J Neurophysiol. 1993a;70:1181–1194. doi: 10.1152/jn.1993.70.3.1181. [DOI] [PubMed] [Google Scholar]
- 16.Clatworthy AL, Walters ET. Activity-dependent depression of mechanosensory discharge and excitability in Aplysia. J Neurophysiol. 1993b;70:1195–1209. doi: 10.1152/jn.1993.70.3.1195. [DOI] [PubMed] [Google Scholar]
- 17.Clatworthy AL, Walters ET. Comparative analysis of hyperexcitability and synaptic facilitation induced by nerve injury in two populations of mechanosensory neurones of Aplysia californica. J Exp Biol. 1994;190:217–238. doi: 10.1242/jeb.190.1.217. [DOI] [PubMed] [Google Scholar]
- 18.Cleary LJ, Byrne JH, Frost WN. Role of interneurons in defensive withdrawal reflexes of Aplysia. Learn Memory. 1995;2:133–151. doi: 10.1101/lm.2.3-4.133. [DOI] [PubMed] [Google Scholar]
- 19.Cohen TE, Henzi V, Kandel ER, Hawkins RD. Further behavioral and cellular studies of dishabituation and sensitization in Aplysia. Soc Neurosci Abstr. 1991;17:1302. [Google Scholar]
- 20.Colebrook E, Lukowiak K. Learning by the Aplysia model system: lack of correlation between gill and gill motor neurone responses. J Exp Biol. 1988;135:411–429. doi: 10.1242/jeb.140.1.273. [DOI] [PubMed] [Google Scholar]
- 21.Cooper B, Loughner B, Friedman RM, Heft MW, LaBanc J, Fonte A. Parallels between properties of high-threshold mechanoreceptors of the goat oral mucosa and human pain report. Exp Brain Res. 1993;94:323–335. doi: 10.1007/BF00230302. [DOI] [PubMed] [Google Scholar]
- 22.Frost WN, Kandel ER. Structure of the network mediating siphon-elicited siphon withdrawal in Aplysia. J Neurophysiol. 1995;73:2413–2427. doi: 10.1152/jn.1995.73.6.2413. [DOI] [PubMed] [Google Scholar]
- 23.Frost WN, Castellucci VF, Hawkins RD, Kandel ER. Monosynaptic connections made by the sensory neurons of the gill- and siphon-withdrawal reflex in Aplysia participate in the storage of long-term memory for sensitization. Proc Natl Acad Sci USA. 1985;82:8266–8269. doi: 10.1073/pnas.82.23.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garell PC, Mcgillis SLB, Greenspan J. Mechanical response properties of nociceptors innervating feline hairy skin. J Neurophysiol. 1996;75:1177–1189. doi: 10.1152/jn.1996.75.3.1177. [DOI] [PubMed] [Google Scholar]
- 25.Hawkins RD, Frost L. Contribution of LE siphon sensory neurons to habituation, dishabituation and sensitization of the gill-withdrawal reflex in Aplysia. Soc Neurosci Abstr. 1995;21:1024. [Google Scholar]
- 26.Hawkins RD, Frost L. Contribution of monosynaptic EPSPs from LE siphon sensory neurons to mediation and habituation of the gill- and siphon-withdrawal reflex in Aplysia. Soc Neurosci Abstr. 1996;22:1445. [Google Scholar]
- 27.Hickie CP (1994) Functional and motor neuronal analysis of defensive siphon responses in Aplysia californica. PhD thesis, University of Texas Houston Graduate School of Biomedical Sciences.
- 28.Hickie C, Walters ET. Motor neuronal control of tail-directed and head-directed siphon responses in Aplysia californica. J Neurophysiol. 1995;74:307–321. doi: 10.1152/jn.1995.74.1.307. [DOI] [PubMed] [Google Scholar]
- 29.Hickie C, Cohen LB, Balaban PM. Voltage-sensitive dye recording from the Aplysia abdominal ganglion indicates modest LE cell response to a light siphon touch. Soc Neurosci Abstr. 1995;21:1679. [Google Scholar]
- 30.Hickie C, Cohen LB, Balaban PM (1997) The contribution of the LE sensory neurons to the Aplysia gill-withdrawal reflex. Eur J Neurosci, in press. [DOI] [PubMed]
- 31.Illich PA, Walters ET. Nociceptive responses and sensitization of LE siphon sensory neurons in Aplysia. Soc Neurosci Abstr. 1995;21:1679. [Google Scholar]
- 32.Illich PA, Joynes RL, Walters ET. Response-specific inhibition during general facilitation of defensive responses in Aplysia. Behav Neurosci. 1994;108:1–10. doi: 10.1037//0735-7044.108.3.614. [DOI] [PubMed] [Google Scholar]
- 33.Krasne FB, Glanzman DL. What we can learn from invertebrate learning. Annu Rev Psychol. 1995;46:585–624. [Google Scholar]
- 34.Kress M, Koltzenburg M, Reeh PW, Handwerker HO. Responsiveness and functional attributes of electrically localized terminals of cutaneous C-fibers in vivo and in vitro. J Neurophysiol. 1992;68:581–595. doi: 10.1152/jn.1992.68.2.581. [DOI] [PubMed] [Google Scholar]
- 35.Kumazawa T. Functions of the nociceptive primary neurons. Jpn J Physiol. 1990;40:1–14. doi: 10.2170/jjphysiol.40.1. [DOI] [PubMed] [Google Scholar]
- 36.Leem JW, Willis WD, Chung JM. Cutaneous sensory receptors in the rat foot. J Neurophysiol. 1993;69:1684–1699. doi: 10.1152/jn.1993.69.5.1684. [DOI] [PubMed] [Google Scholar]
- 37.Light AR. The initial processing of pain and its descending control: spinal and trigeminal systems. Karger; Basel: 1992. [Google Scholar]
- 38.Lynn B. Cutaneous nociceptors. In: Holden AV, Winlow W, editors. The neurobiology of pain. Manchester UP; Manchester: 1984. pp. 97–107. [Google Scholar]
- 39.Lynn B, Faulstroh K, Pierau FK. The classification and properties of nociceptive afferent units from the skin of the anaesthetized pig. Eur J Neurosci. 1995;7:431–437. doi: 10.1111/j.1460-9568.1995.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 40.Meyer RA, Davis KD, Cohen RH, Treede RD, Campbell JN. Mechanically insensitive afferents (MIAs) in cutaneous nerves of monkey. Brain Res. 1991;561:252–261. doi: 10.1016/0006-8993(91)91601-v. [DOI] [PubMed] [Google Scholar]
- 41.Meyer RA, Campbell JN, Raja SN. Peripheral neural mechanisms of nociception. In: Wall PD, Melzack R, editors. Textbook of pain. Churchill Livingstone; Edinburgh: 1994. pp. 13–44. [Google Scholar]
- 42.Pastor J, Bernat S, Belmonte C. Properties of the nociceptive neurons of the leech segmental ganglion. J Neurophysiol. 1996;75:2268–2279. doi: 10.1152/jn.1996.75.6.2268. [DOI] [PubMed] [Google Scholar]
- 43.Pennings SC. Multiple factors promoting narrow host range in the sea hare Aplysia californica. Oecologia. 1990;82:192–200. doi: 10.1007/BF00323535. [DOI] [PubMed] [Google Scholar]
- 44.Pinsker HM, Hening WA, Carew TJ, Kandel ER. Long-term sensitization of a defensive withdrawal reflex in Aplysia. Science. 1973;182:1039–1042. doi: 10.1126/science.182.4116.1039. [DOI] [PubMed] [Google Scholar]
- 45.Reeh PW, Bayer J, Kocher L, Handwerker HO. Sensitization of nociceptive cutaneous nerve fibers from the rat tail by noxious mechanical stimulation. Exp Brain Res. 1987;65:505–512. doi: 10.1007/BF00235973. [DOI] [PubMed] [Google Scholar]
- 46.Sherrington C. The integrative action of the nervous system. Yale UP; New Haven: 1947. [Google Scholar]
- 47.Tanelian DL, Beuerman RW. Responses of rabbit corneal nociceptors to mechanical and thermal stimulation. Exp Neurol. 1984;84:165–178. doi: 10.1016/0014-4886(84)90013-x. [DOI] [PubMed] [Google Scholar]
- 48.Treede RD, Meyer RA, Campbell JN. Comparison of heat and mechanical receptive fields of cutaneous C-fiber nociceptors in monkey. J Neurophysiol. 1990;64:1502–1513. doi: 10.1152/jn.1990.64.5.1502. [DOI] [PubMed] [Google Scholar]
- 49.Walters ET. Site-specific sensitization of defensive reflexes in Aplysia: a simple model of long-term hyperalgesia. J Neurosci. 1987a;7:400–407. doi: 10.1523/JNEUROSCI.07-02-00400.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walters ET. Multiple sensory neuronal correlates of site-specific sensitization in Aplysia. J Neurosci. 1987b;7:408–417. doi: 10.1523/JNEUROSCI.07-02-00408.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walters ET. A functional, cellular, and evolutionary model of nociceptive plasticity in Aplysia. Biol Bull. 1991;180:241–251. doi: 10.2307/1542394. [DOI] [PubMed] [Google Scholar]
- 52.Walters ET. Injury-related behavior and neuronal plasticity: an evolutionary perspective on sensitization, hyperalgesia and analgesia. Int Rev Neurobiol. 1994;36:325–427. doi: 10.1016/s0074-7742(08)60307-4. [DOI] [PubMed] [Google Scholar]
- 53.Walters ET, Ambron RT. Long-term alterations induced by injury and by 5-HT in Aplysia sensory neurons: convergent pathways and common signals? Trends Neurosci. 1995;18:137–142. doi: 10.1016/0166-2236(95)93891-z. [DOI] [PubMed] [Google Scholar]
- 54.Walters ET, Erickson MT. Directional control and the functional organization of defensive responses in Aplysia. J Comp Physiol [A] 1986;159:339–351. doi: 10.1007/BF00603980. [DOI] [PubMed] [Google Scholar]
- 55.Walters ET, Byrne JH, Carew TJ, Kandel ER. Mechanoafferent neurons innervating tail of Aplysia. I. Response properties and synaptic connections. J Neurophysiol. 1983a;50:1522–1542. doi: 10.1152/jn.1983.50.6.1522. [DOI] [PubMed] [Google Scholar]
- 56.Walters ET, Byrne JH, Carew TJ, Kandel ER. Mechanoafferent neurons innervating tail of Aplysia. II. Modulation by sensitizing stimulation. J Neurophysiol. 1983b;50:1543–1559. doi: 10.1152/jn.1983.50.6.1543. [DOI] [PubMed] [Google Scholar]
- 57.Walters ET, Alizadeh H, Castro GA. Similar neuronal alterations induced by axonal injury and learning in Aplysia. Science. 1991;253:797–799. doi: 10.1126/science.1652154. [DOI] [PubMed] [Google Scholar]
- 58.Walters ET, Illich PA, Hickie C. Inking and siphon response plasticity in Aplysia: anti-predator and alarm signal functions. Soc Neurosci Abstr. 1993;19:578. [Google Scholar]
- 59.Wright WG, Kirschman D. Direct comparison of serotonin effects on siphon versus tail sensory neurons in Aplysia. Learn Memory. 1995;2:178–184. doi: 10.1101/lm.2.3-4.178. [DOI] [PubMed] [Google Scholar]
- 60.Wright WG, Marcus EA, Carew TJ. A cellular analysis of inhibition in the siphon withdrawal reflex of Aplysia. J Neurosci. 1991;11:2498–2509. doi: 10.1523/JNEUROSCI.11-08-02498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xin Y, Weiss KR, Kupfermann I. Distribution in the central nervous system of Aplysia of afferent fibers arising from cell bodies located in the periphery. J Comp Neurol. 1995;359:627–643. doi: 10.1002/cne.903590409. [DOI] [PubMed] [Google Scholar]