Abstract
Neuronal voltage-dependent calcium channels undergo inhibitory modulation by G-protein activation, generally involving both kinetic slowing and steady-state inhibition. We have shown previously that the β-subunit of neuronal calcium channels plays an important role in this process, because when it is absent, greater receptor-mediated inhibition is observed (Campbell et al., 1995b). We therefore hypothesized that the calcium channel β-subunits normally may occlude G-protein-mediated inhibition. Calcium channel β-subunits bind to the cytoplasmic loop between transmembrane domains I and II of the α1-subunits (Pragnell et al., 1994). We have examined the hypothesis that this loop is involved in G-protein-mediated inhibition by making chimeras containing the I–II loop of α1B or α1A inserted into α1E (α1EBE and α1EAE, respectively). This strategy was adopted because α1B (the molecular counterpart of N-type channels) and, to a lesser extent, α1A (P/Q-type) are G-protein-modulated, whereas this has not been observed to any great extent for α1E. Although α1B, coexpressed with α2-δ and β1b transiently expressed in COS-7 cells, showed both kinetic slowing and steady-state inhibition when recorded with GTPγS in the patch pipette, both of which were reversed with a depolarizing prepulse, the chimera α1EBE (and, to a smaller extent, α1EAE) showed only kinetic slowing in the presence of GTPγS, and this also was reversed by a depolarizing prepulse. These results indicate that the I–II loop may be the molecular substrate of kinetic slowing but that the steady-state inhibition shown by α1B may involve a separate site on this calcium channel.
Keywords: calcium channel, G-protein, β-subunit, α1B, α1E, modulation
Voltage-dependent calcium channels are hetero-oligomers consisting of a number of subunits: α1, which is the pore-forming subunit, and several accessory subunits, including α2-δ and β (Dolphin, 1995). Cloning has revealed six different α1-subunits, termed A, B, C, D, E, and S (Tanabe et al., 1987; Snutch et al., 1990). C, D, and S correspond to L-type channels (for review, see Dolphin, 1995), and α1B corresponds to the ω-conotoxin (CTX) GVIA-sensitive N-type calcium channel (Dubel et al., 1992). In contrast, the physiological counterparts of the α1A and α1E calcium channels are less clearly established (Sather et al., 1993; Soong et al., 1993; Schneider et al., 1994; Stea et al., 1994; Berrow et al., 1997; Stephens et al., 1997).
Neuronal and neurosecretory subtypes of calcium channels, including N, P, Q, and L, have been shown to be inhibited by various neurotransmitters and modulatory agents (Kleuss et al., 1991;Menon-Johansson et al., 1993; Mintz and Bean, 1993; Zhang et al., 1993). The modulation involves activation of a G-protein that is usually, but not invariably, pertussis toxin-sensitive (Hille, 1992;Dolphin, 1995). In many systems evidence has been obtained that the G-protein involved is Go (Kleuss et al., 1991; Wang et al., 1992; Campbell et al., 1993). Recent expression studies have reconstituted G-protein modulation of cloned α1A and α1B, but not α1E, calcium channels by several receptors (Bourinet et al., 1996;Toth et al., 1996). The main mechanism of modulation is thought to be membrane-delimited (i.e., not involving a soluble second messenger) and to be attributable to a direct interaction between activated G-protein subunits and one of the calcium channel subunits (Hille, 1992). The calcium channel β-subunits are intracellular proteins that bind to the cytoplasmic loop between domains I and II of the α1-subunit of all calcium channels (Pragnell et al., 1994). We have obtained evidence, by antisense depletion of calcium channel β-subunits from cultured rat dorsal root ganglion neurons, that coupling of calcium channels to G-proteins may involve direct or indirect competition between the activated G-protein and the calcium channel β-subunit for binding to the calcium channel α1-subunit (Berrow et al., 1995;Campbell et al., 1995b). This was confirmed in a coexpression study of calcium channel subunits in Xenopus oocytes, in which it was found that G-protein modulation of α1A by activation of expressed opiate receptors was greater in the absence of a coexpressed calcium channel β-subunit (Bourinet et al., 1996). Recent studies also suggest that G-protein subunits involved in interaction with α1A and α1B are the Gβγ-subunits (Herlitze et al., 1996; Ikeda, 1996). It is therefore possible that these G-protein subunits interact with the I–II loop of calcium channel α1-subunits to produce modulation of the channel.
The hypothesis that the I–II loop of α1A and α1B calcium channels is involved in G-protein modulation has been tested in the present study by creating a chimera, which consists of α1E with the I–II loops from α1A or α1B, to determine whether the ability to be modulated by G-protein activation in this way can be conferred on the α1E calcium channel.
MATERIALS AND METHODS
Construction of chimeras. The rat α1A (GenBank accession number M64373), α1E (L15453), and β1b (X61394) cDNAs (Starr et al., 1991; Soong et al., 1993; Tomlinson et al., 1993) were provided by Dr. T. Snutch (University of British Columbia, Vancouver, Canada) in a modified pMT2 expression vector (Genetics Institute, Cambridge, MA). The rabbit α1B (D14157) (Fujita et al., 1993) was provided by Dr. Y. Mori (Seiriken, Okazaki, Japan); the full-length rat α2-δ (neuronal splice variant, M86621) (Kim et al., 1992) was provided by Dr. H. Chin (National Institutes of Health, Bethesda, Maryland). The S65T mutant of GFP was a gift from Dr. S. Moss (University College London, London, UK). All DNAs were subcloned, using standard techniques, into the pMT2 vector for transient expression in COS-7 cells.
To produce chimeras containing the I–II loop of α1A or B substituted for the same region of α1E, we performed PCR on α1E subcloned into the EcoRI site of the pcDNA3 vector (Invitrogen, San Diego, CA). Chimeric primers were directed against regions of the IS6 and IIS1 domains conserved between α1E, α1A, and α1B. Rat α1A and rabbit α1B I–II loops were amplified using the primers GGAACTGGCTGTACTTCATCC (at position 1024 in α1E, 1010 in α1A, and 1112 in α1B) and CACTCAGGACGATCCAGTAGAA (position 1500 in α1E, 1492 in α1A, and 1594 in α1B) to give 482 base pair (bp) fragments. Then the 482 bp products were used as primers in two individual second-stage PCR reactions in the presence of α1E, one containing the pcDNA3 forward primer, CTCACTATAGGGAGACCCAAGC, and the other containing the reverse primer, GACTTCATGGAGCTCATCAAGG (position 1852 in α1E). These PCR products (of 1430 and 834 bp) were combined in a third-stage reaction, in the absence of α1E, and extended to give a full-length product of 1782 bp. To facilitate subcloning, we put a 3314 bp fragment (between XbaI nucleotide 822 and ApaI 4134) into the XbaI–ApaI sites of pcDNA3. The 1782 bp product was digested with the enzymes XbaI andAccB7I, and the 980 bp DNA was subcloned back into the 3314 bp fragment in pcDNA3. All PCR was performed using the proofreadingPfu polymerase (Stratagene, La Jolla, CA) for 30 cycles of 95°C for 30 sec, 54°C for 1 min, and 75°C for 2 min. The sequence of the chimeras between the XbaI (822 bp, α1E) andAccB7I (1802 bp) sites was verified by the SequiTherm Cycle Sequencing kit (Epicenter Technologies, Madison, WI). The 3314 bpXbaI–ApaI DNA was subcloned back into the remainder of the α1E pMT2 vector. This resulted in chimeras with substitution of the I–II loop of α1E for that of α1A or B. Part of IS6 also was substituted, but this is identical in the three sequences, except for V293 in α1E, which is substituted by M in α1A, B, and the chimeras.
Transfection of COS-7 cells. COS-7 cells were cultured and transfected by electroporation essentially as described previously (Campbell et al., 1995a). In all, 15, 10, 5, and 1 μg of the pMT2-α1, α2-δ, β1b, and GFP constructs, respectively, were used for transfection. If all subunits were not transfected, the total 31 μg of cDNA was made up by pMT2 vector. Successfully transfected cells were identified for electrophysiological studies by expression of GFP, and recordings were made between 2 and 4 d after transfection.
Electrophysiology. Recordings were made at room temperature (20–22°C) from COS-7 cells that had been replated between 1 and 16 hr previously, using a nonenzymatic cell dissociation medium (Sigma, St. Louis, MO). Only small cells with a circular morphology were used. Mean cell capacitance was ∼20 pF. Cells were viewed briefly with a fluorescein filter block, and only fluorescent cells expressing GFP, which were spatially isolated and with a compact morphology and smooth surface as visualized by Hoffmann optics, were used in experiments. The internal (pipette) and external solutions and recording techniques are similar to those previously described (Campbell et al., 1995b). The patch pipette solution contained (in mm): Cs aspartate 140, EGTA 5, MgCl2 2, CaCl2 0.1, K2ATP 2, GTP 0.1, and HEPES 10, pH 7.2, 310 mOsm with sucrose. GTPγS (100 μm) was included where stated. The external solution contained (in mm): tetraethylammonium (TEA) bromide 160, KCl 3, NaHCO3 1.0, MgCl2 1.0, HEPES 10, glucose 4, and BaCl2 10, pH 7.4, 320 mOsm with sucrose. Pipettes of resistance 2–4 MΩ were used, and the holding current at −100 mV was normally <20 pA. Cells were used only where series resistance was compensated to 80%, and space clamp was adequate as judged by graded activation of IBa. The voltage errors from the residual uncompensated series resistance were <1 mV for the largest currents, and no further correction was made. An Axopatch 1D or Axon 200A amplifier was used, and data were filtered at 2–5 kHz and digitized at 5–20 kHz. Analysis was performed by pClamp 6 and Origin 3.5. Data are given as mean ± SEM, and current records are shown after leak and residual capacitance current subtraction (P/4 or P/8 protocol).
RESULTS
Characteristics of α1E and α1B expressed in COS-7 cells
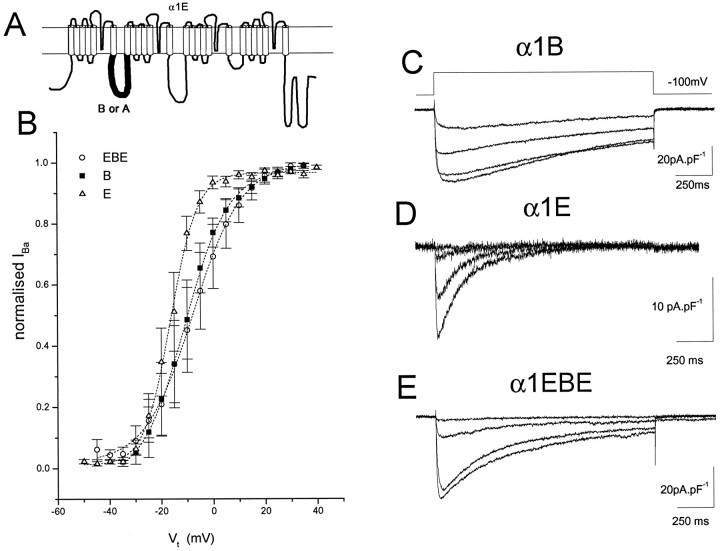
The α1-subunits A, B, and E and the α1EBE chimera (Fig.1A) were transiently expressed with accessory subunits α2-δ and β1b in COS-7 cells. The properties of α1E and α1B were clearly distinct from each other in terms of both voltage dependence of their activation (Fig. 1B) and kinetics of inactivation (Fig. 1C,D). α1E was activated at slightly more negative potentials than α1B, the midpoint for activation being 5 mV more hyperpolarized, and it showed a slightly steeper voltage dependence (Fig. 1B, Table1). Most strikingly, it also showed a much greater degree of inactivation than α1B during 1500 msec steps (Fig.1D compared with Fig. 1C). However, the steady-state inactivation profiles of α1E and α1B were very similar (Table 1). The chimera α1EBE, the sequence of which was identical to that of α1E except for replacement of the entire intracellular loop between domains I and II with that of α1B and one substitution in IS6 (Fig. 1A; see Materials and Methods), showed a more depolarized voltage dependence of activation than α1E, similar to α1B (Fig. 1B). Its steady-state inactivation parameters were similar to α1E and α1B (Table 1); it showed inactivation kinetics intermediate between those of α1B and α1E (Fig. 1E, Table 1). The current densities resulting from expression of α1E, α1B, and α1EBE were similar (Table 1), but the percentage of GFP-positive cells expressing α1E was greater (∼80%) than for α1B (∼40%). The percentage of cells expressing α1EBE was similar to that for α1E.
Fig. 1.
IBa was recorded from cells transfected with α1E, α1B, and α1EBE, together with α2-δ and β1b. A, Schematic diagram of the chimera α1EBE. B, The holding potentialVH was −100 mV, and 20–30 msec steps to increasing test potentials Vt were applied to maximally activate IBa without any inactivation. Tail current amplitudes were measured after repolarization to −80 mV. The tail current I–Vrelationships were normalized to the maximum tail current amplitude, and the mean ± SEM of four, seven, and three experiments for α1B (▪), α1E (▵), and α1EBE (○) are given. The curves were fit (dotted lines) with a Boltzmann equation of the form: Inorm = 1/{1+exp[(Vt−V50)/k]}, in which V50 is the voltage for 50% activation and k is the slope factor. The values for the parameters are given in Table 1 for the mean ± SEM of the individual activation curves. C–E, Cells were held at −100 mV, and 1500 msec steps to voltages between −30 and 0 mV (C, D) or −35 to −5 mV (E) (ΔV 10 mV) were applied to examine the rate of inactivation ofIBa. τinact values are given in Table 1.
Table 1.
Biophysical parameters of calcium channel currents resulting from expression of α1B, α1E, α1EBE, and α1EAE with β1b and α2-δ in COS-7 cells
| Control | α1B | α1E | α1EBE | α1EAE |
|---|---|---|---|---|
| PeakIBapA/pF | −30.9 ± 7.2 (8) | −29.8 ± 7.2 (10) | −25.6 ± 7.6 (8) | −48.5 ± 11.4 (9) |
| Current activation | ||||
| V50 mV | −11.4 ± 3.9 (4) | −16.4 ± 1.9 (7) | −9.1 ± 7.1 (3) | −14.0 ± 3.0 (7) |
| k mV | 6.0 ± 0.7 (4) | 4.3 ± 0.8 (7) | 6.6 ± 0.7 (3) | 6.6 ± 0.7 (n = 7) |
| Steady-state inactivation | ||||
| V50 mV | −61.3 ± 7.1 (3) | −59.7 ± 3.6 (3) | −56.8 ± 4.1 (4) | ND |
| k mV | −6.6 ± 1.1 (3) | −11.5 ± 1.3 (3) | −5.5 ± 1.7 (4) | ND |
| τinact at −10 mV msec | 1021 ± 648 (6) | 210 ± 25 (5) | 503.4 ± 68.8# (7) | 438 ± 65# (10) |
| GTPγS | α1B (GTPγS) | α1E (GTPγS) | α1EBE (GTPγS) | α1EAE (GTPγS) |
|---|---|---|---|---|
| Peak IBapA/pF | −18.7 ± 6.1* (6) | −31.6 ± 7.0 (7) | −20.3 ± 4.3 (12) | −45.0 ± 10.9 (6) |
| Current activation | ||||
| V50 mV | −7.9 ± 5.3 (4) | −16.1 ± 2.5 (6) | −10.7 ± 4.7 (3) | −9.2 ± 5.4 (4) |
| k mV | 7.6 ± 1.1⧫ (4) | 4.7 ± 1.1 (6) | 7.3 ± 0.65♦ (3) | 6.2 ± 0.5 (4) |
| Steady-state inactivation | ||||
| V50 mV | −58.5 ± 4.5 (3) | −58.3 ± 3.2 (3) | −54.7 ± 3.5 (3) | ND |
| k mV | −6.4 ± 0.6 (3) | −9.9 ± 1.3 (3) | −6.6 ± 1.2 (3) | ND |
| τinact at −10 mV msec | 1377 ± 405# (7) | 218 ± 24 (5) | 470.8 ± 91.7⧫ (6) | 513 ± 82# (5) |
Peak IBa was determined fromI–V relationships. Activation data were determined from tail currents as described in the legend to Figure 1B. For steady-state inactivation, cells were held at −100 mV, and depolarizing prepulses of 15 sec duration were applied between −100 and 0 mV (Δ 10 mV) before recording the maximumIBa at −5 to +10 mV. Values were expressed as a fraction of the maximum IBa seen in each cell, and V50 and k were determined from a Boltzmann relationship of the form given in the legend to Figure 1. τinact was determined from 600–2000 msec steps to −10 mV, as shown in Figure 1C–E. Data are given as mean ± SEM, with the number of determinations in parentheses. Significance of difference between data in the presence of GTPγS compared with control data for each calcium channel clone is given by
p< 0.05,
F1-160: p < 0.01. Significance of difference of α1B and the two chimeras from α1E is given by ⧫p< 0.05,
F1-159: p < 0.01. ND, Not determined.
Comparison of the effect of GTPγS on the kinetics of activation of α1B, α1E, and α1EBE
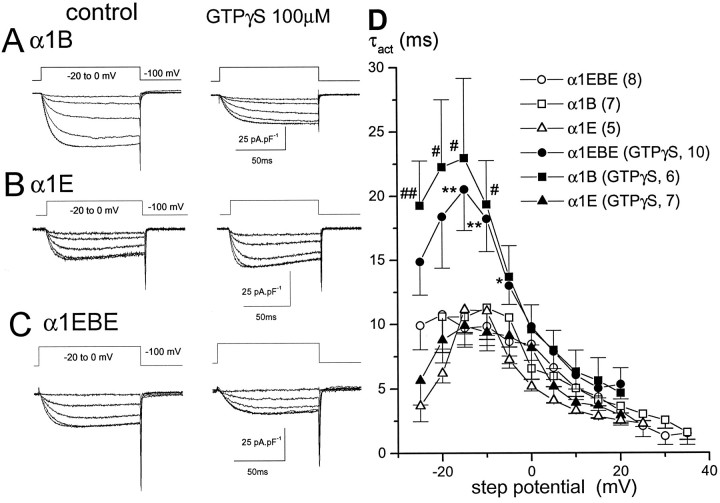
To examine the effect of G-protein activation on the expressed calcium channel currents, we included 100 μm GTPγS in the patch pipette, and currents were recorded after it had diffused into the cell for 2–5 min. GTPγS produced a clear slowing of the activation of α1B, but not α1E, currents as compared with control currents recorded in the absence of GTPγS (Fig.2A compared with 2B), indicative of G-protein modulation of α1B, but not α1E, currents. This was most evident from examination of the time constant of activation (τact) at depolarizations between −20 and 0 mV when the current amplitude is submaximal (Fig.2D).
Fig. 2.
IBa was recorded from cells transfected with α1B (A), α1E (B), and α1EBE (C), together with α2-δ and β1b. Cells were held at −100 mV, and 100 msec steps from −20 mV (Δ 5 mV) were applied to examine the kinetics of activation of IBa. The examples given are from different cells recorded either in the absence or in the presence of GTPγS in the patch pipette. Mean amplitudes of the maximumIBa are given in Table 1. A single exponential was fit to the activation phase of the current, initiated after the transient positive-going current had decayed back to baseline, to quantify the rate of activation. Examples of single exponential fits (heavy dotted lines) are given for the maximum currents at 0 mV for the two families of traces inA. The time constant of activation (τact) is 7.9 msec for control and 11.0 msec for the GTPγS-containing cell.D, The τact values were plotted against voltage for α1EBE (○), α1B (□), and α1E (▵), both under control conditions (open symbols) and in the presence of GTPγS (closed symbols). The mean ± SEM is shown for the number of cells given inparentheses on the figure. It is clear that only α1B and α1EBE show slowed activation in the presence of GTPγS, particularly at submaximal voltages for IBaactivation. Statistical significance (Student’s t test) of GTPγS groups from their respective controls is given by *p < 0.05, **p < 0.01 for α1EBE, #p < 0.05 and ##p < 0.01 for α1B.
Because of the possibility that the site of G-protein modulation of calcium channels resided on the I–II loop of the α1B-subunit, we examined the ability of the α1EBE chimera to be modulated by G-protein activation. GTPγS now produced a slowing of the activation of the α1EBE calcium channel current similar to that found for α1B (Fig. 2C,D). Thus the incorporation of the I–II loop from α1B into α1E endows the chimera with the ability to be modulated by G-proteins. However, a comparison of the current–voltage relationships from cells recorded in the absence or presence of GTPγS in the patch pipette indicates that G-protein activation has had a greater inhibitory effect on the amplitude of α1B currents (Fig.3A) than is evident for the α1EBE chimera (Fig. 3B). No effect was observed of GTPγS on the current–voltage relationships for α1E (Fig. 3C), and there was no effect of G-protein activation on the steady-state inactivation parameters for any of the calcium channel clones (Table1).
Fig. 3.
Mean current–voltage relationships were determined for cells under control conditions (open symbols) and in the presence of GTPγS (closed symbols) for α1B (□, 8; ▪, 6),α1EBE (○, 12; •, 9), and α1E(▵, 6; ▴, 6). The data are the mean ± SEM for thenumbers given in parentheses. Statistical significance between control and GTPγS groups is given by *p < 0.05 (Student’s ttest).
Effect of depolarizing prepulses on activation of parental α1E, α1B, and chimeric α1EBE calcium channels
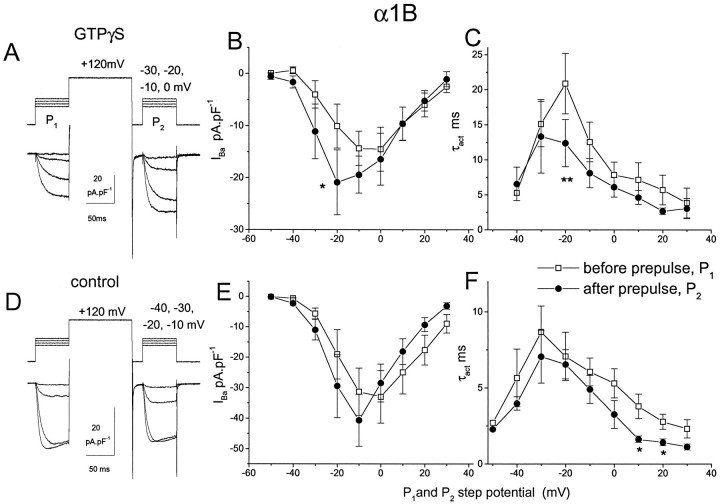
Depolarizing prepulses previously have been shown to reverse the G-protein modulation of calcium currents (Tsunoo et al., 1986; Grassi and Lux, 1989). This protocol was used in the present study to examine the extent of calcium channel current modulation by GTPγS for both parental and chimeric channels. Depolarizing prepulses to varying voltages (+80 to +140 mV) markedly enhanced calcium current activation and amplitude of α1B currents in the presence of GTPγS while having less effect on α1B currents in the absence of GTPγS. The maximum enhancement was observed with 100 msec depolarizing prepulses to +120 mV (results not shown). For subsequent experiments, a constant prepulse to +120 mV was used, and test pulses of increasing amplitude were applied immediately before (P1) and 10 msec after (P2) the depolarizing prepulse. Thus the effect of the depolarizing prepulse on current–voltage and τact–voltage relationships was examined. For α1B, in GTPγS-dialyzed cells, the prepulse produced a marked enhancement of the calcium channel current amplitude and its rate of activation, particularly at small depolarizations (Fig.4A–C). Therefore, the prepulse shifted the voltage for half-activation of the current by approximately −6 mV. At −20 mV, the P2/P1 ratio was 0.61 ± 0.04 (n = 6) for τact and 1.76 ± 0.23 (n = 6) forIBa amplitude. No significant effect was observed either on τact or IBaamplitude in the absence of GTPγS (Fig.4D–F).
Fig. 4.
Cells were transfected with α1B, α2-δ, and β1b and recorded after 3–4 d in culture.IBa was examined immediately before (P1) and 10 msec after (P2) application of a depolarizing prepulse to +120 mV, according to the voltage protocol given in A and D.P1 andP2 both were augmented at 0.05 Hz from −40 mV with Δ 10 mV to activate currents in cells recorded in the presence of GTPγS (A) or in control cells (B). The IBa amplitude and τact were determined for the currents evoked byP1 (□) andP2 (•), and these are plotted against the step potential of P1and P2 forIBa and τact in GTPγS-modulated (B, C) and control (E, F) cells, respectively. The mean ± SEM is given for six GTPγS-modulated and seven control cells. The statistical significance of difference between both τact andIBa amplitude evoked inP1 andP2 was determined by pairedt test; *p < 0.05, **p< 0.01.
For α1E, there was little effect at any potential of a depolarizing prepulse, either on τact or on current amplitude in the presence or absence of GTPγS. For example, at −20 mV in control cells, τact was 5.7 ± 0.6 msec and 4.5 ± 0.4 msec before and after the prepulse, respectively. The P2/P1 ratio was 0.81 ± 0.05 (n = 6; p < 0.05, paired ttest). The corresponding values were 5.4 ± 1.7 msec and 4.6 ± 1.2 msec in the presence of GTPγS, giving a P2/P1 ratio of 0.89 ± 0.04 (n = 5). At the same potential, the P2/P1 ratios for the current amplitudes were 0.99 ± 0.06 in control cells and 1.03 ± 0.04 in GTPγS-dialyzed cells. Thus we conclude that α1E is not subject to G-protein modulation in this system, although there is a small degree of prepulse facilitation of the control activation kinetics.
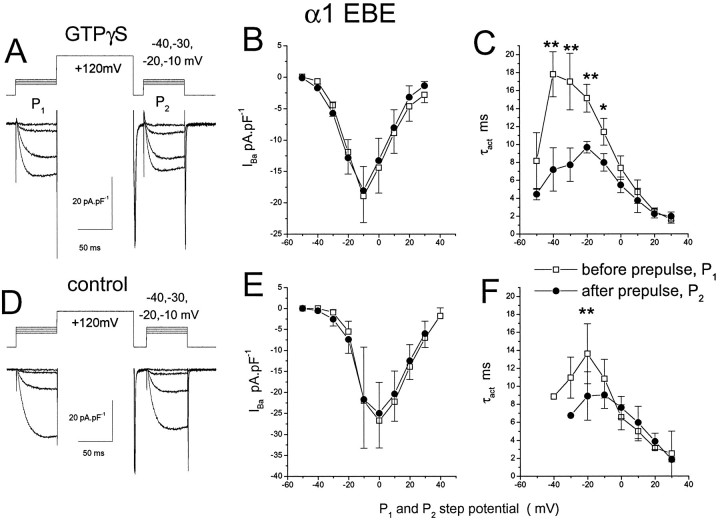
In marked contrast with its effect on the parental α1E, the depolarizing prepulse significantly enhanced the rate of activation of the chimera α1EBE calcium channel current (Fig. 5), particularly in the presence of GTPγS (Fig. 5A,C). For example, at −20 mV, P2/P1 for τact was 0.63 ± 0.05 (n = 8; Fig.5C) in the presence of GTPγS and 0.75 ± 0.08 (n = 5; Fig. 5F) under control conditions. Clearly, there is some prepulse facilitation of the activation kinetics of α1EBE IBa in the absence of GTPγS, but this is increased greatly in its presence. However, there was no effect of the prepulse on current amplitude, either in the presence or absence of GTPγS (Fig.5B,E).
Fig. 5.
Cells were transfected with the α1EBE chimera, together with α2-δ and β1b, and experiments were performed exactly as described in the legend to Figure 4. The mean ± SEM is given for nine GTPγS-modulated and five control cells. The statistical significance of difference between both τactand IBa amplitude evoked inP1 (□), andP2 (•) was determined by pairedt test; *p < 0.01, **p < 0.001.
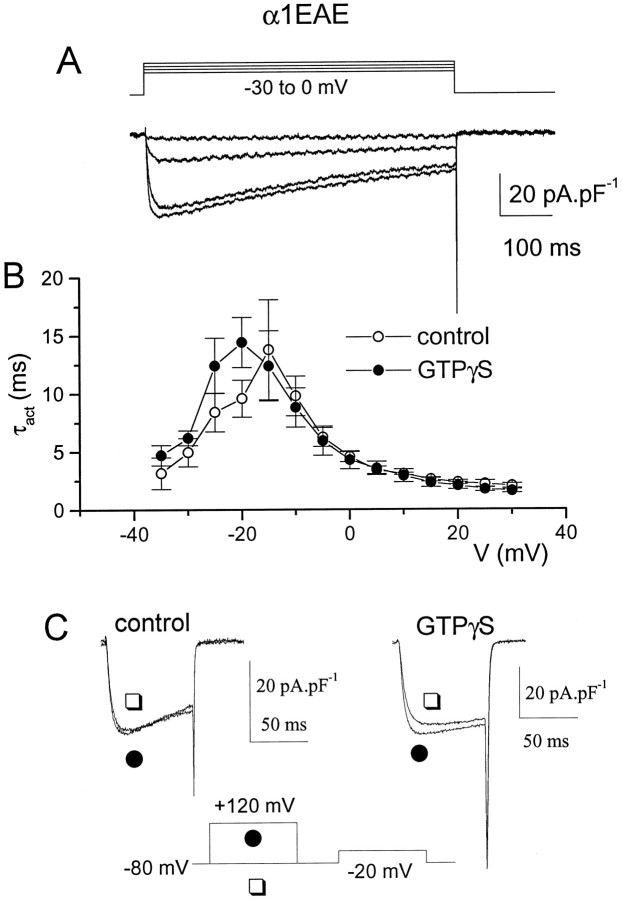
Characteristics of chimeric α1EAE expressed in COS-7 cells
Because there is evidence in the literature that α1A also may be G-protein-modulated, although to a more limited extent than α1B (Bourinet et al., 1996), a similar chimera also was made containing the intracellular I–II loop of α1A, replacing the I–II loop of α1E (α1EAE). We previously have described the properties of the α1A clone expressed in the COS-7 cell expression system (Berrow et al., 1997). It was not examined further in this study, because its low expression levels precluded direct comparison. The properties of the α1EAE chimera are shown in Figure 6 and Table 1. The voltage dependence of activation was similar to α1E (Table 1) and much more negative than α1A (V50 +9.5 mV;Berrow et al., 1997). The inactivation kinetics were intermediate between α1E and α1B, being similar to α1EBE (Fig.6A, Table 1). A comparison of τinactbetween α1EAE and data previously obtained for α1A was difficult because of the differences in their voltage range for activation, given the voltage dependence of inactivation kinetics. However, at +10 mV, τinact was 297 ± 54 msec (n = 8) for α1EAE, as compared with 414 ± 15 msec (n = 5) at +15 mV for α1A (Berrow et al., 1997).
Fig. 6.
Cells were transfected with the α1EAE chimera, together with α2-δ and β1b, and IBawas recorded after 3 d in culture. A,IBa was activated by 600 msec steps to examine the rate of inactivation of α1EAE. B,IBa was activated by 100 msec steps, and τact was measured as described in the legend to Figure 3for cells recorded in the presence of 100 μm GTPγS in the patch pipette (•, n = 5), or in its absence (○, n = 7). C,IBa was recorded in the presence (•) or absence (□) of a +120 mV depolarizing prepulse applied 30 msec before the test pulse to −20 mV for a control cell (left) and a cell containing GTPγS (right). Prepulse facilitation was observed only in the GTPγS-containing cell.
A small effect of GTPγS was observed on the kinetics of activation of α1EAE (Fig. 6B, Table 1), τact for IBa at −20 mV being 9.7 ± 1.6 msec (n = 7) in control cells and 14.4 ± 2.1 msec (n = 5) in cells recorded in the presence of GTPγS. In agreement with this, in GTPγS-dialyzed cells a depolarizing prepulse to +120 mV applied before a test pulse to −20 mV decreased τact from 9.1 ± 2.0 msec to 7.1 ± 2.1 msec (n = 5; p < 0.01, pairedt test; Fig. 6C). However, the same depolarizing prepulse produced no facilitation of the amplitude ofIBa (50.9 ± 20.7 pA/pF to 55.6 ± 20.6 pA/pF; n = 5; Fig. 6C). In control cells, no effect of the same depolarizing prepulse was observed either on the amplitude or τact ofIBa.
DISCUSSION
G-protein regulation of α1B and α1EBE in COS-7 cells
The most significant result of the present study is that the cytoplasmic loop between domains I and II of the B-type calcium channel α1-subunit is sufficient to confer aspects of G-protein sensitivity on the α1E calcium channel clone, which itself shows no or little G-protein modulation (Bourinet et al., 1996; Toth et al., 1996; Yassin et al., 1996). It was first necessary for us to demonstrate classical G-protein modulation of the α1B calcium channel expressed in COS-7 cells. Because of the lack of suitable endogenous receptors in COS-7 cells, we have chosen to produce G-protein activation by dialysis of GTPγS from the patch pipette. The expressed α1B currents exhibited both of the classical characteristics of G-protein modulation: reduced amplitude and slowed activation in the presence of GTPγS. Furthermore, both of these effects could be reversed by a depolarizing prepulse. This has been shown previously for opiate modulation of α1B in oocytes (Bourinet et al., 1996) and for somatostatin modulation of α1B in a stable HEK293 cell line (Toth et al., 1996). Although COS-7 cells contain no Gαo, they have several Gαispecies as well as Gαq and Gα11 (Boyer et al., 1989). GTPγS is able to bypass the specificity for Go of receptor-mediated modulation of calcium channels (McFadzean et al., 1989; Kleuss et al., 1991; Campbell et al., 1993) and will liberate Gβγ from all available sources. Recent evidence suggests that this is the G-protein species responsible for modulation of the neuronal calcium channels α1A and α1B (Herlitze et al., 1996; Ikeda, 1996).
Role of the cytoplasmic I–II loop of α1A and α1B in G-protein modulation
The cytoplasmic I–II loop contains the major binding site for the calcium channel β-subunit (Pragnell et al., 1994; De Waard et al., 1995; Witcher et al., 1995), the association of which modifies the properties of calcium channel α1-subunits (Lory et al., 1993; Neely et al., 1993; Stea et al., 1993; Berrow et al., 1995). We have shown previously that the presence of the calcium channel β-subunit reduces the ability of native neuronal calcium channels to be modulated by G-protein activation, because depletion of calcium channel β-subunits from dorsal root ganglion neurons by antisense oligonucleotide injection enhanced the ability of the calcium current to be modulated by GABAB receptor activation (Campbell et al., 1995b). This result was confirmed in an oocyte expression study (Bourinet et al., 1996) in which the coexpression of a calcium channel β-subunit with α1A decreased the modulation observed as a result of activation of expressed opiate receptors. We put forward the proposal that there is either direct or allosteric competition between the activated G-protein subunits and the calcium channel β-subunits for binding to the calcium channel α1-subunit (Campbell et al., 1995b). It is, therefore, feasible to speculate in the light of the present results that the I–II loop of the calcium channel α1B- and α1A-subunits contains an essential site of interaction required for G-protein modulation. Therefore, because our results indicate that the I–II loop of α1B and, to a lesser extent, α1A confer G-protein sensitivity on the α1E calcium channel, it would seem likely that the G-protein subunits mediating this effect (Herlitze et al., 1996; Ikeda, 1996) bind to a region on the I–II loop of α1B. We have preliminary evidence that Gβγ mediates the observed effects associated with the I–II loop (G. J. Stephens, N. S. Berrow, A. C. Dolphin, unpublished observations).
Kinetic slowing, but not steady-state inhibition, is conferred on α1E by the I–II loop of α1B or α1A
Insertion of the I–II loop of α1B into α1E conferred on the resultant chimeric calcium channel one key characteristic of G-protein modulation, that of slowed activation and reversal of this slowing by depolarizing prepulses. The I–II loop of α1A produced a similar, although less marked, effect. However, the other response observed in α1B, inhibition of the steady-state current amplitude, was not present in α1EBE or α1EAE. Several groups previously have noted differences between these two properties: kinetic slowing and scaled or steady-state inhibition (Ciranna et al., 1993; Diversé-Pierluissi et al., 1995). It has been suggested that the kinetic slowing represents voltage-dependent inhibition, possibly a dissociation of activated G-protein from the channel at depolarized potentials (Boland and Bean, 1993). Others have found the steady-state inhibition to be a voltage-independent process (Luebke and Dunlap, 1994), although in many instances prepulse facilitation of G-protein-modulated currents not only restores the control rate of activation of the current but also markedly increases its amplitude (Ikeda, 1991, 1996). A number of pieces of evidence have been put forward to suggest that the two processes involve different calcium channel subtypes (Ciranna et al., 1993), although this would seem unlikely here, because both effects are observed for cloned α1B. However, it also has been suggested that they involve different mechanisms (Diversé-Pierluissi et al., 1995), kinetic slowing being a direct G-protein-mediated process and steady-state inhibition resulting from Gβγ activation of the protein kinase C pathway. The present results would support the hypothesis of two separate mechanisms and would suggest further that whereas kinetic slowing involves the I–II loop of α1B and, to a lesser extent, α1A, another region apart from this loop may be responsible for the G-protein-mediated steady-state inhibition of calcium channel current. However, it is also clear that the kinetics of inactivation of the channel will affect the ability to observe prepulse potentiation of calcium currents, and we have shown in the present experiments that α1EBE shows more rapid voltage-dependent inactivation than α1B. In this context additional experiments are in progress to examine the properties of the mutant α1BEB, with the I–II loop of α1E inserted into α1B.
Role of the cytoplasmic I–II loop and IS6 in determination of inactivation kinetics
Different calcium channel α1-subunits show different intrinsic inactivation rates (Ellinor et al., 1993), α1E being the most rapidly inactivating. Furthermore, the binding of different calcium channel β-subunits to the α1-subunit modifies inactivation in a subunit-specific manner (Ellinor et al., 1993; Olcese et al., 1994). β2a, in contrast to the other β-subunits, produces a marked reduction in inactivation rate (Ellinor et al., 1993; Olcese et al., 1994). It has been found that the extreme N terminus of the β-subunit is responsible for determining its inactivation properties (Olcese et al., 1994), whereas the binding domain for the interaction with the I–II loop of the α1-subunit is in the center of the β-subunit sequence (De Waard et al., 1994). In a previous study on chimeras between the slowly inactivating α1A and the rapidly inactivating α1E (doe-1), it was found that a region including IS6 and stretching 19 amino acids into the I–II loop was important for determining the inactivation properties of the α1-subunit (Zhang et al., 1994). A subsidiary result observed in the present study is that the I–II loop of α1B, when inserted into α1E, produces a current phenotype with inactivation kinetics intermediate between α1B and α1E, again implicating this loop in determination of inactivation properties. Similar results also were found for the I–II loop of α1A inserted into α1E. The only alteration in transmembrane segment IS6 was V293→M, as described in Materials and Methods. Thus, from the present and previous result (Zhang et al., 1994) it is likely that the inactivation properties of the channel are determined both by the β-subunit and intrinsically by sites in IS6 and on the I–II loop, probably lying N terminal to the β-subunit interaction domain.
Footnotes
We gratefully acknowledge financial support from the Wellcome Trust. We thank the following for generous gifts of cDNAs and reagents: Dr. T. Snutch (University of British Columbia, Vancouver, Canada), α1A, α1E, and β1b; Dr. H. Chin (National Institutes of Health, Bethesda, Maryland), α2-δ; Dr. J. Marshall (Yale University, New Haven, CT), GFP; Dr. Y. Mori (Seiriken, Okazaki, Japan), α1B; Dr. S. Moss (University College London, London, UK), S65→T GFP; Genetics Institute (Cambridge, MA), pMT2. We also thank Ms. A. Odunlami, Mr. I. Tedder, and Mr. D. Bell for technical assistance and Dr. A. Mathie for reading this manuscript. This work benefited from the use of the Seqnet facility (Daresbury, UK).
Correspondence should be addressed to Dr. A. C. Dolphin at the above address.
REFERENCES
- 1.Berrow NS, Campbell V, Fitzgerald EG, Brickley K, Dolphin AC. Antisense depletion of β-subunits modulates the biophysical and pharmacological properties of neuronal calcium channels. J Physiol (Lond) 1995;482:481–491. doi: 10.1113/jphysiol.1995.sp020534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berrow NS, Brice NL, Tedder I, Page K, Dolphin AC (1997) Properties of cloned rat α1A calcium channels transiently expressed in the COS-7 cell line. Eur J Neurosci, in press. [DOI] [PubMed]
- 3.Boland LM, Bean BP. Modulation of N-type calcium channels in bullfrog sympathetic neurons by luteinizing hormone-releasing hormone: kinetics and voltage dependence. J Neurosci. 1993;13:516–533. doi: 10.1523/JNEUROSCI.13-02-00516.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourinet E, Soong TW, Stea A, Snutch TP. Determinants of the G-protein-dependent opioid modulation of neuronal calcium channels. Proc Natl Acad Sci USA. 1996;93:1486–1491. doi: 10.1073/pnas.93.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyer JL, Hepler JR, Harden TK. Hormone and growth factor receptor-mediated regulation of phospholipase C activity. Trends Pharmacol. 1989;10:360–364. doi: 10.1016/0165-6147(89)90008-4. [DOI] [PubMed] [Google Scholar]
- 6.Campbell V, Berrow N, Dolphin AC. GABAB receptor modulation of Ca2+ currents in rat sensory neurones by the G-protein Go: antisense oligonucleotide studies. J Physiol (Lond) 1993;470:1–11. doi: 10.1113/jphysiol.1993.sp019842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell V, Berrow N, Brickley K, Page K, Wade R, Dolphin AC. Voltage-dependent calcium channel β-subunits in combination with alpha-1 subunits have a GTPase activating effect to promote hydrolysis of GTP by Gαo in rat frontal cortex. FEBS Lett. 1995a;370:135–140. doi: 10.1016/0014-5793(95)00813-o. [DOI] [PubMed] [Google Scholar]
- 8.Campbell V, Berrow NS, Fitzgerald EM, Brickley K, Dolphin AC. Inhibition of the interaction of G-protein Go with calcium channels by the calcium channel β-subunit in rat neurones. J Physiol (Lond) 1995b;485:365–372. doi: 10.1113/jphysiol.1995.sp020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciranna L, Mouginot D, Feltz P, Schlichter R. Serotonin inhibits Ca2+ currents in porcine melanotrophs by activating 5-HT1C and 5-HT1A receptors. J Physiol (Lond) 1993;463:17–38. doi: 10.1113/jphysiol.1993.sp019582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Waard M, Pragnell M, Campbell KP. Ca2+ channel regulation by a conserved β-subunit domain. Neuron. 1994;13:495–503. doi: 10.1016/0896-6273(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 11.De Waard M, Witcher DR, Pragnell M, Liu H, Campbell KP. Properties of the α1-β anchoring site in voltage-dependent Ca2+ channels. J Biol Chem. 1995;270:12056–12064. doi: 10.1074/jbc.270.20.12056. [DOI] [PubMed] [Google Scholar]
- 12.Diversé-Pierluissi M, Goldsmith PK, Dunlap K. Transmitter-mediated inhibition of N-type calcium channels in sensory neurons involves multiple GTP-binding proteins and subunits. Neuron. 1995;14:191–200. doi: 10.1016/0896-6273(95)90254-6. [DOI] [PubMed] [Google Scholar]
- 13.Dolphin AC. Voltage-dependent calcium channels and their modulation by neurotransmitters and G-proteins: G. L. Brown prize lecture. Exp Physiol. 1995;80:1–36. doi: 10.1113/expphysiol.1995.sp003825. [DOI] [PubMed] [Google Scholar]
- 14.Dubel SJ, Starr TVB, Hell J, Ahlijanian MK, Enyeart JJ, Catterall WA, Snutch TP. Molecular cloning of the α-1 subunit of an ω-conotoxin-sensitive calcium channel. Proc Natl Acad Sci USA. 1992;89:5058–5062. doi: 10.1073/pnas.89.11.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellinor PT, Zhang J-F, Randall AD, Zhou M, Schwarz TL, Tsien RW, Horne WA. Functional expression of a rapidly inactivating neuronal calcium channel. Nature. 1993;363:455–458. doi: 10.1038/363455a0. [DOI] [PubMed] [Google Scholar]
- 16.Fujita Y, Mynlieff M, Dirksen RT, Kim M-S, Niidome T, Nakai J, Friedrich T, Iwabe N, Miyata T, Furuichi T, Furutama D, Mikoshiba K, Mori Y, Beam KG. Primary structure and functional expression of the ω-conotoxin-sensitive N-type calcium channel from rabbit brain. Neuron. 1993;10:585–598. doi: 10.1016/0896-6273(93)90162-k. [DOI] [PubMed] [Google Scholar]
- 17.Grassi F, Lux HD. Voltage-dependent GABA-induced modulation of calcium currents in chick sensory neurons. Neurosci Lett. 1989;105:113–119. doi: 10.1016/0304-3940(89)90021-9. [DOI] [PubMed] [Google Scholar]
- 18.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein βγ-subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 19.Hille B. G-protein-coupled mechanisms and nervous signaling. Neuron. 1992;9:187–195. doi: 10.1016/0896-6273(92)90158-a. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda SR. Double-pulse calcium channel current facilitation in adult rat sympathetic neurones. J Physiol (Lond) 1991;439:181–214. doi: 10.1113/jphysiol.1991.sp018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein βγ-subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 22.Kim H-L, Kim H, Lee P, King RG, Chin H. Rat brain expresses an alternatively spliced form of the dihydropyridine-sensitive L-type calcium channel α2 subunit. Proc Natl Acad Sci USA. 1992;89:3251–3255. doi: 10.1073/pnas.89.8.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleuss C, Hescheler J, Ewel C, Rosenthal W, Schultz G, Wittig B. Assignment of G-protein subtypes to specific receptors inducing inhibition of calcium currents. Nature. 1991;353:43–48. doi: 10.1038/353043a0. [DOI] [PubMed] [Google Scholar]
- 24.Lory P, Varadi G, Slish DF, Varadi M, Schwartz A. Characterization of β-subunit modulation of a rabbit cardiac L-type Ca2+ channel α1 subunit as expressed in mouse L cells. FEBS Lett. 1993;315:167–172. doi: 10.1016/0014-5793(93)81156-t. [DOI] [PubMed] [Google Scholar]
- 25.Luebke JI, Dunlap K. Sensory neuron N-type calcium currents are inhibited by both voltage-dependent and -independent mechanisms. Pflügers Arch. 1994;428:499–507. doi: 10.1007/BF00374571. [DOI] [PubMed] [Google Scholar]
- 26.McFadzean I, Mullaney I, Brown DA, Milligan G. Antibodies to the GTP binding protein, Go, antagonize noradrenaline-induced calcium current inhibition in NG108-15 hybrid cells. Neuron. 1989;3:177–182. doi: 10.1016/0896-6273(89)90030-5. [DOI] [PubMed] [Google Scholar]
- 27.Menon-Johansson AS, Berrow N, Dolphin AC. Go transduces GABAB-receptor modulation of N-type calcium channels in cultured dorsal root ganglion neurons. Pflügers Arch. 1993;425:335–343. doi: 10.1007/BF00374184. [DOI] [PubMed] [Google Scholar]
- 28.Mintz IM, Bean BP. GABAB receptor inhibition of P-type Ca2+ channels in central neurons. Neuron. 1993;10:889–898. doi: 10.1016/0896-6273(93)90204-5. [DOI] [PubMed] [Google Scholar]
- 29.Neely A, Wei X, Olcese R, Birnbaumer L, Stefani E. Potentiation by the β-subunit of the ratio of the ionic current to the charge movement in the cardiac calcium channel. Science. 1993;262:575–578. doi: 10.1126/science.8211185. [DOI] [PubMed] [Google Scholar]
- 30.Olcese R, Qin N, Schneider T, Neely A, Wei X, Stefani E, Birnbaumer L. The amino terminus of a calcium channel β-subunit sets rates of channel inactivation independently of the subunit’s effect on activation. Neuron. 1994;13:1433–1438. doi: 10.1016/0896-6273(94)90428-6. [DOI] [PubMed] [Google Scholar]
- 31.Pragnell M, De Waard M, Mori Y, Tanabe T, Snutch TP, Campbell KP. Calcium channel β-subunit binds to a conserved motif in the I–II cytoplasmic linker of the α1-subunit. Nature. 1994;368:67–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- 32.Sather WA, Tanabe T, Zhang J-F, Mori Y, Adams ME, Tsien RW. Distinctive biophysical and pharmacological properties of class A (BI) calcium channel α1 subunits. Neuron. 1993;11:291–303. doi: 10.1016/0896-6273(93)90185-t. [DOI] [PubMed] [Google Scholar]
- 33.Schneider T, Wei X, Olcese R, Costantin JL, Neely A, Palade P, Perez-Reyes E, Qin N, Zhou J, Crawford GD, Smith RG, Appel SH, Stefani E, Birnbaumer L. Molecular analysis and functional expression of the human type E neuronal Ca2+ channel α1 subunit. Receptors Channels. 1994;2:255–270. [PubMed] [Google Scholar]
- 34.Snutch TP, Leonard JP, Gilbert MM, Lester HA, Davidson N. Rat brain expresses a heterogeneous family of calcium channels. Proc Natl Acad Sci USA. 1990;87:3391–3395. doi: 10.1073/pnas.87.9.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soong TW, Stea A, Hodson CD, Dubel SJ, Vincent SR, Snutch TP. Structure and functional expression of a member of the low voltage-activated calcium channel family. Science. 1993;260:1133–1136. doi: 10.1126/science.8388125. [DOI] [PubMed] [Google Scholar]
- 36.Starr TVB, Prystay W, Snutch TP. Primary structure of a calcium channel that is highly expressed in the rat cerebellum. Proc Natl Acad Sci USA. 1991;88:5621–5625. doi: 10.1073/pnas.88.13.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stea A, Dubel SJ, Pragnell M, Leonard JP, Campbell KP, Snutch TP. A β-subunit normalizes the electrophysiological properties of a cloned N-type Ca2+ channel α1-subunit. Neuropharmacology. 1993;32:1103–1116. doi: 10.1016/0028-3908(93)90005-n. [DOI] [PubMed] [Google Scholar]
- 38.Stea A, Tomlinson WJ, Soong TW, Bourinet E, Dubel SJ, Vincent SR, Snutch TP. Localization and functional properties of a rat brain α1A calcium channel reflect similarities to neuronal Q- and P-type channels. Proc Natl Acad Sci USA. 1994;91:10576–10580. doi: 10.1073/pnas.91.22.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stephens GJ, Page K, Burley JR, Berrow NS, Dolphin AC. Functional expression of rat brain cloned α1E calcium channels in COS-7 cells. Pflügers Arch. 1997;433:523–532. doi: 10.1007/s004240050308. [DOI] [PubMed] [Google Scholar]
- 40.Tanabe T, Takeshima H, Mikami A, Flockerzi V, Takahashi H, Kangawa K, Kojima M, Matsuo H, Hirose T, Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987;328:313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- 41.Tomlinson WJ, Stea A, Bourinet E, Charnet P, Nargeot J, Snutch TP. Functional properties of a neuronal class C L-type calcium channel. Neuropharmacology. 1993;32:1117–1126. doi: 10.1016/0028-3908(93)90006-o. [DOI] [PubMed] [Google Scholar]
- 42.Toth PT, Shekter LR, Ma GH, Philipson LH, Miller RJ. Selective G-protein regulation of neuronal calcium channels. J Neurosci. 1996;16:4617–4624. doi: 10.1523/JNEUROSCI.16-15-04617.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsunoo A, Yoshii M, Narahashi T. Block of calcium channels by enkephalin and somatostatin in neuroblastoma-glioma hybrid NG108-15 cells. Proc Natl Acad Sci USA. 1986;83:9832–9836. doi: 10.1073/pnas.83.24.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang HY, Pisano MR, Friedman E. Age-related alteration in G-protein function in rat cortex. Ann NY Acad Sci. 1992;663:426–428. doi: 10.1111/j.1749-6632.1992.tb38689.x. [DOI] [PubMed] [Google Scholar]
- 45.Witcher DR, De Waard M, Liu H, Pragnell M, Campbell KP. Association of native Ca2+ channel β-subunits with the α1 subunit interaction domain. J Biol Chem. 1995;270:18088–18093. doi: 10.1074/jbc.270.30.18088. [DOI] [PubMed] [Google Scholar]
- 46.Yassin M, Zong SQ, Tanabe T. G-protein modulation of neuronal class E (α1E) calcium channel expressed in GH3 cells. Biochem Biophys Res Commun. 1996;220:453–458. doi: 10.1006/bbrc.1996.0426. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J-F, Randall AD, Ellinor PT, Horne WA, Sather WA, Tanabe T, Schwarz TL, Tsien RW. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993;32:1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J-F, Ellinor PT, Aldrich RW, Tsien RW. Molecular determinants of voltage-dependent inactivation in calcium channels. Nature. 1994;372:97–100. doi: 10.1038/372097a0. [DOI] [PubMed] [Google Scholar]